Abstract
Objective
Polycystic ovary syndrome (PCOS) is a prevalent endocrine disorder in women of reproductive age. Chemerin has recently been discovered as a novel adipokine associated with obesity and metabolic syndrome. Excessive autophagy activity and overexpression of autophagy-related genes in follicular granulosa cells are important mechanisms of PCOS. This study aimed to investigate the effect of chemerin on autophagy in PCOS.
Methods
A rat model of PCOS was established by subcutaneous injection of testosterone propionate under a high-fat diet. Expression levels of chemerin and its receptor CMKLR1 were determined by real-time polymerase chain reaction and western blot. Proliferation and apoptosis of human granulosa cells in vitro and expression of autophagy-related genes were examined using bafilomycin A1 (autophagy inhibitor) and Torin1 (autophagy inducer).
Results
Chemerin and CMKLR1 expression were significantly increased in the ovary in a rat model of PCOS. Ectopic expression of chemerin promoted the proliferation and inhibited the apoptosis of COV434 cells. Ectopic expression of chemerin also induced autophagy by inhibiting the phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathway.
Conclusions
Chemerin and CMKLR1 were overexpressed in PCOS rats. Chemerin promoted autophagy through inhibiting the PI3K/Akt/mTOR pathway, and may provide a potential target and biomarker of PCOS.
Keywords: Polycystic ovary syndrome, CMKLR1, COV434 granulosa cell, chemerin, autophagy, mammalian target of rapamycin
Introduction
Polycystic ovary syndrome (PCOS) is a multifactorial heterogeneous clinical syndrome characterized by hyperandrogenism and chronic anovulation, and is one of the most prevalent endocrine disorders in women of reproductive age. PCOS is associated with follicle growth arrest, chronic anovulation, minimal granulosa cell proliferation, hyperthecosis with hyperandrogenemia, and insulin resistance. 1 Several studies2–4 have demonstrated that PCOS is a complex and multifactorial disorder involving adipocyte factors. Abnormal lipid metabolism plays an important role in the pathogenesis of many diseases. 2 Adiponectin, together with adipocyte size, is the strongest factor associated with insulin resistance in women with PCOS. 3 Metabolic abnormalities are also more prevalent in obese PCOS women. 4 Androgen excess, insulin resistance, and adipocyte factors are thus the three main factors determining the symptoms of PCOS.
Chemerin is a chemoattractant ligand for the G protein-coupled receptor chemokine receptor-like 1 (CMKLR1). Chemerin has been shown to promote adipogenesis and regulate immunity and glucose metabolism. 5 The chemerin gene, located at 7q361, encodes a precursor protein containing 163 amino acid residues with a molecular weight of 18 kD, which is transformed into active chemerin after digestion of hydrolytic carboxyl by extracellular protease,6,7 then released into the body fluids. Patients with PCOS were shown to have increased serum chemerin concentrations.8,9 In addition, elevated chemerin induced insulin resistance in human granulosa-lutein cells from PCOS patients, 8 and individuals with higher chemerin expression tended to have a higher risk of PCOS. 9 However, chemerin’s function in PCOS is largely unknown.
Autophagy is a major degradative pathway responsible for energy stress and cellular homeostasis to maintain energy production, as well as the synthesis of new macromolecules. Increasing evidence has revealed that increased autophagy is associated with chronic inflammatory behavior in polycystic ovaries. 10 Li et al. 11 reported that 41 autophagy-related genes were differentially expressed in PCOS patients and animal models, including genes involved in four significant pathways: the insulin, ErbB, mammalian target of rapamycin (mTOR) signaling pathways, and protein processing in the endoplasmic reticulum.
In the current study, we aimed to provide evidence for a potential role of chemerin in autophagy disorders in PCOS. We investigated the relationship between chemerin and PCOS and autophagy in a rat model in vivo and in human granulosa cells in vitro, and discussed the potential clinical applications of chemerin.
Materials and methods
Rat model of PCOS
Forty specific-pathogen-free (SPF) female Sprague–Dawley rats (55–70 g, 21 days old) were purchased from the Laboratory Animal Center of Chongqing Medical University. The rats were kept in an SPF-class laboratory animal room in Chongqing Medical University, and housed at a constant temperature of 20°C, with light from 08:00 to 20:00 and dark from 20:00 to 08:00. Standard chow and water were available.
All the rats were fed for 7 consecutive days to adapt to the environment. Forty rats were then divided randomly into four experimental groups (n = 10 per group). Rats in the high-fat diet group (F) were fed a high-fat diet (26.2 g% protein, 26.3 g% carbohydrate, and 34.9 g% fat); rats in the testosterone propionate (#154498, Aike Reagent, Chengdu, China) group (T) were injected subcutaneously with testosterone propionate 10 mg/kg/day (dissolved in 0.2 ml olive oil); rats in the high-fat diet/testosterone propionate group (model group) were fed a high-fat diet and injected subcutaneously with testosterone propionate 10 mg/kg/day (dissolved in 0.2 ml olive oil) at the same time; and control group rats were fed a normal diet and injected subcutaneously with an identical volume of olive oil. All the experimental groups were managed for 56 days. 12 The basic principle of the model was that excessive androgen interferes with follicle growth and maturation resulting in non-ovulation, and a high-fat diet leads to hyperinsulinemia and insulin resistance, similar to PCOS.
Sample collection
All the rats were weighed every 2 days. Vaginal smears were monitored daily for 10 consecutive days to determine the stage of the estrus cycle, starting at day 54. Vaginal smears obtained by vaginal washing were analyzed under a microscope to determine the predominant cell type. Vaginal epithelium with sustained keratosis represented successful establishment of the PCOS model. At the end of the study, all rats were fasted overnight and infused intragastrically with 2 g/kg glucose, and blood samples were collected at 0, 30, 60, and 120 minutes to evaluate blood glucose levels. The rats were then sacrificed using pentobarbital sodium (intravenous 100 mg/kg) and blood samples obtained from rats fasted for 12 hours were centrifuged at 350 × g for 15 minutes and stored at −80°C. Ovary tissues were also obtained from all rats and stored at −80°C. Oral glucose tolerance tests (OGTTs) and smears of exfoliated vaginal cells were used to confirm the creation of the rat model. All animal experimental procedures were approved by the Committee on the Use and Care of Animals of Chongqing Medical University, Chongqing, China (CMU201866, approved on September 1, 2018) and performed in accordance with the institution’s guidelines. 13 All efforts were made to minimize the number of animals used and to decrease their suffering.
Cell culture and transfection
COV434 human ovarian granulosa cells derived from a solid primary granulosa tumor were purchased from Beijing Beina Chuanglian Biotechnology Institute, Beijing, China (No. BNCC338036) and cultured in Dulbecco’s Modified Eagle Medium (10-017-CVR; Corning, NY, USA) containing 10% fetal bovine serum. The cells were incubated under 5% carbon dioxide at 37°C.
Lentiviral vectors expressing short hairpin RNAs targeting chemerin (LV3-590 and LV3-1623) and the chemerin-lentiviral expression vector (LV5-chemerin) were provided by Genepharma (Shanghai, China). The autophagy inhibitor bafilomycin A1 (Cat. No: S1413; Selleck Chemicals) was dissolved in dimethylsulfoxide and diluted with serum-free culture medium to reach a concentration of 100 nM. The mTOR inhibitor, Torin1 (Cat. no: S2827; Selleck Chemicals, Shanghai, China), which induces autophagy, was used to treat cells at a concentration of 10 mM. Cell proliferation of COV434 cells was detected in vitro using Cell-Light™ EdU (C10310; Guangzhou RiboBio Co., Ltd., Guangzhou, China) and apoptosis was detected by flow cytometry (Accuri C6; Biosciences, NY, USA).
Quantitative real-time polymerase chain reaction (RT-qPCR)
Total RNA was isolated using a high-purity Total RNA Rapid Extraction Kit (RP1201; Bioteke Corporation, Beijing, China) according to the manufacturer's instructions. cDNA was synthesized using an Iscript cDNA synthesis kit (4106228; Bio-Rad Laboratories, CA, USA). The primers used for amplifying chemerin and CMKLR1 and β-actin were synthesized by GeneCopoeia (Guangzhou, China). The RT-qPCR kit was purchased from GeneCopoeia. The PCR conditions were as follows: 95°C for 10 s, 60°C for 20 s, and 72°C for 10 s. Each sample was analyzed in triplicate. Relative quantification of mRNA was performed using the comparative threshold cycle (CT) method. This value was used to plot the gene expression using the formula 2−△△CT.
Western blot
Pathways that activate mTOR, such as Akt and mitogen-activated protein kinase (MAPK), inhibit autophagy, while pathways that negatively regulate mTOR, such as AMP-activated protein kinase, promote autophagy. When mTOR inhibition occurs, Unc-51-like autophagy-activating kinase 1 (ULK1) induces autophagy by phosphorylation of beclin-1. 14 We analyzed the protein expression levels of CMKLR1, phosphoinositide 3-kinase (PI3K), Akt, MAPK, ULK-1, extracellular signal-regulated kinase (ERK), phospho-mTOR (p-mTOR), and β-actin by western blot, using the following primary antibodies: polyclonal rabbit anti-chemerin (ab103153; Abcam, Shanghai, China); polyclonal rabbit anti-CMKLR1 (bs-10410R; Bioss Biotechnology Company, Beijing, China); rabbit monoclonal to ULK1 (D8H5) (#8054; Cell Signaling Technology, MA, USA); rabbit monoclonal to p-mTOR (Ser2448) (#5536; Cell Signaling Technology); rabbit monoclonal to MAPK (#8690; Cell Signaling Technology); rabbit monoclonal to Akt (#4691; Cell Signaling Technology); rabbit monoclonal to PI3K Class III (D9A5) (#4263; Cell Signaling Technology); rabbit monoclonal to LC3A/B (D3U4C) (#12741S; Cell Signaling Technology); and polyclonal rabbit anti-β-actin (#4970S; Cell Signaling Technology). Band density was analyzed using a gel imaging system and compared with an internal control, as described previously. 15
Statistical analysis
All the experiments were conducted at least three times individually. Statistical analysis was carried out using GraphPad Prism software version 7.0 (GraphPad Software, CA, USA), and values were expressed as mean ± standard deviation. Means were compared between groups using Student's t-test, and means were compared among multiple groups by one-way ANOVA. Comparisons between groups were made using the Student–Newman–Keuls test. A P value <0.05 was considered significant.
Results
Chemerin/CMKLR1 expression were increased in ovarian tissues in PCOS model rats
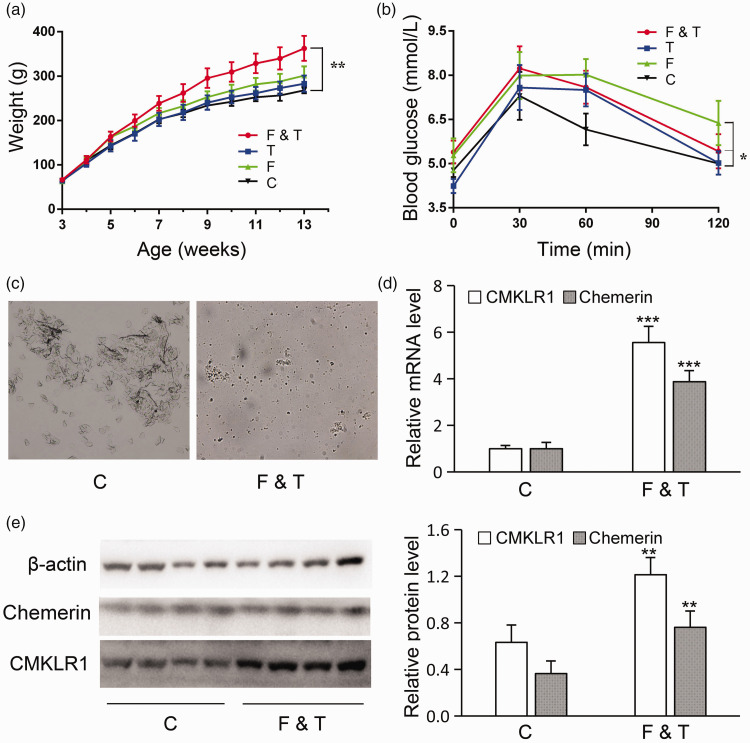
PCOS model rats (n = 10) were injected subcutaneously with testosterone propionate (1 mg/100 g rat) and fed a high-fat diet. Control rats (n = 10) rats were fed an ordinary diet and injected subcutaneously with an equal volume of olive oil. Based on the results of vaginal smears, six successful PCOS model rats were selected for further analysis. Body weights were significantly higher in the model (F&T) compared with the control rats (P < 0.01; Figure 1a). However, the OGTT results showed that rats in the model group also developed significantly impaired glucose tolerance (P < 0.05; Figure 1b). The results of vaginal smears revealed that the estrous cycle was inhibited in the PCOS rats (Figure 1c). In addition, mRNA and protein levels of chemerin and CMKLR1 were significantly increased in PCOS model rats (Figure 1d, e).
Figure 1.
Chemerin/CMKLR1 was increased in ovarian tissues in a rat model of polycystic ovary syndrome (PCOS) (n = 6). (a) Rats in the high-fat diet (F), testosterone propionate (T), model (F&T), and control groups (C) were weighed every 2 days during the modeling. (b) Oral glucose tolerance tests were performed in all the experimental groups to reveal impaired glucose tolerance. (c) Vaginal smears were examined after modeling in each group. (d and e) CMKLR1/chemerin mRNA and protein levels were upregulated in ovarian tissues in the model group. Error bars represent standard error. Bars show mean ± standard deviation of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control group.
Chemerin regulated the proliferation and apoptosis of COV434 cells in vitro
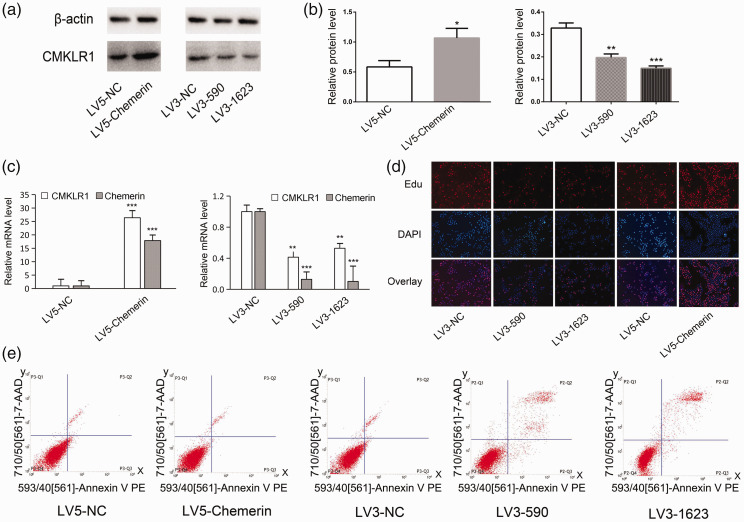
We also examined the function of chemerin in COV434 human ovarian granulosa cells. The mRNA and protein levels of chemerin and its receptor CMKLR1 were increased in LV5-chemerin-transfected COV434 cells compared with control cells (Figure 2a–c), but were reduced in cells transfected with LV3-590 and LV3-1623 (Figure 2a–c). We also examined the effect of chemerin on the proliferation and apoptosis of COV434 cells. Cell proliferation was suppressed in LV3-590-transfected and LV3-1623-transfected cells compared with control cells. In contrast, transfection with LV5-chemerin promoted cell proliferation (Figure 2d). Apoptosis measured by flow cytometry was decreased in LV5-chemerin-transfected cells and increased in LV3-590-transfected and LV3-1623-transfected cells (Figure 2e). These results indicated that chemerin promoted cell proliferation and suppressed apoptosis in COV434 cells.
Figure 2.
Chemerin regulated cell proliferation and apoptosis in vitro. (a and b) CMKLR1 protein levels were upregulated by transfection with LV5-chemerin and downregulated by transfection with LV3-590 and LV3-1623. (c) CMKLR1 mRNA levels were upregulated by transfection with LV5-chemerin and downregulated by transfection with LV3-590 and LV3-1623. (d) COV434 cell proliferation was detected using an EdU fluorescence microscope. (e) Apoptotic cells were measured by flow cytometry. Error bars represent standard error. Bars show mean ± standard deviation of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control group.
Chemerin promoted COV434 cell autophagy
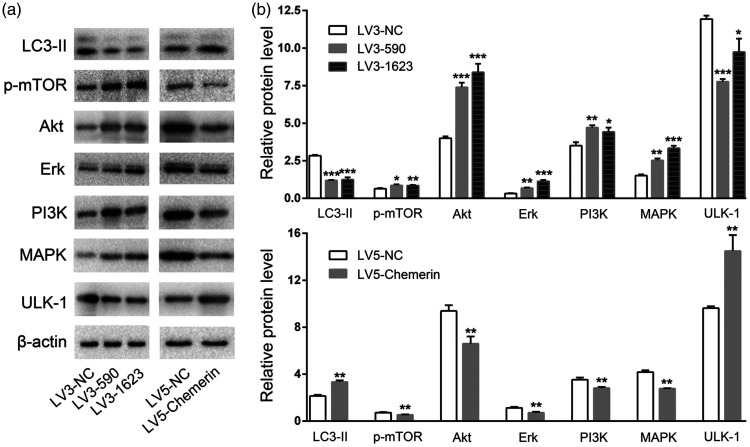
LC3 is a marker of autophagy. During autophagy, cytoplasmic LC3-I is transformed into membrane LC3-II, and the LC3-II/I ratio can thus be used to estimate the level of autophagy. We studied the molecular mechanism of autophagy induced by chemerin. LC3-II expression was significantly decreased in LV3-590- and LV3-1623-transfected COV434 cells (P < 0.001) and increased in LV5-chemerin-transfected COV434 cells compared with control cells (P < 0.01) (Figure 3a, b). Expression of p-mTOR was elevated in LV3-590- (P < 0.05) and LV3-1623-transfected COV434 cells (P < 0.01), but suppressed in LV5-chemerin-transfected COV434 cells compared with the control group (P < 0.01) (Figure 3a, b). Similarly, PI3K, Akt, and ERK expression were up-regulated in LV3-590- and LV3-1623-transfected but down-regulated in LV5-chemerin-transfected COV434 cells (all P < 0.01). As expected, ULK-1 expression was reduced in LV3-590- (P < 0.001) and LV3-1623-transfected cells (P < 0.05) but increased in LV5-chemerin-transfected COV434 cells (P < 0.01), while MAPK was increased in LV3-590- (P < 0.01) and LV3-1623-transfected COV434 cells (P < 0.001) but reduced in LV5-chemerin-transfected COV434 cells (P < 0.01). These data confirmed that chemerin promoted the autophagy of COV434 cells.
Figure 3.
Chemerin promoted COV434 cell autophagy. (a and b) Protein expression levels of LC3-II, p-mTOR, Akt, ERK, PI3K, MAPK, and ULK-1 in COV434 cells were detected by western blot. Error bars represent standard error. Bars show mean ± standard deviation of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control group.
Chemerin promoted autophagy by inhibiting the PI3K/Akt/mTOR signaling pathway
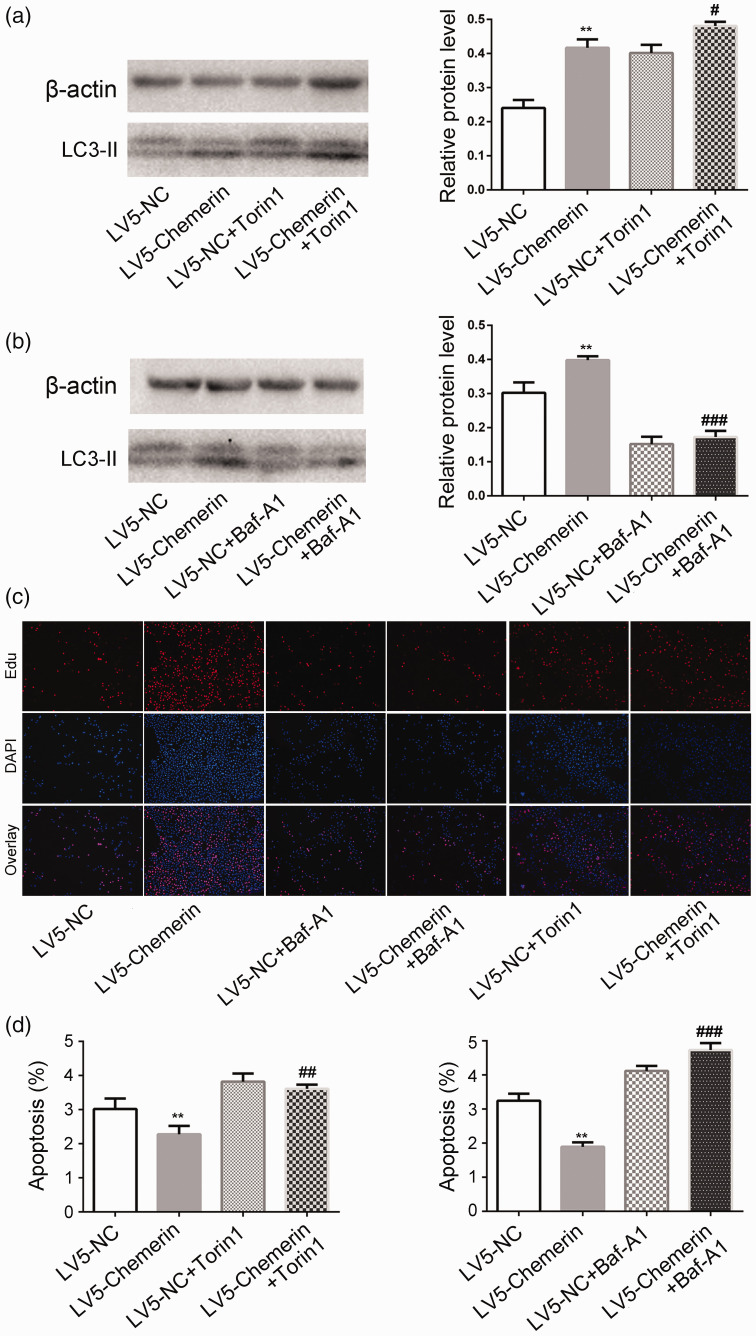
Bafilomycin A1 is an inhibitor of autophagy, while Torin1 inhibits mTOR and induces autophagy. LC3-II expression was enhanced by the addition of Torin1 to LV5-chemerin-transfected COV434 cells (P < 0.05 vs. chemerin) (Figure 4a), but these elevated levels of LC3-II were reversed by the addition of bafilomycin A1 (P < 0.001 vs. chemerin) (Figure 4b). Bafilomycin A1 also inhibited the proliferation and promoted the apoptosis of COV434 cells induced by chemerin, while Torin1 promoted the chemerin-induced proliferation and apoptosis (Figure 4c, d). These results indicate that chemerin promoted autophagy by inhibiting the PI3K/Akt/mTOR pathway, thus promoting cell proliferation and inhibiting apoptosis.
Figure 4.
Chemerin promoted autophagy by inhibiting the PI3K/Akt/mTOR signaling pathway. (a) The effects of (a) Torin1 and (b) bafilomycin A1 on chemerin-induced autophagy were detected by western blot. (c) The effects of Torin1 and bafilomycin A1 on (c) chemerin-induced proliferation and (d) chemerin-induced apoptosis. Bars show mean ± standard deviation of three independent experiments. **P < 0.01 vs. LV5-control, #P < 0.05, ###P < 0.001 vs. LV5-chemerin.
Discussion
The main clinical manifestations of PCOS are obesity, dyslipidemia, and insulin resistance. Numerous studies are currently investigating the pathological mechanism of PCOS, including regulation of the brain–gut axis and hormones. 16 However, the cellular response in the ovary may be the initial pathological mechanism of PCOS, and recent studies found that LC3 protein was mainly expressed in follicular granulosa cells, and that excessive autophagy in ovarian granulosa cells may lead to the occurrence of PCOS. 17
The newly identified adipocytokine chemerin plays an important role in lipid formation and blood glucose regulation. 18 Patients with PCOS had abnormally high serum levels of chemerin, which were reduced by the use of metformin for symptom relief. 19 In addition, clinical data suggest that obesity and type 2 diabetes are also associated with increased chemerin, 20 and this correlation between chemerin and metabolism supports an association between chemerin and PCOS. The current results showed that chemerin and its receptor CMKLR1 were also elevated in ovarian tissues in a PCOS rat model, suggesting that ectopic expression of chemerin may play an important role in PCOS. Chemerin may be involved in regulating the proliferation, apoptosis, and autophagy of COV434 cells. A previous report showed that chemerin promoted the proliferation and invasion of squamous esophageal cancer cells, 21 and the present study similarly showed that chemerin promoted the proliferation and suppressed the apoptosis of COV434 cells.
Autophagy is enhanced in ovarian tissues in both humans and rats with PCOS. Ovarian granulosa cells in a PCOS rat model showed increased expression of the autophagy marker protein light chain 3B (LC3B). 22 In the current study, chemerin promoted the expression of LC3-II, indicating promotion of autophagy. Previous studies demonstrated that autophagy may be regulated by the PI3K, Akt, and ERK pathway.23,24 The current results accordingly indicated that chemerin/CMKLR1 inhibited the expression of PI3K, Akt, and ERK and promoted the expression of ULK-1, thus inducing autophagy. Chemerin also inhibited the expression of MAPK. mTOR acts as an intersection among many pathways, and mTOR activation inhibited autophagy while its inhibition promoted autophagy.25,26 These results revealed that chemerin promoted cell autophagy via inhibiting the PI3K/Akt/mTOR and MAPK signaling pathway. However, inhibition of autophagy decreased proliferation and enhanced apoptosis, indicating that chemerin-induced autophagy promoted cell survival by inhibiting apoptosis during starvation, which may be associated with formation of PCOS.
Although this study provided preliminary confirmation of the role of chemerin in PCOS, further studies are needed to determine its effects on ovarian function during the development of PCOS and its possible use as a target for the diagnosis and treatment of PCOS.
In conclusion, the current results indicated that chemerin and CMKLR1 expression were elevated in a PCOS rat model. Chemerin may promote autophagy by inhibiting the PI3K/Akt/mTOR pathway, and may provide a potential therapeutic target and biomarker of PCOS.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This work was supported by the Natural Science Foundation of Chongqing [grant number CSTC 2015JCYJA10067] and the National Science Foundation of China [grant number 81501220].
ORCID iD: Xiaojing Dong https://orcid.org/0000-0001-8506-9362
References
- 1.Escobar-Morreale HF. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018; 14: 270–284. [DOI] [PubMed] [Google Scholar]
- 2.Dai P, Tang Z, Qi M, et al. The dispersion and utilization of lipid droplets mediates respiratory syncytial virus-induced airway hyperresponsiveness. Pediatr Allergy Immunol. 2021. doi: 10.1111/pai.13651. [DOI] [PubMed] [Google Scholar]
- 3.Benrick A, Chanclón B, Micallef P, et al. Adiponectin protects against development of metabolic disturbances in a PCOS mouse model. Proc Natl Acad Sci U S A. 2017; 114: E7187–E7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Layegh P, Mousavi Z, Farrokh TD, et al. Insulin resistance and endocrine-metabolic abnormalities in polycystic ovarian syndrome: Comparison between obese and non-obese PCOS patients. Int J Reprod Biomed. 2016; 14: 263–270. [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Q, Kim JY, Xue K, et al. Chemerin, a novel regulator of follicular steroidogenesis and its potential involvement in polycystic ovarian syndrome. Endocrinology. 2012; 153: 5600–5611. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Shi B, Li S. Association between serum chemerin concentrations and clinical indices in obesity or metabolic syndrome: A meta-analysis. PLoS One. 2014; 9: e113915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao W, Baecker A, Song Y, et al. Adipokine levels during the first or early second trimester of pregnancy and subsequent risk of gestational diabetes mellitus: A systematic review. Metabolism. 2015; 64: 756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Zhu Q, Wang W, et al. Elevated chemerin induces insulin resistance in human granulosa-lutein cells from polycystic ovary syndrome patients. FASEB J. 2019; 33: 11303–11313. [DOI] [PubMed] [Google Scholar]
- 9.Huang R, Yue J, Sun Y, et al. Increased serum chemerin concentrations in patients with polycystic ovary syndrome: Relationship between insulin resistance and ovarian volume. Clin Chim Acta. 2015; 450: 366–369. [DOI] [PubMed] [Google Scholar]
- 10.Saha P, Kumar S, Datta K, et al. Upsurge in autophagy, associated with mifepristone-treated polycystic ovarian condition, is reversed upon thymoquinone treatment. J Steroid Biochem Mol Biol. 2021; 208: 105823. [DOI] [PubMed] [Google Scholar]
- 11.Li D, You Y, Bi FF, et al. Autophagy is activated in the ovarian tissue of polycystic ovary syndrome. Reproduction. 2018; 155: 85–92. [DOI] [PubMed] [Google Scholar]
- 12.Ding Y, Jiang Z, Xia B, et al. Mitochondria-targeted antioxidant therapy for an animal model of PCOS-IR. Int J Mol Med. 2019; 43: 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br J Pharmacol. 2020; 177: 3617–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Julia SG, Avinash RS. Regulation and repurposing of nutrient sensing and autophagy in innate immunity. Autophagy. 2021; 17: 1571–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin L, Qiu K, Hu C, et al. Respiratory syncytial virus promoted the differentiation of Th17 cells in airway microenvironment through activation of Notch-1/Delta3. J Med Microbiol. 2019; 68: 649–656. [DOI] [PubMed] [Google Scholar]
- 16.Bajinka O, Simbilyabo L, Tan Y, et al. Lung-brain axis. Crit Rev Microbiol. 2021; 1–13. [DOI] [PubMed] [Google Scholar]
- 17.Kumariya S, Ubba V, Jha RK, et al. Autophagy in ovary and polycystic ovary syndrome: Role, dispute and future perspective. Autophagy. 2021; 17: 2706–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Recinella L, Orlando G, Ferrante C, et al. Adipokines: New potential therapeutic target for obesity and metabolic, rheumatic, and cardiovascular diseases. Front Physiol. 2020; 11: 578966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashraf AF, Engy AF, Mohamed AE, et al. Serum chemerin levels in polycystic ovary syndrome after metformin therapy. Diabetes Metab Syndr. 2019; 13: 1309–1315. [DOI] [PubMed] [Google Scholar]
- 20.Roguska J, Zubkiewicz-Kucharska A. Chemerin as an early marker of metabolic syndrome. Pediatr Endocrinol Diabetes Metab. 2018; 24: 45–51. [DOI] [PubMed] [Google Scholar]
- 21.Kumar JD, Kandola S, Tiszlavicz L, et al. The role of chemerin and ChemR23 in stimulating the invasion of squamous oesophageal cancer cells. Br J Cancer. 2016; 114: 1152–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li D, You Y, Bi FF, et al. Autophagy is activated in the ovarian tissue of polycystic ovary syndrome. Reproduction. 2018; 155: 85–92. [DOI] [PubMed] [Google Scholar]
- 23.Zheng W, Wang B, Si M, et al. Zearalenone altered the cytoskeletal structure via ER stress- autophagy-oxidative stress pathway in mouse TM4 Sertoli cells. Sci Rep. 2018; 8: 3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu RF, Fu G, Li J, et al. Roles of autophagy in androgen-induced benign prostatic hyperplasia in castrated rats. Exp Ther Med. 2018; 15: 2703–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell GR, Bruckman RS, Herns SD, et al. Induction of autophagy by PI3K/MTOR and PI3K/MTOR/BRD4 inhibitors suppresses HIV-1 replication. J Biol Chem. 2018; 293: 5808–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Y, Shan X, Safarpour F, et al. Pharmacological inhibition of O-GlcNAcase (OGA) enhances autophagy in brain through an mTOR-independent pathway. ACS Chem Neurosci. 2018; 9: 1366–1379. [DOI] [PubMed] [Google Scholar]