Abstract
Objective:
To analyze the thermogenic effects of footbaths with medicinal powders in oncological patients (ON) and healthy controls (HC).
Intervention and Outcomes:
Thirty-six participants (23 ON, 13 HC; 24 females; 49.9 ± 13.3 years) received 3 footbaths in a random order with cross-over design: warm water only (WA), warm water plus mustard (MU, Sinapis nigra), and warm water plus ginger (GI, Zingiber officinale). Warmth perception of the feet (Herdecke Warmth Perception Questionnaire, HeWEF) at the follow-up (10 minutes after completion of footbaths, t2) was assessed as the primary outcome measure. Secondary outcome measures included overall warmth as well as self-reported warmth (HeWEF) and measured skin temperature (high resolution thermography) of the face, hands and feet at baseline (t0), post immersion (t1), and follow-up (t2).
Results:
With respect to the warmth perception of the feet, GI and MU differed significantly from WA (P’s < .05) with the highest effect sizes at t1 (WA vs GI, d = 0.92, WA vs MU, d = 0.73). At t2, perceived warmth tended to be higher with GI compared to WA (d = 0.46). No differences were detected between ON and HC for self-reported warmth. With respect to skin temperatures, face and feet skin temperatures of ON were colder (at t0 and t1, 0.42 ≥ d ≥ 0.68) and tended to have diametrical response patterns than HC (ON vs HC: colder vs warmer after MU).
Conclusion:
Among adult oncological patients and healthy controls, footbaths with mustard and ginger increased warmth perception of the feet longer than with warm water only. The potential impact of regularly administered thermogenic footbaths over extended periods merits further investigation for the recovery of cancer-related sense of cold.
Keywords: footbath, mustard, ginger, warmth, cancer, oncological patients, thermoregulation
Introduction
Defects in thermoregulation are a frequent cancer-related complaint causing thermal discomfort. 1 Those oncological patients who are affected report excessive overheating similar to menopausal hot flashes, as well as excessive and persistent feelings of being cold. 1 Hot flashes mainly affect patients after hormone suppression treatments, for example, after breast or prostate cancer treatments. 1 Although hot flashes have been reported as a predictor of better disease prognosis in breast cancer, such thermal dysregulation can precipitate sharp declines in self-reported quality of life. 1 Symptom relief can be achieved by administering selective serotonin reuptake inhibitors (SSRIs) for both breast and prostate cancer or clonidine for breast cancer patients. 1
Much less attention is paid to the acquired symptom of feeling cold after cancer diagnosis and treatment, although it is reported by a large percentage of patients with various types of cancer.1,2 Sense of cold is associated with quality-of-life problems, hidden costs arising from the need for extra heating, 1 a negative impact on the immune system,1,3 and a higher risk of patients developing disturbances in sleep behavior.4,5 Thus, persistently feeling cold might be linked to a poorer disease prognosis. 1 These symptoms of feeling cold are often aggregated with menopausal-like symptoms induced by treatment. 1 Although the sense of hot flashes is often followed by chills, 6 the feeling of being cold can outlast those phases and remain for long periods of time. 1 The feeling of being cold might therefore originate from different pathological thermoregulatory mechanisms. 1 Interestingly, it was shown that body temperature correlates with health status, with multimorbid patients having a lower body temperature than healthy individuals of the same age. 1 Mild thermal therapies might help to alleviate thermal discomfort and to positively influence changes in immune function.1,7 Foot bathing is a frequently employed method demonstrated to improve body warmth regulation,8-10 relieve symptoms of fatigue, 10 improve sleep,10-12 and decrease sympathetic activity as well as pain intensity. 13 Previous studies suggest prolonged thermal effects when ginger (Zingiber officinale, GI) or mustard (Sinapis nigra, MU) were added to warm water footbaths (WA).8,9 This thermogenic effect might stem from the binding of the active ingredients of GI (gingerols, shogaols) and MU (allyl isothiocyanate) to temperature sensitive ion channels of the transient receptor potential (TRP) ion channel superfamily.14-16 In addition to the potential therapeutic advantages of external applications of GI and MU, both substances were reported to have cancer preventive and antiproliferative properties when used as dietary agents.17-19 However, little is understood about warming patients with oncological disease with footbaths containing thermoregulatory substances. Moreover, the symptoms of thermal discomfort and their improvement are often underappreciated and neglected by healthcare providers. 1 To address these gaps, our study aimed to investigate and compare the effects of footbaths containing medicinal powders of MU or GI on the self-reported warmth perception and measured skin temperature between oncological patients (ON) and healthy controls (HC).
Materials and Methods
Study Design
The study was an explorative, randomized, vehicle-controlled, 3-armed trial with a cross-over design comparing the effects of MU and GI footbaths on psychophysiological parameters in ON and HC. The sequence in which participants received all 3 footbath conditions (warm water only, WA; warm water plus mustard, MU; warm water plus ginger, GI) was randomized, for a total of 6 possible sequences. Ethical approval was obtained from the ethics committee of the University of Tübingen, Germany (no. 690/2015BO2). The study was registered at the US National Institutes of Health (ClinicalTrials.gov) NCT04271670 and complied with the CONSORT (Consolidated Standards of Reporting Trials) guidelines. 20
Study Population
Participants were recruited through posting notices in a German hospital and through direct contact by the medical staff of the clinic. Potential participants were screened for eligibility with the following inclusion criteria: written informed consent and an age over 18 years. Participants were excluded in case of infectious diseases (core body temperature > 38°C), skin injuries on the lower legs or feet, self-reported hypersensitivity to MU or GI products, cardiac arrhythmia, pregnancy, or insufficient knowledge of the German language. For oncological patient-participants an additional inclusion criterion was the presence of an acute oncological disease, and the additional exclusion criteria of confinement to bed or poor general condition (according to the assessment of the attending physicians). Prior to the study, we initially decided to restrict ON age to 18 to 65 years and to require at least 1 previous chemotherapy or radiation therapy treatment. However, we abandoned this approach after trial commencement in order to reach a higher external validity and included 5 participants with an age over 65 years as well as 1 participant who had not yet received any chemotherapy or radiation therapy. For practical reasons we planned to include only ON who were currently inpatients on the oncology ward of a German clinic, with a minimum stay of 4 days. However, to expand the pool of eligible ON participants, we also enrolled oncology patients who had shorter hospital stay of 3 days (n = 6). Enrolled participants were asked to refrain from consuming nicotine and coffee within 3 hours prior to each measurement.
Study Interventions
Each participant received all 3 footbath interventions according to the randomized sequence. A wash-out period of at least 1 day was required between 2 consecutive measurements (M = 4.60 ± 8.05 days). Participants were instructed to remain in a seated position with unclothed feet and lower legs during each footbath intervention session. HC were provided with hospital gowns for this purpose, while ON wore either hospital gowns or nightgowns. As recommended for the use of human infrared (IR) applications,21,22 we endeavored to maintain a stable room temperature (M = 23.77 ± 1.81°C) and humidity (M = 32.87% ± 7.59%). In order to optimize the stabilization of participants’ body temperature, a ten-minute equilibration period was provided.21,22 Footbaths were prepared with 12 l of water heated to 40°C (M = 39.95 ± 0.28°C), placed within plastic tubs (water depth 15 cm). For the experimental conditions (GI, MU), 80 g of prepared powder (Zingiberis rhizome/Sinapis nigrae semen) were added. After the equilibration period, the participants received 1 of 3 footbath intervention conditions, with a maximum duration of 20 minutes. Participants were instructed to voice any concerns or discomfort during the footbaths. To minimize the potential for harm, footbath interventions were interrupted if and when participants were uncomfortable. The participants remained in a seated position for another ten minutes after taking their feet out of water. Room temperature, humidity, water temperature and the duration of footbath immersion were monitored for subsequent analyses. All measurements were conducted in the afternoon and early evening between 12 and 8 pm. For HC, measurements took place in a laboratory setting, whereas for ON, measurements were performed in the hospital patient rooms.
Study Outcomes
The study’s outcome measures were assessed at 3 specific time-points: directly before the intervention following the 10-minute equilibration period (baseline or t0), directly after the footbath intervention (post immersion or t1), and 10 minutes following the footbath (follow-up or t2). The primary outcome measure was self-reported warmth perception of the feet at t2 as assessed with the Herdecke Warmth Perception Questionnaire (Herdecker Wärmeempfindungs-Fragebogen, HeWEF) in German language.23,24 This is a patient self-report questionnaire assessing the currently perceived overall warmth and warmth distribution for up to 24 body parts (HeWEF state, current) as well as general warmth (HeWEF trait, typical) (Cronbach’s α = .93). Local warmth perceptions for specific areas are rated on a five-point scale from 0 = cold to 4 = hot and were averaged to represent the body regions feet (items feet and toes), hands (items hands and fingers) and face (items forehead and cheeks). The rating for overall warmth is reported as a single item using the same 5-point scale (0 = cold to 4 = hot). The HeWEF scores for the warmth perception of the feet (at t1), face (t1 + t2), and hands (t1 + t2) as well as for overall warmth (t1 + t2) served as secondary outcome measures. Further secondary outcome measures were the actual skin temperature of the feet (dorsum of feet and toes), hands (back and palms of the hands and fingers), and face (forehead, eye area, inner canthus of the eyes, cheeks, nose, mouth, and chin) at t1 and t2, which were assessed with a high-definition IR camera (FLIR SC660, FLIR Systems, Wilsonville, Oregon/USA, image resolution 640 × 480 pixels, thermal sensitivity <30 mK). Pictures were taken with a distance of 2 m between camera and skin and were analyzed with the software ThermaCAM™.
Participants were interviewed about adverse events (AEs) at t1 and t2.
Baseline Measurements
The European Organization for Research and Treatment of Cancer (EORTC) Core Quality of Life Questionnaire (EORTC QLQ-C30) was used to assess the health-related quality of life (QoL) in ON and HC. The EORTC is a validated, 25 self-report questionnaire in which a global health status/QoL-score, 5 function scales (physical, role, social, emotional, and cognitive functions), 3 symptom scales (fatigue, pain, nausea), and 6 single items scores (dyspnea, insomnia, appetite loss, constipation, diarrhea, financial difficulties) are derived from a total of 30 items. All items with the exception of the QoL-score items (seven-point Likert scale from 1 = very poor to 7 = excellent) are answered on a four-point Likert scale (from 1 = not at all to 4 = very much). Based on a linear transformation, each QLQ-30 score ranges between 0 and 100 (function scales: high = better level of functioning; symptom scales/items: high = higher level of problems/symptomatology). 26 We further assessed participants’ general warmth perception (HeWEF trait) using the question, “How do you generally feel with respect to body temperature?” (scale from 1 = cold to 5 = hot).
Sample Size
Due to the hypothesis-generating character of this study, a convenience sample of 18 ON and 18 HC was estimated to be sufficient. We were unable to identify any published studies examining the effects of footbaths with thermogenic substances on psychophysiological parameters in ON. Vagedes et al 8 examined the effects of such footbaths in healthy adults, healthy adolescents and in adolescents with anorexia nervosa. 9 However, the perceived intensity of warmth of the feet and the efficiency of temperature regulation vary not only with age, but also with health.1,27 Kokolus et al 1 reported that body temperature is lower in older individuals with various morbidities compared to young adults and compared to healthy older individuals. We decided not to calculate the sample size based on the study results of younger, healthy individuals8,9 as ON might suffer from additional thermal regulatory issues. 1 The final number of participants differed from the primary estimate (23 ON, 13 HC) for two reasons: (1) the demand to participate among ON was greater than expected, and (2) we were not able to recruit enough healthy individuals during the allocated time.
Randomization
Eligible and willing participants were stratified by gender and randomly assigned to 1 of the 6 possible footbath sequences (1. MU-WA-GI, 2. MU-GI-WA, 3. WA-GI-MU, 4. WA-MU-GI, 5. GI-MU-WA, and 6. GI-WA-MU). For this, we prepared sealed, opaque envelopes containing 1 of the 6 possible sequences. At the first appointment, participants drew one envelope in the presence of the nurse research assistant. The drawn sequence was recorded, and the participants were assigned a study identification number (ID).
Blinding
Participants and analysts were kept blinded to footbath condition. We strove to maintain the blinding of the participants during each intervention by minimizing visual and olfactory cues. For this reason, the footbath tub was covered with towels during the intervention and a room spray containing essential oils was used. The blinding status was queried by asking the participants which olfactory perceptions they perceived predominantly (response options: MU, GI, eucalyptus, lavender, citrus, and peppermint) and by asking, “Which condition did you receive today?” (response options: WA, MU, GI).
Statistical Analysis
We conducted the statistical analysis with R 28 and RStudio. 29 The analysis of the primary and secondary outcome measures was conducted on an intention-to-treat basis with imputation of missing values by use of single imputation based on predictive mean matching (R package: mice 30 ). Forty imputed datasets were generated and averaged in order to obtain single imputation values. A P-value of less than .05 (two-tailed) was considered to indicate statistical significance. We first investigated possible asymmetrical sequence effects (due to the interaction between treatment and carryover effects) for the primary outcome measure using the procedure described by Wellek and Blettner. 31 For this purpose, we calculated the (total) sum of all 3 periods of baseline measurements (HeWEF feet at t0) per subject and conducted a one-factorial analysis of variance (ANOVA) with sequence group as the factor. Failing to find a significant effect within this analysis, the sequence groups were then pooled together for the analysis of intervention effects (WA vs MU vs GI).
The primary outcome measure, HeWEF warmth of feet at t2, was analyzed using a linear mixed-effects model (R package lme4 32 ) allowing for health status (ON, HC), footbath condition (WA, MU, GI), and time (t1, t2) as fixed effects and participants as a random effect. Interaction terms between time and health status, time and footbath condition as well as between health status and footbath condition were included. The baseline (t0) measurement of the primary outcome measure and the baseline room temperature were fitted as covariates. To verify the choice of covariates, a model selection based on likelihood ratio statistics, and the Akaike and Bayesian information criteria (AIC and BIC 33 ) was performed. For the post hoc analysis, p-values were estimated from the model using the package lmerTest 34 and subjected to a Bonferroni correction. Cohen’s d effect sizes were calculated using the model adjusted values. Secondary outcome measures that were not derived from the primary analysis are reported descriptively and with mean differences, 95% CI and Cohen’s d effect sizes (R package: effsize 35 ). No statistical tests were conducted to compare the randomized groups with respect to the baseline measures. Welch’s unequal variance t-tests were used to compare ON and HC with respect to age, BMI, EORTC QLQ-30, and general warmth (HeWEF trait). Potential differences in the baseline room temperature, humidity and water temperature were examined using two-factorial ANOVAs with health status and footbath condition as the factors. The duration of footbath immersion was analyzed using a linear mixed-effects model with health status, footbath condition and their interaction as fixed effects and participants as a random effect.
The success of blinding was verified with a Cochran-Mantel-Haenszel chi-squared statistic. Potential associations between the footbath conditions MU and GI and participants’ olfactory perceptions were examined taking the total number of olfactory perceptions into account as confounder. Data were crosschecked to assess whether they conformed to a normal distribution.
Results
Study Population
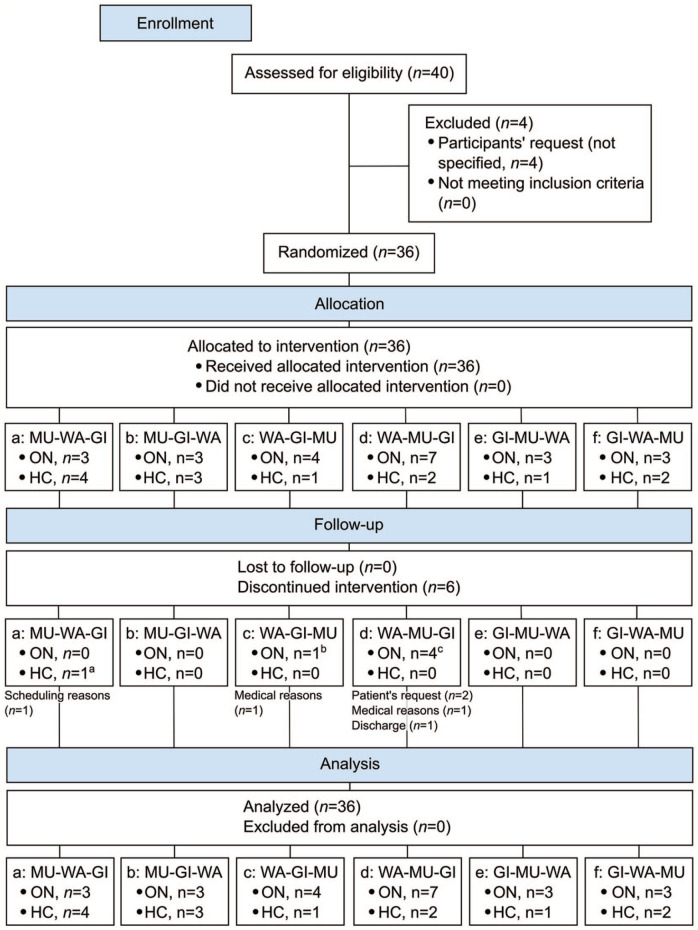
Between January 15, 2014 and September 18, 2017, 40 adults (13 HC, 27 ON) were screened for eligibility and 36 (90%, 13 HC, 23 ON) of them were randomly assigned to 1 of the 6 sequence groups. The sample consisted of 24 women and 12 men between 26 and 77 years of age (M = 49.9 ± 13.3 years) with a mean body-mass-index of 22.73 ± 3.62 kg/m². Six participants discontinued the study protocol (HC: n = 1, ON: n = 5) (Figure 1). The final cases in each footbath condition were n = 35 (HC: n = 12, ON: n = 23) for WA, n = 33 (HC: n = 13, ON: n = 20) for MU, and n = 30 (HC: n = 12, ON: n = 18) for GI. Participants completed all 3 interventions in a mean total time of 10.43 ± 12.17 days (Min = 3, Max = 53).
Figure 1.
CONSORT flow diagram (WA = water only conditions, MU = mustard added to WA, GI = ginger added to WA).
a2 interventions missing (WA, GI).
b2 interventions missing (GI, MU).
c2 interventions missing, n = 2 (MU, GI), 1 interventions missing, n = 2 (GI).
The diagnoses and tumor stages of ON are reported in Table 1. Mean hospital stay was 6.86 ± 5.39 days (Min = 3, Max = 27). During this time, cancer treatments included surgery (n = 1), chemotherapy (n = 12), radiation therapy (n = 1), immunotherapy (n = 3), hyperthermia (n = 9) and mistletoe therapy (n = 13). Footbath treatment schedules were combined with the institution’s standard anthroposophic, holistic care 36 including herbal, anthroposophic or homeopathic remedies (n = 19), external applications (n = 13), psycho-oncology therapy (n = 9), art therapy (n = 7), chromotherapy (n = 5), rhythmic massage (n = 4), eurythmy (n = 4), and physiotherapy (n = 3).
Table 1.
Diagnosis of Participants With Oncological Disorders (ON).
| Sex | Age, years | BMI, kg/m² | Diagnosis | Initial stage | Initial diagnosis | Metastases at study time a | |
|---|---|---|---|---|---|---|---|
| 1 | Female | 50 | 22.68 | Anal cancer | pT3 pN0 cM0 | 2014 | 1 |
| 2 | Female | 47 | 20.08 | Breast cancer | pT2 pN1a cM0 | 2012 | 0 |
| 3 | Female | 52 | 20.18 | Breast cancer | cT4 cN3 cM1 | 2005 | 1 |
| 4 | Female | 67 | 21.72 | Breast cancer | pT1b pN0 cM0 | 1996 | 1 |
| 5 | Female | 58 | 20.57 | Breast cancer | pT3 pN3a cM0 | 2012 | 1 |
| 6 | Female | 50 | 25.52 | Cervical cancer | pT1b pN1 cM0 | 2012 | 1 |
| 7 | Female | 76 | 21.05 | Colorectal carcinoma | pT3 pN2b pM1 | 2015 | 1 |
| 8 | Female | 68 | 32.44 | Colorectal carcinoma | pT4a pN1c pM1 (satellite) | 2013 | 1 |
| 9 | Female | 55 | 20.02 | Colorectal carcinoma | unknown | 2016 | 1 |
| 10 | Male | 43 | 24.90 | Colorectal carcinoma | pT3 pN1a cM0 | 2016 | 1 |
| 11 | Female | 39 | 20.68 | Gastric cancer | cTx cN+ cM1 | 2016 | 1 |
| 12 | Female | 54 | 15.31 | Gastric cancer | cT3 cN+ cM0 | 2014 | 1 |
| 13 | Male | 32 | 19.45 | Lymphoma | Stage III B | 2017 | 0 |
| 14 | Female | 34 | 24.57 | Lymphoma | Stage III EB | 2016 | 0 |
| 15 | Female | 50 | 20.68 | Lymphoma | Stage IIa | 2016 | 0 |
| 16 | Male | 43 | 25.66 | Neuroendocrine tumor | Stage IV | 2017 | 1 |
| 17 | Female | 54 | 16.02 | Ovarian cancer | pT3c pN0 R1 cM0 | 2011 | 1 |
| 18 | Male | 62 | 20.83 | Pancreatic cancer | cT4 cN1 cM1 | 2016 | 1 |
| 19 | Male | 28 | 23.41 | Testicular cancer | pT2 cNX cM0 | 2016 | 1 |
| 20 | Male | 26 | 18.21 | Testicular cancer | pT1 pNx cM0 | 2016 | 1 |
| 21 | Male | 37 | 25.43 | Testicular cancer | pT1 pNx cM0 | 2015 | 1 |
| 22 | Male | 62 | 28.39 | Thyroid cancer | pT4 pN1a cM0 | 1998 | 1 |
| 23 | Female | 61 | 26.23 | Uterine cancer | unknown | 2014 | 1 |
1=Metastatic tumor. 0=Non-metastatic tumor.
Demographic characteristics were similar between HC and ON. Significant differences were found on all EORTC scales with 2 exceptions (the functional scale cognitive functioning and the symptom scale constipation). HC and ON did not differ with respect to participants’ general warmth (HeWEF trait, Table 2), but ON felt significantly colder at the feet and were objectively measured colder at the feet and face (Table 3) at t0.
Table 2.
Personal Characteristics of Participants With Oncological Disorders (ON) and Healthy Controls (HC).
| HC (n = 13) | ON (n = 23) | t | P | ES | |
|---|---|---|---|---|---|
| Demographics | |||||
| Sex female, n (%) | 9 (69.23) | 15 (65.22) | — | — | — |
| Age, years | 50.00 ± 13.96 | 49.91 ± 13.25 | 0.02 | .99 | 0.01 |
| BMI, kg/m² | 23.42 ± 3.02 | 22.35 ± 3.93 | 0.91 | .37 | 0.29 |
| EORTC quality of life questionnaire (QLQ)-C30 [0-100] | |||||
| Global health status/QoL a | 80.77 ± 20.02 | 48.83 ± 20.47 | 4.56 | <.001 | 1.57 |
| Physical functioning a | 92.85 ± 9.48 | 67.30 ± 24.94 | 4.38 | <.001 | 1.23 |
| Role functioning a | 89.77 ± 21.03 | 42.48 ± 32.53 | 5.29 | <.001 | 1.63 |
| Emotional functioning a | 79.54 ± 19.07 | 55.30 ± 24.97 | 3.27 | <.01 | 1.05 |
| Cognitive functioning a | 78.23 ± 24.94 | 66.35 ± 25.46 | 1.36 | .18 | 0.47 |
| Social functioning a | 89.77 ± 23.99 | 44.52 ± 34.20 | 4.64 | <.001 | 1.46 |
| Fatigue b | 29.00 ± 31.96 | 57.17 ± 30.13 | −2.59 | .02 | 0.92 |
| Nausea and vomiting b | 1.31 ± 4.71 | 29.39 ± 31.93 | −4.14 | <.001 | 1.09 |
| Pain b | 6.38 ± 12.71 | 46.22 ± 30.82 | −5.43 | <.001 | 1.54 |
| Dyspnea b | 10.23 ± 21.03 | 42.65 ± 24.08 | −4.21 | <.001 | 1.41 |
| Insomnia b | 20.38 ± 21.64 | 50.13 ± 31.61 | −3.34 | <.01 | 1.04 |
| Appetite loss b | 5.08 ± 12.39 | 44.61 ± 39.45 | −4.43 | <.001 | 1.21 |
| Constipation b | 7.69 ± 20.02 | 21.04 ± 25.91 | −1.72 | 0.09 | 0.56 |
| Diarrhea b | 2.54 ± 9.15 | 36.13 ± 34.21 | −4.44 | <.001 | 1.20 |
| Financial difficulties b | 2.54 ± 9.15 | 40.74 ± 32.77 | −5.24 | <.001 | 1.42 |
| Herdecke warmth perception questionnaire (HeWEF trait) [1 = cold; 5 = hot] | |||||
| General warmth | 3.23 ± 0.93 | 3.04 ± 0.82 | 0.61 | 0.55 | 0.22 |
Note. Data are M ± SD if not otherwise indicated (QoL = quality of life, ES = Cohen’s d effect size). Welch’s unequal variance t-tests were used to calculate t- and P-values.
Bold indicates a P-value <0.05.
High scores represent a high QoL/ a healthy level of functioning.
High scores represent high levels of symptomatology.
Table 3.
Mean Differences Between Oncological Patients (ON, n = 23) and healthy controls (HC, n = 13) as a Function of Time (Footbath Condition Grouped Together).
| HC | ON | Diff | CI | ES | ||
|---|---|---|---|---|---|---|
| Warmth perception (as assessed by the Herdecke warmth perception questionnaire) [0 = cold;4 = hot] | ||||||
| Feet | t0 | 1.84 ± 1.15 | 1.30 ± 0.87 | 0.54 | (0.12;0.96) | 0.55 |
| t1 | 3.18 ± 0.79 | 3.16 ± 0.59 | 0.02 | (−0.27;0.31) | 0.03 | |
| t2 | 2.62 ± 0.92 | 2.65 ± 0.83 | −0.03 | (−0.38;0.33) | 0.03 | |
| Face | t0 | 2.54 ± 0.65 | 2.46 ± 0.54 | 0.08 | (−0.16;0.33) | 0.14 |
| t1 | 2.57 ± 0.61 | 2.65 ± 0.59 | −0.08 | (−0.32;0.16) | 0.14 | |
| t2 | 2.48 ± 0.72 | 2.67 ± 0.56 | −0.18 | (−0.45;0.08) | 0.29 | |
| Hands | t0 | 2.36 ± 0.96 | 2.24 ± 0.92 | 0.12 | (−0.25;0.50) | 0.13 |
| t1 | 2.51 ± 0.96 | 2.66 ± 0.73 | −0.15 | (−0.51;0.20) | 0.19 | |
| t2 | 2.45 ± 0.93 | 2.61 ± 0.78 | −0.16 | (−0.51;0.19) | 0.19 | |
| Overall warmth | t0 | 2.31 ± 0.91 | 2.12 ± 0.75 | 0.19 | (−0.15;0.53) | 0.23 |
| t1 | 2.77 ± 0.54 | 2.75 ± 0.54 | 0.02 | (−0.20;0.23) | 0.03 | |
| t2 | 2.69 ± 0.70 | 2.68 ± 0.62 | 0.01 | (−0.25;0.28) | 0.02 | |
| Skin temperature (as assessed with a high-resolution IR camera in °C) | ||||||
| Feet | t0 | 31.43 ± 3.12 | 30.25 ± 2.58 | 1.18 | (0.003;2.35) | 0.42 |
| t1 | 34.03 ± 0.60 | 33.76 ± 0.62 | 0.28 | (0.03;0.52) | 0.45 | |
| t2 | 32.46 ± 1.80 | 31.87 ± 1.62 | 0.58 | (−0.11;1.28) | 0.35 | |
| Face | t0 | 34.70 ± 0.97 | 34.25 ± 1.12 | 0.46 | (0.05;0.87) | 0.43 |
| t1 | 34.73 ± 0.75 | 34.09 ± 1.05 | 0.65 | (0.30;0.99) | 0.68 | |
| t2 | 34.84 ± 0.83 | 34.10 ± 1.06 | 0.74 | (0.38;1.11) | 0.75 | |
| Hands | t0 | 33.62 ± 2.24 | 32.69 ± 3.03 | 0.93 | (−0.09;1.95) | 0.33 |
| t1 | 33.49 ± 1.87 | 32.72 ± 2.74 | 0.77 | (−0.11;1.66) | 0.31 | |
| t2 | 33.36 ± 2.15 | 32.57 ± 2.85 | 0.78 | (−0.18;1.75) | 0.30 | |
Note. Data are M ± SD (Diff = mean difference, CI = 95% confidence intervals, ES = Cohen’s d effect size, t0 = baseline, t1 = post immersion, t2 = follow-up). Bold indicates CI that do not contain zero.
Baseline Room and Footbath Conditions
Baseline measures of study outcomes were similar in all 3 footbath conditions (Table 4). The initial water temperature of the prepared footbaths did not differ with respect to health status [F(1,320) < 1] or footbath condition [F(2,320) < 1]. However, the duration of footbath immersion differed significantly with respect to the intervention received [footbath condition: F(2,284) = 130.14, P < .001; health status: F(1,34) < 1; interaction: F(2,284) < 1]. Post hoc tests revealed a significantly shorter duration for MU (M = 12.67 ± 5.11 minutes) compared to GI (M = 16.94 ± 3.99 minutes) and WA (M = 18.78 ± 3.03 minutes): MU vs GI t(284) = 11.22, P < .001, d = 0.93; MU vs WA t(284) = 15.65, P < .001, d = 1.46; GI vs WA t(284) = 4.43, P < .001, d = 0.52. Regarding the initial room conditions, the ambient temperature differed significantly between ON and HC [health status: F(1,320) = 8.61, P < .01, d = 0.34; footbath condition: F(2,329) < 1] due to a higher value in HC (M = 24.16 ± 1.95°C) compared to ON (M = 23.55 ± 1.69°C). No differences were found for humidity [health status: F(1,320) < 1; footbath condition: F(2,320) = 1.08, P = .34].
Table 4.
Mean Differences Between the 3 Footbath Conditions WA, MU, and GI (each n = 36) as a Function of Time (Health Status Grouped Together).
| WA | GI | MU | WA vs GI | WA vs MU | GI vs MU | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diff | 95% CI | ES | Diff | 95% CI | ES | Diff | 95% CI | ES | |||||
| Warmth perception (as assessed by the Herdecke warmth perception questionnaire) [0 = cold; 4 = hot] | |||||||||||||
| Feet | t0 | 1.51 ± 1.04 | 1.49 ± 0.94 | 1.49 ± 1.08 | 0.02 | (−0.44;0.49) | 0.02 | 0.02 | (−0.47;0.52) | 0.02 | 0.00 | (−0.47;0.48) | 0.00 |
| t1 | 2.83 ± 0.63 | 3.37 ± 0.56 | 3.30 ± 0.69 | −0.54 | (−0.82;−0.27) | 0.92 | −0.48 | (−0.79;−0.17) | 0.73 | 0.07 | (−0.23;0.36) | 0.11 | |
| t2 | 2.45 ± 0.93 | 2.87 ± 0.89 | 2.59 ± 0.72 | −0.42 | (−0.85;0.01) | 0.46 | −0.15 | (−0.54;0.24) | 0.18 | 0.27 | (−0.11;0.65) | 0.34 | |
| Face | t0 | 2.42 ± 0.55 | 2.51 ± 0.56 | 2.52 ± 0.64 | −0.09 | (−0.35;0.17) | 0.17 | −0.10 | (−0.38;0.18) | 0.17 | −0.01 | (−0.29;0.27) | 0.02 |
| t1 | 2.58 ± 0.61 | 2.62 ± 0.61 | 2.67 ± 0.58 | −0.04 | (−0.32;0.25) | 0.06 | −0.09 | (−0.38;0.19) | 0.16 | −0.06 | (−0.34;0.22) | 0.10 | |
| t2 | 2.57 ± 0.61 | 2.61 ± 0.67 | 2.63 ± 0.61 | −0.04 | (−0.34;0.26) | 0.06 | −0.06 | (−0.35;0.23) | 0.10 | −0.02 | (−0.32;0.28) | 0.03 | |
| Hands | t0 | 2.38 ± 0.97 | 2.23 ± 0.85 | 2.25 ± 0.99 | 0.15 | (−0.28;0.58) | 0.16 | 0.13 | (−0.33;0.59) | 0.13 | −0.02 | (−0.45;0.41) | 0.02 |
| t1 | 2.74 ± 0.87 | 2.51 ± 0.81 | 2.57 ± 0.77 | 0.24 | (−0.16;0.64) | 0.28 | 0.18 | (−0.21;0.57) | 0.22 | −0.06 | (−0.43;0.31) | 0.08 | |
| t2 | 2.52 ± 0.91 | 2.54 ± 0.83 | 2.59 ± 0.78 | −0.01 | (−0.42;0.39) | 0.02 | −0.06 | (−0.46;0.33) | 0.08 | −0.05 | (−0.43;0.33) | 0.06 | |
| Overall warmth | t0 | 2.21 ± 0.75 | 2.08 ± 0.82 | 2.28 ± 0.87 | 0.13 | (−0.24;0.50) | 0.17 | −0.07 | (−0.45;0.31) | 0.09 | −0.20 | (−0.60;0.20) | 0.24 |
| t1 | 2.75 ± 0.60 | 2.74 ± 0.60 | 2.79 ± 0.40 | 0.01 | (−0.27;0.30) | 0.02 | −0.04 | (−0.28;0.20) | 0.08 | −0.06 | (−0.30;0.19) | 0.11 | |
| t2 | 2.67 ± 0.64 | 2.71 ± 0.69 | 2.67 ± 0.62 | −0.04 | (−0.35;0.27) | 0.06 | 0.00 | (−0.30;0.30) | 0.00 | 0.04 | (−0.27;0.35) | 0.06 | |
| Skin temperature (as assessed with a high-resolution IR camera in °C) | |||||||||||||
| Feet | t0 | 30.78 ± 2.89 | 30.52 ± 2.84 | 30.72 ± 2.83 | 0.26 | (−1.09;1.60) | 0.09 | 0.07 | (−1.28;1.41) | 0.02 | −0.19 | (−1.52;1.14) | 0.07 |
| t1 | 33.90 ± 0.60 | 33.80 ± 0.69 | 33.87 ± 0.60 | 0.11 | (−0.20;0.41) | 0.16 | 0.03 | (−0.25;0.31) | 0.05 | −0.07 | (−0.38;0.23) | 0.11 | |
| t2 | 32.11 ± 1.83 | 32.06 ± 1.56 | 32.08 ± 1.75 | 0.05 | (−0.74;0.85) | 0.03 | 0.04 | (−0.81;0.88) | 0.02 | −0.02 | (−0.80;0.76) | 0.01 | |
| Face | t0 | 34.53 ± 1.06 | 34.39 ± 0.97 | 34.31 ± 1.23 | 0.14 | (−0.34;0.62) | 0.14 | 0.23 | (−0.31;0.77) | 0.20 | 0.09 | (−0.43;0.61) | 0.08 |
| t1 | 34.50 ± 0.95 | 34.37 ± 0.82 | 34.10 ± 1.18 | 0.13 | (−0.29;0.55) | 0.15 | 0.40 | (−0.11;0.90) | 0.37 | 0.27 | (−0.21;0.74) | 0.26 | |
| t2 | 34.47 ± 1.03 | 34.37 ± 0.90 | 34.25 ± 1.19 | 0.11 | (−0.35;0.56) | 0.11 | 0.23 | (−0.30;0.75) | 0.20 | 0.12 | (−0.38;0.61) | 0.11 | |
| Hands | t0 | 33.13 ± 2.70 | 32.94 ± 2.97 | 33.00 ± 2.80 | 0.20 | (−1.14;1.53) | 0.07 | 0.14 | (−1.16;1.43) | 0.05 | −0.06 | (−1.42;1.29) | 0.02 |
| t1 | 33.42 ± 2.28 | 32.78 ± 2.81 | 32.79 ± 2.33 | 0.64 | (−0.57;1.84) | 0.25 | 0.63 | (−0.45;1.71) | 0.27 | −0.01 | (−1.22;1.21) | 0.00 | |
| t2 | 33.04 ± 2.58 | 32.67 ± 2.80 | 32.85 ± 2.59 | 0.37 | (−0.90;1.63) | 0.14 | 0.19 | (−1.03;1.40) | 0.07 | −0.18 | (−1.45;1.09) | 0.07 | |
Note. Data are M ± SD (WA = water only condition; MU = mustard added to WA; GI = ginger added to WA; Diff = mean difference; CI = confidence intervals; ES = Cohen’s d effect size; t0 = baseline; t1 = post immersion; t2 = follow-up). Bold indicates CI that do not contain zero.
Analysis of Possible Carry-Over Effects
At t1, the total sum scores for warmth perception of the feet did not differ between the 6 sequence groups [F(5,30) = 2.38, P = .06]. Thus, the possibility for carry-over effects was negligible and groups were pooled together with regard to the intervention received: WA versus MU versus GI (based on the crossover design: n = 36 for WA, MU, and GI).
Model Selection
A model with baseline HeWEF feet (t0) as the only covariate (basic model) was compared to models additionally considering baseline room temperature, humidity, water temperature and duration of the footbaths as covariates. The AIC, BIC and likelihood ratio statistics pointed to a better goodness-of-fit for the model with the covariates baseline HeWEF feet and room temperature. Thus, we decided to apply this model for analyzing the primary outcome measure.
Outcomes and Estimations
A total of 9.26% of the HeWEF and IR data were missing and were imputed.
Primary outcome measure (warmth perception at the feet, HeWEF)
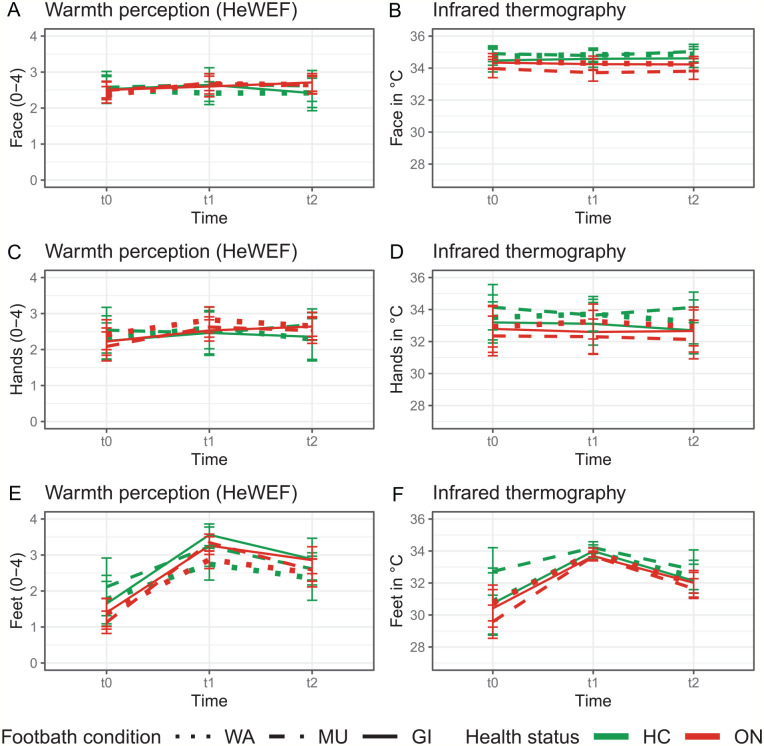
The primary analysis yielded significant main effects of time [F(1,168) = 34.71, P < .001] and footbath condition [F(2,169) = 10.86, P < .001]. These effects were based 1) on a higher perceived warmth post immersion [t1: Madj = 3.15 ± 0.84, t2: Madj = 2.61 ± 0.84, dajd = 0.64], and 2) on a significantly higher warmth perception for GI (Madj = 3.13 ± 0.78) and MU (Madj = 2.90 ± 0.79) compared to WA (Madj = 2.61 ± 0.79): WA versus GI, t(169) = −4.65, padj < 0.001, dadj = 0.66; WA versus MU, t(170) = −2.55, padj = 0.04, dadj = 0.36; GI versus MU, t(169) = 2.09, padj = 0.11, dadj = 0.30. Although the interaction between footbath condition and time did not reach significance [F(2,168) = 1.21, P = .30], the descriptive analysis revealed that the differences between GI and WA as well as between MU and WA were most pronounced at t1 (Table 4). At t2, a trend toward a higher warmth after GI compared to WA can be seen (Table 4, Figure 2).
Figure 2.
Warmth perception (Herdecke warmth perceptions questionnaire, 0 = cold, 4 = hot) and infrared thermography at the face, hands, and feet at baseline (t0), post immersion (t1) and follow-up (t2) (means with 95% confidence interval).
Note. WA = water only condition; MU = mustard added to WA; GI = ginger added to water; ON = participants with oncological disorders; HC = healthy controls.
Neither the factor health status [F(1,32) = 1.07, P = .31] nor the interactions between health status and time [F(1,168) < 1] or between health status and footbath condition [F(2,173) = 1.07, P = .35] reached significance. However, the descriptive analysis showed lower warmth perceptions of the feet at t0 in ON compared to HC (Table 3). No differences between HC and ON were seen with respect to the warmth perception of the feet at t1 and t2 (Table 3) or with respect to the response to WA, GI and MU (Table 5, Figure 3).
Table 5.
Mean Differences Between Oncological Patients (ON, n = 23) and Healthy Controls (HC, n = 13) With Respect to the 3 Footbath Conditions WA, GI, and MU (Time Grouped Together).
| HC | ON | Diff | CI | ES | ||
|---|---|---|---|---|---|---|
| Warmth perception (as assessed by the Herdecke Warmth Perception Questionnaire) [0 = cold;4 = hot] | ||||||
| WA | Feet | 2.29 ± 1.03 | 2.25 ± 1.05 | 0.04 | (−0.37;0.45) | 0.04 |
| Face | 2.45 ± 0.59 | 2.57 ± 0.59 | −0.12 | (−0.35;0.12) | 0.20 | |
| Hands | 2.40 ± 0.96 | 2.63 ± 0.90 | −0.23 | (−0.60;0.15) | 0.25 | |
| Overall | 2.58 ± 0.68 | 2.52 ± 0.72 | 0.06 | (−0.22;0.34) | 0.09 | |
| GI | Feet | 2.70 ± 1.16 | 2.51 ± 1.12 | 0.19 | (−0.27;0.65) | 0.17 |
| Face | 2.53 ± 0.66 | 2.61 ± 0.58 | −0.08 | (−0.33;0.17) | 0.13 | |
| Hands | 2.35 ± 0.97 | 2.46 ± 0.75 | −0.11 | (−0.47;0.25) | 0.13 | |
| Overall | 2.55 ± 0.75 | 2.49 ± 0.78 | 0.06 | (−0.24;0.37) | 0.08 | |
| MU | Feet | 2.65 ± 1.10 | 2.35 ± 1.13 | 0.30 | (−0.14;0.74) | 0.27 |
| Face | 2.62 ± 0.72 | 2.60 ± 0.54 | 0.01 | (−0.25;0.28) | 0.02 | |
| Hands | 2.56 ± 0.90 | 2.41 ± 0.83 | 0.15 | (−0.20;0.50) | 0.18 | |
| Overall | 2.64 ± 0.84 | 2.55 ± 0.59 | 0.09 | (−0.21;0.40) | 0.14 | |
| Skin temperature (as assessed with a high-resolution IR camera in °C) | ||||||
| WA | Feet | 32.38 ± 2.53 | 32.20 ± 2.28 | 0.17 | (−0.80;1.15) | 0.07 |
| Face | 34.81 ± 0.79 | 34.33 ± 1.07 | 0.49 | (0.13;0.84) | 0.50 | |
| Hands | 33.48 ± 2.11 | 33.04 ± 2.71 | 0.45 | (−0.48;1.38) | 0.18 | |
| GI | Feet | 32.28 ± 2.46 | 32.04 ± 2.25 | 0.25 | (−0.71;1.20) | 0.11 |
| Face | 34.55 ± 0.99 | 34.27 ± 0.82 | 0.28 | (−0.09;0.65) | 0.32 | |
| Hands | 33.00 ± 2.21 | 32.68 ± 3.14 | 0.32 | (−0.71;1.35) | 0.11 | |
| MU | Feet | 33.25 ± 1.96 | 31.64 ± 2.33 | 1.62 | (0.78;2.45) | 0.73 |
| Face | 34.91 ± 0.75 | 33.83 ± 1.22 | 1.08 | (0.70;1.45) | 1.00 | |
| Hands | 33.98 ± 1.85 | 32.26 ± 2.70 | 1.72 | (0.84;2.59) | 0.71 | |
Note. Data are M ± SD (WA = water only condition, MU = mustard added to WA, GI = ginger added to WA, Diff = mean difference, CI = 95% confidence intervals, ES = Cohen’s d effect size). Bold indicates CI that do not contain zero.
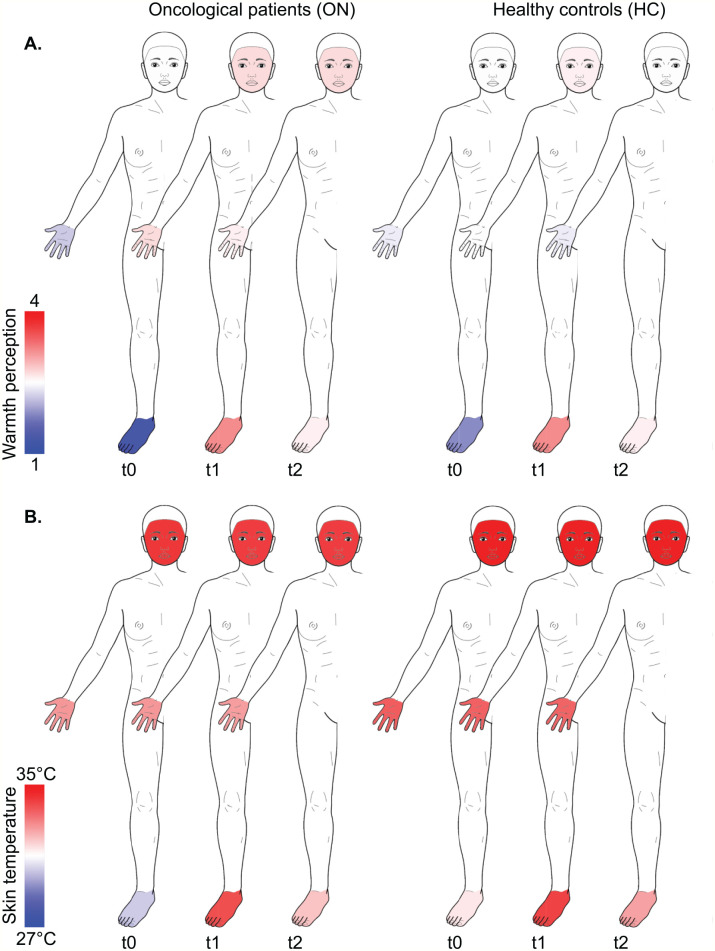
Figure 3.
Course of the warmth perceptions (Herdecke warmth perception questionnaire) (A) and skin temperature (infrared thermography) (B) of participants with oncological disorders (ON) and healthy controls (HC) at the face hands, and feet at baseline (t1), post immersion (t2) and follow-up (t3). Body template modified from Neubert and Beissner (2019): Hannover Body Template. figshare. DOI: 10.6084/m9.figshare.7637387.v5 under CC BY 4.0.
Secondary outcome measures (warmth perception, HeWEF)
The warmth perception of the face and hands as well as overall warmth did not differ between HC and ON at any of the 3 time-points (Table 3, Figure 3) or with respect to the 3 footbath conditions (Table 5). In addition, no differences were detected between WA, GI, and MU at the 3 time-points (Table 4).
Secondary outcome measures (skin temperature)
ON had significantly colder skin temperature of the face (at t0, t1, and t2) and of the feet (at t0 and t1) than HC (Table 3, Figure 3). Moreover, HC and ON showed different responses to the 3 footbath conditions, which was most pronounced after MU. For HC, the skin temperature of the face, hands and feet were highest after MU, whereas it was lowest for ON after the same condition (Table 5).
Success of Blinding
A total of 98 footbaths (WA: n = 35, MU: n = 33, GI: n = 30) were administered. After asking the participants to identify the predominant olfactory perceptions at t0, only one participant was able to name the correct ingredient (GI: n = 1). The most frequently reported olfactory perceptions were citrus (n = 58), eucalyptus (n = 11) and lavender (n = 23). No significant associations were found between GI and ginger olfactory perceptions [Mantel-Haenszel X2(1) < 1, P = .78] or between MU and mustard olfactory perceptions [Mantel-Haenszel X2(1) < 1, P = .98]. At t2, participants named the correct footbath condition in 55 of 98 possible cases (WA: n = 18, MU: n = 22, GI: n = 15).
Harms
A total of 31 AEs (t1: n = 12, t2: n = 19) were recorded. These included burning sensations of the skin (t1, WA: n = 2, MU: n = 3, GI: n = 3; t2, GI: n = 2), fatigue (t1, WA: n = 1, GI: n = 1; t2, WA: n = 2, MU: n = 2, GI: n = 1), circulatory problems (t1, WA: n = 1) and a worsening of chemotherapy side-effects (t2, WA: n = 1). All AEs resolved spontaneously; none required medical intervention.
Discussion
In our randomized controlled trial, we observed a higher warmth perception of the feet after GI and MU compared to WA directly after the footbaths. After the 10-minute follow-up period, the subjective warmth of the feet still tended to be higher with GI compared to WA, which suggests a longer-lasting effect of GI. Although, at baseline, ON were colder (self-reported and measured) at the feet than HC, footbaths enabled a comparable warming in both oncological patients and healthy participants. However, considering the 3 different footbath conditions, ON, and HC tended to have different response patterns of body skin temperature. In ON, skin temperature (at the feet, face and hands) was highest after WA and lowest after MU, whereas in HC, it was highest after MU (diametrical response).
In this study, ON were not generally colder than HC, but oncological patients were colder (self-reported and measured) at the feet potentially indicating a cancer-related sense of cold. 1 Although this sense of cold phenomenon has been often described by breast cancer patients, it was completely out of therapeutic focus for a long time. 37 This might be related to the fact that feeling cold is a normal, thermoregulatory response occurring in the process of thermal adaption under changing ambient temperature. 37 However, a persistent thermal discomfort despite behavioral efforts (e.g., change of clothing) may be indicative of an inability to optimally control body temperature through unconscious mechanisms (e.g., blood flow patterns). 1 The latter is triggered by the anterior hypothalamus that receives information about temperature changes by peripheral (skin) thermoreceptors and internal body temperature sensors. Autonomic thermodefensive responses aiming at heat conservation include cutaneous vasoconstriction and metabolic or shivering thermogenesis, whereas cutaneous vasodilatation, sweating or panting are natural, analogous responses for heat dissipation.38-40
Various pathological conditions can be involved in the establishment of a persistent sense of cold. These include ineffective heat production mechanisms, which can frequently occur in chronic diseases. 37 Maintaining thermal equilibrium is energetically costly.1,37 Thus, the energy base that is normally available for thermoregulation could be reduced in chronically debilitated patients, which could further lead to thermoregulatory defects such as cold extremities. 1 Another potentially contributing condition to thermal dysregulation is an abnormal pro-inflammatory cytokine activity (IL-1, IL-6, TNF-a) in cancer patients,1,37 which is not only linked to self-reported feelings of thermal discomfort, but also to a number of illness behaviors (e.g., fatigue), disease stage and progression. 1 Finally, thermoregulatory pathologies may occur as therapy-related side-effects. 41 In an animal model, an induction of an immune mediator-regulated hypothermia in response to harmful exposures was demonstrated. 1 Such declines in body temperature act as defense mechanisms by decreasing the toxin uptake rate and by limiting its conversion into the active intermediate. 1 Rustemova et al 6 observed that patients had perceptions of cold feelings in their chests and arms directly after chemotherapy, suggesting that chemotherapy is perceived and treated as a toxin by the human body. 1 In this study, 52% of ON received chemotherapy during the study participation, which may have contributed to their lower body temperature compared to HC. The lower values for self-reported warmth in ON may be further related to Endothelin-1 (ET-1), a peptide hormone that is overexpressed by several cancer cell lines1,37,42 and is also released in response to mental stress. 43 Interestingly ET-1 is not only linked to poor prognosis, 37 but also can alter temperature detection thresholds and thermal preferences among patients with cancer. 1
Being persistently cold could negatively impact the anti-tumor immune system potentially amounting to a poorer disease prognosis. 1 Thus, energy conserving interventional strategies such as frequent mild thermal therapies may be recommended in cancer treatment. 1 Warm footbaths are a potent method to induce local vasodilatation,38,44 impact the overall thermal response,8,9 improve immune status, 45 increase the parasympathetic nerve activity,13,45,46 and to contribute to pain relief in hospitalized cancer patients. 13 Thus, footbaths may impact thermoregulatory mechanisms in cancer patients in both the short-term (direct effects on blood flow) and the long-term (indirect effects via immune system adaptions and promotion of relaxation with decreasing levels of the stress hormone ET-1). Previous studies have suggested that warm footbaths with or without thermogenic substances cause a comparable increase in body temperature in healthy adults, adolescents and adolescent patients with anorexia nervosa.8,9 However, footbaths with the addition of the thermogenic agents such as mustard or ginger led to a higher increase in self-reported warmth with longer-lasting effects after GI, results observed in non-oncological subjects.8,9 The results of this study are in line with the previously reported effects of MU and GI, and may be attributed to the skin penetration of the active ingredients gingerols, shogaols, and allyl isothiocyanate.47,48 These ingredients activate cutaneous sensory nerve endings 49 by binding to TRP channels14,16 and through this mechanism can increase warmth perceptions over warm water alone. The active ingredients of ginger activate mainly TRPV1 (TRP vanilloid 1), 16 which is classified as a heat receptor. 15 The active ingredient of MU, however, activates both TRPV1 and TRPA1 (TRP ankyrin 1) 16 with the latter being classified as a cold receptor. 15 The differing TRP activation pattern may explain the slight difference between MU and GI for self-reported warmth perception. The finding of no differences between ON and HC with respect to the increase in self-reported warmth suggests that somatic thermosensitive afferents in ON react normally to the chemical activation of TRP receptors. Interestingly, local TRPV1 and TRPA1 receptors are also involved in the maintenance of ET-1-induced allodynia50,51 and pain-like behaviors.52,53 Despite the lack of health status-effect on changes in self-reported warmth, the response pattern of body temperature differed between ON and HC depending on the footbath condition received. The binding of the biological compounds of ginger and mustard to TRP channels precipitates the release of neuropeptides such as calcitonin gene-related peptide, which increases local cutaneous blood flow by inducing myocyte relaxation and vasodilatation.54,55 As body temperature was lowest after MU in ON, this suggests a differential reaction after TRP binding between ON and HC. Further research is needed to elucidate the exact mechanisms. It remains speculative whether the binding of the active ingredients of ginger and mustard to TRP channels may replace ET-1 from TRP binding sites. As ET-1 acts as a systemic vasoconstrictor, 37 a higher proportion of circulating ET-1 may be involved in the lower body temperature after MU compared to WA in ON.
In the present study, we strove to maintain stable room conditions. It is recommended that room temperatures remain constant between 22°C and 24°C when measuring skin temperature at the extremities with IR.21,56 Based on the mean room temperature of 23.77 ± 1.81°C and a humidity of 32.87% ± 7.59%, it can be assumed that we achieved acceptable conditions for infrared skin temperature measurements. However, as the sensation of thermal comfort differs between individuals, 39 we cannot affirm that all participants felt neutral comfort. For ON, the measurements took place in the hospital patient rooms, where it was more difficult to control the room conditions, for example, the room temperature (compared to the laboratory conditions for HC). Room temperature was considered in the primary analysis, but could have affected the results of the secondary outcome measures. Moreover, the overall difference in environmental conditions between a hospital room and a laboratory could have an impact on warmth perception. Beyond that, water temperature was not kept constant during the duration of the footbaths, as in other studies.8,9,46,57,58 According to Charkoudian, the degree of local vasodilatation is proportional to water temperature with the highest dilatation occurring at 42°C. 59 We applied a lower starting water temperature (M = 39.95 ± 0.28°C), thus, it remains unclear whether a higher and constant temperature would have accentuated or influenced our findings. Although local skin heating can induce rapid vasodilatation, the central autonomic drive dominates over external thermal influences. 38 Thus, in mildly hypothermic (oncological) patients, maximum vasodilatation can only be reached when some levels of hyperthermia are initially induced. 38 In other studies, a temperature shift on the skin surface was observed after footbaths with thermogenic substances (increase in foot, decrease in hand and face skin temperature),8,9 which could have originated from heat dissipation and evaporation from the skin to surrounding environment.44,60 In the current study, the skin temperature of the hands and face remained nearly constant over time, which may point to a limited local effect of footbaths on the body temperature of the treated area.
A study limitation is that we were not able to perform an exact sample size calculation. Given the small sample size and the pilot character of the study, the findings should be interpreted with caution. Due to short inpatient stays and scheduled hospital procedures, some ON received mistletoe therapy (IV) (WA: n = 3, MU: n = 3, GI: n = 1) or chemotherapy infusion (WA: n = 1) on the same day as the footbath intervention and we cannot estimate their direct influence on the outcome measures. In addition, the other treatments that patients received during their hospital stay, such as hyperthermia, might have had an effect on body temperature or warmth perception. With respect to the blinding status of study’s participants, the majority was able to name the correct footbath condition at t2. Previous studies8,9 have described the same challenges associated with blinding, in which direct application of mustard and ginger on the skin probably triggered an unblinding. This could have potentially influenced the assessment of the self-reported parameters at t2. Moreover, the questionnaire HeWEF is the only available measure to assess self-reported sensations of warmth,23,24 although it has limited psychometric support (article on validity has not been published yet). No internal validation for our selected statistical model was performed, which should be carried out in prospective evaluations. Future investigations should examine the long-term effects of footbaths on self-reported and measured warmth and quality of life, the effects of regular footbath applications on cancer pathogenesis, and work to identify key immune patterns that are related to body temperature changes and thermal discomfort in ON.
Conclusions
There is a great need for thermal therapies that alleviate oncological patients’ experience of thermal discomfort without affecting the efficacy of other cancer treatments. 1 Footbaths with thermogenic substances increased perceived warmth of the feet longer than warm water only in cancer patients and in healthy adults. Footbaths could therefore serve as a useful adjunctive treatment for the reduction of cancer-related sense of cold, which can easily and economically be applied in hospital and home settings.
Supplemental Material
Supplemental material, sj-pdf-1-ict-10.1177_15347354211058449 for Increasing Warmth in Oncological Patients: A Randomized Controlled Cross-Over Pilot Trial Examining the Efficacy of Mustard and Ginger Footbaths by Jan Vagedes, Silja Kuderer, Katrin Vagedes, Stefan Hiller, Florian Beissner, Henrik Szőke, Stefanie Joos and Ursula Wolf in Integrative Cancer Therapies
Acknowledgments
The authors thank Bernhard Deckers, Eduard Helmert and the entire team of the oncology ward of the Filderklinik (Filderstadt, Germany) for their significant contribution to this study as well as Elaine C. Meyer (Boston Children’s Hospital, Harvard Medical School, Boston, USA) for valuable discussion and proof-reading of the manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research received no external grant support from any funding agency in the public, commercial, or not-for-profit sectors. The study was financed by the non-profit ARCIM Institute.
ORCID iDs: Jan Vagedes  https://orcid.org/0000-0002-0021-088X
https://orcid.org/0000-0002-0021-088X
Silja Kuderer  https://orcid.org/0000-0001-6621-7219
https://orcid.org/0000-0001-6621-7219
Katrin Vagedes  https://orcid.org/0000-0002-4019-9531
https://orcid.org/0000-0002-4019-9531
Henrik Szőke  https://orcid.org/0000-0002-7243-5789
https://orcid.org/0000-0002-7243-5789
Data Availability: The data used to support the findings of this study are available from the corresponding author upon request.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Kokolus KM, Hong CC, Repasky EA. Feeling too hot or cold after breast cancer: is it just a nuisance or a potentially important prognostic factor? Int J Hyperthermia. 2010;26:662-680. doi: 10.3109/02656736.2010.507235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rudberg L, Carlsson M, Nilsson S, Wikblad K. Self-perceived physical, psychologic, and general symptoms in survivors of testicular cancer 3 to 13 years after treatment. Cancer Nurs. 2002;25:187-195. doi: 10.1097/00002820-200206000-00003 [DOI] [PubMed] [Google Scholar]
- 3. Johnson C, Eccles R. Acute cooling of the feet and the onset of common cold symptoms. Fam Pract. 2005;22:608-613. doi: 10.1093/fampra/cmi072 [DOI] [PubMed] [Google Scholar]
- 4. Lack LC, Gradisar M, Van Someren EJ, Wright HR, Lushington K. The relationship between insomnia and body temperatures. Sleep Med Rev. 2008;12:307-317. doi: 10.1016/j.smrv.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 5. Kräuchi K, Cajochen C, Werth E, Wirz-Justice A. Warm feet promote the rapid onset of sleep. Nature. 1999;401:36-37. doi: 10.1038/43366 [DOI] [PubMed] [Google Scholar]
- 6. Rüstemova D, Genc A, Bora G, Tur BS. A thermal dysregulation problem after breast cancer surgery; what could be? Medicine. 2017;96:e7027. doi: 10.1097/MD.0000000000007027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blazickova S, Rovenský J, Koska J, Vigas M. Effect of hyperthermic water bath on parameters of cellular immunity. Int J Clin Pharmacol Res. 2000;20:41-46. [PubMed] [Google Scholar]
- 8. Vagedes J, Helmert E, Kuderer S, et al. Effects of footbaths with mustard, ginger, or Warm water only on objective and subjective warmth distribution in healthy subjects: a randomized controlled trial. Complement Ther Med. 2018;41:287-294. doi: 10.1016/j.ctim.2018.09.024 [DOI] [PubMed] [Google Scholar]
- 9. Kuderer S, Helmert E, Szöke H, et al. Increasing warmth in adolescents with anorexia Nervosa: a randomized controlled crossover trial examining the efficacy of mustard and ginger footbaths. Evid Based Complement Alternat Med. 2020;2020:2416582. doi: 10.1155/2020/2416582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang HL, Chen XP, Lee KC, Fang FF, Chao YF. The effects of warm-water footbath on relieving fatigue and insomnia of the gynecologic cancer patients on chemotherapy. Cancer Nurs. 2010;33:454-460. doi: 10.1097/NCC.0b013e3181d761c1 [DOI] [PubMed] [Google Scholar]
- 11. Seyyedrasooli A, Valizadeh L, Zamanzadeh V, Nasiri K, Kalantri H. The effect of footbath on sleep quality of the elderly: a blinded randomized clinical trial. J Caring Sci. 2013;2:305-311. doi: 10.5681/jcs.2013.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Valizadeh L, Seyyedrasooli A, Zamanazadeh V, Nasiri K. Comparing the effects of reflexology and footbath on sleep quality in the elderly: a controlled clinical trial. Iran Red Crescent Med J. 2015;17:e20111. doi: 10.5812/ircmj.20111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamamoto K, Nagata S. Physiological and psychological evaluation of the wrapped Warm footbath as a complementary nursing therapy to induce relaxation in hospitalized patients with incurable cancer. Cancer Nurs. 2011;34:185-192. doi: 10.1097/NCC.0b013e3181fe4d2d [DOI] [PubMed] [Google Scholar]
- 14. Vriens J, Nilius B, Voets T. Peripheral thermosensation in mammals. Nat Rev Neurosci. 2014;15:573-589. doi: 10.1038/nrn3784 [DOI] [PubMed] [Google Scholar]
- 15. Castillo K, Diaz-Franulic I, Canan J, Gonzalez-Nilo F, Latorre R. Thermally activated TRP channels: molecular sensors for temperature detection. Phys Biol. 2018;15:021001. doi: 10.1088/1478-3975/aa9a6f [DOI] [PubMed] [Google Scholar]
- 16. Gregersen NT, Belza A, Jensen MG, et al. Acute effects of mustard, horseradish, black pepper and ginger on energy expenditure, appetite, ad libitum energy intake and energy balance in human subjects. Br J Nutr. 2013;109:556-563. doi: 10.1017/S0007114512001201 [DOI] [PubMed] [Google Scholar]
- 17. Rahman S, Salehin F, Iqbal A. In vitro antioxidant and anticancer activity of young Zingiber officinale against human breast carcinoma cell lines. BMC Complement Altern Med. 2011;11:76. doi: 10.1186/1472-6882-11-76 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Rhode J, Fogoros S, Zick S, et al. Ginger inhibits cell growth and modulates angiogenic factors in ovarian cancer cells. BMC Complement Altern Med. 2007;7:44. doi: 10.1186/1472-6882-7-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sávio AL, da Silva GN, Salvadori DM. Inhibition of bladder cancer cell proliferation by allyl isothiocyanate (mustard essential oil). Mutat Res. 2015;771:29-35. doi: 10.1016/j.mrfmmm.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 2010;63:e1-37. doi: 10.1016/j.jclinepi.2010.03.004 [DOI] [PubMed] [Google Scholar]
- 21. Fernández-Cuevas I, Bouzas Marins JC, Arnáiz Lastras J, et al. Classification of factors influencing the use of infrared thermography in humans: a review. Infrared Phys Technol. 2015;71:28-55. doi: 10.1016/j.infrared.2015.02.007 [DOI] [Google Scholar]
- 22. Marins JCB, Moreira DG, Cano SP, et al. Time required to stabilize thermographic images at rest. Infrared Phys Technol. 2014;65:30-35. doi: 10.1016/j.infrared.2014.02.008 [DOI] [Google Scholar]
- 23. Edelhäuser F, Bräuer M, Bovelet M, Büssing A. Entwicklung und Evaluation eines Fragebogens zur Selbstwahrnehmung der Wärmeorganisation. Poster presented at: 4th European Congress for Integrative Medicine; October 7–8, 2011, Berlin, Germany. [Google Scholar]
- 24. Edelhäuser F, Bovelet M, Heusser P, Cysarz D, Büssing A. Measures of physical and emotional warmth and coldness. Eur J Integr Med. 2010;2:208-209. doi: 10.1016/j.eujim.2010.09.077 [DOI] [Google Scholar]
- 25. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. doi: 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 26. Waldmann A, Schubert D, Katalinic A. Normative data of the EORTC QLQ-C30 for the German population: a population-based survey. PLoS One. 2013;8:e74149. doi: 10.1371/journal.pone.0074149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harju E-L. Cold and warmth perception mapped for age, gender, and body area. Somatosens Mot Res. 2002;19:61-75. doi: 10.1080/08990220120113057 [DOI] [PubMed] [Google Scholar]
- 28. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2020. https://www.R-project.org/ [Google Scholar]
- 29. RStudio Team. RStudio: Integrated Development Environment for R. RStudio, PBC; 2020. http://www.rstudio.com/ [Google Scholar]
- 30. Buuren SV, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations inR. J Stat Softw. 2011;45:1-67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 31. Wellek S, Blettner M. On the proper use of the crossover design in clinical trials: part 18 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2012;109:276-281. doi: 10.3238/arztebl.2012.0276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models usinglme4. J Stat Softw. 2015;67:1-48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 33. Fabozzi FJ, Focardi SM, Rachev ST, Arshanapalli BG. Model selection criterion: AIC and BIC—appendix E. The Basics of Financial Econometrics: Tools, Concepts, and Asset Management Applications. John Wiley & Sons, Inc; 2014;399-403. [Google Scholar]
- 34. Kuznetsova A, Brockhoff PB, Christensen RHB. LmerTest package: tests in linear mixed effects models. J Stat Softw. 2017;82:1-26. doi: 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- 35. Torchiano M. Effsize: Efficient Effect Size Computation. R Package Version 0.8.1 [Computer Program]. 2020. https://CRAN.R-project.org/package=effsize.
- 36. Carlsson M, Arman M, Backman M, Flatters U, Hatschek T, Hamrin E. A five-year follow-up of quality of life in women with breast cancer in anthroposophic and conventional care. Evid Based Complement Alternat Med. 2006;3:523-531. doi: 10.1093/ecam/nel042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Golubnitschaja O. Feeling cold and other underestimated symptoms in breast cancer: anecdotes or individual profiles for advanced patient stratification? EPMA J. 2017;8:17-22. doi: 10.1007/s13167-017-0086-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Taylor NA, Machado-Moreira CA, van den Heuvel AM, Caldwell JN. Hands and feet: physiological insulators, radiators and evaporators. Eur J Appl Physiol. 2014;114:2037-2060. doi: 10.1007/s00421-014-2940-8 [DOI] [PubMed] [Google Scholar]
- 39. Yao Y, Lian Z, Liu W, Shen Q. Experimental study on physiological responses and thermal comfort under various ambient temperatures. Physiol Behav. 2008;93:310-321. doi: 10.1016/j.physbeh.2007.09.012 [DOI] [PubMed] [Google Scholar]
- 40. Siemens J, Kamm GB. Cellular populations and thermosensing mechanisms of the hypothalamic thermoregulatory center. Pflugers Arch. 2018;470:809-822. doi: 10.1007/s00424-017-2101-0 [DOI] [PubMed] [Google Scholar]
- 41. Noh GO, Park KS. Effects of aroma self-foot reflexology on peripheral neuropathy, peripheral skin temperature, anxiety, and depression in gynaecologic cancer patients undergoing chemotherapy: a randomised controlled trial. Eur J Oncol Nurs. 2019;42:82-89. doi: 10.1016/j.ejon.2019.08.007 [DOI] [PubMed] [Google Scholar]
- 42. Hans G, Deseure K, Robert D, De Hert S. Neurosensory changes in a human model of endothelin-1 induced pain: a behavioral study. Neurosci Lett. 2007;418:117-121. doi: 10.1016/j.neulet.2007.03.008 [DOI] [PubMed] [Google Scholar]
- 43. Toda N, Nakanishi-Toda M. How mental stress affects endothelial function. Pflugers Arch. 2011;462:779-794. doi: 10.1007/s00424-011-1022-6 [DOI] [PubMed] [Google Scholar]
- 44. Yu L, Su B, Wang X, Li M, Ma W. Experimental study on skin temperature and thermal response of the foot-bather. J Therm Anal Calorim. 2016;123:2507-2516. doi: 10.1007/s10973-015-5063-5 [DOI] [Google Scholar]
- 45. Saeki Y, Nagai N, Hishinuma M. Effects of footbathing on autonomic nerve and immune function. Complement Ther Clin Pract. 2007;13:158-165. doi: 10.1016/j.ctcp.2006.12.006 [DOI] [PubMed] [Google Scholar]
- 46. Yamamoto K, Aso Y, Nagata S, Kasugai K, Maeda S. Autonomic, neuro-immunological and psychological responses to wrapped warm Footbaths–a pilot study. Complement Ther Clin Pract. 2008;14:195-203. doi: 10.1016/j.ctcp.2008.04.001 [DOI] [PubMed] [Google Scholar]
- 47. Doering TJ, Brix J, Steuernagel B, Konitzer M, Schneider B, Fischer GC. Pilot-Senffussbad-Studie unter besonderer Berücksichtigung der zerebralen Blutflussgeschwindigkeit. Complement Med Res. 1998;5:279-282. doi: 10.1159/000021160 [DOI] [Google Scholar]
- 48. Khare CP. Brassica nigra (linn.) koch. Indian Medicinal Plants: An Illustrated Dictionary. Springer; 2007;101. [Google Scholar]
- 49. Jordt SE, Bautista DM, Chuang HH, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260-265. doi: 10.1038/nature02282 [DOI] [PubMed] [Google Scholar]
- 50. Balonov K, Khodorova A, Strichartz GR. Tactile allodynia initiated by local subcutaneous endothelin-1 is prolonged by activation of TRPV-1 receptors. Exp Biol Med. 2006;231:1165-1170. [PubMed] [Google Scholar]
- 51. Nodai T, Hitomi S, Ono K, et al. Endothelin-1 elicits TRP-mediated pain in an acid-induced oral ulcer model. J Dent Res. 2018;97:901-908. doi: 10.1177/0022034518762381 [DOI] [PubMed] [Google Scholar]
- 52. Kawamata T, Ji W, Yamamoto J, et al. Involvement of transient receptor potential vanilloid subfamily 1 in endothelin-1-induced pain-like behavior. Neuroreport. 2009;20:233-237. doi: 10.1097/WNR.0b013e32831befa5 [DOI] [PubMed] [Google Scholar]
- 53. Liang J, Bi H, Ji W. Involvement of TRPA1 in ET-1-induced pain-like behavior in mice. Neuroreport. 2010;21:201-205. doi: 10.1097/WNR.0b013e328335b3c5 [DOI] [PubMed] [Google Scholar]
- 54. Pozsgai G, Hajna Z, Bagoly T, et al. The role of transient receptor potential ankyrin 1 (TRPA1) receptor activation in hydrogen-sulphide-induced CGRP-release and vasodilation. Eur J Pharmacol. 2012;689:56-64. doi: 10.1016/j.ejphar.2012.05.053 [DOI] [PubMed] [Google Scholar]
- 55. Earley S. TRPA1 channels in the vasculature. Br J Pharmacol. 2012;167:13-22. doi: 10.1111/j.1476-5381.2012.02018.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ring E, Ammer K. The technique of infrared imaging in medicine. Thermol. 2000;10:7-14. doi: 10.1088/978-0-7503-1143-4ch1 [DOI] [Google Scholar]
- 57. Saeki Y. The effect of foot-bath with or without the essential oil of lavender on the autonomic nervous system: a randomized trial. Complement Ther Med. 2000;8:2-7. doi: 10.1016/s0965-2299(00)90703-9 [DOI] [PubMed] [Google Scholar]
- 58. Toki K, Yamai T, Fukai K. Skin temperature changes during a footbath in patients who had had a stroke with consequent sensory impairment. Jpn J Nurs Sci. 2015;12:276-286. doi: 10.1111/jjns.12066 [DOI] [PubMed] [Google Scholar]
- 59. Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc. 2003;78:603-612. doi: 10.4065/78.5.603 [DOI] [PubMed] [Google Scholar]
- 60. Ohnari H. Effect of microbubble bathing of lower extremities on peripheral circulation. Bull Yamaguchi Med Sch. 2010;57:25-32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ict-10.1177_15347354211058449 for Increasing Warmth in Oncological Patients: A Randomized Controlled Cross-Over Pilot Trial Examining the Efficacy of Mustard and Ginger Footbaths by Jan Vagedes, Silja Kuderer, Katrin Vagedes, Stefan Hiller, Florian Beissner, Henrik Szőke, Stefanie Joos and Ursula Wolf in Integrative Cancer Therapies