Abstract
As the COVID‐19 pandemic grows, several therapeutic candidates are being tested or undergoing clinical trials. Although prophylactic vaccination against SARS‐CoV‐2 infection has been shown to be effective, no definitive treatment exists to date in the event of infection. The rapid spread of infection by SARS‐CoV‐2 and its variants fully warrants the continued evaluation of drug treatments for COVID‐19, especially in the context of repurposing of already available and safe drugs. Here, we explored the therapeutic potential of melatonin and melatonergic compounds in attenuating COVID‐19 pathogenesis in mice expressing human ACE2 receptor (K18‐hACE2), strongly susceptible to SARS‐CoV‐2 infection. Daily administration of melatonin, agomelatine, or ramelteon delays the occurrence of severe clinical outcome with improvement of survival, especially with high melatonin dose. Although no changes in most lung inflammatory cytokines are observed, treatment with melatonergic compounds limits the exacerbated local lung production of type I and type III interferons, which is likely associated with the observed improved symptoms in treated mice. The promising results from this preclinical study should encourage studies examining the benefits of repurposing melatonergic drugs to treat COVID‐19 and related diseases in humans.
Keywords: agomelatine, COVID‐19, drug repurposing, interferons, melatonin, ramelteon, SARS‐CoV‐2
1. INTRODUCTION
The current global health crisis caused by the rapid spread of SARS‐CoV‐2 infection leading to COVID‐19 poses a huge challenge for the health system and has mobilized the global medical and research community to provide rapidly applicable solutions. During SARS‐CoV‐2 infection, people, particularly with advanced age or comorbid conditions, may experience acute respiratory distress syndrome and an excessive pro‐inflammatory response (cytokine storm) leading in some cases to death. 1 , 2 Promising and diverse therapies have been studied and used against COVID‐19 at the level of viral entry, 3 viral replication 4 or to resolve excessive inflammatory responses. 5 However, due to the multiple and complex facets of the symptoms of COVID‐19 and the various possible mechanisms of action of SARS‐CoV‐2, the identification of effective drugs or combinations of drugs or adjuvants would be required to expand the range of available treatments. Drug development programs are extremely long processes. Repurposing of approved drugs and known molecules with a good safety profile is an attractive and fast alternative to increase the survival rate, while decreasing the number of severe patients requiring hospitalization in intensive care units, as well as limiting the impact of viral infection on long‐term sequelae.
Melatonin is a natural hormone produced during the night and is involved in the synchronization of biological rhythms and sleep regulation. 6 Melatonin acts through a variety of targets, mainly through high‐affinity interaction with two membrane receptors—MT1 and MT2. 7 Additional targets comprise enzymes like calmodulin, quinone reductase 2, and mitochondrial proteins, which render melatonin a potentially highly versatile molecule displaying a wide range of cellular effects that maintains normal physiological activities. 8 In addition to synchronizing biological rhythms and sleep patterns, melatonin and melatonin receptor targeted‐drugs are known to modulate the immune response, regulate the cellular oxidative status, and favor cell survival in the lung under stress and inflammatory conditions. 9 , 10 , 11 Melatonin receptor‐targeted drugs currently available on the market are ramelteon (Rozerem®, Takeda), agomelatine (Valdoxan®, Servier), Tasimelteon Hetlioz® (Vanda), and Circadin® (Neurim Pharmaceuticals). 12 They are indicated for insomnia, “jet‐lag,” and depression. These drugs have been proven to be safe and to display few side effects. 13 , 14 , 15
Since the COVID‐19 crisis, numerous comments and reviews in the literature and media have largely speculated on the potential prophylactic and therapeutic effects of melatonin and melatonin receptor‐targeted drugs in SARS‐CoV‐2‐associated diseases. Contrary to the high number of hypotheses raised, only few experimental data have been generated in this regard. In a system pharmacology study, melatonin was identified among the top 5 molecules with potential anti‐COVID‐19 action that correlated target drug arrays of more than 2,000 FDA approved or experimental drugs to a COVID‐19/human protein interaction network. 16 A more direct observation of potential importance of melatonin in COVID‐19 came from an integrative network medicine analysis, predicting disease manifestations associated with COVID‐19, that identified melatonin among the top repurposing drugs in COVID‐19. The prediction was then reinforced by the analysis of patient data from Cleveland Clinic's COVID‐19 registry revealing that melatonin usage in the general population or in the African‐American population is associated with a 30% and 52% reduced likelihood of being positive for SARS‐CoV‐2 test, respectively. 17 Melatonin and related indole derivatives were recently shown to inhibit the entry of several porcine enteric coronaviruses (porcine epidemic diarrhea virus (PEDV), transmissible gastroenteritis virus (TGEV), and porcine delta coronavirus (PDCoV)) in several cellular models, although at very high concentration (mM range). 18 Currently, melatonin and related drugs are investigated in eleven ongoing clinical trials for COVID‐19, in Spain, USA, Iran, Brazil, and Mexico (EudraCT 2020–001808–42, EudraCT 2020–001530–35, NCT04474483, NCT04531748, NCT04409522, NCT04568863, NCT04530539, NCT04470297, NCT04353128, NCT04570254, IRCT20200506047323N5). However, at present, experimental data on the therapeutic potential of melatonin therapy to treat COVID‐19 are still lacking.
Here, we assessed the effects of melatonin and melatonin receptor‐targeted drugs on the transgenic mice expressing the human angiotensin‐converting enzyme 2 (ACE2) receptor driven by the cytokeratin‐18 (K18) gene promoter (K18‐hACE2) as a model of SARS‐CoV‐2 infection. K18‐hACE2 mice constitutes a model of rapid evolution of SARS‐CoV‐2 infection leading to fast body weight loss, inflammatory manifestations, development of lung lesions, and death as early as 6–7 days after viral infection and recapitulates histopathological and immune findings of COVID‐19 in humans. 19 , 20 , 21 , 22 , 23 , 24 We show that daily treatment with melatonin or with melatonin receptor compounds (agomelatine or ramelteon) delays clinical manifestations and improves survival in SARS‐CoV‐2‐infected K18‐hACE2 mice, in particular with a high dose of melatonin (50µg/kg). These effects are most likely mediated by melatonin‐induced regulation of local production of type I and type III interferon (IFN) in the lungs.
2. MATERIAL AND METHODS
2.1. Murine model
K18‐hACE2 C57BL/6 transgenic mice expressing human ACE2 in airway epithelial cells driven by a human cytokeratin 18 (K18) promoter (Jackson Laboratory, https://www.jax.org/strain/034860) (males, 10‐week‐old) were housed in an animal facility of biosafety level 3 (BSL3) at the French National Veterinary School in Maisons‐Alfort, following a protocol approved by the ANSES/EnvA/UPEC Ethics Committee (CE2A‐16) and authorized by the French ministry of Research under the number APAFIS#25384‐2020041515287655 v6 in accordance with the French and European regulations.
2.2. SARS‐CoV‐2 virus
The strain BetaCoV/France/IDF/200107/20 was supplied by the Urgent Response to Biological Threats (CIBU) hosted by Institut Pasteur (Paris, France) and headed by Dr. Jean‐Claude Manuguerra. The human sample, from which the strain BetaCoV/France/IDF/200107/2020 was isolated, has been provided by Dr O. Paccoud from the La Pitié‐Salpétrière Hospital (Paris, France).
2.3. Protocol for SARS‐COV‐2 infection and treatment with melatonin receptor compounds
Melatonin, agomelatine, and ramelteon were purchased from abcr GmbH (Karlsruhe, Germany). Compounds were reconstituted in vehicle solution (5% ethanol in sterile saline solution). K18‐hACE2 transgenic mice were i.p. injected with melatonin receptor ligands daily, one hour before lights off (to avoid disturbing the natural daily rhythm of MLT production), starting 2 days before virus inoculation. At day of infection (DPI‐0), mice are anesthetized with isoflurane and are infected via intra‐nasal inoculation of SARS‐CoV‐2 (10uL each nostril, 104 TCID50 in total) in Dulbecco's modified Eagle medium. Treatment with vehicle or melatonin receptor‐targeted drugs continued until the end of the experiment (7 days post‐infection, DPI‐7). After infection, mice were randomly divided into the following groups (6 mice/group): vehicle, MLT 10mg/kg (MLT10), MLT 50mg/kg (MLT50), ramelteon (RML, 10 mg/kg), and agomelatine (AgoMLT, 20 mg/kg). Being similar to mice of vehicle group, 2 infected mice and no vehicle administered were included in the vehicle group for the analyses. A group of non‐infected mice was housed under the same conditions and similarly monitored during the whole experiment. Mice were uniquely identified using ear tags and provided an acclimation period of at least 1 week before the experiment. Mice were supplied nutrient gel when weights began to decrease. Mice that met the human endpoint criteria were euthanized to limit suffering. The study was ended at DPI‐7, and surviving mice were killed at that time for comparative analysis. Plasma and lung samples are taken directly after killing and stored at −80°C until analysis.
2.4. Survival rate and clinical score
All mice were examined and weighed daily. After day 4 (DPI‐4), when clinical symptoms started to appear, mice were monitored and scored for clinical symptoms two times per day until DPI‐7. An IACUC‐approved clinical scoring system was utilized to monitor disease progression and establish human endpoints. Categories checked included body weight, posture/fur, activity/ mobility, eye closure, respiratory rate, which were evaluated and defined as clinical score according to standard guidelines 19 with a maximal score of 14. Mice died either naturally from the disease or were killed for ethical reasons when reaching a clinical score of 5 for 2 parameters and for 2 consecutive observation periods, or if weight loss was equal to or greater than 20%.
2.5. Histology
Left lung lobe was dissected and fixed in 4% formalin for 48h and then embedded in paraffin and micro‐sectioned at 5µm on a microtome (Leica). Lung specimens were stained with hematoxylin and eosin (H&E), and tissue slices were imaged on the Lamina Multilabel Slide Scanner (PerkinElmer, Scientific, Massachusetts, USA) and subjected to gross and microscopic pathology analysis by a lung anatomo‐cyto‐pathologist.
2.6. Cytokine mRNA levels and viral load
RNA from frozen lung of non‐infected and infected mice was extracted with TRIzol Plus RNA Purification Kit (Thermo Fisher Scientific). RNA was reverse‐transcribed using the Maxima 1str cDNA Synth kit (Thermo Fisher Scientific), and quantitative PCR was performed using the Taqman fast advance mix (Thermo Fisher Scientific) and Taqman primers for cytokine genes and housekeeping gene HPRT (Thermo Fisher Scientific). Fold changes were determined by comparing treated mice with non‐infected controls. Viral load in the lung is measured in RNA samples by assessing the SARS‐CoV‐2 ORF1 gene copies (specifically the region encoding RNA‐dependent RNA polymerase, RdRp), by RT‐qPCR using the TaqMan RNA‐to‐CT 1‐step Kit (Applied Biosystems) and the TaqMan 2019nCoV Assay Kit v1 (Thermo Fisher Scientific). Human RNase P RPPH1 gene copies were assessed in duplex reactions as an internal positive control. The TaqMan 2019nCoV Control Kit v1 (Thermo Scientific Scientific) was also used as positive control of assay‐specific amplification. Each RT‐qPCR reaction comprises a total volume of 20 μl containing 5 μl of sample RNA, 10 μl of TaqMan PT‐PCR Mix (2x), 0.5 μl of TaqMan RT Enzyme Mix (40x), 1 μl of TaqMan 2019nCoV assay, and 1 μl of RNase P Assay. RT‐qPCR was performed in a LightCycler 480 Instrument II (ROCHE), carried out at 48 °C for 15 min, then at 95 °C for 10 min and followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. The number of gene copies was obtained by the LightCycler 480 Software v1.5.
The correlation between pulmonary cytokines and clinical scores at DPI‐6.5 and DPI‐7 of all groups was analyzed by limiting cytokine value to a threshold corresponding to the mean level of the vehicle group, as beyond a cytokine threshold, the clinical score would reach the same maximum value.
2.7. Plasma cytokine and chemokine protein measurements
Plasma samples were analyzed for cytokines and chemokines using the Mouse ProcartaPlex Panel Thermo Fisher Scientific) and the Bioplex 200 ™(Luminex ®).
2.8. Statistical analysis
Data were shown as the means ±SEM. Sample sizes were designed to give statistical power while minimizing animal use. All statistical comparisons were made using Prism 9 (GraphPad). The specific statistical tests used for each experiment are indicated in the figure legends. One‐ or two‐way ANOVA with two‐stage linear step‐up procedure of Benjamini, Krieger, and Yekutieli as post‐test for multiple comparisons was applied, and statistical significance was determined as p‐value <0.05 (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Statistical details of the one‐way or two‐way ANOVA tests are also indicated in the figure legends: F(DFn, DFd), where F is the F‐value, DFn and DFd are the degrees of freedom, and p is the p‐value determined from the F ratio and the two values for degrees of freedom.
3. RESULTS
To address the therapeutic efficacy of melatonin receptor‐targeted drugs on COVID‐19, we designed a study that evaluated the impact of daily administered melatonin (at 2 doses), agomelatine or ramelteon on SARS‐CoV‐2 infection in the K18‐hACE2 mouse model. K18‐hACE2 mice intranasally infected by SARS‐CoV‐2 develop clinical disease caused by lung pathology, which closely mirrors severe human COVID‐19. 25 , 26 Ten‐week‐old K18‐hACE2 male mice were inoculated via intranasal route with 1×104 TCID 50 (Median Tissue Culture Infectious Dose) of SARS‐CoV‐2 (strain BetaCoV/France/IDF/200107/2020). Starting two days before the viral infection, mice were intra‐peritoneally administered with vehicle or different melatonin receptor compounds (melatonin 10mg/kg (MLT10) or 50mg/kg (MLT50), agomelatine (AgoMLT, 20 mg/kg), ramelteon (RML, 10 mg/kg) in a daily basis until day post‐infection 6 (DPI‐6) as schematically described in Figure 1A. On DPI‐7, all mice were sacrified for analysis and tissues collected for biochemical analysis.
FIGURE 1.
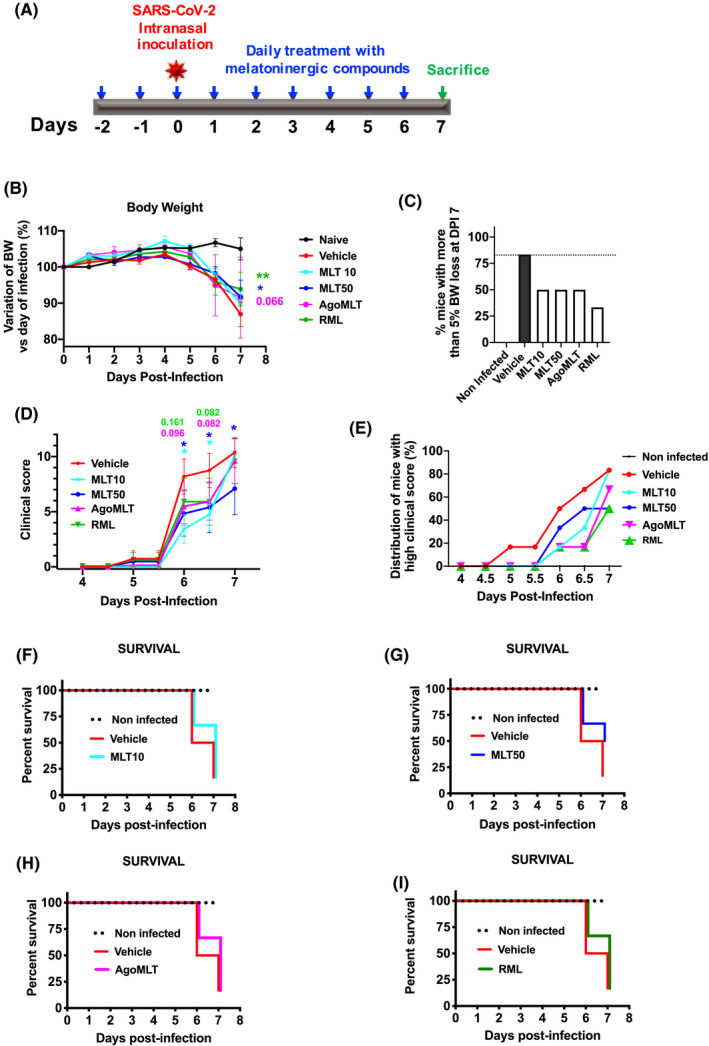
Impact of a treatment with melatonin receptor compounds on body weight, clinical score and survival of SARS‐CoV‐2‐infected mice. A, Protocol for the treatment with melatonin receptor compounds according to the following groups: Vehicle, MLT 10mg/kg (MLT10) or 50mg/kg (MLT50), ramelteon (RML, 10 mg/kg), agomelatine (AgoMLT, 20 mg/kg). Day post‐infection 0 (DPI‐0) is the day of SARS‐CoV‐2 intranasal inoculation (104 PFU). B, Body weight was monitored daily (post‐infection) over 7 days (*p<0.05, **p<0.01 by two‐way ANOVA with two‐stage linear step‐up procedure of Benjamini, Krieger and Yekutieli as post‐test for multiple comparisons). C, Body weight loss was expressed as percentage (%) of mice with more than 5% body weight loss at day 7 post‐infection (DPI‐7) compared to DPI‐0. D, The clinical score was evaluated daily from day 0 to day 4 and twice per day from day 4 to 7. Cumulative clinical scores of all the mice for each group are indicated from DPI5.5 until DPI‐7. *p<0.05 by two‐way ANOVA with two‐stage linear step‐up procedure of Benjamini, Krieger, and Yekutieli as post‐test for multiple comparisons. F‐value, degrees of freedom (DFn and DFd), and p‐value determined from the F ratio and the degrees of freedom are as follows: body weight (F(5,243)=3.339; p=0.0062); clinical score (F(4,196)=2.185; p=0.0721). E, Plot of percentage of mice (%) with a clinical score >6 until DPI‐5.5 and >9 after DPI‐6 for each group. F‐I, Kaplan‐Meier plot of survival curve for infected mice treated with MLT10, MLT50, AgoMLT, and RML is compared to vehicle group
3.1. Treatment with melatonin receptor compounds slightly improves body weight, clinical score, and survival of SARS‐CoV‐2‐infected mice
The clinical score of infected mice was assessed carefully throughout the experiment according to the classical guidelines. 19 Healthy mice have a clinical score of zero (similarly to non‐infected mice), while severely ill mice had a maximal score of fourteen. Symptoms evaluated included weight loss, reduced activity and piloerection, respiratory impairment, lethargy and eye closure (see Methods for details). Infected K18‐hACE2 mice did not show any body weight loss or any other measurable clinical symptoms until day 4 (DPI‐4) (Figure 1B‐E). By day 5, the body weight of all infected mice started to deviate from non‐infected controls, and at DPI‐6 or DPI‐7, infected mice display significant lower body weight (10 to 20%) compared to DPI‐0, the day of infection. Treatment with melatonin receptor ligands significantly limited the body weight loss observed after SARS‐CoV‐2 infection at DPI‐7, mainly for MLT50 and RML (Figure 1B), and reduced the number of mice that lost more than 5% of body weight up to DPI‐7 (Figure 1C). Infected mice exhibited variability in clinical signs of infection, with clinical scores ranging from 0 to 7 at DPI‐5.5 and 0 to 14 at DPI‐7 (Figure 1D). Daily treatment with melatonin receptor ligands delayed the appearance of pathological signs (Figure 1D‐E) and reduced the percentage of mice with high clinical score (Figure 1E). Melatonin receptor ligands retarded the occurrence of death in all groups (Figure 1F‐I) with the group treated with MLT50 displaying the highest percentage of survival of 50%, compared to 16.7% for the vehicle group at the end point of the experiment (DPI‐7) (Figure 1G).
3.2. Treatment with melatonin receptor compounds does not modify the gross histopathological profile of the lungs, the overall plasma cytokines landscape nor the lung cytokine levels of SARS‐CoV‐2‐infected mice at DPI‐7
To verify whether the moderate improvement of treatment with MLT50 translates into preserved lung tissue morphology upon SARS‐CoV‐2 infection, we analyzed the lung architecture of non‐infected and infected mice treated with vehicle or MLT50 (Figure S1). On gross examination, compared to non‐infected mice, most SARS‐CoV‐2‐infected lungs were overall edematous with patchy areas of consolidation. Histological pattern of injury was characterized by diffuse interstitial inflammation and edema in infected mice, with no clear difference between MLT50 and vehicle. Minute foci of parenchymal consolidation, defined as alveolar effacement by a dense inflammatory infiltrate, were observed in both vehicle and MLT50 groups, with a slight increase in the latter. Diffuse hyaline membrane was not observed nor microvascular injury. It should be acknowledged that the lesions and the inflammatory infiltrates in infected mice were heterogeneously distributed in the tissue in a patchy pattern, in accordance with previous reports 20 and the considerable inter‐individual variability between infected mice made the comparative analysis difficult. To gain further insight into the inflammatory status of infected mice and overcome the tissue heterogeneity, we instead determined the overall levels of cytokines in plasma and lungs. An excessive pro‐inflammatory response to SARS‐CoV‐2 infection, referred to as “cytokine storm,” has been described in several COVID‐19 patients and is believed to contribute to severe outcomes including pulmonary pathology, respiratory distress, and death. 1 , 27 Extensive changes in cytokine profiles were also observed in SARS‐CoV‐2‐infected K18‐hACE2 mice. 23 To evaluate the impact of the treatment with melatonin receptor compounds on the immune response, we measured plasma cytokine levels of SARS‐CoV‐2‐infected K18‐hACE2 mice. Infection led expectedly to an increase in plasma IL1β, IL6, TNFα, CXCL10, CCL5, CCL2, IL18, and IFN levels, with a large variability among the mice, resulting in a lack of significant differences between the groups (Figure 2). Daily administration of melatonin receptor compounds did not modify the systemic cytokine levels in SARS‐CoV‐2‐infected mice, with the exception of MLT10 that increases plasma levels of IL6, CXCL10, and IFNβ at DPI‐7 compared to vehicle‐treated group (Figure 2). A local profiling of messenger RNA (mRNA) of relevant cytokines on lung homogenates at DPI‐7 revealed a clear induction of TNFα and Cxcl10 mRNA expression and a tendency of elevation of Cxcl9, IL10, CCL5 mRNA levels at DPI‐7 in the lungs of vehicle‐infected mice compared to non‐infected K18‐hACE2 control mice (Figure 3). The levels of these cytokines were not modified by the treatment with MLT, AgoMLT nor RML. Overall, treatment with melatonin receptor‐targeted drugs did not significantly reshape the global immune response of SARS‐CoV‐2‐infected mice, and the slight improvement of the clinical score observed after drug treatment is likely not attributed to modification of the levels of the pro‐inflammatory cytokines investigated.
FIGURE 2.
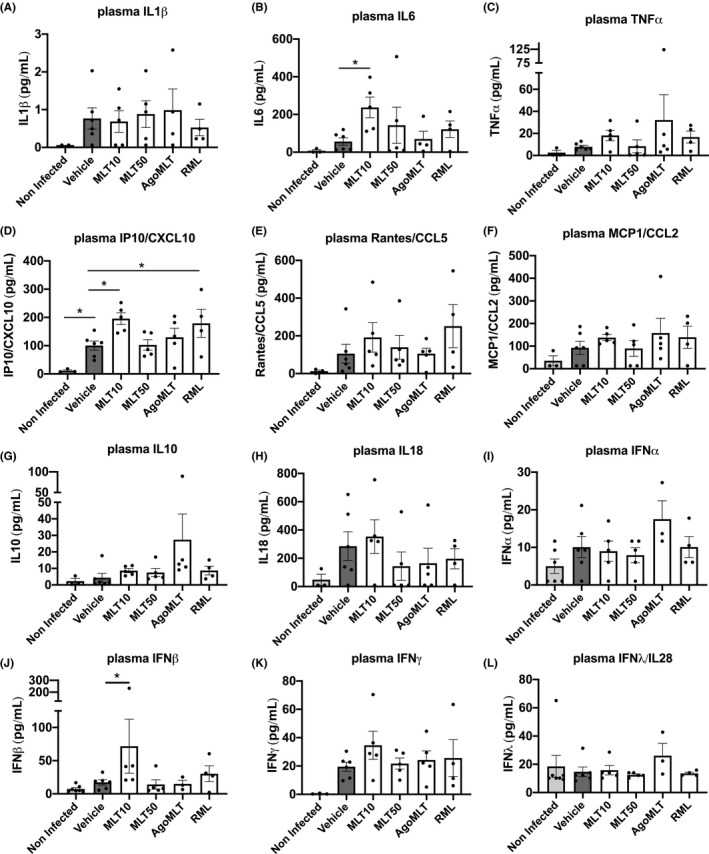
Treatment with melatonin receptor compounds does not modify the plasma cytokines profile of SARS‐CoV‐2‐infected mice at DPI‐7. A‐K, Levels of IL1β, IL6, TNFα, CXCL10, CCL5, CCL2, IL10, IL18, IFNα, IFNβ, IFNλ, and IFNγ in the plasma of SARS‐CoV‐2‐infected mice at sacrifice day DPI‐7 were measured. Data are expressed as mean ±SEM. *p<0.05, by one‐way ANOVA with two‐stage linear step‐up procedure of Benjamini, Krieger, and Yekutieli as post‐test for multiple comparisons. (F(DFn, DFd); and p‐value of the F ratio) are as follows: IL1β (F(5, 20)=0.5345; p=0.7477); IL6 (F(5,21)=2.001; p=0.1203); TNFα (F(5,22)=0.9048; p=0.4956); IP10/CXCL10 (F(5,22)=4.896; p=0.0037); RANTES/CCL5 (F(5,22)=1.221; p=0.3323); MCP1/CCL2 (F(5,22)=1.026; p=0.4267); IL10 (F(5,22)=1.608; p=0.1994); IL18 (F(5,22)=1.002; p=0.4397); IFNα (F(5,23)=1.877; p=0.1376); IFNβ (F(5,24)=1.047; p=0.4063); IFNγ (F(5,22)=1.862; p=0.1422); IFNλ (F(5,24)=0.5846; p=0.7115)
FIGURE 3.
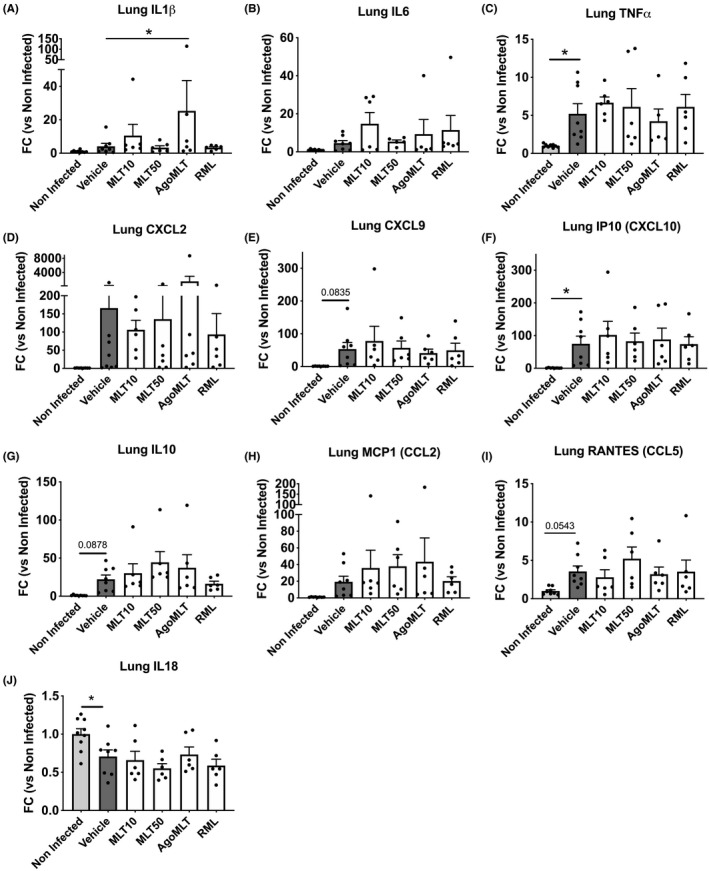
Treatment with melatonin receptor compounds does not modify the cytokine profile in the lung of SARS‐CoV‐2‐infected mice at DPI‐7. A‐J, mRNA levels of IL1β, IL6, TNFα, CXCL2, CXCL9, CXCL10, IL10, CCL2, CCL5, and IL18 in the lung of SARS‐CoV‐2‐infected mice at killing days were determined by RT‐qPCR. Data are presented as fold change (FC) of non‐infected mice. *p<0.05, by one‐way ANOVA with two‐stage linear step‐up procedure of Benjamini, Krieger, and Yekutieli as post‐test for multiple comparisons. F(DFn, DFd) and p‐value of the F ratio are: IL1β (F(5,35)=1.610; p=0.187); IL6 (F(5,32)=1.340; p=0.2730); TNFα (F(5,34)=2.801; p=0.0319); CXCL2 (F(5,34)=1.047; p=0.4063); CXCL9 (F(5,34)=1.385; p=0.2543); IP10/CXCL10 (F(5,34)=2.091; p=0.0906); IL10 (F(5,35)=1.163; p=0.0267); MCP1/CCL2 (F(5,34)=1.269; p=0.3000); RANTES/CCL5 (F(5,34)=1.987; p=0.1058); IL18 (F(5,35)=3.886; p=0.0066)
3.3. Daily injection of melatonin receptor compounds decreases type I and type III interferon levels in the lung of SARS‐CoV‐2‐infected mice
Intranasal inoculation of SARS‐CoV‐2 in the K18‐hACE2 mouse model leads to viral invasion of the lungs and is characterized by a relatively large heterogeneity of viral load between infected animals. 19 , 21 When examining the impact of daily administration of MLT, AgoMLT or RML on lung viral load at DPI‐7 of SARS‐CoV‐2 infection, we also observed a substantial heterogeneity of viral ORF1 RNA level in pulmonary homogenates between mice, regardless of the groups (Figure 4A). Consequently, by examining the mean levels of viral ORF1 RNA of the different groups, there is no significant statistical differences, although melatonin receptor treatments appear to slightly decrease the pulmonary viral load. (Figure 4A). When analyzing the frequency distribution of mice in discrete variables (low, medium, high) of viral load, a modification of the distribution of mice among these groups is observed upon treatment even though no statistical analysis could be performed from the frequency distribution due to the low number of animals. Administration of melatonin receptor compounds led to a decreased percentage of mice with high viral load and a concomitant increase in the number of mice with low viral load, with the most pronounced impact observed for MLT50 and AgoMLT groups (Figure 4B). These data suggest that melatonin receptor therapy, especially with high melatonin doses and AgoMLT, may limit lung infection by the virus.
FIGURE 4.
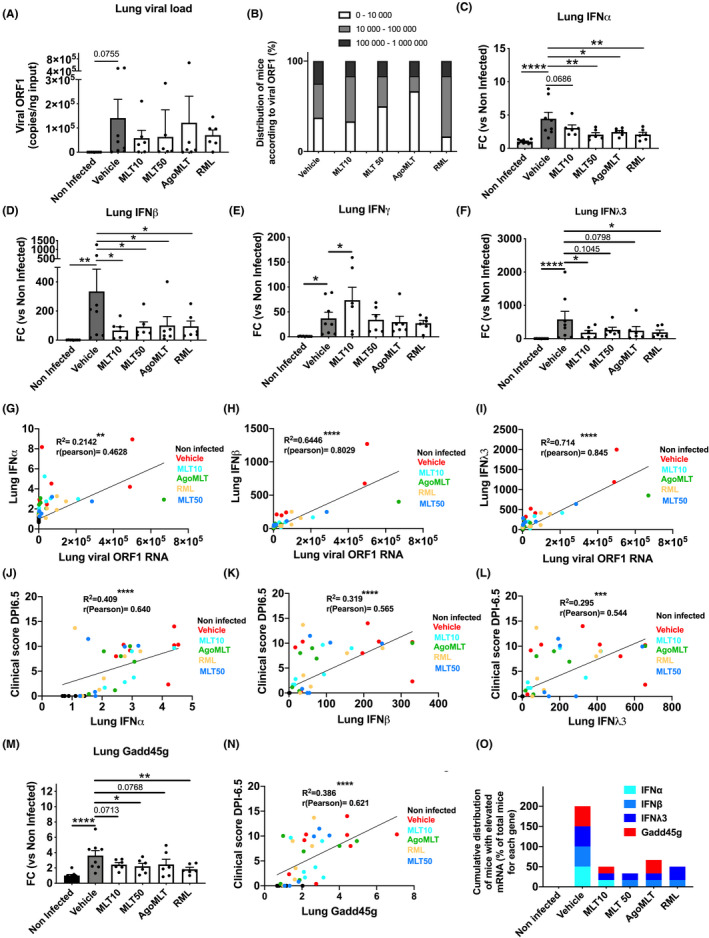
Treatment with melatonin receptor compounds decreases type I and type III interferon levels in the lung of SARS‐CoV‐2‐infected mice. A, Viral ORF1 RNA levels in the lung of SARS‐CoV‐2‐infected mice at DPI‐7. B, Repartition of infected mice (%) according to viral levels in the lungs. C‐F, mRNA levels of IFNα, IFNβ, IFNγ, and IFNλ3 in the lung of SARS‐CoV‐2‐infected mice at sacrifice day DPI‐7. Data are expressed as mean fold change (FC) of non‐infected mice ±SEM. *p<0.05, **p<0.01, ****p<0.0001 by one‐way ANOVA with two‐stage linear step‐up procedure of Benjamini, Krieger, and Yekutieli as post‐test for multiple comparisons. G‐I, Correlation curve between lung IFNα (G), IFNβ (H), or IFNλ3 (I) with viral load. J‐L, Correlation curve between lung IFNα (J), IFNβ (K), or IFNλ3 (L) with clinical score at DPI‐6.5. M, mRNA levels of Growth arrest and DNA‐damage‐inducible protein GADD45 gamma (Gadd45g) in the lung of SARS‐CoV‐2‐infected mice at killing days DPI‐7. Data are expressed as mean fold change (FC) ± SEM. *p<0.05, **p<0.01, ****p<0.0001 by one‐way ANOVA with two‐stage linear step‐up procedure of Benjamini, Krieger and Yekutieli as post‐test for multiple comparisons. L, Correlation curve between lung Gadd45g with clinical score at DPI‐6.5. O, Repartition of SARS‐CoV‐2‐infected mice having elevated mRNA level of IFNα, IFNβ, IFNλ3 or Gadd45g at DPI‐7. Percentage of mice having level of RNA higher than median of vehicle group are represented. Distribution values for each gene are cumulated in the graph. For correlation analysis *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 with two‐tailed p‐value of Pearson correlation based on the assumption that values are sampled from populations that follow a Gaussian distribution. F(DFn, DFd) and p‐value of the F ratio are: viral ORF1 (F(5;35)=0.809; p=0.5516); IFNα (F(5,35)=5.684; p=0.0006); IFNβ (F(5,34)=2.288; p=0.0678); IFNγ (F(5,35)=3.49; p=0.0116); IFNλ (F(5,35)=2.413; p=0.0557); Gadd45g (F(5,35)=4.410; p=0.0032)
As highly elevated and prolonged production of interferons (IFNs) in patients infected with SARS‐CoV‐2 is associated with negative clinical outcomes 28 , 29 and could trigger a more severe disease by impairing lung function, 30 , 31 , 32 we assessed the level of IFN mRNA in the lungs of infected mice. SARS‐CoV‐2 infection strongly triggers exacerbated increase in IFNs expression with heightened levels of IFNα (4‐fold), IFNβ (330‐fold), IFNγ (30‐fold), and IFNλ3 (580‐fold) in the lungs compared to lungs of non‐infected mice (Figure 4C‐F). Treatment with melatonin receptor compounds decreased type I (IFNα and IFNβ) and type III IFN (IFNλ3) levels at approximately 4‐fold for IFNβ and 3‐fold for IFN λ3 (Figure 4C‐F). Interestingly, interferon levels are all significantly correlated with viral load (ORF1 RNA), with the strongest association with IFNβ (R2=489; r (pearson)= 0.699; ****) and IFN λ3 (R2= 0.714, r(pearson)= 0.845, ****) (Figure 4G‐I), while no correlation was observed with other cytokines in the lungs apart from a weak association for IL6 (R2= 0.155, r(pearson)= 0.394, *) and CXCL10 (R2= 0.236, r(pearson)= 0.486, **) (Figure S2). This link evidences an IFN response correlated and driven by the viral infection. Interestingly, a remarkable association of IFNα, IFNβ, and IFNλ3 levels, but not that of IFNγ, with the clinical score at DPI‐6.5 and DPI‐7 is observed, suggesting a harmful impact of uncontrolled production of type I and type III IFN in the lungs (Figure 4J‐L; Figures S3‐4). Of note, in addition to IFNα, IFNβ, and IFNλ3, the expression levels of lung IL6, IL1β, and CXCL2 also correlated with severe clinical score (Figures S3‐4). We then investigated, in the infected lungs, the expression of growth arrest and DNA‐damage‐inducible protein gamma (Gadd45g), which is activated by high levels of interferons and is detrimental to lung function. 31 At DPI‐7, levels of Gadd45g are raised significantly in the lung of SARS‐CoV‐2‐infected mice (Figure 4M), suggesting occurrence of cell death and impairment of cellular regeneration in infected lungs. Accordingly, elevated levels of Gadd45g in the lungs strongly correlated with the severity of the clinical score (R2=386; r (pearson)= 0.621; ****, Figure 4N). Treatment with MLT50 and RML significantly prevented the rise in Gadd45g levels (Figure 4M), suggesting a protective effect of melatonin receptor compounds on limiting the detrimental impact of excessive interferon response in the lower respiratory tract. Accordingly, treatment with melatonin receptor compounds, in particular with MLT50 and RML, reduces the percentage of infected mice having high levels of IFNα, β, γ3, or Gadd45g in the lungs (Figure 4O).
Altogether, our data favor the notion that daily treatment with MLT, AgoMLT, and RML during SARS‐CoV‐2 infection might improve or delay clinical symptoms and severity of the disease by attenuating excessive local production of type I and type III interferons, without reshaping the overall cytokine landscape.
4. DISCUSSION
Several mouse models were generated where hACE2 was introduced in mice by multiple ways including the transient integration of hACE2 via adenoviral vectors, 33 , 34 , 35 the expression of hACE2 under the control of the mouse ACE2 promoter, 36 , 37 the expression of hACE2 driven by the human cytokeratin 18 (K18) promoter (naturally expressed in the lung epithelia) 19 , 20 , 21 , 22 , 23 , 24 , 38 or controlled by a lung ciliated epithelial cell‐specific HFH4/FOXJ1 promoter. 39 , 40 While all of these mouse models can be infected by SARS‐CoV‐2, the best models that recapitulate the disease severity and lethality observed in humans are those expressing hACE2 under the control of K18 19 , 20 , 21 , 22 , 23 , 24 or HFH4 promoters. 40 K18‐hACE2 mice being highly susceptible to SARS‐CoV‐2 and supporting lethal infection, we chose this mouse model available at the Jackson Laboratory to evaluate the impact of a melatonin receptor treatment.
Melatonin receptor ligands, particularly MLT50 and, to a lower extent, AgoMLT and RML showed beneficial effects in infected K18‐hACE2 mice by limiting body weight loss, leading to slight amelioration of clinical scores with retardation of clinical deterioration and improvement of survival rate. Melatonin receptor ligands like MLT at high dose (50mg/kg) and AgoMLT seem to be effective in decreasing the frequency distribution of mice with high viral load, while increasing the proportion of mice with low viral content. On day 7, treatment with melatonin receptor ligands led to decreased lungs IFN levels, and consequent decrease in Gadd45g, an IFN‐related marker of apoptosis and antiproliferative cellular processes, suggesting a potential mechanism of action of melatonin receptor ligands on improving clinical symptoms of SARS‐CoV‐2 infection. Indeed, all melatonin receptor compounds appear to induce a certain degree of improvement in disease progression, with MLT 50 having a more substantial effect on survival rate and clinical score. MLT, AgoMLT, and RML have in common the activation of melatonin MT1 and MT2 receptors. In line with the involvement of melatonin receptors in the observed effects, a recent work observed that administration of ramelteon in rats has a protective effect on ventilator‐induced lung injury that was mitigated by the administration of melatonin receptor specific antagonist, luzindole. 41 The serotonin 5‐HT2C receptors and heterodimers between 5‐HT2C receptors and melatonin receptors have been described as an additional target of AgoMLT. 42 , 43 The fact that AgoMLT has no distinctive effect compared to the other treatments argues against a significant participation of 5‐HT2C receptors in the observed effects. However, a participation of the 5‐HT2C receptors component in the effect of AgoMLT cannot be fully excluded at this point, in particular as our analysis was performed at the final disease stage and did not monitor the effects at the different disease stages. Our data show a clear difference between MLT10 and MLT50 conditions with MLT50 being more effective. MLT10 is normally considered to be high enough to effectively activate both melatonin receptors. The reason for the enhanced effects at higher melatonin doses remains currently unknown. The short plasmatic half‐life of melatonin (approximately 30 minutes) 44 combined with the need for sustained high levels of melatonin could be one possible reason. Inadequate timing of melatonin treatment can be another reason as melatonin is known to display specific action windows around the circadian cycle. 45 The use of a slow‐release melatonin formulation, known as Circadin®, 46 might contribute to answer this question. AgoMLT and RML show more favorable properties in this respect with a plasma half‐life of 1–2 hours. 47 Differences in the ability of these melatonin receptor agonists to promote receptor desensitization might be also a confounding factor. Additional explanations for differences between melatonin doses include the involvement of low affinity melatonin targets that would be expected to be targeted at high doses. 48 Indeed, several additional mechanisms of action of melatonin, mostly based on the antioxidant and anti‐inflammation hypotheses, have been proposed to be of potential relevance for COVID‐19 treatment ( 49 for review). For instance, melatonin has been shown to be protective in several rodent models of inflammation (induced by sepsis or ischemia or acute respiratory distress syndrome) by exerting anti‐inflammatory and anti‐oxidant effects and restoring mitochondrial function for which the participation of receptor‐dependent and independent remains to be established. 10 , 50 , 51 Further potential mechanisms of MLT come from cellular studies indicating that MLT could prevent cell entry and replication of Swine coronavirus at millimolar concentrations, 18 mechanisms that could be envisioned in the case of SARS‐CoV‐2.
The usual prescribed doses in patients range from 2–20 mg/day for melatonin, 8 mg/day for ramelteon, and 25–50 mg/day for agomelatine which are equivalent in mice to 0.41–4.1 mg/kg/day, 1.6 mg/kg/day, and 5–10 mg/kg/day, respectively, dose equivalent conversion calculated according to the FDA guidelines. 52 To better evaluate the therapeutic potential of melatonin receptor drugs in the K18‐hACE2 animal model, we chose to administer higher doses of the compounds (2.5 to 12‐fold higher for MLT, 6‐fold higher for ramelteon and 2–4 times higher for agomelatine), than the recommended doses prescribed against jetlag, insomnia, or depression in humans. Nevertheless, the chosen doses in the current study are still in the lower range of previously tested doses in human studies. For example, in humans, ramelteon has been tested at doses ranging from 4 to 64 mg 53 or even at 160mg, ie, 20 times the recommended therapeutic doses for 18 days 54 without toxicity. Doses of agomelatine at 100mg/day in humans have already been used. 55 , 56 In studies from the 70s, human subjects were treated with very high doses of melatonin ranging from 1–6.6 g/day (equivalent to 205–1350 mg/kg in mice) for 30–45 days. 57 , 58 However, as with any molecule, toxicity is a question of dose and side effects have to be carefully registered by health authorities, as recently commented. 59 If used in SARS‐CoV‐2 infections, appropriate effective doses of melatonin receptor‐targeted drugs in COVID‐19 patients remain to be evaluated.
The function of IFNs in COVID‐19 pathology is ambiguous with opposing effects, either protective or detrimental depending on the timing and site of action. IFNs are very important cytokines in controlling virus infection, acting on both innate and adaptive immune responses, but it can also aggravate inflammatory pathology at late stages of viral infection. 60 There is firm evidence suggesting that an efficient IFN response is essential for protecting against the development of a severe COVID‐19. 61 , 62 , 63 Yet, several studies report that dysregulated and prolonged production of IFN are strongly associated with negative clinical outcomes and impaired lung functions. 28 , 29 , 30 , 31 , 32 Disease severity and morbidity of COVID‐19 patients correlate with the elevated expression of type I and III IFNs in lower respiratory tract. 30 , 32 In line with this, the bronchoalveolar lavage fluid (BALF) derived from the lower airways of COVID‐19 patients is characterized by a markedly higher expression of interferon‐stimulated genes compared to infection by other pathogens. 64 Implication of IFNs was recapitulated in another mouse model of SARS‐CoV‐2 infection revealing the inflammatory role of type I interferon signaling.. 35 To reconcile these discrepancies, a concept has emerged with the notion of proper timing and space for the effective action of interferons. Indeed, the dynamics of IFN should show an early and rapid increase just after viral infection followed by a rapid decline over time avoiding prolonged production. 65 , 66 In addition, local versus systemic IFN levels are opposing. Peripheral blood immune cells from severe and critical COVID‐19 patients have diminished type I IFN, 67 suggesting that in contrast to high local production in the lungs which can be detrimental, 30 , 31 systemic production of IFNs may be beneficial in COVID‐19. In our experimental protocol, DPI‐7 is the time we chose to end the experiment as the K18‐hACE2 mice infected with SARS‐CoV‐2 have a severe course of disease with death around days 6 or 7. At this late time point coinciding with natural death or killing of severely infected mice, lung tissues showed upregulation of IFNs, especially type I (IFNβ) and type III IFN (IFNλ3). Interestingly, we observed that melatonin receptor ligands could limit the overall levels of lung IFNα, IFNβ, and IFNλ3, by 3–4‐fold, seven days after SARS‐CoV‐2 infection, while no decrease in IFN levels was observed in the plasma compared to the vehicle groups. It would be interesting to evaluate the relevance of a modest decrease in lung IFN levels (by 3‐ to 4‐fold) as being beneficial against COVID‐19, by monitoring the evolution of IFN responses in the bronchoalveolar lavage fluid derived from the lower respiratory tract of COVID‐19 patients in therapeutic clinical trials, including those evaluating melatonin therapy. The elevated levels of lung IFN upon infection and diminished by melatonin receptor treatment mirrored the evolution of Gadd45g expression levels. Gadd45g is part of a group of genes that increases following DNA damage and other stressful conditions associated with growth arrest and apoptosis. 68 Gadd45g was suggested to obstruct lung cell proliferation and subsequent lung recovery upon excessive IFN production triggered by viral infection. 31 As augmented levels of type I and type III IFNs correlate with elevated pulmonary viral RNA, with a more severe clinical score and a raise in a marker of apoptosis and of lung anti‐regenerative processes, melatonin receptor compounds, by limiting the production of IFN, would likely restrict excessive lung injury induced by IFNs.
It should be acknowledged that the impact of melatonin receptor treatment was herein assessed in the K18‐hACE2 mouse model with a rapid manifestation of severe symptoms using high viral titer for intranasal inoculation and characterized by a notable variability in response to SARS‐CoV‐2 infection. Although significant, the effects observed upon melatonergic therapy are rather mild in this mouse model highly responsive to SARS‐CoV‐2 with a very rapid and severe disease evolution. Our data suggest that, in the context of the inflammatory response to SARS‐CoV‐2 in K18‐hACE2 mouse lung, many cytokines and chemokines are induced, but no strong improvement of the cytokine storm was observed by the treatment with melatonin receptor compounds, apart from the restriction of lung interferon production. Of note, melatonin and melatonin ligands did not affect cytokine levels here, while there is evidence in in vivo models of bacterial infection or lipopolysaccharide (LPS)‐induced sepsis 11 , 69 , 70 , 71 , 72 and in mouse/rabbit models of viral infection 73 , 74 , 75 , 76 to support an inhibitory effect of melatonin on levels of TNFα and in some cases of Il1β and Il6 by a factor ranging from 1.25–2‐fold. A recent meta‐analysis of clinical trials has also observed an anti‐inflammatory effect of melatonin at the level of IL1β, IL6 and IL8 but not TNFα. 77 The model of K18‐hACE2 mice infected with SARS‐CoV‐2 being an infection model characterized by an excessive and persistent inflammatory response, as well as the measurement of cytokines made here only at a final stage, could explain this lack of overall effect on observed levels of cytokines. Overall, there was no clear histological difference between lungs of vehicle‐ and MLT50‐treated infected mice, with perhaps a slight tendency to see more severe abnormalities in mice treated with MLT50. An explanation for these histological observations may be linked to the patchy pattern and heterogeneity of the tissue, and therefore to the distribution of lesions within the lungs, making comparative analysis difficult. Overall, treatment with MLT50 does not appear to improve the gross histological status of lung tissue after infection with SARS‐CoV‐2. Due to the experimental design, mice were observed until DPI‐7 and killed for analysis; thus, beneficial effects of the treatments that would be translated into higher recovery and survival rates at longer time points could not be investigated here. Future preclinical animal studies with this model should consider lower viral doses for inoculation, a combination with other drugs, and animal follow‐up beyond seven days. The promising results in the current study, especially regarding lung IFN response, are expected to encourage additional inquiries, both in preclinical and human studies. Particularly, studies using melatonin receptor‐targeted drugs with the aim to restrain prolonged production of interferon locally at late stage of the disease should determine the positive potential of melatonin receptor‐targeted drugs in COVID‐19 patients. Association of melatonin receptor compounds with anti‐inflammatory and antiviral drugs might contribute to better resolve disease outcome and especially in the long run to remedy lung damage. Melatonin is a natural hormone, and melatonin receptor‐drugs have a good safety profile, supporting the idea of drug repurposing. In this context, a very small‐scale clinical study conducted in Iran showed promising results, halving the recovery time of COVID‐19 patients who were subjected to combinatory therapy including melatonin, compared to those not treated with melatonin. 78 Larger‐scale studies in humans and further preclinical studies in animals with mechanistic evaluations would determine the effectiveness of melatonergic therapy in COVID‐19.
CONFLICT OF INTERESTS
The authors declare no competing interest.
AUTHOR CONTRIBUTIONS
E.C., J.D., and R.J. were involved in the conceptualization; E.C., C.I, B.K., S.L.P., and J.D. contributed to the in vivo experiments; E.C., C.I., F.R. A.Z., and J.D. contributed to the biochemical investigation; L.T. was involved in the lung histology and expertise; M.R.G was involved in the lung physiopathological evaluation; E.C., J.D., and R.J. analyzed the data; J.D. and R.J. contributed to the writing—original draft; E.C., S.L.P., F.R., M.R.G., M.B., B.K., R.J., and J.D. contributed to the writing—review and editing; M.B. and R.J. acquired the funding; E.C., J.D., and R.J. contributed to the supervision.
Supporting information
Figure S1‐S4
ACKNOWLEDGMENTS
We thank all the members of the Jockers lab for the discussion at the initial phase of the project. This work was supported by Agence Nationale de la Recherche ((ANR‐RA‐COVID‐19 (ANR‐20‐COV4‐0001 to RJ) (ANR‐19‐CE16‐0025‐01 to RJ) (ANR‐16‐CE18‐0013 to JD)), Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS). RJ was supported by the Fondation de la Recherche Médicale (Equipe FRM DEQ20130326503) and La Ligue Contre le Cancer N/Ref: RS19/75‐127 and E.C. by the Association France Alzheimer (grant No. 2042). JD was supported by the Fondation de France. The SARS‐CoV‐2 strain BetaCoV/France/IDF/200107/2020 was kindly provided by Dr JC. Manuguerra, CIBU, Pasteur Institute. The authors greatly acknowledge the Cytometry and Immunobiology Facility (Cybio) (Karine Bailly) of the Cochin Institute for multiplex analysis of plasma cytokines/chemokines, the HistIM core facility (Fabiola Ely‐Marius) of the Cochin Institute for lung paraffin‐embedding, and Mina Ottaviana (team “Pulmonary hypertension pathophysiology and novel therapies” Inserm U999) for micro‐sectioning and hematoxilin and eosin stain of the lung samples. The authors are very grateful to Dr Alice Huertas (MD, PhD, research Associate Professor) and Maryline Favier and Anne Cauvet (Institut Cochin) for their advice and expertise on lung samples.
Cecon E, Izabelle C, Poder SL, et al. Therapeutic potential of melatonin and melatonergic drugs on K18‐hACE2 mice infected with SARS‐CoV‐2. J Pineal Res. 2022;72:e12772. 10.1111/jpi.12772
Ralf Jockers and Julie Dam are Co‐senior authors.
Contributor Information
Ralf Jockers, Email: ralf.jockers@inserm.fr.
Julie Dam, Email: julie.dam@inserm.fr.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN‐COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid‐19. N Engl J Med. 2021;384(3):238‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bravo JPK, Dangerfield TL, Taylor DW, Johnson KA. Remdesivir is a delayed translocation inhibitor of SARS‐CoV‐2 replication. Mol Cell. 2021;81(7):1548‐1552 e1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Group RC , Horby P, Lim WS, et al. Dexamethasone in Hospitalized Patients with Covid‐19. N Engl J Med. 2021;384(8):693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dubocovich ML. Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep Med. 2007;8(Suppl 3):34‐42. [DOI] [PubMed] [Google Scholar]
- 7. Jockers R, Delagrange P, Dubocovich ML, et al. Update on melatonin receptors: IUPHAR Review 20. Br J Pharmacol. 2016;173(18):2702‐2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu L, Labani N, Cecon E, Jockers R. Melatonin Target Proteins: Too Many or Not Enough? Front Endocrinol (Lausanne). 2019;10:791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hardeland R. Aging, Melatonin, and the Pro‐ and Anti‐Inflammatory Networks. Int J Mol Sci. 2019;20(5):1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun CK, Lee FY, Kao YH, et al. Systemic combined melatonin‐mitochondria treatment improves acute respiratory distress syndrome in the rat. J Pineal Res. 2015;58(2):137‐150. [DOI] [PubMed] [Google Scholar]
- 11. Zhang Y, Li X, Grailer JJ, et al. Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome. J Pineal Res. 2016;60(4):405‐414. [DOI] [PubMed] [Google Scholar]
- 12. Liu J, Clough SJ, Hutchinson AJ, Adamah‐Biassi EB, Popovska‐Gorevski M, Dubocovich ML. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu Rev Pharmacol Toxicol. 2016;56:361‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS‐CoV‐2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5(4):562‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Bodinat C, Guardiola‐Lemaitre B, Mocaer E, Renard P, Munoz C, Millan MJ. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9(8):628‐642. [DOI] [PubMed] [Google Scholar]
- 15. McElroy SL, Winstanley EL, Martens B, et al. A randomized, placebo‐controlled study of adjunctive ramelteon in ambulatory bipolar I disorder with manic symptoms and sleep disturbance. Int Clin Psychopharmacol. 2011;26(1):48‐53. [DOI] [PubMed] [Google Scholar]
- 16. Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F. Network‐based drug repurposing for novel coronavirus 2019‐nCoV/SARS‐CoV‐2. Cell Discov. 2020;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou Y, Hou Y, Shen J, et al. A network medicine approach to investigation and population‐based validation of disease manifestations and drug repurposing for COVID‐19. PLoS Biol. 2020;18(11):e3000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhai X, Wang N, Jiao H, et al. Melatonin and other indoles show antiviral activities against swine coronaviruses in vitro at pharmacological concentrations. J Pineal Res. 2021;71(2):e12754. [DOI] [PubMed] [Google Scholar]
- 19. Moreau GB, Burgess SL, Sturek JM, Donlan AN, Petri WA, Mann BJ. Evaluation of K18‐hACE2 Mice as a Model of SARS‐CoV‐2 Infection. Am J Trop Med Hyg. 2020;103(3):1215‐1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Golden JW, Cline CR, Zeng X, et al. Human angiotensin‐converting enzyme 2 transgenic mice infected with SARS‐CoV‐2 develop severe and fatal respiratory disease. JCI . Insight. 2020;5(19):e142032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yinda CK, Port JR, Bushmaker T, et al. K18‐hACE2 mice develop respiratory disease resembling severe COVID‐19. PLoS Pathog. 2021;17(1):e1009195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oladunni FS, Park JG, Pino PA, et al. Lethality of SARS‐CoV‐2 infection in K18 human angiotensin‐converting enzyme 2 transgenic mice. Nat Commun. 2020;11(1):6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Winkler ES, Bailey AL, Kafai NM, et al. SARS‐CoV‐2 infection of human ACE2‐transgenic mice causes severe lung inflammation and impaired function. Nat Immunol. 2020;21(11):1327‐1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arce VM, Costoya JA. SARS‐CoV‐2 infection in K18‐ACE2 transgenic mice replicates human pulmonary disease in COVID‐19. Cell Mol Immunol. 2021;18(3):513‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Munoz‐Fontela C, Dowling WE, Funnell SGP, et al. Animal models for COVID‐19. Nature. 2020;586(7830):509‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee CY, Lowen AC. Animal models for SARS‐CoV‐2. Curr Opin Virol. 2021;48:73‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lucas C, Wong P, Klein J, et al. Longitudinal analyses reveal immunological misfiring in severe COVID‐19. Nature. 2020;584(7821):463‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee JS, Park S, Jeong HW, et al. Immunophenotyping of COVID‐19 and influenza highlights the role of type I interferons in development of severe COVID‐19. Sci Immunol. 2020;5(49):eabd1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Broggi A, Ghosh S, Sposito B, et al. Type III interferons disrupt the lung epithelial barrier upon viral recognition. Science. 2020;369(6504):706‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Major J, Crotta S, Llorian M, et al. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science. 2020;369(6504):712‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sposito B, Broggi A, Pandolfi L, et al. The interferon landscape along the respiratory tract impacts the severity of COVID‐19. Cell. 2021;184(19):4953‐4968.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rathnasinghe R, Strohmeier S, Amanat F, et al. Comparison of transgenic and adenovirus hACE2 mouse models for SARS‐CoV‐2 infection. Emerg Microbes Infect. 2020;9(1):2433‐2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hassan AO, Case JB, Winkler ES, et al. A SARS‐CoV‐2 Infection Model in Mice Demonstrates Protection by Neutralizing Antibodies. Cell. 2020;182(3):744‐753 e744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Israelow B, Song E, Mao T, et al. Mouse model of SARS‐CoV‐2 reveals inflammatory role of type I interferon signaling. J Exp Med. 2020;217(12):e20201241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bao L, Deng W, Huang B, et al. The pathogenicity of SARS‐CoV‐2 in hACE2 transgenic mice. Nature. 2020;583(7818):830‐833. [DOI] [PubMed] [Google Scholar]
- 37. Sun SH, Chen Q, Gu HJ, et al. A Mouse Model of SARS‐CoV‐2 Infection and Pathogenesis. Cell Host Microbe. 2020;28(1):124‐133 e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McCray PB Jr, Pewe L, Wohlford‐Lenane C, et al. Lethal infection of K18‐hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol. 2007;81(2):813‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Menachery VD, Yount BL Jr, Sims AC, et al. SARS‐like WIV1‐CoV poised for human emergence. Proc Natl Acad Sci U S A. 2016;113(11):3048‐3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jiang RD, Liu MQ, Chen Y, et al. Pathogenesis of SARS‐CoV‐2 in Transgenic Mice Expressing Human Angiotensin‐Converting Enzyme 2. Cell. 2020;182(1):50‐58 e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu GC, Peng CK, Liao WI, Pao HP, Huang KL, Chu SJ. Melatonin receptor agonist protects against acute lung injury induced by ventilator through up‐regulation of IL‐10 production. Respir Res. 2020;21(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Millan MJ, Marin P, Kamal M, et al. The melatonergic agonist and clinically active antidepressant, agomelatine, is a neutral antagonist at 5‐HT(2C) receptors. Int J Neuropsychopharmacol. 2011;14(6):768‐783. [DOI] [PubMed] [Google Scholar]
- 43. Gerbier R, Ndiaye‐Lobry D, Martinez de Morentin PB, et al. Pharmacological evidence for transactivation within melatonin MT2 and serotonin 5‐HT2C receptor heteromers in mouse brain. FASEB J. 2021;35(1):e21161. [DOI] [PubMed] [Google Scholar]
- 44. Waldhauser F, Waldhauser M, Lieberman HR, Deng MH, Lynch HJ, Wurtman RJ. Bioavailability of oral melatonin in humans. Neuroendocrinology. 1984;39(4):307‐313. [DOI] [PubMed] [Google Scholar]
- 45. Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, Classification, and Pharmacology of G Protein‐Coupled Melatonin Receptors. Pharmacol Rev. 2010;62(3):343–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zisapel N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br J Pharmacol. 2018;175(16):3190‐3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cardinali DP, Srinivasan V, Brzezinski A, Brown GM. Melatonin and its analogs in insomnia and depression. J Pineal Res. 2012;52(4):365‐375. [DOI] [PubMed] [Google Scholar]
- 48. Liu L, Jockers R. Structure‐Based Virtual Screening Accelerates GPCR Drug Discovery. Trends Pharmacol Sci. 2020;41(6):382‐384. [DOI] [PubMed] [Google Scholar]
- 49. Acuna‐Castroviejo D, Escames G, Figueira JC, de la Oliva P, Borobia AM, Acuna‐Fernandez C. Clinical trial to test the efficacy of melatonin in COVID‐19. J Pineal Res. 2020;69(3):e12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Garcia JA, Volt H, Venegas C, et al. Disruption of the NF‐kappaB/NLRP3 connection by melatonin requires retinoid‐related orphan receptor‐alpha and blocks the septic response in mice. FASEB J. 2015;29(9):3863‐3875. [DOI] [PubMed] [Google Scholar]
- 51. Chen HH, Chen YT, Yang CC, et al. Melatonin pretreatment enhances the therapeutic effects of exogenous mitochondria against hepatic ischemia‐reperfusion injury in rats through suppression of mitochondrial permeability transition. J Pineal Res. 2016;61(1):52‐68. [DOI] [PubMed] [Google Scholar]
- 52. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Karim A, Tolbert D, Cao C. Disposition kinetics and tolerance of escalating single doses of ramelteon, a high‐affinity MT1 and MT2 melatonin receptor agonist indicated for treatment of insomnia. J Clin Pharmacol. 2006;46(2):140‐148. [DOI] [PubMed] [Google Scholar]
- 54. Johnson MW, Suess PE, Griffiths RR. Ramelteon: a novel hypnotic lacking abuse liability and sedative adverse effects. Arch Gen Psychiatry. 2006;63(10):1149‐1157. [DOI] [PubMed] [Google Scholar]
- 55. Cajochen C, Krauchi K, Mori D, Graw P, Wirz‐Justice A. Melatonin and S‐20098 increase REM sleep and wake‐up propensity without modifying NREM sleep homeostasis. Am J Physiol. 1997;272(4 Pt 2):R1189‐1196. [DOI] [PubMed] [Google Scholar]
- 56. Loo H, Dalery J, Macher JP, Payen A. Pilot study comparing in blind the therapeutic effect of two doses of agomelatine, melatonin‐ agonist and selective 5HT2c receptors antagonist, in the treatment of major depressive disorders. Encephale. 2003;29(2):165‐171. [PubMed] [Google Scholar]
- 57. Nordlund JJ, Lerner AB. The effects of oral melatonin on skin color and on the release of pituitary hormones. J Clin Endocrinol Metab. 1977;45(4):768‐774. [DOI] [PubMed] [Google Scholar]
- 58. Papavasiliou PS, Cotzias GC, Duby SE, Steck AJ, Bell M, Lawrence WH. Melatonin and parkinsonism. JAMA. 1972;221(1):88‐89. [PubMed] [Google Scholar]
- 59. Boutin JA, Jockers R. Melatonin controversies, an update. J Pineal Res. 2021;70(2):e12702. [DOI] [PubMed] [Google Scholar]
- 60. King C, Sprent J. Dual Nature of Type I Interferons in SARS‐CoV‐2‐Induced Inflammation. Trends Immunol. 2021;42(4):312‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Laing AG, Lorenc A, Barrio DMD, et al. A dynamic COVID‐19 immune signature includes associations with poor prognosis. Nat Med. 2020;26(10):1623‐1635. [DOI] [PubMed] [Google Scholar]
- 62. Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life‐threatening COVID‐19. Science. 2020;370(6515). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life‐threatening COVID‐19. Science. 2020;370(6515). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhou Z, Ren L, Zhang L, et al. Heightened Innate Immune Responses in the Respiratory Tract of COVID‐19 Patients. Cell Host Microbe. 2020;27(6):883‐890 e882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Snell LM, McGaha TL, Brooks DG. Type I Interferon in Chronic Virus Infection and Cancer. Trends Immunol. 2017;38(8):542‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Acharya D, Liu G, Gack MU. Dysregulation of type I interferon responses in COVID‐19. Nat Rev Immunol. 2020;20(7):397‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID‐19 patients. Science. 2020;369(6504):718‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Salvador JM, Brown‐Clay JD, Fornace AJ Jr. Gadd45 in stress signaling, cell cycle control, and apoptosis. Adv Exp Med Biol. 2013;793:1‐19. [DOI] [PubMed] [Google Scholar]
- 69. Wang H, Wei W, Shen YX, et al. Protective effect of melatonin against liver injury in mice induced by Bacillus Calmette‐Guerin plus lipopolysaccharide. World J Gastroenterol. 2004;10(18):2690‐2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Carrillo‐Vico A, Lardone PJ, Naji L, et al. Beneficial pleiotropic actions of melatonin in an experimental model of septic shock in mice: regulation of pro‐/anti‐inflammatory cytokine network, protection against oxidative damage and anti‐apoptotic effects. J Pineal Res. 2005;39(4):400‐408. [DOI] [PubMed] [Google Scholar]
- 71. Xu DX, Wang H, Ning H, Zhao L, Chen YH. Maternally administered melatonin differentially regulates lipopolysaccharide‐induced proinflammatory and anti‐inflammatory cytokines in maternal serum, amniotic fluid, fetal liver, and fetal brain. J Pineal Res. 2007;43(1):74‐79. [DOI] [PubMed] [Google Scholar]
- 72. Zhou L, Zhao D, An H, Zhang H, Jiang C, Yang B. Melatonin prevents lung injury induced by hepatic ischemia‐reperfusion through anti‐inflammatory and anti‐apoptosis effects. Int Immunopharmacol. 2015;29(2):462‐467. [DOI] [PubMed] [Google Scholar]
- 73. Huang SH, Cao XJ, Liu W, Shi XY, Wei W. Inhibitory effect of melatonin on lung oxidative stress induced by respiratory syncytial virus infection in mice. J Pineal Res. 2010;48(2):109‐116. [DOI] [PubMed] [Google Scholar]
- 74. Crespo I, San‐Miguel B, Sanchez DI, et al. Melatonin inhibits the sphingosine kinase 1/sphingosine‐1‐phosphate signaling pathway in rabbits with fulminant hepatitis of viral origin. J Pineal Res. 2016;61(2):168‐176. [DOI] [PubMed] [Google Scholar]
- 75. Huang S‐H, Liao C‐L, Chen S‐J, et al. Melatonin possesses an anti‐influenza potential through its immune modulatory effect. J Funct Foods. 2019;58:189‐198. [Google Scholar]
- 76. Crespo I, Fernandez‐Palanca P, San‐Miguel B, Alvarez M, Gonzalez‐Gallego J, Tunon MJ. Melatonin modulates mitophagy, innate immunity and circadian clocks in a model of viral‐induced fulminant hepatic failure. J Cell Mol Med. 2020;24(13):7625‐7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cho JH, Bhutani S, Kim CH, Irwin MR. Anti‐inflammatory effects of melatonin: A systematic review and meta‐analysis of clinical trials. Brain Behav Immun. 2021;93:245‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hosseini A, Esmaeili Gouvarchin Ghaleh H, Aghamollaei H, et al. Evaluation of Th1 and Th2 mediated cellular and humoral immunity in patients with COVID‐19 following the use of melatonin as an adjunctive treatment. Eur J Pharmacol. 2021;904:174193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1‐S4


