Abstract
Background:
COVID-19 was initially considered to be a respiratory illness, but current findings suggest that SARS-CoV-2 is increasingly expressed in cardiac myocytes as well. COVID-19 may lead to cardiovascular injuries, resulting in myocarditis, with inflammation of the heart muscle.
Objective:
This systematic review collates current evidence about demographics, symptomatology, diagnostic, and clinical outcomes of COVID-19 infected patients with myocarditis.
Methods:
In accordance with PRISMA 2020 guidelines, a systematic search was conducted using PubMed, Cochrane Central, Web of Science and Google Scholar until August, 2021. A combination of the following keywords was used: SARS-CoV-2, COVID-19, myocarditis. Cohorts and case reports that comprised of patients with confirmed myocarditis due to COVID-19 infection, aged >18 years were included. The findings were tabulated and subsequently synthesized.
Results:
In total, 54 case reports and 5 cohorts were identified comprising 215 patients. Hypertension (51.7%), diabetes mellitus type 2 (46.4%), cardiac comorbidities (14.6%) were the 3 most reported comorbidities. Majority of the patients presented with cough (61.9%), fever (60.4%), shortness of breath (53.2%), and chest pain (43.9%). Inflammatory markers were raised in 97.8% patients, whereas cardiac markers were elevated in 94.8% of the included patients. On noting radiographic findings, cardiomegaly (32.5%) was the most common finding. Electrocardiography testing obtained ST segment elevation among 44.8% patients and T wave inversion in 7.3% of the sample. Cardiovascular magnetic resonance imaging yielded 83.3% patients with myocardial edema, with late gadolinium enhancement in 63.9% patients. In hospital management consisted of azithromycin (25.5%), methylprednisolone/steroids (8.5%), and other standard care treatments for COVID-19. The most common in-hospital complication included acute respiratory distress syndrome (66.4%) and cardiogenic shock (14%). On last follow up, 64.7% of the patients survived, whereas 31.8% patients did not survive, and 3.5% were in the critical care unit.
Conclusion:
It is essential to demarcate COVID-19 infection and myocarditis presentations due to the heightened risk of death among patients contracting both myocardial inflammation and ARDS. With a multitude of diagnostic and treatment options available for COVID-19 and myocarditis, patients that are under high risk of suspicion for COVID-19 induced myocarditis must be appropriately diagnosed and treated to curb co-infections.
Keywords: myocarditis, COVID–19, SARS-CoV-2, symptomatology, biomarkers, adverse events, cytokine storm, systematic review
Introduction
Coronavirus disease 2019 (COVID-19) has led to fright among populations worldwide since it was first reported. 1 The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was initially only considered to be a respiratory illness, but it is now recognized as a complex multi systems disease.2,3 Current literature suggests that the increased expression of angiotensin-converting enzyme 2 (ACE2) receptors of SARS-CoV-2 in cardiac myocytes accounts for the relatively high cardiovascular involvement in COVID-19. 4 Comorbidities such as pre-existing cardiovascular diseases, hypertension and diabetes mellitus have led to worse prognosis among patients infected with COVID-19. 5 However, infected patients may experience added-on cardiovascular injuries, even in the absence of pre-existing cardiac disease. 6 Myocarditis is an inflammation of the heart muscle with symptoms such as chest pain, shortness of breath, and palpitations. 7 A study identified 42 COVID-19 patients with myocarditis, where fever was the most common presenting sign in 57% patients, and hypertension was the most pervasive comorbidity. 8
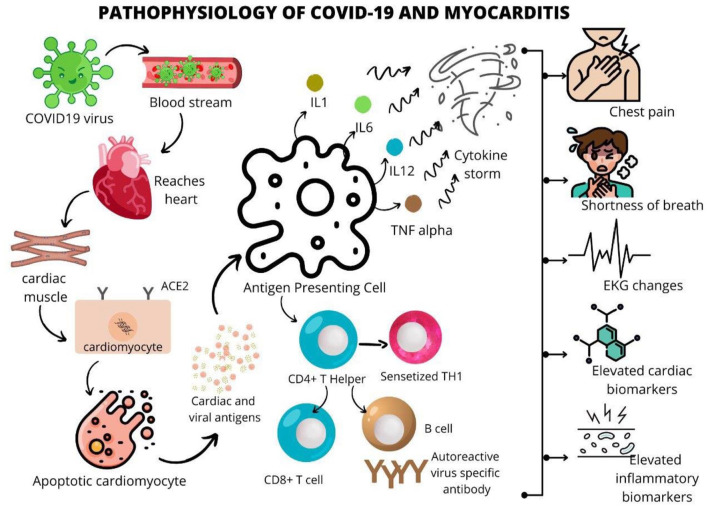
SARS-CoV-2 is posited to gain entry into human cells by binding the spike protein to the membrane protein angiotensin-converting enzyme 2 (ACE2).9,10 As depicted in Figure 1, SARS-CoV-2 gains entry into the bloodstream, making its way to the heart and cardiac muscle. In the cardiomyocytes, the binding to ACE2 upregulates the receptor eventually leading to apoptosis, releasing viral and cardiac antigens. These antigens, when fixed to the antigen presenting cells (APCs), lead to the release of interleukins (IL1, IL6, IL12, TNF alpha), which when presented to CD4+ T helper cells, CD8+ T cells, and B cells, lead to autoreactive virus specific antibodies. The entire mechanism is posited to lead to myocarditis, with elevated inflammatory biomarkers, cardiac biomarkers, EKG changes, and symptoms such as shortness of breath and chest pain (Figure 1).
Figure 1.
A schematic representation of the pathophysiology leading to COVID-19 induced myocarditis.
While our systematic review does not delve into the myocardial effects of COVID-19 vaccines, a report in the New England Journal of Medicine identified 2 cases of histologically confirmed, fulminant myocarditis within 2 weeks of COVID-19 vaccination. 11 As of September 1 2021, the Centers for Disease Control and Prevention writes that the risk of myocarditis is far higher after COVID-19 infection as opposed to the mRNA virus. 12 Based on a study that identified 1.5 million inpatient records with COVID-19, myocarditis was uncommon among patients with or without COVID-19, however, there was a relatively higher risk in the 50 to 75 and over age groups. 12 The under 16 age group could be more prone due to the related multisystem inflammatory syndromes. 12 The paper also noted an 18-fold higher chance of developing myocarditis due to COVID-19. 12
The objective of this systematic review is to collate evidence about demographics, symptomatology, diagnostic techniques, and clinical outcomes of COVID-19 infected patients with myocarditis.
Methods
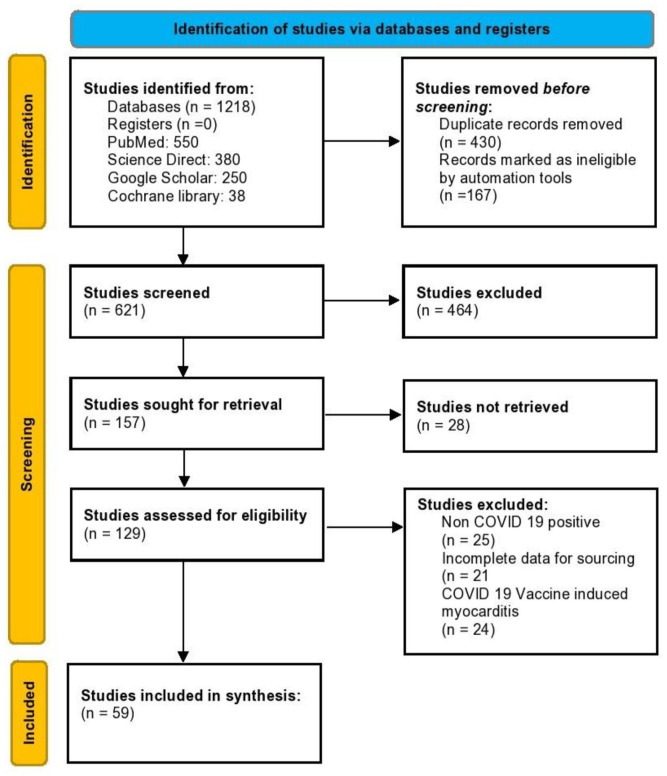
This systematic review was conducted and reported in conformity with the Cochrane and PRISMA (Preferred Reporting Items for Systematic review and Meta-Analyses) 2020 guidelines (Figure 2). A comprehensive literature search was done using the search engines PubMed, Google Scholar, Cochrane CENTRAL, and Web of Science database from their inception up until August 31, 2021. The search terms included “SARS-CoV-2” and/or “COVID 19” and/or “myocarditis.” Reference lists of included studies were also manually screened to identify any relevant studies that may have been missed during the search (umbrella review).
Figure 2.
PRISMA flowchart.
Articles retrieved from the systematic search were exported to EndNote Reference Library software (Clarivate), where duplicates were removed. 2 authors (V.J. and S.Y.) carried out an independent search and screened the titles and abstracts of the identified articles for inclusion. Afterward, full-text articles were reviewed to validate if they satisfied the inclusion criteria. Any discrepancies were resolved by discussion till consensus was achieved. Articles were included if they met all the prespecified eligibility criteria: (1) Patients with confirmed myocarditis in association with COVID-19; (2) Age groups > 18 years; (3) Cohorts, case series and case reports. Studies with post-mortem findings consistent with acute myocarditis were also included. All other studies were excluded.
Data extracted from articles included publication related characteristics (i.e. author/s, study design, number of patients, year of publication, and country) and patient related characteristics. In specific, demographics (age in years, gender, comorbidities), and clinical characteristics along with laboratory findings (particularly, inflammatory markers and cardiac enzymes) were documented (Tables 1-3). Additionally, features of imaging modalities including Chest X-ray/CT scan, ECG, ECHO, CMR, and endomyocardial biopsy were noted. Management pertaining to both COVID-19 and myocarditis, complications, and final clinical outcomes were also recorded. All data was extracted onto a predesigned Excel spreadsheet.
Table 1.
Demographics, Comorbidities, and Presenting Symptoms Among all Patients.
| Authors | Study design | Country | Sample size | Age (y) | Gender | Comorbidities | Presenting symptoms |
|---|---|---|---|---|---|---|---|
| Cizgic et al 13 | Case report | Turkey | 1 | 78 | M | HTN | Chest pain, shortness of breath |
| Yokoo et al 14 | Case report | Brazil | 1 | 81 | M | HTN, Ischemic Stroke | Fever, shortness of breath |
| Pietsch et al 15 | Case report | Germany | 1 | 59 | F | None | ARDS and dyspnea |
| Pavon et al 16 | Case report | Switzerland | 1 | 64 | M | Isolated pulmonary sarcoidosis and epilepsy | Fever, chest pain, shortness of breath, cough |
| Khatri et al 17 | Case report | USA | 1 | 50 | M | HTN, Ischemic stroke | Fever with chills, malaise, shortness of breath, cough, syncope |
| Hussain et al 18 | Case report | USA | 1 | 51 | M | HTN | Cough, shortness of breath, fatigue, fever |
| Dalen et al 19 | Case report | Norway | 1 | 55 | F | None | Fatigue, myalgia, syncope, chest pain |
| Zeng et al 20 | Case report | China | 1 | 63 | M | None | Fever, cough, shortness of breath, chest pain |
| Doyen et al 21 | Case report | France | 1 | 69 | M | HTN | Fever, cough, shortness of breath, vomiting, diarrhea |
| Faircloth et al 22 | Case report | USA | 1 | 60 | M | Multiple sclerosis | Fever, tachycardia, hypotension, shortness of breath, tachypnea, hypoxia |
| Coyle et al 23 | Case report | USA | 1 | 57 | M | HTN | Fever, myalgia, cough, shortness of breath, decrease appetite, nausea, diarrhea |
| Luetkens et al 24 | Case report | Germany | 1 | 79 | M | Asthma | Fatigue, syncope, shortness of breath, wheeze |
| Jain et al 25 | Case report | India | 1 | 60 | M | HTN, DM II | Cough, shortness of breath, hypoxia (75% SpO2) |
| Mustafa et al 26 | Case report | USA | 1 | 56 | M | None | Fatigue, myalgia, chest pain, cough, shortness of breath |
| Mansoor et al 27 | Case report | USA | 1 | 72 | F | HTN | Myalgia, fever, tachycardia, cough, cold, tachypnea, hypoxia (60% Sp02) |
| Al-assaf et al 28 | Case report | UAE | 1 | 58 | M | HTN | Asymptomatic |
| Khalid et al 29 | Case report | USA | 1 | 76 | F | HTN, hyperlipidemia, hypothyroidism | Fever, dyspnea, cough, tachycardia, tachypnea, hypoxia (79%SpO2) |
| Inciardi et al 30 | Case report | Italy | 1 | 53 | F | None | Fatigue, fever, hypotension, cough |
| Fried et al 31 | Case report | USA | 1 | 64 | F | HTN, hyperlipidemia | Asymptomatic |
| Wehit et al 32 | Case report | Argentina | 1 | 68 | M | HTN, obesity, DM II | Fever, fatigue |
| Butler et al. 33 | Case report | USA | 1 | 50 | M | HTN, DM II | Tachycardia, shortness of breath, hypoxia, confusion |
| Lagana et al. 34 | Cohort | Italy | 12 | 76( Mean) | 5M,7F | 75%—Systemic HTN, 66.7% Cardiac, | Fever, cough, shortness of breath |
| Kallel et al 35 | Case report | USA | 1 | 56 | M | Diabetes, obesity | Fever, myalgia, chest pain, cough, hypoxia |
| Ghurge et al. 36 | Case report | Canada | 1 | 62 | M | HTN, dyslipidemia | Fever, fatigue, cough, shortness of breath, tachypnea, lethargy |
| Fath et al 37 | Case report | USA | 1 | 61 | M | HTN, obesity, hyperlipidemia | Fatigue, myalgia, hypotension, tachypnea, hypoxia (spO2 85%), shortness of breath |
| Dabbagh et al 38 | Case report | USA | 1 | 67 | M | Non-ischemic cardiomyopathy with LVEF of 40% | Cough, shortness of breath, left shoulder pain |
| Irabien-Ortiz et al 39 | Case report | Spain | 1 | 59 | F | HTN, lymph node tuberculosis diagnosed by presence of erythema nodosum, and migraine | Fever, squeezing chest pain |
| Albert et al 40 | Case report | USA | 1 | 49 | M | None | Fever, dyspnea |
| Escher et al 41 | Case report | Germany | 1 | 39 | M | None | Fever, dyspnea |
| Ford et al 42 | Case report | USA | 1 | 53 | M | Dyslipidemia | Malaise, fever, chest pain |
| Gauchott et al 43 | Case report | France | 1 | 69 | M | DM II, HTN, IHD | Fever, fatigue, abdominal pain |
| Hua et al 44 | Case report | UK | 1 | 47 | F | None | Fever, dry cough, chest pain, shortness of breath |
| Jacobs et al 45 | Case report | Belgium | 1 | 48 | M | HTN | Diarrhea, cough, dyspnea |
| Labani et al 46 | Case report | French | 1 | 71 | F | Breast Cancer | Flu-like symptoms, chest pain |
| Spano et al 47 | Case report | Switzerland | 1 | 49 | M | None | Dyspnea, fatigue, intermittent epigastric pain, nocturia |
| Tavazzi et al 48 | Case report | Italy | 1 | 69 | M | None | Cough, dyspnea, weakness |
| Trogen et al 49 | Case report | USA | 1 | 69 | M | Obesity, asthma, spondylolysis | Fever, neck pain, diarrhea, vomiting |
| Varga et al 50 | Case report | N/A | 1 | 71 | M | Renal transplant, CAD, HTN | Dyspnea, fever, tachycardia, confusion |
| Warchoł et al. 51 | Case report | Poland | 1 | 74 | M | Atrial fibrillation, arterial HTN | New-onset ventricular tachycardia |
| Sardari et al 52 | Case report | Iran | 1 | 31 | M | None | Dyspnea, fever, |
| Dahl et al 53 | Case report | Norway | 1 | 37 | M | None | Fever, headache, unilateral left painful neck swelling |
| Hu et al. 54 | Case Report | China | 1 | 37 | M | None | Chest pain, dyspnea, diarrhea |
| Volis et al 55 | Case report | Israel | 1 | 21 | M | Smoking | Chest pain, cough, dyspnea, fever |
| Besler et al. 56 | Case report | Turkey | 1 | 20 | M | None | Chest pain, fever |
| Gaine et al 57 | Case report | Ireland | 1 | 58 | M | Smoking | Palpitations, dyspnea |
| Sheikh et al 58 | Case report | USA | 1 | 28 | M | None | Chest pain, cough, dyspnea |
| Salamanca et al 59 | Case report | Spain | 1 | 44 | M | None | Dyspnea, syncope |
| Naneishvili et al 60 | Case report | UK | 1 | 44 | M | None | Syncope, fever, lethargy |
| Kim et al 61 | Case report | Korea | 1 | 21 | F | None | Fever, dyspnea, cough |
| Nikoo et al 62 | Case report | Iran | 1 | 38 | F | None | Chest pain, nausea, vomiting, malaise |
| Sala et al 63 | Case report | Italy | 1 | 43 | F | Unremarkable | Dyspnea, fever, chest pain. |
| Yuan et al 64 | Case report | China | 1 | 33 | M | N/R | Fever, chest pain |
| Warchol et al. 51 | Case report | Poland | 1 | 74 | M | Atrial fibrillation, atrial HTN, type II DM, hypothyroidism | No symptoms |
| Asif and Ali 65 | Case series | USA | 2 | 64,71 | P1:M, P2: F | P1: HTN, Hyperlipidemia, P2: Multiple Myeloma | P1: dyspnea, hypotension. P2: Fever, cough, dyspnea |
| Khalid et al 66 | Case series | USA | 2 | 48, 34 | P1: M, P2: F | P1: Obesity, Diabetes, Obstructive sleep apnea. P2: None | P1: Fever, chills, myalgias, diarrhea, nonproductive cough and shortness of breath. P2: Fever, chills, body ache |
| Ng et al. 67 | Cohort | China | 16 | 68 | 9M,7F | None | All have chest pain, cough, shortness of breath |
| Jirak et al 68 | Cohort | Europe | 76 | 66.8 | 53M,23F | Arterial
hypertension—56.6% CAD—13.2% PVD—5.3% DM II- 26.3% |
N/A |
| Xu yan et al. 69 | Cohort | China | 27 | 69 | 10M, 17F | CHD-11% | Fever (82.4%), chest pain (7,6%), cough (68.1%), shortness of breath (40.3%), diarrhea (31.1%) |
| Kunal et al. 70 | Cohort | India | 28 | 60.9 ± 15.1 | 14M,14F | Diabetes = 71.4%, HTN = 64.3%, | Myalgia, fever, fatigue, chest pain, cold, cough, shortness of breath, confusion, headache, diarrhea |
Table 2.
Biomarkers, Radiographic, Electrocardiography, Echocardiography, and Biopsy Findings.
| Authors | Inflammatory markers | Cardiac markers | Radiographic findings | Electrocardiography | Echocardiography | CMR | Myocardial biopsy |
|---|---|---|---|---|---|---|---|
| Cizgic et al 13 | C reactive protein 94.6 mg/L | Troponin-998.1 ng/L | CT chest-small pericardial effusion and ground-glass opacification with consolidation | Atrial fibrillation besides heart rate of 150 bpm, concave ST elevation except for aVR lead | N/A | N/A | N/A |
| Yokoo et al 14 | N/A | Troponin T-33 pg/ml | Chest CT-small round ground-glass opacities, with multifocal distribution on both lungs | N/A | Reduction in the ejection fraction to 35% | Late enhancement areas with an ischemic pattern on the left ventricle base septum wall, with diffuse hypokinesis, and global systolic function | N/A |
| Pietsch et al 15 | NA | Troponin-83.6 ng/L CK-MB-7.14 ng/ml |
NA | NA | Severe diastolic dysfunction III with an increased wall thickness (inter-ventricular septum, 14 mm), and pericardial effusion | NA | EMB: Intra-myocardial inflammation with absence of signs of necrosis. Increased no. of CD45R0+ T memory cells (96.15 cells/mm2), CD3+ cells (20.54 cells/mm2), CD11a+ cells (24.36 cells/mm2), CD11b+ cells (91.56 cells/mm2), and CD54+ cells (area fraction 1.91%), histology: hypertrophied myocytes (diameter 31 μm) |
| Pavon et al 16 | C-reactive protein-466 mg/L, D-dimer-1210 ng/mL | Troponin (peak)- 1843 ng/L | Chest x-ray bilateral reticulation and ill-defined opacities, indicative of interstitial edema | N/A | Moderately reduced left ventricular ejection fraction of 47%( 72 h after CMR) | Reduced left-ventricular (LV) systolic function (42%), mild hypokinesia of the lateral wall. T2-mapping sequences showed myocardial edema (segmental T2 = 55-57 ms) | N/A |
| Khatri et al 17 | D-dimer-1068 ng/mL, procalcitonin-8.16 ng/mL, C-reactive protein110.85 mg/dL, Ferritin 66 ng/mL | Troponin- 544 ng/L, CK-MB54.3 ng/mL | N/A | Sinus tachycardia along with ST elevation in leads II, III, aVF, and ST depression in I, aVL | Severe global left ventricular systolic dysfunction, right ventricular (RV) enlargement causing its systolic dysfunction, and moderate-to-large pericardial effusion anterior to the Right ventricle | N/A | N/A |
| Hussain et al 18 | Troponin-18 ng/mL and CKMB-14.7 ng/mL | N/A | Diffuse ST elevation | Enlarged heart, marked decrease in ventricular systolic function with an ejection fraction of 20% | N/A | N/A | |
| Dalen et al 19 | C-reactive protein 11 mg/dl | Troponin T-108 ng/L, NTproBNP-1025 ng/L | N/A | Sinus tachycardia, insignificant ST elevation in inferior leads with a T-wave inversion in precordial leads | Left ventricular concentric hypertrophy | T1-mapping exhibited relaxation times of 1260-1270 ms in the anterolateral wall contrasted with 1090 ms in the septum. Late gadolinium enhancement in the anterolateral wall. | N/A |
| Zeng et al 20 | Interleukin-6(peak)- 272.40 pg/mL | Troponin I (peak)- 11.37 g/L, myoglobin (peak) > 390.97 ng/mL, NTpr(peak)- 22 500 pg/mL | Chest X-ray-Typical ground glass changes indicative of viral pneumonia | Sinus tachycardia without ST elevation and left axis deviation | Enlarged LV, diffuse myocardial dyskinesia, LVEF reduced to 32%, pulmonary hypertension, and normal RV function | N/A | N/A |
| Doyen et al 21 | N/A | Troponin I-9002 ng/L | Chest CT-bilateral crazy paving pattern, ground glass opacities and condensation | Diffuse T-wave inversion with the sign of left ventricular hypertrophy | Mild left ventricle hypertrophy, with normal left ventricular ejection fraction and nomal wall motion | Sub-epicardial late gadolinium enhancement of the apex and inferolateral wall | N/A |
| Faircloth et al 22 | C-reactive protein-
20.02 mg/dl, Ferritin-757 ng/ml ESR—78 mm/h |
Troponin-25 000 ng/L | NA | NA | NA | NA | NA |
| Coyle et al 23 | NA | Troponin I(peak) -7.33 on day 3, | N/A | Sinus tachycardia, with normal ST/T wave | Diffuse hypokinesis with relative apical sparing, with a left ventricular ejection fraction of 35–40%, no pericardial effusion | Diffuse edema of both atria and both ventricles along with small foci of late gadolinium enhancement | N/A |
| Luetkens et al 24 | C-reactive protein (pea k)—64.23 mg/L | Troponin T63.5 ng/L, NTproBNP—1178.0 pg/ml | Chest CT pulmonary ground glass peripheral infiltrates in the left upper lobe and discreet pleural and pericardial effusion | Normal | N/R | Diffuse interstitial myocardial edema with an increased T2 signal intensity ratio. T2 mapping showed diffuse myocardial inflammation( on day 10) | N/R |
| Jain et al 25 | Elevated inflammatory markers | Elevated troponin | Chest X-ray showed bilateral diffuse opacities | Age indeterminate inferior infarct versus left anterior fascicular block | EF <30% along with akinesis of the mid to apical myocardial segments | N/A | N/A |
| Mustafa et al 26 | C-reative protein—160 mg/L | Troponin I: 8.6 ng/ml | Chest x-ray was suggestive of increased interstitial prominence | Normal sinus rhythm with ST elevations in the antero-lateral distribution | N/A | N/A | N/A |
| Mansoor et al 27 | C-reactive protein: 27 mg/dl Ferritin: 928 ng/dl ESR: 82 mm/h WBC: 24000/μl, D-dimer: 3455ng/ml |
NT-proBNP: 4639 pg/ml, troponin T (hsTt): 118 ng/L |
N/A | Sinus tachycardia, PR elevation in aVR and PR depression in leads II and aVF on admission | Mildly decreased left ventricular function but no significant segmental wall motion abnormalities, mild mitral regurgitation, mildly enlarged right ventricle with normal right ventricular function, no tricuspid regurgitation, and no pericardial effusion. | N/A | N/A |
| Al-assaf et al 28 | Normal ranges of inflammatory markers and cardiac biomarkers. | N/A | Normal | Sinus bradycardia, no ST-T changes | Unremarkable study showing only a mildly dilated ascending aorta | T1 mapping showing a high value of 1062. T2 mapping showing an abnormal value of 57 |
N/A |
| Khalid et al 29 | C-reactive protein 23.10 mg/L, Interleukin-6 (IL-6) 781.46 mg/L, elevated lactate dehydrogen 334U/L and ferritin 457 ng/ml | Troponin 503 ng/l, proBNP35,000 pg/mL | Chest X-ray-diffuse bilateral pulmonary edema vs infiltrates | Normal sinus rhythm with a short PR interval | Severe left ventricular systolic dysfunction with segmental wall motion anomalies | N/R | N/R |
| Inciardi et al 30 | C reactive protein- 1.3 mg/dl mg/dl, D dimer- 500 U/F | Troponin T(peak)- 0.89 ng/mL, CKMB(peak)- 39.9 ng/mL, BNP(peak)—8465 pg/mL | N/A | Minimal diffuse ST elevation, low voltage in limb leads, ST depression, and T wave inversion in V1 and aV | Increased left ventricular wall thickness with diffuse hypokinesis, and LVEF of 40%. Large circumferential pericardial effusion of size 11 mm with the absence of tamponade | Diffuse biventricular apical hypokinesis, severe LV dysfunction (LVEF of 35%), Short tau inversion recovery and T2-mapping sequences showed marked biventricular myocardial interstitial edema. | N/A |
| Fried et al 31 | C reactive protein: 0.0054 mg/dl, ferritin: 967ng/ml ESR: 166 ng/ml |
Troponin- 7900 ng/L, | N/A | Sinus tachycardia, ST segment elevation in leads I, II, aVL, V2-V6, and PR elevation and ST depression in aVR. Low voltage QRS complexes in the limbs leads. | EF: 30% (reduced) Severe concentric left ventricular hypertrophy, and a dilated, severely hypokinetic right ventricle. Pericardial effusion |
N/A | N/A |
| Wehit et al 32 | LDH-198 UI/l, ferritin- 723 ng/mL, Dimer D- 300 ng/mL | Troponin T- 16 pg/mL, BnP 370 pg/mL | Chest radiography revealed right basal opacities | N/A | Deterioration in both global and segmental longitudinal strain | N/A | N/A |
| Butler et al 33 | N/A | Troponin: 67 ng/L, NT-proBNP: 4529 pg/ml | N/R | N/R | N/R | N/A | N/A |
| Lagana et al 34 | N/A | Troponin:39.9 pg/ml, NT-proBNP: 1557.6 pg/ml | N/R | Ischemic alteration (66.66%) | Diffuse left ventricular hypokinesis 66.66%, 25%QTc prolongation | N/A | N/A |
| Kallel et al 35 | C-reactive protein: 315 mg/l, WBC count: 17 940/UL, Creatinine: 45 mg/l, D-dimer: 1.04 mg/l | Troponin I: 677 ng/L, CPK-MB: 19 UI/l |
CT chest showed typical findings of COVID-19 with ground-glass opacification | Diffuse ST elevation and simple monomorphic supraventricular extrasystoles | Normal systolic function | N/A | N/A |
| Ghurge et al 36 | NA | NA | NA | NA | NA | Normal left ventricular (LV) and right ventricle (RV) size and function, LV ejection fraction was 62%, area of mid myocardial/subepicardial late enhancement in the basal inferolateral wall in a non-ischemic pattern most consistent with a myocarditis type pattern, abnormal hyperintense MRI relaxation associated with the presence of edema, abnormal T2 hyperintense relaxation associated with the presence of edema. | N/A |
| Fath et al 37 | Creatinine-1.16 mg/dL INR-1.5 CRP- 306.8 mg/L LDH- 707U/L IL-6- 23 pg/mL D-dimer-32 563 ng/mL Ferritin-2831.22 ng/mL CK-86U/L |
Elevated Troponin I 7.454 ng/ml |
NA | Diffuse, mainly anterolateral, ST elevation | Reduced ejection fraction | NA | Multiple microscopic sites of myocardial ischemia together with thrombi in the left atrium and pulmonary vasculature and, scattered microscopic cardiomyocyte necrosis. Autopsy also revealed an adherent organizing left atrial thrombus (1.5 cm) and marked thromboembolism of the left pulmonary artery |
| Dabbagh et al 38 | C-reactive protein-15.9 mg/dl, ferritin-593 ng/ml, D-dimer- 6.52 μg/ml and interleukin 6(IL-6)- 8 pg/ml | Troponin I < 18 ng/L, pro-BNP-54 pg/mL | Chest X-ray enlarged cardiac silhouette | Shallow voltage in limb leads, non-specific ST alteration | A decrease in left ventricular ejection fraction to 40%, massive peripheral pleural effusion, an indication of early right ventricular diastolic collapse, dilated but collapsing inferior vena cava | N/A | N/A |
| Irabien-Ortiz et al 39 | C reactive protein- 10 mg/L | Troponin T(peak)- 1100 ng/dL, NTproBNP - 4421 ng/L | Chest X-ray- mild signs of vascular redistribution, with no infiltrations | Diffuse ST elevation and PR-segment depression | Concentric hypertrophy, diminished LV volumes, preserved LVEF, moderate pericardial effusion, absence of tamponade. After 2 h severe biventricular failure and diffuse myocardial edema | N/A | N/A |
| Albert et al 40 | N/A | Elevated troponin, NT-proBNP | No pathological features | Sinus tachycardia, no ST-T changes | Globally depressed LVEFof 20% with LVEDD of 5.8 cm, increased wall thickness | N/A | Inflammatory infiltrates with visualization of viral particles |
| Escher et al 41 | N/A | Troponin-3264 pg/mL, BNP- 12232(pg/mL)- | N/A | N/A | LVEF = 22% | N/A | Active myocarditis with CD3+ 106 cell/mm2 |
| Ford et al 42 | N/A | BNP 588 pg/mL, TnT normal | Left lower lobe consolidation | Wide-complex, irregular tachycardia with a LBBB morphology, as well as a long QT interval | Mild LV dilation with hypokinesis (EF 15%). New transthoracic echo revealed LV thrombus and worsening LV dilation | LV dilation with global hypokinesis, increased T2 signal, hyperemia, and edema |
N/A |
| Gauchotte et al 43 | N/A | Troponin I 8066 pg/mL and CK–MB 2103 UI/L) | Normal | Normal | Severe and diffuse LV hypokinesia, LVEF = 30% | N/A | Post mortem: Multifocal inflammatory infiltration, in both
ventricles and septum, composed in its majority of macrophages
and lymphocytes. The myocardium was edematous, containing
dystrophic cardiomyocytes, without necrosis. Strong presence of
anti-SARS-CoV nucleocapsid protein antibody in the myocardium |
| Hua et al 44 | N/A | Troponin T (peak)-253 ng/L | N/A | Sinus tachycardia, concave inferolateral ST elevation | Left ventricular ejection fraction was normal with pericardial effusion of size 11 mm and absence of cardiac tamponade | N/A | N/A |
| Jacobs et al 45 | Ferritin- 32 401 μg/L, interleukin 6 level- 281 pg/mL |
NTproBNP, 9,223 pg/mL, TnI 14 932 ng/L | Multiple patchy ground-glass opacifications in all lung fields | QRS widening and a positive Deflection at the end of the T wave |
Hyperdynamic ventricular function (inotropes). IVS 12mm, PW 11mm, LV EDD 48mm | NA | Post Mortem: Hypertrophic Cardiac tissue with patchy muscular, sometimes perivascular, and slightly diffuse interstitial mononuclear inflammatory infiltrates, dominated by lymphocytes. Positive immunohistochemical staining with E06 in morphologically degenerating and necrotic cardiomyocytes adjacent to the infiltrate of lymphocytes |
| Labani et al 46 | C-reactive protein 9 mg/L | TnT: 60 ng/L, BNP: 474 ng/L | Mild bilateral peripheral lower pulmonary lobe ground-glass opacities | Diffuse inverted T waves and elongated QT | Infero-septal and infero-apical LV wall hypokinesia, LVEF 56% and a moderate pericardial effusion | LV wall motion, normal LVEF 61% and persistence of a mild pericardial effusion. STIR and T2 map showed suggestive of myocardial edema in the basal inferior LV wall. LGE: multiple areas of inferior subepicardial and mid-wall | N/A |
| Spano et al 47 | Elevated C-reactive protein | Elevated troponin and NT-proBNP levels | CT chest-left heart congestion | Dynamic T- wave inversion | Diffuse hypokinesia with severely decreased left- and right-ventricular function | T2 weighted imaging and T2 mapping revealed diffuse thickening of the myocardium and pericardium attributable to edema | N/A |
| Tavazzi et al 48 | C reactive - protein 52.7 mg/L | Troponin I- 4332 ng/L | N/A | N/A | Dilated left ventricle, severe and diffuse LV hypokinesia with LV ejection fraction of 34% | N/A | N/A |
| Trogen et al 49 | C-reactive protein- 167 mg/L, D-dimer 1218 ng/mL, ferritin 1274.6 ng/mL | Troponin I: 2.97 ng/ml, BNP- 2124 pg/mL | N/R | Sinus tachycardia and T-wave inversion particularly in the inferior leads | Left ventricular ejection fraction mildly depressed without obvious intracardiac clots or pericardial effusion | The normal size of both ventricles along with slightly decreased systolic function. A segment of a mid-wall late gadolinium enhancement at the level of the inferior junction of both ventricles correlative to an area of increased T2 signal, along with an area of hypokinesia | N/R |
| Varga et al 50 | C-reactive protein: 232 mg/l D-dimers: 2.42 mg/l |
Troponin T: 51 ng/l, NTproBNP: 10 456 ng/l | Bilateral infiltration and ground glass opacities with consolidations in the right lung | N/A | Preserved left ventricular ejection fraction, but a severely enlarged left atrium (59ml/m2) indicating longstanding diastolic dysfunction | N/R | Postmortem: accumulation of inflammatory cells associated with endothelium, as well as apoptotic bodies, in the heart |
| Warchoł et al 51 | C-reactive protein levels-94 mg/l,D dimers:1.39 mg/l, lactate dehydrogenase: 369 U/l | Troponin T ranged from 72 ng/l to 102 ng/l, NT-proBNP: 2451 ng/l | N/R | N/R | N/R | Left atrial enlargement and global left ventricular hypokinesia with reduced left ventricular ejection fraction of 20%. Inferior and inferolateral wall large, patchy, and linear non-ischemic pattern of fibrosis with late gadolinium enhancement | N/R |
| Sardari et al 52 | CRP = 105 mg/L, ESR = 70 mm/h | Troponin T = <0.03ng/ml | Bilateral ground glass and consolidative opacities | N/R | Left ventricular dysfunction | Normal LV size, EF of 50 | No |
| Dahl et al 53 | CRP-230 mg/L, procalcitonin-2.1 μg/L | TnT- 90 ng/L, NT-proBNP - 160 ng/L | bibasal consolidations | sinus tachycardia with moderately flattened T-waves | deterioration of the left ventricular function,EF-40% | diffuse myocardial edema suggestive of significant acute myocardial injury. | N/R |
| Hu H et al 54 | N/A | Troponin T-10 000 ng/L,CKMB 112.9 ng/L, BNP—21 025 ng/L | CXR-cardiomegaly, CT-pulmonary infection, enlarged heart | III, AVF ST-segment elevation | enlarged heart and a marked decrease in ventricular systolic function, LVEF-27%,trace 2 mm pericardial effusion | N/R | N/R |
| Volis et al 55 | CRP-3.87 mg/dl | Troponin-I-965 ng/L | chest CT-unremarkable | minimal ST-depressions and T-wave inversions in lead III | Normal left ventricular ejection fraction-65%, normal function, no wall-motion abnormalities. | N/R | N/R |
| Besler et al 56 | CRP-0.0812 g/L | Troponin I-7.621 ng/mL,CKMB-21.92 μg/L,NT-proBNP-1525 ng/L | CXR-focal consolidation on the upper zone of left lung, CHEST CT-subpleural consolidation with ground-glass opacification in the left upper lobe | N/R | N/R | Myocardial wall edema, subepicardial late gadolinium enhancement of the posterolateral wall in the mid ventricle-suggestive of myocarditis,ef-64% | N/R |
| Gaine et al 57 | CRP-7 mg/L | Troponin T -25 ng/L, NTproBNP-3428 pg/mL | CXR-cardiomegaly, increased interstitial lung markings | atrial fibrillation | severely impaired LVEF of 20% and mitral regurgitation | Biventricular oedema suggestive of generalized severe myocarditis | N/R |
| Sheikh et al 58 | CRP-32.5 mg/dL,ESR-88 mm/h | Troponin-0.43 ng/mL, BNP-19 600 pg/mL | CXR-patchy bibasilar opacities | Accelerated junctional rhythm, non specific T wave changes | Left ventricular dysfunction-ejection fraction 30% | N/R | N/R |
| Salamanca et al 59 | N/R | troponin T-745 ng/l, CKMB-30 U/l, NTproBNP-24,167 pg/ml | CXR-bilateral pneumonia | Third-degree atrioventricular block | severely dysfunctional left ventricle,ejection fraction [LVEF] ∼15% | Diffuse edema, negative Late gadolinium enhancement | No significant inflammatory infiltrates |
| Naneishvili et al 60 | CRP-47 mg/L, D-dimer 579 ng/mL |
Troponin I-639 ng/L, CK-1403 U/L |
CXR-bilateral patchy air space shadowing consistent with SARS-CoV-2 pneumonia,CHEST CT-1 cm rim of pericardial fluid and minimal bi-basal lung inflammatory changes. | Atrial fibrillation converted to sinus rhythym by DC cardioversion | Moderate concentric biventricular hypertrophy, diffused left ventricular hypokinesia with moderate to severe left-ventricular systolic dysfunction EF-37% and pericardial effusion with no signs of tamponade | N/R | N/R |
| Kim et al 61 | N/R | Troponin I-1.26 ng/mL,NT-proBNP-1929 pg/mL | CXR-multifocal consolidation on both lung fields and cardiomegaly, CHEST CT-multifocal consolidation and ground-glass opacification in both lungs in the lower lobe. | Multiple premature ventricular complexes | Severe left ventricular systolic dysfunction | Myocardial edema, Extensive transmural late gadolinium enhancement | N/R |
| Nikoo et al 62 | CRP-23 mg/L,ESR-4 mm/h | Troponin I 10.32 Mic gr/L),CKMB-83 IU/L | N/R | Sustained ventricular tachycardia | Biventricular dilation and global hypokinesia with left ventricular ejection fraction-20–25%. | CMR after discharge-normal ventricles size, EF of 52%, diffuse myocardial inflammation of the LV myocardium | N/R |
| Sala et al 63 | CRP: 18 mg/l | Troponin T: 135 ng/L, NT pro BNP: 512 pg/ml | B/L opacity in lungs | Mild ST-segment elevation(V1-V2 and aVR), ST- depression (V4-V6), and diffuse U waves | LVEF = 43%, inferolateral wall hypokinesis and no pericardial effusion | Hypokinesia of left ventricle mid and basal segment, diffuse myocardial oedema | T lymphocytes inflammatory infiltrates and necrosis |
| Yuan et al 64 | N/R | N/R | No ground glass appearance in Lungs. | Ventricular Tachycardia | N/R | Increased left ventricular apical region | N/R |
| Warchol et al 65 | CRP: 94 mg/l, D-dimer: 1.39 mg/l | Trop I: 102 ng/L, NTpro BNP: 2451 ng/l | N/R | 55% | Left atrial enlargement, global left ventricular hypkinesia, myocardial edema with ejection fraction of 20% | N/R | |
| Asif and Ali 65 | P1: N/R, P2: N/R | P1: 0.17ng/ml. P2: 1.6 ng/ml | P1: B/L Diffuse opacity, P2: B/L Diffuse lung opacity | P1: ST- elevation in lead I, aVL and V1-V4 T wave change, P2: ST- elevation in lead V2-V6 and Q waves in lead V4-V6 | P1: 70%, P2: 65% | P1: No regional wall abnormalities, P2: No regional wall abnormalities | N/R |
| Khalid et al 66 | P1: CRP: 8.5 mg/L, D-dimer: 0.73ug/mL, ESR: 29mm/h, Ferritin: 559 ng/mL. P2: Normal | P1: Troponin-I(116 ng/mL), P2: Troponin-I( 2.7 ng/mL), NT pro- BNP(2917) pg/ml | P1: N/R, P2: N/R | P1: Sinus Rhythm,inferioposterior infarct without ST- elevation. P2: Sinus tachycardia, low amplitude QRS, and poor R- wave progression | P1: EF = 45% . P2: EF = 25% | P1: N/R, P2: N/R | N/R |
| Ng et al 67 | Elevated CRP: 4, WBC: 4 | Elevated Troponin: 7 patients | N/R | 14 patients have ECG changes for Myocardial injury | N/R | 14 patients have abnormal CMR finding (High T1 and /or T2, +/- no ischemic LGE) | N/R |
| Jirak et al 68 | C-reactive protein: 27.5 ± 12.2 mg/dl, CK levels: 518 U/L, D-dimer: 6720 ng/ml, Procalcitonin: 1.59 ng/ml WBC count: 14820/μl |
Troponin: 354 ng/L CK-MB: 22 U/L NT-proBNP: 811 pg/ml |
35 Patient shownCardiomegaly (46%), 26 Patient shown Pulmonary venous congestion (34.2%) | N/R | 24 patient shown LVEF, Pericardial effusion in 3 patients. | N/R | N/R |
| Yan et al 69 | CK levels: 80 U/L, Creatinine: 0.72-0.92 mg/dl, Ferritin: 975ng/ml[Male] 748 ng/ml [Female], ESR: 35 mm/h, Procalcitonin: 5850/μl, D-dimer: 1.43ng/ML |
Troponin: 6.9 ng/L, CK-MB: 1.2 ng/ml, NT-proBNP: 221 pg/ml |
N/R | N/R | N/R | N/R | N/R |
| Kunal et al 70 | D-dimer = 84.2% (elevated) | Troponin T = 0.66 ± 1.28 ug/L, CK-MB(U/L) = 55.7 ±3 0.1 | N/R | ST- T change = 32.1%, Max QTc = 457.37 ± 32.7 | N/R | N/R | N/R |
Table 3.
In-Hospital Management, Complications, and Outcomes of Patients.
| Authors | In-hospital management | Complications | Outcomes |
|---|---|---|---|
| Cizgic et al 13 | Furosemide, angiotensin converting enzyme (ACE) inhibitor and, beta-blocker along with Covid-19 specific therapy | ARDS | Transfer red back to Covid19 center |
| Yokoo et al 14 | Antibiotics, steroids | — | Discharged |
| Pietsch et al 15 | N/A | N/R | N/R |
| Pavon et al 16 | Piperacillin-tazobactam, catecholamine, Intubated | N/R | Discharged |
| Khatri et al 17 | Hydroxychloroquine (400 mg twice on the first day, succeeded by 200 mg twice a day for 4 days), IV azithromycin, IV vancomycin, IV cefepime, and methylene blue infusion, IV methylprednisolone (200 mg/d) on 3 day, dobutamine, vasopressin, and norepinephrine | Cardiogenic and distributive shock, with multi-organ failure | Died on day 4 |
| Hussain et al 18 | Remdesivir, hydroxychloroquine and azithromycin, and Indomethacin 7th day, methylprednisolone and colchicine, mechanical ventilation | ARDS on 2nd day | N/R |
| Dalen et al 19 | IV fluids, norepinephrine, and dobutamine | Cardiogenic shock | Recovered |
| Zeng et al 20 | High-flow oxygen, lopinavir-ritonavir, interferon α-1b, immunoglobulin, piperacillin-tazobactam, and continuous renal replacement therapy, IV methylprednisolone, vasopressors used from day 26, ECMO on day 11 | Cardiogenic shock on day 11, Septic shock on day 26, ARDS day 1 | Passed away on day 33 |
| Doyen et al 21 | Aspirin, fondaparinux, IV hydrocortisone for 9 days, Mechanical ventilation | ARDS | Discharged from ICU after 3 weeks |
| Faircloth et al 22 | Norepinephrine, vasopressin, dobutamine, and methylprednisolone | — | Discharged |
| Coyle et al 23 | Hydroxychloroquine, azithromycin, ceftriaxone, and tocilizumab, IV methylprednisolone 500 mg daily x 4 days, followed by decreasing dose and, colchicine, milrinone day 4, norepinephrine day 4, mechanical ventilation on day 3 | ARDS on day 3, Cardiogenic shock on day 4 | Discharged on day 19 |
| Luetkens et al 24 | N/R | N/R | N/R |
| Jain et al 25 | Vasoactive drugs, vancomycin and cefepime, IVIG, pulse dose steroids, and mechanical ventilation. | Cardiogenic shock and multi-organ failure | Discharged on day 46 |
| Renal replacement therapy for acute kidney injury and N-acetylcysteine for acute liver injury, | NA | NA | |
| Mustafa et al 26 | Aspirin, unfractionated heparin and nitroglycerin infusion for acute coronary syndrome. | N/R | Improvement in symptoms over the next few days |
| Azithromycin and hydroxychloroquine | |||
| Mansoor et al 27 | Vancomycin, meropenem, chloroquine, and azithromycin, norepinephrine, phenylephrine, vasopressin, diuretics, and subcutaneous heparin | Multi-organ system failure and pulseless electrical activity. | Mortality on day 6 in ICU |
| Al-assaf et al 28 | Enoxaparin, amlodipine, and scheduled a permanent pacemaker implant. | — | Discharged in stable condition. |
| Khalid et al 29 | Tocilizumab ( 2-dose of 480 mg and 240 mg), intravenous immunoglobulin (25 g for 5 days), ceftriaxone, cefdinir, and cefepime, norepinephrine, Intubated | Cardiogenic shock, ARDS | Recovered |
| Inciardi et al 30 | Hydroxychloroquine (200 mg 2 times a day ), lopinavir/ritonavir (250 every 12 h), kanrenone (50 mg), furosemide(25-50 mg), and bisoprolo(2.5 mg)l, IV methylprednisolone 1 mg/kg for 3 days, dobutamine | Cardiogenic shock on day 1 | Recovered |
| Fried et al 31 | Intraaortic baloon pump was inserted and dobutamine infusion | Cardiogwnic shock | Discharge |
| Wehit et al 32 | Ampicillin/sulbactam, liponavir/ritonavir and hydroxychloroquine, orotracheal intubation and mechanical ventilation | On day 15, bacteraemic sepsis and multi-organ failure | Patient was still in the intensive care unit |
| Butler et al 33 | Rehabilitation | N/R | N/R |
| Lagana et al 34 | Methyl prednisolone (100%), Ace Inhibitor (75%) | Cardiogenic shock (33.33%) | 3(25%) |
| Kallel et al 35 | Oxygen therapy with a high concentration mask (10
litters/minute) for acute respiratory failure on
admission. Dobutamine (5 γ/kg/min) and noradrenaline (3 mg/h). One dose of 80mg of Tocilizumab), corticosteroid, and azithromycin; (500mg the first day then 25 mg/day for 4 days). |
N/R | Discharged 7 days later in-patient management |
| Fath et al 37 | Aspirin and ticagrelor, along with the heparin infusion and inotropic support with norepinephrine, vasopressin, and dobutamine for acute coronary syndrome. | Cardiac arrest | Died |
| Dabbagh et al 38 | Hydroxychloroquine, glucocorticoids, and colchicine; Intubated. | — | Discharged |
| Irabien-Ortiz et 39 | Immunoglobulins (80 mg/day), interferon-B (0.25 mg every 48 h) and ritonavir/lopinavir, IV methylprednisolone 500 mg daily at decreasing doses for 14 days, and norepinephrine, ECMO | Cardiogenic shock on day 1 | N/R |
| Albert et al 40 | Tocilizumab, Methyprednizone, IV immunoglobulin, Inotropes, ECMO. | — | Discharged |
| Escher et al 41 | Cyclophosphamide and steroids. | Recovered | |
| Ford et al 42 | Amiodarone load, ceftriaxone/azithromycin, tissue plasminogen activator, warfarin. | — | Recovered and discharged |
| Gauchotte et al 43 | Vasopressors, Inotropic support, ECMO, intubation. | N/R | Deceased at 6th day of hospitalization |
| Hua et al 44 | Vasopressors | Cardiogenic shock day 1 | Recovered |
| Jacobs et al 45 | Hydroxychloroquine, azithromycin, noradrenaline, adrenaline, and dobutamine | Refractory shock | Died |
| Labani et al 46 | N/R | — | Recovered and discharged |
| Spano et al 47 | N/R | N/R | N/R |
| Tavazzi et al 48 | Adrenaline (0.07 μg/kg/mi n), and noradren aline (0.1 μg/kg/mi n), ECMO and IABP | Cardiogenic shock on day 1 and septic shock | Died |
| Trogen et al 49 | Hydroxychloroquine, piperacillin/tazobactam, enoxaparin | Septic shock | Discharged |
| Varga et al 50 | N/R | N/R | Died |
| Warchoł et al 51 | Azithromycin, oseltamivir, magnesium, and amiodarone | N/R | N/R |
| Sardari et al 52 | Bisoprolol and lisinopril | Plueritic chest pain | N/R |
| Dahl et al 53 | Cefotaxime, clindamycin,3 L/min- oxygen, Furosemide, norepinephrine, Continuous positive airway pressure | respiratory distress,right side bell’s palsy | Discharged on day 11 |
| Hu et al 54 | methylprednisolone,immunoglobulin,norepinephrine,toracemide,furosemidemilrinone,piperacillin,sulbactam, pantoprazole | cardiogenic shock and pulmonary infection | Discharge |
| Volis et al 55 | N/R | Pleuritic chest pain, dyspnea | Discharge |
| Besler et al 56 | Hydroxychloroquine, azithromycin, ceftriaxone, tigecycline, favipiravir, colchicine | chest pain | Discharged on day 7 |
| Gaine et al 57 | Diuretics, rate-control agents, anticoagulants, ACE inhibitor, mineralocorticoid antagonist | Heart failure | discharge |
| Sheikh et al 58 | Metoprolol, lisinopril, low-dose aspirin, hydrochlorthiazide, desmopressin | Diabetes insipidus | Discharge |
| Salamanca et al 59 | Dobutamine, norepinephrine, methylprednisolone, tocilizumab, hydroxychloroquine, azithromycin, lopinavir-ritonavir, temporary pacemaker, extracorporeal membrane oxygenation, intra-aortic balloon pump | Cardiogenic shock | Discharge |
| Naneishvili et al 60 | Methylprednisolone, dobutamine, amiodarone, milrinone, norepinephrine, antibiotics | cardiogenic shock | Discharge |
| Kim et al 61 | N/R | N/R | N/R |
| Nikoo et al 62 | Amiodarone, dexamethasone, standard heart failure therapies (details n/r),therapeutic anticoagulation, temporary pacemaker | Cardiogenic shock | Discharge |
| Long ma et al 3 | NA | NA | NA |
| Sala et al 63 | Lopinavir, Hydroxychloroquine | Chest Pain, dyspnoea | Discharge |
| Yuan et al 64 | N/R | Chest Pain | Discharge |
| Warchol et al | Azithromycin, oseltamivir | Hemodynamically unstable | N/R |
| Asif and Ali 65 | P1: Aspirin, clopidogrel and heparin, azythromycin, hydroxychloroquine, tocilizumab, merooenum, norepinephrine. P2: Azythromycin, tocilizumab, nor epinephrine, midazolam | P1: ARDS, P2: ARDS | P1: Died, P2: ICI |
| Khalid et al 66 | P1: Aspirin, clopidogrel and diuretics P2: Methylprednisone, colchicine | P1: N/R. P2: Refractory shock | P1: Discharge, P2: Discharge |
| Ng et al 67 | N/R | N/R | N/R |
| Jirak et al 68 | Catecholamine, extracorporeal membrane oxygen therapy, Antibiotics. | ARDS, | N/R |
| Yan et al 69 | N/R | N/R | N/R |
| Kunal S et al 70 | Hydroxychloroquine, Azithromycin | N/R | 57.% Died |
Results
In total, 54 case reports and 5 cohorts were identified comprising 215 adult patients. Among the 59 studies, the following comorbidities were noted among 178 patients. Hypertension (n = 92, 51.7%), diabetes mellitus type 2 (n = 47, 46.4%), cardiac comorbidities (n = 26, 14.6%), hyperlipidemia (n = 6, 3.4%), obesity (n = 5, 2.8%), ischemic stroke (n = 2, 1.1%), asthma (n = 2, 1.1%), hypothyroidism (n = 2, 1.1%), smoking (n = 2, 1.1%), cancer (n = 2, 1.1%), sarcoidosis (n = 1, 0.6%), epilepsy (n = 1, 0.6%), multiple sclerosis (n = 1, 0.6%), tuberculosis (n = 1, 0.6%), migraine (n = 1, 0.6%), spondylitis (n = 1, 0.6%), renal transplant (n = 1, 0.6%), and sleep apnea (n = 1, 0.6%) (Table 1).
Presenting symptoms on admission were acquired from 139 of 215 patients. They include cough (n = 86, 61.9%), fever (n = 84, 60.4%), shortness of breath (n = 74, 53.2%), chest pain (n = 61, 43.9%), diarrhea (n = 43, 30.9%), fatigue (n = 37, 26.6%), myalgia (n = 34, 24.5%), dyspnea (n = 17, 12.2%), hypoxia (n = 7, 5%), syncope (n = 6, 4.3%), tachycardia (n = 6 4.3%), hypotension (n = 4, 2.9%), tachypnea (n = 4, 2.9%), malaise (n = 4, 2.9%), vomiting (n = 4, 2.9%), ARDS (n = 1, 0.7%) (Table 1).
Of 215, inflammatory markers were reported among 185 patients. The inflammatory markers were elevated among 181 (97.8%) patients, and were normal in the remaining 4 (2.2%) patients. The cardiac markers were documented in 212 patients, of which 201 (94.8%) had elevated levels, whereas, 11 (5.2%) patients had normal cardiac markers. The mean value of CRP was 91.6 mg/L (Normal: Less than 10 mg/L. High: Equal to or greater than 10 mg/L). 71 The mean D-dimer value was 2419.2 ng/ml (reference concentration of D-dimer is < 500 ng/mL). 72 Mean ferritin laboratory values of 908.9 ng/ml (the normal range for ferritin in blood serum is: 20 to 250 ng/mL for adult males. 10 to 120 ng/mL for adult females) 72 ; and Interleukin 6 of 271.2 pg/mL (normal values: 5-15 pg/ml) were reported. 73 Troponin values were reported as 44.85 ng/ml (normal range for troponin I is between 0 and 0.04 ng/mL but for high-sensitivity cardiac troponin (hs-cTn) normal values are below14ng/L). 74
Radiographic imaging studies particularly, CT and Chest X-ray were indicated in COVID-19 patients (Table 2). Radiographic findings were obtained from 120 individuals with COVID-19 myocarditis. Variable features were noticed, of which cardiomegaly (32.5%) was the most prominent. Precisely, 120 patient radiographic findings were noted, of which cardiomegaly (n = 39, 32.5%) was the most common occurrence. This was followed by pulmonary venous congestion (n = 27, 22.5%), ground glass opacity (n = 23, 19.2%), consolidation (n = 9, 7.5%), pericardial effusion (n = 3, 2.5%), pleural effusion (n = 1, 0.83%), and no abnormal finding (n = 5, 4.2%) were noted in the cohort of included patient (Table 2).
Electrocardiography (ECG) findings were obtained for 96 patients, which were normal in 2 (2%) patients while other patients had varied ECG findings comprising of ST segment elevation among 43 (44.8%) patients, T wave inversion in 7 (7.3%) patients, ST depression in 5 (5.2%) patients, sinus tachycardia in 11 (11.5%) patients, atrial fibrillation in 3 (3.1%) patients, sinus bradycardia in 1 (1%) patient, ventricular tachycardia in 2 (2%) patients, and finally LBBB was reported in 1 (1%) patient as well (Table 2). Echocardiography was conducted in 175 patients, where 9 (51.4%) patients showed normal ejection fractions while 55 (31.4%) patients demonstrated reduced ejection fraction with a mean EF% of 35. Pericardial effusion was demonstrated in 12 (6.9%) patients, left ventricular hypertrophy in 7 (4%) patients, cardiomegaly in 7 (4%) patients, myocardial dyskinesia in 19 (10.9%) patients, and LV thrombus in 1 (0.6%) patient (Table 2).
Cardiovascular magnetic resonance (CMR) imaging is a non-invasive, gold standard test for diagnosing myocarditis. Our synthesis identifies that 42 of 215 patients underwent CMR and 36 of them were diagnosed with Myocarditis by the Lake Louis Criteria. The most common findings were increased signal intensity in T2 weighted imaging that is, myocardial edema (30/36; 83.3%) suggestive of myocardial inflammation and/or ischemia. Late Gadolinium enhancement was observed in 23/36 (63.9%) patients in both ischemic and non ischemic patterns. Hypokinesis and decreased systolic function were present in 8/36 (22.2%) and 6/36 (16.7%) patients respectively. Myocardial fibrosis was found in 1/36 (2.8%) patients. In total, 6 (14.3%) of 42 patients were found to have normal CMR findings (Table 2).
On noting the biopsy and histopathological examination findings, and considering the invasive in nature, these findings were reported in 9 (4.2%) patients out of 215 (Table 2). The most common findings were multifocal or diffuse lymphocytic infiltrates in the myocardium and endothelium along with myocardial edema and necrosis. Other findings included positive myocardial anti-SARS COV nucleocapsid protein antibodies, cardiac hypertrophy, and multiple sites of ischemia and thrombosis with a left atrial and left pulmonary artery thrombus in one patient. 37 Only 1 (11.1%) patient had normal findings on biopsy.
The in-hospital management acquired from 165 patients comprised of azithromycin (n = 42, 25.5%), hydroxychloroquine (n = 41, 24.9%), methylprednisolone/steroid (n = 14, 8.5%), norepinephrine (n = 10, 6%), dobutamine (n = 7, 4.3%), tocilizumab (n = 6, 3.6%), and remdesivir (n = 1, 0.6%) (Table 3). Standard care of treatment for COVID-19 was used for majority of the patients.
Complications during in-hospital stay reported in 128 patients included ARDS (n = 85, 66.4%), cardiogenic shock (n = 18, 14%), pleuritic chest pain (n = 6, 4.7%), multiorgan failure (n = 4, 3.1%), septic shock (n = 3, 2.3%), distributive shock (n = 2, 1.6%), sepsis (n = 2, 1.6%), bells palsy (n = 1, 0.8%) (Table 3). Of 85 patients, 55 (64.7%) survived, whereas 27 (31.8%) died. Three patients (3.5%) were in critical care unit on the last follow-up (Table 3).
Discussion
This systematic review aimed to describe the symptomatology, prognosis, and clinical findings of patients with probable and confirmed COVID-19-related myocarditis. Frequent clinical findings of COVID-19 infection constitute fever, cough, shortness of breath, and fatigue. 75 The World Health Organization has cited fever and cough as striking features of COVID-19. 76 Fever, dyspnea, and/or chest pain are typical manifestations of myocarditis that tend to overlap with COVID-19 symptomatology, thus making the diagnosis challenging.77,78 Laboratory investigations such as rising levels of cardiac biomarkers and electrocardiogram findings may assist in diagnosing COVID-19 induced myocarditis.
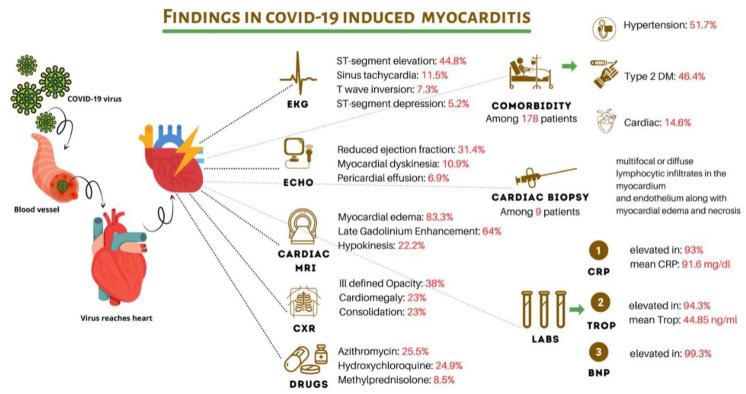
Our systematic review finds hypertension was the most common comorbidity with prevalence among 51.7% patients. This was followed by diabetes mellitus type 2 (46.4%) and cardiac comorbidities (14.6%). Our synthesis also finds that the most common presenting symptoms on admission comprised of 61.9% patients with cough, 60.4% with fever, and 53.2% with shortness of breath. The inflammatory markers were elevated among 97.8% patients, and the cardiac markers were increased in 94.8% of patients. The mean CRP levels were 91.6 mg/L, mean D-dimer values were 2419.2 ng/ml, and mean ferritin was 908.9 ng/ml. Mean Interleukin 6 values were 271.2 pg/mL and troponin values were identified as 44.85 ng/ml. The most distinct radiographic findings were cardiomegaly noted in 32.5% patients, followed by pulmonary venous congestion (22.5%), and ground glass opacity (19.2%). On noting ECG findings, ST segment elevation was reported in 44.8% patients, sinus tachycardia in 11.5%, and T wave inversion in 7.3% patients. Echocardiography noted normal ejection fractions in 51.4% patients, but 31.4% had reduced ejection fraction with a mean percentage of 35%. CMR imaging identified increased signal intensity in T2 weighted imaging, with myocardial edema in 83.3% patients, suggesting myocardial ischemia/inflammation. Late gadolinium enhancement was observed in 63.9% patients. The biopsy and histopathological examination findings found multifocal or diffuse lymphocytic infiltrates in the myocardium and endothelium along with myocardial edema and necrosis. In-hospital management comprised of 22.5% patients treated with azithromycin, 24.9% with hydroxychloroquine, 8.5% with methylprednisolone/steroid and 6% with norepinephrine. Standard of care and treatment was used for the majority of patients. complications during in-hospital stay included 66.4% patients acquiring ARDS and 14% with cardiogenic shock, in addition to others. Overall, 64.7% patients survived. A summary of the findings obtained is depicted in Figure 3.
Figure 3.
A summary of COVID-19 infection induced myocarditis.
Pirzada et al 79 elucidated the features of myocarditis found during the initial waves of the COVID-19 pandemic. The authors write that while the exact pathophysiology of severe COVID-19 was still elusive, a consistent observation of the pro-inflammatory surge, namely the cytokine storm was made. 79 Observations of elevated interleukins (IL-2R, IL-6, IL-10, TNF- α) were presented in a single-center cohort. 80 Viral myocarditis may be considered a direct response of autoimmunity, inflammation, or both. 81 Based on a cohort of 416 patients at the Renmin Hospital of Wuhan University conducted from January 20, 2020 until February 10, 2020, 82 (19.7%) patients had cardiac injury. 82 The cardiac involvement in the cohort of patients had a hazard ratio of 4.26, which is notably high. 82 Mortality in the myocardial injury group versus the general group was significantly higher (51.2% vs 4.5%, P < .001). 82 The symptoms of myocarditis among COVID-19 patients range from mild symptoms such as chest pain, fatigue, and palpitations to life-threatening symptoms such as sudden cardiac death associated with ventricular arrythmia or cardiogenic shock. Classically, myocarditis has a viral prodrome including myalgias, fever, and gastrointestinal/respiratory symptoms, with ranges of 10% to 80%. 79
Sawalha et al 83 identified COVID-19 related myocarditis focusing on management and outcomes until June 30, 2020, including a total of 14 cases. The authors found a male predominance (58%), with a median age of 50.4 years. 83 One thirds of all cases were younger than 40 years, and a majority of patients did not have comorbidities (50%), but among those that did have pre-existing conditions, hypertension was the most prevalent (33%). 83 Among the 14 patients, dyspnea/shortness of breath were the most common presenting features (75%), in addition to fever (75%). 83 On noting the hemodynamic status, 64% patients were in shock, of which 71% of the patients had cardiogenic shock, whereas 29% had a mixed septic and cardiogenic shock. 83 Around 42% of the patients had acute respiratory distress syndrome or developed it during the in-hospital period. 83 ECG findings were variable with ST-segment depression, ST-segment elevation, and T wave inversion occurring at 25% each. 83 Troponin was elevated in 91% of the cases, whereas pro-BNP and CK-MB were less frequently checked. 83 Among the 14 patients, echocardiography was performed in 83% of the case and 60% had a reduced ejection fraction. 83 Cardiac tamponade was reported in 20% of all echocardiograms, where diffuse hypokinesis was prevalent among 30% patients. 83 None of the patients had obstructive coronary disease. Around 50% patients required vasopressor support, with 25% of them warranting inotropic support. 83 Mechanical ventilation was utilized for 17% of the patients, of which ECMO was the most commonly used modality. 83 Many treatment modalities were used to manage myocarditis of which glucocorticoids (58%) were mostly used, followed by immunoglobulin therapy (25%) and colchicine (17%). Therapies to mitigate cytokine storm were interferon and tocilizumab (17% each). 83 Sawalha et al 83 found that 81% survived to discharge whereas 19% did not survive; the patients who did not survive were noted to have both myocarditis and ARDS.
Castiello et al 84 identified 38 case reports of COVID-19 patients with myocarditis based on the WHO/IFSC or ESC criteria. Around 45% of the cases had fever or a mild temperature increase; 21.1% had gastrointestinal symptoms, and 10.5% had a presenting or previous syncope. 84 Troponin levels varied substantially whereas BNP was raised in 57.9% patients. ECG findings were normal in 10.5% patients with variations among the rest. 84 Of 34 patients, only 18.4% patients had no functional or structural abnormality. 84 On noting CMR findings, myocardial inflammation and diffuse edema were captured in 50% patients. 84 EMB was performed only in 21.2% patients, where only 1 case reported the presence of SARS-CoV-2 in the cardiomyocytes. 84 Histological data obtained from autopsies were available for 10.5% patients, of which inflammatory infiltrates, accumulated inflammatory cells in the endothelium and signs of ferroptosis were noted. 84 The medical treatment was variable ranging from hydroxychloroquine (26.3%), tocilizumab (10.5%), lopinavir/ritonavir (7.9%), antibiotics (36.8%), steroids (34.2%), heart failure medications (36.8%), and anticoagulants (21.1%). Of 33 cases with reported outcomes, 84.8% patients survived, whereas 15.2% did not survive. 84
Rathore et al 8 present recent data, until January 5, 2021, of 42 patients with myocarditis and COVID-19, with 71.4% being males, and with a median age of 43.4 years. Hypertension was the most common finding in these patients, where cardiac biomarkers BNP and troponin were raised in 87% and 90% of the patients respectively. 8 ECG findings were non-specific with T-wave and ST-segment changes noted. Echocardiogram commonly showed ventricular systolic dysfunction with cardiomegaly. 8 The commonest histopathological feature was diffuse lymphocytic inflammatory infiltrates. 8 Moreover, corticosteroids and antivirals were most frequently used. Around 40% of the patients required vasopressor support. 8 Of 41 patients, 67% survived, whereas 33% died. 8 Due to the sudden risk of worsening patient conditions and associations with myocarditis, knowledge of this cardiac complication due to COVID-19 is critical for healthcare workers across all settings. Kamarullah et al 85 also conducted a search until January 2021 where 18 patients were included. The findings were suggestive of the beneficial effects of corticosteroids in treating myocarditis associated with COVID-19; the most commonly applied steroids were hydrocortisone (5.5%), methylprednisolone (89%), and prednisolone (5.5%), with the intravenous route being the most common and duration of treatment ranging from 1 to 14 days.85,86
Strengths and Limitations
This systematic review synthesizes the most recent evidence of COVID-19 infection and myocarditis, until August 31, 2021. Published literature obtained during the systematic search presents data collected until January 2021, enabling our collated findings, obtained until August 2021, to be a critical piece of information for healthcare workers worldwide. We present key findings about demographics, COVID-19 and myocarditis symptomatology, essential diagnostic techniques of use to clinicians, and clinical outcomes of interest of COVID-19 infection and myocarditis. The findings further strengthen the benefits of evidence-based healthcare where we gather evidence from reliable published literature to inform healthcare decisions, and reduce variations in healthcare delivery during the COVID-19 pandemic.
This systematic review has certain limitations. First, COVID-19 and myocarditis symptomatology may be overlapping, suggesting difficult clinical demarcations. Second, COVID-19 infections compounded with myocarditis were expected to be underreported as patients who did not previously have comorbidities presented with newly diminished ejection fractions and elevated myocardial markers. Thirdly, our systematic review presents that a low proportion of patents had confirmed myocarditis via MRI/endomyocardial biopsy. A plausible reason was the fear of contracting COVID-19 infection on undergoing MRI/endomyocardial biopsy. Fourthly, ECG and echocardiography were considered to be reliable screening tests, but not diagnostic tests, except for pericardial effusion. Lastly, while biomarkers such as troponin, BNP, and CK-MB were useful in diagnosing myocarditis, they are non-specific because the levels may also rise in other conditions such as demand ischemia and acute heart failure.
Conclusion
This systematic review presents findings about demographics, symptomatology, diagnostic techniques, and clinical outcomes of adult COVID-19 patients with myocarditis. A total of 229 patients were included in this analysis, who were diagnosed with myocarditis. The patients commonly presented with fever, cough, and shortness of breath making the clinical presentations difficult to differentiate. Elevated inflammatory and cardiac marker in addition to ECG and echocardiographic findings were useful indicators of myocardial disease. Gold standard testing such as MRI and endomyocardial biopsy were under-utilized suggesting that a definitive diagnostic approach may be required for those patients who fall under a high risk of suspicion for COVID-19 induced myocarditis. Due to the peaked risk of death among patients contracting both ARDS and myocardial inflammation, it is essential that healthcare workers are aware that myocarditis may be associated with COVID-19 infections. While the treatment approaches were variable across the cohort of patients included in this systematic review, further large-scale randomized controlled trials may help in establishing the best care of treatment for those with a definitive diagnosis of myocarditis with COVID-19.
Acknowledgments
We would like to thank Janelle Tayo, Yoandra Diaz, Furqan Ahmad Jarullah for their early contributions to the manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Vikash Jaiswal  https://orcid.org/0000-0002-2021-1660
https://orcid.org/0000-0002-2021-1660
Zouina Sarfraz  https://orcid.org/0000-0002-5132-7455
https://orcid.org/0000-0002-5132-7455
Azza Sarfraz  https://orcid.org/0000-0001-8206-5745
https://orcid.org/0000-0001-8206-5745
Dattatreya Mukherjee  https://orcid.org/0000-0001-7566-3843
https://orcid.org/0000-0001-7566-3843
Gazala Hitawala  https://orcid.org/0000-0002-5819-4157
https://orcid.org/0000-0002-5819-4157
Ruchika  https://orcid.org/0000-0003-0027-3175
https://orcid.org/0000-0003-0027-3175
Shehar Bano  https://orcid.org/0000-0002-6429-797X
https://orcid.org/0000-0002-6429-797X
Sidra Naz  https://orcid.org/0000-0001-6390-9658
https://orcid.org/0000-0001-6390-9658
References
- 1. Mantica G, Riccardi N, Terrone C, Gratarola A. Re: letter to the editor of Public Health in response to ‘Non-COVID-19 visits to emergency departments during the pandemic: the impact of fear’. Public Health. 2020;186:17. doi: 10.1016/j.puhe.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jaiswal V, Alquraish D, Sarfraz Z, et al. The influence of Coronavirus disease-2019 (COVID-19) On Parkinson’s disease: an updated systematic review. J Prim Care Community Health. 2021;12:21501327211039709. doi: 10.1177/21501327211039709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sarfraz Z, Sarfraz A, Barrios A, et al. Cardio-Pulmonary sequelae in recovered COVID-19 patients: considerations for primary care. J Prim Care Community Health. 2021;12:21501327211023726. doi: 10.1177/21501327211023726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guo J, Huang Z, Lin L, Lv J. Coronavirus disease 2019 (COVID-19) and Cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome Coronavirus 2 infection. J Am Heart Assoc. 2020;9(7):e016219. doi: 10.1161/JAHA.120.016219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45(8):100618. doi: 10.1016/j.cpcardiol.2020.100618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khan IH, Zahra SA, Zaim S, Harky A. At the heart of COVID-19. J Card Surg. 2020;35(6):1287-1294. doi: 10.1111/jocs.14596 [DOI] [PubMed] [Google Scholar]
- 7. Myocarditis and pericarditis following mRNA COVID-19 vaccination | CDC. Accessed September 2, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html [DOI] [PMC free article] [PubMed]
- 8. Rathore SS, Rojas GA, Sondhi M, et al. Myocarditis associated with Covid-19 disease: a systematic review of published case reports and case series. Int J Clin Pract. 2021;75(11):e14470. doi: 10.1111/ijcp.14470 [DOI] [PubMed] [Google Scholar]
- 9. Siripanthong B, Nazarian S, Muser D, et al. Recognizing COVID-19–related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463-1471. doi: 10.1016/j.hrthm.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tissières P, Teboul J-L. SARS-CoV-2 post-infective myocarditis: the tip of COVID-19 immune complications? Ann Intensive Care. 2020;10(1):98-104. doi: 10.1186/s13613-020-00717-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verma AK, Lavine KJ, Lin C-Y. Myocarditis after covid-19 mRNA vaccination. New Engl J Med. 2021;385:1332-1334. doi: 10.1056/NEJMC2109975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data - United States, march 2020-january 2021. MMWR Morb Mortal Wkly Rep. 2021;70(35):1228-1232. doi: 10.15585/mmwr.mm7035e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cizgici AY, Zencirkiran Agus H, Yildiz M. COVID-19 myopericarditis: it should be kept in mind in today’s conditions. Am J Emerg Med. 2020;38(7):1547.e5-1547.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yokoo P, Fonseca EKUN, Neto RS, et al. COVID-19 myocarditis: a case report. Einstein (São Paulo). 2020;18:eRC5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pietsch H, Escher F, Aleshcheva G, et al. Proof of SARS-CoV-2 genomes in endomyocardial biopsy with latency after acute infection. Internet J Infect Dis. 2021;102:70-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pavon AG, Meier D, Samim D, et al. First documentation of persistent SARS-Cov-2 infection presenting with late acute severe myocarditis. Can J Cardiol. 2020;36(8):1326.e5-1326.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khatri A, Wallach F. Coronavirus disease 2019 (Covid-19) presenting as purulent fulminant myopericarditis and cardiac tamponade: a case report and literature review. Heart Lung. 2020;49(6):858-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hussain H, Fadel A, Alwaeli H, Guardiola V. Coronavirus (COVID-19) fulminant myopericarditis and acute respiratory distress syndrome (ARDS) in a middle-aged male patient. Cureus. 2020;12(6):e8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dalen H, Holte E, Guldal AU, et al. Acute perimyocarditis with cardiac tamponade in COVID-19 infection without respiratory disease. BMJ Case Rep. 2020;13(8):e236218. doi: 10.1136/bcr-2020-236218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zeng J-H, Liu Y-X, Yuan J, et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;48(5):773-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Doyen D, Moceri P, Ducreux D, Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;395(10235):1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Faircloth E, Conner C, Dougherty K, Arora S, Thorevska N. Viral heartbreak: a case of covid-19 myocarditis vs stress-induced cardiomyopathy. Chest. 2020;158(4):A173. [Google Scholar]
- 23. Coyle J, Igbinomwanhia E, Sanchez-Nadales A, Danciu S, Chu C, Shah N. A recovered case of COVID-19 myocarditis and ARDS treated with corticosteroids, tocilizumab, and experimental AT-001. Case Rep. 2020;2(9):1331-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luetkens JA, Isaak A, Zimmer S, et al. Diffuse myocardial inflammation in COVID-19 associated myocarditis detected by multiparametric cardiac magnetic resonance imaging. Circ Cardiovasc Imaging. 2020;13(5):e010897. [DOI] [PubMed] [Google Scholar]
- 25. Jain A, Deval N, Paul L. A recovered case of covid-19 myocarditis treated with IV immunoglobulin. Chest. 2020;158(4):A281. [Google Scholar]
- 26. Mustafa S, Zafar M, Agrawal N, Shahbaz A, Al-khafaji N. COVID-19-associated myocarditis mimicking ST elevation myocardial infarction. Chest. 2020;158(4):A572. [Google Scholar]
- 27. Mansoor A, Chang D, Mitra R. Rhythm, conduction, and ST elevation with COVID-19: myocarditis or myocardial infarction? HeartRhythm Case Rep. 2020;6(10):671-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Al-Assaf O, Mirza M, Musa A. Atypical presentation of COVID-19 as subclinical myocarditis with persistent high degree atrio-ventricular block treated with pacemaker implant. HeartRhythm Case Rep. 2020;6(11):884-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khalid Y, Dasu N, Dasu K. A case of novel coronavirus (COVID-19)-induced viral myocarditis mimicking a Takotsubo cardiomyopathy. HeartRhythm Case Rep. 2020;6(8):473-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fried JA, Ramasubbu K, Bhatt R, et al. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141(23):1930-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wehit JM, Sosa FA, Merlo P, Roberti J, Osatnik J. Identification of COVID-19-associated myocarditis by speckle-tracking transesophageal echocardiography in critical care. Acta Colomb Cuidado Intensivo. Published online November 24, 2020. doi: 10.1016/j.acci.2020.11.008 [DOI] [Google Scholar]
- 33. Butler K, Clancy MJ, Adler J, Tevald MA. Acute rehabilitation of a patient with COVID-19 myocarditis: a case report. Phys Ther. 2021;101(1):aa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laganà N, Cei M, Evangelista I, et al. Suspected myocarditis in patients with COVID-19: a multicenter case series. Medicine. 2021;100(8):e24552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kallel O, Bourouis I, Bougrine R, Housni B, El Ouafi N, Ismaili N. Acute myocarditis related to covid-19 infection: 2 cases report. Ann Med Surg. 2021;66:102431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ghugre NR, Orbach A, Biswas L, et al. Suspected subclinical myocarditis detected by cardiac magnetic resonance imaging late post COVID-19 recovery. J Cardiol Cases. Published online May 14, 2021. doi: 10.1016/j.jccase.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fath AR, Aglan A, Varkoly KS, et al. Distinct coagulopathy with myocardial injury and pulmonary embolism in COVID-19. J Investig Med High Impact Case Rep. 2021;9:23247096211019560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dabbagh MF, Aurora L, D’Souza P, Weinmann AJ, Bhargava P, Basir MB. Cardiac tamponade secondary to COVID-19. Case Rep. 2020;2(9):1326-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Irabien-Ortiz Carreras-Mora J, Sionis A, Pàmies J, Montiel J, Tauron M. Fulminant myocarditis due to COVID-19. Rev Esp Cardiol. 2020;73(6):503-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Albert CL, Carmona-Rubio AE, Weiss AJ, Procop GG, Starling RC, Rodriguez ER. The enemy within: sudden-onset reversible cardiogenic shock with biopsy-proven cardiac myocyte infection by severe acute respiratory syndrome coronavirus 2. Circulation. 2020;142(19):1865-1870. [DOI] [PubMed] [Google Scholar]
- 41. Escher F, Pietsch H, Aleshcheva G, et al. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020;7(5):2440-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ford JS, Holmes JF, Jones RF. Cardioembolic stroke in a patient with coronavirus disease of 2019 (COVID-19) myocarditis: a case report. Clin Pract Cases Emerg Med. 2020;4(3):332-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gauchotte G, Venard V, Segondy M, et al. SARS-Cov-2 fulminant myocarditis: an autopsy and histopathological case study. Int J Legal Med. 2021;135(2):577-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hua A, O’Gallagher K, Sado D, Byrne J. Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur Heart J. 2020;41:2130-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jacobs W, Lammens M, Kerckhofs A, et al. Fatal lymphocytic cardiac damage in coronavirus disease 2019 (COVID-19): autopsy reveals a ferroptosis signature. ESC Heart Fail. 2020;7(6):3772-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Labani A, Germain P, Douchet M-P, et al. Acute myopericarditis in a patient with mild SARS-CoV-2 respiratory infection. CJC open. 2020;2(5):435-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Spano G, Fischer K, Maillat C, Vicario G, Huber AT, Gräni C. Delayed isolated peri-myocarditis in a covid-19 patient with respiratory symptoms but without lung involvement. Int J Cardiovasc Imaging. 2020;36(11):2279-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tavazzi G, Pellegrini C, Maurelli M, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911-915. doi: 10.1002/ejhf.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Trogen B, Gonzalez FJ, Shust GF. COVID-19-associated myocarditis in an adolescent. Pediatr Infect Dis J. 2020;39(8):e204-e205. [DOI] [PubMed] [Google Scholar]
- 50. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-1418. doi: 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Warchoł I, Dębska-Kozłowska A, Karcz-Socha I, Książczyk M, Szymańska K, Lubiński A. Terra incognita: clinically suspected myocarditis in a SARS-CoV-2 positive patient. Pol Arch Intern Med. 2020;130(5):446-448. [DOI] [PubMed] [Google Scholar]
- 52. Sardari A, Tabarsi P, Borhany H, Mohiaddin R, Houshmand G. Myocarditis detected after COVID-19 recovery. Eur Heart J - Cardiovasc Imaging. 2021;22(1):131-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dahl EH, Mosevoll KA, Cramariuc D, Vedeler CA, Blomberg B. COVID-19 myocarditis and postinfection bell’s palsy. BMJ Case Rep. 2021;14(1):e240095. doi: 10.1136/BCR-2020-240095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur Heart J. 2021;42(2):206. doi: 10.1093/eurheartj/ehaa190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Volis I, Livneh I, Hussein K, Raz-Pasteur A. COVID-19-Associated suspected myocarditis as the etiology for recurrent and protracted fever in an otherwise healthy adult. Am J Med Sci. 2021;361(4):522-525. doi: 10.1016/j.amjms.2020.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Beşler MS, Arslan H. Acute myocarditis associated with COVID-19 infection. Am J Emerg Med. 2020;38(11):2489.e1-2489.e2. doi: 10.1016/j.ajem.2020.05.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gaine S, Devitt P, Coughlan JJ, Pearson I. COVID-19-associated myocarditis presenting as new-onset heart failure and atrial fibrillation. BMJ Case Rep. 2021;14(7):e244027. doi: 10.1136/BCR-2021-244027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sheikh AB, Javed N, Sheikh AAE, Upadhyay S, Shekhar R. Diabetes insipidus and concomitant myocarditis: a late sequelae of COVID-19 infection. J Investig Med High Impact Case Rep. 2021;9:2324709621999954. doi: 10.1177/2324709621999954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Salamanca J, Díez-Villanueva P, Martínez P, et al. COVID-19 “Fulminant Myocarditis” successfully treated with temporary mechanical circulatory support. JACC Cardiovasc Imaging. 2020;13(11):2457-2459. doi: 10.1016/j.jcmg.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Naneishvili T, Khalil A, O’Leary R, Prasad N. Fulminant myocarditis as an early presentation of SARS-CoV-2. BMJ Case Rep. 2020;13(9):e237553. doi: 10.1136/BCR-2020-237553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim I-C, Kim JY, Kim HA, Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J. 2020;41(19):1859-1859. doi: 10.1093/EURHEARTJ/EHAA288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nikoo MH, Mozaffari R, Hatamnejad MR, Bazrafshan M, Kasaei M, Bazrafshan H. Systolic dysfunction and complete heart block as complications of fulminant myocarditis in a recovered COVID-19 patient. J Cardiol Cases. 2021;24:177-181. doi: 10.1016/J.JCCASE.2021.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sala S, Peretto G, Gramegna M, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41(19):1861-1862. doi: 10.1093/eurheartj/ehaa286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yuan WF, Tang X, Zhao XX. An ‘asymptomatic’ driver with COVID-19: atypical suspected myocarditis by SARS-CoV-2. Cardiovasc Diagn Ther. 2020;10(2):242-243. doi: 10.21037/cdt.2020.03.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Asif T, Ali Z. Transient ST segment elevation in two patients with COVID-19 and a normal Transthoracic echocardiogram. Eur J Case Rep Intern Med. 2020;7(5):001672. doi: 10.12890/2020_001672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Khalid N, Chen Y, Case BC, et al. COVID-19 (SARS-CoV-2) and the heart - an ominous association. Cardiovasc Revasc Med. 2020;21(8):946-949. doi: 10.1016/j.carrev.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ng MY, Ferreira VM, Leung ST, et al. Patients recovered from COVID-19 show ongoing subclinical myocarditis as revealed by Cardiac magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13(11):2476-2478. doi: 10.1016/j.jcmg.2020.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jirak P, Larbig R, Shomanova Z, et al. Myocardial injury in severe COVID-19 is similar to pneumonias of other origin: results from a multicentre study. ESC Heart Fail. 2021;8(1):37-46. doi: 10.1002/EHF2.13136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yan X, Wang S, Ma P, et al. Cardiac injury is associated with inflammation in geriatric COVID-19 patients. J Clin Lab Anal. 2021;35(1):e23654. doi: 10.1002/jcla.23654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kunal S, Sharma SM, Sharma SK, et al. Cardiovascular complications and its impact on outcomes in COVID-19. Indian Heart J. 2020;72(6):593-598. doi: 10.1016/j.ihj.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mayo Clinic. C-reactive protein test - Mayo Clinic. Accessed September 13, 2021. https://www.mayoclinic.org/tests-procedures/c-reactive-protein-test/about/pac-20385228
- 72. Mayo Clinical Laboratories. DIMER. Clinical: D-dimer, plasma. Accessed September 13, 2021. https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/602174
- 73. Alecu M, Geleriu L, Gălăţescu L. The interleukin-1, interleukin-2, interleukin-6 and tumour necrosis factor alpha serological levels in localised and systemic sclerosis. Rom J Intern Med. 1998;36(4):251-259. [PubMed] [Google Scholar]
- 74. University of Rochester Medical Center. Troponin - Health Encyclopedia - University of Rochester Medical Center. Accessed September 13, 2021. https://www.urmc.rochester.edu/encyclopedia/content.aspx?contenttypeid=167&contentid=troponin
- 75. Fu L, Wang B, Yuan T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020;80(6):656-665. doi: 10.1016/j.jinf.2020.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. World Health Organization. Advice for the public. Published 2021. Accessed September 12, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public
- 77. Cooper LT. Myocarditis. New Engl J Med. 2009;360(15):1526-1538. doi: 10.1056/NEJMra0800028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Eichhorn C, Bière L, Schnell F, et al. Myocarditis in athletes Is a challenge: diagnosis, risk stratification, and uncertainties. JACC Cardiovasc Imaging. 2020;13(2 Pt 1):494-507. doi: 10.1016/j.jcmg.2019.01.039 [DOI] [PubMed] [Google Scholar]
- 79. Pirzada A, Mokhtar AT, Moeller AD. COVID-19 and myocarditis: what do we know so far? CJC Open. 2020;2(4):278-285. doi: 10.1016/j.cjco.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Investig. 2020;130(5):2620-2629. doi: 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Reddy J, Massilamany C, Buskiewicz I, Huber SA. Autoimmunity in viral myocarditis. Curr Opin Rheumatol. 2013;25(4):502-508. doi: 10.1097/BOR.0b013e3283620036 [DOI] [PubMed] [Google Scholar]
- 82. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802-810. doi: 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sawalha K, Abozenah M, Kadado AJ, et al. Systematic review of COVID-19 related myocarditis: insights on management and outcome. Cardiovasc Revasc Med. 2020;23:107-113. doi: 10.1016/J.CARREV.2020.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Castiello T, Georgiopoulos G, Finocchiaro G, et al. COVID-19 and myocarditis: a systematic review and overview of current challenges. Heart Fail Rev. 2021;1-11. doi: 10.1007/s10741-021-10087-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kamarullah W, Nurcahyani N, Mary Josephine C, Bill Multazam R, Ghaezany Nawing A, Dharma S. Corticosteroid therapy in management of myocarditis associated with COVID-19; a systematic review of current evidence. Arch Acad Emerg Med. 2021;9(1):e32. doi: 10.22037/aaem.v9i1.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sarfraz A, Sarfraz Z, Sarfraz M, Aftab H, Pervaiz Z. Tocilizumab and COVID-19: a meta-analysis of 2120 patients with severe disease and implications for clinical trial methodologies. Turk J Med Sci. 2021;51(3):890-897. doi: 10.3906/SAG-2010-131 [DOI] [PMC free article] [PubMed] [Google Scholar]