Abstract
Background
Cardiac metastases from thyroid cancers are uncommon with a poor prognosis. There is a lack of long-term follow-up studies.
Cases
We report 2 cases of cardiac metastasis from medullary thyroid cancer (MTC). Both patients presented limited metastatic disease apart from a cardiac metastasis. The initial diagnosis was challenging and was facilitated by functional imaging with an immuno-PET-CT using an anti-CEA bispecific antibody and a <sup>68</sup>Ga-labeled peptide. Both patients were treated with the multitarget kinase inhibitor vandetanib with prolonged stability. The first patient was alive at the last follow-up, 14 years after the diagnosis of cardiac metastasis. The second patient required surgical excision of the cardiac mass because of disease progression under vandetanib.
Conclusion
These cases illustrate long-term survival and effectiveness of clinical management of 2 patients who developed cardiac metastases from MTC, in the current era of personalized medicine with targeted therapy.
Keywords: Medullary thyroid cancers, Cardiac metastasis, Rearranged during transfection inhibitors, Vandetanib, Rearranged during transfection mutation
Established Facts
Cardiac metastases from thyroid cancers are uncommon with a poor prognosis.
Novel Insights
Our work highlights 2 major points:
The diagnostic challenge represented by cardiac metastases: in both cases, the diagnosis was based on conventional imaging and originally confirmed by functional imaging with an immuno-PET scan using anti-CEA bispecific antibody and 68Ga-labeled peptide which is the ultimate evidence of the medullary thyroid origin.
The long-term survival of 2 patients who have developed cardiac metastases from MTC, in the current era of personalized medicine with targeted therapy, has never been described in the previous literature.
Introduction
Cardiac metastases from medullary thyroid cancers (MTC) are uncommon. Their frequency in large autopsy series in patients with thyroid cancers is low, between 0 and 5% [1, 2, 3, 4, 5]. Given the rarity of this metastatic location, the outcome remains unclear. The average length of survival is short, especially in patients with symptomatic and unresectable cardiac metastases [6]. This poor prognosis paradigm may be reconsidered in the light of current available therapies. We report here 2 cases of MTC cardiac metastasis with long-term outcome under the tyrosine kinase inhibitor vandetanib.
Patient 1
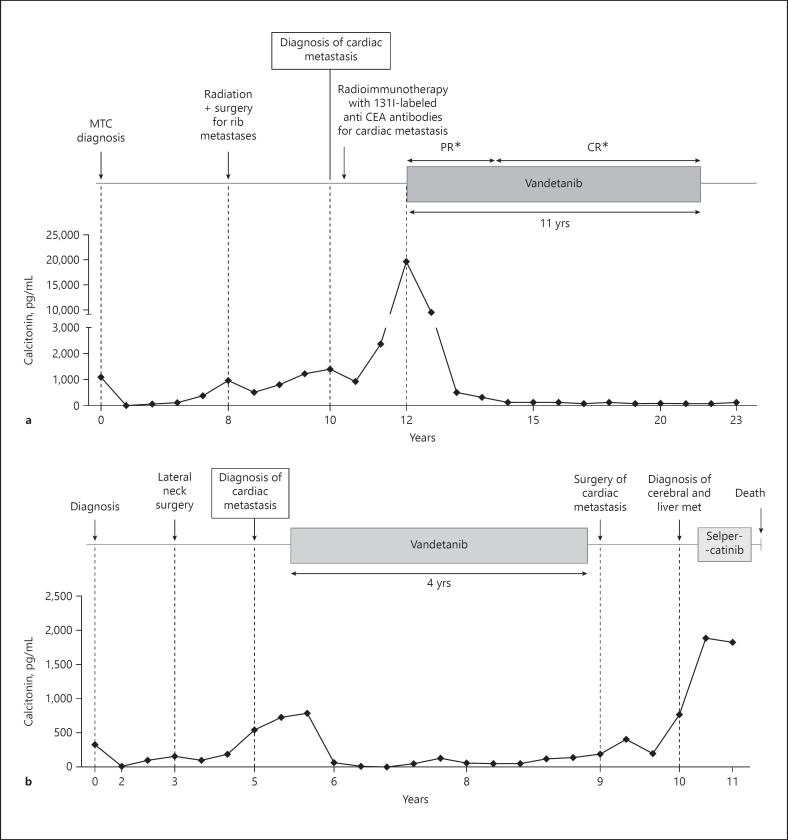
A 45-year-old woman was diagnosed with MTC after total thyroidectomy and neck dissection (Fig. 1a). Histology revealed an 11-mm thyroid tumor with minimal extra-thyroidal extension and massive lymph node metastases pT1b(s)N1b (AJCC/TNM cancer staging system 8th edition) [7]. Germline REarranged during Transfection (RET) gene analysis was negative. After a 3-year period of complete remission (calcitonin: 1 pg/mL), a progressive rise in calcitonin (up to 945 pg/mL, normal value <10 pg/mL) and carcino-embryonic antigen (CEA) (up to 8.5 ng/mL, normal value <5 ng/mL) levels led to the diagnosis of costal metastases treated with external beam radiation and surgery, 8 years after initial diagnosis. Two years later, a rise in calcitonin and CEA levels (1,390 pg/mL and 25 ng/mL, respectively) led to the diagnosis of a 21-mm liver metastasis and a 30-mm cardiac mass in the right ventricular apex. A DOPA (6-[18F]-L-fluoro-L-3, 4-dihydroxyphenylalanine) PET-CT and an immuno-PET-CT using an anti-CEA bispecific antibody and a 68Ga-labeled peptide revealed cardiac uptake, leading to the diagnosis of MTC metastasis. Because of this threatening cardiac location and as an alternative to high-risk surgical treatment, radioimmunotherapy with iodine-131-labeled anti-CEA antibodies was performed. Two years after the cardiac metastasis diagnosis, the liver metastasis increased in size significantly and reached 26 mm with an increase in calcitonin and CEA levels (19,662 pg/mL and 46 ng/mL, respectively), and the patient was treated with vandetanib (300 mg/day), initially in a research protocol [8]. Notwithstanding the comparability between the different images performed, the cardiac metastasis remains globally stable after radioimmunotherapy. The liver metastasis, taken as a target lesion, decreased in size until complete response according to RECIST1.1 assessments, 7 years after the initiation of vandetanib. The cardiac metastasis decreased slightly on CT (−4 mm). No cardiological metastatic-related symptom was reported from the diagnosis. Vandetanib was responsible for moderate toxicity: asthenia, increase in the QTc interval (460–511 ms), and photosensitization with the need for transitory withdrawal and/or reduced dosage. Finally, 10 years after its initiation, vandetanib was stopped because of severe functional renal failure. The evaluation at 2.5 years after vandetanib withdrawal showed a stable disease on imaging with stable tumoral marker levels (calcitonin at 272 pg/mL and CEA at 31 ng/mL), and the patient was still free of cardiological symptoms.
Fig. 1.
Clinical and biochemical evolution of patient 1 (a). Clinical and biochemical evolution of patient 2 (b). MTC, medullary thyroid cancer; PR, partial response; CR, complete response. *The target lesion was a liver metastasis.
Patient 2
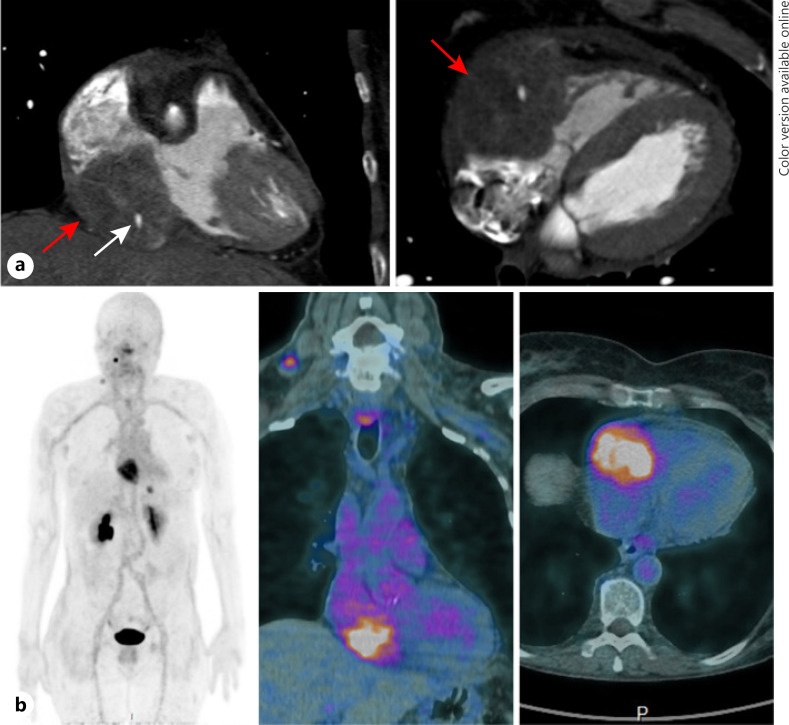
A 63-year-old woman was treated for a sporadic MTC with total thyroidectomy and neck dissection (Fig. 1b). Histology revealed a multifocal tumor with a foci of 20 mm in maximum diameter invading almost the entire lobe with minimal extra-thyroidal extension and massive lymph node metastases pT2(m)N1b [7]. After the initial surgery, serum calcitonin level dropped from 336 to 17 pg/mL. Three years later, calcitonin levels rose to up to 164 pg/mL, leading to the diagnosis of lymph node metastases treated by 2 additional neck surgeries. Two years later, calcitonin and CEA levels increased (751 pg/mL and 14.5 ng/mL, respectively). A chest CT showed micronodular lung and liver metastases and a suspicious 65-mm myocardial mass in the right atrioventricular groove invading the pericardium, the ipsilateral atrial and ventricular cavities, and the tricuspid valve confirmed by a dedicated MRI (Fig. 2a). An F-DOPA PET-CT and 111In-octreoscan revealed a weak uptake of the cardiac mass. An immuno-PET-CT using an anti-CEA bispecific antibody and a 68Ga-labeled peptide (IMP288), performed within a clinical trial (NCT01730638), also revealed a cardiac uptake confirming the metastatic nature of the mass (Fig. 2b). At that time, there was no cardiac metastasis-related consequence. The decision to perform a surgical resection was rejected given the unfavorable benefit-risk balance. Vandetanib (300 mg/day) was initiated, and a partial regression of the cardiac metastasis was observed (−30% in 8 months) and a decrease in cervical lymph node and lung metastases. Vandetanib dosage reduction was necessary because of a prolonged QTc interval 9 months after its introduction. Progressively, calcitonin and CEA levels rose again (422 ng/mL and 13.9 pg/mL, respectively) in parallel with significant progression of the cardiac mass responsible for an atrioventricular obstacle and a low flow. Vandetanib was discontinued 3.5 years after its initiation, and wide surgical resection of the cardiac metastasis was performed with tricuspid bioprosthetic valve replacement. Histological analysis confirmed MTC metastasis with the proliferation marker Ki67 between 5 and 10%. Given the partial metastasis resection, the progression of calcitonin to up to 199 pg/mL, 5 months after the cardiac surgery, and the presence of a RET M918T somatic mutation, a treatment with cabozantinib was initiated (60 mg/day). However, due to a decrease in left ventricular function, cabozantinib was stopped 3 weeks later. As calcitonin and CEA levels decreased after these few weeks of treatment and because of the patient wish, the option of active surveillance was chosen. Unfortunately, 1 year later, new hepatic and brain metastases (right 25-mm frontal-parietal and left occipital lesions of few millimeters) were diagnosed in the workup of myoclonic seizures of the left hand. This diagnosis was accompanied by general progression of the metastatic disease (cardiac, pulmonary, and cervical lymph node metastases) along with calcitonin and CEA levels (respectively, 1,892 ng/mL and 146 pg/mL). Stereotaxic radiation therapy by CyberKnife® was performed on brain metastases. The patient was included in a phase 1–2 trial evaluating the highly specific RET inhibitor selpercatinib [9]. Five weeks after selpercatinib initiation, the patient developed a confusional state. The MRI performed 8 days later revealed a worsening of a perilesional cerebral edema. The patient died 2 weeks later from brain herniation related to an increased vasogenic fronto-parietal edema with a mass effect on the right lateral ventricle.
Fig. 2.
a Cardiac CT in the coronal plane (left panel) and in the sagittal plane (right panel) showing the thyroid metastasis (red arrow) encasing the right ventricle and the right atrium with the right coronary artery (white arrow) encompassed by the tumor. b Planar image (left panel) and fused images (right panel) of an immuno-PET scan using an anti-CEA bispecific antibody and a 68Ga-labeled peptide revealing cardiac uptake of the metastatic lesion with a right lateral neck and mediastinal lymphadenopathies and a liver metastasis.
Discussion
We report here 2 cases of unusual metastatic location of MTC. Giuffrida and Gharib [10] and Catford et al. [6] reviewed the literature of thyroid cancer cardiac metastasis. In the latest review, 59 cases were identified over a 130-year period. The most common histological type is anaplastic thyroid cancer, closely followed by follicular thyroid cancer. Overall, the prognosis would appear poor [6]. Ten other cases of cardiac metastases have been reported since the last review [11, 12, 13, 14, 15, 16, 17, 18, 19, 20], mostly from poorly differentiated or papillary carcinoma with aggressive features.
Only 3 cases of MTC cardiac metastasis have been reported [20, 21, 22]. One of them was our first patient reported in 2010 but without any outcome data [22]. The diagnosis of cardiac metastasis remains challenging. Ultrasonography, CT, or MRI is recommended in the first line [6]. MRI appears to be superior to ultrasound in the detection of cardiac metastases [23, 24]. Some have suggested that echocardiography perfusion imaging could be used to provide information on the vascularization of the mass, helping to differentiate malignant from benign lesions [25]. Others have suggested that 18F-FDG uptake on a PET-CT could be valuable to differentiate between benign, primary malignant, and metastatic cardiac tumors [26, 27]. In the cases reported here, the endocrine nature of the cardiac mass was suggested by abnormal uptake on the F-DOPA PET scan and 111In-octreoscan. In both cases, iodine-131-labeled anti-CEA antibody uptake in the cardiac mass on an immuno-PET scan was the ultimate evidence of the medullary thyroid origin.
Patient-reported outcome in the literature is available for 1 MTC cardiac metastasis, with death 1 year after the diagnosis of the cardiac lesion [21]. The threatening metastatic location of our 2 patients motivated the introduction of a targeted therapy. The prolonged survival under vandetanib is a notable feature which has never been reported in MTC. Surgery initially recused in our second patient became finally mandatory despite the complexity of the procedure, as threatening cardiac-related symptoms occurred. The highly potent and selective RET inhibitors such as pralsetinib [9, 28] and selpercatinib [29] represent promising treatments in patients with driven RET-altered tumors and are currently tested, as first-line therapy, in worldwide phase 3 trials. The risk of cardiac rupture in case of rapid and important tumor regression remains an open question.
The conventional paradigm of short-term prognosis for patients who develop MTC cardiac metastases should be revised in the light of these new targeted therapies. Positive diagnosis remains challenging, but new functional imaging should allow recognizing the metastatic nature of a cardiac mass. In terms of clinical management, based on our experience, cardiac surgery is still indicated whenever technically feasible in case of cardiovascular impairment. However, when necessary, specific RET inhibitors should be considered based on their high efficacy and the lack of cardiovascular toxicity [9].
Statement of Ethics
Informed consent has been obtained from both patients for publication of the case report and accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research did not receive any specific grant.
Author Contributions
Contribution to the conception of the work: C.B., L.G., S.L., and M.S. Analysis or interpretation of data for the work: L.C., F.K.B., C.B.M., A.D., P.L., and O.H. Drafting the work: C.B., L.G., S.L., and M.S. Revising the work for important intellectual content: C.B., L.G., S.L., M.S., L.C., F.K.B., C.B.M., A.D., P.L., and O.H. Final approval of the version to be published: C.B., L.G., S.L., M.S., L.C., F.K.B., C.B.M., A.D., P.L., and O.H. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: C.B., L.G., S.L., M.S., L.C., F.K.B., C.B.M., A.D., P.L., and O.H.
Acknowledgment
The authors acknowledge Liz Atzel for English language editing of the manuscript.
References
- 1.Abraham KP, Reddy V, Gattuso P. Neoplasms metastatic to the heart: review of 3314 consecutive autopsies. Am J Cardiovasc Pathol. 1990;3((3)):195–8. [PubMed] [Google Scholar]
- 2.Berge T, Sievers J. Myocardial metastases. A pathological and electrocardiographic study. Br Heart J. 1968;30((3)):383–90. doi: 10.1136/hrt.30.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klatt EC, Heitz DR. Cardiac metastases. Cancer. 1990;65((6)):1456–9. doi: 10.1002/1097-0142(19900315)65:6<1456::aid-cncr2820650634>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Kline IK. Cardiac lymphatic involvement by metastatic tumor. Cancer. 1972;29((3)):799–808. doi: 10.1002/1097-0142(197203)29:3<799::aid-cncr2820290338>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 5.Pomorski L, Bartos M. Metastasis as the first sign of thyroid cancer. Neoplasma. 1999;46((5)):309–12. [PubMed] [Google Scholar]
- 6.Catford SR, Lee KT, Pace MD, Marasco SF, Longano A, Topliss DJ. Cardiac metastasis from thyroid carcinoma. Thyroid. 2011;21((8)):855–66. doi: 10.1089/thy.2010.0273. [DOI] [PubMed] [Google Scholar]
- 7.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th ed. New York, NY: Springer; 2017. [Google Scholar]
- 8.Wells SA, Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30((2)):134–41. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. 2020;383((9)):825–35. doi: 10.1056/NEJMoa2005651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giuffrida D, Gharib H. Cardiac metastasis from primary anaplastic thyroid carcinoma: report of three cases and a review of the literature. Endocr Relat Cancer. 2001;8((1)):71–3. doi: 10.1677/erc.0.0080071. [DOI] [PubMed] [Google Scholar]
- 11.Bertoldi EG, Severo MD, Scheffel RS, Foppa M, de Azevedo MJ, Maia AL. Left atrial metastases of poorly differentiated thyroid carcinoma diagnosed by echocardiography and magnetic resonance imaging–case report and review of literature. Echocardiography. 2012;29((2)):E30–3. doi: 10.1111/j.1540-8175.2011.01549.x. [DOI] [PubMed] [Google Scholar]
- 12.Giovanella L, Treglia G, Ceriani L, Weidner S, Perriard U, Bongiovanni M. Left atrial metastasis of Hürthle-cell thyroid carcinoma mimicking myxoma. J Nucl Cardiol. 2014;21((2)):406–7. doi: 10.1007/s12350-013-9826-8. [DOI] [PubMed] [Google Scholar]
- 13.Ikeoka T, Ito T, Ando T. Left ventricular infiltration from thyroid papillary carcinoma mimicking the electrocardiographic changes of acute myocardial infarction. Endocrine. 2014;47((2)):652–3. doi: 10.1007/s12020-014-0188-z. [DOI] [PubMed] [Google Scholar]
- 14.Kaul S, Tulchinsky M, Campbell DB, Crist HS, Manni A. Isolated cardiac metastasis from papillary thyroid cancer: prolonged survival with late diagnosis related to inadequate positron emission tomography preparation. Thyroid. 2012;22((4)):443–4. doi: 10.1089/thy.2011.0295. [DOI] [PubMed] [Google Scholar]
- 15.Riva V, Bürgesser MV, Calafat P, Diller A, Ruades Ninfea JI, Caballero Escuti G. Metastatic cardiac tamponade as initial manifestation of papillary thyroid carcinoma. Medicina. 2011;71((6)):550–2. [PubMed] [Google Scholar]
- 16.Rodriguez Caballero MG, Suarez Gutierrez L, Fernandez Fernandez L, Valdes Gallego N, Menendez Torre E. Cardiac tamponade as first sign of papillary thyroid carcinoma. Endocrinol Nutr. 2013;60((8)):e1–2. doi: 10.1016/j.endonu.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Tsoukalas N, Kostakis ID, Demiri S, Koumakis G, Barbounis V, Barbati K, et al. Neoplastic pericarditis as the initial manifestation of a papillary thyroid carcinoma. Ups J Med Sci. 2013;118((3)):196–8. doi: 10.3109/03009734.2013.801541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yanagisawa S, Suzuki Y, Yuasa T, Tanaka T. Right ventricular outflow tract obstruction: metastatic thyroid carcinoma. J Am Coll Cardiol. 2010;55((11)):1159. doi: 10.1016/j.jacc.2009.05.083. [DOI] [PubMed] [Google Scholar]
- 19.Yarmohammadi H, Tacher V, Faulhaber PF, Gilkeson RC, Aktay R, Kamouh A, et al. Imaging of dedifferentiated papillary thyroid carcinoma with left ventricular metastasis: a rare presentation of papillary thyroid metastatic disease. J Cancer Res Ther. 2013 JulSep;9((3)):490–2. doi: 10.4103/0973-1482.119307. [DOI] [PubMed] [Google Scholar]
- 20.El Ghannudi S, Ben Abdelghani M, Germain P, Blondet C, Romain B, Imperiale A. Cardiac metastases of small-bowel carcinoidadded value of (18)F-fluorodihydroxyphenylalanine positron emission tomography combined to magnetic resonance imaging. Circ Cardiovasc Imaging. 2019;12((2)):e008405. doi: 10.1161/CIRCIMAGING.118.008405. [DOI] [PubMed] [Google Scholar]
- 21.Bertagna F, Giubbini R, Savelli G, Pizzocaro C, Rodella C, Biasiotto G, et al. A patient with medullary thyroid carcinoma and right ventricular cardiac metastasis treated by (90)Y-Dotatoc. Hell J Nucl Med. 2009 MayAug;12((2)):161–4. [PubMed] [Google Scholar]
- 22.Morel O, Giraud P, Cahouet A, Lacoeuille F, Girault S. Visualization of cardiac metastasis from medullary thyroid carcinoma on F-18 DOPA PET/CT scan. Clin Nucl Med. 2010;35((4)):253–5. doi: 10.1097/RLU.0b013e3181d18ede. [DOI] [PubMed] [Google Scholar]
- 23.Pavel M, Grossman A, Arnold R, Perren A, Kaltsas G, Steinmüller T, et al. ENETS consensus guidelines for the management of brain, cardiac and ovarian metastases from neuroendocrine tumors. Neuroendocrinology. 2010;91((4)):326–32. doi: 10.1159/000287277. [DOI] [PubMed] [Google Scholar]
- 24.Pun SC, Plodkowski A, Matasar MJ, Lakhman Y, Halpenny DF, Gupta D, et al. Pattern and prognostic implications of cardiac metastases among patients with advanced systemic cancer assessed with cardiac magnetic resonance imaging. J Am Heart Assoc. 2016;5((5)):e003368. doi: 10.1161/JAHA.116.003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moustafa SE, Sauvé C, Amyot R. Assessment of a right ventricular metastasis using contrast echocardiography perfusion imaging. Eur J Echocardiogr. 2008;9((2)):326–8. doi: 10.1093/ejechocard/jen067. [DOI] [PubMed] [Google Scholar]
- 26.Meng J, Zhao H, Liu Y, Chen D, Hacker M, Wei Y, et al. Assessment of cardiac tumors by (18)F-FDG PET/CT imaging: histological correlation and clinical outcomes. J Nucl Cardiol. 2020 doi: 10.1007/s12350-019-02022-1. [DOI] [PubMed] [Google Scholar]
- 27.Qin C, Shao F, Hu F, Song W, Song Y, Guo J, et al. 18F-FDG PET/CT in diagnostic and prognostic evaluation of patients with cardiac masses: a retrospective study. Eur J Nucl Med Mol Imaging. 2020;47((5)):1083–93. doi: 10.1007/s00259-019-04632-w. [DOI] [PubMed] [Google Scholar]
- 28.Subbiah V, Gainor JF, Rahal R, Brubaker JD, Kim JL, Maynard M, et al. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov. 2018;8((7)):836–49. doi: 10.1158/2159-8290.CD-18-0338. [DOI] [PubMed] [Google Scholar]
- 29.Subbiah V, Velcheti V, Tuch BB, Ebata K, Busaidy NL, Cabanillas ME, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Annal Oncol. 2018;29((8)):1869–76. doi: 10.1093/annonc/mdy137. [DOI] [PMC free article] [PubMed] [Google Scholar]