Abstract
Objective
Methicillin-resistant (MR) Staphylococcus aureus bacteremia (SAB) is associated with higher mortality rates than methicillin-susceptible (MS) SAB. This study assessed potential predictors of mortality and evaluated the association of methicillin resistance with mortality in patients with SAB.
Methods
We conducted a retrospective cohort study in patients with hospital-acquired SAB, from 2009 to 2018. Clinical features of patients with MR-SAB were compared with those of patients with MS-SAB and predictors of 30-day mortality were determined using Cox regression analysis.
Results
Among 162 patients, 56.8% had MR-SAB. Overall 30-day mortality was 19.1%; MR-SAB had higher mortality (25.0%) than MS-SAB (11.4%). Univariate analysis highlighted long-term hospitalization, prior antibiotics use, and delayed initiation of appropriate antibiotics as risk factors. Cox regression analysis showed that respiratory tract source, Pitt bacteremia score, Charlson comorbidity index, and appropriate antibiotic therapy within 24 hours were independently and significantly associated with 30-day mortality outcome.
Conclusions
Methicillin resistance was not an independent risk factor for mortality in patients with SAB. Early, appropriate antibiotic treatment is an important prognostic factor.
Keywords: Appropriate antibiotics, bacteremia, methicillin resistance, mortality, Staphylococcus aureus, Methicillin-resistant Staphylococcus aureus
Introduction
Bacteremia, one of the most serious hospital-acquired infections, prolongs the length of hospital stay, increases the cost of healthcare, and has a high fatality rate. Bacteremia caused by Staphylococcus aureus, the second most common cause of bacteremia after coagulase-negative staphylococci, has a crude mortality rate of 20% to 40%, indicating a poor prognosis.1–5 According to differences in antimicrobial susceptibility, S. aureus is classified into methicillin-susceptible S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA). In Japan, the proportion of MRSA and MSSA in blood culture isolates is approximately 50%, and the mortality rate of bacteremia owing to MRSA is 1.5- to 2-fold higher than that of bacteremia caused by MSSA.2,6–8
Although numerous studies have been conducted to investigate the association of methicillin resistance with mortality rates in patients with S. aureus bacteremia (SAB), conflicting results have been reported.2,6,9 Increased mortality associated with MRSA bacteremia may be partially owing to delays caused by inappropriate antibiotic selection, which can occur when causative species and antibiotic susceptibilities are not known. Whereas several studies have shown that appropriate empirical antimicrobial therapy can have a positive impact on patient outcomes,10–12 other publications have indicated that appropriate empirical therapy does not significantly affect patient outcomes.13–15 Therefore, it is unclear whether the increase in mortality associated with MRSA bacteremia is owing to a delay in administering appropriate antimicrobial therapy, differences in strain virulence, or other pathogen–host factors.
Accordingly, in this study, our aim was to determine the clinical features of patients with MRSA bacteremia to identify the predictors of mortality and to evaluate the association of methicillin resistance with mortality in patients with SAB.
Ethical considerations
This study was approved by the institutional review board of the Faculty of Medical Sciences, University of Fukui (approval number: 20120089). The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The requirement for informed consent was waived owing to the retrospective nature of the study.
Study design and population
This retrospective cohort study was conducted at the University of Fukui Hospital, a 600-bed secondary and tertiary care center in Japan. All hospitalized patients at least 16 years of age whose blood cultures tested positive for S. aureus between January 2009 and December 2018 were included, provided the infection occurred more than 48 hours after hospital admission. Only the first episode for each patient was included in the analysis. Patients with polymicrobial bloodstream infection and those diagnosed with microbial contamination were excluded.
Data collection
The following data were collected from medical charts to evaluate the severity of illness at onset of SAB: age; sex; body weight; serum albumin level; underlying diseases; intensive care unit stay; neutrophil count; presence of a central venous catheter (CVC); use of immunosuppressants, corticosteroids, anticancer chemotherapy, or antimicrobial agents (within 30 days prior to SAB); source of bacteremia; length of hospital stay before SAB onset; survival at 14, 30, and 90 days after SAB onset; time to administration of appropriate antibiotics; time to blood culture positivity (TTP); 16 Pitt bacteremia score (PBS); 17 and Charlson comorbidity index (CCI). 18
Definitions
SAB was defined as the presence of at least one S. aureus-positive blood culture in patients with signs and symptoms consistent with an infection. 19 The onset of SAB was defined as the date of sampling for the first S. aureus-positive blood culture. The source of bacteremia was determined using criteria set by the Centers for Disease Control. 20 Primary bacteremia indicates the condition when the source of bacteremia cannot be identified. Antimicrobial therapy was considered appropriate if the antimicrobial agent had in vitro activity against the isolated S. aureus strain. Vancomycin, teicoplanin, arbekacin, linezolid, and daptomycin are suggested as appropriate antibiotics for MRSA treatment. The time to initiation of appropriate antibiotics was defined as the interval between SAB onset and initiation of appropriate antibiotics.
Statistical analysis
All-cause 30-day mortality was used as a prognostic index. Statistical analysis was performed using the χ2 test or Fisher’s exact test for categorical variables and the Student t-test or Mann–Whitney U test for continuous variables. Multivariate Cox regression analysis was used to control the effects of confounding variables so as to identify variables independently associated with 30-day mortality. Variables with P values <0.1 in the univariate analysis were included in the multivariate analysis. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were calculated. All P values were two-tailed, and results with P values <0.05 were considered statistically significant. Statistical analyses were performed using SPSS version 17.0 (SPSS Japan Inc., Tokyo, Japan).
Results
Patient characteristics
In total, 315 patients with SAB were identified during the study period. Of the total patients, 162 fulfilled the inclusion criteria and were enrolled. We excluded 153 patients; 123 patients developed SAB within 48 hours after hospital admission, 9 were less than 16 years of age, 6 had polymicrobial infection, and 15 patients were diagnosed with microbial contamination. The mean patient age was 69.1 years, and 42.0% of patients were women. The overall 14-, 30-, and 90-day mortality rates were 10.5%, 19.1%, and 27.8%, respectively.
Comparison of clinical features in patients with MRSA and MSSA bacteremia
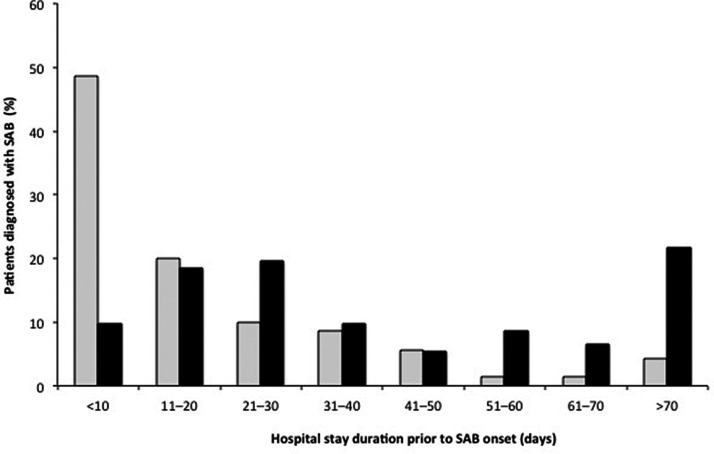
Of the 162 included patients, 70 (43.2%) had MSSA bacteremia and 92 (56.8%) had MRSA bacteremia. The population characteristics for both groups are specified in Table 1. Significant differences in clinical characteristics between the MSSA and MRSA groups were found for several variables. The proportion with prior use of antimicrobial agents in the MRSA group was significantly higher than that in the MSSA group (P < 0.001). As shown in Figure 1, the length of hospital stay before onset of bacteremia was significantly longer in the MRSA group than that in the MSSA group (P < 0.01). The PBS for the MRSA group was significantly higher than that for the MSSA group (P = 0.038). The 14-, 30-, and 90-day mortality rates of patients with MRSA bacteremia were higher than those of patients with MSSA bacteremia (2.9% vs. 16.3%, P = 0.006; 11.4% vs. 25.0%, P = 0.030; 18.6% vs. 34.8%, P = 0.022, respectively). Moreover, initiation of appropriate antibiotic therapy was significantly delayed in the MRSA group at 24 and 48 hours after onset (98.6% vs. 56.5%; 100% vs. 72.8%, respectively; both P < 0.001).
Table 1.
Comparison of clinical features in patients with MSSA and MRSA bacteremia.
| Characteristic | MSSA (N = 70) | MRSA (N = 92) | P |
|---|---|---|---|
| Age (years), mean ± SD | 67.6 ± 13.9 | 70.3 ± 14.5 | 0.128 |
| Female sex | 31 (44.3) | 37 (40.2) | 0.603 |
| Body weight (kg), mean ± SD | 55.5 ± 13.4 | 55.1 ± 10.5 | 0.601 |
| Serum albumin (g/dL), mean ± SD | 2.9 ± 0.6 | 2.7 ± 0.6 | 0.099 |
| Underlying disease | |||
| Malignancy | 34 (48.6) | 40 (43.6) | 0.519 |
| Chronic heart disease | 23 (32.9) | 34 (37.2) | 0.588 |
| Diabetes mellitus | 20 (28.6) | 31 (33.0) | 0.487 |
| Chronic renal failure | 14 (20.0) | 16 (17.0) | 0.688 |
| Liver cirrhosis | 8 (11.4) | 7 (8.5) | 0.406 |
| Respiratory disease | 6 (8.6) | 13 (13.8) | 0.276 |
| Intensive care unit stay (days) | 6 (8.6) | 11 (12.0) | 0.645 |
| Neutropenia (<500/μL) | 3 (4.3) | 7 (7.4) | 0.517 |
| Presence of CVC | 35 (50.0) | 60 (64.9) | 0.064 |
| Immunosuppressants | 2 (2.9) | 2 (2.1) | 1.000 |
| Corticosteroids | 9 (12.9) | 8 (8.5) | 0.392 |
| Anticancer chemotherapy | 21 (30.0) | 19 (20.2) | 0.172 |
| Antimicrobial agents | 17 (24.3) | 66 (72.3) | <0.001 |
| Source of bacteremia | |||
| Primary | 23 (32.9) | 30 (31.9) | 0.973 |
| Catheter-related | 35 (50.0) | 41 (44.7) | 0.492 |
| Skin/soft tissue | 6 (8.6) | 9 (9.6) | 0.792 |
| Respiratory tract | 3 (4.3) | 2 (3.2) | 0.653 |
| Urinary tract | 2 (2.9) | 3 (3.2) | 1.000 |
| Other | 1 (1.4) | 7 (7.6) | 0.139 |
| Length of stay before bacteremia onset (days), mean ± SD | 19.9 ± 21.4 | 48.5 ± 43.6 | <0.001 |
| Mortality | |||
| 14-day | 2 (2.9) | 15 (16.3) | 0.006 |
| 30-day | 8 (11.4) | 23 (25.0) | 0.030 |
| 90-day | 13 (18.6) | 32 (34.8) | 0.022 |
| Initiation of appropriate antibiotic therapy | |||
| ≤24 hours | 69 (98.6) | 52 (56.5) | <0.001 |
| ≤48 hours | 70 (100) | 67 (72.8) | <0.001 |
| TTP (hours), mean ± SD | 15.1 ± 13.3 | 17.6 ± 14.4 | 0.280 |
| PBS, mean ± SD | 1.7 ± 1.4 | 2.3 ± 1.8 | 0.038 |
| CCI, mean ±SD | 2.9 ± 2.0 | 2.8 ± 1.8 | 0.755 |
Data are n (%), unless otherwise indicated.
MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant S. aureus; SD, standard deviation; CVC, central venous catheter; TTP, time to blood culture positivity; PBS, Pitt bacteremia score; CCI, Charlson comorbidity index.
Figure 1.
Duration of hospital stay versus bacteremia onset. Distribution of patients with MSSA (gray bars) and MRSA (black bars) bacteremia according to length of hospital stay (number of days post-admittance) at the time of bacteremia onset. The duration of pre-SAB hospital stay in patients with MRSA bacteremia was significantly longer than for those with MSSA bacteremia (mean ± standard deviation 19.9 ± 21.4 vs. 48.5 ± 43.6; median [interquartile range] 12 [6–27] vs. 33 [17–65]; P < 0.001).
MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant S. aureus; SAB, S. aureus bacteremia.
Risk factors associated with 30-day mortality
Table 2 shows the results of univariate analysis of risk factors for 30-day mortality of SAB in patients who died and those who survived. The proportion of patients with MRSA bacteremia (P = 0.030) and respiratory tract source (P < 0.001) among patients who died (“non-surviving” group) were significantly higher than those in who survived (“surviving” group). Patients who died had higher PBS (P = 0.018) and CCI (P = 0.002) values than surviving patients. The number of patients in the surviving group who received appropriate antibiotics within 24 hours was higher than that in the non-surviving group (78.6% vs. 58.1%; P = 0.018). However, in the surviving and non-surviving groups, there were no significant differences between the number of patients who had received appropriate antibiotics within 48 hours (85.5% vs. 77.4%).
Table 2.
Univariate analysis of risk factors for 30-day mortality in Staphylococcus aureus bacteremia.
| Characteristic | Survival(N = 131) | Death (N = 31) | P |
|---|---|---|---|
| Age (years), mean ± SD | 67.9 ± 13.9 | 72.2 ± 13.2 | 0.056 |
| Female sex | 56 (42.7) | 12 (38.7) | 0.682 |
| Body weight (kg), mean ± SD | 56.1 ± 11.9 | 54.0 ± 11.0 | 0.491 |
| Serum albumin (g/dL), mean ± SD | 2.8 ± 0.6 | 2.7 ± 0.6 | 0.307 |
| MRSA | 69 (52.7) | 23 (74.2) | 0.030 |
| Underlying disease | |||
| Malignancy | 57 (43.5) | 17 (54.8) | 0.317 |
| Heart disease | 41 (31.3) | 9 (29.0) | 0.977 |
| Diabetes mellitus | 44 (33.6) | 7 (22.6) | 0.235 |
| Chronic renal failure | 22 (16.8) | 8 (25.8) | 0.303 |
| Liver cirrhosis | 10 (7.6) | 5 (16.1) | 0.167 |
| Respiratory disease | 13 (9.9) | 6 (19.4) | 0.209 |
| Intensive care unit stay (days) | 14 (10.7) | 3 (9.7) | 0.869 |
| Neutropenia (<500/μL) | 9 (6.9) | 1 (3.2) | 0.448 |
| Presence of CVC | 77 (58.8) | 18 (58.1) | 0.902 |
| Immunosuppressants | 4 (3.1) | 0 | 0.325 |
| Corticosteroids | 14 (10.7) | 3 (9.7) | 0.869 |
| Anticancer chemotherapy | 31 (23.7) | 9 (29.0) | 0.643 |
| Antimicrobial agents | 67 (51.1) | 16 (51.6) | 0.963 |
| Source of bacteremia | |||
| Primary | 36 (27.5) | 13 (41.9) | 0.115 |
| Catheter-related | 66 (50.4) | 10 (32.3) | 0.069 |
| Skin/soft tissue | 14 (10.7) | 1 (3.2) | 0.197 |
| Respiratory tract | 1 (0.8) | 4 (12.9) | <0.001 |
| Urinary tract | 5 (3.8) | 0 | 0.269 |
| Other | 9 (6.9) | 3 (9.7) | 0.701 |
| Length of stay before bacteremia onset (days) | 31.4 ± 29.8 | 48.2 ± 42.0 | 0.072 |
| Initiation of appropriate antibiotics | |||
| ≤24 hours | 103 (78.6) | 18 (58.1) | 0.018 |
| ≤48 hours | 112 (85.5) | 24 (77.4) | 0.271 |
| TTP (hours), mean ± SD | 17.5 ± 15.4 | 13.6 ± 6.2 | 0.239 |
| PBS, mean ± SD | 1.8 ± 1.4 | 2.9 ± 2.4 | 0.018 |
| CCI, mean ± SD | 2.75 ± 1.87 | 3.86 ± 2.0 | 0.002 |
Data are n (%), unless otherwise indicated.
MRSA, methicillin-resistant S. aureus; SD, standard deviation; CVC, central venous catheter; TTP, time to blood culture positivity; PBS, Pitt bacteremia score; CCI, Charlson comorbidity index.
Multivariate analysis showed that respiratory tract source (HR, 5.26; 95% CI, 1.75–15.79; P = 0.003), initiation of appropriate antibiotics within 24 hours (HR, 0.35; 95% CI, 0.16–0.74; P = 0.006), PBS (HR, 1.22; 95% CI, 1.03–1.45, P = 0.019), and CCI (HR, 1.32; 95% CI, 1.09–1.60, P = 0.004) were independent factors associated with 30-day mortality outcome (Table 3). HRs for CCI and PBS are given for 1-point increments in the index and score, respectively. MRSA, however, was not identified as an independent risk factor for 30-day mortality after adjusting for other variables.
Table 3.
Multivariate analysis related to 30-day mortality.
| Variable | Adjusted HR (95% CI) | P |
|---|---|---|
| Age | 1.02a (0.98–1.05) | 0.322 |
| MRSA | 1.67 (0.63–4.38) | 0.301 |
| Source of bacteremia | ||
| Catheter-related | 0.58 (0.27–1.28) | 0.179 |
| Respiratory tract | 5.26 (1.75–15.79) | 0.003 |
| Initiation of appropriate antibiotics (≤24 hours) | 0.35 (0.16–0.74) | 0.006 |
| PBS | 1.22b (1.03–1.45) | 0.019 |
| CCI | 1.32b (1.09–1.60) | 0.004 |
HR, hazard ratio; CI, confidence interval; MRSA, methicillin-resistant S. aureus; PBS, Pitt bacteremia score; CCI, Charlson comorbidity index.
aPer 1-year increment. bPer 1-point increment.
Discussion
In this study, we observed significant differences in clinical outcomes between patients with MRSA and MSSA bacteremia. Consistent with the results of previous studies,2,6–8 the mortality rates of patients with MRSA bacteremia were 2- to 5-fold higher than those of patients with MSSA bacteremia. In the current study, respiratory tract source, PBS, and CCI were identified as independent risk factors for 30-day mortality. Furthermore, early appropriate antibiotic therapy (<24 hours) was identified as an independent factor for an improved prognosis of SAB. This is in accordance with recommended SAB management strategies and current knowledge that timely administration of appropriate antibiotics may improve outcomes in some patients.21,22
Univariate analysis of risk factors for 30-day mortality of SAB in surviving patients and in those who died showed that the proportion of patients with MRSA bacteremia was significantly higher in the non-surviving group. However, in multivariate analysis, MRSA bacteremia was not identified as an independent risk factor for 30-day mortality of SAB. This observation is not entirely surprising when considering current data on MRSA and mortality. The association of antibacterial resistance with mortality is complex and remains poorly understood. This association is likely influenced by various host factors and host–pathogen interactions. Whether the observed increased mortality is attributable to pathogen-specific factors, such as increased virulence, poor response to antibiotic therapy, or both, requires further study.21,23
We compared the clinical characteristics of patients with MSSA and MRSA bacteremia. Current knowledge on the impact of patient factors on SAB outcome is limited, with the only consistent predictors of SAB outcome being the presence of comorbidities and patient age 21 . In the current study, there was no significant difference in age between the MSSA and MRSA groups; however, the cohort mostly included older patients (age 69.1 ± 14.3 years) and was not representative of a broad age range. No significant difference in the severity of comorbidities, as measured by the CCI, was observed between patients with MRSA and those with MSSA. Although the severity of acute illness (as measured by the PBS) was significantly greater in patients with MRSA, the mean PBS score of this group (as with that of the MSSA group) was less than 4, and the proportion of patients with a PBS of more than 4 was not significantly different between the two groups (7.1% MSSA vs. 12.0% MRSA, P = 0.452). A score greater than 4 is routinely considered indicative of critical illness or increased risk of death. Univariate analysis of risk factors for 30-day mortality of SAB in surviving patients and in those who died showed that PBS and CCI were both significantly greater in non-surviving patients. Liao et al. 24 reported that the mean PBS for the non-surviving group of 59 patients with SAB was 5.9; among those patients, 24 died within 3 days of hospitalization. In the current study, the mean PBS for the non-surviving group was 2.3; that is, the severity of bacteremia was relatively low in many patients. Previous conflicting findings regarding the comparison of mortality rates in patients with MRSA and MSSA bacteremia may be explained by differences in patient condition, patient background, or suboptimal response to vancomycin compared with beta-lactams.25,26 Furthermore, methodological differences related to study design, definitions, and analyses should be considered when comparing different studies. Another potential factor for poorer prognosis of MRSA bacteremia and conflicting data is the possibility that MRSA strains are more virulent. Although results from various clinical studies support this notion, in vitro investigations have yielded contradictory results. 23 The most likely cause of these conflicting data is heterogeneity of the bacterial population, with MSSA populations containing resistance cells, which are then selected for on treatment with non-MRSA-targeting antibiotics; this may influence the subsequent clinical response. 23
In our study, we found that the number of patients with low serum albumin levels and the presence of CVC was higher in the MRSA group than in the MSSA group, suggesting that more patients in the MRSA group were malnourished; this is consistent with findings that malnutrition is associated with poor clinical outcomes among hospitalized patients. 27 Furthermore, the number of patients with prior use of antibiotics (within 30 days before SAB) and longer hospitalization was higher in the MRSA group than in the MSSA group. These data are in accordance with previous studies showing that these are considered risk factors for inducing drug-resistant bacterial infections, such as MRSA bacteremia.2,28–34
Current SAB management strategies emphasize the value of timely initiation of appropriate antibiotic therapy 21 . In the current study, we found that initiation of appropriate antibiotic therapy was delayed in most patients with MRSA bacteremia. Only 52 (56.5%) of 92 patients were administered appropriate antibiotics within 24 hours in the MRSA group, compared with 69 (98.6%) of 70 patients in the MSSA group. This could be because beta-lactam antibiotics, which are routinely used as first-line therapy in SAB, exhibit antibacterial activity against MSSA but not MRSA. Therefore, anti-MRSA antibiotics were only administered after obtaining in vitro culture results, causing a delay in the initiation of appropriate antibiotic therapy. Furthermore, we found that the initiation of appropriate antibiotic therapy within 24 hours improved patient outcomes. This is in accordance with previous studies in which timely administration (within 48 hours) of empiric antibiotics was associated with decreased mortality and was positively associated with improved clinical outcomes.10,11,21 Nevertheless, the benefits and importance of early appropriate empirical antibiotic treatments of MRSA are contradicted by many studies showing no benefit with early administration of appropriate empirical antibiotics for MRSA treatment.13–15 These contrasting results could be attributed to differences in the definition of appropriate antibiotic therapy and patient backgrounds. In previous studies, initiation of appropriate antimicrobial treatment varied from 24 to 72 hours after blood culture collection.4,11,13,14 In other studies, the time to initiation of appropriate antibiotic treatment was defined either as the time since a positive blood culture was reported or the time after bacteremia had developed.12,15 In the current study, multivariate analysis was performed using appropriate antibiotic treatment within 24 hours of blood culture collection as a variable, based on the results of univariate analysis. Furthermore, some previous studies were conducted without distinguishing between community onset and hospital onset; moreover, whether SAB was owing to community onset may not be known. Because this study was limited to in-hospital onset, the time from bacteremia onset to the initiation of appropriate antimicrobial treatment could be accurately evaluated. Furthermore, the poorer prognosis of MRSA bacteremia compared with MSSA bacteremia could be owing to the inefficacy of vancomycin in addition to the delay in appropriate antibiotic treatment. Several studies have suggested that vancomycin is less effective than beta-lactam antibiotics against S. aureus.25,26 Although early initiation of appropriate antibiotics may improve prognosis, further research is still required to clarify the relationship between the timing of antimicrobial initiation and prognosis.
The current study highlighted the importance of initiating early therapy using anti-MRSA agents in patients with MRSA bacteremia. It is necessary to carefully evaluate the risk of MRSA infection at the onset of bacteremia, taking advantage of early diagnosis tools such as detection of the mecA gene, and initiating anti-MRSA antibiotic therapy if the risk of MRSA infection is high. Identifying patient factors that can be used as indicators of MRSA bacteremia could be valuable in ensuring timely identification and treatment of patients with MRSA.
This study had some limitations. First, the data were collected retrospectively and therefore could have been misinterpreted. Moreover, other unknown confounders may have been omitted. Second, this was a single-center study with a small number of patients and limited information. Third, in our cohort, the proportion of patients with MRSA bacteremia exceeded 50% versus those with MSSA bacteremia. Furthermore, most patients were older, with mean (± SD) age 69.1 (± 14.3) years; thus, the data may not be generalizable to other age groups. Furthermore, because the most frequently administered anti-MRSA drug was vancomycin, we could not evaluate differences in efficacy between vancomycin and other anti-MRSA drugs.
In conclusion, methicillin resistance was not a significant predictor of mortality in patients with SAB in a hospital setting with a high prevalence of MRSA. Respiratory tract source, PBS, and CCI were identified as risk factors associated with mortality in patients with SAB, and early appropriate antibiotic therapy (<24 hours) was identified as an independent factor to improve SAB prognosis. Furthermore, prior antibiotic exposure, long-term hospitalization, and a high PBS could serve as indicators of an increased risk of MRSA in patients with suspected SAB.
Acknowledgement
Editorial support, in the form of medical writing, assembling tables, and creating high-resolution images based on authors’ detailed directions, collating author comments, copyediting, fact checking, and referencing, was provided by Editage, Cactus Communications.
Footnotes
Author contributions: T. Aratani, H. Tsukamoto, and N. Goto conceptualized and designed the study. T. Aratani, H. Tsukamoto, T. Higashi, T. Kodawara, and Y. Hida acquired the data. T. Aratani, H. Tsukamoto, N. Goto, R. Yano, and H. Iwasaki analyzed and interpreted the data. T. Aratani, H. Tsukamoto, and N. Goto drafted and critically revised the manuscript. All authors approved the final version of the manuscript.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This work was supported by JSPS KAKENHI (grant number JP18K14979). The funding agency had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
ORCID iDs: Tomonori Aratani https://orcid.org/0000-0002-4208-3137
Hitoshi Tsukamoto https://orcid.org/0000-0002-9254-1781
Ryoichi Yano https://orcid.org/0000-0002-2813-6992
References
- 1.Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39: 309–317. 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Wang FD, Chen YY, Chen TL, et al. Risk factors and mortality in patients with nosocomial Staphylococcus aureus bacteremia. Am J Infect Control 2008; 36: 118–122. 10.1016/j.ajic.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Mejer N, Westh H, Schønheyder HC, et al. Danish Staphylococcal Bacteraemia Study Group. Stable incidence and continued improvement in short term mortality of Staphylococcus aureus bacteraemia between 1995 and 2008. BMC Infect Dis 2012; 12: 260. 10.1186/1471-2334-12-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishara J, Goldberg E, Leibovici L, et al. Healthcare-associated vs. hospital-acquired Staphylococcus aureus bacteremia. Int J Infect Dis 2012; 16: e457–e463. 10.1016/j.ijid.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Kovacs CS, Fatica C, Butler R, et al. Hospital-acquired Staphylococcus aureus primary bloodstream infection: a comparison of events that do and do not meet the central line-associated bloodstream infection definition. Am J Infect Control 2016; 44: 1252–1255. 10.1016/j.ajic.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 6.Cosgrove SE, Sakoulas G, Perencevich EN, et al. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: A meta-analysis. Clin Infect Dis 2003; 36: 53–59. 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 7.Blot SI, Vandewoude KH, Hoste EA, et al. Outcome and attributable mortality in critically ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch Intern Med 2002; 162: 2229–2235. 10.1001/archinte.162.19.2229. [DOI] [PubMed] [Google Scholar]
- 8.Holmes NE, Turnidge JD, Munckhof WJ, et al. Antibiotic choice may not explain poorer outcomes in patients with Staphylococcus aureus bacteremia and high vancomycin minimum inhibitory concentrations. J Infect Dis 2011; 204: 340–347. 10.1093/infdis/jir270. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Gregson DB, Ross T, et al. Time to blood culture positivity in Staphylococcus aureus bacteremia: association with 30-day mortality. J Infect 2010; 61: 197–204. 10.1016/j.jinf.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Khatib R, Saeed S, Sharma M, et al. Impact of initial antibiotic choice and delayed appropriate treatment on the outcome of Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis 2006; 25: 181–185. 10.1007/s10096-006-0096-0. [DOI] [PubMed] [Google Scholar]
- 11.Paul M, Kariv G, Goldberg E, et al. Importance of appropriate empirical antibiotic therapy for methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother 2010; 65: 2658–2665. 10.1093/jac/dkq373. [DOI] [PubMed] [Google Scholar]
- 12.Lodise TP, McKinnon PS, Swiderski L, et al. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis 2003; 36: 1418–1423. 10.1086/375057. [DOI] [PubMed] [Google Scholar]
- 13.Ammerlaan H, Seifert H, Harbarth S, et al. European Practices of Infections with Staphylococcus aureus (SEPIA) Study Group. Adequacy of antimicrobial treatment and outcome of Staphylococcus aureus bacteremia in 9 Western European countries. Clin Infect Dis 2009; 49: 997–1005. 10.1086/605555. [DOI] [PubMed] [Google Scholar]
- 14.Kaasch AJ, Rieg S, Kuetscher J, et al. preSABATO study group. Delay in the administration of appropriate antimicrobial therapy in Staphylococcus aureus bloodstream infection: a prospective multicenter hospital-based cohort study. Infection 2013; 41: 979–985. 10.1007/s15010-013-0428-9. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Park WB, Lee CS, et al. Outcome of inappropriate empirical antibiotic therapy in patients with Staphylococcus aureus bacteraemia: analytical strategy using propensity scores. Clin Microbiol Infect 2006; 12: 13–21. 10.1111/j.1469-0691.2005.01294.x. [DOI] [PubMed] [Google Scholar]
- 16.Durmaz G, Us T, Aydinli A, et al. Optimum detection times for bacteria and yeast species with the BACTEC 9120 aerobic blood culture system: Evaluation for a 5-year period in a Turkish university hospital. J Clin Microbiol 2003; 41: 819–821. 10.1128/jcm.41.2.819-821.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study Klebsiella pneumoniae bacteremia: Implications of extended-spectrum beta-lactamase production in nosocomial infections. Ann Intern Med 2004; 140: 26–32. 10.7326/0003-4819-140-1-200401060-00008. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987; 40: 373–383. 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Bassetti M, Peghin M, Trecarichi EM, et al. Characteristics of Staphylococcus aureus bacteraemia and predictors of early and late mortality. PLoS One 2017; 12: e0170236. 10.1371/journal.pone.0170236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horan TC Andrus M andDudeck MA.. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36: 309–332. 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Van Hal SJ, Jensen SO, Vaska VL, et al. Predictors of mortality in Staphylococcus aureus Bacteremia. Clin Microbiol Rev 2012; 25: 362–386. 10.1128/CMR.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52: e18–e55. 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 23.Rozgonyi F, Kocsis E, Kristóf K, et al. Is MRSA more virulent than MSSA? Clin Microbiol Infect 2007; 13: 843–845. 10.1111/j.1469-0691.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 24.Liao CH, Chen SY, Huang YT, et al. Outcome of patients with methicillin-resistant Staphylococcus aureus bacteraemia at an emergency department of a medical centre in Taiwan. Int J Antimicrob Agents 2008; 32: 326–332. 10.1016/j.ijantimicag.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez C, Rubio M, Romero-Vivas J, et al. Bacteremic pneumonia due to Staphylococcus aureus: a comparison of disease caused by methicillin-resistant and methicillin-susceptible organisms. Clin Infect Dis 1999; 29: 1171–1177. 10.1086/313440. [DOI] [PubMed] [Google Scholar]
- 26.Kim SH, Kim KH, Kim HB, et al. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2008; 52: 192–197. 10.1128/AAC.00700-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lew CCH, Yandell R, Fraser RJL, et al. Association between malnutrition and clinical outcomes in the intensive care unit: A systematic review. JPEN J Parenter Enteral Nutr 2017; 41: 744–758. 10.1177/0148607115625638. [DOI] [PubMed] [Google Scholar]
- 28.Furuno JP, McGregor JC, Harris AD, et al. Identifying groups at high risk for carriage of antibiotic-resistant bacteria. Arch Intern Med 2006; 166: 580–585. 10.1001/archinte.166.5.580. [DOI] [PubMed] [Google Scholar]
- 29.Rogers C, Sharma A, Rimland D, et al. Duration of colonization with methicillin-resistant Staphylococcus aureus in an acute care facility: a study to assess epidemiologic features. Am J Infect Control 2014; 42: 249–253. 10.1016/j.ajic.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Raman G, Avendano EE, Chan J, et al. Risk factors for hospitalized patients with resistant or multidrug-resistant Pseudomonas aeruginosa infections: A systematic review and meta-analysis. Antimicrob Resist Infect Control 2018; 7: 79. 10.1186/s13756-018-0370-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lautenbach E, Patel JB, Bilker WB, et al. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: Risk factors for infection and impact of resistance on outcomes. Clin Infect Dis 2001; 32: 1162–1171. 10.1086/319757. [DOI] [PubMed] [Google Scholar]
- 32.Karanika S, Karantanos T, Arvanitis M, et al. Fecal colonization with extended-spectrum beta-lactamase-producing Enterobacteriaceae and risk factors among healthy individuals: A systematic review and meta-analysis. Clin Infect Dis 2016; 63: 310–318. 10.1093/cid/ciw283. [DOI] [PubMed] [Google Scholar]
- 33.Harris AD, McGregor JC, Johnson JA, et al. Risk factors for colonization with extended-spectrum beta-lactamase-producing bacteria and intensive care unit admission. Emerg Infect Dis 2007; 13: 1144–1149. 10.3201/eid1308.070071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dickstein Y, Edelman R, Dror T, et al. Carbapenem-resistant Enterobacteriaceae colonization and infection in critically ill patients: A retrospective matched cohort comparison with non-carriers. J Hosp Infect 2016; 94: 54–59. 10.1016/j.jhin.2016.05.018. [DOI] [PubMed] [Google Scholar]