Abstract
This study aimed to systematically assess COVID‐19 patient background characteristics and pre‐existing comorbidities associated with hospitalisation status. The meta‐analysis included cross‐sectional, cohort, and case‐series studies with information on hospitalisation versus outpatient status for COVID‐19 patients, with background characteristics and pre‐existing comorbidities. A total of 1,002,006 patients from 40 studies were identified. Significantly higher odds of hospitalisation were observed in Black individuals (OR = 1.33, 95% CI: 1.04–1.70), males (OR = 1.59, 95% CI: 1.43–1.76), and persons with current/past smoking (OR = 1.59, 95% CI: 1.34–1.88). Additionally, individuals with pre‐existing comorbidities were more likely to be hospitalised [asthma (OR = 1.22, 95% CI: 1.02–1.45), COPD (OR = 3.68, 95% CI: 2.97–4.55), congestive heart failure (OR = 6.80, 95% CI: 4.97–9.31), coronary heart disease (OR = 4.40, 95% CI: 3.15–6.16), diabetes (OR = 3.90, 95% CI: 3.29–4.63), hypertension (OR = 3.89, 95% CI: 3.34–4.54), obesity (OR = 1.98, 95% CI: 1.59–2.46) and renal chronic disease (OR = 5.84, 95% CI: 4.51–7.56)]. High heterogeneity and low publication bias among all factors were found. Age was not included due to the large variability in the estimates reported. In this systematic review/meta‐analysis for patients with COVID‐19, Black patients, males, persons who smoke, and those with pre‐existing comorbidities were more likely to be hospitalised than their counterparts. Findings provide evidence of populations with higher odds of hospitalisation for COVID‐19.
Keywords: background characteristics, co‐morbidities, COVID‐19, demographics, hospitalisation, meta‐analysis
Abbreviations
- COPD
chronic obstructive pulmonary disease
- COVID‐19
Coronavirus
- CVD
cardiovascular diseases
- ICU
intensive care unit
- PRISMA
Preferred Reporting for Systematic Review and Meta‐Analysis
- SARS‐CoV‐2
Severe Acute Respiratory Syndrome Coronavirus 2
1. INTRODUCTION
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) is a novel beta coronavirus that can be manifested as a mild to severe respiratory infection in humans along with asymptomatic transmission. 1 Emergence of the SARS‐CoV‐2 in late 2019, which became referred to as coronavirus disease‐2019 or COVID‐19, originated in Wuhan, China, and proceeded to escalate into a global pandemic, leaving more than 154 million people infected and 3.23 million people deceased. 2 Transmission of COVID‐19 has been identified to be primarily facilitated through close contact air droplets and physical contact in addition to aerosol exposure in enclosed spaces. 3 Of particular concern is the number of infected individuals who require hospitalisation as debilitating and potentially deadly clinical complications such as acute respiratory distress and respiratory failure are more prevalent among hospitalised individuals. 4
Recorded hospitalisation rates due to COVID‐19 are prone to significant variation week‐to‐week, which may be characterised by certain time intervals with larger peaks in hospitalisations. 5 The burden placed on local hospital systems due to increased rates of patients requiring admittance for COVID‐19 complications is a pressing matter as this creates an additional burden on the available capacity for hospitals to treat new patients. 6 Furthermore, the burden placed upon the healthcare systems and the exposure to healthcare workers due to rates of hospitalisations and ICU transfers is not identical across the world, and identifying methods for minimising this burden are key. 7 , 8
Several characteristics of healthcare systems have been identified that may be at play when examining burden of COVID‐19 hospitalisations on individual healthcare systems, such as timing of outbreaks and more isolated, rural locations of health centres. 9 Prior evidence has suggested that patients admitted due to COVID‐19 may experience longer durations of hospitalisation, increased reliance on oxygen therapy or invasive mechanical ventilation, and more intensive care unit (ICU) need compared to seasonal influenza patients. 10
In addition, COVID‐19 hospitalisation has been identified to vary significantly depending on several predictors such as background characteristics and the presence of certain pre‐existing comorbidities. 11 The range of factors that have been identified among COVID‐19 patients requiring hospitalisation is extensive; background characteristics have been primarily based on differences in age, ethnicity/race, smoking history, and sex. Prior research conducted on hospitalised COVID‐19 patients has shown that individuals requiring admission are typically of older age, from an ethnic or racial minority group, and male. 12
For example, research shows an independent predictor of COVID‐19 hospitalisation includes age 65 years or older. 13 A variety of factors ranging from immune system strength to other chronic health conditions that are more prevalent among older individuals may increase the susceptibility to infection and subsequent hospitalisation. 14 Higher infection rates and need for hospitalisation among specific ethnic and racial groups, such as Hispanic and Black individuals, has been attributed to socio‐economic differences, including living situations, employment status, and associations with pre‐existing conditions. 15 Furthermore, male patients have also been shown to have higher risk for hospitalisation in multiple samples. 16
Observations of COVID‐19 hospitalisation in association with pre‐existing comorbidities has been well‐documented in existing COVID‐19 research. Prior systematic reviews have identified chronic conditions including cardiovascular conditions, diabetes mellitus, respiratory diseases, and kidney diseases, as critical chronic conditions associated with hospitalisation for COVID‐19. 17 Additionally, elevated body mass index (BMI) has been associated to COVID‐19 hospitalisation; individuals with BMI over 25 kg/m2 (overweight) and over 30 kg/m2 (obese) have been shown to require hospitalisation from COVID‐19 at higher odds in comparison to individuals at healthy weights. 18
Considering the preliminary results found in the literature for COVID‐19 patients, a robust systematic review and meta‐analysis was conducted to determine the background characteristics and pre‐existing comorbidities associated with hospitalisation for COVID‐19 patients. This will help identify the most vulnerable populations for severe COVID‐19 infections that would require hospitalisation.
2. METHODS
2.1. Study selection and inclusion criteria
This study focused on the clinical outcome (hospitalisation vs outpatient) of COVID‐19 in association with background characteristics (i.e., ethnicity/race, sex, and smoking status) and preexisting comorbidities (i.e., diabetes mellitus, cardiovascular diseases, respiratory diseases, obesity, hypertension, and renal chronic disease). We conducted a broad literature search in three datasets: (1) PubMed, (2) Web of Science, and (3) Cochrane Library, that comprised pre‐print and published papers from December 2019 to December 2020. The search was not geographically limited, and included papers written in English, Spanish, or Chinese. Additionally, we searched the reference lists of relevant systematic reviews and meta‐analyses to identify potential supplemental studies (one paper was included from this search). This systematic review and meta‐analysis followed the Preferred Reporting for Systematic Review and Meta‐Analysis (PRISMA) consensus statement. 19 The protocol of this study is registered in PROSPERO (CRD42021235460).
The keywords and MESH terms in English comprised in this search included: ‘coronavirus’, ‘COVID‐19’, ‘novel coronavirus’, ‘sars‐cov‐2’, ‘2019‐ncov’, ‘severe acute respiratory syndrome related coronavirus’ AND ‘clinical characteristics’, ‘epidemiologic characteristics’, ‘clinical features’, ‘epidemiologic features’, ‘demographic features’, ‘demographic characteristics’, ‘comorbidities’, ‘diabetes mellitus’, ‘cardiovascular’, ‘respiratory, ‘obesity’, ‘hypertension’, and ‘renal chronic disease’, AND ‘outpatient’, ‘ambulatory’, ‘inpatient’ and ‘hospitalised’.
Cross‐sectional, cohort (retrospective/prospective), and case‐series studies with laboratory‐confirmed diagnoses for COVID‐19 (according to World Health Organisation guidance), which examined patient demographic factors such as ethnicity/race, sex, smoking status, and pre‐existing comorbidities including cardiovascular diseases (CVD) (coronary heart disease and congestive heart failure), diabetes mellitus, hypertension, obesity, renal chronic disease, and respiratory diseases (asthma and chronic obstructive pulmonary disease (COPD)) were included in this meta‐analysis. Age was not included in this analysis due to the variability in the estimates reported.
For external validity and relevance to the general population, inclusion criteria encompassed studies conducted in patients aged 18 years or older. Studies in subpopulation specific samples were excluded (e.g., children, adolescents, pregnant women, nursing homes, institutionalised individuals, HIV‐only, COPD‐only, cardiomyopathic‐only, renal‐only, hepatic‐only).
2.2. Data collection and quality assessment
Following the PRISMA guidelines, one investigator (PMM) identified the studies that potentially met the inclusion criteria, by screening the title and abstract for the searching results, after excluding duplicate papers. Non‐relevant studies and those that met exclusion criteria were removed. This initial review was inclusive to reduce the chance of omitting eligible studies (Figure 1).
FIGURE 1.
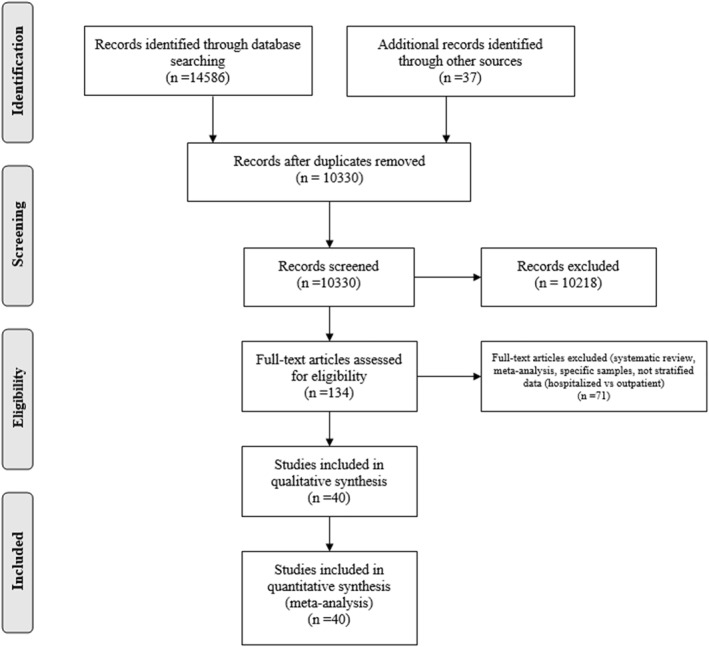
Flowchart for literature search
Two investigators (PMM and CB), independently assessed the full text of initially screened studies and identified the potentially eligible studies based on inclusion and exclusion criteria. Additionally, two investigators (CC and CO) assessed the reference lists of published systematic reviews and meta‐analyses to capture any potential study that could have been missed. The investigators (PMM and CB), independently, then proceeded with the methodological qualitative assessment of the studies using the NIH (National Institutes of Health) Study Quality Assessment Tools. These tools were based on quality assessment methods, concepts, and other tools developed by researchers in the Agency for Healthcare Research and Quality's, the Cochrane Collaboration, the U.S. Preventive Services Task Force, the Scottish Intercollegiate Guidelines Network, and the National Health Service Centre for Reviews and Dissemination. NIH quality assessment tools include specific scales for observational, case‐series and experimental studies. The scales were applied based on the study design. 20 For observational designs, papers with scores of 0–4 were graded as poor, 5–8 were graded as fair, and more than 9 were graded as good. For case‐series and reports, papers with scores of 0–3 were graded as poor, 4–6 were graded as fair, and more than 7 were graded as good. The scores of each study were compared, and any discrepancies were assessed by a third investigator (CC). After the quality assessment, the two investigators proceeded with the data extraction, comparing between the investigators for validity and accuracy. Any discrepancies were again assessed by a third investigator (CC). Data extraction included the following information from each study: title, study design, publication stage, study period, location, first author, publication year, total positive cases, and total number for hospitalised patients and outpatients. For each condition we collected the number of events for hospitalised and outpatients. Crude odds ratios were included when available. The PRISMA checklist of the mansucript is available in the supplementary material.
2.3. Statistical analysis
The association of background characteristics and pre‐existing comorbidities with hospitalisation was assessed. Exposure variables were demographics (i.e., ethnicity/race, sex, smoking) and pre‐existing comorbidities extracted from the papers. Each ethnicity/race category was coded as a non‐mutually exclusive binary outcome due to the variability in definitions among the included studies (e.g., White vs Non‐White). Our outcome variable was hospitalisation status (hospitalised vs outpatients). We estimated the odds ratios (ORs) with 95% confidence interval (95% CI) for COVID‐19 hospitalised patients to one of the potential factors compared to COVID‐19 outpatients from the extracted raw data or reported crude ORs. A random effect model was utilised to calculate pooled odds ratios when the test of heterogeneity (I 2 statistic) was moderate (50%–74%) or high (≥75%) in the pooled estimates. 21 Due to high heterogeneity between studies for all the background characteristics and pre‐existing comorbidities, random‐effects models were used for the meta‐analysis of hospitalisation. In addition, subgroup and sensitivity analyses were conducted to look into heterogeneity. These analyses were determined a priori by (1) study design (i.e., case series, cohort, cross‐sectional) and (2) publication stage (i.e., published, pre‐print), as these could have been potential causes of heterogeneity in the results. Publication bias and small study effect were assessed with the Egger's regression test and the Harbord's modified test which accounted for the heterogeneity and binary outcomes. 22 , 23 To reduce publication bias, we included both published studies and literature published in medRxiv. All statistical analyses were conducted with Stata, Version 15 24 with an alpha at the 0.05 level.
3. RESULTS
3.1. Study characteristics
A total of 14,623 records were identified in databases, during the initial search. We identified 14,586 papers on PubMed, 35 new papers from Cochrane, and 2 papers from the meta‐analyses/systematic review references. After removing duplicates and applying our exclusion criteria, a title and abstract analysis was performed for 134 papers. Only 40 papers included the number of events for both hospitalised patients and outpatients. The 40 papers 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 underwent the quality assessment and were included in our meta‐analysis (Figure 1).
The systematic review identified a total of 1,002,006 patients (188,597 hospitalised patients and 813,409 outpatients). The studies included 26 cohort studies, 26 , 27 , 28 , 29 , 30 , 32 , 34 , 35 , 36 , 37 , 42 , 44 , 45 , 46 , 48 , 49 , 51 , 52 , 53 , 54 , 55 , 56 , 60 , 62 , 63 , 64 7 cross‐sectional studies, 33 , 38 , 39 , 41 , 47 , 50 , 61 and 7 reports and case series 25 , 31 , 40 , 43 , 57 , 58 , 59 that were developed between December 2019 and December 2020. The included studies were conducted in the United States (27 studies), 25 , 26 , 28 , 30 , 31 , 32 , 33 , 35 , 36 , 38 , 40 , 42 , 43 , 44 , 45 , 46 , 48 , 49 , 52 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 62 Mexico, 39 , 51 United Kingdom, 37 , 63 Spain 29 , 41 (2 studies conducted in each country), Brazil, 47 Chile, 61 Denmark, 53 France, 50 Honduras, 64 Italy, 34 and Turkey, 27 (1 study conducted in each country). A total of 4 pre‐print articles 28 , 37 , 46 , 54 were included in the meta‐analysis (Table 1). Age was not assessed in our analysis, due to high variation in the descriptive statistics on age. For example, a total of 11 papers reported mean age (range 44–68.8 years), 32 , 37 , 39 , 41 , 46 , 49 , 55 , 56 , 57 , 60 , 63 11 papers reported median age (range: 41–66.1 years), 25 , 30 , 35 , 36 , 44 , 48 , 51 , 52 , 53 , 54 , 58 and 18 papers did not have available data on age for the total sample. 26 , 27 , 28 , 29 , 31 , 33 , 34 , 38 , 40 , 42 , 43 , 45 , 47 , 50 , 59 , 61 , 62 , 64
TABLE 1.
Main Characteristics of the studies included in the analyses
| Author | Date | Stage of printing | Type of study | Location | Included positive cases (n) | Hospitalised patients (n) | Outpatients (n) | Age of total positive cases (Median/Mean [IQR/SD]) |
|---|---|---|---|---|---|---|---|---|
| Argenziano, et al. | March 11th–April 6th | Case series | US | 1,000 | 850 | 150 | 63.0 [50.0–75.0] | |
| Argyropoulos, et al. | March 12th–March 18th | Retrospective cohort | US | 205 | 40 | 165 | NA | |
| Avci, et al. | March 11th–April 21st | Retrospective cohort | Turkey | 1,197 | 215 | 982 | NA | |
| Baidal, et al. | March 1–May 14 | Pre‐print | Retrospective cohort | US | 8,055 | 5,136 | 2919 | NA |
| Bermejo‐Martin, et al. | March 16th–April 15th | Prospective cohort | Spain | 250 | 200 | 50 | NA | |
| Blair, et al. | April 21st–June 23rd | Prospective cohort | US | 118 | 9 | 109 | 56.0 [50.0–63.0] | |
| CDC COVID‐19 response team | February 12th–March 28th | Report | US | 6,637 | 1,494 | 5143 | NR | |
| Ebinger, et al. | March 8th–March 21st | Retrospective cohort | US | 442 | 214 | 228 | 52.7 [19.6] | |
| Fan, et al. | February 28th–May 14th | Cross‐sectional | US | 88,747 | 27,062 | 61,685 | NR | |
| Giorgi Rossi, et al. | February 27th–April 2nd | Prospective cohort | Italy | 2,653 | 1,075 | 1578 | NR | |
| Gottlieb, et al. | March 4th–June 21st | Retrospective cohort | US | 8,673 | 1,483 | 7190 | 41.0 [29.0–54.0] | |
| Gu, et al. | March 10th–April 22th | Retrospective cohort | US | 1,139 | 523 | 616 | 53.0 [39.0–66.0] | |
| Hamer, et al. | March 16th–April 26th | Pre‐print | Prospective cohort | UK | 387,109 | 760 | 386,349 | 57.1 [9.0] |
| Hao, et al. | March 4th–April 13th | Cross‐sectional | US | 2,566 | 929 | 1637 | NA | |
| Hernandez‐Galdamez, et al. | June | Cross‐sectional | Mexico | 211,003 | 65,495 | 145,508 | 45.7 [16.3] | |
| Hsu, et al. | March 1st to May 18th | Report | US | 2,631 | 1,088 | 1543 | NR | |
| Izquierdo‐Dominguez, et al. | March 21st–April 18th | Cross‐sectional | Spain | 846 | 649 | 197 | 56.8 [15.7] | |
| Jehi, et al. | March 8th to June 5th | Retrospective cohort | US | 4,536 | 958 | 3578 | NA | |
| Killerby, et al. | March 1st–April 7th | Report | US | 531 | 220 | 311 | NA | |
| Marcello, et al. | March 5th–April 9th | Retrospective cohort | US | 13,442 | 6,248 | 7194 | 52.7 [39.5–64.5] | |
| McPadden, et al. | March 1st–April 30th | Pre‐print | Retrospective cohort | US | 7,995 | 2,154 | 5841 | NR |
| Mendy, et al. | March 13th–May 31st | Retrospective cohort | US | 689 | 216 | 473 | 44.4 [1.2] | |
| Menezes Soares, et al. | February 29th–June 11th | Cross‐sectional | Brazil | 10,713 | 1,152 | 9561 | NR | |
| Mikami, et al. | March 13th–April 17th | Retrospective cohort | US | 6,493 | 3,708 | 2785 | 59.0 [43.0–72.0] | |
| Miller, et al. | March 7th–April 30th | Retrospective cohort | US | 3,633 | 2,316 | 1317 | 58.4 [18.1] | |
| Nouchi, et al. | March 23rd–March 27th | Cross‐sectional | France | 390 | 198 | 192 | NA | |
| Ortiz‐Brizuela, et al. | February 26th–March 23rd | Prospective cohort | Mexico | 309 | 140 | 169 | 43.0 [33.0–54.0] | |
| Petrilli, et al. | March 1st–May 5th | Prospective cohort | US | 5,279 | 2,741 | 2538 | 54.0 [38.0–66.0] | |
| Reilev, et al. | February 27th–May 19th | Retrospective cohort | Denmark | 11,122 | 2,254 | 8868 | 48.0 [33.0–62.0] | |
| Rentsch, et al. | February 8th–March 30th | Pre‐print | Retrospective cohort | US | 585 | 297 | 288 | 66.1 [60.4–71.0] |
| Shah, et al. | March 20th–April 22nd | Prospective cohort | US | 77 | 22 | 55 | 44.0 [19.0] | |
| Singer, et al. | March 12th–April 14th | Retrospective cohort | US | 1,651 | 737 | 914 | 50.0 [18.0] | |
| Suleyman, et al. | March 9th–March 27th | Case series | US | 463 | 355 | 108 | 57.5 [16.8] | |
| Tenforde, et al. | April 15th–May 24th | Report | US | 350 | 79 | 271 | 43.0 [32.0–57.0] | |
| Thompson, et al. | February 29th–June 1st | Report | US | 203,792 | 54,211 | 149,581 | NR | |
| Van Gerwen, et al. | March–May | Retrospective cohort | US | 3,703 | 2,015 | 1688 | 56.8 [18.2] | |
| Vial, et al. | March 3rd–April 4th | Cross‐sectional | Chile | 381 | 88 | 293 | NA | |
| Yan, et al. | March 3rd–April 8th | Retrospective cohort | US | 128 | 26 | 102 | NA | |
| Zhang, X., et al. | March 16th–June 29th | Prospective cohort | UK | 1,596 | 1,020 | 576 | 68.8 [9.2] | |
| Zuniga‐Moya, et al. | March 17th–May 4th | Retrospective cohort | Honduras | 877 | 220 | 657 | NR |
Note: NA: the data was not available the total sample, but reported stratified by group (hospitalised, Intensive Care Unit (ICU), outpatient); NR: not reported means or medians. Data was collected in categories.
Overall, 33 papers were graded as fair, 25 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 36 , 37 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 54 , 56 , 57 , 59 , 60 , 61 , 62 , 64 and 7 papers were graded as good 26 , 35 , 38 , 53 , 55 , 58 , 63 (Table S1).
3.2. Patient background characteristics and comorbidities
Black patients (OR = 1.33, 95% CI:1.04–1.70), males (OR = 1.59, 95% CI: 1.43–1.76), and patients with a current or past smoking status had higher odds of hospitalisation (Table 2; Figures S1, S2, and S3). No significant association was found for Hispanic or White patients and hospitalisation (Table 2; Figures S4 and S5). All pre‐existing comorbidities were significantly associated with hospitalisation [asthma (OR = 1.22, 95% CI: 1.02–1.45), COPD (OR = 3.68, 95% CI: 2.97–4.55), congestive heart failure (OR = 6.80, 95% CI:4.97–9.31), coronary heart disease (OR = 4.4, 95% CI: 3.15–6.16), diabetes (OR = 3.90, 95% CI: 3.29–4.63), hypertension (OR = 3.89, 95% CI: 3.34–4.54), obesity (OR = 1.98, 95% CI: 1.59–2.46), and renal chronic disease (OR = 5.84, 95% CI: 4.51–7.56)] (See Table 3 and Figures S6, S7, S8, S9, S10, S11, S12, and S13).
TABLE 2.
Quantitative data synthesis for the association of background characteristics and hospitalisation of COVID‐19
| Background characteristic | Number of studies | Total cases | OR | CI | p value a | I 2 (%) | Tau2 |
|---|---|---|---|---|---|---|---|
| Ethnicity/race | |||||||
| Black versus non‐Black | 27 | 377,636 | 1.33 | 1.04–1.70 | 0.03 | 99.2% | 0.37 |
| Hispanic versus non‐Hispanic | 22 | 361,413 | 1.01 | 0.76–1.33 | 0.97 | 99.2% | 0.39 |
| White versus non‐White | 27 | 764,312 | 0.92 | 0.80–1.05 | 0.22 | 97.3% | 0.10 |
| Sex | |||||||
| Male versus female | 38 | 785,233 | 1.59 | 1.43–1.76 | <0.001 | 95.4% | 0.08 |
| Smoking | |||||||
| Current and former versus never | 24 | 740,896 | 1.59 | 1.34–1.88 | <0.001 | 96.8% | 0.13 |
Note: Each Ethnicity/race category was used as a binary variable due to the variability of how ethnic groups and races were captured across all 40 studies (i.e., not mutually exclusive groups).
Abbreviations: CI, confidence interval; OR, odds ratio.
p < 0.05.
TABLE 3.
Quantitative data synthesis for the association of comorbidities and hospitalisation of COVID‐19
| Comorbidity | Number of studies | Total cases | OR | CI | p value a | I 2 (%) | Tau2 |
|---|---|---|---|---|---|---|---|
| Asthma | 21 | 350,437 | 1.22 | 1.02–1.45 | 0.03 | 92.5% | 0.11 |
| Chronic obstructive pulmonary disease | 24 | 361,466 | 3.68 | 2.97–4.55 | <0.001 | 94.8% | 0.17 |
| Congestive heart failure | 17 | 161,879 | 6.80 | 4.97–9.31 | <0.001 | 95.9% | 0.35 |
| Coronary heart disease | 15 | 135,539 | 4.40 | 3.15–6.16 | <0.001 | 96.8% | 0.36 |
| Diabetes | 34 | 786,373 | 3.90 | 3.29–4.63 | <0.001 | 98.3% | 0.21 |
| Hypertension | 31 | 768,334 | 3.89 | 3.34–4.54 | <0.001 | 98.0% | 0.15 |
| Obesity | 26 | 372,468 | 1.98 | 1.59–2.46 | <0.001 | 99.0% | 0.27 |
| Renal disease | 27 | 391,522 | 5.84 | 4.51–7.56 | <0.001 | 97.4% | 0.35 |
Abbreviations: CI, confidence interval; OR, odds ratio.
p < 0.05.
After conducting the subgroup analyses by study design and printing stage, we found that the association for Black patients in case‐series and cohort studies remained significant but was not the case for cross‐sectional studies (Figure S14). For asthma, the association remained positive in cohort studies, but we found no significant association in case‐series, and a significant inverse association in cross‐sectional studies. It is worth noting that only 2 cross‐sectional studies included asthma (Figure S15). All other associations did not change.
We found a potential small‐study effect bias in the analysis for White race and Hispanic ethnicity, for both the Egger's and Harbord's test (p < 0.01 respectively). In addition, publication bias was found for congestive heart failure (Egger's p = 0.006, Harbord's p = 0.04) and asthma (Egger's p = 0.04, Harbord's p = 0.03). A discrepancy was found between tests for coronary heart disease and renal disease (with a significant publication with the Egger's test only) (Table S2).
4. DISCUSSION
This meta‐analysis compared background characteristics and pre‐existing comorbidities between COVID‐19 hospitalised patients and outpatients. There were higher odds for hospitalisation with Covid‐19 for Black patients, males, and persons with current and former smoking, compared to the reference groups. Assessment of how ethnic and racial groups differ in hospitalisation incidence indicates that minority groups present with higher odds of inpatient status compared to non‐Hispanic Whites. 65 Prior studies have indicated that higher hospitalisation ratios for minority groups may be due in part to relevant social determinants of health including geographic locations, living environments, employment settings, or underlying health conditions. 66 Additionally, chronic cardiovascular diseases such as hypertension and heart disease are more prevalent in the Black community and may play a key role in observing higher hospitalisation from COVID‐19. 67 Nonetheless, this meta‐analysis did not indicate similar findings from previous studies and meta‐analyses for Hispanic patients and odds of hospitalisation. Previous studies indicated that Hispanic patients show higher odds of hospitalisation when compared to non‐Hispanic White patients in addition to higher mortality risk, higher rates of ICU transfers, and higher need for more invasive interventions such as mechanical ventilation in COVID‐19. 68 The evidence of publication bias within this study for Hispanic COVID‐19 patients could be reflected of smaller study sizes for Hispanic populations, and therefore, of the difference in the results when compared to previous studies.
The observation of higher odds for COVID‐19 hospitalisation in males is similarly supported through prior meta‐analyses. 69 This may be explained through a combination of immunological and humoural differences, including a more robust T cell response in females compared to males, as well as a higher cytokine response (e.g., interleukins IL‐6 and IL‐1). 70 Additionally, associations between males and several chronic conditions ranging from cardiovascular complications to obesity may be important to consider in this observation, as these conditions have been associated with higher odds of COVID‐19 hospitalisation. 71
Smoking status also showed a significantly positive association with COVID‐19 hospitalisation, as persons with current or former smoking had higher odds of hospital admission versus those who had never smoked. Associations between smoking and respiratory illness based upon prior research has supported the findings of smoking showing significant increase in the odds of COVID‐19 hospitalisation due to increased possibility of inflammation in the respiratory system. 72 While some researchers have discussed a smoker's paradox in COVID‐19, the limited data about this effect is dubious, and the results in this meta‐analysis are supported by the previous findings about the association of smoking and COVID‐19 susceptibility and worse prognosis. 73
In addition, hospitalised individuals were at higher odds of presenting pre‐existing comorbidities compared to outpatients. The findings of this meta‐analysis are supported by similar meta‐analyses; a relative risk for poor COVID‐19 clinical outcomes have been shown to be significantly associated with hypertension, CVD, chronic kidney disease, obesity, and diabetes. 74
Potential biological explanations have been generally focused on the impact that cardiovascular complications, diabetes, renal disease, obesity, and respiratory disease can have on immune system strength and respiratory functioning. 75 Functioning of cardiovascular tissues and organs has significant interplay with innate immune components and consistent communication between both systems is often facilitated via cytokines and hormones. 76 Cytokines are utilised in the body to assist with intercellular communication and inflammatory responses, 76 which may have significant impact on clinical severity of SARS‐CoV‐2 infection and an increased risk of hospitalisation. This connection is key to consider, as risk of comorbidities, and particularly, cardiovascular diseases, which have been linked to higher activity involving innate immune system functions. 76 Significant interplay can also be observed in prior studies, as pre‐existing chronic conditions such as obesity may additionally have strong associations with other prognostic factors for worse COVID‐19 outcomes such as CVD, respiratory disease, diabetes, and renal disease. 77
Due to the heterogeneity and variability among the factors in the included studies, one of the limitations of this study is the estimation of unadjusted odds ratios when analysing the potential factors. Our pooled estimates account for the crude odds ratios, as it was not possible to account for potential confounders. There was also a high level of heterogeneity between the included studies, which might reduce the strength the precision of the pooled point estimates even after conducting the subgroup analysis. Several factors could account for this heterogeneity, including sample size, location, time during the pandemic, and individual variability which could have increased the variation of estimates for hospitalisation. 78 Furthermore, some of the investigated factors had smaller samples size in some of the strata. In addition, discrepancies were found between the coefficients and confidence intervals of both tests for bias due to unknown potential sources of bias and confounding that were not considered in this study. 79 Lastly, we expect the COVID‐19 pandemic to continue to evolve and expect our findings to only generalise to 2020 (e.g., due to advancement in vaccination and/or newer and more effective treatments).
5. CONCLUSION
To our knowledge, this is the first meta‐analysis to report the pooled estimates for factors and hospitalisation status, which helps to provide stronger support for findings from individual studies that examined background characteristics and pre‐existing comorbidities associated with COVID‐19 hospitalisation. Black individuals, males, and persons with former and current smoking had a higher odds of COVID‐19 hospitalisation. Persons with chronic conditions were significantly associated with COVID‐19 hospitalisation as well. As vaccinations are slowly underway around the world, social restrictions by local governments are reduced, new strains emerged, and the burden on the healthcare systems remain, it is vital to identify the most vulnerable populations that will require hospitalisation due to COVID‐19.
CONFLICT OF INTEREST STATEMENT
The authors whose names are listed in this document, certify that they have no affiliation with or involvement in any organisation or entity with financial interest or non‐financial interest in the subject matter or materials discussed in this manuscript.
AUTHOR CONTRIBUTIONS
Every person who meets the authorship criteria are listed as authors, and all authors certify their participation in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Additionally, the authors certify that this material has not been published and not will be submitted or published in any other journal. Author Parker designed the study. Authors Begle, Mattey‐Mora, Owusu, and Chen conducted the systematic review and participated in all drafts of the paper. Author Mattey‐Mora performed the data analysis. All authors have approved the final article.
Supporting information
Supporting Information S1
Supporting Information S2
ACKNOWLEDGEMENTS
We would like to acknowledge the meaningful contributions of content and revisions to the initial draft by Dr. Kevin C. Maki.
Mattey‐Mora PP, Begle CA, Owusu CK, Chen C, Parker MA. Hospitalised versus outpatient COVID‐19 patients' background characteristics and comorbidities: a systematic review and meta‐analysis. Rev Med Virol. 2022;32(3):e2306. 10.1002/rmv.2306
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS‐CoV‐2 and COVID‐19. Nat Rev Microbiol. 2021;19(3):141‐154. doi: 10.1038/s41579-020-00459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johns Hopkins University & Medicine . COVID‐19 Map. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html
- 3. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID‐19): a review. J Am Med Assoc. 2020;324(8):782‐793. doi: 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 4. Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with in‐hospital mortality in a US national sample of patients with COVID‐19. JAMA Netw Open. 2020;3(12):e2029058. doi: 10.1001/jamanetworkopen.2020.29058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. CDC . COVIDdata tracker. Centers for Disease Control and Prevention. https://covid.cdc.gov/covid‐data‐tracker
- 6.Khera R, Jain S, Lin Z, Ross JS, Krumholz H. Evaluation of the Anticipated Burden of COVID‐19 on Hospital‐Based Healthcare Services Across the United States. Preprint. medRxiv. 2020;2020.04.01.20050492. Published 2020 Apr 3. doi:10.1101/2020.04.01.20050492
- 7. Sen‐Crowe B, Sutherland M, McKenney M, Elkbuli A. A closer look into global hospital beds capacity and resource shortages during the COVID‐19 pandemic. J Surg Res. 2021;260:56‐63. doi: 10.1016/j.jss.2020.11.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gómez‐Ochoa SA, Franco OH, Rojas LZ, et al. COVID‐19 in health‐care workers: a living systematic review and meta‐analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol. 2021;190(1):161‐175. doi: 10.1093/aje/kwaa191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller IF, Becker AD, Grenfell BT, Metcalf CJE. Disease and healthcare burden of COVID‐19 in the United States. Nat Med. 2020;26(8):1212‐1217. doi: 10.1038/s41591-020-0952-y [DOI] [PubMed] [Google Scholar]
- 10. Brehm TT, van der Meirschen M, Hennigs A, et al. Comparison of clinical characteristics and disease outcome of COVID‐19 and seasonal influenza. Sci Rep. 2021;11(1):5803. doi: 10.1038/s41598-021-85081-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Piroth L, Cottenet J, Mariet AS, et al. Comparison of the characteristics, morbidity, and mortality of COVID‐19 and seasonal influenza: a nationwide, population‐based retrospective cohort study. Lancet Respir Med. 2021;9(3):251‐259. doi: 10.1016/S2213-2600(20)30527-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalised with COVID‐19 in the New York City area. J Am Med Assoc. 2020;323(20):2052‐2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alamri F, Alsofayan Y, AlRuthia Y, et al. Predictors of hospitalisation among older adults with COVID‐19 in Saudi arabia: a cross‐sectional study of a nationally representative sample. Risk Manag Healthc Pol. 2021;14:875‐886. doi: 10.2147/RMHP.S294786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klein SL, Pekosz A, Park HS, et al. Sex, age, and hospitalisation drive antibody responses in a COVID‐19 convalescent plasma donor population. J Clin Invest. 2020;130(11):6141‐6150. doi: 10.1172/JCI142004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalisation and mortality in patients with COVID‐19 in New York City. JAMA Netw Open. 2020;3(12):e2026881. doi: 10.1001/jamanetworkopen.2020.26881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID‐19 meta‐analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(1):6317. doi: 10.1038/s41467-020-19741-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fathi M, Vakili K, Sayehmiri F, et al. The prognostic value of comorbidity for the severity of COVID‐19: a systematic review and meta‐analysis study. PLoS One. 2021;16(2):e0246190. doi: 10.1371/journal.pone.0246190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kompaniyets L, Goodman AB, Belay B, et al. Body mass index and risk for covid‐19‐related hospitalisation, intensive care unit admission, invasive mechanical ventilation, and death – United States, March–December 2020. MMWR Morb Mortal Wkly Rep. 2021;70(10):355‐361. doi: 10.15585/mmwr.mm7010e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Institutes of Health . Study Quality Assessment Tools. NHLBI, NIH. https://www.nhlbi.nih.gov/health‐topics/study‐quality‐assessment‐tools
- 21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443‐3457. doi: 10.1002/sim.2380 [DOI] [PubMed] [Google Scholar]
- 24. Stata Corporation . Stata Statistical Software: Release 15. StataCorp LLC; 2019. [Google Scholar]
- 25. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 Patients with COVID‐19 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Argyropoulos KV, Serrano A, Hu J, et al. Association of initial viral load in severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) patients with outcome and symptoms. Am J Pathol. 2020;190(9):1881‐1887. doi: 10.1016/j.ajpath.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Avcı H, Karabulut B, Farasoglu A, Boldaz E, Evman M. Relationship between anosmia and hospitalisation in patients with coronavirus disease 2019: an otolaryngological perspective. J Laryngol Otol. 2020;134(8):710‐716. doi: 10.1017/S0022215120001851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baidal JW, Wang AY, Zumwalt K, et al. Social determinants of health and COVID‐19 among patients in New York City. Preprint. Res Sq. 2020;rs.3.rs‐70959. doi: 10.21203/rs.3.rs-70959/v1 [DOI] [Google Scholar]
- 29. Bermejo‐Martin JF, González‐Rivera M, Almansa R, et al. Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID‐19. Crit Care. 2020;24(1):691. doi: 10.1186/s13054-020-03398-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blair PW, Brown DM, Jang M, et al. The clinical course of COVID‐19 in the outpatient setting: a prospective cohort study. Open Forum Infect Dis. 2021;8(2):ofab007. doi: 10.1093/ofid/ofab007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. CDC COVID‐19 Response Team . Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 – United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382‐386. doi: 10.15585/mmwr.mm6913e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ebinger JE, Achamallah N, Ji H, et al. Pre‐existing traits associated with Covid‐19 illness severity. PLoS One. 2020;15(7):e0236240. doi: 10.1371/journal.pone.0236240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fan VS, Dominitz JA, Eastment MC, et al. Risk Factors for testing positive for SARS‐CoV‐2 in a national US healthcare system. Clin Infect Dis. 2020;ciaa1624. doi: 10.1093/cid/ciaa1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giorgi Rossi P, Marino M, Formisano D, Venturelli F, Vicentini M, Grilli R. Characteristics and outcomes of a cohort of COVID‐19 patients in the province of Reggio Emilia, Italy. PLoS One. 2020;15(8):e0238281. doi: 10.1371/journal.pone.0238281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gottlieb M, Sansom S, Frankenberger C, Ward E, Hota B. Clinical course and factors associated with hospitalisation and critical illness among COVID‐19 patients in Chicago, Illinois. Acad Emerg Med. 2020;27(10):963‐973. doi: 10.1111/acem.14104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gu T, Mack JA, Salvatore M, et al. Characteristics associated with racial/ethnic disparities in covid‐19 outcomes in an academic health care system. JAMA Netw Open. 2020;3(10):e2025197. doi: 10.1001/jamanetworkopen.2020.25197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hamer M, Kivimäki M, Gale CR, Batty GD. Lifestyle risk factors for cardiovascular disease in relation to COVID‐19 hospitalisation: a community‐based cohort study of 387,109 adults in UK. medRxiv. Published May 13, 2020. doi:10.1101/2020.05.09.20096438 [DOI] [PMC free article] [PubMed]
- 38. Hao B, Sotudian S, Wang T, et al. Early prediction of level‐of‐care requirements in patients with COVID‐19. Elife. 2020;9:e60519. doi: 10.7554/eLife.60519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hernández‐Galdamez DR, González‐Block MÁ, Romo‐Dueñas DK, et al. Increased risk of hospitalisation and death in patients with COVID‐19 and pre‐existing noncommunicable diseases and modifiable risk factors in Mexico. Arch Med Res. 2020;51(7):683‐689. doi: 10.1016/j.arcmed.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hsu HE, Ashe EM, Silverstein M, et al. Race/ethnicity, underlying medical conditions, homelessness, and hospitalisation status of adult patients with COVID‐19 at an urban safety‐net medical center – Boston, Massachusetts, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(27):864‐869. doi: 10.15585/mmwr.mm6927a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Izquierdo‐Domínguez A, Rojas‐Lechuga MJ, Chiesa‐Estomba C, et al. Smell and taste dysfunction in COVID‐19 is associated with younger age in ambulatory settings: a multicenter cross‐sectional study. J Investig Allergol Clin Immunol. 2020;30(5):346‐357. doi: 10.18176/jiaci.0595 [DOI] [PubMed] [Google Scholar]
- 42. Jehi L, Ji X, Milinovich A, et al. Development and validation of a model for individualized prediction of hospitalisation risk in 4536 patients with COVID‐19. PLoS One. 2020;15(8):e0237419. doi: 10.1371/journal.pone.0237419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Killerby ME, Link‐Gelles R, Haight SC, et al. Characteristics associated with hospitalisation among patients with COVID‐19 – metropolitan Atlanta, Georgia, March–April 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):790‐794. doi: 10.15585/mmwr.mm6925e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kalyanaraman Marcello R, Dolle J, Grami S, et al. Characteristics and outcomes of COVID‐19 patients in New York City's public hospital system. PLoS One. 2020;15(12):e0243027. doi: 10.1371/journal.pone.0243027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McPadden J, Warner F, Young HP, et al. Clinical characteristics and outcomes for 7995 patients with SARS‐CoV‐2 infection. PLoS One. 2021;16(3):e0243291. doi: 10.1371/journal.pone.0243291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mendy A, Apewokin S, Wells AA, Morrow AL. Factors associated with hospitalisation and disease severity in a racially and ethnically diverse population of COVID‐19 patients. medRxiv. Published June 27, 2020. doi: 10.1101/2020.06.25.20137323 [DOI]
- 47. Soares RCM, Mattos LR, Raposo LM. Risk factors for hospitalisation and mortality due to COVID‐19 in Espírito Santo State, Brazil. Am J Trop Med Hyg. 2020;103(3):1184‐1190. doi: 10.4269/ajtmh.20-0483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mikami T, Miyashita H, Yamada T, et al. Risk factors for mortality in patients with COVID‐19 in New York City. J Gen Intern Med. 2021;36(1):17‐26. doi: 10.1007/s11606-020-05983-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miller J, Fadel RA, Tang A, et al. The impact of sociodemographic factors, comorbidities, and physiologic responses on 30‐day mortality in coronavirus disease 2019 (COVID‐19) patients in metropolitan Detroit. Clin Infect Dis. 2021;72(11):e704‐e710. doi: 10.1093/cid/ciaa1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nouchi A, Chastang J, Miyara M, et al. Prevalence of hyposmia and hypogeusia in 390 COVID‐19 hospitalised patients and outpatients: a cross‐sectional study. Eur J Clin Microbiol Infect Dis. 2021;40(4):691‐697. doi: 10.1007/s10096-020-04056-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ortiz‐Brizuela E, Villanueva‐Reza M, González‐Lara MF, et al. Clinical and epidemiological characteristics of patients diagnosed with COVID‐19 IN a tertiary care center IN Mexico city: a prospective cohort study. Rev Invest Clin. 2020;72(3):165‐177. doi: 10.24875/RIC.20000211 [DOI] [PubMed] [Google Scholar]
- 52. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reilev M, Kristensen KB, Pottegård A, et al. Characteristics and predictors of hospitalisation and death in the first 11,122 cases with a positive RT‐PCR test for SARS‐CoV‐2 in Denmark: a nationwide cohort. Int J Epidemiol. 2020;49(5):1468‐1481. doi: 10.1093/ije/dyaa140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rentsch CT, Kidwai‐Khan F, Tate JP, et al. Covid‐19 testing, hospital admission, and intensive care among 2,026,227 United States veterans aged 54–75 years. Preprint. medRxiv. Published April 14, 2020. doi: 10.1101/2020.04.09.20059964 [DOI]
- 55. Shah S, Majmudar K, Stein A, et al. Novel use of home pulse oximetry monitoring in COVID‐19 patients discharged from the emergency department identifies need for hospitalisation. Acad Emerg Med. 2020;27(8):681‐692. doi: 10.1111/acem.14053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Singer AJ, Morley EJ, Meyers K, et al. Cohort of four thousand four hundred four persons under investigation for COVID‐19 in a New York hospital and predictors of ICU care and ventilation. Ann Emerg Med. 2020;76(4):394‐404. doi: 10.1016/j.annemergmed.2020.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Suleyman G, Fadel RA, Malette KM, et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Netw Open. 2020;3(6):e2012270. doi: 10.1001/jamanetworkopen.2020.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tenforde MW, Billig Rose E, Lindsell CJ, et al. Characteristics of adult outpatients and inpatients with COVID‐19 – 11 academic medical centers, United States, March–May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(26):841‐846. doi: 10.15585/mmwr.mm6926e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Thompson CN, Baumgartner J, Pichardo C, et al. COVID‐19 outbreak – New York City, February 29–June 1, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(46):1725‐1729. doi: 10.15585/mmwr.mm6946a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van Gerwen M, Alsen M, Little C, et al. Risk factors and outcomes of COVID‐19 in New York City; a retrospective cohort study. J Med Virol. 2021;93(2):907‐915. doi: 10.1002/jmv.26337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vial MR, Peters A, Pérez I, et al. Covid‐19 in South America: clinical and epidemiological characteristics among 381 patients during the early phase of the pandemic in Santiago, Chile. BMC Infect Dis. 2020;20(1):955. doi: 10.1186/s12879-020-05665-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yan CH, Faraji F, Prajapati DP, Ostrander BT, DeConde AS. Self‐reported olfactory loss associates with outpatient clinical course in COVID‐19. Int Forum Allergy Rhinol. 2020;10(7):821‐831. doi: 10.1002/alr.22592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang X, Li X, Sun Z, et al. Physical activity and COVID‐19: an observational and Mendelian randomisation study. J Glob Health. 2020;10(2):020514. doi: 10.7189/jogh-10-020514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zuniga‐Moya JC, Norwood DA, Romero Reyes LE, et al. Epidemiology, outcomes, and associated factors of coronavirus disease 2019 (COVID‐19) reverse transcriptase polymerase chain reaction‐confirmed cases in the San Pedro Sula metropolitan area, Honduras. Clin Infect Dis. 2021;72(10):e476‐e483. doi: 10.1093/cid/ciaa1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chang MH, Moonesinghe R, Truman BI. COVID‐19 hospitalisation by race and ethnicity: association with chronic conditions among medicare beneficiaries, January 1–September 30, 2020. J Racial Ethn Health Disparities. 2021:1‐10. doi: 10.1007/s40615-020-00960-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Romano SD, Blackstock AJ, Taylor EV, et al. Trends in racial and ethnic disparities in COVID‐19 hospitalizations, by region – United States, March–December 2020. MMWR Morb Mortal Wkly Rep. 2021;70(15):560‐565. doi: 10.15585/mmwr.mm7015e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Moore JT, Pilkington W, Kumar D. Diseases with health disparities as drivers of COVID‐19 outcome. J Cell Mol Med. 2020;24(19):11038‐11045. doi: 10.1111/jcmm.15599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mackey K, Ayers CK, Kondo KK, et al. Racial and ethnic disparities in COVID‐19‐related infections, hospitalizations, and deaths : a systematic review. Ann Intern Med. 2021;174(3):362‐373. doi: 10.7326/M20-6306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nasiri MJ, Haddadi S, Tahvildari A, et al. COVID‐19 clinical characteristics, and sex‐specific risk of mortality: systematic review and meta‐analysis. Front Med. 2020;7:459. doi: 10.3389/fmed.2020.00459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Takahashi T, Ellingson MK, Wong P, et al. Sex differences in immune responses that underlie COVID‐19 disease outcomes. Nature. 2020;588(7837):315‐320. doi: 10.1038/s41586-020-2700-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mosca L, Barrett‐Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124(19):2145‐2154. doi: 10.1161/CIRCULATIONAHA.110.968792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sanchez‐Ramirez DC, Mackey D. Underlying respiratory diseases, specifically COPD, and smoking are associated with severe COVID‐19 outcomes: a systematic review and meta‐analysis. Respir Med. 2020;171:106096. doi: 10.1016/j.rmed.2020.106096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Usman MS, Siddiqi TJ, Khan MS, et al. Is there a smoker's paradox in COVID‐19? BMJ Evid Based Med. 2020. doi: 10.1136/bmjebm-2020-111492 [DOI] [PubMed] [Google Scholar]
- 74. Dorjee K, Kim H, Bonomo E, Dolma R. Prevalence and predictors of death and severe disease in patients hospitalised due to COVID‐19: a comprehensive systematic review and meta‐analysis of 77 studies and 38,000 patients. PLoS One. 2020;15(12):e0243191. doi: 10.1371/journal.pone.0243191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. O'Hearn M, Liu J, Cudhea F, Micha R, Mozaffarian D. Coronavirus disease 2019 hospitalizations attributable to cardiometabolic conditions in the United States: a comparative risk assessment analysis. J Am Heart Assoc. 2021;10(5):e019259. doi: 10.1161/JAHA.120.019259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Amin MN, Siddiqui SA, Ibrahim M, et al. Inflammatory cytokines in the pathogenesis of cardiovascular disease and cancer. SAGE Open Med. 2020;8. doi: 10.1177/2050312120965752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Földi M, Farkas N, Kiss S, et al. Obesity is a risk factor for developing critical condition in COVID‐19 patients: a systematic review and meta‐analysis. Obes Rev. 2020;21(10):e13095. doi: 10.1111/obr.13095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Imrey PB. Limitations of meta‐analyses of studies with high heterogeneity. JAMA Netw Open. 2020;3(1):e1919325. doi: 10.1001/jamanetworkopen.2019.19325 [DOI] [PubMed] [Google Scholar]
- 79. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Supporting Information S2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


