Abstract
Objectives
Human milk (HM) is the optimal diet for neonates, but it does not provide enough nutrients for preterm infants. HM fortifiers based on highly processed mature bovine milk (BMFs) are routinely used for preterm infants despite risks of causing gut dysfunction and systemic infection. Gently‐processed bovine colostrum as a fortifier (BCF) may better protect against infection and inflammation. We hypothesized that BCF‐fortified HM has enhanced antimicrobial activity against pathogens that commonly cause neonatal sepsis, relative to BMF‐fortified HM.
Methods
Holder‐pasteurized HM samples (10 mothers) were aliquoted into 3 fractions: unfortified HM and HM fortified with either BMF or BCF. The samples were analyzed for pH, lactoferrin concentrations, and antimicrobial activities against Staphylococcus epidermidis, Escherichia coli, and Enterococcus faecalis.
Results
HM+BCF had a lower pH and higher lactoferrin levels than HM+BMF, with HM being intermediate. Relative to infant formula, HM decreased the growth of S epidermidis, E coli, and E faecalis, with no difference between preterm and term HM. Addition of BMF abolished the antimicrobial effect of HM against S epidermidis and E faecalis but not E coli. By contrast, addition of BCF into HM enhanced antimicrobial activity against S epidermidis and E coli, relative to unfortified HM. HM+BCF was superior to HM+BMF in inhibiting growth of all tested bacteria.
Conclusion
BMF fortification decreased whereas BCF fortification enhanced in vitro antimicrobial activity of HM. This effect may partly be derived from the high levels of antimicrobial factors found in BCF, including lactoferrin. BCF may be a better fortifier than BMF for preterm infants.
Keywords: antibacterial activity, bovine colostrum, fortification, human milk, preterm infant
Clinical Relevancy Statement
Human milk (HM) fortifiers are routinely supplemented into HM to provide sufficient nutrients and to ensure appropriate growth in preterm infants. Most of the commercial fortifiers are based on mature bovine milk, and they have been found to suppress antimicrobial activity of HM. Bovine colostrum (BC) may be a better fortifier to provide protection against infection and inflammation, as it contains high levels of bioactive proteins and antimicrobial factors. We now demonstrate that a BC fortifier, following mild thermal processing, enhances the antimicrobial effect of HM in vitro.
Introduction
Mother's own milk (MOM) is the optimal nutrition source for newborn infants, especially for preterm infants (born before completing 37 weeks of gestation) with high risks of neonatal sepsis and necrotizing enterocolitis (NEC). 1 , 2 The protective effects of MOM have been thought to be derived from its multiple bioactive components, including immune cells and antimicrobial proteins and peptides. 3 , 4 Unfortunately, MOM for preterm infants is often delayed or insufficient, especially during the first few weeks of life, partly because mothers after preterm delivery have difficulties in expressing milk or receive various medications. 5 In such conditions, Holder‐pasteurized human donor milk (HDM) is suggested to be the best alternative. 6 Evidenced from multiple previous clinical trials, exclusive HDM or any use of HDM is more beneficial to preterm infants, relative to exclusive preterm infant formula (IF), with regard to decreased incidence of NEC, bronchopulmonary dysplasia, late‐onset sepsis (LOS), and death. 7 , 8
HDM alone does not provide sufficient nutrition for appropriate growth for preterm infants, and inadequate nutrition and/or postnatal growth is associated with impaired neurodevelopment. 6 , 9 Therefore, human milk (HM) fortifiers are routinely added to HM to provide additional energy, protein, vitamins, and minerals. 6 Most of the currently available products are HM fortifiers based on highly processed mature bovine milk (BMFs). Relative to HM, bovine milk has a very different protein composition, including lower levels of whey proteins and the presence of β‐lactoglobulin, a unique bovine milk factor that causes food allergy. 11 Several clinical trials have reported increased incidence of NEC, mortality, and allergy in preterm infants fed bovine milk‐based formula or BMF. 12 , 13 , 14 The reason for these detrimental effects remains elusive, but it is possible that the harsh thermal‐processing conditions of BMF may destroy its bioactive components and generate compounds with inflammatory properties, including advanced glycation end‐products formed by reaction between reducing sugars and free amino acid groups of proteins. 15 Addition of BMF into HM also affects the final product properties, such as pH, osmolality, lipase activity, and antibacterial activity. 16 , 17 Recently, fortifier based on HM has become available, but the product is very costly. Evidence from clinical trials is also controversial, with a study showing that HM‐based fortifier is superior to BMF in protecting against NEC in preterm infants, 18 contrary to a conclusion from a cohort of extremely low‐birth‐weight infants with no differences in NEC incidence between HM‐based fortifier–fed vs BMF‐fed groups. 19
Bovine colostrum (BC) contains high levels of bioactive and immune factors, including immunoglobulin G, lactoferrin (LF), osteopontin, insulin‐like growth factor, and epidermal growth factor. 20 BC consumption reduces the frequency and relieves the symptoms of childhood infectious diarrhea. 21 A specific BC product with gentle thermal processing (pasteurization) has been shown to stimulate gut maturation and body growth and prevent NEC development in the preterm piglet, which is widely accepted as the most clinically relevant model for preterm infants. 22 , 23 , 24 This BC product can also inhibit growth of pathogens and stimulate intestinal epithelial cell proliferation in vitro. 25 In an open‐label, randomized, controlled pilot safety trial, MOM supplementation with BC during the first weeks of life appeared to be safe and reduced time to total enteral feeding in preterm infants. 26 This BC product is currently tested as a fortifier (BCF) into HDM in a randomized controlled trial. 20 However, the protective mechanisms of this fortifier remain elusive. With the above background, we hypothesized in this study that BC with gentle thermal processing used as a fortifier in HDM enhances levels of bioactive proteins and antimicrobial activity against pathogens that commonly cause neonatal sepsis (including Staphylococcus epidermidis, Escherichia coli, and Enterococcus faecalis), 27 , 28 relative to unfortified HDM or HDM fortified with BMF.
Materials and Methods
Collection of HDM and Measurements of pH, Osmolality, and LF Concentration
Frozen Holder‐pasteurized HDM samples were obtained anonymously from 10 Danish mothers at the National Human Milk Bank, Hvidovre Hospital, Denmark (5 preterm and 5 term milk samples). Owing to anonymous sample collection, ethical approval from the Danish National Committee on Health Research Ethics was not required for the study. Each milk sample was aliquoted into 3 fractions: an unfortified HM fraction, a fraction fortified with a commercial BMF (HM+BMF), and a fraction fortified with gently processed BCF (HM+BCF). The protein levels of each group are shown in Table 1. To mimic the clinical practice, 4 g of powder of BMF (PreNAN FM 85, Nestlé, Vevey, Switzerland) or 2.8 g of powder of BCF (ColoDan, Biofiber Damino, Gesten, Denmark) was mixed with 100 mL of HM to ensure a similar amount of protein of the added fortifiers (1.4 g/100 mL). BCF was devoid of iron, whereas BMF contained 0.45 mg of iron per gram of powder. Preterm ready‐to‐feed IF (PreNAN partially hydrolyzed preterm formula, Nestlé, Switzerland) was used as negative control. All samples were then stored in aliquots of 1 mL at −20 °C. In some experiments, HM+BCF was also supplemented with iron, using ferrous sulfate heptahydrate (SigmaAldrich, Copenhagen, Denmark) to reach iron levels similar to those found in HM+BMF.
Table 1.
Protein and Lactoferrin levels, pH, and Osmolality in Unfortified HM and HM Fortified with BMF and BCF
| HM | HM+BMF | HM+BCF | ||||
|---|---|---|---|---|---|---|
| Parameters | Preterm (n = 5) | Term (n = 5) | Preterm (n = 5) | Term (n = 5) | Preterm (n = 5) | Term (n = 5) |
| Protein, g/100 mL | 1.6 ± 0.1 | 1.2 ± 0.03* | 3.0 ± 0.1 | 2.6 ± 0.03 * | 3.0 ± 0.1 | 2.6 ± 0.03* |
| 1.4 ± 0.1a | 2.8 ± 0.1b | 2.8 ± 0.1b | ||||
| Lactoferrin, mg/L | 175 ± 24 | 115 ± 22 | 154 ± 30 | 105 ± 8 | 225 ± 34 | 183 ± 20 |
| 145 ± 18ab | 130 ± 17a | 204 ± 20b | ||||
| pH | 6.89 ± 0.09 | 6.91 ± 0.11 | 6.99 ± 0.07 | 6.96 ± 0.08 | 6.73 ± 0.07 | 6.76 ± 0.09 |
| 6.90 ± 0.07 ab | 6.98 ± 0.05a | 6.75 ± 0.05b | ||||
| Osmolality, mOsm/kg H2O | 307 ± 1.56 | 298 ± 0.74 * | 415 ± 3.01 | 410 ± 3.25 | 342 ± 2.54 | 337 ± 2.97 |
| 303 ± 1.65a | 412.50 ± 2.22b | 339.40 ± 1.98c | ||||
Values are mean ± SD; groups in the same row not sharing the same letters are significantly different (P < .05).
BCF, bovine colostrum processed by mild thermal treatment as a fortifier; BMF, HM fortifier based on highly processed mature bovine milk; HM, human milk.
* P < .05, comparing preterm vs term milk samples, with or without fortification.
pH was measured by Mettler‐Toledo GmbH instrument (Griefensee, Switzerland). Osmolality was measured using cryoscopic osmometer (OSMOMAT, Gonotec, Berlin, Germany). The concentration of LF was determined by a sandwich enzyme‐linked immunosorbent assay (ELISA) method. The milk samples were centrifuged at 3000 g for 15 minutes at 4 °C to remove milk fat, and skimmed milk samples (100 μL) were diluted 5000 times prior to ELISA measurement using rabbit anti‐human LF antibody (MyBioSource, San Diego, CA, USA).
Preparation of Bacterial Stocks
Three bacterial strains isolated from the blood culture of septic patients were used for the antimicrobial assay, including E coli, E faecalis (kindly provided by Dr Henrik Westh, Hvidovre Hospital, Copenhagen, Denmark), and S epidermidis (kindly provided by Dr Carina Mallard, University of Gothenburg, Gothenburg, Sweden). The frozen stock of these bacteria was used to prepare mid‐log stocks as previously described. 25 , 29 Briefly, each bacterium was streaked out onto blood agar and incubated overnight at 37 °C; then, 1–2 pure bacterial colonies were transferred into 10 mL of heart infusion broth medium and incubated overnight with continuous shaking at 120 rpm at 37 °C. The overnight cultures were diluted with broth media to reach optical density (OD) = 0.05 (measured at a wavelength of 600 nm) and incubated for another 24 hours with frequent OD measurement to determine the bacterial growth curve (Figure 1). Bacterial cultures at mid‐log phase were then mixed with sterile glycerol (final concentration of 15% [vol/vol] glycerol) and stored at −80 °C in 1‐mL aliquots. The concentration of bacterial stock was determined prior to storage to calculate the theoretical dose of stimulation with milk samples following triplicated spotting of 20‐μL stock samples onto a blood agar with stock dilution of 100–106 times, overnight incubation at 37 °C, and manual counting. The viability and actual concentrations of mid‐log stocks were assessed for each experiment by a similar plating method.
Figure 1.
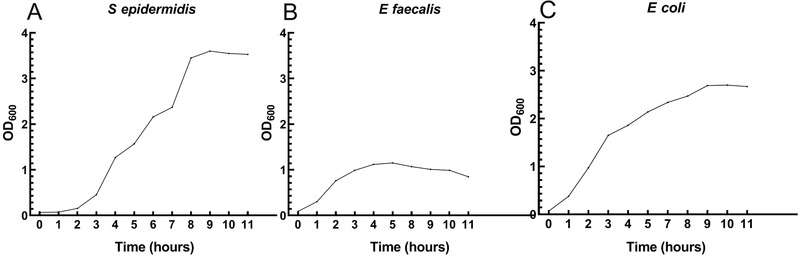
The growth curve of S epidermidis, E faecalis, and E coli. Pure bacterial stock was inoculated in fresh heart infusion broth medium at OD = 0.05 and incubated at 37 °C overnight. Bacterial density was monitored every 1–2 hours by optical density (OD) measurement.
Antimicrobial Assays
Endogenous bacterial levels of individual HM samples and IF were identified following serial dilution of samples from 100 to 103 times in sterile saline, spotting onto blood agar, and overnight incubation at 37 °C, as mentioned in the previous section. Samples with endogenous bacterial detection were excluded from the antibacterial assay.
For the assay, the frozen bacterial stocks were thawed, centrifuged at 3200 g for 10 minutes at room temperature, and washed twice with sterile saline. The stock bacterial concentrations were then adjusted, and 10 μL of bacteria was added into 190 μL of milk samples in sterile 96‐well polypropylene plates to reach an inoculation level of 106 colony‐forming units (CFU)/mL for S epidermidis, 104 CFU/mL for E faecalis, and 103 CFU/mL for E coli. IF was used as negative control and run in duplicates. After 0, 2, 4, 6, and 24 hours of incubation at 37 °C, aliquots of incubated samples were used to identify the bacterial levels by using the plating methods on blood agar plates as mentioned previously with bacterial stock.
Statistics
Data were analyzed using GraphPad Prism version 5.0 (San Diego, CA, USA). pH, osmolality, and LF concentrations were compared between preterm and term milk samples, as well as among HM vs HM+BCF vs HM+BMF, using analysis of variance (ANOVA) with post hoc Tukey test. Differences in bacterial levels in the antimicrobial assays were analyzed using 2‐way ANOVA with repeated measurement (treatment and time of incubation as 2 main factors), followed by Tukey multiple comparisons tests. A P‐value of <.05 was considered statistically significant.
Results
pH, Osmolality, and LF Content in HM and Fortified HM
The concentration of LF and pH were similar in preterm and term milk, as well as in fortified preterm vs fortified term milk. However, the total protein levels and osmolality were higher in preterm milk than in term milk (P < .05, Table 1). When pooling preterm and term milk for comparisons among fortified groups, the pH of HM+BCF was significantly lower than that of HM+BMF (P < .05), with HM as the intermediate (Table 1). The osmolality of HM was 303 ± 2 mOsm/kg H2O, and after fortification with BMF and BCF, it increased to 413 ± 2 and 339 ± 2 mOsm/kg H2O, respectively (P < .05). HM+BCF had lower osmolality than HM+BMF (P < .05, Table 1). The concentration of LF was higher in HM+BCF than in HM+BMF (P < .05), with HM being intermediate (Table 1).
Bacterial Growth–Inhibitory Effects of HM and Fortified HM
Endogenous bacteria were detected in 1 term HM sample, and this sample was excluded from the assay. Over the period of 24 hours, all 3 types of bacteria grew quickly in both preterm and term HM, with concentration of S epidermidis increasing 100‐fold after 6 hours and 1000‐fold after 24 hours, E faecalis increasing 100‐fold after 6 hours and 105‐fold for 24 hours, and E coli increasing 107‐fold after 6 hours and 109‐fold after 24 hours (Figure 2A–C). There were no differences in the growth of all 3 bacteria after incubation with preterm vs term HM, although each group of preterm or term HM showed overall lower bacterial growth than did formula (Figure 2A–C). For all remaining comparisons among fortified groups, we pooled data from preterm and term milk samples to compare HM, HM+BCF, and HM+BMF.
Figure 2.
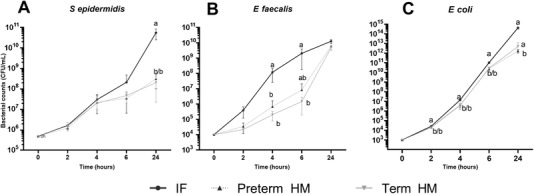
S epidermidis (A), E faecalis (B), and E coli (C) growth over time following incubation with preterm and term HM (n = 5 and 4, respectively). The inoculated concentrations were 106, 104, and 103 CFU/mL for S epidermidis, E faecalis, and E coli, respectively. Values are mean ± SD. Values at the same incubation time point that do not share the same letters are significantly different (P < .05). CFU, colony‐forming units; HM, human milk; IF, infant formula.
For S epidermidis, the concentration of bacteria following incubation with IF increased 2‐fold after 2 hours, 100‐ to 200‐fold after 4–6 hours, and drastically (∼105‐fold) after 24 hours (P < .01, Figure 3A). No effects of HM, HM+BCF, or HM+BMF, relative to IF, were observed against S epidermidis growth following 2–4 hours of incubation. Only HM+BCF, not HM or HM+BMF, showed inhibitory effects against S epidermidis after 6 hours of incubation, relative to IF (P < .01, Figure 3A). After 24 hours of incubation, HM exerted better bacterial growth inhibition than both HM+BCF and HM+BMF (P < .01), but the HM+BCF group was still more efficient at inhibiting bacterial growth than HM+BMF (P < .001, Figure 3A).
Figure 3.
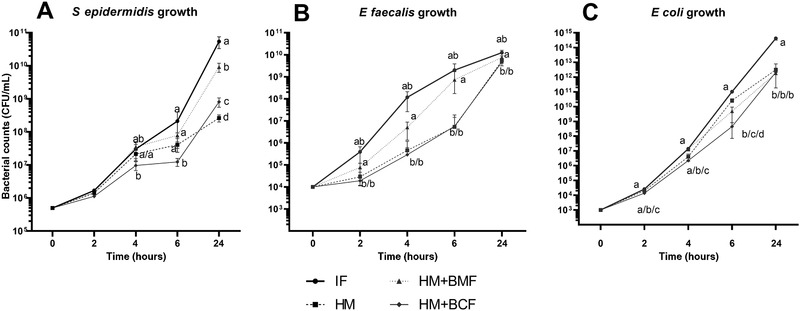
S epidermidis (A), E faecalis (B), and E coli (C) growth over time following incubation with infant formula (IF, n = 2), unfortified donor human milk (HM, n = 9), HM fortified with a bovine milk–based fortifier (HM+BMF, n = 9), or HM fortified with bovine colostrum (HM+BCF, n = 9). The inoculated concentrations were 106, 104, and 103 CFU/mL for S epidermidis, E faecalis, and E coli, respectively. Values are mean ± SD. Values at each incubation time point that do not share the same letters are significantly different (P < .05). CFU, colony‐forming units.
For E faecalis, the concentration of bacteria increased drastically following incubation with IF, ∼20 times after 2 hours and 104–106 times after 4–24 hours (P < .01, Figure 3B). Among HM, HM+BCF, and HM+BMF products, HM and HM+BCF showed similar capacity of bacterial inhibition across 2–24 hours, and both groups were better than the HM+BMF group (P < .001, Figure 3B). The bacterial concentration in HM+BMF was similar to that in IF at all incubation time points.
Similar to E faecalis, E coli grew very quickly in IF, 10 times after 2 hours and 104–1011 times after 4–24 hours, with a higher concentration than that in the remaining 3 groups at all time points (P < .01, Figure 3C, except 2–4 hours vs BMF). The bacterial growth significantly differed in HM, HM+BCF, and HM+BMF after 2–4 hours of incubation, with the lowest concentration being in HM+BCF, followed by HM, and the highest being in HM+BMF (P < .01, Figure 3C). At 6 hours of incubation, the bacterial concentration was still lowest in HM+BCF and higher in HM vs HM+BMF (P < .001, Figure 3C). No difference in bacterial levels at 24 hours was detected among HM, HM+BCF, and HM+BMF.
Effects of Iron on the Antimicrobial Activity of BCF
BCF did not contain iron, which is an important factor in facilitating bacterial growth 30 and affecting antimicrobial activity of LF, the protein with the highest levels in HM+BCF. 31 In this follow‐up experiment, we added iron in the form of ferrous sulfate in BCF to reach the levels in BMF and compare bacterial growth inhibition among HM+BMF, HM+BCF, and HM+BCF+iron. After 4 hours of incubation with E coli, the bacterial levels in HM+BCF were 1.5 times lower than in HM+BMF (P < .05, Figure 4) but not different from those in HM+BCF+iron. This suggests that the bacterial growth–inhibitory effects of BCF are partly independent of its iron levels and probably derived from multiple antimicrobial proteins and peptides rather than only LF.
Figure 4.
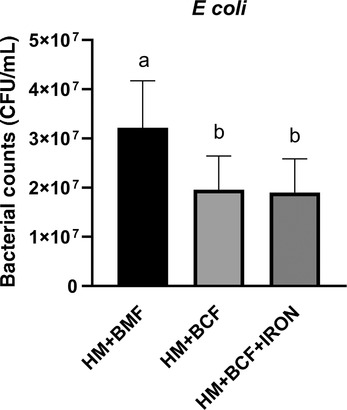
E coli growth following 4 hours of incubation with human milk fortified with either a bovine milk–based fortifier (HM+BMF, n = 9) or bovine colostrum without (HM+BCF, n = 9) or with iron supplementation (HM+BCF+IRON, n = 9). Values are means ± SD. Groups not sharing the same letters are significantly different (P < .05). CFU, colony‐forming units.
Discussion
Following years of preclinical investigations using the preterm piglet model, BC processed by mild thermal treatment has been suggested as a novel alternative to bovine milk–based formula to be supplemented into HM during the first few days of life or to be used as an HM fortifier for preterm infants. 20 , 23 , 26 The detailed mechanistic protective effects of BC, however, have remained elusive. Now, based on in vitro assays in the current study, we have provided evidence for plausible protective effects of BC against gut dysfunction and gut‐derived systemic infection/sepsis via its bioactive components with inhibitory effects against gut bacterial overgrowth.
Prior to antimicrobial assays, we performed physiochemical analyses of the fortified products to evaluate the appropriate product osmolality and pH for preterm infants. We observed that the pH of HM was slightly acidic, similar to previous studies, 32 and HM+BCF showed lower pH than HM+BMF. This may be due to the low pH of the original fortifier BCF, being ∼6.0. 16 It has been shown that highly acidified fortifiers, designed by artificial pH adjustment (pH 4.5–5.0), may cause metabolic acidosis, 33 but from our ongoing clinical trials, preterm infants fed HM+BCF during the first 10 days of life did not have adverse effects, 20 , 26 , 34 , 35 , 36 indicating the safety of this slightly acidic colostrum fortifier. Further, we examined the osmolality of HM after fortification because hyperosmolar IF may be a risk factor for NEC and because preterm IF has been recommended with osmolality not exceeding 400 mOsm/L. 37 We found that HM fortified with BMF or BCF showed increased osmolality from 302 to 330–412 mOsm/kg H2O, though still in a safe range, as previously suggested. 38
It is commonly thought that the antimicrobial activity of a milk product and its protective effects against gut and systemic infection are mainly derived from their bioactive components, including LF. 3 LF is an iron‐binding globular glycoprotein that is present in various secretory fluids, such as milk, tears, and saliva. Its concentration in postpartum bovine milk and HM declines over time, 39 , 40 with levels lower in mature milk than in colostrum. 3 , 40 , 41 , 42 This natural decline explains the highest levels of LF in HM+BCF among the 3 groups in this study. The effects of BMF fortification on the antimicrobial activity of HM have been investigated, 17 , 43 but the antimicrobial effects of BCF fortification have remained unclear. Now in the present study, we compared HM with HM+BCF and HM+BMF for their inhibitory effects against the growth of S epidermidis, E faecalis, and E coli, the common pathogens found in the blood cultures of infants with LOS. Similar to a previous study, 17 addition of BMF into HM decreased the antibacterial activity against all 3 tested bacteria but did not alter levels of LF. Low levels of bioactive components but enriched nutrition contents in BMF may facilitate faster bacterial growth when incubated with HM+BMF vs HM, leading to decreased antimicrobial activity of HM+BMF. 17 By contrast, BCF addition to HM markedly increased antimicrobial activity for all 3 tested bacteria. Unlike BMF, BCF contains high levels of bioactive and antimicrobial factors such as LF, osteopontin, insulin‐like growth factor, and epidermal growth factor. We speculate that the enhanced antimicrobial activity via these bioactive components, especially LF, was much more significant than the effects of increased nutrition content in facilitating bacterial growth following BCF fortification. Collectively, the antimicrobial effects of BCF may directly or indirectly modulate gut microbial composition and strengthen gut barrier function, thereby preventing bacterial gut translocation and reducing systemic infection in preterm infants fed HM fortified with BCF.
It is noteworthy that the antimicrobial activity of LF is dependent on iron levels of the products, as iron chelation turns LF from apo (no iron binding) into holo form (iron binding) and reduces the capacity of LF to interact with and kill bacteria. 29 In the current study, BMF and BCF supplemented HM with 1.8 mg 20 and 0 mg of iron per 100 mL, respectively, as BCF was completely devoid of iron. The iron content may have contributed to the decreased antibacterial activity in HM+BMF. 17 Thus, we attempted to investigate whether HM+BCF with iron levels similar to those of HM+BMF would still have better bacterial growth–inhibitory effects. Interestingly, E coli growth in HM+BCF with iron addition was similar to the product without iron addition and still lower than that in HM+BMF. This suggests that BCF is indeed superior to BMF with regard to the antimicrobial activity, independent of iron levels, probably because of the contributing effects from other bioactive factors in BCF, such as immunoglobulins or osteopontin.
In conclusion, to our knowledge, this was the first study investigating the antibacterial effect of HM fortified with BCF. It appeared that BCF addition to HM modulated physicochemical properties and prevented in vitro overgrowth of bacteria involved in neonatal sepsis, and therefore, BCF may be a superior fortifier for preterm infants. It remains to be elucidated whether similar effects can be observed in fresh MOM fortified with BCF. The clinical implication of enhanced in vitro antibacterial activity of BCF requires careful consideration, and in vivo efficacy of BCF as a nutrient fortifier to tackle other gut pathogens involved in LOS and to modulate gut microbiome in preterm infants requires further investigation.
Statement of Authorship
X. Gao contributed to the study design, performed the experiments, acquired and analyzed the data, and drafted the manuscript. Y. Li contributed to study design and statistical analysis and critically revised the manuscript. A. B. Olin contributed to the study design, acquired milk samples, and critically revised the manuscript. D. N. Nguyen contributed to the study design, supervised the experiments, and critically revised the manuscript. All authors approved the final version of the manuscript.
Acknowledgments
The authors would like to thank Dr. Peter Damborg for providing the laboratory facilities, Drs. Henrik Torkil Westh and Carina Mallard for donating bacterial strains, and Karoline Aasmul‐Olsen for laboratory assistance.
Financial disclosure: The study was funded by the Innovation Fund Denmark (NEOCOL, grant number 6150‐00004B), and Xiaoyan Gao was also supported by Foshan Science and Technology Foundation, China (2017AB002901).
Conflicts of interest: University of Copenhagen holds a patent on the use of colostrum for pediatric patients. All authors declare no potential conflicts of interest.
References
- 1. Maffei D, Schanler RJ. Human milk is the feeding strategy to prevent necrotizing enterocolitis! Semin Perinatol. 2017;41:36‐40. [DOI] [PubMed] [Google Scholar]
- 2. Corpeleijn WE, Kouwenhoven SM, Paap MC, et al. Intake of own mother's milk during the first days of life is associated with decreased morbidity and mortality in very low birth weight infants during the first 60 days of life. Neonatology. 2012;102:276‐281. [DOI] [PubMed] [Google Scholar]
- 3. Trend S, Strunk T, Hibbert J, et al. Antimicrobial protein and Peptide concentrations and activity in human breast milk consumed by preterm infants at risk of late‐onset neonatal sepsis. PLoS One. 2015;10:e0117038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lonnerdal B. Bioactive Proteins in Human Milk‐Potential Benefits for Preterm Infants. Clin Perinatol. 2017;44:179‐191. [DOI] [PubMed] [Google Scholar]
- 5. Ikonen R, Paavilainen E, Helminen M, Kaunonen M. Preterm infants' mothers' initiation and frequency of breast milk expression and exclusive use of mother's breast milk in neonatal intensive care units. J Clin Nurs. 2018;27:e551‐e558. [DOI] [PubMed] [Google Scholar]
- 6. Arslanoglu S, Boquien CY, King C, et al. Fortification of Human Milk for Preterm Infants: Update and Recommendations of the European Milk Bank Association (EMBA) Working Group on Human Milk Fortification. Front Pediatr. 2019;7:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller J, Tonkin E, Damarell RA, et al. A Systematic Review and Meta‐Analysis of Human Milk Feeding and Morbidity in Very Low Birth Weight Infants. Nutrients. 2018;10(6):707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Canizo Vazquez D, Salas Garcia S, Izquierdo Renau M, Iglesias‐Platas I. Availability of donor milk for very preterm infants decreased the risk of necrotizing enterocolitis without adversely impacting growth or rates of breastfeeding. Nutrients. 2019;11(8):1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stephens BE, Walden RV, Gargus RA, et al. First‐week protein and energy intakes are associated with 18‐month developmental outcomes in extremely low birth weight infants. Pediatrics. 2009;123:1337‐1343. [DOI] [PubMed] [Google Scholar]
- 10. Pozzo L, Cirrincione S, Russo R, et al. Comparison of Oxidative Status of Human Milk, Human Milk Fortifiers and Preterm Infant Formulas. Foods. 2019;8(10):458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luyt D, Ball H, Makwana N, et al. BSACI guideline for the diagnosis and management of cow's milk allergy. Clin Exp Allergy. 2014;44:642‐672. [DOI] [PubMed] [Google Scholar]
- 12. Nakamura T, Hatanaka D, Kashima K, et al. A male preterm infant with cow's milk allergy to human milk fortifier showing only severe respiratory symptoms. Fukushima J Med Sci. 2019;65:50‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vlieghe V, Des Roches A, Payot A, Lachance C, Nuyt AM. Human milk fortifier in preterm babies: source of cow's milk protein sensitization? Allergy. 2009;64:1690‐1691. [DOI] [PubMed] [Google Scholar]
- 14. Abrams SA, Schanler RJ, Lee ML, Rechtman DJ. Greater mortality and morbidity in extremely preterm infants fed a diet containing cow milk protein products. Breastfeed Med. 2014;9:281‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pischetsrieder M, Henle T. Glycation products in infant formulas: chemical, analytical and physiological aspects. Amino Acids. 2012;42:1111–1118. [DOI] [PubMed] [Google Scholar]
- 16. Donovan R, Kelly SG, Prazad P, et al. The effects of human milk fortification on nutrients and milk properties. J Perinatol. 2017;37:42‐48. [DOI] [PubMed] [Google Scholar]
- 17. Chan GM. Effects of powdered human milk fortifiers on the antibacterial actions of human milk. J Perinatol. 2003;23:620‐623. [DOI] [PubMed] [Google Scholar]
- 18. Sullivan S, Schanler RJ, Kim JH, et al. An exclusively human milk‐based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk‐based products. J Pediatr. 2010;156:562‐567.e561. [DOI] [PubMed] [Google Scholar]
- 19. Eibensteiner F, Auer‐Hackenberg L, Jilma B, Thanhaeuser M, Wald M, Haiden N. Growth, Feeding Tolerance and Metabolism in Extreme Preterm Infants under an Exclusive Human Milk Diet. Nutrients. 2019;11(7):1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahnfeldt AM, Hyldig N, Li Y, et al. FortiColos ‐ a multicentre study using bovine colostrum as a fortifier to human milk in very preterm infants: study protocol for a randomised controlled pilot trial. Trials. 2019;20:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li J, Xu YW, Jiang JJ, Song QK. Bovine colostrum and product intervention associated with relief of childhood infectious diarrhea. Sci Rep. 2019;9:3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rasmussen SO, Martin L, Ostergaard MV, et al. Bovine colostrum improves neonatal growth, digestive function, and gut immunity relative to donor human milk and infant formula in preterm pigs. Am J Physiol Gastrointest Liver Physiol. 2016;311:G480‐491. [DOI] [PubMed] [Google Scholar]
- 23. Sun J, Li Y, Pan X, et al. Human Milk Fortification with Bovine Colostrum Is Superior to Formula‐Based Fortifiers to Prevent Gut Dysfunction, Necrotizing Enterocolitis, and Systemic Infection in Preterm Pigs. JPEN J Parenter Enteral Nutr. 2019;43:252‐262. 10.1002/jpen.1422 [DOI] [PubMed] [Google Scholar]
- 24. Sty AC, Sangild PT, Skovgaard K, et al. Spray Dried, Pasteurised Bovine Colostrum Protects Against Gut Dysfunction and Inflammation in Preterm Pigs. J Pediatr Gastroenterol Nutr. 2016;63:280‐287. [DOI] [PubMed] [Google Scholar]
- 25. Nguyen DN, Currie AJ, Ren S, Bering SB, Sangild PT, Heat treatment and irradiation reduce anti‐bacterial and immune‐modulatory properties of bovine colostrum. J Funct Foods. 2019;57:182‐189. [Google Scholar]
- 26. Juhl SM, Ye X, Zhou P, et al. Bovine Colostrum for Preterm Infants in the First Days of Life: A Randomized Controlled Pilot Trial. J Pediatr Gastroenterol Nutr. 2018;66:471‐478. [DOI] [PubMed] [Google Scholar]
- 27. Dong Y, Speer CP, Glaser K. Beyond sepsis: Staphylococcus epidermidis is an underestimated but significant contributor to neonatal morbidity. Virulence. 2018;9:621‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nguyen DN, Stensballe A, Lai JC, et al. Elevated levels of circulating cell‐free DNA and neutrophil proteins are associated with neonatal sepsis and necrotizing enterocolitis in immature mice, pigs and infants. Innate Immun. 2017;23:524‐536. [DOI] [PubMed] [Google Scholar]
- 29. Woodman T, Strunk T, Patole S, Hartmann B, Simmer K, Currie A. Effects of lactoferrin on neonatal pathogens and Bifidobacterium breve in human breast milk. PLoS One. 2018;13:e0201819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. 2013;13:509‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rosa L, Cutone A, Lepanto MS, Paesano R, Valenti P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int J Mol Sci. 2017;18(9):1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ogundele MO. Effects of storage on the physicochemical and antibacterial properties of human milk. Br J Biomed Sci. 2002;59:205‐211. [DOI] [PubMed] [Google Scholar]
- 33. Thoene M, Hanson C, Lyden E, Dugick L, Ruybal L. Anderson‐Berry A Comparison of the effect of two human milk fortifiers on clinical outcomes in premature infants. Nutrients. 2014;6:261‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aunsholt L, Jeppesen PB, Lund P, et al. Bovine colostrum to children with short bowel syndrome: a randomized, double‐blind, crossover pilot study. JPEN J Parenter Enteral Nutr. 2014;38:99‐106. [DOI] [PubMed] [Google Scholar]
- 35. Aunsholt L, Qvist N, Sangild PT, et al. Minimal Enteral Nutrition to Improve Adaptation After Intestinal Resection in Piglets and Infants. JPEN J Parenter Enteral Nutr. 2018;42:446‐454. [DOI] [PubMed] [Google Scholar]
- 36. Li Y, Juhl SM, Ye X, et al. A Stepwise, Pilot Study of Bovine Colostrum to Supplement the First Enteral Feeding in Preterm Infants (Precolos): Study Protocol and Initial Results. Front Pediatr. 2017;5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barness LA, Mauer AM, Holliday MA, et al. Commentary on breast‐feeding and infant formulas, including proposed standards for formulas. Pediatrics. 1976;57(2):278‐285. [PubMed] [Google Scholar]
- 38. Kreissl A, Zwiauer V, Repa A, et al. Effect of fortifiers and additional protein on the osmolarity of human milk: is it still safe for the premature infant? J Pediatr Gastroenterol Nutr. 2013;57:432‐437. [DOI] [PubMed] [Google Scholar]
- 39. Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. 2013;60:49‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mastromarino P, Capobianco D, Campagna G, et al. Correlation between lactoferrin and beneficial microbiota in breast milk and infant's feces. Biometals. 2014;27:1077–1086. [DOI] [PubMed] [Google Scholar]
- 41. Yang Z, Jiang R, Chen Q, et al. Concentration of Lactoferrin in Human Milk and Its Variation during Lactation in Different Chinese Populations. Nutrients. 2018;10(9):1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Albenzio M, Santillo A, Stolfi I, et al. Lactoferrin Levels in Human Milk after Preterm and Term Delivery. Am J Perinatol. 2016;33:1085‐1089. [DOI] [PubMed] [Google Scholar]
- 43. Santiago MS, Codipilly CN, Potak DC, Schanler RJ. Effect of human milk fortifiers on bacterial growth in human milk. J Perinatol. 2005;25:647‐649. [DOI] [PubMed] [Google Scholar]


