Abstract
Objective
To establish the relationship between pulse wave transit time (PWTT) before anaesthesia induction and blood pressure variability (BPV) during anaesthesia induction.
Methods
This prospective observational cohort study enrolled consecutive patients that underwent elective surgery. Invasive arterial pressure, electrocardiography, pulse oximetry, heart rate and bispectral index were monitored. PWTT and BPV were measured with special software. Anaesthesia was induced with propofol, sufentanil and rocuronium.
Results
A total of 54 patients were included in this study. There was no correlation between BPV and the dose of propofol, sufentanil and rocuronium during anaesthesia induction. Bivariate linear regression analysis demonstrated that PWTT (r = –0.54), age (r = 0.34) and systolic blood pressure (r = 0.31) significantly correlated with systolic blood pressure variability (SBPV). Only PWTT (r = –0.38) was significantly correlated with diastolic blood pressure variability (DBPV). Patients were stratified into high PWTT and low PWTT groups according to the mean PWTT value (96.8 ± 17.2 ms). Compared with the high PWTT group, the SBPV of the low PWTT group increased significantly by 3.4%. The DBPV of the low PWTT group increased significantly by 2.1% compared with the high PWTT group.
Conclusions
PWTT, assessed before anaesthesia induction, may be an effective predictor of haemodynamic fluctuations during anaesthesia induction.
Keywords: Pulse wave transit time, blood pressure variation, anaesthesia induction
Introduction
Cardiovascular events can occur during the anaesthesia induction period, mainly due to unstable haemodynamics including severe hypotension that may lead to postoperative stroke and renal failure.1–3 Blood pressure also increases during anaesthesia induction due to sympathetic excitation caused by endotracheal intubation. 4 Previous studies have shown that the increase of blood pressure variability (BPV) is closely related to the damage inflicted on target organs and the occurrence of cardiovascular complications, which suggests that BPV could be used as a strong predictor of cardiovascular complications independent of blood pressure and a reduction of BPV could decrease the occurrence of target organ injury events.5–7 Therefore, equal attention should be paid to BPV and hypotension during the whole anaesthesia process. Increased BPV leads to cardiovascular and cerebrovascular events, which are associated with increased arterial stiffness and vascular remodelling and increased arterial stiffness impairs the ability to regulate blood pressure. 8 The current anaesthesia risk assessment tools, including the American Society of Anesthesiologists (ASA) classification, echocardiography and electrocardiogram (ECG), which have been used for predicting postoperative cardiovascular complications, are not reliable predictors of the risk of cardiovascular events. 9 A few studies have reported on the preoperative assessment of hypotension during anaesthesia induction while the preoperative assessment of BPV during induction has not been reported. 9,10
Pulse wave transit time (PWTT) is a good indicator of arterial stiffness, which is sensitive to vascular dilatation and is a simple and non-invasive technique for evaluating vascular changes. 11 Increase in vascular tone will shorten the PWTT, while loss of vascular tone will prolong the PWTT.12,13 Thus, it could be hypothesized that patients with a shorter PWTT would have greater variability in blood pressure during anaesthesia induction. The aim of this current study was to establish whether increased arterial stiffness as assessed in preoperative assessments was associated with increased BPV during induction of anaesthesia.
Patient and methods
Patient population
This prospective observational cohort study enrolled consecutive patients that underwent elective surgery in the Department of Anaesthesiology, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Taizhou, Zhejiang Province, China between June 2020 and January 2021. Health information was obtained from each patient by medical history, current medication, echocardiography, ECG and physical examination. The inclusion criteria were as follows: (i) aged 30–80 years; (ii) ASA I or II; (iii) general anaesthesia with endotracheal intubation; (iv) elective surgery patients. The exclusion criteria were as follows: (i) vascular stenosis; (ii) arrhythmia; (iii) heart failure; (iv) heart valve disease; (v) upper limb paralysis.
The study was approved by the Medical Ethics Committee of Taizhou Hospital of Zhejiang Province (no. K20200105). The study was also registered with the Chinese Clinical Trial Registry (registration no. ChiCTR2000033431). Before each interview in the prospective section of this study, interviewees were given information about the study, voluntary participation and the confidential handling of all data. Patients and their family members provided written informed consent for participation and the use of their clinical data in publications. The writing of this report followed the STROBE guidelines. 14
Anaesthesia induction
Electrocardiography, pulse oximetry, invasive arterial pressure (AP), bispectral index (BIS; BISTM; Covidien, Mansfield, MA, USA), end-tidal carbon dioxide and oxygen concentration were monitored after entering the operating room. Patients were placed in the supine position for 10 min before anaesthesia induction that enabled physical and sensorial rest. A computer with special software (Haoju Company, Shanghai, China) was used to capture ECG and invasive AP wave forms on the monitor (B650; GE Healthcare, Helsinki, Finland) via a USB cable 1 min before anaesthesia induction. ECG and AP waveforms were captured at 300 Hz and 100 Hz, respectively. The time period of acquisition included 1 min before anaesthesia induction and 5 min after endotracheal intubation. The time and amplitude of each point on the wave forms were saved in an Excel® spreadsheet in a folder. Anaesthesia was induced using sufentanil, propofol and rocuronium as follows: 0.4 µg/kg sufentanil was given as a single slowly injected intravenous injection to avoid the cough caused by rapid injection; propofol was administered via target-controlled infusion selected according to the population pharmacokinetic data set of Schnider 9 and initiated to achieve an effect-site concentration of 3 µg/ml, and subsequently titrated to maintain BIS values of 40–60; and 0.8 mg/kg rocuronium was given as a single intravenous injection when the BIS value was <60. Endotracheal intubation was performed under video laryngoscope after 90 s. During anaesthesia induction, 6 mg ephedrine was administered intravenously when the AP decreased ≥30% of baseline; and this was repeated every 5 min until the AP decreased <30%.
Data collection
Demographic data including sex, age, height and weight of all patients were recorded before anaesthesia induction. The doses of propofol, sufentanil, rocuronium and ephedrine were recorded for each patient. The PWTT value was defined as the time between the apex of the R wave in the ECG and the next starting point of the ascending branch in the invasive AP wave. BPV in this study refers to the degree of blood pressure fluctuation during the whole collection process. Patients with cough occurrence during anaesthesia induction that disrupted the ECG or AP waveform were recorded but excluded. Systolic blood pressure variation (SBPV) and diastolic blood pressure variation (DBPV) were obtained by collecting all the recorded AP on a computer and using software to calculate the values (Haoju Company). PWTT, SBP and DBP 1 min before anaesthesia induction were obtained from an Excel® spreadsheet of ECG and AP waveform stored in the computer. In this study, the mean of five consecutive PWTT values and invasive AP 1 min before anaesthesia induction were used as PWTT values and invasive AP. The correlation between PWTT, SBPV and DBPV during induction were analysed. The patients were stratified into high PWTT and low PWTT groups according to the mean PWTT value; and the BPV values between the two groups were compared to further clarify the relationship between them.
Statistical analyses
All statistical analyses were performed using GraphPad Prism 6 (Graphpad Software Inc., San Diego, CA, USA). The sample size was calculated from data collected in a pilot study. The Kolmogorov–Smirnov test was used to measure the normality of the data. Continuous variables that were normally distributed are expressed as mean ± SD with the range. Categorical variables are presented as n of patients (%). Relationships between the variables were assessed using bivariate linear regression analysis. The mean ± SD values were compared using Student’s t-test. Categorical data were compared using χ2-test. The association between PWTT and BPV during induction of anaesthesia was assessed by first considering PWTT as continuous variable and then as a categorical variable (high versus low). The patients were stratified into high PWTT and low PWTT groups according to a mean PWTT value of 96.8 ms. A P-value <0.05 was considered statistically significant.
Results
A total of 60 patients were enrolled in this study. Three patients had cough during induction, one patient had tracheal intubation difficulties and two patients had blood pressure wave forms disturbed by external factors. A total of 54 patients were included in the analyses. Of the 54 patients, 19 were ASA I and 35 were ASA II, 15 patients had hypertension, 11 had atherosclerosis and three had diabetes mellitus. Table 1 presents the demographic and clinical characteristics of the study population.
Table 1.
Demographic and clinical characteristics of patients (n = 54) that underwent elective surgery and were enrolled in a study to determine if increased arterial stiffness as assessed in preoperative assessments was associated with increased blood pressure variability during induction of anaesthesia.
| Characteristics | Study cohort n = 54 |
|---|---|
| Age, years | 59.2 ± 10.2 (34–80) |
| Height, cm | 162.5 ± 7.8 (148–180) |
| Weight, kg | 65.2 ± 9.7 (43–85) |
| BMI, kg/m2 | 24.7 ± 3.0 (17.0–34.2) |
| SBP, mmHg | 170.8 ± 21.8 (132–222) |
| DBP, mmHg | 80.3 ± 8.6 (62–98) |
| ASA I/ASA II | 19/35 |
| Male/female | 27/27 |
| Hypertension | 15 (28%) |
| Atherosclerosis | 11 (20%) |
| Diabetes mellitus | 3 (6%) |
| Ephedrine use | 3 (6%) |
| Propofol, mg | 149.9 ± 32.3 (81–221) |
| Sufentanil, µg | 26.6 ± 4.1 (20–35) |
| Rocuronium, mg | 53.0 ± 10.1 (40–100) |
| SBPV, % | 18.3 ± 4.4 (9.7–28.0) |
| DBPV, % | 16.1 ± 5.4 (8.3–40.4) |
| PWTT, ms | 96.8 ± 17.2 (61–132) |
Data presented as mean ± SD (range) or n of patients (%).
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; ASA, American Society of Anesthesiologists; SBPV, systolic blood pressure variability; DBPV, diastolic blood pressure variability; PWTT, pulse wave transit time.
The PWTT, SBP and DBP were assessed 1 min before induction of anaesthesia and their mean ± SD values were 96.8 ± 17.2 ms, 170.8 ± 21.8 mmHg and 80.3 ± 8.6 mmHg, respectively (Table 1). SBPV and DBPV were calculated from the blood pressure 1 min before induction to 5 min after endotracheal intubation. Bivariate linear regression analyses were performed to test the relationship between SBPV and DBPV and total sufentanil dose, total rocuronium dose, total propofol dose, age, height, weight, body mass index (BMI), SBP, DBP and PWTT. The analyses demonstrated that SBPV was significantly correlated with PWTT (r = –0.54, P < 0.01), age (r = 0.34, P < 0.05) and SBP (r = –0.31, P < 0.05) (Table 2). DBPV was significantly correlated with PWTT (r = –0.38, P < 0.01). The total doses of sufentanil, rocuronium and propofol were not significantly correlated with SBPV or DBPV. The height, weight and BMI were also not significantly correlated with SBPV or DBPV.
Table 2.
Bivariate linear regression analyses of systolic blood pressure variability (SBPV), diastolic blood pressure variability (DBPV) and related variables during anaesthesia induction in patients (n = 54) that underwent elective surgery.
| SBPV |
DBPV |
|||
|---|---|---|---|---|
| P-value | r | P-value | r | |
| Total dose of sufentanil, µg | NS | −0.1900 | NS | −0.1491 |
| Total dose of rocuronium, mg | NS | −0.1616 | NS | −0.1349 |
| Total dose of propofol, mg | NS | −0.2913 | NS | −0.0176 |
| Age, years | P = 0.0124 | 0.3381 | NS | 0.0201 |
| Height, cm | NS | −0.2095 | NS | −0.0785 |
| Weight, kg | NS | −0.2023 | NS | −0.0492 |
| BMI, kg/m2 | NS | −0.1000 | NS | −0.0515 |
| SBP, mmHg | P = 0.0215 | 0.3122 | NS | 0.0352 |
| DBP, mmHg | NS | −0.0081 | NS | −0.0153 |
| PWTT, ms | P < 0.0001 | −0.5432 | P = 0.0048 | −0.3781 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; PWTT, pulse wave transit time; NS, no significant association (P ≥ 0.05).
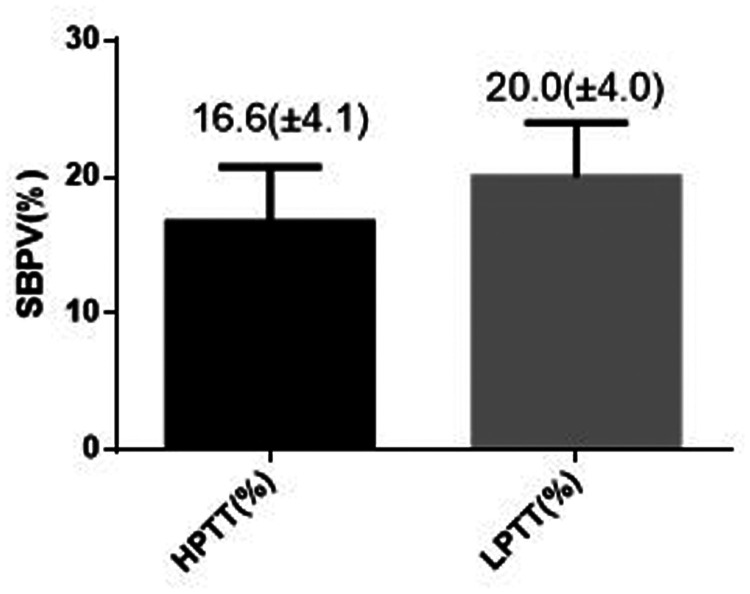
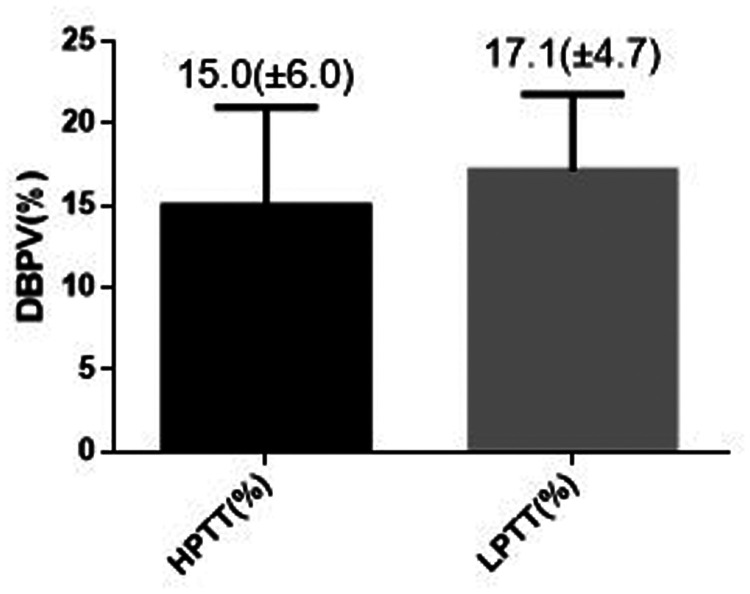
In order to evaluate the correlation between PWTT and BPV during anaesthesia induction, the study population was divided into a high PWTT group (PWTT ≥96.8 ms; n = 27) and a low PWTT group (PWTT <96.8 ms; n = 27) for comparison. There were no significant differences between the two groups in terms of sex, age, height, weight, BMI, SBP, DBP and dose of propofol per kg of body weight (Table 3). The SBPV and DBPV were significantly different between the high PWTT and low PWTT groups; compared with the high PWTT group, the SBPV of the low PWTT group increased significantly by 3.4% (Figure 1; P < 0.01). The DBPV of the low PWTT group increased significantly by 2.1% compared with the high PWTT group (Figure 2; P < 0.05).
Table 3.
Demographic and clinical characteristics of patients (n = 54) that underwent elective surgery stratified into a high pulse wave transit time (PWTT) group (PWTT ≥96.8 ms) and a low PWTT group (PWTT <96.8 ms).
| Characteristic | High PWTT group n = 27 | Low PWTT group n = 27 |
|---|---|---|
| Male/female | 15/12 | 12/15 |
| Age, years | 59.3 ± 11.3 | 59.1 ± 9.2 |
| Height, cm | 163.7 ± 8.4 | 161.3 ± 7.2 |
| Weight, kg | 67.4 ± 10.7 | 63.0 ± 8.2 |
| BMI, kg/m2 | 25.1 ± 3.5 | 24.2 ± 2.5 |
| SBP, mmHg | 165.1 ± 18.8 | 176.5 ± 23.4 |
| DBP, mmHg | 79.0 ± 8.4 | 81.6 ± 8.7 |
| Propofol, mg/kg | 2.35 ± 0.37 | 2.24 ± 0.28 |
Data presented as mean ± SD or n of patients.
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
No significant between-group differences (P ≥ 0.05); continuous data were compared using Student’s t-test; and categorical data were compared using χ2-test.
Figure 1.
Comparison of systolic blood pressure variability (SBPV) in patients (n = 54) that were stratified into high (HPTT) and low (LPTT) pulse wave transit time groups. Data presented as mean ± SD.
Figure 2.
Comparison of diastolic blood pressure variability (DBPV) in patients (n = 54) that were stratified into high (HPTT) and low (LPTT) pulse wave transit time groups. Data presented as mean ± SD.
Discussion
Blood pressure during anaesthesia induction undergoes short-term fluctuations, which are mainly affected by the dose of the induction drugs, the autonomic nervous system, arterial stiffness, baroreflex regulation and other cardiovascular regulatory mechanisms.15–18 As a good indicator of arterial stiffness, PWTT is sensitive to vascular dilatation. 11 Reduced SBP during anaesthesia induction could be detected by tracking changes in PWTT simultaneously; and changes in PWTT indicate the onset of changes in blood pressure and a 15% decrease in PWTT could predict a ≥30% decrease in SBP. 12 PWTT is also a useful indicator of the state of the body, which could reflect the activities of the autonomic nervous system during sevoflurane anaesthesia. 19 A previous study found that PWTT was related to the influence of the autonomic nervous system on the cardiovascular system. 20 The baroreceptors are sensory nerve endings that are sensitive to mechanical distension, which are mainly located in the aortic arch and carotid sinus. The baroreceptor reflex is an important mechanism for regulating short-term BPV, because it can sense changes in vascular tension and shape, convert mechanical signals into electrical signals and provide important feedback to regulate blood pressure. 21 A previous study found that increased arterial stiffness was independently associated with impaired baroreceptors; and PWTT was an indicator of arterial stiffness, so there was a correlation between PWTT and baroreceptors. 22 Therefore, PWTT may be a predictor of BPV during anaesthesia induction.
To the best of our knowledge, this is the first study to demonstrate that PWTT assessed before anaesthesia induction was associated with BPV during induction. This current study also found that age and SBP were associated with SBPV during anaesthesia induction. Only PWTT was associated with both SBPV and DBPV. The mean PWTT of the study participants determined the threshold value and the cohort was divided into high and low PWTT groups. The SBPV and DBPV were significantly different between the high and low PWTT groups. The SBPV of the low PWTT group was 3.4% higher than that of the high PWTT group. The DBPV increased by 2.1% in the low PWTT group compared with the high PWTT group. These current results suggest that arterial stiffness as assessed by PWTT before anaesthesia induction might be a useful indicator for predicting BPV during induction.
The target-controlled infusion mode was applied for the propofol administration, which takes several factors into account such as age, weight and sex in order to calculate the concentration of drug at the affected site and the total dose administered during anaesthesia induction. 9 For the induction of anaesthesia, etomidate might be more appropriate in elderly patients, but the side-effect of muscle tremor interferes with the arterial blood pressure waveform. 23 Thus, propofol induction was used in the current study. In this current study, ECG and AP were recorded using a computer to avoid potential human interference. BPV was obtained by calculating the values of each invasive AP during the induction process. By using a computer, it was possible to analyse a large amount of data, resulting in no omissions and more accurate results. The ECG and AP waveforms were captured at 300 Hz and 100 Hz, respectively, rather than continuously transmitted, so there might be some deviation between the time of the apex of the R wave in the ECG and the starting point of the ascending branch in the invasive AP wave. The higher the capture frequency, the more accurate the data. The study period time was from 1 min before anaesthesia induction administration to 5 min after endotracheal intubation; the whole process being approximately 10 min. There was no expected external disturbance interference during this period except for the effects of the anaesthetic drugs and endotracheal intubation on blood pressure.
This current study had several limitations. First, three patients were treated with ephedrine because their blood pressure decreased by ≥30% after anaesthesia induction. Ephedrine was used to maintain stable haemodynamics, which would interfere with BPV and reduce BPV. The PWTT values for these three patients were 88 ms, 88 ms and 100 ms, which were at medium and low levels in this study. These findings for these three patients were consistent with the results of this current study. Previous studies on PWTT were all small sample studies and the data were not sufficient to show a reference value of PWTT in the normal range.13,24Therefore, the mean used in this current study as the threshold to stratify the patients into a high PWTT group and a low PWTT group lacked accuracy. Secondly, due to the small sample size of this study, the predictive value of PWTT before anaesthesia induction on haemodynamics during the whole induction period needs to be further confirmed by increasing the sample size. Thirdly, this current study only included ASA I or II noncardiac surgery patients, so the results of this study might not be directly applicable to high-risk patients.
In conclusion, these current results suggest that arterial stiffness as assessed by PWTT prior to induction may be a useful indicator for predicting BPV during induction.
Footnotes
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Guoqiang Hu https://orcid.org/0000-0003-2706-922X
References
- 1.POISE Study Group, Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371: 1839–1847. [DOI] [PubMed] [Google Scholar]
- 2.Ko SB. Perioperative stroke: pathophysiology and management. Korean J Anesthesiol 2018; 71: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brienza N, Giglio MT, Marucci M, et al. Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study. Crit Care Med 2009; 37: 2079–2090. [DOI] [PubMed] [Google Scholar]
- 4.Baddal N, Conkbayir C, Erdemli O, et al. Comparison of fentanil and remifentanil for coronary artery surgery with low ejection fraction. Arch Med Sci Atheroscler Dis 2020; 5: e20–e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okada H, Fukui M, Tanaka M, et al. Visit-to-visit variability in systolic blood pressure is correlated with diabetic nephropathy and atherosclerosis in patients with type 2 diabetes. Atherosclerosis 2012; 220: 155–159. [DOI] [PubMed] [Google Scholar]
- 6.Gosmanova EO, Mikkelsen MK, Molnar MZ, et al. Association of Systolic Blood Pressure Variability With Mortality, Coronary Heart Disease, Stroke, and Renal Disease. J Am Coll Cardiol 2016; 68: 1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masugata H, Senda S, Murao K, et al. Visit-to-visit variability in blood pressure over a 1-year period is a marker of left ventricular diastolic dysfunction in treated hypertensive patients. Hypertens Res 2011; 34: 846–850. [DOI] [PubMed] [Google Scholar]
- 8.Zhou TL, Henry R, Stehouwer C, et al. Blood Pressure Variability, Arterial Stiffness, and Arterial Remodeling. Hypertension 2018; 72: 1002–1010. [DOI] [PubMed] [Google Scholar]
- 9.Alecu C, Cuignet-Royer E, Mertes PM, et al. Pre-existing arterial stiffness can predict hypotension during induction of anaesthesia in the elderly. Br J Anaesth 2010; 105: 583–588. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J andCritchley LA.. Inferior Vena Cava Ultrasonography before General Anesthesia Can Predict Hypotension after Induction. Anesthesiology 2016; 124: 580–589. [DOI] [PubMed] [Google Scholar]
- 11.Zhang YL, Zheng YY, Ma ZC, et al. Radial pulse transit time is an index of arterial stiffness. Hypertens Res 2011; 34: 884–887. [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, Song JG, Park JH, et al. Beat-to-beat tracking of systolic blood pressure using noninvasive pulse transit time during anesthesia induction in hypertensive patients. Anesth Analg 2013; 116: 94–100. [DOI] [PubMed] [Google Scholar]
- 13.Kang JE, Song IK, Lee JH, et al. Pulse transit time shows vascular changes caused by propofol in children. J Clin Monit Comput 2015; 29: 533–537. [DOI] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 15.Scherpereel PA andTavernier B.. Perioperative care of diabetic patients. Eur J Anaesthesiol 2001; 18: 277–294. [DOI] [PubMed] [Google Scholar]
- 16.Lankhorst S, Keet SW, Bulte CS, et al. The impact of autonomic dysfunction on peri-operative cardiovascular complications. Anaesthesia 2015; 70: 336–343. [DOI] [PubMed] [Google Scholar]
- 17.Schillaci G, Bilo G, Pucci G, et al. Relationship between short-term blood pressure variability and large-artery stiffness in human hypertension: findings from 2 large databases. Hypertension 2012; 60: 369–377. [DOI] [PubMed] [Google Scholar]
- 18.Vullo PA Real Navacerrada MI andNavarro Suay R.. Hemodynamic impact of increasing time between fentanyl and propofol administration during anesthesia induction: a randomised, clinical trial. Braz J Anesthesiol 2021: S0104-0014(21)00290-6. [DOI] [PubMed] [Google Scholar]
- 19.Baik SW Ye SY andJeon GR. Comparison of heart rate variability with pulse transit time during general anesthesia. Proceedings of the 9th WSEAS International Conference on SIGNAL PROCESSING, 2010; 25–28.
- 20.Weiss T, Del Bo A, Reichek N, et al. Pulse transit time in the analysis of autonomic nervous system effects on the cardiovascular system. Psychophysiology 1980; 17: 202–207. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi K andNaruse K.. Response to mechanical stimulus and cardiovascular homeostasis. Clin Calcium 2016; 26: 1671–1676 [Article in Japanese; English abstract]. [PubMed] [Google Scholar]
- 22.Michas F, Manios E, Stamatelopoulos K, et al. Baroreceptor reflex sensitivity is associated with arterial stiffness in a population of normotensive and hypertensive patients. Blood Press Monit 2012; 17: 155–159. [DOI] [PubMed] [Google Scholar]
- 23.Xu JS, Chen HX, Chen H, et al. Effect of etomidate on hemodynamics in elderly and shock patients during general anesthesia induction. Di Yi Jun Yi Da Xue Xue Bao 2005; 25: 1060–1061 [Article in Chinese, English abstract]. [PubMed] [Google Scholar]
- 24.Kortekaas MC, Niehof SP, van Velzen MH, et al. Pulse transit time as a quick predictor of a successful axillary brachial plexus block. Acta Anaesthesiol Scand 2012; 56: 1228–1233. [DOI] [PubMed] [Google Scholar]