Abstract
In 2020, Council Directive 2005/94/EC required EU Member States (MSs) to carry out surveillance for avian influenza (AI) in poultry and wild birds and notify the results to the responsible authority. Based on this, MSs, Iceland, Norway, Switzerland and the United Kingdom implemented ongoing surveillance programmes to monitor incursions of AI viruses in poultry and wild birds. EFSA received a mandate from the European Commission to collate, validate, analyse and summarise the data resulting from the avian influenza surveillance programmes in an annual report. This is the second such report produced using data directly submitted to EFSA by MSs. This report summarises the results of the surveillance activities carried out in poultry and wild birds in 2020. Overall, 24,768 poultry establishments (PEs) were sampled, of which 46 were seropositive for H5 virus strains and seven for H7 strains. Seropositive PEs were found in nine MSs (Belgium, Denmark, Finland, France, Italy, the Netherlands, Poland, Spain and Sweden) and the United Kingdom. As per previous years, the highest percentages of seropositive PEs were found in establishments raising waterfowl game birds and breeding geese. Out of the 53 PEs with positive serological tests for H5/H7, seven tested positive in polymerase chain reaction (PCR) or virology for H5/H7 virus strains: six for Low Pathogenic Avian Influenza (LPAI) and one for Highly Pathogenic Avian Influenza (HPAI). In addition, 13 countries also reported PCR results from 748 PEs which did not correspond to the follow‐up testing of a positive serology event (e.g. in some PEs, PCR tests were used for screening). Twenty‐five of these PEs were found positive for AI viral RNA. These positive PEs were located in Bulgaria, Estonia, Germany, Romania and Slovakia. A total of 18,968 wild birds were sampled, with 878 birds testing positive to HPAI virus. Fourteen countries reported HPAI‐positive wild birds, with all HPAI strains identified as H5. Most positive birds were infected with H5N8, with a smaller number of N1, N3, N5 and unidentified NA subtypes. In addition, there were 317 birds testing positive for LPAI H5 or H7 virus and 429 birds testing positive for non‐H5/H7 AI virus, reported by 31 countries. The surveillance findings for poultry and wild birds for 2020 are discussed in relation to the current knowledge of the epidemiology of AI in Europe, in particular the H5N8 epidemic which has been identified late 2020.
Keywords: Avian Influenza, HPAI, LPAI, surveillance, poultry, wild birds
1. Summary
The European Union's Member States (MSs), Iceland, Norway, Switzerland and the United Kingdom (together referred to as reporting countries, RCs) implement surveillance programmes to detect incursions of avian influenza viruses (AIVs) in poultry and wild birds, particularly migratory wild birds, which are considered the main source of introduction of AIVs to poultry. The present report summarises the results of the EU co‐funded surveillance activities conducted in 2020, which consisted of:
Serological surveys to monitor the circulation of AIV subtypes H5 and H7 in poultry (active surveillance).
Passive surveillance aiming at the virological detection of AI in wild birds found dead or moribund.
In addition, some MSs also reported the results of active surveillance performed by testing live and hunted birds. AI surveillance in some RCs is based on targeted sampling. Therefore, comparisons of seropositivity rates between different groups presented in this report relate to the specific observations recorded (surveillance samples) only. They cannot be extrapolated to the source populations because sampling was targeted at higher risk groups and the targeting approach may be different between countries, between groups and between years. Risk‐based surveillance is designed for early detection and should not be used to measure changes in disease prevalence or incidence.
1.1. Serological surveys in poultry
A total of 31 reporting countries (RCs) reported data on sampling and AI testing in poultry establishments (PEs). In some RCs, establishments were sampled several times throughout the year. For the purpose of this report, each sampling exercise taking place on a specific date and targeting a different poultry category was considered as an independent event and counted as one PE sampled. Therefore, the numbers reported in this report as PEs sampled should be interpreted as the number of sampling events taking place in an RC for each of the reported categories.
Figures on the size of the poultry population under surveillance in the RCs were not available at the time of writing of the present report. In 2020, a total of 24,767 PEs were sampled, roughly the same number of PEs as the previous year. The total number of PEs sampled and reported in each RC ranged from 28 in Malta to 5,035 in Italy.
Sixteen poultry categories have been used to report surveillance results in the present document. None of them were sampled by all RCs. However, laying hen (conventional and free‐range), fattening turkey, breeding chicken and gallinaceous game bird establishments were sampled by at least 20 RCs each. Growers and breeding geese were targeted by only few countries. In terms of the number of PEs sampled, backyard flocks were the most sampled category (n = 4,740), followed by conventional and free‐range laying hens (n = 4,404 and 3,487, respectively).
A total of 53 PEs (0.21%) were seropositive to either H5 or H7 (hereafter ‘H5/H7’), including 46 H5 and 7 H7. The H5/H7 seropositivity rate was around half of that observed in 2019 (0.45%). Ten countries reported H5 seropositive PEs: Belgium, Denmark, Finland, France, Italy, the Netherlands, Poland, Spain, Sweden and the United Kingdom. Spain also reported H7 seropositive PEs.
Most H5/H7 detections (45 PEs out of 53) occurred in countries, which sampled a number of PEs larger than the median number of PEs sampled. The 2020 results confirm an overall decreasing trend in the proportion of H5/H7 seropositive establishments noted since the 2016 H5 outbreaks (with the significant exception of 2019). The number of H5 seropositive PEs detected remained higher than H7 detections, as per previous years.
As observed in previous years, waterfowl game birds and breeding geese were the categories with the highest proportion of H5/H7 seropositive establishments (9.5% and 3.3%, respectively). The proportion of H5/H7 seropositive PEs was 1.8% in breeding duck establishments and below 1% in all other poultry categories. No positive PE were found in the following categories: conventional laying hens, turkeys (fattening and breeding), broilers (heightened risk) and breeding chickens. While backyard establishments and conventional laying hens had the largest numbers tested, one seropositive PE only was identified in the former category, and none in the latter. Ten of the H5/H7 seropositive PEs were identified in June in Spain, among waterfowl game birds, associated with a larger sampling effort in this category at the end of the hunting season. December was the month with the second highest seropositivity rate and did not appear to be associated with a particular category or country.
Serological results for AI subtypes other than H5 and H7 were also reported for some PEs. However, due to the non‐mandatory reporting, the results presented in this report do not represent the complete picture of the distribution of these subtypes in reporting countries. In addition, 13 countries also reported PCR results from 748 PEs carried out either as a screening test or subsequent to a negative serological test result. Twenty‐five of these PEs were found positive for H5 AI viral RNA (Bulgaria, Germany, Romania and Slovakia) or non‐H5/H7 AI viral RNA (Estonia).
In Commission Delegated Regulation (EU) 2020/6891, MSs are required from April 2021 to carry out complementary risk‐based surveillance aiming to detect clusters of establishments (in time and geographical proximity) infected with LPAI viruses. The poultry categories in which this surveillance is recommended to be carried out include, among others, the categories where most of the serological positive results were found in 2020. In order to better understand the data resulting from this complementary surveillance (and poultry surveillance in general), RCs are encouraged to report the link between seropositive establishments, and the results of further follow‐up sampling and/or testing carried out in the same or surrounding establishments. Finally, understanding the underlying poultry population will help to better understand the efficiency of the surveillance carried out at a European level. The estimated poultry population could be submitted to EFSA in an aggregated form (by poultry category and NUTS3 level) as a once‐off exercise, with updates reported by RC when available.
1.2. Surveillance in wild birds
All 27 EU MSs, Iceland, Norway, Switzerland and the United Kingdom reported results from passive surveillance of AI in wild birds in 2020. Although not mandatory, ten countries also reported results from their active surveillance programmes. Wild bird surveillance in some RCs is not based on representative sampling, and therefore, the results presented here cannot be extrapolated to the source populations. Comparisons are only valid for the specific observations recorded (surveillance samples) and cannot be used to imply differences between years, species or locations.
Results were reported for a total of 18,968 wild birds, including 12,418 birds sampled by passive surveillance. This is a similar total number of birds as in 2019, but with a larger contribution of passive surveillance. The total number of birds tested by passive surveillance by RC ranged from 3 birds in Estonia to 3,041 birds in Germany. As active surveillance results in wild birds are reported to EFSA on a non‐mandatory basis, the numbers presented in this document do not represent the full extent of surveillance activities conducted by some RCs.
The distribution of number of birds by quarter was relatively consistent from January to September, with an increase in the last quarter (41% of the total). The distribution within specific countries was highly variable. Almost all birds were fully identified with a species name (9,905 birds). These birds belonged to 259 species distributed in 22 orders. As expected, most samples originated from birds in the order Anseriformes (n = 3,578). The orders Passeriformes, Columbiformes, Accipitriformes and Charadriiformes were also sampled in high numbers (n > 1,000). Forty‐four of the 50 species listed by EFSA as target for HPAI surveillance were sampled in 2020. The proportion of birds belonging to target species was 35% and 49% among passive and active surveillance samples, respectively.
A total of 1,624 wild birds tested positive to AI: 878 for HPAI and 746 with LPAI. Most HPAI strains were identified as H5N8 (737 out of 878 positive birds). Three species made up 44% of the HPAI‐infected birds (Branta leucopsis,Cygnus olor and Anas penelope). HPAI was identified much more frequently than previous years (163 and 1 HPAI positive wild birds reported in 2018 and 2019, respectively). HPAI‐positive birds were reported by 14 countries: Belgium, Denmark, France, Germany, Hungary, Ireland, Italy, the Netherlands, Norway, Poland, Slovenia, Spain, Sweden and the United Kingdom. Almost all positive birds were detected from mid‐October onwards. These results are in accordance with the widespread epidemic of H5N8 reported in Europe since late 2020, affecting both poultry and wild birds. The last large HPAI epidemic in Europe had been reported in 2016–2017. After a relatively low circulation of HPAI in Europe in 2018 and 2019, it appears that the risk of AI has substantially increased late 2020 throughout the continent.
The 932 wild birds positive for non‐HPAI viruses were reported by 16 of the 30 RCs. A total of 21 wild bird species as well as birds from four genera with unknown species were detected as positive for non‐HPAI AIVs. Positivity rates were lowest in spring (March to July). Most positive birds were detected from September onwards. The majority of positive LPAI detections were found by active surveillance (93%). Most LPAI‐positive birds belonged to the order Anseriformes, which was expected given that this is the order most sampled by both active and passive surveillance.
The report also presents summary data of wild bird observations in the RCs by voluntary contributors, obtained from the EuroBirdPortal project. Despite the limitations of such data, and until further spatial modelling of the distribution and abundance of wild birds in Europe is readily available, the maps presented in this report could help to shed light on areas where the birds of the species belonging to the target list may gather, supporting RCs in carrying out more targeted surveillance activities. Further maps of the distribution of the 50 target species and the number of samples taken by RCs for those species by month and NUTS3 have been uploaded in Zenodo.2 Considering the seasonality attached to the circulation of avian influenza viruses, these maps may be of help in improving the timing of sampling within targeted surveillance activities.
2. Introduction
Since late 2020, several European countries have been experiencing outbreaks of avian influenza (AI) in domestic poultry, mainly farmed ducks, due to an H5N8 virus subtype.3 In addition to this virus strain and other high pathogenic avian influenza (HPAI) virus strains identified over the years, low pathogenic avian influenza (LPAI)4 viruses are regularly isolated from both domestic and wild birds in the EU. To implement appropriate measures to prevent incursions of AI and control the spread of the disease when incursions occur, Member States (MSs) have implemented surveillance programmes in poultry and wild birds, including serological and virological surveillance activities. These activities include sampling of biological materials from different origins, detection of Avian Influenza A viruses (AIV) by various laboratory methods and typing of different antigenic subtypes based on their surface glycoproteins: haemagglutinin (H) and neuraminidase (N). The development and implementation of these surveillance programmes was supported by a legislative frame, which is presented below. Please note that this frame was in place until the 21st of April 2021, date in which the new Animal Health Law was implemented. The Terms of Reference of the European Commission mandate to the European Food Safety Authority (EFSA) related to the production of the present report are also described.
2.1. Background and Terms of Reference
In 2020, EU legislation on avian influenza required Member States to carry out compulsory surveillance programmes in poultry and wild birds.
The objective of the surveillance programme for AI in poultry stated in Annex I of Commission Decision 2010/367/EU was:
to inform the competent authority of circulating avian influenza virus with a view to controlling the disease in accordance with Directive 2005/94/EC by the annual detection through active surveillance for:
a ‐ LPAI of subtypes H5 and H7 in gallinaceous birds (chickens, turkeys, guinea fowl, pheasants, partridges and quails) and ratites thereby complementing other existing early detection systems.
b ‐ LPAI of subtypes H5 and H7 and HPAI in domestic waterfowl (ducks, geese and mallards for re‐stocking supplies of game).
The objective of the surveillance programme for AI in wild birds, as stated in Annex II of Commission Decision 2010/367/EU is:
the timely detection of HPAI of the subtype H5N1 in wild birds in order to protect poultry in poultry holdings and safeguard veterinary public health.
Also, as described in Decision 2018/1136/EU, the identification and review of areas that are at particular risk for the introduction of HPAI viruses into poultry establishments, had to be carried out by MSs, ensuring that increased passive surveillance of the wild bird populations took place in these higher risk areas.
Guidelines for the implementation of the surveillance programmes have been provided by the EC. The EC guidelines also include a list of wild bird target species which is under constant review as new evidence is generated when HPAI epidemics occur in Europe. As a result, EFSA published a scientific report providing further guidance to adjust wild bird surveillance of susceptible European species for the detection of H5 HPAI by passive surveillance (EFSA AHAW Panel, 2017).
Under Directive 2005/94/EC, MSs were requested to submit the results of these surveillance programmes to the competent authority. Late in 2017, EFSA received a mandate with the Terms of Reference being to: ‘collect, collate, validate, analyse and summarise in an annual report the results from avian influenza surveillance carried out by Member States in poultry and wild birds’. In the context of Article 31 of Regulation (EC) No 178/2002, from 2019 onwards, EFSA was requested to provide the technical and scientific assistance to the Commission to deliver on this mandate. This implies that EFSA is in charge of producing the annual surveillance report on AI since 2019.5 In addition, the collation of all data relevant to the surveillance activities taking place in MSs has been conducted by EFSA since January 2019.
3. Results
3.1. Poultry
3.1.1. Number of poultry establishments sampled
Twenty‐seven MSs as well as Iceland, Norway, Switzerland and the United Kingdom, here referred to as reporting countries (RCs), reported their serological surveillance activities in 2020. Data on the total number of poultry establishments present in each RC and on the distribution of poultry categories within RCs were not available for this report. For this reason, the number of samples by poultry category reported below does not include information on the proportion of the population sampled in each RC and poultry category.
A total of 24,768 poultry establishments (PEs) were sampled as part of the RCs’ surveillance programmes. In this report, the numbers reported as ‘PEs sampled’ should be treated with caution as they refer to the total number of poultry sampling events taking place on a specific date, in a specific establishment and for a specific poultry category (see Methods section for further details). Thus, the number of distinct poultry establishments where sampling occurred in each country may be lower than the total number of PEs sampled reported here, where poultry establishments have been sampled more than once in 2020. The reason PEs are defined in this way is because not all RCs submit surveillance data in a non‐aggregated manner.
Surveillance in RCs varied in both the number of PEs sampled and the poultry categories targeted for surveillance (Figure 1). Some countries conducted testing in a limited number of poultry categories (e.g. backyard flocks), while others distributed their sampling effort over a larger number of categories. An overview of the total number of PEs sampled by each RC and for each poultry category is provided in Figures 5A and 9A, respectively.
Figure 1.
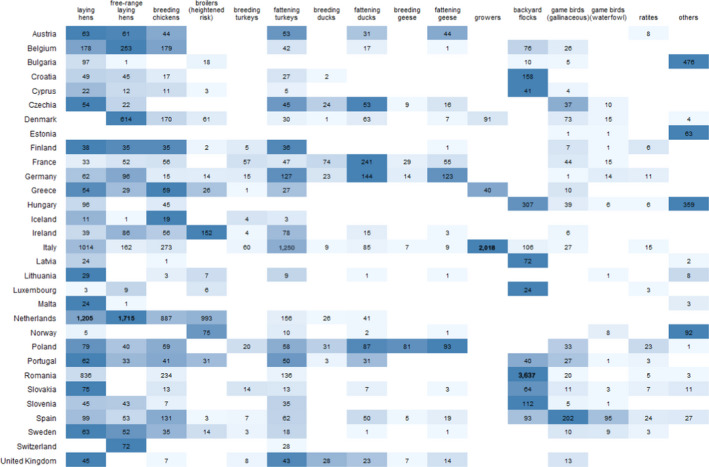
Total number of PEs sampled, presented by RC and poultry category, according to 16 poultry categories. The colours are used to indicate the poultry categories with the smallest (lightest blue shade) to the largest (darkest blue shade) number of PEs sampled within a given RC
Figure 5.
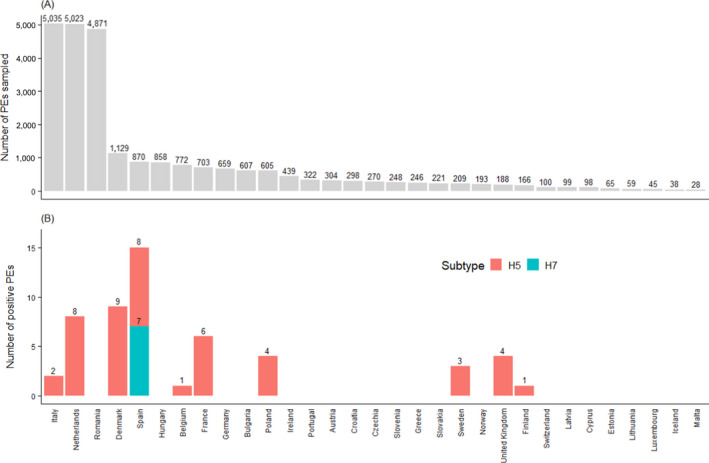
(A) Total number of PEs sampled in 2020 per RC shown in descending order and (B) total number of serologically positive PEs found by H subtype
Figure 9.
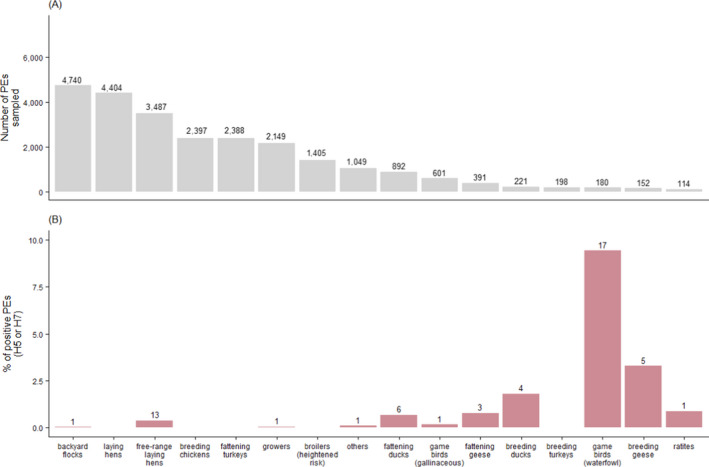
(A) Total number of PEs sampled by poultry category with values above the bars referring to the number of PEs sampled; (B) percentage (y‐axis) and number (above bars) of PEs sampled that tested serologically positive to H5 or H7 AI virus by poultry category
When looking at the poultry categories among which the largest number of samples were taken by RCs, backyard flocks and conventional and free‐range laying hens were the three most sampled poultry categories (Figure 1). In addition, Figure 1 also shows the poultry categories which are most frequently targeted (i.e. tested by the largest number of RCs). There were five categories for which surveillance results were reported by at least 20 RCs: laying hens (conventional and free‐range), fattening turkeys, breeding chickens and gallinaceous game birds. Only 3 and 7 countries reported taking samples from growers6 and breeding geese, respectively. Between 10 and 17 countries reported surveillance results for the remaining categories (others, breeding and fattening ducks, breeding turkeys, backyard flocks, waterfowl game birds, ratites, broilers at heightened risk and fattening geese).
The mapping between current, more detailed reporting categories and the 16 reporting categories used in this report (for consistency with previous reports) is presented in Appendix A (Tables A.1 and A.2).
Table A.1.
Total number of PEs sampled and testing positive in 2020, according to the 16 poultry categories used in this report and to the detailed reporting categories available to MSs
| Reporting category used in this report | Detailed reporting category | Number of sampling events | Number of H5 or H7 positive events |
|---|---|---|---|
| Backyard flocks | Backyard | 4,740 | 51 |
| Breeding chickens | Breeding chickens | 2,393 | 34 |
| Free‐range breeding chickens | 4 | 0 | |
| Breeding ducks | Breeding ducks | 203 | 4 |
| Ducks | 18 | 0 | |
| Breeding geese | Breeding geese | 151 | 7 |
| Geese | 1 | 0 | |
| Breeding turkeys | Breeding turkeys | 198 | 3 |
| Broilers (heightened risk) | Broilers | 1,234 | 1 |
| Free‐range broilers | 171 | 0 | |
| Fattening ducks | Fattening ducks | 858 | 23 |
| Free‐range fattening ducks | 34 | 1 | |
| Fattening geese | Fattening geese | 343 | 9 |
| Free‐range fattening geese | 48 | 1 | |
| Fattening turkeys | Fattening turkeys | 2,363 | 9 |
| Free‐range fattening turkeys | 25 | 0 | |
| Free‐range laying hens | Free‐range laying hens | 3,487 | 65 |
| Game birds (gallinaceous) | Farmed game birds (Gallinaceous) | 323 | 5 |
| Guinea‐fowl | 17 | 0 | |
| Partridges | 40 | 0 | |
| Pheasants | 184 | 1 | |
| Quails | 37 | 0 | |
| Game birds (waterfowl) | Farmed game birds (Waterfowl) | 155 | 45 |
| Mallard ducks | 25 | 3 | |
| Growers | Chickens | 128 | 0 |
| Generic poultry | 2,021 | 1 | |
| Laying hens | Laying hens | 4,404 | 38 |
| Others | Chickens | 189 | 10 |
| Ducks | 601 | 1 | |
| Geese | 112 | 1 | |
| Other | 34 | 0 | |
| Parrots | 3 | 0 | |
| Turkeys | 110 | 0 | |
| Ratites | Free‐range ostriches | 11 | 0 |
| Ostriches | 44 | 0 | |
| Ratites | 59 | 1 |
Table A.2.
Detailed mapping of the 16 poultry categories used in this report and the detailed reporting categories available to MSs, comprising the species, production method and purpose of raising poultry
| Reporting category used in this report | Detailed reporting category | Poultry species | Purpose of raising | Production methods |
|---|---|---|---|---|
| Backyard flocks | Backyard | Anseriformes (as animal) | Not Available | Backyard farming – growing |
| Duck (as animal) | Growers | Backyard farming – growing | ||
| Duck (as animal) | Not Available | Backyard farming – growing | ||
| Duck breeding flock (as animals) | Not Available | Backyard farming – growing | ||
| Duck fattening animal (as animal) | Not Available | Backyard farming – growing | ||
| Gallus gallus (chicken) (as animal) | Growers | Backyard farming – growing | ||
| Gallus gallus (chicken) (as animal) | Not Available | Backyard farming – growing | ||
| Gallus gallus breeding flock (as animals) | Not Available | Backyard farming – growing | ||
| Gallus gallus broiler (as animal) | Not Available | Backyard farming – growing | ||
| Gallus gallus laying hens (as animal) | Not Available | Backyard farming – growing | ||
| Generic poultry (as animal) | Growers | Backyard farming – growing | ||
| Generic poultry (as animal) | Not Available | Backyard farming – growing | ||
| Goose (as animal) | Not Available | Backyard farming – growing | ||
| Goose breeding flock (as animals) | Not Available | Backyard farming – growing | ||
| Goose fattening animal (as animal) | Not Available | Backyard farming – growing | ||
| Guinea‐fowl (as animal) | Not Available | Backyard farming – growing | ||
| Turkey (as animal) | Not Available | Backyard farming – growing | ||
| Turkey breeding flock (as animals) | Not Available | Backyard farming – growing | ||
| Turkey fattening animal (as animal) | Not Available | Backyard farming – growing | ||
| Breeding chickens | Breeding chickens | Gallus gallus breeding flock (as animals) | Breeding purpose | Not Available |
| Gallus gallus breeding flock (as animals) | Not Available | Not Available | ||
| Free‐range breeding chickens | Gallus gallus breeding flock (as animals) | Not Available | Outdoor/free‐range growing condition | |
| Breeding ducks | Breeding ducks | Duck breeding flock (as animals) | Breeding purpose | Not Available |
| Duck breeding flock (as animals) | Game purpose | Not Available | ||
| Duck breeding flock (as animals) | Not Available | Not Available | ||
| Ducks | Duck (as animal) | Breeding purpose | Not Available | |
| Duck laying hens (as animal) | Breeding purpose | Not Available | ||
| Breeding geese | Breeding geese | Goose breeding flock (as animals) | Breeding purpose | Not Available |
| Goose breeding flock (as animals) | Not Available | Not Available | ||
| Free‐range breeding geese | Goose breeding flock (as animals) | Not Available | Outdoor/free‐range growing condition | |
| Geese | Goose laying hens (as animal) | Breeding purpose | Not Available | |
| Breeding turkeys | Breeding turkeys | Turkey breeding flock (as animals) | Breeding purpose | Not Available |
| Turkey breeding flock (as animals) | Not Available | Not Available | ||
| Broilers (heightened risk) | Broilers | Gallus gallus broiler (as animal) | Breeding purpose | Not Available |
| Gallus gallus broiler (as animal) | Meat production purpose | Not Available | ||
| Gallus gallus broiler (as animal) | Not Available | Not Available | ||
| Free‐range broilers | Gallus gallus broiler (as animal) | Not Available | Outdoor/free‐range growing condition | |
| Fattening ducks | Fattening ducks | Duck fattening animal (as animal) | Breeding purpose | Not Available |
| Duck fattening animal (as animal) | Game purpose | Not Available | ||
| Duck fattening animal (as animal) | Meat production purpose | Not Available | ||
| Duck fattening animal (as animal) | Not Available | Not Available | ||
| Free‐range fattening ducks | Duck fattening animal (as animal) | Not Available | Outdoor/free‐range growing condition | |
| Fattening geese | Fattening geese | Goose fattening animal (as animal) | Meat production purpose | Not Available |
| Goose fattening animal (as animal) | Not Available | Not Available | ||
| Free‐range fattening geese | Goose fattening animal (as animal) | Not Available | Outdoor/free‐range growing condition | |
| Fattening turkeys | Fattening turkeys | Turkey fattening animal (as animal) | Breeding purpose | Not Available |
| Turkey fattening animal (as animal) | Meat production purpose | Not Available | ||
| Turkey fattening animal (as animal) | Not Available | Not Available | ||
| Free‐range fattening turkeys | Turkey fattening animal (as animal) | Not Available | Outdoor/free‐range growing condition | |
| Free‐range laying hens | Free‐range laying hens | Gallus gallus laying hens (as animal) | Not Available | Outdoor/free‐range growing condition |
| Game birds (gallinaceous) | Farmed game birds (Gallinaceous) | Galliformes (as animal) | Game purpose | Not Available |
| Galliformes (as animal) | Not Available | Not Available | ||
| Peafowl (as animal) | Not Available | Not Available | ||
| Free‐range partridges | Partridge (as animal) | Game purpose | Outdoor/free‐range growing condition | |
| Free‐range pheasants | Pheasant (as animal) | Game purpose | Outdoor/free‐range growing condition | |
| Guinea‐fowl | Guinea‐fowl (as animal) | Not Available | Not Available | |
| Other | Game or wild bird (as animal) | Game purpose | Not Available | |
| Partridges | Partridge (as animal) | Breeding purpose | Not Available | |
| Partridge (as animal) | Not Available | Not Available | ||
| Partridge breeding flock (as animals) | Game purpose | Not Available | ||
| Partridge breeding flock (as animals) | Not Available | Not Available | ||
| Pheasants | Pheasant (as animal) | Breeding purpose | Not Available | |
| Pheasant (as animal) | Game purpose | Not Available | ||
| Pheasant (as animal) | Not Available | Not Available | ||
| Pheasant breeding flock (as animals) | Breeding purpose | Not Available | ||
| Pheasant breeding flock (as animals) | Game purpose | Not Available | ||
| Pheasant breeding flock (as animals) | Not Available | Not Available | ||
| Pheasant laying hens (as animal) | Not Available | Not Available | ||
| Quails | Common Quail (as animal) | Not Available | Not Available | |
| Grey Partridge (as animal) | Not Available | Not Available | ||
| Quail (as animal) | Not Available | Not Available | ||
| Quail breeding flock (as animals) | Breeding purpose | Not Available | ||
| Quail fattening animal (as animal) | Not Available | Not Available | ||
| Quail laying hens (as animal) | Not Available | Not Available | ||
| Turkeys | Turkey (as animal) | Game purpose | Not Available | |
| Game birds (waterfowl) | Ducks | Duck (as animal) | Game purpose | Not Available |
| Farmed game birds (Waterfowl) | Anas (as animal) | Not Available | Not Available | |
| Anseriformes (as animal) | Game purpose | Not Available | ||
| Anseriformes (as animal) | Not Available | Not Available | ||
| Anseriformes (as animal) | Not Available | Outdoor/free‐range growing condition | ||
| Common Goldeneye (as animal) | Not Available | Not Available | ||
| Velvet Scoter (as animal) | Not Available | Not Available | ||
| Wood Duck (as animal) | Not Available | Not Available | ||
| Free‐range mallard ducks | Mallard (as animal) | Game purpose | Outdoor/free‐range growing condition | |
| Mallard ducks | Mallard (as animal) | Game purpose | Not Available | |
| Mallard (as animal) | Not Available | Not Available | ||
| Growers | Chickens | Gallus gallus (chicken) (as animal) | Growers | Not Available |
| Generic poultry | Generic poultry (as animal) | Growers | Not Available | |
| Turkeys | Turkey (as animal) | Growers | Not Available | |
| Laying hens | Laying hens | Gallus gallus laying hens (as animal) | Breeding purpose | Not Available |
| Gallus gallus laying hens (as animal) | Not Available | Not Available | ||
| Others | Chickens | Gallus gallus (chicken) (as animal) | Not Available | Not Available |
| Ducks | Duck (as animal) | Meat production purpose | Not Available | |
| Duck (as animal) | Not Available | Not Available | ||
| Duck laying hens (as animal) | Not Available | Not Available | ||
| Free‐range chickens | Gallus gallus (chicken) (as animal) | Not Available | Outdoor/free‐range growing condition | |
| Free‐range ducks | Duck (as animal) | Not Available | Outdoor/free‐range growing condition | |
| Geese | Goose (as animal) | Not Available | Not Available | |
| Goose laying hens (as animal) | Not Available | Not Available | ||
| Other | Cattle Egret (as animal) | Not Available | Not Available | |
| Common Cuckoo (as animal) | Not Available | Not Available | ||
| Eurasian Spoonbill (as animal) | Not Available | Not Available | ||
| Falco (as animal) | Not Available | Not Available | ||
| Greater Flamingo (as animal) | Not Available | Not Available | ||
| Pigeon (as animal) | Not Available | Backyard farming – growing | ||
| Pigeon (as animal) | Not Available | Not Available | ||
| Saker Falcon (as animal) | Not Available | Not Available | ||
| Parrots | Parrots (as animal) | Not Available | Not Available | |
| Psittaciformes (as animal) | Not Available | Backyard farming – growing | ||
| Psittaciformes (as animal) | Not Available | Not Available | ||
| Pigeon breeding flock | Pigeon breeding flock (as animals) | Not Available | Not Available | |
| Turkeys | Turkey (as animal) | Not Available | Not Available | |
| Ratites | Free‐range ostriches | Ostrich (as animal) | Not Available | Outdoor/free‐range growing condition |
| Free‐range ratites | Ratite (as animal) | Not Available | Outdoor/free‐range growing condition | |
| Ostriches | Ostrich (as animal) | Game purpose | Not Available | |
| Ostrich (as animal) | Not Available | Not Available | ||
| Ostrich breeding flock (as animals) | Not Available | Not Available | ||
| Ostrich fattening animal (as animal) | Not Available | Not Available | ||
| Other | Emu (as animal) | Not Available | Not Available | |
| Ratites | Ratite (as animal) | Not Available | Not Available |
Within MSs and in addition to the sampling carried out under European funding (‘EU co‐funded active surveillance’, in blue in Figure 2), five countries reported surveillance results from their national programmes (Estonia, Lithuania, Luxembourg, Slovakia and Spain) and one from a private industry sampling programme (Slovakia) (Figure 2). Norway, Switzerland and Iceland reported results from their national programmes, with Iceland also reporting some results obtained via private industry sampling.
Figure 2.
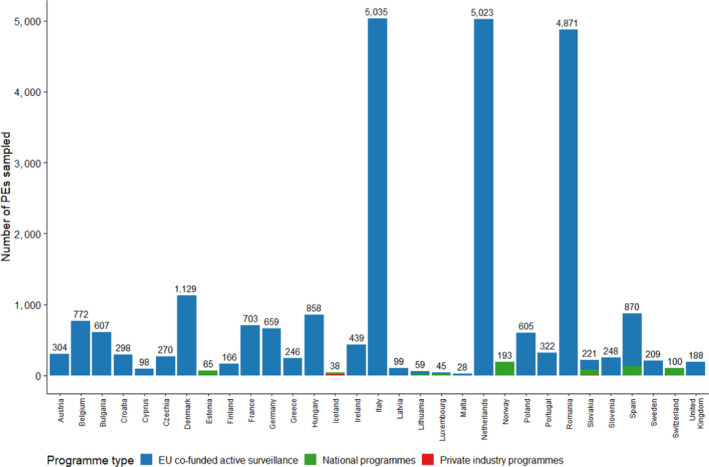
Number of PEs sampled by RCs in 2020 according to the type of active surveillance programme and for which results were reported to EFSA
Please note that it is not mandatory for MSs to report surveillance results from surveillance activities other than the EU co‐funded active surveillance.
3.1.2. Timing of sampling in poultry
In terms of the timing of the sampling, 57% of the sampling took place in the second half of the year (July–December). All countries but France conducted sampling activities during both semesters (Figure 3). A total of 14,068 PEs were reported as sampled from July to December 2020, while 10,700 PEs were reported as sampled in the reporting period going from January to June. Figure 3 shows the monthly distribution of poultry sampling in each RC.
Figure 3.
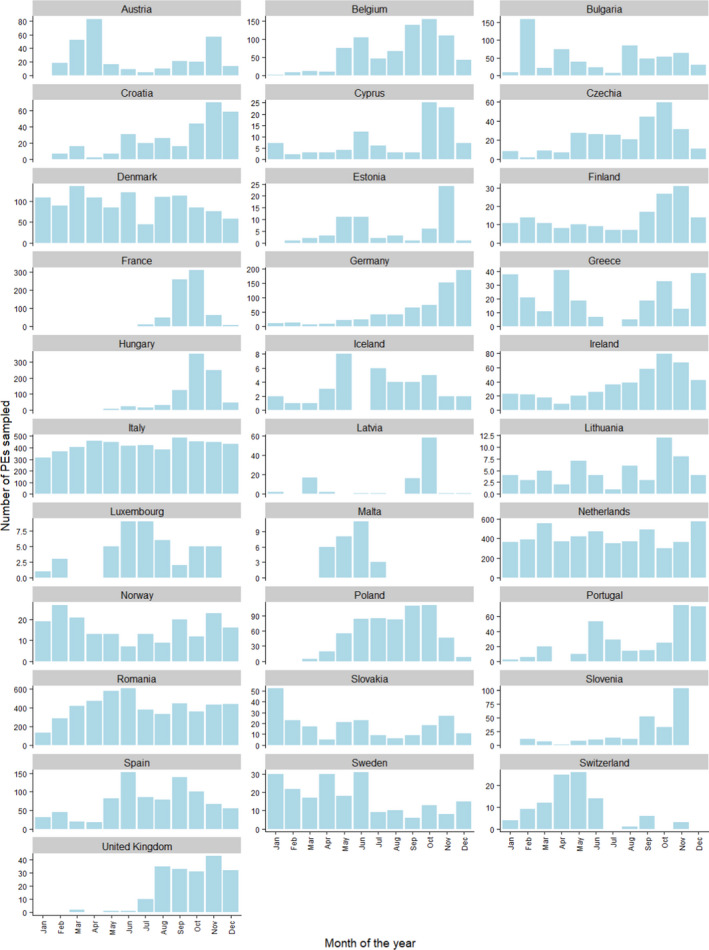
Monthly number of PEs sampled in 2020, presented by RC. Note that the scale of the vertical axes is specific to each country
3.1.3. Avian influenza in poultry
3.1.3.1. Serological results overview
In this section, comparisons of seropositivity rates between different groups relate to the sampling results. They cannot be extrapolated to the source populations because:
the sampling was targeted at higher‐risk groups (non‐representative sampling strategy) in some RCs,
the targeting approaches may differ between countries, between groups and between years.
Therefore, the percentages and trends provided in this report relate only to the surveillance samples, not to the underlying population. Temporal trends are based on the assumption that sampling strategies and targeting remain constant over time in all RCs.
In 2020, 46 PEs tested positive for AI H5 and 7 for H7 (Figure 4). None of the PEs sampled tested positive for both H5 and H7. The combined H5/H7 seropositive percentage was 0.21%, lower than the seropositive percentage in 2019 (0.45%). The percentage of AI H5 seropositive PE was 0.19%. This number is lower than that of the previous year (0.36%). The percentage of AI H7 seropositive PEs was 0.03%, lower than the proportion found in 2019 (0.09%). In 2020, the total number of PEs sampled (n = 24,768) was at its highest since 2014. It was lower than the number of PEs sampled in 2013 and in previous years. The downward trend in the number of PEs sampled observed since 2008 may be reverting (Figure 4A).
Figure 4.
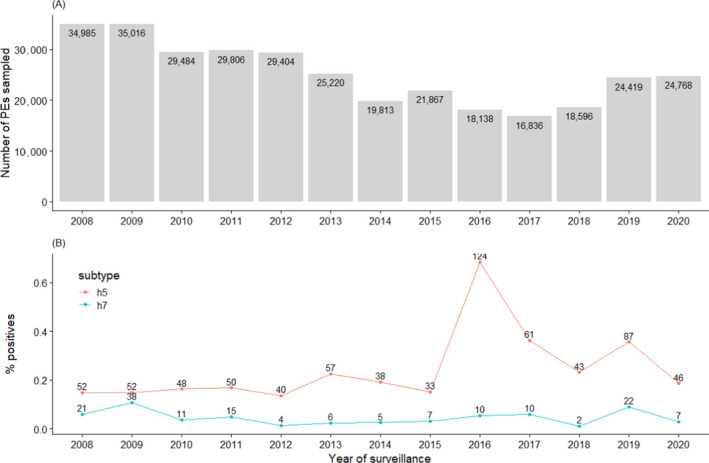
(A) Total number of PEs sampled per year and (B) line graph of the percentage of the AI seropositive PEs of the H5 and H7 subtypes, with the number of seropositive PEs shown per year as labels
3.1.3.2. Serological results by reporting countries
Considerable variation in the number of PEs sampled7 among RCs was observed in 2020 (Figure 5), as already noted in previous years. Three countries (Italy, the Netherlands and Romania) reported 60% of all PEs sampled over the course of 2020. The total number of PEs sampled among RCs ranged from 28 in Malta, to 5,035 in Italy, with the median number of PEs sampled among RC being 270 (Figure 5). Variation among RCs in terms of the number of PEs testing seropositive to either H5 or H7 AI was also noticed. Ten RCs reported the detection of seropositive PEs for H5 or H7. All ten countries reported detection of AI H5 (total of 46 PEs) and only Spain reported the detection of AI H7 seropositive PEs (total of 7 PEs) (Figure 5). Most H5/H7 detections (45 PEs out of 53) occurred in countries which sampled a number of PEs larger than the median number of PEs sampled.
3.1.3.3. Serological results by administrative units
Surveillance activities in poultry were reported for 32 NUTS2 (Nomenclature of Territorial Units for Statistics, level 2) units and 798 NUTS3 units in 2020. Reporting at NUTS2 level was linked to surveillance activities in Belgium, Germany, Italy and the United Kingdom. Out of the 24,768 PEs, 5,830 and 18,938 were reported at NUTS2 and NUTS3 level, respectively. Out of 53 seropositive PEs, 3 and 50 were reported at NUTS2 and NUTS3 level, respectively.
Figure 6 shows the geographical distribution of the surveillance activities that took place in 2020, as well as the number of H5 or H7 seropositive detections. Data are represented at the NUTS level they were reported at (i.e. the maps show a combination of NUTS2 and NUTS3 units). The sampling density, estimated as the number of PEs sampled per 100 km2 within an NUTS region, and the distribution of the seropositive PEs for AI H5 or H7 are presented in Figure 6 in the upper and lower maps, respectively.
Figure 6.
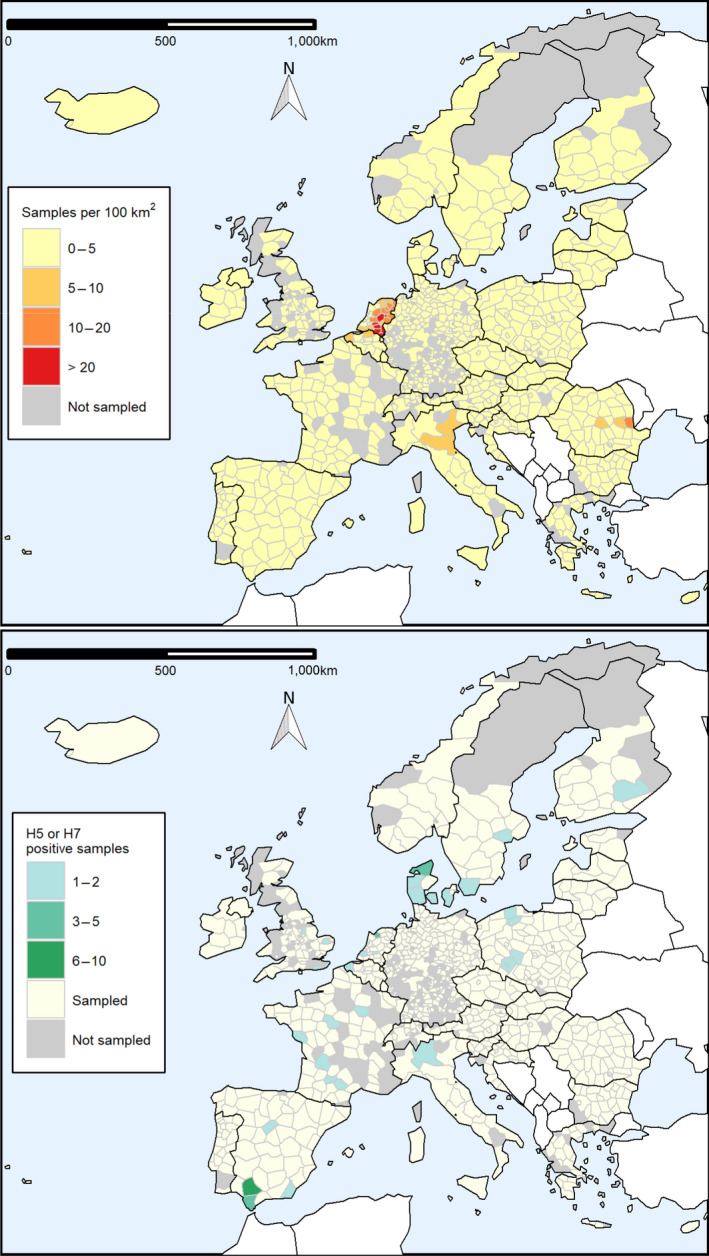
Sampling density expressed as the number of PEs sampled per 100 km2 (upper map) and geographical distribution of AI H5 and H7 seropositive PEs (lower map) by administrative unit. Non‐reporting countries are shown in white
3.1.3.4. Serological results by month
Since 2019, poultry surveillance data have been reported on a monthly basis. The distribution of PEs testing positive for H5 or H7 by month shows that the months with the highest seropositivity rates (and the highest number of seropositive PEs) were February, June, October and December 2020 (Figure 7). During these months, 6, 12, 6 and 9 PEs, respectively, were reported positive, compared to between 1 and 3 PEs during other months of the year. There was no apparent correlation between higher seropositivity rates and higher number of PEs sampled. However, as noted in the previous report, the month with the highest number of positives corresponded to the month where most of the PEs from the category ‘game birds (waterfowl)’ were sampled. In 2019, this occurred in April, while in 2020, it occurred in June: 76 PEs sampled in June compared to 104 during the remaining 11 months. Out of 17 positive PEs in waterfowl game birds, 10 were identified in June.
Figure 7.
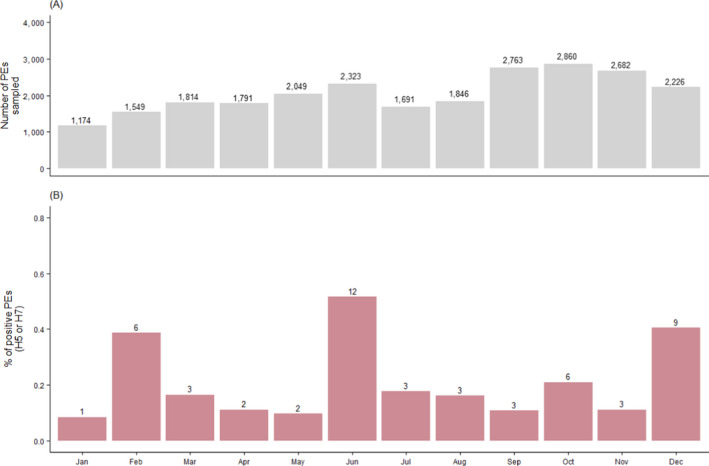
(A) Total number of PEs sampled by month with values above the bars referring to the number of PEs sampled. (B) percentage (y‐axis) and number (above bars) of PEs sampled that tested serologically positive to H5 or H7 AI virus by month
For the ten countries reporting H5 or H7 seropositive PEs, the distribution of these events by month is shown in Figure 8.
Figure 8.
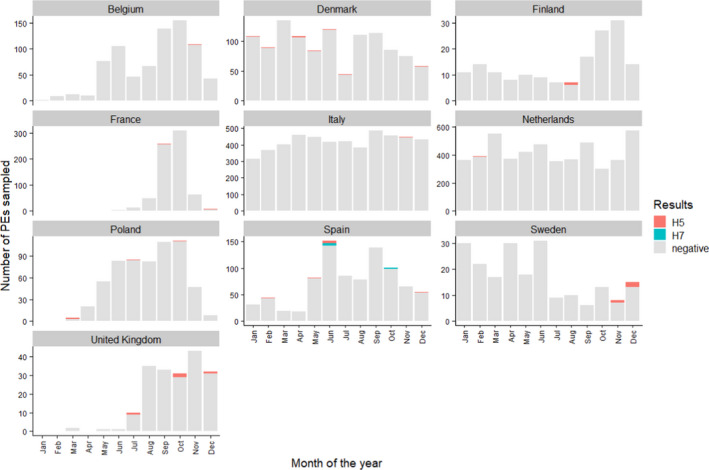
Monthly number of PEs sampled and positive in serology (H5 or H7 only) in 2020, presented for RCs with at least one H5 or H7 positive PE only. Note that the scale of the vertical axes is specific to each country
3.1.3.5. Serological results by poultry category
The highest numbers of PEs sampled by RCs in 2020 were from the backyard and conventional laying hen categories (n = 4,740 and 4,404, respectively) (Figure 9A). These two most sampled categories were the same as in previous years. Other categories sampled in large numbers were the free‐range laying hens, breeding chickens, fattening turkeys and growers (Figure 9A).
As in 2019 and earlier, the highest percentage of AI H5 or H7 seropositive PEs in 2020 was found in the waterfowl game bird category (9.4% out of 180 waterfowl game bird PEs sampled), followed by breeding geese (3.3% out of 152 PEs) and breeding ducks (1.8% out of 221 PEs). The proportion of seropositive PEs was under 1% in all other poultry categories. The ‘other’ category had a lower proportion of seropositive PEs compared to the previous year (0.1% out of 1,049 PEs sampled). When considering only gallinaceous species, the highest percentage of H5 or H7 seropositive PEs was observed in the free‐range laying hen category (0.4% out of 3,487 PEs sampled). No H5 or H7 seropositive results were found in turkeys (fattening or breeding), broilers (heightened risk), breeding chickens and conventional laying hens. One positive PE was found in each of the growers and ratite categories, unlike in 2019 where no positive PE had been found in these categories.
In addition to H5 and H7 positive results, ten RCs reported non‐H5/H7 positive results in poultry (Austria, Belgium, Denmark, Estonia, Germany, Latvia, Luxembourg, Norway, Sweden and Spain). There were 261 PEs seropositive for AI virus strains other than H5 or H7.8 The categories with the largest numbers of non‐H5/H7 seropositive PEs were the laying hens (free‐range and conventional), backyard flocks, waterfowl game birds and breeding chickens. Proportions of non‐H5/H7 seropositive PEs by poultry category could not be reliably estimated, as not all RCs reported these results. For this reason, Figure 9 does not display the non‐H5/H7 results.
For each poultry category, detailed results by month are shown in Figure 10. In addition, surveillance results by bird species and order are shown in Figure B.1 – Appendix B. The figure shows that, regardless of management system, positive PEs were found in Anseriformes (domestic and Mallard ducks as well as geese), chickens, ratites and pheasants. A large number of positive samples were identified in PEs raising game birds from the order Anseriformes for which the bird species was not available.
Figure 10.
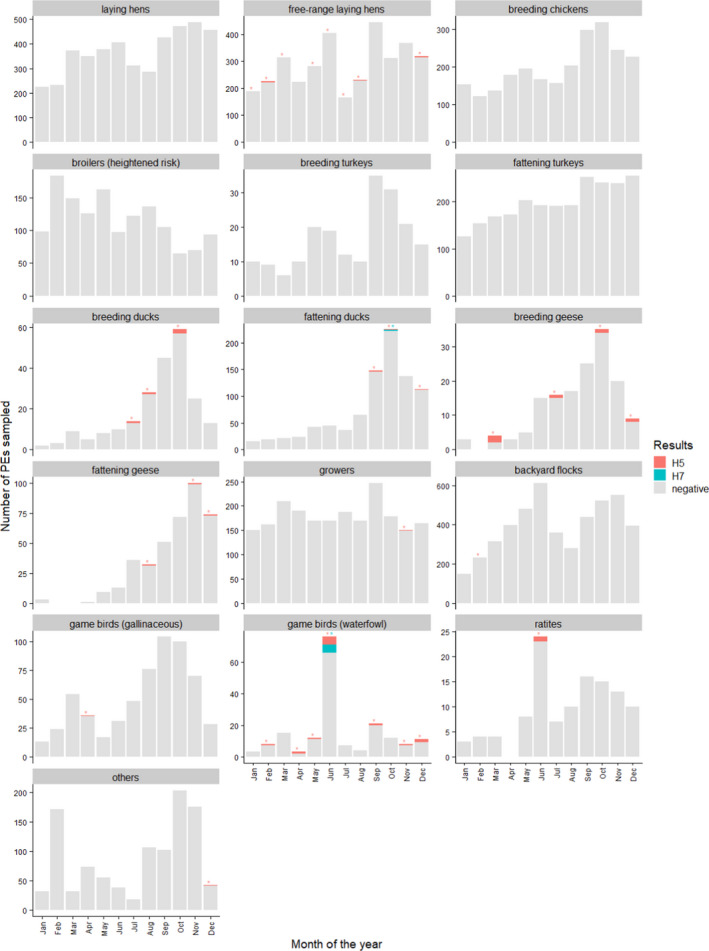
Monthly number of PEs sampled and positive in serology (H5 or H7 only) in 2020, presented by poultry category. Note that the scale of the vertical axes is specific to each category. Some positive results (e.g. in laying hens) are not visible due to the small number of positive PE that month (e.g. 1 H5‐positive PE only). The asterisks indicate whether there was at least one positive PE in that category and month
Figure B.1.
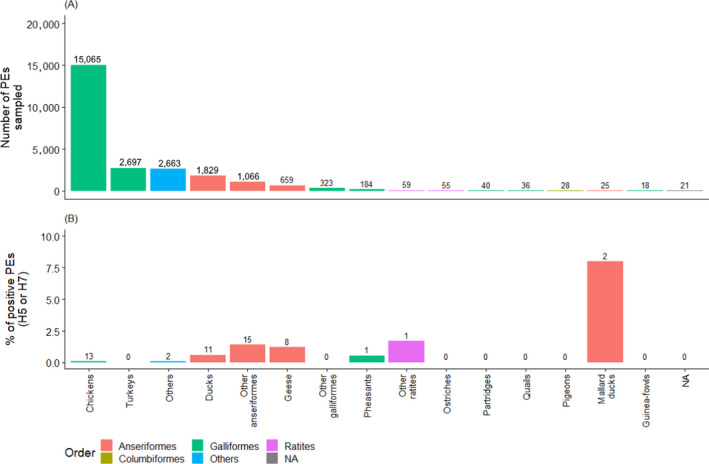
(A) Number of PEs sampled by poultry species; (B) Proportion of PE sampled that tested positive for H5 or H7 AI virus in serology. The numbers above the bars indicate the number of seropositive PEs. Bars are colour coded to identify the order to which these species belong to. The species name was not reported for some PEs, which were only identified at the bird order level. Ostriches, emus and other ratites were classified under the term ‘ratites’ which is not an order, given that species names were not always available
3.1.3.6. Serological results: summary
Figure 11 shows the countries and poultry categories in which H5 seropositive birds were detected. Spain, the Netherlands and Denmark were the countries reporting the most H5‐positive PEs. Those PEs were reported mainly in free‐range laying hens in Denmark and the Netherlands, and mainly in waterfowl game birds in Spain. Spain also reported the detection of H7 seropositive PEs (waterfowl game birds and fattening ducks).
Figure 11.
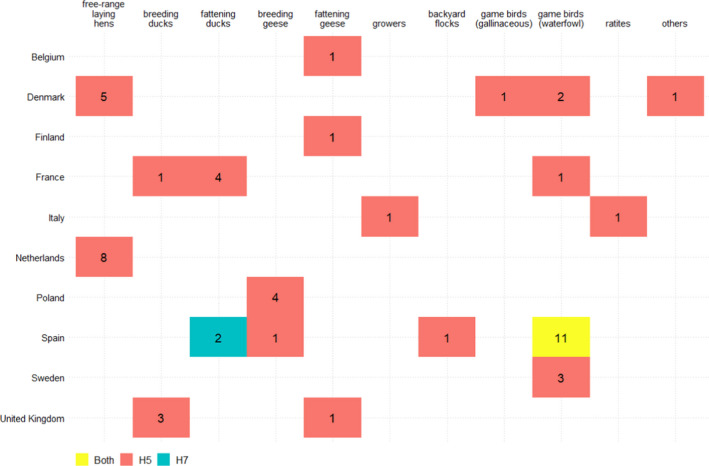
Number of H5 and H7 seropositive PEs by RC and poultry category in 2020, presented for RCs and categories with at least one H5 or H7 positive PE only
The sensitivity of serological surveillance activities to detect HPAI in RCs depends on several parameters, including the number of poultry establishments in each country, the number of establishments sampled, the sensitivity of within‐establishment sampling and the design prevalence (proportion of establishments which is expected to be infected should HPAI be present in the country).
3.1.3.7. PCR and virological results
Out of the 53 PEs with positive serological tests for H5 or H7, samples from 46 PEs were tested further for AI viral RNA using polymerase chain reaction (PCR), and seven of these PEs tested positive in PCR:
two positives in France, one for H5 LPAI and one for non‐H5/H7 LPAI, both in fattening ducks.
one free‐range laying hen PE tested positive in the Netherlands for H5 LPAI.
in Denmark, one laying hen PE tested positive for H5 LPAI and one PE (category ‘others’) tested positive for H5 HPAI.
one waterfowl game bird PE tested positive for non‐H5/H7 LPAI in Sweden.
and, last, one breeding duck PE tested positive for H5 LPAI in the United Kingdom.
Most of the seropositive PEs were tested by PCR on the same day (n = 36), while the remainder were re‐sampled for PCR testing on average 11 days after the serological tests. No virus isolation results were available for the PEs with positive serological or PCR tests. Virus isolation results were available for samples from four PEs (all in Spain) and were all negative.
In addition, 13 countries also reported PCR results from 748 PEs which did not correspond to the follow‐up testing of a positive serology event (e.g. in some PEs, PCR tests were used for screening). Twenty‐five of these PEs were found positive for AI viral RNA, including 16 PEs with H5 HPAI in Bulgaria and Germany. The pathogenicity of the virus identified in the other PEs was not available (H5 in Romania and Slovakia, non‐H5/H7 in Estonia).
3.2. Wild birds
3.2.1. Number of birds sampled
In 2020, a total of 18,968 wild birds were sampled by 27 MSs as well as Iceland, Norway, Switzerland and the United Kingdom (31 RCs) either by active or passive surveillance.
Within MSs and in addition to the sampling carried out under European funding (‘EU co‐funded passive surveillance’, in blue in Figure 12), four countries reported surveillance results from their national programmes (non‐EU co‐funding programmes) (Belgium, Estonia, Germany and Spain). Norway, Switzerland and Iceland reported results from their national programmes.
Figure 12.
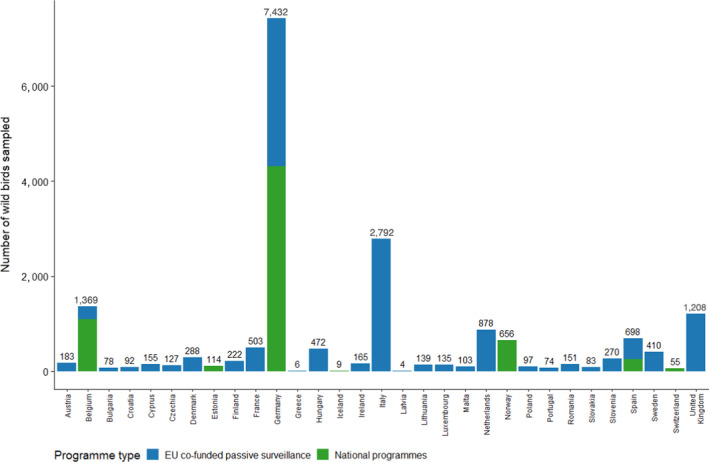
Number of wild birds sampled by RCs in 2020 according to the type of surveillance programme
For the purpose of this report, birds ‘found dead’ or ‘live with clinical signs’ were classified under passive surveillance (the latter including injured birds), while birds reported as ‘hunted with clinical signs’, ‘hunted without clinical signs’ and ‘live without clinical signs’ were considered as birds sampled via active surveillance. This is consistent with the classification method followed in previous reports. Passive surveillance is assumed to be undertaken by voluntary contributors.
All 31 RCs reported results from their passive surveillance. From the total number of birds sampled, 12,418 were sampled by passive surveillance in 2020, more than in 2018 or 2019, but less than in 2017 (Table 1). The sensitivity of passive surveillance for AI in wild birds is highly dependent on the probability of contributors discovering and reporting birds found dead, injured or with clinical signs.
Table 1.
Number of wild birds sampled by RC in 2020 (light grey background), with active and passive surveillance presented separately and combined as a total, and number of wild birds sampled by passive surveillance from 2017 to 2019 (no background colour). Small figures or no data for active surveillance do not mean that no active surveillance was carried out in that RC, rather, little or no data were reported to EFSA from that RC
| Reporting Country | Passive surveillance | Active surveillance 2020 | Total 2020 | |||
|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | |||
| Austria | 897 | 109 | 85 | 183 | 0 | 183 |
| Belgium | 367 | 237 | 423 | 275 | 1,094 | 1,369 |
| Bulgaria | 47 | 58 | 65 | 70 | 8 | 78 |
| Croatia | 279 | 223 | 160 | 92 | 0 | 92 |
| Cyprus | 117 | 109 | 87 | 137 | 18 | 155 |
| Czechia | 330 | 94 | 104 | 127 | 0 | 127 |
| Denmark | 154 | 148 | 111 | 288 | 0 | 288 |
| Estonia | 38 | 16 | 8 | 3 | 111 | 114 |
| Finland | 316 | 195 | 174 | 222 | 0 | 222 |
| France | 766 | 113 | 158 | 503 | 0 | 503 |
| Germany | 8,533 | 1,711 | 1,392 | 3,041 | 4,391 | 7,432 |
| Greece | 90 | 13 | 12 | 6 | 0 | 6 |
| Hungary | 703 | 371 | 338 | 472 | 0 | 472 |
| Iceland | – | – | 2 | 9 | 0 | 9 |
| Ireland | 137 | 142 | 78 | 165 | 0 | 165 |
| Italy | 2,019 | 2,109 | 2,719 | 2,791 | 1 | 2,792 |
| Latvia | 11 | 14 | 15 | 4 | 0 | 4 |
| Lithuania | 131 | 70 | 63 | 139 | 0 | 139 |
| Luxembourg | 61 | – | 50 | 135 | 0 | 135 |
| Malta | – | – | – | 9 | 94 | 103 |
| Netherlands | 509 | 663 | 643 | 878 | 0 | 878 |
| Norway | – | – | 28 | 128 | 528 | 656 |
| Poland | 209 | 36 | 33 | 97 | 0 | 97 |
| Portugal | 54 | 82 | 126 | 74 | 0 | 74 |
| Romania | 528 | 244 | 201 | 107 | 44 | 151 |
| Slovakia | 513 | 84 | 45 | 83 | 0 | 83 |
| Slovenia | 556 | 178 | 231 | 270 | 0 | 270 |
| Spain | 370 | 344 | 281 | 437 | 261 | 698 |
| Sweden | 452 | 455 | 456 | 410 | 0 | 410 |
| Switzerland | 162 | 45 | 30 | 55 | 0 | 55 |
| United Kingdom | 1,194 | 1,282 | 816 | 1,208 | 0 | 1,208 |
| Total | 19,543 | 9,145 | 8,934 | 12,418 | 6,550 | 18,968 |
Some RCs (n = 10) also performed and reported results from active surveillance data (non‐EU co‐funding programmes for which reporting is non‐mandatory), particularly, Belgium, Estonia, Germany and Norway who sampled a higher number of birds by active than by passive surveillance (Table 1). Although active surveillance was carried out in other RCs, the data shown in the report represent the data submitted to EFSA only. As reporting active surveillance results in wild birds to EFSA is not mandatory, the numbers reported below for active surveillance do not represent the full extent of activities conducted by some RCs. Consequently, this report contains complete data for passive surveillance only and mainly focuses on summarising the sampling activities and results obtained by passive surveillance.
3.2.2. Timing of sampling in wild birds
In Figure 13, the quarterly distribution of the number of birds sampled by passive surveillance in 2020 is shown by RC. The highest number of samples were taken during the last quarter (October‐December). The distribution of sampling was lower but relatively consistent during the first three quarters:
Quarter 1: 2,152 birds, 17%
Quarter 2: 2,352 birds, 19%
Quarter 3: 2,789 birds, 22%
Quarter 4: 5,125 birds, 41%
Figure 13.
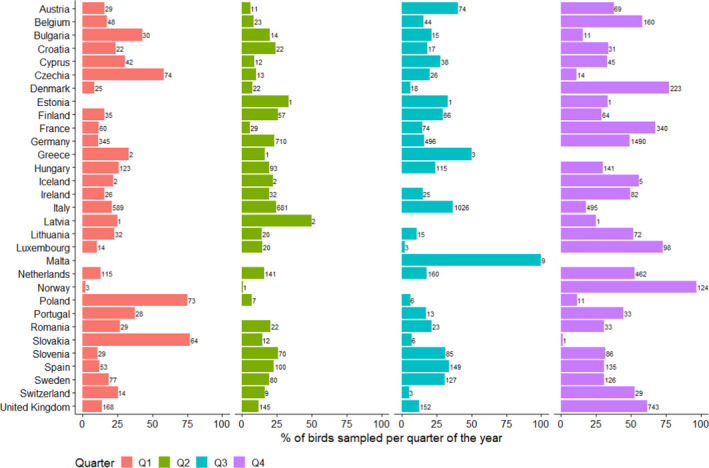
Quarterly percentage (bars) and total number (values) of wild birds sampled by passive surveillance by RC in 2020, with quarter 1 starting in January 2020
Figure 13 shows some variation among RCs in terms of the sampling distribution throughout the year (percentage of samples taken at each quarter by each RC). For example, around 75% of samples collected in Poland and Slovakia over the year were taken during the first quarter. All samples collected in Malta were reported for the third quarter. Finally, the sampling was most intensive in the fourth quarter for Denmark, France, Luxembourg and Norway.
3.2.3. Species distribution in wild birds
Among wild birds sampled via passive surveillance, there were:
9,905 birds fully identified with a species name. These samples belonged to a total of 259 wild bird species belonging to 22 orders.
2,162 birds for which only the genus was identified but not the species (14 orders).
123 birds for which only the family was identified but not the species (7 orders).
37 birds for which only the order was identified (5 orders).
191 birds for which identification information was completely missing. Birds from this category are shown under the group name ‘Species unknown’ in Figure 14.
Figure 14.
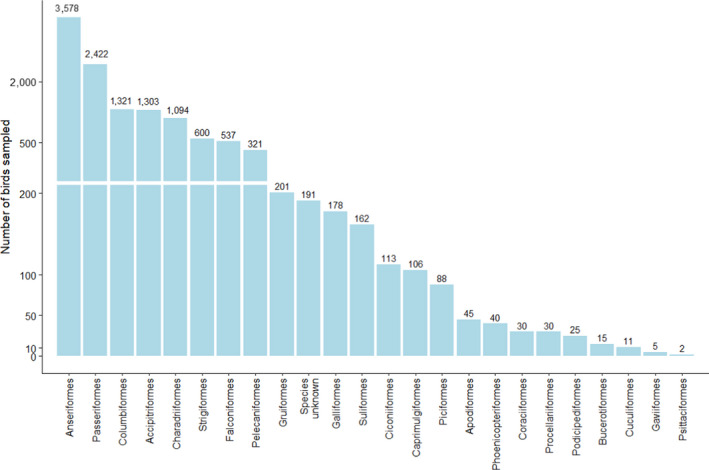
Total number of wild birds of the different orders, sampled by passive surveillance in 2020 (n = 12,418). The Y‐axis is presented on a non‐linear scale to improve visibility
The most sampled order was Anseriformes (n = 3,578), which accounted for 28.8% of the total number of birds sampled by passive surveillance. The orders Passeriformes, Columbiformes, Accipitriformes and Charadriiformes were also sampled in high numbers (n > 1,000) (Figure 14).
Active surveillance samples were also mostly taken from birds of the order Anseriformes. A total of 5,153 samples from this order were tested by active surveillance, out of a total of 6,550 samples tested (78.7%). The distribution of birds sampled by order is shown jointly for active and passive surveillance in Figure C.1 – Appendix C.
Figure C.1.
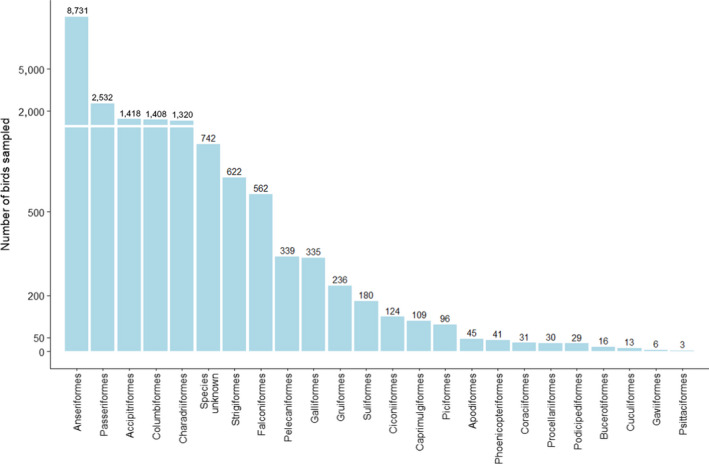
Total number of wild birds of the different orders sampled by passive and active surveillance by RCs in 2020. The group ‘Species unknown’ includes all birds for which data on species and order were not available. The Y‐axis is presented on a non‐linear scale to improve visibility
The majority of the species sampled by passive surveillance belonged to the orders Passeriformes (n = 84 species), Charadriiformes (n = 47), Anseriformes (n = 46) and Accipitriformes (n = 26). In Figure 15, the 40 species with the most birds sampled in 2020 are shown (out of 259 fully identified species).
Figure 15.
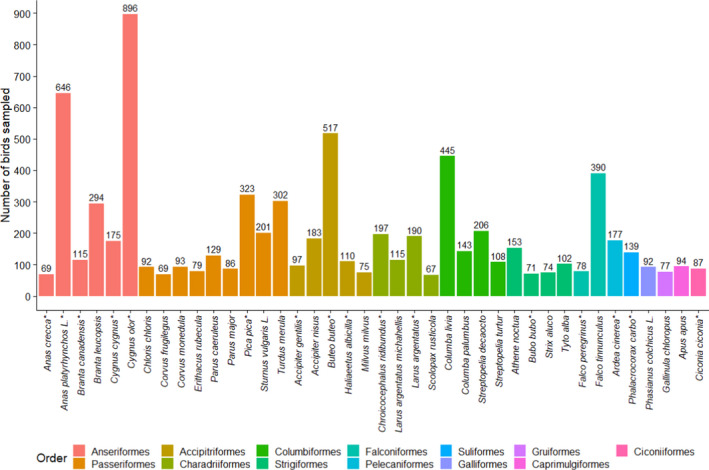
Total number of birds sampled for the 40 most sampled wild bird species reported by passive surveillance in 2020 (7,556 birds out of 9,905 fully identified birds). The bar colours refer to the bird orders. English common names for the species shown are provided in Appendix D
The four most sampled species (passive surveillance) were Cygnus olor (mute swan), Anas platyrhynchos (mallard), Buteo buteo (common buzzard) and Columba livia (common pigeon), similarly to the 2019 results, albeit with a different ranking. All English common names for the species shown in Figure 15 are listed in Table D.1 – Appendix D.
Table D.1.
English common names and scientific names of wild bird species sampled in 2020
| Latin name | English common name |
|---|---|
| Accipiter gentilis | Northern Goshawk |
| Accipiter nisus | Sparrowhawk |
| Aegolius funereus | Boreal Owl |
| Aegypius monachus | Cinereous Vulture |
| Aix galericulata | Mandarin Duck |
| Aix sponsa | Wood Duck |
| Alauda arvensis | Eurasian skylark |
| Alca torda | Razorbill |
| Alcedo atthis | Common Kingfisher |
| Alectoris chukar | Chukar partridge |
| Alectoris rufa | Red legged partridge |
| Alle alle | Little Auk |
| Alopochen aegyptiacus | Egyptian Goose |
| Anas acuta | Northern Pintail |
| Anas clypeata | Shoveler |
| Anas crecca | Common Teal |
| Anas penelope | Eurasian Wigeon |
| Anas platyrhynchos L. | Mallard |
| Anas strepera | Gadwall |
| Anser albifrons | Greater White‐fronted Goose |
| Anser anser | Greylag goose |
| Anser brachyrhynchus | Pink‐footed Goose |
| Anser cygnoides | Swan Goose |
| Anser fabalis | Taiga Bean Goose |
| Anthus trivialis | Tree Pipit |
| Apus apus | Common swift |
| Apus melba | Alpine swift |
| Apus pallidus | Pallid swift |
| Aquila adalberti | Spanish Imperial Eagle |
| Aquila chrysaetos | Golden Eagle |
| Ardea alba | Great White Egret |
| Ardea cinerea | Grey Heron |
| Ardea purpurea | Purple Heron |
| Ardeola ralloides | Squacco Heron |
| Arenaria interpres | Ruddy Turnstone |
| Asio flammeus | Short‐Eared Owl |
| Asio otus | Long‐Eared Owl |
| Athene noctua | Little Owl |
| Aythya ferina | Common Pochard |
| Aythya fuligula | Tufted Duck |
| Aythya marila | Greater scaup |
| Aythya nyroca | Ferruginous Duck |
| Bombycilla garrulus | Bohemian Waxwing |
| Branta bernicla | Brant Goose |
| Branta canadensis | Canada goose |
| Branta leucopsis | Barnacle Goose |
| Branta ruficollis | Red‐Breasted Goose |
| Bubo bubo | Eurasian Eagle‐Owl |
| Bubo scandiacus | Snowy Owl |
| Bubulcus ibis | Cattle Egret |
| Bucephala clangula | Common Goldeneye |
| Burhinus oedicnemus | Eurasian Stone‐curlew |
| Buteo buteo | Common Buzzard |
| Buteo lagopus | Rough‐legged Hawk |
| Buteo rufinus | Long‐legged Buzzard |
| Cairina moschata | Muscovy duck |
| Calidris alba | Sanderling |
| Calidris alpina | Dunlin |
| Calidris canutus | Red Knot |
| Calidris ferruginea | Curlew Sandpiper |
| Calidris minuta | Little Stint |
| Calonectris diomedea | Scopoli's Shearwater |
| Caprimulgus europaeus | European Nightjar |
| Carduelis carduelis | European goldfinch |
| Carduelis flammea | Common redpoll |
| Carduelis spinus | Eurasian Siskin |
| Cepphus grylle | Black Guillemot |
| Certhia familiaris | Eurasian Treecreeper |
| Charadrius alexandrinus | Kentish Plover |
| Charadrius hiaticula | Common ringed plover |
| Chloris chloris | European greenfinch |
| Chroicocephalus ridibundus | Black‐headed Gull |
| Ciconia ciconia | White Stork |
| Ciconia nigra | Black Stork |
| Circaetus gallicus | Short‐toed Snake Eagle |
| Circus aeruginosus | Western Marsh Harrier |
| Circus cyaneus | Hen harrier |
| Circus pygargus | Montagu's Harrier |
| Clamator glandarius | Great Spotted Cuckoo |
| Coccothraustes coccothraustes | Hawfinch |
| Columba livia | Pigeon |
| Columba oenas | Stock dove |
| Columba palumbus | Common woodpigeon |
| Corvus corax | Common Raven |
| Corvus corone | Carrion Crow |
| Corvus corone cornix | Hooded crow |
| Corvus corone corone | Carrion Crow |
| Corvus frugilegus | Rook |
| Corvus monedula | Jackdaw |
| Coturnix coturnix | Common Quail |
| Coturnix japonica | Japanese Quail |
| Crex crex | Corn Crake |
| Cuculus canorus | Common Cuckoo |
| Cyanopica cyanus | Azure‐winged Magpie |
| Cygnus atratus | Black Swan |
| Cygnus bewickii | Bewick's Swan |
| Cygnus columbianus | Tundra Swan |
| Cygnus cygnus | Whooper swans |
| Cygnus olor | Mute swan |
| Delichon urbica | House Martin |
| Dendrocopos major | Great spotted woodpecker |
| Dendrocopos syriacus | Syrian Woodpecker |
| Dryocopus martius | Black Woodpecker |
| Egretta garzetta | Little Egret |
| Emberiza citrinella | Yellowhammer |
| Erithacus rubecula | European robin |
| Estrilda astrild | Common Waxbill |
| Falco cherrug | Saker Falcon |
| Falco columbarius | Merlin |
| Falco naumanni | Lesser Kestrel |
| Falco peregrinus | Peregrine Falcon |
| Falco rusticolus | Gyrfalcon |
| Falco subbuteo | Eurasian Hobby |
| Falco tinnunculus | Common Kestrel |
| Falco vespertinus | Red‐Footed Falcon |
| Ficedula albicollis | Collared Flycatcher |
| Ficedula hypoleuca | European Pied Flycatcher |
| Fringilla coelebs | Chaffinch |
| Fringilla montifringilla | Brambling |
| Fulica cristata | Red‐Knobbed Coot |
| Gallinago gallinago | Common snipe |
| Gallinula chloropus | Moorhen |
| Garrulus glandarius | Eurasian Jay |
| Gavia arctica | Black‐throated loon |
| Gavia immer | Common Loon |
| Gavia stellata | Red‐Throated Loon |
| Geronticus eremita | Northern Bald Ibis |
| Glaucidium passerinum | Eurasian Pygmy Owl |
| Grus grus | European crane |
| Grus virgo | Demoiselle Crane |
| Gypaetus barbatus | Bearded Vulture |
| Gyps fulvus | Griffon Vulture |
| Haematopus ostralegus | Eurasian Oystercatcher |
| Haliaeetus albicilla | White‐tailed eagle |
| Hieraaetus fasciatus | Bonelli's Eagle |
| Hieraaetus pennatus | Booted Eagle |
| Himantopus himantopus | Black‐winged Stilt |
| Hippolais icterina | Icterine Warbler |
| Hirundo rustica | Barn swallow |
| Ixobrychus minutus | Little Bittern |
| Lanius collurio | Red‐backed Shrike |
| Lanius excubitor | Great Grey Shrike |
| Lanius minor | Lesser Grey Shrike |
| Larus argentatus | European Herring Gull |
| Larus argentatus argentatus | European Herring Gull |
| Larus argentatus cachinnans | Caspian gull |
| Larus argentatus michahellis | Yellow‐legged Gull |
| Larus canus | Mew Gull |
| Larus fuscus | Lesser black backed gull |
| Larus marinus | Great Black‐backed Gull |
| Larus melanocephalus | Mediterranean gull |
| Limosa lapponica | Bar‐tailed Godwit |
| Limosa limosa | Black‐tailed godwit |
| Linaria cannabina | Common Linnet |
| Loxia curvirostra | Red Crossbill |
| Luscinia megarhynchos | Common Nightingale |
| Lymnocryptes minimus | Jack Snipe |
| Marmaronetta angustirostris | Marbled Duck |
| Melanitta fusca | Velvet Scoter |
| Melanitta nigra | Common Scoter |
| Mergus albellus | Smew |
| Mergus merganser | Common Merganser |
| Mergus serrator | Red breasted merganser |
| Merops apiaster | European Bee‐eater |
| Microcarbo niger | Little Cormorant |
| Milvus migrans | Black Kite |
| Milvus milvus | Red kite |
| Monticola saxatilis | Rufous‐Tailed Rock Thrush |
| Morus capensis | Cape Gannet |
| Motacilla alba | White Wagtail |
| Motacilla cinerea | Grey Wagtail |
| Muscicapa striata | Spotted Flycatcher |
| Myiopsitta monachus | Monk Parakeet |
| Netta rufina | Red‐crested Pochard |
| Numenius arquata | Eurasian Curlew |
| Nycticorax nycticorax | Night heron |
| Oenanthe oenanthe | Northern Wheatear |
| Oriolus oriolus | Eurasian Golden Oriole |
| Otus scops | Eurasian Scops Owl |
| Oxyura jamaicensis | Ruddy Duck |
| Oxyura leucocephala | White‐headed Duck |
| Pandion haliaetus | Osprey |
| Parus ater | Coal tit |
| Parus caeruleus | Blue tit |
| Parus major | Great tit |
| Passer domesticus | House sparrow |
| Passer montanus | Eurasian tree sparrow |
| Pelecanus crispus | Dalmatian Pelican |
| Pelecanus onocrotalus | Great white pelican |
| Perdix perdix | Grey Partridge |
| Pernis apivorus | European Honey‐buzzard |
| Phalacrocorax aristotelis | European Shag |
| Phalacrocorax carbo | Great Cormorant |
| Phasianus colchicus L. | Pheasant |
| Phoenicopterus roseus | Greater Flamingo |
| Phoenicopterus ruber | American Flamingo |
| Phoenicurus ochruros | Black Redstart |
| Phoenicurus phoenicurus | Common Redstart |
| Phylloscopus collybita | Common Chiffchaff |
| Phylloscopus sibilatrix | Wood Warbler |
| Phylloscopus trochilus | Willow Warbler |
| Pica pica | Eurasian Magpie |
| Picus viridis | European Green Woodpecker |
| Platalea leucorodia | Eurasian Spoonbill |
| Plegadis falcinellus | Glossy Ibis |
| Pluvialis squatarola | Grey Plover |
| Podiceps auritus | Horned Grebe |
| Podiceps cristatus | Great crested grebe |
| Poecile palustris | Marsh Tit |
| Porzana parva | Little Crake |
| Porzana porzana | Spotted Crake |
| Prunella modularis | Dunnock |
| Psittacula krameri | Rose‐Ringed Parakeet |
| Puffinus puffinus | Manx Shearwater |
| Pyrrhula pyrrhula | Eurasian Bullfinch |
| Rallus aquaticus | Water rail |
| Recurvirostra avosetta | Pied Avocet |
| Regulus ignicapillus | Firecrest |
| Regulus regulus | Goldcrest |
| Riparia riparia | Sand Martin |
| Rissa tridactyla | Black‐legged Kittiwake |
| Scolopax rusticola | Eurasian woodcock |
| Serinus serinus | European Serin |
| Sitta europaea | Eurasian Nuthatch |
| Somateria mollissima | Common Eider |
| Stercorarius parasiticus | Parasitic Jaeger |
| Stercorarius skua | Great Skua |
| Sterna hirundo | Common tern |
| Sterna paradisaea | Arctic Tern |
| Streptopelia decaocto | Collared Dove |
| Streptopelia turtur | European turtle dove |
| Strix aluco | Tawny Owl |
| Strix nebulosa | Great Grey Owl |
| Strix uralensis | Ural Owl |
| Sturnus unicolor | Spotless Starling |
| Sturnus vulgaris L. | Starling |
| Sula bassana | Northern Gannet |
| Surnia ulula | Northern Hawk‐Owl |
| Sylvia atricapilla | Eurasian Blackcap |
| Sylvia borin | Garden Warbler |
| Tachybaptus ruficollis | Little grebe |
| Tadorna ferruginea | Ruddy Shelduck |
| Tadorna tadorna | Common Shelduck |
| Tetrao tetrix | Black Grouse |
| Tetrao urogallus | Western Capercaillie |
| Tetrastes bonasia | Hazel grouse |
| Tringa erythropus | Spotted Redshank |
| Tringa glareola | Wood Sandpiper |
| Tringa totanus | Common redshank |
| Troglodytes troglodytes | Eurasian wren |
| Turdus iliacus | Redwing |
| Turdus merula | Common blackbird |
| Turdus philomelos | Song Thrush |
| Turdus pilaris | Fieldfare |
| Tyto alba | Barn Owl |
| Upupa epops | Eurasian Hoopoe |
| Uria aalge | Common murre |
| Vanellus vanellus | Northern Lapwing |
Forty‐four out of the 50 recommended target species by EFSA (EFSA, 2017) are included in the 259 species reported (see Table E.1 – Appendix E). Respectively, 34.9% and 49% of the birds sampled by passive and active surveillance belonged to target species (n = 4,334 and 3,207).
Table E.1.
List of target wild bird species published in December 2017 as part of the EFSA‐ECDC‐EURL scientific report (species not sampled in 2020 are highlighted in grey)
| Family | Subfamily, tribe or genus | Species |
|---|---|---|
| Coots, crakes and rails (Rallidae) | Western swamphen (Porphyrio porphyrio) | |
| Cormorants and shags (Phalacrocoracidae) | Great cormorant (Phalacrocorax carbo) | |
| Corvids (Corvidae) | Eurasian magpie (Pica pica) | |
| Ducks, geese and swans (Anatidae) | Dabbling ducks (Anatinae) | Eurasian teal (Anas crecca) |
| Eurasian wigeon (Anas penelope) | ||
| Gadwall (Anas strepera) | ||
| Mallard (Anas platyrhynchos) | ||
| Northern pintail (Anas acuta) | ||
| Diving ducks (Aythyini) | Common pochard (Aythya ferina) | |
| Greater scaup (Aythya marila) | ||
| Red‐crested pochard (Netta rufina) | ||
| Tufted duck (Aythya fuligula) | ||
| Sea ducks (Mergini) | Common eider (Somateria mollissima) | |
| Common goldeneye (Bucephala clangula) | ||
| Goosander (Mergus merganser) | ||
| Smew (Mergus albellus) | ||
| Shelducks and sheldgeese (Tadorninae) | Common shelduck (Tadorna tadorna) | |
| Shelducks and sheldgeese (Tadorninae) | Egyptian goose (Alopochen aegyptiacus) | |
| Swans (Cygnus) | Black swan (Cygnus atratus) | |
| Mute swan (Cygnus olor) | ||
| Whooper swan (Cygnus cygnus) | ||
| True geese (Anser, Branta, Chen) | Brant goose (Branta bernicla) | |
| Canada goose (Branta canadensis) | ||
| Greater white‐fronted goose (Anser albifrons) | ||
| Greylag goose (Anser anser) | ||
| Lesser white‐fronted goose (Anser erythropus) | ||
| Pink‐footed goose (Anser brachyrhynchus) | ||
| Taiga bean Goose (Anser fabalis) | ||
| Grebes (Podicipedidae) | Black‐necked grebe (Podiceps nigricollis) | |
| Great crested grebe (Podiceps cristatus) | ||
| Little grebe (Tachybaptus ruficollis) | ||
| Gulls, terns and allies (Laridae) | Black‐headed gull (Chroicocephalus ridibundus) | |
| European herring gull (Larus argentatus) | ||
| Great black‐backed gull (Larus marinus) | ||
| Mew gull (Larus canus) | ||
| Herons (Ardeidae) | Eurasian bittern (Botaurus stellaris) | |
| Great white egret (Egretta alba) | ||
| Grey heron (Ardea cinerea) | ||
| Little egret (Egretta garzetta) | ||
| Pelicans (Pelecanidae) | Dalmatian pelican (Pelecanus crispus) | |
| Great white pelican (Pelecanus onocrotalus) | ||
| Raptors (Accipitridae, Falconidae, Strigidae) | Common buzzard (Buteo buteo) | |
| Eurasian eagle‐owl (Bubo bubo) | ||
| Northern goshawk (Accipiter gentilis) | ||
| Peregrine falcon (Falco peregrinus) | ||
| Rough‐legged buzzard (Buteo lagopus) | ||
| White‐tailed eagle (Haliaeetus albicilla) | ||
| Sandpipers (Scolopacidae) | Green sandpiper (Tringa ochropus) | |
| Storks (Ciconiidae) | White stork (Ciconia ciconia) | |
| Thrushes (Turdidae) | Fieldfare (Turdus pilaris) |
3.2.4. Avian influenza in wild birds
3.2.4.1. Detection of avian influenza virus in samples
When analysing data from both active and passive surveillance, a total of 1,624 (8.6%) birds, out of the 18,968 sampled by RCs, tested positive to AI (Table 2). This proportion was about twice as high as in 2019 (4.7%) or 2018 (3.8%). Of the 1,624 positive birds, 878 were infected with HPAI virus and 746 with LPAI virus.9
Table 2.
Avian influenza diagnostic results for birds sampled by passive (no background) and active (light grey background) surveillance by all RCs in 2020, by bird status. The column ‘All positive’ includes all AI positive birds obtained by polymerase chain reaction (PCR) or virus isolation (VI). All birds with a successful AI virus isolation (column ‘Positive in VI’) had previously tested positive by PCR
| Bird status | No. of birds sampled | No. of AI positive birds | ||||
|---|---|---|---|---|---|---|
| All positive | Positive in VI | HPAI positive | LPAI positive | |||
| Active | Hunted with clinical signs | 84 | 33 | 0 | 30 | 3 |
| Hunted without clinical signs | 2,403 | 313 | 10 | 31 | 282 | |
| Live without clinical signs | 4,063 | 107 | 35 | 9 | 98 | |
| Subtotal | 6,550 | 453 | 45 | 70 | 383 | |
| Passive | Found dead | 11,904 | 1,157 | 11 | 797 | 360 |
| Live with clinical signs | 514 | 14 | 1 | 11 | 3 | |
| Subtotal | 12,418 | 1,171 | 12 | 808 | 363 | |
| Total | 18,968 | 1,624 | 57 | 878 | 746 | |
Most AI‐positive birds were found dead (1,157 birds tested AI positive, including 797 testing positive for HPAI). In 2020, the majority of AI‐positive birds were found by passive surveillance (72%), a major difference from the previous year (e.g. 7% of AI detections by passive surveillance in 2019). The proportions of positive birds in active and passive surveillance were 7% and 9%, respectively.
Wild bird sampling was reported for 19 NUTS2 units, 188 NUTS3 units and 9,865 individual coordinate locations in 2020. Italy reported surveillance results at NUTS2 level, while Czechia, Hungary, Iceland, Ireland, Latvia, Lithuania, Malta, Netherlands, Poland, Romania and Spain reported results at NUTS3 level. Norway reported some results at NUTS3 level and some by location coordinates. Other countries reported results by location coordinates only.
Out of the 18,968 wild birds sampled, 2,792 and 3,440 were reported at NUTS2 and NUTS3 level, respectively, while 12,736 were reported by location coordinates. Out of the 878 H5 HPAI‐positive birds, 2 and 148 were reported at NUTS2 and NUTS3 level, respectively, while 728 were reported by location coordinates.
Figure 16 shows the geographical distribution of AI surveillance activities conducted by RCs in wild birds in 2020. Data are represented at the NUTS level they were reported at (i.e. the maps show a combination of NUTS2 and NUTS3 units). Data reported with location coordinates were aggregated at NUTS3 level.
Figure 16.
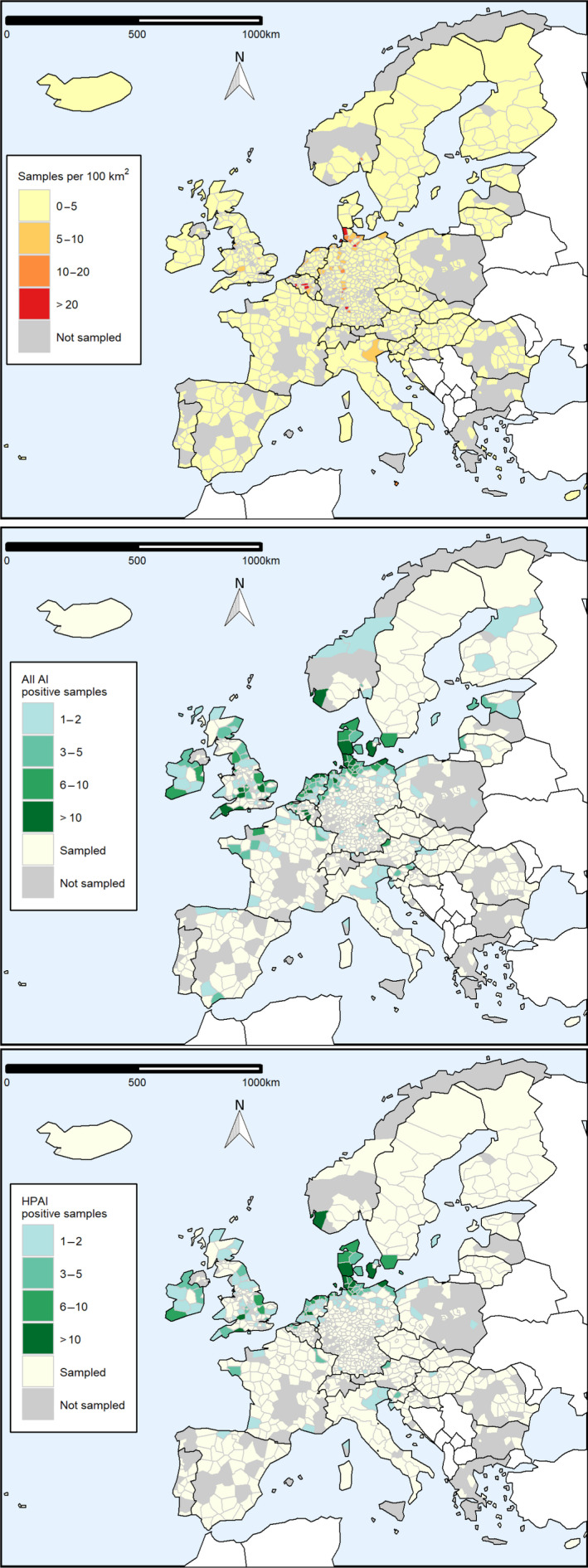
Sampling density, expressed as the number of wild birds sampled per area of 100 km2 (upper map), and geographical distribution of all AI positive birds (middle map) and HPAI positive birds (lower map), by administrative unit. Non‐reporting countries are shown in white
3.2.4.2. High pathogenic avian influenza in wild birds
3.2.4.2.1. HPAI results by neuraminidase type
A total of 878 birds tested positive for HPAI in 2020, more than in 2019 (1 positive bird) and 2018 (163 positive birds). HPAI‐positive birds were reported by 14 RCs. All HPAI strains were identified as H5, and most were identified as H5N8 (84%). Figure 21 summarises the reported N subtypes for these positive samples.
Figure 21.
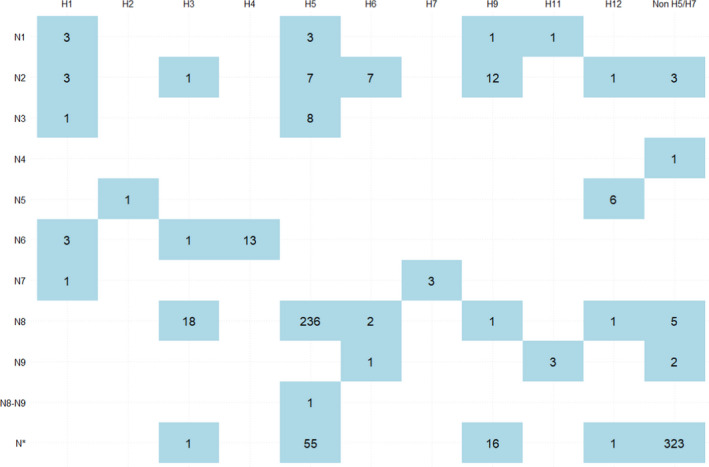
AI virus haemagglutinin (H) and neuraminidase (N) types identified in LPAI‐positive wild birds. Note: birds for which positive samples could not all be typed (e.g., one sample was characterised as H5 and another sample from the same bird as H‐antigen unknown) are classified under the available H or N type (in this example, H5). There were no birds with more than one H antigen identified
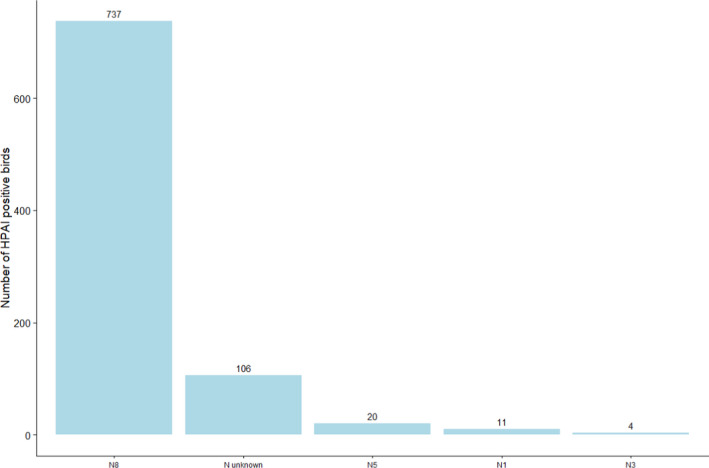
Figure 17: Virus neuraminidase (N) type identified in HPAI‐positive wild birds (all HPAI strains were identified as H5). Values are provided above the bars. There were no birds with more than one N antigen identified
3.2.4.2.2. HPAI results by species
A total of 51 wild bird species, birds from 8 genera with unknown species and birds from 2 families with unknown species were detected as positive for HPAI, as well as 30 birds with no species identification (no order, family, genus or species). The HPAI infected birds belonged to the 12 orders as well as unknown orders, as shown in Figures 18 and 19. These two figures show combined data for passive and active surveillance. The same data is presented separately by type of surveillance in Appendices G and H: Figures G.1 and G.2 (passive surveillance), Figures H.1 and H.2 (active surveillance).
Figure 18.
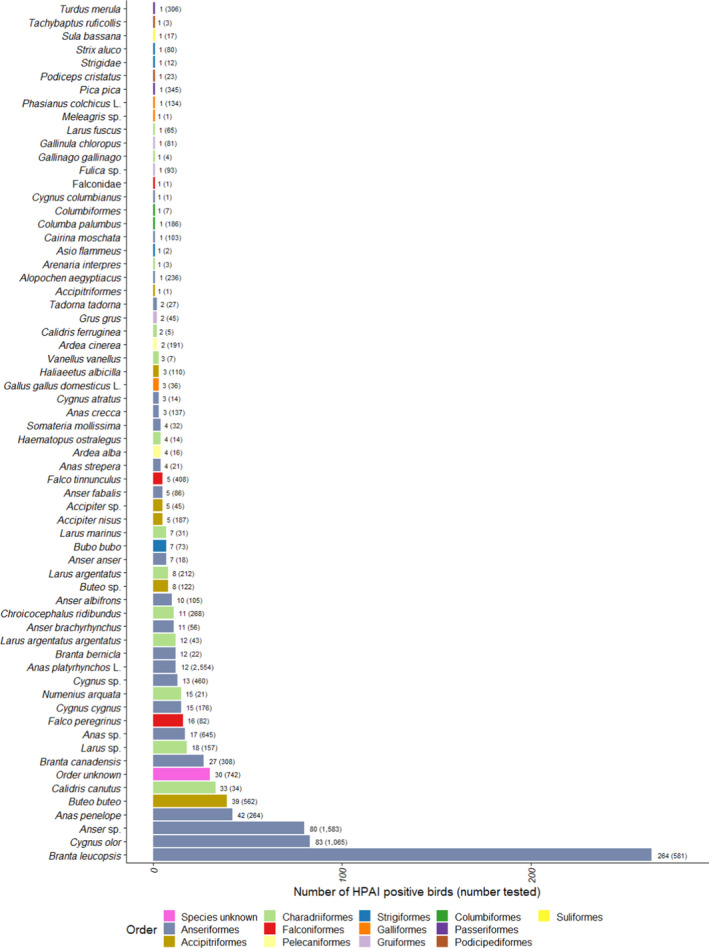
Number of HPAI‐positive wild birds detected by both passive and active surveillance, for species with at least one HPAI positive sample. The number of wild birds tested is indicated in brackets. Bars are ordered by increasing number of positives and colour coded to identify the order to which these species belong to. English common names are provided in Appendix D
Figure 19.
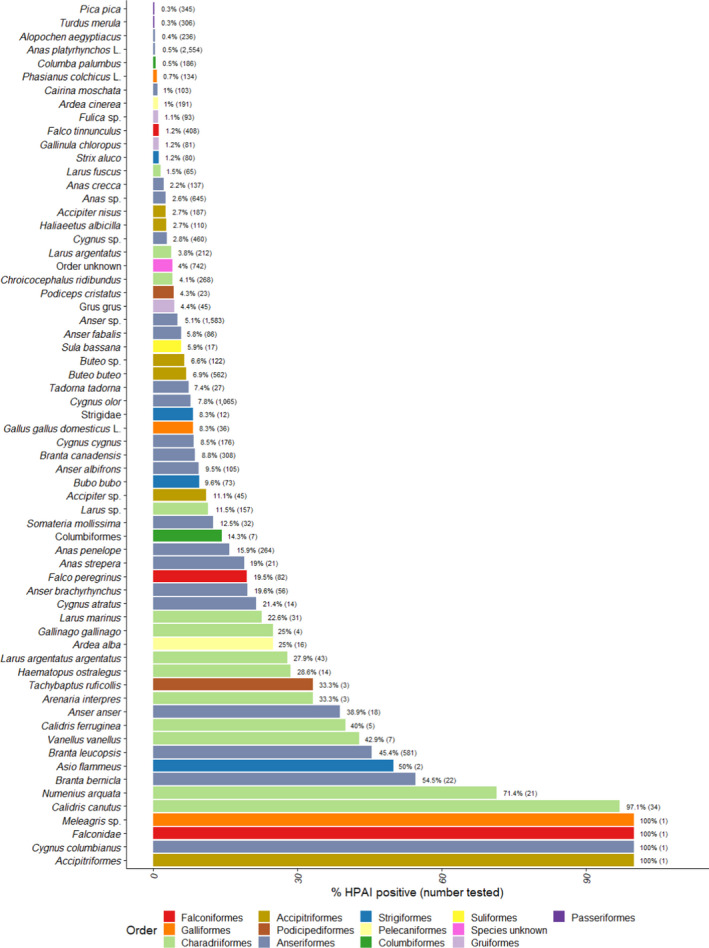
Proportion of HPAI‐positive (all types) wild birds detected among birds tested by both passive and active surveillance, for species with at least one HPAI positive sample. The number of wild birds tested is indicated in brackets. Bars are ordered by increasing proportion of positives and colour coded to identify the order to which these species belong to. English common names are provided in Appendix D
Figure G.1.
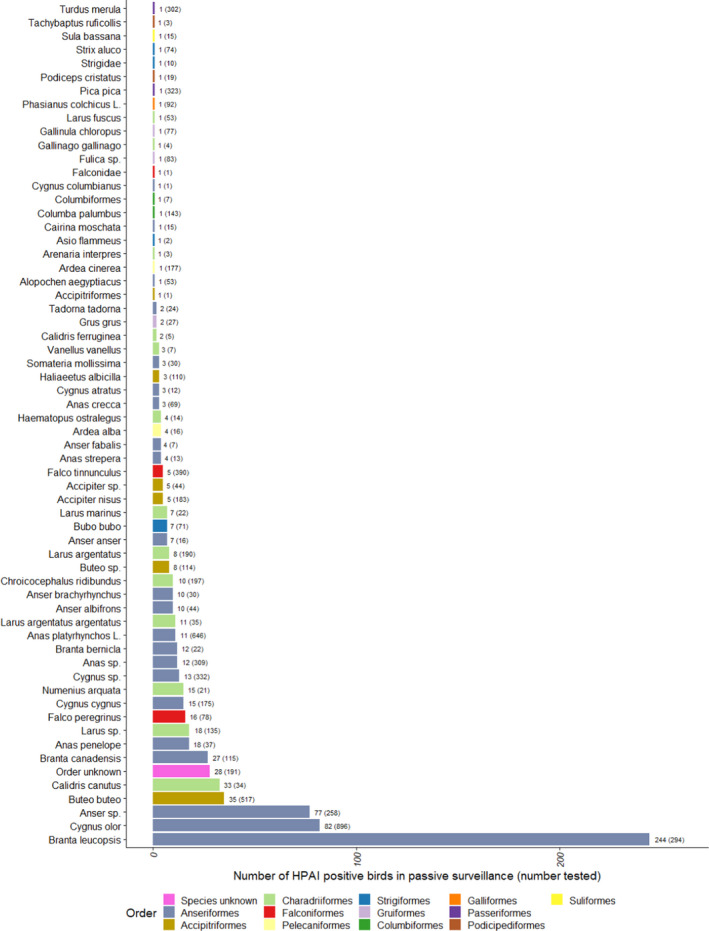
Number of HPAI‐positive wild birds detected by passive surveillance, for species with at least one HPAI positive sample. The number of wild birds tested is indicated in brackets. Bars are ordered by increasing number of positives and colour coded to identify the order to which these species belong to
Figure G.2.
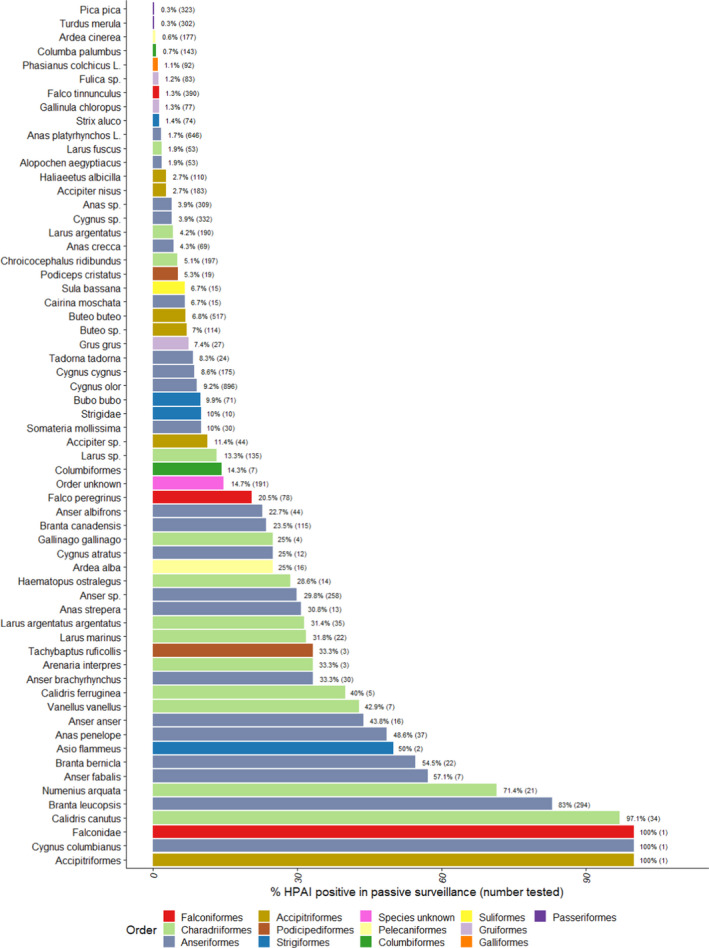
Proportion of HPAI‐positive (all types) wild birds detected among birds tested by passive surveillance, for species with at least one HPAI positive sample. The number of wild birds tested is indicated in brackets. Bars are ordered by increasing proportion of positives and colour coded to identify the order to which these species belong to
Figure H.1.
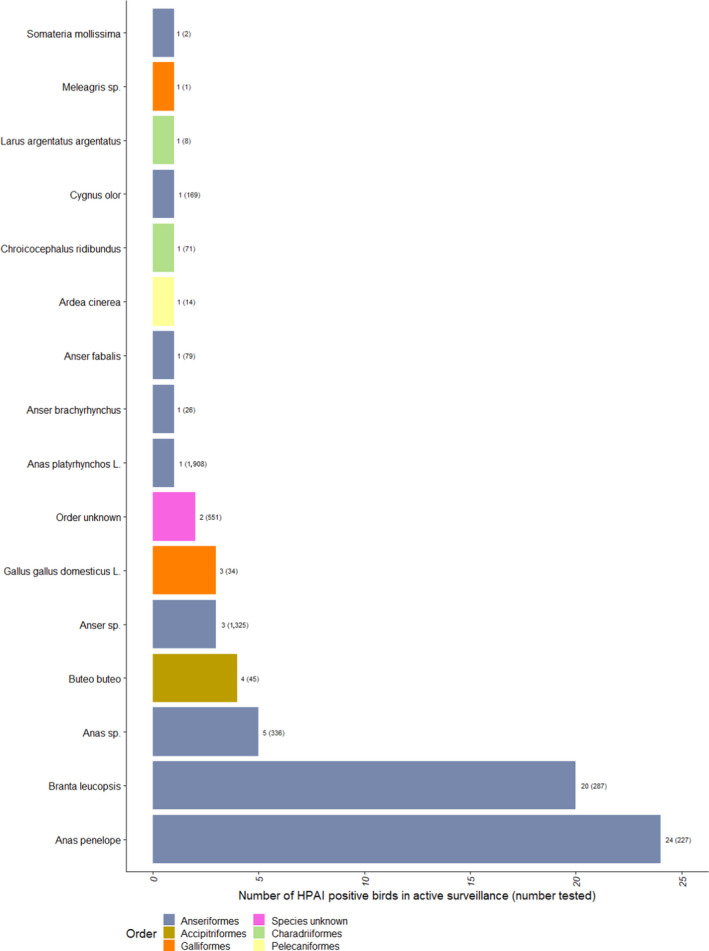
Number of HPAI‐positive wild birds detected by active surveillance, for species with at least one HPAI positive sample. The number of wild birds tested is indicated in brackets. Bars are ordered by increasing number of positives and colour coded to identify the order to which these species belong to
Figure H.2.
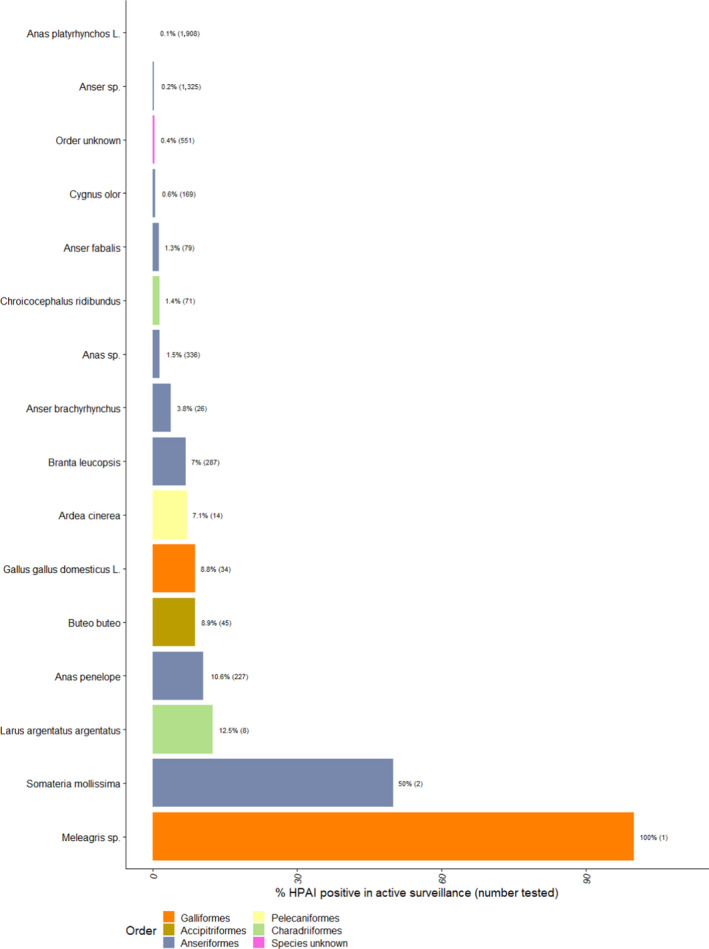
Proportion of HPAI‐positive (all types) wild birds detected among birds tested by active surveillance, for species with at least one HPAI positive sample. The number of wild birds tested is indicated in brackets. Bars are ordered by increasing proportion of positives and colour coded to identify the order to which these species belong to
Less than half of the HPAI positive birds belonged to the EFSA target species (n = 337, 38%). In particular, the species with the largest number of HPAI‐positive samples identified in passive surveillance was barnacle goose (Branta leucopsis, n = 264), which is not listed as a target species (Figure 18). The two other species with the largest numbers of HPAI‐infected birds were Cygnus olor (n = 83) and Anas penelope (n = 42), while 80 positive birds were identified as geese at the genus level only (Anser sp.).
The percentage of HPAI‐positive birds by species shown in Figure 19 must be interpreted carefully, as the number of birds sampled for a given species may be very small. For instance, only one Cygnus columbianus was sampled and tested positive, yielding a percentage of 100% for that species.
3.2.4.2.3. HPAI results by type of surveillance
Table 3 shows the proportion of HPAI‐positive birds by type of surveillance. Active surveillance yielded positive results in Germany and Norway only, while passive surveillance yielded positive results in 14 countries. The highest percentages of HPAI positive birds found by passive surveillance were in Denmark (32% of samples), Germany (14%), the Netherlands (12%) and Ireland (12%).
Table 3.
Total number of wild birds sampled and positive for HPAI by passive and active surveillance in each RC. Cells with a grey background indicate that no positive birds were detected in that country via the corresponding surveillance activity
| Country | Passive surveillance | Active surveillance | ||
|---|---|---|---|---|
| No. of birds | No. HPAI positive (%) | No. of birds | No. HPAI positive (%) | |
| Austria | 183 | 0 (0%) | 0 | – |
| Belgium | 275 | 18 (6.5%) | 1,094 | 0 (0%) |
| Bulgaria | 70 | 0 (0%) | 8 | 0 (0%) |
| Croatia | 92 | 0 (0%) | 0 | – |
| Cyprus | 137 | 0 (0%) | 18 | 0 (0%) |
| Czechia | 127 | 0 (0%) | 0 | – |
| Denmark* | 288 | 92 (31.9%) | 0 | – |
| Estonia | 3 | 0 (0%) | 111 | 0 (0%) |
| Finland | 222 | 0 (0%) | 0 | – |
| France | 503 | 11 (2.2%) | 0 | – |
| Germany | 3,041 | 436 (14.3%) | 4,391 | 61 (1.4%) |
| Greece | 6 | 0 (0%) | 0 | – |
| Hungary | 472 | 1 (0.2%) | 0 | – |
| Iceland | 9 | 0 (0%) | 0 | – |
| Ireland | 165 | 19 (11.5%) | 0 | – |
| Italy | 2,791 | 2 (0.1%) | 1 | 0 (0%) |
| Latvia | 4 | 0 (0%) | 0 | – |
| Lithuania | 139 | 0 (0%) | 0 | – |
| Luxembourg | 135 | 0 (0%) | 0 | – |
| Malta | 9 | 0 (0%) | 94 | 0 (0%) |
| Netherlands | 878 | 109 (12.4%) | 0 | – |
| Norway | 128 | 5 (3.9%) | 528 | 9 (1.7%) |
| Poland | 97 | 5 (5.2%) | 0 | – |
| Portugal | 74 | 0 (0%) | 0 | – |
| Romania | 107 | 0 (0%) | 44 | 0 (0%) |
| Slovakia | 83 | 0 (0%) | 0 | – |
| Slovenia | 270 | 6 (2.2%) | 0 | – |
| Spain | 437 | 1 (0.2%) | 261 | 0 (0%) |
| Sweden | 410 | 7 (1.7%) | 0 | – |
| Switzerland | 55 | 0 (0%) | 0 | – |
| United Kingdom | 1,208 | 96 (7.9%) | 0 | – |
Active surveillance data were not reported to EFSA, nonetheless, Denmark confirmed that active surveillance took place in 2020, with two positive HPAI H5 results being reported in ADNS.
3.2.4.2.4. HPAI results in time
Figure 20 presents a timeline of the detection of HPAI in RCs in 2020, for passive and active surveillance separately (blue and red colours, respectively). HPAI was detected from week 42 onwards (mid‐October), with the highest proportion of positive birds in weeks 45 and 46. During these two weeks, respectively 24% and 21% of samples (passive and active surveillance combined) tested positive for HPAI virus. As noted above, an increase in the sampling effort was also observed in the last quarter associated to the mass mortality events observed in this epidemic.
Figure 20.
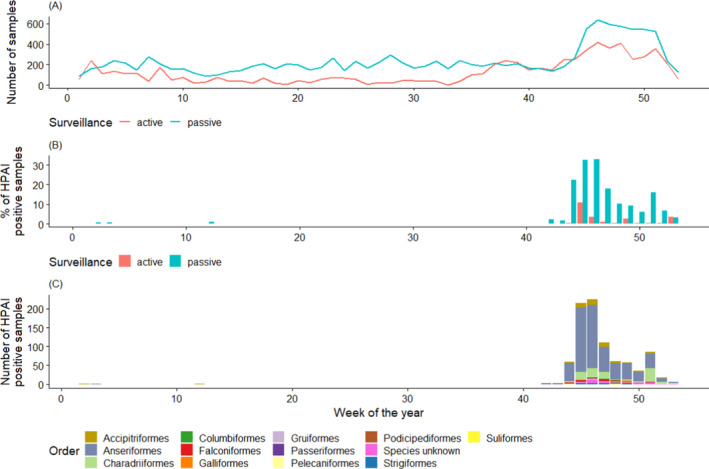
(A) Weekly number of wild birds sampled by both, passive and active surveillance, (B) weekly percentage of HPAI‐positive wild birds found and (C) weekly number of HPAI‐positive wild birds by taxonomic order
3.2.4.3. Low pathogenic avian influenza in wild birds
Among the 746 birds which tested positive for non‐HPAI virus, 101 birds were infected with viruses reported as low pathogenic, while no virus pathogenicity results were available for the remaining 645 birds. Out of the 645 birds for which information on the pathogenicity was not available, there were 277 birds positive for H5 and 1 bird positive for H7. For the remainder of this section, ‘LPAI‐positive’ birds include all positive birds which were not positive for HPAI (n = 746). This is consistent with previous reports.
LPAI‐positive birds were reported by 17 RCs. Among these positives, 310 were subtyped as H5 and 3 as H7. The majority of the LPAI viruses detected were reported as non‐H5/H7 (n = 334), without further information on the virus subtype provided. Figure 21 summarises all the identified and reported LPAI subtypes.
As shown in Figure 22, most LPAI‐positive results were found from August onwards (starting week 29) for both types of surveillance. There were very few positive birds between March and July. Most LPAI‐positive birds belonged to the order Anseriformes (Figure 22C), which is the order most sampled by both active and passive surveillance.
Figure 22.
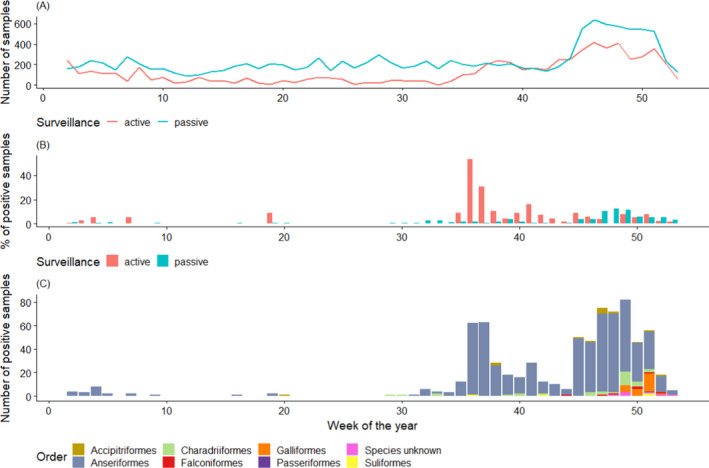
(A) Weekly number of wild birds sampled by both, passive and active surveillance, (B) weekly percentage of LPAI‐positive wild birds found and (C) weekly number of LPAI‐positive wild birds by taxonomic order
3.3. Abundance and distribution of wild birds in Europe
Voluntary contribution data on abundance and distribution of the wild bird species have been made available to EFSA by the EuroBird Portal (EBP). EBP10 is one of the three major monitoring projects run by the Euro Bird Census Council (EBCC). This project mobilises year‐round observational data submitted by volunteer birdwatchers to the online bird recording portals operating across Europe (c. 50 million bird records from c. 100,000 bird observers annually). Information on the distribution of the 50 species included in the EFSA target list of wild bird species is now submitted to EFSA annually, aggregated at NUTS3 and monthly level. The data come to EFSA with two measures for each NUTS3 and month:
the total number of birds observed at that specific location during that month, and,
the number of birds of each of the 50 species included in the target list of species observed at that location during that month.
The total number of birds observed is a function of abundance and observation effort. This value may be used as an indirect measure of the effort that took place in a given location. However, it cannot be directly interpreted as the observation effort, as this would assume that abundance is constant across locations.
Figure F.1 in Appendix F shows the density of birds observed in a specific location (upper map), as well as the density of birds of the 50 target species (lower map), each estimated as the total number of observations in the NUTS3 region divided by the surface of the area (also available in Zenodo11). This figure shows that the highest densities of observations of wild birds (all species, i.e. an indirect measure of observation effort) were mostly in Belgium, the Netherlands and some regions of France, Germany, Switzerland and the United Kingdom. The density was lowest in Bulgaria, Croatia, Greece, Hungary, Ireland and Romania. No data were available for Lithuania. In 2020, some data were also available for Iceland and Slovenia, unlike in 2019. Within countries, the variability between NUTS3 regions was high. During the course of the year, wild bird observations were reported at least once for 1,314 NUTS3 in total in the countries for which EBP data were available. Birds from the EFSA target list were reported in all but 1 of these NUTS3 (Figure F.1, lower map).
Figure F.1.
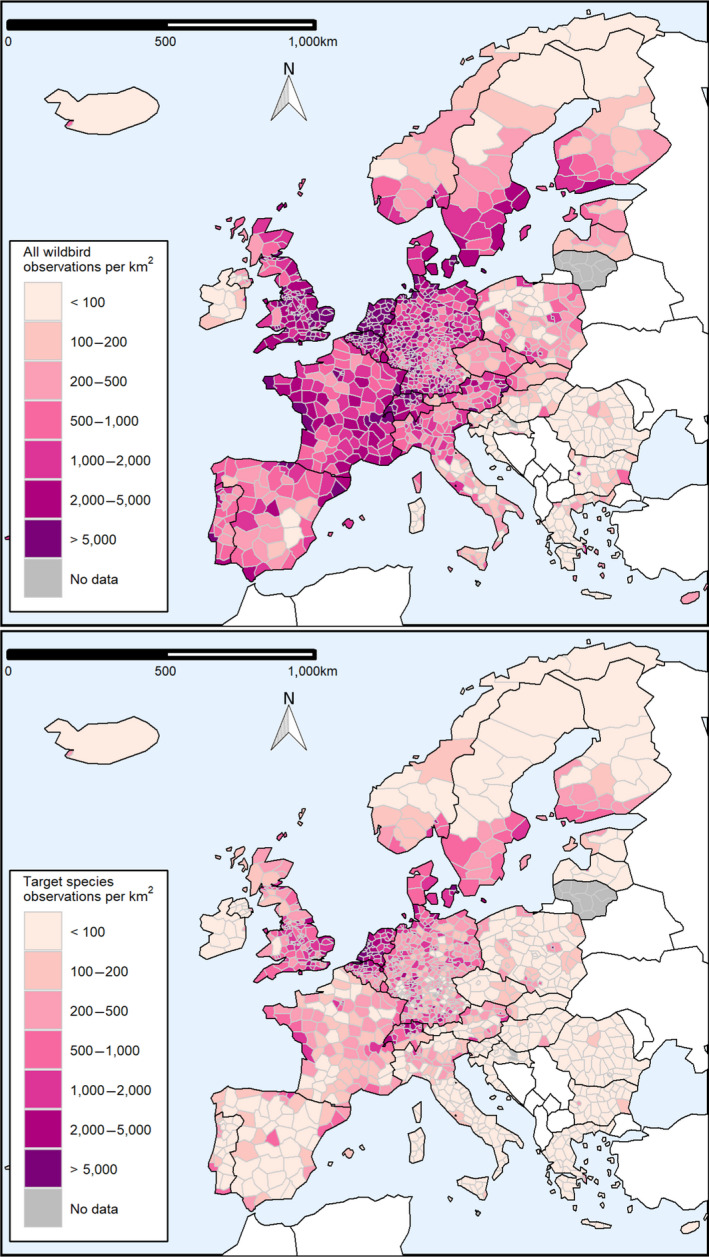
Density of wild bird observations for 2020 by NUTS3 region, as per data provided by the EuroBirdPortal project. The density of observations was estimated as the total number of observations in the NUTS3 region divided by the surface of the area. The upper map shows all bird species, while the lower map is restricted to species from the EFSA target list
Showing these two types of records, observation effort and density for given species, provides an indicator of the reliability of the data presented. For example, if a small number of wild birds of the species included in the list of target species is observed for a certain NUTS3 and month, in an area where the observation effort is high (large number of total observations), our confidence in the reliability of that information would be higher than if the total number of observations was low.
Additional maps are available in Zenodo12 at the monthly‐level: these maps display both the number of birds from target species observed in each NUTS3 (EBP data) and the number of birds from target species sampled by passive surveillance (RC data).
Figures F.2 and F.3 (Appendix F) show the distribution of bird observations according to the EBP data, by bird orders and species for the entire year, for the 50 species included in the EFSA target list (Appendix E). Almost half of the observations reported concerned Anseriformes, followed by Pelecaniformes, Charadriiformes, Accipitriformes and Passeriformes. It was not possible to compare these distributions with the distribution of orders and species sampled for AI surveillance, given that detailed data was only available for the target list species. For instance, Columbiformes ranked 3rd in terms of sampling, but were not reported in the available EBP data.
Figure F.2.
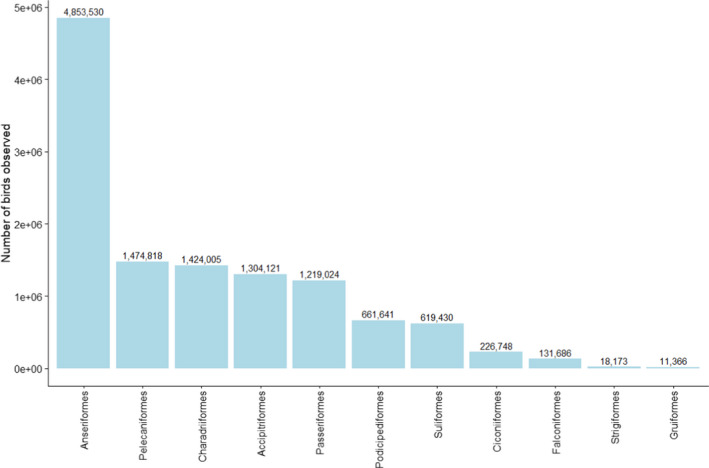
Number of wild birds from the EFSA list of target wild bird species (N = 50) observed in 2020 and recorded in the EuroBirdPortal project, aggregated by bird order
Figure F.3.
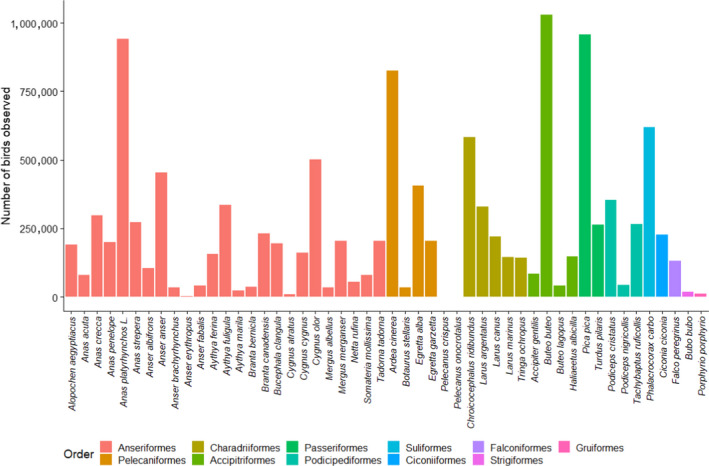
Number of wild birds from the EFSA list of target wild bird species (N = 50) observed in 2020 and recorded in the EuroBirdPortal project, aggregated by bird species
Last, there were also some discrepancies between the wild birds reported as observed and the dead wild birds collected by the passive surveillance programmes. There were 2,438 records of dead bird samples from EFSA target species for a given species, NUTS3 and month. Among those, 442 were not associated with a corresponding observation in the EBP data. Therefore, it is difficult to use the EBP observation data to assess the quality of the passive surveillance in reporting countries.
4. Discussion and conclusions
It is important to note that risk‐based sampling strategies are used for AI surveillance in some RCs, and that these strategies may vary between countries. Therefore, the differences observed between countries in this report in terms of AI incidence, both in poultry and wild birds, should be interpreted with caution and direct comparisons between countries avoided.
A targeted (non‐representative) sampling approach helps to increase the efficiency of detection of AIV, but prevents valid assessments of measures of disease, differences between locations, categories or species, or trends over time. Comparisons of seropositivity rates between different time periods, categories, species or locations are valid for the specific observations (surveillance results) only and cannot be extrapolated to the source populations. The seropositivity rates are not only influenced by disease, but also the efficiency of targeting of the risk‐based sampling approach. Therefore, increases in rates overtime may be due to changes in the disease situation, but also to improved targeting. As the risk‐based surveillance is designed for early detection, it should not be used to measure changes in prevalence or incidence. If such an interpretation is required, representative sampling would need to be undertaken using methodologies that have been standardised between RCs.
4.1. Poultry
An increasing trend in the number of PEs sampled is observed since 2017, after a decrease observed over the previous years. Both the number and the proportion of H5/H7 seropositive establishments were lower than observed in 2019, and similar to those observed in 2018. However, variations in sampling activities among RCs and between years mean that it is difficult to make valid inferences about the detection percentages. In 2020, 46 PEs tested positive for H5 virus and 7 for H7 virus. This suggests a more active circulation of H5 viruses compared to H7 viruses in Europe, consistently with previous years.
The two months with the highest H5/H7 seropositivity rates were June and December 2020. As noted for April 2019, the large number of positive PEs in June 2020 coincided with intensive sampling in waterfowl game bird holdings in Spain. Although this poultry category is sampled throughout the year, a large proportion of the sampling takes place at the end of the hunting season in the spring. On the other hand, the higher seropositivity rate in December did not appear to be associated with a particular category or country.
The serosurveillance results by species from 2020 are consistent with findings from previous years. The highest risk of circulation of LPAI remains in aquatic birds (game birds, geese and ducks), while gallinaceous birds (in particular chickens and turkeys) were at low risk overall. While backyard establishments and conventional laying hens had the largest numbers tested, one seropositive PE only was identified in the former category, and none in the latter. In Commission Delegated Regulation (EU) 2020/68913, from April 2021 MSs are required to carry out a complementary risk‐based surveillance aiming to detect clusters of establishments (in time and geographical proximity) infected with LPAI virus. The poultry categories where this surveillance is recommended include the categories where most of the serological positive results have been found in recent years.
Active surveillance provides useful insights into the circulation of AIVs in poultry establishments, in particular for LPAI and for poultry species or categories who exhibit little or no symptoms when infected. However, the sensitivity of such a surveillance approach remains limited as it does not provide a high coverage in terms of population and time. Therefore, the results obtained from other surveillance approaches should always be considered when interpreting the present results.
According to Commission Decision 2010/367/EU, MSs should follow up PEs with positive serology results by performing PCR testing on the same flock and/or neighbouring flocks.
Follow‐up PCR results were not available for 7 of the seropositive PEs at the time of writing of the present report. This value shows a significant improvement from the 2019 surveillance reporting (29 seropositive PEs were not followed by PCR in 2019). Reasons for the lack of follow‐up included birds sampled at the slaughterhouse and premises which had been fully investigated in the previous months and tested positive again for the same flock. It is important to note that no investigation identifiers were available at the time of analysis. Therefore, if follow‐up testing was conducted on neighbouring flocks rather than on the same flock (i.e. with a different holding identifier), these events could not be linked and the seropositive event would have been classified as not followed up. The data collection allows to report follow‐up activities (‘sampInfo_origSampId’), and it is recommended that RCs use this feature accordingly.
Finally, it is important to note that no data on the underlying poultry population were available to EFSA. This poultry population data could be submitted to EFSA in an aggregated manner (by poultry category and NUTS3 level) as a once‐off exercise, with updates reported when available. Understanding the underlying population of the different poultry categories would improve the interpretation of the AI surveillance results at the European level.
4.2. Wild birds
The number of wild birds tested by passive surveillance in 2020 was substantially higher than in 2019 and 2018. Twenty‐two countries, out of 31 RCs, sampled more birds by passive surveillance as during the previous year. Some countries also reported a large number of birds sampled under active surveillance activities (e.g. Belgium, Germany).
While one bird sample had tested positive for HPAI in 2019, a large number of birds tested positive for HPAI in 2020. Out of the 878 HPAI‐positive birds, 797 were birds found dead identified by the passive surveillance system. These values continue to support the importance of this surveillance approach for AI surveillance in wild bird species. A large proportion of both the sampling and HPAI positive results occurred in the fourth quarter of 2020. This can be linked with the large epidemic of H5N8 virus which started in October 2020 in EU/EAA countries and the UK. This event has been associated with over 1,000 outbreaks to date, in both domestic poultry and wild birds and is the largest H5N8 HPAI epidemic recorded in the EU since the 2016/2017 epidemic14 . The outbreaks in Europe appear to be linked with a wider epidemic including Russia, Iraq and Kazakhstan (Lewis et al., 2021; Verhagen et al., 2021). Details about the HPAI cases in poultry, captive birds and wild birds have been reported by EFSA in the quarterly reports describing the AI situation in Europe and outside EU, during the last quarter of 202015 and at the beginning of 2021.16
The respective proportions of birds sampled by passive surveillance and of HPAI‐positive birds belonging to the list of target species recommended by EFSA remain relatively low (35% and 38%, respectively). As this list includes species that are more likely to die if infected with HPAI, reporting countries are encouraged to target these species in their passive surveillance activities when possible. The present results suggest that the list could be adjusted with recent knowledge about the species of interest depending on their likelihood of dying when infected with HPAI.
Summary data provided by the EuroBirdPortal project are presented (Appendix F) to describe the number of wild bird observations reported by voluntary contributors in 2020. These data may provide some context regarding the performance of passive surveillance of AI in wild birds in the EU. However, it is important to note that the density of wild bird observations is the product of two factors:
the density of wild birds (which depends on species‐specific factors such as the location, biotope, time of the year, etc.)
the probability that a wild bird is observed by someone and reported in a relevant database, given that it is present. This is also known as the ‘effort’ put into wild bird observations.
As a consequence, areas with low density of observations may correspond to areas where the sensitivity of passive surveillance is low due to a lower ‘effort’ or to habitats which are simply not favourable to birds (low density of birds) or both. A previous study in Sweden warned that contributor‐based data should be used with care, given the limitations of this data collection method (Snäll et al., 2011). Despite the limitations of the voluntary observation data presented in this report, and until further spatial modelling of the distribution of wild birds in Europe by species is readily available, the maps presented in this report (and also those linked to this report and shown in Zenodo), could help to shed light on areas where the birds of the species belonging to the target list may gather, supporting RCs in carrying out more targeted surveillance activities.
5. Methods
5.1. Framework for reporting
Directive 2005/94/EC on Community measures to control avian influenza established in its Article 4 the legal basis for the obligatory conduct of surveillance programmes in poultry and wild bird populations. Both surveillance programmes must be carried out following harmonised guidelines which were laid down in 2010/367/EU.
Surveillance programmes of the MSs are evaluated and approved for co‐financing by Commission's procedures that are detailed on the Commission's website: http://ec.europa.eu/dgs/health_food-safety/funding/cff/animal_health/vet_progs_en.htm.
Diagnostic procedures for testing the samples collected within the surveillance programmes are outlined in Diagnostic Manual for avian influenza as set out in Decision 2006/437/EC17.
Previous Annual Reports and more information on surveillance for avian influenza in poultry and wild birds can be found at: https://ec.europa.eu/food/animals/animal-diseases/diseases-and-control-measures/avian-influenza_en.
5.2. Survey design
5.2.1. Poultry
The epidemiological unit for reporting surveillance in poultry is the holding, which is defined in Council Directive 2009/158/EC18 as: ‘a facility used for the rearing or keeping of breeding or productive poultry. For the purposes of avian influenza surveillance, this may include facilities that only contain poultry during certain months of the year (i.e. poultry do not need to be present all year round)’. In this report, the word ‘holding’ was replaced by ‘poultry establishment’19 to be aligned with the Regulation (EU) 2016/429 (Animal Health Law). Detailed guidelines for the design of surveillance based on representative sampling or risk‐based surveillance as well as the identification of the target population (poultry species and production categories) and guidelines for calculation of sample size at holding and bird level are described in Annex I of the Commission Decision 2010/367/EU.
5.2.2. Wild birds
The epidemiological unit for surveillance in wild birds is the bird. Procedures for surveillance design are outlined in Annex II of the Commission Decision 2010/367/EU.
5.3. Sampling procedures and laboratory testing
Sampling and laboratory testing procedures for both poultry and wild birds are described in Annex I and II, respectively, of Commission Decision 2010/367/EU. In this Commission Decision, the procedures to carry out epidemiological investigations following positive detections are also outlined.
Following the events of previous years (2014–2017), when HPAI virus with N subtype other than N1 were detected in poultry and wild birds, it was expected, particularly in the case of wild bird samples, that MSs would proceed to identify the specific N subtype, either by using national reference laboratories or submitting the samples to the EU reference laboratory for its identification. The only HPAI subtype identified in wild birds in 2018 and 2019 was H5N6.
The definition of Low Pathogenic Avian Influenza (LPAI) provided by Annex I of Directive 2005/94/CE includes any H5 or H7 AI virus not classified as HPAI and excludes all other subtypes of Influenza A viruses. For the purpose of the present report, and for consistency with the previous report, birds reported positive for subtypes other than H5/H7 and not classified as HPAI are also included as LPAI.
5.4. Data and data processing
Data collation and validation as well as exploratory and statistical analysis were carried out using the statistical software R (R Core Team, 2020).
In some RCs, establishments were sampled several times throughout the year, this was the case for establishments containing one or different poultry categories. For the purpose of this report, each sampling exercise taking place on a specific date, at a specific establishment and targeting a specific poultry category was considered as an independent event and counted as an establishment sampled. As a result, an overestimation of the total number of establishments sampled could occur for some RCs, with this number being higher than the total number of establishments of a specific poultry category in a specific RC. Therefore, the numbers reported in this report as ‘poultry establishments (PEs) sampled’ should be interpreted as the number of sampling events taking place in a RC for each of the reported categories. Throughout the report, the term ‘number of PEs sampled’ refers to all PEs sampled, regardless of the type of tests conducted on the samples (serology or virology).
For the wild bird data analysis, data submitted by RCs as the year of sampling’ (‘sampY’), month of sampling (‘sampM’) and day of sampling (‘sampD’) were used as sampling date. As for the 2018 and 2019 reports, the updated EFSA list of target species (EFSA, 2017) was used instead of the target list provided in the Commission Decision 2010/367/EU. Pooled testing takes place in some MSs when more than one wild bird from the same species are collected at the same time and location (as indicated by variable ‘sampMethod’). In such cases, the variable ‘sampSize’ was used to report the number of birds from which samples were pooled. When positive results were obtained from pooled samples (this occurred with pools of up to five birds), all the birds included in the pool were considered positive, given that no further information was available.20
Eurostat reference shapefiles were used to create the maps: ‘Countries 2016’ (version 3/6/2019) and ‘NUTS 2016’ (version 14/3/2019). These versions were used to match the units reported in the surveillance data for 2020. Maps plotting the geographical distribution of the sampling events and the location of positive results were aggregated at NUTS2 level for both poultry and wild birds in the present report. However, maps at NUTS3 level are also provided as high‐quality images in the Zenodo repository for this report,21 for countries which provided data at NUTS3 level. To summarise sampling activities, the intensity of sampling, calculated as the number of samples taken within a NUTS2 region per 100 km2, was displayed, given that the total number of poultry establishments present in a given region was not available. Samples with geocoordinates which could not be match to a NUTS region from the country reporting the data are not displayed in the maps, but are accounted for by all other figures and tables in the document.
The results presented in this report are based on the data reported by RCs under Commission Decision 2010/367/EU. As a result, data may differ, particularly with regard to HPAI detections in wild birds, from data reported to the Animal Disease Notification System (ADNS), the World Animal Health Information Database (WAHID) or individual national surveillance databases.
5.5. Uncertainty
The assessment of uncertainty was undertaken following the EFSA ‘Guidance on Uncertainty Analysis in Scientific Assessments’ (EFSA Scientific Committee, 2018a), the EFSA scientific opinion on ‘The principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessments’ (EFSA Scientific Committee, 2018b) and the checklist for applying EFSA's uncertainty guidance in a case‐specific assessment. As in this document a summary of the data reported by RCs is made, with no further risk assessment, specific notes of caution were explicitly described for some of the conclusions made in the report.
Abbreviations
- AI
Avian Influenza
- AIV
Avian Influenza A Virus
- H
Haemagglutinin
- HPAI
High Pathogenic Avian Influenza
- LPAI
Low Pathogenic Avian Influenza
- MS
Member State
- N
Neuraminidase
- NUTS
Nomenclature of Territorial Units for Statistics
- PE
Poultry Establishment
- RC
Reporting Country
Appendix A – Comparison of detailed poultry establishment categories with previous reporting categories
1.
Appendix B – Serological results by poultry species
1.
Appendix C – Total number of wild birds of the different orders sampled by passive and active surveillance
1.
Appendix D – Scientific and common names of wild bird species
1.
Appendix E – EFSA list of target wild bird species for avian influenza surveillance
1.
Appendix F – Wild bird observations by voluntary contributors
1.
Appendix G – Wild bird species detected with HPAI virus in passive surveillance
1.
Appendix H – Wild bird species detected with HPAI virus in active surveillance
1.
Appendix I – Country data sets
Table I.1: Links to the avian influenza data sets for 2020 by reporting country. All country data sets containing the tables on the occurrence of avian influenza per country are available on the EFSA Knowledge Junction community on Zenodo. The countries that submitted data sets on the 2020 surveillance data year are: the 27 EU Member States and 4 non‐EU Member States
Suggested citation: EFSA (European Food Safety Authority) , Aznar I, Baldinelli F, Papanikolaou A, Stoicescu A and Van der Stede Y, 2021. Annual Report on surveillance for avian influenza in poultry and wild birds in Member States of the European Union in 2020. EFSA Journal 2021;19(12):6953, 57 pp. 10.2903/j.efsa.2021.6953
Requestor: European Commission
Question number: EFSA‐Q‐2021‐00340
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: EFSA wishes to thank the following for the support provided to this scientific output: AUSVET, Member State representatives and Verena Oswaldi. EFSA also wishes to thank the AHAW Panel of Experts for endorsing this report.
Approved: 23 June 2021
This article was originally published on the EFSA website http://www.efsa.europa.eu on 20 July 2021 as part of EFSA's publication procedures.
Notes
Monthly observations and samples from wild birds on the EFSA list of target species for 2020 by NUTS3 region. The green colour scale represents the number of wild bird observations from the target species, as per data provided by the EuroBirdPortal project. The black dots represent the number of wild bird samples from target species tested within the countries’ AI passive surveillance programmes. Wild bird samples reported at NUTS2 level are not shown on these maps. https://doi.org/10.5281/zenodo.4967481
Avian influenza overview December 2020 – February 2021, https://doi.org/10.2903/j.efsa.2021.6497
In the present report, LPAI‐positive birds include both birds reported positive for an H5 or H7 AI virus not classified as HPAI and birds reported positive for subtypes other than H5 or H7.
The annual report on surveillance for avian influenza in poultry and wild birds in 2019 is available at https://doi.org/10.2903/j.efsa.2020.6349
For the purpose of this report, growers are defined as poultry establishments (different species) in which birds are reared for only part of their productive cycle, to be then sold to other farms belonging to the rural sector where birds will end their production cycle for meat/eggs (Brouwer et al., 2018).
Please note that throughout this report, “number of PEs sampled” refers to all PEs sampled, regardless of the type of tests conducted on the samples (serology or virology).
Reporting of non‐H5 or H7 seropositive PEs by MSs is not compulsory.
For some AI‐positive birds, one or more samples tested positive for HPAI while virus pathogenicity results were not available for one or more of the other positive samples. These birds are considered as HPAI‐positive in the present report.
Avian influenza overview October 2016–August 2017. https://doi.org/10.2903/j.efsa.2017.5018
Avian influenza overview August–December 2020. https://doi.org/10.2903/j.efsa.2020.6379
Avian influenza overview December 2020–February 2021. https://doi.org/10.2903/j.efsa.2021.6497
Commission Decision 2006/437/EC of 4 August 2006 approving a Diagnostic Manual for avian influenza as provided for in Council Directive 2005/94/EC. OJ L 237, 31.8.2006, p. 1–27.
Council Directive 2009/158/EC of 30 November 2009 on animal health conditions governing intra‐Community trade in, and imports from third countries of, poultry and hatching eggs. OJ L 343, 22.12.2009, p. 74–113.
According to Regulation (EU) 2016/429 ‘establishment’ means any premises, structure or, in the case of open‐air farming, any environment or place, where animals or germinal products are kept, on a temporary or permanent basis, except for: (a) households where pet animals are kept; (b) veterinary practices or clinics. (Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘Animal Health Law’). OJ L 84, 31.3.2016, p. 1–208.
This assumption very likely resulted in an over‐estimation of the bird‐level prevalence of LPAI. To address this issue, either samples in positive pools should be re‐tested individually, or, if available, more detailed data on pooling strategies and results could be used for statistical estimation of bird‐level prevalence using a tool such as EpiTools (https://epitools.ausvet.com.au/pooledprevalence).
References
- Brouwer A, Huneau A, Kuiken T, Staubach C, Stegeman A, Baldinelli F, Verdonck F and Aznar I, 2018. Reporting Avian Influenza Surveillance. EFSA Journal 2018;16(11):e05493. 10.2903/j.efsa.2018.5493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European and Food Safety Authority), 2017. Avian Influenza Overview September 2017. EFSA Journal 2017;15(12):5141. 10.2903/j.efsa.2017.5141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), More S, Bicout D, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gort Azar Schmidt C, Michel V, Miranda MA, Nielsen SS, Raj M, Sihvonen L, Spoolder H, Thulke H‐H, Velarde A, Willeberg P, Winckler C, Breed A, Brouwer A, Guillemain M, Harder T, Monne I, Roberts H, Baldinelli F, Barrucci F, Fabris C, Martino L, Mosbach‐Schulz O, Verdonck F, Morgado J and Stegeman JA, 2017. Scientific Opinion on Avian Influenza. EFSA Journal 2017;15(8):4991, 233 pp. 10.2903/j.efsa.2017.4991 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2018a. Guidance on uncertainty analysis in scientific assessments. EFSA Journal 2018;16(1):5123. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018b. The principles and methods behind EFSA's guidance on uncertainty analysis in scientific assessment. EFSA Journal 2018;16(1):e05122. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis NS, Banyard AC, Whittard E, Karibayev T, Al Kafagi T, Chvala I, Byrne A, Meruyert S, King J, Harder T, Grund C, Essen S, Reid SM, Brouwer A, Zinyakov NG, Tegzhanov A, Irza V, Pohlmann A, Beer M, Fouchier RAM, Akhmetzhan (Akievich) S and Brown IH, 2021. Emergence and spread of novel H5N8, H5N5 and H5N1 Clade 2.3.4.4 highly pathogenic avian influenza in 2020. Emerging Microbes & Infections, 10, 148–151. 10.1080/22221751.2021.1872355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team , 2020. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Snäll T, Kindvall O, Nilsson J and Pärt T, 2011. Evaluating citizen‐based presence data for bird monitoring. Biological Conservation, 144, 804–810. 10.1016/j.biocon.2010.11.010 [DOI] [Google Scholar]
- Verhagen JH, Fouchier RAM and Lewis N, 2021. Highly pathogenic avian influenza viruses at the WildDomestic bird interface in Europe: future directions for research and surveillance. Viruses, 13, 212. 10.3390/v13020212 [DOI] [PMC free article] [PubMed] [Google Scholar]


