Abstract
Two high-copy-number insertion sequences, IS2404 and IS2606, were recently identified in Mycobacterium ulcerans and were shown by Southern hybridization to possess restriction fragment length polymorphism between strains from different geographic origins. We have designed a simple genotyping method that captures these differences by PCR amplification of the region between adjacent copies of IS2404 and IS2606. We have called this system 2426 PCR. The method is rapid, reproducible, sensitive, and specific for M. ulcerans, and it has confirmed previous studies suggesting a clonal population structure of M. ulcerans within a geographic region. M. ulcerans isolates from Australia, Papua New Guinea, Malaysia, Surinam, Mexico, Japan, China, and several countries in Africa were easily differentiated based on an array of 4 to 14 PCR products ranging in size from 200 to 900 bp. Numerical analysis of the banding patterns suggested a close evolutionary link between M. ulcerans isolates from Africa and southeast Asia. The application of 2426 PCR to total DNA, extracted directly from M. ulcerans-infected tissue specimens without culture, demonstrated the sensitivity and specificity of this method and confirmed for the first time that both animal and human isolates from areas of endemicity in southeast Australia have the same genotype.
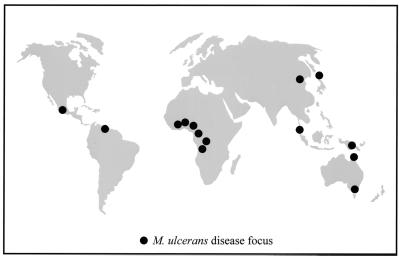
Mycobacterium ulcerans is an environmental mycobacterium with worldwide distribution (Fig. 1) and causes chronic, necrotizing skin lesions in otherwise healthy humans. The incidence of M. ulcerans disease has been increasing worldwide, particularly throughout rural west Africa (11, 22). The reasons for this increase are unknown. Epidemiological and PCR-based evidence suggests that swamps and slow-flowing water are sources of the organism (1, 6, 8, 17, 25), and recent PCR data from Benin and Ghana have identified M. ulcerans DNA in aquatic insects (12). So far M. ulcerans has never been isolated by culture from any of these environments. It has therefore been difficult to clearly identify reservoirs or modes of transmission. One approach to improving our understanding of the ecology of this organism has been to study the molecular epidemiology of M. ulcerans, for which two methods have been reported. The first of these identified restriction fragment length polymorphism (RFLP) between isolates by probing with the polymorphic GC-rich repeat sequence contained in plasmid pTBN12 (7). This approach distinguished 11 distinct RFLP types among isolates from four countries and suggested a clonal population structure within a geographic region. Supporting this conclusion, nucleotide sequence analysis of the 3′ region of the 16S rRNA gene identified three alleles that correlated with the geographic origin of an isolate (13). However, the methods used in these studies were limited by requiring high-concentration, purified DNA or by offering limited discriminative capability.
FIG. 1.
World map showing regions where the isolates used in the study were obtained.
PCR genotyping of mycobacteria by targeting insertion sequences (IS) has been well described (10, 16). The recent discovery of two high-copy-number IS elements in M. ulcerans that displayed RFLP between strains (21) suggested that PCR amplification between adjacent copies of these elements (IS2404 and IS2606) may be suitable for genotyping this species.
The aims of this study were twofold. The first was to develop a method that would offer strain discrimination in a simple, reproducible format. Second, using this method, we hoped to improve our understanding of the molecular epidemiology of M. ulcerans by analyzing a selection of isolates from disease foci around the world.
MATERIALS AND METHODS
Mycobacterial strains, clinical specimens, and culture conditions.
The origins of the M. ulcerans strains used in this study are listed in Table 1. The origins of the other species of mycobacteria that were used and general culture conditions have been described previously (21).
TABLE 1.
Origins of the M. ulcerans isolates used in this study
| Isolate | Origin | Year isolated | Sourcea | 2426 type |
|---|---|---|---|---|
| 96-658 | Angola | 1996 | ITM | African |
| 94-856 | Benin | 1994 | ITM | African |
| 97-111 | Benin | 1997 | ITM | African |
| 5152 | Congo | 1976 | ITM | African |
| 5155 | Congo | 1976 | ITM | African |
| 97-610 | Ghana | 1997 | ITM | African |
| 97-680 | Togo | 1997 | ITM | African |
| 98-912 | China | 1997 | ITM | Asian |
| ATCC 33728 | Japan | 1980 | Asian | |
| 186510 | Malaysia | 1992 | VIDRL | Malaysian |
| 5114 | Mexico | 1953 | ITM | Mexican |
| 5143 | Mexico | 1967 | ITM | Mexican |
| 11878/70 | Papua New Guinea | 1971 | QDRLMD | PNG I |
| 94-1331 | Papua New Guinea | 1994 | ITM | PNG II |
| 186463 | Papua New Guinea | 1992 | VIDRL | PNG II |
| 13822/70 | North Queensland, Australia | 1971 | QDRLMD | Queensland |
| 6537/78 | North Queensland, Australia | 1978 | QDRLMD | Queensland |
| 95016102 | North Queensland, Australia | 1994 | VIDRL | Queensland |
| 842 | Surinam | 1986 | ITM | Suriname |
| 95023625 | East Gippsland, Victoria, Australia | 1994 | VIDRL | Victorian |
| ATCC 19423 | East Gippsland, Victoria, Australia | 1948 | Victorian | |
| 95045548 | East Gippsland, Victoria, Australia | 1995 | VIDRL | Victorian |
| 144727 | East Gippsland, Victoria, Australia | 1989 | VIDRL | Victorian |
| 186453 | Langwarrin, Victoria, Australia | 1992 | VIDRL | Victorian |
| 187636 | Langwarrin, Victoria, Australia | 1992 | VIDRL | Victorian |
| 189182 | Langwarrin, Victoria, Australia | 1992 | VIDRL | Victorian |
| 93112352 | Langwarrin, Victoria, Australia | 1992 | VIDRL | Victorian |
| 95046437 | Langwarrin, Victoria, Australia | 1995 | VIDRL | Victorian |
| 95063471 | Langwarrin, Victoria, Australia | 1995 | VIDRL | Victorian |
| 97029961 | Langwarrin, Victoria, Australia | 1997 | VIDRL | Victorian |
| 93133170 | Phillip Island, Victoria, Australia | 1993 | VIDRL | Victorian |
| 93138796 | Phillip Island, Victoria, Australia | 1993 | VIDRL | Victorian |
| 93147172 | Phillip Island, Victoria, Australia | 1993 | VIDRL | Victorian |
| 93160339 | Phillip Island, Victoria, Australia | 1993 | VIDRL | Victorian |
| 93170008 | Phillip Island, Victoria, Australia | 1993 | VIDRL | Victorian |
| 93171525 | Phillip Island, Victoria, Australia | 1993 | VIDRL | Victorian |
| 94100241 | Phillip Island, Victoria, Australia | 1993 | VIDRL | Victorian |
| 94114510 | Phillip Island, Victoria, Australia | 1994 | VIDRL | Victorian |
| 94128817 | Phillip Island, Victoria, Australia | 1994 | VIDRL | Victorian |
| 94151667 | Phillip Island, Victoria, Australia | 1994 | VIDRL | Victorian |
| 94155376 | Phillip Island, Victoria, Australia | 1994 | VIDRL | Victorian |
| 94161099 | Phillip Island, Victoria, Australia | 1994 | VIDRL | Victorian |
| 94162101 | Phillip Island, Victoria, Australia | 1994 | VIDRL | Victorian |
| 94171428 | Phillip Island, Victoria, Australia | 1994 | VIDRL | Victorian |
| 95001022 | Phillip Island, Victoria, Australia | 1995 | VIDRL | Victorian |
| 95067597 | Phillip Island, Victoria, Australia | 1995 | VIDRL | Victorian |
| 96042434 | Phillip Island, Victoria, Australia | 1996 | VIDRL | Victorian |
| 94179419 | Victoria, Australia | 1994 | VIDRL | Victorian |
| 96035870 | Victoria, Australia | 1996 | VIDRL | Victorian |
| 94112961 | Westernport, Victoria, Australia | 1993 | VIDRL | Victorian |
| 95060263 | Westernport, Victoria, Australia | 1995 | VIDRL | Victorian |
ITM, Institute for Tropical Medicine; VIDRL, Victorian Infectious Diseases Reference Laboratory; QDRLMD, Queensland Diagnostic and Reference Laboratory for Mycobacterial Diseases.
DNA preparation.
An approximately 10-μl loopful of cells was scraped from an egg yolk agar slope and resuspended in 500 μl of 1% Triton X-100. This cell suspension was added to a 2-ml skirted, screw cap tube containing 200 μl of washed 100-μ-m-diameter glass beads and 500 μl of chloroform-isoamylalcohol (24:1). The tube was placed in a Fastprep cell disrupter (Savant Instruments, Holbrook, N.Y.) at speed setting 6 for 40 s. After cell disruption, the tube was cooled on ice for 5 min and then centrifuged at 17,000 × g for 5 min. In some instances DNA was also prepared by resuspending cells in 300 μl of water, heating cells to 100°C for 20 min, and then centrifuging as described above. The aqueous phases were retained and stored at −20°C. A 2-μl volume of DNA from either preparation was used as a template for the PCR.
For primer specificity testing, DNA was extracted from a panel of 23 species of mycobacteria and pooled into five groups. Each pool of DNA was then tested by 2426 PCR. The composition of each group was as follows: pool 1, Mycobacterium heidelbergense, M. intermedium, M. lentiflavum, M. obuense, and M. parafortuitum; pool 2, M. pulveris, M. rhodesiae, M. shimoidei, M. tokaiense, and M. triplex; pool 3, M. vaccae, M. xenopi, M. gordonae, M. simiae, and M. flavescens; pool 4, M. terrae, M. aurum, M. chelonae, and M. smegmatis; pool 5, M. fortuitum, M. marinum, M. tuberculosis, and M. haemophilum.
For method sensitivity experiments, purified DNA was extracted by the method of Boddinghaus et al. (2) from 50 mg (wet weight) of M. ulcerans cells harvested from egg yolk agar slopes. The DNA was diluted as previously described to represent M. ulcerans genome equivalents, where 4 to 5 fg of DNA approximates one mycobacterial genome (9).
2426 PCR.
Reaction conditions used for 2426 PCR were as follows: each PCR mixture (20 μl) contained 1× PCR buffer II (10× PCR buffer II contained 500 mM KCl and 100 mM Tris-HCl [pH 8.3]), 2.5 mM MgCl2, 0.2 mM deoxynucleoside triphosphates (0.2 mM [each] dATP, dTTP, dCTP, and dGTP), an 0.5 μM concentration of each primer (MU4, 5′ ATCGCCGAAGCCTGCCGGAT 3′, positions 1119 to 1138, and MU9, 5′ TCTTCGTGGTTTTGTGATGGC 3′, positions 1306 to 1326), 1 U of Ampli-Taq DNA polymerase (Perkin-Elmer, Melbourne, Australia), and 2 μl of DNA. PCR was performed in an FTS-960 thermal sequencer (Corbett Research, Sydney, Australia) with the following protocol, optimized for this application: 5 cycles of 95°C for 1 min, 60°C for 1 min, and 72°C for 1 min and 25 cycles (30 cycles for tissue specimens) of 95°C for 20 s, 58°C for 30 s, and 72°C for 40 s, followed by a final extension step at 72°C for 5 min. The PCR products were held at 4°C until analyzed and detected with 7.5% (0.75 mM) native polyacrylamide gel electrophoresis at 200 V for 38 min in a Mini-Protean II apparatus (Bio-Rad) with rapid silver staining. A 100-bp ladder was used as a size marker (Gibco, BRL). The silver stain procedure was adapted from Caetano-Anoelles and Breshoff (Promega Notes 45:13–18, 1994) as follows. After electrophoresis, gels were fixed in 7.5% acetic acid for 6 min, rinsed three times in distilled water, and then stained for 8 min with 50 ml of a 0.075% silver nitrate solution containing 75 μl of formaldehyde. Gels were rinsed twice in distilled water and developed in 50 ml of developing solution, which contained 1.5 g of sodium carbonate, 0.2 μg of sodium thiosulfate, and 75 μl of formaldehyde. The developing reaction was terminated with 7.5% acetic acid.
The banding patterns from all strains were compared visually. A binary matrix was constructed by scoring each pattern for the presence or absence of the 29 PCR products that constituted the total pool of fragments between 200 and 900 bp. It was assumed that comigrating bands were of identical sequence and therefore an indication of genetic relatedness. The patterns were compared by the method of unweighted pair group average linkage and the simple matching coefficient (19). The goodness of fit of the cluster analysis was tested with the cophenetic correlation coefficient, where r values greater than 0.9 suggest a very good fit (18). All analyses were performed with NTSYS, version 1.8, software (Applied Biostatistics Inc., Setauket, N.Y.).
RESULTS
A test panel of M. ulcerans clinical isolates was assembled from many of the known M. ulcerans disease foci in the world (Fig. 1), a collection that represented both temporal and spatial diversity. All isolates were found to contain IS2404 and IS2606 as determined by PCR, and a selection of these results is shown in Fig. 2A.
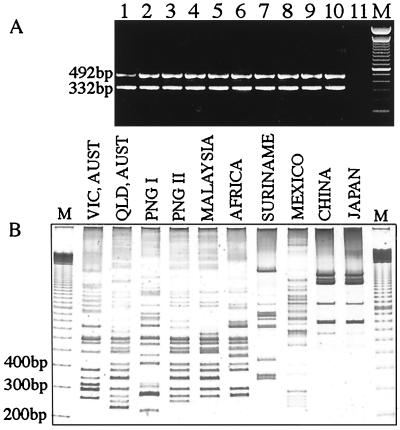
FIG. 2.
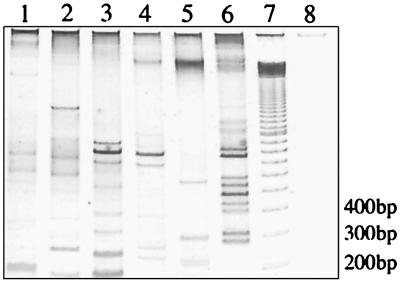
(A) PCR detection of IS2404 (492 bp) and IS2606 (332 bp) in M. ulcerans isolates from different geographic regions. (B) 2426 PCR genotype analysis of M. ulcerans isolates from different geographic regions. Lanes (A and B), 1, ATCC 19423; 2, 13822/70; 3, 11878/70; 4, 94-1331; 5, 186510; 6, 5155; 7, 842; 8, 5114; 9, 98-912; 10, ATCC 33728; lane 11, no-template control; lane M, 100-bp size ladder. Vic., Victoria; QLD, Queensland; Aust., Australia.
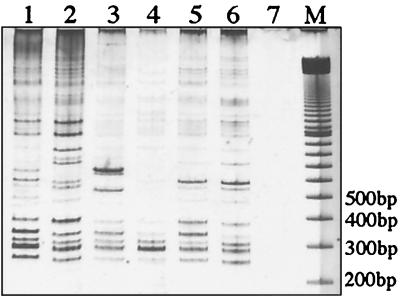
Several combinations of outward-priming oligonucleotides for IS2404 and IS2606 were then evaluated in order to obtain reproducible and discriminatory patterns of bands for different strains of M. ulcerans. Primers MU4 and MU9 gave the largest number of clearly resolvable bands when PCR products were separated by nondenaturing polyacrylamide gel electrophoresis and detected by silver staining (Fig. 2B). The use of each primer alone in a PCR did not produce a useful banding pattern. Nine different 2426 PCR genotypes were observed (Fig. 2B). All isolates were tested at least twice with the same Triton X-100 DNA preparation, and identical profiles were produced. For the strains from Africa, Queensland, Malaysia, and Papua New Guinea and for two of the Victorian isolates, DNA was also extracted by boiling cells in water. The crude DNA preparations from this method produced banding patterns identical to that obtained from the detergent-extracted DNA (data not shown). A single pattern for all seven African isolates was obtained despite the large distances and long times separating some of these isolates (Table 1; Fig. 1 and 3). The 35 Australian isolates produced two profiles, representing one genotype from Queensland (northern Australia) and one from Victoria (southeastern Australia). No variation was detected among the 32 isolates from Victoria (Fig. 4), but there were two distinct genotypes among the 3 isolates from Papua New Guinea (PNG I and PNG II). The Malaysian genotype was nearly identical to PNG II, differing only by the positions of two bands between 200 and 900 bp. Distinct profiles were observed for the isolates from Surinam and Mexico; however, the patterns for isolates from Japan and China were identical.
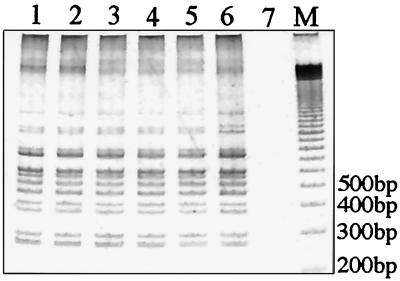
FIG. 3.
2426 PCR genotype analysis of M. ulcerans isolates from five countries in Africa. Lane 1, 96-658; lane 2, 94-856; lane 3, 97-111; lane 4, 5155; lane 5, 97-610; lane 6, 97-680; lane 7, no-template control; lane 8, 100-bp ladder size marker.
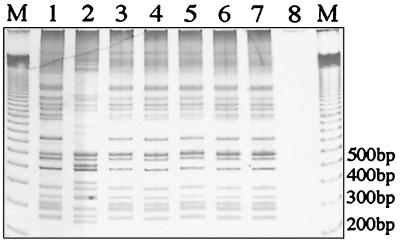
FIG. 4.
2426 PCR genotype analysis of M. ulcerans isolates from southeast Australia and Papua New Guinea. Lanes 1 and 3 to 7, strains 94114510, 95046437, 95067597, 94161099, 94171428, 94151667, respectively; lane 2, 186463; lane 8, no-template control; lane M, 100-bp ladder size marker.
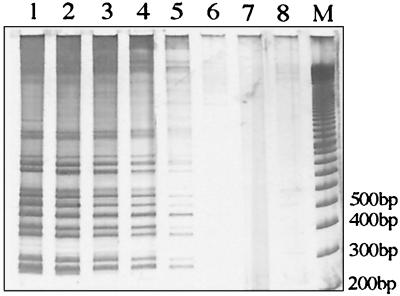
To investigate primer specificity 24 other species of mycobacteria were tested by 2426 PCR. Some PCR products from other mycobacteria were observed, but these were generally faint, and none produced profiles similar to any of the patterns obtained from the different M. ulcerans strains (Fig. 5).
FIG. 5.
Determination of primer specificity for M. ulcerans by 2426 PCR of 23 other species of mycobacteria as described in Materials and Methods. Lane 1, mycobacterial DNA pool 1; lane 2, pool 2; lane 3, pool 3; lane 4, pool 4; lane 5, pool 5; lane 6, M. ulcerans Malaysian strain 186510; lane 7, 100-bp ladder size marker; lane 8, no-template control.
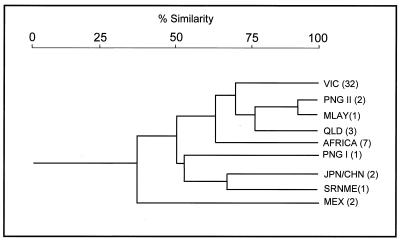
A dendrogram was constructed to display the relatedness between strains based on banding pattern similarity (Fig. 6). The clustering of the southeast Asian genotypes and the African genotype is suggestive of an evolutionary link among these strains; however, the PNG I genotype was an interesting exception to this cluster. The genotypes representing the other regions appeared less related to each other, displaying 30 to 60% similarity (r = 0.91) (Fig. 6).
FIG. 6.
Cluster analysis of the genetic relationships among 51 isolates based on banding pattern similarity displayed by 2426 PCR. Percentage similarity is indicated by a scale at the top. Genotypes codes are given on the right and reflect the origin of the isolates. Codes are as follows: VIC; Victoria, Australia; PNG I and PNG II, Papua New Guinea; MLAY, Malaysia; QLD, Queensland, Australia; JPN/CHN, Japan and China; SRNME, Surinam, South America; MEX, Mexico. The numbers in parentheses denote the numbers of isolates displaying the genotypes.
The detection sensitivity of 2426 PCR was tested with a dilution series of purified M. ulcerans genomic DNA from an African isolate. This demonstrated a detection limit of 100 genomes (Fig. 7).
FIG. 7.
Detection sensitivity of 2426 PCR for genotyping M. ulcerans (strain 5152) with a dilution series of DNA. Lane 1, 106 genomes; lane 2, 105 genomes; lane 3, 104 genomes; lane 4, 103 genomes; lane 5, 102 genomes; lane 6, 10 genomes; lane 7, 1 genome; lane 8, no-template control; lane M, 100-bp ladder size marker.
The relatively low concentration of cells required to produce a profile and the specificity of the target sequences to M. ulcerans suggested that this method may be useful for genotyping directly from clinical samples. To investigate this, DNA extracted from 50 patient specimens was subjected to 2426 PCR. These samples included swabs and formalin-fixed and paraffin-embedded tissue specimens that had been screened previously by IS2404 PCR for M. ulcerans diagnosis (unpublished data). Of these 50 samples, 40 were IS2404 positive and the remainder were IS2404 negative. All 50 samples were then tested by 2426 PCR. An example of some profiles obtained is shown in Fig. 8. A recognizable genotype was obtained for 20 of the 40 IS2404-positive specimens. All samples that were IS2404 negative were also 2426 PCR negative. The specificity of the 2426 PCR was 100% (calculated as the number of true negatives divided by the number of true negatives plus false positives), and the sensitivity was 66.7% (calculated as the number of true positives divided by the number of true positives plus false negatives). From the 20 recognizable profiles, 13 were identified as the Victorian genotype and 7 as the Queensland genotype. These profiles also correctly predicted the geographic origin of the specimen. Analysis of tissue samples from an M. ulcerans-infected alpaca (Quechua alpako) and possum (Trichosurus vulpecula) clearly demonstrated that these animals were infected with the same genotype as the human cases from these areas (Fig. 8). Both these animals inhabited regions in southeast Australia where M. ulcerans disease is known to be endemic.
FIG. 8.
2426 PCR genotype analysis of M. ulcerans from DNA extracted directly from human and animal specimens. Lanes 1 to 3, dry swab, paraffin-embedded tissue, and formaldehyde-preserved tissue specimens, respectively, from a possum captured on Phillip Island; lane 4, formaldehyde-preserved human tissue specimen from the Langwarrin focus; lane 5, formaldehyde-preserved alpaca tissue from the east Gippsland focus; lane 6, Victorian isolate ATCC 19423; lane 7, no-template control; lane M, 100-bp ladder size marker.
DISCUSSION
In mycobacteria, genetic diversity is driven by the activity of mobile DNA such as IS rather than nucleotide sequence drift (20). Thus IS elements are potentially ideal targets for indexing rapidly evolving change in mycobacterial populations (10, 26). M. ulcerans has at least two high-copy-number IS elements, IS2404 and IS2606, that demonstrate RFLP between strains (21) and that appear to be present in isolates of diverse geographic origin (Fig. 2A) (4). However, M. ulcerans is a slow-growing organism, and extraction of a sufficient quantity and quality of DNA to perform a Southern analysis is a time-consuming and impractical process for routine analysis.
The PCR typing method we have developed is a simple and robust tool for quickly determining the origin of an isolate, requiring a minimum of 100 genomic equivalents of DNA (Fig. 7). The high detection sensitivity and specificity permitted typing directly from patient specimens without requiring culture. Other mycobacteria tested by 2426 PCR did produce some bands, but none reproduced any of the profiles seen with M. ulcerans (Fig. 5). The sensitivity of 2426 PCR for genotyping directly from tissue specimens was, however, found to be somewhat low (66.7%). This was most likely due to the low numbers of target cells in some samples; however, DNA extracted from swabs or tissue specimens served equally well as a template. The detection sensitivity of 2426 PCR in this application may be improved by ensuring that specimens are taken from areas of ulceration likely to contain large numbers of bacilli.
Analysis by 2426 PCR of IS2404-positive swab and tissue specimens, obtained from animals and humans in areas of southeast Australia where M. ulcerans disease is endemic identified the same genotype in all specimens, suggesting that animals are infected from the same environmental source as humans (Fig. 8). Genotyping directly from tissue specimens will be particularly useful for studying the epidemiology of M. ulcerans, as culture of this organism is slow and relatively insensitive (4).
To investigate the discriminating capability of 2426 PCR, 55 M. ulcerans isolates were tested (Table 1). The combination of primers MU4 and MU9 produced nine distinct profiles that correlated absolutely with the geographic source of the isolates, i.e., the origin of an isolate could be unambiguously assigned based on its banding pattern. This result is somewhat surprising as other mycobacteria, such as Mycobacterium avium, exhibit considerable IS diversity even within a geographic region when analyzed by a similar genotyping technique (26). The lack of genotype variation in M. ulcerans within a region may be a reflection of its slow generation time and perhaps a low population density within its environmental niche. PCR-based incidence data, gathered during a disease outbreak, suggested a very low abundance of bacteria in the environment (17).
A lack of IS mobility as a cause of the limited genotype diversity is possible but unlikely, as the different 2426 PCR genotypes are indicative of ongoing transposition events or IS-associated genome rearrangements. Furthermore, these events appear to have occurred relatively recently, considering (i) the absence of IS2404 and IS2606 from the closely related M. marinum and (ii) the very high nucleotide sequence similarity between the 16S rRNA and groEL genes of M. ulcerans and M. marinum (13–15, 23), which is indicative of the recent evolutionary divergence of these species. The inferred close genetic relationship among the five genotypes obtained from the southeast Asian isolates (Fig. 6) suggests that M. ulcerans is introduced into an area and then is isolated or sequestered, undergoing occasional transposition or genome rearrangement events to produce a unique genotype in a particular region. However, rapid dispersal over large distances also seems possible considering that only one genotype was observed among the isolates from five countries throughout central and west Africa (Fig. 3). This analysis suggests that the spread of disease across Africa has occurred after its spread throughout southeast Asia and Australia if the rate of genome rearrangements displayed by 2426 PCR is assumed to be the same for all strains.
The 2426 PCR method is highly reproducible. The DNA from all isolates was tested at least twice, and each set of tests produced identical profiles. In addition, 2426 PCR performed with DNA extracted from cells in crude form by boiling produced profiles identical to those produced by PCR performed with purified DNA. The reproducibility of this method is further reinforced by the ability to produce the same pattern from DNA over a 5-log-unit dilution range of template DNA (Fig. 7) and by the fact that the same pattern was obtained for the 32 Victorian isolates and 7 African isolates (Fig. 4).
The findings of previous genotype studies of M. ulcerans by Southern hybridization with the pTBN12 probe (7) and nucleotide sequence analysis of the 16SrRNA gene (13) generally concurred with the results from 2426 PCR analysis, where identical genotypes were detected within a region. However the pTBN12 probe method also identified some additional RFLP among African isolates. The apparent increased discriminatory capability of the pTBN12 probe is countered by the length of time taken to perform a Southern hybridization (3 to 4 days) compared with that taken by 2426 PCR (4 to 6 h) and the ability of 2426 PCR to obtain a genotype directly from a clinical specimen.
Interestingly the pTBN12 probe offered no additional discrimination among Victorian isolates, thus supporting the suggestion of clonality of M. ulcerans within a region. Analysis of additional African isolates with the pTBN12 probe and 2426 PCR is warranted to more thoroughly examine evidence for clonality between endemic foci and to better compare the capabilities of each method.
It is of interest that two genotypes were detected in Papua New Guinea (Fig. 1B). The PNG II pattern appears closely related those of the Malaysian and Australian strains. By comparison, the PNG I pattern is quite distinct and appears, at least by this method, less related to those of the other southeast Asian strains than to those of the African isolates (Fig. 6). Unfortunately further details regarding the origin of this strain were unavailable but, it is likely to represent an endemic focus distant to the PNG II isolates. We are currently sequencing multiple gene loci from M. ulcerans to better establish the genetic relatedness between all strains.
It has been proposed that the global distribution of M. ulcerans may be linked to the breakup of the supercontinents at the end of the Jurassic period (5). However, the data presented in this study, previous 16S rRNA sequence comparisons of M. ulcerans and M. marinum, and the discovery of M. ulcerans in Japan and China (3, 24) argue against this hypothesis and suggest a far more recent mechanism of dispersal. More-extensive nucleotide sequence analyses of M. ulcerans strains are now required to investigate intraspecies and interspecies evolution and to further our understanding of the molecular epidemiology of M. ulcerans.
ACKNOWLEDGMENTS
We thank David Dawson for the provision of mycobacterial isolates.
This work was supported in part by funding from the Government of Victoria through the Department of Human Services.
REFERENCES
- 1.Anonymous. Epidemiology of Mycobacterium ulcerans infection (Buruli ulcer) at Kinyara, Uganda. Trans R Soc Trop Med Hyg. 1971;65:763–775. doi: 10.1016/0035-9203(71)90090-3. [DOI] [PubMed] [Google Scholar]
- 2.Boddinghaus B, Rogall T, Flohr T, Blocker H, Bottger E C. Detection and identification of mycobacteria by amplification of rRNA. J Clin Microbiol. 1990;28:1751–1759. doi: 10.1128/jcm.28.8.1751-1759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faber, W. R., L. M. Pereira Arias-Bouda, J. E. Zeegelaar, A. H. J. Kolk, P. A. Fonteyne, J. Toonstra, and F. Portaels. 1999. First case of Mycobacterium ulcerans infection in the Peoples Republic of China. Trans. R. Soc. Trop. Med. Hyg., in press. [DOI] [PubMed]
- 4.Guimaraes-Peres A, Portaels F, de Rijk P, Fissette K, Pattyn S R, van Vooren J, Fonteyne P. Comparison of two PCRs for detection of Mycobacterium ulcerans. J Clin Microbiol. 1999;37:206–208. doi: 10.1128/jcm.37.1.206-208.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayman J. Mycobacterium ulcerans: an infection from Jurassic time? Lancet. 1984;ii:1015–1016. doi: 10.1016/s0140-6736(84)91110-3. [DOI] [PubMed] [Google Scholar]
- 6.Hayman J. Postulated epidemiology of Mycobacterium ulcerans infection. Int J Epidemiol. 1991;20:1093–1098. doi: 10.1093/ije/20.4.1093. [DOI] [PubMed] [Google Scholar]
- 7.Jackson K, Edwards R, Leslie D E, Hayman J. Molecular method for typing Mycobacterium ulcerans. J Clin Microbiol. 1995;33:2250–2253. doi: 10.1128/jcm.33.9.2250-2253.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson P D, Veitch M G, Leslie D E, Flood P E, Hayman J A. The emergence of Mycobacterium ulcerans infection near Melbourne. Med J Aust. 1996;164:76–78. doi: 10.5694/j.1326-5377.1996.tb101352.x. [DOI] [PubMed] [Google Scholar]
- 9.Mangiapan G, Vokurka M, Schouls L, Cadranel J, Lecossier D, van Embden J, Hance A J. Sequence capture-PCR improves detection of mycobacterial DNA in clinical specimens. J Clin Microbiol. 1996;34:1209–1215. doi: 10.1128/jcm.34.5.1209-1215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Picardeau M, Vincent V. Typing of Mycobacterium avium isolates by PCR. J Clin Microbiol. 1996;34:389–392. doi: 10.1128/jcm.34.2.389-392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Portaels F. Historical overview of the Buruli ulcer. In: Asiedu K, Raviglione M, Scherpbier R, editors. Proceedings of the International Conference on Buruli Ulcer Control and Research. Geneva, Switzerland: World Health Organization; 1998. p. 17. [Google Scholar]
- 12.Portaels F, Elsen P, Guimares-Peres A, Fonteyne P A, Meyers W M. Insects in the transmission of Mycobacterium ulcerans infection. Lancet. 1999;353:986. doi: 10.1016/S0140-6736(98)05177-0. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 13.Portaels F, Fonteyne P A, de Beenhouwer H, de Rijk P, Guedenon A, Hayman J, Meyers M W. Variability in 3′ end of 16S rRNA sequence of Mycobacterium ulcerans is related to geographic origin of isolates. J Clin Microbiol. 1996;34:962–965. doi: 10.1128/jcm.34.4.962-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts B, Hirst R. Immunomagnetic separation and PCR for detection of Mycobacterium ulcerans. J Clin Microbiol. 1997;35:2709–2711. doi: 10.1128/jcm.35.10.2709-2711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogall T, Wolters J, Flohr T, Bottger E C. Towards a phylogeny and definition of species at the molecular level within the genus Mycobacterium. Int J Syst Bacteriol. 1990;40:323–330. doi: 10.1099/00207713-40-4-323. [DOI] [PubMed] [Google Scholar]
- 16.Ross B C, Dwyer B. Rapid, simple method for typing isolates of Mycobacterium tuberculosis by using the polymerase chain reaction. J Clin Microbiol. 1993;31:329–334. doi: 10.1128/jcm.31.2.329-334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross B C, Johnson P D, Oppedisano F, Marino L, Sievers A, Stinear T, Hayman J A, Veitch M G, Robins-Browne R M. Detection of Mycobacterium ulcerans in environmental samples during an outbreak of ulcerative disease. Appl Environ Microbiol. 1997;63:4135–4138. doi: 10.1128/aem.63.10.4135-4138.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sneath P H A. Classification of microorganisms. In: Norris J R, Richmond M H, editors. Essays in microbiology. Chichester, United Kingdom: John Wiley; 1978. pp. 9/1–9/31. [Google Scholar]
- 19.Sneath P H A, Sokal R R. Numerical taxonomy. W. H. San Francisco, Calif: Freeman & Co.; 1973. [Google Scholar]
- 20.Sreevatsan S, Pan X, Stockbauer K E, Connell N D, Kreiswirth B N, Whittam T S, Musser J M. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc Natl Acad Sci USA. 1997;94:9869–9874. doi: 10.1073/pnas.94.18.9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stinear T, Ross B C, Davies J K, Marino L, Robins-Browne R M, Oppedisano F, Sievers A, Johnson P D R. Identification and characterization of IS2404 and IS2606: two distinct repeated sequences for detection of Mycobacterium ulcerans by PCR. J Clin Microbiol. 1999;37:1018–1023. doi: 10.1128/jcm.37.4.1018-1023.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thangaraj H S, Evans M R W, Wansborough-Jones M H. Mycobacterium ulcerans disease; Buruli ulcer. Trans R Soc Trop Med Hyg. 1999;93:337–340. doi: 10.1016/s0035-9203(99)90104-9. [DOI] [PubMed] [Google Scholar]
- 23.Tonjum T, Welty D B, Jantzen E, Small P L. Differentiation of Mycobacterium ulcerans, M. marinum, and M. haemophilum: mapping of their relationships to M. tuberculosis by fatty acid profile analysis. DNA-DNA hybridization, and 16S rRNA gene sequence analysis. J Clin Microbiol. 1998;36:918–925. doi: 10.1128/jcm.36.4.918-925.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsukamura M, Kaneda K, Imaeda T, Mikoshiba H. A taxonomic study on a mycobacterium which caused a skin ulcer in a Japanese girl that resembled Mycobacterium ulcerans. Kekkaku. 1989;64:691–697. [PubMed] [Google Scholar]
- 25.Veitch M G, Johnson P D, Flood P E, Leslie D E, Street A C, Hayman J A. A large localized outbreak of Mycobacterium ulcerans infection on a temperate southern Australian island. Epidemiol Infect. 1997;119:313–318. doi: 10.1017/s0950268897008273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoder S, Argueta C, Holtzman A, Aronson T, Berlin O G W, Tomasek P, Glover N, Froman S, Stelma G., Jr PCR comparison of Mycobacterium avium isolates obtained from patients and foods. Appl Environ Microbiol. 1999;65:2650–2653. doi: 10.1128/aem.65.6.2650-2653.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]