Abstract
COVID‐19‐associated cutaneous manifestations are one of the most important and relatively common extra‐respiratory presentations of SARS‐COV‐2 infection. The exact identification and classification of these lesions can facilitate the accurate diagnosis and treatment. There are several case reports and small case series which describe cutaneous lesions in hands and feet. Currently, there is no scoping review about acral skin manifestations associated with COVID‐19. This paper covers the COVID‐related acral skin manifestations in 10 entities including acral papulo‐vesicular eruption, acral urticarial lesion, acral non‐inflammatory purpura and necrosis, acro‐ischemia associated COVID‐19, acral vasculitis, chilblain‐like lesion (COVID Toe), acral erythema multiform (EM) like lesion, hand and foot skin lesions associated with multisystem inflammatory syndrome in children (MISC), acral peeling conditions and red half‐moon nail sign. Future studies should focus on exact investigation of etiologies of these lesions including role of immune senescence, environment, gender, immunogenetics and relation of these lesion with major organ involvements.
Keywords: Acral, COVID‐19, cutaneous manifestations, SARS‐CoV‐2, skin
1. INTRODUCTION
Cutaneous manifestations associated with COVID‐19 are one of the extra‐respiratory manifestations of SARS‐CoV‐2 infection. These manifestations are important and relatively common presentations of SARS‐COV‐2 infection. 1 , 2 , 3 , 4
Among cutaneous manifestations, acral skin manifestations of COVID‐19 can be divided into at least 10 discrete categories based on their clinicopathological presentations.
Occasionally, acral skin lesions may represent the initial presentation of SARS‐CoV‐2 infection prior to fever or respiratory manifestations. Moreover, several acral cutaneous manifestations could be associated with poor prognosis or additional medical conditions. 5
Thus, recognition of covid‐19 related acral signs and symptoms can help clinicians to consider COVID‐19 in the differential diagnoses of acral skin lesions.
Some of the most common acral skin presentations of COVID‐19 include: acral papulo‐vesicular eruption, acral urticarial lesion, acral non‐inflammatory purpura and necrosis, acro‐ischemia associated COVID‐19, acral vasculitis, chilblain‐like lesion (COVID Toe), acral erythema multiform (EM) like lesion, hand and foot skin lesions associated with multisystem inflammatory syndrome in children (MISC), acral peeling conditions and red half‐moon nail sign.
As explained above, we conducted a scoping review to summarize the different acral skin lesions associated with COVID‐19 in children and adults as to recognize distinct cutaneous signs in COVID‐19 patients for accurate use of each entity and to prevent confusion among clinicians in similar entities. We also provided a table to categorize these findings more (Table 1).
TABLE 1.
Summary of clinical manifestations, ethiopathogenesis, histopathological findings and clinical management of 10 acral skin manifestations in COVID‐19
| No. | Lesion | Clinical presentation | Etiopathogenesis | Histopathological findings | Clinical management |
|---|---|---|---|---|---|
| 1 | Acral papulo‐vesicular eruption | Widespread chickenpox‐like polymorphic pattern/ Monomorphic acral papulo‐vesicular variant | Immune system hyperactivity/direct cytopathic effect of virus on endotheliual dermal vessels | Prominent acantholysis/supra‐basal dyskeratosis with intraepidermal vesicles | Watchful waiting/symptomatic therapy |
| 2 | Acral urticarial lesion | Erythematous papule or plaque ± Angioedema and intense pruritus | Direct cutaneous effect of virus/drug‐induced exanthema/immune system over activity | Superficial perivascular infiltration of lymphocytes/few eosinophilic infiltration/marked dermal edema in upper dermis | Non‐sedating antihistamines/short course of low‐dose systemic corticosteroids in severe cases |
| 3 | Acral Non‐inflammatory purpura and skin necrosis | Lace‐like reddish‐blue to purple mottled discolorations/ Hemorrhagic blisters/ Necrotic‐ulcerative lesion/ Dry gangrene | Hypercoagulative state/pauci‐inflammatory micro thrombotic vasculopathy/drug‐induced | Pauci‐inflammatory thrombogenic vasculopathy/extensive deposition of complement components in cutaneous microvasculature | Therapeutic‐dose of anticoagulation |
| 4 | Acro‐ischemia | Blue to gray discoloration without relation to the cold | Cytokine storm/hypercoagulative state/thrombotic events/ DIC | Microthrombosis in dermal vessels/endothelial cell damage/extravasation of RBCs/superficial and deep perivascular lymphocytic infiltration/vacuolar degeneration in basal layer | Therapeutic anticoagulation + Intravenous unfractionated heparin |
| 5 | Acral vasculitis | Symmetric palpable inflammatory purpura with necrotic center/ Blisters formation | Direct damage of endothelial cells by the virus/indirect damage of endothelial cells by immune dysregulation | Perivascular neutrophilic infiltration/fibrin deposition/fibrinoid necrosis/leucocytoclasis/endothelial swelling | Topical or systemic corticosteroids |
| 6 | Chilblain‐Like Lesion (“COVID Toe”) | Erythematous edematous painful pruritic skin resembling perniosis/ Blister formation/ Digital swelling | Damage of skin capillaries/immune dysregulation/immunologic response to cutaneous vessels/drug‐induced | Diffuse dense lymphoid infiltration in the dermis and hypodermis/perivascular pattern/endothelial activation | Following public health guidelines for COVID‐19 testing and isolation |
| 7 | Acral EM‐like lesion | Targetoid lesions/ Two or three concentric circles with a small necrotic/ Hemorrhagic area | Immune response to virus/hypersensivity/potential delayed immune response to the virus | Mild superficial perivascular infiltration /microthrombi formation/Granular positivity in endothelial and epithelial cells of eccrine glands in Immunohistochemistry | Spontaneous improvement in 1–3 weeks |
| 8 | Acral lesions associated with MISC in children | Acral erythema and edema | Macrophage activation and T‐helper stimulation/cytokine release/overproduction of antibodies/hyper immune response | Perivascular cuffing with cytotoxic CD8+ lymphocytes and eosinophil that can be seen in Kawasaki syndrome | Single dose of 2 g/kg of IVIG over 8–12 h ± Systemic corticosteroids |
| 9 | Acral peeling lesions | Superficial desquamation of the distal phalanges of hands and feet | Alternation in regulation of expression of acral keratins |
Skin biopsy is usually not performed. |
Spontaneous improvement in 1–4 weeks |
| 10 | Red half‐moon nail sign | Half‐moon‐shaped transversal red band at the distal margin of the lunula as single crescent erythronychia on the nail bed | Complement‐mediated microvascular injury of the nail bed/capillary network damage of the distal sub ungual arcade/subsequent thrombus formation in small vessels | No histopathological data | Treatment is unnecessary. |
Abbreviations: EM, erythema multiform; MISC, multisystem inflammatory syndrome in children.
2. CUTANEOUS MANIFESTATIONS
2.1. Acral papulo‐vesicular eruption
2.1.1. Clinical presentation
COVID‐19‐associated papulo‐vesicular lesions have two distinct clinical patterns; a widespread chickenpox‐like polymorphic pattern and monomorphic acral papulo‐vesicular variant. 6 , 7 , 8 Papulo‐vesicular lesions are associated with intermediate severity of COVID‐19. 6 (Figure 1).
FIGURE 1.

Acral papulovesicular and large blister formation associated with COVID‐19
2.1.2. Etiopathogenesis
The possible explanation for development of acral papulo‐vesicular lesions is immune system hyperactivity and direct cytopathic effect of SARS‐CoV‐2 on endothelial dermal vessels. 9
2.1.3. Histopathological findings
Histological features include prominent acantholysis and dyskeratosis associated with intraepidermal vesicles in supra‐basal location. In some cases, epidermal necrosis, swelling of keratinocytes, ballooning degeneration of keratinocytes and endotheliitis may be present. 10 , 11
2.1.4. Clinical management
There is no standard treatment for COVID‐19‐associated papulo‐vesicular lesions. A “watchful waiting” strategy and symptomatic therapy is recommended. 12
2.2. Acral urticarial lesion
2.2.1. Clinical presentation
Urticarial lesions typically present as an erythematous papule or plaque with or without angioedema and intense pruritus. Among the most common sites of involvement are extremities including acral sites. 13 , 14 (Figure 2).
FIGURE 2.
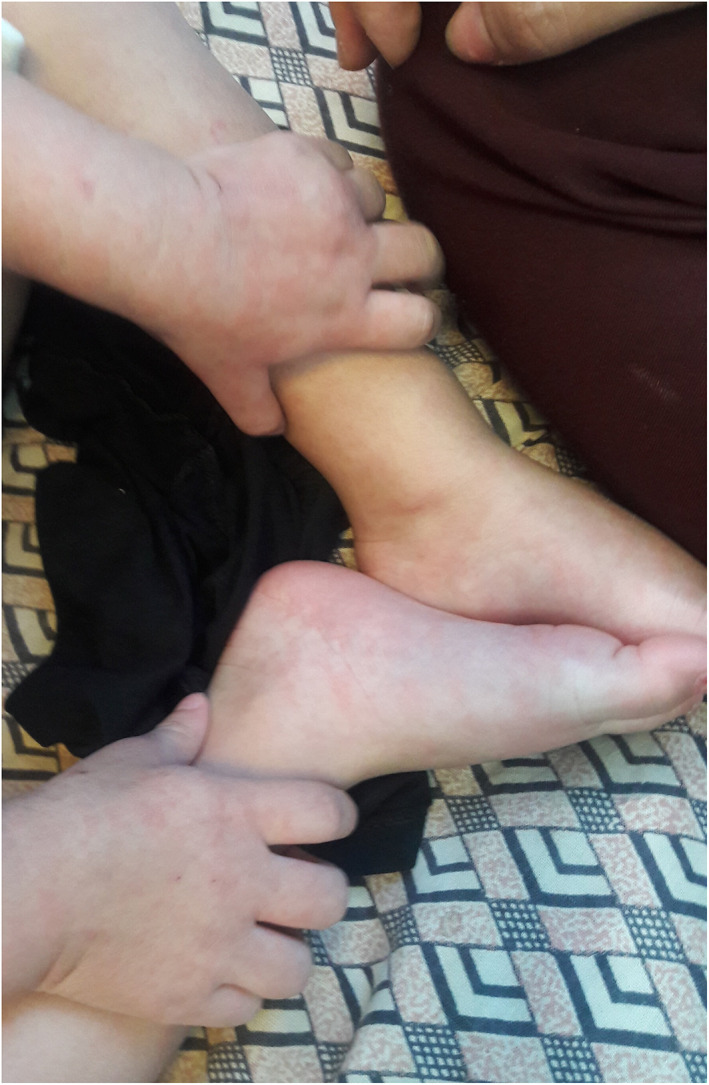
Acral urticarial lesion
2.2.2. Etiopathogenesis
Possible explanations for urticaria associated with COVID‐19 are direct cutaneous effect by SARS‐CoV‐2 virus, drug‐induced exanthema and over activity of the immune system. 13
2.2.3. Histopathological findings
Pathological findings of urticarial lesions in COVID‐19 patients consist of superficial perivascular infiltration of lymphocytes with few eosinophilic infiltrations associated with marked dermal edema in upper dermis. 6
2.2.4. Clinical management
Urticarial lesions are usually associated with moderate to severe COVID‐19. Non‐sedating antihistamines can be used for treatment of COVID‐19‐associated urticarial lesions. In more severe cases, a short course of low‐dose systemic corticosteroids can be used. 13
2.3. Acral non‐inflammatory purpura and skin necrosis
2.3.1. Clinical presentation
Most cases of purpura associated with COVID‐19 are non‐inflammatory retiform purpura presented as a lace‐like reddish‐blue to purple mottled discolorations on extremities and acral sites. These purpuric lesions may evolve into hemorrhagic blisters or necrotic‐ulcerative lesions and dry gangrene. 15 , 16 (Figure 3).
FIGURE 3.
Acral non‐inflammatory purpura associated with COVID‐19
2.3.2. Etiopathogenesis
Hypercoagulative state associated with COVID‐19 and pauci‐inflammatory micro thrombotic vasculopathy are proposed as the underlying mechanisms for purpuric lesions. 12 In addition, the cutaneous side effects of anti‐COVID‐19 agents like high‐dose intravenous immunoglobulin (IVIG) should be considered as skin manifestations of microvascular occlusion syndrome. 17
2.3.3. Histopathological findings
Pauci‐inflammatory thrombogenic vasculopathy with extensive deposition of complement components within the cutaneous microvasculature are dominant histopathological features of non‐inflammatory petechial and purpural lesions. 18
2.3.4. Clinical management
Petechia, purpura and acral necrotic lesions are usually associated with greater severity of COVID‐19. 18 Since acral non‐inflammatory purpura and skin necrosis are cutaneous signs of thrombotic microangiopathy, therapeutic‐dose of anticoagulants is appropriate for documented evidence of thrombosis or ongoing thrombosis formation. 19
2.4. Acro‐ischemia associated with COVID‐19
2.4.1. Clinical presentation
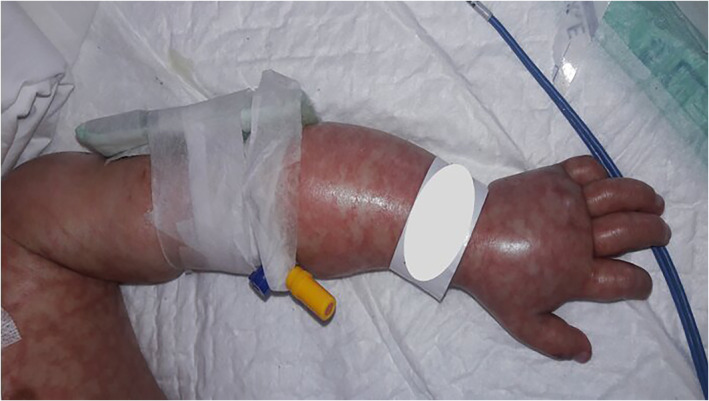
Distal acute ischemia presents with blue to gray discoloration without relation to the cold. Acro‐ischemic lesions usually have been reported in ICU‐admitted patients. 8 , 20 , 21 (Figure 4).
FIGURE 4.
Acro‐cyanosis associated with COVID‐19
2.4.2. Etiopathogenesis
Acro‐ischemic lesions are attributed to the cytokine storm when innate and adaptive immunity fails to get rid of the virus. Compensatory mechanism in COVID‐19 patients can be associated with hypercoagulative state, thrombotic events and disseminated intravascular coagulation. 8 , 20 , 21
2.4.3. Histopathological findings
Histological features of acro‐ischemic lesions are compatible with microthrombosis formation in dermal vessels, endothelial cell damage and extravasation of red blood cells. Superficial and deep perivascular lymphocytic infiltration with vacuolar degeneration in the basal layer can be seen. 22
2.4.4. Clinical management
Therapeutic anticoagulation combined with intravenous unfractionated heparin should be provided for patients with diagnosis of acro‐ischemia. Option for vascular intervention and revascularization depends on the extension of lesion and general condition of patients. 23
2.5. Acral vasculitis
2.5.1. Clinical presentation
Cutaneous small vessel vasculitis (CSVV), also known as Leucocytoclastic vasculitis, is characterized by symmetrically distributed palpable inflammatory purpura usually in the lower extremities. In addition to palpable papules, inflammatory purpuric lesions with necrotic center and blisters formation can be seen. 13 , 24 , 25 (Figure 5).
FIGURE 5.
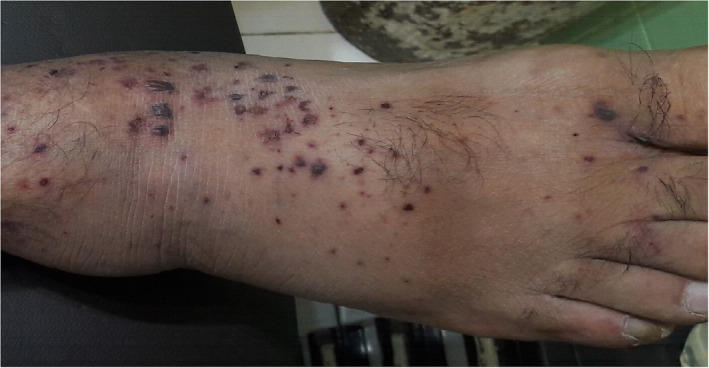
Cutaneous small vessels cellulitis associated with COVID‐19
2.5.2. Etiopathogenesis
Possible mechanisms of CSVV associated with COVID‐19 include direct damage of endothelial cells by the virus and indirect damage of endothelial cells by immune dysregulation induced by SARS‐COV2 13 , 25 .
2.5.3. Histopathological findings
Histopathological findings of CSVV in COVID‐19 patients consists of perivascular neutrophilic infiltration, fibrin deposition, fibrinoid necrosis, leucocytoclasis and endothelial swelling. 13 , 25 , 26
2.5.4. Clinical management
CSVV usually occurs in severe cases of COVID‐19 and is associated with higher mortality rate. Topical corticosteroids can be used for treating mild cases. Patients with generalized necrotic/ulcerative lesions may be treated with systemic corticosteroids. 27
2.6. Chilblain‐like lesion (“COVID Toe”)
2.6.1. Clinical presentation
Patients present with erythematous edematous painful pruritic skin resembling perniosis on the extremities occasionally associated with blister formation and digital swelling. They are considered as “COVID toes.” These lesions are a common feature in children with positive nasopharyngeal and rectal swabs for COVID‐19 with mild symptomatic or asymptomatic conditions. 8 , 28 , 29 These perniosis‐like lesions differ from acro‐ischemic lesions associated with severe COVID‐19 conditions. 29 (Figure 6).
FIGURE 6.

Chilblain‐like lesion on the finger (A) and foot (B)
2.6.2. Etiopathogenesis
Chilblain‐like lesions are caused by skin capillaries damage. Possible mechanisms for the damage are immune dysregulation and an immunologic response targeting cutaneous vessels. 8 , 16 Chilblain‐like lesions can also be cutaneous manifestation of strong type I interferon (IFN‐I) response in the setting of an effective immune response. 30 , 31 , 32
2.6.3. Histopathological findings
Histology of these acral lesions shows diffuse dense lymphoid infiltration in dermis and hypodermis, with a prevalent perivascular pattern and signs of endothelial activation. 8
2.6.4. Clinical management
Although most patients with COVID‐19‐associated chilblain‐like lesions have none or mild symptoms, they should follow public health guidelines for COVID‐19 testing and isolation. 33
2.7. Acral EM‐like lesion
2.7.1. Clinical presentation
Acral EM‐like lesions associated with COVID‐19 usually present as a conventional EM associated with other causes. This finding presents with targetoid acral lesions with two or three concentric circles with a small necrotic/hemorrhagic area. Lesions may be observed on extremities, dorsal of hands and feet. 34 (Figure 7).
FIGURE 7.
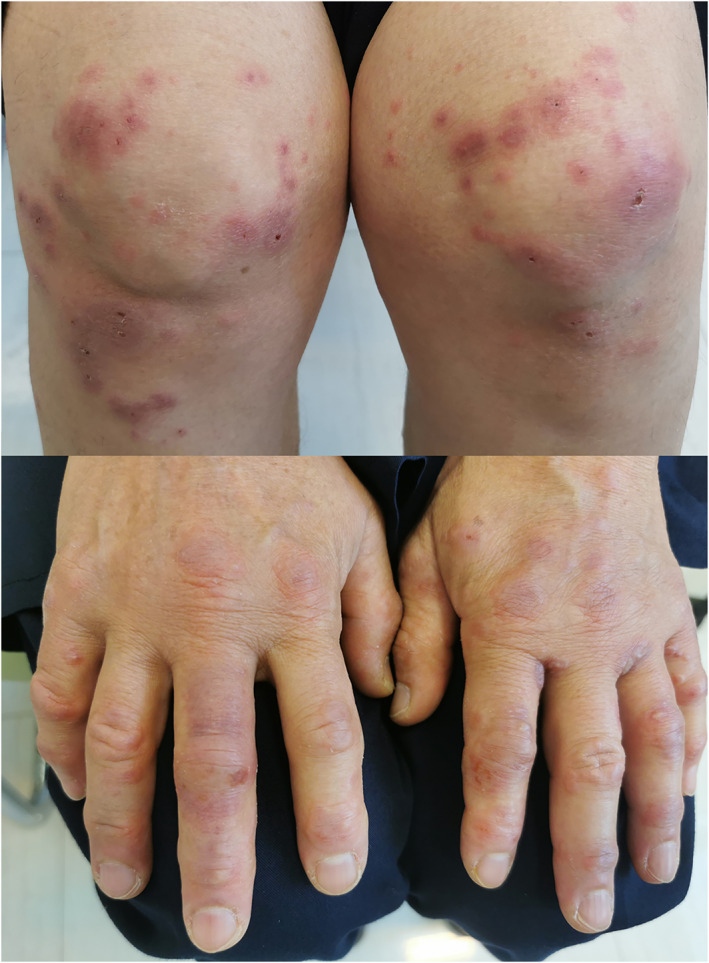
Erythema multiform‐like lesion associated with COVID‐19. In addition to the acral site, similar bulous lesions were seen on knees and elbows
2.7.2. Etiopathogenesis
Although the exact mechanism of EM‐like lesions in COVID ‐19 is uncertain, immune response to the SARS‐COV‐2, hypersensivity by cytokines cascade triggered by SARS‐COV‐2 infection and a potential delayed immune response to the virus can be proposed as possible etiopathogenesis of COVID‐associated EM‐like lesion. 17 , 35
2.7.3. Histopathological findings
Mild superficial perivascular infiltration and microthrombi formation are common features of these lesions. 34 In some cases, immunohistochemistry for SARS‐CoV/SARS‐CoV‐2 spike protein shows granular positivity in endothelial and epithelial cells of eccrine glands. 34
2.7.4. Clinical management
Skin lesions usually improve spontaneously in 1–3 weeks. 34
2.8. Acral lesions associated with multisystem inflammatory syndrome in children (MISC)
2.8.1. Clinical presentation
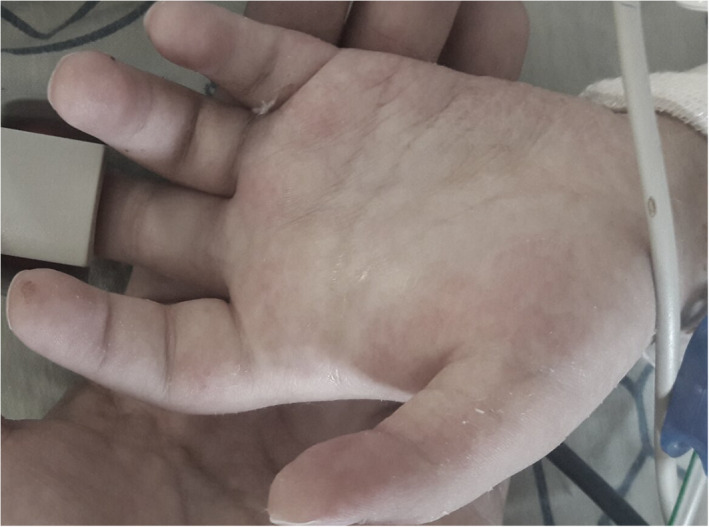
Muco‐cutaneous manifestations are common in MISC. One of the most important features of MISC which is considered as a sign of organ involvement, is involvement of hands and/or feet. Acral involvement in the setting of MISC presents with acral erythema and edema. 36 , 37 (Figure 8).
FIGURE 8.
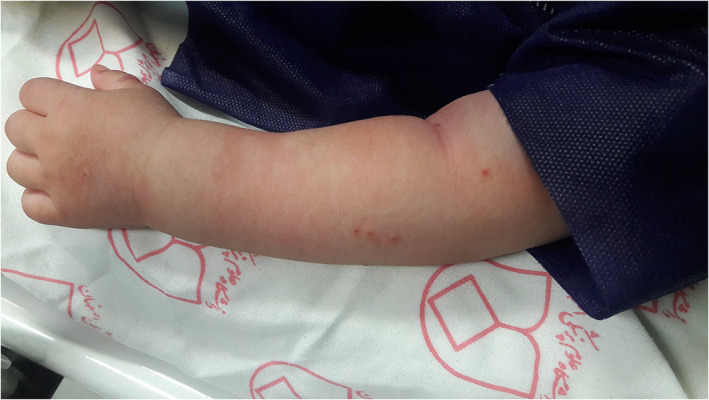
Acral edema associated with multisystem inflammatory syndrome in children
2.8.2. Etiopathogenesis
The early infection of SARS‐COV2 triggers macrophage activation and T‐helper stimulation. These events lead to cytokine release, stimulation of leucocytes (including B‐cells) and plasma cells which lead to overproduction of antibodies and induction of hyper immune response which is characteristic for MISC. 38
2.8.3. Histopathological findings
Perivascular cuffing with cytotoxic CD8+ lymphocytes and eosinophils that can be seen in Kawasaki syndrome, has also been reported in MISC. 39 , 40 , 41
2.8.4. Clinical management
Although MISC can be a potentially serious condition with significant complications and organ involvements, most children will survive from acute phase with prompt diagnosis and treatment. Single dose of 2 g/kg IVIG administered over 8–12 h with or without systemic corticosteroids according to the severity of condition and organ failure, should be used for all patients who meet diagnostic criteria for MISC. 42
2.9. Acral peeling lesions
2.9.1. Clinical presentation
Superficial desquamation of distal phalanges of hands and feet can occur in association with COVID‐19. The lesions are asymptomatic or slightly symptomatic without preceding edema, in contrast to MISC‐associated acral edema, erythema and subsequent desquamation. 43 , 44 , 45 (Figure 9).
FIGURE 9.
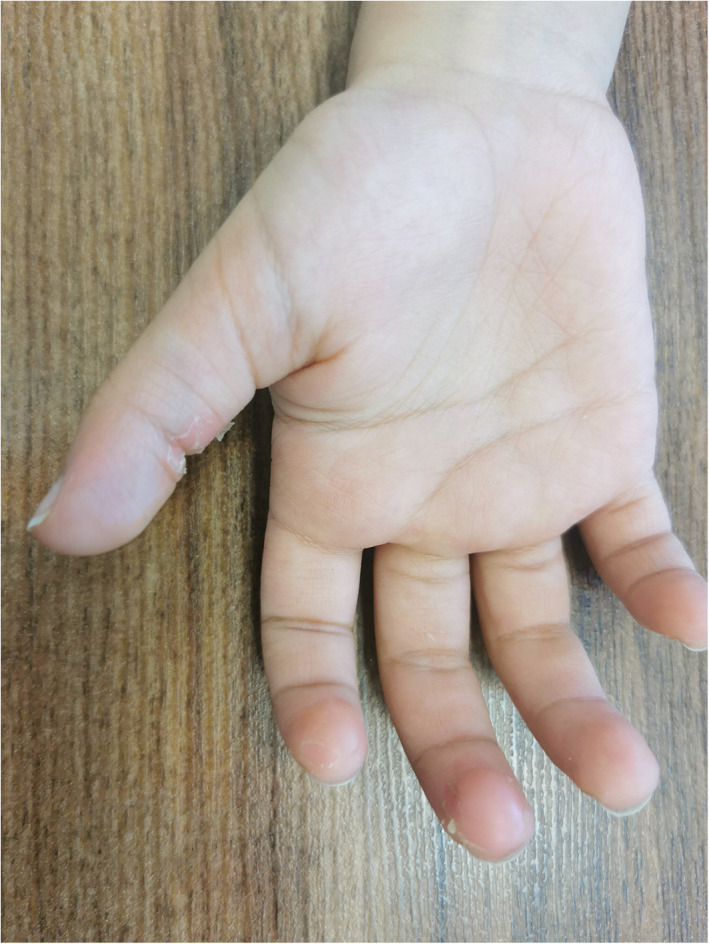
Acral peeling syndrome associated with COVID‐19
2.9.2. Ethiopathogenesis
The exact mechanism of acral peeling lesions in association with COVID‐19 is unclear. Similar to other post infections with significant inflammatory response, alternation in regulation of expression of acral keratins can be considered as possible mechanism. 46
2.9.3. Histopathological findings
Due to mild and self‐limiting nature of these lesions, skin biopsy is usually not performed.
2.9.4. Clinical management
Treatment is usually not necessary as lesions spontaneously resolve within 1–4 weeks. 46
2.10. Red half‐moon nail sign associated with C OVID‐19
2.10.1. Clinical presentation
Half‐moon‐shaped transversal red band at the distal margin of the lunula as single crescent erythronychia on the nail bed is considered as a pathognomonic nail finding in COVID‐19 infection. 47 , 48
2.10.2. Etiopathogenesis
The exact pathogenesis of the red half‐moon nail sign associated with COVID‐19 in unknown. Complement‐mediated microvascular injury of the nail bed, distal sub ungual arcade capillary network damage and subsequent thrombus formation in small vessels have been proposed as possible mechanisms. 47 , 48
2.10.3. Histopathological findings
There is no histopathological data up to date about histology of red half‐moon nail sign associated with COVID‐19.
2.10.4. Clinical management
Since the lesions are asymptomatic, treatment is unnecessary.
3. CONCLUSION
Acral skin changes associated COVID‐19 may provide valuable insight into underlying systemic manifestations of SARS‐CoV‐2. Clinicians should continue to document these changes since they may become important diagnostic clues and help clinicians better understand the pathophysiologic basis for this viral illness. Future studies are needed to investigate etiopathogenesis and management of these skin lesions.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Saeedeh Farajzadeh, Maryam Khalili, Shakiba Dehghani and Bahareh Abtahi‐Naeini contributed in study conception and design. Data collection was done by Sharareh Babaie and Bahareh Abtahi‐Naeini. Analysis and interpretation of results was carried out by Mahdi Fattah and Bahareh Abtahi‐Naeini. Shakiba Dehghani and Bahareh Abtahi‐Naeini prepared the manuscript draft and Saeedeh Farajzadeh, Maryam Khalili, Sharareh Babaie and Mahdi Fattah did the manuscript revision. All authors reviewed and approved the final version of manuscript and agreed with all aspects of the work.
ACKNOWLEDGMENTS
The authors declare that none of the authors are employed by a government agency that has a primary function other than research and/or education. None of the authors is an official representative or on behalf of the government. Besides, the authors declare that written informed consents were obtained from the patients or their guardians for publication of this review and any accompanying images.
Farajzadeh S, Khalili M, Dehghani S, Babaie S, Fattah M, Abtahi‐Naeini B. Top 10 acral skin manifestations associated with COVID‐19: A scoping review. Dermatologic Therapy. 2021;34(6):e15157. doi: 10.1111/dth.15157
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Tan SW, Tam YC, Oh CC. Skin manifestations of COVID‐19: A worldwide review. JAAD Int. 2021;2:119‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khalili M, Iranmanesh B, Mohammadi S, Aflatoonian M. Cutaneous and histopathological features of coronavirus disease 2019 in pediatrics: A review article. Dermatol Ther. 2021;34(1):e14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abtahi‐Naeini B, Fattah M. COVID‐19 and dermatological manifestations. In: Smoller B, Bagherani N, eds. Atlas of Dermatology, Dermatopathology and Venereology. Springer International Publishing; 2020:1‐34. [Google Scholar]
- 4. Conforti C, Dianzani C, Agozzino M, et al. Cutaneous manifestations in confirmed COVID‐19 patients: a systematic review. Biology (Basel). 2020;9(12):449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clawson RC, Tabata MM, Ko JM. Management of a child vs an adult presenting with acral lesions during the COVID‐19 pandemic: a practical review. Cutis. 2021;107(3):139‐142. [DOI] [PubMed] [Google Scholar]
- 6. Fernandez‐Nieto D, Ortega‐Quijano D, Jimenez‐Cauhe J, et al. Clinical and histological characterization of vesicular COVID‐19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol. 2020;45(7):872‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marzano AV, Genovese G, Fabbrocini G, et al. Varicella‐like exanthem as a specific COVID‐19‐associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83(1):280‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34(5):e212‐e213. [DOI] [PubMed] [Google Scholar]
- 9. Criado PR, Abdalla BMZ, De Assis IC, Van Blarcum de Graaff Mello C, Caputo GC, Vieira IC. Are the cutaneous manifestations during or due to SARS‐CoV‐2 infection/COVID‐19 frequent or not? Revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69(8):745‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mahé A, Birckel E, Merklen C, et al. Histology of skin lesions establishes that the vesicular rash associated with COVID‐19 is not ‘varicella‐like’. J Eur Acad Dermatol Venereol. 2020;34(10):e559‐e561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trellu LT, Kaya G, Alberto C, Calame A, McKee T, Calmy A. Clinicopathologic aspects of a papulovesicular eruption in a patient with COVID‐19. JAMA Dermatol. 2020;156(8):922‐924. [DOI] [PubMed] [Google Scholar]
- 12. Genovese G, Moltrasio C, Berti E, Marzano AV. Skin manifestations associated with COVID‐19: current knowledge and future perspectives. Dermatology. 2021;237(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Masson A, Bouaziz JD, Sulimovic L, et al. Chilblains is a common cutaneous finding during the COVID‐19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83(2):667‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bosch‐Amate X, Giavedoni P, Podlipnik S, et al. Retiform purpura as a dermatological sign of coronavirus disease 2019 (COVID‐19) coagulopathy. J Eur Acad Dermatol Venereol. 2020;34(10):e548‐e549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bouaziz JD, Duong TA, Jachiet M, et al. Vascular skin symptoms in COVID‐19: a French observational study. J Eur Acad Dermatol Venereol. 2020;34(9):e451‐e452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rubio‐Muniz CA, Puerta‐Peña M, Falkenhain‐López D, et al. The broad spectrum of dermatological manifestations in COVID‐19: clinical and histopathological features learned from a series of 34 cases. J Eur Acad Dermatol Venereol. 2020;34(10):e574‐e576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Giorgi V, Recalcati S, Jia Z, et al. Cutaneous manifestations related to coronavirus disease 2019 (COVID‐19): a prospective study from China and Italy. J Am Acad Dermatol. 2020;83(2):674‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cuker A, Tseng EK, Nieuwlaat R, et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID‐19. Blood Adv. 2021;5(3):872‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mazzotta F, Troccoli T. Acute acro‐ischemia in the child at the time of COVID‐19. Eur J Pediatr Dermatol. 2020;30(2):71‐74. [Google Scholar]
- 21. Romaní J, Baselga E, Mitjà O, et al. Chilblain and acral purpuric lesions in Spain during Covid confinement: retrospective analysis of 12 cases. Actas Dermo‐Sifiliograficas. 2020;111(5):426‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. García‐Gil MF, Monte Serrano J, García García M, Pascual‐del‐Riquelme JA, Ara‐Martín M. Acro‐ischemic lesions associated with extremely elevated D‐dimer in a child during the COVID‐19 pandemic. Australas J Dermatol. 2021;62(1):80‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patel P, Yu Y, Zia S, Padberg F, Curi M, Huang J. Systemic thrombolysis as initial treatment of COVID‐19 associated acute Aortoiliac and lower extremity arterial thrombosis. Ann Vasc Surg. 2021;70:297‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Damsky W, Peterson D, King B. When interferon tiptoes through COVID‐19: Pernio‐like lesions and their prognostic implications during SARS‐CoV‐2 infection. J Am Acad Dermatol. 2020;83(3):e269‐e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mayor‐Ibarguren A, Feito‐Rodriguez M, Quintana Castanedo L, Ruiz‐Bravo E, Montero Vega D, Herranz‐Pinto P. Cutaneous small vessel vasculitis secondary to COVID‐19 infection: a case report. J Eur Acad Dermatol Venereol. 2020;34(10):e541‐e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koutkia P, Mylonakis E, Rounds S, Erickson A. Leucocytoclastic vasculitis: an update for the clinician. Scand J Rheumatol. 2001;30:315‐322. [DOI] [PubMed] [Google Scholar]
- 27. Karaca Z, Yayli S, Çalışkan O. A unilateral purpuric rash in a patient with COVID‐19 infection. Dermatol Ther. 2020;33(4):e13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kelvin AA, Halperin S. COVID‐19 in children: the link in the transmission chain. Infect Dis. 2020;20(6):633‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baeck M, Herman A. COVID toes: where do we stand with the current evidence? Int J Infect Dis. 2021;102:53‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lipsker D. A chilblain epidemic during the COVID‐19 pandemic. A sign of natural resistance to SARS‐CoV‐2? Med Hypotheses. 2020;144:109959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Battesti G, el Khalifa J, Abdelhedi N, et al. New insights in COVID‐19‐associated chilblains: a comparative study with chilblain lupus erythematosus. J Am Acad Dermatol. 2020;83(4):1219‐1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mazzotta F, Troccoli T, Caselli D, Bonifazi E. Acral rash in a child with COVID‐19. Eur J Pediatr Dermatol. 2020;2:79‐82. [Google Scholar]
- 33. Ladha MA, Luca N, Constantinescu C, Naert K, Ramien ML. Approach to chilblains during the COVID‐19 pandemic. J Cutan Med Surg. 2020;24(5):504‐517. [DOI] [PubMed] [Google Scholar]
- 34. Torrelo A, Andina D, Santonja C, et al. Erythema multiforme‐like lesions in children and COVID‐19. Pediatr Dermatol. 2020;37(3):442‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Farinazzo E, Dianzani C, Zalaudek I, Conforti C, Grabbe S, Goldust M. Synthesis of the data on COVID‐19 skin manifestations: underlying mechanisms and potential outcomes. Clin Cosmet Investig Dermatol. 2021;14:991‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yozgat CY, Uzuner S, Bursal Duramaz B, et al. Dermatological manifestation of pediatrics multisystem inflammatory syndrome associated with COVID‐19 in a 3‐year‐old girl. Dermatol Ther. 2020;33(4):e13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aronoff SC, Hall A, Del Vecchio MT. The natural history of severe acute respiratory syndrome coronavirus 2‐related multisystem inflammatory syndrome in children: a systematic review. J Pediatric Infect Dis Soc. 2020;9(6):746‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakra NA, Blumberg DA, Herrera‐Guerra A, Lakshminrusimha S. Multi‐system inflammatory syndrome in children (MIS‐C) following SARS‐CoV‐2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children (Basel, Switzerland). 2020;7(7):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kobayashi M, Matsumoto Y, Ohya M, Harada K, Kanno H. Histologic and Immunohistochemical evaluation of infiltrating inflammatory cells in Kawasaki disease arteritis lesions. Appl Immunohistochem Mol Morphol. 2021;29(1):62‐67. [DOI] [PubMed] [Google Scholar]
- 40. Gianotti R, Veraldi S, Recalcati S, et al. Cutaneous Clinico‐pathological findings in three COVID‐19‐positive patients observed in the metropolitan area of Milan, Italy. Acta Derm Venereol. 2020;100(8):adv00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gianotti R, Zerbi P, Dodiuk‐Gad RP. Clinical and histopathological study of skin dermatoses in patients affected by COVID‐19 infection in the northern part of Italy. J Dermatol Sci. 2020;98(2):141‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ahmed M, Advani S, Moreira A, et al. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine. 2020;26:100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rotulo GA, Signa S, Rosina S, Pastorino C, Bondi E, Maghnie M. Giant Urticaria and Acral peeling in a child with coronavirus disease 2019. J Pediatr. 2021;230:261‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Young TK, Shaw KS, Shah JK, et al. Mucocutaneous manifestations of multisystem inflammatory syndrome in children during the COVID‐19 pandemic. JAMA Dermatol. 2021;157(2):207‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rekhtman S, Tannenbaum R, Strunk A, Birabaharan M, Wright S, Garg A. Mucocutaneous disease and related clinical characteristics in hospitalized children and adolescents with COVID‐19 and multisystem inflammatory syndrome in children. J Am Acad Dermatol. 2021;84(2):408‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Andina‐Martínez D, Villaizán‐Perez C, Pavo‐García MR, et al. Acral peeling as the sole skin manifestation of COVID‐19 in children. Pediatr Dermatol. 2021;38(3):664‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neri I, Guglielmo A, Virdi A, Gaspari V, Starace M, Piraccini BM. The red half‐moon nail sign: a novel manifestation of coronavirus infection. J Eur Acad Dermatol Venereol. 2020;34(11):e663‐e665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Méndez‐Flores S, Zaladonis A, Valdes‐Rodriguez R. COVID‐19 and nail manifestation: be on the lookout for the red half‐moon nail sign. Int J Dermatol. 2020;59(11):1414‐1414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


