Abstract
Background
Clostridioides difficile infection (CDI) is a considerable healthcare burden, and now identified as the leading cause of acquired diarrheal illness in patients receiving antibiotics. Patients with malignancies are more prone to acquire CDI, owing to their frequent exposure to risk factors.
Objective
This study aims to investigate the factors affecting the outcome of Clostridioides Difficile Infection in patients with solid tumors at our community healthcare center.
Methods
This is a retrospective study that included a total of 59 patients with solid tumors who were hospitalized for Clostridioides difficile infection.
Results
The median age of the study population was 79 years with 39 males and 20 females. The patients had a diagnosis of a malignancy involving the following sites: prostate (25), lung (19), colon (7), bladder (4), breast (3), and renal (1). There were 52 cases of first time and 7 cases of recurrent CDI admissions. 40 patients were detected to have CDI at presentation while 19 patients were diagnosed with CDI after admission. CDI was categorized as follows: non-severe (29), severe (28), and very severe (2). There were 33 patients on chemotherapy and 20 patients undergoing radiotherapy. Twenty-seven patients had a recent history of cancer care-related procedures or interventions. Twenty-nine patients were from either a rehabilitation center or a long-term nursing care facility. There were 39 recent hospitalizations with 29 patients receiving antibiotics. Almost half of the patients were on proton pump inhibitors (29) and 12 were on steroids (20.3%) at the time of developing CDI. Patients with a high-risk qSOFA score of 2 or more (p-value = 0.008) or a high white blood cell count of >15 × 109/L (p-value = 0.016) at the time of admission were found to have higher in-hospital mortality. Critical care data suggested that 9 patients required intensive care, 7 patients required vasopressor support, and 6 needed mechanical ventilation. Patients were treated with either vancomycin alone (13), or metronidazole alone (25), or combination therapy with vancomycin + metronidazole (21). The median duration of hospital stay was 6 days with 11 fatalities (18.64%).
Conclusions
CDI causes significant morbidity in patients with malignancies. A high qSOFA score and leukocytosis are significantly associated with high morbidity and thus should be used to prioritize and intensify inpatient care of these patients.
Keywords: Clostridioides difficile infection, Cancer, Chemotherapy, Immunotherapy, Antimicrobials
Clostridioides difficile infection; Cancer; Chemotherapy; Immunotherapy; Antimicrobials.
1. Introduction
The last few decades have seen exponential growth in cancer treatment. Newer diagnostic tools and drugs have improved the overall morbidity and mortality of patients with malignancies. Treatment modalities including immunotherapy, targeted therapy, and focused radiation therapy have provided a wide spectrum of the armamentarium for oncologists to treat their patients [1]. Unfortunately, anticancer medications have their associated side effects including the risk of superadded infections [2].
Among the infections, Clostridioides difficile (C. difficile) infection is an extremely lethal infection with increased morbidity and mortality [3]. Data from the United States have shown that amongst the healthcare-associated infections, C. difficile accounts for approximately 15% of all infections [4]. Patients with cancer are at an elevated risk to acquire C. difficile infection because of multiple visits to chemotherapy centers, infusion centers, hospitalizations, and increased chemo/radiotherapy associated risk factors like myelosuppression and change in gut flora microbiota. These risk factors can be roughly stratified to three major categories-(1) medications/pharmacology related (2) Host related, and (3) Intervention related [5, 6]. Data gathered from the hospitalized adults diagnosed with cancer in United States from 2001–2010 showed overall incidence of C. difficile to be 8.6 discharges per 1000 adult cancer discharges [7, 8].
C. difficile infection not only poses a huge financial burden but also increases the non–cancer-related mortality rates in patients with malignancies.
2. Methodology
2.1. Patients and methodology
Search strategy: This is a retrospective study conducted at a community hospital, Worcester, Massachusetts, United States that included all hospital admissions for C. difficile infection in cancer patients who presented to us between January 2009 to December 2019. This is a 381 bedded hospital which is one of the busiest community hospitals in Central Massachusetts. It has a full-fledged inpatient service including an intensive care unit and associated outpatient cancer wellness centre. The detection method for C. difficile infection in our hospital was done by using stool sample with Nucleic Acid Amplification Test (NAAT). The study was approved by the institutional review board (IRB# 2019-102).
2.1.1. Selection and inclusion criteria
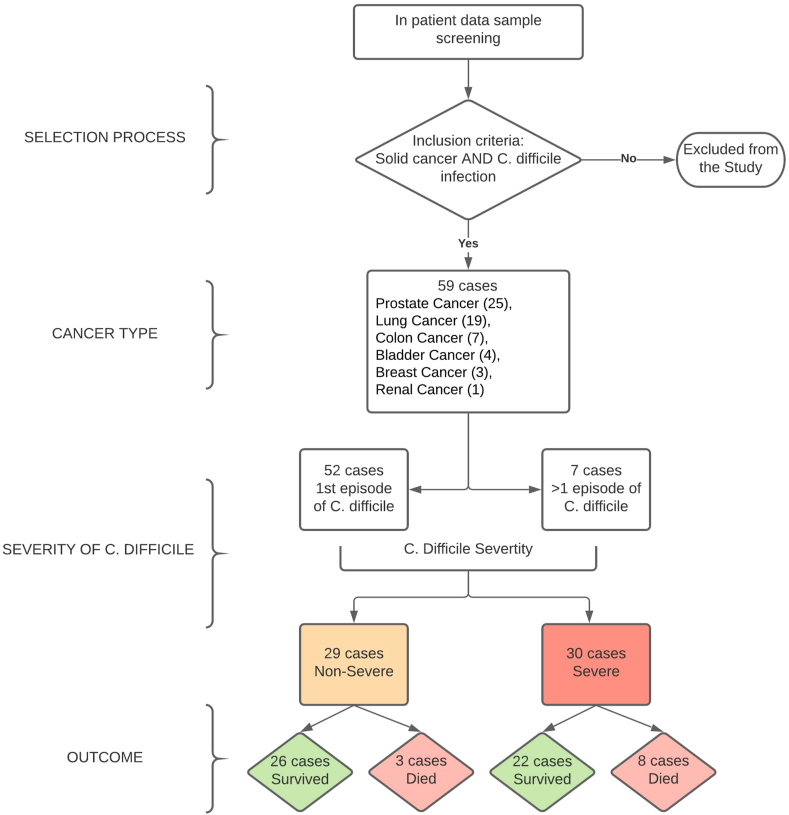
All adult (age greater than 18 years) patients with malignancies namely solid tumors who presented to our hospital and were diagnosed with C. difficile were included in this retrospective analysis. Oncology patients with diagnoses other than C. difficile infection were excluded from the study (Figure 1).
Figure 1.
Synopsis of the selection of patients, their cancer types, the severity of infection with the outcome.
Data extraction: All selected patients’ case records were thoroughly reviewed, data were extracted and entered in a predefined excel sheet. For the defined period of the study: age of the patient, risk factors for infection, details on the usage of anti-microbiological agents in recent past, first versus recurrence C. Difficile infection, duration of hospital stay, hemodynamic instability, the requirement of vasopressors, type of treatment, mortality and outcome were recorded retrospectively.
Data analysis: Statistical analysis was done using SPSS, version 16. Means and percentages were reported for continuous and categorical variables, respectively. Laboratory results (worst value documented in the hospital) were tabulated, and variables were studied with in-hospital mortality as the primary outcome. To compare the differences between categorical patient variables, chi-square test was used. A p value of less than 0.05 was considered statistically significant. We studied other variables in our patient set for their impact on mortality. We stratified patients for greater risk for a poor outcome using the qSOFA score system which is a bedside prompt that may identify patients with suspected infection who are outside the intensive care unit (ICU). It comprises of three criteria scoring system, with one point each for hypotension (SBP ≤100 mmHg), tachypnea (≥22 breaths per min), or impaired mentation (GCS score <15). 41 patients fell into the low-risk category (0–1 score), while 18 patients fell into the high risk (2–3 score) category. The qSOFA score was calculated on the day of diagnosis of C. Difficile was made.
Stratification of C. difficile: Patients with C. difficile infection were divided into categories (non-severe, severe, and very severe) based on the following established criteria: Non-severe is defined as patients with white blood cell count less than 15.00 × 109/L and serum creatinine less than 1.5 mg/dL. Severe C. difficile infection was defined as white blood cell count more than 15.00 × 109/L or serum creatinine equal or more than 1.5 mg/dL. Very severe/fulminant colitis was defined as patients with shock, ileus, or megacolon [9].
3. Results
3.1. Demographics of patients and cancer descriptions
A total number of 59 patients were found to have solid cancers and C. difficile infection. The median age of the study population was 79 years with 39 males and 20 females. The patients were suffering from malignancies located at the following sites: prostate (25 patients), lung (19 patients), colon (7 patients), bladder (4 patients), breast (3 patients), and renal (1 patient).
Data on C. Difficile infection: We reviewed the electronic medical records and found 52 cases with the first episode of C. difficile infection-related admissions. The other 7 cases had 2 or more C. difficile infection-related admissions.
Amongst the 59 patients, 40 patients were detected to have C. difficile infection at presentation while the remaining 19 patients were diagnosed with C. difficile infection after admission and during the course of hospitalization (Table 1). Among the 19 patients, who developed infection during hospitalization, our review showed that 7 patients had hospital acquired infections (that is the occurrence of symptoms were after 3 days of admission to hospital). In our patient dataset, based on the abovementioned criteria, we had non-severe (29 cases), severe (28 cases), and very severe/fulminant (2 cases). There were 33 patients and 20 patients actively receiving chemotherapy and radiotherapy, respectively. Six patients were not on any treatment at the time of admission. Table 2 mentions descriptive data of the cohort including age, gender, and proportions of variables that were evaluated as risk factors.
Table 1.
Indication of the hospitalization of C. difficile patients.
| Reason for admission | |
|---|---|
| C. difficile related | 40 |
| Pneumonia | 7 |
| Dehydration, chemotherapy side effects | 4 |
| Urinary tract infection | 4 |
| Congestive heart failure | 2 |
| COPD | 2 |
| Total patient | 59 |
Table 2.
Descriptive data of the cohort including age, gender, and proportions of variables that were evaluated as risk factors.
| Median Age | 79 years | |
| Patient population | Total | 59 |
| Males | 39 | |
| Females |
20 |
|
| Malignancy types | Prostate | 25 |
| Lung | 19 | |
| Colon | 7 | |
| Bladder | 4 | |
| Breast | 3 | |
| Renal |
1 |
|
| C. Difficile severity | Non-Severe | 29 |
| Severe | 28 | |
| Very Severe |
2 |
|
| Treatment | Chemotherapy | 33 |
| Radiotherapy |
20 |
|
| Variables | Cancer related procedures | 27 |
| Nursing home residents | 29 | |
| Recent hospitalizations | 39 | |
| Recent Antibiotic use | 29 | |
| Proton Pump use | 29 | |
| Steroid use |
12 |
|
| Comorbidities | COPD | 15 |
| Congestive heart failure | 11 | |
| Chronic Kidney Disease | 10 | |
| Diabetes mellitus | 5 | |
| Inflammatory bowel disease | 2 | |
| Asthma | 2 | |
| Interstitial Lung disease |
1 |
|
| Laboratory Data | Parameters | Mean value |
| WBC count | 16.95 × 10 9/L | |
| Hemoglobin | 9.66 gm/dL | |
| Platelet count | 210,650/microlitre | |
| Creatinine | 1.41 mg/dL | |
| Albumin | 2.56 gm/dL | |
| Lactate level | 2.45 mmol/L | |
Associated comorbidities: We also studied our patients for the various comorbidities. The most commonly associated comorbidities were COPD (15 cases), congestive heart failure (11 cases), chronic kidney disease (10 cases), diabetes mellitus (5 cases), inflammatory bowel disease, asthma (2 cases), and interstitial lung disease (1 case). We did not compare these factors with the mortality outcome due to the small sample size.
Predisposing risk factors: In our study, we found that 27 patients had a recent history of cancer care-related procedures or interventions done within 3 months of admission to our hospital. 29 patients were from either a rehabilitation center or resident of a long-term nursing facility. There were 39 recent hospitalizations (<90 days) with 29 patients receiving antibiotics for various reasons (during prior hospitalization or as outpatient treatment). Almost half of the patients were on proton pump inhibitors (29 patients) and 12 were on steroids (20.3%) at the time of developing C. difficile infection.
Laboratory data: We also studied various laboratory parameters in our study population. Mean WBC count of the patients was 16.95 × 109/L (59 patients, Range: 0.5 to 40.3 × 109/L). Mean hemoglobin value was 9.66 g/dL (59 patients, Range: 6.2–14.9 g/dL). The mean platelet count was 210,650 per microliter (59 patients, Range 9000 to 435,000 per microliter). Analysis of the CBC data suggested that the impact of leukocytosis (59 patients, WBC >15 × 109/L) was significant with higher in-hospital mortality (P value: 0.016, CI: 0.030–0.815). There was no significant impact of anemia (defined as < 9 g/dL) and thrombocytopenia (defined as < 150,000 per microliter) on the survival outcome of our patients.
Similarly, we studied the comprehensive metabolic profile of our patients. Mean creatinine value was noted to be 1.41 mg/dL (59 patients, Range: 0.39–7.02 mg/dL). The mean serum albumin was 2.56 g/dL (49 patients, Range 1.4–4.1 g/dL). The mean serum lactate was 2.45 mmol/L (32 patients, Rane 0.6–14.1 mmol/L). Analysis of the comprehensive metabolic profile data suggested that there was no impact of lactic acidosis, deranged renal function, or hypoalbuminemia on the survival outcome of our patients.
Other parameters: We found that patients with a high-risk qSOFA score of 2 or more (p-value = 0.008) were found to have higher in-hospital mortality. Recent hospitalization, use of proton pump inhibitors, steroids, staying in rehabilitation/nursing home were not associated with clinically significant mortality (Table 3).
Table 3.
Analyzing various laboratory and clinical parameters with the in-hospital mortality.
| Impact of ICU stay on mortality | ||||
| Required ICU stay |
Survived |
Died |
Marginal Row Totals |
P value |
| Yes | 7 (7.32) [0.01] | 2 (1.68) [0.06] | 9 | 0.764 |
| No | 41 (40.68) [0] | 9 (9.32) [0.01] | 50 | |
| Marginal Column Totals |
48 |
11 |
59 (Grand Total) |
|
|
Impact of hospital stay on mortality | ||||
|
Hospital stay |
Alive |
Died |
Marginal Row Totals |
P value |
| <5 days | 20 (18.71) [0.09] | 3 (4.29) [0.39] | 23 | 0.370 |
| >5 days | 28 (29.29) [0.06] | 8 (6.71) [0.25] | 36 | |
| Marginal Column Totals |
48 |
11 |
59 (Grand Total) |
|
|
Impact of qSOFA stratification on mortality | ||||
|
qSOFA risk category |
Survived |
Died |
Marginal Row Totals |
P value |
| Low risk (0–1 score) | 37 (33.36) [0.4] | 4 (7.64) [1.74] | 41 | 0.008 OR-0.636 (CI:0.041–0.689) Significant |
| High risk (2–3 score) | 11 (14.64) [0.91] | 7 (3.36) [3.96] | 18 | |
| Marginal Column Totals |
48 |
11 |
59 (Grand Total) |
|
|
Impact of cancer type on mortality | ||||
|
Cancer type |
Survived |
Died |
Marginal Row Totals |
P value |
| Colon cancer | 6 (5.69) [0.02] | 1 (1.31) [0.07] | 7 | |
| Cancers other than colon cancer | 42 (42.31) [0] | 10 (9.69) [0.01] | 52 | 0.107 |
| Marginal Column Totals |
48 |
11 |
59 (Grand Total) |
|
|
Impact of severity of C. difficile on mortality | ||||
|
C. difficile severity |
Survived |
Died |
Marginal Row Totals |
P value |
| Non severe | 26 (23.59) [0.25] | 3 (5.41) [1.07] | 29 | 0.752 |
| Severe/Very Severe | 22 (24.41) [0.24] | 8 (5.59) [1.04] | 30 | |
| Marginal Column Totals |
48 |
11 |
59 (Grand Total) |
|
|
Impact of severity of chemotherapy on mortality | ||||
|
Chemotherapy |
Survived |
Died |
Marginal Row Totals |
P value |
| Yes | 29 (26.85) [0.17] | 4 (6.15) [0.75] | 33 | 0.147 |
| No | 19 (21.15) [0.22] | 7 (4.85) [0.96] | 26 | |
| Marginal Column Totals |
48 |
11 |
59 (Grand Total) |
|
|
Impact of rehab/nursing facility stay on mortality | ||||
|
Rehab/Nursing facility |
Survived |
Died |
Marginal Row Totals |
P value |
| Yes | 22 (23.59) [0.11] | 7 (5.41) [0.47] | 29 | 0.286 |
| No | 26 (24.41) [0.1] | 4 (5.59) [0.45] | 30 | |
| Marginal Column Totals |
48 |
11 |
59 (Grand Total) |
|
|
Impact of recent hospitalization stay on mortality | ||||
|
Recent hospitalization |
Survived |
died |
Marginal Row Totals |
P value |
| Yes | 29 (31.73) [0.23] | 10 (7.27) [1.02] | 39 | 0.053 |
| No | 19 (16.27) [0.46] | 1 (3.73) [2] | 20 | |
| Marginal Column Totals |
48 |
11 |
59 (Grand Total) |
|
|
Impact of recent antibiotics use on mortality | ||||
|
Recent antibiotic use |
Survived |
Died |
Marginal Row Totals |
P value |
| Yes | 21 (23.59) [0.29] | 8 (5.41) [1.24] | 29 | 0.082 |
| No | 27 (24.41) [0.28] | 3 (5.59) [1.2] | 30 | |
| Marginal Column Totals |
48 |
11 |
59 (Grand Total) |
|
|
Impact of recent proton pump inhibitors use on mortality | ||||
|
Proton pump inhibitors |
Survived |
Died |
Marginal Row Totals |
P value |
| Yes | 23 (23.59) [0.01] | 6 (5.41) [0.07] | 29 | 0.691 |
| No | 25 (24.41) [0.01] | 5 (5.59) [0.06] | 30 | |
| Marginal column totals |
48 |
11 |
59 (Grand Total) |
|
|
Impact of leukocytosis (WBC >15 x109/L) on mortality | ||||
|
WBC >15 x109/L |
Survived |
Died |
Marginal Row Totals |
P value |
| WBC <15 | 28 (24.41) [0.53] | 2 (5.59) [2.31] | 30 | 0.016 OR-0.1587 (CI: 0.030–0.815) Significant |
| WBC >15 | 20 (23.59) [0.55] | 9 (5.41) [2.39] | 29 | |
| Marginal Column Totals |
48 |
11 |
59 (Grand Total) |
|
|
Impact of anemia (< 90 g/L) on mortality | ||||
|
Hemoglobin (< 90 g/L) |
Survived |
Died |
Marginal Row Totals |
P value |
| Yes | 19 (18.71) [0] | 4 (4.29) [0.02] | 23 | 0.843 |
| No | 29 (29.29) [0] | 7 (6.71) [0.01] | 36 | |
| Marginal Column Totals |
48 |
11 |
59 (Grand Total) |
|
|
Impact of renal dysfunction (S. creatinine >1.5 mg/dL) on mortality | ||||
|
S. creatinine |
Survived |
Dead |
Marginal Row Totals |
P value |
| <1.5 | 33 (33.36) [0] | 8 (7.64) [0.02] | 41 | 0.796 |
| >1.5 | 15 (14.64) [0.01] | 3 (3.36) [0.04] | 18 | |
| Marginal Column Totals |
48 |
11 |
59 (Grand Total) |
|
|
Impact of hypoalbuminemia on mortality | ||||
|
S. albumin level (g/dL) |
Survived |
Died |
Marginal Row Totals |
P value |
| <3 g/dL | 27 (28.69) [0.1] | 10 (8.31) [0.35] | 37 | 0.177 |
| >3 g/dL | 11 (9.31) [0.31] | 1 (2.69) [1.07] | 12 | |
| Marginal Column Totals |
38 |
11 |
49 (Grand Total) |
|
|
Impact of lactic acidosis on mortality | ||||
|
S. Lactate (mmol/L) |
Survived |
Died |
Marginal Row Totals |
P value |
| <2 | 16 (15.75) [0] | 5 (5.25) [0.01] | 21 | 0.829 |
| >2 | 8 (8.25) [0.01] | 3 (2.75) [0.02] | 11 | |
| Marginal Column Totals | 24 | 8 | 32 (Grand Total) | |
3.2. Treatment and outcome
Critical care data suggested that 9 patients required intensive care therapy, 7 patients required vasopressor support, and 6 needed mechanical ventilation. Patients were treated per guideline-directed therapy (following Infectious Diseases Society of America guidelines; https://www.idsociety.org/practice-guideline/clostridium-difficile/) with either oral vancomycin alone (13 patients), or intravenous metronidazole alone (25 patients), or combination therapy with oral vancomycin and intravenous metronidazole (21 patients). The median duration of hospital stay was 6 days with 11 fatalities (18.64%). None of the patients were given fidaxomicin or fecal transplant treatment.
4. Discussion
Clostridioides difficile is the most common cause of nosocomial diarrhea [10]. It is associated with significant mortality and morbidity especially in old, frail individuals who are immunocompromised and have multiple comorbidities. As per the latest data from the Centers for Disease Control and Prevention (CDC), nearly half a million Clostridioides difficile infections are reported just from the United States. Of these, 15,000 deaths were assessed to be causally related to C. difficile infections [11]. In addition, recurrence rate is significantly high which adds to both health care and financial burden. Musher et al is their study of 207 patients reported a recurrence rate of 28% [12]. The problem of recurrence worsens in patients with cancer than non-cancer patients and this was reported by Chung et al in their recent study [13]. Unfortunately, with the advent of newer therapies like immunotherapy, cellular therapy and various target molecules, the incidence of opportunistic and nosocomial infections are expected to rise further [14, 15].
Studies in the recent past have suggested that patients with malignancies have higher mortality and longer duration of hospital stay as compared to patients without cancer [8, 16]. Delgado et al studied the data of Clostridioides difficile hospital discharges from 2001 to 2010. They found that cancer patients had 9.4% mortality rates as compared to non-cancer patients (7.5%, p < 0.0001). Also, the hospital stay was longer in patients with malignancies than those without that diagnosis (9 days vs. 4 days, p < 0.0001) [7]. We did not do a similar comparative study between cancer and non-cancer patients due to the small sample size. 67% of our patient cohort had Clostridioides difficile infection at presentation while the remaining 33% developed Clostridioides difficile infection during their hospital stay. Various opportunistic infections like the Clostridioides difficile infections acquired during the hospital stay is a huge challenge for oncologists because patients undergoing therapy for malignancies often have prolonged hospital stays due to several reasons and hence are more prone to acquire Clostridioides difficile infection [17]. Kamboj et al in their multicenter survey of 11 cancer centers found that pooled rates to develop hospital-onset Clostridioides difficile infection in cancer patients were twofold when compared to all US general patient population (15.8 vs 7.4 per 10,000 patient-days) [8].
Various factors have been very well studied and are known to be associated with an increased risk of developing Clostridioides difficile infection. These include prolonged use of broad-spectrum antibiotics, frequent hospitalizations, long-term residence at nursing care facilities, use of proton pump inhibitors, and steroids [18, 19, 20]. There are various other factors specific to oncology care patients that make them more vulnerable including (10 the use of chemotherapy, (2) newer immunotherapy and targeted drugs, (3) high and prolonged doses of steroids, (4) use of feeding tube, (5) multiple visits to hospitals and infusion centers and, (6) mucositis to list a few [16, 21, 22, 23, 24, 25, 26]. As mentioned in our study as well, we found that 45% of patients (27/59) underwent cancer care-related procedures or interventions in the recent past. Yeom et al in their recent study on 219 patients with colorectal cancer found Preoperative metallic stent insertion and age more than 60 years as risk factors for postoperative C. difficile-associated colitis [29]. It is being postulated that there may be a potential change in intestinal microbiota following colorectal surgery which could predispose to develop C. colitis [28].
Almost half of our study population were (29/59) patients who were either from a rehabilitation center or long-term care nursing facility. Also, there was a significant number of recent hospitalizations (39 hospitalization events in total <90 days). Twenty-nine patients received a recent course of antibiotics for various reasons. These findings are in alignment with the established data on predisposing/risk factors for Clostridioides difficile infection in non-cancer patients. These findings were also confirmed in the recent study conducted in nine hospitals from seven European countries. In this study by Czepiel et al, a total of 624 patients were included. The frequency of co-morbidities was studied as a potential risk factor for CDI mortality. Malignancy was found to be associated as risk factor for CDI related mortality [27].
Studies at the microbiological level have shown that anti-cancer medications can alter the intestinal microbiota and have a deleterious impact on the intestinal ecology [26]. For instance, 5-Fluorouracil (5-FU) which is commonly used to treat solid cancers has been found to alter the intestinal microbiota [30,31,]. Recently, there have been frequent reports on the association of newer molecular therapies with Clostridioides difficile infection [32]. In our patient data, we could not retrieve detailed information regarding the chemotherapy/immunotherapy protocols used due to the inaccessibility to patient oncological treatment charts.
Another important fact to note is the wide differentials of gastrointestinal symptoms that an oncology patient can have; chemotherapy-induced mucositis, immune colitis, and non- Clostridioides difficile colitis. Garzotto et al did a retrospective study on 225 patients with solid tumors who were hospitalized with diarrhea. They found 39 of 225 patients (17.3 %) were subsequently diagnosed with Clostridioides difficile infection. They did not find any association between specific chemotherapy and risk to develop Clostridioides difficile infection. Interestingly, they found that patients with breast cancer had a greater predisposition to Clostridioides difficile infection. In contrast, patients with gastrointestinal malignancy had a lesser predisposition [23]. Leukocytosis (WBC >15 × 109/L), and high Q SOFA score (High risk, 2–3 score) were the only two factors noted in our patient study group which was associated with high inpatient mortality.
In our patients, the treatment protocol was decided by the infectious disease specialists depending on the severity of the disease and guideline-directed treatment protocols. The use of fecal microbiota transplantation (FMT) in cancer patients has also recently been explored. However, the major challenge remains the risk of donor-derived infection in the recipients [33].
4.1. Limitation of the study
We acknowledge a few potential shortcomings of the study due to the design of a retrospective model and associated potential confounders. Other important limitation noted was in determining symptomatic severity of CDI and temporal association with risk factors. In addition, the details of cancer treatment of our patients could not be confirmed because of the inaccessibility of chemotherapy electronic medical records. Only patients with solid malignancies were included in the study since our center does not have a bone marrow transplant unit and inpatient leukemia service unit.
5. Conclusion
Clostridioides difficile remains a major hurdle to improve the overall survival of patients with cancer. This interrupts the normal cycles of chemotherapy and radiotherapy and hence can have a serious impact on achieving or maintaining remission. Through this study, we emphasize the importance of early recognition, diagnosis, and treatment of Clostridioides difficile infections in patients with malignancies.
Declarations
Author contribution statement
Kamal Kant Sahu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Susan George: Performed the experiments; Wrote the paper.
Ahmad Daniyal Siddiqui: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ajay Mishra and Vishal Jindal: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
None.
References
- 1.Sahu K.K., Mishra A., Chastain I. Novel anticancers and dermatological adversities: old rivals but new challenges. BMJ Case Rep. 2018 Dec 14;11(1) doi: 10.1136/bcr-2018-227790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghanem S., Kim C.J., Dutta D., Salifu M., Lim S.H. Antimicrobial therapy during cancer treatment: beyond antibacterial effects. J. Intern. Med. 2020 Dec 29 doi: 10.1111/joim.13238. [DOI] [PubMed] [Google Scholar]
- 3.Marra A.R., Perencevich E.N., Nelson R.E., Samore M., Khader K., Chiang H.-Y., et al. Incidence and outcomes associated with Clostridium difficile infections: a systematic review and meta-analysis. JAMA Netw Open. 2020 Jan 3;3(1) doi: 10.1001/jamanetworkopen.2019.17597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dutta D., Jafri F., Stuhr D., Knoll B.M., Lim S.H. A contemporary review of Clostridioides difficile infections in patients with haematologic diseases. J. Intern. Med. 2020 Sep 10 doi: 10.1111/joim.13173. [DOI] [PubMed] [Google Scholar]
- 5.Legenza L.M., Barnett S.G., Rose W.E. Vaccines in development for the primary prevention of Clostridium difficile infection. J. Am. Pharm. Assoc. JAPHA. 2017 Aug;57(4):547–549. doi: 10.1016/j.japh.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Eze P., Balsells E., Kyaw M.H., Nair H. Risk factors for Clostridium difficile infections - an overview of the evidence base and challenges in data synthesis. J. Glob. Health. 2017 Jun;7(1) doi: 10.7189/jogh.07.010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delgado A., Reveles I.A., Cabello F.T., Reveles K.R. Poorer outcomes among cancer patients diagnosed with Clostridium difficile infections in United States community hospitals. BMC Infect. Dis. [Internet] 2017 Jun 23;17 doi: 10.1186/s12879-017-2553-z. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5481960/ [cited 2021 Jan 16] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamboj M., Son C., Cantu S., Chemaly R.F., Dickman J., Dubberke E., et al. Hospital-onset Clostridium difficile infection rates in persons with cancer or hematopoietic stem cell transplant: a C3IC network report. Infect. Control Hosp. Epidemiol. 2012 Nov;33(11):1162–1165. doi: 10.1086/668023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kachrimanidou M., Malisiovas N. Clostridium difficile infection: a comprehensive review. Crit. Rev. Microbiol. 2011 Aug;37(3):178–187. doi: 10.3109/1040841X.2011.556598. [DOI] [PubMed] [Google Scholar]
- 10.Lal A., Davaro R., Mishra A.K., Sahu K.K., Abraham G.M. Detection of coexisting toxigenic Clostridium difficile and nontyphoidal Salmonella in a healthcare worker with diarrhea: a therapeutic dilemma. J. Fam. Med. Prim. Care. 2019 Aug;8(8):2724–2727. doi: 10.4103/jfmpc.jfmpc_227_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC Press Releases [Internet] CDC; 2016. https://www.cdc.gov/media/releases/2015/p0225-clostridium-difficile.html [cited 2021 Jan 16]. Available from: [Google Scholar]
- 12.Musher D.M., Aslam S., Logan N., Nallacheru S., Bhaila I., Borchert F., et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005 Jun 1;40(11):1586–1590. doi: 10.1086/430311. [DOI] [PubMed] [Google Scholar]
- 13.Chung M.S., Kim J., Kang J.O., Pai H. Impact of malignancy on Clostridium difficile infection. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2016 Nov;35(11):1771–1776. doi: 10.1007/s10096-016-2725-6. [DOI] [PubMed] [Google Scholar]
- 14.Sahu K.K., Mahagaokar K., Patel B., Winokur D., Suzuki S., Daly J.S., et al. Strongyloides stercoralis hyperinfection syndrome in mantle cell lymphoma in post-transplant setting. Ann. Hematol. 2020 May 6 doi: 10.1007/s00277-020-04049-8. [DOI] [PubMed] [Google Scholar]
- 15.Mishra A.K., Sahu K.K., James A. Disseminated herpes zoster following treatment with benralizumab. Clin. Res. J. 2019 Mar;13(3):189–191. doi: 10.1111/crj.12998. [DOI] [PubMed] [Google Scholar]
- 16.Neemann K., Freifeld A. Clostridium difficile-associated diarrhea in the oncology patient. J. Oncol. Pract. 2017 Jan;13(1):25–30. doi: 10.1200/JOP.2016.018614. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S., Singh P., Sahu K.K., Rajwanshi A., Malhotra P., Naseem S. Histoplasmosis in pleural effusion in a 23-year-old man with mixed-phenotype acute leukemia. Lab. Med. 2017 Aug 1;48(3):249–252. doi: 10.1093/labmed/lmx021. [DOI] [PubMed] [Google Scholar]
- 18.Djuikoue I.C., Tambo E., Tazemda G., Njajou O., Makoudjou D., Sokeng V., et al. Evaluation of inpatients Clostridium difficile prevalence and risk factors in Cameroon. Infect. Dis. Poverty. 2020 Aug 31;9(1):122. doi: 10.1186/s40249-020-00738-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerding D.N., Johnson S. Advances in pathogenesis, diagnosis and management of CDI. Nat. Rev. Gastroenterol. Hepatol. 2011 Feb;8(2):67–68. doi: 10.1038/nrgastro.2010.215. [DOI] [PubMed] [Google Scholar]
- 20.Ananthakrishnan A.N. Clostridium difficile infection: epidemiology, risk factors and management. Nat. Rev. Gastroenterol. Hepatol. 2011 Jan;8(1):17–26. doi: 10.1038/nrgastro.2010.190. [DOI] [PubMed] [Google Scholar]
- 21.Hautmann M.G., Hipp M., Kölbl O. Clostridium difficile-associated diarrhea in radiooncology: an underestimated problem for the feasibility of the radiooncological treatment? Radiat. Oncol. 2011 Aug 1;6(1):89. doi: 10.1186/1748-717X-6-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stringer A.M. Interaction between Host cells and microbes in chemotherapy-induced mucositis. Nutrients. 2013 May;5(5):1488–1499. doi: 10.3390/nu5051488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez Garzotto A., Mérida García A., Muñoz Unceta N., Galera Lopez M.M., Orellana-Miguel M.Á., Díaz-García C.V., et al. Risk factors associated with Clostridium difficile infection in adult oncology patients. Support. Care Cancer. 2015 Jun 1;23(6):1569–1577. doi: 10.1007/s00520-014-2506-7. [DOI] [PubMed] [Google Scholar]
- 24.McCaleb R.V., Gandhi A.S., Clark S.M., Clemmons A.B. Clinical outcomes of Acid suppressive therapy use in hematology/oncology patients at an academic medical center. Ann. Pharmacother. 2016 Jul 1;50(7):541–547. doi: 10.1177/1060028016644469. [DOI] [PubMed] [Google Scholar]
- 25.Bishop K.D., Castillo J.J. Risk factors associated with Clostridium difficile infection in adult oncology patients with a history of recent hospitalization for febrile neutropenia. Leuk. Lymphoma. 2012 Aug 1;53(8):1617–1619. doi: 10.3109/10428194.2012.654472. [DOI] [PubMed] [Google Scholar]
- 26.Anand A., Glatt A.E. Clostridium difficile infection associated with antineoplastic chemotherapy: a review. Clin. Infect. Dis. 1993 Jul 1;17(1):109–113. doi: 10.1093/clinids/17.1.109. [DOI] [PubMed] [Google Scholar]
- 27.Czepiel J., Krutova M., Mizrahi A., et al. Mortality following Clostridioides difficile infection in Europe: a retrospective multicenter case-control study. Antibiotics (Basel) 2021;10(3):299. doi: 10.3390/antibiotics10030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koliarakis I., Athanasakis E., Sgantzos M., et al. Intestinal microbiota in colorectal cancer surgery. Cancers (Basel) 2020;12(10):3011. doi: 10.3390/cancers12103011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeom C.H., Cho M.M., Baek S.K., Bae O.S. Risk factors for the development of Clostridium difficile-associated colitis after colorectal cancer surgery. J. Korean Soc. Coloproctol. 2010;26(5):329–333. doi: 10.3393/jksc.2010.26.5.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin X.B., Dieleman L.A., Ketabi A., Bibova I., Sawyer M.B., Xue H., et al. Irinotecan (CPT-11) chemotherapy alters intestinal microbiota in tumour bearing rats. PLoS One. 2012 Jul 26;7(7) doi: 10.1371/journal.pone.0039764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bültzingslöwen I von, Adlerberth I., Wold A.E., Dahlén G., Jontell M. Oral and intestinal microflora in 5-fluorouracil treated rats, translocation to cervical and mesenteric lymph nodes and effects of probiotic bacteria. Oral Microbiol. Immunol. 2003;18(5):278–284. doi: 10.1034/j.1399-302x.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 32.Babacan N.A., Tanvetyanon T. Superimposed Clostridium difficile infection during checkpoint inhibitor immunotherapy-induced colitis. J. Immunother. Hagerstown Md. 1997;42(9):350–353. doi: 10.1097/CJI.0000000000000270. 2019 Dec. [DOI] [PubMed] [Google Scholar]
- 33.Sabus A., Merrow M., Heiden A., Boster J., Koo J., Franklin A.R.K. Fecal microbiota transplantation for treatment of severe Clostridioides difficile colitis in a pediatric patient with non-Hodgkin Lymphoma. J. Pediatr. Hematol. Oncol. 2020 Dec 2 doi: 10.1097/MPH.0000000000002023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.