Abstract
Background:
Ventricular assist devices (VAD) simulation-based mastery learning (SBML) results in better patient and caregiver self-care skills compared to usual-training.
Objective:
To evaluate the effect of SBML on driveline exit site infections.
Methods:
We compared the probability of remaining infection free at 3 and 12 months between patients randomized to SBML- or usual-training.
Results:
The SBML-training group had no infections at 3 months and 2 at 12 months, yielding a Kaplan-Meier estimate of the probability of remaining infection free of .857 (95% CI 0.692–1.00) at 12 months. The usual-training group had 6 infections at 3 months with no additional infections by 12 months. Kaplan-Meier estimates of remaining infection free at 3 and 12 months were 0.878 (95% CI 0.758–1.00) and 0.748 (95% CI 0.591–0.946), respectively. Time-to-infection distributions for SBML- vs. usual-training showed a difference in 12-month infection rates of 0.109 (p=0.07).
Conclusions:
VAD self-care SBML resulted in fewer 12-month infections.
Trial Registration:
INTRODUCTION
Heart Failure (HF) affects over 5 million U.S. adults, with 825,000 incident cases annually.1 Advanced HF occurs in 50,000 to 100,000 patients annually, with a two-year survival of 13 to 40%.2,3 Approximately 2,500 heart transplants are performed each year in North America due to a fixed supply of available donor organs.4 Ventricular assist device (VAD) implantation is an alternative surgical option for patients with advanced HF. VADs are surgically implanted and connect to the heart ventricle and aorta to help pump blood to the body. A driveline from the VAD pump passes through the skin and connects to a system controller that is connected to power.
Driveline exit site (DLES) infection is a serious complication of ventricular assist device (VAD) therapy.5 DLES infections are the most common type of VAD-related complication,6 occurring because the DLES creates a conduit for bacterial entry. VAD DLES infections can be local or systemic (i.e., bacteremia), and can lead to strokes and VAD failure.7 VAD self-care is critical to prevent infections, as wound healing around the driveline can take up to 1 month. Thus, maintaining a sterile dressing at the DLES is important to prevent infection. Nationally, the 12 month DLES infection rate approaches 20%, which partially accounts for the post VAD implantation 30-day readmission rate of ~30%.8–10 Strategies to reduce DLES infections include improved surgical techniques, anchoring devices to reduce trauma, and driveline self-care education.11 However, VAD self-care training is highly variable due to time, availability, and lack of standardization.
The authors use VAD self-care simulation-based mastery learning (SBML) to train patients and caregivers at their own institution.12,13 SBML is a rigorous form of competency-based education featuring deliberate practice and individualized feedback. In SBML, all learners must meet or exceed a minimum passing standard (MPS) before completing training. Several studies have shown that SBML for physicians in training improves clinical skills and patient outcomes in advanced cardiac life support,14,15 thoracentesis,16 paracentesis,17 laparoscopic common bile duct exploration,18 and central venous catheter (CVC) insertion.19,20 An SBML intervention used to train internal medicine and emergency medicine residents on CVC insertion showed that patients who had CVCs inserted by SBML-trained residents had an 84% reduction in central line-associated bloodstream infections (CLABSI) compared to patients who had catheters inserted by “traditionally-trained” residents (who learned the techniques vicariously).19 However, to date, no SBML training inteventions have demonstrated improved patient outcomes for patients or their caregivers.
In a randomized controlled trial (RCT), patients and caregivers completing VAD self-care SBML had significantly better skills at discharge from the initial hospitalization for VAD implant than those receiving usual training.12 As an exploratory aim, we evaluated the effect of SBML on DLES infections given SBML had previously been shown to reduce CLABSI. We hypothesized that more patients would be infection free at 3 and 12 months in the VAD self-care SBML group compared to the usual-training group.
METHODS
Study Design
The authors report secondary analyses from an RCT conducted at a large volume VAD implantation center from June 2017-July 2020.3 We compared the probability of remaining DLES infection free at 3 and 12 months after VAD implant hospitalization between patients randomized to SBML or usual training. The Northwestern University Institutional Review Board approved this study.
Participants
Patients who received a VAD implant (HeartWare™, HeartMate II™, or HeartMate 3™) and their caregivers were eligible to participate in the RCT.3 After providing written informed consent, patients and their caregivers were randomized (1:1) to the SBML- or usual-trained group. Patients were followed for at least one year after VAD implant hospitalization discharge and censored at time of death, transplant, or voluntarily withdrawal. Of note caregivers were included in the study because they are responsible for performing DLES dressing changes on patients.
Procedure
All participants randomized to SBML took a pretest on controller, power source, and dressing changes; watched videos; participated in deliberate practice on a VAD simulator; and were required to meet or exceed the MPS for each skill at posttest.3 Participants who did not meet the MPS at initial posttest participated in more deliberate practice until they met this standard at retesting. The usual-trained group received a program-approved VAD self-care training protocol, which did not include formal pre- or post-testing.3
Measurement
Sociodemographic and clinical characteristics of participants were collected in the original RCT. Sociodemographic data included age, sex, race, ethnicity, marital status, number of children, maximum education level achieved, employment status, and medical insurance type (patient only). Clinical data included the patient’s body mass index (BMI), VAD type (HeartWare™ or HeartMate II ™ and HeartMate 3 ™ devices), implant strategy (bridge to transplant or destination therapy), ventricle(s) supported, reason for implant, Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profile, New York Heart Association (NYHA) class, glomerular filtration rate, and presence or absence of diabetes or lung disease.
Electronic medical records (EMR) were evaluated by two independent reviewers (RSH, VS; VS was blinded to group assignment) to determine if patients met the International Society for Heart and Lung Transplantation definition of DLES infection at least a year after VAD implant hospitalization discharge (unless the patient met censorship criteria earlier).21,22 This methodology is consistent with the standard reporting of DLES infections.23,24
Analysis
Time-to-infection distributions were estimated using Kaplan-Meier curves, as were probabilities of infection occurring specifically after 3- and 12-month time points with Greenwood’s formula for variance estimation. Time-to-infection distributions were compared across treatment groups using a logrank test. Observations were censored on September 28, 2020 for each study participant if infection was not observed and they did not meet censorship criteria earlier. As evaluating DLES infections was an exploratory aim, the original RCT was not powered to evaluate between-group differences. Exploratory analyses were also performed using logrank tests to examine associations between time-to-infection and sociodemographic and clinical variables including sex, race (African-American vs. white; Asian and American Indian not evaluated due to small sample size), ethnicity (Hispanic/Latinx vs. non-Hispanic/Latinx), marital status, number of children (yes vs. no), maximum education level achieved (>high school vs. ≤high school), employment status, VAD type (HeartWare vs. HeartMate), implant strategy (thoracotomy vs. sternotomy), INTERMACS profile (1, 2 vs. 3, 4), glomerular filtration rate (> 60 vs. <60) and presence or absence of diabetes or lung disease. A Cox proportional hazards model was used to explore the association between age and BMI as continuous variables and time-to-infection. A multivariate Cox model including sex and treatment arm was used to examine adjusted associations with time-to-infection since age was the only statically significant variable in the logrank tests. Nominal p<0.05 was used to indicate statistical significance for these exploratory analyses.
RESULTS
Twenty-six patients and caregivers completed the SBML intervention while 27 completed usual-training during VAD implant hospitalization. Two patient and caregiver pairs in each group were lost to follow-up leaving 24 assigned to the SBML intervention and 25 assigned to usual-training in the final analysis. Patient and caregiver demographic and clinical information were similar between groups as reported in our original RCT.12 Baseline demographics are shown in the Table 1. Patients with VAD were more likely to be male, while caregivers were more likely to be female. More patients and caregivers self-identified as Caucasian, and non-Hispanic. Additionally, more patients were implanted using a destination therapy strategy (i.e., palliative care) as opposed to a bridge to transplant, which is consistent with national rates.8
Table 1.
Demographic and Clinical Information for Patients and Caregivers in the Simulation-based Mastery Learning (SBML)-trained and Usual-trained Groups with Standardized Differences between Groups. Interagency Registry for Mechanically Assisted Circulatory Support = INTERMACS.
| Characteristic | Patients n=49 |
Caregivers n=49 |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| SBML trained n=24 |
Usual trained n=25 |
Standardized difference | SBML trained n=24 |
Usual trained n=25 |
Standardized difference | |
|
| ||||||
| Age, years, mean (SD) | 53.8 (13.8) | 55.6 (13.8) | .275 | 48.5 (14.6) | 54.8(15.0) | .42 |
|
| ||||||
| Sex, no. (%) | .12 | .22 | ||||
| Female | 7 (29%) | 6 (24%) | 18 (75%) | 21 (84%) | ||
| Male | 17 (71%) | 19 (76%) | 6 (25%) | 4 (16%) | ||
|
| ||||||
| Race, no. (%) | .43 | .30 | ||||
| African American/Black | 9 (38%) | 8 (32%) | 9 (38%) | 9 (36%) | ||
| American Indian/Alaskan Native | 1 (4%) | 0 | 1 (4%) | 0 | ||
| Asian | 1 (4%) | 1 (4%) | 1 (4%) | 1 (4%) | ||
| Caucasian/White | 13 (54%) | 16 (64%) | 13 (54%) | 15 (60%) | ||
|
| ||||||
| Ethnicity, no. (%) | .18 | .43 | ||||
| Hispanic or Latino/a | 2 (8%) | 1 (4%) | 2 (8%) | 0 | ||
| Non-Hispanic or Latino/a | 22 (92%) | 24 (96%) | 22 (92%) | 25 (100%) | ||
|
| ||||||
| Marital status, no. (%) | .49 | .81 | ||||
| Married/Partner | 18 (75%) | 15 (60%) | 19 (79%) | 16 (64%) | ||
| Separated/Divorced | 3 (13%) | 6 (24%) | 0 | 3 (12%) | ||
| Single | 3 (13%) | 3 (12%) | 5 (21%) | 5 (20%) | ||
| Widowed | 0 | 1 (4%) | 0 | 1 (4%) | ||
|
| ||||||
| Relationship to patient, no. (%) | .31 | |||||
| Spouse/Partner | 14 (58%) | 14 (56%) | ||||
| Son or daughter | 4 (17%) | 4 (16%) | ||||
| Parent | 1 (4%) | 3 (12%) | ||||
| Sibling | 4 (17%) | 3 (12%) | ||||
| Other | 1 (4%) | 1 (4%) | ||||
|
| ||||||
| Education level, no. (%) | .13 | .56 | ||||
| < High school | 1 (4%) | 1 (4%) | 0 | 0 | ||
| High school graduate | 6 (25%) | 7 (28%) | 6 (25%) | 9 (36%) | ||
| Technical school, some college, or associate degree | 9 (38%) | 10 (40%) | 8 (33%) | 9 (36%) | ||
| Bachelor’s degree | 6 (25%) | 5 (20%) | 8 (33%) | 3 (12%) | ||
| Graduate/Professional degree | 2 (8%) | 1 (4%) | 2 (8%) | 4 (16%) | ||
|
| ||||||
| Work, no. (%) | .41 | .29 | ||||
| Not currently working | 18 (75%) | 14 (56%) | 9 (38%) | 13 (52%) | ||
|
| ||||||
| Home life, no. (%) | .02 | |||||
| Living alone | 3 (13%) | 3 (12%) | ||||
|
| ||||||
| Insurance, no. (%) * | .92 | |||||
| Private | 15 (63%) | 10 (48%) | ||||
| Medicaid | 8 (33%) | 9 (41%) | ||||
| Medicare | 8 (33%) | 12 (55%) | ||||
| Other (Cobra) | 1 (4%) | 0 | ||||
|
| ||||||
| Body Mass Index, mean (SD) | 29.7 (9.0) | 29.9 (8.1) | .02 | |||
|
| ||||||
| Ventricular Assist Device, no. (%) | .27 | |||||
| HeartMate 3™ | 4 (17%) | 2 (8%) | ||||
| HeartWare™ | 20 (83%) | 23 (92%) | ||||
|
| ||||||
| Implant strategy, no. (%) | 31 | |||||
| Bridge to transplant | 8 (33%) | 5 (20%) | ||||
| Destination therapy | 16 (67%) | 20 (80%) | ||||
|
| ||||||
| Ventricle(s) supported, no. (%) | - | |||||
| Left ventricle | 24 (100%) | 25 (100%) | ||||
|
| ||||||
| INTERMACS Profile, no. (%) | .46 | |||||
| 1 | 5 (25%) | 3 (15%) | ||||
| 2 | 11 (46%) | 16 (64%) | ||||
| 3 | 7 (29%) | 6 (24%) | ||||
| 4 | 1 (5%) | 0 | ||||
|
| ||||||
| Etiology of heart failure, no. (%) | .24 | |||||
| Dilated-included non-ischemic | 15 (63%) | 13 (52%) | ||||
| Ischemic | 8 (33%) | 10 (40%) | ||||
| Other | 1 (4%) | 2 (8%) | ||||
|
| ||||||
| New York Heart Class, no. (%) | - | |||||
| Class IV | 24 (100%) | 25 (100%) | ||||
|
| ||||||
| Surgical approach, no. (%) | .06 | |||||
| Sternotomy | 16 (67%) | 16 (64%) | ||||
| Thoracotomy | 8 (33%) | 9 (36%) | ||||
|
| ||||||
| Glomerular Filtration Rate, no (%) | .32 | |||||
| <15 | 0 | 1 (4%) | ||||
| 15–29 | 2 (8%) | 3 (12%) | ||||
| 30–59 | 8 (33%) | 8 (32%) | ||||
| ≥60 | 14 (58%) | 13 (52%) | ||||
|
| ||||||
| Diabetes, no. (%) | 10 (42%) | 14 (56%) | .29 | |||
|
| ||||||
| Lung disease (Chronic obstructive lung disease, emphysema, chronic bronchitis), no. (%) | 4 (17%) | 6 (24%) | .18 | |||
Option to select more than 1 response
EMR reviewers had complete agreement on infection data. In the SBML group, no infections were observed at 3 months and 2 were observed at 12 months, yielding a Kaplan-Meier estimate of the probability of remaining infection free at 12 months of .857 (95% CI .692–1.00; Figure 1). In contrast, the usual-training group had 6 infections at 3 months with no additional infections by 12 months with Kaplan-Meier estimates of probabilities of remaining infection free at 3 and 12 months of .878 (95%CI .758–1.00) and .748 (95% CI .591–.946), respectively (Figure 1). A logrank test showed a difference in time-to-infection distributions for SBML- vs. usual-training in 12-month infection rates of .109 (p=0.07).
Figure 1.
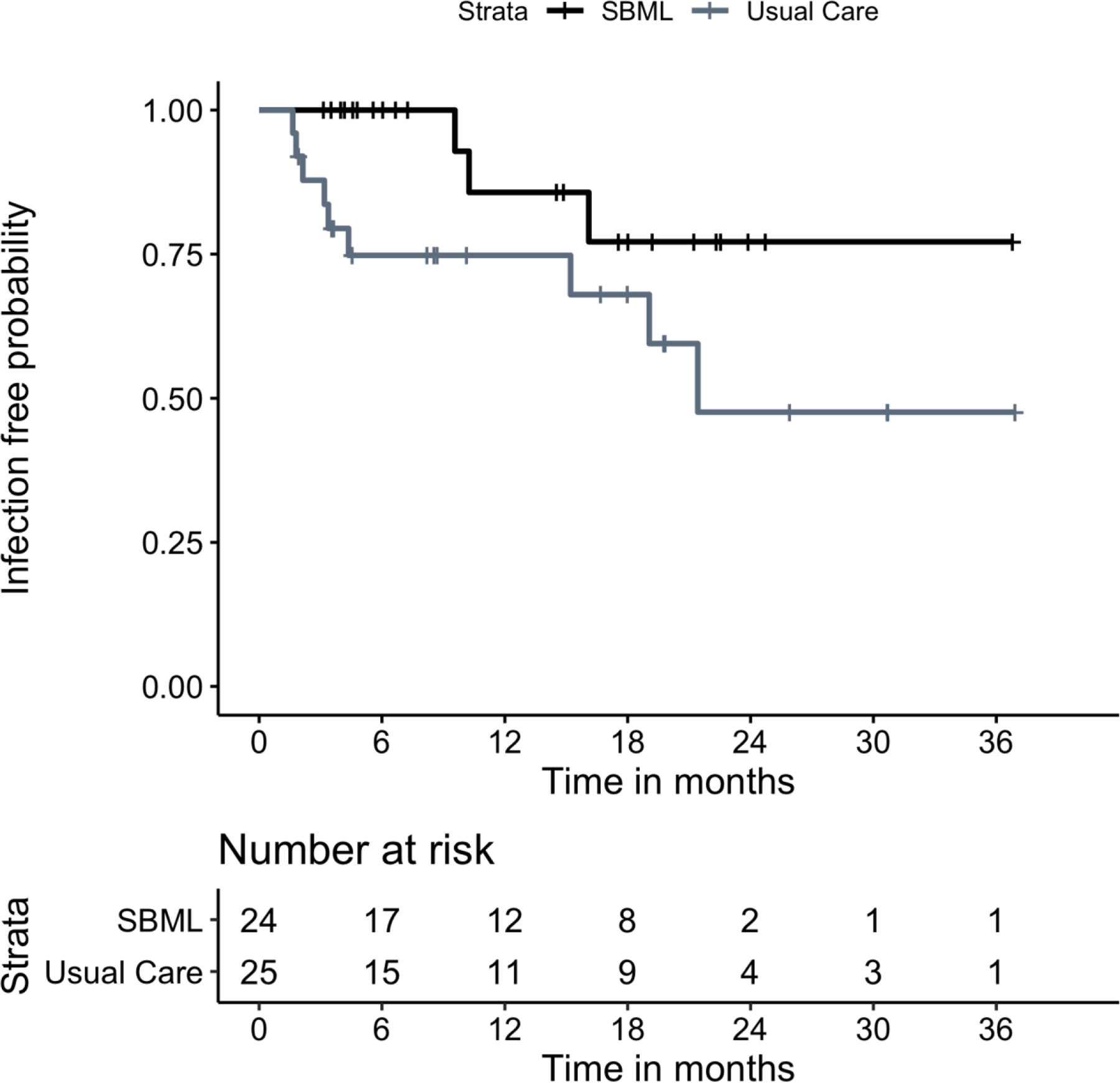
Kaplan-Meier Curves Showing the Probabilities of Remaining Driveline Exit Site Infection Free During the Study Period.
Sex was the only sociodemographic or clinical variable showing a significant association with time to infection. Kaplan Meier estimates yielded a probability of remaining infection free at 12 months of .887 (95% CI .772–1.00) for males and .577 (95% CI .347-.960) for females. The logrank test showed a reduction in time-to-infection distributions for male vs. female in 12-month infection rates of .310 (p=0.02). In a Cox model with additional adjustment for treatment assignment (SBML- vs usual-training), the association remained significant with the same p=0.02. The effect of treatment assignment did not change.
DISCUSSION
Patients assigned to VAD self-care SBML demonstrated a trend toward fewer DLES infections during follow-up compared to the usual-training group, with potentially meaningful differences at 3- and 12-months. To our knowledge, this is the first study demonstrating that patient and caregiver self-care SBML may improve clinical outcomes. The SBML intervention was likely successful because it used adult learning strategies including simulation (hands-on training), deliberate practice, explanations of why certain dressing change methods were critical, and rigorous assessments to ensure near flawless techniques. Additionally, the SBML intervention included educational content that was considered best practice for DLES dressing changes (i.e., training on proper sterile technique, how to properly sterilize the skin with chlorhexidine, and placing driveline anchors).25
The VAD self-care SBML intervention might also provide return on investment. While cost data on DLES infections is lacking, VAD-related infections incrementally increase implantation costs by $37,721.26 The cost of our intervention was low because VAD coordinator training time was only 4 hours, the SBML curriculum did not require resources other than an inexpensive plastic mannequin, and training time for patients and caregivers was equivalent between the SBML- and usual-trained groups.12 The SBML model also provides structure, which leads to efficient handoffs between trainers and decreases preparation time before training sessions. Additionally, SBML training has a definitive completion given the training is objective and does not allow for the subjectivity or differences in evaluation by different trainers.
DLES infection is often referred to as the “Achilles heel” of VAD therapy because of the associated patient morbidity and mortality.27 INTERMACS categorizes DLES as early (e.g., within 3 months of implantation) or late (after 3 months).23 In addition to these registry reports, other single center studies have measured time to DLES infection.24 Reporting infections as using the time to event methodology allows for all individuals to contribute to DLES endpoint competing risks of death and/or transplant to be accounted for and. Many efforts have been made to reduce these infections, including surgical technique (e.g. burying the velour portion of driveline),28 securing the DLES with anchoring devices, and changing dressing protocols.29 However, DLES infection rates remain elevated even in clinical trials, and there is no clear signal of reduced risk of DLES in one pump type vs. another.30 This provided further rationale for evaluating the effects of the VAD self-care SBML intervention on DLES infection rates.
In the exploratory analyses, female sex was associated with a lower probability of DLES infection. This is the first report showing an association of sex with DLES infections. However, in long term follow-up of the MOMENTUM 3 trial, female sex was associated with higher risk of overall VAD-related infections.30 This study highlights the importance of pre-specified sex-specific analyses of outcomes in future trials as females are typically underrepresented in the VAD literature. Other established risk factors for DLES include obesity, presence of diabetes, and younger age.23 Interestingly, we did not find an association between BMI, diabetes, or age and risk of DLES infection; this may be due to the small numbers of infection events.
Our study has limitations. First it was a single center RCT, limiting generalizability. Second, there were a small number of DLES infections in each of the study groups and one additional infection could have significantly changed our results. Finally, evaluation of infections was an exploratory aim of our RCT; we were not powered to show differences in DLES infections. However, results from this study will inform planning of a larger, multi-center clinical trial.
In conclusion, 12-month follow-up after VAD self-care SBML suggests fewer infections, and may improve patient outcomes, compared to usual-training. Patients with VADs and their caregivers at our own institution are now trained exclusively using SBML. Further research is planned to demonstrate successful transfer of the SBML curriculum to other institutions.
Supplementary Material
Acknowledgments:
We would like to acknowledge Drs. Douglas Vaughan, Kevin O’Leary, Duc Pham, and Clyde Yancy for their support and encouragement of this work. We thank the patients, caregivers, VAD coordinators, and physicians at Northwestern Memorial Hospital for their dedication to education and patient safety.
Funding:
This work was supported by the National Institutes of Health, National Institute of Nursing Research [grant number 1R21NR016745–01].
Footnotes
Conflict of Interest Disclosures: Dr. Wilcox receives consulting honoraria from Boehringer Ingelheim, Medtronic, and serves on the scientific advisory board for Cytokinetics. The other authors have no relevant conflicts of interest to report.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399–410. [DOI] [PubMed] [Google Scholar]
- 2.Park SJ, Tector A, Piccioni W, et al. Left ventricular assist devices as destination therapy: a new look at survival. J Thorac Cardiovasc Surg. 2005;129(1):9–17. [DOI] [PubMed] [Google Scholar]
- 3.Stevenson LW, Miller LW, Desvigne-Nickens P, et al. Left ventricular assist device as destination for patients undergoing intravenous inotropic therapy: a subset analysis from REMATCH (Randomized Evaluation of Mechanical Assistance in Treatment of Chronic Heart Failure). Circulation. 2004;110(8):975–981. [DOI] [PubMed] [Google Scholar]
- 4.Stehlik J, Stevenson LW, Edwards LB, et al. Organ allocation around the world: insights from the ISHLT International Registry for Heart and Lung Transplantation. J Heart Lung Transplant. 2014;33(10):975–984. [DOI] [PubMed] [Google Scholar]
- 5.O’Horo JC, Abu Saleh OM, Stulak JM, Wilhelm MP, Baddour LM, Rizwan Sohail M. Left Ventricular Assist Device Infections: A Systematic Review. ASAIO J. 2018;64(3):287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein DJ, Naftel D, Holman W, et al. Continuous-flow devices and percutaneous site infections: clinical outcomes. J Heart Lung Transplant. 2012;31(11):1151–1157. [DOI] [PubMed] [Google Scholar]
- 7.Pereda D, Conte JV. Left ventricular assist device driveline infections. Cardiol Clin. 2011;29(4):515–527. [DOI] [PubMed] [Google Scholar]
- 8.Kormos RL, Cowger J, Pagani FD, et al. The Society of Thoracic Surgeons Intermacs database annual report: Evolving indications, outcomes, and scientific partnerships. J Heart Lung Transplant. 2019;38(2):114–126. [DOI] [PubMed] [Google Scholar]
- 9.Casida J, Aikens J, Pagani F, et al. Advancing the Science of Self-Management in Adults With Long-Term Left Ventricular Assist Devices. Artif Organs. 2018;42(11):1095–1103. [DOI] [PubMed] [Google Scholar]
- 10.Trachtenberg BH, Cordero-Reyes AM, Aldeiri M, et al. Persistent blood stream infection in patients supported with a continuous-flow left ventricular assist device is associated with an increased risk of cerebrovascular accidents. J Card Fail. 2015;21(2):119–125. [DOI] [PubMed] [Google Scholar]
- 11.Yarboro LT, Bergin JD, Kennedy JL, et al. Technique for minimizing and treating driveline infections. Ann Cardiothorac Surg. 2014;3(6):557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barsuk JH, Wilcox JE, Cohen ER, et al. Simulation-Based Mastery Learning Improves Patient and Caregiver Ventricular Assist Device Self-Care Skills: A Randomized Pilot Trial. Circ Cardiovasc Qual Outcomes. 2019;12(10):e005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barsuk JH, Cohen ER, Harap RS, et al. Patient, Caregiver, and Clinician Perceptions of Ventricular Assist Device Self-care Education Inform the Development of a Simulation-based Mastery Learning Curriculum. J Cardiovasc Nurs. 2020;35(1):54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wayne DB, Didwania A, Feinglass J, Fudala MJ, Barsuk JH, McGaghie WC. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133(1):56–61. [DOI] [PubMed] [Google Scholar]
- 15.Didwania A, McGaghie WC, Cohen ER, et al. Progress toward improving the quality of cardiac arrest medical team responses at an academic teaching hospital. Journal of graduate medical education. 2011;3(2):211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barsuk JH, Cohen ER, Williams MV, et al. Simulation-Based Mastery Learning for Thoracentesis Skills Improves Patient Outcomes: A Randomized Trial. Acad Med. 2018;93(5):729–735. [DOI] [PubMed] [Google Scholar]
- 17.Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. The American journal of medicine. 2013;126(4):349–356. [DOI] [PubMed] [Google Scholar]
- 18.Schwab B, Teitelbaum EN, Barsuk JH, Soper NJ, Hungness ES. Single-stage laparoscopic management of choledocholithiasis: An analysis after implementation of a mastery learning resident curriculum. Surgery. 2018;163(3):503–508. [DOI] [PubMed] [Google Scholar]
- 19.Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Archives of internal medicine. 2009;169(15):1420–1423. [DOI] [PubMed] [Google Scholar]
- 20.Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749–756. [DOI] [PubMed] [Google Scholar]
- 21.Hannan MM, Husain S, Mattner F, et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J Heart Lung Transplant. 2011;30(4):375–384. [DOI] [PubMed] [Google Scholar]
- 22.Kusne S, Mooney M, Danziger-Isakov L, et al. An ISHLT consensus document for prevention and management strategies for mechanical circulatory support infection. J Heart Lung Transplant. 2017;36(10):1137–1153. [DOI] [PubMed] [Google Scholar]
- 23.Hannan MM, Xie R, Cowger J, et al. Epidemiology of infection in mechanical circulatory support: A global analysis from the ISHLT Mechanically Assisted Circulatory Support Registry. J Heart Lung Transplant. 2019;38(4):364–373. [DOI] [PubMed] [Google Scholar]
- 24.Rahal A, Ruch Y, Meyer N, et al. Left ventricular assist device-associated infections: incidence and risk factors. J Thorac Dis. 2020;12(5):2654–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilcox JE, Cameron KA, Harap RS, et al. Ventricular Assist Device Driveline Dressing-Change Protocols: A Need for Standardization. A Report from the SimVAD Investigators. J Card Fail. 2019;25(8):695–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slaughter MS, Bostic R, Tong K, Russo M, Rogers JG. Temporal changes in hospital costs for left ventricular assist device implantation. J Card Surg. 2011;26(5):535–541. [DOI] [PubMed] [Google Scholar]
- 27.Zierer A, Melby SJ, Voeller RK, et al. Late-onset driveline infections: the Achilles’ heel of prolonged left ventricular assist device support. Ann Thorac Surg. 2007;84(2):515–520. [DOI] [PubMed] [Google Scholar]
- 28.Wert L, Hanke JS, Dogan G, et al. Reduction of driveline infections through doubled driveline tunneling of left ventricular assist devices-5-year follow-up. J Thorac Dis. 2018;10(Suppl 15):S1703–S1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lander MM, Kunz N, Dunn E, et al. Substantial Reduction in Driveline Infection Rates With the Modification of Driveline Dressing Protocol. J Card Fail. 2018;24(11):746–752. [DOI] [PubMed] [Google Scholar]
- 30.Patel CB, Blue L, Cagliostro B, et al. Left ventricular assist systems and infection-related outcomes: A comprehensive analysis of the MOMENTUM 3 trial. J Heart Lung Transplant. 2020;39(8):774–781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


