Abstract
The coronavirus disease of 2019 (COVID-19) has caused significant morbidity and mortality among infected individuals across the world. High transmissibility rate of the causative virus – Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) – has led to immense strain and bottlenecking of the health care system. While noteworthy advances in vaccine development have been made amid the current global pandemic, most therapeutic agents are repurposed from use in other viral infections and are being evaluated for efficacy in COVID-19. Favipiravir, an orally administered drug originally developed in Japan against emerging influenza viral strains, has been shown to have widespread application and safety across multiple ribonucleic acid (RNA) viral infections. With a strong affinity toward the viral RNA-dependent RNA polymerase (RdRp), favipiravir could be a promising therapy against SARS-CoV-2, by targeting downstream viral RNA replication. Initial trials for usage in COVID-19 have suggested that favipiravir administration during initial infection stages, in individuals with mild to moderate infection, has a strong potential to improve clinical outcomes. However, additional well-designed clinical trials are required to closely examine ideal timing of drug administration, dosage, and duration, to assess the role of favipiravir in COVID-19 therapy. This review provides evidence-based insights and throws light on the current clinical trials examining the efficacy of favipiravir in tackling COVID-19, including its mechanism, pharmacodynamics, and pharmacokinetics.
Keywords: antiviral, COVID-19, favipiravir, influenza, SARS-CoV-2
Introduction
SARS-CoV-2 and its anatomy
The coronavirus disease of 2019 (COVID-19) has infected over 242 million people, leading to over 4.9 million deaths worldwide, at the time of this publication. 1 It is caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2),2,3 closely related to Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and Middle East Respiratory Syndrome Coronavirus (MERS-CoV).3,4 SARS-CoV-2 is a single-stranded positive sense ribonucleic acid (RNA) virus, 50–200 nm in diameter, consists of four structural proteins (about 33% of viral genome) – Spike (S), Envelope (E), Membrane (M), and Nucleocapsid (N). 5 The S protein, comprising of S1 and S2 subunits, mediates viral entry into the host cell membrane via the human cell angiotensin converting enzyme 2 (ACE2) receptor. 5 The remaining 67% of the viral genome encodes polyproteins and precursors that further cleave into 16 non-structural proteins (nsp), like the RNA-dependent RNA polymerase (RdRp), critical for viral life cycle – replication and host immune system regulation. 6 RdRp complex like nsp-12, with assistance of other cofactor proteins (like nsp7-nsp-8 heterodimer), handles the viral replication and transcription process of the virus.6,7 Interestingly, across RNA viral classes, various sequence motifs and tertiary structures of RdRps are conserved.4,7–11 These conserved RdRp motifs are essential for its catalytic function, hence useful as potential drug targets.4,12
COVID-19 pathogenesis and symptoms
COVID-19 is a multisystemic viral disease and commonly affects the respiratory tract. 13 SARS-CoV-2 is transmitted mainly through droplets and exhibits pulmonary tropism, which is attributable to ACE2 receptors being widely distributed across lung epithelial cells. 14 Clinical manifestations in human hosts can be wide ranging from asymptomatic to symptomatic disease with potential for sudden symptom evolution.12,14 Symptoms of mild disease may include fever, myalgia, cough, fatigue, anosmia, dysgeusia, and shortness of breath while moderate-severe or critical illness may lead to prolonged hospitalization, cytokine storm, viral pneumonia, secondary bacterial or fungal pneumonia, arrhythmias, acute kidney injury, neurological illness, and so on. 12 The COVID-19 response in some individuals can lead to a complex immunological cascade leading to a cytokine storm with damage to multiple organs.13,15 Symptomatic disease could eventually lead to post-acute COVID-19 syndrome.16,17 The exact duration for which these symptoms persist after resolution of acute disease remains to be fully understood.
COVID-19 management
While COVID-19 prevention is largely associated with targeting viral spike and structural proteins by highly efficacious vaccination – generating robust humoral antibody response – hospitalization-associated patient management and therapy is critically symptom associated. Identification of clinical disease staging – clinical symptoms combined with laboratory and radiologic assessment – are critical for risk stratification, triage, and early therapeutic intervention of patients who progress to severe disease, requiring hospitalization.13,18 In hospitalized patients, mechanical ventilation, oxygen therapy, and symptomatic treatment have been the mainstay of therapy. 19 While there is no clinically effective therapy for COVID-19 in singular, several therapeutic agents such as corticosteroids, antivirals, monoclonal antibodies, and convalescent plasma are currently being administered and evaluated, many a times, in conjunction. Antivirals have a special role to play, repurposed through their role in other viral infections, preventing viral entry into the host cells and causing suppression of viral replication at various steps. 20 Some key antivirals of interest are listed in Table 1.
Table 1.
Repurposed antivirals being evaluated for therapeutic activity in COVID-19.
| Drug | Mechanism | Primary application |
|---|---|---|
| Remdesivir | Targets viral proteases and RdRp | Hepatitis C, Respiratory Syncytial Virus (RSV), Human Immunodeficiency Virus (HIV), Ebola and Marburg viral disease 21 |
| Favipiravir | Inhibits RdRp (purine nucleoside analog) | Multiple RNA viral diseases – Influenza, Ebola, Yellow fever, etc. 22 |
| Lopinavir and ritonavir | Inhibits viral proteases | HIV, SARS, MERS 23 |
| Molnupiravir | Competitive substrate of viral RdRp | Influenza, Chikungunya virus, Respiratory syncytial virus, Ebola, SARS, MERS 24 |
| Ribavirin | Acts on viral RdRp in replicating viral genome (guanine analog) | RSV, Hepatitis C, SARS, MERS 25 |
| Umifenovir | Inhibits fusion of viral S protein to ACE2 | Influenza, SARS 26 |
ACE2, angiotensin converting enzyme 2; HIV, Human Immunodeficiency Virus; MERS, Middle East Respiratory Syndrome; RNA, ribonucleic acid; RSV, Respiratory Syncytial Virus; SARS, Severe Acute Respiratory Syndrome.
Several clinical trials recruiting patients across the disease stages are trying to identify the most efficacious drug(s) for efficacious COVID-19 therapy, and retroviral therapies have been of most interest.21,23 Remdesivir has been used under emergency use authorization in several countries, demonstrating some efficacy in shortening recovery time in COVID-19 patients. 27 The combination of lopinavir with ritonavir showed some favorable outcome, but a clinical trial was discontinued due to side effects. 19 Ribavirin, a guanine analog targeting viral RdRp was found to be ineffective in improving COVID-19 recovery outcomes, although in combination triple therapy with lopinavir and ritonavir, with interferon beta-1b, some improvement in efficacy was observed to decrease recovery in hospitalized patients with mild to moderate COVID-19. 25 In addition, singular therapy with umifenovir (arbidol) did not improve patient outcomes. 26 Favipiravir, similar in action to remdesivir, inhibits transcription and replication of multiple RNA viruses by targeting RdRp. 28
Favipiravir
Drug development and pharmacodynamics
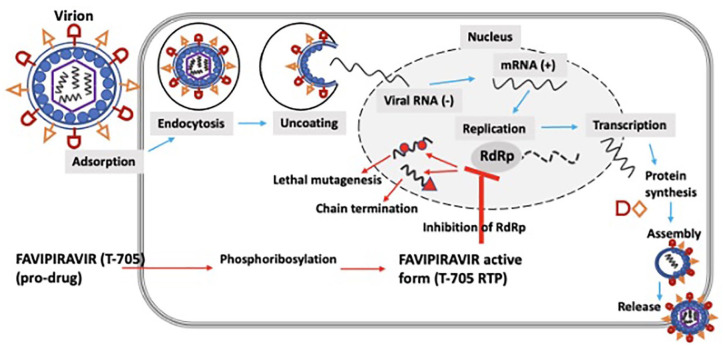
In the process of viral replication, RdRp is a critical element, conserved across RNA viruses but absent in human cells, making it a promising antiviral target. 12 Favipiravir was developed by Toyama Chemical Company Limited and was first approved for clinical use in Japan in 2014, for novel and re-emerging influenza virus infections. It was discovered through a library screen of chemical candidates utilizing a plaque reduction assay.28,29 Understanding efficacy of favipiravir in COVID-19 is of particularly high interest,30,31 as it has been shown to have effective in vitro and in vivo antiviral activity against RNA viruses of multiple families – orthomyxoviridae, bunyaviridae, arenaviridae, filoviridae, rhabdoviridae, paramyxoviridae, flaviviridae, togaviridae, picornaviridae, and caliciviridae.22,32,33 Favipiravir is a pyrazine carboxamide derivative and is a prodrug; its active metabolite, favipiravir ribofuranosyl 5′ triphosphate (T-705 RTP), is formed by phosphoribosylation and is a nucleoside analog causing disruption of viral replication.29,33–35 It was observed that neither favipiravir nor favipiravir-ribofuranosyl-5′-monophosphate inhibited RdRp activity, instead the triphosphate metabolism of favipiravir to T-705 RTP lead to active influenza viral inhibition, through concentration dose-dependent studies. 28 Plaque reduction with time of addition assays were utilized to define the mechanism of action by exposing influenza infected cells to favipiravir. It was observed that plaque inhibitory activity was observed during the replication phase and not adsorption or release phase. 28 While competitive assays were used to investigate the mechanism of inhibition, 28 it was observed that the antiviral activity of favipiravir was significantly decreased in presence of natural purine nucleoside and not pyrimidine bases, thus indicating its mechanism of action as a pseudo purine, through RdRp.28,36 Although the precise interaction is not known, it is hypothesized that this active form either binds to the catalytic format of RdRp or gets misincorporated to the nascent growing viral RNA, leading to chain termination by ambiguous base-pairing, in turn causing abrupt break in viral replication36,37 (Figure 1). Sequencing of nucleoprotein clones revealed a higher mutation frequency and associated shift in the nucleotide profiles of the nucleoprotein gene. In addition, no viable favipiravir-resistant mutants were observed, suggesting a ‘virucidal’ effect.28,34 The wide-spectrum activity of favipiravir may be associated with this misincorporation effect. The lethal mutagenesis and subsequent chain termination mechanism has been suggested against multiple RNA viral infections as well.34,35,38,39 One study indicated that binding of the T-705 RTP to RdRp may not lead to sufficient inhibition of norovirus activity. 40 Although, it may be possible that variable concentration and availability of the drug may be key in impacting the mechanism of action. 37 In addition, as this mechanism of action is vastly different to some other antivirals that inhibit cellular RNA or DNA synthesis – as with ribavirin and the neuraminidase inhibitors – favipiravir, instead, specifically acting on viral RdRp may be responsible for observed lower toxicity while being target sensitive and efficacious across multiple classes of RNA viruses. 36 At 50% inhibitory concentration, favipiravir was about 2650 times more selective for influenza RdRp, while lacking inhibition of human RNA or DNA synthesis.36,41
Figure 1.
Schematic showing the mechanisms of action associated with favipiravir.
Pharmacokinetics and safety
Favipiravir is metabolized mainly by aldehyde oxidase (AO) and partly metabolized to a hydroxylated form by xanthine oxidase (XO). A glucuronate conjugate was observed in human plasma and urine as a metabolite other than the hydroxylated form. It is predominantly excreted into the urine as a hydroxylated form, with minimal unchanged drug. The pharmacokinetic profile of favipiravir has been studied through dose-escalated studies, with oral dosage for adults at 1600 mg twice daily on day 1 (loading dose), followed by 600 mg twice daily for 4 days, with the total duration of therapy being 5 days. 29 The maximum plasma concentration was achieved within 2 h after administration of a single dose, although plasma concentration quickly decreased, with half-life between 2 and 6 h.29,42 Interestingly, administration of multiple doses led to an increase in the time to maximum plasma concentration and half-life. 42 Favipiravir is associated with dose- and time-dependent pharmacokinetics, possibly explainable by saturation of the main enzymatic pathway as it was shown to inhibit AO in vitro.29,42 In Ebola-infected patients, the favipiravir plasma concentration surprisingly decreased between treatments, potentially due to severe disease conditions or intrinsic properties of favipiravir metabolism. 43 In variable disease conditions, favipiravir bioavailability and the hepatic first pass can be altered, through an increase in the activity of the AO with temperature.32,43
The literature suggests that favipiravir is a well-tolerated drug. 32 The most frequently reported adverse effects of favipiravir when used for influenza treatment, are mild to moderate diarrhea, asymptomatic increase of blood uric acid and transaminases, and a decrease in the neutrophil count. 29 In a study evaluating the inhibition properties of favipiravir against human and mouse norovirus RNA polymerases, it was found that favipiravir ribonucleoside triphosphate was recognized as a substrate of the human mitochondrial RNA polymerase, but did not result in inhibition of the DNA-dependent RNA polymerase nor cause mitochondrial toxicity in cells.32,40 Despite good tolerance observed, a major concern to safety of clinical favipiravir use is related to its teratogenic tendencies. In studies with fetal/embryo models of multiple animal species, teratogenicity was observed across all. 44 The exposure dosage causing teratogenicity was comparable with the proposed human use dosage. Therefore, it is strongly recommended that favipiravir not be administered to pregnant or possibly pregnant individuals.29,44 Due to this high risk of teratogenicity and embryotoxicity, favipiravir is conditionally marketed with strict regulation in Japan. 32 Hence the clinical use of favipiravir is restricted to novel or re-emerging influenza viruses, only when that virus is resistant to other influenza antivirals, while being manufactured and distributed only upon request by the Minister of Health, Labor and Welfare in Japan.29,32
Use in influenza
Early literature on favipiravir dates to 2001. Also known as T-705, favipiravir was tested in vitro for its antiviral properties against Influenza A, B, and C, against all of which it was found effective. It was also reported to have activity against oseltamivir and amantadine-resistant Influenza A. The Influenza A viral strains originally studied included H1N1, H2N2, and H3N2. 45 Selective viral RNA polymerase inhibition without inhibition of cellular RNA or DNA synthesis was reported as its proposed mechanism of action. 36 Takahashi et al. 46 reported that T-705 retained its efficacy against influenza-infected mice when compared with oseltamivir despite a delay of treatment initiation of up to 25-h post infection. Sidwell et al. 47 reported activity of T-705 against mice infected with avian H5N1 influenza. Antiviral drug regimens studied in mice yielded statistically significant protection against death at doses of or higher than 30 mg/kg/day given twice a day for 5 days. The effect of delaying therapy by up to 72 h after infection was also studied and Furuta et al. reported that T-705 retained its antiviral property in protecting mice against death at doses of 300 mg/kg/day given every 6 h for 5 days.41,48 Favipiravir resistance was first reported by Goldhill et al. 49 who stated that a K229R mutation in RdRp would confer drug resistance to favipiravir among pandemic 2009 H1N1 Influenza A viral strains. In one study, the combination of favipiravir with oseltamivir showed synergy at low doses in animal models infected with H1N1, H3N2, and H5N1 Influenza A viral strains. 50 Wang et al. 51 found accelerated clinical recovery among patients with severe Influenza on favipiravir and oseltamivir combination therapy compared with oseltamivir alone.
The approved favipiravir drug regimen in Japan for the treatment of Influenza is 1600 mg (loading doses) on day 1 followed by 600 mg twice a day, with the total duration of therapy being 5 days.29,52 Other experimental clinical studies in influenza53–56 used a regimen of 1800 mg (loading doses) followed by 800 mg twice a day, for a total duration of therapy of 5 days. Evidence from clinical trials assessing over 2000 patients suggests that favipiravir is well-tolerated.51,53–56 Adverse drug reactions reported include elevation in serum uric acid levels, diarrhea, elevation of liver enzymes, and reduction in neutrophil count. 52
Use in other viral diseases
Viral hemorrhagic fevers
The causative agents of viral hemorrhagic fevers (VHFs) belong to Arenaviridae, Bunyaviridae, Filoviridae, and Flaviviridae families of viruses, all of which are RNA viruses. Prominent VHFs caused by these viruses include Ebola viral disease and Yellow Fever among others. Favipiravir was trialed in rural Guinea during the Ebola virus disease (EVD) outbreak in 2014 as a multicenter proof-of-concept non-comparative trial. Nuanced conclusions were drawn about the efficacy of favipiravir as the trial was nonrandomized and all study participants received the drug given the public health crisis at the time. The authors reported that although favipiravir monotherapy may not be effective in patients with very high viremia, its clinical utility in intermediate to high viremia would merit investigation. 57 Another retrospective observational study on outcomes of patients treated with compassionate use favipiravir versus untreated patients showed a lower-case fatality ratio among those treated with favipiravir thereby conferring survival benefit. 58 Translational research on the efficacy of favipiravir against EVD done in macaques showed survival benefit among the infected animals that received ⩾ 150 mg/kg of favipiravir. The authors observed inhibition of viral replication inhibition in a concentration-dependent manner and concluded that favipiravir plasma trough concentrations > 70–80 μg/ml were linked to lower viral loads and improved survival. 59
Favipiravir has been tested in vitro against other etiological agents of VHFs including Bunyaviridae and Arenaviridae. Gowen et al. 60 studied the in vitro inhibitory effects of favipiravir (T-705) and ribavirin in monkey kidney Vero 76 cells against La Crosse virus, Punta Toro virus, sandfly fever virus, and rift valley fever virus (Bunyaviruses) and found that the drug inhibited the growth of the tested viral strains and was less toxic than ribavirin. The same authors examined the in vitro activity of favipiravir (T-705) against Junin virus, Pichinde virus, and Tacaribe virus (Arenaviruses) and found that the drug was highly active against the tested viral strains in cell cultures. The drug also afforded protection to hamsters infected with Pichinde virus and mice infected with Punta Toro virus.
Yellow fever is a VHF caused by the yellow fever virus (YFV), a flavivirus. The YFV distribution runs through tropical and subtropical Africa and South America. Clinical disease could be asymptomatic or may manifest with fever, chills, headache, myalgia, jaundice, bleeding, shock, and organ failure. The mortality rate of those with severe symptomatic disease can be > 30%. Julander et al. found T-705 to be moderately effective against YFV in cell culture. It was also found to be an effective treatment in YFV infected hamsters conferring survival benefit among the infected animals treated with T-705. 61
West Nile Virus
West Nile Virus (WNV) is a vector-borne (mosquito) disease caused by the West Nile Virus, a flavivirus. The spectrum of disease can vary from asymptomatic infection to mild febrile illness to severe viral meningitis or encephalitis. To date, there is no approved antiviral for the treatment of WNV. Morrey et al. studied the efficacy of orally administered favipiravir (T-705) among rodents infected with WNV and found the drug to be active against WNV. The drug’s lack of activity past the second day on viral infection was attributed to either the lack of a standard drug regimen or insufficient bioavailability in the brain or lack of neuronal metabolism to convert favipiravir to T-705 RTP. 62
Nipah virus
Nipah virus is an emerging zoonotic infection caused by the Nipah virus which belongs to the Paramyxoviridae family of viruses. Outbreaks have been reported out of Malaysia, Bangladesh, and India periodically. Fruit bats are its natural reservoir, and the virus can be transmitted to humans either from bats or via an intermediate host such as pigs or via human-to-human transmission. Clinically, the disease manifests as viral encephalitis with features of respiratory compromise. To date, there is no approved treatment or vaccine for Nipah virus. The disease has a high mortality rate, ranging upward of 40%. 63 Favipiravir has shown in vitro activity against vero cells infected with Nipah virus and prevented viral replication. 64 Dawes et al. 64 also found benefit of favipiravir administration among hamsters infected with Nipah virus and reported that treated hamsters infected with the virus did not develop any signs of clinical disease.
Respiratory viral diseases (other than influenza and COVID-19)
Human metapneumovirus (hMPV) and RSV, previously classified under Paramyxoviridae, belong to the family Pneumoviridae and can cause upper or lower respiratory tract disease in humans. Parainfluenza virus can cause similar disease and belongs to the Paramyxoviridae family of viruses. Favipiravir (T-705) demonstrated activity against all these viruses in vitro in a study done by Jochmans et al. The authors also reported in vivo drug activity in hamsters infected with hMPV. 65
Use in COVID-19
Early genomic sequencing of SARS-CoV-2 virion suggested over 90% homology, across several essential enzymes, with other coronaviruses, particularly those causing SARS and MERS. 66 In a quest for identifying effective therapy amid a catastrophic global pandemic, this knowledge helped trigger the ‘repurposing’ of multiple antiviral drugs including broad-spectrum antivirals and more specifically, drugs previously directed toward therapy of SARS, MERS, and other viral infections. The mechanism of action of favipiravir against SARS-CoV-2 is similar to that previously described in literature. After viral incorporation of T-705 RTP by RdRp, the nascent coronaviral RNA undergoes slowed RNA synthesis, chain termination, and viral mutagenesis, while evading the viral RNA repair mechanism. The SARS-CoV-2 RdRp has been described to have 10-fold higher activity than other viral counterparts, while simultaneously being prone to high nucleotide incorporation and error rates. 67 These processes significantly bottleneck the RNA replication by causing nucleotide shifts in an unbalanced viral environment with already low cytosine levels. 67 These critical hallmarks of the SARS-CoV-2 virion machinery make it vulnerable to RdRp inhibitors.
Alongside an effective vaccine, antivirals have a critical role in containing local spread of infection by curtailing viral load and shedding, at an early infection stage. 68 Studies suggest viral load in the upper and lower respiratory tracts in patients with mild infection peak in the early stage of infection, days 4 and 6, respectively, while in moderate-severely infected patients they peak in later stages, days 8 and 11. 69 Thus, the role of antivirals like favipiravir may be higher in mild-moderate infections at an early stage of the disease, to control the viral load, shedding, and infectivity rates, 70 to help reduce the burden of hospitalization and associated patient care on an already overwhelmed system.
An early study (February 2020) showed that favipiravir at higher concentrations (EC50 = 61.88 μM) was able to minimize viral infection in vero E6 cells in vitro. 71 While these in vitro studies suggested a need for further evaluation, the efficacy of favipiravir in patients infected with COVID-19 was initially assessed through two separate clinical trials in China (February 2020). 72 The inclusion criteria for both the trials included patients within the early stage of disease, from manifestation of symptoms and positive test result with reverse transcription polymerase chain reaction (RT-PCR), and patients with severe disease were excluded. In one of the trials, patients with COVID-19 were treated with a combination of favipiravir and alpha interferon (n = 30), while the other treatment arm was treated with lopinavir and ritonavir combination with alpha interferon. 72 The second clinical trial assessed the efficacy of favipiravir against therapy with baloxavir marboxil. 72 In addition, after initial approval of favipiravir for treatment of COVID-19 in China (February 2020), another study with 80 patients showed it had promise as an antiviral therapeutic, with lower side effects when compared with the lopinavir/ritonavir treatment arm. 73 While these initial studies showed some favorable results, there were safety concerns for use due to side effects like teratogenicity, prolongation of QTc interval and hyperuricemia as evidenced in use for other pathologies. 74 Interestingly, in a randomized controlled trial conducted on patients to receive either favipiravir or arbidol, with a primary outcome for recovery on day 7, favipiravir did improve latency to relief of cough and fever but did not improve clinical recovery rate, while the most frequently observed side effect was hyperuricemia. 75
Subsequently, other studies have been conducted to understand the efficacy of favipiravir in COVID-19. Studies have suggested that favipiravir could lead to viral clearance by day 7 and improve clinical outcome by 2 weeks when administered in early stages of infection.76,77 In one study, the median time to viral clearance was about 12.8 days in patients when favipiravir therapy was initiated on day 1, and increased to 17.8 days when therapy was started on day 6. 78 In addition, there were significant differences in duration of hospitalization, with need for oxygenation and mechanical ventilation, in patients with mild or asymptomatic infection when favipiravir therapy initiated late stage versus early. Thus, it may be important to initiate favipiravir therapy at an early stage of disease in asymptomatic or mildly infected COVID-19 patients, to achieve reduction in viral load and disease progression.76,77 Importantly, dosing regimens could significantly change the clinical outcome of the patient, lower doses indicating to have lower association with better outcomes. 76 Comparison of multiple trials suggests that favipiravir may not have a significant beneficial effect on improving patient mortality rate in subjects with mild to moderate COVID-19, and this may be associated with the timeline of therapy initiation. 77 However, it is necessary to assess the favipiravir therapy initiation timeline, dosage, duration, and safety through trials with larger sample size.
The use of favipiravir on a compassionate or approved basis for the indication of COVID-19 treatment has been put through clinical trials across the globe. Table 2 summarizes key published clinical trials (including from preprint servers) to date. The drug was trialed in cases of asymptomatic and mild-moderate disease as well as in moderate-severe cases of COVID-19 pneumonia. While most trialed a regimen of 1600 mg loading dose (two doses) on day 1 followed by 600 mg twice daily, other regimens used 1800 mg loading dose (two doses) followed by 800 mg twice a day. The length of drug therapy with favipiravir was varied and ranged from 7 to 14 days. At the present time, favipiravir is not approved for use in COVID-19 by the United States Food and Drug Administration or its European counterpart, the European Medicines Agency. The formally recommended dose and duration of therapy remain awaited based on results of the ongoing clinical trials. However, it is noteworthy that 600 mg twice daily maintenance dose has been used to manage COVID-19 in some international markets. 79 Doi et al. studied the effect of early versus late favipiravir use among adolescents and adults with asymptomatic or mild COVID-19 and noted that viral clearance by day 6, which was their primary endpoint, was similar between the two groups. Secondary and exploratory endpoints, that is, viral load reduction by day 6 and time to defervescence were met in the early treatment arm. 80 Another group of researchers who studied the drug’s efficacy versus supportive care alone, among patients with mild to moderate COVID-19, found shorter time to cessation of viral shedding with favipiravir but this was not statistically significant. The median time to clinical cure among symptomatic patients at baseline was reportedly significantly faster with favipiravir. 81 Ivashchenko et al. observed statistically significant differences between favipiravir treatment groups compared with standard of care at the time (hydroxychloroquine or chloroquine or lopinavir/ritonavir); in terms of viral PCR clearance, Cai et al. suggested favorable radiographic outcomes in the favipiravir treatment arm compared with control (lopinaivr/ritonavir) in addition to shorter median time for viral clearance.82,83 The optimal dosages and duration of drug therapy with favipiravir for COVID-19 remain indefinite. Further clinical trials with specific aims to guide these aspects of treatment are warranted.
Table 2.
Summary of published (including preprint) literature from favipiravir clinical trials to date.
| Authors | Study period | Geographic region of study origin | Trial design | Patient population | Intervention | Favipiravir dose(s) | Duration of therapy | Primary outcome | Secondary outcome(s) | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al. 75 | February–March 2020 | China | Prospective, randomized, controlled, open-label multicenter | Adults with COVID-19 pneumonia | Conventional therapy + umifenovir versus conventional therapy + favipiravir | 1600/600 | 10 days | Clinical recovery on day 7 | Latency to relief for fever and cough, the rate of auxiliary oxygen therapy or noninvasive mechanical ventilation | Favipiravir did not significantly improve the clinically recovery rate at day 7 compared with umifenovir. Significantly improved latency to relief for fever and cough |
| Lou et al. 78 | February 2020 | China | Exploratory, single-center, open-label, randomized, controlled, 1:1:1 | Hospitalized adults with COVID-19 | Baloxavir, favipiravir, control (lopinavir/ritonavir, umifenovir, darunavir/cobicistat) | 1600 or 2200/600 | 14 days | PCR negativity by day 14 | Percentage of subjects with negative PCR by day 7, the incidence of mechanical ventilation by day 14, ICU admission by day 14, and all-cause mortality by day 14 | Adding either baloxavir marboxil or favipiravir to the current standard treatment did not provide additional benefits |
| Doi et al. 80 | March–May 2020 | Japan | Prospective, randomized, open-label | Adolescents and adults with asymptomatic or mild COVID-19 | Early versus late favipiravir (day 1 versus day 6) | 1800/800 | 10 days | Viral clearance by day 6 | Change in viral load by day 6 | Viral clearance by day 6 was not significantly different, associated with numerical reduction in time to defervescence |
| Udwadia et al. 81 | May–July 2020 | India | Randomized, open-label, parallel arm, multicenter, phase 3 | Pts with mild-moderate COVID-19 | Favipiravir plus supportive care versus supportive care alone | 1800/800 | 14 days | Time to cessation of viral shedding (OP and NP swabs) | Time to clinical cure | Shorter time to cessation of viral shedding with favipiravir but not statistically significant. Median time to clinical cure among pts symptomatic at baseline was significantly faster with favipiravir |
| Ivashchenko et al. 82 | April–May 2020 | Russia | Adaptive, multicenter, open-label, randomized, Phase II/III | Hospitalized pts with moderate COVID-19 pneumonia | Favipiravir versus standard of care (HCQ/CQ & lopinavir/ritonavir) | 1600/600, 1800/800 | 14 days | Elimination of SARS-CoV-2 by day 10 | – | Proportion of pts who achieved negative PCR on day 5 on both dosing regimens of favipiravir was twice as high as in the control group (p < 0.05) |
| Zhao et al. 84 | February–March 2020 | China | Multicenter, randomized 3:1:1 | Adults with COVID-19 and increased IL-6 | Favipiravir + Tocilizumab, favipiravir, tocilizumab | 1600/600 | 7 days | Cumulative lung lesion remission rate | Improvement of clinical symptoms, changes in results of routine blood tests and IL-6, changes of oxygen therapy | Cumulative lung lesion remission rate at day 14 was significantly higher in combination group as compared with favipiravir group (p = 0.019), significant difference between tocilizumab and favipiravir (p = 0.034) |
| Khamis et al. 85 | June–Aug 2020 | Muscat, Oman | Open-label, randomized controlled | Adults (18–75 years) hospitalized with moderate to severe COVID-19 pneumonia | Combination of favipiravir with inhaled interferon beta-1b versus hydroxychloroquine | 1600/600 | 10 days | Time to clinical recovery, normalization of inflammatory markers and improvement in oxygen saturation | Deterioration of pneumonia, ICU admission rate and mortality within 14 days | No differences in overall length of hospital stay, ICU transfers, discharges, oxygen saturation at discharge, overall mortality and changes in the inflammatory markers at discharge |
| Solaymani-Dodaran et al. 86 | April–August 2020 | Iran | Multicenter randomized open-labeled | Pts with PCR confirmed SARS-CoV-2 or GGOs on CT Chest, ages 16–100, oxygen saturation < 93% (moderate-severe cases) | Favipiravir + HCQ versus lopinavir/ritonavir + HCQ | 1600/600 | 7 days | Number of ICU admissions | Length of hospital stay, in-hospital mortality, time to clinical recovery | No reduction in number of ICU admissions or intubations or in-hospital mortality compared with lopinavir/ritonavir. Time to clinical recovery and length of hospital stay similar in two groups |
| Dabbous et al. 87 | April–May 2020 | Egypt | Randomized, controlled, interventional, Phase 3 | Mild-moderate COVID-19, NP swab PCR positive pts | Oseltamivir + HCQ versus favipiravir | 1600/600 | 10 days | SARS-CoV-2 viral clearance on days 3, 7, and 14 | Clinical outcomes on days 3, 7, and 14. | No statistically significant difference in PCR negativity between groups by day 7 and ~90% of both groups achieved viral clearance by day 14 |
| Dabbous et al. 88 | April–August 2020 | Egypt | Multicenter, randomized, controlled | Mild-moderate COVID-19, ages 18–18, hospitalized pts | CQ versus favipiravir | 1600/600 | 10 days | Mortality rate and need for mechanical ventilation | – | No statistically significant differences between two groups although lower length of stay in favipiravir arm |
COVID-19, coronavirus disease of 2019; CQ, chloroquine; CT, computed tomography; HCQ, hydroxychloroquine; ICU, Intensive Care Unit; NP, nasopharyngeal; OP, oropharyngeal; PCR, polymerase chain reaction; Pts, patients; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2. Retracted papers were excluded from the table. Ages 18-80, hospitalized pts.
Future directions
Table 3 summarizes publicly available information about ongoing favipiravir clinical trials.89–95 The GETAFIX, FLARE, COVERAGE, and VIRCO trials are focused on drug efficacy early in the treatment of COVID-19 and the COVERAGE trial will be looking specifically at geriatric patients. The PRINCIPLE trial collaborative group evaluated the use of azithromycin among adults 50 years of age or more with medical comorbidities and all adults above 65 years of age with COVID-19 and found that its use did not shorten the time to clinical recovery or reduce the risk of hospitalization. 96 Interim results of the PRINCIPLE trial also reported clinical utility of inhaled budesonide among patients with COVID-19 at risk for adverse outcomes. 97 The findings from the PRINCIPLE trial about favipiravir’s clinical utility remain awaited. Bosaeed et al. 89 have proposed a multicentric randomized controlled trial to evaluate efficacy of favipiravir and hydroxychloroquine combination in comparison with control. Hassaniazad et al. 90 also published about an upcoming single-center, randomized, open-label clinical trial among moderately ill patients with COVID-19 evaluating the safety and efficacy of favipiravir and interferon beta-1a combination in comparison with lopinavir/ritonavir and interferon beta-1 combination.
Table 3.
Summary of ongoing favipiravir clinical trials.
| Name of trial, authors, and region | Trial design | Participants | Intervention & comparator | Outcome |
|---|---|---|---|---|
| FACCT A Trial of Favipiravir and Hydroxychloroquine combination in Adults Hospitalized with moderate and severe COVID-19 Bosaeed et al. 89 Saudi Arabia |
Open-label, multicenter, randomized controlled clinical trial | • Age 18 and above • Male or nonpregnant female • PCR positive • Moderate or severe COVID-19 • Within 10 days of disease onset |
Favipiravir (1800/800) × 10 days + HCQ ×5 days versus standard of care | Time to clinical improvement Other outcome: Time to PCR negativity |
| Evaluation of the efficacy and safety of favipiravir and interferon compared with lopinavir/ritonavir and interferon in moderately ill patients with COVID-19 Hassaniazad et al. 90 Iran |
Phase 3, single-center, randomized, open-label, controlled trial | •Age 20 and above ○ PCR positive ○ Moderate COVID-19 pneumonia on CT or CXR requiring hospitalization ○ Hospitalized ⩽ 48 h |
Favipiravir (1600/600) × 5 days + IFN beta-1a versus lopinavir/ritonavir + IFN beta-1a | Viral load in NP swab samples assessed by PCR after 7 days and clinical improvement of fever and O2 saturation within 7 days |
| GETAFIX Glasgow Early Treatment Arm Favipiravir for adults with early stage COVID-19 Hanna et al. 91 United Kingdom |
Multi center, open-label, phase II/III randomized trial | • Age 16 and above • Exhibiting symptoms • Positive for SARS-CoV-2 • Point 1, 2, 3, or 4 on the WHO COVID-19 ordinal severity scale at time of randomization (asymptomatic with positive valid COVID-19 test, symptomatic independent, symptomatic assistance needed, hospitalized, with no oxygen therapy) • Have ⩾ 10% risk of death if admitted to hospital • Able to swallow oral medication |
Favipiravir (1800/800) × 10 days + standard of care versus standard of care alone | Drug efficacy |
| COVERAGE Home Treatment of Older People with Symptomatic COVID-19 Duvignaud et al. 92 France |
Multicenter, randomized, controlled clinical trial 1:1:1: 1:1 Arm 1: Control Arms 2–5: Treatment |
• Age 65 and above ○ Positive NP swab PCR ○ Symptom onset within 3 days ○ No hospitalization |
(1) HCQ (2) Imatinib (3) Favipiravir 200 mg, 12 tablets BID on day 0, 6 tablets BID from days 1–9 (4) Telmisartan Comparator: AZINC Forme et Vitalité®, 1 capsule BID for 10 days |
Efficacy of drugs to prevent hospitalization/death in seniors > 65 years of age with recent COVID-19 |
| VIRCO An adaptive randomized placebo-controlled phase II trial of antivirals for COVID-19 infection McMahon et al. 93 Australia |
Randomized, placebo-controlled phase II clinical trial | • Age ⩾ 18 years • PCR positive within 5 days • Within 5 days of symptom onset |
Favipiravir (1800/800) × 14 days versus placebo | Drug efficacy based on time to PCR negativity within 14 days. Other outcome measures: Drug safety, clinical benefit, effect on biomarkers of inflammation and immune system activity |
| FLARE Early antiviral treatment in outpatients with COVID-19 Brown et al. 94 United Kingdom |
Phase IIA, randomized, double-blind, placebo-controlled trial | • Ages 18–70 • Symptoms of COVID-19 disease within the first 5 days of symptom onset OR symptoms of COVID-19 and tested positive for within the first 7 days of symptom onset OR no symptoms but tested positive within the last 48 h |
1:1:1:1 (1) Favipiravir (1800/400 QID) + lopinavir/ritonavir × 7 days (2) Favipiravir + lopinavir/ritonavir placebo (3) Favipiravir placebo + lopinavir/ritonavir (4) Favipiravir placebo + lopinavir/ritonavir placebo |
Upper respiratory tract viral load on day 5 |
| PRINCIPLE Platform Randomized trial of Interventions against COVID-19 in older people Hayward et al. 95 United Kingdom |
Open-label, multi-arm, prospective, adaptive platform, randomized clinical trial in community care | • Symptoms of possible COVID-19 with a positive SARS-CoV-2 test – Within the last 14 days • Age ⩾ 65 years OR Patients aged ⩾ 50–64 years with comorbidities |
Favipiravir/ivermectin/HCQ/azithromycin/doxycycline/inhaled budesonide versus standard of care | Time to recovery/hospitalization/death within 28 days |
BID, twice a day; COVID-19, coronavirus disease of 2019; CT, computed tomography; CXR, chest X-ray; HCQ, hydroxychloroquine; IFN, interferon; NP, nasopharyngeal; PCR, polymerase chain reaction; QID, four times a day; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; WHO, World Health Organization.
Conclusion
Favipiravir, among other drugs, has gained prominence since 2020 because of the COVID-19 pandemic and its versatility as a broad-spectrum antiviral that inhibits RdRp and targets viral replication. While its utility has shown benefit in a few clinical trials, others yielded mixed results. The drug’s full potential as a therapy for COVID-19 remains to be determined, including its optimal timing of administration, dosage, and duration of therapy. It is fairly well-tolerated with a major safety concern being its teratogenic potential. Adverse drug reactions among patients included hyperuricemia, QTc interval prolongation, and elevation in hepatic enzymes. Finally, favipiravir’s prospect as a post-exposure prophylactic agent in COVID-19 remains to be tested.
Footnotes
Author contributions: KS: Conceptualiztion, manuscript preparation, review and editing.
MR: Conceptualization, manuscript preparation, review, editing and submission.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Kritika Srinivasan  https://orcid.org/0000-0003-2877-3542
https://orcid.org/0000-0003-2877-3542
Contributor Information
Kritika Srinivasan, Department of Biomaterials and Pathology, Vilcek Institute, New York University School of Medicine, New York, NY, USA.
Mana Rao, Essen Medical Associates, 2015 Grand Concourse, Bronx, NY 10453, USA.
References
- 1. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Coronavirus Resource Center, https://coronavirus.jhu.edu/map.html
- 2. Gorbalenya AE, Baker SC, Baric RS, et al. The species severe acute respiratory syndrome-related coronavirus : classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 2020; 5: 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579: 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu W, Chen CZ, Gorshkov K, et al. RNA-dependent RNA polymerase as a target for COVID-19 drug discovery. SLAS Discov 2020; 25: 1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tai W, He L, Zhang X, et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol 2020; 17: 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020; 579: 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hillen HS, Kokic G, Farnung L, et al. Structure of replicating SARS-CoV-2 polymerase. Nature 2020; 584: 154–156. [DOI] [PubMed] [Google Scholar]
- 8. Kirchdoerfer RN, Ward AB. Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nat Commun 2019; 10: 2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shu B, Gong P. Structural basis of viral RNA-dependent RNA polymerase catalysis and translocation. Proc Natl Acad Sci USA 2016; 113: E4005–E4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gong P, Peersen OB. Structural basis for active site closure by the poliovirus RNA-dependent RNA polymerase. Proc Natl Acad Sci USA 2010; 107: 22505–22510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Appleby TC, Perry JK, Murakami E, et al. Structural basis for RNA replication by the hepatitis C virus polymerase. Science 2015; 347: 771–775. [DOI] [PubMed] [Google Scholar]
- 12. Venkataraman S, Prasad B, Selvarajan R. RNA dependent RNA polymerases: insights from structure, function and evolution. Viruses 2018; 10: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eskian M, Rezaei N. Clinical manifestations of COVID-19. In: Rezaei N. (ed.) Coronavirus disease – COVID-19. Cham: Springer International Publishing, 2021, pp. 179–196, https://link.springer.com/10.1007/978-3-030-63761-3_11 (accessed 2 July 2021). [DOI] [PubMed] [Google Scholar]
- 14. Bombardini T, Picano E. Angiotensin-converting enzyme 2 as the molecular bridge between epidemiologic and clinical features of COVID-19. Can J Cardiol 2020; 36: 784.e1–784.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shen W-X, Luo R-C, Wang J-Q, et al. Features of cytokine storm identified by distinguishing clinical manifestations in COVID-19. Front Public Health 2021; 9: 671788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yelin D, Margalit I, Yahav D, et al. Long COVID-19 – it’s not over until? Clin Microbiol Infect 2021; 27: 506–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021; 27: 601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McRae MP, Simmons GW, Christodoulides NJ, et al. Clinical decision support tool and rapid point-of-care platform for determining disease severity in patients with COVID-19. Lab Chip 2020; 20: 2075–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ita K. Coronavirus disease (COVID-19): current status and prospects for drug and vaccine development. Arch Med Res 2021; 52: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Majumder J, Minko T. Recent developments on therapeutic and diagnostic approaches for COVID-19. AAPS J 2021; 23: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Verma HK, Merchant N, Verma MK, et al. Current updates on the European and WHO registered clinical trials of coronavirus disease 2019 (COVID-19). Biomed J 2020; 43: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B 2017; 93: 449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu R, Gao Y, Robert S-H, et al. Systematic review of the registered clinical trials for coronavirus disease 2019 (COVID-19). J Transl Med 2020; 18: 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Painter GR, Natchus MG, Cohen O, et al. Developing a direct acting, orally available antiviral agent in a pandemic: the evolution of molnupiravir as a potential treatment for COVID-19. Curr Opin Virol 2021; 50: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hung IF-N, Lung K-C, Tso EY-K, et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 2020; 395: 1695–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lian N, Xie H, Lin S, et al. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect 2020; 26: 917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of covid-19 – final report. N Engl J Med 2020; 383: 1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Furuta Y, Gowen BB, Takahashi K, et al. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 2013; 100: 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toyama Chemical Co., Ltd. Report on the deliberation results – Avigan (favipiravir) Tablet 200 mg, 2014, https://www.pmda.go.jp/files/000210319.pdf
- 30. Kaptein SJF, Jacobs S, Langendries L, et al. Favipiravir at high doses has potent antiviral activity in SARS-CoV-2−infected hamsters, whereas hydroxychloroquine lacks activity. Proc Natl Acad Sci USA 2020; 117: 26955–26965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Agrawal U, Raju R, Udwadia ZF. Favipiravir: a new and emerging antiviral option in COVID-19. Med J Armed Forces India 2020; 76: 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Delang L, Abdelnabi R, Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral Res 2018; 153: 85–94. [DOI] [PubMed] [Google Scholar]
- 33. Shiraki K, Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther 2020; 209: 107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baranovich T, Wong S-S, Armstrong J, et al. T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J Virol 2013; 87: 3741–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Ávila AI, Gallego I, Soria ME, et al. Lethal mutagenesis of hepatitis C virus induced by favipiravir. PLoS ONE 2016; 11: e0164691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Furuta Y, Takahashi K, Kuno-Maekawa M, et al. Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother 2005; 49: 981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jin Z, Smith LK, Rajwanshi VK, et al. The ambiguous base-pairing and high substrate efficiency of T-705 (favipiravir) ribofuranosyl 5′-triphosphate towards influenza A virus polymerase. PLoS ONE 2013; 8: e68347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Escribano-Romero E, Jiménez de, Oya N, Domingo E, et al. Extinction of West Nile Virus by favipiravir through lethal mutagenesis. Antimicrob Agents Chemother 2017; 61: e01400–17, https://journals.asm.org/doi/10.1128/AAC.01400-17 (accessed 5 July 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Avila AI, Moreno E, Perales C, et al. Favipiravir can evoke lethal mutagenesis and extinction of foot-and-mouth disease virus. Virus Res 2017; 233: 105–112. [DOI] [PubMed] [Google Scholar]
- 40. Jin Z, Tucker K, Lin X, et al. Biochemical evaluation of the inhibition properties of favipiravir and 2′-C-methyl-cytidine triphosphates against human and mouse norovirus RNA polymerases. Antimicrob Agents Chemother 2015; 59: 7504–7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kiso M, Takahashi K, Sakai-Tagawa Y, et al. T-705 (favipiravir) activity against lethal H5N1 influenza A viruses. Proc Natl Acad Sci USA 2010; 107: 882–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Madelain V, Nguyen THT, Olivo A, et al. Ebola virus infection: review of the pharmacokinetic and pharmacodynamic properties of drugs considered for testing in human efficacy trials. Clin Pharmacokinet 2016; 55: 907–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nguyen THT, Guedj J, Anglaret X, et al. Favipiravir pharmacokinetics in Ebola-infected patients of the JIKI trial reveals concentrations lower than targeted. PLoS Negl Trop Dis 2017; 11: e0005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nagata T, Lefor AK, Hasegawa M, et al. Favipiravir: a new medication for the Ebola virus disease pandemic. Disaster Med Public Health Prep 2015; 9: 79–81. [DOI] [PubMed] [Google Scholar]
- 45. Furuta Y, Takahashi K, Fukuda Y, et al. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother 2002; 46: 977–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takahashi K, Furuta Y, Fukuda Y, et al. In vitro and in vivo activities of T-705 and oseltamivir against influenza virus. Antivir Chem Chemother 2003; 14: 235–241. [DOI] [PubMed] [Google Scholar]
- 47. Sidwell RW, Barnard DL, Day CW, et al. Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice. Antimicrob Agents Chemother 2007; 51: 845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Furuta Y, Takahashi K, Shiraki K, et al. T-705 (favipiravir) and related compounds: novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res 2009; 82: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goldhill DH, te Velthuis AJW, Fletcher RA, et al. The mechanism of resistance to favipiravir in influenza. Proc Natl Acad Sci USA 2018; 115: 11613–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Smee DF, Hurst BL, Wong M-H, et al. Effects of the combination of favipiravir (T-705) and oseltamivir on influenza A virus infections in mice. Antimicrob Agents Chemother 2010; 54: 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang Y, Fan G, Salam A, et al. Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically ill patients with influenza virus infection. J Infect Dis 2020; 221: 1688–1698. [DOI] [PubMed] [Google Scholar]
- 52. Avigan Tablets 200 mg, https://www.cdc.gov.tw/File/Get/ht8jUiB_MI-aKnlwstwzvw
- 53. Wang Y, Zhong W, Salam A, et al. Phase 2a, open-label, dose-escalating, multi-center pharmacokinetic study of favipiravir (T-705) in combination with oseltamivir in patients with severe influenza. EBioMedicine 2020; 62: 103125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. MDVI. Dose-finding study of favipiravir in the treatment of uncomplicated influenza, 2014, https://clinicaltrials.gov/show/NCT01068912
- 55. Phase 3 efficacy and safety study of favipiravir for treatment of uncomplicated influenza in adults – T705US316, https://clinicaltrials.gov/show/NCT02026349
- 56. Phase 3 efficacy and safety study of favipiravir for treatment of uncomplicated influenza in adults, https://clinicaltrials.gov/show/NCT02008344
- 57. Sissoko D, Laouenan C, Folkesson E, et al. Experimental treatment with favipiravir for Ebola virus disease (the JIKI trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med 2016; 13: e1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kerber R, Lorenz E, Duraffour S, et al. Laboratory findings, compassionate use of favipiravir, and outcome in patients with Ebola virus disease, Guinea, 2015 – a retrospective observational study. J Infect Dis 2019; 220: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guedj J, Piorkowski G, Jacquot F, et al. Antiviral efficacy of favipiravir against Ebola virus: a translational study in cynomolgus macaques. PLoS Med 2018; 15: e1002535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gowen BB, Wong M-H, Jung K-H, et al. In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob Agents Chemother 2007; 51: 3168–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Julander JG, Shafer K, Smee DF, et al. Activity of T-705 in a hamster model of yellow fever virus infection in comparison with that of a chemically related compound, T-1106. Antimicrob Agents Chemother 2009; 53: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Morrey J, Taro B, Siddharthan V, et al. Efficacy of orally administered T-705 pyrazine analog on lethal West Nile virus infection in rodents. Antiviral Res 2008; 80: 377–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. World Health Organization. Nipah virus infection, https://www.who.int/news-room/fact-sheets/detail/nipah-virus
- 64. Dawes BE, Kalveram B, Ikegami T, et al. Favipiravir (T-705) protects against Nipah virus infection in the hamster model. Sci Rep 2018; 8: 7604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jochmans D, van Nieuwkoop S, Smits SL, et al. Antiviral activity of favipiravir (T-705) against a broad range of paramyxoviruses in vitro and against human metapneumovirus in hamsters. Antimicrob Agents Chemother 2016; 60: 4620–4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Morse JS, Lalonde T, Xu S, et al. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. ChemBioChem 2020; 21: 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shannon A, Selisko B, Le N, et al. Favipiravir strikes the SARS-CoV-2 at its Achilles heel, the RNA polymerase. bioRxiv 2020, http://biorxiv.org/lookup/doi/10.1101/2020.05.15.098731 (accessed 8 July 2021).
- 68. Mitjà O, Clotet B. Use of antiviral drugs to reduce COVID-19 transmission. Lancet Glob Health 2020; 8: e639–e640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Magleby R, Westblade LF, Trzebucki A, et al. Impact of severe acute respiratory syndrome coronavirus 2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin Infect Dis. Epub ahead of print 30 June 2020. DOI: 10.1093/cid/ciaa851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Joshi S, Parkar J, Ansari A, et al. Role of favipiravir in the treatment of COVID-19. Int J Infect Dis 2021; 102: 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020; 30: 269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov 2020; 19: 149–150. [DOI] [PubMed] [Google Scholar]
- 73. Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther 2020; 14: 58–60. [DOI] [PubMed] [Google Scholar]
- 74. Pilkington V, Pepperrell T, Hill A. A review of the safety of favipiravir – a potential treatment in the COVID-19 pandemic? J Virus Erad 2020; 6: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen C, Zhang Y, Huang J, et al. Favipiravir versus Arbidol for COVID-19: a randomized clinical trial. medRxiv. Epub ahead of print 15 April 2020. DOI: 10.1101/2020.03.17.20037432. [DOI] [Google Scholar]
- 76. Manabe T, Kambayashi D, Akatsu H, et al. Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis. BMC Infect Dis 2021; 21: 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hassanipour S, Arab-Zozani M, Amani B, et al. The efficacy and safety of favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials. medRxiv 2021, http://medrxiv.org/lookup/doi/10.1101/2021.02.14.21251693 (accessed 16 May 2021). [DOI] [PMC free article] [PubMed]
- 78. Lou Y, Liu L, Yao H, et al. Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in COVID-19 patients: an exploratory randomized, controlled trial. Eur J Pharm Sci 2021; 157: 105631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jirjees F, Saad AK, Al Hano Z, et al. COVID-19 treatment guidelines: do they really reflect best medical practices to manage the pandemic? Infect Dis Rep 2021; 13: 259–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Doi Y, Hibino M, Hase R, et al. A prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19. Antimicrob Agents Chemother 2020; 64: e0189720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Udwadia ZF, Singh P, Barkate H, et al. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int J Infect Dis 2021; 103: 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ivashchenko AA, Dmitriev KA, Vostokova NV, et al. AVIFAVIR for treatment of patients with moderate coronavirus disease 2019 (COVID-19): interim results of a phase II/III multicenter randomized clinical trial. Clin Infect Dis 2021; 73: 531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cai Q, Yang M, Liu D, et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering 2020; 6: 1192–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhao H, Zhu Q, Zhang C, et al. Tocilizumab combined with favipiravir in the treatment of COVID-19: a multicenter trial in a small sample size. Biomed Pharmacother 2021; 133: 110825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Khamis F, Al Naabi H, Al Lawati A, et al. Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta-1b in hospitalized patients with moderate to severe COVID-19 pneumonia. Int J Infect Dis 2021; 102: 538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Solaymani-Dodaran M, Ghanei M, Bagheri M, et al. Safety and efficacy of favipiravir in moderate to severe SARS-CoV-2 pneumonia. Int Immunopharmacol 2021; 95: 107522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dabbous HM, El-Sayed MH, El Assal G, et al. Safety and efficacy of favipiravir versus hydroxychloroquine in management of COVID-19: a randomised controlled trial. Sci Rep 2021; 11: 7282. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88. Dabbous HM, Abd-Elsalam S, El-Sayed MH, et al. Efficacy of favipiravir in COVID-19 treatment: a multi-center randomized study. Arch Virol 2021; 166: 949–954. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89. Bosaeed M, Mahmoud E, Hussein M, et al. A trial of favipiravir and hydroxychloroquine combination in adults hospitalized with moderate and severe Covid-19: a structured summary of a study protocol for a randomised controlled trial. Trials 2020; 21: 904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hassaniazad M, Bazram A, Hassanipour S, et al. Evaluation of the efficacy and safety of favipiravir and interferon compared to lopinavir/ritonavir and interferon in moderately ill patients with COVID-19: a structured summary of a study protocol for a randomized controlled trial. Trials 2020; 21: 886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hanna CR, Blyth KG, Burley G, et al. Glasgow Early Treatment Arm Favirpiravir (GETAFIX) for adults with early stage COVID-19: a structured summary of a study protocol for a randomised controlled trial. Trials 2020; 21: 935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Duvignaud A, Lhomme E, Pistone T, et al. Home treatment of older people with symptomatic SARS-CoV-2 infection (COVID-19): a structured summary of a study protocol for a multi-arm multi-stage (MAMS) randomized trial to evaluate the efficacy and tolerability of several experimental treatments to reduce the risk of hospitalisation or death in outpatients aged 65 years or older (COVERAGE trial). Trials 2020; 21: 846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. McMahon JH, Lau JSY, Roney J, et al. An adaptive randomised placebo controlled phase II trial of antivirals for COVID-19 infection (VIRCO): a structured summary of a study protocol for a randomised controlled trial. Trials 2020; 21: 847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Brown L-AK, Freemantle N, Breuer J, et al. Early antiviral treatment in outpatients with COVID-19 (FLARE): a structured summary of a study protocol for a randomised controlled trial. Trials 2021; 22: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hayward G, Butler CC, Yu L-M, et al. Platform Randomised trial of INterventions against COVID-19 In older peoPLE (PRINCIPLE): protocol for a randomised, controlled, open-label, adaptive platform, trial of community treatment of COVID-19 syndromic illness in people at higher risk. BMJ Open 2021; 11: e046799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Butler CC, Dorward J, Yu L-M, et al. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet 2021; 397: 1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yu L-M, Bafadhel M, Dorward J, et al. Inhaled budesonide for COVID-19 in people at higher risk of adverse outcomes in the community: interim analyses from the PRINCIPLE trial. medRxiv 2021, http://medrxiv.org/lookup/doi/10.1101/2021.04.10.21254672 (accessed 25 July 2021).