Abstract
The germinal centre (GC) response is critical for the generation of affinity-matured plasma cells and memory B cells capable of mediating long-term protective immunity. Understanding whether severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or vaccination elicits a GC response has profound implications for the capacity of responding B cells to contribute to protection against infection. However, direct assessment of the GC response in humans remains a major challenge. Here we summarize emerging evidence for the importance of the GC response in the establishment of durable and broad immunity against SARS-CoV-2 and discuss new approaches to modulate the GC response to better protect against newly emerging SARS-CoV-2 variants. We also discuss new findings showing that the GC B cell response persists in the draining lymph nodes for at least 6 months in some individuals following vaccination with SARS-CoV-2 mRNA-based vaccines.
Subject terms: Germinal centres, Immunological memory
In this Review, Brian Laidlaw and Ali Ellebedy outline our current understanding of the germinal centre response in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and its importance for establishing protective immunity against the virus. They also consider the germinal centre responses seen following vaccination and how germinal centre responses may be modulated to induce broad protection against new variants of SARS-CoV-2.
Introduction
During an immune response, B cells that encounter their cognate antigen become activated and migrate to the centre of the B cell follicle, where they form structures known as germinal centres (GCs)1. Within the GC, B cells compete for a limiting amount of T cell-derived signals, such as cytokines and CD40 ligand, that promote their migration from the light zone to the dark zone2. The magnitude of T cell help received by a B cell in the light zone dictates the extent of cell division and somatic hypermutation that occurs within the dark zone3,4. B cells that accrue productive mutations within their B cell receptor preferentially capture and present antigens to T cells upon their return to the light zone, facilitating their eventual differentiation into memory B cells or plasma cells2,5,6.
Data generated in mouse models suggest that memory B cells tend to emerge from the GC before plasma cells and, accordingly, display reduced levels of somatic hypermutation7–9. Memory B cells persist for years to decades and rapidly differentiate into antibody-secreting cells upon antigen re-encounter10,11. Following antigen re-encounter, memory B cells can also re-enter the GC, where they undergo further affinity maturation11. The reduced mutational load of memory B cells could facilitate their ability to recognize and respond to viral variants, with the memory B cell population in humans containing clones that are broadly reactive to several pathogens, including influenza virus and HIV12,13. By contrast, plasma cells are a terminally differentiated population of cells that tend to be specific for the subtype of virus previously encountered. Plasma cells persist in sites such as the bone marrow and serve as a first line of defence against pathogen reinfection through constitutive secretion of antibodies14–16. In this manner, memory B cells and plasma cells cooperate to provide overlapping layers of protection against reinfection by the pathogen or related variants.
The quality of the B cell response following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection determines the duration and breadth of protective immunity. While SARS-CoV-2 infection induces a durable B cell response, antibody levels decay over time, raising the risk that immunity may wane as the neutralizing antibody titre decreases below the threshold needed to protect against reinfection17–21. SARS-CoV-2 reinfection has been observed among some previously infected individuals, raising the possibility that infection-induced immunity against SARS-CoV-2 may be short-lived, as is the case for seasonal coronaviruses22–28. However, it is not yet clear whether the dynamics of immunity to SARS-CoV-2 will follow the same patterns reported for other coronaviruses.
Additionally, the development and widespread use of mRNA-based and vector-based vaccines against SARS-CoV-2 is likely to profoundly impact the duration of protective immunity29–32. It is particularly important to determine the B cell response following mRNA-based and adenovirus vector-based vaccinations considering that these platforms have not previously been widely used in humans33 (Box 1). mRNA-based vaccines against SARS-CoV-2 have shown 94–95% efficacy against symptomatic disease and 90% efficacy in preventing asymptomatic infection at 12 weeks after vaccination29,30,34–37. While total SARS-CoV-2-specific antibody titres wane over time following vaccination, neutralizing antibody titres and protection against hospitalization and death persist at high levels for at least 6 months38,39. However, key questions remain regarding the duration of protective immunity following mRNA-based vaccination and whether antibodies induced by vaccination will protect against reinfection by SARS-CoV-2 variants.
Considerable progress has been made in elucidating the B cell response following SARS-CoV-2 infection and vaccination. Here we examine emerging evidence that establishment of a robust GC response is critical for the induction of durable protective immunity. We also summarize new data showing that SARS-CoV-2 vaccination induces a GC B cell response that persists for at least 6 months in some individuals. Finally, we discuss the importance of the memory B cell response in protecting against newly emerging viral variants and examine how the GC response can be modulated to induce a more broadly protective B cell response.
Box 1 mRNA-based vaccines.
mRNA-based vaccines use lipid nanoparticles to transport mRNA encoding viral proteins to the cell membrane of host cells123. The nanoparticles are then endocytosed into the cell, where they subsequently escape the endosome and release the enclosed mRNA into the cytoplasm to be subsequently translated into antigenic protein (for example, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike glycoprotein). Exogenous mRNA is inherently immunostimulatory and is recognized by numerous pattern recognition receptors expressed in different locations in the cell173. This allows mRNA-based vaccines to induce a robust T cell and B cell response against the viral protein without requiring additional adjuvants. The lipid nanoparticles used in mRNA-based vaccines are conjugated to several lipids, including polyethylene glycol, to increase their stability and lifespan. The mRNA transcribed by the vaccine has a short half-life and remains in human tissues for only a few days174. While the half-life of the viral protein produced by mRNA-based vaccines is unclear, the persistence of the germinal centre response following vaccination suggests that viral antigen is present for at least 30 weeks after vaccination in some individuals99. mRNA-based vaccines are easily modifiable, allowing rapid production of vaccines containing mRNA encoding proteins expressed by viral variants.
B cell response to SARS-CoV-2 infection
SARS-CoV-2 infection induces a robust humoral immune response in most individuals17,40,41. While the magnitude of the serum antibody response against SARS-CoV-2 is heterogeneous, it generally declines rapidly over the first 4 months after infection, with a more gradual decline evident from that point onwards17–19,40,42–48. Conversely, the SARS-CoV-2-specific memory B cell response increases over the first 4–5 months after infection before plateauing17,40,49. We found that SARS-CoV-2-specific plasma cells are stably maintained in the bone marrow between 7 and 11 months after infection, consistent with a model in which long-lived bone marrow plasma cells maintain serum antibody levels at later time points39,42.
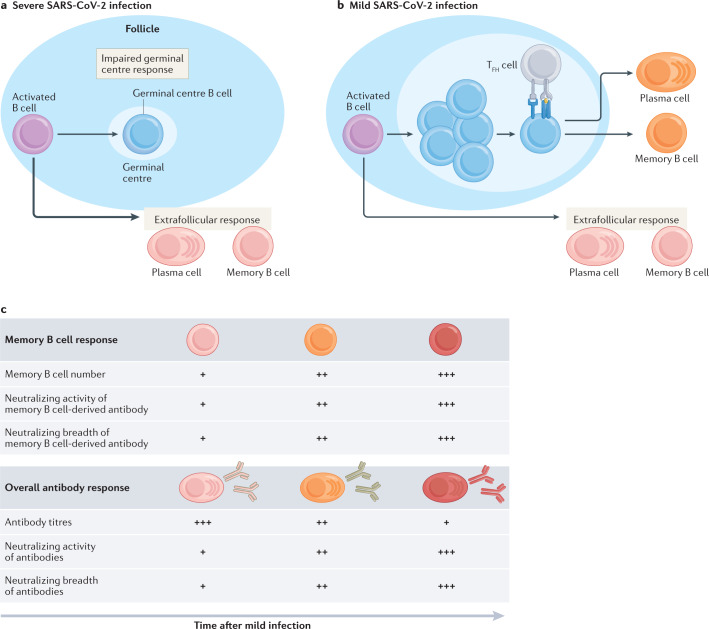
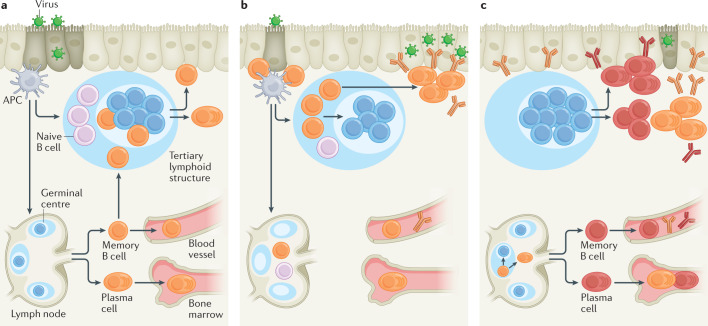
Severe SARS-CoV-2 infection is associated with an elevated antibody and memory B cell response compared with milder infections17,50,51 (Fig. 1a). This could be explained by the fact that severely ill individuals generate a robust extrafollicular B cell response that correlates with an increase in proinflammatory cytokine levels and neutralizing antibody titres52. Severely ill individuals may also be failing to form functional GCs as evidenced by the marked decrease in the number of T follicular helper T (TFH cells) present in the draining lymph nodes and spleen53. The low levels of somatic hypermutation among responding B cells following severe infection is consistent with an impaired GC response and may lead to the production of antibodies that are unable to mediate disease resolution. However, a robust circulating TFH cell response is detectable in the blood of many severely infected individuals, suggesting that the GC response is not defective in all cases54–56. Impaired disease resolution in severely infected individuals may also be caused in part by impaired T cell-mediated clearance of virally infected cells19,52,57–60.
Fig. 1. B cell response to SARS-CoV-2 infection.
a | Severe infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) induces a robust extrafollicular response but an impaired germinal centre (GC) B cell response in some individuals. Antigen-engaged B cells differentiate into memory B cells and short-lived plasmablasts that give rise to antibodies with a low level of somatic hypermutation. b | Mild SARS-CoV-2 infection induces both an extrafollicular response and GC B cell response. The GC B cell response gives rise to affinity-matured memory B cells and long-lived plasma cells. c | There is an increase in the number of SARS-CoV-2-specific memory B cells over time following mild infection. Memory B cells derived from the GC undergo continued clonal evolution for at least 1 year, with memory B cells found at later time points displaying increased levels of somatic hypermutation and encoding antibodies with enhanced neutralizing activity and breadth. The SARS-CoV-2-specific antibody titre decreases over the first 6 months following infection owing to the loss of antibodies derived from short-lived plasmablasts. The loss in protection attributable to this decrease in antibody titre is partially offset by a per antibody increase in neutralizing titre and breadth, likely owing the emergence of clonally evolved plasma cells from the GC. TFH cell, T follicular helper cell.
Mild SARS-CoV-2 infection also induces an early extrafollicular response in which naive and seasonal coronavirus-specific memory B cells differentiate into activated B cells and short-lived plasmablasts61,62 (Fig. 1b). While early SARS-CoV-2-specific memory B cells have near germ line sequences, these cells progressively accrue somatic mutations in their Vh genes, suggesting that they are a product of an ongoing GC response40,41,61,63 SARS-CoV-2 nucleic acids have been detected in the intestine of some individuals for at least 3 months after mild infection and may fuel an ongoing GC response40. The presence of long-lived plasma cells in the bone marrow of SARS-CoV-2-infected individuals further supports this model, as high-affinity plasma cells are predominantly derived from GCs42,64. Indeed, a robust GC and TFH cell response that persists for up to 6 months has been identified in humans and rhesus macaques following SARS-CoV-2 infection65,66.
Antibodies expressed from somatically mutated SARS-CoV-2-specific memory B cells display enhanced antigen binding, neutralizing potency and neutralizing breadth relative to those from memory B cells present at earlier time points40,61,63,67. B cells encoding SARS-CoV-2-specific antibodies that fail to neutralize the virus, including those that cross-react with seasonal coronaviruses, are less detectable at later time points40,41,48,61,68,69. Somatic mutations of the Vh genes in memory B cells are associated with a sustained antibody response and rapid recovery from SARS-CoV-2 infection40,46. Additionally, SARS-CoV-2-specific antibodies in the serum at 10 months after infection display enhanced neutralizing activity and breadth48. Together, these data suggest that the GC response, which is necessary for the development of affinity-matured memory cells and plasma cells, is important for the development of B cells capable of protecting against SARS-CoV-2 infection70–72 (Fig. 1c).
Numerous monoclonal antibodies have been derived from responding B cells in convalescent patients that are capable of neutralizing SARS-CoV-2, with some of these monoclonal antibodies already being used therapeutically to resolve SARS-CoV-2 infection. An antibody titre of 20% of the mean convalescent level has been proposed as sufficient for 50% protection against detectable SARS-CoV-2 infection, while only 3% is necessary for 50% protection from severe infection73. Therefore, an individual who starts with 80% protection against mild disease will have more than 50% protection against severe infection with an antigenically similar strain of SARS-CoV-2 for at least 3 years73. However, the widespread emergence of viral variants that evade neutralization by preformed antibodies present in convalescent serum will likely significantly decrease the duration of antibody-mediated immunity elicited by SARS-CoV-2 infection74,75. In these cases, protection against severe disease will be reliant on the reactivation of somatically mutated memory B cells that recognize antigenically distinct viral variants, which is discussed further in the next section40,76,77.
B cell response to SARS-CoV-2 vaccination
There are several dozen SARS-CoV-2 vaccines that are in use globally. Currently, only the mRNA-based Pfizer–BioNTech and Moderna vaccines and the viral vector-based Johnson & Johnson Janssen vaccine are authorized for use in the United States. mRNA-based vaccination induces a robust SARS-CoV-2-specific antibody response that is strongly enhanced upon administration of a second dose in individuals who were not previously infected69,78–83 (Fig. 2a). About half of vaccinated individuals develop neutralizing antibodies to SARS-CoV-2 after a single dose, with almost all individuals having neutralizing antibodies after the second dose78. mRNA-based vaccination also induces a robust memory B cell response that is further enhanced after the second dose69. There is an increase in the percentage of class-switched memory B cells following the second dose78.
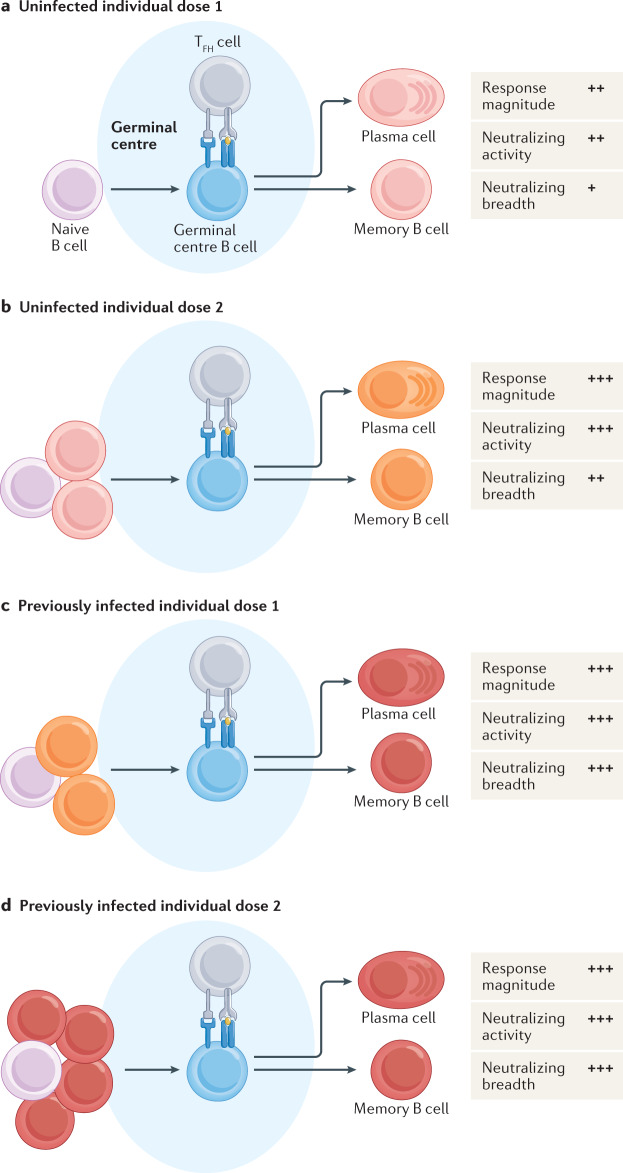
Fig. 2. Germinal centre B cell response to SARS-CoV-2 vaccination.
a | The first dose of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA-based vaccine induces a robust germinal centre response that gives rise to plasma cells and memory B cells in previously uninfected individuals. Primary immunization induces a significant increase in the number of SARS-CoV-2-specific antibodies, with about half of individuals developing neutralizing antibodies. However, these antibodies are largely unable to neutralize SARS-CoV-2 variants. b | The second dose of the SARS-CoV-2 mRNA-based vaccine induces a significant increase in the antibody response in previously uninfected individuals. Antibodies derived from the secondary immunization have robust neutralizing activity, although there is a decrease in the ability to neutralize SARS-CoV-2 variants compared with the wild-type (D614G) virus. c | The first dose of the SARS-CoV-2 mRNA-based vaccine induces a significant increase in the antibody response in individuals who have recovered from previous SARS-CoV-2 infection. Antibodies induced by primary immunization have robust neutralizing activity, with equivalent ability to neutralize wild-type and variant viruses. The number of pre-existing SARS-CoV-2-specific memory B cells strongly correlates with the magnitude of the antibody response following vaccination. d | The second dose of the SARS-CoV-2 mRNA-based vaccine does not induce any further increase in antibody titre or neutralizing activity in individuals who have recovered from previous SARS-CoV-2 infection. TFH cell, T follicular helper cell.
mRNA-based vaccination also induces a significant increase in the numbers of SARS-CoV-2-specific antibodies and memory B cells in individuals who were previously infected with SAR-CoV-263,78,84,85 (Fig. 2b). The magnitude of this increase strongly correlates with the number of pre-existing SARS-CoV-2 memory B cells, indicating that memory B cells are critical in driving a recall response upon re-exposure to SARS-CoV-2 antigens78. Consistent with this model, vaccination results in an increase in the number of all pre-existing SARS-CoV-2-specific memory B cell clones63. No further increase in the SARS-CoV-2-specific antibody or memory B cell response is observed upon administration of a second dose to previously infected individuals, suggesting that only one dose of the mRNA-based vaccine is needed to reach peak humoral immunity in previously infected individuals78,83,86–88. However, this does not exclude a potential beneficial role for the second dose in promoting SARS-CoV-2-specific B cell survival or affinity maturation. Additionally, mRNA-based vaccination induces a robust SARS-CoV-2-specific T cell response, which could be further boosted upon administration of a second dose85,89–91. The importance of T cells in the establishment of protective immunity against SARS-CoV-2 infection is a subject of active investigation (Box 2). While previously infected individuals have elevated numbers of SARS-CoV-2-specific memory B cells at 3 months after vaccination compared with vaccinated uninfected individuals, there is a similar number of memory B cells in both groups at 6 months92.
SARS-CoV-2 mRNA-based vaccines induce a robust GC and TFH cell response in mice93,94. However, the role of the GC response in the human B cell response to vaccination was until recently unclear owing to an inability to directly sample draining lymph nodes following vaccination. We used ultrasound-guided fine needle aspiration to serially sample the draining lymph node following influenza vaccination and found that vaccination induced a robust GC response in which B cells undergo somatic hypermutation95,96. We then used this approach to assess the B cell response in the draining axillary draining lymph nodes of individuals who received the Pfizer–BioNTech SARS-CoV-2 vaccine97. We found that vaccination induced a robust SARS-CoV-2-specific GC B cell and TFH cell response, with administration of a second dose further increasing the percentage of GC B cells97,98. The GC response was composed of both pre-existing memory B cell clones specific for seasonal coronaviruses and newly recruited naive B cells that were specific for unique epitopes within the SARS-CoV-2 spike protein97. Remarkably, SARS-CoV-2-specific GC B cells were maintained in the lymph node at near peak frequency for at least 15 weeks following vaccination, indicating that these cells are likely undergoing affinity maturation97. Memory B cells that bind to SARS-CoV-2 variants display elevated levels of somatic hypermutation compared with cells that bind only wild-type SARS-CoV-2, suggesting a role for the GC in the acquisition of broadly protective immunity92.
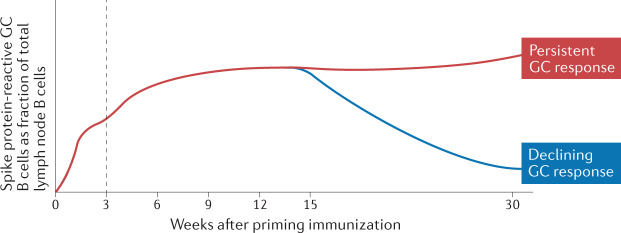
We have now extended these findings to assess the GC response at 30 weeks following vaccination (Fig. 3). We recently reported that 10 of 15 individuals analysed displayed a persistent SARS-CoV-2-specific GC B cell response in the lymph node, with spike protein-binding GC B cells not detected in the other five individuals99. These findings indicate that there is heterogeneity in the duration of the SARS-CoV-2-specific GC response induced by vaccination. It will be important to determine whether GC persistence is associated with enhanced quality of the B cell response and if spike protein antigen is detectable in individuals with persistent GCs. A requirement for antigen in the persistence of the GC response would indicate that antigen availability is an important mechanism governing the development of broadly protective immunity.
Fig. 3. The germinal centre B cell response at 30 weeks following vaccination.
Following vaccination with the Pfizer–BioNTech severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA-based vaccine, the fraction of B cells in the axillary lymph node that are spike protein-reactive germinal centre (GC) B cells increases rapidly. This population further increases upon administration of a second vaccine dose (indicated by the dotted line) and is stably maintained for at least 15 weeks. We have now found that 10 of 15 individuals tested maintain a persistent spike protein-reactive GC B cell response at 30 weeks after vaccination, with the remaining five individuals having no detectable response at this time point. This finding indicates that there is heterogeneity in the longevity of the GC response elicited by vaccination.
Box 2 Cellular immunity to SARS-CoV-2.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection induces a T cell response in almost all individuals, with the magnitude of this response correlated with control of primary infection17,175–177. SARS-CoV-2-specific T cells persist for at least 6 months following infection and maintain polyfunctionality upon peptide simulation178. SARS-CoV-2 vaccination also induces a robust T cell response with broad specificity to peptides present in the spike protein, with the magnitude of this response bolstered by the administration of a second vaccine dose82,91,179–181. Epitopes recognized by SARS-CoV-2-specfic CD4+ T cells and CD8+ T cells were 93% and 97% conserved, respectively, in emerging viral variants89,182. Accordingly, the T cell response to peptide restimulation was minimally impacted by variant mutations182. T cells recognizing conserved epitopes are more abundant in individuals with mild disease and display enhanced expression of markers associated with long-lived memory183. While SARS-CoV-2-specific T cells alone are not expected to be able to prevent reinfection by variant viruses, they may have an important contribution in limiting disease severity184. For example, memory CD4+ T cells can provide accelerated help to B cells upon antigen re-encounter, facilitating the induction of antibodies capable of mediating viral clearance185. Memory T cells that establish residence in mucosal tissues following SARS-CoV-2 infection may also contribute to protective immunity by limiting viral spread beyond the site of reinfection186. Finally, circulating SARS-CoV-2-specific T cells may promote long-term immunity through direct killing of infected cells.
B cell response to SARS-CoV-2 variants
Coronaviruses have a reduced mutation rate and frequency of escape from antibody neutralization compared with smaller RNA viruses owing to their expression of a proofreading 3′-5′ exoribonuclease100,101. Nevertheless, thousands of mutations have been identified in circulating SARS-CoV-2 particles, including a host of mutations in the spike protein that impact disease pathogenesis and susceptibility to antibody neutralization75,102. While convalescent serum is capable of neutralizing viruses expressing the wild-type spike protein, there is a marked decrease in neutralization sensitivity for several of the spike proteins expressing mutations that map to the ACE2-binding site and that are found in variant viruses75,103–108. High concentrations of convalescent serum are still capable of neutralizing viruses expressing escape mutations. However, the existence of confirmed cases of SARS-CoV-2 reinfection suggests that some previously infected individuals may not have sufficient antibody titres to protect against reinfection by SARS-CoV-2 variants75,109. It is important to note that there has been no evidence of antibody-dependent enhancement following infection of vaccinated individuals by escape variants.
Therefore, vaccination is critical to bolster the SARS-CoV-2-specific antibody response to a level sufficient to protect against infection by emerging SARS-CoV-2 variants. One dose of an mRNA-based vaccine was sufficient to significantly enhance the titre of neutralizing antibodies specific for the Alpha (B.1.1.7) and Beta (B.1.351) viral variants in previously infected individuals78. Conversely, two vaccine doses were required in SARS-CoV-2-naive individuals to reliably elicit neutralizing antibodies against the Alpha, Beta and Delta (B.1.617) variants78,85,110. The neutralizing antibody response to viruses expressing escape mutations is reduced relative to that to the wild-type virus in SARS-CoV-2-naive individuals, although it is still present at significant levels63,78,110–116. Viruses expressing mutations in E484 were particularly adept at escaping neutralization from vaccine-induced serum69,105,107,111. The E484 mutation is in the receptor-binding domain of the spike protein and is important for ACE2 binding102. Interestingly, antibodies from previously infected individuals who were vaccinated did not show a reduction in neutralizing titre against the Beta variant63,78. The neutralizing titre against both the wild-type virus and variant viruses is increased in previously infected individuals who were vaccinated relative to vaccinated SARS-CoV-2-naive individuals78,85,117. There is not a significant increase in the mutational load of memory B cells following vaccination in previously infected individuals63. These data suggest that previously infected individuals who were vaccinated may have enhanced immunity to escape variants compared with vaccinated SARS-CoV-2-naive individuals. This may be a result of ongoing clonal evolution increasing the breadth of the B cell response following infection63,118. Broader and/or more persistent exposure to SARS-CoV-2 antigens, as well as differences in the specificity and phenotype of the memory T cell response, likely contribute to the enhanced protective immunity to viral variants in previously infected individuals40,119.
High neutralizing antibody levels are associated with lower infectivity and reduced likelihood of reinfection by SARS-CoV-2 (refs73,120,121). However, individuals who do not have a sufficiently high neutralizing antibody titre to prevent reinfection may still exhibit significant protection from hospitalization and death owing to the memory B cell response. Memory B cells continue to undergo clonal evolution for at least 1 year following infection, with antibodies encoded by these cells capable of binding to the Alpha, Beta and Delta variants63,92. Antibodies derived from 10 of 15 memory B cell clones present at 12 months following infection were able to neutralize all variants tested (including E484-expressing variants) compared with only 1 of 15 clones present at 1.3 months63. The rapid differentiation of cross-reactive SARS-CoV-2-specific memory B cells into antibody-secreting cells may therefore represent a critical mechanism limiting disease pathogenesis upon reinfection with variant viruses.
The persistence of the GC response induced by vaccination suggests that vaccine-induced memory B cells may also increase their neutralizing breadth over time97. Indeed, memory B cells continue to undergo clonal evolution for at least 5 months following vaccination and contain levels of mutations in antibody genes similar to those of memory B cells present at 6 months following infection118. However, there was no significant increase in the neutralizing activity of antibodies encoded by memory B cells between 1.3 and 5 months after vaccination118. Additionally, antibodies encoded by vaccine-induced memory B cells displayed minimal increase in affinity and breadth, with only 4 of 19 antibody pairs conserved between 1.3 and 5 months showing increased potency against pseudotyped viruses expressing the spike protein with mutations found in the Delta variant compared with 11 of 16 antibody pairs conserved between 1.3 and 6 months after infection118. It will be important to expand this analysis to consider additional antibodies encoded by memory B cells and to examine how the duration of the GC response induced by vaccination influences the clonal evolution of memory B cells. The administration of an additional booster vaccine encoding a protein expressed by variant viruses was effective in increasing neutralizing antibody titres against the variant virus in mice and may be necessary to increase the breadth of antibodies encoded by vaccine-induced memory B cells122.
Approaches to induce a broadly reactive memory B cell response
The efficacy of mRNA-based vaccines in protecting against SARS-CoV-2 highlights the importance of continuing to develop new approaches to combat emerging viruses. Development of mRNA-based vaccines was initiated as early as 1990 and was intended to protect against pathogens such Ebola virus, Zika virus, rabies virus and influenza virus before being adapted to target SARS-CoV-2 (refs123,124). Given the likelihood that the rate at which novel diseases emerge from the environment will continue to increase, there is an urgent need to develop new approaches to proactively combat potential sources of future pandemics125. In particular, the development of new strategies to drive a broadly protective memory B cell response against rapidly mutating pathogens would be a valuable tool to combat the threat of future pandemics.
Novel vaccination approaches
One approach to drive a more broadly reactive memory B cell response is to alter the viral regions that are targeted by vaccination. Traditional vaccines induce antibodies specific for the immunodominant viral regions, which tend to undergo frequent mutation to allow the virus to escape antibody neutralization126. However, regions that are conserved between different viral strains, such as the influenza virus haemagglutinin (HA) stalk, undergo much slower mutation, with the mutations that do occur less likely to result in immune evasion127. Therefore, the development of a vaccine that induces antibodies targeting conserved viral regions would be an attractive option to elicit heterosubtypic immunity.
Multiple approaches have been designed to induce broadly reactive memory B cells. A chimeric HA-based influenza vaccine recently completed a phase I trial and was shown to safely elicit cross-reactive antibodies to the HA stalk region expressed by group 1 influenza viruses128. This approach relies on sequential administration of chimeric HA proteins expressing divergent head regions but the same stalk regions to selectively boost stalk-reactive memory B cells. Antibodies induced in humans using this vaccination approach were sufficient to protect mice against a lethal challenge with a divergent strain of influenza virus128. Alternative approaches under development include vaccination with HA proteins in which the head region is masked or removed to drive stalk-specific B cell responses, as well as vaccination with divergent HA proteins129–133. However, significant work remains to optimize these approaches to reliably elicit antibodies capable of providing long-term immunity against group 1 influenza, group 2 influenza and influenza B viruses. Extending these approaches to other pathogens will require identification of surface protein regions that are broadly conserved between different variants and that lead to viral neutralization upon antibody binding. The lack of protective efficacy of antibodies that cross-react between seasonal coronaviruses and SARS-CoV-2 highlights the challenge of identifying the optimal surface region to target to induce heterosubtypic immunity68.
Memory B cell subsets
Another approach to elicit broadly reactive memory B cells following vaccination is to induce an immune response that gives rise to more memory B cells capable of undergoing affinity maturation upon re-exposure to viral antigens. Memory B cells develop through both GC-dependent and GC-independent pathways, with both populations contributing to protective immunity70,134. Atypical memory B cells, which are distinguished on the basis of their expression of T-bet and CD11c, are expanded following severe SARS-CoV-2 infection and may develop independently of the GC50,52,135–138. However, GC-independent memory B cells generally do not go through class switching or affinity maturation, and may not be able to undergo the clonal evolution necessary to neutralize emerging viral variants63,71,134. Therefore, the GC response is an attractive target for efforts to induce a memory B cell response capable of ongoing clonal evolution.
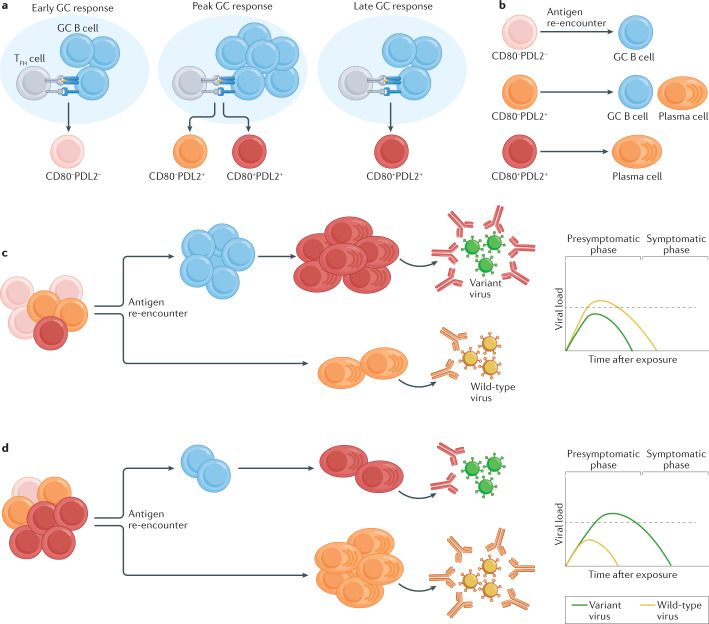
The memory B cell population is composed of multiple functionally and transcriptionally distinct subsets that emerge from the GC at different times9,11,139 (Fig. 4a). While the markers used to identify these subsets differ between mice and humans, transcriptionally distinct memory B cell subsets have been identified in numerous immune contexts, including following SARS-CoV-2 infection136,138,140–142. Memory B cells expressing CD80 and PDL2 in mice tend to differentiate into plasma cells upon antigen re-encounter, while those lacking CD80 and PDL2 re-enter the GC and undergo further somatic hypermutation11,143 (Fig. 4b). Bolstering the number of memory B cells capable of re-entering the GC may therefore enhance the capacity of the memory B cell pool to evolve to neutralize emerging viral variants (Fig. 4c,d). CD80−PDL2− memory B cells display differential expression of numerous cytokine receptors and downstream transcription factors11,143. A better understanding of how these pathways shape the composition of the memory B cell pool is needed to design vaccines that can modulate these pathways to favour the development of a particular memory B cell subset.
Fig. 4. Memory B cell subset development and function.
a | During an immune response, memory B cells emerging from the early germinal centre (GC) response are predominantly CD80−PDL2−. As the GC response matures, memory B cells begin to express PDL2 and CD80, with the memory B cells emerging from the late GC response predominantly being CD80+PDL2+. b | Upon antigen re-encounter, CD80−PDL2− memory B cells predominantly differentiate into GC B cells, CD80−PDL2+ memory B cells differentiate into either GC B cells or plasma cells and CD80+PDL2+ memory B cells differentiate into plasma cells. c | A memory B cell population that is skewed towards CD80−PDL2− memory B cells would be predisposed to differentiate into GC B cells upon antigen re-encounter. This response would promote the development of neutralizing antibodies to variant viruses but would generate a delayed response to wild-type viruses. d | A memory B cell population that is skewed towards CD80+PDL2+ memory B cells would be predisposed to differentiate into plasma cells upon antigen re-encounter. This response would promote the rapid clearance of wild-type viruses but would result in a delay in the induction of antibodies capable of neutralizing variant viruses. The dotted line in the plots on the right in parts c and d indicates the viral load necessary to enable spread of the virus to uninfected individuals. TFH cell, follicular helper T cell.
Mucosal memory B cell response
Many viruses that are potential sources of future pandemics, including SARS-CoV-2 and influenza virus, primarily infect mucosal surfaces and induce a local immune response144,145 (Fig. 5). While antibodies present in the serum are capable of mediating viral clearance in mucosal sites, the establishment of a mucosal memory B cell response would enable a rapid increase in local antibody titre that could mediate rapid viral clearance following reinfection146. Additionally, the induction of a robust mucosal antibody response is critical in preventing viral spread. A subset of SARS-CoV-2-vaccinated individuals who were infected by the Delta variant were recently found to have a similar level of viral transcripts in their upper respiratory tract as infected unvaccinated individuals147. While vaccinated individuals who are reinfected still display reduced duration of viral shedding and infectivity compared with unvaccinated individuals, the development of vaccination approaches that induce sterilizing immunity at mucosal sites is critical to limit the spread of variants that have increased transmission risk148,149.
Fig. 5. The B cell response following mucosal infection.
a | Viruses such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza virus infect respiratory epithelial cells, leading to uptake of antigens by antigen-presenting cells (APCs). Activated APCs migrate to the draining lymph node, where they and lymph node-resident APCs present antigens to cognate T cells and B cells, leading the induction of a germinal centre (GC) response. Tertiary lymphoid structures may also form in response to mucosal inflammation and serve as sites for local GCs. The GC response in the draining lymph node typically precedes the response occurring in the mucosal tissue, with memory B cells that develop in the lymph node capable of migrating to mucosal tissues. Plasma cells derived from the GC response migrate to the bone marrow, where they reside long term. b | Upon antigen re-encounter, memory B cells residing in the mucosal tissues differentiate into plasma cells or re-enter the local GC. Antibodies derived from mucosal plasma cells cooperate with circulating antibodies to mediate early viral control. APCs that take up antigens will migrate to the draining lymph node, where they and lymph node-resident APCs will present antigens to cognate T cells and B cells. c | At later time points, antibodies derived from memory B cells that become reactivated in the draining lymph node contribute to viral clearance. Memory B cells and plasma cells that have undergone further affinity maturation in the mucosal and lymph node GCs will also help mediate viral clearance at later time points.
Memory B cells have been identified in multiple mucosal tissues, including in the lungs of mice following influenza virus infection150–152. Lung-resident memory B cells were associated with enhanced protective immunity upon challenge infection and displayed increased cross-reactivity relative to cells present in the draining lymph node150,153. B cells are found in many mucosal tissues in humans154. SARS-CoV-2 infection induces virus-specific memory B cells in the bone marrow, spleen, lungs and lymph nodes66. Residual antigen depots are also present in mucosal tissues, including in the gut, following SARS-CoV-2 infection, and may fuel the continued clonal evolution of B cells in these sites40,63,155. It is currently unclear whether tissue-resident memory B cells arise from GC responses occurring in the tertiary lymphoid structures of mucosal tissues or whether they migrate to the mucosal tissue after developing in the draining lymph node156–158. Tertiary lymphoid structures are induced by inflammation and are key sites in which memory B cells become reactivated and give rise to antibodies capable of mediating rapid viral clearance159,160.
The development of vaccination approaches that induce a mucosal GC and memory B cell response may therefore significantly enhance the development of protective immunity against newly emerging viruses. For example, an intranasal adenovirus-based vaccine against SARS-CoV-2 that induced mucosal B cells had an enhanced ability to prevent upper and lower airway infection in mice and hamsters compared with the same vaccine administered intramuscularly161,162. An intranasal adenovirus vaccine also induced a cellular and humoral immune response in rhesus macaques and was protective against SARS-CoV-2 infection163. Intranasal delivery of SAR-CoV-2-specific IgM was also effective in protecting against infection in mice164. Together, these studies indicate that vaccines that induce immune responses at mucosal surfaces (that is, vaccines delivered orally or intranasally) may represent an effective strategy to induce protective immunity165.
Concluding remarks and perspective
Remarkable progress had been made in elucidating the B cell response following SARS-CoV-2 infection. However, there remain many key knowledge gaps that will shape the public health response in the years ahead. One central question is whether additional ‘booster’ vaccines expressing mRNA from variant strains will be necessary to induce a B cell response with sufficient breadth and affinity to neutralize future SARS-CoV-2 variants. While the administration of a third vaccine dose of the same formulation will likely result in an increase in antibody titres, it is unlikely to profoundly alter the specificity of the memory B cell response92. Variant-based booster vaccines may be necessary to engage naive B cells that recognize variant-specific epitopes and reshape the composition of the memory B cell response. The memory B cell response continues to undergo clonal evolution even 12 months after SARS-CoV-2 infection, with these mutations critical in increasing the potency and breadth of antibodies derived from these cells63. While vaccine-induced B cells also undergo clonal evolution, likely owing to the persistence of the GC response, it is unclear whether mutations that accumulate over time lead to enhanced neutralizing activity118. It is important to note that vaccination is still essential to bolster the infection-induced B cell response to levels sufficient to protect against reinfection. Determining whether the persistence of the GC following vaccination is associated with enhanced protective immunity as well as understanding the mechanisms underlying the persistence of the GC will be important in evaluating the necessity of additional booster vaccines.
Another key unanswered question is whether SARS-CoV-2 vaccination induces a B cell response in mucosal tissues. While SARS-CoV-2-specific antibodies are detectable in mucosal compartments such as the saliva and breast milk following vaccination, it is unclear whether these antibodies are a product of a local B cell response or how long they persist166–168. SARS-CoV-2 vaccines are administered intramuscularly and therefore are unlikely to induce sufficient levels of antigen expression or inflammation in mucosal tissues to support a local GC response. In the absence of a mucosal B cell response, protection from reinfection will be reliant on maintaining a high enough titre of circulating antibodies to neutralize viruses that infect the airways. The reduced capacity of serum antibodies induced solely by vaccination to neutralize variant viruses relative to antibodies induced by vaccination of previously infected individuals is therefore a significant concern for the long-term prospect of maintaining a sufficient neutralizing antibody titre at mucosal sites to prevent SARS-CoV-2 reinfection, especially against variants such as the Delta variant that induce a significantly higher viral load63,78,148.
Relatedly, understanding how the SARS-CoV-2-specific IgA response differs between vaccinated and infected individuals will be important going forwards. The serum IgA response rapidly declines following both SARS-CoV-2 vaccination and SARS-CoV-2 infection and is less potent at neutralizing SARS-CoV-2 than IgG169,170. However, SARS-CoV-2 infection also elicits a virus-specific IgG, IgA and IgE antibody response in the saliva and bronchoalveolar fluid. Dimeric SARS-CoV-2-specific IgA, the primary form of IgA present in the nasopharynx, has an enhanced ability to neutralize the virus compared with IgG and may have an important role in preventing reinfection171,172. While it is not known whether SARS-CoV-2 vaccination induces a mucosal IgA response in humans, intramuscular vaccination of mice drove a minimal mucosal IgA response and was not as good at mediating viral clearance at mucosal sites as intranasal vaccination161. Therefore, developing vaccination approaches that induce dimeric IgA at mucosal surfaces may be an important tool to limit reinfection.
Acknowledgements
The authors would like to thank J. S. Turner, J. Zhou, W. Kim, S. Teefey, W. Middleton, I. Pusic, J. O’Halloran, R. Presti and the rest of their research teams for their contributions to the unique studies performed at Washington University School of Medicine to understand human immune responses to SARS-CoV-2 infection and vaccination. The Laidlaw laboratory is supported by NIAID grants DP2AI169978 and K22AI153015. The Ellebedy laboratory is supported by NIAID grants U01AI141990 and U01AI150747, NIAID Centers of Excellence for Influenza Research and Surveillance contracts HHSN272201400006C and HHSN272201400008C and NIAID Collaborative Influenza Vaccine Innovation Centers contract 75N93019C00051. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIAID or NIH.
Author contributions
The authors contributed equally to all aspects of the article.
Competing interests
The Ellebedy laboratory received funding under sponsored research agreements that are unrelated to the data presented in the current study from Emergent BioSolutions and from AbbVie. A.H.E. has received consulting payments from Mubadala Investment Company, InBios International LLC and Fimbrion Therapeutics and is the founder of ImmuneBio Consulting LLC. B.J.L. declares no competing interests.
Footnotes
Peer review information
Nature Reviews Immunology thanks G. Gorochov and S. Tangye for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Brian J. Laidlaw, Email: brian.laidlaw@wustl.edu
Ali H. Ellebedy, Email: ellebedy@wustl.edu
References
- 1.Cyster JG, Allen CDC. B cell responses: cell interaction dynamics and decisions. Cell. 2019;177:524–540. doi: 10.1016/j.cell.2019.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Victora GD, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gitlin AD, Shulman Z, Nussenzweig MC. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature. 2014;509:637–640. doi: 10.1038/nature13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gitlin AD, et al. T cell help controls the speed of the cell cycle in germinal center B cells. Science. 2015;349:643–646. doi: 10.1126/science.aac4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kräutler NJ, et al. Differentiation of germinal center B cells into plasma cells is initiated by high-affinity antigen and completed by Tfh cells. J. Exp. Med. 2017;214:1259–1267. doi: 10.1084/jem.20161533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen CDC, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weisel FJ, Zuccarino-Catania GV, Chikina M, Shlomchik MJ. A temporal switch in the germinal center determines differential output of memory b and plasma cells. Immunity. 2016;44:116–130. doi: 10.1016/j.immuni.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shinnakasu R, et al. Regulated selection of germinal-center cells into the memory B cell compartment. Nat. Immunol. 2016;17:861–869. doi: 10.1038/ni.3460. [DOI] [PubMed] [Google Scholar]
- 9.Laidlaw BJ, Duan L, Xu Y, Vazquez SE, Cyster JG. The transcription factor Hhex cooperates with the corepressor Tle3 to promote memory B cell development. Nat. Immunol. 2020;21:1082–1093. doi: 10.1038/s41590-020-0713-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crotty S, et al. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J. Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 11.Zuccarino-Catania GV, et al. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat. Immunol. 2014;15:631–637. doi: 10.1038/ni.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarthy KR, et al. Memory B Cells that cross-react with group 1 and group 2 influenza A viruses are abundant in adult human repertoires. Immunity. 2018;48:174–183.e9. doi: 10.1016/j.immuni.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams LD, et al. Potent and broad HIV-neutralizing antibodies in memory B cells and plasma. Sci. Immunol. 2017;2:eaal2200. doi: 10.1126/sciimmunol.aal2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammarlund E, et al. Plasma cell survival in the absence of B cell memory. Nat. Commun. 2017;8:1781. doi: 10.1038/s41467-017-01901-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zehentmeier S, et al. Static and dynamic components synergize to form a stable survival niche for bone marrow plasma cells. Eur. J. Immunol. 2014;44:2306–2317. doi: 10.1002/eji.201344313. [DOI] [PubMed] [Google Scholar]
- 16.Brynjolfsson SF, Mohaddes M, Kärrholm J, Wick M-J. Long-lived plasma cells in human bone marrow can be either CD19+ or CD19–. Blood Adv. 2017;1:835–838. doi: 10.1182/bloodadvances.2017004481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dan JM, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371:eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wajnberg A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robbiani DF, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584:437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibarrondo FJ, et al. Rapid decay of anti–SARS-CoV-2 antibodies in persons with mild Covid-19. N. Engl. J. Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long Q-X, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 22.Edridge AWD, et al. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 2020;26:1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
- 23.Galanti M, Shaman J. Direct observation of repeated infections with endemic coronaviruses. J. Infect. Dis. 2020;223:jiaa392. doi: 10.1093/infdis/jiaa392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397:1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santos LAD, et al. Recurrent COVID-19 including evidence of reinfection and enhanced severity in thirty Brazilian healthcare workers. J. Infect. 2021;82:399–406. doi: 10.1016/j.jinf.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiyuka PK, et al. Human coronavirus NL63 molecular epidemiology and evolutionary patterns in rural coastal Kenya. J. Infect. Dis. 2018;217:1728–1739. doi: 10.1093/infdis/jiy098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monto AS, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J. Infect. Dis. 2020;222:9–16. doi: 10.1093/infdis/jiaa161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Callow KA, Parry HF, Sergeant M, Tyrrell DAJ. The time course of the immune response to experimental coronavirus infection of man. Epidemiol. Infect. 1990;105:435–446. doi: 10.1017/s0950268800048019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baden LR, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2020;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polack FP, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logunov DY, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voysey M, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anand P, Stahel VP. The safety of Covid-19 mRNA vaccines: a review. Patient Saf. Surg. 2021;15:20. doi: 10.1186/s13037-021-00291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang L, et al. Asymptomatic and symptomatic SARS-CoV-2 infections after BNT162b2 vaccination in a routinely screened workforce. JAMA. 2021;325:2500–2502. doi: 10.1001/jama.2021.6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angel Y, et al. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA. 2021;325:2457–2465. doi: 10.1001/jama.2021.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haas EJ, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Regev-Yochay G, et al. Decreased infectivity following BNT162b2 vaccination. SSRN Electron J. 2021 doi: 10.2139/ssrn.3815668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chemaitelly H, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N. Engl. J. Med. 2021 doi: 10.1056/nejmoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levin EG, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N. Engl. J. Med. 2021 doi: 10.1056/nejmoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaebler C, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakharkar M, et al. Prolonged evolution of the human B cell response to SARS-CoV-2 infection. Sci. Immunol. 2021;6:eabg6916. doi: 10.1126/sciimmunol.abg6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner JS, et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. 2021;595:421–425. doi: 10.1038/s41586-021-03647-4. [DOI] [PubMed] [Google Scholar]
- 43.Ripperger TJ, et al. Orthogonal SARS-CoV-2 serological assays enable surveillance of low prevalence communities and reveal durable humoral immunity. Immunity. 2020;53:925–933.e4. doi: 10.1016/j.immuni.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muecksch F, et al. Longitudinal analysis of serology and neutralizing antibody levels in COVID19 convalescents. J. Infect. Dis. 2020;223:jiaa659. doi: 10.1093/infdis/jiaa659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang K, et al. Longitudinal dynamics of the neutralizing antibody response to SARS-CoV-2 infection. Clin. Infect. Dis. 2020;73:e1143–e1539. doi: 10.1093/cid/ciaa1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y, et al. Quick COVID-19 healers sustain anti-SARS-CoV-2 antibody production. Cell. 2020;183:1496–1507.e16. doi: 10.1016/j.cell.2020.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaisman-Mentesh A, et al. SARS-CoV-2 specific memory B cells frequency in recovered patient remains stable while antibodies decay over time. Preprint at. medRxiv. 2020 doi: 10.1101/2020.08.23.20179796. [DOI] [Google Scholar]
- 48.Moriyama S, et al. Temporal maturation of neutralizing antibodies in COVID-19 convalescent individuals improves potency and breadth to circulating SARS-CoV-2 variants. Immunity. 2021;54:1841–1852.e4. doi: 10.1016/j.immuni.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodda LB, et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell. 2021;184:169–183.e17. doi: 10.1016/j.cell.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuri-Cervantes L, et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 2020;5:eabd7114. doi: 10.1126/sciimmunol.abd7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Long Q-X, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 52.Woodruff MC, et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat. Immunol. 2020;21:1506–1516. doi: 10.1038/s41590-020-00814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaneko N, et al. Loss of Bcl-6-expressing T follicular helper cells and germinal centers in COVID-19. Cell. 2020;183:143–157.e13. doi: 10.1016/j.cell.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fenoglio D, et al. Characterization of T lymphocytes in severe COVID-19 patients. J. Med. Virol. 2021;93:5608–5613. doi: 10.1002/jmv.27037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adamo S, et al. Profound dysregulation of T cell homeostasis and function in patients with severe COVID-19. Allergy. 2021;76:2866–2881. doi: 10.1111/all.14866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang J, et al. Spike-specific circulating T follicular helper cell and cross-neutralizing antibody responses in COVID-19-convalescent individuals. Nat. Microbiol. 2021;6:51–58. doi: 10.1038/s41564-020-00824-5. [DOI] [PubMed] [Google Scholar]
- 57.Nielsen SCA, et al. Human B cell clonal expansion and convergent antibody responses to SARS-CoV-2. Cell Host Microbe. 2020;28:516–525.e5. doi: 10.1016/j.chom.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ju B, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 59.Hoehn KB, Kleinstein SH. Cutting edge: distinct B cell repertoires characterize patients with mild and severe COVID-19. J. Immunol. 2021;206:2785–2790. doi: 10.4049/jimmunol.2100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Z, Wherry EJ. T cell responses in patients with COVID-19. Nat. Rev. Immunol. 2020;20:529–536. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sokal A, et al. Maturation and persistence of the anti-SARS-CoV-2 memory B cell response. Cell. 2021;184:1201–1213.e14. doi: 10.1016/j.cell.2021.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ellebedy AH, et al. Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat. Immunol. 2016;17:1226–1234. doi: 10.1038/ni.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Z, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595:426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phan TG, et al. High affinity germinal center B cells are actively selected into the plasma cell compartment. J. Exp. Med. 2006;203:2419–2424. doi: 10.1084/jem.20061254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lakshmanappa YS, et al. SARS-CoV-2 induces robust germinal center CD4 T follicular helper cell responses in rhesus macaques. Nat. Commun. 2021;12:541. doi: 10.1038/s41467-020-20642-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poon MML, et al. SARS-CoV-2 infection generates tissue-localized immunological memory in humans. Sci. Immunol. 2021 doi: 10.1126/sciimmunol.abl9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dugan HL, et al. Profiling B cell immunodominance after SARS-CoV-2 infection reveals antibody evolution to non-neutralizing viral targets. Immunity. 2021;54:1290–1303.e7. doi: 10.1016/j.immuni.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anderson EM, et al. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell. 2021;184:1858–1864.e10. doi: 10.1016/j.cell.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Z, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592:616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krishnamurty AT, et al. Somatically hypermutated plasmodium-specific IgM+ memory B cells are rapid, plastic, early responders upon malaria rechallenge. Immunity. 2016;45:402–414. doi: 10.1016/j.immuni.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pritchard GH, et al. The development of optimally responsive plasmodium-specific CD73+CD80+IgM+ memory B cells requires intrinsic BCL6 expression but not CD4+ Tfh cells. Preprint at. bioRxiv. 2019 doi: 10.1101/564351. [DOI] [Google Scholar]
- 72.Toyama H, et al. Memory B cells without somatic hypermutation are generated from Bcl6-deficient B cells. Immunity. 2002;17:329–339. doi: 10.1016/s1074-7613(02)00387-4. [DOI] [PubMed] [Google Scholar]
- 73.Khoury DS, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 74.Zhou D, et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184:2348–2361.e6. doi: 10.1016/j.cell.2021.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Z, et al. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe. 2021;29:477–488.e4. doi: 10.1016/j.chom.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tong P, et al. Memory B cell repertoire for recognition of evolving SARS-CoV-2 spike. Cell. 2021;184:4969–4980.e15. doi: 10.1016/j.cell.2021.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DiMuzio JM, et al. Unbiased interrogation of memory B cells from convalescent COVID-19 patients reveals a broad antiviral humoral response targeting SARS-CoV-2 antigens beyond the spike protein. Vaccine X. 2021;8:100098. doi: 10.1016/j.jvacx.2021.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goel RR, et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci. Immunol. 2021;6:eabi6950. doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krammer F, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N. Engl. J. Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saadat S, et al. Single dose vaccination in healthcare workers previously infected with SARS-CoV-2. Preprint at. medRxiv. 2021 doi: 10.1101/2021.01.30.21250843. [DOI] [Google Scholar]
- 81.Bradley T, Grundberg E, Selvarangan R. Antibody responses boosted in seropositive healthcare workers after single dose of SARS-CoV-2 mRNA vaccine. Preprint at. medRxiv. 2021 doi: 10.1101/2021.02.03.21251078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Samanovic MI, et al. Robust immune responses after one dose of BNT162b2 mRNA vaccine dose in SARS-CoV-2 experienced individuals. Preprint at. medRxiv. 2021 doi: 10.1101/2021.02.07.21251311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Levi R, et al. One dose of SARS-CoV-2 vaccine exponentially increases antibodies in recovered individuals with symptomatic COVID-19. J. Clin. Invest. 2021 doi: 10.1172/jci149154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stamatatos L, et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021;372:1413–1418. doi: 10.1126/science.abg9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reynolds CJ, et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science. 2021;372:1418–1423. doi: 10.1126/science.abh1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lozano-Ojalvo D, et al. Differential effects of the second SARS-CoV-2 mRNA vaccine dose on T cell immunity in naïve and COVID-19 recovered individuals. Cell Rep. 2021;36:109570. doi: 10.1016/j.celrep.2021.109570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mazzoni A, et al. First-dose mRNA vaccination is sufficient to reactivate immunological memory to SARS-CoV-2 in recovered COVID-19 subjects. J. Clin. Invest. 2021;131:e149150. doi: 10.1172/JCI149150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ebinger JE, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021;27:981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tarke A, et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2021;2:100355. doi: 10.1016/j.xcrm.2021.100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gallagher KME, et al. SARS -CoV-2 T-cell immunity to variants of concern following vaccination. Preprint at. bioRxiv. 2021 doi: 10.1101/2021.05.03.442455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Woldemeskel BA, Garliss CC, Blankson JN. SARS-CoV-2 mRNA vaccines induce broad CD4+ T cell responses that recognize SARS-CoV-2 variants and HCoV-NL63. J. Clin. Invest. 2021;131:e149335. doi: 10.1172/JCI149335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goel RR, et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021 doi: 10.1126/science.abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lederer K, et al. SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity. 2020;53:1281–1295.e5. doi: 10.1016/j.immuni.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.DiPiazza AT, et al. COVID-19 vaccine mRNA-1273 elicits a protective immune profile in mice that is not associated with vaccine-enhanced disease upon SARS-CoV-2 challenge. Immunity. 2021;54:1869–1882.e6. doi: 10.1016/j.immuni.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Turner JS, et al. Human germinal centres engage memory and naive B cells after influenza vaccination. Nature. 2020;586:127–132. doi: 10.1038/s41586-020-2711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hoehn KB, et al. Human B cell lineages engaged by germinal centers following influenza vaccination are measurably evolving. Preprint at. bioRxiv. 2021 doi: 10.1101/2021.01.06.425648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Turner JS, et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596:109–113. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mudd PA, et al. SARS-CoV-2 mRNA vaccination elicits robust and persistent T follicular helper cell response in humans. Preprint at. bioRxiv. 2021 doi: 10.1101/2021.09.08.459485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim W, et al. Germinal centre-driven maturation of B cell response to SARS-CoV-2 vaccination. Preprint at. bioRxiv. 2021 doi: 10.1101/2021.10.31.466651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith EC, Blanc H, Surdel MC, Vignuzzi M, Denison MR. Coronaviruses lacking exoribonuclease activity are susceptible to lethal mutagenesis: evidence for proofreading and potential therapeutics. PLoS Pathog. 2013;9:e1003565. doi: 10.1371/journal.ppat.1003565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ferron F, et al. Structural and molecular basis of mismatch correction and ribavirin excision from coronavirus RNA. Proc. Natl Acad. Sci. USA. 2018;115:E162–E171. doi: 10.1073/pnas.1718806115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Greaney AJ, et al. Complete mapping of mutations to the SARS-CoV-2 spike receptor-binding domain that escape antibody recognition. Cell Host Microbe. 2021;29:44–57.e9. doi: 10.1016/j.chom.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weisblum Y, et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. eLife. 2020;9:e61312. doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Andreano E, et al. SARS-CoV-2 escape in vitro from a highly neutralizing COVID-19 convalescent plasma. Preprint at. bioRxiv. 2020 doi: 10.1101/2020.12.28.424451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Collier DA, et al. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature. 2021;593:136–141. doi: 10.1038/s41586-021-03412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cele S, et al. Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma. Nature. 2021;593:142–146. doi: 10.1038/s41586-021-03471-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jangra S, et al. The E484K mutation in the SARS-CoV-2 spike protein reduces but does not abolish neutralizing activity of human convalescent and post-vaccination sera. Preprint at. medRxiv. 2021 doi: 10.1101/2021.01.26.21250543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang P, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 109.Nonaka CKV, et al. Genomic evidence of a SARS-CoV-2 reinfection case with E484K spike mutation in Brazil. Emerg. Infect. Dis. 2021;596:1522–1524. doi: 10.3201/eid2705.210191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Planas D, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 111.Garcia-Beltran WF, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184:2372–2383.e9. doi: 10.1016/j.cell.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Muik A, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine–elicited human sera. Science. 2021;371:1152–1153. doi: 10.1126/science.abg6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu K, et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. Preprint at. bioRxiv. 2021 doi: 10.1101/2021.01.25.427948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Edara V-V, et al. Infection and vaccine-induced neutralizing antibody responses to the SARS-CoV-2 B.1.617.1 variant. N. Engl. J. Med. 2021;385:664–666. doi: 10.1056/NEJMc2107799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu C, et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184:4220–4236.e13. doi: 10.1016/j.cell.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wall EC, et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397:2331–2333. doi: 10.1016/S0140-6736(21)01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lucas C, et al. Impact of circulating SARS-CoV-2 variants on mRNA vaccine-induced immunity. Nature. 2021 doi: 10.1038/s41586-021-04085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cho A, et al. Anti-SARS-CoV-2 receptor binding domain antibody evolution after mRNA vaccination. Nature. 2021 doi: 10.1038/s41586-021-04060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zuo J, et al. Robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nat. Immunol. 2021;22:620–626. doi: 10.1038/s41590-021-00902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bergwerk M, et al. Covid-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021 doi: 10.1056/nejmoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Earle KA, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu K, et al. Variant SARS-CoV-2 mRNA vaccines confer broad neutralization as primary or booster series in mice. Preprint at. bioRxiv. 2021 doi: 10.1101/2021.04.13.439482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines — a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wolff J, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 125.Morens DM, Fauci AS. Emerging pandemic diseases: how we got to COVID-19. Cell. 2020;182:1077–1092. doi: 10.1016/j.cell.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Heaton NS, Sachs D, Chen C-J, Hai R, Palese P. Genome-wide mutagenesis of influenza virus reveals unique plasticity of the hemagglutinin and NS1 proteins. Proc. Natl Acad. Sci. USA. 2013;110:20248–20253. doi: 10.1073/pnas.1320524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kirkpatrick E, Qiu X, Wilson PC, Bahl J, Krammer F. The influenza virus hemagglutinin head evolves faster than the stalk domain. Sci. Rep. 2018;8:10432. doi: 10.1038/s41598-018-28706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nachbagauer R, et al. A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat. Med. 2021;27:106–114. doi: 10.1038/s41591-020-1118-7. [DOI] [PubMed] [Google Scholar]
- 129.Impagliazzo A, et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science. 2015;349:1301–1306. doi: 10.1126/science.aac7263. [DOI] [PubMed] [Google Scholar]
- 130.Yassine HM, et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 2015;21:1065–1070. doi: 10.1038/nm.3927. [DOI] [PubMed] [Google Scholar]
- 131.Eggink D, Goff PH, Palese P. Guiding the immune response against influenza virus hemagglutinin toward the conserved stalk domain by hyperglycosylation of the globular head domain. J. Virol. 2014;88:699–704. doi: 10.1128/JVI.02608-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ellebedy AH, et al. Adjuvanted H5N1 influenza vaccine enhances both cross-reactive memory B cell and strain-specific naive B cell responses in humans. Proc. Natl Acad. Sci. USA. 2020;117:17957–17964. doi: 10.1073/pnas.1906613117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nachbagauer R, et al. Induction of broadly reactive anti-hemagglutinin stalk antibodies by an H5N1 vaccine in humans. J. Virol. 2014;88:13260–13268. doi: 10.1128/JVI.02133-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Viant C, et al. Germinal center–dependent and –independent memory B cells produced throughout the immune response. J. Exp. Med. 2021;218:e20202489. doi: 10.1084/jem.20202489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Winslow GM, Papillion AM, Kenderes KJ, Levack RC. CD11c+T-bet+ memory B cells: immune maintenance during chronic infection and inflammation? Cell Immunol. 2017;321:8–17. doi: 10.1016/j.cellimm.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oliviero B, et al. Expansion of atypical memory B cells is a prominent feature of COVID-19. Cell Mol. Immunol. 2020;17:1101–1103. doi: 10.1038/s41423-020-00542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wildner NH, et al. B cell analysis in SARS-CoV-2 versus malaria: increased frequencies of plasmablasts and atypical memory B cells in COVID-19. J. Leukoc. Biol. 2021;109:77–90. doi: 10.1002/JLB.5COVA0620-370RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mathew D, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369:eabc8511. doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tomayko MM, Steinel NC, Anderson SM, Shlomchik MJ. Cutting edge: hierarchy of maturity of murine memory B cell subsets. J. Immunol. 2010;185:7146–7150. doi: 10.4049/jimmunol.1002163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stamper C, Wilson P. Distinct B cell subsets give rise to antigen-specific antibody responses against SARS-CoV-2. Res. Sq. 2010 doi: 10.21203/rs.3.rs-80476/v1. [DOI] [Google Scholar]
- 141.Ogega CO, et al. Durable SARS-CoV-2 B cell immunity after mild or severe disease. J. Clin. Invest. 2021;131:e145516. doi: 10.1172/JCI145516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Laidlaw BJ, Cyster JG. Transcriptional regulation of memory B cell differentiation. Nat. Rev. Immunol. 2021;21:209–220. doi: 10.1038/s41577-020-00446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.He J-S, et al. IgG1 memory B cells keep the memory of IgE responses. Nat. Commun. 2017;8:641. doi: 10.1038/s41467-017-00723-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Russell MW, Moldoveanu Z, Ogra PL, Mestecky J. Mucosal immunity in COVID-19: a neglected but critical aspect of SARS-CoV-2 infection. Front. Immunol. 2020;11:611337. doi: 10.3389/fimmu.2020.611337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sungnak W, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Palladino G, Mozdzanowska K, Washko G, Gerhard W. Virus-neutralizing antibodies of immunoglobulin G (IgG) but not of IgM or IgA isotypes can cure influenza virus pneumonia in SCID mice. J. Virol. 1995;69:2075–2081. doi: 10.1128/jvi.69.4.2075-2081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Centers for Disease Control and Prevention Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings — Barnstable County, Massachusetts, July 2021. MMWR. 1995;70:1059–1062. doi: 10.15585/mmwr.mm7031e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li B, et al. Viral infection and transmission in a large, well-traced outbreak caused by the SARS-CoV-2 Delta variant. Preprint at. medRxiv. 2021 doi: 10.1101/2021.07.07.21260122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shamier MC, et al. Virological characteristics of SARS-CoV-2 vaccine breakthrough infections in health care workers. Preprint at. medRxiv. 2021 doi: 10.1101/2021.08.20.21262158. [DOI] [Google Scholar]
- 150.Allie SR, et al. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat. Immunol. 2019;20:97–108. doi: 10.1038/s41590-018-0260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Allie SR, Randall TD. Resident memory B cells. Viral Immunol. 2020;33:282–293. doi: 10.1089/vim.2019.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Weisel NM, et al. Comprehensive analyses of B-cell compartments across the human body reveal novel subsets and a gut-resident memory phenotype. Blood. 2020;136:2774–2785. doi: 10.1182/blood.2019002782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Adachi Y, et al. Distinct germinal center selection at local sites shapes memory B cell response to viral escape. J. Exp. Med. 2015;212:1709–1723. doi: 10.1084/jem.20142284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sathaliyawala T, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim TS, Hufford MM, Sun J, Fu Y-X, Braciale TJ. Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. J. Exp. Med. 2010;207:1161–1172. doi: 10.1084/jem.20092017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tan H-X, et al. Inducible bronchus-associated lymphoid tissues (iBALT) serve as sites of B cell selection and maturation following influenza infection in mice. Front. Immunol. 2019;10:611. doi: 10.3389/fimmu.2019.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Moyron-Quiroz JE, et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25:643–654. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 158.Moyron-Quiroz JE, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 159.Onodera T, et al. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. Proc. Natl Acad. Sci. USA. 2012;109:2485–2490. doi: 10.1073/pnas.1115369109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.GeurtsvanKessel CH, et al. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus–infected mice. J. Exp. Med. 2009;206:2339–2349. doi: 10.1084/jem.20090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hassan AO, et al. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183:169–184.e13. doi: 10.1016/j.cell.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bricker TL, et al. A single intranasal or intramuscular immunization with chimpanzee adenovirus-vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters. Cell Rep. 2021;36:109400. doi: 10.1016/j.celrep.2021.109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Doremalen NV, et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models. Sci. Transl. Med. 2021;13:eabh0755. doi: 10.1126/scitranslmed.abh0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ku Z, et al. Nasal delivery of an IgM offers broad protection from SARS-CoV-2 variants. Nature. 2021;595:718–723. doi: 10.1038/s41586-021-03673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lavelle EC, Ward RW. Mucosal vaccines — fortifying the frontiers. Nat. Rev. Immunol. 2021 doi: 10.1038/s41577-021-00583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mades A, et al. Detection of persistent SARS-CoV-2 IgG antibodies in oral mucosal fluid and upper respiratory tract specimens following COVID-19 mRNA vaccination. Preprint at. medRxiv. 2021 doi: 10.1101/2021.05.06.21256403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ketas TJ, et al. Antibody responses to SARS-CoV-2 mRNA vaccines are detectable in saliva. Pathog. Immun. 2021;6:116–134. doi: 10.20411/pai.v6i1.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Perl SH, et al. SARS-CoV-2–specific antibodies in breast milk after COVID-19 vaccination of breastfeeding women. JAMA. 2021;325:2013–2014. doi: 10.1001/jama.2021.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wisnewski AV, Luna JC, Redlich CA. Human IgG and IgA responses to COVID-19 mRNA vaccines. PLoS ONE. 2021;16:e0249499. doi: 10.1371/journal.pone.0249499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sterlin D, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021;13:eabd2223. doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang Z, et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci. Transl. Med. 2021;13:eabf1555. doi: 10.1126/scitranslmed.abf1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Corthésy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 2013;4:185. doi: 10.3389/fimmu.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen N, et al. RNA sensors of the innate immune system and their detection of pathogens. Iubmb Life. 2017;69:297–304. doi: 10.1002/iub.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Probst J, et al. Spontaneous cellular uptake of exogenous messenger RNA in vivo is nucleic acid-specific, saturable and ion dependent. Gene Ther. 2007;14:1175–1180. doi: 10.1038/sj.gt.3302964. [DOI] [PubMed] [Google Scholar]
- 175.Moderbacher CR, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Peng Y, et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bergamaschi L, et al. Longitudinal analysis reveals that delayed bystander CD8+ T cell activation and early immune pathology distinguish severe COVID-19 from mild disease. Immunity. 2021;54:1257–1275.e8. doi: 10.1016/j.immuni.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Breton G, et al. Persistent cellular immunity to SARS-CoV-2 infection. J. Exp. Med. 2021;218:e20202515. doi: 10.1084/jem.20202515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sahin U, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 180.Jackson LA, et al. An mRNA vaccine against SARS-CoV-2-preliminary report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Painter MM, et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immune responses to SARS-CoV-2 mRNA vaccination. Immunity. 2021 doi: 10.1016/j.immuni.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tarke A, et al. Comprehensive analysis of T cell immunodominance and immunoprevalence of SARS-CoV-2 epitopes in COVID-19 cases. Cell Rep. Med. 2021;2:100204. doi: 10.1016/j.xcrm.2021.100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mallajosyula V, et al. CD8+ T cells specific for conserved coronavirus epitopes correlate with milder disease in COVID-19 patients. Sci. Immunol. 2021;6:eabg5669. doi: 10.1126/sciimmunol.abg5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lipsitch M, Grad YH, Sette A, Crotty S. Cross-reactive memory T cells and herd immunity to SARS-CoV-2. Nat. Rev. Immunol. 2020;20:709–713. doi: 10.1038/s41577-020-00460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.MacLeod MKL, et al. Memory CD4 T cells that express CXCR5 provide accelerated help to B cells. J. Immunol. 2011;186:2889–2896. doi: 10.4049/jimmunol.1002955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Grau-Expósito J, et al. Peripheral and lung resident memory T cell responses against SARS-CoV-2. Nat. Commun. 2021;12:3010. doi: 10.1038/s41467-021-23333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]