Abstract
Background
COVID‐19 frequently presents with acute gastrointestinal (GI) symptoms, but it is unclear how common these symptoms are after recovery. The purpose of this study was to estimate the prevalence and characteristics of GI symptoms after COVID‐19.
Methods
The medical records of patients hospitalized with COVID‐19 between March 1 and June 30, 2020, were reviewed for the presence of GI symptoms at primary care follow‐up 1 to 6 months later. The prevalence of new GI symptoms was estimated, and risk factors were assessed. Additionally, an anonymous survey was used to determine the prevalence of new GI symptoms among online support groups for COVID‐19 survivors.
Key Results
Among 147 patients without pre‐existing GI conditions, the most common GI symptoms at the time of hospitalization for COVID‐19 were diarrhea (23%), nausea/vomiting (21%), and abdominal pain (6.1%), and at a median follow‐up time of 106 days, the most common GI symptoms were abdominal pain (7.5%), constipation (6.8%), diarrhea (4.1%), and vomiting (4.1%), with 16% reporting at least one GI symptom at follow‐up (95% confidence interval 11 to 23%). Among 285 respondents to an online survey for self‐identified COVID‐19 survivors without pre‐existing GI symptoms, 113 (40%) reported new GI symptoms after COVID‐19 (95% CI 33.9 to 45.6%).
Conclusion and inferences
At a median of 106 days after discharge following hospitalization for COVID‐19, 16% of unselected patients reported new GI symptoms at follow‐up. 40% of patients from COVID survivor groups reported new GI symptoms. The ongoing GI effects of COVID‐19 after recovery require further study.
Keywords: coronavirus, COVID‐19, functional gastrointestinal disorders, irritable bowel syndrome
Abbreviations
- COVID‐19
coronavirus disease 2019
- GI
gastrointestinal
- IBS
Irritable bowel syndrome
- IBS‐SSS
irritable bowel syndrome symptom severity score
- IQR
interquartile range
1. INTRODUCTION
The SARS‐CoV‐2 virus has infected nearly 70 million people worldwide, including nearly 15 million in the United States. 1 While over 1.5 million patients have died, many more have recovered. The medical community continues to grapple with the long‐term effects of coronavirus disease 2019 (COVID‐19). It is clear that persistent symptoms are common after recovery, in particular fatigue and dyspnea. 2 , 3 , 4 , 5
COVID‐19 often presents with gastrointestinal (GI) symptoms such as diarrhea, abdominal pain, and nausea or vomiting. 6 , 7 , 8 , 9 Other infections that cause acute GI symptoms frequently lead to persistent symptoms such as post‐infectious irritable bowel syndrome (IBS) and post‐viral gastroparesis. 10 , 11 It has been estimated that post‐infectious IBS occurs in approximately 10% of patients after acute infectious gastroenteritis. 12 , 13 Given the millions of people affected by COVID‐19, even a small percentage with persistent GI symptoms would have important public health implications for patients and their providers.
In this study, we sought to identify the prevalence, severity, and risk factors for GI symptoms in the months following initial infection with COVID‐19, through both a retrospective analysis of patients hospitalized for COVID‐19 with primary care follow‐up at our institution, and through an online survey of “long‐haulers” drawn from two COVID‐19 support groups.
2. METHODS
This was a two‐part study in which we determined the prevalence of new gastrointestinal symptoms during the months following diagnosis of COVID‐19 using two fundamentally different cohorts. First, we performed a retrospective study of patients hospitalized with COVID‐19 at our institution, using manual chart review to determine the prevalence of GI symptoms 1–6 months after hospital discharge. Second, we administered an online survey to users of two support groups for survivors of COVID‐19 assessing the prevalence, type, and severity of GI symptoms, as well as risk factors and demographic information.
In the first study cohort, we included all adult patients who were hospitalized for COVID‐19 at Columbia University Irving Medical Center between March 1 and June 30, 2020, and had at least one primary care follow‐up appointment at our institution between 30 and 180 days post‐discharge. Patients were excluded if they had a pre‐existing history of irritable bowel syndrome, inflammatory bowel disease, or other chronic GI conditions, determined by both manual chart review and the presence of International Classification of Disease diagnostic codes identifying these conditions at the time of COVID‐19 diagnosis. They were also excluded if they had a diagnosis of any other acute enteric infection (eg, Clostridioides difficile infection) during hospitalization for COVID‐19. Patients who were not admitted for COVID‐19 based on the discharge diagnosis (such as women admitted for labor and delivery found to screen positive for SARS‐CoV‐2) were also excluded. Thus, the cohort included all adults admitted for COVID‐19, whether or not they had any gastrointestinal symptoms at the time of admission. The primary outcome was the documentation of prespecified GI symptoms (diarrhea, abdominal pain, nausea or vomiting, and constipation) based on manual chart review at follow‐up. Other covariates including age, sex, hospital length of stay, symptoms at presentation, intensive care unit or ventilator requirement, and history of anxiety or depression were also extracted from the electronic medical record.
In the second study cohort, we administered an anonymous, online survey using Qualtrics to adult (≥18 years of age) users of two support groups for survivors of COVID‐19 (www.reddit.com/r/covid19positive, and Survivor Corps). The survey consisted of questions related to demographics, COVID‐19 severity, and pre‐COVID and post‐COVID GI symptoms (complete survey available in the online Methods Supplement). Questions were designed to determine whether respondents met the Rome IV criteria for irritable bowel syndrome (IBS) with IBS questions pertaining to both pre‐–COVID‐19 and post–COVID‐19 symptoms so that we could clearly identify whether symptoms were new. 14 , 15 Additional questions related to the current presence of gastrointestinal symptoms including abdominal pain, diarrhea, constipation, and nausea/vomiting were used to provide additional information about specific symptoms outside of the Rome criteria for IBS. Respondents also completed the IBS Symptom Severity Score (IBS‐SSS), a validated survey used to determine the severity of IBS symptoms on a 0–500 point scale, with ≤175 indicating mild, 175–300 moderate, and ≥300 severe IBS. 16 The survey was administered between November 12 and December 15, 2020. The primary outcome was again the presence of post‐COVID GI symptoms, defined as the report of any GI symptoms (same symptoms as above) post–COVID‐19 that were not reported pre–COVID‐19. A secondary outcome was the presence of new symptoms meeting Rome IV criteria for IBS post–COVID‐19, among those who did not meet criteria for IBS pre–COVID‐19.
Participation in the survey was voluntary and anonymous with no financial or medical incentive. The survey was distributed by email and by posting on the relevant forum with the permission of the group administrators. The Columbia University Institutional Review Board approved this study.
2.1. Statistical analysis
For both parts of the study, continuous variables were compared in patients with and without persistent GI symptoms using the Student's t test or Mann‐Whitney U test for non‐parametric data. Categorical variables were compared using chi‐square tests. A multivariable logistic regression model was also used to identify independent risk factors for ongoing GI symptoms, adjusting for predictors that were significant at alpha level 0.20. Among the unselected patients coming for routine follow‐up visits, we sought to test whether the prevalence of GI symptoms diminished with increasing time from hospital discharge. To do this, the cohort was divided a priori into those presenting for follow‐up from 30 to 89 days after hospitalization for COVID‐19, 90–119 days after hospitalization, 120–149 days after hospitalization, and 150–180 days after hospitalization. The trend across the proportions of those with GI symptoms within each time interval was then assessed using the Cochran‐Armitage test. Among the second study cohort of online survey respondents, the prevalence of GI symptoms meeting criteria for irritable bowel syndrome was compared before and after COVID‐19 diagnosis using McNemar's test for paired categorical data. Confidence interval for proportions was calculated using the exact binomial (Clopper‐Pearson) method. All statistical analyses were performed with Stata version 16 (College Station, Tx). Alpha.05 was considered statistically significant for all analyses.
3. RESULTS
3.1. Unselected patients hospitalized for COVID‐19
There were 187 adult patients hospitalized for COVID‐19 at Columbia University Irving Medical Center between March 1 and June 30, 2020, with at least one primary care outpatient clinic appointment at least 30 days after hospital discharge. Of these, 37 were excluded for pre‐existing GI symptoms (documentation of chronic diarrhea, constipation, abdominal pain, celiac disease, irritable bowel syndrome, inflammatory bowel disease, or gastroparesis). An additional 3 patients were excluded for C. difficile infection during the COVID‐19 hospitalization, leaving 147 patients in the cohort. The most common GI symptoms at initial presentation with COVID‐19 among this group were diarrhea (23%), nausea/vomiting (21%), and abdominal pain (6.1%), with 51 patients (35%) having at least one GI symptom at the time of hospital admission.
3.2. Unselected patients: persistent new GI symptoms at routine follow‐up
After a median follow‐up time of 106 days (IQR 78–141), 24/147 (16%) of patients had at least one ongoing GI symptom (95% confidence interval 11%–23%) at the time of their most recent follow‐up appointment after hospitalization for COVID‐19, which had not been present prior to their diagnosis of COVID‐19. These included 10 patients with constipation (6.8%), 6 with diarrhea (4.1%), 6 with nausea/vomiting (4.1%), and 11 with abdominal pain (7.5%), with 14 patients having more than one symptom. An additional 5% of patients experienced transient GI symptoms at follow‐up, which had resolved by the time of their latest clinic appointment. Among the 113 patients with at least 3 months of follow‐up, the prevalence of ongoing abdominal pain in association with bowel disturbance (diarrhea or constipation) was 6.2%. As a point of comparison, the prevalence of post‐infectious IBS after infectious gastroenteritis has been estimated at 10%, 12 , 13 though it should be noted that this estimate varies widely depending on pathogen and definition used. Furthermore, the diagnosis of IBS requires at least 6 months of symptoms, which patients in this cohort did not have.
3.3. Unselected patients: GI symptom characteristics and baseline risk factors
Among patients hospitalized for COVID‐19, the prevalence of constipation increased from 0% at hospital admission to 6.8% at most recent outpatient follow‐up (p < 0.01). The prevalence of diarrhea decreased from 23% at admission to 4.1% at follow‐up (p < 0.01). The prevalence of abdominal pain was similar at 6.1% at admission and 7.5% at follow‐up (p = 0.64). The prevalence of nausea/vomiting decreased from 21% at admission to 4.1% at follow‐up (p < 0.01). The prevalence of each GI symptom present at the time of the most recent follow‐up is shown in Table 1. Women were significantly more likely to report new abdominal pain compared to men (14% vs. 1.3%, p < 0.01), though there was no significant difference in the prevalence of overall GI symptoms by sex (21% in women vs. 12% in men, p = 0.15). There were no significant differences in GI symptoms by age, race, or ethnicity. Prior history of depression was also a risk factor for persistent GI symptoms (28% vs. 13%, p = 0.04).
TABLE 1.
Prevalence of gastrointestinal symptoms among 147 patients hospitalized for COVID‐19 after a median 106 days of follow‐up
| Gastrointestinal symptoms at most recent follow‐up appointment | |||||
|---|---|---|---|---|---|
| Constipation | Diarrhea | Abdominal pain | Nausea or vomiting | Any symptoms | |
| Sex | |||||
| Female (n = 72) | 4 | 4 | 10* | 3 | 15 (21%) |
| Male (n = 75) | 6 | 2 | 1* | 3 | 9 (12%) |
| Age | |||||
| 18–44 (n = 24) | 3 | 0 | 4 | 0 | 5 (21%) |
| 45–59 (n = 36) | 3 | 3 | 4 | 2 | 9 (25%) |
| 60–69 (n = 34) | 0 | 1 | 1 | 1 | 3 (8.8%) |
| ≥70 (n = 53) | 4 | 2 | 2 | 3 | 7 (13%) |
| Race | |||||
| White (n = 37) | 2 | 2 | 2 | 2 | 5 (14%) |
| Black (n = 27) | 0 | 2 | 0 | 0 | 2 (7.4%) |
| Asian (n = 3) | 1 | 0 | 1 | 0 | 2 (67%) |
| Other/decline (n = 80) | 7 | 2 | 8 | 4 | 15 (19%) |
| Ethnicity | |||||
| Hispanic (n = 83) | 7 | 4 | 8 | 3 | 15 (18%) |
| Non‐Hispanic (n = 38) | 2 | 2 | 1 | 1 | 5 (13%) |
| Unknown/decline (n = 26) | 1 | 0 | 2 | 2 | 4 (15%) |
| Depression | |||||
| No (n = 115) | 7 | 3 | 7 | 4 | 15 (13%)* |
| Yes (n = 32) | 3 | 3 | 4 | 2 | 9 (28%)* |
| Anxiety | |||||
| No (n = 120) | 7 | 5 | 7 | 5 | 19 (16%) |
| Yes (n = 27) | 3 | 1 | 4 | 1 | 5 (19%) |
| Fibromyalgia | |||||
| No (n = 128) | 9 | 5 | 7* | 6 | 19 (15%) |
| Yes (n = 19) | 1 | 1 | 4* | 0 | 5 (26%) |
p < 0.05.
3.4. Unselected patients: hospital risk factors for persistent new GI symptoms
The prevalence of persistent GI symptoms stratified by hospitalization characteristics is shown in Table 2. While patients who had GI symptoms at initial presentation had a higher prevalence of persistent GI symptoms at follow‐up, this difference was not statistically significant (22% vs. 14%, p = 0.21). Length of stay, intensive care unit admission, and use of antibiotics, steroids, or hydroxychloroquine were not associated with a higher prevalence of GI symptoms at follow‐up. The peak laboratory values of inflammatory markers during hospitalization were also identified for each patient, and there were no significant differences in the median erythrocyte sedimentation rate, C‐reactive protein, white blood cell count, ferritin, or interleukin‐6 levels in patients with and without persistent GI symptoms at follow‐up (Table S1).
TABLE 2.
Hospitalization characteristics of patients with GI symptoms at follow‐up
| GI Symptoms at most recent follow‐up appointment | |||||
|---|---|---|---|---|---|
| Constipation | Diarrhea | Abdominal pain | Nausea or vomiting | Any symptoms | |
| GI symptoms at diagnosis | |||||
| No (n = 96) | 5 | 3 | 8 | 3 | 13 (14%) |
| Yes (n = 51) | 5 | 3 | 3 | 3 | 11 (22%) |
| Length of Stay | |||||
| 0 to 3 days (n = 33) | 4 | 3 | 4 | 1 | 8 (24%) |
| 4–7 days (n = 34) | 0 | 0 | 1 | 2 | 3 (8.8%) |
| 8–14 days (n = 43) | 3 | 0 | 3 | 1 | 5 (12%) |
| >14 days (n = 37) | 3 | 3 | 3 | 2 | 8 (22%) |
| ICU admission | |||||
| No (n = 128) | 8 | 4 | 9 | 5 | 19 (15%) |
| Yes (n = 19) | 2 | 2 | 2 | 1 | 5 (26%) |
| Hydroxychloroquine | |||||
| No (n = 46) | 5 | 3 | 2 | 3 | 9 (20%) |
| Yes (n = 101) | 5 | 3 | 9 | 3 | 15 (15%) |
| Steroids | |||||
| No (n = 111) | 5 | 3 | 7 | 4 | 15 (14%) |
| Yes (n = 36) | 5 | 3 | 4 | 2 | 9 (25%) |
| Antibiotics | |||||
| No (n = 43) | 4 | 3 | 1 | 3 | 9 (21%) |
| Yes (n = 104) | 6 | 3 | 10 | 3 | 15 (14%) |
p < 0.05.
3.5. Unselected patients: multivariable analysis of predictors of ongoing GI symptoms at follow‐up
A multivariable logistic regression model was also used to evaluate for independent risk factors of ongoing GI symptoms at the time of follow‐up (Table 3). After adjusting for age, sex, and admission to intensive care unit (predictors that were significant at alpha level 0.20), only a history of depression remained significantly associated with the presence of GI symptoms at follow‐up, with adjusted odds ratio 3.07, 95% confidence interval 1.04–9.04, p = 0.042).
TABLE 3.
Multivariable logistic regression of predictors of gastrointestinal symptom at follow‐up
| Variable | Any GI Symptom at most recent follow‐up appointment | ||
|---|---|---|---|
| Adjusted odds ratio | 95% confidence interval | p‐value | |
| Age | |||
| 18–44 | 1 (reference) | ||
| 45–59 | 1.52 | 0.40–5.81 | 0.54 |
| 60–69 | 0.37 | 0.07–1.97 | 0.24 |
| ≥70 | 0.64 | 0.15–2.67 | 0.54 |
| Sex | |||
| Male | 1 (reference) | ||
| Female | 2.13 | 0.74–6.13 | 0.16 |
| ICU admission | |||
| No | 1 (reference) | ||
| Yes | 3.25 | 0.81–13.00 | 0.096 |
| Depression | |||
| No | 1 (reference) | ||
| Yes | 3.07 | 1.04–9.04 | 0.042 |
3.6. Unselected patients: gastrointestinal symptoms over time
The prevalence of GI symptoms was 24% among patients whose most recent follow‐up was 30–89 days after discharge, 20% for those 90–119 days post‐discharge, 17% for those 120–149 days post‐discharge, and 11% for those 150–180 days post‐discharge (Figure 1, Cochran‐Armitage test for trend p = 0.10). Of the 24 patients with ongoing GI symptoms at follow‐up, 5 had undergone evaluation with upper endoscopy and 3 with colonoscopy at the time of chart review, with only minor findings noted (one patient with grade A esophagitis and 1 with esophageal candidiasis).
FIGURE 1.
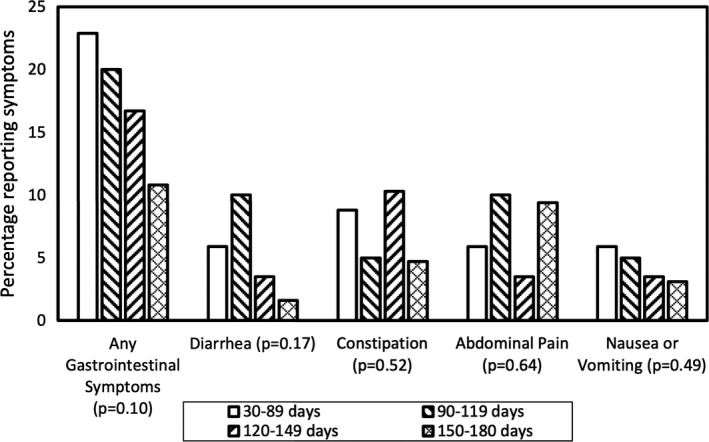
Prevalence of gastrointestinal symptoms by time period of most recent follow‐up among patients hospitalized with COVID‐19: p‐value refers to trend of symptom prevalence over time within each symptom category
3.7. Respondents to an online survey
There were 355 respondents to the online survey (229 from Survivor Corps and 126 from the COVID19positive Reddit group) who were ≥18 years of age and answered “yes” to the question “Have you been diagnosed with COVID‐19.” There were 70 respondents (20%) who reported a history of IBS or symptoms consistent with IBS prior to their COVID‐19 diagnosis leaving 285 in the primary analysis. Among these, 91 (32%) reported diagnosis less than 1 month prior to the survey, 38 (13%) 1–3 months prior, 44 (15%) 3–6 months prior, and 112 (39%) more than 6 months prior.
3.8. Survey respondents: post–COVID‐19 abdominal pain with bowel disturbance
After excluding survey respondents with pre‐existing IBS, there were 113/285 (40%) respondents with post–COVID‐19 abdominal pain with bowel disturbance at the time of the survey (ie, with weekly abdominal pain associated with at least two of the following: related to defecation, change in bowel frequency, or change in stool form). In the most recent 10 days prior to survey completion, 165/285 (58%) respondents reported diarrhea, 173 (61%) reported abdominal pain, 130 (46%) reported constipation, and 134 (47%) reported nausea or vomiting. Among all survey respondents, 123/355 (35%) reported taking a new medication for a new gastrointestinal symptom after diagnosis with COVID‐19.
3.9. Survey respondents: risk factors for abdominal pain with bowel disturbance
The characteristics of the survey respondents, excluding those with pre‐existing IBS, is shown in Table 4, comparing those with and without weekly abdominal pain with bowel disturbance after recovery from COVID‐19. Women had a higher prevalence of GI symptoms, but the difference was not statistically significant (42% vs. 35%, p = 0.25). There was no association between post‐COVID GI symptoms and race/ethnicity, or history of depression, anxiety, or fibromyalgia. There was no significant association with the method of COVID‐19 diagnosis. Those who had GI symptoms at the time of diagnosis were significantly more likely to report GI symptoms at follow‐up (45% vs. 27%, p = 0.01), as were those who received antibiotics (51% vs. 35%, p = 0.02) or hydroxychloroquine (69% vs. 38%, p = 0.03).
TABLE 4.
Prevalence of Post‐COVID GI symptoms in an anonymous survey of survivors.
| All respondents (n = 285) | No post‐COVID GI symptoms (n = 172) | Post‐COVID GI symptoms* (n = 113) | p‐value | |
|---|---|---|---|---|
| Age | ||||
| 18–29 | 48 (17%) | 35 (73%) | 13 (27%) | 0.31 |
| 30–39 | 67 (24%) | 41 (61%) | 26 (39%) | |
| 40–49 | 49 (17%) | 26 (53%) | 23 (47%) | |
| 50–59 | 58 (21%) | 31 (53%) | 27 (47%) | |
| 60–69 | 47 (17%) | 29 (62%) | 18 (38%) | |
| ≥70 | 12 (4.3%) | 6 (50%) | 6 (50%) | |
| Sex | ||||
| Female | 198 (71%) | 114 (58%) | 84 (42%) | 0.25 |
| Male | 80 (29%) | 52 (65%) | 28 (35%) | |
| Race | ||||
| White | 242 (86%) | 141 (58%) | 101 (42%) | 0.36 |
| Black | 9 (3.2%) | 7 (78%) | 2 (22%) | |
| Asian | 10 (3.6%) | 8 (80%) | 2 (20%) | |
| Other | 21 (7.5%) | 13 (62%) | 8 (38%) | |
| Ethnicity | ||||
| Hispanic | 25 (9.4%) | 14 (56%) | 11 (44%) | 0.73 |
| Non‐Hispanic | 240 (91%) | 143 (60%) | 97 (40%) | |
| Depression | ||||
| No | 202 (71%) | 125 (62%) | 77 (38%) | 0.41 |
| Yes | 83 (29%) | 47 (57%) | 36 (43%) | |
| Anxiety | ||||
| No | 192 (67%) | 118 (61%) | 74 (39%) | 0.58 |
| Yes | 93 (33%) | 54 (58%) | 39 (42%) | |
| Fibromyalgia | ||||
| No | 272 (95%) | 166 (61%) | 106 (39%) | 0.28 |
| Yes | 13 (4.6%) | 6 (46%) | 7 (54%) | |
| Diagnosis Type | ||||
| PCR | 192 (67%) | 111 (58%) | 81 (42%) | 0.57 |
| Antibody | 21 (7.4%) | 15 (71%) | 6 (29%) | |
| Symptoms | 63 (22%) | 40 (63%) | 23 (37%) | |
| Unsure | 9 (3.2%) | 6 (67%) | 3 (33%) | |
| GI symptoms at diagnosis | ||||
| No | 85 (30%) | 62 (73%) | 23 (27%) | 0.01 |
| Yes | 200 (70%) | 110 (55%) | 90 (45%) | |
| Hospitalized | ||||
| No | 249 (88%) | 150 (60%) | 99 (40%) | 0.87 |
| Yes | 34 (12%) | 20 (59%) | 14 (41%) | |
| Intubated | ||||
| No | 279 (98%) | 169 (61%) | 110 (39%) | 0.60 |
| Yes | 6 (2.1%) | 3 (50%) | 3 (50%) | |
| Received Antibiotics | ||||
| No | 206 (72%) | 133 (65%) | 73 (35%) | 0.02 |
| Yes | 79 (28%) | 39 (49%) | 40 (51%) | |
| Received Hydroxychloroquine | ||||
| No | 272 (95%) | 168 (62%) | 104 (38%) | 0.03 |
| Yes | 13 (4.6%) | 4 (31%) | 9 (69%) | |
| Received Steroids | ||||
| No | 233 (82%) | 141 (61%) | 92 (39%) | 0.91 |
| Yes | 52 (18%) | 31 (60%) | 21 (40%) | |
| Time from diagnosis | ||||
| < 1 month | 91 (32%) | 64 (70%) | 27 (30%) | 0.01 |
| 1–3 months | 38 (13%) | 15 (39%) | 23 (61%) | |
| 3–6 months | 44 (15%) | 25 (57%) | 19 (43%) | |
| >6 months | 112 (39%) | 68 (61%) | 44 (39%) | |
Presence of at least weekly abdominal pain, associated with altered bowel habits, among all respondents without pre‐existing IBS. Patients with any follow‐up length of time were included in this analysis.
3.10. Survey respondents: new symptoms consistent with IBS lasting ≥6 months
There were 134 survey respondents who were diagnosed with COVID‐19 at least 6 months prior to the survey (including 22 with and 112 without pre‐COVID symptoms of IBS). These respondents thus could be evaluated for chronic symptoms of post‐infectious IBS by the Rome IV criteria. Among them, the prevalence of IBS symptoms (abdominal pain with altered bowel habits) increased from 22/134 (16%) pre–COVID‐19 to 55/134 (41%) post–COVID‐19 (p < 0.01). Among these 134 respondents, there were 92 who answered all questions from the IBS‐SSS pertaining to their current symptoms, as well as to their symptoms prior to developing COVID‐19. The mean IBS‐SSS score pre‐COVID was 61 and increased to 248 post‐COVID (p < 0.01). Among the 112 respondents with at least 6 months of follow‐up, excluding 22 with pre‐existing history or symptoms of IBS, the prevalence of post‐COVID IBS symptoms was 44/112 or 39%.
4. DISCUSSION
This study evaluated the prevalence of new gastrointestinal symptoms after diagnosis of COVID‐19 using two methods: (1) during a routine primary care visit a median 3 months after hospitalization for COVID‐19 and (2) using an anonymous survey of COVID‐19 survivors drawn from two online support groups. In the unselected cohort of hospitalized patients, among those without previous chronic GI symptoms, the prevalence of at least one new GI symptom at follow‐up was 16%. In contrast, the prevalence of new GI symptoms among self‐identified COVID‐19 survivors (after excluding those with pre‐existing GI symptoms) was 40%. Routine primary care visits may under‐estimate the true prevalence of GI symptoms due to under‐documentation whereas selection bias among COVID long‐haulers is likely to over‐estimate the true prevalence of GI symptoms. We believe the true prevalence of persistent GI symptoms (meaning any symptoms for one month or more) after COVID‐19 is likely between 16 and 40%, but much closer to the former than the latter.
The prevalence of post‐infectious IBS after acute gastroenteritis has been estimated at 10%. 12 , 13 There are important caveats to recognize that prevent the direct comparison of this study's 16% prevalence of GI symptoms at a median of 3 months after COVID‐19 to the 10% estimate of post‐infectious IBS after acute gastroenteritis. First, patients in this study did not all have the length of follow‐up time to meet the Rome IV criteria for IBS. When the definition for “persistent GI symptoms” within the unselected cohort was restricted to patients with both persistent abdominal pain and altered bowel habits for ≥3 months, the prevalence of abdominal pain with bowel disturbance fell to 6.2%. Second, the prevalence of GI symptoms appeared to decrease with increasing time since discharge, although this trend was not statistically significant. A subset of patients may have had lingering symptoms from COVID‐19 rather than a true post‐infectious IBS, and it is likely that estimates for symptom prevalence would decline if the duration of follow‐up was extended.
In the second study cohort, we evaluated self‐identified COVID‐19 survivors via online survey of COVID‐19 support groups and found a higher prevalence of ongoing GI symptoms. In this survey, we directly asked questions to determine whether patients met the Rome IV criteria for IBS, as well as questions regarding the severity of their GI symptoms using the IBS‐SSS, which was not possible through a retrospective chart review (as clinicians may document diarrhea without specifically asking about the presence of abdominal pain, for example). Among 134 patients diagnosed with COVID‐19 at least 6 months prior to the survey, the prevalence of symptoms consistent with IBS increased from 16% pre–COVID‐19 to 41% post–COVID‐19. The severity of GI symptoms as measured by the IBS Symptom Severity Score also increased significantly from a mean of 61 pre–COVID‐19 to 248 post–COVID‐19 (corresponding to moderate IBS symptoms). A recent study found that 21% of patients with gastroenteritis due to Campylobacter developed post‐infectious IBS, in most cases diarrhea predominant or mixed. 17 Among patients with pre‐existing IBS, 38% experienced an increase in frequency of abdominal pain after Campylobacter infection, similar to our finding that IBS symptom severity score increased after COVID‐19.
These data are intriguing, but it is unknown how well these self‐identified COVID‐19 survivors represent the general population. “Long hauler” patients with persistent symptoms are more likely to complete surveys evaluating such symptoms and have the potential to create a powerful selection bias. Nonetheless, given the high numbers of patients with so‐called “long COVID,” it remains valuable to determine the prevalence and severity of gastrointestinal symptoms in this population. Our results suggest that in addition to dyspnea, fatigue, and “brain fog” symptoms, abdominal pain and altered bowel habits are common and can be severe. 4 , 18
Our study did not uncover any surprising risk factors for new GI symptoms after COVID‐19. Markers of more serious illness such as length of stay or intensive care unit admission were not associated with new GI symptoms. Among the hospitalized group, women were more likely to have abdominal pain at follow‐up, and patients with a pre‐existing history of depression were more likely to have at least one GI symptom, consistent with previous literature demonstrating an association of functional GI disorders with these groups. 19 , 20 , 21 , 22 Post‐COVID GI symptoms may be related to ongoing psychological factors such as post‐traumatic stress disorder, disturbance of the gut microbiome, or persistent intestinal inflammation, 23 and future studies should aim to elucidate the mechanism underlying this association.
There are few studies reporting on the frequency and severity of new GI symptoms post–COVID‐19 in detail. A study of 100 patients in Italy reported that 3% had “new bowel control problems” a mean of 48 days post‐discharge for COVID‐19. 24 Another study of 150 non‐critically ill COVID‐19 patients in France found that 30 days post‐discharge, 17% reported ongoing diarrhea or vomiting, which decreased to 12% at 60 days. 25 A recent study of hospitalized patients in China found that 80/1,655 (5%) reported diarrhea or vomiting six months later, which was similar to our finding of 4.1% for both diarrhea and vomiting as individual symptoms among hospitalized patients. 5 However, they did not survey patients for abdominal pain or constipation. These studies were relatively limited in their assessment of persistent GI symptoms, so it is challenging to compare their results to ours.
Some limitations should be mentioned. The retrospective cohort included only patients who were hospitalized during the early phase of the pandemic, when testing for SARS‐CoV‐2 was limited and often reserved for those with more severe symptoms. Therefore, it may not be generalizable to the broader population of all COVID‐19 patients who typically have a milder course. As mentioned, due to the retrospective nature of the study, it was not possible to assess for the Rome IV criteria for post‐infectious IBS. On the contrary, the online survey allowed for more precise questioning on symptom type, chronicity, and severity, in keeping with Rome IV. But this part of the study was limited by selection bias, as patients suffering from ongoing symptoms are more likely to be users of online support groups.
In summary, new GI symptoms were common after COVID‐19. This was true in unselected patients coming for routine primary care visits and more so among self‐identified COVID‐19 survivors in an online survey. Outpatient providers caring for COVID‐19 survivors should be aware that GI symptoms may persist or develop after COVID‐19 and the long‐term GI impact of COVID‐19 merits additional study.
CONFLICT OF INTEREST
All authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
JWB, DJ, and DEF contributed to study concept and design. JWB and JL involved in acquisition of data. JWB, DJ, and DEF involved in analysis and interpretation of data. JWB and DEF involved in drafting of the manuscript. JWB, JL, DJ, and DEF involved in critical revision of the manuscript for important intellectual content.
ETHICS APPROVAL
The Columbia University Institutional Review Board approved this study.
Supporting information
Table S1
Supplementary Material
Blackett JW, Li J, Jodorkovsky D, Freedberg DE. Prevalence and risk factors for gastrointestinal symptoms after recovery from COVID‐19. Neurogastroenterology & Motility. 2022;34:e14251. 10.1111/nmo.14251
DATA AVAILABILITY STATEMENT
Anonymized data are available from the corresponding author upon reasonable request.
REFERENCES
- 1. COVID‐19 Dashboard by the Center for Systems Science and Engineering. Johns Hopkins University. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6. Published 2020. Accessed December 8.
- 2. Carfi A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID‐19. JAMA. 2020;324(6):603‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davido B, Seang S, Tubiana R, de Truchis P. Post‐COVID‐19 chronic symptoms: a postinfectious entity? Clin Microbiol Infect. 2020;26(11):1448‐1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garrigues E, Janvier P, Kherabi Y, et al. Post‐discharge persistent symptoms and health‐related quality of life after hospitalization for COVID‐19. J Infect. 2020;81(6):e4‐e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Redd WD, Zhou JC, Hathorn KE, et al. Prevalence and characteristics of gastrointestinal symptoms in patients with severe acute respiratory syndrome coronavirus 2 infection in the United States: a multicenter cohort study. Gastroenterology. 2020;159(2):765‐767 e762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou Z, Zhao N, Shu Y, Han S, Chen B, Shu X. Effect of gastrointestinal symptoms in patients with COVID‐19. Gastroenterology. 2020;158(8):2294‐2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nobel YR, Phipps M, Zucker J, et al. Gastrointestinal symptoms and coronavirus disease 2019: a case‐control study from the United States. Gastroenterology. 2020;159(1):373‐375 e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pan L, Mu M, Yang P, et al. Clinical characteristics of COVID‐19 patients with digestive symptoms in Hubei, China: A Descriptive, Cross‐Sectional, Multicenter Study. Am J Gastroenterol. 2020;115(5):766‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Camilleri M, Chedid V, Ford AC, et al. Gastroparesis. Nat Rev Dis Primers. 2018;4(1):41. [DOI] [PubMed] [Google Scholar]
- 11. Card T, Enck P, Barbara G, et al. Post‐infectious IBS: defining its clinical features and prognosis using an internet‐based survey. United European Gastroenterol J. 2018;6(8):1245‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta‐analysis. Gastroenterology. 2017;152(5):1042‐1054 e1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome–a meta‐analysis. Am J Gastroenterol. 2006;101(8):1894‐1899. quiz 1942. [DOI] [PubMed] [Google Scholar]
- 14. Palsson OS, Whitehead WE, van Tilburg MA, et al. Rome IV diagnostic questionnaires and tables for investigators and clinicians. Gastroenterology. 2016;150(6):1481–1491. [DOI] [PubMed] [Google Scholar]
- 15. Palsson OS, Whitehead W, Tornblom H, Sperber AD, Simren M. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology. 2020;158(5):1262‐1273 e1263. [DOI] [PubMed] [Google Scholar]
- 16. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11(2):395‐402. [DOI] [PubMed] [Google Scholar]
- 17. Berumen A, Lennon R, Breen‐Lyles M, et al. Characteristics and risk factors of post‐infection irritable bowel syndrome after Campylobacter Enteritis. Clin Gastroenterol Hepatol. 2020;19(9):1855–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rubin R. As their numbers grow, COVID‐19 "Long Haulers" Stump Experts. JAMA. 2020;324(14):1381. [DOI] [PubMed] [Google Scholar]
- 19. Ballou S, Katon J, Singh P, et al. Chronic diarrhea and constipation are more common in depressed individuals. Clin Gastroenterol Hepatol. 2019;17(13):2696‐2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee SP, Sung IK, Kim JH, Lee SY, Park HS, Shim CS. The effect of emotional stress and depression on the prevalence of digestive diseases. J Neurogastroenterol Motil. 2015;21(2):273‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123(5):1686‐1701. [DOI] [PubMed] [Google Scholar]
- 22. Heitkemper MM, Jarrett ME. Update on irritable bowel syndrome and gender differences. Nutr Clin Pract. 2008;23(3):275‐283. [DOI] [PubMed] [Google Scholar]
- 23. Schmulson M, Ghoshal UC, Barbara G. Managing the inevitable surge of post‐COVID‐19 functional gastrointestinal disorders. Am J Gastroenterol. 2021;116(1):4‐7. [DOI] [PubMed] [Google Scholar]
- 24. Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID‐19 infection: a cross‐sectional evaluation. J Med Virol. 2020;93(2):1013‐1022. [DOI] [PubMed] [Google Scholar]
- 25. Carvalho‐Schneider C, Laurent E, Lemaignen A, et al. Follow‐up of adults with noncritical COVID‐19 two months after symptom onset. Clin Microbiol Infect. 2020;27(2):258‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Supplementary Material
Data Availability Statement
Anonymized data are available from the corresponding author upon reasonable request.


