Abstract
Rationale
Understanding the mechanisms responsible for stress-induced relapse is important for guiding treatment strategies aimed at minimizing the contribution of stress to addiction. Evidence suggests that these mechanisms involve interactions between noradrenergic systems and the neuropeptide corticotropin-releasing factor (CRF).
Objectives
The interaction between β-adrenergic receptors (ARs) and CRF as it relates to the reinstatement of cocaine-conditioned reward in response to a stressor was examined in mice. We hypothesized that β2 ARs are required for stress-induced activation of CRF pathways responsible for reinstatement.
Methods
Stress-induced relapse was examined based on the re-establishment of cocaine-induced conditioned place preference (CPP; 4 x 15 mg/kg cocaine, ip) after extinction using forced swim (6 min at 22°C) or an injection of the β2-AR agonist, clenbuterol (4 mg/kg, i.p.). The CRF-R1 antagonist antalarmin (10 mg/kg, i.p.) or the β2-AR antagonist ICI-118,551 (1 mg/kg, i.p.) were given 30 min prior to reinstating stimuli. Quantitative PCR was conducted in dissected BNST and amygdala, putative sources of CRF that contribute to reinstatement, to examine the effects of ICI-118,551 on swim-induced increases in crf mRNA in mice with a cocaine history.
Results
Pretreatment with ICI-118,551 or antalarmin blocked swim-induced reinstatement of CPP. Reinstatement by clenbuterol was also blocked by antalarmin. ICI-118,551 pretreatment prevented swim-induced increases in crf mRNA in the BNST. Effects in the amygdala were not observed.
Conclusions
These findings indicate that, during stress, norepinephrine, via β2-ARs, either directly or indirectly activates CRF-releasing neurons in the BNST that interface with motivational neurocircuitry to induce reinstatement of cocaine-conditioned reward.
Keywords: addiction, cocaine, stress, reinstatement, relapse, noradrenergic, CRF, conditioned place preference, bed nucleus of the stria terminalis, amygdala, β2-adrenergic receptor
INTRODUCTION
Understanding the mechanisms through which stress promotes relapse to drug use by cocaine addicts continues to be a critical research objective. Outcome measures for current treatment approaches aimed at relapse prevention remain poor and stress is a pervasive trigger for relapse as it is typically unavoidable in daily life. In addition, there is currently no FDA-approved medication for cocaine addiction, making it a critical unmet need. Stress-induced relapse to cocaine use can be modeled in mice using a cocaine-induced conditioned place preference (CPP) approach in which, after extinction, preference can be re-established (i.e., reinstated) using stressors such as forced swim (Kreibich and Blendy 2004; Mantsch et al. 2010).
A role for noradrenergic signaling in stress-induced reinstatement of cocaine-conditioned reward has been established. Using the CPP/reinstatement approach in mice, our laboratory has found that functional antagonism of noradrenergic neurotransmission via administration of the α2-adrenergic receptor (AR) agonist clonidine blocks swim-induced reinstatement of extinguished CPP (Mantsch et al. 2010), while disinhibition of noradrenergic activity via administration of yohimbine or the highly selective α2-AR antagonist BRL 44,408 is sufficient to reinstate (Mantsch et al. 2010; Vranjkovic et al. 2012). These findings parallel those in rats demonstrating α2-adrenergic AR agonist blockade of shock-induced reinstatement following cocaine self-administration (SA; Erb et al. 2000) and sufficiency of central administration of norepinephrine or systemic delivery of yohimbine for reinstatement of cocaine seeking (Brown et al. 2011; Brown et al. 2009; Feltenstein and See 2006). More importantly, they are consistent with reports that α2-AR agonists attenuate drug craving in human cocaine addicts upon presentation of stress-associated imagery (Jobes et al. 2011).
Stress-induced increases in noradrenergic activity appear to induce reinstatement of cocaine-conditioned reward in part via activation of β AR. We have found that swim-induced reinstatement of cocaine-induced CPP is blocked by the non-selective β-AR antagonist propranolol (Mantsch et al. 2010) and is not observed in β-AR-deficient mice (Vranjkovic et al. 2012), while the non-selective β-AR agonist isoproterenol induces reinstatement (Vranjkovic et al. 2012). More specifically, our findings suggest that β2-ARs are necessary for stress-induced reinstatement of cocaine-conditioned reward, as swim-induced reinstatement of CPP is blocked by the selective β2-AR antagonist ICI-118,551 and administration of the selective β2-AR agonist clenbuterol is sufficient for reinstatement of CPP (Vranjkovic et al. 2012).
The sites at which stress-induced increases in β-AR activation promote reinstatement behavior appear to include the bed nucleus of the stria terminalis (BNST) and central amygdala (CeA), both of which receive dense noradrenergic projections (Ricardo and Koh 1978; Woulfe et al. 1988), express β ARs (Asanuma et al. 1991; Cecchi et al. 2007; Rainbow et al. 1984), and are required for stress-induced reinstatement of lever pressing following SA in rats (Erb et al. 2001; Leri et al. 2002; McFarland et al. 2004) or cocaine-induced CPP in mice (Briand et al. 2010). In rats, delivery of a cocktail of β1- and β2-AR antagonists directly into either the BNST or the CeA prevents shock-induced reinstatement of cocaine seeking following self-administration (Leri et al. 2002). The BNST and CeA are components of the extended amygdala and serve as key interfaces between the stress and reward systems. Both regions are highly interconnected with the mesocorticolimbic system through which they likely regulate drug-seeking behavior and reinstatement of cocaine-conditioned reward, although the precise pathways and mechanisms through which this regulation occurs are not fully understood.
The link between the mesocorticolimbic circuitry that has been implicated in relapse and β-AR actions in the BNST and amygdala likely involves the neuropeptide, corticotropin-releasing factor (CRF). CRF is a key mediator of stress-induced reinstatement of cocaine seeking following self-administration in rats (Buffalari et al. 2012; Erb et al. 2006; Erb et al. 1998; Graf et al. 2011; Mantsch et al. 2008; Shaham et al. 1998) and has been reported to contribute to stress-induced reinstatement of lever pressing following SA via actions in the BNST (Erb et al. 2001) and the ventral tegmental area (VTA; Wang et al. 2005; Blacktop et al. 2011). In the BNST, evidence suggests that stress-induced increases in CRF arise in part from local release from intrinsic cell populations (Silberman et al. 2013). Moreover, CRF release into the VTA is thought to involve projections from the BNST and the CeA (Rodaros et al. 2007). In light of the requirement for β-AR signaling in these regions for stress-induced cocaine seeking in rats following SA (Leri et al. 2002) and evidence suggesting CRF-dependence of β-AR actions in BNST (Nobis et al. 2011; Silberman et al. 2013), we hypothesized that β2-AR activation is required for stress-induced regulation of CRF-releasing neurons in one or both of these regions and for relapse to drug use during periods of stress. In support of this hypothesis, it has been reported that reinstatement in response to central norepinephrine is CRF-dependent (Brown et al. 2009).
Here we further test this hypothesis by 1) investigating the ability of the CRF-R1 receptor antagonist, antalarmin, to prevent reinstatement of cocaine-induced CPP in mice by a stressor, forced swim, and administration of the β2-AR agonist clenbuterol, and 2) testing for the ability of the β2-AR selective antagonist ICI-118,551, which blocks swim-induced cocaine-conditioned reward, to also prevent swim-induced activation of CRF neurons in the BNST and amygdala, as assessed by increases in crf mRNA measured using qPCR quantification in dissected tissue from mice with a prior history of cocaine administration.
METHODS
Subjects
A total of 116 male C57BL/6 mice (8–10 weeks old; 20 mice for CPP testing and 96 for qPCR testing), purchased from Harlan Laboratories, were housed individually in a humidity- and temperature- controlled, AAALAC- accredited animal facility under a 12h/12h light/dark cycle (lights on at 0700h) with food and water available ad libitum, except when in the experimental chambers. All procedures were carried out in compliance with the NIH guidelines and the Guide for Care and Use of Laboratory Animals and were approved by Marquette University.
Conditioned Place Preference Apparatus
Behavioral testing was conducted as previously described (Mantsch et al. 2010; Vranjkovic et al. 2012). Six ENV-3013 mouse CPP chambers from Med Associates, Inc. were used. The stainless-steel and polyvinyl chloride chambers consisted of three distinct compartments separated by 5 cm wide × 5.9 cm high manual guillotine doors. The two 46.5 × 12.7 × 12.7-cm side compartments consisted of a white compartment with a 6.35 × 6.35-mm stainless-steel mesh floor and a black compartment with a stainless-steel grid rod floor consisting of 3.2-mm rods spaced 7.9 mm apart. The side compartments were attached via a gray-colored 7.2-cm-long center compartment with a smooth floor. The clear tops of the compartments were hinged to permit placement and removal of the mice. Ceiling lights were attached to each top. To balance unconditioned side preferences, only the light in the black compartment was illuminated during the training, testing and extinction phases. Automated data collection was accomplished by using photobeams (six beams for the white and black test areas and two beams for the center gray area) that were evenly spaced across the length of the chamber and interfaced with a computer containing MED-PC software (MED Associates). Using this automated photobeam system, entry into a side compartment was defined as consecutive breaks of the first two photocell beams in that compartment located adjacent to the door separating that compartment from the center compartment. Exiting of a side compartment (and entry into the center compartment) was indicated by occlusion of the beams in the center compartment.
Cocaine- Induced Conditioned Place Preference
Cocaine-induced conditioned place preference (CPP) was conducted using an unbiased approach by pairing one compartment with cocaine and the other with saline as previously described (Mantsch et al. 2010). These assignments were made randomly. On the first day of CPP, mice were placed into the center compartment of the chamber and given free access to all three compartments for 30 min in the absence of cocaine or saline to determine preconditioning preference. During the 8-day conditioning phase of the experiment, mice received cocaine (15mg/kg, i.p.) on the odd days and saline injections on the even days. Immediately after the injection, mice were confined (guillotine door closed) to the drug-appropriate compartment for 30 min. A day after the final conditioning session, mice were tested for the expression of cocaine-induced CPP by placing them in the center compartment and allowing them full access to the apparatus for 30 min. CPP was defined as the change in time spent (sec) in the cocaine-paired compartment after conditioning compared with the initial preconditioning session. Mice were determined to exhibit a CPP if they spent more time in the cocaine-paired compartment during the post-conditioning session compared to the preconditioning session.
Extinction
Daily extinction training was conducted after conditioning. During the extinction sessions, the mice had free access to the entire apparatus for 30 min following placement in the center compartment with both guillotine doors open. Mice underwent daily extinction training until the extinction criterion was met (50% reduction in the preference for the cocaine-paired compartment). Mice were tested for reinstatement 24 hrs after this criterion was reached.
Reinstatement
Aside from exposure to a reinstating stimulus, the reinstatement test sessions were identical to extinction conditions: mice were provided free access to the entire apparatus for 30 min following placement in the center compartment with both guillotine doors open. Most mice were tested multiple times for reinstatement. The sequence of reinstatement test conditions was counter-balanced using a Latin Square design. Mice underwent additional extinction prior to each subsequent test and were not tested again until the criterion for extinction was once again reached. Reinstatement was defined as the difference in the time spent (sec) in the cocaine-paired compartment between the reinstatement test day and the prior extinction day.
Drugs
Cocaine HCl (15mg/kg) was obtained from the National Institute on Drug Abuse (NIDA) through the NIDA Drug Supply Program. The CRF-R1 antagonist antalarmin (10mg/kg), the β2-adrenergic receptor (AR) agonist clenbuterol (4mg/kg), and the β2-AR antagonist ICI-118,551 (1mg/kg) were purchased from Sigma-Aldrich. Cocaine, clenbuterol, and ICI-118,551 were dissolved in saline (0.9% bacteriostatic saline). Antalarmin was dissolved in 5% DMSO. All drugs were administered i.p. in a volume of 0.1ml per 25g body weight.
Experiment 1: Role of β-2 AR and CRF-R1 receptors in stress-induced reinstatement of CPP
Forced swim-induced reinstatement
Stress-induced reinstatement was induced using a forced swim (FS) protocol as previously reported (Kreibich and Blendy 2004; Mantsch et al. 2010). Briefly, mice were placed into a 30 cm high × 20 cm deep cylindrical polypropylene container filled with water (20–25°C) for 6 min. After the forced swim, mice were placed back into their home cages for 3 to 4 min before being introduced into the center compartment of the CPP apparatus with free access to the entire apparatus for reinstatement testing, as described above.
The role of β2-ARs and CRF-R1 receptors in swim-induced reinstatement
We have previously reported that either pharmacological blockade of the β2-AR or genetic β2-AR deletion prevents stress-, but not cocaine-induced reinstatement of extinguished cocaine-induced CPP in mice (Mantsch et al. 2010; Vranjkovic et al. 2012). To confirm the role of β2-ARs in swim-induced reinstatement and to determine the role of CRF-R1 receptors, mice (n=8) underwent cocaine-induced CPP and extinction as described above before testing for swim-induced reinstatement following pretreatment with the β2-AR antagonist ICI-118,551 (1mg/kg i.p.), the CRF-R1 antagonist antalarmin (10mg/kg i.p.), or vehicle. Mice received injections 30 min prior to forced swim. The sequence of the treatments was counter-balanced using a Latin Square design.
Experiment 2: Effects of CRF-R1 antagonism on β2-AR agonist-induced reinstatement
We previously reported that the β2-AR agonist clenbuterol reinstates extinguished cocaine-induced CPP (Mantsch et al. 2010; Vranjkovic et al. 2012). To determine if the β2-AR induces cocaine-conditioned reward via a CRF-dependent mechanism, mice (n=12) were tested for reinstatement in response to administration of the β2-AR agonist clenbuterol (4mg/kg, i.p.) or vehicle 30 min after pretreatment with CRF-R1 receptor antagonist antalarmin (10 mg/kg, i.p.; Kreibich et al., 2009) or vehicle. Mice were introduced into the experimental chambers 30 min after clenbuterol administration and tested for reinstatement. The sequence of the treatments was counter-balanced using a Latin Square design. Notably, we have found that reinstatement of extinguished CPP by this dose of clenbuterol is not attenuated by pretreatment with the beta-1 AR antagonist, betaxolol (20 mg/kg, ip), suggesting that its agonist actions are selective for the beta-2 AR and/or that beta-1 AR, if activated, do not contribute to reinstatement (Ext: 558.28 ± 72.45; Clen/Betax: 1075.11 ± 94.03; Significant reinstatement, paired t-test; P<0.001).
Experiment 3: Role of β2-ARs in swim-induced effects on crf mRNA in the BNST and amygdala
In order to examine the role of β2-ARs on stress-induced crf mRNA, mice received an injection of cocaine (15 mg/kg, i.p.) or saline on alternating days for 8 days. Following the cocaine treatment, mice were given an 8-day withdrawal period before exposure to the stressor. Mice were pre-treated with either ICI-118,551 (1 mg/kg, i.p.) or vehicle 30 min prior to forced swim. After forced swim, mice were placed back in their home cages for 30 min prior to sacrifice. The remaining mice did not undergo forced swim but instead were euthanized 60 min after the ICI-118,551 or vehicle injection. The groups were as follows: Vehicle, Vehicle + Forced Swim, ICI, ICI + Forced Swim.
Tissue extraction
Mice were killed by cervical dislocation and their brains were rapidly removed and flash frozen by submersion for 30 s in a beaker filled with 2-methylbutane sitting in dry ice and brains were then stored at −80°C. A cryostat was used to cut one 500-μm-thick coronal section from frozen brain tissue at the level of the BNST (+0.45 mm to −0.05 mm from Bregma; Fig. 3A) and tissue punches were taken from the BNST using a tissue punch kit (1.00 mm in diameter), with hemispheres pooled into one sample for each mouse. In the same brain, a cryostat was used to cut two 500-μm-thick coronal sections from frozen brain tissue at the level of the amygdala (−0.82 mm to −1.82 mm from Bregma; Fig. 3B) and tissue punches were taken from the amygdala, with hemispheres pooled into one sample for each mouse. The tissue punches were stored at −80° C for later quantitative real-time polymerase chain reaction (qPCR) analysis.
FIGURE 3. Tissue dissection schematic for qPCR analysis.
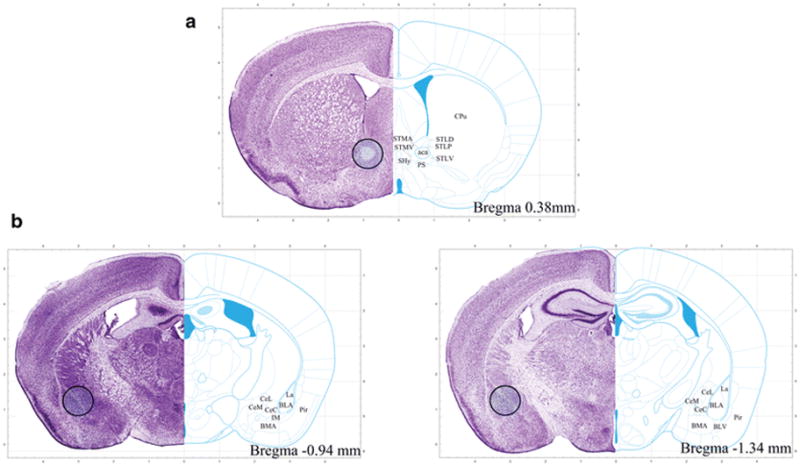
A) For the BNST, a tissue punch (1.00 mm in diameter) was taken from one 500-μm section. The punch site is shown in the shaded circle and both the dorsal and ventral portions of the BNST were collected. B) For the amygdala, a tissue punch (1.00 mm in diameter) was taken from two 500-μm sections. The punch site is shown in the shaded circle and the majority of the amygdala was collected. For both regions, hemispheres were pooled into one sample. Adapted from Franklin and Paxinos (2007).
RNA was extracted from frozen brain punches using a phenol: chloroform method. Due to the small amount of tissue, tissue from two mice was pooled for each sample. Briefly, tissue punches were homogenized in Trizol using a 20G followed by a 25G needle. Homogenized samples were incubated at room temperature with chloroform and then centrifuged at 11,000 x G for 15 min. The upper aqueous phase was separated into a new tube and isopropranolol was added. Following incubation at room temperature, samples were centrifuged at 12,000 x G for 10 min. The isopropranolol was poured off and samples were washed twice in 70 % ethanol followed by centrifugation at 12,000 x G for 5 min. The pellet was finally stored in 70% ethanol at −20°C.
Reverse transcription and qPCR
For cDNA production, the ethanol was poured off and the RNA pellet was allowed to air dry before being re-suspended in RNAse free water. Samples were then treated with DNAse (Invitrogen) to remove genomic DNA. Following DNAse treatment, cDNA was created using Oligo(dT) primers and a Superscript II reverse transcription kit (Invitrogen). cDNA was diluted 1:3 and stored at −20°C for qPCR analysis.
All qPCR was run using the StepOne Real-Time PCR system (Applied Biosystem). For qPCR analysis, reactions containing PerfeCTa SYBR Green FastMix with ROX (Quanta Biosciences), 20 μM of forward and reverse primers, and cDNA were loaded into the wells of a 48 well plate on ice. The cycling parameters were 95°C for 30s followed by 40 cycles of 95°C (5s), 60°C (15s), and 70°C (10s) followed by a melting curve to ensure amplification of a single product. All reactions were performed in triplicate and the mean threshold cycle for each gene product for each sample was used for analysis. The mRNA levels of crf were normalized to the housekeeping gene, TATA binding protein (TBP). Primer sequences were generously provided by Dr. Julie Blendy (Cleck et al. 2008).
RESULTS
Prior to testing for reinstatement, mice underwent CPP and extinction as previously described (Mantsch et al. 2010). All mice displayed cocaine-induced CPP and met criteria for extinction. Five mice were excluded from testing because they did not display CPP (2 mice) or did not extinguish (3 mice). The mean time spent in the cocaine-paired compartment prior to and after conditioning and during extinction for each CPP experiment (Exp. 1 and 2) is shown in Table 1.
Table 1. Cocaine-induced conditioned place preference (CPP) and extinction.
Mice underwent CPP using an unbiased 8-day (4 x alternating 15 mg/kg, ip cocaine and vehicle administration) approach. Data represent the mean time (sec ± S.E.) spent in the cocaine compartment prior to conditioning (Pre-Cond.), after conditioning (Post-Cond.), and on the first and last (prior to the first reinstatement test) days of extinction (First and Last Ext) in mice used for Experiments 1 and 2. Mice used in both experiments displayed significant CPP (*p<0.01; paired t-test Post-Cond. vs. Pre-) and showed extinction (**p<0.001; paired t-test, First Ext. vs. Last).
| Time Spent in Cocaine-Paired Compartment (sec ± S.E) | ||||
|---|---|---|---|---|
|
| ||||
| Pre-Cond. | Post-Cond | First Ext | Last Ext | |
|
|
||||
| Experiment 1 (n=8) | 528.12 (±106.68) | 957.47* (±37.99) | 927.34 (±120.56) | 639.84** (±33.10) |
|
|
||||
| Experiment 2 (n=12) | 558.58 (±72.59) | 995.80* (±80.67) | 1012.45 (±167.34) | 573.97** (±53.85) |
|
|
||||
Experiment 1: Role of β2-AR and CRF-R1 receptors in stress-induced reinstatement of CPP
For this experiment, mice (n=8) were tested for the effects of vehicle, the CRF-R1 antagonist antalarmin, and the β2-AR antagonist ICI-118,551 on reinstatement of extinguished CPP following forced swim. The pretreatments in combination with forced swim were tested in counterbalanced sequence determined using a Latin square design. The effects are shown in Figure 1.
FIGURE 1. Stress- (swim-) induced reinstatement of extinguished cocaine-induced CPP requires β2-adrenergic and CRF-R1 receptor activation.
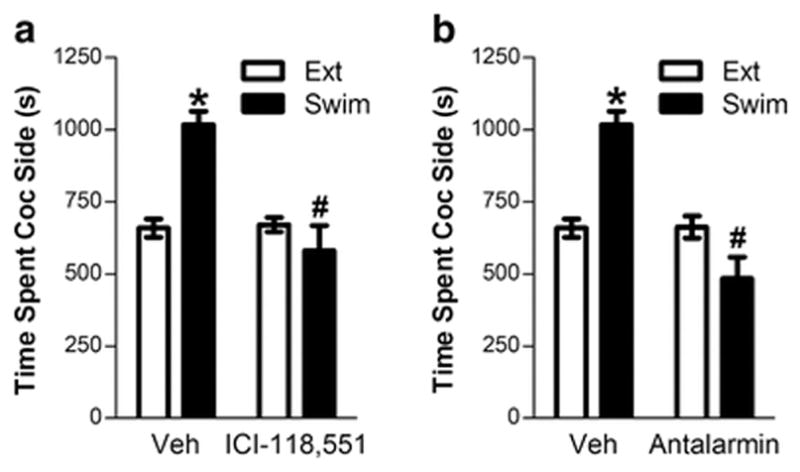
Data represent the time spent in the compartment previously paired with cocaine (s ± S.E.) during the preceding extinction session (Ext) or during reinstatement testing following a 6-min forced swim (22°C) in mice (n=8) pretreated with the β2-AR antagonist, ICI-118,551 (1 mg/kg, ip; 1A), the CRF-R1 receptor antagonist, antalarmin (10 mg/kg, ip; 1B) or Veh. Both ICI-118,551 and antalarmin prevented swim-induced reinstatement. In both cases, significant reinstatement was observed in vehicle- but not antagonist-pretreated mice (*p<0.01 vs. Ext) and reinstatement was significantly lower following antagonist pretreatment (#p<0.01 vs. Veh).
1.1: Effects of the β2-AR antagonist, ICI-118,551, on stress-induced reinstatement of CPP
We previously reported that the β2-AR antagonist, ICI-118,551, prevented swim-induced reinstatement of CPP in mice (Vranjkovic et al. 2012). This effect of ICI-118,551 (1 mg/kg, ip) was also observed in the present study (Fig. 1A). Consistent with our earlier report, two-way repeated measures ANOVA showed significant overall effects of forced swim (F(1,7) = 22.423; p<0.01) and ICI-118,551 (F(1,7) = 8.829; p<0.05) and a significant interaction between swim-induced reinstatement and ICI-118,551 pretreatment (F(1,7)=14.272; p<0.01). ICI-118,551 prevented swim-induced reinstatement. Post-hoc testing showed that significant reinstatement was observed in vehicle but not ICI-118,551 pretreated mice (p<0.01 vs. the prior extinction session) and was significantly reduced following ICI-118,551 pretreatment (p<0.01 vs. vehicle).
1.2: Effects of the CRF-R1 receptor antagonist, antalarmin on stress-induced reinstatement of CPP
The neuropeptide CRF has also been implicated in stress-induced cocaine seeking (Erb et al. 1998; Shaham et al. 1998). To test for the involvement of CRF in swim-induced reinstatement of CPP, mice received pretreatment with the CRF-R1 receptor antagonist, antalarmin (10 mg/kg, ip). Antalarmin prevented reinstatement following forced swim (Fig. 1B). Two-way repeated measures ANOVA showed significant overall effects of forced swim (F(1,7) =5.710; p<0.05) and antalarmin (F(1,7) =13.642; p<0.01) and a significant interaction between swim-induced reinstatement and antalarmin pretreatment (F(1,7) =67.375; p<0.01). Post-hoc testing showed that significant reinstatement was observed in vehicle- but not antalarmin-pretreated mice (p<0.01 vs. extinction) and was significantly reduced following antalarmin pretreatment (p<0.01 vs. vehicle).
Experiment 2: Effects of CRF-R1 antagonism on β2-AR agonist-induced reinstatement
We previously reported that the administration of β2-AR agonist, clenbuterol (4 mg/kg, ip), is sufficient to reinstate extinguished cocaine-induced CPP in mice (Vranjkovic et al. 2012). Here we demonstrate that, like forced swim, clenbuterol-induced reinstatement is blocked by pretreatment with the CRF-R1 receptor antagonist, antalarmin. Pretreatment with antalarmin (10 mg/kg, ip) prevented clenbuterol-induced reinstatement (Fig. 2). Twelve mice were tested for reinstatement following vehicle pretreatment. Nine were tested for reinstatement following antalarmin. Since some mice were not tested for effects of both vehicle and antalarmin on clenbuterol-induced reinstatement, a mixed two-way clenbuterol reinstatement (repeated measure) x antalarmin pretreatment condition (between subjects measure) ANOVA was used. A significant overall effect of clenbuterol administration (F(1,19) = 26.335; p <0.001) and a significant interaction between antalarmin pretreatment and clenbuterol-induced reinstatement (F(1,19) = 4.294; p = 0.050) were observed. Antalarmin prevented clenbuterol-induced reinstatement. Significant reinstatement was observed in vehicle but not antalarmin-pretreated mice (p<0.01 vs. extinction) and time spent in the cocaine-paired compartment was increased in mice pretreated with vehicle compared to mice pre-treated with antalarmin (p=0.05).
FIGURE 2. Clenbuterol-induced reinstatement is significantly decreased by pretreatment with the CRF-R1 receptor antagonist, antalarmin.
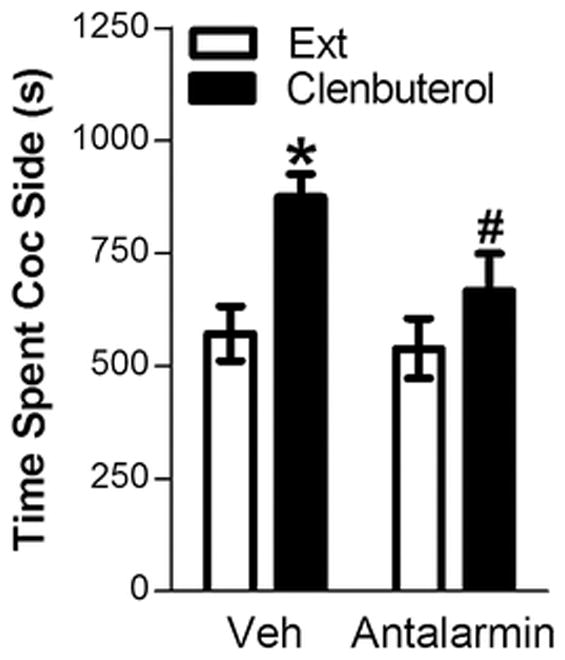
Data represent the time spent in the compartment previously paired with cocaine (s ± S.E.) during the preceding extinction session (Ext) or during reinstatement testing following administration of clenbuterol (4 mg/kg, ip) after pretreatment with the CRF-R1 receptor antagonist, antalarmin (10 mg/kg, ip; n=9) or vehicle (n=12). Significant reinstatement was observed in vehicle- but not antalarmin-pretreated mice (*p<0.01 vs. Ext) and reinstatement was significantly lower following antalarmin pretreatment (#p<0.05 vs. Veh).
Experiment 3: Role of β2-ARs stress-induced effects on crf mRNA in the BNST and amygdala
Since reinstatement of CPP following administration of the β2-AR agonist, ICI-118,551 was dependent on CRF receptor activation, we hypothesized that β2-AR receptors regulate, either directly or indirectly, CRF-producing neurons in brain regions previously implicated in CRF-dependent stress-induced cocaine seeking. To test this hypothesis we quantified CRF mRNA in BNST and amygdala dissected from cocaine-treated mice 30 min following the termination of a 6-min forced swim using qPCR after pretreatment with ICI-118,551 (1 mg/kg, ip) or vehicle. Schematics depicting the areas targeted for dissection are included in Figure 3.
3.1: Effects on crf mRNA in the BNST
The BNST is involved in stress-induced cocaine seeking following SA in rats (Leri et al. 2002; McFarland et al. 2004), and both β-AR signaling and CRF actions in the BNST are important for stress-induced reinstatement (Erb and Stewart 1999; Leri et al. 2002; Wang et al. 2006), suggesting that the BNST is a critical site in which β2-ARs can either directly or indirectly regulate CRF. Here, we examined the effect of pretreatment with the β2-AR antagonist ICI-118,551 (1 mg/kg, ip) on swim-induced levels of crf mRNA in the BNST following cocaine treatment and withdrawal (Fig. 4A). Two-way ANOVA did not show a significant main effect of forced swim (F(1,36) = 3.737, p = 0.061) or ICI-118,551 (F(1,36) = 1.919, p = 0.174) on crf mRNA levels in the BNST but did show a significant interaction between forced swim and ICI-118,551 (F(1,36) = 4.851, p < 0.05). Mice that received vehicle pretreatment and forced swim had significantly higher crf mRNA levels in the BNST compared to the vehicle treatment alone (p < 0.05). However, when mice were pretreated with ICI-118,551, forced swim no longer increased crf mRNA levels in the BNST (p > 0.05 compared to vehicle treatment) while the treatment with ICI-118,551 alone had no significant effect on crf mRNA levels (p > 0.05, compared to vehicle treatment alone).
FIGURE 4. Stress- (swim-) induced increases in crf mRNA in the BNST, but not the amygdala, are blocked by pretreatment with a β2-adrenergic receptor antagonist.
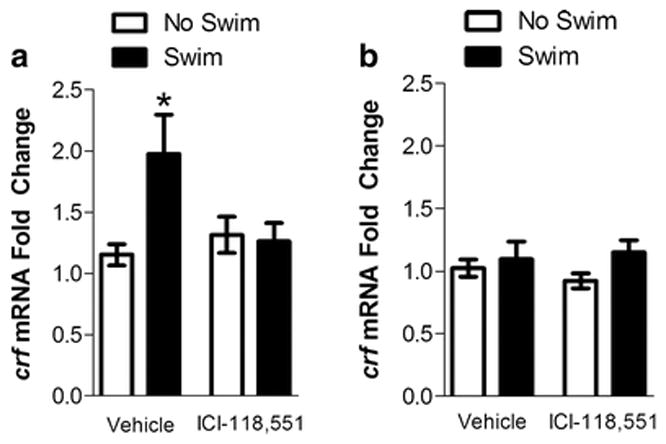
Data represent the fold change in crf mRNA ± S.E. The mRNA changes were assessed using quantitative real-time polymerase chain reaction (qPCR) on tissue from the BNST (A) and amygdala (B) of mice exposed to a 6-min forced swim (22°C) after cocaine treatment and withdrawal. The mean threshold cycle for crf was normalized to the endogenous control TATA binding protein (tbp). Forced swim significantly increased crf mRNA levels in the BNST in mice that received a vehicle pretreatment (*p<0.05, compared to vehicle alone). However, in mice that received a pretreatment the β2-AR antagonist, ICI-118,551 (1 mg/kg, ip), forced swim no longer increased crf mRNA levels in the BNST while the pretreatment of ICI-118,551 alone had no effect on crf mRNA. In contrast, in the amygdala, neither forced swim nor ICI-118,551 had a significant effect on crf mRNA.
3.2: Effects on crf mRNA in the amygdala
Like the BNST, the amygdala has been implicated in stress-induced cocaine seeking following SA in rats (McFarland et al. 2004). Moreover, β-AR signaling in the central nucleus of the amygdala contributes to stress-induced reinstatement (Leri et al. 2002) and forced swim following protracted withdrawal after binge cocaine access has been reported to increase crf mRNA levels in the amygdala (Cleck et al. 2008). Thus, the amygdala is a potential site at which CRF is either directly or indirectly regulated by β2-ARs to promote cocaine use. Here, we examined the effect of the β2-AR antagonist ICI-118,551 (1 mg/kg, ip) on swim-induced levels of crf mRNA in the amygdala following cocaine treatment and withdrawal (Fig. 4B). In contrast to the BNST, a two-way ANOVA did not show a significant main effect of forced swim (F(1,39) = 2.033, p = 0.162), ICI-118,551 (F(1,39) = 0.046, p = 0.83) or an interaction between forced swim and ICI-118,551 on crf mRNA in the amygdala (F(1,39) = 0.558, p = 0.459).
CONCLUSIONS
The main finding of this study is that an interaction between β2-ARs and CRF-R1 receptors is critical for stress-induced reinstatement of extinguished cocaine-induced CPP in mice. Additionally, β2-AR signaling is required for the stress-induced regulation of crf mRNA levels in the BNST. We found that not only is CRF-R1 activation necessary for swim-induced reinstatement of cocaine-induced CPP but it is also necessary for reinstatement in response to the β2-AR agonist clenbuterol, suggesting that β2-AR signaling is upstream from CRF actions in the neural pathway responsible for stress-induced reinstatement of cocaine-conditioned reward. In support of this hypothesis, we also found that forced swim can significantly increase crf mRNA levels in the BNST, a region necessary for stress-induced reinstatement, and that this effect can be prevented by pretreatment with the β2-AR antagonist ICI-118,551. Surprisingly, this effect does not occur in the amygdala. Taken altogether, these data raise the possibility that β2-AR-dependent activation of CRF-expressing neurons in the BNST mediates stress-induced reinstatement of cocaine-conditioned reward, a process that likely contributes to relapse.
The BNST is an important site for integration of stress and reward networks (Flavin and Winder 2013). The BNST receives heavy innervation from most subregions of the amygdala (Dong et al. 2001a; Weller and Smith 1982), a structure known to be involved in stress, fear and anxiety, and projects to regions involved in neuroendocrine, autonomic and behavioral control (Dong and Swanson 2006) such as the PVN and the amygdala (Cullinan et al. 1993; Dong et al. 2001a; Dong et al. 2001b). In addition to its connectivity with stress-related structures, BNST efferent projections target regions involved in motivation and reward, such as the nucleus accumbens (Dong et al. 2001b) and the VTA (Georges and Aston-Jones 2001; 2002). The BNST is activated during stress-induced reinstatement (Briand et al. 2010; Brown et al. 2011) and is necessary for stress-induced reinstatement in mice and rats (Briand and Blendy 2010; Erb and Stewart 1999; Leri et al. 2002; McFarland et al. 2004). In addition, it is an important region for CRF actions that mediate stress-induced reinstatement following SA, as CRF-R1 activation in the BNST is both necessary (Erb et al. 2001; Erb and Stewart 1999) and sufficient (Erb and Stewart 1999) for reinstatement, and stress can increase crf mRNA in the BNST in rats (Funk et al. 2006). Indeed, our own findings support this hypothesis by demonstrating that CRF-R1 receptor activation is necessary for forced swim induced reinstatement of CPP in mice and that the same stressor increases crf mRNA levels in the BNST of mice with a prior history of cocaine, albeit in the absence of CPP. These data suggest that the BNST is a major site of regulation of CRF actions during stress-induced reinstatement of cocaine-conditioned reward.
In addition to CRF, the noradrenergic system plays an important role in reward and stress (Flavin and Winder 2013; Weinshenker and Schroeder 2007). In particular, this system is heavily involved in stress-induced reinstatement behaviors. For example, reducing norepinephrine levels through α2-AR agonism blocks stress-induced reinstatement of cocaine seeking following SA in rats (Erb et al. 2000) and reinstatement of cocaine-conditioned reward in mice (Mantsch et al. 2010), as well as stress-associated cocaine craving in humans (Jobes et al. 2011). Norepinephrine is also sufficient for reinstatement as elevation of norepinephrine levels through central administration of norepinephrine or α2-AR antagonism promotes reinstatement behaviors in both rats and mice (Brown et al. 2011; Brown et al. 2009; Feltenstein and See 2006; Mantsch et al. 2010).
We have previously demonstrated, using the mouse CPP/reinstatement approach that, specifically, β2-ARs are required for stress-induced reinstatement (Mantsch et al. 2010; Vranjkovic et al. 2012). Reinstatement of CPP by either forced swim or yohimbine is blocked by the β-2 AR-selective antagonist, ICI-118,551, but not the β1-AR-selective antagonist, betaxolol, while administration of the β2-AR-selective agonist, clenbuterol, is sufficient to reinstate CPP. Our current findings confirm this role for β2-ARs. In rats, the BNST, which receives dense noradrenergic innervation through the ventral noradrenergic bundle (Ricardo and Koh 1978; Woulfe et al. 1988) and displays increased norepinephrine levels following stress (Pacak et al. 1995), appears to be a key site for β-AR regulation of cocaine seeking during stress. Blockade of β1- and β2-ARs in the BNST using a cocktail of ICI-118,551 and betaxolol prevents footshock-induced reinstatement of cocaine seeking following SA in rats (Leri et al. 2002).
The precise mechanism through which β2-ARs regulate reinstatement of cocaine seeking and CPP is unclear, but we hypothesize that it involves effects on CRF neurons in the BNST. Beta AR activation increases excitatory transmission in the BNST (Egli et al. 2005) and these effects appear to be mediated in part via a CRF-R1 dependent mechanism (Nobis et al. 2011). In addition, norepinephrine-induced reinstatement is prevented by CRF receptor antagonism but CRF-induced reinstatement is not blocked by inhibition of noradrenergic neurotransmission (Brown et al. 2009), suggesting that noradrenergic signaling is upstream from CRF in the chain of events that lead to reinstatement. Our own findings support this possibility, as we found that reinstatement of CPP induced by the β2-AR agonist clenbuterol is blocked by the CRF-R1 antagonist antalarmin and that stress-induced increases in crf mRNA in the BNST are prevented by the β2-AR antagonist ICI-118,551. CRF released into the BNST may originate from CRF-releasing afferent projections to the BNST or from local cell populations (Veinante et al. 1997). The latter possibility is supported by findings from Silberman et al. (2013) suggesting that norepinephrine depolarizes CRF-containing neurons intrinsic to the BNST, thereby releasing CRF locally which then enhances glutamatergic neurotransmission via presynaptic effects.
CRF neurons in the BNST also send efferent projections to a number of brain regions, most notably the VTA (Rodaros et al. 2007). The VTA is a heterogeneous region that is the source of dopamine neurons that comprise the mesocorticolimbic system and has a well-established role in stress-induced cocaine seeking (Mantsch et al. 2014; McFarland et al. 2004). CRF actions in the VTA are necessary for stress-induced reinstatement and are mediated by activation of CRF-R1 receptors (Blacktop et al. 2011). Emerging evidence suggests that the BNST-VTA pathway is essential for stress-induced cocaine seeking and reinstatement of CPP (Mantsch et al. 2014). For example, reinstating stimuli such as forced swim increase c-fos expression specifically in BNST neurons that project to the VTA (Briand et al. 2010). The BNST sends both glutamatergic and GABAergic projections to the VTA (Georges and Aston-Jones 2001; Kudo et al. 2012), which appear to have distinct roles in reward and aversion (Jennings et al. 2013). However, the relative roles of GABAergic vs. glutamatergic projections from the BNST to the VTA in the regulation of stress-induced reinstatement behaviors are not clear. Notably, withdrawal from chronic ethanol exposure enhances basal glutamatergic regulation of VTA-projecting BNST neurons in a CRF-R1 dependent manner (Silberman et al. 2013). Understanding how projections from the BNST to the VTA promote relapse to drug use during stress and delineation of the processes in the BNST that regulate these projections represent important goals for future research.
In general, the role for CRF and NE in stress-induced reinstatement behaviors, as demonstrated in the present study, extends to other classes of abused drugs. Antagonism of CRF receptors prevents stress-induced reinstatement following alcohol (Lê et al., 2000), heroin (Shaham et al., 1997), and nicotine (Zislis et al., 2007; Bruijnzeel et al., 2009) SA, as does suppression of noradrenergic neurotransmission using alpha-2 AR agonist drugs (Lê et al., 2005, alcohol; Shaham et al., 2000, heroin; Zislis et al., 2007; nicotine). However, subtle differences in the mechanisms underlying stress-induced reinstatement appear to exist. For example, the alpha-1 AR antagonist, prazosin blocks stress-induced reinstatement (Lê et al., 2011), while the highly selective alpha-2 AR antagonist, RS-79948, fails to evoke reinstatement following alcohol SA in rats (Dzung Lê et al., 2009). Thus, application of the current findings to other drugs of abuse will require further investigation.
There is some evidence that suggests that targeting ARs in human cocaine addicts may be an effective treatment strategy. In humans, α2-AR agonists, which functionally antagonize noradrenergic neurotransmission, reduce stress-induced (Jobes et al. 2011) and cue-induced (Fox et al. 2012) cocaine craving. The effectiveness of beta blockers in cocaine users classified as dependent has also been examined (Kampman et al. 2001). It is noteworthy that, while the beta blocker propranolol did not universally improve treatment outcome measures, positive responses were observed in subjects who displayed more severe cocaine withdrawal symptoms. Considering that such symptoms are largely stress-related, we view these findings as supportive of a role for β ARs in stress-induced relapse.
Similar to the BNST, the amygdala is a region known to be involved in both reward and stress. The amygdala is comprised of a number of subnuclei that are highly interconnected with regions involved in stress-related responses such as the PVN and BNST, as well those involved in reward-related processes, such as the NAc, PFC, and the VTA (McDonald 1991a; b; Rodaros et al. 2007; Silverman et al. 1981). As is the case for the BNST, it has been reported that the amygdala is essential for stress-induced reinstatement (McFarland et al. 2004) and that the CeA sends CRF-positive projections to the VTA (Rodaros et al 2007). Moreover, β2-AR signaling in the CeA (Leri et al. 2002) and a projection from the CeA that regulates CRF release in the BNST (Erb et al. 2001) have been reported to be necessary for stress-induced cocaine seeking following SA in rats, suggesting that β2-AR regulation of CRF neurons may occur in the amygdala as well. Surprisingly, we found that forced swim stress did not alter crf mRNA levels in the amygdala. This is in contrast to a prior study demonstrating swim-induced increases in crf mRNA levels in the amygdala of mice with a prior history of cocaine (Cleck et al. 2008). However, in that study, mice received greater amounts of cocaine and the increase in crf mRNA levels was only observed following prolonged withdrawal (Cleck et al. 2008). Importantly, in the present study, mice were tested for crf mRNA responses after repeated exposure to cocaine but in the absence of the CPP context. It is not clear how conditioning to cocaine and testing for swim effects on crf mRNA measured following exposure to the CPP chambers could have influenced our results. It should also be noted that since our dissections were gross dissections of the amygdala due to the small amount of tissue yielded from a mouse brain, we cannot exclude the possibility that a more specific dissection of the CeA may yield different results. In contrast to its regulatory role in anxiety, CRF actions in the CeA do not appear to be necessary for stress-induced reinstatement following SA or CPP (Erb and Stewart 1999; Wang et al. 2006). Our findings also suggest that, while CeA regulation of CRF release into the BNST is necessary for stress-induced reinstatement behaviors (Erb et al. 2001), the source of CRF may consist of CRF projections from regions other than the CeA or cells that intrinsic to the BNST and not CRF neurons originating in the CeA.
Our current findings support a hypothesis that places β2-AR signaling upstream of CRF actions in the BNST in the neural pathway that mediates stress-induced reinstatement of cocaine-conditioned reward. While there are long-established roles for AR and CRF individually in stress-induced reinstatement, it has become clear that an interaction between β2-AR signaling and CRF producing neurons, possibly in the BNST is likely critical. For this reason, further investigation of the precise mechanisms through which β2-AR contribute to the stress-induced regulation of CRF neurons in the BNST is needed. Finally, our findings also suggest that medications targeting β2-ARs hold some promise for potential treatment to minimize the contribution of stress to relapse.
Acknowledgments
This research was funded by NIH grant DA15758 to JR Mantsch. The authors declare no conflicts of interest.
References
- Asanuma M, Ogawa N, Mizukawa K, Haba K, Hirata H, Mori A. Distribution of the beta-2 adrenergic receptor messenger RNA in the rat brain by in situ hybridization histochemistry: effects of chronic reserpine treatment. Neurochem Res. 1991;16:1253–6. doi: 10.1007/BF00966654. [DOI] [PubMed] [Google Scholar]
- Blacktop JM, Seubert C, Baker DA, Ferda N, Lee G, Graf EN, Mantsch JR. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J Neurosci. 2011;31:11396–403. doi: 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Blendy JA. Molecular and genetic substrates linking stress and addiction. Brain Res. 2010;1314:219–34. doi: 10.1016/j.brainres.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Vassoler FM, Pierce RC, Valentino RJ, Blendy JA. Ventral tegmental afferents in stress-induced reinstatement: the role of cAMP response element-binding protein. J Neurosci. 2010;30:16149–59. doi: 10.1523/JNEUROSCI.2827-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ZJ, Nobrega JN, Erb S. Central injections of noradrenaline induce reinstatement of cocaine seeking and increase c-fos mRNA expression in the extended amygdala. Behav Brain Res. 2011;217:472–6. doi: 10.1016/j.bbr.2010.09.025. [DOI] [PubMed] [Google Scholar]
- Brown ZJ, Tribe E, D’Souza NA, Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology. 2009;203:121–30. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Prado M, Isaac S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol Psychiatry. 2009;66:110–7. doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, Baldwin CK, Feltenstein MW, See RE. Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats. Physiol Behav. 2012;105:209–14. doi: 10.1016/j.physbeh.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi M, Capriles N, Watson SJ, Akil H. Beta1 adrenergic receptors in the bed nucleus of stria terminalis mediate differential responses to opiate withdrawal. Neuropsychopharmacology. 2007;32:589–99. doi: 10.1038/sj.npp.1301140. [DOI] [PubMed] [Google Scholar]
- Cleck JN, Ecke LE, Blendy JA. Endocrine and gene expression changes following forced swim stress exposure during cocaine abstinence in mice. Psychopharmacology. 2008;201:15–28. doi: 10.1007/s00213-008-1243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001a;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001b;436:430–55. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J Comp Neurol. 2006;494:75–107. doi: 10.1002/cne.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzung Lê A, Funk D, Harding S, Juzytsch W, Fletcher PJ. The role of noradrenaline and 5-hydroxytryptamine in yohimbine-induced increases in alcohol-seeking in rats. Psychopharmacology. 2009;204:477–88. doi: 10.1007/s00213-009-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD, Winder DG. Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30:657–68. doi: 10.1038/sj.npp.1300639. [DOI] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–50. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Erb S, Petrovic A, Yi D, Kayyali H. Central injections of CRF reinstate cocaine seeking in rats after postinjection delays of up to 3 h: an influence of time and environmental context. Psychopharmacology. 2006;187:112–20. doi: 10.1007/s00213-006-0392-5. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2001;158:360–5. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–36. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Flavin SA, Winder DG. Noradrenergic control of the bed nucleus of the stria terminalis in stress and reward. Neuropharmacology. 2013;70:324–30. doi: 10.1016/j.neuropharm.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, Morgan PT, Sinha R. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcohol Clin Exp Res. 2012;36:351–60. doi: 10.1111/j.1530-0277.2011.01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3. Academic Press; London: 2007. [Google Scholar]
- Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–43. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci. 2001;21:RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–87. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf EN, Hoks MA, Baumgardner J, Sierra J, Vranjkovic O, Bohr C, Baker DA, Mantsch JR. Adrenal activity during repeated long-access cocaine self-administration is required for later CRF-Induced and CRF-dependent stressor-induced reinstatement in rats. Neuropsychopharmacology. 2011;36:1444–54. doi: 10.1038/npp.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–8. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobes ML, Ghitza UE, Epstein DH, Phillips KA, Heishman SJ, Preston KL. Clonidine blocks stress-induced craving in cocaine users. Psychopharmacology. 2011;218:83–8. doi: 10.1007/s00213-011-2230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, Mulvaney F, Alterman AI, Cornish J, Gariti P, Cnaan A, Poole S, Muller E, Acosta T, Luce D, O’Brien C. Effectiveness of propranolol for cocaine dependence treatment may depend on cocaine withdrawal symptom severity. Drug Alcohol Depend. 2001;63:69–78. doi: 10.1016/s0376-8716(00)00193-9. [DOI] [PubMed] [Google Scholar]
- Kreibich AS, Blendy JA. cAMP response element-binding protein is required for stress but not cocaine-induced reinstatement. J Neurosci. 2004;24:6686–92. doi: 10.1523/JNEUROSCI.1706-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibich AS, Briand L, Cleck JN, Ecke L, Rice KC, Blendy JA. Stress-induced potentiation of cocaine reward: a role for CRF R1 and CREB. Neuropsychopharmacol. 2009;34:2609–17. doi: 10.1038/npp.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Uchigashima M, Miyazaki T, Konno K, Yamasaki M, Yanagawa Y, Minami M, Watanabe M. Three types of neurochemical projection from the bed nucleus of the stria terminalis to the ventral tegmental area in adult mice. J Neurosci. 2012;32:18035–46. doi: 10.1523/JNEUROSCI.4057-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacol. 2000;150:317–24. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Lê AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacol. 2005;179:366–73. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology. 2011;218:89–99. doi: 10.1007/s00213-011-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–8. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Francis DM, Katz ES, Hoks MA, Serge JP. Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacology. 2008;195:591–603. doi: 10.1007/s00213-007-0950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Vranjkovic O, Twining RC, Gasser PJ, McReynolds JR, Blacktop JM. Neurobiological mechanisms that contribute to stress-related cocaine use. Neuropharmacology. 2014;76(Pt B):383–94. doi: 10.1016/j.neuropharm.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for beta-2 adrenergic receptors. Neuropsychopharmacology. 2010;35:2165–78. doi: 10.1038/npp.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Lê AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacol. 2007;195:345–55. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991a;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience. 1991b;44:15–33. doi: 10.1016/0306-4522(91)90248-m. [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–60. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobis WP, Kash TL, Silberman Y, Winder DG. beta-Adrenergic receptors enhance excitatory transmission in the bed nucleus of the stria terminalis through a corticotrophin-releasing factor receptor-dependent and cocaine-regulated mechanism. Biol Psychiatry. 2011;69:1083–90. doi: 10.1016/j.biopsych.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak K, McCarty R, Palkovits M, Kopin IJ, Goldstein DS. Effects of immobilization on in vivo release of norepinephrine in the bed nucleus of the stria terminalis in conscious rats. Brain Res. 1995;688:242–6. doi: 10.1016/0006-8993(95)00566-9. [DOI] [PubMed] [Google Scholar]
- Rainbow TC, Parsons B, Wolfe BB. Quantitative autoradiography of beta 1- and beta 2-adrenergic receptors in rat brain. Proc Natl Acad Sci U S A. 1984;81:1585–9. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–14. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology. 1998;137:184–90. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2000;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Matthews RT, Winder DG. A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis. J Neurosci. 2013;33:950–60. doi: 10.1523/JNEUROSCI.2949-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman AJ, Hoffman DL, Zimmerman EA. The descending afferent connections of the paraventricular nucleus of the hypothalamus (PVN) Brain Res Bull. 1981;6:47–61. doi: 10.1016/s0361-9230(81)80068-8. [DOI] [PubMed] [Google Scholar]
- Veinante P, Stoeckel ME, Freund-Mercier MJ. GABA- and peptide-immunoreactivities co-localize in the rat central extended amygdala. Neuroreport. 1997;8:2985–9. doi: 10.1097/00001756-199709080-00035. [DOI] [PubMed] [Google Scholar]
- Vranjkovic O, Hang S, Baker DA, Mantsch JR. beta-adrenergic receptor mediation of stress-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: roles for beta1 and beta2 adrenergic receptors. J Pharmacol Exp Ther. 2012;342:541–51. doi: 10.1124/jpet.112.193615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–96. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fang Q, Liu Z, Lu L. Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology. 2006;185:19–28. doi: 10.1007/s00213-005-0262-6. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32:1433–51. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- Weller KL, Smith DA. Afferent connections to the bed nucleus of the stria terminalis. Brain Res. 1982;232:255–70. doi: 10.1016/0006-8993(82)90272-4. [DOI] [PubMed] [Google Scholar]
- Woulfe JM, Hrycyshyn AW, Flumerfelt BA. Collateral axonal projections from the A1 noradrenergic cell group to the paraventricular nucleus and bed nucleus of the stria terminalis in the rat. Exp Neurol. 1988;102:121–4. doi: 10.1016/0014-4886(88)90084-2. [DOI] [PubMed] [Google Scholar]
- Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist D-Phe CRF(12–41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacol. 2007;53:958–66. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


