Abstract
Untreated individuals with antithrombin (AT) deficiency are at higher risk of thrombosis and adverse pregnancy outcomes. The present recommendations are mostly empirical for treating patients with AT deficiency during pregnancy because of the absence of guidelines. We report a rare case of heparin resistance due to AT deficiency in a pregnant 32-year-old Chinese woman. We also reviewed the English medical literature for AT deficiency and its association with thromboembolism and treatment. This patient suffered two early miscarriages because of thrombosis due to AT deficiency. The patient was administered the combination of adequate low molecular weight heparin with fresh frozen plasma and warfarin because of her heparin resistance. She delivered a healthy female newborn without any adverse effects of the anticoagulation therapy. Our findings suggest that the combination of adequate low molecular weight heparin with fresh frozen plasma and warfarin is effective for preventing thrombus during pregnancy.
Keywords: Antithrombin deficiency, recurrent miscarriage, heparin resistance, low molecular weight heparin, fresh frozen plasma, pregnancy, thrombus
Introduction
Antithrombin (AT) is regarded as a natural anticoagulant that inhibits factor Xa, thrombin (factor IIa), and other serine proteases. 1 Inherited AT deficiency is closely related to the occurrence of venous thromboembolism. 2 Egeberg first reported a case of AT deficiency associated with venous thromboembolism in 1965. 3 During pregnancy or delivery, untreated individuals with AT deficiency are at a higher risk of thrombosis. 4 These women also have a high risk of adverse pregnancy outcomes, such as spontaneous miscarriage, intrauterine growth restriction, early-onset severe preeclampsia, and placental abruption owing to a physiological hypercoagulable state that is aggravated with pregnancy. 5
Congenital AT deficiency is a rare autosomal dominant disease due to mutation of the serpin family C member 1 (SERPINC1) gene. To date, more than 330 various mutations have been reported. 5 AT deficiency is classified as two types as follows. Type I (quantitative) deficiency is described as a low amount and activity of AT, which is related to a 20-fold increased risk of venous thrombosis. 6 Type II is a functional defect, which has three subtypes according to the site of the key mutations. The type II reactive site is due to a flaw in the AT reactive site. The type II heparin-binding site (HBS) is a mutation of HBS and pleiotropic mutations that lead to a type II pleiotropic effect. 7
There are currently no guidelines for managing AT deficiency in patients during pregnancy. However, LMWH has been widely used as a prophylactic therapy by the recommendation of the Royal College of Obstetricians and Gynecologists and the American Congress of Obstetricians and Gynecologists.8,9 The Royal College of Obstetricians and Gynecologists recommends 50% to 100% low molecular weight heparin (LMWH) throughout pregnancy. 9 Heparin cannot exert its anticoagulant effect alone. 10 Therefore, because of heparin resistance (HR) present in many AT-deficient patients, the therapeutic effect of LMWH is not uniformly accepted. Furthermore, little data are available on the ideal dosage of LMWH and the appropriate use of substitution therapy. Therefore, focusing on this rare, but severe coagulopathy, is challenging. We report here a pregnant woman with AT deficiency who had a successful pregnancy outcome after therapeutic treatment with an adequate dose of LMVH or warfarin with fresh frozen plasma (FFP) supplementation.
Case report
We report the case of a 32-year-old Chinese patient who had a history of two pregnancy-related thromboembolic events. These events were cerebral vein-venous sinus thrombosis at 5 gestational weeks (GW) when she was 27 years old and left lower thrombosis following a miscarriage in her second pregnancy in the first trimester at 29 years old. At that point, she was then treated with warfarin.
After routine assessments were performed in the patient at 32 years old, no inherited or acquired risk factors for thrombosis or related family history were discovered, except for AT activity. Her AT activity was only 37% (normal range: 75%–125%) (Berichrom Antithrombin III; Siemens, Erlangen, Germany) while the AT antigen (BN ProSpec system AT-III; Siemens) was 93% (normal range: 80%–120%). The D-dimer concentration was 0.2 µg/mL D-dimer units (normal range: 0–0.5 µg/mL D-dimer units), platelet aggregation in response to arachidonic acid was 82% (normal range: 60%–80%), the prothrombin time was 13.5 s (normal range: 10.3–16.6 s), and the activated partial thromboplastin time was 32 s (normal range: 25.1–36.5 s). Her fibrinogen concentration was 1.13 µg/mL (normal range: 0–5 µg/mL), protein C activity was 98.8% (normal range: 70%–140%), protein S activity was 93.8% (normal range: 63.5%–149%), and homocysteine concentration was 9.8 µmol/L (normal range: 3–17 µmol/L). Antiphospholipid antibodies, including lupus anticoagulant, anticardiolipin, and beta-2 glycoprotein I-dependent anticardiolipin antibodies, were negative.
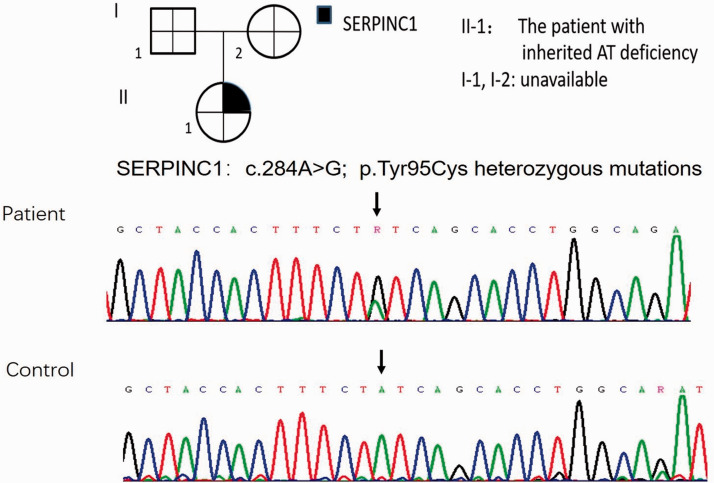
The SERPINC1 gene in this patient was then analyzed by the gold standard Sanger fluorescent sequencing method in which all exons, exon-intron boundaries, and the promoter region were examined (ABI PRISM 3130; Applied Biosystems, Foster City, CA, USA). Molecular analysis showed a heterozygous mutation, which led to a tyrosine to cysteine change at amino acid position 95, compatible with a type II HBS defect (Figure 1). On the basis of the findings of a low AT activity level, normal AT antigen level, and genetic result, we made the diagnosis of type II hereditary AT deficiency. Unfortunately, her parents were not available for laboratory assessment for AT activity and genetic testing.
Figure 1.
Gene sequence of the patient.
SERPINC1, serpin family C member 1; AT, antithrombin.
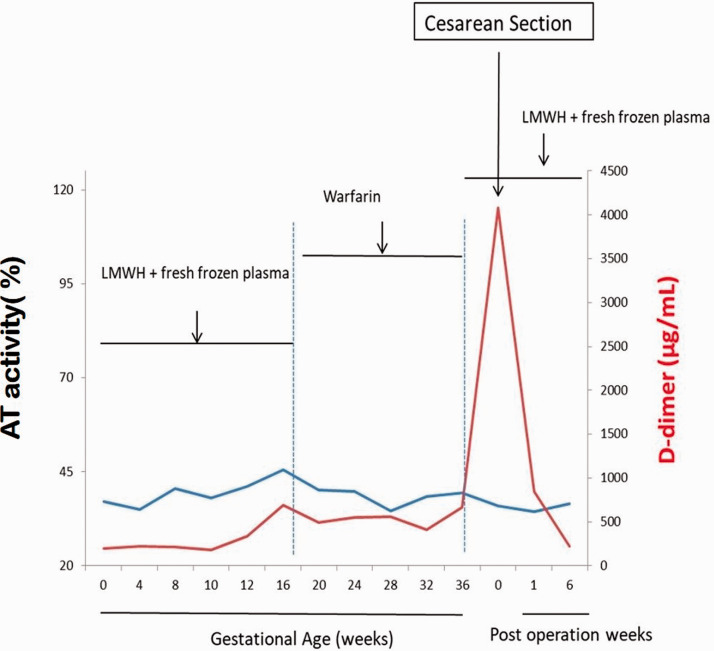
When the patient was 31 years old, she consulted her doctor because she wanted to become pregnant again. At the start of her pregnancy, thromboprophylaxis with self-injection of LMWH at 4100 units every 12 hours with FFP 1 unit weekly was prescribed to replace warfarin because of its adverse effects on the fetus. She had arachidonic acid and D-dimer concentrations measured fortnightly, while her AT activity and anti-Xa measurements were checked monthly throughout pregnancy (Figure 2). Her serum AT activity level remained at 30% to 40%, but no thrombosis occurred during pregnancy. Her plasma D-dimer concentrations ranged from 0.2 to 1.5 µg/mL D-dimer units, which were within the expected range for pregnant women. Nevertheless, peak anti-Xa activity levels ranged from 0 to 0.29 IU/mL in the second trimester. This activity was not consistent with the required dose ranging from 0.5 to 1 U/mL 2 to 4 hours following the injection. This finding indicated the phenomenon of HR.
Figure 2.
AT and D-dimer concentrations during and after pregnancy.
AT, antithrombin; LMWH, low molecular weight heparin.
After 14 GW, she used LMWH as a bridge to warfarin, then she continued warfarin therapy before delivery. The international normalized ratio was monitored weekly to achieve a level between 2.0 and 3.0. She resumed LMWH and FFP from 37 GW. A cesarean section was scheduled at 37+4 GW. The patient delivered a healthy, 3165-g girl with Apgar scores of 10 at both 1 and 5 minutes. There were no adverse effects of the anticoagulation therapies. The patient and her newborn were discharged without morbidity 7 days later. She continued the same doses of LMWH with FFP, which was replaced with oral anticoagulant therapy from 6 weeks after delivery. The patient and her infant were healthy with no sequelae or adverse events at a follow-up visit 6 weeks after delivery.
The reporting of this study conforms to the CARE guidelines. 11
Discussion
Hereditary AT deficiency is related to mutation of the SERPINC1 gene at the position 1q23-q25. 5 Many studies have shown that the variety of clinical manifestations and severity of this disease depend on the type of SERPINC1gene mutation. Deep venous thrombosis and pulmonary embolism are the most common occurrences in AT-deficient patients. 12 Heterozygous type II HBS is usually thought to have a low thrombotic risk, 7 while the homozygous form leads to early onset of venous and arterial thrombosis. 13 We report a case of a 32-year-old Chinese woman who was diagnosed with heterozygous type II hereditary AT deficiency. 14 and suffered from pregnancy-related thrombosis twice. Data on women with the severe form of heterozygous type II hereditary AT deficiency are scarce. 7 The findings in our case suggest a variety of type II AT deficiency, which may affect the prevention and treatment of pregnant women with AT deficiency.
LMWH is widely regarded as a safe and effective substitute for oral anticoagulant therapy, such as warfarin, during the early and late stages of pregnancy. 15 Unlike unfractionated heparin, LMWH has greater activity against factor Xa than thrombin. Therefore, LMWH has a reduced risk of bleeding, heparin-induced thrombocytopenia, and osteoporosis. 16 In addition to the anticoagulant properties of LMWH, it has other beneficial effects on the placenta, such as enhanced angiogenesis in endothelial cells, inhibition of the tissue factor pathway, and decreased platelet aggregation, trophoblast apoptosis, and complement activation. 17
In AT deficiency, less antithrombin–heparin complexes are in the circulation. Therefore, women with HR need to take more heparin to prevent thrombosis. 18 HR might be regarded as a state of response to a variable dose of heparin. Therefore, a combination of supplementary LMWH, FFP, or AT concentrate is usually recommended. 19 However, few well-designed clinical studies have investigated the appropriate dosage of heparin and substitution treatment in patients with AT deficiency during pregnancy. 13
Sabbagh et al. was the first to treat patients with HR who underwent cardiopulmonary bypass with FFP. 20 Barnette et al. showed that FFP contained some other coagulation factors, such as heparin cofactor 2, and it was an optional source of AT to neutralize HR that resulted in an increased heparin effect. 21 A low dosage of LWMH is better for managing the anticoagulation status during pregnancy. In this case, based on the patient’s history, clinical manifestations, laboratory tests, and the cost–benefit ratio, we treated her with the combination of prophylactic LWMH with FFP weekly at the early and late stages of pregnancy as a substitute for warfarin. This patient successfully delivered a healthy newborn without any adverse effects of the anticoagulation therapy.
Although AT concentrate is regarded as an efficient supplement that decreases volume administration, there is a risk of transfusion-related acute lung injury and transfusion-related infections, 19 and AT concentrate costs much more than FFP. There is also a large amount of uncertainty regarding the amount of AT concentrate and when to administer this concentrate. Furthermore, AT concentrate is not available in our area. On the basis of these reasons, we do not routinely treat AT-deficient patients with AT concentrate in our hospital.
Our study has some limitations that should be discussed. Because of technical limitations, we were not able to perform polymerase chain reaction analysis for the prothrombotic mutations factor V Leiden and FII G20210A in this patient. Additionally, data supporting a potential type II HBS deficiency (e.g., crossed immunoelectrophoresis, heparin cofactor, and progressive activity) were not available. However, because of the rarity of AT deficiency during pregnancy, we consider that reporting its management and course during pregnancy is important because it might lead to a better understanding of this rare disease.
Conclusions
We report a pregnant woman with AT deficiency who had a successful pregnancy outcome after therapeutic treatment with an adequate dose of LMVH or warfarin with FFP supplementation. Our findings might be helpful for preventing maternal complications and complications in the fetus in patients with congenital AT deficiency. Further investigations are necessary regarding this issue, but this will be challenging because of the rarity of this disorder.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Ethics statement: All procedures were performed in accordance with the ethical standards of the Ethics Committee of RenJi Hospital, School of Medicine, Shanghai Jiao Tong University (approval number: KY2020-082) and with the declaration of Helsinki (as revised in Edinburgh 2000). Informed written consent was obtained from the patient.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This work was supported by the Bethune Foundation (X-J-2019-024).
ORCID iD: Aimin Zhao https://orcid.org/0000-0002-8022-5175
References
- 1.Paidas MJ, Triche EW, James AH, et al . Recombinant human antithrombin in pregnant patients with hereditary antithrombin deficiency: integrated analysis of clinical data. Am J Perinatol 2016; 33: 343–349. [DOI] [PubMed] [Google Scholar]
- 2.Di Minno MN, Dentali F, Veglia F, et al. Antithrombin levels and the risk of a first episode of venous thromboembolism: a case-control study. Thromb Haemost 2013; 109: 167–169. [DOI] [PubMed] [Google Scholar]
- 3.Egeberg O. Inherited antithrombin deficiency causing thrombophilia. Thromb Diath Haemorrh 1965; 13: 516–530. [PubMed] [Google Scholar]
- 4.James AH, Jamison MG, Brancazio LR, et al. Venous thromboembolism during pregnancy and the postpartumperiod: incidence, risk factors, and mortality. Am J Obstet Gynecol 2006; 194: 1311–1315. [DOI] [PubMed] [Google Scholar]
- 5.Kovac M, Mitic G, Mikovic Z, et al. The influence of specific mutations in the AT gene (SERPINC1) on the type of pregnancy related complications. Thromb Res 2019; 173: 12–19. [DOI] [PubMed] [Google Scholar]
- 6.Abbattista M, Gianniello F, Novembrino C, et al. Risk of pregnancy-related venous thromboembolism and obstetrical complications in women with inherited type I antithrombin deficiency: a retrospective, single-centre, cohort study. Lancet Haematol 2020; 7: e320–e328. [DOI] [PubMed] [Google Scholar]
- 7.Kovac M, Mitic G, Mikovic Z, et al. Pregnancy related stroke in the setting of homozygous type-II HBS antithrombin deficiency. Thromb Res 2016; 139: 111–113. [DOI] [PubMed] [Google Scholar]
- 8.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 197: inherited thrombophilias in pregnancy. Obstet Gynecol 2018; 132: e18–e34. [DOI] [PubMed] [Google Scholar]
- 9.Gabbay-Benziv R. Venous thromboembolism during pregnancy and the puerperium – Who? when? and how to treat? Harefuah 2019; 158: 53–59. [PubMed] [Google Scholar]
- 10.Bharadwaj J Jayaraman C andShrivastava R.. Heparin resistance. Lab Hematol 2003; 9: 125–131. [PubMed] [Google Scholar]
- 11.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Glob Adv Health Med 2013; 2: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper PC, Coath F, Daly ME, et al. The phenotypic and genetic assessment of antithrombin deficiency, Int J Lab Hematol 2011; 33: 227–237. [DOI] [PubMed] [Google Scholar]
- 13.Kraft J, Sunder-Plassmann R, Mannhalter C, et al. Women with homozygous AT deficiency type II heparin-binding site (HBS) are at high risk of pregnancy loss and pregnancy complications. Ann Hematol 2017; 96: 1023–1031. [DOI] [PubMed] [Google Scholar]
- 14.http://www.hgmd.cf.ac.uk.
- 15.Rogenhofer N, Bohlmann MK, Beuter-Winkler P, et al. Prevention, management and extent of adverse pregnancy outcomes in women with hereditary antithrombin deficiency. Ann Hematol 2014; 93: 385–392. [DOI] [PubMed] [Google Scholar]
- 16.Khalafallah AA, Ibraheem ARO, Teo QY, et al. Review of management and outcomes in women with thrombophilia risk during pregnancy at a single institution. ISRN Obstet Gynecol 2014; 2014; 381826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greer IA Brenner B andGris JC.. Antithrombotic treatment for pregnancy complications: Which path for the journey to precision medicine? Br J Haematol 2014; 165: 585–599. [DOI] [PubMed] [Google Scholar]
- 18.Sniecinski RM Bennett-Guerrero E andShore-Lesserson L.. Anticoagulation Management and Heparin Resistance During Cardiopulmonary Bypass: A Survey of Society of Cardiovascular Anesthesiologists Members. Anesth Analg 2019; 129: e41–e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beattie GW andJeffrey RR.. Is there evidence that fresh frozen plasma is superior to antithrombin administration to treat heparin resistance in cardiac surgery? Interact Cardiovasc Thorac Surg 2014; 18: 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabbagh AH, Chung GK, Shuttleworth P, et al . Fresh frozen plasma: a solution to heparin resistance during cardiopulmonary bypass. Ann Thorac Surg 1984; 37: 466–468. [DOI] [PubMed] [Google Scholar]
- 21.Barnette RE, Shupak RC, Pontius J, et al . In vitro effect of fresh frozen plasma on the activated coagulation time in patients undergoing cardiopulmonary bypass. Anesth Analg 1988; 67: 57–60. [PubMed] [Google Scholar]