Abstract
Objective
To determine whether patients with inflammatory autoimmune diseases treated with rituximab (RTX) have more severe forms of COVID-19 compared with patients treated with anticytokine therapies, such as Tumour Necrosis Factor (TNF) inhibitors.
Methods
We included all patients who were on either RTX or infliximab (IFX) in two Swiss cantons during the first wave of the COVID-19 pandemic. We collected self-reported symptoms compatible with COVID-19, PCR-confirmed diagnoses of COVID-19 and the evolution of COVID-19 infections. We computed the raw and propensity score-adjusted incidence of COVID-19 by treatment group.
Results
190 patients were enrolled, of whom 121 (64%) were in the RTX group and 69 (36%) were in the IFX group. Twenty-one patients (11%) reported symptoms compatible with COVID-19 (RTX: 10, IFX: 11, p=0.14). Among patients with COVID-19 symptoms, four developed severe forms of the disease, with life-threatening pulmonary manifestations requiring intensive mechanical ventilation (RTX: 4 of 10, IFX: 0 of 11, Fisher’s exact test p=0.04). The incidence rate of COVID-19 symptoms was 0.73 (95% CI 0.39 to 1.37) cases per 1000 patient-days on RTX vs 1.52 (95% CI 0.82 to 2.85) cases per 1000 patient-days on IFX (crude p=0.10, adjusted p=0.07). The incidence rate of severe COVID-19 was 0.28 (95% CI 0.08 to 0.7.2) cases per 1000 patient-days on RTX compared with null on IFX (95% CI 0.0 to 0.44) (p=0.13). A replication in an independent validation cohort confirmed these findings, with consistent results in the Swiss Clinical Quality Management registry.
Conclusion
While the incidence of symptoms compatible with COVID-19 was overall similar in patients receiving RTX or IFX, the incidence of severe COVID-19 tended to be higher in the RTX group.
Keywords: rituximab, infliximab, COVID-19, epidemiology
Key messages.
What is already known about this subject?
While patients with chronic inflammatory rheumatic and musculoskeletal diseases (RMDs) have an increased risk of developing severe forms of COVID-19, it is still unclear if this increased risk is related to the underlying disease or to immunosuppressive treatments.
The risk of severe evolution of SARS-CoV-2 infection may vary depending on the mechanism of action of immunosuppressive treatments.
What does this study add?
The incidence of severe COVID-19 evolution appears to be increased in patients treated with rituximab compared with patients treated with TNF inhibitors.
How might this impact on clinical practice or further developments?
These results suggest prudence should be observed when using immunosuppressive biotherapies, in particular rituximab, in patients with RMDs during the SARS-CoV-2 pandemic.
Introduction
Since the end of 2019, COVID-19 has spread to all continents. In Switzerland, the prevalence of anti-SARS-CoV-2 IgG antibodies in the Geneva population was estimated to be 9.7% at the end of April 2020 and over 20% in early 2021.1 2 Patients with any immunosuppressive condition are at higher risk of developing severe forms of COVID-19 infection. In the UK, patients with rheumatic and musculoskeletal diseases (RMDs) had an increased relative risk (RR=1.19) of dying from COVID-19 compared with the general population.3 However, it is currently still unclear whether this increased risk of death is due to the underlying inflammatory rheumatism, the associated comorbidities or to the treatments received by patients with these conditions. Several studies suggest an increased risk of severe COVID-19 in patients with RMDs associated with active disease on one hand and associated with specific immunosuppressive antirheumatic agents, such as Janus kinase inhibitor and RTX, on the other hand.4 5 Some mechanisms linking increased risk of severe COVID-19 with rituximab (RTX) have been proposed. For instance, by causing, RTX leads to long-lasting immunosuppression due to B lymphocyte depletion, which may explain the increased risk of viral infections. Interestingly, RTX has also been associated with an increased risk of JC polyomavirus infection, responsible for an often lethal multifocal leucoencephalopathy.6
We hypothesised that long-lasting, cell-depleting therapies may increase the risk of developing a severe COVID-19 infection compared with targeted anticytokinic therapies, such as TNF inhibitors. The aim of this study was to determine whether patients treated with B cell-depleting therapy RTX have more severe forms of COVID-19 compared with patients treated with TNF inhibitors.
Methods
Population and study design
We conducted a retrospective observational cohort study in the rheumatology departments of Geneva University Hospital and the Hospital Network of Neuchatel.
Both institutions administer intravenous antirheumatic drugs nearly exclusively for their respective cantons, which implies that the patient population can be considered a representative population-based sample. Because patients receiving intravenous antirheumatic drugs tend to differ from patients who auto-administer their biotherapy, we chose an intravenous administered anti-TNF agent infliximab (IFX) to compare with RTX in order to limit confounding by indication. We identified all patients who received either RTX or IFX at the two study sites. For patients on RTX, we included those who received their treatment in the last 12 months prior to the first wave of the pandemic, between 1 March 2019 and 29 February 2020. For patients on IFX, because its biologic half-life is shorter, we included all patients who received at least one dose of IFX during the last 6 months prior to the first wave of the pandemic, between 1 September 2019 and 29 February 2020. We excluded patients who refused to participate and patients who could not be reached by phone after a minimum of three calls. Patients who agreed to answer the questions responded to a standardised questionnaire on the occurrence of symptoms compatible with COVID-19 or a diagnosis of COVID-19 by nasopharyngeal swabs and positive PCR test for SARS-CoV-2.7 For patients not answering their phone, we consulted the electronic health records to verify if the patient was hospitalised or had died in the mean time.
We planned a priori a replication cohort (see paragraph below) in an independent setting to assess the robustness of our findings.
Primary outcome
The standardised questionnaire filled out by all participants included the following:
Symptoms suggestive of COVID-19, such as presence of ageusia or anosmia, headache, fever, abdominal pain, sore throat, rhinorrhoea, cough, dyspnoea, asthenia and diarrhoea (see online supplemental table 1 and 2).
COVID-19 test results and the type of test used.
Known risk factors and comorbidities associated with severe forms of COVID-19.
Hospitalisation due to COVID-19.
rmdopen-2021-001711supp001.pdf (51.8KB, pdf)
We operationally defined symptoms compatible with COVID-19 as having at least two self-declared symptoms suggestive of COVID-19 in the absence of an alternative diagnosis. Severe COVID-19 was operationally defined as patients requiring hospitalisation in an intensive care unit (ICU) with mechanical ventilation or death related to COVID-19.
Statistical analysis
We compared baseline patient characteristics using Wilcoxon test for continuous variables and Fisher’s exact test for categorical variables. We computed the incidence rate of the various forms of COVID-19 and crude and adjusted incidence rate ratios by generalised estimating equations (GEE). Because patients in the RTX and IFX groups differed in important risk factors, we adjusted the analysis for potential key confounding factors using propensity score adjustment. Bias reduction was evaluated by comparing the coefficients with and without adjustment. Coefficients remained quite similar, as could be expected since propensity score distributions did not differ between treatment outcomes (eg, COVID-19-like symptoms). The propensity score was computed using a logistic regression with treatment as the outcome and age, gender, type of disease (rheumatoid arthritis (RA) vs other), smoking, concomitant corticosteroid therapy and presence of comorbidities as potential confounders.
Validation cohort
Because our sample size was limited, we planned to validate our findings in an independent larger cohort. As this study population originated only from a small part of the French-speaking regions of Switzerland (two cantons, representing less than 10% of the Swiss population), we obtained data from the larger national registry of RMDs (the Swiss Clinical Quality Management (SCQM) cohort). Because the potential overlap between the two study populations is limited to only approximately 10%, the national SCQM population represents an interesting validation cohort. We could not remove the individual patients from the original cohort out of the SCQM cohort because patient identification and place of origin were unavailable (anonymised data). The SCQM launched an independent study of prevalent COVID-19 cases using the same questionnaire. Patients were invited to answer the questionnaire via the SCQM telephone app recording information between March and December 2020.7 We used the same outcome definitions as in the main analysis, but had to use as a proxy outcome for severe COVID-19 any hospitalisation for COVID-19, as data on ICU stays, mechanical ventilation or death related to COVID-19 were not available in the SCQM app data. Prevalence of COVID-19 was calculated using GEE. As in the main analysis, the propensity score for adjustment included age, type of disease (RA vs other), concomitant corticosteroid therapy but not smoking and presence of comorbidities, as this information was not available in the SCQM app data.
Results
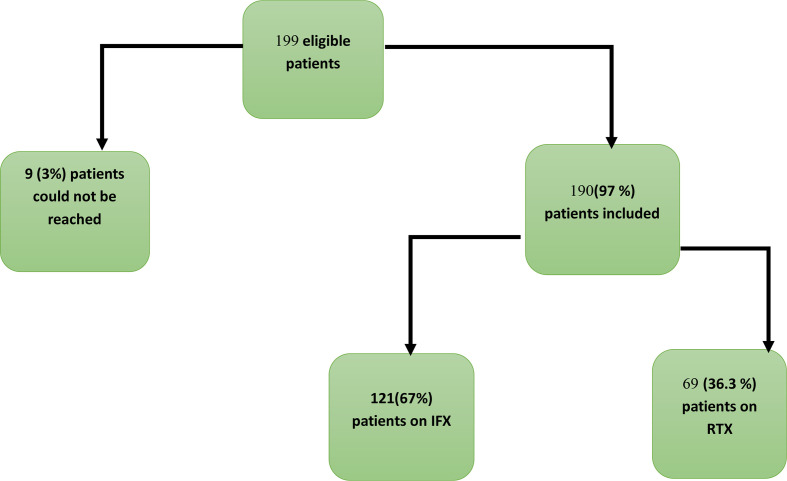
Given our study inclusion criteria, 199 patients were eligible, of whom 190 (97.0%) provided complete information (see figure 1): 63.7% (n=121) in the RTX group and 36.3% (n=69) in the IFX group. Patients on RTX were older and had somewhat different underlying diagnoses, with more RA in the RTX group and more spondyloarthritis (SpA) in the IFX group (table 1).
Figure 1.
Study flow chart shows the number of patients eligible for this study and the number of patients who were included per treatment group. IFX, infliximab; RTX, rituximab.
Table 1.
Patient baseline demographic and disease characteristics by treatment group
| Characteristics | IFX (n=69) | RTX (n=121) | P value |
| Age (years), median (IQR) | 51 (40–60) | 59 (50–72) | <0.001 |
| Sex (male), n (%) | 30 (43.5) | 20 (16.5) | <0.001 |
| At least one comorbidity, n (%) | 4 (5.8) | 15 (12.4) | 0.209 |
| Type of immune-mediated disease, n (%) | <0.001 | ||
| Rheumatoid arthritis | 17 (24.6) | 76 (62.8) | |
| Spondyloarthritis | 45 (65.2) | 0 (0) | |
| Other | 7 (10.2) | 45 (37.2) |
IFX, infliximab; IQR, Interquartile Range; RTX, rituximab.
Twenty-one patients (11.1%) reported symptoms consistent with COVID-19, of whom 10 (8.3 %) were in the RTX group and 11 (15.9 %) were in the IFX group (p=0.14). Because viral confirmation testing for SARS-CoV-2 was initially not widely available, only patients with risk factors or admitted to hospital were formally diagnosed using PCR testing. Of all patients, 26 (13.7%) were tested for SARS-CoV-2 using nasopharyngeal swab PCR tests (RTX=16, IFX=10) and a total of 11 patients were positive (RTX=7, IFX=4).
Four patients, all in the RTX group, developed severe pulmonary complications requiring acute respiratory assistance (one patient died, three patients had long stays in the ICU) and none in IFX group (Fisher’s exact test, p=0.04). The patients with severe forms of COVID-19 were relatively young, with a median age of 59 years. None had major risk factors for severe COVID-19 other than immunosuppressive therapy, but for a history of smoking in two patients and a pulmonary rheumatoid nodulosis that had required surgical treatment in one patient (see table 2).
Table 2.
Characteristics of patients with a severe course of COVID-19
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
| Age range | 40–50 | 50–60 | 50–60 | 70–80 |
| Sex | Male | Female | Female | Female |
| Underlying disease | RA + Sjogren’s syndrome | Systemic lupus erythematosus + Sjogren’s syndrome | RA | RA |
| Treatment | RTX | RTX | RTX | RTX |
| Time interval between last RTX dose and occurrence of COVID-19 (months) | 7 | 5 | 2 | 7 |
| Risk factors for severe evolution of COVID-19 | ||||
| Tobacco | Former | Former | Active | No |
| Other comorbidities | None | None | Pulmonary rheumatoid nodule with lobectomy | None |
| Intensive care | Yes | Yes | Yes | Yes |
| Death | No | No | Yes | No |
RA, rheumatoid arthritis; RTX, rituximab.
The incidence rate of symptoms consistent with COVID-19 was 0.73 (95% CI 0.39 to 1.37) cases per 1000 patient-days in the RTX group compared with 1.52 (95% CI 0.82 to 2.85) cases per 1000 patient-days in the IFX group (crude p=0.10, adjusted p=0.07).
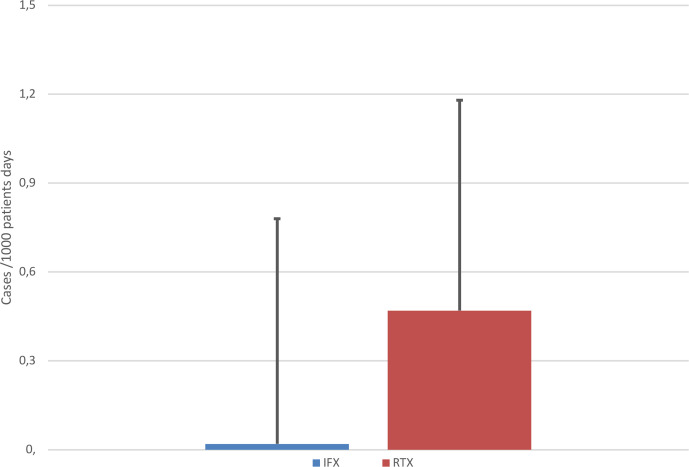
The incidence rate of severe COVID-19 was 0.28 (95% CI 0.08 to 0.72) cases per 1000 patient days on RTX compared with null (95% CI 0.0 to 0.44) on IFX (exact rate ratio p=0.10, adjusted p=0.13) (see figure 2).
Figure 2.
Incidence of severe COVID-19 in patients hospitalised with COVID-19 in the rituximab (RTX) group compared with the infliximab (IFX) group.
Validation cohort
We analysed the data from a population-based cohort using the same exposure and outcome definitions as above to confirm our findings in an independent cohort, calculating a prevalence. Forty-three patients (45.7%) in the RTX group had symptoms consistent with COVID-19 and 53 patients (47.3%) in the IFX group (p=0.9). The patients were relatively young, with an average age of 62 (IQR: 52–67) in the RTX group and 52 (IQR: 43–58) in the IFX group (see table 3).
Table 3.
Patient characteristics by treatment group in the validation cohort
| Characteristics | IFX (n=112) | RTX (n=94) | P value |
| Age (years), median (IQR) | 52 (43–58) | 62 (52–67) | <0.001 |
| Sex (male), n (%) | 52 (46.4) | 27 (28.7) | 0.010 |
| Concomitant csDMARD, n (%) | 58 (51) | 42 (46.7) | 0.330 |
| Concomitant corticosteroid, n (%) | 3 (2.7) | 16 (12) | 0.001 |
| Diagnoses, n (%) | <0.001 | ||
| Rheumatoid arthritis | 25 (22.3) | 94 (100.0) | |
| Axial spondyloarthritis | 67 (59.8) | 0 (0.0) | |
| Psoriatic arthritis | 20 (17.9) | 0 (0.0) |
Baseline patient characteristics were compared using Wilcoxon test for continuous variables and Fisher’s exact test for categorical variables.
csDMARD, conventional synthetic disease-modifying antirheumatoid drug; IFX, infliximab; RTX, rituximab.
The prevalence of symptoms compatible with COVID-19 in the previous month (precise date was unknown) was 0.46 (95% CI 0.37 to 0.57) for RTX vs 0.47 (95% CI 0.39 to 0.58) for IFX (crude p=0.82, adjusted p=0.066, adjusted prevalence ratio RTX vs IFX: 1.89, 95% CI 0.958 to 3.750). Twenty-four patients (55.8%) had a PCR test in the RTX group and 31 (58.5%) in the IFX group. None of the IFX patients developed a severe form of COVID-19, compared with three patients who had to be hospitalised in the RTX group (Fisher’s exact test, crude p=0.09). The prevalence of hospitalisation for COVID-19 was 0.032 (95% CI 0.011 to 0.097) for RTX and 0 for IFX. All three patients with severe COVID-19 had RA as an underlying disease, with one patient also on concomitant low-dose oral corticosteroids.
Discussion
Our study suggests that despite a similar incidence of COVID-19 in the IFX group, RTX patients tended to have more severe forms of SARS-CoV-2 infection. Similar trends were found in the SCQM validation cohort regarding the prevalence of hospitalisations. In our sample, patients with severe forms of COVID-19 were relatively young and had limited risk factors for severe disease, suggesting that anti-CD20 therapy could be an independent risk factor, even though the limited sample size precludes us from making definitive conclusions.
Few studies have examined the incidence of severe forms of COVID-19 with respect to specific antirheumatic therapies and data on COVID-19 in patients with RMDs are still scarce.8 9 Monti and colleagues8 suggested early on that patients with RA and SpA who received biologic disease-modifying antirheumatic drugs (bDMARDs) or targeted synthetic disease-modifying antirheumatic drugs were not at increased risk of developing severe forms of COVID-19. However, several recent publications have suggested an increased severity of COVID-19 in patients receiving RTX.10–12 A recent study using data from the Rheumatology Alliance physician registry demonstrated that RTX increased the risk of developing a severe form of COVID-19 by four times compared with patients on anti-TNF agents including IFX.13
Some case reports have also suggested a possible association between RTX treatment and poor prognosis in patients infected with SARS-CoV-2.11 12 Loarce-Martos et al14 described 76 patients who had received RTX in the last 12 months; of these 13 (17.1%) developed symptoms suggestive of COVID-19 or had a confirmed diagnosis of COVID-19, 8 of the infected patients (62%) developed severe forms of COVID-19 and 3 of these (23%) died. The patients who died in this series each had at least three comorbidities, which is different from the patients in our series, as three of these patients had no comorbidities and the patient who died in our series had lung damage due to RA that was operated on.14 A cross-sectional, case-reporting registry including 3729 COVID-19 cases suggested a fourfold increase in the probability of death from COVID-19 on RTX.15
In Spain, one of the European countries most affected by COVID-19, 10% (38 of 3711 patients) of all hospitalised patients had a rheumatic or musculoskeletal pathology. Ten out of the 38 hospitalised patients with RMDs died, of whom 1 patient was on RTX. The authors concluded that the number of patients on biotherapy in their sample was too small to draw a valid conclusion.16 Nuño and colleagues17 found that the seven patients who received RTX in their sample were hospitalised for COVID-19 compared with zero patients in the outpatient group and this finding was statistically significant. The authors concluded that taking RTX increases the risk of hospital admission.
A French cohort study of patients with RMDs compared mortality between patients hospitalised for COVID-19 with and without RMDs. They found that 34 out of 694 patients (4.9%) were on RTX, of whom 11 (32%) developed a severe form of COVID-19. The authors concluded that regarding treatments for RMDs, a more frequent severe disease was observed with use of corticosteroid, mycophenolate mofetil and RTX (OR=4.34, 95% CI 1.77 to 10.63).18 Overall, published results suggest an increased risk of developing severe forms of COVID-19 in patients on RTX.10
During the previous global pandemic with H1N1 influenza, studies had also demonstrated that patients treated with RTX had severely reduced capacity of developing an immune response to vaccination for at least 4–6 months.19 Taken together, available data suggest that RTX may increase the risk of severe viral infections, particularly during the first months following administration.
Our study has several limitations. First, we cannot exclude some residual confounding as patients on RTX and anti-TNF may differ. To address this issue, we restricted our analysis to only intravenously administered drugs and adjusted for known risk factors of severe evolution. The limited sample size also did not allow adjusted analyses for severe COVID-19. Also, in the validation cohort, some misclassification bias of severe COVID-19 may have occurred due to the fact that very ill or dead patients would not have answered the app questionnaire, which would have biased the results towards the null.
The strength of this study is that it is one of the first studies to determine the incidence and prevalence of mild and severe forms of COVID-19 in patients treated with RTX, compared with an alternative bDMARD (IFX), with the findings confirmed in an (mostly) independent confirmation cohort. Strikingly, patients on RTX who evolved to severe forms of COVID-19 were relatively young and had no relevant comorbidities other than past tobacco use, suggesting a potential causative role of anti-CD20 therapy.
Conclusion
While the incidence of COVID-19-compatible symptoms was overall similar in patients receiving RTX or IFX, severe PCR-confirmed cases of COVID-19 tended to occur more often in patients on RTX. Similar results were suggested in the analysis of the national registry, with more COVID-19 hospitalisations on RTX compared with IFX, suggesting that anti-CD20 agents may represent a possible risk factor for severe COVID-19 evolutions. While these results require replication, prudence would recommend using RTX sparingly in patients with RMDs until COVID-19 vaccination has been completed.
Acknowledgments
We would like to thank all the patients who agreed to participate in this study and all the colleagues who participated in this work. This work was awarded a price for the best abstract presentation by the Swiss Society of Rheumatology. We thank the SCQM registry for providing the data for the validation study. The SCQM cohort COVID-19 study is study is investigator initiated and financially supported by Biogen, Bristol-Myers Squibb, Gilead, Novartis, Pfizer and Sanofi.
Footnotes
Contributors: Material preparation and data collection and analysis were performed by all authors. The first draft of the manuscript was written by CMMPT and AF, and all authors commented on subsequent version of the manuscript. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article or uploaded as supplementary information. We would consider sharing raw data with researchers in a scientific collaboration. Please contact the corresponding author.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was approved by the Cantonal Commission for Research Ethics (CCER 2020-00932).
References
- 1.Pascarella G, Strumia A, Piliego C, et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med 2020;288:192–206. 10.1111/joim.13091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stringhini S, Wisniak A, Piumatti G, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet 2020;396:313–9. 10.1016/S0140-6736(20)31304-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–6. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regierer AC, Hasseli R, Schäfer M, et al. TNFi is associated with positive outcome, but JAKi and rituximab are associated with negative outcome of SARS-CoV-2 infection in patients with RMD. RMD Open 2021;7:e001896. 10.1136/rmdopen-2021-001896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulze-Koops H, Krueger K, Vallbracht I, et al. Increased risk for severe COVID-19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann Rheum Dis 2021;80:e67. 10.1136/annrheumdis-2020-218075 [DOI] [PubMed] [Google Scholar]
- 6.Multani A, Ho DY. Jc polyomavirus infection potentiated by biologics. Infect Dis Clin North Am 2020;34:359–88. 10.1016/j.idc.2020.02.007 [DOI] [PubMed] [Google Scholar]
- 7.Ciurea A, Papagiannoulis E, Bürki K, et al. Impact of the COVID-19 pandemic on the disease course of patients with inflammatory rheumatic diseases: results from the Swiss clinical quality management cohort. Ann Rheum Dis 2021;80:238–41. 10.1136/annrheumdis-2020-218705 [DOI] [PubMed] [Google Scholar]
- 8.Monti S, Balduzzi S, Delvino P, et al. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis 2020;79:667–8. 10.1136/annrheumdis-2020-217424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez-Piedra C, Diaz-Torne C, Manero J, et al. Clinical features and outcomes of COVID-19 in patients with rheumatic diseases treated with biological and synthetic targeted therapies. Ann Rheum Dis 2020;79:988–90. 10.1136/annrheumdis-2020-217948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharmeen S, Elghawy A, Zarlasht F, et al. COVID-19 in rheumatic disease patients on immunosuppressive agents. Semin Arthritis Rheum 2020;50:680–6. 10.1016/j.semarthrit.2020.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haberman R, Axelrad J, Chen A, et al. Covid-19 in immune-mediated inflammatory diseases — case series from New York. N Engl J Med Overseas Ed 2020;383:85–8. 10.1056/NEJMc2009567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sparks JA, Wallace ZS, Seet AM, et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 global rheumatology alliance physician registry. Ann Rheum Dis 2021;80:1137–46. 10.1136/annrheumdis-2021-220418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loarce-Martos J, García-Fernández A, López-Gutiérrez F, et al. High rates of severe disease and death due to SARS-CoV-2 infection in rheumatic disease patients treated with rituximab: a descriptive study. Rheumatol Int 2020;40:2015–21. 10.1007/s00296-020-04699-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis 2021;80:930–42. 10.1136/annrheumdis-2020-219498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santos CS, Morales CM, Álvarez ED, et al. Determinants of COVID-19 disease severity in patients with underlying rheumatic disease. Clin Rheumatol 2020;39:2789–96. 10.1007/s10067-020-05301-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nuño L, Novella Navarro M, Bonilla G, et al. Clinical course, severity and mortality in a cohort of patients with COVID-19 with rheumatic diseases. Ann Rheum Dis 2020;79:1659–61. 10.1136/annrheumdis-2020-218054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Florence A, Nassim AA, Jean-David A, et al. Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: data from the French RMD COVID-19 cohort of 694 patients. Ann Rheum Dis 2020;80:527–38. 10.1136/annrheumdis-2020-218310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapetanovic MC, Kristensen L-E, Saxne T, et al. Impact of anti-rheumatic treatment on immunogenicity of pandemic H1N1 influenza vaccine in patients with arthritis. Arthritis Res Ther 2014;16:R2. 10.1186/ar4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2021-001711supp001.pdf (51.8KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article or uploaded as supplementary information. We would consider sharing raw data with researchers in a scientific collaboration. Please contact the corresponding author.