Summary
The full 2‐month lockdown to fight the coronavirus disease 2019 (COVID‐19) pandemic in 2020 led to substantial disruption of daily life and routines. The present study aimed to comprehensively identify the lockdown’s effects on sleep, daily rhythms and emotions of the French population. A survey was published online during the last week of the 2‐month full lockdown and 1,627 individuals completed the online survey. The survey was self‐administered and included standardised questionnaires. Sleep schedules were delayed during lockdown in more than half of the participants. New severe delayed sleep phase affected 10% of participants with sleep schedules delayed by ≥3 hr during the lockdown compared to before. A significant decrease in exposure to morning (p < 0.001) and evening natural light (p < 0.001), a significant increase in screen exposure time (with a significant screen exposure >3 hr during the evening for 45% of the participants during lockdown versus 18% before lockdown, p < 0.001), an increase in substance use for one‐quarter of participants, a poorer sleep quality in 56% of participants, and less regular sleep schedules in 48% of participants were observed. We also found a poorer sleep quality in women than men during lockdown (p = 0.004). The French full lockdown had a severe impact on sleep quality, sleep–wake rhythms, and sleep behaviours. The implementation of public health strategies for the prevention and care of sleep–wake cycles during lockdown are therefore essential.
Keywords: chronobiology, public health, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), sleep–wake cycles
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), emerged in December 2019 in Wuhan, in the Chinese province of Hubei (Wang, Horby, Hayden, & Gao, 2020). The World Health Organization declared the coronavirus situation a “pandemic” on March 11, 2020 (Geoffroy, Le Goanvic, & Sabbagh, 2020). On March 17, 2020, President Emmanuel Macron announced a complete national lockdown plan, which was extended to May 11, 2020, to contain the spread of COVID‐19 (Geoffroy, Le Goanvic, et al., 2020). This full lockdown led to substantial disruption of daily life. In addition to the stress induced by the pandemic and its consequences (economic, social, and health wise), this context may induce significant disturbances of biological rhythms, sleep quality and emotions regulation, which in turn can lead to mental disorders such as depressive or anxiety disorders (Baglioni et al., 2011; Wirz‐Justice & Benedetti, 2020). A previous survey conducted in the general Chinese population reported that 54% of subjects had a psychological impact of the epidemic, 16.5% had depressive symptoms and 28.8% had anxiety symptoms (Wang, Pan, et al., 2020). The impact of a full lockdown on sleep quality, rhythms and emotions is thus of major interest and needs to be better characterised.
During a full lockdown, main sleep–wake circadian rhythm synchronisers (exposure to daylight, diurnal physical activity, regular meals, and social interactions) are strongly modified or even abolished. Consequently, sleep disorders are likely to develop in this at‐risk context. In addition, exposure to some of these environmental stimuli can also act as “desynchronisers” by occurring at the wrong time of day (e.g. exposure to screens late at night), which may have a negative impact on sleep–wake rhythms similar to a jet lag syndrome: difficulty falling asleep, emotional disturbances, and daytime sleepiness (Altena et al., 2016). Furthermore, the stress caused by this context may have a direct impact on the sleep–wake cycle and circadian rhythms (Lo Martire, Caruso, Palagini, Zoccoli, & Bastianini, 2020) and may also induce insomnia symptoms via cognitive and somatic hyperarousal related to the hyperactivation of the stress system (Riemann et al., 2015). Indeed, Riemann et al. showed that the arousal system overactivity caused by stress could result in an hybrid sleep–wake state, characterised by different and independent sleep and wake states in different brain regions (Riemann et al., 2015). This full lockdown and context‐related stress factors can therefore lead to a vicious circle, wherein the loss of habits and the addition of new desynchronisers, might lead to sleep–wake rhythm disorders and psychiatric disorders. A survey of the general population appears necessary in order to better characterise these disorders, to be able to address appropriate public health messages, and to develop targeted preventive actions, especially as intermittent lockdowns are considered by many countries in the ongoing fight against the pandemic.
The objective of the present survey conducted in the French general population was to identify the effects of the full 2‐month lockdown on sleep, circadian rhythms, and emotions.
2. METHODS
2.1. Population
To reach volunteers from the general population, without exclusion criteria, the survey was published online (emails, newsletter from French sleep and psychiatric societies, Facebook, and Twitter) during the last week of the lockdown, starting on May 6, 2020. This first full lockdown in France lasted from March 17 to May 11, over a 2‐month period. Article 1 of decree n°2020–260 of March 16, 2020 stated the prohibition to leave homes with certain exceptions (journeys between home and professional activities and professional trips that cannot be delayed; purchases of supplies necessary for the professional activity and purchases of basic necessities in establishments whose activities remain authorised by order of the Minister in charge of health, issued on the basis of the provisions of the article L. 3131‐1 of the French Public Health Code; travel for health reasons; travel for compelling family reasons, for the assistance of vulnerable persons or for childcare; short trips, close to the home, related to the individual physical activity of persons, excluding any collective sports practice, and for pets’ needs).
During this last week of full lockdown, 1,627 subjects answered all the questions of the online survey. We did not consider the 1,146 participants who logged on to the platform and did not respond to the survey or who only partially completed the survey.
2.2. Survey
The purpose of this online questionnaire was to assess the participants’ sociodemographic characteristics, the impact of the lockdown on work activities and lifestyle habits, sleep, and circadian rhythm characteristics before and during the lockdown, medical history, treatments, and substance use (Appendix 1). Among the questions, we asked participants to rate their sleep quality on a scale of 1–10 (1 = very bad sleep, 10 = very good sleep), to quantify their sleep duration in hours, and to rate their sleep schedule regularity on a scale of 1–10 (1 = very irregular, 10 = very regular), both before and during the lockdown. We also asked whether they had shifted their bedtimes and wake‐up times during the lockdown, and if so, by how much. We then compared these scores between before and during lockdown. We asked the participants to estimate how much physical activity they did per week (“how much physical activity did you do per week?”), as well as their exposure time to natural light (“evaluate your exposure to natural light”) and screens (“evaluate your screen exposure time”), before and during the lockdown. The survey asked the participants about meal regularity and weight changes as follows: “What impact did the lockdown have on the regularity of your meals?” and “Did you lose or gain weight during the lockdown?”. Several standardised questionnaires were also included: the Hospital Anxiety and Depression Scale (HADS; Snaith, 2003), the Post‐traumatic stress disorder (PTSD) CheckList‐5 (PCL‐5; Blevins, Weathers, Davis, Witte, & Domino, 2015), the Insomnia Severity Index (ISI; Bastien, 2001), the Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) and the Epworth Sleepiness Scale (ESS; Johns, 1991). The HAD‐A anxiety and HAD‐D depression scales are considered significant when the total score is equal to 11, a threshold value of 38 on the total score of the PCL‐5 scale suggests PTSD, a total score of >5 on the PSQI indicates sleep disturbance, and a total score of ≥11 on the ESS indicates pathological sleepiness. Concerning the ISI, a total score of <7 is not in favour of insomnia, a score between 8 and 14 indicates mild insomnia, a score between 15 and 21 suggests moderate insomnia, and a total score between 22 and 28 is in favour of severe insomnia.
This French‐language survey was conducted on the Assistance Publique‐Hôpitaux de Paris (AP‐HP) Lime Survey platform with anonymised data storage on the AP‐HP intranet, in partnership with AP‐HP‐Enquêtes (https://questionnaire.aphp.fr/). The survey was carried out by the SOPSY section (sleep and biological rhythms in psychiatry) of the French Association of Biological Psychiatry and Neuropsychopharmacology (AFPBN) and the French Society for Sleep Research and Medicine (SFRMS). Data storage was carried out on an AP‐HP computer at the Bichat hospital. The duration of the survey was 10–15 min and subjects were free to interrupt their participation at any time without justification. The questionnaire was anonymous, contact details and identities of the participants were not required. Participants were informed that the data would be analysed for research purposes.
This research project titled “Study of biological Rhythms and Emotions during the COVID‐19 lockdown (Covid‐RythmE)” was approved (N°CER‐2020–40) by the Comité d’Evaluation de l’Ethique des projets de Recherche Biomédicale (CEERB) Paris Nord (Institutional Review Board ‐ IRB 00006477‐ of HUPNVS, Paris 7 University, AP‐HP).
2.3. Statistical analysis
The minimum response target was set at 1,000 in order to have sufficient power for analyses and to reflect the general population’s sleep and rhythms. A total of 1,627 complete responses were obtained. The results are given in mean ± standard deviation (SD) and the categorical variables are expressed as a percentage. A Spearman’s correlation and a Mann–Whitney U test were performed to compare the sleep quality variable to age and gender. To investigate more precisely which age groups showed differences during the lockdown, we made Dwass–Steel–Critchlow–Fligner pairwise comparisons. A McNemar test and a Pearson correlation were performed to compare different variables before and during lockdown. Then, we conducted multivariate analyses, using a binomial logistic regression, to confirm independent associations adjusting for age, gender, and main occupation. Statistical analyses were conducted using the JAMOVI software (Version 0.9.5.12).
3. RESULTS
3.1. Sociodemographic characteristics
Sociodemographic characteristics are summarised in Table 1. The vast majority of participants were aged between 26 and 65 years (79%), 13.4% were <25 years, 7.5% were >65 years, and 74.3% were women. In all, 77.4% of the participants reported being confined the majority of the time (i.e. no daily business travel). Only 11% of responders changed their place of residence during the lockdown. In all, 45% of the participants lived in a large‐sized city, 29% in a medium‐sized city, and 26% in a rural area or village. In all, 8.7% of the participants did not work, 45.3% teleworked, and 31% were present at work.
TABLE 1.
Sociodemographic characteristics
| N (%) | |
|---|---|
| Age, years | |
| <18 | 7 (1.0) |
| 18–25 | 202 (12.4) |
| 26–45 | 787 (48.4) |
| 46–65 | 497 (30.6) |
| >65 | 122 (7.5) |
| Gender | |
| Female | 1,204 (74.3) |
| Male | 417 (25.7) |
| Main occupation | |
| Farmer | 1 (0) |
| Craftsman, trader, business manager, liberal profession | 141 (8.7) |
| Executive, higher intellectual profession | 544 (33.5) |
| Staff and service personnel | 187 (11.5) |
| Student | 181 (11.1) |
| Worker | 9 (0.5) |
| Intermediate profession, middle management | 213 (13.1) |
| Retired | 132 (8.1) |
| Unemployed | 81 (4.9) |
| Others | 136 (8.4) |
| Lockdown at home | |
| Yes | 1,258 (77.4) |
| No | 367 (22.6) |
| COVID‐19 status | |
| Evocative symptoms | 279 (17.2) |
| Confirmed disease: positive test | 26 (1.6) |
| COVID‐19 hospitalisation | 5 (0.3) |
| Absence of COVID‐19 symptoms | 1,235 (76) |
| Close contact with someone with COVID‐19 | 179 (11) |
| Family/friend with COVID‐19 | 226 (13.9) |
| Family/Friend deceased from COVID‐19 | 32 (2) |
| History of psychiatric disorder | |
| No | 1,367 (84.1) |
| Depression | 107 (6.6) |
| Anxiety disorder | 179 (11) |
| Bipolar disorder | 17 (1) |
| Psychotic disorder | 1 (0.1) |
| Addictive disorder | 26 (1.6) |
| Other | 29 (1.7) |
| History of non‐psychiatric comorbidities | |
| No | 1,246 (76.7) |
| Diabetes | 23 (1.4) |
| High blood pressure | 110 (6.8) |
| Obesity | 66 (4.1) |
| Cancer | 22 (1.4) |
| Sleep apnea syndrome | 44 (2.7) |
| Respiratory disease | 61 (3.8) |
In all, 84.1% of the participants had no history of psychiatric comorbidity, and 6.6% had a depression, 11% had an anxiety disorder, 1% had a bipolar disorder, 0.1% had a psychotic disorder, and 1.6% had an addictive disorder. In all, 76.7% of the participants had no history of a general medical disorder, and there was the presence of diabetes in 1.4%, hypertension in 6.8%, obesity in 4.1%, cancer in 1.4%, sleep apnea syndrome in 2.7%, respiratory disease in 3.8%, and other pathology in 3.1%.
3.2. Behavioural rhythms and life habits
3.2.1. Comparison of behavioural rhythms and life habits between before and during lockdown
A majority (56%) of the participants reported having a poorer sleep quality during the lockdown compared to the period before the lockdown (Figure 1a). In all, 30% of the participants reported sleeping less during the lockdown compared to before and 33% indicated that they slept more during the lockdown (Figure 1b). Many of the participants (48%) reported having less regular sleep schedules during the lockdown than before (Figure 1c).
FIGURE 1.
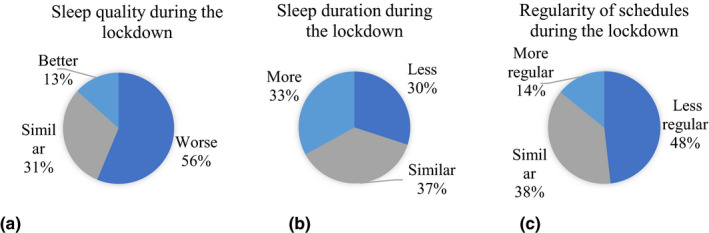
Changes in sleep quality, sleep duration and regularity of schedules during the lockdown. (a) Changes in sleep quality during the lockdown (percentage of individuals). (b) Changes of sleep duration during the lockdown (percentage of individuals). (c) Changes in the regularity of schedules during the lockdown (percentage of individuals) [Colour figure can be viewed at wileyonlinelibrary.com]
A total of 54% of the participants reported going to bed later during the lockdown (Figure 2a). Regarding those who did change their bedtime, the delay was 1–3 hr in 68% and >3 hr in 11%, constituting a marked phase delay pattern (Figure 2b). In all, 57% of the participants reported getting up later during the lockdown (Figure 2a), and the delay was 1–3 hr in 59% and >3 hr in 9% (Figure 2b).
FIGURE 2.
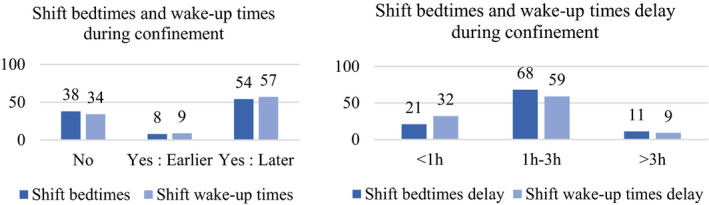
(a) Shift bedtimes and wake‐up times during confinement (percentage of individuals). (b) Shift bedtimes and wake‐up times delay during the lockdown (percentage of individuals) [Colour figure can be viewed at wileyonlinelibrary.com]
We compared the sleep quality between before and during the lockdown in the different age groups using the sleep quality scale from 1 to 10 described above. Globally all age groups altered their sleep quality during the lockdown (18–25 years: Spearman’s rho = 0.206, p = 0.003; 26–45 years: Spearman’s rho = 0.414, p < 0.001; 46–65 years: Spearman’s rho = 0.467, p < 0.001; >65 years: Spearman’s rho = 0.733, p < 0.001), except individuals aged <18 years (Spearman’s rho = 0.119, p = 0.650; Table 2).
TABLE 2.
Sleep quality differences between before and after lockdown by age group: descriptive variables and Spearman’s correlation
| Age, years | Sleep quality before the lockdown, mean (SD) | Sleep quality during the lockdown, mean (SD) | Spearman’s rho | p |
|---|---|---|---|---|
| <18 | 7.12 (1.62) | 7.76 (1.39) | 0.119 | 0.650 |
| 18–25 | 7.14 (1.68) | 5.97 (2.14) | 0.206 | 0.003 |
| 26–45 | 7.30 (1.66) | 5.96 (2.17) | 0.414 | <0.001 |
| 46–65 | 7.15 (1.73) | 6.04 (2.19) | 0.467 | <0.001 |
| >65 | 7.14 (1.92) | 6.57 (2.23) | 0.733 | <0.001 |
Bold values indicate a statistically significant difference with p < 0.05.
We also compared sleep quality by gender. Both men and women had poorer sleep quality during lockdown compared to before (men: Spearman’s rho = 0.478, p < 0.001; women: Spearman’s rho = 0.414, p < 0.001; Table 3). But the results showed a poorer sleep quality in women than in men during lockdown (t = 227483; p = 0.004), while there was no significant difference before lockdown (t = 245943, p = 0.528; Table 3).
TABLE 3.
Sleep quality by gender: descriptive variables, Mann‐Whitney U test and Spearman’s correlation
| Sleep quality before the lockdown, mean (SD) | Sleep quality during the lockdown, mean (SD) | Spearman’s rho (before versus during the lockdown) | p | |
|---|---|---|---|---|
| Male | 7.24 (1.69) | 6.27 (2.23) | 0.478 | <0.001 |
| Female | 7.22 (1.71) | 5.97 (2.16) | 0.414 | <0.001 |
| Mann‐Whitney U test (male versus female) | 245943 | 227483 | ||
| p | 0.528 | 0.004 |
Bold values indicate a statistically significant difference with p < 0.05.
We then compared sleep quality, sleep duration and sleep schedule regularity between before and during the lockdown and we found a significant decrease in sleep quality (Spearman’s rho = 0.429, p < 0.001), an increase in sleep duration (Spearman’s rho = 0.363, p < 0.001) and less regular sleep schedules during lockdown (Spearman’s rho = 0.379, p < 0.001; Table 4).
TABLE 4.
Comparisons before and during the full lockdown regarding sleep quality, sleep duration, sleep schedule regularity, physical activity, natural light exposure, and screen exposure
| Variable | Before the lockdown | During the lockdown | chi‐square or Spearman’s rho | p | Estimatea (95% CI) | p b |
|---|---|---|---|---|---|---|
| Sleep quality, mean (SD) | 7.22 (1.70) | 6.05 (2.18) | 0.429 | <0.001 | −0.313 (−0.352, −0.275) | <0.001 |
| Sleep duration, mean (SD) | 6.66 (0.863) | 6.71 (1.16) | 0.363 | <0.001 | 0.0482 (−0.0212, 0.118) | 0.173 |
| Sleep schedule regularity, mean (SD) | 7.35 (1.95) | 6.30 (2.34) | 0.379 | <0.001 | −0.235 (−0.269, −0.2) | <0.001 |
| Physical activity (weekly), n (%) | ||||||
| None | 254 (15.6) | 390 (24) | 127 | <0.001 | −0.279 (−0.354, −0.205) | <0.001 |
| <1 hr | 299 (18.4) | 419 (25.8) | ||||
| 1−4 hr | 835 (51.4) | 588 (36.2) | ||||
| >4 hr | 237 (14.6) | 228 (14) | ||||
| Natural light exposure, n (%) | ||||||
| Morning | 1392 (85.7) | 1221 (75.1) | 121 | <0.001 | −0.696 (−0.876, −0.515) | <0.001 |
| Evening | 1475 (90.8) | 1375 (84.6) | 102 | <0.001 | −0.584 (−0.801, −0.367) | <0.001 |
| Screen exposure in the morning, n (%) | ||||||
| None | 235 (14.5) | 128 (7.9) | 545 | <0.001 | 0.408 (0.349, 0.466) | <0.001 |
| <30 min | 560 (34.5) | 299 (18.4) | ||||
| 30 min−1 hr | 363 (22.3) | 375 (23.1) | ||||
| 1−3 hr | 249 (15.3) | 426 (26.2) | ||||
| >3 hr | 218 (13.4) | 397 (24.2) | ||||
| Screen exposure in the evening, n (%) | ||||||
| None | 9 (0.6) | 13 (0.8) | 601 | <0.001 | 0.807 (0.708, 0.906) | <0.001 |
| <30 min | 101 (6.2) | 31 (1.9) | ||||
| 30 min−1 hr | 363 (22.3) | 145 (8.9) | ||||
| 1−3 hr | 855 (52.6) | 709 (43.6) | ||||
| >3 hr | 297 (18.3) | 727 (44.7) | ||||
Bold values indicate a statistically significant difference with p < 0.05.
Estimate represent the log odds of “Before the lockdown” versus “During the lockdown”.
p from multivariate analyses (binomial logistic regression) including age, gender, and main occupation.
The distribution of the amount of physical activity was significantly different before and during the lockdown (chi‐square = 127, p < 0.001). Indeed, there was a tendency to decrease the amount of physical activity per week during the lockdown (Table 4, Figure S1).
We also found significant differences between before and during lockdown in terms of exposure to natural light in the morning (chi‐square = 121, p < 0.001) and evening (chi‐square = 102, p < 0.001). Indeed, during lockdown there was a decrease in the number of participants exposed to natural light, both during the morning and evening hours (Table 4, Figure S2).
Screen exposure times were also significantly different before and during the lockdown, both during the morning (chi‐square = 545, p < 0.001) and the evening (chi‐square = 601, p < 0.001). For screen exposure during the morning, there was a major increase in the proportion of participants exposed to the screens for >1 hr, i.e. 50.4% of the participants during lockdown versus 28.7% before lockdown. Thus, overall, the participants were exposed more to screens in the morning during lockdown. For exposure to screens during the evening, there was a considerable increase in the percentage of participants having increased their time spent in front of the screen (Table 4, Figure S3).
All univariate associations, comparing before and during the lockdown, remained significant in multivariate analyses including age, gender and main occupation, except for the sleep duration (Table 4). So, associations with sleep quality, sleep schedule regularity, physical activity, natural light exposure, and screen exposure all remained significant (Table 4).
For the regularity of meals during the lockdown, 31% reported less regularity and 27% reported better regularity (Figure 3a). For the impact of the lockdown on weight, 26% reported weight loss and 41% weight gain (Figure 3b).
FIGURE 3.
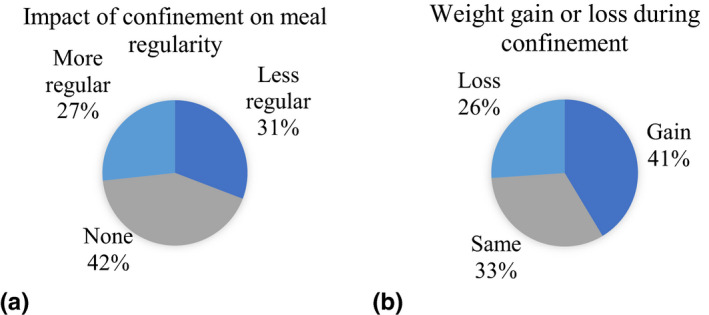
(a) Impact of confinement on meal regularity (percentage of individuals). (b) Weight gain or loss during confinement (percentage of individuals) [Colour figure can be viewed at wileyonlinelibrary.com]
For substance use, 49% did not use any, 41.3% used alcohol, 15.2% tobacco, 1.6% cannabis, 0.1% cocaine, 0.1% amphetamines, and 0.8% opiates (multiple choice). In all, 24% of the participants reported an increase in substance use during the lockdown (Figure S4).
For online gambling and video games, 3% of the participants reported online gambling, which was an increase for 31% of them. In all, 27% of individuals reported playing video games, and 35% of them declared an increase between 1 and 3 hr/day (Figure S5).
3.2.2. Assessment of mood, anxiety, trauma, and sleep symptoms during the lockdown
During the lockdown, the average HADS‐A was 7.77, the average HADS‐D was 4.91, and the average total HADS was 12.7. The HADS‐A score was ≥11 (in favour of an anxiety disorder) in 24% of the participants and the HADS‐D score was ≥11 (in favour of a depressive disorder) in 8%. The average PCL‐5 scale was 14. The PCL‐5 scale was at least equal to 38 in 6.5% of the participants, suggesting the presence of PTSD. For the ISI score, 49% of the participants did not have significant insomnia symptoms (i.e. a score between 0 and 7); 31% had a score between 8 and 14, suggesting mild insomnia; 17% had a score between 15 and 21, suggesting moderate insomnia; and 3% had a score between 22 and 28, in favour of severe insomnia. The average ISI score was 8.59. Thus, half of the study population (51%) had a score in favour of at least mild insomnia. The PSQI score assessing sleep quality was >5 in 58.1% of the participants, indicating sleep disturbances. The average PSQI score was 7.03 for all the participants. The ESS score was ≥11 in 22% of the participants, reflecting pathological sleepiness, and the mean ESS score was 7.06 (Table 5, Figure S6).
TABLE 5.
Scales: Hospital Anxiety and Depression Scale (HADS), Post‐traumatic stress disorder CheckList‐5 (PCL‐5), Insomnia Severity Index (ISI), Pittsburgh Sleep Quality Index (PSQI), and Epworth Sleepiness Scale (ESS)
| Scale | Score, mean (SD) | N (%) |
|---|---|---|
| HADS‐A | 7.77 (3.89) | |
| HADS‐D | 4.91 (3.63) | |
| HADS | 12.7 (6.63) | |
| HADS‐A ≥ 11 | 394 (24.2) | |
| HADS‐D ≥ 11 | 136 (8.4) | |
| PCL‐5 | 14 (12.9) | |
| PCL‐5 score ≥38 | 105 (6.5) | |
| ISI | 8.59 (6.27) | |
| ISI score 0–7 (no insomnia) | 803 (49.4) | |
| ISI score 8–14 (mild insomnia) | 497 (30.6) | |
| ISI score15–21 (moderate insomnia) | 275 (16.9) | |
| ISI score 22–28 (severe insomnia) | 50 (3.1) | |
| PSQI | 7.03 (3.99) | |
| PSQI score >5 | 944 (58.1) | |
| ESS | 7.06 (4.48) | |
| ESS score ≥11 | 351 (21.6) |
4. DISCUSSION
4.1. Comparison of these results with the existing scientific literature
The gender effect in sampling (74.3% of women) was similar to previous studies: 73.1% (Franceschini et al., 2020), 72% (Alfonsi et al., 2021), 71% (Gorgoni et al., 2021) and 70.9% (Duran & Erkin, 2021). The proportion of participants with poor sleep quality, as assessed by the PSQI (58.1%) strictly reflects what has been reported by others: 55.3% (Franceschini et al., 2020), 51% (Alfonsi et al., 2021), 59% (Gorgoni et al., 2021), 55.1% (Duran & Erkin, 2021), 52.4% (Cellini, Canale, Mioni, & Costa, 2020), 57.1% (Casagrande, Favieri, Tambelli, & Forte, 2020), but it does not correspond to China’s data from to February 18 to 25 (36.38%; Zhao, Lan, Li, & Yang, 2021). The mean score on the ISI, which was 8.59 in our present study, was similar to the score found in another French study, which was 9.2 (Kokou‐Kpolou, Megalakaki, Laimou, & Kousouri, 2020). The irregular rhythms and phase delay interestingly confirmed observations from previous studies (Innocenti, Puzella, Mogavero, Bruni, & Ferri, 2020; Li et al., 2020; Targa, Benítez, & Moncusí‐Moix, 2020), and the increase in time spent in front of screens confirms observations from an American study, which found an increase for 77.7% of participants. (Bigalke, Greenlund, & Carter, 2020). In addition, the lockdown effect on sleep quality and duration varied between individuals, and other studies also reported this non‐uniform effect too (Alfonsi et al., 2021; Kocevska, Blanken, Van Someren, & Rösler, 2020). Finally, we observed anxious (HADS‐A score ≥11) and depressive (HADS‐D score ≥11) symptoms in 24.2% and 8.4% of the participants, compared with 8.7% and 8.8% in another French study (Peretti‐Watel, Alleaume, Léger, Beck, & Verger, 2020), 34.3% and 27.8% in Italy (Marelli et al., 2020), and 35.1% and 20.1% in China (Huang & Zhao, 2020). Thus, the prevalence of depressive and anxiety symptoms appears to be higher in Italy and China than in France.
4.2. Underlying possible mechanisms
In the present study, we observed the consequences of the lockdown due to COVID‐19 on both biological, behavioural daily rhythms, and emotions. The lockdown caused a disruption in lifestyle habits and significant stress, profoundly affecting sleep–wake rhythms. We could observe its impact on the main synchroniser of the circadian rhythm, which is light exposure. We have indeed observed a drastic decrease in natural light exposure. Beyond its circadian effect, light also has a direct alerting effect, independent of the circadian system (Cajochen, 2007); and we observed an increase in exposure to screens, and therefore to blue light during the evening, which is responsible for an inhibition of melatonin secretion, thus further delaying sleep phase. Other external circadian rhythms synchronisers were similarly impacted, e.g. the loss of meal rhythms regularity, decreased physical activity, decreased social contact, and lack of daily work transit. The loss of these external synchronisers has a deleterious impact on wakefulness and sleep patterns due to the lack of synchronisation of the circadian rhythm with the environment. This leads to an alteration in sleep, which was reported in more than half of the participants and objectified by the PSQI scale in 58.1% of the participants, with a decreased quality of wakefulness, with pathological daytime sleepiness reported in 22%. Women’s sleep was more impaired during lockdown than men’s, and it could be explained by the shorter circadian period in women, which would make them more vulnerable to insomnia (Eastman, Tomaka, & Crowley, 2017). The participants also reported more irregular meals (31%) and weight gain (41%), and it has been shown that irregular mealtimes can lead to food clock disruption, a condition that favours gaining weight, especially in case of decreased physical activity (Engin, 2017). In addition, the circadian system modulates arousal, neurocognitive and affective functions and its desynchronisation can have important consequences on complex daytime functions such as learning, memory, and emotions (Wright, Lowry, & LeBourgeois, 2012). Indeed, sleep has an important role in immunity (Prather, 2019), mood (Stephenson, Schroder, Bertschy, & Bourgin, 2012), and cognitive functions such as judgment and concentration (Cassé‐Perrot et al., 2016), but also in emotional processing (Altena et al., 2016), and the consequences of their alterations are crucial to consider in this context, particularly as a reduction in sleep time can have a deleterious impact on vulnerability to infections (Irwin, 2015) and on the occurrence of psychiatric and addictive disorders (Geoffroy, Tebeka, Blanco, Dubertret, & Le Strat, 2020). Several works showed that insomnia, sleep loss, and altered circadian rhythms may induce a state of chronic inflammation by enhancing neuroinflammation, both directly and indirectly, via microglia and astrocytes activation (Palagini et al., 2021). Even a few days of sleep deprivation or circadian shift in young healthy subjects is sufficient to increase levels of pro‐inflammatory cytokines, sympathetic tone, blood pressure, and evening cortisol levels (McEwen & Karatsoreos, 2015). These sleep disturbances act as neurobiological stressors and induce the accumulation of neurotoxic proteins, oxidative stress, and also a deficit in neuroprotection, hence contributing to neurodegeneration and neuroprogression (Palagini et al., 2021). Finally, a two‐way relationship exist between stress and sleep, and so may be responsible for persistent insomnia (McEwen & Karatsoreos, 2015) but also neurotoxicity and neuroprogression (Palagini et al., 2021).
The implementation of behavioural sleep measures appears to be essential in this context, and recommendations from sleep experts have been widely distributed (https://www.afpbn.org/‐les‐experts‐du‐sommeil‐vous‐conseillent/) to prevent, in particular, the appearance or chronicisation of insomnia or other sleep disorders (Geoffroy, Bénard, & Amad, 2020). These recommendations consist of respecting regular sleeping and eating schedules, favouring exposure to natural light at the beginning of the day, avoiding exposure to screens and blue light during the evening, and maintaining a favourable sleeping environment, among others. Finally, we emphasised several possible links between the different studied parameters and observed how they could affect each other, directly or indirectly. Further studies are needed to better disentangle these multiple interactions between the sleep–wake cycle, circadian rhythms, stress, emotional regulation, mood, light exposure, lifestyle habits, physical activity, social contacts, and immunity.
4.3. Limitations and strengths
The limitations of the present study reside mainly in the fact that it was conducted as a self‐administered survey with no medical assessments. In addition, no sleep measurement tools were used, such as actigraphy. In addition, it was conducted on a voluntary basis, which may have induced a bias in the subjects’ selection.
Regarding the strengths of the present study, we note the participation of a large number of subjects, allowing a better representation of the general population. In addition, it was conducted during the last week of the lockdown, which made it possible to consider the whole lockdown period. In addition, standardised scales were used for the analysis of anxiety, depression, insomnia, sleep quality, somnolence and PTSD, thus providing access to more accurate validated data.
5. CONCLUSION
The French 2‐month complete lockdown in March–May 2020 highlighted important changes in behavioural circadian rhythms and lifestyle habits, with a severe impact on the sleep quality, sleep–wake circadian rhythm, and sleep behaviours. These alterations were similarly reported in several other European countries, e.g. Italy and Spain, as well as in China and in the United States, with some variances probably due to different lockdown measures and public health strategies. The present study is a call for the implementation of public health strategies for the prevention and care of sleep–wake cycles during lockdown in order to preserve cognitive and mental health of the population.
CONFLICT OF INTEREST
Authors have no conflicts of interest related to this work.
AUTHOR CONTRIBUTIONS
LB and PAG designed the study analysis, wrote the first draft of the manuscript, made the tables and figures, and made the statistical analyses. LB, CS, PB, JM, YA, MPdO, ML, PAG participated in the results interpretation, the manuscript redaction and approved the final version of the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We thank all participants of this survey, the AP‐HP‐Enquetes MissionWeb team, the SOPSY section (sleep and biological rhythms in psychiatry) of the French Association of Biological Psychiatry and Neuropsychopharmacology (AFPBN) and the French Society for Sleep Research and Medicine (SFRMS).
Bertrand, L. , Schröder, C. , Bourgin, P. , Maruani, J. , Atoui, Y. , d’Ortho, M.‐P. , Lejoyeux, M. , & Geoffroy, P. A. (2022). Sleep and circadian rhythm characteristics in individuals from the general population during the French COVID‐19 full lockdown. Journal of Sleep Research, 31, e13480. 10.1111/jsr.13480
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Contributor Information
Léa Bertrand, Email: lea.h.bertrand@outlook.fr.
Pierre A. Geoffroy, Email: pierre.a.geoffroy@gmail.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Alfonsi, V. , Gorgoni, M. , Scarpelli, S. , Zivi, P. , Sdoia, S. , Mari, E. , … De Gennaro, L. (2021). COVID‐19 lockdown and poor sleep quality: Not the whole story. Journal of Sleep Research, Online ahead of print, e13368. 10.1111/jsr.13368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altena, E. , Micoulaud‐Franchi, J.‐A. , Geoffroy, P.‐A. , Sanz‐Arigita, E. , Bioulac, S. , & Philip, P. (2016). The bidirectional relation between emotional reactivity and sleep: From disruption to recovery. Behavioral Neuroscience, 130(3), 336–350. 10.1037/bne0000128 [DOI] [PubMed] [Google Scholar]
- Baglioni, C. , Battagliese, G. , Feige, B. , Spiegelhalder, K. , Nissen, C. , Voderholzer, U. , … Riemann, D. (2011). Insomnia as a predictor of depression: A meta‐analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders, 135(1‐3), 10–19. 10.1016/j.jad.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Bastien, C. (2001). Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine, 2, 297–307. 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- Bigalke, J. A. , Greenlund, I. M. , & Carter, J. R. (2020). Sex differences in self‐report anxiety and sleep quality during COVID‐19 stay‐at‐home orders. Biology of Sex Differences, 11, 56. 10.1186/s13293-020-00333-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins, C. A. , Weathers, F. W. , Davis, M. T. , Witte, T. K. , & Domino, J. L. (2015). The posttraumatic stress disorder checklist for DSM‐5 (PCL‐5): Development and initial psychometric evaluation. Journal of Traumatic Stress, 28, 489–498. [DOI] [PubMed] [Google Scholar]
- Buysse, D. J. , Reynolds, C. F. , Monk, T. H. , Berman, S. R. , & Kupfer, D. J. (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Cajochen, C. (2007). Alerting effects of light. Sleep Medicine Reviews, 11, 453–464. 10.1016/j.smrv.2007.07.009 [DOI] [PubMed] [Google Scholar]
- Casagrande, M. , Favieri, F. , Tambelli, R. , & Forte, G. (2020). The enemy who sealed the world: effects quarantine due to the COVID‐19 on sleep quality, anxiety, and psychological distress in the Italian population. Sleep Medicine, 75, 12–20. 10.1016/j.sleep.2020.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassé‐Perrot, C. , Lanteaume, L. , Deguil, J. , Bordet, R. , Auffret, A. , Otten, L. , … Micallef, J. (2016). Neurobehavioral and cognitive changes induced by sleep deprivation in healthy volunteers. CNS & Neurological Disorders: Drug Targets, 15(7), 777–801. 10.2174/1871527315666160518125156 [DOI] [PubMed] [Google Scholar]
- Cellini, N. , Canale, N. , Mioni, G. , & Costa, S. (2020). Changes in sleep pattern, sense of time and digital media use during COVID‐19 lockdown in Italy. Journal of Sleep Research, 29(4), 13074. 10.1111/jsr.13074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran, S. , & Erkin, Ö. (2021). Psychologic distress and sleep quality among adults in Turkey during the COVID‐19 pandemic. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 107, 110254. 10.1016/j.pnpbp.2021.110254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman, C. I. , Tomaka, V. A. , & Crowley, S. J. (2017). Sex and ancestry determine the free‐running circadian period. Journal of Sleep Research, 26(5), 547–550. 10.1111/jsr.12521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin, A. (2017). Circadian rhythms in diet‐induced obesity. Advances in Experimental Medicine and Biology, 960, 19–52. [DOI] [PubMed] [Google Scholar]
- Franceschini, C. , Musetti, A. , Zenesini, C. , Palagini, L. , Scarpelli, S. , Quattropani, M. C. , … Castelnuovo, G. (2020). Poor sleep quality and its consequences on mental health during the COVID‐19 lockdown in Italy. Frontiers in Psychology, 11, 574475. 10.3389/fpsyg.2020.574475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy, P.A. , Bénard, V. , Amad, A. , Royant‐Parola, S. , Poirot, I. , Guichard, K. , … Schroder, C. (2020). Conseils d’experts du sommeil pour bien dormer et garder le rythme chez les adultes et les enfants en cette période de confinement liée au CoVid‐19. Médecine Sommeil. [Google Scholar]
- Geoffroy, P. A. , Le Goanvic, V. , Sabbagh, O. , Richoux, C. , Weinstein, A. , Dufayet, G. , & Lejoyeux, M. (2020). Psychological support system for hospital workers during the Covid‐19 outbreak: Rapid design and implementation of the Covid‐Psy hotline. Frontiers in Psychiatry, 11, 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy, P. A. , Tebeka, S. , Blanco, C. , Dubertret, C. , & Le Strat, Y. (2020). Shorter and longer durations of sleep are associated with an increased twelve‐month prevalence of psychiatric and substance use disorders: Findings from a nationally representative survey of US adults (NESARC‐III). Journal of Psychiatric Research, 124, 34–41. 10.1016/j.jpsychires.2020.02.018 [DOI] [PubMed] [Google Scholar]
- Gorgoni, M. , Scarpelli, S. , Alfonsi, V. , Annarumma, L. , Cordone, S. , Stravolo, S. , & De Gennaro, L. (2021). Pandemic dreams: quantitative and qualitative features of the oneiric activity during the lockdown due to COVID‐19 in Italy. Sleep Medicine, 81, 20–32. 10.1016/j.sleep.2021.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. , & Zhao, N. (2020). Generalized anxiety disorder, depressive symptoms and sleep quality during COVID‐19 outbreak in China: a web‐based cross‐sectional survey. Psychiatry Research, 288, 112954. 10.1016/j.psychres.2020.112954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti, P. , Puzella, A. , Mogavero, M. P. , Bruni, O. , & Ferri, R. (2020). Letter to editor: CoVID‐19 pandemic and sleep disorders‐a web survey in Italy. Neurological Sciences, 41(8), 2021–2022. 10.1007/s10072-020-04523-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin, M. R. (2015). Why sleep is important for health: a psychoneuroimmunology perspective. Annual Review of Psychology, 66(1), 143–172. 10.1146/annurev-psych-010213-115205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns, M. W. (1991). A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep, 14(6), 540–545. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- Kocevska, D. , Blanken, T. F. , Van Someren, E. J. W. , & Rösler, L. (2020). Sleep quality during the COVID‐19 pandemic: not one size fits all. Sleep Medicine, 76, 86–88. 10.1016/j.sleep.2020.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokou‐Kpolou, C. K. , Megalakaki, O. , Laimou, D. , & Kousouri, M. (2020). Insomnia during COVID‐19 pandemic and lockdown: Prevalence, severity, and associated risk factors in French population. Psychiatry Research, 290, 113128. 10.1016/j.psychres.2020.113128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Qin, Q. , Sun, Q. , Sanford, L. D. , Vgontzas, A. N. , & Tang, X. (2020). Insomnia and psychological reactions during the COVID‐19 outbreak in China. Journal of Clinical Sleep Medicine, 16(8), 1417–1418. 10.5664/jcsm.8524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Martire, V. , Caruso, D. , Palagini, L. , Zoccoli, G. , & Bastianini, S. (2020). Stress & sleep: A relationship lasting a lifetime. Neuroscience and Biobehavioral Reviews, 117, 65–77. [DOI] [PubMed] [Google Scholar]
- Marelli, S. , Castelnuovo, A. , Somma, A. , Castronovo, V. , Mombelli, S. , Bottoni, D. , … Ferini‐Strambi, L. (2020). Impact of COVID‐19 lockdown on sleep quality in university students and administration staff. Journal of Neurology, 268(1), 8–15. 10.1007/s00415-020-10056-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen, B. S. , & Karatsoreos, I. N. (2015). Sleep Deprivation and Circadian Disruption. Sleep Medicine Clinics, 10(1), 1–10. 10.1016/j.jsmc.2014.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palagini, L. , Geoffroy, P. A. , Miniati, M. , Perugi, G. , Biggio, G. , Marazziti, D. , & Riemann, D. (2021). Insomnia, sleep loss, and circadian sleep disturbances in mood disorders: a pathway toward neurodegeneration and neuroprogression? A theoretical review. CNS Spectrums, online ahead of print, 1–11. 10.1017/S1092852921000018 [DOI] [PubMed] [Google Scholar]
- Peretti‐Watel, P. , Alleaume, C. , Léger, D. , Beck, F. , & Verger, P. (2020). Anxiety, depression and sleep problems: a second wave of COVID‐19. General Psychiatry, 33(5), e100299. 10.1136/gpsych-2020-100299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather, A. A. C. (2019). Sleep, stress, and immunity. In Grandner M. A. (Ed.), Sleep and Health (Vol. 24, pp. 319–330). Cambridge, MA: Academic Press. [Google Scholar]
- Riemann, D. , Nissen, C. , Palagini, L. , Otte, A. , Perlis, M. L. , & Spiegelhalder, K. (2015). The neurobiology, investigation, and treatment of chronic insomnia. The Lancet Neurology, 14(5), 547–558. 10.1016/S1474-4422(15)00021-6 [DOI] [PubMed] [Google Scholar]
- Snaith, R. P. (2003). The hospital anxiety and depression scale. Health and Quality of Life Outcomes, 1, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson, K. M. , Schroder, C. M. , Bertschy, G. , & Bourgin, P. (2012). Complex interaction of circadian and non‐circadian effects of light on mood: shedding new light on an old story. Sleep Medicine Reviews, 16(5), 445–454. 10.1016/j.smrv.2011.09.002 [DOI] [PubMed] [Google Scholar]
- Targa, A. D. S. , Benítez, I. D. , Moncusí‐Moix, A. , Arguimbau, M. , de Batlle, J. , Dalmases, M. , & Barbé, F. (2020). Decrease in sleep quality during COVID‐19 outbreak. Sleep Breath, 25(2), 1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Horby, P. W. , Hayden, F. G. , & Gao, G. F. (2020). A novel coronavirus outbreak of global health concern. The Lancet, 395(10223), 470–473. 10.1016/S0140-6736(20)30185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Pan, R. , Wan, X. , Tan, Y. , Xu, L. , Ho, C. S. , & Ho, R. C. (2020). Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID‐19) Epidemic among the General Population in China. International Journal of Environmental Research and Public Health, 17(5), 1729. 10.3390/ijerph17051729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz‐Justice, A. , & Benedetti, F. (2020). Perspectives in affective disorders: Clocks and sleep. European Journal of Neuroscience, 51(1), 346–365. 10.1111/ejn.14362 [DOI] [PubMed] [Google Scholar]
- Wright, K. P. , Lowry, C. A. , & LeBourgeois, M. K. (2012). Circadian and wakefulness‐sleep modulation of cognition in humans. Frontiers in Molecular Neuroscience, 5, 50. 10.3389/fnmol.2012.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X. , Lan, M. , Li, H. , & Yang, J. (2021). Perceived stress and sleep quality among the non‐diseased general public in China during the 2019 coronavirus disease: a moderated mediation model. Sleep Medicine, 77, 339–345. 10.1016/j.sleep.2020.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


