Abstract
Background
The mammalian target of rapamycin (mTOR) pathway is upregulated in nearly half of hepatocellular carcinoma (HCC) tumors and is associated with poor prognosis. In preclinical models of HCC, the combination of mTOR pathway inhibition with the multikinase inhibitor sorafenib improves treatment efficacy. A prior phase I study of the allosteric mTOR inhibitor temsirolimus combined with sorafenib demonstrated acceptable safety at the recommended phase II dose.
Methods
We conducted a single-arm, multicenter phase II trial of the combination of temsirolimus 10 mg intravenously weekly plus sorafenib 200 mg b.i.d. The primary endpoint was time to progression (TTP) with efficacy target of median TTP of at least 6 months; secondary endpoints included overall survival (OS), objective response rate, safety, and alpha-fetoprotein (AFP) tumor marker response. Next-generation tumor sequencing was performed as an exploratory endpoint.
Results
Twenty-nine patients were enrolled, including 48% with hepatitis C virus infection and 28% with hepatitis B virus; 86% had Barcelona clinic liver cancer stage C disease. Among 28 patients evaluable for efficacy, the median TTP was 3.7 (95% confidence interval [CI]: 2.2, 5.3) months, with 14% of patients achieving TTP of at least 6 months. The median OS was 8.8 (95% CI: 6.8, 14.8) months. There were no complete or partial responses; 75% of patients had stable disease as best response. AFP decline by at least 50% was associated with prolonged TTP and OS. Serious adverse events occurred in 21%; the most common treatment-related adverse events of CTCAE grade 3 or higher were hypophosphatemia (36%), thrombocytopenia (14%), and rash (11%). There were no grade 5 events attributed to sorafenib or temsirolimus. Tumor next-generation sequencing (NGS) was performed in a subgroup of 24 patients with adequate tumor samples. Tumor mTOR pathway mutations were identified in 42%. There was no association between tumor mutation profile and OS or TTP.
Conclusions
The combination of temsirolimus and sorafenib demonstrated acceptable safety but did not achieve the target threshold for efficacy in this phase II study. Tumor NGS including the presence of mTOR pathway mutations was not associated with treatment response in an exploratory subgroup analysis.
Keywords: Sorafenib, Temsirolimus, Hepatocellular carcinoma, Mammalian target of rapamycin, Next-generation sequencing
Introduction
Hepatocellular carcinoma (HCC) is rising in incidence and represents the third leading cause of death worldwide [1]. Heterogeneous tumor biology without prevalent driver mutations poses a challenge to identifying effective therapies in HCC [2]. Despite recent advances in systemic therapies, the prognosis for advanced stages of HCC remains poor, and unlike most other common cancers, there are no established tumor genetic biomarkers to guide treatment decisions.
The multikinase inhibitor sorafenib targets a variety of oncogenic pathways in HCC, including vascular endothelial growth factor receptors 2 and 3 and RAF kinase [3]. Sorafenib became a global standard of care for unresectable and advanced stages of HCC after 2 randomized, phase III trials demonstrated improvement in survival over placebo [4, 5]. Despite consistent prolongation of survival and progression-free survival, the benefit from sorafenib is modest in most patients, and no clinical or genetic biomarkers have been validated as predictive of differential response [6, 7].
The mammalian target of rapamycin (mTOR) pathway demonstrates upregulation in around 40–50% of HCC tumors and is associated with poorer prognosis [2, 8, 9]. The potential mechanisms of mTOR pathway activation are heterogeneous and include inactivating mutations or deletions in tumor suppressors, such as the tuberous sclerosis complex (TSC1, 2) or phosphatase and tensin homolog, activating mutations in oncogenes, such as AKT or phosphatidylinositol-3′ kinase, or ligand-dependent growth factor receptor activation [2, 9, 10]. Despite the prevalence of mTOR pathway activation in HCC; however, the allosteric inhibitor of mTOR complex 1 (mTORC1) everolimus did not improve overall survival (OS) in the randomized, phase III EVOLVE-1 trial in an unselected, advanced HCC population, though a subgroup of patients with underlying hepatitis B virus (HBV) infection demonstrated prolongation of survival compared to placebo [11]. An exploratory subanalysis of EVOLVE-1 identified decreased levels or loss of TSC2 expression by immunohistochemistry in 10.8% (15/139) of evaluable patients' tumor samples [12]. Low levels or loss of TSC2 expression showed a nonsignificant trend toward longer OS with everolimus treatment compared with placebo in this study, though interpretation is limited by small sample size.
The combination of mTOR inhibition with antiangiogenic therapies, such as sorafenib confers potential for additive benefit or synergy [13, 14]. We previously conducted a multicenter, phase I, dose-escalation study of the combination of the allosteric mTORC1 inhibitor, temsirolimus, combined with sorafenib in 25 patients [15]. The maximum tolerated dose and recommended phase II dose were temsirolimus 10 mg intravenously (IV) administered weekly plus sorafenib 200 mg by mouth twice a day (BID). Pharmacokinetic analyses showed that the temsirolimus dose of 10 mg IV weekly in the phase I study produced similar area under the curve and slower clearance than a 25 mg IV weekly dose in studies of non-HCC patients, suggesting potential diminished metabolism in HCC patients, even those with preserved, Child-Pugh A liver function [15]. The disease control rate in the phase I study was 68%, motivating further investigation in a phase II trial.
Here we report results from the ensuing multicenter, phase II trial of the combination of mTOR inhibition with temsirolimus and sorafenib for patients with unresectable and advanced stages of HCC. Tumor next-generation DNA sequencing (NGS) was performed to explore for candidate biomarkers of response.
Materials and Methods
Study Design and Ethics Oversight
This investigator-initiated study was designed as an open-label, single-arm, phase II trial with enrollment at the Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco (UCSF) and the Robert H. Lurie Comprehensive Cancer Center at Northwestern University (NWU). All patients provided written informed consent. The trial was approved by the 2 participating centers' Institutional Review Boards (UCSF IRB 12-09445, NWU IRB STU00071124) and the NCCN Temsirolimus Scientific Review Committee. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice and was registered on clinicaltrials.gov (NCT01687673).
Patient Eligibility
Patients must have been of age of at least 18 years with histologically confirmed diagnosis of HCC; patients with histology of mixed HCC-cholangiocarcinoma were eligible with study chair approval if the treating investigator assessed that HCC-directed therapy was appropriate based upon pathology and clinical characteristics. Tumors were required to be AJCC stage II, III, or IV [16] at study entry, not amenable to curative resection, transplantation, or ablative therapies, and without any prior systemic therapy for HCC. Tumors must have been measurable by RECIST v1.1 [17]. Patients had to have adequate organ function, including hemoglobin at least 8.5 g/dL, platelets at least 75,000/mcL, bilirubin ≤2 mg/dL or 1.5 times upper limits of normal, albumin at least 2.8 g/dL, cholesterol <350 mg/dL, triglycerides <300 mg/dL, and glycosylated hemoglobin <7.5%. Active HBV was required to have treatment antiviral therapy according to institutional standard of care. Child-Pugh score of A or B7 was required. Blood pressure at entry was required to be ≤150/90 mm Hg. Eastern Cooperative Oncology Group (ECOG) score of 0–1 and life expectancy of at least 3 months were required. Primary biliary tract cancers or fibrolamellar histology were excluded. Patients with nonhealing wounds, prior liver transplant, uncontrolled intercurrent illness, or requirement for therapeutic anticoagulation were excluded.
Treatment
Patients were treated with the combination of temsirolimus plus sorafenib according to the recommended phase II dose established in the prior phase I study [18]. Cycle length was 28 days. Temsirolimus was administered at a fixed dose of 10 mg IV with in-line filter over 60 min weekly during cycle 1, then over 30 min during subsequent cycles if no evidence of hypersensitivity. Diphenhydramine premedication was administered approximately 30 min prior to each temsirolimus infusion. Sorafenib was administered at a dose of 200 mg orally b.i.d. starting cycle 1, day 1 after temsirolimus infusion. Dose modifications in temsirolimus to 7.5 mg or 5 mg IV and in sorafenib to 200 mg once daily or every other day were permitted for management of toxicity along with optimal supportive care.
Study Assessments
Adverse events (AEs) were assessed using CTCAE v.4.0 [19] at clinic visits weekly during cycle 1 and monthly thereafter, along with weekly vital sign and laboratory monitoring for the duration of the study. Efficacy was assessed with multiphase (including arterial and portal venous contrast phases) computed tomography, or magnetic resonance imaging of abdomen and pelvis, along with computed tomography scans of chest every 8 weeks on treatment along with alpha-fetoprotein (AFP) tumor marker each cycle. Baseline pre-treatment archival tumor samples with adequate tumor cell content underwent NGS using the UCSF500 Cancer Gene Panel Test (UCSF500), as previously described [20, 21].
Study Endpoints and Statistical Analysis Plan
The primary endpoint was time to progression (TTP) [22]. Enrollment of 28 patients was planned to achieve a sample size of at least 25 efficacy-evaluable patients to test the alternate hypothesis of median TTP of at least 6 months, with null hypothesis of median TTP of 3 months or less with 1-sided alpha of 10% and power of 88% under the exact test. Patients who did not complete at least 1 dose of protocol therapy and/or were determined ineligible after enrollment were replaced. Secondary endpoints included objective response rate by RECIST v.1.1 [17], OS, AFP response defined as a 50% decrease at time of best response in patients with an elevated baseline AFP of at least 20 ng/mL [23, 24], and safety, characterized by adverse event profile and dose modifications or discontinuations for toxicity. Tumor genotype by NGS was explored for relationship with TTP and OS using log-rank tests, and for association with clinical covariates using Fisher's exact tests. There was no correction for multiple comparisons owing to exploratory nature of these analyses.
Results
Patient Characteristics
Twenty-nine patients were enrolled (24 at UCSF, 5 at NWU) between October 2012 and October 2015. One patient enrolled at NWU was replaced due to discontinuation for elevated liver function prior to starting treatment. The most common cause of underlying liver disease was hepatitis C virus (HCV) in 48%, with HBV as the cause of liver disease in 28%. Most patients (86%) had advanced, Barcelona clinic liver cancer stage C disease, with portal vein thrombosis in 41% and extrahepatic spread in 69%. Serum AFP level was at least 400 ng/mL in 31%. A majority of patients (72%) had received at least 1 prior liver-directed therapy for HCC. Patient characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Characteristic (N = 29 enrolled) | N or median (range) | % |
|---|---|---|
| Gender, n | ||
| Male | 25 | 86 |
| Female | 4 | 14 |
| Age, years | ||
| Median (range) | 61 (33–78) | |
| Baseline body weight, kg | ||
| Median (range) | 76.75 (41.17–130) | |
| Race | ||
| African-American | 1 | 3 |
| Asian | 10 | 34 |
| Caucasian | 18 | 62 |
| Ethnicity | ||
| Non-Hispanic/Latino | 25 | 86 |
| Hispanic/Latino | 4 | 14 |
| ECOG | ||
| 0 | 21 | 72 |
| 1 | 8 | 28 |
| Etiology of liver disease | ||
| HBV sAg+ | 8 | 28 |
| HCV Ab+ | 14 | 48 |
| HCV Ab+ with HBV cAb total+ | 8 | 28 |
| Alcoholic liver disease | 1 | 3 |
| NASH | 1 | 3 |
| Other/Unknown/Idiopathic | 5 | 17 |
| Child-Pugh class | ||
| A5 or A6 | 26 | 90 |
| B7 | 3 | 10 |
| Tumor histology | ||
| HCC | 27 | 93 |
| Mixed HCC-cholangiocarcinoma | 2 | 7 |
| BCLC stage | ||
| B | 4 | 14 |
| C | 25 | 86 |
| Portal vein thrombosis present | 12 | 41 |
| Extrahepatic spread present | 20 | 69 |
| Baseline albumin, g/dL | ||
| Median (range) | 3.8 (2.8–4.7) | |
| Baseline AFP, ng/mL | ||
| <400 | 20 | 69 |
| ≥400 | 9 | 31 |
| Median (range) | 156 (1–98,622) | |
| Treatment site | ||
| UCSF | 24 | 83 |
| NWU | 5 | 17 |
| Previous therapy | ||
| Surgical resection | 6 | 21 |
| Bland embolization | 1 | 3 |
| Chemoembolization (TACE) | 16 | 55 |
| Radioembolization | 3 | 10 |
| Ablation (radiofrequency or microwave) | 7 | 24 |
| None | 8 | 28 |
ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; NASH, nonalcoholic steatohepatitis; BCLC, Barcelona clinic liver cancer; NWU, Northwestern University; UCSF, University of California, San Francisco; AFP, alpha-fetoprotein.
Patient Disposition
The median number of cycles received was 3 (range: 1–11), as shown in Table 2. One patient enrolled at NWU did not receive any temsirolimus infusions and discontinued from study due to elevated bilirubin attributed to rapid disease progression on subsequent imaging, rendering the patient nonevaluable for safety or efficacy and requiring replacement per protocol. Dose reductions were required in sorafenib in 7%, in temsirolimus in 29%, or in both drugs in 11%; drug discontinuation for toxicity occurred in 14% for temsirolimus and in 11% for both drugs. Most patients (79%) discontinued treatment due to disease progression, though 21% discontinued due to AEs without progression.
Table 2.
Patient disposition
| Patients treated (N = 28) | N or median (range or %) |
|---|---|
| Median cycles received | 3 (1–11) |
| Evaluable for safety | 28 (100) |
| Evaluable for efficacy | 28 (100) |
| Dose reduction for AE | |
| TEM | 8 (29) |
| SOR | 2 (7) |
| Both | 3 (11) |
| Dose discontinuation for AE | |
| TEM | 4 (14) |
| SOR | 0 |
| Both | 3* (11) |
| Reason for discontinuation | |
| Tumor progression | 22* (79) |
| Adverse event/toxicity | 6 (21) |
One patient discontinued due to clinical progression and at least possibly treatment-related toxicity; principal reason for discontinuation was adjudicated as progression. AE, adverse event.
Efficacy Outcomes
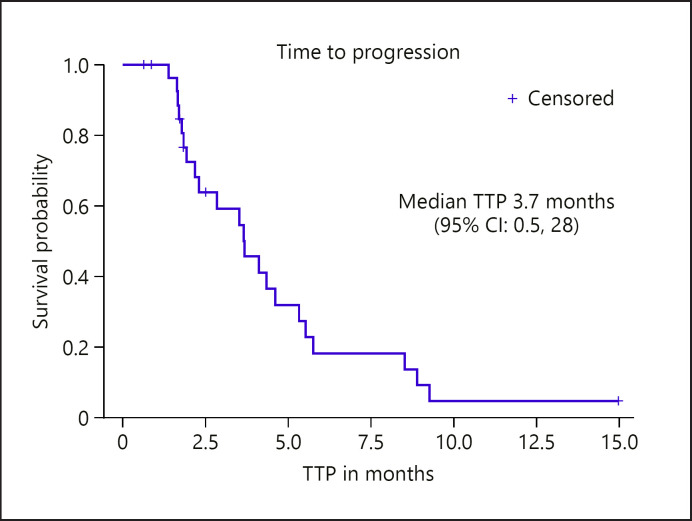
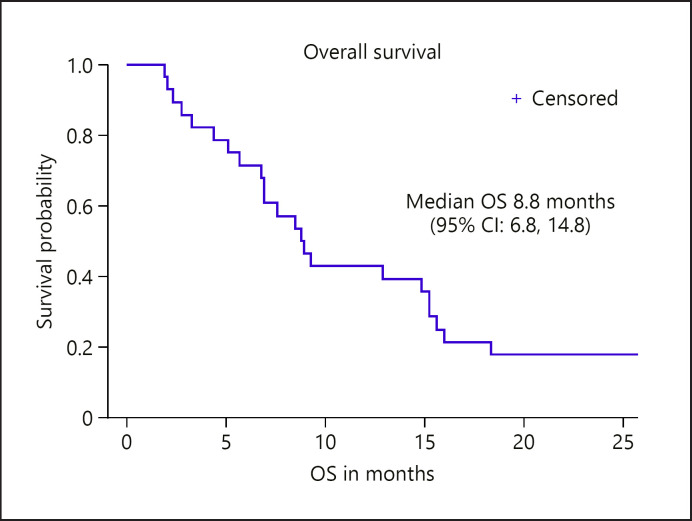
Twenty-eight patients (97%) were evaluable for the primary endpoint of TTP. The median TTP was 3.7 (95% CI: 2.2, 5.3) months, with 14% (95% CI: 0.5, 28) achieving TTP of at least 6 months (see Fig. 1). The median OS was 8.8 (95% CI: 6.8, 14.8) months (see Fig. 2). There were no partial or complete responses on study. A best response of stable disease occurred in 21 (75%), while 4 (14%) had best response of progressive disease (Table 3). There was no significant relationship between HBsAg + or HCV Ab + status and TTP or OS outcomes.
Fig. 1.
TTP in efficacy-evaluable patients (n = 28). One patient was not evaluable for TTP due to hepatic decompensation requiring removal before receipt of any dose of temsirolimus. TTP was censored according to protocol for 6 patients who discontinued treatment for adverse events without progression. TTP, time to progression; CI, confidence interval.
Fig. 2.
OS for enrolled study population (n = 29). OS was censored for 1 patient still alive at time of data lock. OS, overall survival; CI, confidence interval.
Table 3.
ORR
| Best response by RECIST v.1.1 | (N = 28 efficacy-evaluable), n (%) |
|---|---|
| Complete response | 0 |
| Partial response | 0 |
| Stable disease | 21 (75) |
| Progressive disease | 4 (14) |
| Not available* | 3 (11) |
Objective radiographic response for 3 patients was not available due to discontinuation due to toxicity prior to restaging imaging [3]. One patient was not evaluable for efficacy due to discontinuation for hepatic decompensation before receiving a dose of temsirolimus and not included. ORR, objective radiographic response.
Safety
Serious adverse events (SAEs) occurred in 6 of 28 (21%) safety-evaluable patients, with at least possibly treatment-related SAE occurring in 2 patients (7%) (1 patient with an SAE of grade 3 diarrhea and grade 3 dehydration requiring hospitalization, and 1 patient with SAE of grade 3 cellulitis and grade 2 extremity pain requiring hospitalization). The most common treatment-related AEs of CTCAE grade 3 or higher were hypophosphatemia, thrombocytopenia, and rash. Adverse events adjudicated as at least possibly related to treatment with incidence of at least 10% are displayed in Table 4.
Table 4.
Summary of treatment-related AE in ≥10% of patients
| Category and CTCAE term (18) | Grade 1–2, n (%) | Grade 3–4, n (%) | All grades (N = 28), n (%) |
|---|---|---|---|
| Blood and lymphatic system disorders | |||
| Anemia | 3 (11) | 2 (7) | 5 (18) |
| Neutropenia | 4 (14) | 2 (7) | 6 (21) |
| Thrombocytopenia | 15 (54) | 4 (14) | 19 (68) |
| Constitutional disorders | |||
| Anorexia | 12 (43) | 0 | 12 (43) |
| Fatigue or malaise | 14 (60) | 0 | 14 (60) |
| Weight loss | 13 (46) | 0 | 13 (56) |
| Gastrointestinal disorders | |||
| Abdominal pain | 7 (25) | 0 | 7 (25) |
| Constipation | 3 (11) | 0 | 3 (11) |
| Diarrhea | 14 (60) | 1 (4) | 15 (54) |
| Dry mouth | 4 (14) | 0 | 4 (14) |
| Dysgeusia | 7 (25) | 0 | 7 (25) |
| Dyspepsia/heartburn | 3 (11) | 0 | 3 (11) |
| Nausea | 10 (36) | 0 | 10 (36) |
| Stomatitis/sore mouth | 6 (21) | 0 | 6 (21) |
| General disorders and administration site conditions | |||
| Chills/diaphoresis | 4 (14) | 0 | 4 (14) |
| Fever | 4 (14) | 0 | 4 (14) |
| Infusion reaction | 3 (11) | 1 (4) | 4 (14) |
| Hemorrhage and bleeding | 7 (25) | 1 (4) | 8 (29) |
| Infections and infestations | 3 (11) | 1 (4) | 4 (14) |
| Investigations and metabolic disorders | |||
| Cholesterol/triglyceride elevation | 14 (60) | 1 (4) | 15 (54) |
| Hyperglycemia | 4 (14) | 1 (4) | 5 (18) |
| Hypoalbuminemia | 9 (32) | 0 | 9 (32) |
| Hypokalemia/hypomagnesemia | 7 (25) | 2 (7) | 9 (32) |
| Hypophosphatemia | 13 (46) | 10 (36) | 23 (82) |
| Transaminase elevation | 5 (18) | 1 (4) | 6 (21) |
| Musculoskeletal disorders | |||
| Edema | 6 (21) | 0 | 6 (21) |
| Musculoskeletal pain | 7 (25) | 0 | 7 (25) |
| Myalgias/cramping | 5 (18) | 0 | 5 (18) |
| Nervous system disorder | |||
| Headache | 4 (14) | 0 | 4 (14) |
| Respiratory disorders | |||
| Hoarseness/voice changes | 7 (25) | 0 | 7 (25) |
| Pneumonitis | 3 (11) | 0 | 3 (11) |
| Skin and subcutaneous tissue disorders | |||
| Dry skin | 4 (14) | 0 | 4 (14) |
| Palmar-plantar erythrodysesthesia | 8 (29) | 0 | 8 (29) |
| Rash | 17 (61) | 3 (11) | 20 (71) |
| Vascular | |||
| Hypertension | 4 (14) | 1 (4) | 5 (18) |
There were no grade 5 events attributed to be at least possibly related to temsirolimus, sorafenib, or both. AEs, adverse events
Biomarker Analyses
AFP response, defined as decline in AFP from baseline AFP level of at least 50%, occurred in 10 of 21 patients (48%) who were evaluable with baseline AFP elevation of at least 20 ng/mL and at least 1 serial AFP measurement on treatment. Patients who experienced an AFP response had longer TTP (median 5.8 months, 95% CI: 4.1, 9.3) by comparison to patients without AFP response (n = 11), for whom the median TTP was 2.3 months (95% CI: 1.6, 3.5) (p < 0.0001 by log-rank test) (see online suppl. Fig 1a; see www.karger.com/doi/10.1159/000518297 for all online suppl. material). Median OS was also longer in patients who experienced AFP response (median 11.1 months, 95% CI: 1.9, 18.3) and median OS was 7.6 months (95% CI: 2.3, 8.9) (p = 0.10 by log-rank test) (see online suppl. Fig. 1b).
Next-Generation Sequencing
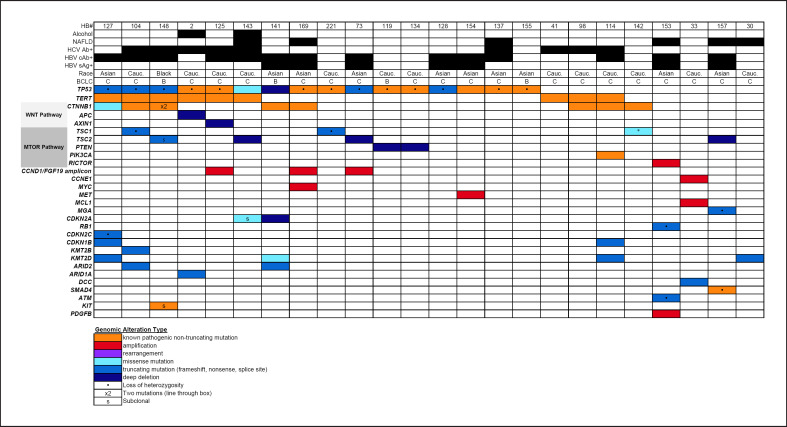
Archival tumor samples were collected and evaluated for specimen adequacy for tumor NGS using the UCSF500 panel. Twelve samples had sufficient tumor content for UCSF500 sequencing; alteration data from clinical NGS with the FoundationOne panel was included from 1 additional patient. An additional 11 samples with sufficient tumor samples for NGS were procured from the preceding phase I trial of the same treatment combination [18], resulting in a total sample size of 24 patients with available tumor NGS results (n = 23 by UCSF500, n = 1 by FoundationOne) (“NGS cohort”). Pathogenic alterations were identified in the NGS cohort with frequencies summarized in Table 5, along with co-mutations and clinical covariates in Figure 3. The most prevalent alterations were in TP53 (67%), TERT promoter (38%), CTNNB1 (33%), TSC1 or TSC2 (30%), KMT2D (17%), and CCND1/FGF19/4/3 amplicon (13%). Wnt pathway mutations (including CTNNB1, APC, and AXIN1) were present in 42%, and mTOR pathway mutations (including TSC1, TSC2, phosphatase and tensin homolog, phosphatidylinositol-3′ kinase, and RICTOR) were present in 46%. There was no significant relationship between any of the prevalent tumor mutations or groups with incidence ≥10% and TTP or OS. There was a nonsignificant trend toward shorter TTP and OS in patients with mTOR pathway mutations. TERT promoter mutations were associated with HCV Ab+ (p = 0.0001), non-HBsAg+ (p = 0.223), and non-Asian race (p = 0.033) by Fisher's exact tests.
Table 5.
Summary of high-frequency tumor mutations (≥10%) by NGS with UCSF500 panel
| Alteration in UCSF500 panel (19, 20) | Incidence of mutation in pooled phase I and II study NGS cohort | Median TTP (95% CI), months | p value | Median OS (95% CI), months | p value |
|---|---|---|---|---|---|
| Overall NGS cohort (n = 24) | − | 2.88 (1.71, 4.67) | − | 13.87 (7.59, 24.39) | − |
| TP53 | 0.67 | 2.07 (1.38, 5.32) | 0.55 | 13.87 (6.93, 24.39) | 0.80 |
| TERT promoter | 0.38 | 3.45 (0.85, 4.67) | 0.33 | 14.86 (3.32, 40.80) | 0.77 |
| mTOR pathway | 0.46 | 1.94 (0.85, 5.52) | 0.49 | 9.27 (2.99, 15.22) | 0.12 |
| WNT pathway | 0.42 | 3.45 (0.85, 4.67) | 0.95 | 7.7 (2.99, 26.79) | 0.06 |
| KMT2D | 0.17 | 3.57 (2.30, 4.31) | 0.61 | 24.49 (8.78, 29.42) | 0.84 |
| CCND1/FGF19/4/3 amplicon | 0.13 | 5.33 (1.15, 9.27) | 0.22 | 12.89 (3.65, 32.12) | 0.73 |
NGS, next-generation sequencing; UCSF, University of California, San Francisco; OS, overall survival; TTP, time to progression; CI, confidence interval; mTOR, mammalian target of rapamycin.
Fig. 3.
Tile plot of tumor mutations and clinical covariates. HBV, hepatitis B virus; HCV, hepatitis C virus; BCLC, Barcelona clinic liver cancer; TSC, tuberous sclerosis complex; PTEN, phosphatase and tensin homolog; PIK3CA, phosphatidylinositol-3′ kinase
Discussion
This phase II study of the combination of temsirolimus plus sorafenib originated from preclinical rationale, suggesting potential for synergy between mTOR inhibition and vascular endothelial growth factor pathway inhibition, as well as promising disease control in the preceding phase I trial [18]. Among the 28 evaluable patients treated with the combination of temsirolimus plus sorafenib; however, the median TTP of 3.7 months failed to disprove the null hypothesis, and only 14% of patients were free of progression at 6 months. The median OS of 8.8 months did not differentiate the combination from sorafenib as monotherapy [4, 5], or the combination of atezolizumab plus bevacizumab which has emerged as a new standard of care for first-line therapy in advanced HCC [25].
Contemporary to this study, the randomized, phase II SAKK 77/08 and SASL 29 trial reported on the outcomes of sorafenib 400 mg twice daily combined with everolimus 5 mg once daily in 106 patients (46 treated with sorafenib alone and 60 treated with the combination of sorafenib plus everolimus) [26]. The median TTP was 7.6 months for sorafenib monotherapy and 6.3 months for the combination; OS was similar in both arms (10 and 12 months, respectively). The authors concluded that there was no evidence for improved efficacy for the combination, and that the rate of AEs was higher. The SAKK/SASL study efficacy outcomes for sorafenib in combination with everolimus are similar to the findings from our study, reinforcing the impression that the combination of sorafenib with an mTORC1 inhibitor does not improve upon outcomes for sorafenib alone in an unselected population.
The adverse event profile for the combination of temsirolimus with sorafenib in this study indicate overall acceptable safety and tolerability, with all-cause SAE occurring in 21% of patients, and discontinuation for AEs required in 14% for temsirolimus and in 11% for both drugs. These rates are lower than the SAE and dose discontinuation rates reported for the combination arm of the randomized, phase II trial of sorafenib with or without everolimus, perhaps owing to the lower starting dose of sorafenib of 200 mg twice daily in our study based upon the preceding phase I study MTD [18]. These safety findings reinforce the importance of dose finding studies to be conducted in HCC cohorts for combination therapies with potential for overlapping toxicity and/or reliance on hepatic metabolism.
AFP response, defined in this study as 50% reduction occurring at any time after start of treatment in patients with baseline AFP of at least 20 ng/mL, was a secondary efficacy endpoint. AFP response was associated with significantly prolonged TTP, and a trend toward longer OS. Similarly, associations between AFP response and progression-free survival and OS also have been reported from randomized trials of targeted therapies in patients with advanced stages of HCC, including sorafenib, cabozantinib, and ramucirumab, as well as for the combination of atezolizumab plus bevacizumab [23, 24, 27, 28]. The consistency of this association between AFP response and efficacy outcomes across studies of systemic therapy in HCC warrants further prospective study of AFP response as a surrogate for radiographic response assessment in advanced HCC, noting the potential for radiographic assessments to be confounded by infiltrative tumor patterns, scarring from prior liver-directed therapies, or nodularity due to cirrhosis in this population.
A planned exploratory analysis was tumor NGS and association with clinical endpoints. Evaluable tumor samples were available from 13 patients enrolled in this phase II study along with 11 additional patients from the preceding phase I trial of the same treatment combination. The proportion of patients with TP53 mutations in this advanced HCC cohort was 67%, numerically higher than in earlier stage patients studied in TCGA (30.1%) [29], consistent with the known association of TP53 mutation with poor prognosis [30]. None of the prevalent mutations or pathway groupings were associated with TTP or OS in this subanalysis; patients with mTOR pathway mutations demonstrated a trend toward shorter TTP and OS compatible with known poor prognostic impact of mTOR pathway activation and suggesting against substantial differential benefit from temsirolimus in the mTOR pathway subgroup. TERT promoter mutations were associated with the presence of HCV infection and absence of HBV infection, as has been previously reported [31].
A potential limitation of this study was the choice of temsirolimus, an allosteric mTORC1 inhibitor, as the combination partner for sorafenib. Effective mTOR pathway targeting may require both mTORC1 and mTORC2 inhibition to address distinct downstream effectors of mTOR activation, as well as the potential for upregulation of mTORC2 signaling as an escape mechanism [32]. A phase I study of the dual mTORC1/2 inhibitor CC-223/ATG-008 achieved objective responses in 11% of patients in the HCC expansion cohort [32, 33]. Additional studies of dual mTORC1/2 inhibitors, including CC-223/ATG-008 as monotherapy in an Asian population with HBV + HCC (NCT03591965) and MLN0128 (NCT02575339) are now ongoing in advanced HCC; CC-223/ATG-008 is also being studied in combination with the anti-PD-1 immune checkpoint inhibitor, toripalimab in a phase I/II study in advanced solid tumors, including HCC (NCT04337463). Demonstration of safety and efficacy as monotherapy will be required before pursuing combination approaches, and the optimal combination partner remains unknown. Limitations of the exploratory tumor NGS subanalyses of this study include the small sample size; population heterogeneity, owing to pooling of data from patients in the prior phase I study; and the possibility that susceptibility to mTOR inhibition may not be predictable by tumor genotype, owing to the propensity for ligand-dependent mTOR pathway activation in the absence of recurring mutations. The lack of adequate HCC tumor tissue for biomarker analyses in over half of patients in this study, despite requirement for histologic diagnosis for study entry, represents a recurring challenge in advanced HCC systemic therapy trials, underscoring the need to increase tumor biospecimen collection from HCC patients enrolled in prospective clinical trials.
Conclusion
The regimen of temsirolimus combined with sorafenib does not demonstrate sufficient efficacy to warrant further exploration in the expanding landscape of treatment options for advanced HCC. Tumor genomic biomarkers of response remain an important unmet need to guide choice of targeted therapies in this biologically heterogeneous cancer.
Statement of Ethics
This phase II clinical trial was conducted in accordance with the World Medical Association Declaration of Helsinki and with approval from the Institutional Review Board (IRB) of both institutions (UCSF IRB 12-09445, NWU IRB STU00071124). All patients provided written informed consent.
Conflict of Interest Statement
R.K.K. reports the following conflicts of interest: research funding to institution from Agios, Astra Zeneca, Bayer, Bristol-Myers Squibb, Eli Lilly, EMD Serono, Exelixis, Merck, Novartis, Partner Therapeutics, QED, Taiho; consulting/advisory fees to self from Exact Sciences, Genentech/Roche, and Gilead; travel support for satellite symposium from Ipsen. H.S.N. reports the conflicts of interest: employment by Foundation Medicine, Inc. L.M.K. reports the following conflicts of interest: Advisory board consultant for Bayer, Eisai, Exelixis, Genentech; consultant for Merck; speaker for Eisai, Gilead, Target HCC, and Peerview CME. Z.N. reports the conflicts of interest: employment by Nektar Therapeutics. S.C.B. reports the conflicts of interest: consulting/advisory fees to self from AAA Novartis and Progenics. A.B.B. reports the following conflicts of interest: research funding to institution from Celgene, Infinity Pharmaceuticals, Merck Sharp and Dohme, Taiho Pharmaceutical, Rafael Pharmaceuticals, Medimmune/AstraZeneca, Xencor, ST Pharm, Elevar Therapeutics, Lexicon Pharmaceuticals/TerSera; advisor or consultant for Bristol-Myers Squibb, Therabionic, Guardant, Merck (advisory), Lexicon, Amgen (DMC/advisor), Artemida Pharma, Array/Pfizer (advisory), AbbVie, Apexigen, Tyme, SynCore, Samsung, HalioDx, Janssen, Natera; Research Data Monitoring Committee member for Bristol-Myers Squibb, Astellas, Amgen, Syncore, Tyme. A.P.V. reports the following conflicts of interest: research funding to institution from Amgen Novartis; consulting/advisory fees from Array, QED and Glaxo Smith Kline. J.D.G. reports the conflicts of interest: consulting/advisory fees to self from Genentech/Roche. N.M.J., J.H., C.O., K.Z., and A.G.B. report no conflict of interest to declare.
Funding Sources
This work was supported by a grant from the National Comprehensive Cancer Network (NCCN) through general research support provided by Pfizer, Inc. Temsirolimus was provided by Pfizer, Inc. Support for biospecimen collection was provided by the Bili Project Foundation, Inc. Tumor NGS was supported by the UCSF HDFCCC 500 Cancer Genes Sequencing Pilot Program. The funding sources were not involved in collection or analysis of the data or preparation of the manuscript. J.D.G. is supported by a Burroughs Wellcome Fund Career Award.
Author Contributions
R.K.K. contributed to conception and design of study; contributed to acquisition, analysis, and interpretation of data; drafted, revised, and approved manuscript; and agrees to be accountable for accuracy and integrity of work. J.D.G. contributed to acquisition and interpretation of data; contributed to revision of, and approved submitted manuscript; and agrees to be accountable for accuracy and integrity of work. H.S.N. contributed to design of study; contributed to acquisition and interpretation of data; contributed to revision of and approved submitted manuscript; and agrees to be accountable for accuracy and integrity of work. N.M.J. contributed to interpretation of data; contributed to revision of and approved submitted manuscript; and agrees to be accountable for accuracy and integrity of work. L.M.K. contributed to acquisition and interpretation of data; contributed to revision of and approved submitted manuscript; and agrees to be accountable for accuracy and integrity of work. Z.N. contributed to acquisition of data; contributed to revision of and approved submitted manuscript; and agrees to be accountable for accuracy and integrity of work. J.H. contributed to design of study; contributed to analysis and interpretation of data; contributed to revision of and approved submitted manuscript; and agrees to be accountable for accuracy and integrity of work. S.C.B. contributed to acquisition and interpretation of data; contributed to revision of and approved submitted manuscript; and agrees to be accountable for accuracy and integrity of work. C.O. contributed to interpretation of data; contributed to revision of and approved submitted manuscript; and agrees to be accountable for accuracy and integrity of work. K.Z. contributed to acquisition of data; contributed to revision of and approved submitted manuscript; and agrees to be accountable for accuracy and integrity of work. A.G.B. contributed to acquisition of data; contributed to revision of and approved submitted manuscript; and agrees to be accountable for accuracy and integrity of work. A.B.B. contributed to conception and design of study; contributed to interpretation of data; contributed to revision of and approved submitted manuscript; and agrees to be accountable for accuracy and integrity of work. A.P.V. contributed to conception and design of study; contributed to interpretation of data; contributed to revision of and approved submitted manuscript; and agrees to be accountable for accuracy and integrity of work.
Data Availability Statement
All data analyzed during this study are included in this article and its online suppl. files. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68((6)):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15((10)):599–616. doi: 10.1038/s41571-018-0073-4. [DOI] [PubMed] [Google Scholar]
- 3.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64((19)):7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 4.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10((1)):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359((4)):378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol. 2017;67((5)):999–1008. doi: 10.1016/j.jhep.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 7.Pinyol R, Montal R, Bassaganyas L, Sia D, Takayama T, Chau GY, et al. Molecular predictors of prevention of recurrence in HCC with sorafenib as adjuvant treatment and prognostic factors in the phase 3 STORM trial. Gut. 2019;68((6)):1065–75. doi: 10.1136/gutjnl-2018-316408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matter MS, Decaens T, Andersen JB, Thorgeirsson SS. Targeting the mTOR pathway in hepatocellular carcinoma: current state and future trends. J Hepatol. 2014;60((4)):855–65. doi: 10.1016/j.jhep.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135((6)):1972–11. doi: 10.1053/j.gastro.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol. 2018;15((5)):273–91. doi: 10.1038/nrclinonc.2018.28. [DOI] [PubMed] [Google Scholar]
- 11.Zhu AX, Kudo M, Assenat E, Cattan S, Kang YK, Lim HY, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014;312((1)):57–67. doi: 10.1001/jama.2014.7189. [DOI] [PubMed] [Google Scholar]
- 12.Huynh H, Hao HX, Chan SL, Chen D, Ong R, Soo KC, et al. Loss of tuberous sclerosis complex 2 (TSC2) is frequent in hepatocellular carcinoma and predicts response to mTORC1 inhibitor everolimus. Mol Cancer Ther. 2015;14((5)):1224–35. doi: 10.1158/1535-7163.MCT-14-0768. [DOI] [PubMed] [Google Scholar]
- 13.Zhu AX, Sahani DV, Duda DG, di Tomaso E, Ancukiewicz M, Catalano OA, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27((18)):3027–35. doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin B, Edeline J, Patard JJ, Oger E, Jouan F, Boulanger G, et al. Combination of Temsirolimus and tyrosine kinase inhibitors in renal carcinoma and endothelial cell lines. J Cancer Res Clin Oncol. 2012;138((6)):907–16. doi: 10.1007/s00432-012-1162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelley RK, Nimeiri HS, Munster PN, Vergo MT, Huang Y, Li CM, et al. Temsirolimus combined with sorafenib in hepatocellular carcinoma: a phase I dose-finding trial with pharmacokinetic and biomarker correlates. Ann Oncol. 2013;24((7)):1900–7. doi: 10.1093/annonc/mdt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benson AB, 3rd, D'Angelica MI, Abbott DE, Abrams TA, Alberts SR, Saenz DA, et al. NCCN guidelines insights: hepatobiliary cancers, Version 1.2017. J Natl Compr Canc Netw. 2017;15((5)):563–73. doi: 10.6004/jnccn.2017.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45((2)):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Kelley RK, Nimeiri HS, Munster PN, Vergo MT, Huang Y, Li CM, et al. Temsirolimus combined with sorafenib in hepatocellular carcinoma: a phase I dose-finding trial with pharmacokinetic and biomarker correlates. Ann Oncol. 2013;24((7)):1900–7. doi: 10.1093/annonc/mdt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Cancer Institute, Division of Cancer Treatment and Diagnosis; Common terminology criteria for adverse events (CTCAE) [updated 2018 Jan 3; cited 2019 Jan 24]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm. [Google Scholar]
- 20.UCSF 500 cancer gene panel test (UCSF500/UC500) The Regents of the University of California; 2020. [cited 2020 Sep 27]. Available from: https://genomics.ucsf.edu/content/ucsf-500-cancer-gene-panel-test-ucsf500-uc500. [Google Scholar]
- 21.Joseph NM, Tsokos CG, Umetsu SE, Shain AH, Kelley RK, Onodera C, et al. Genomic profiling of combined hepatocellular-cholangiocarcinoma reveals similar genetics to hepatocellular carcinoma. J Pathol. 2019;248((2)):164–78. doi: 10.1002/path.5243. [DOI] [PubMed] [Google Scholar]
- 22.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100((10)):698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 23.Chau I, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, et al. Alpha-fetoprotein kinetics in patients with hepatocellular carcinoma receiving ramucirumab or placebo: an analysis of the phase 3 REACH study. Br J Cancer. 2018;119((1)):19–26. doi: 10.1038/s41416-018-0103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelley RK, Meyer T, Rimassa L, Merle P, Park JW, Yau T, et al. Serum alpha-fetoprotein levels and clinical outcomes in the Phase III CELESTIAL Study of cabozantinib versus placebo in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2020;26((18)):4795–804. doi: 10.1158/1078-0432.CCR-19-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finn RS, Cheng AL. Atezolizumab and bevacizumab in hepatocellular carcinoma. Reply. N Engl J Med. 2020;383((7)):695. doi: 10.1056/NEJMc2021840. [DOI] [PubMed] [Google Scholar]
- 26.Koeberle D, Dufour JF, Demeter G, Li Q, Ribi K, Samaras P, et al. Sorafenib with or without everolimus in patients with advanced hepatocellular carcinoma (HCC): a randomized multicenter, multinational phase II trial (SAKK 77/08 and SASL 29) Ann Oncol. 2016;27((5)):856–61. doi: 10.1093/annonc/mdw054. [DOI] [PubMed] [Google Scholar]
- 27.Galle PR, Foerster F, Kudo M, Chan SL, Llovet JM, Qin S, et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39((12)):2214–29. doi: 10.1111/liv.14223. [DOI] [PubMed] [Google Scholar]
- 28.Personeni N, Bozzarelli S, Pressiani T, Rimassa L, Tronconi MC, Sclafani F, et al. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2012;57((1)):101–7. doi: 10.1016/j.jhep.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Liver hepatocellular carcinoma (TCGA, PanCancer Atlas) [cited 2020 Oct 11]. Available from: https://www.cbioportal.org/study/summary?id=lihc_tcga_pan_can_atlas_2018.
- 30.Woo HG, Wang XW, Budhu A, Kim YH, Kwon SM, Tang ZY, et al. Association of TP53 mutations with stem cell-like gene expression and survival of patients with hepatocellular carcinoma. Gastroenterology. 2011;140((3)):1063–70. doi: 10.1053/j.gastro.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cancer Genome Atlas Research Network Comprehensive and Integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169((7)):1327–41.e23. doi: 10.1016/j.cell.2017.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bendell JC, Kelley RK, Shih KC, Grabowsky JA, Bergsland E, Jones S, et al. A phase I dose-escalation study to assess safety, tolerability, pharmacokinetics, and preliminary efficacy of the dual mTORC1/mTORC2 kinase inhibitor CC-223 in patients with advanced solid tumors or multiple myeloma. Cancer. 2015;121((19)):3481–90. doi: 10.1002/cncr.29422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varga A, Mita MM, Wu JJ, Nemunaitis JJ, Cloughesy TF, Mischel PS, et al. Phase I expansion trial of an oral TORC1/TORC2 inhibitor (CC-223) in advanced solid tumors. J Clin Oncol. 2013 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
All data analyzed during this study are included in this article and its online suppl. files. Further inquiries can be directed to the corresponding author.