Abstract
TDP‐43 is an RNA‐binding protein that forms ribonucleoprotein condensates via liquid‐liquid phase separation (LLPS) and regulates gene expression through specific RNA interactions. Loss of TDP‐43 protein homeostasis and dysfunction are tied to neurodegenerative disorders, mainly amyotrophic lateral sclerosis (ALS) and frontotemporal dementia. Alterations of TDP‐43 LLPS properties may be linked to protein aggregation. However, the mechanisms regulating TDP‐43 LLPS are ill‐defined, particularly how TDP‐43 association with specific RNA targets regulates TDP‐43 condensation remains unclear. We show that RNA binding strongly promotes TDP‐43 LLPS through sequence‐specific interactions. RNA‐driven condensation increases with the number of adjacent TDP‐43‐binding sites and is also mediated by multivalent interactions involving the amino and carboxy‐terminal TDP‐43 domains. The physiological relevance of RNA‐driven TDP‐43 condensation is supported by similar observations in mammalian cellular lysate. Importantly, we find that TDP‐43‐RNA association maintains liquid‐like properties of the condensates, which are disrupted in the presence of ALS‐linked TDP‐43 mutations. Altogether, RNA binding plays a central role in modulating TDP‐43 condensation while maintaining protein solubility, and defects in this RNA‐mediated activity may underpin TDP‐43‐associated pathogenesis.
Keywords: amyotrophic lateral sclerosis, liquid‐liquid phase separation, ribonucleoprotein (RNP) granules, RNA‐binding protein, TDP‐43
Subject Categories: Molecular Biology of Disease, Neuroscience, RNA Biology
Liquid‐liquid phase separation of the RNA‐binding protein TDP‐43 is regulated by specific RNA sequences. In addition to modulating phase separation, RNA is crucial for maintaining the liquid state of TDP‐43 condensates.

Introduction
The intracellular organization of RNA‐binding proteins into ribonucleoprotein (RNP) granules is mediated by a process of condensation or phase separation. Based on evidence emerging over the last decade, these protein and RNA‐rich granules are formed via liquid‐liquid phase separation (LLPS) that often show dynamic, liquid‐like properties (Brangwynne et al, 2009; Wippich et al, 2013; Feric et al, 2016). The high concentration of the components while retaining dynamic properties within the droplets may be essential to determine function, cellular organization, and rapid response to cellular stimuli. Formation of RNP granules is mediated by multivalent interactions, including RNA binding and self‐assembly through different protein domains, such as low complexity regions (Li et al, 2012; Lin et al, 2015; Zhang et al, 2015; Garcia‐Jove Navarro et al, 2019). Although RNA is a fundamental component of RNP granules, how it regulates the biogenesis and metabolism of RNP condensates is not well understood (Zhang et al, 2015; Garcia‐Jove Navarro et al, 2019). Recent studies suggest that RNA targets may modulate the LLPS properties of RNA‐binding proteins through specific interactions. Numerous reports also suggest that defects in LLPS homeostasis are associated with disease, such as in the case of TDP‐43 (TAR DNA‐binding protein), FUS (Fused in sarcoma), and other RNA‐binding proteins linked to neurodegeneration (Lin et al, 2015; Molliex et al, 2015; Patel et al, 2015). Specifically, conversion of the condensates into fibrils or complexes with solid‐like properties may lead to the accumulation of protein aggregates associated with pathology. Aggregation and loss of TDP‐43 function are hallmarks of amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD) (Arai et al, 2006; Neumann et al, 2006), and limbic‐predominant age‐related TDP‐43 encephalopathy (LATE) (Nelson et al, 2019). In addition, TDP‐43 inclusions are associated with multisystem proteinopathy (MSP) (Weihl et al, 2009) and other neurodegenerative disorders, including Alzheimer’s disease (AD) and chronic traumatic encephalopathy (CTE) (McKee et al, 2010).
TDP‐43 is a multi‐domain heterogeneous ribonucleoprotein (hnRNP) whose function is essential for the regulation of hundreds of mRNA transcripts to which it binds (Polymenidou et al, 2011; Tollervey et al, 2011). The best‐characterized TDP‐43 function is regulation of splicing, however, TDP‐43 also participates in different mechanisms of RNA processing and transport (Buratti & Baralle, 2010; Alami et al, 2014). For example, TDP‐43 plays a more global role in gene regulation as an inhibitor of cryptic exon inclusion (Ling et al, 2015) and regulates the alternative polyadenylation of > 1,000 genes through direct binding (Koyama et al, 2016; Rot et al, 2017; Melamed et al, 2019). TDP‐43 shows a strong preference for binding dinucleotide guanosine and uridine (GU) RNA motifs as seen in vitro and through the identification of coding and noncoding transcripts that bind TDP‐43 in mouse brain and in human cells (Polymenidou et al, 2011; Tollervey et al, 2011). Purified TDP‐43 binds GU repeat sequences tightly through specific interactions that are evolutionarily conserved throughout higher eukaryotes (Ayala et al, 2005; Li et al, 2017). TDP‐43 forms RNP granules in different cell types, including Cajal bodies and paraspeckles in the nucleus (West et al, 2016; Izumikawa et al, 2019; Modic et al, 2019) and is recruited to mRNA transport granules in neurons (Alami et al, 2014). TDP‐43‐positive granules may form under conditions of proteotoxic stress as nuclear or cytoplasmic stress granules (Dewey et al, 2012; Udan‐Johns et al, 2014). In addition, our lab showed that specific phosphorylation in RRM1 results in nucleolar localization (Li et al, 2017), suggesting that posttranslational modifications may regulate TDP‐43 RNP body dynamics.
To investigate the mechanisms underlying RNA‐mediated assembly of TDP‐43 RNP granules, which remain largely unknown, we asked whether TDP‐43 LLPS properties are modulated by RNA molecules depending on sequence and number of proximal protein‐binding sites. We found that specific interactions of TDP‐43 with RNA consisting of multiple binding sites readily promote phase separation into droplets that exhibit the liquid‐like properties associated with LLPS. Moreover, we observe that specific RNA‐binding increases the liquid properties of TDP‐43 droplets. With this experimental paradigm in place, we were also able to determine the impact of disease‐associated TDP‐43 mutations on RNA‐mediated LLPS properties of TDP‐43. We found that specific mutations reduced the liquid properties of RNA‐driven TDP‐43 condensates. Importantly, we found that specific RNA binding also triggered TDP‐43 phase separation in mammalian cell lysate in the presence of multiple binding sites. Based on our observations, we speculate that physiological RNA recruitment of TDP‐43 nucleates phase separation and, at the same time, prevents the conversion of the droplets into solid‐like complexes.
Results
RNA binding increases the liquid‐like properties of TDP‐43 condensates
A major question in the field is whether TDP‐43 condensation necessarily promotes protein aggregation, and what factors prevent TDP‐43 loss of proteostasis in the condensed phase. We and others previously showed that specific RNA binding inhibits TDP‐43 aggregation (French et al, 2019; Mann et al, 2019). The chaperone‐like activity of GU‐rich RNA in maintaining TDP‐43 solubility depends on specific interactions with the RNA‐binding domains (French et al, 2019). Here, we explored whether RNA binding increases TDP‐43 solubility during phase separation. For these studies, we established reconstitution assays using purified TDP‐43. Until recently, these experiments using full‐length TDP‐43 have been challenging, compared with studying related proteins, such as FUS and hnRNP A1, because the condensates rapidly convert into aggregates (Molliex et al, 2015). We established purification and assay conditions whereby TDP‐43 formed small droplets at 150 mM of salt (Fig 1A). These droplets failed to coalesce for up to 2 h of incubation and formed clusters or chain‐like assemblies, suggesting that these complexes are more viscous and have gel or solid‐like properties. This is consistent with our previous report showing increased misfolded TDP‐43 oligomeric species when the droplets were incubated for 2 h (French et al, 2019). We then asked whether association with A(GU)6 RNA oligonucleotide (Appendix Table S1), which strongly inhibits TDP‐43 aggregation in vitro (French et al, 2019), alters the dynamic properties of TDP‐43 droplets. Addition of A(GU)6 RNA dramatically increased the size of TDP‐43 droplets compared to no RNA conditions (Fig 1B). The TDP‐43 droplets in the presence of A(GU)6 maintained liquid‐like properties, as shown by fluorescence recovery after photobleaching (FRAP) (Fig EV1). As additional control, we used an RNA oligonucleotide of similar length, A(CA)6, which previously showed no significant binding activity for TDP‐43 (Buratti & Baralle, 2001). In contrast with the GU‐rich RNA molecule, the control RNA oligonucleotide did not promote TDP‐43 droplet coalescence (Fig 1B). A(GU)6 is predicted to interact with one TDP‐43 molecule, based on biochemical and structural studies (Ayala et al, 2006; Lukavsky et al, 2013). Therefore, we tested a longer RNA sequence of GU‐repeats that potentially interacts with three TDP‐43 molecules, A(GU)18 (Appendix Table S1), and observed a dramatic increase in the size of TDP‐43 droplets compared to those formed in the presence of A(GU)6 (Fig 1B). Together, these data indicate that RNA is a strong modulator of TDP‐43 phase separation, promoting fusion and coalescence into large droplets, depending on sequence and length. In addition, our findings suggest that GU‐rich oligonucleotides promote liquid‐like behavior of TDP‐43 condensates. We speculate that this previously unexplored function of RNA may be linked to the ability of these RNA sequences to prevent TDP‐43 misfolding (French et al, 2019).
Figure 1. RNA binding promotes liquid‐like properties of TDP‐43 in the dense phase.
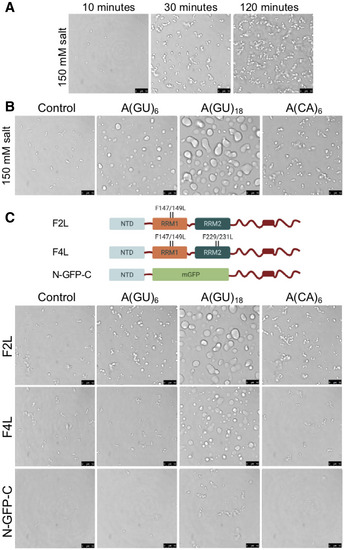
- Condensates formed by purified TDP‐43 at 150 mM of salt observed by brightfield microscopy at different time points.
- TDP‐43 condensates without RNA control or in the presence of A(GU)6 (8 μM), A(GU)18 (2.6 μM), or A(CA)6 (8 μM) (Appendix Table S1) at 150 mM of NaCl (4 μM TDP‐43).
- RNA binding‐deficient TDP‐43 mutants generated by site‐directed substitutions in RRM1 (F2L) or in both RRM domains (F4L) and substitution of RRM1 and RRM2 with monomeric GFP (N‐GFP‐C). Phase separation of the mutants without added RNA (control) or in the presence A(GU)6 (8 μM), A(GU)18 (2.6 μM), or A(CA)6 (8 μM) at 150 mM of NaCl (4 µM protein).
Data information: Representative images for three independent experiments with two different protein preparations. Scale bars, 10 μm.
Figure EV1. RNA‐driven LLPS of TDP‐43 observed by fluorescence recovery after photobleaching (FRAP).
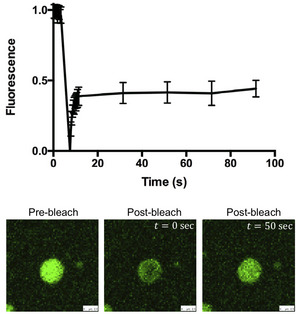
FRAP of TDP‐43‐A(GU)6 RNA condensates formed after mixing 4 μM TDP‐43 with 8 μM A(GU)6 at 150 mM of NaCl. Mean values and SD from three biological replicates comprising 14 bleached droplets, using two different protein purifications. Scale bar 2.5 μm.
Source data are available online for this figure.
To further test the role of specific RNA binding in promoting dynamic, liquid properties of TDP‐43‐RNA droplets, we used three RNA binding‐deficient TDP‐43 mutants (Fig 1C; Buratti & Baralle, 2001; French et al, 2019). TDP‐43 is composed of two tandem RNA recognition motifs (RRMs), which are important for specific binding to RNA and single‐stranded DNA. Of the two RNA binding domains, RRM1 confers most of the binding affinity and sequence specificity to RNA (Buratti & Baralle, 2001; Lukavsky et al, 2013). The mutations studied carry substitutions in RRM1 alone, F2L (Phe147/149Leu); mutations in RRM1 and RRM2, F4L (Phe147/149/229/231Leu); and a substitution of both RRMs with monomeric GFP (N‐GFP‐CTD; French et al, 2019). To confirm the expected loss in RNA‐binding affinity of these mutants, we measured the apparent dissociation constant (Kd,app ) of wild‐type (WT) and mutants for GU‐repeat RNA by fluorescence anisotropy (Appendix Fig S1). These assays showed 11 and 92‐fold loss in GU‐rich RNA‐binding affinity for F2L and F4L compared to WT, respectively (Table 1). We were unable to measure binding in the case of N‐GFP‐CTD. In the absence of RNA, F2L and F4L formed small droplets that were not significantly different from WT droplets shown in Fig 1B (Fig 1C). However, in the presence of A(GU)6 RNA, F2L formed smaller droplets and decreased coalescence, compared to WT, that were not different from those in the presence of A(CA)6 RNA. Furthermore, addition of A(GU)6 did not significantly alter the behavior of F4L and N‐GFP‐CTD, compared to no RNA and A(CA)6 control, forming small condensates and fibrillar structures. Addition of the longer A(GU)18 RNA rescued phase separation in the case of F2L and partially recovered it in the case of F4L (Fig 1C). In contrast, complete substitution of the RRMs in the N‐GFP‐CTD chimera abolished the ability of RNA to modulate condensation even in the presence of A(GU)18. Collectively, these results suggest that disrupting RNA‐TDP‐43 interactions in the RRM1/2 region greatly impairs the ability of RNA composed of a single binding module, A(GU)6, to promote droplet liquid‐like properties. However, in the presence of RNA with multiple binding sites, which bring multiple TDP‐43 molecules in close proximity, LLPS of F2L and F4L is rescued and partially recovered, respectively. This may be explained by our data showing that these mutations substantially lower the affinity of TDP‐43 for the chosen GU‐rich RNA sequences, but still maintain nanomolar affinities for these sequences (Table 1), perhaps explaining the incomplete inhibition of A(GU)18‐driven LLPS. This process is no longer possible if specific RNA binding through the RRMs is entirely absent, as shown in the case of N‐GFP‐C, where the RRM1‐2 region is substituted by mGFP. Our observations with the mGFP chimera also suggest that the N and C‐terminal TDP‐43 domains play a minimal role in GU‐driven LLPS in the absence of the RRMs because of the lack of liquid droplets in any of the conditions (Fig 1C).
Table 1.
RNA‐binding affinity for A(GU)6 RNA.
| Protein | Kd,app (nM) |
|
|
|---|---|---|---|
| Wild‐type (WT) | 0.8 ± 0.1 | 1 | |
| F2L (F147/149L) | 9 ± 1 | 11 | |
| F4L (F147/149/F229/231L) | 74 ± 20 | 92 | |
| ΔN (a.a. 102–414) | 0.7 ± 0.1 | 0.8 | |
| Y4R/E17R | 1.7 ± 0.5 | 2 | |
| RRM1‐2 (a.a. 102–269) | 0.80 ± 0.16 | 1 | |
| ΔC (a.a. 1–269) | 1.1 ± 0.2 | 1.4 | |
| K181E | 3.6 ± 0.5 | 5 | |
| A315E | 1.1 ± 0.3 | 1.4 | |
| A315T | 0.6 ± 0.1 | 0.8 | |
| A321G | 1.3 ± 0.2 | 1.6 | |
| Q331K | 1.6 ± 0.3 | 2 | |
| M337V | 2.4 ± 0.5 | 3 | |
| A382T | 0.7 ± 0.2 | 0.9 |
Estimated loss (> 1) or gain (< 1) in binding affinity relative to WT.
RNA binding to multiple sites strongly induces TDP‐43 phase separation
Based on the results described above, we sought to further investigate how RNA sequences that recruit multiple TDP‐43 molecules affect phase separation. In particular, we were interested in surveying RNA molecules representative of natural TDP‐43 targets, including GU repeat tracks (Polymenidou et al, 2011; Tollervey et al, 2011). We previously showed that a minimum of five GU repeats is required for binding one molecule of TDP‐43 and that GU repeats may be interrupted by nonspecific residues without a significant loss in binding affinity (Ayala et al, 2005). This was confirmed by structural studies in which binding of one TDP‐43 molecule covers approximately 10–12 nucleotides through specific interactions with the RRMs (Lukavsky et al, 2013). Therefore, the natural TDP‐43‐bound RNA sequences, consisting of long stretches of GU‐rich sequences (GU‐rich clusters), indicate extended regions of multiple TDP‐43‐binding sites (Polymenidou et al, 2011; Tollervey et al, 2011). Examples of these sequences include a GT repeat polymorphism (9–15 repeats) in the cystic fibrosis transmembrane conductance regulator (CFTR) modulating exon 9 splicing (Buratti et al, 2004) and GT‐repeat stretches (10 up to 40 repeats) found in introns and 3’ UTR of Cdk6 (Ayala et al, 2008; Tollervey et al, 2011). To explore how RNA molecules similarly composed of extended GU‐rich sequences affect LLPS properties of TDP‐43, we studied droplet formation at higher salt (250 mM of NaCl). Under these conditions, TDP‐43 did not phase separate on its own (Fig 2A and B), however, addition of A(GU)18 led to the spontaneous formation of protein droplets, which entirely colocalized with RNA (Fig EV2A). We tested the dynamic behavior of the A(GU)18‐mediated TDP‐43 droplets to determine whether these were consistent with LLPS properties. We reproducibly observed spherical condensates that fused and coalesced into larger droplets (Movie EV1). In addition, increasing salt concentration to the preformed droplets dissolved the dense phase (Movie EV2), indicating the reversibility of the multicomponent complex.
Figure 2. RNA composed of multiple binding sites potently induces TDP‐43 phase separation.
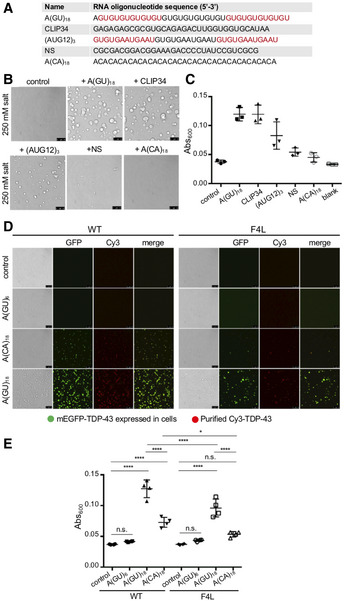
- Description of RNA oligonucleotide sequences composed of multiple TDP‐43‐binding sites, A(GU)18, CLIP34 and (AUG12)3, NS, or A(CA)18 Sequence in different colors separates regions that accommodate one TDP‐43 molecule, if known. NS, nonspecific control RNA.
- TDP‐43 condensates in the presence of the different RNA sequences. TDP‐43 and RNA oligonucleotide concentration is 4 μM and 3.9 μM, respectively. Representative images for three biological replicates using two different protein preparations. Scale bars, 10 μm.
- LLPS measured by turbidity in the same experimental conditions as panel B. Mean and SD from three biological replicates with two different protein preparations.
- Lysate from HEK 293 cells expressing a mEGFP‐tagged copy of either wild‐type (WT) or F147/149/229/231L (F4L) TDP‐43 was added to a mixture of recombinant WT or F4L TDP‐43 (5.3 μM, 10% Cy3‐labeled protein) and A(GU)6 (22.8 μM), A(CA)18 (7.6 μM), A(GU)18 (7.6 μM), or no RNA control at 250 mM of NaCl. Droplets were imaged by brightfield and fluorescence microscopy. Representative images for three biological replicates using two protein preparations. Scale bars, 10 μm.
- LLPS measured by turbidity in the same experimental conditions as panel D. Mean and SD of four biological replicates using two recombinant protein preparations. Analyzed by one‐way ANOVA (F(7,24) = 69.30, P < 0.0001). Sidak’s multiple comparisons test was used to compare selected groups. *P ≤ 0.05, ****P ≤ 0.0001.
Source data are available online for this figure.
Figure EV2. RNA‐driven TDP‐43 phase separation is sequence dependent.
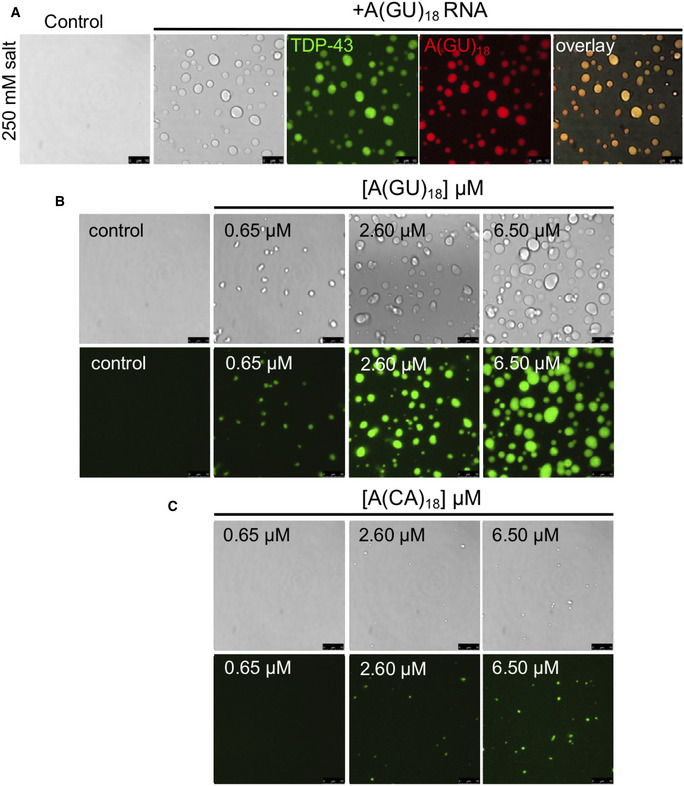
-
ADroplets observed by brightfield and fluorescence microscopy when Oregon green‐labeled TDP‐43 was mixed with no RNA control or Alexa 594‐labeled A(GU)18 RNA at 250 mM of salt. Representative images for three biological replicates using two protein preparations. Scale bars, 10 μm.
-
B, CLiquid droplets observed by brightfield and fluorescence microscopy when Oregon green‐labeled TDP‐43 (4 μM, 10% labeled protein) was mixed with no RNA control or increasing A(GU)18 or A(CA)18 RNA concentration at 250 mM of salt. Representative images from three biological replicates using two different protein preparations. Scale bars, 10 μm.
We conducted the next set of experiments to test the specificity of GU‐rich RNA molecules in modulating TDP‐43 phase separation. To facilitate our experiments using purified full‐length TDP‐43, which is highly aggregation‐prone, the protein was N‐terminally tagged with the yeast SUMO ortholog Smt3 (Li et al, 2017; French et al, 2019). Therefore, it was important to evaluate whether the tag influenced TDP‐43 condensation driven by GU‐rich RNA by removing SUMO, as described in methods. Appendix Fig S2A shows that A(GU)18 RNA triggered condensation of untagged TDP‐43 at 250 mM of NaCl, while no LLPS was observed upon mixing with A(CA)18, or in control in the absence of RNA. The amount of protein recovered following SUMO cleavage and isolation of TDP‐43 was considerably less than that used for the experiments with tagged TDP‐43, estimated at 0.2–0.5 μM vs. 4 μM, respectively. These lower concentrations were probably caused by a drastic loss of soluble protein upon removal of the N‐terminal SUMO. The differences in concentration may explain the smaller sized condensates observed for the A(GU)18‐induced condensates formed by the untagged protein relative to SUMO‐tagged TDP‐43 (e.g., shown in Fig 2B). Nevertheless, while the final yield of the untagged protein was low, untagged TDP‐43 droplets were generated only in the presence of long GU‐repeat RNA. We also wanted to determine the effect of charged polymers other than long GU‐rich RNA oligonucleotides on TDP‐43 condensation. Heparin or a nonspecific single‐stranded DNA (ssDNA) oligonucleotide did not affect TDP‐43 phase separation under the conditions tested (Appendix Fig S2B). ssDNA composed of GT repeats A(GT)18, which binds TDP‐43 through RRM interactions with approximately 200‐fold lower affinity than the analogous RNA sequence (Kuo et al, 2009; Kitamura et al, 2018), did not induce TDP‐43 droplet formation at concentrations that strongly promoted phase separation in the case of A(GU)18 (Appendix Fig S2B). For our studies, we used modified RNA oligonucleotides to increase stability. The modification did not alter the ability of RNA to drive TDP‐43 LLPS under the conditions tested, as seen by using analogous nonmodified RNA (Appendix Fig S2B).
Next, we analyzed the regulation of TDP‐43 LLPS by alternative RNA sequences that may potentially bind multiple protein molecules, but are not composed of GU‐repeats (Fig 2A). These sequences are equivalent to those found in physiological targets. The consensus sequence found among natural TDP‐43‐bound RNA transcripts in human cells, AUG12 (Tollervey et al, 2011) binds one molecule of TDP‐43 (Lukavsky et al, 2013). We generated an RNA oligonucleotide composed of AUG12 repeated three times, (AUG12)3. In addition, we analyzed the effect of CLIP34, a 34‐nucleotide sequence derived from the Tardbp 3’ UTR that recruits TDP‐43 during autoregulation (Ayala et al, 2011; Polymenidou et al, 2011; Tollervey et al, 2011). This sequence differs from GU repeat RNA as it is not particularly abundant in GU motifs. We found that the CLIP34 oligonucleotide used here binds multiple TDP‐43 molecules, accommodating at least three as estimated by electromobility shift assays (EMSA) (Appendix Fig S3). At 250 mM of salt, CLIP34 triggered spontaneous TDP‐43 phase separation into large droplets, similar to A(GU)18 (Fig 2B). Addition of (AUG12)3 also induced droplets, although these were smaller compared to those driven by A(GU)18 and CLIP34 at similar RNA concentrations (Fig 2B). In contrast, an alternative RNA oligonucleotide of similar length, NS (32 nucleotides), consistently showed few small condensates mixed with fibrillar structures (Fig 2B). Additionally, the sequence A(CA)18 showed no effect on TDP‐43 LLPS. Furthermore, turbidity measurements taken as absorbance at 600 nm showed readings consistent with what was observed microscopically (Fig 2C). Addition of A(GU)18 and CLIP34 generated the greatest increase in turbidity while the nonspecific sequences NS and A(CA)18 showed values closer to control in the absence of RNA. In all, our observations provide strong evidence that RNA‐drives the formation of TDP‐43 condensates exhibiting liquid‐like properties. In addition, the ability to promote TDP‐43 demixing is unique to RNA sequences that are established to interact with TDP‐43 specifically and may bind to more than one TDP‐43 molecule. These RNA oligonucleotides bind TDP‐43 with high affinity in vitro and are composed of sequences commonly found among physiological targets (Buratti & Baralle, 2001; Ayala et al, 2005; Polymenidou et al, 2011; Tollervey et al, 2011).
Long GU‐repeat RNA promotes LLPS of TDP‐43 in mammalian cell lysate
Our assays to this point had tested whether RNA could induce TDP‐43 LLPS in a simplified system combining primarily purified protein and RNA. However, the cellular milieu is much more complex. We asked whether we could reconstitute RNA‐driven TDP‐43 condensates in an environment composed of cellular cytoplasmic and nuclear components. To this end, we established a new protocol to analyze cellular extract from HEK293 cells that stably express a single copy of either mEGFP‐TDP‐43 WT or the RNA binding‐deficient mutant mEGFP‐TDP‐43 F4L. Our methods were adapted from recent studies that reconstitute stress granules and nucleoli in cellular lysates (Freibaum et al, 2021). We prepared cellular lysate after inducing the expression of GFP‐tagged TDP‐43 and added Cy3‐labeled recombinant wild‐type TDP‐43. In the absence of RNA, the mixture appeared homogenous and lacked visible foci (Fig 2D). Likewise, when A(GU)6 RNA, which favors binding one TDP‐43 molecule, was added, the mixture remained free of liquid droplets and showed no significant increase in turbidity compared to control (Fig 2D and E). However, when A(GU)18, which consists of three potential TDP‐43‐binding sites, was added, spherical droplets containing both mEGFP‐labeled cellular and Cy3‐labeled recombinant TDP‐43 formed (Fig 2D). Consistently, addition of A(GU)18 led to an almost 4‐fold increase in turbidity compared to control or in the presence of A(GU)6 RNA (Fig 2E). Unlike in our LLPS assay in the absence of cellular lysate, A(CA)18 RNA also generated a number of droplets, albeit far fewer than A(GU)18. A corresponding significant increase in turbidity was also seen upon mixing with A(CA)18 RNA (Fig 2E), however this was 50% lower than in the presence of A(GU)18. We hypothesize that the effect of A(CA)18 could be due to the increased concentration of both TDP‐43 and RNA in this assay, or to the contribution of other RNA‐binding proteins present in the cell lysate. Lysate from cells expressing mEGFP‐TDP‐43 F4L combined with purified Cy3‐labeled TDP‐43 F4L in the presence of A(GU)18 showed a readily noticeable decrease in the number of droplets, compared to WT (Fig 2D). Correspondingly, the TDP‐43 F4L lysate showed decreased turbidity relative to WT TDP‐43 lysate (Fig 2E) under phase separation‐promoting conditions. In agreement with our observations using purified protein and RNA, these experiments show that specific TDP‐43 interactions with RNA composed of multiple binding sites induces LLPS in a cellular environment. Furthermore, we show that this new technique may be used to reconstitute TDP‐43 RNP granules and interrogate their regulation in the presence of other cellular components.
RNA‐driven TDP‐43 phase separation is sequence‐dependent and is modulated by the number of binding sites
Next, we sought to further establish the role of GU‐rich RNA as a modulator of TDP‐43 phase properties. First, we observed that TDP‐43 droplets grew larger as A(GU)18 concentration increased (quantified by droplet area), suggesting that the volume of the dense phase increased as a function of RNA (Fig 3A and B). We also quantified the concentration of TDP‐43 in the light phase (Cout ) by Bradford protein analysis following separation of dense and light phases. We found that as the size of the droplets grew with increasing A(GU)18 concentration, Cout decreased (Fig 3C). The highest A(GU)18 concentration led to approximately 70% reduction of TDP‐43 concentration in the light phase relative to control, in the absence of RNA. Additionally, we tested the effect of increasing concentrations of A(CA)18 on TDP‐43 LLPS and observed substantially fewer and smaller condensates than were present in corresponding A(GU)18 conditions (Fig EV2B and C). In accordance with this data, Cout of A(CA)18 samples showed substantially less change from baseline than did A(GU)18, suggesting less TDP‐43 accumulation in condensates that do form under nonspecific RNA conditions (Fig 3C). These data strongly indicate that TDP‐43 accumulation in the dense phase is upregulated as a function of GU‐rich RNA presence specifically. To explore whether A(GU)18 altered the TDP‐43 phase boundary, we analyzed droplet formation at different salt concentrations as a function of A(GU)18 concentration. At equal TDP‐43 concentration, the increase in salt inhibited droplet formation regardless of RNA presence (Fig 3D). However, A(GU)18 promoted droplet formation at salt concentrations that were not permissive in the absence of RNA. A schematic representation of droplet formation as a function of salt indicates a shift in the TDP‐43 phase boundary driven by RNA (Fig 3E), suggesting a decrease in the saturation concentration (Csat ) of TDP‐43 necessary to initiate condensation. These results are consistent with the effect of GU‐rich RNA on driving TDP‐43 LLPS and provide strong evidence that RNA composed of multiple binding sites modifies TDP‐43 phase behavior by favoring condensation. Previous studies showed that at high concentrations, nonspecific RNA drives TDP‐43 away from phase separation (Maharana et al, 2018; Mann et al, 2019), thus we investigated whether high concentrations of specific RNA would have the same effect. Using A(GU)15, we increased the concentration of RNA while keeping the TDP‐43 concentration constant, up to a 50‐fold molar excess of RNA. TDP‐43 liquid droplets and turbidity continued to increase up to 50 μM A(GU)15 and sharply declined at the highest RNA concentration (Fig EV3). These results support the idea that at very high concentrations, RNA may act as a suppressor of TDP‐43 phase separation, as previously suggested. It is also possible that at the concentrations generally used in our studies, long GU repeat RNA recruits multiple TDP‐43 molecules, driving phase separation. However, LLPS may be reversed at very high RNA concentrations, where excess RNA results in binding one TDP‐43 per RNA molecule. While we consider this possible scenario, we also note that reentry into a single‐phase system only occurred at the highest A(GU)15 concentration tested, and this may be due to the preference of these RNA oligonucleotides to bind multiple TDP‐43 molecules. Our speculation is based on the binding cooperativity of TDP‐43 upon association with long GU repeat RNA, as recently reported by Rengifo‐Gonzales et al (2021) and previously suggested by Flores et al (2019).
Figure 3. RNA with multiple binding sites modulates TDP‐43 phase properties.
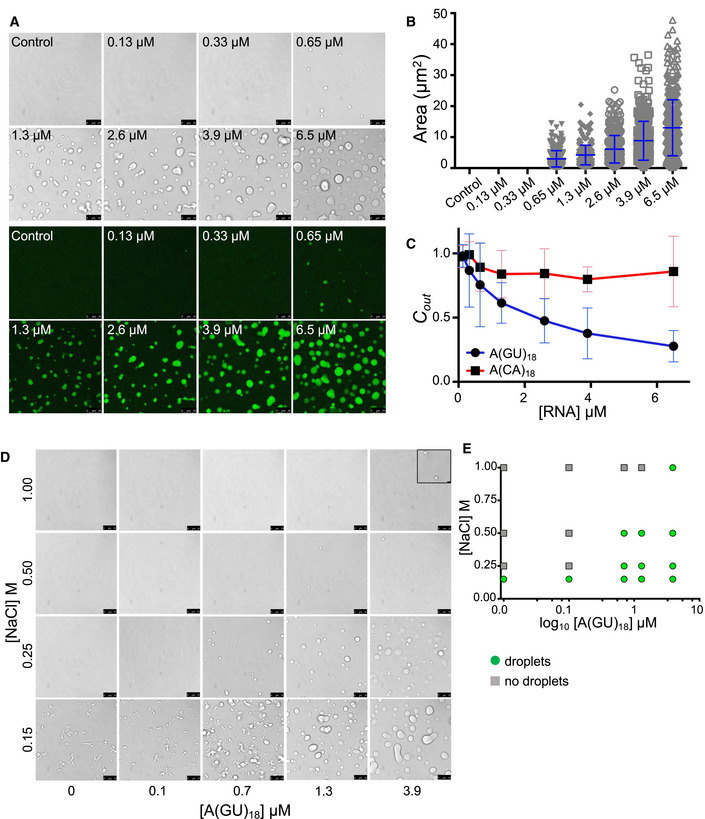
- Liquid droplets observed by brightfield and fluorescence microscopy when Oregon green labeled TDP‐43 (4 μM, 10% labeled protein) was mixed with no RNA control or increasing A(GU)18 RNA concentration at 250 mM of salt. Representative images from three biological replicates using two different protein preparations. Scale bars, 10 μm.
- Area of droplets plotted at different A(GU)18 concentrations. Mean and SD of > 300 droplets from three biological replicates using two different protein preparations.
- TDP‐43 concentration in the light phase measured as a function of A(GU)18 (blue) or A(CA)18 (red) concentration measured by Bradford protein assay. Mean and SD from ≥ 6 biological replicates using two different protein preparations (250 mM of NaCl, 4 µM TDP‐43).
- Phase separation of TDP‐43 (4 μM) at increasing salt concentration without RNA or in the presence of increasing A(GU)18 concentration. Scale bars, 10 μm. Inlay added to top right panel for increased visibility, scale bar 1 μm.
- Phase diagram derived from panel D, denoting either no droplets or droplet formation for each condition.
Source data are available online for this figure.
Figure EV3. Increasingly high concentrations of GU repeat RNA drive TDP‐43 out of liquid‐liquid phase separation.
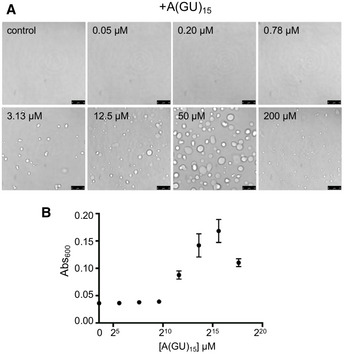
- Droplets observed by brightfield when TDP‐43 was mixed with no RNA control or increasing concentrations of A(GU)15 RNA at 250 mM of NaCl. Representative images for three biological replicates using two protein preparations. Scale bars, 10 μm.
- LLPS measured by turbidity in the same experimental conditions as panel A. Mean and SD from three biological replicates using two different protein preparations.
Source data are available online for this figure.
We next asked whether increasing multivalent association of TDP‐43 molecules on an RNA template increases protein condensation. For these experiments, we studied LLPS as a function of the number of contiguous potential TDP‐43‐binding sites. The concentration of GU‐ or CA‐rich RNA oligonucleotides was modified for each sequence to maintain the number of TDP‐43‐binding sites constant across samples. In doing so, we ensured that the absolute amount of RNA did not change from one condition to the next, rather the only change was the number of potential TDP‐43‐binding sites that were linked together. At 250 mM of NaCl, addition of A(GU)6 RNA, which favors binding of one TDP‐43 molecule, did not promote TDP‐43 liquid droplet formation (Fig 4A). Small condensates started to form in the presence of A(GU)9 and these grew larger as a function of GU‐repeat length, while condensates were not visible microscopically with CA‐rich sequences until a repeat number of 24 (Fig EV4). We also analyzed TDP‐43 LLPS by measuring turbidity in the presence of the different GU‐repeat RNA oligonucleotides (Appendix Fig S4). In agreement with our imaging data, starting with A(GU)9, turbidity increased as the length of GU repeats in the sample increased. Further quantification of TDP‐43 condensation triggered by increasing GU‐repeat length, showed that droplet area directly increased with the number of TDP‐43‐binding sites (Fig 4B). In addition, TDP‐43 concentration in the light phase (Cout ) decreased proportionally with greater GU‐repeat number (Fig 4C), suggesting a corresponding increase of TDP‐43 in the dense phase as the number of GU‐repeats increased. In stark contrast, TDP‐43 Cout only moderately decreased with increasing CA repeat number, compared to control in the absence of RNA (Fig 4C). Collectively, our observations indicate that TDP‐43 condensation is strongly regulated by number of proximal RNA‐binding sites. These findings open avenues to investigate how different TDP‐43 RNA targets, which are composed of varying numbers of binding modules and varying binding affinity, may differentially regulate protein phase separation.
Figure 4. TDP‐43 phase separation is regulated by the number of binding sites on RNA.
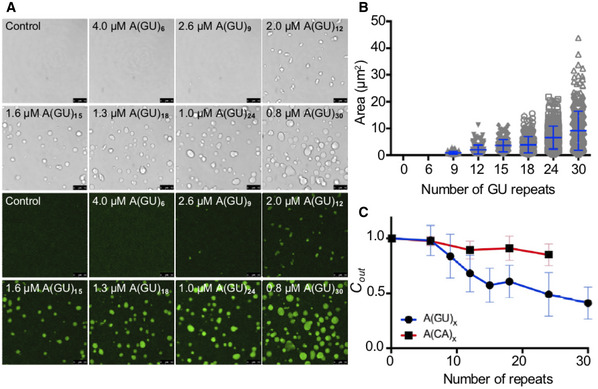
- TDP‐43 droplets observed by brightfield and fluorescence microscopy when Oregon green labeled TDP‐43 (4 μM, 10% labeled protein) was mixed with no RNA control or GU‐repeat RNA oligonucleotides of increasing length at 250 mM of salt. As indicated, RNA concentration varied to maintain a constant total number of TDP‐43‐binding sites. Scale bars, 10 μm.
- The plot shows droplet area quantified as a function of GU‐repeat length. Mean and SD of > 150 droplets from three biological replicates using three different protein preparations.
- TDP‐43 concentration in the light phase (Cout ) as a function of GU (blue) or CA (red)‐repeat length quantified by Bradford protein assay. Mean and SD from ≥ 5 biological replicates using two different protein preparations. (250 mM of NaCl, 4 µM TDP‐43).
Source data are available online for this figure.
Figure EV4. RNA induction of TDP‐43 phase separation is length dependent and sequence specific.
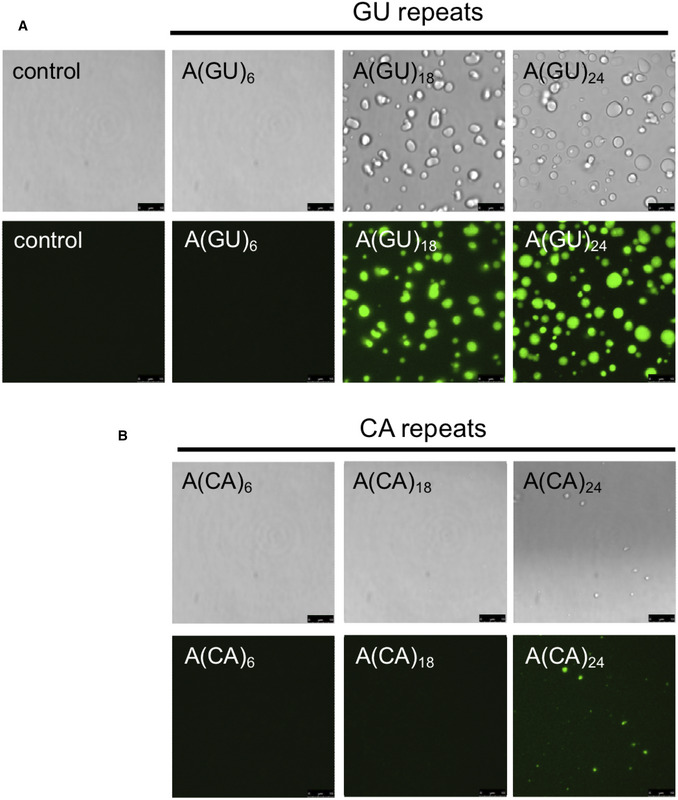
-
A, BTDP‐43 (4 μM) was mixed with RNA composed of GU or CA repeats, in panel A and B, respectively, at 250 mM of NaCl. To maintain the concentration of binding modules, the concentration of the separate oligonucleotides varied as follows: 4 μM A(GU)6 and A(CA)6, 1.3 μM A(GU)18 and A(CA)18, and 1 μM A(GU)24 and A(CA)24. Imaged by brightfield and fluorescence microscopy. Representative images of three biological replicates using two different protein preparations. Scale bars, 10 μm.
RNA‐driven TDP‐43 phase separation is mediated by N‐terminal and low complexity domain interactions
To further investigate the mechanism of TDP‐43 RNA‐driven phase separation, we explored whether this process is also affected by TDP‐43 domains that participate in protein‐protein interactions and self‐assembly. TDP‐43 is organized into three independently folded domains followed by a mostly disordered region at the carboxyl terminus (Fig 5A). These four domains all contribute to TDP‐43 self‐assembly and RNA processing function. The N‐terminal domain (NTD) folds into a homeobox‐like structure that promotes TDP‐43 oligomerization (Wang et al, 2013, 2018; Afroz et al, 2017; Mompean et al, 2017). NTD‐driven complexes are necessary for protein function in RNA processing, phase separation, and for preventing the accumulation of RNA:DNA hybrid structures (Wang et al, 2013, 2018; Afroz et al, 2017; Mompean et al, 2017; Wood et al, 2020). This region is followed by the two RRMs and the carboxy‐terminal domain (CTD), which is referred to as low complexity or prion‐like domain for its similarity to yeast prion domains. The CTD is a mostly disordered region that undergoes phase separation alone, suggesting that it plays an important role in full‐length TDP‐43 condensation (Conicella et al, 2016). At the same time, this domain is a primary driver of protein aggregation (Johnson et al, 2009) and is also where almost all ALS and FTD‐linked mutations are found. We investigated droplet formation using WT, mutant, and TDP‐43 fragments targeting the NTD and CTD (Fig 5A) at 250 mM of salt. In the absence of RNA, none of the constructs showed phase separation (Fig 5B). We found that deletion of both NTD and CTD (RRM1‐2) precluded TDP‐43 phase separation in the presence of A(GU)30, in contrast to WT (Fig 5B). A fragment lacking the entire NTD (ΔN) or two site substitutions that prevent NTD‐driven oligomerization (Y4R/E17R) (Wang et al, 2018) completely blocked TDP‐43 phase separation in the presence of RNA (Fig 5B). Similarly, RNA did not promote TDP‐43 LLPS in the absence of the low complexity domain (Fig 5B). We measured the Kd,app of WT and all the mutants for GU‐repeat RNA (Appendix Fig S5, Table 1) and obtained values consistent with those previously measured for full‐length WT and fragments, including ΔC and RRM1‐2, using other methods (Ayala et al, 2005; Li et al, 2017; Flores et al, 2019). In all cases, the mutations did not significantly alter the binding affinity for GU‐repeats, relative to WT. This suggests that the lack of A(GU)30‐induced droplet formation by the mutants is unlikely to be caused by reduced RNA interactions. Instead, the data indicate that binding of RRM1 and RRM2 to RNA is essential, but under the conditions tested is not sufficient to promote RNA‐driven TDP‐43 LLPS. The N‐terminal domain oligomerization as well as CTD interactions play a major role in the RNA‐mediated process, which is consistent with the importance of multivalent interactions in biomolecular condensation.
Figure 5. RNA‐induced phase separation requires multivalent interactions mediated by TDP‐43 domains.
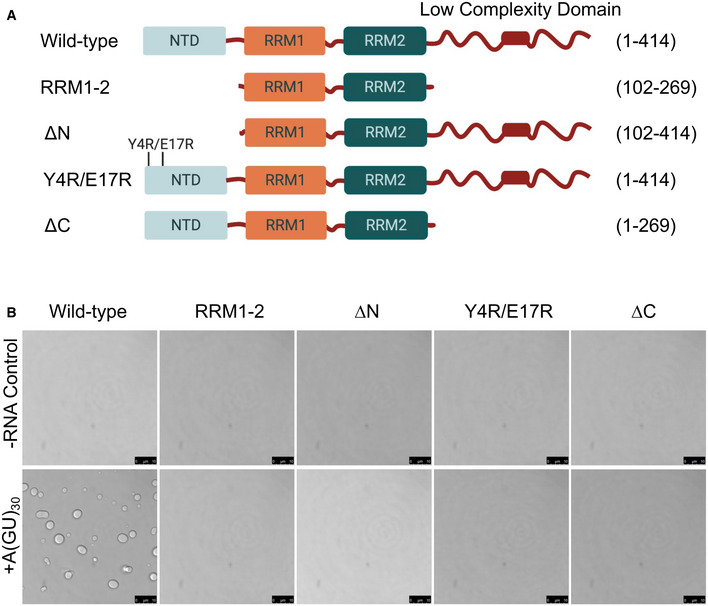
- Full‐length TDP‐43, mutant, and deletion fragments targeting regions that mediate self‐assembly. Amino acid residue positions are indicated for each.
- Phase separation of full‐length and TDP‐43 variants (4 μM) mixed with A(GU)30 RNA (0.8 μM) or no RNA control at 250 mM of NaCl observed by brightfield microscopy. Representative images for three biological replicates using two different protein preparations. Scale bars, 10 μm.
Source data are available online for this figure.
ALS‐linked mutations compromise RNA‐driven liquid‐like behavior of TDP‐43 condensates
TDP‐43 phase separation has been the focus of much investigation because disruption of LLPS homeostasis may underlie pathogenesis linked to protein aggregation and loss of function in neurodegeneration (Lin et al, 2015; Molliex et al, 2015; Patel et al, 2015; Mackenzie et al, 2017). To shed light on disease mechanisms we analyzed the behavior of TDP‐43 mutations causative of ALS/FTD. We selected a group of these, including K181E, A315E, A315T, A321G, Q331K, M337V, and A382T to determine whether they altered RNA‐driven TDP‐43 condensation (Gitcho et al, 2008; Sreedharan et al, 2008; Baumer et al, 2009; Chio et al, 2011; Fujita et al, 2011; Chen et al, 2019). The recently identified K181E mutation is positioned in the linker region between RRM1 and RRM2 and was shown to increase aggregation in cells (Chen et al, 2019). All the other substitutions are in the vicinity or within an evolutionarily conserved region of the CTD (a.a. 320–343) which forms an alpha‐helical structure (a.a. 321–330, boxed region in Fig 6A) that extends further (up to a.a. 340) upon interaction with the same region of a second CTD molecule (Conicella et al, 2016). This structure and the helix‐helix association is central for TDP‐43 LLPS and previous studies showed that these ALS mutations disrupt the intermolecular CTD interactions, drastically reducing LLPS (Conicella et al, 2016, 2020; Schmidt & Rohatgi, 2016). In addition, we and others previously showed that some of these mutants, e.g., M337V and A315T, accelerate TDP‐43 aggregation (Johnson et al, 2009; Conicella et al, 2016; French et al, 2019) and increase intracellular aggregate seeding (French et al, 2019). We compared RNA‐driven LLPS of WT and the mutants in control conditions without RNA or in the presence of A(GU)18 RNA at 250 mM of salt. Our microscopy analyses showed no significant changes in the RNA‐driven LLPS of K181E, A315E, A315T, Q331K, and A382T relative to WT (Fig 6A). In contrast, we found that A321G and M337V strongly changed the phase separation dynamics in the presence of A(GU)18 (Fig 6A, marked by red boxes). The A321G and M337V condensates induced by the addition of A(GU)18 were noticeably smaller than those formed by WT and assembled in clusters without fusing into larger droplets. This is highlighted by the movie monitoring M337V droplets over time showing a stark contrast to WT behavior (Movies EV3 and EV4). We measured the binding affinity for GU‐rich RNA of all the mutations tested and did not find significant changes relative to WT (Appendix Fig S6, Table 1). Therefore, our results suggest that impaired liquid properties of the RNA‐mediated M337V and A321G condensates are not caused by defects in RNA association. In addition, we tested whether M337V condensates could interact with WT and impact the liquid properties of WT RNA‐mediated assemblies. We mixed equal concentrations of fluorescently labeled WT (Oregon green) and M337V (Cy3) proteins in the presence of A(GU)18 RNA. This led to the formation of both larger and small chain‐like droplets that consisted of WT and M337V proteins (Fig 6B). These results suggest that WT and mutant TDP‐43 may assemble together to form condensates, however, WT protein may not transfer liquid‐like properties to M337V complexes. Instead, mutant TDP‐43 was able to disrupt WT LLPS behavior. These observations may be relevant to the ALS cases affected by the M337V mutation, caused by the heterozygous substitution c. 1009A>G, which has been independently found in families in a number of countries (Rutherford et al, 2008; Sreedharan et al, 2008; Tamaoka et al, 2010). Our results demonstrate that one intact copy of TDP‐43 is likely insufficient to prevent the maturation of liquid RNP granules to more solid structures in these patients. The assays established for these studies shed light on mechanisms essential for TDP‐43 function that may be disrupted by ALS mutations and may underpin TDP‐43‐linked disease. Our analysis also indicates that these mutations affect protein/gene function differently. The defects observed in the case of A321G and M337V may be caused by structural alterations that inhibit LLPS and/or result in condensates with gel or more solid‐like properties. For this reason, mutant condensates may be more likely to increase fibrilization, as observed previously (French et al, 2019). These conclusions are also supported by previous reports of similar condensate clusters formed by the CTD harboring M337V (Conicella et al, 2016). Interestingly, A321G and M337V are located within the alpha‐helical region in the CTD. Our observations that long GU‐rich RNA is unable to promote liquid TDP‐43 condensation in the case of these mutants aligns with earlier reports, highlighting the importance of alpha‐helix‐mediated intermolecular contacts on LLPS (Conicella et al, 2016, 2020). This model warrants further investigation as it may not fully explain our observations with the Q331K mutation. Q331K also disrupts the CTD interactions of the isolated fragment (Conicella et al, 2016), but behaves similar to WT in our assays with RNA‐driven LLPS of full length TDP‐43 (Fig 6B), which is consistent with recently published work showing minimal differences in the cellular condensation properties of this mutant relative to WT (Hallegger et al, 2021).
Figure 6. ALS‐linked TDP‐43 mutations A321G and M337V impair RNA‐driven phase separation.
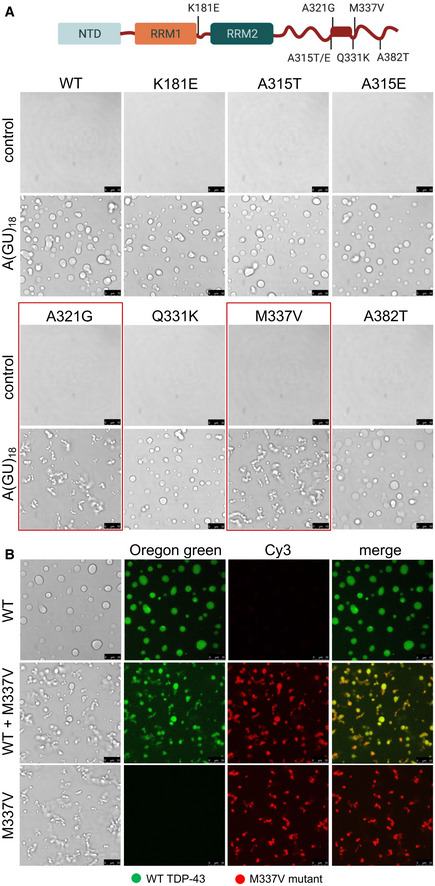
- TDP‐43 ALS‐linked amino acid substitutions K181E, A315T/E, A321G, Q331K, M337V, and A382T. Boxed in the C‐terminal domain is the α‐helical structure within the conserved region (a.a. 320–343). Droplets formed by TDP‐43 wild‐type (WT) and the different mutants (4 μM) observed by brightfield microscopy in the presence of no RNA control or A(GU)18 RNA (3.9 μM) at 250 mM of NaCl. Mutations A321G and M337V, showing decreased liquidity of the condensates in the presence of A(GU)18, are boxed in red. Representative images for three biological replicates using three different protein preparations of WT and M337V, 2 preparations of A321G, Q331K, K181E and one preparation of A315T/E and A382T. Scale bars, 10 μm.
- Phase separation of WT (4 μM, 10% Oregon green‐labeled protein) and M337V (4 μM, 10% Cy‐3‐labeled protein) observed by brightfield and fluorescence microscopy in the presence of A(GU)18 (3.9 μM) at 250 mM of NaCl. The middle panel shows mixing of these WT (2 μM) and M337V (2 μM) samples. Representative images for three biological replicates using two protein preparations. Scale bars, 10 μm.
Discussion
The self‐assembly of TDP‐43 plays a central role in determining protein function and cellular dynamics while at the same time, aberrant self‐association may lead to protein aggregation and pathology associated with numerous neurodegenerative disorders. Our goal was to investigate the control of TDP‐43 condensation by RNA molecules for their role as a principal TDP‐43 interacting partner. Previous studies showed that full‐length TDP‐43 LLPS is inhibited in the presence of total yeast RNA, suggesting that RNA may prevent condensation and thereby counteract aggregation (Maharana et al, 2018; Mann et al, 2019). We studied RNA molecules with specific sequence composition, which represent natural TDP‐43 targets. For these studies, we used a simplified system with full‐length TDP‐43 and find that RNA strongly promotes TDP‐43 phase separation in a sequence and length dependent manner. This process is mediated by specific binding, suggesting that TDP‐43 condensation may be regulated by the number of proximal binding sites on RNA targets. However, at very high concentrations of specific‐binding RNA this observation is reversed and this is in line with previous observations studying the CTD fragment condensation (Conicella et al, 2016). We speculate a scenario in which lower concentrations of GU‐rich RNA allow multiple TDP‐43 molecule binding to each oligonucleotide, promoting droplet formation, whereas saturating RNA concentrations result in each RNA molecule binding to a single TDP‐43 molecule. Similar to A(GU)6 being unable to promote LLPS at 250 mM of NaCl, this will drive the system back toward a single‐phase regime. Our experiments in cell lysates showing that RNA composed of multiple TDP‐43‐binding sites promotes TDP‐43 phase separation, whereas RNA composed of a single binding module does not induce TDP‐43 granule formation, strongly supports the physiological relevance of this RNA‐mediated function. Importantly, we show that specific interactions of the RNA‐binding domains with GU‐rich RNA are crucial to maintain liquid properties of the TDP‐43 condensates. Furthermore, our results indicate that specific TDP‐43 disease‐linked mutations greatly impair this function of RNA, which may help explain the connection between disease‐associated mechanisms and TDP‐43 homeostasis.
Our studies focused on RNA composed of sequences representative of TDP‐43 targets found in cells, which are characterized by long GU‐rich stretches (Polymenidou et al, 2011; Tollervey et al, 2011). We find that these RNA sequences that are predicted to bind multiple TDP‐43 molecules are potent modulators of TDP‐43 condensation. Increasing the number of TDP‐43‐binding modules enhances protein condensation, as seen by the size of droplets and increased enrichment of protein in the dense phase (Fig 4). This process is impaired upon disruption of RNA binding using either RRM mutations (Fig 1), or when using RNA molecules with no measurable TDP‐43‐binding affinity (Figs 1 and 2). Our findings indicate that: (i) the ability of RNA to regulate TDP‐43 phase separation depends on sequence‐specific interactions between the RNA‐binding domains of the protein and RNA; and (ii) the size of the TDP‐43‐binding region is a strong determinant of TDP‐43 LLPS. These results suggest that we may predict TDP‐43 LLPS based on the sequence composition of RNA targets. Furthermore, this may provide insight into how TDP‐43 function is modulated depending on whether or not phase separation is involved. These conclusions are supported by reports published while this manuscript was being revised. The group led by Jernej Ule found that specific RNA sequences in the transcriptome and TDP‐43 condensation are tightly linked, affecting RNA processing function of the transcripts involved (Hallegger et al, 2021). Among these RNA regions are long GU‐rich sequences and the 3’ UTR of Tardbp, which contains CLIP34. In addition, Rengifo‐Gonzalez et al present data suggesting that long GU‐repeat RNA binds multiple TDP‐43 molecules cooperatively and that these interactions are important to maintain TDP‐43 solubility (Rengifo‐Gonzalez et al, 2021).
To further investigate whether the RNA‐driven condensation is mediated by multivalent interactions, which characterize LLPS, we disrupted domains that mediate TDP‐43 oligomerization and self‐assembly. Mutations either in the amino terminal or carboxyl low complexity domain block droplet formation in the presence of RNA, under the conditions tested (Fig 5). While we observed no LLPS of these mutants in these conditions, it is important to note that at different protein, salt, or RNA concentrations, it is certainly possible that these constructs would enter into a two‐phase regime. In addition, the ALS‐associated mutations A321G and M337V, which affect a conserved region in the CTD (Conicella et al, 2016, 2020; Schmidt & Rohatgi, 2016), show reduced liquid properties of the RNA‐mediated complexes (Figs 6 and 7A). Like the mutant fragments, the ALS mutations do not disrupt GU‐rich RNA‐binding affinity (Table 1), but impair self‐assembly and accelerate aggregation (Johnson et al, 2009; Conicella et al, 2016; French et al, 2019). These results underscore the importance of the interactions mediated by this C‐terminal region during RNA‐triggered condensation. We propose a model in Fig 7B whereby GU‐rich RNA or RNA sequences that bind multiple TDP‐43 molecules (orange lines) act as a scaffold bringing multiple TDP‐43 molecules in close proximity. This spatial organization may increase formation of the meshwork through N‐terminal and CTD‐mediated interactions highlighted in our model (Fig 7B). These interactions constitute a multivalent network by increasing the number of crosslinks between RNA‐bound TDP‐43. This is in contrast with conditions in which RNA molecules (blue lines) do not significantly interact with TDP‐43 and fail to concentrate the protein. In this scenario, NTD and CTD interactions may still occur, however, the number of possible linked interactions is considerably less.
Figure 7. RNA binding is a key driver of TDP‐43 phase separation maintaining liquid properties of the dense state.
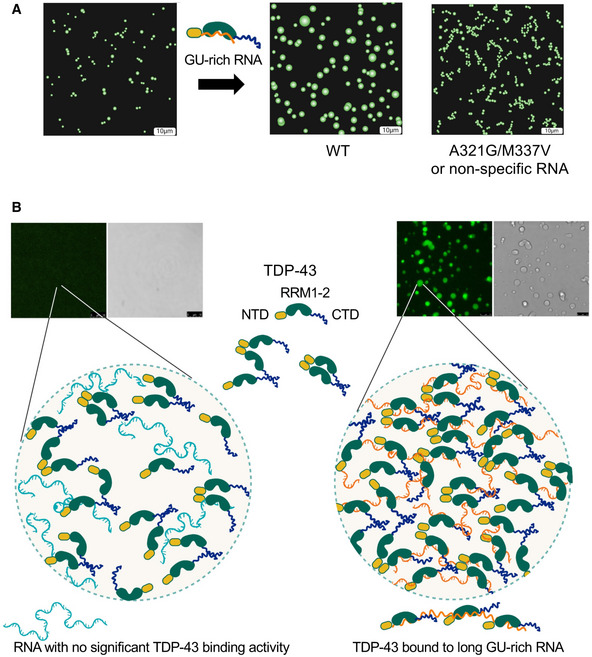
- Binding of TDP‐43 to GU‐rich RNA promotes liquid properties of TDP‐43 condensates, as shown by the fusion of small condensates into larger droplets. This fluidity is impaired by the loss of RNA‐binding affinity or disease‐linked mutations that alter phase separation, such as M337V and A321G, which could explain increased TDP‐43 aggregation.
- RNA molecules that bind multiple TDP‐43 molecules (orange lines) provide a scaffold and increase multivalent interactions, including N‐ and C‐terminal domains, creating a meshwork that results in phase separation. In contrast, nonspecific RNA (blue lines) fails to sufficiently concentrate TDP‐43 to generate the multivalent network necessary for condensation. Scale bars, 10 μm.
We and others have shown that RNA exerts chaperone‐like functions which increase solubility of RNA‐binding proteins in cells (Maharana et al, 2018), including in the case of TDP‐43 (French et al, 2019; Mann et al, 2019). Here, we find that RNA maintains the liquid properties within the droplets, decreasing maturation into solid‐like assemblies (Fig 1B). This RNA activity is dependent on specific RNA binding and RNA composed of a single TDP‐43‐binding site, A(GU)6, is sufficient to increase the liquid properties of TDP‐43 droplets (Fig 1B). This observation is in agreement with our previous findings that A(GU)6 is a potent inhibitor of TDP‐43 aggregation using the purified protein (French et al, 2019). Therefore, we postulate that the RNA‐bound state stabilizes the RRMs of TDP‐43 in soluble conformations preventing the initial steps of protein aggregation that we previously showed to be triggered by RRM misfolding (French et al, 2019). These findings may provide insight into TDP‐43 proteostasis in cells, particularly in the cytoplasm where TDP‐43 pathology is predominantly found (Arai et al, 2006; Neumann et al, 2006). The pathological inclusions in the cytoplasm may result from decreased RNA levels in this compartment relative to the nucleus, as previously suggested (Maharana et al, 2018). Thus, RNA binding and recruitment to RNP granules may play a key role in maintaining soluble TDP‐43 particularly in the cytoplasm. Cytosolic stress granules, which have been viewed as crucibles for TDP‐43 pathology may actually be protective in preventing TDP‐43 aggregation as they accumulate high levels of RNA molecules. This is supported by findings showing that TDP‐43 aggregation is increased when the protein is excluded from stress granules (McGurk et al, 2018; Gasset‐Rosa et al, 2019). Based on our work, we predict that the dynamic properties and reversibility of stress granules and other ribonucleoprotein granules, such as axonal RNA transport granules (Gopal et al, 2017), depend on specific TDP‐43‐RNA interactions.
In summary, our findings provide evidence for a model in which RNA binding drives TDP‐43 phase separation and that this activity is modulated by the number of proximal protein‐binding sites (Fig 7B). These findings carry important implications on the mechanisms by which TDP‐43 controls RNA metabolism. Furthermore, our work lays the foundation for the development of new therapeutic strategies that utilize RNA molecules to alter TDP‐43 phase properties and regulate protein homeostasis.
Materials and Methods
Reagents were purchased from Sigma unless otherwise noted. All experiments, unless otherwise noted were performed at room temperature (approximately 22°C).
Expression and purification of recombinant TDP‐43
Recombinant His6‐SUMO N‐terminally tagged TDP‐43 was expressed in BL21(DE3) Escherichia coli cells from the SUMO‐TDP43 plasmid (pET28b(+)‐SUMO‐TDP43; Li et al, 2017). Mutants were generated by QuickChange site‐directed mutagenesis (Agilent) using DNA oligonucleotides listed in Appendix Table S2. The protocol for protein preparation was modified from previously described methods (Li et al, 2017). Briefly, induction was carried out with 0.3 mM of isopropyl β‐D‐thiogalactopyranoside (IPTG) and cells were grown at 16°C for 16 h. Cell pellets were resuspended in lysis buffer (1 M of NaCl, 50 mM of HEPES, pH 7.5, 2% TRITON X‐100, 5% glycerol, SIGMAFAST EDTA‐free protease inhibitor cocktail, 2.5 μg/ml of lysozyme, 10 μg/ml of DNase I, 1 mM (tris(2‐carboxyethyl)phosphine) (TCEP)). Lysates were centrifuged for 20 min at 20,000 g at 4°C following sonication on ice. Protein was bound to nickel‐nitrilotriacetic acid resin (McLab), and the resin was loaded into a column for purification (AKTA‐Start, GE Healthcare). The column was washed with wash buffer 1 (500 mM of NaCl, 50 mM of HEPES, pH 7.5, 5% glycerol, 1 mM of TCEP), followed by wash buffer 2 (500 mM of NaCl, 50 mM of HEPES, pH 7.5, 5% glycerol, 50 mM of Imidazole, 1 mM of TCEP), and eluted in elution buffer (500 mM of NaCl, 50 mM of HEPES, pH 7.5, 5% glycerol, 300 mM of Imidazole, 5 mM of DTT). Protein was flash frozen in liquid nitrogen and stored in aliquots at −80°C.
Purification of untagged TDP‐43
This protocol was modified from previously described methods (Li et al, 2017; French et al, 2019). As above, His6‐SUMO‐TDP‐43 was expressed in BL21(DE3) Escherichia coli cells. Cells were lysed in lysis buffer. Lysates were sonicated and centrifuged. Protein was bound to nickel‐nitrilotriacetic acid resin, washed with wash buffer 1, wash buffer 2, and again with wash buffer 1. Recombinant His6‐ULP1 was added to the protein‐bound resin and incubated at room temperature for 30 min. The supernatant containing TDP‐43 was collected.
Oligonucleotide development
All oligonucleotides were modified with a 2’OMe group as well as a phosphorothioate backbone unless otherwise noted. Oligonucleotides were synthesized and modified by Integrated DNA Technologies (IDT).
LLPS assay
Thawed recombinant protein was centrifuged for 35 min at 98,400 g at 4°C to remove any preformed aggregates and was diluted to the required starting concentration using elution buffer. LLPS buffer (50 mM of HEPES, pH 7.5, 5% glycerol, 5 mM of DTT) containing appropriate NaCl and RNA concentrations was spotted onto a Lab‐Tek Chambered #1.0 borosilicate cover glass slide, followed by the addition of protein. Droplets were imaged by brightfield and fluorescence microscopy using a Leica TCS SP5 confocal microscope with an HCX APO U‐V‐I 100.0×/1.30 oil immersion objective and 2.5× digital zoom at room temperature. For experiments involving fluorescently labeled protein, recombinant protein was labeled with Oregon Green 488 or Cy3 maleimide dye (Thermo Fisher Scientific, OG‐TDP‐43, Cy3‐TDP‐43) according to manufacturer's protocol, purified with Zeba Spin columns (Thermo Fisher Scientific), and mixed with unlabeled protein in a ratio of 1:10. For experiments involving fluorescently labeled RNA, Alexa Fluor 594‐labeled RNA was mixed with unlabeled RNA in a ratio of 1:10. Images were taken approximately 45 min after beginning the reaction, unless otherwise noted. OG‐TDP‐43 was excited using a 488 nm laser line, Cy3‐TDP‐43 was excited using a 514 nm laser line, and labeled RNA was excited using a 543 nm laser line. For droplet area measurements, droplet regions of interest were manually selected and analyzed using ImageJ. Fluorescent channel intensity values were universally increased within experiments by the same amount for each image, and brightfield images were linearly contrasted using Leica LAS AF software.
Fluorescence recovery after photobleaching
TDP‐43 and RNA liquid droplets were first generated as described above. Ten baseline images were taken in 0.376 s intervals, and ROIs were bleached for 3.76 s using 30% laser power from a 488 nm laser line. Eleven post‐bleach images were then taken in 0.376 s intervals, followed by 4 images taken in 20 s intervals. Data points were normalized using the equation where Fr(t) is fluorescence recovery at a given time point, I(t) is intensity at a given time point, Ibleach is the intensity immediately following bleaching, and Ipre is the prebleach intensity.
High salt droplet reversal assay
TDP‐43/RNA droplets were formed as described above. As soon as droplets began to settle on the glass, high salt buffer (4 M of NaCl, 50 mM of HEPES, pH 7.5, 5% glycerol, 5 mM of DTT) was added to a final NaCl concentration of 1.3 M in order to promote dissolution of condensates.
Turbidity assay
Thawed recombinant protein was centrifuged for 35 min at 98,400 g at 4°C and was diluted to the required starting concentration using elution buffer. LLPS buffer containing appropriate RNA concentration was added to clear 96‐well clear‐bottom plates (Greiner Bio‐One), followed by the addition of protein. Plates were read at room temperature for 1 h using a SpectraMax i3 microplate reader (Molecular Devices). Turbidity was measured as maximum absorbance at 600 nm λ.
Measurement of light phase TDP‐43 concentration
Thawed recombinant protein was centrifuged for 35 min at 98,400 g at 4°C and was diluted to the required starting concentration using elution buffer. LLPS buffer containing appropriate RNA concentration was mixed with protein and incubated for 30 min. The samples were centrifuged at 21,000 g for 10 min to pellet the dense phase. The concentration of protein in the supernatant, or light phase (Cout ), was then measured by Bradford assay using a Nanodrop spectrophotometer.
Fluorescence anisotropy assay
Recombinant TDP‐43 wild‐type (WT) and constructs previously purified were serially diluted in a 1:2 ratio (from 75 to 0 nM) into a 300 mM of NaCl, 10 mM of Tris (pH 8.0), 5% glycerol, 5% sucrose, 1 mM of TCEP buffer solution. A(GU)6 RNA labeled with fluorescein isothiocyanate (FITC) from IDT was used at a final concentration of 0.6, 1.3, 2.5, and 5 nM. The protein preparations were mixed with A(GU)6‐FITC and added in triplicate in a 384‐well black flat bottom plate (Corning) protected from light. The anisotropy measurements were performed in a Biotek SYNERGY Neo2 (BioSPX) plate reader with excitation and emission wavelengths of 480 and 520 nm, respectively. To assess the binding curves, the data were fitted as previously described (Li et al, 2017). Data analysis was performed using GraphPad Prism 5.
Electromobility shift assay
The EMSA protocol was adapted from Flores et al (2019). Briefly, purified full‐length TDP‐43 was centrifuged 98,400 g, 30 min, 4°C. Varying protein (0–7 μM range) was incubated with 10 nM of CLIP‐34 oligonucleotide labeled with 5,700 nm IR, 5’ minutes on ice and 25’ at room temperature in binding buffer (150 mM of NaCl; 10 mM of Tris pH 8; 2 mM of MgCl2; 5% glycerol; 1 mM of DTT). Electrophoresis of 6% polyacrylamide gels was performed at 100 V. Images were acquired using the LI‐COR Odyssey platform.
Cell constructs
HA‐mEGFP‐TDP constructs were created by subcloning TDP‐43 cDNA into the mEGFP‐C1 vector (Addgene) using the restriction enzymes XhoI and HindIII. The resulting mEGFP‐TDP‐43 gene was then subcloned into the pCDNA5 (Thermo) vector using the restriction enzymes EcoRV and NotI downstream of the HA‐tag.
Cell culture and induction of mEGFP‐tagged TDP‐43
HEK Flp‐In™ T‐REX™ 293 cells (Thermo) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum. Stable incorporation of mEGFP‐TDP‐43 was achieved using the pcDNA™5/FRT/TO Vector system (Thermo). Cells were plated in cell‐culture treated 10‐cm dishes. Expression of GFP‐tagged TDP‐43 protein was induced at 70% confluency by addition of 1 μg/ml of tetracycline for 24 h prior to harvesting.
Cell collection, lysis, and induction of LLPS using cell lysates
The following protocol was adapted from Freibaum et al (2021). Cells were washed with PBS and pelleted by centrifugation at 300 g for 5 min. PBS was aspirated and cell pellets were frozen at −80°C. Prior to use, pellets were thawed at room temperature for 2 min. 200 µl of lysis buffer (50 mM of HEPES, pH 7.5, 500 mM of NaCl, 0.5% NP‐40, 5% glycerol, 5 mM of DTT, and SigmaFast EDTA‐free protease inhibitor cocktail) was added to cell pellets. Pellets were lysed by pipetting up and down and transferred to Eppendorf tubes, incubated for 3 min at room temperature, centrifuged at 21,000 g for 5 min at room temperature and the supernatant was collected. Microscopy and turbidity experiments were conducted as described above, with cell lysate as 10% by volume added as the final component.
Author contributions
ZRG, ACSB, TMM, and YMA designed the study; ZRG generated recombinant protein and performed the phase separation analysis. ACSB generated recombinant protein and measured RNA‐binding affinity. LDM generated DNA constructs and mutagenesis and helped with protein purification. RLF helped with the initial solubility experiments and contributed with discussion. YMA wrote the manuscript. ZRG, ACSB, LDM, RLF, TMM, and YMA edited and revised the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Movie EV1
Movie EV2
Movie EV3
Movie EV4
Source Data for Expanded View and Appendix
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Acknowledgments
The authors thank Dr. Alessandro Vindigni for his careful and close reading of the manuscript. This work was supported in part by funding from the National Institutes of Health (NIH) National Institute for Neurological Disorders and Stroke (NINDS) and National Institute for Aging (NIA) R01 NS114289 and NINDS R56 NS105806 to Y.M.A.; the Department of Defense CDMRP/ALSRP W81XWH‐20‐1‐0241 to Y.M.A. and T.M.M.; and the NIH/NINDS R01 NS078398 to T.M.M. Some of the figures were created with BioRender.com.
EMBO reports (2021) 22: e53632.
Data availability
This study includes no data deposited in external repositories.
References
- Afroz T, Hock E‐M, Ernst P, Foglieni C, Jambeau M, Gilhespy LAB, Laferriere F, Maniecka Z, Plückthun A, Mittl P et al (2017) Functional and dynamic polymerization of the ALS‐linked protein TDP‐43 antagonizes its pathologic aggregation. Nat Commun 8: 45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alami N, Smith R, Carrasco M, Williams L, Winborn C, Han S, Kiskinis E, Winborn B, Freibaum B, Kanagaraj A et al (2014) Axonal transport of TDP‐43 mRNA granules is impaired by ALS‐causing mutations. Neuron 81: 536–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y et al (2006) TDP‐43 is a component of ubiquitin‐positive tau‐negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351: 602–611 [DOI] [PubMed] [Google Scholar]
- Ayala YM, De Conti L, Avendaño‐Vázquez SE, Dhir A, Romano M, D'Ambrogio A, Tollervey J, Ule J, Baralle M, Buratti E et al (2011) TDP‐43 regulates its mRNA levels through a negative feedback loop. EMBO J 30: 277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala YM, Misteli T, Baralle FE (2008) TDP‐43 regulates retinoblastoma protein phosphorylation through the repression of cyclin‐dependent kinase 6 expression. Proc Natl Acad Sci USA 105: 3785–3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala YM, Pagani F, Baralle FE (2006) TDP43 depletion rescues aberrant CFTR exon 9 skipping. FEBS Lett 580: 1339–1344 [DOI] [PubMed] [Google Scholar]
- Ayala YM, Pantano S, D'Ambrogio A, Buratti E, Brindisi A, Marchetti C, Romano M, Baralle FE (2005) Human, Drosophila, and C.elegans TDP43: nucleic acid binding properties and splicing regulatory function. J Mol Biol 348: 575–588 [DOI] [PubMed] [Google Scholar]
- Baumer D, Parkinson N, Talbot K (2009) TARDBP in amyotrophic lateral sclerosis: identification of a novel variant but absence of copy number variation. J Neurol Neurosurg Psychiatry 80: 1283–1285 [DOI] [PubMed] [Google Scholar]
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Julicher F, Hyman AA (2009) Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324: 1729–1732 [DOI] [PubMed] [Google Scholar]
- Buratti E, Baralle FE (2001) Characterization and functional implications of the RNA binding properties of nuclear factor TDP‐43, a novel splicing regulator of CFTR exon 9. J Biol Chem 276: 36337–36343 [DOI] [PubMed] [Google Scholar]
- Buratti E, Baralle FE (2010) The multiple roles of TDP‐43 in pre‐mRNA processing and gene expression regulation. RNA Biol 7: 420–429 [DOI] [PubMed] [Google Scholar]
- Buratti E, Brindisi A, Pagani F, Baralle FE (2004) Nuclear factor TDP‐43 binds to the polymorphic TG repeats in CFTR intron 8 and causes skipping of exon 9: a functional link with disease penetrance. Am J Hum Genet 74: 1322–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H‐J, Topp SD, Hui HS, Zacco E, Katarya M, McLoughlin C, King A, Smith BN, Troakes C, Pastore A et al (2019) RRM adjacent TARDBP mutations disrupt RNA binding and enhance TDP‐43 proteinopathy. Brain 142: 3753–3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chio A, Borghero G, Pugliatti M, Ticca A, Calvo A, Moglia C, Mutani R, Brunetti M, Ossola I, Marrosu MG et al (2011) Large proportion of amyotrophic lateral sclerosis cases in Sardinia due to a single founder mutation of the TARDBP gene. Arch Neurol 68: 594–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conicella AE, Dignon GL, Zerze GH, Schmidt HB, D'Ordine AM, Kim YC, Rohatgi R, Ayala YM, Mittal J, Fawzi NL (2020) TDP‐43 alpha‐helical structure tunes liquid‐liquid phase separation and function. Proc Natl Acad Sci USA 117: 5883–5894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conicella AE, Zerze GH, Mittal J, Fawzi NL (2016) ALS mutations disrupt phase separation mediated by α‐helical structure in the TDP‐43 low‐complexity C‐terminal domain. Structure 24: 1537–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey CM, Cenik B, Sephton CF, Johnson BA, Herz J, Yu G (2012) TDP‐43 aggregation in neurodegeneration: Are stress granules the key? Brain Res 1462: 16–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM, Kriwacki RW, Pappu RV, Brangwynne CP (2016) Coexisting liquid phases underlie nucleolar subcompartments. Cell 165: 1686–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores BN, Li X, Malik AM, Martinez J, Beg AA, Barmada SJ (2019) An intramolecular salt bridge linking TDP43 RNA binding, protein stability, and TDP43‐dependent neurodegeneration. Cell Rep 27: 1133–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freibaum BD, Messing J, Yang P, Kim HJ, Taylor JP (2021) High‐fidelity reconstitution of stress granules and nucleoli in mammalian cellular lysate. J Cell Biol 220: e202009079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French RL, Grese ZR, Aligireddy H, Dhavale DD, Reeb AN, Kedia N, Kotzbauer PT, Bieschke J, Ayala YM (2019) Detection of TAR DNA‐binding protein 43 (TDP‐43) oligomers as initial intermediate species during aggregate formation. J Biol Chem 294: 6696–6709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Ikeda M, Yanagisawa T, Senoo Y, Okamoto K (2011) Different clinical and neuropathologic phenotypes of familial ALS with A315E TARDBP mutation. Neurology 77: 1427–1431 [DOI] [PubMed] [Google Scholar]
- Garcia‐Jove Navarro M, Kashida S, Chouaib R, Souquere S, Pierron G, Weil D, Gueroui Z (2019) RNA is a critical element for the sizing and the composition of phase‐separated RNA‐protein condensates. Nat Commun 10: 3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasset‐Rosa F, Lu S, Yu H, Chen C, Melamed Z, Guo L, Shorter J, Da Cruz S, Cleveland DW (2019) Cytoplasmic TDP‐43 de‐mixing independent of stress granules drives inhibition of nuclear import, loss of nuclear TDP‐43, and cell death. Neuron 102: 339–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitcho MA, Baloh RH, Chakraverty S, Mayo K, Norton JB, Levitch D, Hatanpaa KJ, White CL III, Bigio EH, Caselli R et al (2008) TDP‐43 A315T mutation in familial motor neuron disease. Ann Neurol 63: 535–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal PP, Nirschl JJ, Klinman E, Holzbaur EL (2017) Amyotrophic lateral sclerosis‐linked mutations increase the viscosity of liquid‐like TDP‐43 RNP granules in neurons. Proc Natl Acad Sci USA 114: E2466–E2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallegger M, Chakrabarti AM, Lee FCY, Lee BL, Amalietti AG, Odeh HM, Copley KE, Rubien JD, Portz B, Kuret K et al (2021) TDP‐43 condensation properties specify its RNA‐binding and regulatory repertoire. Cell 184: 4680–4696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa K, Nobe Y, Ishikawa H, Yamauchi Y, Taoka M, Sato K, Nakayama H, Simpson RJ, Isobe T, Takahashi N (2019) TDP‐43 regulates site‐specific 2'‐O‐methylation of U1 and U2 snRNAs via controlling the Cajal body localization of a subset of C/D scaRNAs. Nucleic Acids Res 47: 2487–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BS, Snead D, Lee JJ, McCaffery JM, Shorter J, Gitler AD (2009) TDP‐43 is intrinsically aggregation‐prone, and amyotrophic lateral sclerosis‐linked mutations accelerate aggregation and increase toxicity. J Biol Chem 284: 20329–20339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura A, Shibasaki A, Takeda K, Suno R, Kinjo M (2018) Analysis of the substrate recognition state of TDP‐43 to single‐stranded DNA using fluorescence correlation spectroscopy. Biochem Biophys Rep 14: 58–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama A, Sugai A, Kato T, Ishihara T, Shiga A, Toyoshima Y, Koyama M, Konno T, Hirokawa S, Yokoseki A et al (2016) Increased cytoplasmic TARDBP mRNA in affected spinal motor neurons in ALS caused by abnormal autoregulation of TDP‐43. Nucleic Acids Res 44: 5820–5836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo PH, Doudeva LG, Wang YT, Shen CK, Yuan HS (2009) Structural insights into TDP‐43 in nucleic‐acid binding and domain interactions. Nucleic Acids Res 37: 1799–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Banjade S, Cheng H‐C, Kim S, Chen B, Guo L, Llaguno M, Hollingsworth JV, King DS, Banani SF et al (2012) Phase transitions in the assembly of multivalent signalling proteins. Nature 483: 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Reeb AN, Lin B, Subramanian P, Fey EE, Knoverek CR, French RL, Bigio EH, Ayala YM (2017) Heat shock‐induced phosphorylation of TAR DNA‐binding protein 43 (TDP‐43) by MAPK/ERK kinase regulates TDP‐43 function. J Biol Chem 292: 5089–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Protter DS, Rosen MK, Parker R (2015) Formation and maturation of phase‐separated liquid droplets by RNA‐binding proteins. Mol Cell 60: 208–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling JP, Pletnikova O, Troncoso JC, Wong PC (2015) TDP‐43 repression of nonconserved cryptic exons is compromised in ALS‐FTD. Science 349: 650–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukavsky PJ, Daujotyte D, Tollervey JR, Ule J, Stuani C, Buratti E, Baralle FE, Damberger FF, Allain FH (2013) Molecular basis of UG‐rich RNA recognition by the human splicing factor TDP‐43. Nat Struct Mol Biol 20: 1443–1449. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Nicholson AM, Sarkar M, Messing J, Purice MD, Pottier C, Annu K, Baker M, Perkerson RB, Kurti A et al (2017) TIA1 mutations in amyotrophic lateral sclerosis and frontotemporal dementia promote phase separation and alter stress granule dynamics. Neuron 95: 808–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharana S, Wang J, Papadopoulos DK, Richter D, Pozniakovsky A, Poser I, Bickle M, Rizk S, Guillén‐Boixet J, Franzmann TM et al (2018) RNA buffers the phase separation behavior of prion‐like RNA binding proteins. Science 360: 918–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JR, Gleixner AM, Mauna JC, Gomes E, DeChellis‐Marks MR, Needham PG, Copley KE, Hurtle B, Portz B, Pyles NJ et al (2019) RNA binding antagonizes neurotoxic phase transitions of TDP‐43. Neuron 102: 321–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk L, Gomes E, Guo L, Mojsilovic‐Petrovic J, Tran V, Kalb RG, Shorter J, Bonini NM (2018) Poly(ADP‐Ribose) prevents pathological phase separation of TDP‐43 by promoting liquid demixing and stress granule localization. Mol Cell 71: 703–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW, Perl DP, Hedley‐Whyte ET, Price B, Sullivan C et al (2010) TDP‐43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol 69: 918–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed Ze’ev, López‐Erauskin J, Baughn MW, Zhang O, Drenner K, Sun Y, Freyermuth F, McMahon MA, Beccari MS, Artates JW et al (2019) Premature polyadenylation‐mediated loss of stathmin‐2 is a hallmark of TDP‐43‐dependent neurodegeneration. Nat Neurosci 22: 180–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modic M, Grosch M, Rot G, Schirge S, Lepko T, Yamazaki T, Lee FCY, Rusha E, Shaposhnikov D, Palo M et al (2019) Cross‐Regulation between TDP‐43 and Paraspeckles Promotes Pluripotency‐Differentiation Transition. Mol Cell 74: 951–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, Mittag T, Taylor JP (2015) Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163: 123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mompean M, Romano V, Pantoja‐Uceda D, Stuani C, Baralle FE, Buratti E, Laurents DV (2017) Point mutations in the N‐terminal domain of transactive response DNA‐binding protein 43 kDa (TDP‐43) compromise its stability, dimerization, and functions. J Biol Chem 292: 11992–12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, Rademakers R, Alafuzoff I, Attems J, Brayne C et al (2019) Limbic‐predominant age‐related TDP‐43 encephalopathy (LATE): consensus working group report. Brain 142: 1503–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM et al (2006) Ubiquitinated TDP‐43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314: 130–133 [DOI] [PubMed] [Google Scholar]
- Patel A, Lee H, Jawerth L, Maharana S, Jahnel M, Hein M, Stoynov S, Mahamid J, Saha S, Franzmann T et al (2015) A liquid‐to‐solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162: 1066–1077 [DOI] [PubMed] [Google Scholar]
- Polymenidou M, Lagier‐Tourenne C, Hutt KR, Huelga SC, Moran J, Liang TY, Ling S‐C, Sun E, Wancewicz E, Mazur C et al (2011) Long pre‐mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP‐43. Nat Neurosci 14: 459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengifo‐Gonzalez JC, El Hage K, Clément M‐J, Steiner E, Joshi V, Craveur P, Durand D, Pastré D, Bouhss A (2021) The cooperative binding of TDP‐43 to GU‐rich RNA repeats antagonizes TDP‐43 aggregation. eLife 10.7554/elife.67605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rot G, Wang Z, Huppertz I, Modic M, Lence T, Hallegger M, Haberman N, Curk T, von Mering C, Ule J (2017) High‐resolution RNA maps suggest common principles of splicing and polyadenylation regulation by TDP‐43. Cell Rep 19: 1056–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford NJ, Zhang Y‐J, Baker M, Gass JM, Finch NA, Xu Y‐F, Stewart H, Kelley BJ, Kuntz K, Crook RJP et al (2008) Novel mutations in TARDBP (TDP‐43) in patients with familial amyotrophic lateral sclerosis. PLoS Genet 4: e1000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HB, Rohatgi R (2016) In vivo formation of vacuolated multi‐phase compartments lacking membranes. Cell Rep 16: 1228–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E et al (2008) TDP‐43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319: 1668–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoka A, Arai M, Itokawa M, Arai T, Hasegawa M, Tsuchiya K, Takuma H, Tsuji H, Ishii A, Watanabe M et al (2010) TDP‐43 M337V mutation in familial amyotrophic lateral sclerosis in Japan. Intern Med 49: 331–334 [DOI] [PubMed] [Google Scholar]
- Tollervey JR, Curk T, Rogelj B, Briese M, Cereda M, Kayikci M, König J, Hortobágyi T, Nishimura AL, Župunski V et al (2011) Characterizing the RNA targets and position‐dependent splicing regulation by TDP‐43. Nat Neurosci 14: 452–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udan‐Johns M, Bengoechea R, Bell S, Shao J, Diamond MI, True HL, Weihl CC, Baloh RH (2014) Prion‐like nuclear aggregation of TDP‐43 during heat shock is regulated by HSP40/70 chaperones. Hum Mol Genet 23: 157–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Conicella AE, Schmidt HB, Martin EW, Rhoads SN, Reeb AN, Nourse A, Ramirez Montero D, Ryan VH, Rohatgi R et al (2018) A single N‐terminal phosphomimic disrupts TDP‐43 polymerization, phase separation, and RNA splicing. EMBO J 37: e97452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y‐T, Kuo P‐H, Chiang C‐H, Liang J‐R, Chen Y‐R, Wang S, Shen JCK, Yuan HS (2013) The truncated C‐terminal RNA recognition motif of TDP‐43 protein plays a key role in forming proteinaceous aggregates. J Biol Chem 288: 9049–9057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihl CC, Pestronk A, Kimonis VE (2009) Valosin‐containing protein disease: inclusion body myopathy with Paget's disease of the bone and fronto‐temporal dementia. Neuromuscul Disord 19: 308–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JA, Mito M, Kurosaka S, Takumi T, Tanegashima C, Chujo T, Yanaka K, Kingston RE, Hirose T, Bond C et al (2016) Structural, super‐resolution microscopy analysis of paraspeckle nuclear body organization. J Cell Biol 214: 817–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wippich F, Bodenmiller B, Trajkovska MG, Wanka S, Aebersold R, Pelkmans L (2013) Dual specificity kinase DYRK3 couples stress granule condensation/dissolution to mTORC1 signaling. Cell 152: 791–805 [DOI] [PubMed] [Google Scholar]
- Wood M, Quinet A, Lin YL, Davis AA, Pasero P, Ayala YM, Vindigni A (2020) TDP‐43 dysfunction results in R‐loop accumulation and DNA replication defects. J Cell Sci 133: jcs244129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Elbaum‐Garfinkle S, Langdon EM, Taylor N, Occhipinti P, Bridges AA, Brangwynne CP, Gladfelter AS (2015) RNA controls PolyQ protein phase transitions. Mol Cell 60: 220–230 [DOI] [PMC free article] [PubMed] [Google Scholar]


