Obesity and impaired metabolic health are important risk factors for severe COVID-19. Novel data indicate that these risk factors might also promote vaccine-breakthrough SARS-CoV-2 infections in fully vaccinated people. Here, these relationships are discussed and post-acute sequelae of COVID-19 that are related to obesity and impaired metabolic health are addressed.
Subject terms: Diabetes, Infectious diseases, Obesity
Key advances
Novel data reveal a causal role for increased BMI in the development of severe COVID-19 (ref.2).
Solid scientific evidence indicates that there is a linearly increased risk of hospital admission or death due to COVID-19 at a BMI >23 kg/m2, particularly in younger people3.
Novel data indicate not only that hyperglycaemia and insulin resistance might drive immunosenescence, but also that hyperinsulinaemia might promote increased risk of SARS-CoV-2 infection and complicated COVID-19 (ref.6).
Studies indicate that obesity and impaired metabolic health might increase the risk of vaccine-breakthrough SARS-CoV-2 infections7–9.
Advanced age is associated with the highest risk of severe COVID-19. It is now well established that the risk of infection with SARS-CoV-2, as well the risk of severe COVID-19 with complications, is also increased in people with chronic medical conditions1. However, as some of these conditions (for instance, obesity and diseases associated with impaired metabolic health, such as diabetes mellitus, hypertension, cardiovascular disease, chronic kidney disease and liver disease) are often tightly related, the question is to what extent they affect COVID-19 independently of each other.
A large study on behalf of NHS England, OpenSAFELY, links primary care records of 17,278,392 adults to 10,926 COVID-19-related deaths; this study found that obesity, diabetes mellitus, cardiovascular disease, kidney disease and liver disease were independently associated with increased COVID-19-related mortality1. Nevertheless, obesity and diseases associated with impaired metabolic health share the important underlying pathomechanisms of insulin resistance, subclinical inflammation and fatty liver, which might explain their relationship with severe COVID-19 (ref.1). Therefore, it is important to better understand the individual contribution of these comorbidities to COVID-19. In this respect, published in July 2021, a study mapping the human genetic architecture of COVID-19 and applying Mendelian randomization analyses found a causalrole for increased body mass index (BMI) for severe COVID-19 (ref.2). Furthermore, published in June 2021, a very large community-based cohort study from the UK that evaluated data from 6,910,695 patients with a positive SARS-CoV-2 test result found that a high BMI was associated with adverse COVID-19 outcomes, independently of several other COVID-19 risk factors, including type 2 diabetes mellitus (T2DM). A linearly increased risk of hospital admission or death due to COVID-19 was observed at a BMI >23 kg/m2. Interestingly, the risk of severe COVID-19 outcomes related to an elevated BMI was highest in the youngest age groups (20–39 years old) and decreased progressively with increasing age3.
Related to the role of hyperglycaemia, increased levels of glucose were found to regulate viral replication and cytokine production in monocytes, and glycolysis was found to sustain SARS-CoV-2-induced monocyte response and viral replication4. However, it might not only be hyperglycaemia impairing adequate immune function and inducing immunosenescence. Premature immunosenescence (that is, accelerated ageing of the immune system), particularly of the CD4+ and CD8+ T cell compartments, has been found in people with obesity or T2DM1. Intriguingly, a paper published in 2018 described a mechanism explaining this observation; impaired insulin signalling was observed to have an important role in modulating the body’s immune response. In rodents, insulin-receptor-deficient T cells were found to have reduced inflammatory potential and poor protective immunity against H1N1 influenza infection5. A paper published in October 2021 provided support for the role of insulin resistance in promoting severe COVID-19. Hyperinsulinaemia, which is commonly observed in insulin resistance, was found to increase the expression of GRP78 as a binding partner of the SARS-CoV-2 spike protein and ACE2 in adipocytes6. This effect might induce increased infection rates of adipocytes, resulting in progression of COVID-19 in people with SARS-CoV-2 infection.
Vaccination against COVID-19 is highly effective in addressing the COVID-19 pandemic7. However, because patients with obesity and/or T2DM show immunosenescence (for example, of the CD4+ and CD8+ T cell compartments) and increased HbA1c levels are associated with a reduced immune response to an influenza A (H1N1) vaccine1, the question arises as to whether obesity and impaired metabolic health might adversely influence the efficacy of vaccines against SARS-CoV-2. In this respect, a study published in September 2021 that included patients who were fully vaccinated against COVID-19 and were admitted to the Yale New Haven Health system hospital investigated parameters associated with vaccine-breakthrough COVID-19 infections. Among the pre-existing comorbidities, overweight, T2DM and cardiovascular disease were frequently seen in patients with severe or critical illness8.
In addition, a study published in September 2021 that investigated the effectiveness of COVID-19 vaccination in Scotland’s nationwide platform EAVE II, T2DM, coronary heart disease and chronic kidney disease were associated with increased risk of severe COVID-19 outcomes9. These findings agree with results from a study in Israel showing that COVID-19 vaccine effectiveness might be slightly lower among people with a higher number of coexisting conditions, such as obesity, T2DM and hypertension, compared with people with a low number of coexisting conditions. For example, in patients with no coexisting conditions, vaccine effectiveness 7 days after the second dose of the BNT162b2 mRNA vaccine to end of follow-up was 91% (95% CI 83–96) for documented infection and 93% (95% CI 78–100) for symptomatic illness. The effectiveness was reduced to 86% (95% CI 72–95) and 89% (95% CI 68–98), respectively, in patients with three or more coexisting conditions7. Whether the risk of breakthrough infections can be estimated by the level of spike-antibody levels after COVID-19 vaccination has not yet been investigated. However, studies indicate that a time-dependent decline in antibody levels might increase the risk of breakthrough infections10.
What is the bigger picture about the relationships of obesity and impaired metabolic health with COVID-19? First, more than a year after the first reports about these relationships were published, novel data support the strong effect of obesity and impaired metabolic health on the severity of COVID-19. Second, new studies highlight that obesity, and possibly diabetes mellitus, strongly promote a severe course of the disease, specifically in younger people. Third, mechanistic studies highlight the role of hyperglycaemia and hyperinsulinaemia in this process. Fourth, as COVID-19 might become an endemic disease, the role of obesity and impaired metabolic health in promoting vaccine-breakthrough SARS-Cov-2 infections needs to be taken very seriously (Fig. 1). This issue is particularly important as, beyond the acute illness, a substantial health loss, including that associated with disorders of lipid metabolism, diabetes mellitus and obesity, has been observed in people who have had COVID-19 (ref.1). Thus, to combat the COVID-19 pandemic, weight loss in people with obesity or overweight, and improvement of hyperglycaemia and insulin resistance, should be promoted. Furthermore, national and international programmes on the political level and in the public health sector need to be initiated to achieve the goal to reduce the prevalence of obesity and improve metabolic health.
Fig. 1. Obesity, impaired metabolic health, COVID-19 and vaccine-breakthrough SARS-CoV-2 infection.
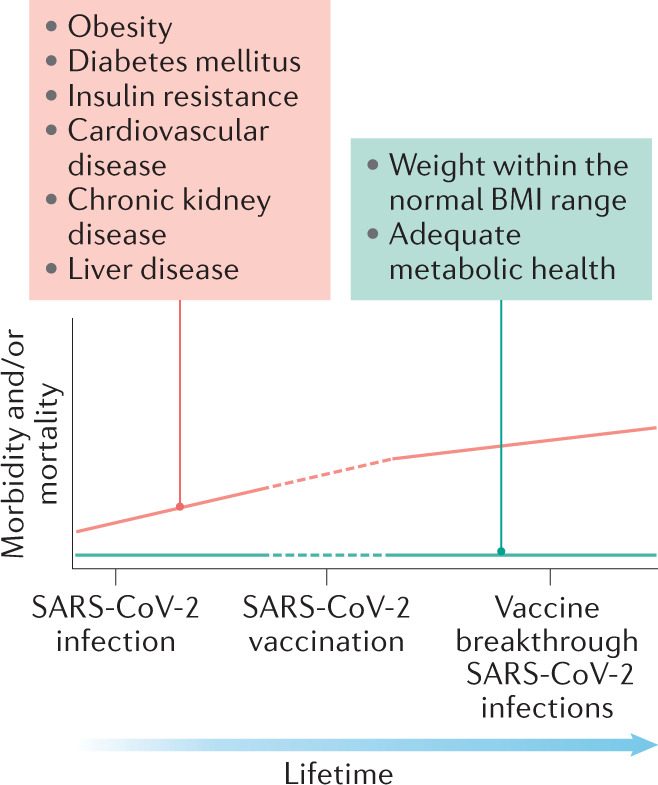
Relationships between obesity and impaired metabolic health and morbidity and mortality, based on SARS-CoV-2 infections and vaccine-breakthrough SARS-CoV-2 infections.
Competing interests
The author declares no competing interests.
References
- 1.Stefan N, Birkenfeld AL, Schulze MB. Global pandemics interconnected - obesity, impaired metabolic health and COVID-19. Nat. Rev. Endocrinol. 2021;17:135–149. doi: 10.1038/s41574-020-00462-1. [DOI] [PubMed] [Google Scholar]
- 2.COVID-19 Host Genetics Initiative Mapping the human genetic architecture of COVID-19. Nature. 2021 doi: 10.1038/s41586-021-03767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao M, et al. Associations between body-mass index and COVID-19 severity in 6·9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 2021;9:350–359. doi: 10.1016/S2213-8587(21)00089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Codo AC, et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 2020;32:498–499. doi: 10.1016/j.cmet.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai S, et al. Insulin receptor-mediated stimulation boosts T cell immunity during inflammation and infection. Cell Metab. 2018;28:922–934. doi: 10.1016/j.cmet.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Shin J, et al. Possible involvement of adipose tissue in patients with older age, obesity, and diabetes with coronavirus SARS-CoV-2 infection (COVID-19) via GRP78 (BIP/HSPA5): significance of hyperinsulinemia management in COVID-19. Diabetes. 2021 doi: 10.2337/db20-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagan N, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juthani PV, et al. Hospitalisation among vaccine breakthrough COVID-19 infections. Lancet Infect. Dis. 2021;21:1485–1486. doi: 10.1016/S1473-3099(21)00558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agrawal U, et al. COVID-19 hospital admissions and deaths after BNT162b2 and ChAdOx1 nCoV-19 vaccinations in 2·57 million people in Scotland (EAVE II): a prospective cohort study. Lancet Respir. Med. 2021 doi: 10.1016/S2213-2600(21)00380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrotri M, et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398:385–387. doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


