Abstract
Objective
This prospective study aimed to evaluate the safety of improved transurethral plasma kinetic enucleation of the prostate (iTUPKEP) in the perioperative period in high-risk patients with benign prostatic hyperplasia (BPH) and coronary artery disease.
Methods
Patients with BPH underwent surgical treatment with transurethral vapour resection of the prostate (TUVP) or iTUPKEP. Serum endothelin-1, cardiac troponin-I, and high-sensitivity C-reactive protein concentrations were evaluated in the short term after surgery. The postvoid residual urine volume, maximum urinary flow rate, international prostate symptom score, and quality of life indicators were evaluated in the long term after surgery.
Results
Endothelin-1 concentrations were lower in the iTUPKEP group than in the TUVP group at 1 and 2 days postoperatively. The iTUPKEP group had lower cardiac troponin-I and high-sensitivity C-reactive protein concentrations at all time points postoperatively. The postvoid residual urine volume, international prostate symptom score, and quality of life values were lower, but the maximum urinary flow rate was higher, in the iTUPKEP group than in the TUVP group.
Conclusions
The iTUPKEP procedure has a smaller effect on vascular endothelial function compared with TUVP. Therefore, iTUPKEP may reduce the incidence of postoperative cardiovascular adverse events in high-risk patients with BPH and coronary artery disease.
Keywords: Benign prostatic hyperplasia, coronary artery disease, transurethral vapour resection of the prostate, transurethral plasma kinetic enucleation of the prostate, endothelin-1, quality of life
Introduction
Benign prostatic hyperplasia (BPH) is a common benign tumour that is mainly found in elderly men. The prevalence of BPH in men older than 50 years is as high as 50%, and its incidence increases with age.1–3 Because of the anatomical position of BPH, it can compress the urethra, resulting in a series of lower urinary tract symptoms, including difficulty urinating and irritable urination. 4 In patients with moderate to severe lower urinary tract symptoms, surgical treatment can be considered to improve symptoms. The postvoid residual urine volume (PVR), maximum urinary flow rate (Qmax), international prostate symptom score (IPSS), quality of life (QOL) score, and other indicators should be measured and recorded before surgery to allow for further evaluation of the efficacy of surgery. Twenty years previously, transurethral resection of the prostate (TURP) was the gold standard for surgical treatment of BPH because it led to a significant improvement in symptoms.5,6 TURP also has a good curative effect in treating BPH in patients with renal transplantation.7,8 However, TURP causes some common adverse events after surgery, including postoperative urinary retention, bleeding, and urinary tract infection. 6
BPH and coronary artery disease (CAD) may be inextricably linked. As early as the 1950s, Reis et al reported that there was a correlation between BPH and CAD. 9 Some studies have shown that BPH and CAD are affected by hormone concentrations in the body.10,11 Furthermore, Weisman et al showed that smooth muscle proliferation affected by hormones may play an important role in the occurrence and development of CAD and BPH in elderly men. 12 Endothelin-1 (ET-1) is a peptide composed of 21 amino acids and is mainly produced by vascular endothelial cells, but also by vascular smooth muscle cells and epithelial cells.13,14 Studies have shown that ET-1 causes endothelial dysfunction, and inflammation and may be involved in the formation of atherosclerotic plaques, thereby accelerating the progression of CAD. 15 Elevated cardiac troponin-I (cTn-I) concentrations are associated with an increased risk of adverse cardiovascular events. 16 Many factors can cause fluctuations in serum cTn-I concentrations, but their peak is different from the increase in cTn-I concentrations after myocardial infarction.17–19 C-reactive protein (CRP) concentrations rapidly increase in the blood when the body is infected or injured, which may play a role in the process of atherosclerosis. 20 With the advancement of new high-sensitivity CRP (hs-CRP) detection technology, some studies have shown that hs-CRP is significantly related to the presence of atherosclerosis.21,22
In recent years, adverse cardiovascular events after prostate surgery have received increasing attention. 23 We speculate that the trauma caused by surgery may cause the concentrations of ET-1, cTn-I, and hs-CRP in the body to greatly fluctuate. This in turn leads to the occurrence and development of cardiovascular adverse events. Transurethral vapour resection of the prostate (TUVP) is a minimally invasive method for the treatment of BPH that was developed on the basis of TURP, but with less intraoperative bleeding. 24 Transurethral plasma kinetic enucleation of the prostate (TUPKEP) is the latest generation of minimally invasive surgery for treating BPH. The vascular supply of the prostate is blocked early in this procedure, and therefore, it is effective in reducing bleeding and safety.25,26 After long-term practice and exploration, we improved the classic TUPKEP surgical method (iTUPKEP) to further shorten the operation time, reduce bleeding, and reduce the probability of urethral sphincter injury. In this study, we aimed to determine the dynamic changes in serum ET-1, cTn-I, and hs-CRP concentrations before and after surgery, and discuss the safety of iTUPKEP in the perioperative period of high-risk patients with BPH and CAD.
Methods
Patients
A total of 146 patients with BPH who underwent surgical treatment in the No. 907 Hospital of PLA Joint Logistics Support Force from November 2017 to June 2020 were randomly selected as the study population. This prospective study was approved by the Ethics Committee of the No. 907 Hospital of PLA Joint Logistics Support Force (approval number: 2017-H-14MS076-01, approval date: September 2017). Written informed consent was obtained from the patients or their families before the study. The inclusion criteria were patients diagnosed with BPH by Doppler colour ultrasound, prostate-specific antigen, and other auxiliary examinations before surgery, and no use of cardiovascular disease treatment drugs within 24 hours before enrolment. The exclusion criteria were patients with certain lifestyle habits, such as smoking and drinking, and chronic diseases, such as infections, tumours, and diabetes. Additionally, patients with chronic and underlying diseases that may respond to fluctuations in inflammatory indicators were excluded from this study. According to the medical history, an electrocardiogram (ECG), 24-hour dynamic ECG, cardiac colour Doppler, blood biochemical indicators, and the diagnostic criteria of the International Heart Association and the World Health Organization, the patients were divided into the CAD group (patients with coronary artery disease, 33 cases) and the NCAD group (patients with no coronary artery disease, 113 cases). For patients with BPH in our hospital with surgical indications, we assessed the patient’s condition in detail (the severity of BPH and whether it was associated with cardiovascular disease), the doctor’s recommended procedure, and the advantages and disadvantages of the two procedures (e.g., safety, effectiveness, and cost). We then chose the operation method according to the patient’s wishes, and the patient signed an informed consent form for the operation. According to the patient’s condition and wishes, we kept the personal information of all patients confidential, and we have de-identified all patients’ details. The reporting of this study conforms to the STROBE guidelines. 27
Surgical procedures
To maintain the rigour of the study, all patients’ surgical operations were performed by the same surgeon. The patients completed all routine examinations before surgery. During the operation, combined spinal–epidural anaesthesia was used, and ECG monitoring and blood oxygen saturation monitoring were performed at the same time.
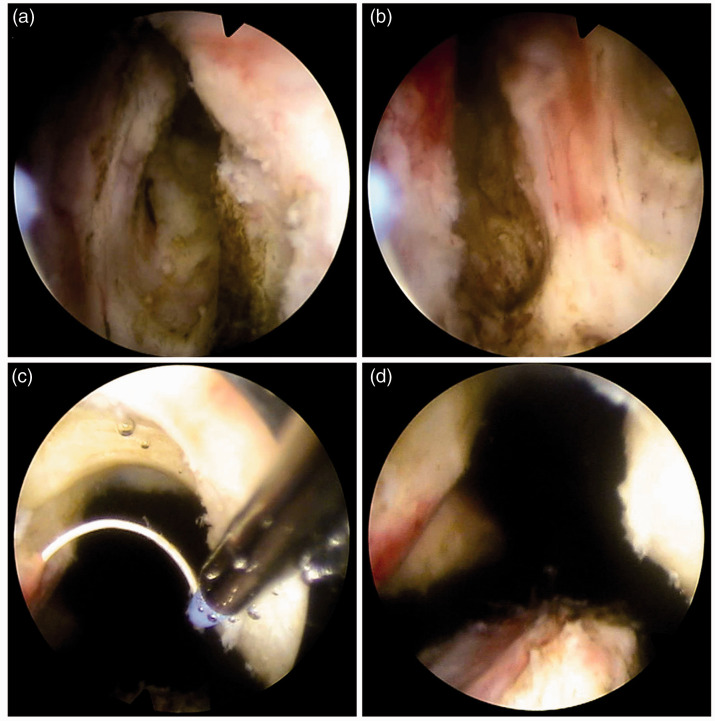
The iTUPKEP procedure uses the British Gyrus plasma bipolar electrocuting system (744000; Gyrus Acmi, Cardiff, UK), with an electrocoagulation power of 60 to 80 W, a cutting power of 80 to 160 W, and continuous washing with 0.9% sodium chloride as the rinsing solution. The relative positional relationship between the bladder triangle and prostate, the position of the bladder neck, posterior urethra, and seminal caruncle, and the morphology and degree of prostatic hyperplasia were observed by transurethral resectoscopy. Based on the classic TUPKEP, the iTUPKEP procedure was as follows. First, the prostatic mucosa was incised at the tip of the prostate near the seminal caruncle, and then a wedge-shaped groove was cut between the proliferative gland and the surgical capsule by push cutting. Subsequently, marker grooves at the 5, 7, and 12 o’clock positions were cut at the lithotomy position to the prostate surgical capsule and communicated with the sharp wedge-shaped groove (see Figure 1). The gland tissue of each lobe was then peeled from the surgical capsule layer upward with the ceramic head of the inner sheath of the resectoscope. The supply blood vessels and fibre belts of the glands were cut off, and haemostasis was performed by electrocoagulation so that the prostate lobes were stripped from the surgical capsule layer to the bladder neck. Finally, the gland tissue was harvested and rapidly cut by an electric cutting loop. After the operation, a 22-F three-chamber balloon catheter was inserted, 0.9% sodium chloride was used for continuous washing for 1 to 2 days, and the catheter was retained for 3 to 6 days. The catheter was removed without obvious haematuria.
Figure 1.
Marker grooves during the surgical operation of improved transurethral plasma kinetic enucleation of the prostate. (a) A marker groove at a 5 o’clock cut at the lithotomy position. (b) A marker groove at a 7 o’clock cut at the lithotomy position. (c) A marker groove at a 12 o’clock cut at the lithotomy position. (d) The three completed marking grooves in the same surgical field of view.
TUVP was performed with a 24-F electric cutting mirror and vapourisation electrode system produced by Shenda® (Shenyang, China). The electric coagulation power was 70 W, the cutting power was 220 W, and 5% mannitol solution was used as the flushing fluid for continuous flushing. The relative positional relationship between the bladder triangle and prostate, the position of the bladder neck, posterior urethra, and seminal caruncle, and the morphology and degree of prostatic hyperplasia were observed by transurethral resectoscopy. Different cutting methods were chosen depending on the gland size. If the patient mainly had hyperplasia of the middle lobe of the prostate, a wedge-shaped marking groove was cut at the tip of the prostate near the seminal caruncle to the prostate surgical capsule. Longitudinal marking grooves were then cut at the 5 and 7 o’clock positions to reach the surgical capsule of the prostate and connect with the wedge-shaped marking groove at the proximal end of the seminal caruncle. The middle lobe and the two lateral lobes were then cut in turn. If BPH mainly occurred on both sides of the prostate, a wedge-shaped marking groove was cut at the proximal end of the seminal caruncle first, and then a longitudinal mark groove was cut at 12 o’clock to reach the surgical capsule. The starting point, ending point, and depth of cutting were determined. The bilateral lobes, middle leaves, and other parts were then removed in turn, and finally, the prostate apex was carefully removed. At the end of the operation, a 22-F three-lumen balloon catheter was inserted, and 0.9% sodium chloride was used for continuous washing for 1 to 3 days. A urinary catheter was inserted for 3 to 6 days, and the urinary catheter was then removed without obvious haematuria.
Outcome measures
To measure ET-1 concentrations, venous blood was collected from the elbow forearm of the patients in a fasting state 2 hours before surgery, and 1, 2, and 7 days after surgery. ET-1 concentrations were measured by a specific radioimmunoassay (Shanghai Bogu Biotechnology Co., Ltd., Shanghai, China). The entire procedure was completed in strict accordance with the reagent instructions.
To detect cTn-I and hs-CRP, venous blood was collected from the elbow forearm of the patients in a fasting state 2 hours before surgery, 12 hours after surgery, and 1, 2, and 7 days after surgery. Concentrations of cTn-I and hs-CRP were measured by transmission immunoturbidimetry using a kit (HUMAN Company, Wiesbaden, Germany). The entire procedure was completed in strict accordance with the manufacturer’s instructions.
The patients’ PVR, Qmax, IPSS, and QOL score were measured and recorded before and 1 month after surgery. Additionally, the patients’ operation time, intraoperative blood loss during the operation, and postoperative catheter retention time were recorded.
Statistical analysis
Data are expressed as the mean ± standard deviation (SD). For measurement data with equal variance, the independent samples t-test and one-way analysis of variance were used to analyse the differences between groups. For data with unequal variance, the Wilcoxon rank-sum test was used. All statistical analyses were performed with IBM SPSS 22.0 software (IBM Corp., Armonk, NY, USA). P values <0.05 were considered significant.
Results
Patients and baseline parameters
Fourteen patients were treated with TUVP and 19 patients were treated with iTUPKEP in the CAD group. In the NCAD group, 26 patients were treated with TUVP and 87 patients were treated with iTUPKEP. Baseline data, such as age, PVR, Qmax, IPSS, and QOL, were not significantly different between the groups (Table 1).
Table 1.
Baseline data of 146 patients with benign prostatic hyperplasia.
| Group | Case | Age (years) | PVR (mL) | Qmax (mL/s) | IPSS (score) | QOL (score) |
|---|---|---|---|---|---|---|
| CAD | 33 | |||||
| TUVP | 14 | 68.13 ± 5.22 | 168.38 ± 20.47 | 7.74 ± 2.28 | 25.64 ± 2.45 | 4.37 ± 1.13 |
| iTUPKEP | 19 | 68.92 ± 7.75 | 159.75 ± 18.24 | 7.52 ± 2.87 | 24.31 ± 2.27 | 4.56 ± 0.92 |
| NCAD | 113 | |||||
| TUVP | 26 | 67.94 ± 5.83 | 156.62 ± 16.33 | 8.71 ± 1.35 | 23.82 ± 3.48 | 4.34 ± 1.27 |
| iTUPKEP | 87 | 68.16 ± 6.54 | 162.18 ± 19.62 | 8.68 ± 2.96 | 24.62 ± 2.55 | 4.61 ± 1.38 |
Data are mean ± standard deviation. There were no significant differences in baseline data between the groups.
PVR, postvoid residual urine volume; Qmax, maximum urinary flow rate; IPSS, international prostate symptom score; QOL, quality of life; CAD, coronary artery disease; TUVP, transurethral resection of the prostate; iTUPKEP, improved transurethral plasma kinetic enucleation of the prostate; NCAD, non-coronary artery disease.
Serum ET-1, cTn-I, and hs-CRP concentrations and intraoperative parameters
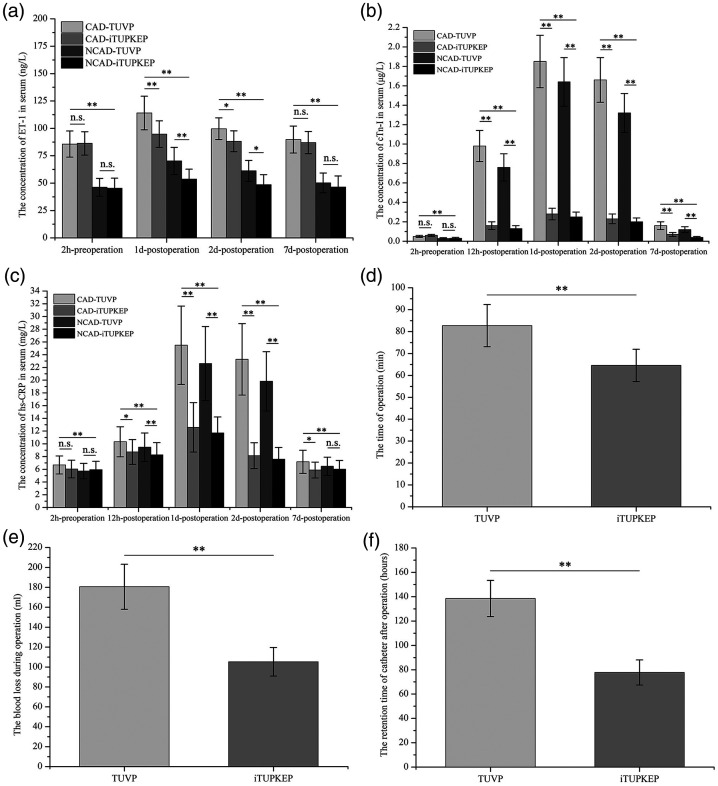
All patients were successfully discharged after surgery. Two hours before the operation and 7 days postoperatively, mean ET-1 concentrations were not significantly different between the CAD-TUVP and CAD-iTUPKEP groups or between the NCAD-TUVP and NCAD-iTUPKEP groups (Figure 2). However, mean ET-1 concentrations were significantly higher in the CAD-TUVP group than in the CAD-iTUPKEP group at 1 and 2 days postoperatively (all P < 0.05). Additionally, mean ET-1 concentrations were significantly higher in the NCAD-TUVP group than in the NCAD-iTUPKEP group at 1 and 2 days postoperatively (all P < 0.05). Mean serum ET-1 concentrations in the CAD groups were significantly higher than those in the NCAD groups at four time points (2 hours preoperatively, and 1, 2, and 7 days postoperatively) (all P < 0.01). However, serum cTn-I and hs-CRP concentrations, which are mainly affected by surgical methods, showed different trends compared with serum ET-1 concentrations. At the five time points (2 hours preoperatively, and 12 hours, 1, 2, and 7 days postoperatively) when cTn-I concentrations were measured, there were significant differences between the groups. At the four time points postoperatively, mean serum cTn-I concentrations in patients in the CAD-iTUPKEP and NCAD-iTUPKEP groups were significantly lower than those in the corresponding CAD-TUVP and NCAD-TUVP groups (all P < 0.01). There was no significant difference in cTn-I concentrations between patients with different surgical procedures within the CAD and NCAD groups 2 hours before surgery. Similarly, serum hs-CRP concentrations in patients in each group showed the same trends of change as those in serum cTn-I concentrations. Notably, there was no significant difference in serum hs-CRP concentrations between patients with different surgical procedures in the NCAD groups 7 days after surgery. Additionally, intraoperative blood loss and the postoperative catheter retention time were significantly lower in the iTUPKEP group than in the TUVP group (both P < 0.01), with no relationship with the presence of concomitant CAD (see Figure 2 and additional Tables 1, 2, and 3).
Figure 2.
Comparison of intraoperative parameters and serum ET-1, cTn-I, and hs-CRP concentrations between four groups. (a) Mean ET-1 concentrations at 2 hours preoperatively, and 1, 2, and 7 days postoperatively. (b) Mean cTn-I at 2 hours preoperatively, 12 hours postoperatively, and 1, 2, and 7 days postoperatively. (c) Mean hs-CRP concentrations at 2 hours preoperatively, 12 hours postoperatively, and 1, 2, and 7 days postoperatively. (d) Mean operation time in the TUVP and iTUPKEP groups. (e) Mean blood loss during the operation in the TUVP and iTUPKEP groups. (f) Mean retention time of the catheter after the operation in the TUVP and iTUPKEP groups.
*P < 0.05, **P < 0.01.
CAD, coronary artery disease; NCAD, non-coronary artery disease; TUVP, transurethral resection of the prostate; iTUPKEP, improved transurethral plasma kinetic enucleation of the prostate; ET-1, endothelin-1; cTn-I, cardiac troponin-I; hs-CRP, high-sensitivity C-reactive protein; n.s., no significant difference.
Improvement of BPH symptoms 1 month after the operation
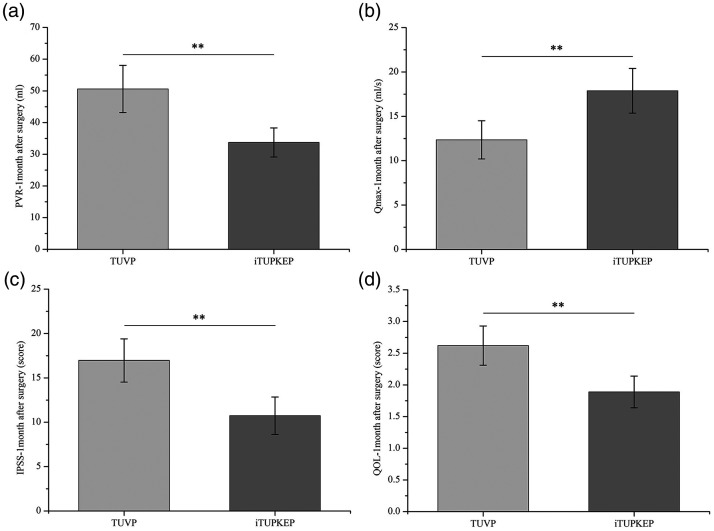
To further evaluate the effect of iTUPKEP on the improvement of patients’ BPH symptoms, we measured the PVR, Qmax, IPSS, and QOL score 1 month after surgery. The mean PVR, IPSS, and QOL score in the iTUPKEP group were significantly lower than those in the TUVP group (all P < 0.01). Additionally, the mean Qmax in the iTUPKEP group was significantly higher than that in the TUVP group (P < 0.01) (Figure 3).
Figure 3.
Comparison of improvement of symptoms of BPH between two groups at 1 month after the operation. (a) Mean PVR 1 month after the operation in the TUVP and iTUPKEP groups. (b) Mean Qmax 1 month after the operation in the TUVP and iTUPKEP groups. (c) Mean IPSS 1 month after the operation in the TUVP and iTUPKEP groups. (d) Mean QOL score 1 month after the operation in the TUVP and iTUPKEP groups.
**P < 0.01.
PVR, postvoid residual urine volume; TUVP, transurethral resection of the prostate; iTUPKEP, improved transurethral plasma kinetic enucleation of the prostate; Qmax, maximum urinary flow rate; IPSS, international prostate symptom score; QOL, quality of life.
Postoperative adverse events
Five patients with CAD who underwent TUVP surgery had ischaemic ST-T changes early in the postoperative period (within 2 days). Additionally, three patients developed angina pectoris, all of whom were controlled by timely treatment. No serious complications, such as acute myocardial infarction (AMI), acute heart failure, or sudden cardiac death, occurred. After our team performed strict postoperative care and close dynamic evaluation of postoperative inflammation indicators for patients, no other complications were reported in each group.
Discussion
With the ageing of the population, the incidence of BPH is increasing annually. 28 Therefore, the medical cost associated with BPH treatment is increasing, which has resulted in a serious economic burden on patients and society. Moderate and severe BPH has a serious effect on the QOL of patients and often requires surgical treatment. 29 Fortunately, minimally invasive transurethral surgery plays an important role in the treatment of BPH. 23 Compared with other substantial organs in the body, the urinary system has a natural lumen structure. Therefore, minimally invasive transurethral surgery can avoid the severe trauma associated with open surgery. However, although minimally invasive surgery causes relatively minor postoperative urinary retention and bleeding, the perioperative safety of high-risk patients with BPH and CAD is an important factor that requires more attention. BPH and CAD are two common diseases in elderly men that are related and influenced by each other. A study by Bruno et al showed that the incidence of CAD was higher in countries with a higher incidence of BPH and vice versa, and the incidence of AMI was significantly increased after TURP and open prostatectomy. 30 Hahn et al conducted a series of studies on the relationship between BPH surgery and the occurrence of postoperative AMI, and found that the incidence of AMI significantly increased after TURP. 31 Weisman et al found that the incidence of AMI and cardiovascular death in patients with BPH after surgery was significantly higher than that in non-surgical patients.12,32 In this study, we investigated 146 patients with BPH, including 33 patients with CAD (incidence rate: 22.6%).
Although the development of modern medical technology has made great progress, CAD still has high morbidity and mortality rates worldwide. 33 In the occurrence and development of CAD, the endothelin system is associated with unavoidable problems. ET-1 is the main subtype in the endothelin system, and its genes are located on chromosome 6. 14 ET-1 is a vasoconstrictor with the strongest and longest active time among the vasoactive substances, and its effect is 10 times higher than that of angiotensin. 15 Moreover, the vasoconstriction effect of ET-1 is more intense on coronary arteries compared with that on peripheral arteries. 34 ET-1 can also induce endothelial dysfunction in coronary arteries through a variety of mechanisms, such as oxidative stress, inflammation, and decreased activity of the nitric oxide pathway. 35 Studies have shown that ET-1 accelerates the progression of atherosclerosis by upregulating lipid metabolism genes and the mitogen-activated protein kinase pathway.36,37 Researchers have found that plasma ET-1 concentrations began to rise within 10 minutes of the onset of ischaemia in animal models. 38 Similarly, clinical studies have shown that plasma ET-1 concentrations increase significantly shortly after AMI and are related to the infarct size. 39 In general, ET-1 plays an important role in different stages of CAD. Therefore, large fluctuations in ET-1 concentrations in the body will inevitably have adverse effects on patients with CAD and may even lead to sudden death. Additionally, the increased expression of endothelin receptors in hyperplastic prostate tissues indicates that ET-1 promotes the growth of prostate cells and may play an important role in prostate diseases.40,41
Inflammation plays a major role in all stages of atherosclerosis, such as plaque formation, recruitment, and chemotaxis of inflammatory cells and plaque stability.42,43 CRP is a non-specific inflammation marker, which suggests systemic inflammation and tissue damage. CRP synthesised in the liver can play a role in atherosclerosis by affecting the synthesis of key molecules. Several recent studies have shown that CRP downregulates endothelial nitric oxide synthase or upregulates nuclear factor κB, which is a key nuclear transcription factor that can affect numerous pro-atherosclerotic genes.20,44,45 Additionally, cTn-I is a marker of myocardial injury and has important clinical significance for the diagnosis and risk stratification of AMI. 46
In this study, we compared the changes in plasma ET-1, cTn-I, and hs-CRP concentrations before and after TUVP or iTUPKEP in patients with BPH. Serum ET-1, cTn-I, and hs-CRP concentrations increased after the two operation methods, which indicated that all patients had vascular endothelial dysfunction and inflammatory reactions. However, mean serum ET-1, cTn-I, and hs-CRP concentrations in patients who underwent TUVP were significantly higher in the early postoperative period compared with those in patients who underwent iTUPKEP, especially in patients with CAD. Furthermore, serum cTn-I and hs-CRP concentrations postoperatively were significantly associated with the type of operation. These results suggested that surgery further aggravated endothelial dysfunction in high-risk patients with BPH and CAD, but the effect of iTUPKEP on vascular endothelial function was relatively mild. The reason for this finding may be that iTUPKEP has a shorter operation time and is associated with less intraoperative bleeding compared with TUVP. Therefore, iTUPKEP has a smaller effect on vascular endothelial function than TUVP. A shorter retention time of the catheter after the operation also reflects reduced intraoperative bleeding in iTUPKEP. The PVR, Qmax, IPSS, and QOL score were measured 1 month after the operation. These parameters also showed that iTUPKEP improved the symptoms of patients with BPH. In patients with BPH without CAD, our team’s recommendation is that patients should choose the iTUPKEP procedure because it has a better effect in improving the symptoms of BPH (PVR, Qmax, IPSS, and QOL) after surgery. Additionally, the iTUPKEP procedure had advantages regarding the operation time, intraoperative blood loss, and postoperative catheter indwelling time. Many studies have shown that, under normal circumstances, the concentration of ET-1 in the blood vessel wall is 100 times that in plasma. 47 An abnormal increase in ET-1 and cTn-I concentrations in vivo is an important cause of AMI or aggravation of ischaemia and may even induce cardiovascular accidents. Therefore, in high-risk patients with BPH and CAD, iTUPKEP is a safer surgical method than TUVP.
This study has the following limitations. First, observation of the postoperative improvement in symptoms of the patients in this study was only for 1 month. Therefore, long-term dynamic evaluation for a longer period is required in these patients. Second, in the grouping of patients, we mainly considered cardiovascular disease and different surgical procedures. In the future, we need to study the prostate volume as a grouping factor. Finally, we focussed on the effect of changes in postoperative inflammation indicators on cardiovascular disease, but did not address other possible complications.
Conclusion
Higher concentrations of ET-1, cTn-I, and hs-CRP caused by postoperative vascular endothelial injury are common in patients with BPH, especially in high-risk patients with BPH and CAD. These high concentrations may be one of the causes of postoperative cardiovascular adverse events. The iTUPKEP procedure has a smaller effect on vascular endothelial function than that on TUVP, thereby reducing the incidence of postoperative cardiovascular adverse events in high-risk patients with BPH and CAD.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605211060890 for Evaluation of efficacy and safety of improved transurethral plasma kinetic enucleation of the prostate in high-risk patients with benign prostatic hyperplasia and coronary artery disease by Qingchao Meng, Jingmei Li, Mingfeng Li and Rangxue Qiu in Journal of International Medical Research
Acknowledgements
We thank the patients for their participation in this study.
Availability of data and materials: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contributions: Study design and drafting the manuscript: QCM and JML; data collection and analysis: QCM and MFL; experimental guidance and manuscript revision: JML and RXQ; and selection of study methods and technical support: MFL and RXQ.
ORCID iD: Rangxue Qiu https://orcid.org/0000-0003-2214-0656
References
- 1.Langan RC. Benign Prostatic Hyperplasia. Prim Care 2019; 46: 223–232. [DOI] [PubMed] [Google Scholar]
- 2.Berry SJ, Coffey DS, Walsh PC, et al. The development of human benign prostatic hyperplasia with age. J Urol 1984; 132: 474–479. [DOI] [PubMed] [Google Scholar]
- 3.Verhamme KM, Dieleman JP, Bleumink GS, et al. Incidence and prevalence of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in primary care–the Triumph project. Eur Urol 2002; 42: 323–328. [DOI] [PubMed] [Google Scholar]
- 4.Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992; 148: 1549–1557; discussion 64. [DOI] [PubMed] [Google Scholar]
- 5.Fowler FJ, Jr, Wennberg JE, Timothy RP, et al. Symptom status and quality of life following prostatectomy. JAMA 1988; 259: 3018–3022. [PubMed] [Google Scholar]
- 6.Wasson JH, Reda DJ, Bruskewitz RC, et al. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. The Veterans Affairs Cooperative Study Group on Transurethral Resection of the Prostate. N Engl J Med 1995; 332: 75–79. [DOI] [PubMed] [Google Scholar]
- 7.Sarier M, Tekin S, Duman İ, et al. Results of transurethral resection of the prostate in renal transplant recipients: a single center experience. World J Urol 2018; 36: 99–103. [DOI] [PubMed] [Google Scholar]
- 8.Sarier M, Duman I, Kilic S, et al. Comparative Results of Transurethral Incision with Transurethral Resection of The Prostate in Renal Transplant Recipients with Benign Prostate Hyperplasia. Urol J 2018; 15: 209–213. [DOI] [PubMed] [Google Scholar]
- 9.Van Der Reis L. Benign prostatic hypertrophy and coronary heart disease: correlation or coincidence? J Am Geriatr Soc 1959; 7: 866–869. [DOI] [PubMed] [Google Scholar]
- 10.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998; 280: 605–613. [DOI] [PubMed] [Google Scholar]
- 11.McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med 1998; 338: 557–563. [DOI] [PubMed] [Google Scholar]
- 12.Weisman KM, Larijani GE, Goldstein MR, et al. Relationship between benign prostatic hyperplasia and history of coronary artery disease in elderly men. Pharmacotherapy 2000; 20: 383–386. [DOI] [PubMed] [Google Scholar]
- 13.Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988; 332: 411–415. [DOI] [PubMed] [Google Scholar]
- 14.Lüscher TF andBarton M.. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation 2000; 102: 2434–2440. [DOI] [PubMed] [Google Scholar]
- 15.Kolettis TM, Barton M, Langleben D, et al. Endothelin in coronary artery disease and myocardial infarction. Cardiol Rev 2013; 21: 249–256. [DOI] [PubMed] [Google Scholar]
- 16.Willeit P, Welsh P, Evans JDW, et al. High-Sensitivity Cardiac Troponin Concentration and Risk of First-Ever Cardiovascular Outcomes in 154,052 Participants. J Am Coll Cardiol 2017; 70: 558–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skadberg Ø, Kleiven Ø, Ørn S, et al. The cardiac troponin response following physical exercise in relation to biomarker criteria for acute myocardial infarction; the North Sea Race Endurance Exercise Study (NEEDED) 2013. Clin Chim Acta 2018; 479: 155–159. [DOI] [PubMed] [Google Scholar]
- 18.Weil BR, Suzuki G, Young RF, et al. Troponin Release and Reversible Left Ventricular Dysfunction After Transient Pressure Overload. J Am Coll Cardiol 2018; 71: 2906–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laugaudin G, Kuster N, Petiton A, et al. Kinetics of high-sensitivity cardiac troponin T and I differ in patients with ST-segment elevation myocardial infarction treated by primary coronary intervention. Eur Heart J Acute Cardiovasc Care 2016; 5: 354–363. [DOI] [PubMed] [Google Scholar]
- 20.Koenig W. High-sensitivity C-reactive protein and atherosclerotic disease: from improved risk prediction to risk-guided therapy. Int J Cardiol 2013; 168: 5126–5134. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997; 336: 973–979. [DOI] [PubMed] [Google Scholar]
- 22.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004; 350: 1387–1397. [DOI] [PubMed] [Google Scholar]
- 23.Sinanoglu O, Ekici S, Balci MC, et al. Comparison of plasmakinetic transurethral resection of the prostate with monopolar transurethral resection of the prostate in terms of urethral stricture rates in patients with comorbidities. Prostate Int 2014; 2: 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Küpeli S, Yilmaz E, Soygür T, et al. Randomized study of transurethral resection of the prostate and combined transurethral resection and vaporization of the prostate as a therapeutic alternative in men with benign prostatic hyperplasia. J Endourol 2001; 15: 317–321. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Zheng S, Li H, et al. Transurethral enucleation and resection of prostate in patients with benign prostatic hyperplasia by plasma kinetics. J Urol 2010; 184: 2440–2445. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Z, Zeng G, Zhong W, et al. A prospective, randomised trial comparing plasmakinetic enucleation to standard transurethral resection of the prostate for symptomatic benign prostatic hyperplasia: three-year follow-up results. Eur Urol 2010; 58: 752–758. [DOI] [PubMed] [Google Scholar]
- 27.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344–349. [DOI] [PubMed] [Google Scholar]
- 28.Casabé A, Roehrborn CG, Da Pozzo LF, et al. Efficacy and Safety of the Coadministration of Tadalafil Once Daily with Finasteride for 6 Months in Men with Lower Urinary Tract Symptoms and Prostatic Enlargement Secondary to Benign Prostatic Hyperplasia. J Urol 2014; 191: 727–733. [DOI] [PubMed] [Google Scholar]
- 29.Girman CJ, Jacobsen SJ, Rhodes T, et al. Association of health-related quality of life and benign prostatic enlargement. Eur Urol 1999; 35: 277–284. [DOI] [PubMed] [Google Scholar]
- 30.Bruno AN andSummers JL.. Ischemic heart disease in patients with large gland prostatic hypertrophy. Urology 1985; 25: 239–241. [DOI] [PubMed] [Google Scholar]
- 31.Hahn RG. Acute myocardial infarction after transurethral resection of the prostate. Biomed Pharmacother 2001; 55: 144–147. [DOI] [PubMed] [Google Scholar]
- 32.Weisman KM Larijani GE andGoldberg ME.. Incidence of acute myocardial infarction and cause-specific mortality after transurethral treatments of prostatic hypertrophy. Urology 2000; 56: 544. [DOI] [PubMed] [Google Scholar]
- 33.Roger VL. Epidemiology of myocardial infarction. Med Clin North Am 2007; 91: 537–552; ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thorin E andWebb DJ.. Endothelium-derived endothelin-1. Pflugers Arch 2010; 459: 951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lerman A, Holmes DR, Jr, Bell MR, et al. Endothelin in coronary endothelial dysfunction and early atherosclerosis in humans. Circulation 1995; 92: 2426–2431. [DOI] [PubMed] [Google Scholar]
- 36.Piechota A Polańczyk A andGoraca A.. Role of endothelin-1 receptor blockers on hemodynamic parameters and oxidative stress. Pharmacol Rep 2010; 62: 28–34. [DOI] [PubMed] [Google Scholar]
- 37.Simeone SM, Li MW, Paradis P, et al. Vascular gene expression in mice overexpressing human endothelin-1 targeted to the endothelium. Physiol Genomics 2011; 43: 148–160. [DOI] [PubMed] [Google Scholar]
- 38.Wang QD, Hemsén A, Li XS, et al. Local overflow and enhanced tissue content of endothelin following myocardial ischaemia and reperfusion in the pig: modulation by L-arginine. Cardiovasc Res 1995; 29: 44–49. [PubMed] [Google Scholar]
- 39.Yasuda M, Kohno M, Tahara A, et al. Circulating immunoreactive endothelin in ischemic heart disease. Am Heart J 1990; 119: 801–806. [DOI] [PubMed] [Google Scholar]
- 40.Walden PD, Ittmann M, Monaco ME, et al. Endothelin-1 production and agonist activities in cultured prostate-derived cells: implications for regulation of endothelin bioactivity and bioavailability in prostatic hyperplasia. Prostate 1998; 34: 241–250. [DOI] [PubMed] [Google Scholar]
- 41.Le Brun G, Moldovan F, Aubin P, et al. Identification of endothelin receptors in normal and hyperplasic human prostate tissues. Prostate 1996; 28: 379–384. [DOI] [PubMed] [Google Scholar]
- 42.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 1999; 340: 115–126. [DOI] [PubMed] [Google Scholar]
- 43.Galkina E andLey K.. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol 2009; 27: 165–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bisoendial RJ Kastelein JJ andStroes ES.. C-reactive protein and atherogenesis: from fatty streak to clinical event. Atherosclerosis 2007; 195: e10–e18. [DOI] [PubMed] [Google Scholar]
- 45.Verma S, Wang CH, Li SH, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation 2002; 106: 913–919. [DOI] [PubMed] [Google Scholar]
- 46.Apple FS, Sandoval Y, Jaffe AS, et al. Cardiac Troponin Assays: Guide to Understanding Analytical Characteristics and Their Impact on Clinical Care. Clin Chem 2017; 63: 73–81. [DOI] [PubMed] [Google Scholar]
- 47.Battistini B D'Orléans-Juste P andSirois P. . Endothelins: circulating plasma levels and presence in other biologic fluids. Lab Invest 1993; 68: 600–628. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605211060890 for Evaluation of efficacy and safety of improved transurethral plasma kinetic enucleation of the prostate in high-risk patients with benign prostatic hyperplasia and coronary artery disease by Qingchao Meng, Jingmei Li, Mingfeng Li and Rangxue Qiu in Journal of International Medical Research