Abstract
Background & Aims:
Resting Energy Expenditure (REE) quantitatively describes the calories used to support body function (e.g. breathing, blood circulation, etc.) at resting condition. Assessment of the REE is essential for successful weight management and the understanding of metabolic health. REE is typically determined via indirect calorimetry. Current biomedical indirect calorimetry technologies, utilizing assessment of oxygen consumption (VO2) and carbon dioxide production (VCO2) rates (which are typically in the form factor of a metabolic cart) are bulky and require on-site calibration and/or trained professionals to operate. We introduce a novel wearable medical device with FDA clearance to determine REE accurately, portable, and user-friendly format, which can be used both by health professionals in a clinical environment and by the patient at home. Previously, we have reported the validation of Breezing Med (also named as Breezing Pro™) through Douglas Bag Method, a gold standard for gas exchange measurement, and excellent agreement has been found between the two methods for the determination of REE, VO2, and VCO2 rates.1 Now we present the validation of Breezing Med against Medical Graphics (MGC) CPX Ultima™, a FDA 510k cleared metabolic cart, which principle is based on breath-by-breath analysis. In addition, we present Breezing Med as a tool for daily measurement of metabolic rate by the lay person at home.
Methods:
A) The validation study was executed via parallel measurement of 20 healthy participants under resting conditions using both the Breezing Med and the MGC Ultima CPX™ (10 min test). B) Breezing Med measurements were carried out by six subjects at home during stay-at-home order due to COVID-19 for 30 days.
Results:
A) The resulting measurements from both devices was compared with correlation slope’s and R-squared coefficients close to 1. B) Results were recorded and analyzed for variability. The pilot study demonstrated the advantage of Breezing Med device to be easy-to-use at home by lay people, which make the valuable device for telemedicine applications related to weight management from home.
Conclusions:
This result shows that the MGC Ultima CPX™ and Breezing Med are substantially equivalent for REE measurement; and an advantage of this device for metabolic assessment under the current COVID-19 pandemic situation, for people with impaired physical mobility, and for those who lives in rural areas or face impediments that limit physical access to care.
Keywords: Metabolic tracker, Resting energy expenditure, Telemedicine, Point of Care, Breezing Med, Breezing Pro
1. Introduction
Since December 2019, the pandemic due to the novel COVID-19 virus has introduced the world to certain lifestyle changes, where losing, controlling or gaining weight could be a challenge.2,3 It is been proven that people with overweight or obesity present a higher risk of severe symptoms with COVID-19 infection, and consequently need to be hospitalized.4–7 In order to prevent the spread of the virus and for protection of the population, stay-at-home, quarantine and isolation were implemented around the world. Working, studying, exercising, and all normal daily activities must be at home, which promoted the demand for innovative technological solutions to meet the current needs of the time. These technological solutions should bring three main associated requirements: accuracy, friendly use, and capability to perform secure data connection via well-known protocols such as HIPAA compliant protocols.
Weight management during lockdown has presented several issues, poor eating habits, stress eating, snacking, and changes in the routine have generally resulted in weight gain and higher incidences of obesity in the general population.8–10 Total Energy Expenditure (TEE) is the total number of calories burned per day, involving basal energy expenditure, diet-induced thermogenesis, non-exercise activity thermogenesis, and physical activity.11–15 TEE varies with age, gender, weight, height, and the amount of activity realized as sedentary, moderate, or strenuous. TEE can even be different from person to person having same physical parameters.16,17 For sedentary population, Resting Energy Expenditure (REE, kcal per day burned during non-active periods), corresponds in average up to 70-80% of the TEE,18 and its measurement is important to accurately assess a more accurate daily total energy expenditure.1,19 Values of REE calculated from epidemiological equations can differ up to 900 kcal/day, and therefore, measurements of REE are essential20–22 to guide caloric intake, and assess metabolic changes such as the metabolic adaptation.
Telehealth technology is not new but has seen significantly increased adoption as a result of the pandemic.23 This tool offers an expansion of health services while protecting healthcare personnel and patients. In addition, Remote patient monitoring (RPM) systems or devices have won popularity for patients due to eliminating patient transportation to a facility, increased data collection, and presents improved patient outcomes.24,25
In this work, we present the analytical and usability validation of Breezing Med as a wearable medical device with sensing technology capable of determining Resting Energy Expenditure, REE. This device can be used by a professional or the patient. The device performs gold-standard indirect calorimetry, in which the REE is determined by the measured oxygen consumption rate (VO2) and carbon dioxide production (VCO2) rate in breath. In comparison with other devices commercially available, Breezing Med uses a mask specially designed to prevent leaks and provide an accurate VO2 and VCO2 measurement while breathing by mouth and/or nose. In addition, we avoid the use of nose clips which is very uncomfortable for the patients.
Figure 1 shows a schematic representation of how this device works. Through the user-friendly app, the user, either a professional in his/her office or the patient at home, can perform the measurement and share/manage the data via HIPAA compliant systems. Using this application, a time-stamped and graphical interface is displayed for visualization of longitudinal EE data, which can be used for getting accurate professional care advice. In addition, the Breezing App’s records can be exported to CSV (most recent or entire history records) and can be shared to remote locations.
Figure 1.
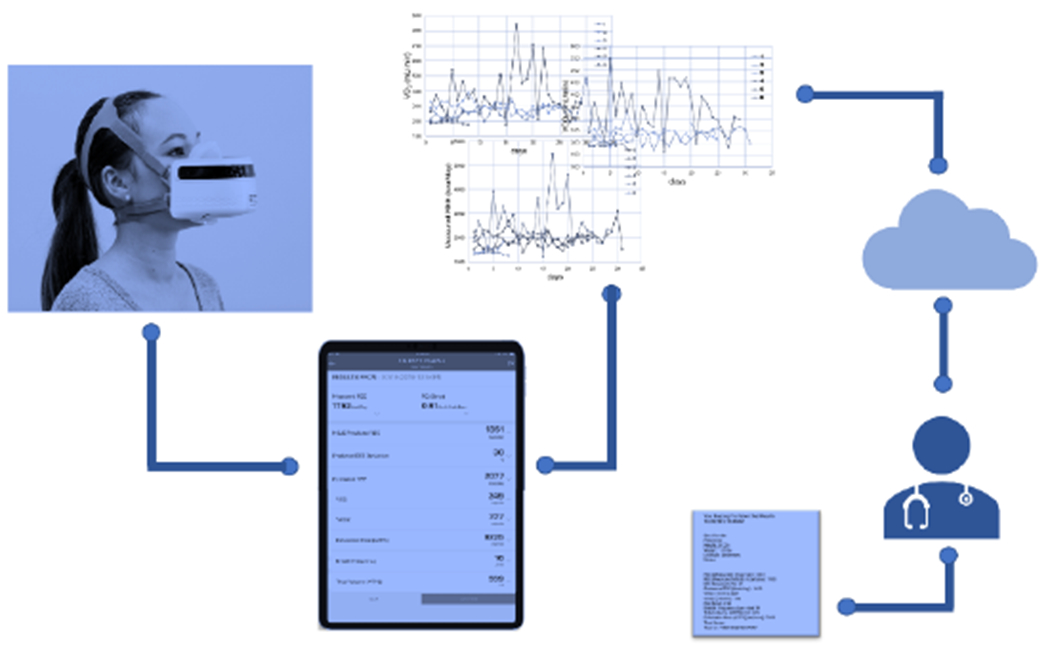
Schematic representation of Breezing Med Technology
Validation of medical devices must be precise and reliable. Metabolic carts such as the MGC CPX Ultima™, provides a highly accurate determination of REE analyzed breath-by-breath.26–28 In this work, MGC high technology was implemented for the analytical validation of Breezing Med. In addition, we present the usability validation comprising self-testing results from six subjects during the pandemic, under the stay-at-home orders, which provides evidence of efficacy for the Breezing Med to determine REE of patients as a telemedicine tool.
2. Materials and methods
2.1. Analytical validation’s Participants
A total of 20 healthy subjects between 23 and 60 years old were tested. This study included 12 females and 8 males. Table 1 shows the list of individual physical characteristic of the subjects. The subject’s heights were ranged from 152 to 189 cm, their weights from 45 to 95 kg, and the body mass indices (BMI) were ranged from 16.9 to 35.3 kg/m2 (see Table 2). As can be seen from Table 1 and 2, the patient’s BMI values ranges from 16.9 to 35.3 which is considered by the Centers for Disease Control and Prevention (CDC) as underweight and obesity Class 2, respectively.29
Table 1:
Individual physical characteristics of the study’s participants
| Subject # | Height (cm) | Weight (kg) | Age | Gender | BMI (kg/m2) |
|---|---|---|---|---|---|
| 1 | 158 | 70 | 34 | F | 28.04 |
| 2 | 168 | 57 | 48 | F | 20.20 |
| 3 | 180 | 95 | 28 | F | 29.32 |
| 4 | 152 | 45 | 42 | F | 19.48 |
| 5 | 176 | 67 | 60 | F | 21.63 |
| 6 | 170 | 49 | 24 | F | 16.96 |
| 7 | 164 | 76 | 46 | F | 28.26 |
| 8 | 158 | 51 | 34 | F | 20.43 |
| 9 | 170 | 66 | 36 | F | 22.84 |
| 10 | 170 | 92 | 58 | F | 31.83 |
| 11 | 167 | 63 | 31 | F | 22.59 |
| 12 | 164 | 95 | 23 | F | 35.32 |
|
| |||||
| 13 | 178 | 66 | 38 | M | 20.83 |
| 14 | 189 | 82 | 37 | M | 22.96 |
| 15 | 178 | 92 | 28 | M | 29.04 |
| 16 | 173 | 65 | 38 | M | 21.72 |
| 17 | 178 | 84 | 23 | M | 26.51 |
| 18 | 167 | 62 | 31 | M | 22.23 |
| 19 | 173 | 73 | 30 | M | 24.39 |
| 20 | 176 | 86 | 32 | M | 27.76 |
Table 2:
Averaged physical characteristics of the study’s participants*
| Gender | N | Height (cm) | Weight (kg) | Age | BMI (Kg/m2) |
|---|---|---|---|---|---|
| Women | 12 | 166.4 ± 7.5 | 68.8 ± 16.9 | 38.6 ± 11.8 | 24.7 ± 5.4 |
| (152 - 180) | (45 - 95) | (23 - 60) | (16.9 - 35.3) | ||
| Men | 8 | 176.5 ± 5.8 | 76.2 ± 10.5 | 32.1 ± 5 | 24.4 ± 2.8 |
| (167 - 189) | (62 - 92) | (23 - 38) | (20.8 - 29) | ||
| Total | 20 | 170.4 ± 8.5 | 71.8 ± 15.1 | 36.0 ± 10.1 | 24.6 ± 4.5 |
| (152 - 189) | (45 - 95) | (23 - 60) | (16.9 - 35.3) | ||
parameters including mean ± SD, and minimum and maximum values.
The 10 min tests for determining REE were taken at resting state under specific instructions as no food or caffeine intake in the past 4 hours, no moderate exercise performed 4 hours before the test, and no strenuous exercise performed for the past 12 hours. All participants (except one of the home participants) adhered to testing instructions.
All subjects participated voluntarily. Before the test, each person was informed and introduced to the propose of our study, and a consent form was signed.
The tests were carried from September to November 2019, in Mayo Clinic by Arizona State University researchers. The study was approved by the Institutional Review Board of Arizona State University; IRB reference protocols # STUDY00006562.
2.2. Measurements Collected Using the Breezing Med and MGC CPX Ultima™
In collaboration with the Department of Cardiovascular Medicine of Mayo Clinic in Scottsdale, Arizona, a Cardiorespiratory Diagnostic Systems (CPX) Ultima Series™ (MGC Diagnostics Corporation, Saint Paul, MN USA)30 was used for the determination of VO2, VCO2 and REE simultaneously with Breezing Med.
MGC CPX Ultima has been selected as reference instrument because it has received 510k-clearance, which means that had been compared with other formerly cleared or approved FDA-instrument. In addition, this instrument is used for clinical guidance of diets and weight management and has received clinical validity.
MGC Diagnostics’ equipment uses advanced gas exchange analysis technology and is an established breath-by-breath reference instrument. Before the use, the equipment was allowed to warm-up for 30 minutes, the flow was manually calibrated using a calibration syringe, and the O2 and CO2 sensors (galvanic cell and NDIR absorption, respectively) were calibrated using standard reference gas cylinders that are part of the equipment. In addition, MGC CPX Ultima includes a preVent® flow sensor, which meets ATS/ERS standards and specifications.27
A serial connection between the two MGC and the Breezing Med (TF Health, Tempe, AZ US)31 was designed to guarantee there was no air leakage between the two devices during breathing, and the data was collected simultaneously for the two methods. As can be seen from Figure 2, the mask was connected to the MGC through a custom-made 2 one-way valve adapter followed by a preVent flow sensor. The preVent flow sensor was directly connected to the MGC by an umbilical adapter, and to the mouthpiece with a saliva trap. The Breezing Med analyzer was attached to the subject by a headgear strap and a laboratory jack was used to hold the device at the corresponding height of each subject’s face. In this way, discomfort or nuisance during the test was avoided. The subject wore a disposable nose clip to avoid air leaks thorough the nose during the test.
Figure 2.
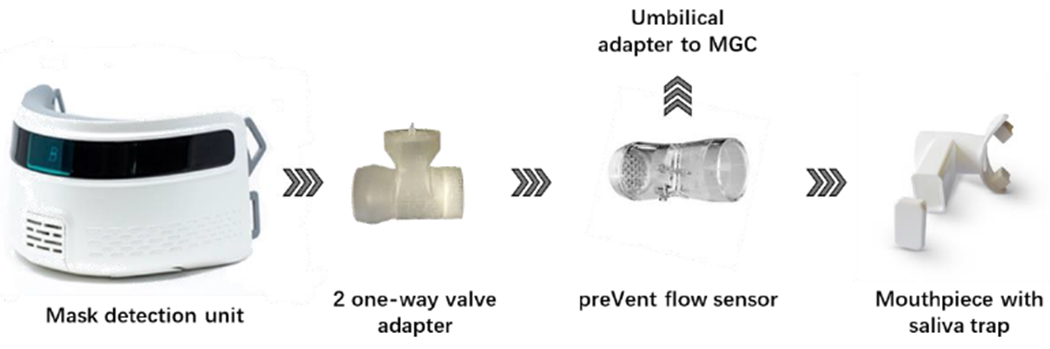
Schematic representation of the connection between Breezing Med device MCG equipment.
A new preVent flow sensor and mouthpiece were used for each subject. The device was clean and disinfected with 70% isopropanol solution between tests, and a period of 20 minutes was awaited between users.
The Breezing Med device works via measurement of color change from single-use sensors previously calibrated via a QR code. This pre-calibrated QR-code mechanism allows the user to complete the device setting prior to the measurement without use of calibration gases. Similarly to MGC, Breezing Med device used oxygen consumption rate and carbon dioxide production rate (VCO2) to assess the Resting Energy Expenditure through the well-known Weir equation (Equation 1):32
Two different batches of sensors were used for Breezing Med in this study. Each batch was packaged with a desiccant in a plastic bag with a QR code printed on the outside, which was scanned right before its use following the step-by-step and user-friendly procedure indicated by the Breezing-for-professional App (Breezing Pro). The procedure is well explained and indicated photographically in the Breezing iOS App, in order to make this device easy to use. At the end of the test, the screen shows the metabolic parameters obtained as measured REE, respiratory quotient, Mifflin St. Jeor Equation (MSJE) predicted and estimated total energy expenditure, VO2, VCO2, exhalation rate, breath frequency, and tidal volume. For each patient, a test history diagram will show the variation of REE along the time the tests were taken. From the app, a pdf with the results can be send by email to the patient, and/or exported to an excel file for further analysis of the professional. Patients and professionals assess their information via HIPPA–compliant methods of data reporting and transmission.
2.3. Data and statistical analysis
The data collected from MGC was analyzed in Microsoft Excel, by taking the average and standard deviation of the last 5 minutes of the breathing test. The first half of the time is required to reach an equilibrium in the breathing frequency. Those results were compared and the correlation between the two methods was analyzed using linear regression for VO2, VCO2 and, REE. In addition, the results were analyzed by paired t-test to determine the statistical difference by the two methods using GraphPad Prism.33 Finally, data were reported as Mean ± SD.
2.4. Breezing Med and Telemedicine assessment
A total of 6 subjects, 2 females and 4 males performed the measurements of metabolic parameters by using Breezing Med for self-monitoring for 1 and 4 weeks. The device, mask, sensors and manual were sent to their homes and they were asked to their indirect calorimetry measurement daily. They were also provided with a Samsung tablets (Tab A, 10 inches) or iPads (Mini 1 &2, Pro 2019), where the mobile app was used. Each patient followed the corresponding indications from the app with no additional training.
3. Results and Discussion
3.1. Analytical Validation
The linear regression calculated from the graph for VO2 and VCO2 from Breezing Med vs MGC is shown in Fig. 3. As can be seen, a value of 1.02 was find for the slope (R2 = 0.8435) for VO2 and 0.96 for VCO2 (R2 = 0.9194). Similarly, from Fig. 4, the lineal regression for REE was graphed and calculated the slope and R2, resulting in a value of 1.00 and 0.8817, respectively. The values obtained for ppaired t-test were 0.9524, 0.0612 and 0.5745 for VO2, VCO2, and REE respectively. In addition, mean of the difference and standard deviation between the Breezing Med device and MGC method were calculated and are summarized in Table 3.
Figure 3.
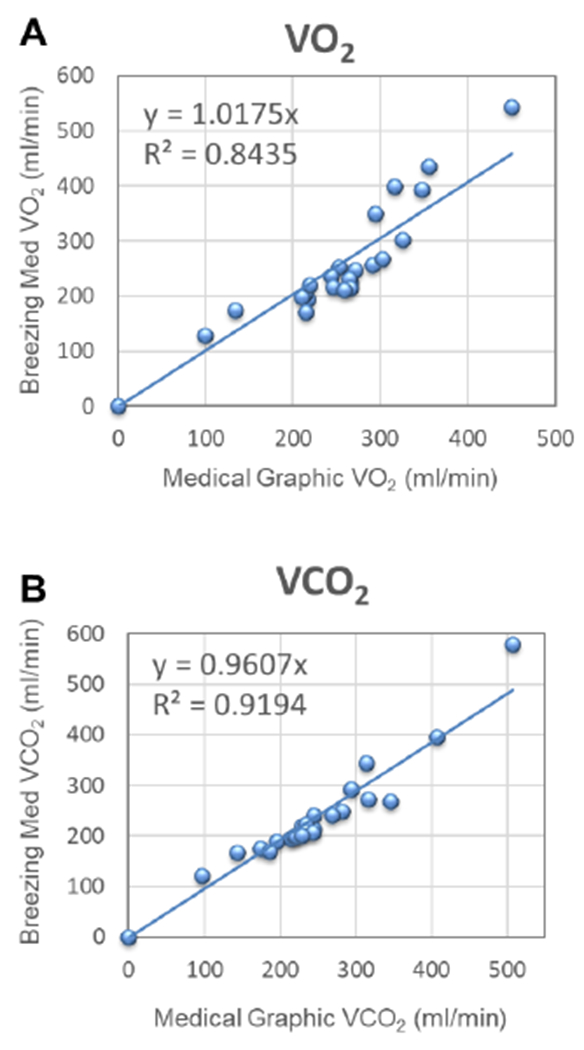
Validation study for VO2 (A) and VCO2 (B), Breezing Med vs. Medical Graphic Method (two sensor batches); N=20 subjects; 23 tests.
Figure 4.
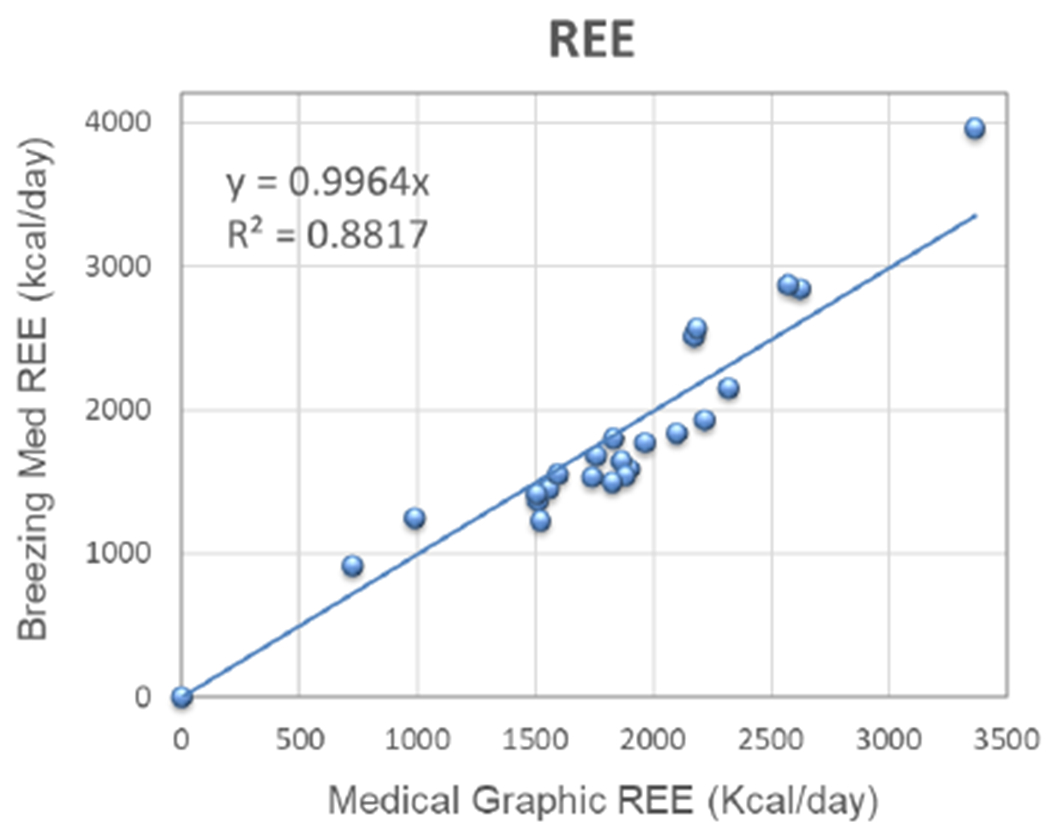
Validation Study for REE, Breezing Med vs. Medical Graphic. N=20 subjects; 23 tests.
Table 3.
Summary and comparison between metabolic parameters Measured by Breezing Med and MGC Ultima CPX™
| VO2 | VCO2 | REE | |
|---|---|---|---|
| y | 1.02 | 0.96 | 1.00 |
| R2 | 0.8435 | 0.9194 | 0.8817 |
| p paired t-test | 0.9524 | 0.0612 | 0.5745 |
| % Mean difference | −0.1 | 4 | 2 |
| SD | ± 16 | ± 11 | ± 14 |
a = Correlation slope; R2 = Squared correlation coefficient; Mean difference and ±SD (standard deviation) expressed as mL/min for VO2 and VCO2, and kcal/day for REE.
An accurate value for REE is directly proportional to accurate measurement of exhalation rate and the fraction of O2 and CO2 in the exhaled gas. In our previous work, the exhalation rate was determined to be accurate for real-time breath flow measurements.1 The results for VO2, VCO2 and, REE over this study’s 20 healthy patients were within the expected range of 100-350 ml/min for VO2 and VCO2 and 1000-3500 kcal/day for REE, similarly to those values found in a group of 66 subjects in the analytical validation study using the Douglas Bag Method.1 Therefore, is important to denote that there are not limitations when the test is implemented in an adult population with a wide range of BMI values. As can be seen from Table 1 and 2, the patient’s BMI values ranges from 16.9 to 35.3 which is considered by the Centers for Disease Control and Prevention (CDC) as underweight and obesity Class 2, respectively.29 In addition, by conventional criteria from ppaired t-test no statistically significant difference between the two data groups was observed.
As was shown in Table 3, the mean difference (%) of the measured VO2, VCO2, and REE between the two methods indicated there is no significant difference between them.
3.2. Usability validation for remote patient assessment
All the subjects expressed to be more convenient to take the test in the morning before breakfast, to accomplish the necessary requirements of fasting conditions. All of them reported when a test was forgotten or taken under certain conditions not specified by the manual, as for example after exercising. In that case, REE value out of the normal range was found and was not shown in the final data or analysis. No complaints or issues were reported when using the device or app.
Our study demonstrates that the device can be used properly by users at home guided by the manual and the app. None of the subjects required professional help, indicating the advantage of this medical device for assessing accurate REE measures, and suitability for telemedicine conditions of use.
We could assess the subjects average REE, and other pulmonary parameters captured by the Breezing Med device, in addition to physiological parameters of oxygen saturation (SpO2) and heart rate (no shown). REE can vary from person to person depending on age, BMI, health, and particular assessment conditions.34 Typical day-by-day variability of REE for subjects is +/−10%.16. As can be seen from Figure 5, we could analyze subject variability of the indirect calorimeter parameters. For the subjects with higher variability than 10%, we interviewed them and determined that exercise conditions and physical activities were identified as factors of REE increase. In this case, we could evaluate, the subject’s energy expenditure variability due to specific conditions of testing such us thermogenic effect of food and post-exercise oxygen consumption.
Figure 5.
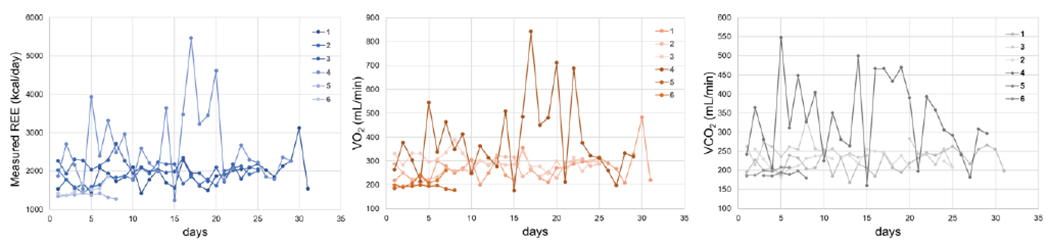
Usability test of Breezing Med for patient’s remote assessment of indirect calorimetry parameter as REE, VO2 and VCO2. The figure shows the information collected through the HIPAA-compliant Breezing App by the six subjects for a period of 1-4 weeks.
5. Conclusion
Breezing Med is a FDA 510k cleared medical device designed to perform indirect calorimetry in a mobile, patient-friendly manner. REE is essential for personalized nutrition assessments and individual diet plan creation. Measurements from Breezing Med were compared to measurements from the selected reference instrument, CPX Ultima Series™ metabolic cart from MGC Diagnostics Co., and the comparative analysis concluded that both instruments have equivalent accuracy for measures taken at resting conditions in adults subjects. In addition, Breezing Med device was probed to be user-friendly and useful to assess REE profiles of users at home, which makes the device appropriate for remote metabolic rate assessment, and remote patient monitoring.
Acknowledgments
The authors acknowledge the participants for compliance to protocols. In addition, we acknowledge NIH R03 funding from NIBIB (EB027336-02) and, A. J. and Sigismunda Palumbo Charitable Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors, excepting Erica Forzani and Xiaojun Xian, currently with Breezing Co., declare no conflict of interest.
References
- 1.Mora SJ, Mann S, Bridgeman D, Quach A, Balsells L, Garcia A, Lind M-L, Robbins R, Xian X Validation of a wearable metabolic tracker (Breezing Pro™) for Resting Energy Expenditure (REE) measurement via Douglas bag method. Glob J Obes Diabetes Metab Syndr 2020, 7(1): 001–008. 10.17352/2455-8583.000039 [DOI] [Google Scholar]
- 2.Ramachandran D, Gill T Impact of COVID-19 lockdown on self-managed weight loss journeys. Obes Res Clin Pract 2020, 14, 386–387. 10.1016/j.orcp.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kushner RF What COVID-19 is Teaching Us About Counseling for Weight Management. Obesity 2020, 28(11), 2035–2037. 10.1002/oby.22988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wadman M Why COVID-19 is more deadly in people with obesity-even if they’re young. Science 2020, September. 10.1126/science.abe7010 [DOI] [Google Scholar]
- 5.Obesity, Race/Ethnicity, and COVID-19. https://www.cdc.gov/obesity/data/obesity-and-covid-19.html
- 6.Woo Baidal JA, Chang J, Hulse E, Turetsky R, Parkinson K, Rausch JC Zooming Toward a Telehealth Solution for Vulnerable Children with Obesity During Coronavirus Disease 2019. Obesity 2020, 28, 1184–1186. 10.1002/oby.22860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao M, Piernas C, Astbury NM, Hippisley-Cox J, O’Rahilly S, Aveyard P, Jebb SA Associations between body-mass index and COVID-19 severity in 6·9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol 2021, 9, 350–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pellegrini M, Ponzo V, Rosato R, Scumaci E, Goitre I, Benso A, Belcastro S, Crespi C, De Michieli F, Ghigo E, Broglio F, Bo S Changes in Weight and Nutritional Habits in Adults with Obesity during the “Lockdown” Period Caused by the COVID-19 Virus Emergency. Nutrients 2020, 12, 2016. 10.3390/nu12072016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson E, Boyland E, Chisholm A, Harrold J, Maloney NG, Marty L, Mead BR, Noonan R, Hardman CA Obesity, eating behavior and physical activity during COVID-19 lockdown: A study of UK adults. Appetite 2021, 156, 104853. 10.1016/j.appet.2020.104853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ALMughamis NS, AlAsfour S, Mehmood S Poor Eating Habits and Predictors of Weight Gain During the COVID-19 Quarantine Measures in Kuwait: A Cross Sectional Study. Research Square 2020. 10.21203/rs.3.rs-29219/v1 [DOI] [Google Scholar]
- 11.Heydenreich J, Kayser B, Schutz Y, Melzer K Total Energy Expenditure, Energy Intake, and Body Composition in Endurance Athletes Across the Training Season: A Systematic Review. Sports Med Open 2017, 3(1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pontzer H, Durazo-Arvizu R, Dugas LR, Plange-Rhule J, Bovet P, Forrester TE, Lambert EV, Cooper RS, Schoeller DA, Luke A Constrained Total Energy Expenditure and Metabolic Adaptation to Physical Activity in Adult Humans. Carr Biol 2016, 26(3), 410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinha S, Kurpad AV, Kuriyan R Total energy expenditure (TEE) of young adults from urban South India: revisiting their daily energy requirement. Eur. J. Clin. Nutr 2021, 75, 845–851. [DOI] [PubMed] [Google Scholar]
- 14.Westerterp KR. Control of energy expenditure in humans. Eur. J. Clin. Nutr 2017, 340–344. [DOI] [PubMed] [Google Scholar]
- 15.Komura K, Nakae S, Hirakawa K, Ebine N, Suzuki K, Ozawa H, Yamada Y, Kimura M, Ishii K Total energy expenditure of 10- to 12-year-old Japanese children measured using the doubly labeled water method. Nutr Metab 2017, 14, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McArdle WD, Katch FI, Katch VL (2007) Exercise Physiology: Nutrition, Energy, and Human Performance. Lippincott Williams Wilkins. [Google Scholar]
- 17.Heimburger D, Ard J (2006) In Handbook of Clinical Nutrition. 4th Edition. Link: http://bit.ly/2VKFM1K [Google Scholar]
- 18.Schulz L, Schoeller D A compilation of total daily energy expenditures and body weights in healthy adults. Am J Clin Nutr 1994, 60, 676–81. [DOI] [PubMed] [Google Scholar]
- 19.Zhao D, Xian X, Terrera M, Krishnan R, Miller D, Bridgeman D, Tao K, Zhang L, Tsowa F, Forzani E, Tao N A pocket-sized metabolic analyzer for assessment of resting energy Expenditure. Clin Nutr 2014, 33, 341–347. 10.1016/j.clnu.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 20.Deng Y, Scott BJ. Comparison of Resting Metabolic Rates: Calculated using predictive equation and measured using Portable Indirect Calorimeter. Glob J Obes Diabetes Metab Syndr 2019, 6(1), 010–016. 10.17352/gjodms [DOI] [Google Scholar]
- 21.Frankenfield D, Roth-Yousey L, Compher C Comparison of Predictive Equations for Resting Metabolic Rate in Healthy Nonobese and Obese Adults: A Systematic Review. J Acad Nutr Diet 2005, 105, 775–789. 10.1016/j.jada.2005.02.005 [DOI] [PubMed] [Google Scholar]
- 22.Cancello R, Soranna D, Brunani A, Scacchi M, Tagliaferri A, Mai S, Marzullo P, Zambon A, Invitti C Analysis of Predictive Equations for Estimating Resting Energy Expenditure in a Large Cohort of Morbidly Obese Patients. Front Endocrinol 2018, 9(367). 10.3389/fendo.2018.00367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayhurst C A turning point for Telehealth: COVTD-19 Spurs Rapid Uptake of connected care. Biomed Instrum Technol 2020, 54(4), 243–250. 10.2345/0899-8205-54.4.242 [DOI] [PubMed] [Google Scholar]
- 24.Cliffe M, Di Battista E, Bishop S Can you see me? Participant experience of accessing a weight management programme via group videoconference to overcome barriers to engagement. Health Expectations 2020, 00:1–11. 10.1111/hex.13148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stump C, Jackemeyer D, Abidov Y, Herbst K, Tao N, Forzani E Study of the Effect of Mobile Indirect Calorimeter on Weight Management. Glob J Obes Diabetes Metab Syndr 2017, 4(2): 044–050. 10.17352/2455-8583.000022 [DOI] [Google Scholar]
- 26. https://fda.report/GUDID/B368800840001.
- 27.Porszasz J, Barstow T, Wasserman K Evaluation of a symmetrically disposed Pitot tube flowmeter for measuring gas flow during exercise. J Appl Physiol 1994, 77(6), 2659–65. doi: 10.1152/jappl.1994.77.6.2659. [DOI] [PubMed] [Google Scholar]
- 28.Huszczuk A, Whipp B, Wasserman K A respiratory gas exchange simulator for routine calibration in metabolic studies. Eur Respir J. 1990, 3(4), 465–8. PMTD: 2114308. [PubMed] [Google Scholar]
- 29.Defining Adult Overweight and Obesity. https://www.cdc.gov/obesity/adult/defining.html
- 30. https://mgcdiagnostics.com/products/ultima-ccm-indirect-calorimeter.
- 31. www.breezing.com.
- 32.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949, 109, 1–9. 10.1113/jphysiol.1949.sp004363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. https://www.graphpad.com/ [Google Scholar]
- 34.Manore M, Meyer N, Thompson J (2009) “Sport Nutrition for Health and Performance” Human Kinetics (Ed.), vol. Second Edition. [Google Scholar]


