Abstract
Introduction
Follow‐up studies of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in kidney transplant recipients (KTR) are scarcely reported.
Methods
We studied 142 hospitalized KTR for a median (interquartile range) follow‐up of 9 (8–11) months who recovered from SARS‐CoV‐2 during May 2020 to Dec 2020. The outcomes were to assess persistent symptoms post‐discharge; EuroQoL visual analogue score (EQ‐VAS); EuroQoL 5‐dimension score (E5‐QD‐5L) score and modified medical research dyspnea score (mMRC) at 1 month, 3‐month, and beyond 6 months. Graft outcome was also analyzed.
Results
The age of the cohort was 43 (34–69) years and COVID‐19 severity ranged from asymptomatic (4%), mild (50%), moderate (35%) to severe (12%).
The most common persistent symptom was fatigue which significantly decreased in the follow‐up (n = 45 [32.3] vs. 10 [7.4] vs. 4 [2.9]; p‐value = 0.001) at 1‐month, 3‐month, and beyond 6 months respectively. Decrement in the mean (standard deviation) EQ‐VAS score from baseline was also improved (28.6 [13] vs. 10.4 [12.5] vs. 7.5 [12.0]; p‐value = 0.012). There was significant improvement in all EQ‐5D‐5L scores in follow‐up. There was no deterioration in mMRC scores during the follow‐up (n = 4, 3% vs. 7, 5% vs. 3, 2%; p‐value = 0.86). Cases requiring oxygen had significantly poorer overall scores initially, but there was no difference at 6 months. All 10 graft losses had oxygen requirement and chronic graft dysfunction at baseline.
Conclusion
Our initial assessment reports significant improvement in the quality of life in follow‐up. The majority recovered from allograft dysfunction. Further research is warranted to study the full spectrum of follow‐up.
Keywords: COVID‐19 recovered, follow‐up, kidney transplantaion, post‐COVID‐19, quality of life
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has devastated all health communities across the globe. The issue of long‐term effects of coronavirus disease (COVID‐19) on survivors is incompletely understood, 1 and the data are still emerging. In the general population, there are various studies of post‐COVID‐19 sequelae demonstrating functional disability after recovery. 2 , 3 , 4 Organ transplant recipients are more severely affected by COVID‐19 5 , 6 as compared to the general patients, 7 , 8 , 9 , 10 and hence studies demonstrating long‐term SARS‐CoV‐2 sequelae should trigger an alarm for the need of similar studies in transplant recipients. This entity may require a protocol for post‐COVID‐19 follow‐up and rehabilitation clinic. A recent study raised concerns about chronic COVID‐19 in patients on chronic immunosuppression. 11 The questions which need to be answered are as follows: What is the burden and spectrum of persistent symptoms in the transplant population? How does COVID‐19 affect the quality of life of kidney transplant recipients (KTR) in follow‐up? What is the long‐term graft outcome of COVID‐19‐recovered KTR? Are there any multisystem complications during follow‐up?
Thus, we investigated the short‐term and long‐term follow‐up course of hospitalized KTR discharged from our center. The novelty of this study lies in the fact that comprehensive follow‐up of COVID‐19‐recovered KTR has been scantly described in the literature so far. This study will further help transplant physicians across the world in understanding SARS‐CoV‐2 sequelae and guide them in further planning and management for potential future waves.
2. METHODOLOGY
2.1. Design, settings, and ethics
This is a retrospective cohort study conducted from May 2020 to 27July 2021 at the Institute of Kidney Diseases and Research Centre, Dr. HL Trivedi Institute of Transplantation Sciences (IKDRC‐ITS), Ahmedabad, India. This study was approved by the institutional ethics board (ECR/143/Inst/GJ/2013/RR‐19) and conducted as per the rules of declaration of Helsinki and declaration of Istanbul.
2.2. Study participants
SARS‐CoV‐2‐affected KTR patients who tested positive by reverse transcriptase‐polymerase test on the nasopharyngeal sample (nRT‐PCR) and had survived the acute COVID‐19 phase (defined as period from hospital admission to discharge) were included for analysis in the study. A total of 142 hospitalized COVID‐19 survivors were analyzed, and their clinical course, treatment modalities, inflammatory profile, and other relevant data were retrieved from electronic institutional records and case files. The patients were classified based on COVID‐19 severity as follows: Mild: No need of oxygen therapy; Moderate: Maintaining oxygen saturation of 90%–94% at room air; Severe: Maintaining oxygen saturation of below 90% at room air. 12
2.3. Follow‐up testing in SARS‐CoV‐2‐discharged KTR
The institutional protocol was to do a follow‐up at 1‐month, 3‐month, and beyond 6 months. A dedicated team of doctors conducted the follow‐up of discharged patients through telephonic and in‐person visits as feasible. A comprehensive evaluation of physical, clinical, and psychological domains was done on follow‐up visits. Routine tests which include complete blood counts, random blood sugar, blood urea, serum creatinine, electrocardiogram, and liver functions test were done in all cases. Urine analysis was performed in every visit for detection of any new onset hematuria or albuminuria. In the case of a sensitized patient, donor‐specific antibody (DSA) testing was done. DSA testing was done cases with incomplete recovery (declining creatinine but not reaching baseline) of acute kidney injury (AKI) in follow‐up. Cytomegaloviruses, BK polyomavirus (BKV), Epstein Barr virus, Parvovirus B19 were screened for the cases which had concomitant viral infections with COVID‐19 or had a history of these viral infections in past.
Repeat Chest x‐ray was done for all in the first follow‐up visit and on subsequent visits based on any symptoms. A repeat high resolution computed tomography (HRCT) thorax was done only in cases with complaints of breathing difficulty or worsening report in chest imaging done in the follow‐up.
nRT‐PCR repeat testing was not mandatory, and there was no fixed protocol on the timing of a repeat test, as it was logistically not possible and clinically not meaningful. For measuring the quality of life, EuroQoL5‐dimension score (EQ‐5D‐5L) 13 and EuroQoL visual analogue score (EQVAS) score were used. The modified medical research council 14 scale (mMRC) for breathlessness was used for assessing dyspnoea. In patients scoring poorly on mMRC scale, pulmonary function tests were done. In case of any significant psychiatric problems, patients underwent counselling and were referred to the psychiatry unit for expert review and further management.
2.4. Statistical analysis
For analysis of data, patients were grouped based on the duration of follow‐up, and three groups were formed: 1‐month, 3‐month, and ≥6 months follow‐up. Categorical data were expressed as numbers and percentages in parenthesis, and continuous data were expressed as mean, range, median, standard deviation, and interquartile range (IQR). A t‐test was used to compare the median baseline creatinine of graft loss and normal grafts in the cohort. The three group variables were compared with the analysis of variance (ANOVA) test for parametric variables and the Kruskal–Wallis test for non‐parametric variables. For assessing the graft outcome, a spaghetti plot, scatter plot, Kaplan–Meier plot, and box whisker plots are derived. A two‐tailed p‐value of less than 0.05 was considered statistically significant. Statistical analysis was done using IBM SPSS 21 software.
3. RESULTS
From May 2020 to December 2020, a total of 142 KTR with COVID‐19 were discharged of whom the follow‐up was done from discharge to 27 July 2021.
A total of 139, 134, and 128 KTR completed 1‐month, 3‐month, and ≥6‐month follow‐up, respectively. The median (IQR) follow‐up duration of the cohort was 9 (8–11) months. Figure 1 summarizes the course of the study. One case died at home before 1 month of discharge. Two deaths occurred as aspergillus pneumonia within 3 months. One case of re‐infection resulted in death. Two deaths occurred as post‐COVID‐19 mucormycosis. 15 There were no admissions for the viral infections which were screened in selected cases.
FIGURE 1.
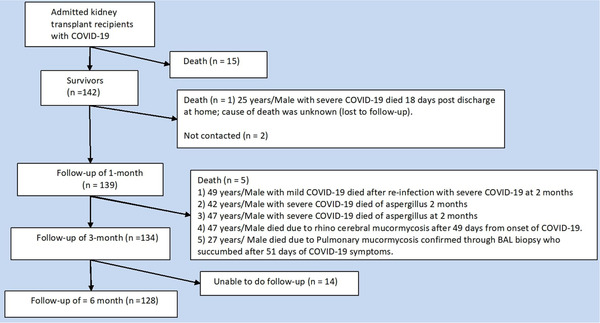
Summary of the cases
3.1. Baseline characteristics and acute COVID‐19 course
Tables S1 and S2 demonstrate the baseline and demographic features of the cohort and acute COVID‐19 course, respectively. The median age of the cohort was 42 (32–49) years with a predominance of male sex (84%). The bulk of the cohort was live‐related transplants (n = 120, 84%) and only a few cases (n = 22, 16%) presented with COVID‐19 within 1 year of transplantation. The comorbid conditions reported were hypertension (n = 104, 73%) as the most common followed by diabetes (n = 33, 23%), heart disorders (n = 14, 9.8%), and obesity (n = 30, 21%).
The COVID‐19 severity during the admission spanned from asymptomatic (n = 9, 4%), mild (n = 71, 50%), moderate (n = 46, 35%) to severe (n = 18, 12%). The cohort had no mechanical ventilation cases, as all admissions with mechanical ventilation had mortality. Radiological changes (n = 96, 68%) were observed in most of the cases. Allograft dysfunction (n = 64, 45%) was reported in almost half of the cases. Most patients were treated with steroids (n = 64, 45%) and remdesivir (n = 63, 44%). Antimetabolite was discontinued (n = 108, 72%) or reduced (n = 31, 22%) in most of the cohort. Calcineurin inhibitors (CNIs) was stopped (n = 19, 16%) or reduced (n = 26, 21%) in moderate to severe cases. The protocol for the timing of reintroduction for antimetabolite was 7–10 days post‐discharge, while CNI was resumed on the day of discharge in a half dose.
3.2. Clinical symptoms post‐discharge
Table 1 describes the clinical signs and symptoms of the cohort during the follow‐up. Overall, 37.5% of the cohort reported one or more than 1 symptom at 1‐month follow‐up. The most common symptoms observed were fatigue (n = 45, 32.3%), muscle pain (n = 35, 25.1%), decreased appetite (n = 24, 17.2%), and altered sleep (n = 22, 15.8%) at 1‐month follow‐up. Fatigue (n = 45, 32.3% vs. 10, 7.4% vs. 4, 2.9%; p‐value = 0.001) and muscle pain (n = 35, 25.1% vs. 10, 7.4% vs. 3, 2.2%; p‐value = 0.002) which were the main symptoms at 1‐month resolved significantly at longer follow‐up. Overall, there were very less symptoms at extended follow‐up. Disturbed sleep (n = 9, 6.6%) and appetite loss (n = 7, 5.1%) were most common symptom at ≥6‐month follow‐up.
TABLE 1.
Symptoms and quality of life in follow‐up stratified by oxygen requirement during COVID‐19
| 1 month | 3 month (n = 134) | ≥6 month (n = 128) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ordinal scale ≤3, | Ordinal scale ≥4, | Ordinal scale ≤3 | Ordinal scale ≥4 | Ordinal scale ≤3 | Ordinal scale ≥4 | |||||||||
| n = 139 | n = 78 | n = 61 | p‐value** | n = 134 | n = 75 | n = 59 | p‐value** | n = 128 | n = 71 | n = 57 | p‐value** | H‐statistic | p‐value* | |
| Cumulative symptoms | ||||||||||||||
| Subjective fever | 6 (4.3) | 4 (5.1) | 2 (3.2) | 0.6 | 2 (1.4) | 1 (1.3) | 1 (1.7) | 1 | 0 (0) | 0 (0) | 0 (0) | ‐ | 0.38 | 0.82 |
| Appetite loss | 24 (17.2) | 10 (12.8) | 20 (32.7) | <0.01 | 17 (12.6) | 7 (9.3) | 10 (16.9) | 0.20 | 7 (5.4) | 3 (4.2) | 4 (7) | 0.69 | 2.8 | 0.24 |
| Anosmia/Parosmia | 12 (8.6) | 8 (10.2) | 4 (6.5) | 0.55 | 3 (2.2)) | 1 (1.3) | 2 (3.3) | 0.58 | 0 (0) | 0 (0) | 0 (0) | ‐ | 1.6 | 0.44 |
| Chest tightness | 12 (8.6) | 2 (2.5) | 10 (16.4) | <0.01 | 3 (2.2) | 0 (0) | 3 (5) | 0.08 | 1 (0) | 0 (0) | 1 (1.7) | 0.44 | 1.6 | 0.44 |
| Palpitations | 8 (5.7) | 4 (5.1) | 4 (6.5) | 0.73 | 2 (1.4) | 1 (.3) | 1 (1.7) | 1 | 1 (1) | 0 (0) | 1 (1.7) | 0.44 | 0.58 | 0.74 |
| Alopecia | 10 (7.1) | 6 (7.7) | 4 (6.5) | 1 | 9 (6.7) | 3 (3.9) | 6 (10) | 0.18 | 6 (4.4) | 2 (2.8) | 4 (7) | 0.40 | 0.13 | 0.93 |
| Skin rash | 4 (2.8) | 1 (1.3) | 3 (4.9) | 0.31 | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | ‐ | ‐ |
| Impaired memory | 9 (6.4) | 2 (2.5) | 7 (11.4) | 0.04 | 4 (2.9) | 2 (2.7) | 2 (3.3) | 1 | 2 (1.5) | 0 (0) | 2 (3.5) | 0.19 | 0.51 | 0.77 |
| Impaired concentration | 9 (6.4) | 2 (2.5) | 7 (11.4) | 0.04 | 3 (2.2) | 1 (1.3) | 2 (3.3) | 0.58 | 3 (2.3) | 1 (1.4) | 2 (3.5) | 0.58 | 0.57 | 0.74 |
| Joint pain | 12 (8.6) | 4 (5.1) | 8 (13.1) | 0.12 | 10 (7.4) | 4 (5.3) | 6 (10) | 0.33 | 3 (2.3) | 1 (1.4) | 2 (3.5) | 0.58 | 0.87 | 0.644 |
| Muscle pain | 35 (25.1) | 14 (17.9) | 21 (34.4) | 0.03 | 10 (7.4) | 3 (3.9) | 7 (11.8) | 0.10 | 3 (2.3) | 1 (1.4) | 2 (3.5) | 0.58 | 11 | 0.002 |
| Disturbed sleep | 22 (15.8) | 8 (10.2) | 14 (22.9) | 0.059 | 12 (8.9) | 4 (5.3) | 8 (13.5) | 0.12 | 9 (7) | 1 (1.4) | 8 (14) | 0.01 | 1.73 | 0.42 |
| Bleeding | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | ‐ | ‐ |
| Sore throat | 14 (10) | 8 (10.2) | 6 (9.8) | 0.059 | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | ‐ | ‐ |
| Nausea | 9 (6.4) | 5 (6.4) | 4 (6.5) | 1 | 3 (2.2) | 1 (1.3) | 2 (3.3) | 0.58 | 3 (2.2) | 1 (1.4) | 2 (3.5) | 0.58 | 0.57 | 0.74 |
| Gastritis | 10 (7.1) | 3 (3.8) | 7 (11.4) | 0.1 | 9 (6.7) | 2 (2.7) | 7 (11.8) | 0.04 | 6 (4.4) | 2 (2.8) | 4 (7) | 0.40 | 0.13 | 0.93 |
| Dizziness | 6 (4.3) | 3 (3.8) | 3 (4.9) | 1 | 2 (1.4) | 1 (1.3) | 1 (1.7) | 1 | 0 (0) | 0 (0) | 0 (0) | ‐ | 0.38 | 0.82 |
| Fatigue | 45 (32.3) | 10 (12.8) | 35 (57.3) | <0.01 | 10 (7.4) | 2 (2.7) | 8 (13.5) | 0.02 | 4 (2.9) | 0 (0) | 4 (7) | 0.03 | 20 | 0.0001 |
| Syncope | 3 (2.1) | 1 (1.3) | 3 (4.9) | 0.31 | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | ‐ | ‐ |
| Loss of libido | 10 (7.1) | 5 (6.4) | 5 (81) | 0.74 | 6 (4.5) | 3 (3.9) | 3 (5) | 1 | 3 (2.2) | 1 (1.4) | 2 (3.5) | 0.58 | 0.47 | 0.78 |
| Asymptomatic | 87 (62.5) | 61 (78.2) | 26 (42.6) | <0.01 | 114 (85) | 65 (86.6) | 49 (83) | 0.62 | 118 (92) | 68 (95.8) | 50 (87.7) | 0.47 | 19 | 0.0008 |
| EQ‐5D‐5L scores | ||||||||||||||
| Motility | ||||||||||||||
| No problems in walking | 104 (75) | 66 (84.6) | 38 (62.3) | <0.01 | 120 (90) | 71 (94.6) | 49 (83) | 0.04 | 119 (93) | 68 (95.8) | 51 (89.5) | 0.18 | 7.53 | 0.023 |
| Slight problems in walking | 31 (22) | 11 (14.1) | 20 (32.9) | 0.01 | 4 (3) | 2 (2.7) | 2 (3.3) | 1 | 3 (2) | 1 (1.4) | 2 (3.5) | 0.58 | 10 | 0.004 |
| Moderate problems in walking | 3 (2) | 1 (1.3) | 2 (3.2) | 0.58 | 10 (7) | 2 (2.7) | 8 (13.6) | 0.02 | 6 (4.4) | 2 (2.8) | 4 (7) | 0.40 | 0.57 | 0.75 |
| Severe problems in walking | 1 (1) | 0 (0) | 1 (1.6) | 0.43 | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | 0.01 | 0.99 |
| Confined to bed | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ‐ | ‐ | ‐ | |
| Self‐care | ||||||||||||||
| No problems with self‐care | 111 (80) | 67 (85.9) | 44 (72.2) | 0.06 | 127 (95) | 72 (96) | 55 (93.4) | 0.69 | 125 (98) | 71 (100) | 54 (94.8) | 0.08 | 7.4 | 0.024 |
| Slight problems with self‐care | 24 (17) | 10 (12.8) | 14 (22.9) | 0.17 | 3 (2) | 1 (1.3) | 2 (3.3) | 0.58 | 2 (1.4) | 0 (0) | 2 (3.5) | 0.19 | 6.4 | 0.04 |
| Moderate problems with self‐care | 4 (3) | 1 (1.3) | 3 (4.9) | 0.31 | 4 (3) | 2 (2.7) | 2 (3.3) | 1 | 1 (1) | 0 (0) | 1 (1.7) | 0.44 | 0.12 | 0.94 |
| Severe problems in bathing or dressing | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | ‐ | ‐ |
| Unable to bathe or dress | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | ‐ | ‐ |
| Usual activities (e.g., work, study, household work, family or leisure activities) | ||||||||||||||
| No problems in performing usual activities | 95 (68) | 62 (79.5) | 29 (52.5) | <0.01 | 117 (87) | 69 (92) | 48 (81.3) | 0.07 | 120 (93) | 71 (100) | 49 (86) | <0.01 | 14 | 0.001 |
| Slight problems in performing usual activities | 28 (20) | 10 (12.8) | 18 (29.5) | 0.02 | 8 (6) | 3 (4) | 5 (8.5) | 0.29 | 4 (3.1) | 0 (0) | 4 (7) | 4 (2.9) | 6.7 | 0.03 |
| Moderate problems in performing usual activities | 14 (10) | 5 (6.4) | 9 (14.8) | 0.15 | 8 (6) | 3 (4) | 5 (8.5) | 0.29 | 4 (3.1) | 0 (0) | 4 (7) | 4 (2.9) | 0.97 | 0.67 |
| Severe problems in performing usual activities | 2 (2) | 1 (1.3) | 2 (3.2) | 0.58 | 1 (1) | 0 (0) | 1 (1.7) | 0.44 | 0 (0) | 0 (0) | 0 (0) | ‐ | 0.04 | 0.97 |
| Unable to perform my usual activities | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | ‐ | ‐ |
| Pain/Discomfort | ||||||||||||||
| No pain or discomfort | 118 (85) | 71 (91) | 47 (77.1) | 0.03 | 125 (93) | 71 (94.6) | 54 (91.6) | 0.50 | 121 (94) | 69 (97.2) | 52 (91.3) | 0.24 | 2.2 | 0.32 |
| Slight pain or discomfort | 16 (11) | 6 (7.7) | 10 (16.4) | 0.17 | 5 (4) | 2 (2.7) | 3 (5.1) | 0.65 | 6 (4.4) | 2 (2.8) | 4 (7) | 0.40 | 1.6 | 0.43 |
| Moderate pain or discomfort | 4 (3) | 1 (1.3) | 3 (4.9) | 0.31 | 4 (3) | 2 (2.7) | 2 (3.3) | 1 | 1 (1) | 0 (0) | 1 (1.7) | 0.44 | 0.12 | 0.94 |
| Severe pain or discomfort | 1 (1) | 0 (0) | 1 (1.6) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | 0.01 | 0.99 |
| Extreme pain or discomfort | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | ‐ | ‐ |
| Anxiety/Depression | ||||||||||||||
| Not anxious or depressed | 65 (47) | 47 (60.3) | 18 (29.5) | <0.01 | 89 (66) | 57 (76) | 32 (54.2) | 0.01 | 84 (65) | 54 (76.1) | 30 (52.7) | <0.01 | 10 | 0.006 |
| Slightly anxious or depressed | 51 (37) | 21 (26.9) | 30 (49.2) | <0.01 | 22 (16) | 8 (10.7) | 14 (23.7) | 0.06 | 18 (14) | 7 (9.9) | 11 (19.3) | 0.20 | 12 | 0.001 |
| Moderately anxious or depressed | 22 (15) | 10 (12.8) | 12 (19.7) | 0.35 | 22 (17) | 10 (13.3) | 12 (20.4) | 0.34 | 25 (20) | 10 (14) | 15 (26.3) | 0.07 | 1.1 | 0.57 |
| Severely anxious or depressed | 1 (1) | 0 (0) | 1 (1.6) | 0.43 | 1 (1) | 0 (0) | 1 (1.7) | 0.44 | 1 (1) | 0 (0) | 1 (1.7) | 0.44 | 0.0001 | 0.99 |
| Extremely anxious or depressed | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | ‐ | ‐ |
| EQVAS score* change from pre‐COVID‐19 | 28.6 (13) | 10.4 (12.5) | 7.5 (12.0) | 111 | 0.001 | |||||||||
| mMRC scoring for breathlessness | ||||||||||||||
| 0 | 135 (97) | 77 (98.7) | 58 (95.1) | 0.31 | 127 (94) | 73 (97.3) | 53 (89.8) | 0.13 | 124 (97) | 70 (98.6) | 54 (94.8) | 0.35 | 0.1 | 0.9 |
| 1 | 4 (3) | 1 (1.3) | 3 (4.9) | 0.31 | 7 (5) | 2 (2.7) | 5 (8.5) | 0.23 | 3 (2) | 1 (1.4) | 2 (3.5) | 0.58 | 0.24 | 0.88 |
| 2 | 0 (0) | 0 (0) | 0 (0) | ‐ | 1 (1) | 0 (0) | 1 (1.7) | 0.44 | 1 (1) | 0 (0) | 1 (1.7) | 0.44 | 0.01 | 0.99 |
| 3 | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | ‐ | ‐ |
| 4 | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | 0 (0) | 0 (0) | 0 (0) | ‐ | ‐ | ‐ |
Note: Data are expressed as numbers (percentage), mean (standard deviation).
Abbreviations: EQ5D5L, EuroQoL 5 dimension score; EQVAS, EuroQoL visual analogue score which ranged from 0 (worst health) to 100 (best health); mMRC, modified medical research for dyspnea scale (0 = no complaint of breathlessness, 1 = difficulty in breathing when running or upstairs, 2 = difficulty in breathing while walking for longer time, 3 = has to stop to breath after waking even few steps, 4 = too breathless to leave house).
*Statistical significance (p‐value < 0.05) for all parameters was calculated from Kruskal–Wallis test (H‐statistic) except for EQVAS scale where analysis of variance (K‐statistic) test was used.
**p‐value was calculated by Fisher test. Ordinal scale of 3 means no oxygen requirement and above 3 implies oxygen need during COVID‐19 admission.
3.3. Quality of life assessment and perceived breathing
Table 1 also shows the results of screening tests performed in the follow‐up. Overall, there was an improvement in all of the domains of EQ‐5D‐5L. The most affected parameter at both 1‐month and ≥6 months was anxiety/depression, although the severity was less in majority and improved significantly at extended follow‐up (n = 51, 37% vs. 22, 16% vs. 18, 14%; p‐value = 0.001). As described in E5QD5L scores, only a few cases had slight difficulty in walking at home at 1‐month which improved significantly at ≥6‐months (n = 31, 22% vs. 4, 3% vs. 3, 2%; p‐value = 0.004). There was slight problem in routine activities which also resolved at extended follow‐up (n = 24, 17% vs. n = 3, 2% vs. n = 2, 1%; p‐value = 0.03). There were only a few cases of difficulty in breathing in the cohort assessed through mMRC, and there was no increase in the number of such cases in subsequent follow‐up (n = 4, 3% vs. 7, 5% vs. 3, 2%; p‐value = 0.86). All such patients had graft loss. Decrement in the EQ‐VAS score from baseline was improved in subsequent follow‐up (28.6 [13] vs. 10.4 [12.5] vs. 7.5 [12.0]; p‐value: 0.012). After stratification of scores by oxygen requirement, it was observed that cases with oxygen need during COVID‐19 had poorer scores at 1 month and 3 months, but no significant differences at more than 6‐month follow‐up
4. GRAFT OUTCOME DURING FOLLOW‐UP
Table 2 describes the summary of cases with graft losses or rejection. Sixty‐four (45%) cases developed AKI during COVID‐19 admission, of which 10 resulted in graft loss in follow‐up. Half of the cases which were dialysis‐dependent during COVID‐19 admission never had any renal improvement. The baseline creatinine and estimated glomerular filtration rate (eGFR) of the cases with graft loss was 4.3 (3.1–6.75) mg/dl and 16.5 (8.2–23) ml/min/1.73m2 respectively. All graft loss cases had significantly higher baseline serum creatinine compared to the remaining cohort (4.3 [3.1–6.75] mg/dl vs. 1.4 [2–1.1]; p value = 0.01).
TABLE 2.
Graft loss and rejection episodes
| Number | Age/Sex | Baseline creatinine (mg/dl) | eGFR ml/min/1.73 m2 | COVID‐19 severity | Time from transplant to COVID‐19 (years) | Course |
|---|---|---|---|---|---|---|
| 1 | 31/M | 4.5 | 16 | Moderate | 2 | HD requirement since COVID‐19, biopsy showed chronic rejection changes only |
| 2 | 35/M | 7 | 9 | Moderate | 2.5 | HD requirement since COVID‐19 |
| 3 | 54/M | 9 | 6 | Severe | 7 | HD requirement since COVID‐19 |
| 4 | 32/M | 4.2 | 17 | Severe | 3 | HD requirement since COVID‐19 |
| 5 | 49/M | 3.4 | 20 | Moderate | 4 | 1.5 months post‐discharge: Biopsy showed FSGS similar to previous biopsy/chronic changes |
| 6 | 29/M | 8 | 8 | Severe | 1.5 | HD requirement since COVID‐19 |
| 7 | 25/M | 1.9 | 48 | Severe | 2 | Pseudo pancreatic cyst infection with severe pancreatitis post‐discharge requiring prolonged hospital stay, graft loss, re‐infection at 10‐month follow‐up with mild COVID‐19. |
| 8 | 38/M | 1.79 | 47 | Moderate | 2 | Invasive aspergillus, post‐discharge, prolonged hospital stay |
| 9 | 36/F | 6 | 8 | Moderate | 3 | Chronic rejection at baseline |
| 10 | 44/M | 3 | 24 | Severe | 5 | Drug non‐adherence, already had chronic graft dysfunction. Biopsy not done, no DSA |
Note: Bold cases underwent biopsy.
Abbreviations: DSA, donor‐specific antibodies; eGFR, estimated glomerular filtration rate; F, female; FSGS, focal segmental sclerosis; HD, hemodialysis; M, male.
Figure 2 shows the detailed analysis of the graft outcome in the study. Half of the cases with graft losses had severe COVID‐19, and the other half had moderate COVID‐19. No graft loss was reported in mild or asymptomatic cases. The plasma levels of CNI were measured routinely, and in all cases their values was optimal. Graft biopsy was performed in two (Table 2) of the 10 cases (one case showed focal segmental glomerulosclerosis which had already a history of similar biopsy reports; the second case showed chronic antibody rejection). The cohort who did not develop AKI (55%) had normal graft function throughout the follow‐up. DSA was tested in 23 (16%) patients who had a prior history of antirejection therapy and none showed the development of antibodies. Urine routine and microscopy were done in the entire cohort during every visit, and none reported new‐onset proteinuria/hematuria.
FIGURE 2.
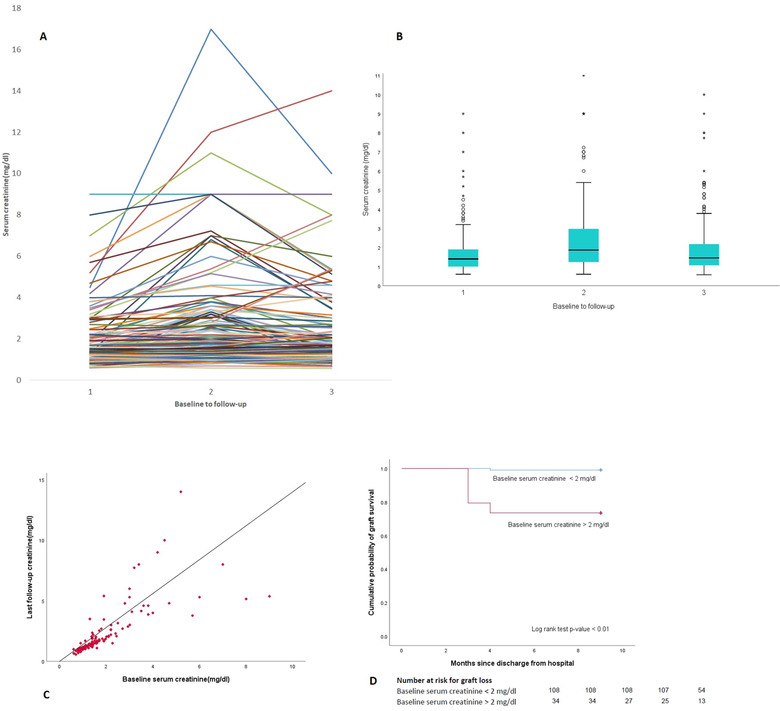
(A) Spaghetti plot showing trend of serum creatinine of individual cases. The cases with incomplete recovery or non‐recovery of acute kidney injury had higher baseline serum creatinine. (B) Box and whisker plot showing the differences in median (IQR) serum creatinine levels with follow‐up. Note the median creatinine at follow‐up nearing baseline levels (x‐axis values in graphs (A) and (B) depict 1 as serum creatinine value prior to COVID‐19, 2 as the peak creatinine levels, and 3 as the last follow‐up creatinine levels. The median follow‐up was 9 months). (C) Correlation matrix analysis of baseline and last follow‐up creatinine. Note a linear trend especially in cases with baseline creatinine below 2 mg/dl. (D) Kaplan–Meier curve for analysis of graft loss in two divided groups based on baseline serum creatinine of 2 mg/dl
5. DISCUSSION
The study reports the follow‐up outcome of SARS‐CoV‐2 survivors in KTR. Initially, when reports of COVID sequelae were published, we decided to do the follow‐up of recovered patients, a dedicated team of doctors was assigned for follow‐up of the patients. We explore here the follow‐up course of post‐transplant SARS‐CoV‐2 survivors with all grades of severity and duration of follow‐up from 1 month to more than 6 months. The authors have previously documented the acute COVID‐19 course of KTR from the center. 16
Even in mild cases, there are reports of persistent symptoms. 17 , 18 , 19 The most common persistent symptoms in our study were fatigue and malaise as reported in the general population. Chronic fatigue syndrome (CFS) has been linked with survivors of the SARS pandemic, 20 but it was not observed in our study in COVID‐19. The symptoms and signs resolved before 6 months, so none fulfilled the criteria for CFS. As contrary to concerning reports of persistent cough, breathlessness previously described in general patients, we did not find any evidence of these persistent symptoms in our study. Our report is similar to the long‐term report of a 6‐month follow‐up of a large study from China. 21 Duration from the onset of COVID‐19 symptoms to symptom‐free was 14 days. In our report, there were no complaints of parosmia or anosmia at 1‐month discharge unlike reported in the general population. 22 , 23 A recent meta‐analysis 24 reported a high possibility of anxiety and depression in COVID‐19 survivors which simulates our report in both initial and later stages of follow‐up. In our center, all cases with anxiety and depression were given counselling and psychiatric consultation, but only four cases required anti‐depressants for improvement. The high rate of anxiety/depression was mostly contributed to unprecedented times of COVID‐19, fear of graft loss, and vaccine hesitancy.
In our report, we have found that the majority of the cases showed improvement in their quality‐of‐life scores as the follow‐up duration was increased.
Anxiety/depression was the worst affected component in the long‐term follow‐up. The mental well‐being of surviving KTR should be considered for the rehabilitative measures. This problem is similar to that reported in the general population. 23 , 31 Likewise, the KTRs should also be assessed for physical rehabilitative measures especially in the initial month of discharge. The quality‐of‐life parameters in the first 3 months of discharge were most severely affected in COVID‐19 cases which required oxygen therapy and in those which suffered graft losses. Our study highlights the progress in the quality of life with time and provides acceptable reassurance that a significant number of cases will improve irrespective of the disease severity.
AKI has been found in almost half the cases of SARS‐CoV‐2 in KTR in most of the published literature; however, follow‐up recovery and graft outcome are less extensively studied. With reports of renal injury among COVID‐19 survivors case series before, 25 , 26 rigorous follow‐up of graft function needs to be done. In our center, most of the patients had normal graft functions in the follow‐up. We did screen for any de nova or increase in previous titres of DSA also and found no increased incidence of antibodies as such. Theoretically, due to stopping or reducing immunosuppression during acute COVID‐19 and delay in restoration to baseline can cause the development of antibodies. Still, these are early days to call that there will be no sequelae of the allograft dysfunction in SARS‐CoV‐2. A recent study showed an association of lower eGFR at baseline with incomplete graft recovery following AKI in follow‐up 27 Our report also showed similar findings, with graft losses occurring in cases with statistically significant baseline creatinine compared to patients with the favourable course. In our center, a previous study of invasive fungal infection reported 25% graft loss. 28 Similarly dengue cohort 29 from our center reported 6.4% acute rejection and 6.4% graft loss. Our preliminary report hence do not suggest any increased risk of graft loss or rejection compared to other infections
Recently, a few studies in general patients have shown pulmonary sequelae in considerable cases. 30 , 31 Initial reports in the general population warned the global health system about the risk of pulmonary complications in COVID‐19 survivors like persistent breathing difficulty and non‐resolution or progression of radiological abnormalities. 32 , 33 In our cohort, the proportion of patients with radiological abnormality at acute COVID‐19 admission was high, but in follow‐up, we had only one case of pulmonary fibrosis. Due to resource limitations and unprecedented second COVID‐19 wave, HRCT scans and pulmonary function testing surveillance in all cases were not done. We did a subjective screening through mMRC, clinical screening, and a repeat x‐ray chest at a regular follow‐up to rule out any evidence of pulmonary fibrosis, and hence the likelihood of missing pulmonary fibrosis cases is less. A large population‐based study has reported neurological and psychiatric manifestations in COVID‐19 survivors. 34 In our cohort, there were no neurological events like stroke, new‐onset tremor disorder, or Parkinsonism. We did not specifically screen for a possibility of the thromboembolic episode, but there were no such obvious reports during the study period.
The strength of the study is enlisted. Firstly, this is the first study of its kind in KTR to describe the follow‐up course of post‐COVID‐19. Secondly, a large sample size was studied, so the applicability of results in other settings is considerable. Thirdly, all grades of COVID‐19 severity were explored, so the study can be interpolated for asymptomatic to critical cases. Fourthly, we did not fix a particular time frame to collect data, so the results encompass follow‐up duration of diverse lengths, which further adds to the power of the study.
The study has few inherent and worth mentioning limitations. Firstly, the sample size was small, and also the number of severe and critical cases was relatively less in the cohort, so the results should be very cautiously applied in severe cases. Secondly, the study did not objectively assess for pulmonary functions or thromboembolic phenomena. However, no clinical symptoms in follow‐up were suggestive of such complications. There could be also some reporting bias in the subjective assessment. Thirdly, protocol biopsy was not done in acute COVID‐19 to see any direct effect of the virus in the graft. The justification behind this was the initial observations of recovering graft shortly after discharge; hence we waited for the kidney function to normalize within few weeks of discharge. Fourthly, the immune response to COVID‐19 was not studied.
CONCLUSION
We describe the largest cohort of SARS‐CoV‐2 sequelae in KTR with the longest follow‐up of 9 (8–11) months. Fatigue was the most prominent symptom post‐discharge. Oxygen requiring cases had poorer quality of life compared to non‐oxygen requiring cases, but the assessment scores significantly improved during the follow‐up course. Ten graft losses reported were mostly in patients with chronic graft dysfunction. Our initial reports are having reassuring outcomes. Still, these are early days to cut loose, and the follow‐up studies should be continued in the transplant population to avoid any possible complications till further high‐level scientific data are available.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Supporting information
Table S1 Baseline characteristics of the cohort of renal transplant patients with recovered COVID‐19
Table S2 Characteristics of the cohort during acute COVID‐19
ACKNOWLEDGMENTS
We would like to express our sincere gratitude to the hospital staff for their committed efforts in managing the patients during the COVID‐19 crisis.
Chauhan S, Meshram HS, Kute V, Patel H, Desai S, Dave R. Long‐term follow‐up of SARS‐CoV‐2 recovered renal transplant recipients: A single‐center experience from India. Transpl Infect Dis. 2021;23:e13735. 10.1111/tid.13735
All authors have contributed equally to the work.
REFERENCES
- 1. Iqbal FM, Lam K, Sounderajah V, Clarke JM, Ashrafian H, Darzi A. Characteristics and predictors of acute and chronic post‐COVID syndrome: a systematic review and meta‐analysis. EClinicalMedicine. 2021;36:100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hay JW, Gong CL, Jiao X, et al. A US population health survey on the impact of COVID‐19 using the EQ‐5D‐5L. J Gen Intern Med. 2021;36(5):1292‐1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Orrù G, Bertelloni D, Diolaiuti F, et al. Long‐COVID Syndrome? A study on the persistence of neurological, psychological, and physiological symptoms. Healthcare (Basel). 2021;9(5):575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID‐19: a systematic review. JAMA Netw Open. 2021;4(5):e2111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moris D, Kesseli SJ, Barbas AS. Kidney transplant recipients infected by COVID‐19: Review of the initial published experience. Transpl Infect Dis. 2020;22(6):e13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moosavi SA, Mashhadiagha A, Motazedian N, Hashemazar A, Hoveidaei AH, Bolignano D. COVID‐19 clinical manifestations and treatment strategies among solid‐organ recipients: a systematic review of cases. Transpl Infect Dis. 2020;22(6):e13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caillard S, Chavarot N, Francois H, et al. Is COVID‐19 infection more severe in kidney transplant recipients?. Am J Transplant. 2021;21(3):1295‐1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kute VB, Bhalla AK, Guleria S, et al. Clinical profile and outcome of COVID‐19 in 250 kidney transplant recipients: a multicenter cohort study from India. Transplantation. 2021;105(4):851‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jager KJ, Kramer A, Chesnaye NC, et al. Results from the ERA‐EDTA Registry indicate a high mortality due to COVID‐19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98(6):1540‐1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coll E, Fernández‐Ruiz M, Sánchez‐Álvarez JE, et al. COVID‐19 in transplant recipients: the Spanish experience. Am J Transplant. 2021;21(5):1825‐1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abbasi J. Researchers tie severe immunosuppression to chronic COVID‐19 and virus variants. JAMA. 2021;325:2033‐2035. [DOI] [PubMed] [Google Scholar]
- 12.Clinical management protocol: covid‐19. Government of India Ministry of Health & Family Welfare Directorate General of Health Services (EMR Division) . June 13, 2020. Accessed July 27, 2021. https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19.pdf
- 13. Cleemput I, Kesteloot K, Moons P, et al. The construct and concurrent validity of the EQ‐5D in a renal transplant population. Value Health. 2004;7(4):499‐509. [DOI] [PubMed] [Google Scholar]
- 14. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580‐586. [DOI] [PubMed] [Google Scholar]
- 15. Meshram HS, Kute VB, Chauhan S, Desai S. Mucormycosis in post‐COVID‐19 renal transplant patients: a lethal complication in follow‐up. Transpl Infect Dis. 2021;23:e13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meshram HS, Kute VB, Patel H, et al. Feasibility and safety of remdesivir in SARS‐CoV2 infected renal transplant recipients: a retrospective cohort from a developing nation. Transpl Infect Dis. 2021;23:e13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Havervall S, Rosell A, Phillipson M, et al. Symptoms and functional impairment assessed 8 months after mild COVID‐19 among health care workers. JAMA. 2021;325:2015‐2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carvalho‐Schneider C, Laurent E, Lemaignen A, et al. Follow‐up of adults with noncritical COVID‐19 two months after symptom onset. Clin Microbiol Infect. 2021;27(2):258‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID‐19 survivors in Wuhan, China: a single‐center longitudinal study. Clin Microbiol Infect. 2021;27(1):89‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lam MH, Wing YK, Yu MW, et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long‐term follow‐up. Arch Intern Med. 2009;169(22):2142‐2147. [DOI] [PubMed] [Google Scholar]
- 21. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hopkins C, Surda P, Vaira LA, et al. Six month follow‐up of self‐reported loss of smell during the COVID‐19 pandemic. Rhinology. 2021;59(1):26‐31. [DOI] [PubMed] [Google Scholar]
- 23. Makaronidis J, Firman C, Magee CG, et al. Distorted chemosensory perception and female sex associate with persistent smell and/or taste loss in people with SARS‐CoV‐2 antibodies: a community based cohort study investigating clinical course and resolution of acute smell and/or taste loss in people with and without SARS‐CoV‐2 antibodies in London, UK. BMC Infect Dis. 2021;21(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta‐analysis with comparison to the COVID‐19 pandemic. Lancet Psychiatry. 2020;7(7):611‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Akilesh S, Nast CC, Yamashita M, et al. Multicenter clinicopathologic correlation of kidney biopsies performed in COVID‐19 patients presenting with acute kidney injury or proteinuria. Am J Kidney Dis. 2021;77(1):82‐93.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sharma P, Uppal NN, Wanchoo R, et al. COVID‐19‐associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol. 2020;31(9):1948‐1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bajpai D, Deb S, Bose S, et al. Recovery of kidney function after AKI due to COVID‐19 in kidney transplant recipients. Transpl Int. 2021;34:1074‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patel MH, Patel RD, Vanikar AV, et al. Invasive fungal infections in renal transplant patients: a single center study. Ren Fail. 2017;39(1):294‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meshram HS, Kute V, Patel H, Banerjee S, Chauhan S, Desai S. Successful management of dengue in renal transplant recipients: a retrospective cohort from a single center. Clin Transplant. 2021;35(7):e14332. [DOI] [PubMed] [Google Scholar]
- 30. Liu M, Lv F, Huang Y, Xiao K. Follow‐up study of the chest CT characteristics of COVID‐19 survivors seven months after recovery. Front Med (Lausanne). 2021;8:636298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID‐19 four months after hospital discharge. JAMA Netw Open. 2021;4(1):e2036142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Han X, Fan Y, Alwalid O, et al. Six‐month follow‐up chest CT findings after severe COVID‐19 pneumonia. Radiology. 2021;299(1):E177‐E186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guler SA, Ebner L, Aubry‐Beigelman C, et al. Pulmonary function and radiological features 4 months after COVID‐19: first results from the national prospective observational Swiss COVID‐19 lung study. Eur Respir J. 2021;57(4):2003690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6‐month neurological and psychiatric outcomes in 236 379 survivors of COVID‐19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Baseline characteristics of the cohort of renal transplant patients with recovered COVID‐19
Table S2 Characteristics of the cohort during acute COVID‐19


