Summary
Background
Helicobacter pylori infection is a major cause of non-cardia gastric cancer (NCGC), but its causal role in cardia gastric cancer (CGC) is unclear. Moreover, the reported magnitude of association with NCGC varies considerably, leading to uncertainty about population-based H pylori screening and eradication strategies in high-risk settings, particularly in China, where approximately half of all global gastric cancer cases occur. Our aim was to assess the associations of H pylori infection, both overall and for individual infection biomarkers, with the risks of NCGC and CGC in Chinese adults.
Methods
A case-cohort study was done in adults from the prospective China Kadoorie Biobank study, aged 30–79 years from ten areas in China (Qingdao, Haikou, Harbin, Suzhou, Liuzhou, Henan, Sichuan, Hunan, Gansu, and Zhejiang), and included 500 incident NCGC cases, 437 incident CGC cases, and 500 subcohort participants who were cancer-free and alive within the first two years since enrolment in 2004–08. H pylori biomarkers were measured in stored baseline plasma samples using a sensitive immunoblot assay (HelicoBlot 2.1), with adapted criteria to define H pylori seropositivity. Cox regression was used to estimate adjusted hazard ratios (HRs) for NCGC and CGC associated with H pylori infection. These values were used to estimate the number of gastric cancer cases attributable to H pylori infection in China.
Findings
Of the 512 715 adults enrolled in the China Kadoorie Biobank between June, 2004, and July, 2008, 500 incident NCGC cases, 437 incident CGC cases, and 500 subcohort participants were selected for analysis. The seroprevalence of H pylori was 94·4% (95% CI 92·4–96·4) in NGCG, 92·2% (89·7–94·7) in CGC, and 75·6% (71·8–79·4) in subcohort participants. H pylori infection was associated with adjusted HRs of 5·94 (95% CI 3·25–10·86) for NCGC and 3·06 (1·54–6·10) for CGC. Among the seven individual infection biomarkers, cytotoxin-associated antigen had the highest HRs for both NCGC (HR 4·41, 95% CI 2·60–7·50) and CGC (2·94, 1·53–5·68). In this population, 78·5% of NCGC and 62·1% of CGC cases could be attributable to H pylori infection. H pylori infection accounted for an estimated 339 955 cases of gastric cancer in China in 2018.
Interpretation
Among Chinese adults, H pylori infection is common and is the cause of large numbers of gastric cancer cases. Population-based mass screening and the eradication of H pylori should be considered to reduce the burden of gastric cancer in high-risk settings.
Funding
Cancer Research UK, Wellcome Trust, UK Medical Research Council, British Heart Foundation, Kadoorie Charitable Foundation, National Key Research and Development Program of China, and National Natural Science Foundation of China.
Introduction
Gastric cancer is the fifth most frequently diagnosed cancer and the second leading cause of cancer death globally, causing more than 1 million new cases and approximately 770 000 deaths in 2020, with China alone accounting for approximately half (478 000) of the number of global new cases.1 One of the most notable and preventable causes of gastric cancer is Helicobacter pylori infection, which caused an estimated 0·8 million new gastric cancer cases globally in 2018, based mainly on relative risk (RR) estimates from studies in populations from Europe, the USA, and Australia.2
The prevalence of H pylori infection varies greatly between and within countries, from 20% to 50% in high-income countries to more than 80% in many low-income countries.3 Although several epidemiological studies have consistently shown the strong association of non-cardia gastric cancer (NCGC) with H pylori infection in diverse populations, the reported RR estimates varied considerably between the studies.4, 5, 6 Moreover, there is substantial uncertainty about the role of H pylori infection in the cause of cardia gastric cancer (CGC), which accounts for approximately a third of gastric cancer cases globally. By contrast to a few east Asian studies showing a positive association of CGC with H pylori infection,7, 8 albeit more modest than that for NCGC, most studies of populations in Europe, the USA, and Australia have reported either null or reduced risks of CGC associated with H pylori infection.5, 9
Research in context.
Evidence before this study
We searched for articles in PubMed published in English, Chinese, and other languages (including Japanese and Korean with English abstracts available, but with a focus on papers published in English) from Jan 1, 1990, to Dec 8, 2020, for individual studies or pooled or meta-analyses on Helicobacter pylori infection and risks of gastric cancer. The search terms (“H. pylori” or “Helicobacter pylori”) and (“gastric” or “non-cardia” or “cardia” or “stomach”) and (“cancer” or “carcinoma”) and (“risk” or “association”) were used. Overall, we identified six pooled analyses or meta-analyses that included approximately 20 prospective studies and a larger number of retrospective studies, with approximately a third involving east Asian populations. For non-cardia gastric cancer (NCGC), most studies have consistently shown a positive association with H pylori infection, but the relative risk (RR) estimates varied by more than 20 times, partly because of differences in study design, assay methods used, and exclusion criteria applied to reduce the effects of gastric atrophy on H pylori seropositivity. In a pooled analysis undertaken by the Helicobacter and Cancer Collaborative Group that included 12 prospective studies, H pylori infection as established by ELISA was associated with a 3·0-fold (95% CI 2·3–3·8; n=762 cases) increased risk of NCGC, with a higher RR (5·9, 95% CI 3·4–10·3; n=223 cases) after restricting analyses to cases with blood samples collected over 10 years before cancer diagnosis. More recently, in a pooled analysis of three nested case-control studies of populations in Europe, the USA, and Australia using a more sensitive immunoblot assay (HelicoBlot 2·1) for H pylori infection, the reported RR was 17·0 (11·6–25·0; n=189 cases) for NCGC, much higher than that obtained using ELISA in the same study population. Previous studies of H pylori infection and cardia gastric cancer (CGC), which accounts for approximately 30% of gastric cancer, have produced conflicting findings, especially between studies in east Asia and Europe, the USA, and Australia. In the aforementioned pooled analysis by HCCG (n=274 CGC cases) and a subsequent meta-analysis of 34 studies that also included many retrospective studies, H pylori infection showed either a null or negative association with CGC in populations in Europe, the USA, and Australia, but a positive, albeit more modest compared with NCGC, association in east Asian populations. In mainland China, where approximately half of all global gastric cancer cases currently occur, only four prospective studies have examined the associations of H pylori infection with gastric cancer. None have used immunoblot assays, excluded cases diagnosed within the first few years after sample collection, or covered multiple diverse areas. Although they showed positive associations of H pylori infection with overall gastric cancer, or with NCGC and CGC if available, there was substantial uncertainty about the magnitude of the associations.
Added value of this study
Our study had a case-cohort design within the large prospective China Kadoorie Biobank of 0·5 million adults recruited from ten geographically diverse areas in China. In addition to the inclusion of many adjudicated NCGC and CGC cases that occurred at least 2 years after sample collection, it also used a sensitive immunoblot assay to detect H pylori infection. Our study showed that H pylori infection was associated with 6-times higher risk of NCGC and 3-times higher risk of CGC.These RR estimates were approximately twice those previously reported among Chinese adults. We further estimated that approximately 80% of cases of NCGC and more than 60% of cases of CGC currently occurring in China each year could be attributable to H pylori infection. In a combined analysis of the present study with published data from three studies in Europe, the USA, and Australia that used the same HelicoBlot assay, the pooled RR for NCGC was 8·95 (95% CI 5·59–14·33), which can inform the future estimation of the disease burden associated with H pylori infection regionally and globally.
Implications of all the available evidence
The reliable estimation of the risk and burden of gastric cancer due to H pylori infection will help policy makers to develop and implement suitable strategies for cancer prevention locally and globally. The new evidence from this study should help to refine the estimation of the global gastric cancer burden attributable to H pylori infection. More importantly, our findings, combined with evidence from a randomised controlled trial of the effects of H pylori treatment on gastric cancer risks in China, suggest that population-based H pylori screening and eradication should be considered as a key strategy for gastric cancer prevention, before considering mass gastric cancer screening by barium photofluorography or endoscopy in China and other high-risk settings.
As a result of chronic H pylori infection, a high proportion of people with gastric cancer might develop severe gastric atrophy several years before cancer development,10 which could reduce antibody concentrations and substantially underestimate the risk of H pylori infection in case-control studies or prospective studies with a short follow-up, in which H pylori infection is measured after or shortly before cancer diagnosis.5 Moreover, the risk estimates might also be affected by the sensitivity of the assay used to measure H pylori antibodies. In populations in Europe, the USA, and Australia, the immunoblot assay has proven to be more sensitive than conventional ELISA to detect H pylori antibodies, with some studies reporting 2–5 times higher risks for NCGC with an immunoblot than with ELISA in the same study populations.6, 11, 12, 13 However, these previous studies tended to be small, typically involving fewer than 100 patients with cancer. To our knowledge, no large prospective study using an immunoblot assay has been done in China where H pylori infection is highly prevalent, with different H pylori strain-specific features from populations in Europe, the USA, and Australia.14
Using an immunoblot assay and a case-cohort study design within the prospective China Kadoorie Biobank study of more than 0·5 million adults from ten geo-graphically diverse areas, we aimed to assess the associations of H pylori infection, both overall and for individual infection biomarkers, with risks of NCGC and CGC in Chinese adults. We also estimated the number of gastric cancer cases attributable to H pylori infection in China.
Methods
Study population
Details of the design, methods, and study participants in the China Kadoorie Biobank study have been previously described.15 Briefly, the baseline survey was done between June, 2004, and July, 2008, in ten (five urban and five rural) geographically diverse areas across China (Qingdao, Haikou, Harbin, Suzhou, Liuzhou, Henan, Sichuan, Hunan, Gansu, and Zhejiang) and enrolled 512 715 adults aged 30–79 years. The ten regions were selected with the aim to maximise the diversity from the geographical locations, socioeconomic characteristics, risk exposures, and disease patterns, while considering the quality of death and disease registries and local capacity. In each study area, a regional coordinating centre and survey team were set up and all permanent residents aged 30–79 years identified from local residential records were invited to participate. Extensive data were collected through a laptop-based questionnaire (eg, sociodemographic, lifestyle and dietary factors, and medical history) and physical measurements (eg, blood pressure and adiposity), along with the collection of a 10 mL blood sample for long-term storage. Ethics approval was obtained from relevant international, national, and local ethics committees (in the UK, Oxford Tropical Research Ethics Committee; in China, the Chinese Centre for Disease Control and Prevention Ethical Review Committee and the Chinese Academy of Medical Sciences and Peking Union Medical College Ethical Committee). All participants provided written informed consent.
Follow-up of participants in the China Kadoorie Biobank study was done through linkage, via unique personal identification numbers, to established mortality and morbidity registries (for cancer, stroke, ischaemic heart disease, and diabetes) that were already available in the study areas since the beginning of the baseline survey, and to the nationwide health insurance system, which records any episodes of patients being admitted to and staying in hospital as inpatients. By Jan 1, 2017, 44 037 (8·6%) participants had died, 4781 (<1%) were lost to follow-up, and 27 903 (5·4%) developed cancer, including 3464 gastric cancers (International Classification of Disease 10 [ICD-10]: C16). For any reported cancer cases, systematic validation was done through a review of the original medical records (including histopathological reports) retrieved from hospitals. Among the 1355 reported gastric cancer cases that were adjudicated, 1246 (92%) were confirmed, and among the confirmed cases with pathology results available, 1060 (85%) of 1246 were adenocarcinomas.
Design of case-cohort study
To reduce the potential effects of lower H pylori antibody concentrations due to gastric atrophy, the selection of incident gastric cancer cases were confined to participants who were alive and with no history of cancer 2 years after study entry. All 437 reported or confirmed CGC cases (ICD-10: C16·0) were selected, and we randomly selected 500 cases from the 762 confirmed NCGC (of 2035 reported) cases. Since CGC arises in the oesophageal-gastric junction and is often difficult to distinguish from oesophageal adenocarcinoma, which is rare in China, we also included all confirmed oesophageal adenocarcinoma cases (n=27). A subcohort of 500 participants was sampled from the modified baseline cohort (ie, participants who were alive with no history of cancer 2 years after study entry) using stratified random sampling with the sizes of the strata based on the proportions of age categories and sex of all selected participants with gastric cancer (appendix p 2). This modified baseline subcohort included approximately 100 000 previously genotyped participants who were randomly selected from the whole cohort.
Immunoblot assay
A qualitative western blot kit assay, HelicoBlot 2.1 (MP Diagnostics; Bordeaux, France), was used to detect IgG antibodies to H pylori proteins in the baseline plasma samples retrieved from storage in liquid nitrogen. The assay detects seven diagnostic antibodies binding to H pylori proteins separated on a nitrocellulose membrane and has been shown to have a sensitivity of 96% and a specificity of 93% for the detection of H pylori infections in populations in Europe.16 The immunoblot assay results were independently classified and reviewed by two laboratory staff (one of which was DC) who were masked to the baseline information and case status of the samples. Each H pylori biomarker band was classified as positive, negative, or ambiguous. These results were then used to define H pylori seropositivity according to two different criteria. The clinical criteria, as defined by the manufacturer of the western blot kit, were the presence of one or more of the 89kD (vacuolating cytotoxin), 37kD, or 35kD bands; or both the 30kD (urease enzyme light subunit) and 19·5kD bands; or both the 116kD (cytotoxin-associated antigen) and the current infection marker bands. For this study, we modified the criteria by removing the requirement to have the current infection marker band alongside the cytotoxin-associated antigen band (ie, the cytotoxin-associated antigen band alone can define seropositivity). This choice was made for two reasons: first, the current infection marker band is more likely to be absent in patients with atrophic stomach or precancerous lesions; and second, the presence of antibodies to cytotoxin-associated antigen is the most sensitive marker of a past infection,17 which makes it relevant for investigating the role of H pylori infection in patients progressing towards cancer. These modified criteria are thereafter referred to as epidemiological criteria (appendix p 3).
Statistical analysis
Seroprevalence estimates and their 95% CIs were calculated among the NCGC, CGC, and subcohort groups. Spearman's correlations between H pylori biomarkers were calculated. Cox proportional hazards models were fitted using the pseudo-partial likelihood for the Borgan III estimator18 to estimate hazard ratios (HRs) for risk of NCGC, CGC, and oesophageal adenocarcinoma associated with H pylori infection, both overall and by individual H pylori biomarkers, with adjustments for age (numeric), sex, individual study regions, and education (six categories: no formal school, primary school, middle school, high school, technical school or college, and university). Time in the study (time from 2 years after entry into the study to cancer diagnosis, loss to follow-up, death because of other causes, or Jan 1, 2017, whichever occurred first) was used as the timescale in the model. Models were also fitted additionally and sequentially adjusting for other gastric cancer risk factors, including smoking, alcohol drinking, a family history of cancer, body-mass index, and the consumption of some dietary factors (eg, preserved vegetables or fresh fruits). We fitted Cox regression models with piecewise constant time-varying coefficients using the Prentice estimator, with stratification by the design stratification variables (age groups and sex) and adjustment for age (years), individual study regions, and education. We additionally assessed the proportional hazards assumption using Schoenfeld residuals.
We did sensitivity analyses by assuming that all ambiguous bands were either seropositive or seronegative. Further analyses were done in subgroups defined by age (30–59 and 60–79 years), sex, area (rural or urban), education (two groups: no formal education or primary school, and middle school or higher), and body-mass index (<25 or ≥25 kg/m2); and among men only, by regular smoking and alcohol drinking. HRs were also calculated for different gastric cancer histopathological subtypes and by the time since blood collection (individuals with ambiguous H pylori status were excluded from this analysis). To facilitate comparisons with previous studies, additional sensitivity analyses were done using the clinical criteria to define H pylori seropositivity.
Population attributable fractions (PAFs) were calculated as PAF=Pc (R–1) / R, where Pc is the observed seroprevalence among the cases and R is the estimated HR associated with H pylori infection in the study.2 The number of new gastric cancer cases attributable to H pylori infection was estimated by multiplying the PAF by the national incidence rates of gastric cancer in China in 2018.19 Statistical analysis was done with R (version 4.0.2).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
After a median 10·1 years (IQR 9·2–11·1) of follow-up, the incidence in the whole cohort was 57·6 per 100 000 person-years for NCGC and 10·5 per 100 000 person-years for CGC, both of which increased with age. In each age group, men had a nearly 3 times higher rate than did women (appendix p 4). One case of CGC and one case of NCGC had missing H pylori data and were not included in analyses.
Compared with the subcohort participants, participants with NCGC were more likely to be urban residents; to have a higher socioeconomic status (measured by both education and household income); to consume meat, dairy products, and fresh fruits; to be regular smokers or alcohol drinkers (among men); and to report good self-reported health at baseline (table 1). For participants with CGC, however, the converse appeared to be true, except for preserved vegetables and spicy food, for which both cancer groups showed similar patterns to each other compared with subcohort participants (table 1). Because of the higher retrieval rates of medical notes in urban than in rural hospitals, disproportionally more participants with NCGC were selected from urban areas, which is reflected in the slightly better socioeconomic and health status among the selected participants with NCGC compared with those not selected for the study (appendix p 5).
Table 1.
Baseline characteristics of patients with gastric cancer (NCGC and CGC) and subcohort participants
| NCGC (n=499) | CGC (n=436) | Subcohort (n=500) | ||
|---|---|---|---|---|
| Demographic factors | ||||
| Age (years) | 59·0 (9·5) | 61·2 (8·6) | 59·1 (9·9) | |
| Sex | ||||
| Men | 327 (66%) | 325 (75%) | 347 (69%) | |
| Women | 172 (34%) | 111 (25%) | 153 (31%) | |
| Urban residents | 351 (70%) | 159 (36%) | 252 (50%) | |
| ≥6 years of education | 225 (45%) | 141 (32%) | 217 (43%) | |
| Household income (>¥20 000 per year) | 231 (46%) | 132 (30%) | 210 (42%) | |
| Lifestyle factors | ||||
| Current regular smoking | ||||
| Men | 230 (70%) | 186 (57%) | 202 (58%) | |
| Women | 5 (3%) | 0 | 6 (4%) | |
| Current regular alcohol drinking | ||||
| Men | 134 (41%) | 89 (27%) | 111 (32%) | |
| Women | 2 (1%) | 0 | 7 (5%) | |
| Physical activity (MET-h per day) | 19·5 (15·2) | 18·1 (15·5) | 17·9 (14·0) | |
| Medical history and health status | ||||
| Poor self-rated health at baseline | 46 (9%) | 53 (12%) | 55 (11%) | |
| Diabetes (self-reported or screen detected) | 32 (6%) | 25 (6%) | 44 (9%) | |
| A history of peptic ulcer | 31 (6%) | 23 (5%) | 27 (5%) | |
| A history of cirrhosis or chronic hepatitis | 3 (1%) | 3 (1%) | 6 (1%) | |
| A history of CHD, stroke, or TIA | 28 (6%) | 31 (7%) | 52 (10%) | |
| A history of emphysema or bronchitis | 12 (2%) | 18 (4%) | 21 (4%) | |
| Anthropometry and blood pressure | ||||
| Body mass index (kg/m2) | 23·6 (3·4) | 24·0 (3·5) | 23·7 (3·5) | |
| Underweight (<18·5 kg/m2) | 25 (5%) | 14 (3%) | 26 (5%) | |
| Normal (18·5–24·9 kg/m2) | 314 (63%) | 266 (61%) | 301 (60%) | |
| Overweight (25·0–29·9 kg/m2) | 136 (27%) | 135 (31%) | 150 (30%) | |
| Obese (≥30 kg/m2) | 24 (5%) | 21 (5%) | 23 (5%) | |
| Waist circumference (cm) | 81·1 (10·1) | 82·3 (10·2) | 81·4 (10·3) | |
| SBP (mm Hg) | 135·3 (22·8) | 138·0 (23·1) | 135·8 (22·5) | |
| Daily dietary consumption | ||||
| Rice | 337 (68%) | 183 (42%) | 358 (72%) | |
| Wheat | 221 (44%) | 271 (62%) | 188 (38%) | |
| Meat | 174 (35%) | 69 (16%) | 150 (30%) | |
| Poultry | 6 (1%) | 0 | 2 (0%) | |
| Dairy | 86 (17%) | 41 (9.4) | 64 (12.8) | |
| Fresh vegetables | 474 (95%) | 424 (97%) | 481 (96%) | |
| Soybean | 24 (5%) | 14 (3%) | 18 (4%) | |
| Preserved vegetables | 160 (32%) | 105 (24%) | 86 (17%) | |
| Fresh fruit | 125 (25%) | 32 (7%) | 97 (19%) | |
| Spicy food | 86 (17%) | 41 (9%) | 122 (24%) | |
Data are mean (SD) or number (%). CGC=cardia gastric cancer. CHD=coronary heart disease. MET-h=metabolic equivalent of task-hours. NCGC=non-cardia gastric cancer. SBP=systolic blood pressure. TIA=transient ischaemic attack.
Overall, the seroprevalence of H pylori infection was 94·4% (95% CI 92·4–96·4) in participants with NCGC, 92·2% (89·7–94·7) in participants with CGC, and 75·6% (71·8–79·4) in subcohort participants, with a further 12 (2·4%) of those with NCGC, 7 (1·6%) of those with CGC, and 21 (4·2%) in the subcohort participants classified as seroambiguous (appendix p 6). In the subcohort, the seroprevalence of H pylori infection was higher among urban or highly educated participants, but did not vary substantially by age or sex (appendix p 7). Among the seven biomarkers measured, cytotoxin-associated antigen had the highest seroprevalence in each study group (465 [93·2%, 95% CI 91·0–95·4] for NCGC, 400 [91·7%, 89·2–94·3] for CGC, and 370 [74·1%, 70·3–78·0] for the subcohort; appendix p 6). Moderate correlations between different H pylori biomarkers were observed (eg, r=0·58 between cytotoxin-associated antigen and vacuolating cytotoxin, and r=0·48 between cytotoxin-associated antigen and urease enzyme light subunit; appendix p 8).
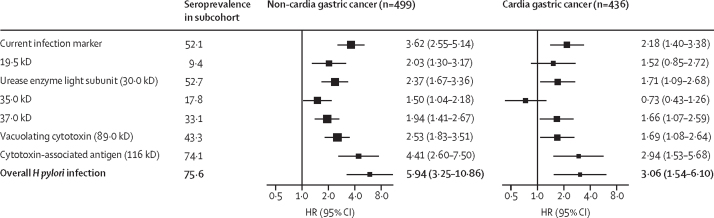
Compared with H pylori seronegative participants, those who were seropositive had adjusted HRs of 5·94 (95% CI 3·25–10·86) for NCGC and 3·06 (1·54–6·10) for CGC (figure 1). Additional adjustments for other risk factors did not materially alter the risk estimates (appendix p 9). The HRs did not differ significantly between women and men for both NCGC (16·96 vs 4·25) and CGC (7·16 vs 2·55) or between younger and older age groups (appendix p 10). Furthermore, the HRs did not differ significantly in population subgroups defined by other factors such as area, education, smoking, alcohol drinking, body-mass index, and family history of cancer (appendix p 10).
Figure 1.
Associations of Helicobacter pylori infection, overall and by individual biomarkers, with risks of non-cardia gastric cancer and cardia gastric cancer
H pylori infection was established using epidemiological criteria. Cox regression, with the time in the study as the timescale fitted using the Borgan III estimator, was used to estimate HRs, with adjustment for age, sex, area, and education. The black squares represent the adjusted HRs, with the area inversely proportional to the variance of the log HRs, and the horizontal lines representing their corresponding 95% CIs. HR=hazard ratio.
In sensitivity analyses using the clinical criteria to define H pylori infection, the seroprevalence in the NCGC group was 418 (84%) of 499, in the CGC group, 347 (80%) of 436, and in the subcohort, 288 (58%) of 500, with adjusted HRs of 4·68 (95% CI 3·13–6·98) for NCGC and 2·36 (1·42–3·94) for CGC (appendix p 11).
Among the seven individual biomarkers, cytotoxin-associated antigen showed the strongest association with the risks of NCGC and CGC, with HRs of 4·41 (95% CI 2·60–7·50) for NCGC and 2·94 (1·53–5·68) for CGC (figure 1). The other biomarkers were each associated with an approximately 2 times increased risk of both cancers, except for a null association of the 35·0 kD band with CGC risk (figure 1). For both NCGC and CGC there were also indications of positive dose–response associations with the number of positive H pylori biomarkers. Compared with individuals who were seronegative for all relevant biomarkers, individuals who were seropositive for both cytotoxin-associated antigen and vacuolating cytotoxin had HRs of 6·29 (95% CI 3·56–11·12) for NCGC and 3·23 (1·59–6·56) for CGC, whereas for those who were seropositive for both cytotoxin-associated antigen and urease enzyme light subunit, the HRs were 5·01 (2·70–9·29) for NCGC and 2·54 (1·20–5·39) for CGC. Being seropositive for cytotoxin-associated antigen, vacuolating cytotoxin, and urease enzyme light subunit did not further increase the risk estimates (appendix p 12).
For oesophageal adenocarcinoma, although the adjusted HRs for overall or individual biomarkers seropositivity were generally similar to those for CGC, the 95% CIs were wide because of the small number of cases involved (appendix p 13).
Although no statistically significant time interaction was detected, for both NCGC and CGC the HRs at 3–4 years were approximately half of those at 5–6 or 7 years or more since sample collection (table 2). The associations did not differ significantly between major histopathological subtypes of cancer (appendix p 14).
Table 2.
Adjusted HRs for NCGC and CGC associated with Helicobacter pylori infection stratified by time since blood sample collection
| Number of cases | HR (95% CI)* | |
|---|---|---|
| NCGC | ||
| 3–4 years | 123 | 3·12 (1·38–7·07) |
| 5–6 years | 156 | 7·55 (2·66–21·45) |
| ≥7 years | 208 | 8·05 (3·12–20·75) |
| CGC | ||
| 3–4 years | 97 | 1·78 (0·74–4·26) |
| 5–6 years | 109 | 5·11 (1·59–16·35) |
| ≥7 years | 223 | 3·24 (1·52–6·87) |
CGC=cardia gastric cancer. HR=hazard ratio. NCGC=non-cardia gastric cancer.
HRs estimated using Cox regression fitted using the Prentice estimator, with stratification by the design stratification variables (age groups and sex) and adjustment for age (years), area, and education.
Compared with seronegative participants, those with ambiguous H pylori infection status had adjusted HRs of 4·08 (95% CI 1·56–10·66) for NCGC and of 0·99 (95% CI 0·23–4·21) for CGC. When classifying all ambiguous results as seropositive or seronegative, the HRs were attenuated for both NCGC and CGC (appendix p 15).
In this population, we estimated that the PAF for H pylori was 78·5% for NCGC and 62·1% for CGC. We estimated that based on the 2018 China cancer statistics,19 H pylori infection would have caused approximately 339 955 new cases of gastric cancer (271 389 NCGC cases and 68 566 CGC cases) in China annually (table 3).
Table 3.
The estimated numbers of cases attributable to Helicobacter pylori in China
| Seropositive (%, 95% CI) | HR (95% CI)* | Population attributable fractions (%) | Attributable cases | |
|---|---|---|---|---|
| NCGC | 471/499 (94·4%, 92·4–96·4%) | 5·94 (3·25–10·86) | 78·5% | 271 389 |
| CGC | 402/436 (92·2%, 89·7–94·7%) | 3·06 (1·54–6·10) | 62·1% | 68 566 |
CGC=cardia gastric cancer. HR=hazard ratio. NCGC=non-cardia gastric cancer.
HRs estimated using the Borgan III estimator and adjusted for age (years), sex, individual study regions, and education.
Discussion
This large prospective case-cohort study shows clearly that H pylori infection was a strong risk factor not only for NCGC but also for CGC in Chinese adults. Using a sensitive immunoblot assay together with the exclusion of the first 2 years after sample collection, we showed that H pylori infection was associated with a 6 times higher risk of NCGC and a 3 times higher risk of CGC. It is likely that the observed associations are largely causal, hence H pylori infection would have caused approximately 80% of NCGC and more than 60% of CGC in this population.
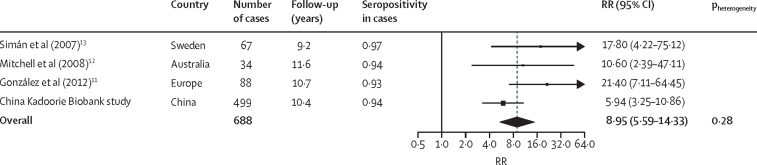
Previous studies of H pylori infection and gastric cancer were mainly based on the less sensitive ELISA5, 7, 9, 20 or, more recently, multiplex serology assays.21, 22, 23, 24 Although an increased risk of NCGC with H pylori has been shown consistently, the magnitude of the association varied greatly between studies, and was generally smaller in Chinese populations than in populations in Europe, the USA, and Australia. In a pooled analysis of 12 prospective studies,5 H pylori infection established by ELISA was associated with an odds ratio (OR) of 3·0 (95% CI 2·3–3·8) for NCGC (n=762 cases), increasing to 5·9 (3·4–10·3) among individuals with blood samples collected more than 10 years before cancer diagnosis (n=223 cases). There is evidence from studies in Europe and Australia that the use of immunoblot assay leads to greater risk estimates than those based on ELISA. In the Eurogast-EPIC study involving 88 NCGC cases and 338 controls,11 the OR for NCGC was 21·4 (95% CI 7·1–64·4) using similar seropositivity criteria to those in the present study, which was three times as high as that based on ELISA (6·8 [95% CI 3·0–15·1]). Similar but less striking differences were also shown in another two small studies comparing immunoblot assays with ELISA assays in the same study populations.12, 13 Overall, these three studies in Europe and Australia based on immunoblot assays with a total of just 189 participants with NCGC yielded a pooled RR of 17·0 (95% CI 11·6–25·0),6 which has been used to estimate the global burden of NCGC attributable to H pylori infection.2 For comparison, in our study using the same immunoblot assay and with more than 2·5 times as many participants as in all the three studies combined, the RR estimate for NCGC was only approximately a third of those of the previous studies. The reasons for such a large difference in the risk estimates are not fully understood, but might reflect the differences in the background prevalence of H pylori infection in China (75% in the China Kadoorie Biobank study vs 59% in Eurogast-EPIC11), and potentially a comparatively more notable role of other factors in the cause of gastric cancer in Chinese populations than other populations. Although not significant, we observed higher HRs in women, who rarely smoked or drank alcohol, than in men. In a combined analysis of the present study and the published data from these three studies in Europe and Australia,6 the pooled RR was 8·95 (5·59–14·33; figure 2), and there was no significant heterogeneity in the risk estimates from these four different studies. This updated risk estimate for NCGC should help inform the future estimation of the disease burden associated with H pylori infection, both regionally and globally.
Figure 2.
Meta-analysis of the association of Helicobacter pylori with non-cardia gastric cancer in the China Kadoorie Biobank study and three published studies using the same immunoblot assay
The combined estimate is an inverse-variance weighted average. The black squares represent RR, with the area inversely proportional to the variance of the log RR, and the horizontal lines represent their corresponding 95% CIs. The dotted vertical line indicates the overall RR, and the black diamond indicates it and its 95% CI. pheterogeneity denotes the p value for heterogeneity across the four studies. RR=relative risk.
The existing evidence linking CGC with H pylori infection remains conflicting, with studies from Europe, the USA, and Australia generally reporting null or inverse associations, whereas studies from east Asian countries tended to show modest positive associations. CGC is defined as a lesion with its centre located within 1 cm proximal and 2 cm distal to the oesophageal-gastric junction. However, there might be differences in the anatomical locations of CGC lesions between different populations, with CGC in populations in Europe, the USA, and Australia mainly involving the distal oesophagus, and in east Asians, mainly involving the proximal stomach.7, 9 In the aforementioned pooled analyses of 12 prospective studies (eight in Europe, the USA, and Australia and four in east Asian countries), overall there was no clear association of H pylori infection with CGC (274 cases; OR 0·99 [95% CI 0·72–1·35]), with one study in China (n=99) showing a significant 77% excess risk.5 In another meta-analysis of 30 studies (ten prospective and 20 retrospective),9 there was a significantly lower risk of CGC (OR 0·78, 95% CI 0·63–0·97) in 16 studies in Europe, the USA, and Australia but a significantly higher risk of CGC with H pylori infection (RR 1·98, 95% CI 1·38–2·83) in 14 east Asian studies, driven mainly by large RRs in retrospective studies. The present study provides important new evidence about the role of H pylori infection in the cause of CGC in the Chinese population. Our RR estimates are approximately twice those in previous studies of Chinese and other Asian populations that used less sensitive assays and suboptimal study designs.4, 7, 21, 22, 23, 24 Our findings are also consistent with the notion that in east Asians, CGC might mainly involve the proximal stomach.7, 9 Moreover, the associations were similar when the analyses were further confined to clinically adjudicated CGC cases (appendix p 16). Furthermore, the association of H pylori with oesophageal adenocarcinoma, which is rare in the Chinese population, was similar to the association with CGC. Further studies are warranted to further substantiate (or refute) the seemingly discrepant role of H pylori in the cause of CGC between populations in east Asia and in Europe, the USA, and Australia.
There is evidence that the development of atrophic gastritis occurs in late stage pre-malignant cases and not accounting for this occurrence might result in an underestimation of the risk associated with H pylori infection.25, 26 Indeed, our study showed that although there was no evidence that the proportional hazards assumption is inconsistent with the data, the RRs of both NCGC and CGC during the early period of follow-up (even after excluding the first 2 years of follow-up as part of our study design) tended to be lower than those during the subsequent years of follow-up. This result suggests that the exclusion of the first 2 years of follow-up might have not fully accounted for this occurrence. If this assumption were true, the real risk estimates (and the attributable fractions) for both NCGC and CGC associated with H pylori infection would be higher (appendix p 17).
A few studies have also shown the predominant role of cytotoxin-associated antigen-positive H pylori strains, in which nearly all participants with NCGC were cytotoxin-associated antigen-positive.11, 12 Among all H pylori biomarkers, antibodies to cytotoxin-associated antigen have the strongest immunoreactivity and are the last to disappear after H pylori eradication or in the precancerous stomach.25 As such, the epidemiological criteria used in the present study (and the Eurogast-EPIC study11) should provide a good indicator of past infection. Consistent with previous studies of populations in Europe, the USA, and Australia,4, 13, 23, 27, 28 our study showed that cytotoxin-associated antigen had the strongest associations with the risks of both NCGC and CGC of all the measured biomarkers among Chinese adults. Moreover, there were also higher RRs associated with larger numbers of other specific biomarkers (eg, vacuolating cytotoxin and urease enzyme light subunit) in addition to cytotoxin-associated antigen.
Apart from the prospective study design and inclusion of the large number of well characterised cases, the key strengths of our study were the use of a sensitive immunoblot assay, the exclusion of the first few years of follow-up to reduce the possible effects of atrophic gastritis on seropositivity, the ability to adjust for many known or potential risk factors for gastric cancer, and for individual study regions to account for large regional variations in H pylori seroprevalence and cancer rates.16 Furthermore, our study was the first to show separate results from seroambiguous bands; misclassification of those as seropositive or seronegative is likely to bias the risk estimates. However, the study also has limitations. First, H pylori has approximately 1500 proteins,10 and the HelicoBlot 2.1 assay only covers seven of them. Hence, the effects of other H pylori proteins and their interactions could not be investigated. Moreover, the immunoblot assay used was developed on the basis of the H pylori strains typically seen in populations in Europe, the USA, and Australia, with no available data for separate validation nor direct comparison with conventional ELISA assays in Chinese populations. Second, because of the high retrieval rates of medical notes in urban hospitals, more participants with NCGC in urban areas were selected in our study. However, with comprehensive adjustment for confounding, this difference is unlikely to affect the RR estimates. For participants with CGC, given the low proportion of adjudicated cases, we also included reported cases, all of which were specifically ICD-10 coded as C16·0 when first reported, thus minimising the risk of misclassification. Third, we did not investigate simultaneously the role of other pathogens (eg, Epstein-Barr virus) and their potential interactions with H pylori in the causation of gastric cancer. Lastly, although various measures were taken in analyses, residual confounding and other unknown biases might persist.
In summary, the present study provides robust new evidence about the causal role of H pylori infection in both NCGC and CGC in China, where H pylori infection is widespread. The observed associations are probably largely causal and suggest that H pylori infection causes approximately 0·34 million gastric cancer cases annually in China. In addition to improving the estimation of the global burden of cancer because of H pylori infection, our study findings should also inform prevention strategies in China and elsewhere. To date, there is no prophylactic or therapeutic vaccination against H pylori. Moreover, despite the availability of highly cost-effective anti-H pylori treatments, population-based screening and eradication have not yet been included in the national cancer primary prevention programme in China. Our findings, together with evidence from a randomised controlled trial of reduced gastric cancer risk after H pylori eradication,29 suggest that population-based H pylori mass screening and eradication should be considered as a key strategy for gastric cancer prevention, before considering mass gastric cancer screening by barium photofluorography or endoscopy in China and high-risk settings globally.
Data sharing
Anonymised baseline, resurvey, and cause-specific mortality and morbidity data are available for access through a formal application on the China Kadoorie Biobank website. The application will then be reviewed by a Data Access Committee. Further details about the access policy and procedures can be found on the website.
For the China Kadoorie Biobank website see www.ckbiobank.org
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This study was funded by Cancer Research UK Prevention and Population Research Committee Project Award (C56488/A24504). The China Kadoorie Biobank baseline survey was supported by the Kadoorie Charitable Foundation (Hong Kong). The China Kadoorie Biobank long-term follow-up was supported by grants from the UK Wellcome Trust (212946/Z/18/Z, 202922/Z/16/Z, 104085/Z/14/Z, and 088158/Z/09/Z), National Natural Science Foundation of China (91843302), and National Key Research and Development Programme of China (2016YFC 0900500, 0900501, 0900504, and 1303904). The UK Medical Research Council (MC_UU_00017/1, MC_UU_12026/2, and MC_U137686851), Cancer Research UK (C16077/A29186 and C500/A16896), and British Heart Foundation (CH/1996001/9454) provided core funding for the project. We thank the participants, project staff, and China National Centre for Disease Control and Prevention and its regional offices for access to death and disease registries. We also wish to thank Andrew Gordon at the Nuffield Department of Population Health Wolfson Laboratories at the University of Oxford (Oxford, UK) and Francis Megraud and his group at Laboratoire de Bacteriologie (Bordeaux, France) for technical assistance.
Contributors
LY, CK, IYM, LL, and ZC had full access to the study data and verified the underlying study data. DC, SC, and MH did all the laboratory tests including the initial interpretation of the assay data in the Nuffield Department of Population Health Wolfson Laboratories. LY, CK, IYM, PY, CdM, MP, RGW, RJ, TW, GMC, SF, and RP contributed to the conception of this paper, interpretation of the results, and the revision of the manuscript. YG, YC, PP, JL, CY, and LL contributed to data acquisition. All authors were involved in study design, analysis of data, interpretation, and writing of the report, and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8:e180–e190. doi: 10.1016/S2214-109X(19)30488-7. [DOI] [PubMed] [Google Scholar]
- 3.Franceschi S, Herrero R. In: World Cancer Report 2014. Steward BW, Wild CP, editors. International Agency for Research on Cancer; Lyon: 2014. Infections. [Google Scholar]
- 4.Cai H, Ye F, Michel A, et al. Helicobacter pylori blood biomarker for gastric cancer risk in east Asia. Int J Epidemiol. 2016;45:774–781. doi: 10.1093/ije/dyw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helicobacter and Cancer Collaborative Group Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–353. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487–490. doi: 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- 7.Kamangar F, Qiao YL, Blaser MJ, et al. Helicobacter pylori and oesophageal and gastric cancers in a prospective study in China. Br J Cancer. 2007;96:172–176. doi: 10.1038/sj.bjc.6603517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan JM, Yu MC, Xu WW, Cockburn M, Gao YT, Ross RK. Helicobacter pylori infection and risk of gastric cancer in Shanghai, China: updated results based upon a locally developed and validated assay and further follow-up of the cohort. Cancer Epidemiol Biomarkers Prev. 1999;8:621–624. [PubMed] [Google Scholar]
- 9.Cavaleiro-Pinto M, Peleteiro B, Lunet N, Barros H. Helicobacter pylori infection and gastric cardia cancer: systematic review and meta-analysis. Cancer Causes Control. 2011;22:375–387. doi: 10.1007/s10552-010-9707-2. [DOI] [PubMed] [Google Scholar]
- 10.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 11.González CA, Megraud F, Buissonniere A, et al. Helicobacter pylori infection assessed by ELISA and by immunoblot and noncardia gastric cancer risk in a prospective study: the Eurgast-EPIC project. Ann Oncol. 2012;23:1320–1324. doi: 10.1093/annonc/mdr384. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell H, English DR, Elliott F, et al. Immunoblotting using multiple antigens is essential to demonstrate the true risk of Helicobacter pylori infection for gastric cancer. Aliment Pharmacol Ther. 2008;28:903–910. doi: 10.1111/j.1365-2036.2008.03792.x. [DOI] [PubMed] [Google Scholar]
- 13.Simán JH, Engstrand L, Berglund G, Forsgren A, Florén CH. Helicobacter pylori and CagA seropositivity and its association with gastric and oesophageal carcinoma. Scand J Gastroenterol. 2007;42:933–940. doi: 10.1080/00365520601173863. [DOI] [PubMed] [Google Scholar]
- 14.Cover TL. Helicobacter pylori diversity and gastric cancer risk. MBio. 2016;7:e01869–e01875. doi: 10.1128/mBio.01869-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z, Chen J, Collins R, et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol. 2011;40:1652–1666. doi: 10.1093/ije/dyr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monteiro L, de Mascarel A, Sarrasqueta AM, et al. Diagnosis of Helicobacter pylori infection: noninvasive methods compared to invasive methods and evaluation of two new tests. Am J Gastroenterol. 2001;96:353–358. doi: 10.1111/j.1572-0241.2001.03518.x. [DOI] [PubMed] [Google Scholar]
- 17.Lu C-Y, Kuo C-H, Lo Y-C, et al. The best method of detecting prior Helicobacter pylori infection. World J Gastroenterol. 2005;11:5672–5676. doi: 10.3748/wjg.v11.i36.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borgan O, Langholz B, Samuelsen SO, Goldstein L, Pogoda J. Exposure stratified case-cohort designs. Lifetime Data Anal. 2000;6:39–58. doi: 10.1023/a:1009661900674. [DOI] [PubMed] [Google Scholar]
- 19.Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69:1564–1571. doi: 10.1136/gutjnl-2020-321600. [DOI] [PubMed] [Google Scholar]
- 20.Abnet CC, Zheng W, Ye W, et al. Plasma pepsinogens, antibodies against Helicobacter pylori, and risk of gastric cancer in the Shanghai Women's Health Study Cohort. Br J Cancer. 2011;104:1511–1516. doi: 10.1038/bjc.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butt J, Varga MG, Wang T, et al. Smoking, Helicobacter Pylori serology, and gastric cancer risk in prospective studies from China, Japan, and Korea. Cancer Prev Res (Phila) 2019;12:667–674. doi: 10.1158/1940-6207.CAPR-19-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epplein M, Zheng W, Xiang YB, et al. Prospective study of Helicobacter pylori biomarkers for gastric cancer risk among Chinese men. Cancer Epidemiol Biomarkers Prev. 2012;21:2185–2192. doi: 10.1158/1055-9965.EPI-12-0792-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao L, Michel A, Weck MN, Arndt V, Pawlita M, Brenner H. Helicobacter pylori infection and gastric cancer risk: evaluation of 15 H pylori proteins determined by novel multiplex serology. Cancer Res. 2009;69:6164–6170. doi: 10.1158/0008-5472.CAN-09-0596. [DOI] [PubMed] [Google Scholar]
- 24.Murphy G, Freedman ND, Michel A, et al. Prospective study of Helicobacter pylori antigens and gastric noncardia cancer risk in the nutrition intervention trial cohort. Int J Cancer. 2015;137:1938–1946. doi: 10.1002/ijc.29543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plummer M, van Doorn LJ, Franceschi S, et al. Helicobacter pylori cytotoxin-associated genotype and gastric precancerous lesions. J Natl Cancer Inst. 2007;99:1328–1334. doi: 10.1093/jnci/djm120. [DOI] [PubMed] [Google Scholar]
- 26.You WC, Zhang L, Gail MH, et al. Gastric dysplasia and gastric cancer: Helicobacter pylori, serum vitamin C, and other risk factors. J Natl Cancer Inst. 2000;92:1607–1612. doi: 10.1093/jnci/92.19.1607. [DOI] [PubMed] [Google Scholar]
- 27.Shakeri R, Malekzadeh R, Nasrollahzadeh D, et al. Multiplex H pylori serology and risk of gastric cardia and noncardia adenocarcinomas. Cancer Res. 2015;75:4876–4883. doi: 10.1158/0008-5472.CAN-15-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song H, Michel A, Nyrén O, Ekström AM, Pawlita M, Ye W. A CagA-independent cluster of antigens related to the risk of noncardia gastric cancer: associations between Helicobacter pylori antibodies and gastric adenocarcinoma explored by multiplex serology. Int J Cancer. 2014;134:2942–2950. doi: 10.1002/ijc.28621. [DOI] [PubMed] [Google Scholar]
- 29.Li WQ, Zhang JY, Ma JL, et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial. BMJ. 2019;366 doi: 10.1136/bmj.l5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymised baseline, resurvey, and cause-specific mortality and morbidity data are available for access through a formal application on the China Kadoorie Biobank website. The application will then be reviewed by a Data Access Committee. Further details about the access policy and procedures can be found on the website.
For the China Kadoorie Biobank website see www.ckbiobank.org