Abstract
Background
Coronavirus Disease‐19 (COVID‐19) has high mortality in kidney transplant recipients (KTR), and vaccination against severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2) is vital for this population. Although the humoral response to messenger RNA vaccines was shown to be impaired in KTR, there is a lack of data regarding the antibody response to inactivated vaccines. We investigated the antibody response to two consequent doses of the inactivated SARS‐CoV‐2 vaccine (CoronaVac; Sinovac Biotech, China).
Methods
A total of 118 patients from two centers were included. The levels of anti‐SARS‐CoV‐2 immunoglobulin‐G antibodies against the nucleocapsid and spike antigens were determined with enzyme immunoassay (DIA.PRO; Milano, Italy) before the vaccine and one month after the second dose of the vaccine. Thirty‐three patients were excluded due to antibody positivity in the serum samples obtained before vaccination.
Results
Eighty‐five patients, 47 of whom were female, with a mean age of 46 ± 12, were included in the statistical analysis. The maintenance immunosuppressive therapy comprised tacrolimus (88.2%), mycophenolate (63.6%), and low‐dose steroids (95.3%) in the majority of the patients. After a median of 31 days following the second dose of the vaccine, only 16 (18.8%) patients developed an antibody response. The median (IQR) antibody level was 52.5 IU/ml (21.5–96). Age (48 vs. 38, p = .005) and serum creatinine levels (1.14 vs. 0.91, p = .04) were higher in non‐responders and were also found to be independently associated with the antibody response (odds ratio (OR): 0.93, p = 0.012 and 0.15, p = 0.045, respectively) in multivariate analysis.
Conclusion
In this study, we found the antibody response to the inactivated vaccine to be considerably low (18.8%) in KTR. Increased age and impaired renal function were associated with worse antibody response. Based on the knowledge that mRNA vaccines yield better humoral responses, this special population might be considered for additional doses of mRNA vaccination.
Keywords: antibody response, COVID‐19, inactivated vaccine, renal transplantation, SARS‐CoV‐2
1. INTRODUCTION
Chronic kidney disease patients, dialysis patients, and kidney transplant recipients (KTR) have a high risk of getting infected and developing severe coronavirus disease‐19 (COVID‐19). 1 , 2 Mortality in these patients particularly in KTR, 3 , 4 , 5 , 6 is higher than it is in the normal population. Therefore, preventive measures and vaccination against COVID‐19 are pivotal for this immunocompromised population. 7 Although impaired vaccine response is a matter of concern, most of the transplant centers have continued to perform kidney transplantation activities under the presumed protection of vaccination rather than leaving the patients on dialysis. 8 , 9
ABBREVIATIONS
- COVID‐19
coronavirus disease‐19
- IQR
interquartile range
- KTR
kidney transplant recipients
- SARS‐CoV‐2
severe acute respiratory syndrome‐coronavirus‐2
Regarding the responses to inactivated vaccines, the results of a phase 3 trial in Turkey showed that CoronaVac (Sinovac Biotech, China) vaccine prevented the reverse‐transcriptase polymerase chain reaction‐confirmed symptomatic cases of COVID‐19 disease 2 weeks after the second dose, with a rate of 83.5% in 11 303 healthy volunteers. 10 Additionally, when 1413 participants were further analyzed for immunogenicity in Turkey's phase 3 trial, 89.7% of the vaccine group were found to be seropositive for IgG and IgM antibodies against SARS‐CoV‐2 spike protein. 10 On the other hand, inactivated SARS‐CoV‐2 vaccine effectiveness was reported to be 65.9% in 10.2 million people in Chile. 11 In Turkey, however, because immunosuppressive patients were excluded in the phase 3 trial, there are no data regarding the responses to the inactivated vaccine in this group. Even so, vaccine responses to SARS‐CoV‐2 mRNA vaccines are well known to be impaired in transplantation settings. 12 , 13 , 14
In Turkey, mRNA vaccines were not available for the public vaccination program until mid‐2021, so nearly all of the transplant patients were vaccinated with an inactivated vaccine. Although there is evidence that responses to inactivated SARS‐CoV‐2 vaccine were poor in the patients who were on an immunosuppressant therapy due to immune‐mediated diseases, 15 it is not clear whether the humoral response would be sufficient in KTR. In this study, we investigated the antibody response and possible risk factors affecting this response to two doses of the CoronaVac vaccine.
2. METHODS
In Turkey, the vaccination campaign started on January 14, 2021, with healthcare workers and continued with elderly people and patients suffering from chronic diseases. Until April 12, 2021, inactivated SARS‐CoV‐2 CoronaVac vaccine of the Sinovac company (Beijing, China) was the only available vaccine in Turkey. According to the regulations of the Ministry of Health, two doses of CoronaVac—applied 4 weeks apart—was the scheme of the vaccination program.
This two‐center cross‐sectional prospective observational study was conducted at the transplantation units of Ankara University School of Medicine and Istanbul University Istanbul School of Medicine between January 22, 2021, and June 21, 2021.
This study was approved by the Ankara University School of Medicine Ethics Committee for Clinical Studies (Approval Number: I3—207‐21). Written informed consents had been obtained from all patients before the blood samples were collected.
Before vaccination, blood samples were drawn during regular control visits before the vaccination program was initiated for transplant recipients. After vaccination, blood samples were obtained from the same patients one month following their second vaccine shots. All blood samples were centrifugated at room temperature (23°C), and the sera samples were kept at –80°C until the tests were studied. Antibody levels against spike and nucleocapsid proteins were simultaneously detected by using the COVID‐19 IgG antibody enzyme immunoassay kit (DIA.PRO, Milano, Italy). The test was performed according to the manufacturer's instructions and a cut of the value of >10 IU/ml was accepted as positive. Positive results were categorized as equivocal (10–12 IU/ml), low positive (12–50 IU/ml), medium positive (50–250 IU/ml), high positive (250–1000 IU/ml), and very high positive (≥1000 IU/ml) according to the manual. After vaccination, serum samples of six patients which yielded equivocal results in the initial testing were re‐tested in duplicate wells after re‐centrifugation of the serum samples and all of them gave negative results in the second study.
2.1. Statistical analysis
Clinical and laboratory data are expressed as percentages, means (±SD), or medians [interquartile range (IQR)], as appropriate. Continuous variables in the characteristics of the two groups were compared by t‐test or Mann–Whitney U test and categorical variables with Pearson's chi‐square or Fischer exact tests. Logistic regression analyses were performed to study associations between positive antibody results (dependent variable) and predictor variables. Multivariate logistic regression analysis incorporated age, gender, mycophenolate treatment, and serum creatinine level as possible independent risk factors due to their p‐values being <0.1 in univariate analysis or their clinical relevance of humoral vaccine response in previous studies. The final model was reached by the backward stepwise regression (Backward LR) method incorporating constant. The quality of adjustment of the model was tested with the Hosmer‐Lemeshow statistic. Odds ratios (ORs) were expressed with 95% confidence intervals (CIs). A threshold value of p < .05 was considered statistically significant. The calculations were carried out with IBM SPSS 23 (IBM SPSS v.23, Armonk, NY, USA).
3. RESULTS
A total of 373 patients’ samples were collected before vaccination. However, due to an increase in COVID‐19 case numbers and the partial lock‐down periods during our study, most of the patients could not come to the hospital to give their post‐vaccination blood samples. We also excluded the patients who had their second vaccine dose less than 30 days prior. Therefore, 118 adult patients whose post‐vaccination serum samples were available were included in the study (78 from Ankara University and 40 from Istanbul University). Of these 118 patients, 33 patients, who had positive COVID‐19 antibody response in their initial samples taken before vaccination, and thus were excluded from the study. Of these 33 patients, nine were diagnosed with PCR‐confirmed COVID‐19. Two patients had pneumonia and required hospitalization; however, seven of them had mild disease. The rest of the patients did not report any symptoms related to COVID‐19 or any contact with individuals diagnosed with COVID‐19. Regarding the serological results of those 33 patients that were excluded, 28 of the patients’ COVID‐19 IgG antibody results remained positive after two doses of inactivated vaccine. We did not observe any significant increase in antibody titer after two doses of the vaccine (median COVID‐19 IgG (IU/ml), IQR: 43 (17.2–69.9) vs. 43.4 (20.2–100), p = .43).
In the end, statistical analysis was performed with 85 patients (Figure 1).
FIGURE 1.
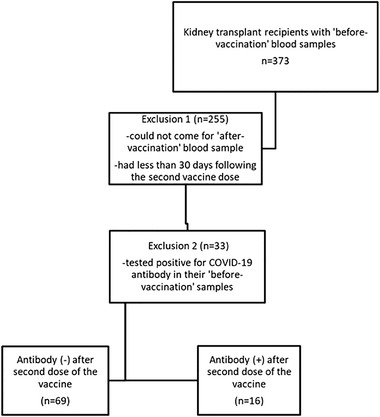
Flowchart of the patients in the study
In total, out of 85 patients whose mean age was 46 ± 12 only 16 (18.8%) patients developed an antibody response to the inactivated SARS‐CoV‐2 vaccine 4 weeks following the second dose of the vaccine.
There were 47 (55.3%) female patients and gender rates were not different among the patients who had a positive antibody response. The patients who had a positive antibody response were younger than the non‐responders (Table 1). There was no difference regarding donor type, human leukocyte antigen mismatch, the time elapsed after transplantation, renal replacement therapy history, or renal replacement therapy duration between the two groups (Table 1). Immunosuppressive therapy regimens including induction or maintenance therapy and history of rejection or antirejection therapy were not different among the groups (Table 1).
TABLE 1.
Baseline characteristics and comparisons of the patients regarding antibody response
| All patients n = 85 | Antibody (–) n = 69 | Antibody (+) n = 16 (18.8%) | p‐value | |
|---|---|---|---|---|
| Gender, F/M (n, %) | 47/38, 55.3/44.7 | 25/34, 50.7/49.3 | 12/4, 75/25 | .07 |
| Age, year (mean ±SD) | 46.4 ± 12.5 | 48±11 | 38 ± 12 | .005 |
| Primary kidney disease | .48 | |||
| Diabetic nephropathy | 9, 10.5% | 9, 13% | – | |
| Hereditary kidney diseases | 7, 8.2% | 4, 5.7% | 3, 18.7% | |
| Hypertension | 4, 4.7% | 4, 5.7% | – | |
| Chronic glomerulonephritis | 28, 32.9% | 24, 34.7% | 4, 25% | |
| Chronic TIN | 19, 22.3% | 15, 21.7% | 4, 25% | |
| Unknown | 18, 21.1% | 13, 18.8% | 5, 31.2% | |
| Donor type (n,%) | .23 | |||
| Deceased | 22, 25.9% | 16, 23.2% | 10, 62.5% | |
| Living | 63, 74.1% | 53, 76.8% | 6, %37.5 | |
| HLA mismatch (median, IQR) | 3 (2–4) | 3 (2–4) | 3 (1–4) | .65 |
| Time after transplantation, month (mean ± SD) | 82 ± 68 | 86 ± 72 | 69 ± 42 | .71 |
| Preemptive transplantation (n, %) | 22, 25.9% | 20, 28.9% | 2, 12.5% | .16 |
| RRT history | .39 | |||
| HD | 44, 51.8% | 35, 50.7% | 9, 56.3% | |
| PD | 9, 10.6% | 5, 7.2% | 4, 25% | |
| TX | 1, 1.2% | 1, 1.4% | – | |
| Multiple | 8, 9.4% | 7, 10.1% | 1, 6.3% | |
| Second transplantation (n, %) | 9, %10.6 | 8, 11.6% | 1, 6.3% | .52 |
| RRT duration, month (mean ± SD) | 44.8 ± 7 | 39 ± 7 | 65 ± 20 | .11 |
| Induction therapy (n, %) | .29 | |||
| No | 25, 29.4% | 22, 31.9% | 3, 18.8% | |
| ATG | 28, 32.9% | 21, 30.4% | 7, 43.7% | |
| Basiliksimab | 19, 22.3% | 15, 21.7% | 4, 25% | |
| History of ATG (n, %) | 30, 35.3% | 23, 33.3% | 7, 43.8% | .8 |
| Maintenance immunosuppression (n, %) | ||||
| Tacrolimus | 75, 88.2% | 61, 88.4% | 14, 87.5% | .57 |
| Cyclosporine | 5, 5.9% | 4, 5.8% | 1, 6.3% | .89 |
| MMF‐MPA | 54, 63.6% | 45, 65.2% | 9, 56.3% | .7 |
| AZA | 28, 32.9% | 22, 31.9% | 6, 37.5% | .54 |
| mTOR inhibitors | 1, 1.1% | 1, 2.9% | – | – |
| Steroids | 81, 95.3% | 67, 97.1% | 14, 87.5% | .1 |
| History of Rejection (n, %) | 12, 14,1% | 10, 14.5% | 2, 12.5% | .83 |
| AMR | 4, 33.3% | 4, 5.8% | – | |
| TCR | 4, 33.3% | 3, 4.3% | 1, 6.3% | |
| Mixt | 4, 33.3% | 3, 4.3% | 1, 6.3% | |
| Serum Creatinine level, mg/dL (median, IQR) | 1.11 (0.84–1.33) | 1.14 (0.92–1.36) | 0.91 (0.62–1.27) | .04 |
| eGFR, ml/min/1.73m2 (median, IQR) | 70 (53–88) | 67.4 (53–81) | 93.3 (55–116) | .04 |
| Proteinuria, mg/day (median, IQR) | 129 (100–254) | 131 (100–234) | 100 (100–297) | .72 |
| Other vaccines in last 2 years, (n, %) | ||||
| Pneumococcal vaccine | 28, 32,9% | 24, 34.8% | 4, 25% | 1 |
| Influenza vaccine | 35, 41,2% | 32, 46.4% | 3, 18.8% | .15 |
| BCG vaccine | 55, 64,7% | 47, 68.1% | 8, 50% | .56 |
| COVID‐19 IgG level after vaccination, IU/mL (median, IQR) | 3.27 (0.85–7.27) (0–99.9) | 2.04 (0.58–5.02) | 52.5 (21.5–96) | – |
| COVID‐19 IgG level after vaccination, IU/ml | – | – | ||
| Negative (<10 IU/ml) | 69, 81.2% | |||
| Equivocal (10–12 IU/ml) | – | |||
| Low Positive (12–50 IU/ml) | 8, 9.4% | |||
| Medium Positive (50–250 IU/ml) | 8, 9.4% | |||
| High Positive (250–1000 IU/ml) | – | |||
| Adverse events | .35 | |||
| Arthralgia, myalgia, fatigue | 6, 7% | 5, 7.2% | 1, 6.2% | |
| Headache | 2, 2.3% | 2, 2.8% | – | |
| Inoculation site pain | 2, 2.3% | 1, 1.4% | 1, 6.2% | |
| Fever | 1, 1.1% | 1, 1.4% | – | |
| Hypertension | 1, 1.1% | 1, 1.4% | – | |
| Dizziness | 2, 2.3% | 2, 2.8% | – | |
| Sore throat | 1, 1.1% | 1, 1.4% | – | |
| Chest pain | 1, 1.1% | 1, 1.4% | – |
Abbreviations: AMR, antibody‐mediated rejection; ATG, anti‐thymocyte globulin; AZA, azathioprine; BCG, Bacillus Calmette‐Guerin; COVID‐19, coronavirus disease 2019.; eGFR; estimated glomerular filtration rate; F, female; HD, hemodialysis; HLA, human leukocyte antigen; IQR, interquartile range; IVIG, intravenous immune globulin; M, male; MMF/MPA, mycophenolate mofetil/mycophenolic acid; mTOR, mammalian target of rapamycin; PD, peritoneal dialysis; RRT, renal replacement therapy; SD, standard deviation; TCR, T‐cell mediated rejection; TIN, tubulointerstitial nephritis; TX, transplantation.
Serum creatinine level, which was obtained when the post‐vaccination blood samples were collected, was lower in the antibody responder group than in the non‐responder group (median, IQR: 0.91 mg/dl (0.62–1.27) vs. 1.14 mg/dl (0.92–1.36), p = .04). Unsurprisingly, the estimated glomerular filtration rate was higher in the antibody‐positive group (93.3 ml/min/1.73m2 (55–116) vs. 67.4 ml/min/1.73m2 (53–81), p = .04). Proteinuria levels were not different between the two groups.
Additionally, despite the missing data, the vaccination profile regarding influenza, pneumococcus, and Bacillus Calmette‐Guerin was similar between the two groups, and the majority of the patients had had a Bacillus Calmette‐Guerin vaccine (64.7%) (Table 1).
Four patients were diagnosed with COVID‐19 in a median of 98 days following the two doses of the SARS‐CoV‐2 vaccine. Two patients had pneumonia requiring hospitalization, and one of them died in the intensive care unit. The other two had mild COVID‐19 infection and were treated at home. Only one of them was tested positive for SARS‐CoV‐2 antibody after the two doses of the vaccine and had a favorable course of COVID‐19 with mild infection.
The median anti‐SARS‐CoV‐2 IgG level was 52.5 IU/ml (IQR: 21.5–96) one month after the second dose of the vaccine (Figure 2). None of the patients gave a high antibody response (Table 1).
FIGURE 2.
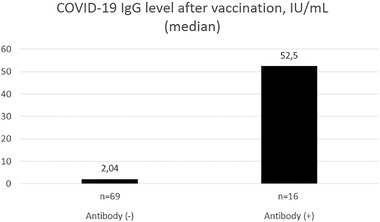
Median COVID‐19 IgG levels after vaccination
The most common adverse events related to the vaccination were arthralgia, myalgia, and fatigue (7%) in the entire patient population. There was no allergic reaction or anaphylaxis.
To assess possible factors that might have affected the antibody response, we conducted a multivariate logistic regression analysis incorporating age, gender, mycophenolate treatment, and serum creatinine level as candidate independent risk factors depending on their univariate analysis results and their clinical relevance to an antibody response. In the last multivariate model, we found that higher age (OR: 0.93 (0.88–0.98), p = .012) and higher creatinine level (OR: 0.15 (0.02–0.95), p = .045) were inversely associated with the antibody response to the vaccine even if the differences regarding these parameters were small between the two groups (Table 2).
TABLE 2.
Multivariate logistic regression analysis regarding positive antibody response to SARS‐CoV‐2
| Univariate results | Last multivariate model | |||
|---|---|---|---|---|
| Parameter | p‐value | Odds ratio (95% CI) | p‐value * | Odds ratio (95% CI) |
| Age, years | .008 | 0.93 (0.88–0.98) | .012 | 0.932 (0.883–0.985) |
| Gender (Reference: Female) | .087 | 0.343 (0.10–1.16) | ||
| MMF/MPA | .703 | 0.8 (0.25–2.51) | ||
| Serum creatinine level (mg/dL) | .051 | 0.18 (0.032–1.009) | .045 | 0.155 (0.025–0.956) |
Abbreviations: CI, confidence interval; MMF/MPA, mycophenolate mofetil/mycophenolic acid.
Final step of logistic regression analysis using Backward LR method.
4. DISCUSSION
This study indicated that, in the KTR population, humoral response to inactivated SARS‐CoV‐2 vaccine was very low (18.8%). Age and impaired renal function were negatively affecting the antibody response to vaccination. Additionally, in 33 (27.9%) of the 118 patients, SARS‐CoV‐2 antibodies were positive in the pre‐vaccination serum samples. Interestingly, of these 33 patients, only nine had been diagnosed with PCR‐confirmed COVID‐19. This shows that a considerable amount (about 20%) of our KTR patients had asymptomatic and/or undiagnosed COVID‐19 in our study.
Immunosuppressed patients including KTR are known to have poor vaccine responses compared to a healthy population. 16 Seyahi et al., 15 in their study, showed that patients with immune‐mediated diseases, especially the ones who were on immunosuppressive drugs and those aged ≥60 years, had less antibody response to CoronaVac vaccine than the healthy controls did. Bertrand et al. 14 showed that both humoral (anti‐spike antibody 88.9% vs. 17.8%) and spike‐specific T cell responses (100% vs. 57.8%) to mRNA BNT162b2 (BioNTech; Pfizer, Germany) vaccine were higher in maintenance dialysis patients than in KTR. Longlune et al. 17 and Broseta et al. 18 reported very high seroconversion rates (88.7% and 95.4%, respectively) among dialysis patients who had had at least two doses of mRNA vaccines. On this basis, transplant recipient candidates should be recommended to get vaccinated before the transplantation. Additionally, these results showed that, even if the humoral response rates were very low, the T cell response could still be induced to a certain extent. 14 The presence of a cellular response without a humoral response might also provide protection to the virus as indicated by Litjens et al. 19 in cytomegalovirus infection setting.
It is known that, especially in the early post‐transplantation period, vaccine responses become impaired with an advanced age, with an impaired kidney function, and with some particular drugs such as rituximab, belatacept, or mycophenolate mofetil. 14 , 20 , 21 , 22 Regarding the COVID‐19 vaccines, Boyarsky et al. 12 reported a 54% antibody response to two doses of the mRNA vaccine, and they concluded that poor response was a concern in the patients who were on antimetabolite immunosuppression. In line with these findings, we found that a worsening graft function and advanced age were risk factors for a poor antibody response to the vaccine. Antiproliferative usage was also less frequent in antibody‐positive patients in our study. However, this did not reach statistical significance, which was probably due to the limited number of cases.
Kamar et al. 23 reported that the antibody response rate was 40% after the second dose, and increased to 68% after the third dose of the mRNA vaccine in KTR. Forty‐four percent of the patients, who had been seronegative before the third dose, developed antibodies after the third dose. 23 From a different perspective, Chen et al. 24 showed that neutralizing antibody elicited by CoronaVac was lower for certain variants of the virus in healthy volunteers. As antibody response rate (18.8%) to CoronaVac was much lower in our patients than those achieved with mRNA vaccines, and considering the increasing numbers of the infected people and the introduction of new variants to the population, it seems crucial that KTR who received the inactivated vaccine, be vaccinated with at least one additional dose of SARS‐CoV‐2 mRNA vaccine. In Turkey, the option of getting the third dose of the vaccine has been available since June 2021 and all immunosuppressive patients should be recommended to get it.
It remains so far unknown what level of antibody titer would be preventive against SARS‐CoV‐2, but it was shown that dialysis patients had lower titers which peaked later than in normal controls, 25 and so did the KTR. 26 , 27 Although these studies were conducted with the patients who had mRNA vaccines, we found that antibody titers were very low in our study.
From an immunological point of view, it has been shown in adults that the previous vaccinations for influenza and/or pneumococcal disease do not hamper the specific immune response to SARS‐CoV‐2 BNT162b2 vaccination. 28 Here in the present study, we found similar findings with the inactivated SARS‐CoV‐2 vaccine.
There were many limitations of our study. The sample size was small and, unfortunately, we had no control group to compare the antibody response to the inactivated vaccine. However, Tanriover et al. 10 reported the seropositivity rate as 89.7% in inactivated vaccine recipients of CoronaVac in the phase 3 trial in healthcare workers. Therefore, we might assume that, in the KTR population, the humoral vaccine response to the inactivated vaccine is weaker than in healthy individuals. There were difficulties in performing multivariate models due to the small sample size. We did not have the chance to study the neutralizing activity of the antibodies or to determine the cellular vaccine response.
In conclusion, our study showed that the antibody response rate to two doses of the inactivated SARS‐CoV‐2 (CoronaVac) vaccine in KTR patients was very low (18.8%). Increased age and serum creatinine levels were associated with non‐responsiveness to the vaccine. The third dose of the vaccine, especially that of mRNA vaccines or vaccines with adjuvants, should be strongly recommended to the transplant recipients.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGMENTS
This study was funded by the Turkish Transplantation Immunology and Genetics Association (Transplantasyon İmmünolojisi ve Genetiği Derneği). We would like to thank Bio. Burcu Uysal for her technical contribution to the study.
Eren Sadioğlu R, Demir E, Evren E, et al. Antibody response to two doses of inactivated SARS‐CoV‐2 vaccine (CoronaVac) in kidney transplant recipients. Transpl Infect Dis. 2021;23:e13740. 10.1111/tid.13740
REFERENCES
- 1. Thaunat O, Legeai C, Anglicheau D, et al. IMPact of the COVID‐19 epidemic on the moRTAlity of kidney transplant recipients and candidates in a French Nationwide registry sTudy (IMPORTANT). Kidney Int. 2020;98(6):1568‐1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hsu CM, Weiner DE, Aweh G, et al. COVID‐19 among US dialysis patients: risk factors and outcomes from a national dialysis provider. Am J Kidney Dis. 2021;77(5):748‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ozturk S, Turgutalp K, Arici M, et al. Mortality analysis of COVID‐19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: a nationwide analysis from Turkey. Nephrol Dial Transplant. 2020;35(12):2083‐2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hilbrands LB, Duivenvoorden R, Vart P, et al. COVID‐19‐related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35(11):1973‐1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akalin E, Azzi Y, Bartash R, et al. Covid‐19 and kidney transplantation. N Engl J Med. 2020;382(25):2475‐2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Demir E, Uyar M, Parmaksiz E, et al. COVID‐19 in kidney transplant recipients: a multicenter experience in Istanbul. Transpl Infect Dis. 2020;22(5):e13371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Combe C, Kirsch AH, Alfano G, et al. At least 156 reasons to prioritize COVID‐19 vaccination in patients receiving in‐centre haemodialysis. Nephrol Dial Transplant. 2021;36(4):571‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Azzi Y, Bartash R, Scalea J, Loarte‐Campos P, Akalin E. COVID‐19 and solid organ transplantation: a review article. Transplantation. 2021;105(1):37‐55. [DOI] [PubMed] [Google Scholar]
- 9. Khairallah P, Aggarwal N, Awan AA, et al. The impact of COVID‐19 on kidney transplantation and the kidney transplant recipient ‐ One year into the pandemic. Transplant international. Transplant Int. 2021;34(4):612‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanriover MD, Doganay HL, Akova M, et al. Efficacy and safety of an inactivated whole‐virion SARS‐CoV‐2 vaccine (CoronaVac): interim results of a double‐blind, randomised, placebo‐controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jara A, Undurraga EA, Gonzalez C, et al. Effectiveness of an inactivated SARS‐CoV‐2 Vaccine in Chile. N Engl J Med. 2021;385(10):946‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2‐Dose SARS‐CoV‐2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204‐2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marion O, Del Bello A, Abravanel F, et al. Safety and immunogenicity of anti‐sars‐cov‐2 messenger RNA vaccines in recipients of solid organ transplants. Ann Intern Med. 2021;174(9):1336‐1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bertrand D, Hamzaoui M, Lemee V, et al. Antibody and T cell response to SARS‐CoV‐2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32(9):2147‐2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seyahi E, Bakhdiyarli G, Oztas M, et al. Antibody response to inactivated COVID‐19 vaccine (CoronaVac) in immune‐mediated diseases: a controlled study among hospital workers and elderly. Rheumatol Int. 2021;41(8):1429‐1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS‐CoV‐2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21(8):2719‐2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Longlune N, Nogier MB, Miedouge M, et al. High immunogenicity of a messenger RNA based vaccine against SARS‐CoV‐2 in chronic dialysis patients. Nephrol Dial Transplant. 2021;36(9):1704‐1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Broseta JJ, Rodriguez‐Espinosa D, Rodriguez N, et al. Humoral and cellular responses to mRNA‐1273 and BNT162b2 SARS‐CoV‐2 vaccines administered to hemodialysis patients. Am J Kidney Dis. 2021;78(4):571‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Litjens NHR, Huang L, Dedeoglu B, Meijers RWJ, Kwekkeboom J, Betjes MGH. Protective cytomegalovirus (CMV)‐Specific T‐Cell immunity is frequent in kidney transplant patients without serum anti‐CMV antibodies. Front Immunol. 2017;8:1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krueger KM, Ison MG, Ghossein C. Practical guide to vaccination in all stages of CKD, including patients treated by dialysis or kidney transplantation. Am J Kidney Dis. 2020;75(3):417‐425. [DOI] [PubMed] [Google Scholar]
- 21. Eckerle I, Rosenberger KD, Zwahlen M, Junghanss T. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS One. 2013;8(2):e56974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Danziger‐Isakov L, Kumar D, AICo Practice. Vaccination of solid organ transplant candidates and recipients: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33(9):e13563. [DOI] [PubMed] [Google Scholar]
- 23. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA covid‐19 vaccine in solid‐organ transplant recipients. N Engl J Med. 2021;385(7):661‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen Y, Shen H, Huang R, Tong X, Wu C. Serum neutralising activity against SARS‐CoV‐2 variants elicited by CoronaVac. Lancet Infect Dis. 2021;21(8):1071‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Danthu C, Hantz S, Dahlem A, et al. Humoral response after SARS‐CoV‐2 mRNA vaccination in a cohort of hemodialysis patients and kidney transplant recipients. J Am Soc Nephrol. 2021;32(9):2153‐2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rincon‐Arevalo H, Choi M, Stefanski AL, et al. Impaired humoral immunity to SARS‐CoV‐2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6(60):eabj1031. [DOI] [PubMed] [Google Scholar]
- 27. Korth J, Jahn M, Dorsch O, et al. Impaired humoral response in renal transplant recipients to SARS‐CoV‐2 vaccination with BNT162b2 (Pfizer‐BioNTech). Viruses. 2021;13(5):756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Puro V, Castilletti C, Agrati C, et al. Impact of prior influenza and pneumoccocal vaccines on humoral and cellular response to SARS‐CoV‐2 BNT162b2 vaccination. Vaccines (Basel). 2021;9(6):615. [DOI] [PMC free article] [PubMed] [Google Scholar]


