Abstract
Coronavirus disease 19 (COVID‐19) is considered a multisystemic disease. Several studies have reported persistent symptoms or late‐onset complications after acute COVID‐19, including post‐COVID‐19 hematological disorders. COVID‐19‐induced coagulopathy, an immunothrombotic state, has been linked to thromboembolic and hemorrhagic events. Late‐onset thrombocytopenia related to immune system dysregulation has also been reported as a rare manifestation post COVID‐19. Close monitoring of laboratory dynamics is considered essential to identify timely abnormal values that need further investigation, providing supportive care whenever indicated. The role of hematologists is essential in terms of the multidisciplinary approach of long COVID‐19. This review summarizes all the available evidence on post‐acute COVID‐19 hematological complications.
1. INTRODUCTION
Coronavirus disease 19 (COVID‐19) pandemic had spread rapidly with devastating consequences worldwide. The mortality rate ranges from 3% to 5%, while approximately 80% of patients hospitalized with COVID‐19 and 60% of those admitted to intensive care units (ICUs) survive. 1 Older people and those with comorbidities are at increased risk of death and complications from COVID‐19. 2 , 3 COVID‐19 survivors might experience multiple organ impairment with a significant impact on their quality of life post recovery. 4 , 5 , 6 Although it is well known that the disease primarily manifests as a respiratory tract infection, several studies have demonstrated that it should be considered a multisystemic disease including cardiovascular impairment, respiratory illness, gastrointestinal disorders, neurological symptoms, as well as hematopoietic and immune system dysregulation. 6 , 7 , 8 , 9 The multisystemic aspects of acute COVID‐19 have been thoroughly evaluated; however, the long‐term complications are still an underexplored area. “Long (haul)‐covid” is used to describe the ongoing effects of COVID‐19 infection, which possibly encompasses different entities; post‐intensive care syndrome, post‐viral fatigue syndrome, and long‐term COVID‐19 syndrome. 10 During COVID‐19 pandemic, micro and macrovascular thrombotic complications have emerged as common clinical complications of the disease, particularly among critically ill and hospitalized patients. 11 , 12 The risk of pulmonary microvascular thrombosis and arterial thrombotic events (infarction, stroke, limb ischemia) is also increased, albeit to a lesser extent than venous thrombosis (up to 17% in a recent meta‐analysis). 13 , 14 , 15 , 16 , 17
Here we comprehensively review the long‐term hematological complications that have been described post COVID‐19 recovery, providing guidance for their prevention and management.
2. METHODS
A comprehensive literature search was performed in PubMed using both controlled vocabulary and free text terms for COVID‐19 and late/long‐term hematologic complications. Updates were performed at regular intervals throughout the review process until September 1, 2021. The search strategy was as follows: (“Hematologic” OR “Hematologic Disease” OR “Blood Disease” OR “Blood Diseases” OR “Blood Hematological Diseases”) AND (“COVID‐19” OR COVID19 OR “COVID 2019” OR “novel coronavirus” OR “SARS‐CoV” or “SARS‐CoV‐2” OR “SARS2” or “2019‐nCoV” OR ncov19 OR ncov‐19 OR “2019‐novel CoV” OR sarscov2 OR sarscov‐2 OR Sars‐coronavirus2 OR Sars‐coronavirus‐2 OR SARS‐like coronavirus* OR coronavirus‐19) AND (complication* OR damag* OR delayed OR after or post* OR survivor* OR following OR secondary OR chronic OR late* OR “new onset” OR long* OR permanent* OR sequence OR persist* OR “follow up” OR “follow‐up”). Articles were initially screened based on title and abstract. Relevant articles were assessed by full‐text reading. All types of studies (case reports, case series, retrospective and prospective studies, and clinical trials) were considered. Two independent reviewers (E.K. and D.F.) screened the literature. All relevant papers reporting on hematological complications related to long COVID‐19 were included. Studies reporting only complications during the acute phase of the disease were excluded. Available evidence is summarized and discussed.
3. POST‐ACUTE COVID‐19 HEMATOLOGICAL COMPLICATIONS
3.1. Thrombotic and hemorrhagic events
COVID‐19‐induced coagulopathy (CIC) is an immunothrombotic state that appears to be more prothrombotic than hemorrhagic (Table 1). 25 The optimal thromboprophylactic practice across the spectrum of the disease has not been established yet, but multiple ongoing randomized controlled trials (RCTs) are addressing this question (Table 2). 26 Individual institutions and professional societies have published interim recommendations to guide anticoagulation strategies. 27 , 28 , 29
TABLE 1.
Representative studies reporting thromboembolic/hemorrhagic manifestations of subacute and/or chronic COVID‐19
| Study | Design | Study population | Type of event | Anticoagulation | Time interval between diagnosis and time of event |
|---|---|---|---|---|---|
| Patell et al. 18 | Retrospective observational |
n = 163 F: 52.2% Obesity: NR |
VTE 2.5% 1 PE 1 ICT 1 thrombosed AV fistula 1 ischemic stroke Hemorrhagic events: 3.7% 2 MB 4 CRNMB |
No |
Median duration to thrombotic event post discharge 23 days (IQR: 12–33) Median duration to event post discharge: 27 days (IQR 16–31 days) |
| Roberts et al. 19 | Retrospective observational |
n = 1877 F: NR Obesity: NR |
VTE n = 9 (11%) 2 proximal DVT 7 PE |
No | Median duration to event post discharge 8 days (IQR, 3–33 days) |
| Engelen et al. 20 | Prospective cohort study Systematic post‐discharge screening |
n = 102 F: NR Obesity: NR |
0 symptomatic events 1 asymptomatic VTE |
8% on prophylactic LMWH | Outpatient follow‐up screening at 6 weeks post discharge |
| Bourguignon et al. 21 | Retrospective cohort study |
n = 140 Discharged from medical ward F: 46% Obesity: NR n = 35 discharged from rehab ward F: 63% Obesity: NR |
0.71% n = 1 PE 0% |
10% on anticoagulation at discharge 29% on anticoagulation at discharge |
Event at 9 days post discharge |
| Huang et al. 22 | Cohort study with systematic screening |
n = 2469 F: 48% n = 390 F: NR Obesity: NR |
Three ischemic strokes one PE (only events requiring re‐admission reported) No asymptomatic VTE in screening |
NR NR |
NR NA |
| Giannis et al. 23 | Prospective registry |
n = 4906 F: 46% Obesity: 18.9% |
n = 76 (1.55%) VTE DVT 44 PE 42 Splanchnic VT 2 n = 84 (1.71%) ATE stroke 22 MI 24 MLE 26 S. embolism 16 n = 85 (1.73%) MB |
12.7% on thromboprophylaxis n = 62 (1.3%) enoxaparin n = 3 (0.06%) UFH n = 180 (3.7%) apixaban n = 336 (6.9%) rivaroxaban |
Outcomes at 90 days post discharge |
| Spyropoulos et al. 24 | Case series |
n = 4 M: 100% Obesity: 0% |
Two large‐vessel ischemic strokes one acute ischemic limb (emboli from aortic thrombosis) one AL STEMI |
No | Median time to event post diagnosis: 72 days |
Abbreviations: AL STEMI, anteriolateral ST‐elevation myocardial infarction; ATE, arterial thromboembolism; CRNMB, clinically relevant non‐major bleed; DVT, deep vein thrombosis; ICT, intracardiac thrombus; MB, major bleeding; MLE, major limb event; PE, pulmonary embolism; S. embolism, systemic embolism; UFH, unfractionated heparin; VTE, venous thromboembolism.
TABLE 2.
Summary of ongoing clinical trials addressing prevention strategies of thromboembolic events at the post‐acute phase of COVID‐19
| Clinical trial title/NCT | Intervention/treatment | Description | Primary outcome |
|---|---|---|---|
|
COVID‐19 Positive Outpatient Thrombosis Prevention in Adults Aged 40–80 ACTIV4 |
Apixaban 2.5 mg apixaban 5 mg Aspirin versus placebo |
Double‐blind placebo‐controlled platform trial to compare the effectiveness of anticoagulation with antiplatelet agents and with placebo to prevent thrombotic events in patients diagnosed with COVID‐19 not admitted to hospital | Hospitalization for cardiovascular/pulmonary events (time frame: 45 days) |
|
COVID‐19 Thrombosis Prevention Trials: Post‐hospital Thromboprophylaxis ACTIV4c |
Apixaban 2.5 mg versus placebo | Adaptive, prospective, randomized platform Phase III trial designed to compare the effectiveness and safety of antithrombotic therapy with no antithrombotic therapy after hospitalization for 48 h or longer for COVID‐19. For Stage 1 of this study, participants will be randomized to either prophylactic anticoagulation or matching placebo for 30 days, and then followed for an additional 60 days after the completion of treatment (total duration of follow‐up 90 days). | Composite outcome of symptomatic deep vein thrombosis, pulmonary embolism, other venous thromboembolism, ischemic stroke, myocardial infarction, other arterial thromboembolism, and all‐cause mortality as measured by hospital records (time frame: 30 days after hospital discharge) |
|
Medically Ill Hospitalized Patients for COVID‐19 THrombosis Extended ProphyLaxis With Rivaroxaban ThErapy THe MICHELLE Trial |
Rivaroxaban 10 mg versus no intervention after discharge | Randomized Phase III trial evaluating the safety and efficacy of rivaroxaban 10 mg OD for 35 ± 4 days versus no intervention after hospital discharge in COVID‐19 patients who were at increased risk for VTE and have received standard parenteral VTE prophylaxis during hospitalization |
Venous thromboembolism (VTE) and VTE related‐death (time frame: at day 35 ± post hospital discharge). A composite efficacy end point of symptomatic VTE, VTE‐related death, and/or VTE detected by mandatory bilateral lower limb venous duplex scan and computed tomography pulmonary angiogram on day 35 ± 4 post hospital discharge |
|
Effect of Anticoagulation Therapy on Clinical Outcomes in COVID‐19 COVID‐PREVENT |
Rivaroxaban versus standard of care | Multicenter, prospective, randomized, Phase II, event‐driven study. Thromboprophylaxis therapy will be given for 28 days up to day 35 post randomization or even longer. If the patient cannot be discharged from the hospital prior to day 35 post randomization, the thromboprophylaxis phase will also start upon hospital discharge, but is then shorter than 28 days, because the study ends at day 60 post randomization | Composite end point of venous thromboembolism (DVT and/or fatal or non‐fatal PE), arterial thromboembolism, new myocardial infarction, non‐hemorrhagic stroke, all‐cause mortality or progression to intubation and invasive ventilation (time frame: 35 days post randomization) |
|
Effect of the Use of Anticoagulant Therapy During Hospitalization and Discharge in Patients With COVID‐19 Infection XACT |
Enoxaparine (prophylactic or therapeutic dose) |
Randomized clinical trial in patients with confirmed COVID‐19 who require hospital treatment and subsequent ambulatory surveillance |
Identifying the benefit of different doses of low‐molecular‐weight heparin (enoxaparin) on ventilatory support time, length of hospital stay, and mortality in patients requiring hospital care for COVID‐19 infection. Comparing oral anticoagulation therapy by administering rivaroxaban 10 mg PO every 24 h on early thrombotic complications |
|
Hamburg Edoxaban for Anticoagulation in COVID‐19 Study (HERO‐19) |
Edoxaban and/or high‐dose LMWH versus low‐dose low‐molecular‐weight heparin or placebo | Prospective, randomized, assessor‐blinded, multicenter, placebo‐controlled, interventional Phase III trial that will investigate whether therapeutic anticoagulation on top of SOC compared to prophylactic anticoagulation as part of SOC can improve objective patient‐relative end points, relevant for prognosis in patients with COVID‐19 |
All‐cause mortality and/or venous/arterial thromboembolism (time frame: 42 days) All‐cause mortality and/or venous/arterial thromboembolism during follow‐up (42 days). Thromboembolisms will be detected by duplex ultrasonography of arms and legs |
|
A Study of Rivaroxaban to Reduce the Risk of Major Venous and Arterial Thrombotic Events, Hospitalization and Death in Medically Ill Outpatients With Acute, Symptomatic Coronavirus Disease 2019 (COVID‐19) Infection PREVENT‐HD trial (NCT04508023) |
Rivaroxaban versus placebo |
Multicenter, randomized, placebo‐controlled, pragmatic Phase III study investigating the efficacy and safety of rivaroxaban to reduce the risk of major venous and arterial thrombotic events, hospitalization, and eeath in medically ill outpatients with acute, symptomatic COVID‐19 infection. |
Time to first occurrence of a composite end point of symptomatic venous thromboembolism (VTE), myocardial infarction (MI), ischemic stroke, acute limb ischemia, noncentral nervous system (non‐CNS) systemic embolization, all‐cause hospitalization, and all‐cause mortality will be assessed (time frame up to day 35) |
|
Protective Effect of Aspirin on COVID‐19 Patients (PEAC) |
Aspirin 100 mg | Randomized, parallel‐assignment, open label, Phase II–III trial, assessing the early use of aspirin in COVID‐19 patients, which has the effects of inhibiting virus replication, antiplatelet aggregation, anti‐inflammatory and anti‐lung injury, is expected to reduce the incidence of severe and critical patients, shorten the length of hospital duration, and reduce the incidence of cardiovascular complications. | Clinical recovery time (TTCR) (time frame: not more than 14 days) |
|
Aggrenox To Treat Acute Covid‐19 (ATTAC‐19) ATTAC‐19 |
Dipyridamole ER 200 mg/aspirin 25 mg orally/enterally AND SOC vs. SOC | Randomized controlled trial to evaluate the outcomes with aggrenox in patients with SARS‐CoV‐2 infection | Change in composite COVID ordinal scale at day 15 |
With regard to pathophysiology, SARS‐CoV‐2 invades vascular endothelial cells directly and induces a suitable environment for the migration and aggregation of immune cells via negative regulation of the ACE‐2 receptor activity and angiotensin II accumulation. 30 , 31 Proinflammatory and procoagulant cytokine release and endothelial injury lead to the subsequent activation of the coagulation cascade, impaired fibrinolysis and thrombin generation, and modification of the hemostatic environment. 31 , 32 , 33 , 34 Heparin has demonstrated significant effects in reducing the procoagulant environment and the relevant vascular inflammation. 30 The hyperinflammatory response induces endothelitis, 30 , 35 but it is not clear how long endothelitis can persist in the convalescent phase of the disease. It has been postulated that low‐grade endothelial activation and subsequent downstream signaling of prothrombotic pathways and low‐level inflammation may persist after the acute phase of the disease. 36 The risk of thrombotic complications in the post‐acute COVID‐19 phase is probably linked to the duration and severity of a hyperinflammatory state, although how long this persists is unknown. B cells may also play a role in long COVID‐19 autoimmunity. Antiphospholipid antibodies were detected in 52% of patients (24% anti‐phosphatidylserine/prothrombin IgG, 18%, anti‐phosphatidylserine/prothrombin IgG, and 23% anti‐cardiolipin IgM) in one hospitalized cohort, and there was an association with increased neutrophil activity and more severe outcomes. 37 , 38
It is hypothesized that endothelial cell (EC) pathway activation may contribute in long COVID‐19 pathogenesis (Figure 1). In a recent study, 50 patients were evaluated in a median of 68 days post COVID‐19. EC activation, thrombin generation, and NETosis compounds were assessed. Thrombin generation assays demonstrated shorter lag times in combination with increased endogenous thrombin potential and peak thrombin. EC biomarkers such as VWF:Ag, VWF propeptide (VWFpp), and Factor VIII (FVIII:C) were significantly increased as measured in the convalescent COVID‐19 plasma. Plasma‐soluble thrombomodulin (sTM) levels were also significantly elevated. Patients with advanced age or suffering from comorbidities, or those who required hospitalization, revealed sustained endotheliopathy. These findings strongly indicate that the presence of endotheliopathy might be involved in the underlying pathogenesis of long COVID‐19. 39
FIGURE 1.
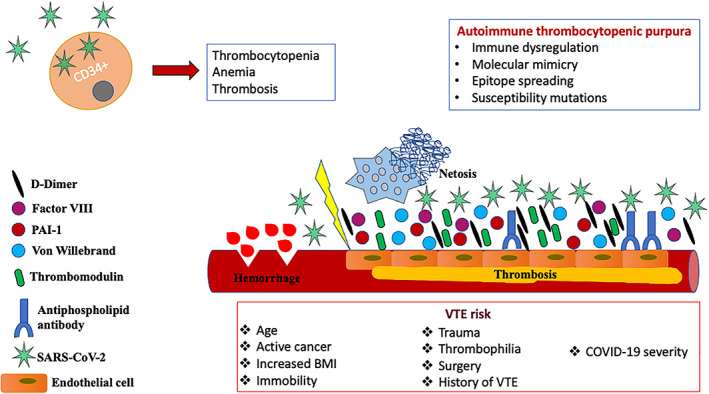
Schematic overview of late‐onset hematologic complications post COVID‐19 (see text for details) [Color figure can be viewed at wileyonlinelibrary.com]
A recent study showed that SARS‐CoV‐2 can cause potential breakdown of the hematopoietic stem cells differentiation, leading to acute anemia of inflammation, thrombocytopenia, and thrombosis (Figure 1). The Sars‐CoV‐2 RNA enters ex vivo the primary CD34+ HSCs, inducing the formation of defective megakaryocytic and erythroid cells. Viral particles from affected CD34+ HSCs confer the greatest risk for severe thrombosis related to Sars‐CoV‐2 infection. 40
Epidemiological data examining the risk of thrombosis post hospital discharge are limited, but the overall the risk is similar to that of patients hospitalized with acute medical illnesses other than COVID‐19. Based on data from studies with variability in outcome ascertainment, the rate of venous thromboembolism (VTE) in the post‐acute phase of COVID‐19 disease is well below 5% (Table 1). 41
In a single‐center retrospective report of 163 patients who did not receive thromboprophylaxis post discharge, the cumulative incidence of thrombosis (segmental pulmonary embolism, intracardiac thrombus, ischemic stroke, VTE) at 30 days was 2.5%, for VTE alone 0.6% (median time to event 23 days), and for hemorrhage 3.7%. 18 Similar rates are reported in other small retrospective studies. 19
One prospective study assessed 146 patients (28% received thromboprophylaxis) at 6 weeks post discharge; one asymptomatic deep vein thrombosis (DVT) (0.7%) and one symptomatic pulmonary embolism (0.7%) were diagnosed. No bleedings were reported. At 6 weeks, 32% of ward patients and 42% of ICU patients had elevated D‐dimer levels. 20 In another outpatient cohort study of 175 patients, at 60 days the incidence of arterial events was 0% and venous events 0.71% (1 PE at 9 days post discharge), and no bleedings were reported. 21 Similar rates were also reported from a Chinese study of post‐acute COVID‐19 . 22 In another study, the VTE rate was 1.6% 23 among 4906 individuals following hospital discharge (VTE rate was lower among patients on thromboprophylaxis). The rate of major bleeding was 1.7%. Among 1877 individuals who did not use post‐discharge thromboprophylaxis, nine VTE events were ported (0.48%) and the median time to event was 8 days (3–33). 19 Ongoing larger studies such as CORONA‐VTE, CISCO‐19, and CORE‐19 will provide more insight to this post‐acute COVID‐19 complication. 24 , 42
Townsend et al., 43 in a retrospective study on 150 patients, reported increased D‐Dimer levels in 25.3% of convalescent patients at a median of 80.5 (range 44–155) days post COVID‐19. D‐dimer elevation was associated with vascular events, a bilateral PE, and an acute coronary syndrome requiring coronary artery bypass graft surgery (CABG) in this cohort.
Fan et al. 44 reported four cases of catastrophic large arterial thromboses. Patients were young with no pre‐existing cardiovascular risk factors and asymptomatic SARS‐CoV‐2 infection, and there was a long latency (median time 78 days from seroconversion) between positive serology and the event. Two patients had large‐vessel ischemic strokes, one had acute limb ischemia due to emboli from an aortic thrombosis, and one had a myocardial infarction. In addition, all patients had a hypercoagulable state, defined by markedly raised Factor VIII, elevated von Willebrand factor antigen, increased D‐dimer levels, and hyperfibrinogenemia.
The risk of thrombotic complications in the post‐acute setting is associated with the severity of COVID‐19 infection (ICU stay, duration of hospital stay) and standard risk factors for thrombosis and adverse outcomes. 29 In addition, events during the acute phase of the disease increase the risk for recurring events. 18 , 45
Patients hospitalized for acute medical illness, also at the acute phase of COVID‐19, are at risk for VTE for up to 90 days following discharge. Extended thromboprophylaxis reduces this risk but increases the bleeding risk and is therefore not routinely recommended. Given the low rates of thrombotic complications in the post‐acute phase of the disease, it is currently 46 unclear and under investigation whether extended thromboprophylaxis should be used. Enrolment in clinical trials is encouraged. Given the lack of prospective data, professional societies and institutions have published interim recommendations on anticoagulation management for COVID19 patients. 28 , 29 Post discharge, the Global COVID‐19 Thrombosis Collaborative group (endorsed by ISTH, NATF, ESVM, IUA) recommends extending VTE prophylaxis in appropriately selected patients, home INR (international normalized ratio) testing in patients on vitamin K antagonists (VKAs), and encouraging home exercise. 29
Standard tools for VTE risk assessment should be used to identify patients at high risk for thrombosis in the post‐acute period 29 who should be considered for extended prophylaxis (up to 45 days). 27 , 29 Standard risk factors such as active cancer, immobility, age, high BMI (body mass index), recent trauma, thrombophilia, surgery, and personal or familial history of VTE should be taken into account. The severity of COVID‐19 disease is also included in risk assessment, and some authors also include elevated D‐dimer levels (>2 times the upper limit of normal). 47 , 48 Low‐molecular‐weight heparin and direct oral anticoagulants are generally preferred over VKAs due to the lack of need to monitor therapeutic levels. 29 Potential drug–drug interactions should also be considered.
The role of antiplatelet agents as an alternative for thromboprophylaxis (or together with anticoagulation agents) has not yet been determined. It is being assessed as a prolonged primary thromboprophylaxis strategy in those managed as outpatients (ACTIV‐4 (NCT04498273). At the same time, maintaining physical activity and ambulation should be recommended to all patients when appropriate.
The area of post‐acute COVID‐19 disease thromboembolic and hemorrhagic risk is under active investigation. 49 ACTIV‐4c will evaluate the impact of apixaban 2.5 mg BID (twice a day) on the rate of all‐cause mortality and arterial and venous thromboembolism on 5320 post‐discharge patients. The MICHELLE Trial (NCT04662684) is an open‐label RCT with 320 participants; it aims to evaluate the safety and efficacy of rivaroxaban 10 mg QD (everyday) versus no intervention after hospital discharge. In the INSPIRATION study, an intermediate or standard prophylactic dose of enoxaparin will be continued after discharge in 600 patients randomized in the ICU. In the COVID‐PREVENT trial (NCT04416048), rivaroxaban at prophylactic doses will be compared with the standard of care in COVID‐19 outpatients. In XACT, Factor Xa Inhibitor versus Standard of Care Heparin in Hospitalized Patients with COVID‐19 during hospitalization and discharge will be assessed (NCT04508439). In the HERO‐19 study, edoxaban 60 mg QD or placebo will continue after discharge in 172 patients randomized to treatment to evaluate all‐cause mortality rate and VTE incidence at 42 days. Finally, aspirin in the PEAC study and extended‐release dipyridamole plus aspirin in the ATTAC‐19 study will be continued after discharge. The PREVENT‐HD trial (NCT04508023) will compare rivaroxaban 10 mg tablet orally once daily for 35 days along with the standard‐of‐care treatment versus placebo along with standard of care.
3.2. Autoimmune hematological disorders
Autoimmune thrombocytopenic purpura (ITP), although rarely, has been recognized as a late manifestation of COVID‐19. Potential mechanisms underlying SARS‐CoV‐2‐mediated immune thrombocytopenia include immune system dysregulation, molecular mimicry, epitope spreading, and susceptibility mutations in SOCS 1 (Figure 1). Delayed‐onset ITP has been reported in the post‐acute phase, between 3 and 4 weeks after COVID‐19 symptom onset. 50 Chen et al. reported, in a large cohort of 271 hospitalized COVID‐19 patients in China, a delayed‐phase thrombocytopenia of presumed immune origin in 11.8% of patients. 51 A recent systematic review reported that among 45 patients with ITP secondary to COVID‐19, 20% developed thrombocytopenia 3 weeks after the onset of COVID‐19 symptoms and 9% had relapse following treatment response during follow‐up. 52 Most patients were effectively treated with glucocorticoids and intravenous immunoglobulin, and just a few received thrombopoietin receptor agonist as a second‐line agent. A recent systematic review on hematological autoimmune disorders in the course of COVID‐19 reported 12 cases of delayed ITP at least 20 days after COVID‐19 diagnosis. 53
4. POST‐COVID‐19 HEMATOLOGICAL LABORATORY FINDINGS
CIC is a hypercoagulable but also a hyperinflammatory state. Lymphopenia is a consistent and typical laboratory finding of severe acute COVID‐19, along with increased inflammatory markers and a hypercoagulable state reflecting cytokine storm, endothelitis, and microangiopathy. 54 , 55 A prothrombotic state documented upon increased plasma levels of factor VIII and plasminogen‐activator inhibitor type 1 (PAI‐1) is maintained for 4 months post discharge, as shown in a follow‐up study of 52 COVID‐19 survivors discharged from hospital. 56 These findings could partially explain the hypercoagulable and hypofibrinolytic status post COVID‐19, although sustained endothelitis may also play a role.
COVID‐19 patients with severe disease and fatal outcomes present with a decreased lymphocyte/white blood cell ratio at admission and during hospitalization compared with the survivors. 57 , 58 Survivors demonstrated a lymphocyte nadir on day 7 and then a subsequent restoration. 59 A prognostic model based on lymphocyte counts at two different time points has been proposed; patients with less than 20% lymphocytes at days 10–12 and less than 5% at days 17–19 seem to have the worst prognosis. 60 Lymphocytes express the ACE‐2 receptor, enabling SARS‐CoV‐2 to bind directly and, consequently, leading to their lysis. 61 The cytokine storm is characterized by extremely high levels of interleukins (mainly IL‐6, IL‐2, IL‐7, granulocyte colony‐stimulating factor, interferon‐γ inducible protein 10, MCP‐1, and MIP1‐a) and tumor necrosis factor (TNF)‐alpha, which may also induce lymphocyte apoptosis. 62 , 63 , 64 Persistent lymphocytopenia may be evident even at 5 weeks from disease onset, especially in patients with severe acute COVID‐19 disease. 65 A study from China on 435 hospitalized COVID‐19 patients reported that lymphocytopenia was particularly relevant for CD3+, CD4+, CD8+, CD19+, and CD16/56+ lymphocyte subsets. 65 Lymphocytes increased between the third and fifth week, returning gradually to normal levels, but remained lower compared to healthy controls. Although this was a retrospective study based on electronic medical records, the observation period was long and the sample size at the key time points was large. However, the number of patients at each time point was not consistent and some clinical data were lacking. Nevertheless, the trend in variation of cellular immune function during treatment is not affected by the factors reported above. Thus, the study provides useful evidence and highlights important results on this topic.
A longitudinal follow‐up study of 25 convalescent COVID‐19 survivors showed that CD8+ T lymphocytes returned to normal within 3 months after symptom onset, although CD4+ T lymphocytes were lower than in healthy controls at the first month of follow‐up. 66
Neutropenia is a complication in many viral illnesses, but it is uncommon during the course of COVID‐19. Neutropenia following COVID‐19 infection has been associated with bone marrow suppression secondary to inflammatory response. It may resolve by itself following a wait‐and‐watch approach, especially in young healthy patients. 67 Bouslama et al. reported a case of a pediatric patient with neutropenia successfully treated with filgrastim, which persisted 1 month after GCSF (granulocyte colony stimulating factor) discontinuation. 68 Lufti et al. reported GSCF‐responsive neutropenia combined with thrombocytosis 1 week after resolution of COVID‐19 symptoms. 69 Therapeutic agents used at the acute phase of COVID‐19 such as tocilizumab have been associated with prolonged neutropenia, even after the resolution of acute symptoms. 70
Thrombocytopenia is a common complication of viral infections. The exact mechanisms by which SARS‐CoV‐2 infection induces thrombocytopenia are not fully understood and require further research. Various potential mechanisms have been proposed: immune system dysfunction and autoimmunity mediated by the virus; dysfunctional bone marrow milieu or direct infection of platelets by SARS‐CoV‐2, via CD‐13 receptors, leading to inhibition of platelet synthesis; decreased thrombopoietin production by a dysfunctional liver; and platelet aggregation and platelet consumption in microthrombi formations in the lungs. A meta‐analysis of nine studies has shown that thrombocytopenia is strongly associated with the severity of the disease. 71 Chen et al. 51 reported in a cohort of 271 hospitalized COVID‐19 patients that 11.8% developed delayed‐phase thrombocytopenia. More prone were elderly patients or patients with lymphopenia on admission. In this study, delayed thrombocytopenia had a significant negative correlation with the proportion of B cells. Bone marrow aspiration performed in three patients revealed impaired growth of megakaryocytes.
Elevated D‐dimer levels may serve as a marker of the prothrombotic state. A retrospective study including 150 patients showed increased D‐dimer levels in 25.3% of convalescent patients at a median of 80.5 (range 44–155) days post SARS‐CoV‐2 infection. Other coagulation and inflammation markers were normal in the vast majority (>90%) of patients. D‐dimer elevation was associated with major vascular events in two patients. The pathophysiological mechanisms underlying D‐dimer elevation and its implication to late COVID‐19 manifestations remain poorly understood. Extravascular pulmonary fibrinolysis has been proposed as a potential mechanism to explain elevated D‐dimers post COVID‐19; however, further research is needed. An observational, prospective, multicenter trial evaluated cardiopulmonary recovery after COVID‐19 at 6 and 100 days post COVID‐19 diagnosis. The results of the study indicate a significant improvement in symptoms and cardiopulmonary status over time. Blood samples were received from 145 patients at the first follow‐up and 134 patients at the second follow‐up. With regard to the hematological long COVID findings, it was demonstrated that D‐dimer and ferritin levels were significantly decreased and hemoglobin levels were significantly increased from first to second follow‐up. 72
COVID‐19 has been associated with hyperferritinemia, which is linked to increased mortality. A recent prospective observational study tried to clarify whether the iron metabolism dysregulation is correlated with the pathogenesis of the disease. A total of 109 patients were enrolled and evaluated 60 days post COVID‐19. Thirty percent of the subjects had still iron deficiency and 9% had anemia classified as inflammation anemia. These patients had increased inflammation biomarkers such as IL‐6 and CRP. Hyperferritinemia remained present in 38% of the enrolled subjects and was more common in patients with severe or critical disease. The mRNA expression of peripheral blood mononuclear cells was investigated, which showed that there is an association between the increased ferritin levels and the cytokine mRNA expression. Furthermore, persisting hyperferritinemia correlated with severe lung disease and impaired performance status. 73
5. CONCLUSIONS
In conclusion, COVID‐19 is a multisystemic disease with significant manifestations from the hematopoietic system both at the acute and post‐acute or chronic phases. Regular monitoring of blood results and evaluation of the individualized thrombotic risk based on comorbidities and coagulation profile are essential for both post‐acute and chronic COVID‐19. Careful evaluation of laboratory dynamics at baseline, during the disease course, and at specific time points post symptoms resolution can lead to a tailored therapeutic approach. We would like to stress that patients who recover from COVID‐19 need special attention and medical care in post‐COVID clinics for the early identification of hematological and other late manifestations of SARS‐CoV‐2. The role of the hematologist in such clinics is essential for the multidisciplinary approach of late manifestations of COVID‐19.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Korompoki E, Gavriatopoulou M, Fotiou D, Ntanasis‐Stathopoulos I, Dimopoulos MA, Terpos E. Late‐onset hematological complications post COVID‐19: An emerging medical problem for the hematologist. Am J Hematol. 2022;97(1):119-128. doi: 10.1002/ajh.26384
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Bedford J, Enria D, Giesecke J, et al. COVID‐19: towards controlling of a pandemic. Lancet. 2020;395(10229):1015‐1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Madjid M, Safavi‐Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: areview. JAMA Cardiol. 2020;5(7):831‐840. [DOI] [PubMed] [Google Scholar]
- 4. Balachandar V, Mahalaxmi I, Subramaniam M, et al. Follow‐up studies in COVID‐19 recovered patients—is it mandatory? Sci Total Environ. 2020;729:139021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Korompoki E, Gavriatopoulou M, Hicklen RS, et al. Epidemiology and organ specific sequelae of post‐acute COVID19: a narrative review. J Infect. 2021;83(1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID‐19) pandemic. J Am Coll Cardiol. 2020;75(18):2352‐2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bangash MN, Patel J, Parekh D. COVID‐19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5(6):529‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Understanding the long‐term health effects of COVID‐19. EClinicalMedicine. 2020;26:100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Piazza G, Campia U, Hurwitz S, et al. Registry of arterial and venous thromboembolic complications in patients with COVID‐19. J Am Coll Cardiol. 2020;76(18):2060‐2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID‐19. J Am Coll Cardiol. 2020;76(16):1815‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pillai P, Joseph JP, Fadzillah NHM, Mahmod M. COVID‐19 and major organ thromboembolism: manifestations in neurovascular and cardiovascular systems. J Stroke Cerebrovasc Dis. 2021;30(1):105427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vernuccio F, Lombardo FP, Cannella R, et al. Thromboembolic complications of COVID‐19: the combined effect of a pro‐coagulant pattern and an endothelial thrombo‐inflammatory syndrome. Clin Radiol. 2020;75(11):804‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vinayagam S, Sattu K. SARS‐CoV‐2 and coagulation disorders in different organs. Life Sci. 2020;260:118431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Silva Andrade B, Siqueira S, de Assis Soares WR, et al. Long‐COVID and post‐COVID health complications: an up‐to‐date review on clinical conditions and their possible molecular mechanisms. Viruses. 2021;13(4):700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jimenez S, Miro O, Llorens P, et al. Incidence, risk factors, clinical characteristics and outcomes of deep venous thrombosis in patients with COVID‐19 attending the emergency department: results of the UMC‐19‐S8. Eur J Emerg Med. 2021;28(3):218‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patell R, Bogue T, Koshy A, et al. Postdischarge thrombosis and hemorrhage in patients with COVID‐19. Blood. 2020;136(11):1342‐1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roberts LN, Whyte MB, Georgiou L, et al. Postdischarge venous thromboembolism following hospital admission with COVID‐19. Blood. 2020;136(11):1347‐1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Engelen MM, Vandenbriele C, Balthazar T, et al. Venous thromboembolism in patients discharged after COVID‐19 hospitalization. Semin Thromb Hemost. 2021;47(4):362‐371. [DOI] [PubMed] [Google Scholar]
- 21. Bourguignon A, Beaulieu C, Belkaid W, Desilets A, Blais N. Incidence of thrombotic outcomes for patients hospitalized and discharged after COVID‐19 infection. Thromb Res. 2020;196:491‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;97(10270):220‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giannis D, Allen SL, Tsang J, et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID‐19: the CORE‐19 registry. Blood. 2021;137(20):2838‐2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18(8):1859‐1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Connors JM, Levy JH. COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033‐2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Talasaz AH, Sadeghipour P, Kakavand H, et al. Recent randomized trials of antithrombotic therapy for patients with COVID‐19: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2021;77(15):1903‐1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID‐19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50(1):72‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost. 2020;18(5):1023‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;75(23):2950‐2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Escher R, Breakey N, Lammle B. Severe COVID‐19 infection associated with endothelial activation. Thromb Res. 2020;190:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pavoni V, Gianesello L, Pazzi M, Stera C, Meconi T, Frigieri FC. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID‐19 pneumonia. J Thromb Thrombolysis. 2020;50(2):281‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chaudhary R, Kreutz RP, Bliden KP, Tantry US, Gurbel PA. Personalizing antithrombotic therapy in COVID‐19: role of Thromboelastography and Thromboelastometry. Thromb Haemost. 2020;120(11):1594‐1596. [DOI] [PubMed] [Google Scholar]
- 34. Giustino G, Pinney SP, Lala A, et al. Coronavirus and cardiovascular disease, myocardial injury, and arrhythmia: JACC focus seminar. J Am Coll Cardiol. 2020;76(17):2011‐2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zachariah U, Nair SC, Goel A, et al. Targeting raised von Willebrand factor levels and macrophage activation in severe COVID‐19: consider low volume plasma exchange and low dose steroid. Thromb Res. 2020;192:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dalan R, Boehm BO. The implications of COVID‐19 infection on the endothelium: a metabolic vascular perspective. Diabetes Metab Res Rev. 2021;37(3):e3402. [DOI] [PubMed] [Google Scholar]
- 37. Yong SJ. Long COVID or post‐COVID‐19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond). 2021;53(10):737‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zuo Y, Estes SK, Ali RA, et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID‐19. Sci Transl Med. 2020;12(570):eabd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fogarty H, Townsend L, Morrin H, et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost. 2021;19(10):2546‐2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Balzanelli MG, Distratis P, Dipalma G, et al. Sars‐CoV‐2 virus infection may interfere CD34+ hematopoietic stem cells and megakaryocyte‐Erythroid progenitors differentiation contributing to platelet defection towards insurgence of thrombocytopenia and thrombophilia. Microorganisms. 2021;9(8):1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nalbandian A, Sehgal K, Gupta A, et al. Post‐acute COVID‐19 syndrome. Nat Med. 2021;27(4):601‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mangion K, Morrow A, Bagot C, et al. The Chief Scientist Office cardiovascular and pulmonary imaging in SARS coronavirus disease‐19 (CISCO‐19) study. Cardiovasc Res. 2020;116(14):2185‐2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Townsend L, Fogarty H, Dyer A, et al. Prolonged elevation of D‐dimer levels in convalescent COVID‐19 patients is independent of the acute phase response. J Thromb Haemost. 2021;19(4):1064‐1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fan BE, Umapathi T, Chua K, et al. Delayed catastrophic thrombotic events in young and asymptomatic post COVID‐19 patients. J Thromb Thrombolysis. 2021;51(4):971‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Adams D, Gonzalez‐Duarte A, O'Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11‐21. [DOI] [PubMed] [Google Scholar]
- 46. Di Castelnuovo A, Costanzo S, Antinori A, et al. Heparin in COVID‐19 patients is associated with reduced in‐hospital mortality: the multicenter Italian CORIST study. Thromb Haemost. 2021;121(8):1054‐1065. [DOI] [PubMed] [Google Scholar]
- 47. Schindewolf M, Weitz JI. Broadening the categories of patients eligible for extended venous thromboembolism treatment. Thromb Haemost. 2020;120(1):14‐26. [DOI] [PubMed] [Google Scholar]
- 48. Spyropoulos AC, Lipardi C, Xu J, et al. Modified IMPROVE VTE risk score and elevated D‐dimer identify a high venous thromboembolism risk in acutely ill medical population for extended thromboprophylaxis. TH Open. 2020;4(1):e59‐e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bajaj NS, Vaduganathan M, Qamar A, et al. Extended prophylaxis for venous thromboembolism after hospitalization for medical illness: a trial sequential and cumulative meta‐analysis. PLoS Med. 2019;16(4):e1002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mahevas M, Moulis G, Andres E, et al. Clinical characteristics, management and outcome of COVID‐19‐associated immune thrombocytopenia: a French multicentre series. Br J Haematol. 2020;190(4):e224‐e229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen W, Li Z, Yang B, et al. Delayed‐phase thrombocytopenia in patients with coronavirus disease 2019 (COVID‐19). Br J Haematol. 2020;190(2):179‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bhattacharjee S, Banerjee M. Immune thrombocytopenia secondary to COVID‐19: a systematic review. SN Compr Clin Med. 2020. Published online Sepember 19, 2021. 10.1007/s42399-020-00521-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Taherifard E, Taherifard E, Movahed H, Mousavi MR. Hematologic autoimmune disorders in the course of COVID‐19: a systematic review of reported cases. Hematology. 2021;26(1):225‐239. [DOI] [PubMed] [Google Scholar]
- 54. Terpos E, Ntanasis‐Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID‐19. Am J Hematol. 2020;95(7):834‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Merrill JT, Erkan D, Winakur J, James JA. Emerging evidence of a COVID‐19 thrombotic syndrome has treatment implications. Nat Rev Rheumatol. 2020;16(10):581‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. von Meijenfeldt FA, Havervall S, Adelmeijer J, et al. Sustained prothrombotic changes in COVID‐19 patients 4 months after hospital discharge. Blood Adv. 2021;5(3):756‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID‐19) in Wuhan, China: a retrospective study. Chin Med J (Engl). 2020;133(11):1261‐1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020;71(15):762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Singh S, Sharma A, Arora SK. High producer haplotype (CAG) of ‐863C/a, −308G/a and ‐238G/a polymorphisms in the promoter region of TNF‐alpha gene associate with enhanced apoptosis of lymphocytes in HIV‐1 subtype C infected individuals from North India. PLoS One. 2014;9(5):e98020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liao YC, Liang WG, Chen FW, Hsu JH, Yang JJ, Chang MS. IL‐19 induces production of IL‐6 and TNF‐alpha and results in cell apoptosis through TNF‐alpha. J Immunol. 2002;169(8):4288‐4297. [DOI] [PubMed] [Google Scholar]
- 64. Aggarwal S, Gollapudi S, Gupta S. Increased TNF‐alpha‐induced apoptosis in lymphocytes from aged humans: changes in TNF‐alpha receptor expression and activation of caspases. J Immunol. 1999;162(4):2154‐2161. [PubMed] [Google Scholar]
- 65. Deng Z, Zhang M, Zhu T, et al. Dynamic changes in peripheral blood lymphocyte subsets in adult patients with COVID‐19. Int J Infect Dis. 2020;98:353‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang Z, Yang L, Chen Y, Xu Z, Wang H, Zhang X. A longitudinal follow‐up of COVID‐19 patients in the convalescent phase showed recovery in radiological results, the dynamics of lymphocytes, and a decrease in the level of IgG antibody: a single‐Centre, observational study. J Thorac Dis. 2021;13(5):2986‐3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mank VMF, Mank J, Ogle J, Roberts J. Delayed, transient and self‐resolving neutropenia following COVID‐19 pneumonia. BMJ Case Rep. 2021;14(5):e242596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bouslama B, Pierret C, Khelfaoui F, Bellanné‐Chantelot C, Donadieu J, Héritier S. Post‐COVID‐19 severe neutropenia. Pediatr Blood Cancer. 2021;68(5):e28866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lutfi F, Benyounes A, Farrukh N, Bork J, Duong V. Agranulocytosis following COVID‐19 recovery. Cureus. 2020;12(7):e9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Atallah B, El Nekidy W, Mallah SI, et al. Thrombotic events following tocilizumab therapy in critically ill COVID‐19 patients: a facade for prognostic markers. Thromb J. 2020;18:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a meta‐analysis. Clin Chim Acta. 2020;506:145‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sonnweber T, Sahanic S, Pizzini A, et al. Cardiopulmonary recovery after COVID‐19: an observational prospective multicentre trial. Eur Respir J. 2021;57(4):2003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sonnweber T, Boehm A, Sahanic S, et al. Persisting alterations of iron homeostasis in COVID‐19 are associated with non‐resolving lung pathologies and poor patients' performance: a prospective observational cohort study. Respir Res. 2020;21(1):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


