Abstract
Objective
Associations between serum lipids and their individual components with premenopausal breast cancer risk are unclear. This meta-analysis summarized the literature on serum lipids and premenopausal breast cancer risk to elucidate their relationship.
Methods
Eligible studies were identified by searching the PubMed, Embase, China National Knowledge Infrastructure, and Wanfang databases until 31 December 2020. Standardized mean difference (SMD) scores with 95% confidence intervals (95%CIs) were used to assess the impact of serum lipids on premenopausal breast cancer risk. The I2 statistic was calculated to measure the percentage of heterogeneity, and Egger’s test was performed to measure publication bias.
Results
Thirteen studies were included. The SMD scores of triglycerides (TG) and low-density lipoprotein cholesterol (LDL-C) were 12.90 (95%CI: 7.19–18.61) and 31.43 (95%CI: 8.72–54.15), respectively. The SMD scores of total cholesterol (TC) and high-density lipoprotein cholesterol (HDL-C) were not significantly different between the groups. The included studies were highly heterogeneous. There were no publication biases found in TC, LDL-C, or HDL-C analyses, whereas publication bias was present in the TG analysis.
Conclusions
TG and LDL-C were higher in premenopausal breast cancer patients than in women without breast cancer. However, no significant differences were found in TC or HDL-C levels.
Keywords: Premenopausal breast cancer, serum lipid, meta-analysis, triglyceride, low-density lipoprotein cholesterol, tumor biomarker
Introduction
Carcinoma of the breast is the most common cancer in women worldwide. 1 Epidemiologic evidence has suggested that metabolic syndrome is associated with a higher risk of breast cancer.2–4 Metabolic syndrome represents a complex group of metabolic disorders including obesity, hyperglycemia, hypertension, and dyslipidemia. 5 Although dyslipidemia is a component of metabolic syndrome, its relationship with breast cancer risk remains unclear.
Dyslipidemia is a cluster of lipoprotein abnormalities that is defined by the presence of one of the following: high levels of triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), or low levels of high-density lipoprotein cholesterol (HDL-C). The primary metabolite of cholesterol, 27-Hydroxycholesterol (27-HC), is an endogenous selective estrogen receptor modulator (SERM) with estrogen-like effects; it can induce the proliferation of estrogen receptor-positive breast cancer cells and can facilitate metastasis.6,7 Laboratory studies have indicated that cholesterol is a component of lipid rafts, which act as platforms for cell signaling, invasion, and migration.8,9 LDL-C and HDL-C regulate cholesterol levels by mediating cholesterol transport between the liver and blood and the transport of 27-HC through esterification; 10 thus, they may have an indirect effect on breast cancer progression. Meanwhile, LDL-C can trigger the proliferation and migration of cancer cells by activating the P38 signaling pathway, which is related to breast cancer development. 11 Furthermore, increased HDL-C content can lead to increased expression of anti-inflammatory cytokines such as interleukin-10 and decreased expression of insulin-like growth factor-I; the former protects against breast cancer, whereas the latter promotes carcinogenesis.12–14 The diversity of lipid components and the complex mechanisms of each component has made elucidating the relationship between blood lipids and breast cancer difficult.
Menstrual status can also affect serum lipids. LDL-C and TG levels are lower in premenopausal women compared with in postmenopausal women, whereas HDL-C levels show the opposite trend.15,16 Therefore, the role of lipoproteins in breast cancer risk may vary according to menopausal status, and several studies support this conclusion.17,18 To our knowledge, this is the first meta-analysis to explore the relationship between serum lipids and breast cancer risk only in premenopausal women.
Materials and methods
This meta-analysis was registered with INPLASY in September 2021 (registration number ID: INPLASY202190011), and the DOI number is 10.37766/inplasy2021.9.0011. This meta-analysis was performed in accordance with the PRISMA 2020 guidelines. 19
Search strategy
We searched the PubMed, Embase, China National Knowledge Infrastructure, and Wanfang databases without restrictions for articles published until 31 December 2020. The following keywords were used for the search: “breast cancer,” “breast carcinoma,” “breast neoplasm,” “serum lipid,” “blood lipid,” “dyslipidemia,” “lipoprotein,” “triglyceride,” and “cholesterol.” Furthermore, we also reviewed the reference lists of all retrieved articles to identify additional studies.
Inclusion and exclusion criteria
Relevant studies were selected if they fulfilled the following criteria: 1) all subjects in the case group were histopathologically diagnosed as having primary breast cancer before menopause and had not started any antitumor treatments; and 2) the risk factors explored included at least one of the selected lipid components (TG, TC, LDL-C, and HDL-C). The study was excluded if included patients had primary hyperlipidemia, metabolic syndrome, or were taking drugs or hormones that affect lipid metabolism.
Data extraction and quality assessment
Two authors (JW and WL) independently assessed study eligibility and extracted data, any disagreements were resolved by consensus. If the study met the inclusion criteria, the following data were extracted: author, year of publication, country, study design, sample size, and mean ± standard deviation (SD) of serum lipids in the case and control groups.
The nine-star Newcastle–Ottawa Scale (NOS) 20 for case–control studies was used to assess methodological quality. NOS consists of three major aspects: selection, comparability, and exposure/outcome. High-quality studies were defined as those with seven or more stars.
Statistical analysis
We used STATA version 15.1 (STATA Corp., College Station, TX, USA) for all analyses. As the explored exposure factors (serum lipids) were measurement data, standardized mean difference (SMD) scores with 95% confidence intervals (CIs) were calculated. The I2 statistic was calculated to measure the percentage of heterogeneity, with I2 values of 25%, 50%, and 75% corresponding to low, medium, and high data heterogeneity, respectively. When substantial heterogeneity was detected, random effect models were used. Otherwise, fixed effect models were used to assess the effect of the size of the included studies in the meta-analysis. Egger’s tests were performed to measure publication bias. A p-value <0.05 was considered statistically significant.
Ethical approval
This study was a meta-analysis, and all data were extracted from previously published articles. Thus, obtaining informed consent was not required.
Results
Literature search results
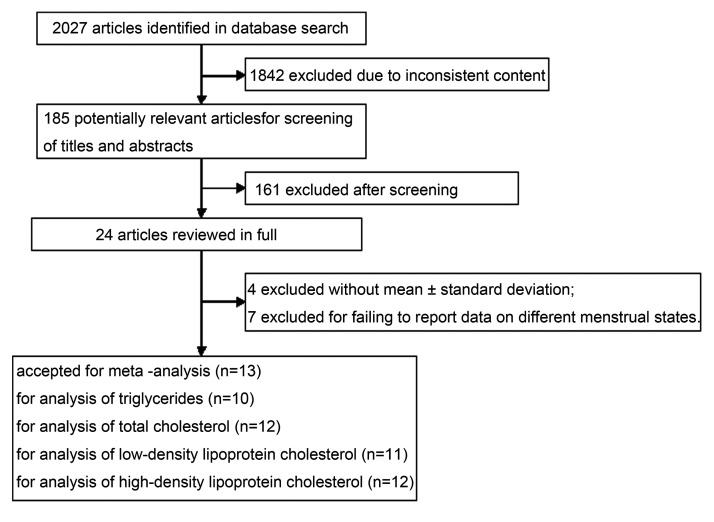
The process for the literature search and study selection is displayed in a flow diagram in Figure 1. A total of 2027 articles were identified through the search strategy, among which 1842 were excluded because their contents did not meet the purpose of our study. After screening the titles and abstracts of 185 potentially relevant articles, 24 full texts were reviewed for eligibility. Ultimately, after excluding articles whose data did not contain the mean ± standard deviation or did not report the blood lipid data of premenopausal and postmenopausal women separately, 13 studies were included in this systematic review.
Figure 1.
Flow diagram depicting the literature search and study selection.
Study characteristics
Characteristics of the 13 included articles21–33 are listed in Table 1. NOS scores of all included articles were seven stars; thus, they were all considered to be of high quality. Four articles21–23,25 were published in Chinese, and the remaining seven were published in English. The 10 articles that analyzed TG included 915 premenopausal women with breast cancer as the case group and 1779 healthy premenopausal women as the control group. The 12 articles that analyze TC included 1202 women in the case group and 1943 women in the control group. The 11 studies that analyzed LDL-C included 1142 and 1913 women in the case and control groups, respectively. For HDL-C analysis, there were 1279 cases enrolled in 12 studies with 2051 control subjects.
Table 1.
Characteristics of the 13 included articles.
| Author | Country | Design | Number of cases | Number of controls | Exposure | Case group mean ± SD (mg/dL) |
Control group mean ± SD (mg/dL) |
NOS score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TG | TC | LDL-C | HDL-C | TG | TC | LDL-C | HDL-C | |||||||
| Yang LN (2020) | China | case–control study | 99 | 91 | TG, TC, LDLC, HDLC | 120.50 ± 50.50 | 190.21 ± 33.25 | 102.45 ± 41.37 | 56.44 ± 12.76 | 100.12 ± 51.39 | 154.64 ± 21.56 | 96.26 ± 30.16 | 61.47± 15.85 | 7 |
| Jing GM (2019) | China | case–control study | 38 | 37 | TG, TC, LDLC, HDLC | 185.17 ± 46.07 | 247.04 ± 23.58 | 111.34 ± 11.21 | 62.63 ± 10.05 | 155.05 ± 52.27 | 247.04 ± 18.94 | 97.42 ± 11.21 | 72.29 ± 11.60 | 7 |
| Huang Z (2019) | China | case–control study | 190 | 104 | TC, LDLC, HDLC | NR | 178.61 ± 36.34 | 100.90 ± 17.78 | 39.05 ± 11.21 | NR | 190.59 ± 33.63 | 97.81 ± 19.72 | 48.33 ± 13.92 | 7 |
| Akalanka HMK (2018) | Sri Lanka | case–control study | 50 | 30 | TG, TC, LDLC, HDLC | 122 ± 58 | 226 ± 40 | 158 ± 34 | 43 ± 10 | 108 ± 45 | 178 ± 23 | 111 ± 23 | 44 ± 8.3 | 7 |
| Wei LJ (2016) | China | case–control study | 498 | 1423 | TG, TC, LDLC, HDLC | 100.12 ± 3.54 | 185.18 ± 1.55 | 127.58 ± 22.42 | 61.86 ± 0.39 | 97.46 ± 0.89 | 185.18 ± 0.77 | 88.92 ± 0.77 | 62.24 ± 0.39 | 7 |
| Kumar V (2015) | India | case–control study | 50 | 50 | TG, TC, LDLC, HDLC | 120.94 ± 26.338 | 183.76 ± 40.485 | 105.22 ± 23.406 | 40.20 ± 5.115 | 105.60 ± 37.460 | 142.04 ± 29.965 | 82.72 ± 29.552 | 38.06 ± 6.264 | 7 |
| Kakaiya A (2013) | India | case–control study | 27 | 27 | TG, TC, LDLC, HDLC | 110.96 ± 33.82 | 164.42 ± 22.90 | 95.654 ± 19.99 | 46.58 ± 10.893 | 94.28 ± 40.063 | 173.06 ± 32.124 | 101.172 ± 25.2600 | 52.59 ± 10.933 | 7 |
| Abdelsalam KE (2012) | Sudan | case–control study | 97 | 60 | TC, LDLC, HDLC | NR | 283.7 ± 8.4 | 193.1 ±7.3 | 50 ± 2.7 | NR | 153.6 ±1.5 | 100.1 ± 2.2 | 48.3 ± 4.1 | 7 |
| Peela JR (2012) | Libya | case–control study | 25 | 21 | TG, TC, LDLC, HDLC | 111.6 ± 49.17 | 177.5 ± 29.65 | 109.62 ± 29.23 | 44.3 ± 12.19 | 99.28 ± 31.10 | 152.38 ± 35.39 | 98.33 ± 33.92 | 33.33 ± 9.99 | 7 |
| Yadav NK (2012) | Nepal | case–control study | 25 | 25 | LDLC, HDLC | 120.52 ± 12.92 | 192.24 ± 10.60 | 112.24±8.55 | 45.76 ± 3.82 | 108.28 ± 13.08 | 159.80 ± 6.69 | 111.80 ± 24.44 | 46.12 ± 4.08 | 7 |
| Owiredu WK (2009) | Ghana | case–control study | 43 | 45 | TG, TC, LDLC, HDLC | 102.65 ± 51.14 | 202 ± 64.7 | 116.62 ± 49.73 | 55.61 ± 19.62 | 92.01 ± 37.01 | 173.52 ± 34 | 95.20 ± 32.20 | 59.33 ± 21.41 | 7 |
| Abu-Bedair FA (2003) | Egypt | case–control study | 35 | 15 | TG, TC | 107 ± 6.5 | 234 ± 5.6 | NR | NR | 94 ± 6.7 | 207 ± 6.8 | NR | NR | 7 |
| Abu-Bedair FA (2003) | Egypt | case–control study | 25 | 15 | TG, TC | 116 ± 6.8 | 244 ± 8.5 | NR | NR | 103 ± 8.6 | 201 ± 6.6 | NR | NR | 7 |
| Ferraroni M (1993) | Italy | case–control study | 137 | 138 | HDLC | NR | NR | NR | 56.8 ± 5.08 | NR | NR | NR | 57.2 ± 4.79 | 7 |
TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; NOS, Newcastle–Ottawa scale; NR, not reported.
Relationship between serum lipids and premenopausal breast cancer risk
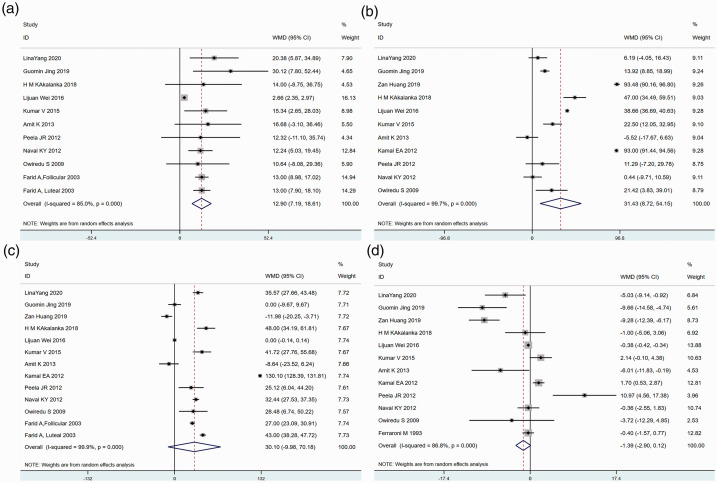
Figure 2 shows the relationships between the four serum lipid components and the risk of premenopausal breast cancer from our meta-analyses. The SMD of TG was 12.90 (95% CI: 7.19–18.61) (Figure 2a), indicating that TG levels of premenopausal breast cancer patients were significantly higher than those of healthy premenopausal women. Similarly, a significant increase in LDL-C (SMD 31.43, 95% CI: 8.72–54.15) was detected in the case group compared with the control group (Figure 2b). Although premenopausal women with breast cancer tended to have lower TC and higher HDL-C than those without breast cancer, no significant differences were found in TC (SMD 31.10, 95% CI: −9.98–70.18) or HDL-C (SMD −1.39, 95% CI: −2.90–0.12) between the two groups (Figure 2c and 2d).
Figure 2.
Forest plot depicting the standardized mean difference (SMD) scores and 95% confidence intervals for studies comparing the serum lipid levels of premenopausal breast cancer patients with those of healthy women: (a) triglycerides (TG), (b) low-density lipoprotein cholesterol (LDL-C), (c) total cholesterol (TC), and (d) high-density lipoprotein cholesterol (HDL-C).
According to demographic characteristics described in the 13 included articles, four studies included an African population,28,29,31,32 eight included an Asian population,21–27,30 and one had a European population. 33 Subgroup analyses were performed based on the African and Asian populations (Table 2). In the African population, only TG levels of premenopausal breast cancer patients were significantly higher than in the healthy premenopausal population. However, in the Asian population, TG, TC and LDL-C levels were all significantly higher in the case group than in the control group, while HDL-C levels showed the opposite trend.
Table 2.
Subgroup analysis based on different ethnic groups.
| Subgroup | Number of studies | Number of patients |
SMD (95% CI) | Heterogeneity |
||
|---|---|---|---|---|---|---|
| Case | Control | I2 (%) | P | |||
| TG | ||||||
| African | 3 | 128 | 91 | 12.92 (9.84–16.01) | 0.00 | 0.996 |
| Asian | 7 | 787 | 1683 | 13.54 (5.57–21.51) | 76.0 | <0.001 |
| LDL-C | ||||||
| African | 3 | 165 | 126 | 42.40 (−18.89–103.69) | 98.5 | <0.001 |
| Asian | 8 | 977 | 1787 | 27.26 (2.49–52.03) | 99.4 | <0.001 |
| TC | ||||||
| African | 4 | 225 | 156 | 50.98 (−7.08–109.04) | 99.9 | <0.001 |
| Asian | 8 | 977 | 1787 | 16.94 (2.00–31.87) | 97.9 | <0.001 |
| HDL-C | ||||||
| African | 3 | 165 | 126 | 3.22 (−3.47–9.91) | 78.8 | 0.009 |
| Asian | 8 | 977 | 1787 | −3.18 (−5.64–−0.72) | 88 | <0.001 |
TG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; SMD, standardized mean difference; CI, confidence interval.
Percentage of heterogeneity and publication bias
The studies selected in this analysis were performed in different countries and with different populations. The I2 values of all four blood lipid components were >75% (TG: 85.0%, LDL-C: 99.9%, TC: 99.9%, and HDL-C: 86.8%) (Figure 2). Thus, the studies included in this meta-analysis were highly heterogeneous, and random effect models were used to estimate summary hazard ratios (HRs) and 95% CIs. Considering the small study effect, publication bias was tested using Egger’s test. No evidence of publication bias was found in the TC (P = 0.189), LDL-C (p = 0.107), or HDL-C (p = 0.575) analyses, whereas evidence of publication bias was found in the TG (p = 0.001) analysis.
Discussion
We performed this meta-analysis to determine whether serum lipids are a risk factor for premenopausal breast cancer. The results support the hypothesis that a significant positive association exists between dyslipidemia (high levels of TG and LDL-C) and premenopausal breast cancer risk. In Asian populations, all four indicators of dyslipidemia (high levels of TG, LDL-C, TC, and low levels of HDL-C) were considered risk factors for premenopausal breast cancer. Most of the studies included in this meta-analysis included Asian or African populations, probably because the peak age of breast cancer varies in different regions. Epidemiological evidence has shown that the peak age of breast cancer in Asia and Africa is earlier than in western countries.34,35 For example, in Asian countries, the peak age of breast cancer ranges from 40 to 50 years old, whereas in western countries, the peak age ranges from 60 to 70 years old. 36
Compared with the other lipid components, relatively few studies have been conducted on the relationship between breast cancer risk and TG. Some studies have indirectly reflected the relationship between TG and breast cancer risk by confirming that a high-fat diet can increase breast cancer risk.37,38 As early as the 1990s, Goodwin et al. 39 found that elevated triglyceride levels were an independent risk factor for premenopausal breast cancer. Han et al. 40 also reported that elevated serum TG levels might be correlated with an increased risk of breast cancer. Four studies included in our meta-analysis reached the same conclusion.22,26,30,31 A statistically significant difference was found in the TG concentrations between premenopausal breast cancer patients and healthy women, with patients having higher TG levels; this was consistent in African and Asian populations.
Cholesterol plays an important role in the etiology of cardiovascular disease. High levels of TC and LDL and low levels of HDL are adverse factors that lead to cardiovascular disease.41,42 Changes in these three lipids are also risk factors for breast cancer, However, a review of related studies still cannot provide a clear picture because the results are inconsistent. A large prospective study showed a correlation between serum TC and breast cancer. Women with TC levels >240 mg/dL reportedly had a higher risk of developing breast cancer than those with TC levels <160 mg/dL. 43 In contrast, another large prospective study that included 288,057 female participants suggested that low serum TC was a favorable factor for the prevention of breast cancer. 44 A study of 38,823 Norwegian women found a 25% increased risk of breast cancer in the low HDL-C group compared with in the high HDL-C group. 45 A meta-analysis conducted by Melvin et al. 46 showed that increasing TC and decreasing HDL were associated with an increased cancer risk (including breast cancer) of 18% and 15%, respectively.
In 2015, two meta-analyses arrived at different conclusions regarding the relationship between these three lipids and breast cancer risk. One observed a modest inverse association between TC and HDL-C and the risk of breast cancer after considering studies that eliminated preclinical bias, and no association was found between LDL-C and breast cancer risk. 47 The other meta-analysis suggested that serum HDL-C may protect against breast carcinogenesis among postmenopausal women but not premenopausal women, whereas serum TC and LDL-C levels may be not associated with breast cancer risk. 48 Unfortunately, most of these articles did not separately discuss the premenopausal and postmenopausal populations. In this meta-analysis, elevated LDL-C was found to be an adverse factor for premenopausal women, but higher TC and lower HDL levels were not related to the risk of premenopausal breast cancer. Notably, our subgroup analyses confirmed that TC and LDL-C were modest but statistically significant unfavorable factors, whereas HDL-C was a favorable factor for premenopausal breast cancer patients in the Asian population. No significant relationship was found between TC, LDL-C, or HDL-C and premenopausal breast cancer risk in the African population.
Our meta-analysis had several limitations. First, all studies included were of high quality according to the NOS assessment; however, the outcomes in the four components of serum lipids were moderate either because of high heterogeneity or the limited number of case–control trials available for analysis. Additionally, the populations studied were mostly from Asia, followed by Africa. Data from European and American countries were lacking. Second, because studies with positive results tended to be published, especially case–control studies, publication bias might be a concern. In particular, when the number of articles included in the meta-analysis was small, tests for publication bias tended to lack statistical power. Finally, some relevant articles might not have been included in the analysis due to the different ways data are evaluated in various articles. For example, studies were excluded if the data did not report the mean ± SD or failed to separately list the premenopausal and postmenopausal women.
The etiology of breast cancer is multifactorial. Thus, an increase or decrease in isolated cholesterol may not be the decisive factor for carcinogenesis. The results of our meta-analysis should be interpreted with caution, because the number of studies was extremely limited. Many studies have focused on postmenopausal women with breast cancer. We hope to find more large-scale prospective clinical studies that are focused on breast cancer in premenopausal women in the future.
Conclusion
To our knowledge, no previous meta-analysis has specifically evaluated the role of dyslipidemia in assessing breast cancer risk in premenopausal women. TG and LDL-C levels were higher in premenopausal breast cancer patients than in healthy premenopausal women. High serum TG and LDL-C levels may be associated with increased premenopausal breast cancer risk. Among Asian women, serum HDL-C may play a protective role, whereas TG, TC, and LDL-C may promote carcinogenesis in premenopausal breast.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This work was financially supported by the Chongqing Municipal Education Commission Science and Technology Research Project (KJQN201800109), Chongqing Science and Health Joint Medical Research Project (2021MSXM222), National Cancer Center Climbing Fund (NCC201922B01), Central University’s basic scientific research business fee medical integration project (2019CDYGYB004), and Chongqing Science and Technology Bureau Project (cstc2018jxjl130044).
ORCID iD: Wei Li https://orcid.org/0000-0002-9003-732X
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Lubian Lopez DM, Castillo Lara M, Rodriguez B, et al. Metabolic syndrome and prognostic factors in postmenopausal breast cancer patients. Breast J 2019; 25: 548–551. [DOI] [PubMed] [Google Scholar]
- 3.Ekinci O, Eren T, Kurtoglu Yakici M, et al. Relationship between metabolic syndrome and postmenopausal breast cancer. Cir Esp (Engl Ed) 2020; 98: 540–546. [DOI] [PubMed] [Google Scholar]
- 4.Agnoli C, Grioni S, Sieri S, et al. Metabolic syndrome and breast cancer risk: a case-cohort study nested in a multicentre Italian cohort. PLoS One 2015; 10: e0128891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 6.McDonnell DP Chang CY andNelson ER.. The estrogen receptor as a mediator of the pathological actions of cholesterol in breast cancer. Climacteric 2014; 17: 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cedo L, Reddy ST, Mato E, et al. HDL and LDL: potential new players in breast cancer development. J Clin Med 2019; 8: 853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danilo C andFrank PG.. Cholesterol and breast cancer development. Curr Opin Pharmacol 2012; 12: 677–682. [DOI] [PubMed] [Google Scholar]
- 9.Vona R Iessi E andMatarrese P.. Role of cholesterol and lipid rafts in cancer signaling: a promising therapeutic opportunity? Front Cell Dev Biol 2021; 9: 622908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burkard I, Von Eckardstein A, Waeber G, et al. Lipoprotein distribution and biological variation of 24S- and 27-hydroxycholesterol in healthy volunteers. Atherosclerosis 2007; 194: 71–78. [DOI] [PubMed] [Google Scholar]
- 11.Bulat N Waeber G andWidmann C.. LDLs stimulate p38 MAPKs and wound healing through SR-BI independently of Ras and PI3 kinase. J Lipid Res 2009; 50: 81–89. [DOI] [PubMed] [Google Scholar]
- 12.Esteve E Ricart W andFernandez-Real JM.. Dyslipidemia and inflammation: an evolutionary conserved mechanism. Clin Nutr 2005; 24: 16–31. [DOI] [PubMed] [Google Scholar]
- 13.Langsenlehner U, Krippl P, Renner W, et al. Interleukin-10 promoter polymorphism is associated with decreased breast cancer risk. Breast Cancer Res Treat 2005; 90: 113–115. [DOI] [PubMed] [Google Scholar]
- 14.Schernhammer ES, Holly JM, Pollak MN, et al. Circulating levels of insulin-like growth factors, their binding proteins, and breast cancer risk. Cancer Epidemiol Biomarkers Prev 2005; 14: 699–704. [DOI] [PubMed] [Google Scholar]
- 15.Inaraja V, Thuissard I, Andreu-Vazquez C, et al. Lipid profile changes during the menopausal transition. Menopause 2020; 27: 780–787. [DOI] [PubMed] [Google Scholar]
- 16.Izumida T, Nakamura Y, Sato Y, et al. Association among age, gender, menopausal status and small dense low-density lipoprotein cholesterol: a cross-sectional study. BMJ Open 2021; 11: e041613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y, Park SK, Han W, et al. Serum high-density lipoprotein cholesterol and breast cancer risk by menopausal status, body mass index, and hormonal receptor in Korea. Cancer Epidemiol Biomarkers Prev 2009; 18: 508–515. [DOI] [PubMed] [Google Scholar]
- 18.Kucharska-Newton AM, Rosamond WD, Mink PJ, et al. HDL-cholesterol and incidence of breast cancer in the ARIC cohort study. Ann Epidemiol 2008; 18: 671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021; 372: n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 21.Yang LN andRui YY.. Combined detection of serum lipids and tumor markers in diagnosing breast cancers. Chin J Biomed Eng 2020; 26: 263–267. [Google Scholar]
- 22.Jing GM, Wu LR, Liu JS, et al . Relationship between serum lipid level and the development of breast cancer. Lab Med Clin 2019; 16: 3563–3565,3568. [Google Scholar]
- 23.Huang Z. Correlation of lipid metabolism, body mass index and breast cancer. China Prac Med 2019; 14: 3–5. [Google Scholar]
- 24.Akalanka HMK Ekanayake S andSamarasinghe K.. Could anthropometric and lipid parameters reflect susceptibility to breast cancer? Comparison of newly diagnosed breast cancer and apparently healthy women. Asian Pac J Cancer Prev 2018; 19: 2475–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei LJ, Zhang C, Zhang H, et al. A case-control study on the association between serum lipid level and the risk of breast cancer. Chin J Prev Med 2016; 50: 1091–1095. [DOI] [PubMed] [Google Scholar]
- 26.Kumar V, Singh A, Sidhu DS, et al. A comparitive study to evaluate the role of serum lipid levels in aetiology of carcinoma breast. J Clin Diagn Res 2015; 9: PC01–PC03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kakaiya A, Modi G, Kakaiya A, et al. Lipid abnormalities in breast cancer of women. GRA 2013; 2: 30–32. [Google Scholar]
- 28.Abdelsalam KE Hassan IK andSadig IA.. The role of developing breast cancer in alteration of serum lipid profile. J Res Med Sci 2012; 17: 562–565. [PMC free article] [PubMed] [Google Scholar]
- 29.Peela JR, Jarari AM, Alsoaeiti SO, et al. The relationship between serum lipids and breast cancer in Libya. Biochem Anal Biochem 2012; 1. doi: 10.4172/2161-1009.1000117. [Google Scholar]
- 30.Yadav NK, Poudel B, Thanpari C, et al. Assessment of biochemical profiles in premenopausal and postmenopausal women with breast cancer. Asian Pac J Cancer Prev 2012; 13: 3385–3388. [DOI] [PubMed] [Google Scholar]
- 31.Owiredu WK, Donkor S, Addai BW, et al. Serum lipid profile of breast cancer patients. Pak J Biol Sci 2009; 12: 332–338. [DOI] [PubMed] [Google Scholar]
- 32.Abu-Bedair FA, El-Gamal BA, Ibrahim NA, et al. Serum lipids and tissue DNA content in Egyptian female breast cancer patients. Jpn J Clin Oncol 2003; 33: 278–282. [DOI] [PubMed] [Google Scholar]
- 33.Ferraroni M, Gerber M, Decarli A, et al. HDL-cholesterol and breast cancer: a joint study in northern Italy and southern France. Int J Epidemiol 1993; 22: 772–780. [DOI] [PubMed] [Google Scholar]
- 34.Toi M, Ohashi Y, Seow A, et al. The Breast Cancer Working Group presentation was divided into three sections: the epidemiology, pathology and treatment of breast cancer. Jpn J Clin Oncol 2010; 40: i13–i18. [DOI] [PubMed] [Google Scholar]
- 35.Bhikoo R, Srinivasa S, Yu TC, et al. Systematic review of breast cancer biology in developing countries (part 1): Africa, the Middle East, Eastern Europe, Mexico, the Caribbean and South America. Cancers (Basel) 2011; 3: 2358–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leong SP, Shen ZZ, Liu TJ, et al. Is breast cancer the same disease in Asian and Western countries? World J Surg 2010; 34: 2308–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dela Cruz R, Park SY, Shvetsov YB, et al. Diet quality and breast cancer incidence in the multiethnic cohort. Eur J Clin Nutr 2020; 74: 1743–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu AH. Diet and breast carcinoma in multiethnic populations. Cancer 2000; 88: 1239–1244. [DOI] [PubMed] [Google Scholar]
- 39.Goodwin PJ, Boyd NF, Hanna W, et al. Elevated levels of plasma triglycerides are associated with histologically defined premenopausal breast cancer risk. Nutr Cancer 1997; 27: 284–292. [DOI] [PubMed] [Google Scholar]
- 39.Han C, Zhang HT, Du L, et al. Serum levels of leptin, insulin, and lipids in relation to breast cancer in China. Endocrine 2005; 26: 19–24. [DOI] [PubMed] [Google Scholar]
- 41.Pedro-Botet J andPinto X.. LDL-cholesterol: the lower the better. Clin Investig Arterioscler 2019; 31: 16–27. [DOI] [PubMed] [Google Scholar]
- 42.Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J 2016; 37: 2999–3058. [DOI] [PubMed] [Google Scholar]
- 43.Kitahara CM, Berrington De Gonzalez A, Freedman ND, et al. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol 2011; 29: 1592–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strohmaier S, Edlinger M, Manjer J, et al. Total serum cholesterol and cancer incidence in the Metabolic syndrome and Cancer Project (Me-Can). PLoS One 2013; 8: e54242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furberg AS, Veierod MB, Wilsgaard T, et al. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J Natl Cancer Inst 2004; 96: 1152–1160. [DOI] [PubMed] [Google Scholar]
- 46.Melvin JC, Holmberg L, Rohrmann S, et al. Serum lipid profiles and cancer risk in the context of obesity: four meta-analyses. J Cancer Epidemiol 2013; 2013: 823849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Touvier M, Fassier P, His M, et al. Cholesterol and breast cancer risk: a systematic review and meta-analysis of prospective studies. Br J Nutr 2015; 114: 347–357. [DOI] [PubMed] [Google Scholar]
- 48.Ni H Liu H andGao R.. Serum lipids and breast cancer risk: a meta-analysis of prospective cohort studies. PLoS One 2015; 10: e0142669. [DOI] [PMC free article] [PubMed] [Google Scholar]