To the Editor:
Systemic light chain (AL) amyloidosis is caused by a usually small plasma cell clone that produces amyloidogenic immunoglobulin light chains. This clone may affect immune competence and immune system fitness but, in addition, organ dysfunction, leading to heart failure, nephrotic range proteinuria, malabsorption, etc. further increases susceptibility to infections and risk of complications. Anti‐plasma cell therapies cause immunosuppression and have been associated with increased risk of infectious complications, including COVID‐19. 1 , 2 Vaccination against Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is the best strategy to avoid severe Coronavirus disease 2019 (COVID‐19), 3 however, response to vaccines is compromised in patients with plasma cell malignancies or other B‐cell lymphoproliferative disorders. 4 , 5 , 6 , 7 , 8
Data on the humoral responses to vaccination against COVID‐19, for patients with AL amyloidosis is limited. Subjects with asymptomatic plasma cell dyscrasias (monoclonal gammopathy of undetermined significance [MGUS] or smoldering myeloma) may have attenuated responses to vaccination, 6 but although the clones in these patients may be similar in extent to the plasma cell clone in AL amyloidosis, these patients are not receiving active therapy. We reported that after the first dose of vaccine in 59 patients with AL amyloidosis, there was a blunted humoral response; but results following the second dose of the vaccine were not available. 9 We also observed that patients with transthyretin‐related amyloidosis (ATTRwt), despite their old age could mount a post‐vaccination humoral response similar to their matched controls. We prospectively measured the titers of neutralizing antibodies (NAbs) against SARS‐CoV‐2 after vaccination with the Pfizer‐BioNTech BNT162b2 mRNA vaccine and analyzed for factors that were related to seroconversion.
This report is part of larger prospective study (NCT04743388) assessing the kinetics of anti‐SARS‐CoV‐2 antibodies development after COVID‐19 vaccination. The major inclusion criteria for this analysis include: (i) a prior diagnosis of AL or ATTR amyloidosis and, (ii) eligibility for vaccination. As a control group, we used volunteers matched for age, gender (1:2), who had (i) no autoimmune or active malignant disease; (ii) no Human Immunodeficiency Virus (HIV) or active hepatitis B, C infection. Serum was separated within 4 h from blood collection and stored at −80°C until the day of measurement. Collected samples refer to day 1 (D1; first BNT162b2 dose), day 22 (D22; second dose), and day 50 (D50; i.e., 4 weeks after the second dose of the vaccine). NAbs against SARS‐CoV‐2 were measured using an FDA approved surrogate assay (ELISA, cPass™ SARS‐CoV‐2 NAbs Detection Kit; GenScript, Piscataway, NJ). We used the 30% inhibition cut‐off for this surrogate NAbs test as previously suggested 10 ; a titer of at least 50% is considered a clinically relevant threshold for viral inhibition. 11 The study was approved by the respective Ethical Committees in accordance with the Declaration of Helsinki and all patients and controls have provided written informed consent prior to enrollment. The primary end point of the study was the NAbs titer on D50. Details of the analysis are provided in the online supplement.
The final analysis included 126 fully monitored patients who were included in the final analysis (68 males/58 females, median age: 66, and range 35–86). The control group included 252 (ratio 1:2) fully matched controls (for age, gender) (136 male/116 females; median age: 66, and range 35–86 years). The median body mass index (BMI) of patients with AL amyloidosis was 25.4 kg/m2 (range 17.9–42.4) and of controls was 26.2 kg/m2 (range 17.4–42.7) (p = .704).
At the time of vaccination, 66 (52%) patients with AL amyloidosis were on active therapy, 29 (24%) were on daratumumab‐based therapy, 8 had completed therapy but <3 months from the first dose of the vaccine and 52 (43%) had discontinued therapy >3 months from the date of the first vaccine shot, 33 (27%) had prior exposure to daratumumab (at least 3 months had passed since the last dose), and 92 (75%) were in hematologic remission (hematologic Complete response [CR] or hematologic very good partial response [VGPR]). Among those not on therapy, median time since last dose was 26 months (range 3–118). More patients' characteristics are shown in Table S1.
Prior to the first dose (D1), the NAb titers were similar between patients and controls (median 14.9% [IQR 7.8%–23.1%] vs. 14% [IQR 6.8%–22.9%], p = .439). Eight (6.5%) patients with AL amyloidosis patients had baseline NAbs ≥30% (positivity cutoff), of which five reported a history of prior COVID‐19 infection. Among controls, 24 (9.9%) had NAb titers of ≥30% (Figure 1A).
FIGURE 1.
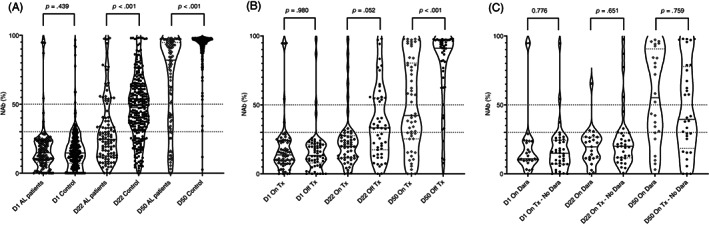
(A) Kinetics of neutralizing antibodies (NAbs) in patients with AL amyloidosis and age and sex‐matched controls before and after the vaccination with BNT162b2. (B) Kinetics of neutralizing antibodies (NAbs) in patients with AL amyloidosis on active therapy (On Tx) versus those not currently receiving anti‐clonal therapy (Off Tx). (C) Kinetics of neutralizing antibodies (NAbs) in patients with AL amyloidosis on active treatment with daratumumab (On Dara) or on active treatment that does not contain daratumumab (On Tx—No Dara)
On D22 after the first dose of the BNT162b2 vaccine, there was a significant increase of NAbs titers both in controls and AL patients (both p < .001); however, the median NAbs titer was 23.6% (IQR 12.4%–37.7%) in patients with AL amyloidosis versus 47.5% (IQR 32.1%–62.7%) in the control group (p < .001). Thus, 18% of AL patients versus 44.7% of controls (p < .001) developed NAb titers ≥50%; this level of Nabs is associated with clinically relevant viral inhibition 11 (Figure 1A).
On D50 after the initial vaccine shot (4 weeks after the second shot), there was further increase in NAbs titers both in controls and AL patients (for both p < .001) and median NAbs titer for AL patients was 83.1% (IQR 41.5%–94.9%) versus 95.6% (IQR 91.7%–97.2%) in controls (p < .001). Thus, after completion of the two doses, 71% of patients with AL amyloidosis versus 98% of matched controls (p < .001) developed NAb titers ≥50% (Figure 1A).
In univariate analysis, factors associated with NAb titers on D50 included age (p < .001), lymphocyte counts (p < .001), serum albumin (p < .001) and amount of proteinuria at the time of vaccination (p = .047), renal involvement (p = .047), use of steroids (p < .001), active treatment (p < .001), treatment‐free interval (p = .001) and remission status (CR/VGPR vs. PR/NR) (p = .018). There was no significant association of D50 Nabs titers with gender (p = .092), BMI (p = .198), serum IgG (0.099), IgA (p = .789), or IgM levels (p = .687) or low (i.e., below lower limit of normal) of at least one of the noninvolved immunoglobulin (p = .179) at the time of vaccination, as well as of liver (p = .521) or heart involvement by amyloidosis (p = .141).
Patients on active therapy had lower NAb titers at D50 (median 51.5% [IQR 25.3%–84.1%] vs. 91.6% [IQR 74.5%–96.5%]) compared to those not on active treatment (p < .001). Specifically, 51% of patients on anti‐clonal therapy had a D50 NAb titer ≥50% versus 87% of those who were not on therapy. There was no difference in the baseline NAb titers among those on or off active therapy, while on D22 (i.e., after 1st dose) the NAbs titer was higher among those on therapy but statistical difference was marginal (median 30% vs. 19.6%, p = .052). Thus, after the second dose, the response among those not on therapy was substantially more robust than in patients receiving anti‐clonal therapy (Figure 1B). Notably, on D22, NAb titers among AL patients not on active therapy were still significantly lower as compared to non‐AL controls (median 27.9 vs. 46.7%, p < .001) as well as on D50 (median 91.6% vs. 95.6%, p < .001). Using receiver operating characteristic (ROC) analysis, we found that at least 3 months since the last dose of therapy were associated with significantly higher probability of ≥50% NAbs on D50 (sensitivity: 62%, specificity: 80%, AUC: 0.690, p = .001).
Among patients on treatment, there was no significant difference between those on bortezomib‐based therapy (VCD) versus those on daratumumab‐alone therapy, those on daratumumab with VCD or those on IMiDs, or those with current or prior use of cyclophosphamide. Active therapy containing daratumumab did not affect NAbs titers among patients on active therapy (median NAb titer was 52.1% vs. 46.4% for those not receiving treatment with daratumumab, p = .486). Similarly, among patients not on active treatment, prior exposure to daratumumab did not affect D50 NAb titers (92.1% vs. 91.2%, p = .966) (Figure 1C). Six (5%) patients had a history of prior autologous stem cell transplantation (ASCT) with a median time since transplant of 61 months, (range 6–184); median NAb titer at D50 was 89% for this group of patients with two patients having NAbs titers below 50%.
Generalized linear models after normalization of NAb titers, were used for evaluation of multiple factors associated with D50 NAb titers (Table S3). Specifically, at least 3 months since the last dose of anticlonal therapy (p < .001), lymphocyte counts (p = .001) and serum albumin levels at the time of vaccination (p = .020) were independent predictors of NAb titers on D50.
Focusing on significant (i.e., protective) seroconversion (defined as NAbs titer ≥50% at D50), we performed a multiple logistic regression analysis. In this analysis, >3 months of treatment‐free interval (OR: 7.75, p < .001), was the strongest predictive factor associated with activation of humoral immune responses (Table S2).
Among the patients in the study, one was infected and developed very mild symptoms after the first and before the second dose and two patients were infected but remained completely asymptomatic more than 2 weeks after the second dose (in both testing was performed because of close home contact with an infected individual).
We also compared NAbs titers of patients with ATTRwt (N = 22) to age and gender matched controls (N = 44, 1:2 matching, median age 84, range 72–92 in both) (Table S4) that were vaccinated with BNT162b2; 18 (82%) of the patients were receiving tafamidis (61 mg dose). The median baseline NAb titers were similar (23% for patients with ATTRwt vs. 16% for controls, p = .717) as well as on D22 (40.5% for ATTRwt vs. 41.2% for controls, p = .596) and on D50 (92.2% vs. 94.2%, p = .546) (Figure S1).
The data presented in this report indicate that patients with AL amyloidosis have an attenuated humoral response to vaccination with BNT162b2, as compared to matched controls. Our analysis indicates that the most important factor that negatively affects the development of NAbs after vaccination for COVID‐19 is the administration of active anti‐clonal therapy. This data point to the major immunosuppressive role of anti‐plasma cell therapy but also to the immunosuppressive effect of the underlying plasma cell clone, even though the impact may be low. Thus, patients with AL amyloidosis do not differ significantly in their response to vaccination than patients with myeloma (data published from our group 5 , 6 and others 7 , 8 ). Specifically, we recently reported that antibody response after vaccination with BNT162b2 in patients with myeloma depends on the type of anti‐myeloma treatment. 6 In the current study, in patients with AL amyloidosis, we observed that the use of daratumumab as part of the treatment regimen did not significantly affect antibody response, among those on active therapy, while prior exposure to daratumumab, also did not have any additive immunosuppressive effect. Data from our group in patients with myeloma indicated that daratumumab‐based therapy was associated with lower NAb titers 6 ; however, myeloma patients were more heavily pretreated than patients with AL amyloidosis and with more extensive and aggressive clones. In addition, weekly bortezomib with cyclophosphamide (the most widely used regimen in AL amyloidosis) is also associated with significant immunosuppression. Time from the last dose of therapy was a significant factor to predict an adequate antibody response; however, some patients may require more time to restore a fully functional immune system. We observed that a duration of at least 3 months after last dose of anti‐clonal therapy before vaccination may affect humoral immune response. In support, in an exploratory analysis, not all patients had recovered lymphocyte counts at the time of vaccination (10% of those not in therapy had lymphocyte counts <1000/μL). Although it is recommended that a treatment‐free period should precede and follow vaccination, this may not be feasible; in fact, although vaccination is an urgent need for those most vulnerable subjects, it may not be possible to hold treatment for a disease like AL amyloidosis. Nevertheless, patients on therapy who are in deep hematologic remission may still mount adequate NAbs titers. We also evaluated a small number of elderly patients with non‐AL amyloidosis and no clonal disease (i.e., patients with ATTRwt amyloidosis) and observed that they developed an antibody response that was similar to their age/gender‐matched controls.
These results clearly support the need for further evaluation of possible booster doses in patients that fail to develop adequate antibody responses. Across these lines of possible interventions, recent data 12 from a small randomized study support that among transplant recipients receiving immunosuppression a third dose of the mRNA‐1273 vaccine (Moderna) triggered substantially higher immunogenicity than placebo. Since there are similarities in the level of immunosuppression caused by anti‐clonal therapies for AL amyloidosis (as well as for myeloma and lymphomas), a similar strategy is probably a likely indication, but has not yet been approved by the authorities. Nonetheless, our data provide evidence that there is an urgent need to adopt strategies that can enhance a clinically meaningful protective seroconversion in patients with AL amyloidosis (either on therapy or after therapy). Given the ongoing spread of the SARS‐CoV‐2 B.1.617.2 (or delta) variant with increased infectivity, this need becomes urgent. Recently, a booster dose has been recommended for immunocompromised patients in many countries, including those who had been vaccinated with BNT162b2. Our data indicate that such a strategy could indeed be beneficial for many patients who failed to achieve seroconversion, or who may have waning antibody levels.
Our study was not designed to assess clinical efficacy of vaccination in patients with AL amyloidosis. Among the 126 vaccinated patients, there were only two cases of asymptomatic infection, despite the increasing infection rates in Greece. For the evaluation of efficacy, we used the previously reported threshold of at least 50% titer for neutralizing antibodies. However, even lower levels of NAb titers may be protective against severe infection, 13 while new variants of SARS‐CoV‐2 may be associated with resistance to antibodies requiring higher Nab titers for effective clinical protection. There is evidence that NAbs against Wuhan strain, elicited after vaccination with current vaccines display significant activity against other variants of concern, including the delta variant, although neutralizing serum responses may be weaker. 14 , 15 In a recent paper by Rosati et al., 16 the authors found that anti‐spike antibodies from the vaccine recipients (naïve and convalescent) and SARS‐CoV‐2 convalescent patients show a strong ability to recognize and neutralize the autologous WA1, as well as the Alpha and Delta Spike variants but that they show greatly impaired recognition of Beta, in agreement with others. Furthermore, they found a strong direct correlation between NAbs against WA1 and Delta variant, supporting the notion that individuals with robust NAb against WA1 also strongly neutralize Delta. This data indicates that a title of NAb >50% may not be as protective against delta variant infection, but the clinically relevant threshold is difficult to define. Finally, in the current study, we did not evaluate the antiviral T and B cell memory responses after vaccination, which may also be affected in patients with AL amyloidosis, and which are also important for protection from the infection.In conclusion, patients with AL amyloidosis have a less robust response to anti‐SARS‐CoV‐2 vaccination as compared to matched controls. Our data point to the critical role of concomitant anti‐clonal therapy, which significantly reduced the probability of an adequate antibody response and protective seroconversion. Moreover, our data support the need for additional booster doses, especially for those on active therapy.
CONFLICT OF INTEREST
Efstathios Kastritis: Janssen: Consultancy, Honoraria, Other: Travel/accommodations/expenses, Research Funding; Pfizer: Consultancy; Genesis Pharma: Consultancy, Honoraria, Other: Travel/accommodations/expenses; Amgen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Other: Travel/accommodations/expenses. Evangelos Terpos: Bristol‐Myers Squibb: Honoraria; Sanofi: Honoraria; Celgene: Honoraria; Genesis: Honoraria, Other: Travel expenses, Research Funding; Takeda: Honoraria, Other: Travel expenses, Research Funding; Amgen: Honoraria, Research Funding; Janssen: Honoraria, Research Funding. Meletios A. Dimopoulos: Beigene: Honoraria; Bristol‐Myers Squibb: Honoraria; Amgen: Honoraria; Takeda: Honoraria; Celgene: Honoraria; Janssen: Honoraria.
Supporting information
Appendix S1: Supporting Information.
ACKNOWLEDGMENTS
We thank Ioanna Charitaki, RN; Christine Ivy Liacos, MSc; Nikoletta‐Aikaterini Kokkali, RN; Nefeli Mavrianou‐Koutsoukou, MSc; Dimitrios Patseas, MSc; and Mrs Stamatia Skourti, MSc for administrative, technical, or material support. We also thank SYN‐ENOSIS (Greece), AEGEAS (Greece), and IEMBITHEK (Greece) for partially funding this study, as well as all of the study participants for donating their time and samples.
DATA AVAILABILITY STATEMENT
Raw data are available at reasonable request by direct contact with the corresponding author.
REFERENCES
- 1. Kastritis E, Wechalekar A, Schonland S, et al. Challenges in the management of patients with systemic light chain (AL) amyloidosis during the COVID‐19 pandemic. Br J Haematol. 2020;190(3):346‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chari A, Samur MK, Martinez‐Lopez J, et al. Clinical features associated with COVID‐19 outcome in multiple myeloma: first results from the international myeloma society data set. Blood. 2020;136(26):3033‐3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trougakos IP, Stamatelopoulos K, Terpos E, et al. Insights to SARS‐CoV‐2 life cycle, pathophysiology, and rationalized treatments that target COVID‐19 clinical complications. J Biomed Sci. 2021;28(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165‐3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Terpos E, Trougakos IP, Gavriatopoulou M, et al. Low neutralizing antibody responses against SARS‐CoV‐2 in older patients with myeloma after the first BNT162b2 vaccine dose. Blood. 2021;137(26):3674‐3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Terpos E, Gavriatopoulou M, Ntanasis‐Stathopoulos I, et al. The neutralizing antibody response post COVID‐19 vaccination in patients with myeloma is highly dependent on the type of anti‐myeloma treatment. Blood Cancer J. 2021;11(8):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Avivi I, Balaban R, Shragai T, et al. Humoral response rate and predictors of response to BNT162b2 mRNA COVID19 vaccine in patients with multiple myeloma. Br J Haematol. 2021;195(2):186‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bird S, Panopoulou A, Shea RL, et al. Response to first vaccination against SARS‐CoV‐2 in patients with multiple myeloma. Lancet Haematol. 2021;8(6):e389‐e392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kastritis E, Terpos E, Sklirou A, et al. Antibody response after initial vaccination for SARS‐CoV‐2 in patients with amyloidosis. HemaSphere. 2021;5(8):e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trougakos IP, Terpos E, Zirou C, et al. Comparative kinetics of SARS‐CoV‐2 anti‐spike protein RBD IgGs and neutralizing antibodies in convalescent and naive recipients of the BNT162b2 mRNA vaccine versus COVID‐19 patients. BMC Med. 2021;19(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA‐based Covid‐19 vaccine candidates. N Engl J Med. 2020;383(25):2439‐2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA‐1273 vaccine in transplant recipients. N Engl J Med. 2021;385(13):1244‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat Med. 2021;27(7):1205‐1211. [DOI] [PubMed] [Google Scholar]
- 14. Sokal A, Barba‐Spaeth G, Fernandez I, et al. mRNA vaccination of naive and COVID‐19‐recovered individuals elicits potent memory B cells that recognize SARS‐CoV‐2 variants. Immunity. 2021;S1074‐7613(21)00396‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mlcochova P, Kemp SA, Dhar MS, et al. SARS‐CoV‐2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599(7883):114‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosati M, Terpos E, Agarwal M, et al. Distinct neutralization profile of spike variants by antibodies induced upon SARS‐CoV‐2 infection or vaccination. Am J Hematol. 2021;97(1):E3‐E7. 10.1002/ajh.26380 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information.
Data Availability Statement
Raw data are available at reasonable request by direct contact with the corresponding author.


