Abstract
The emergence of COVID-19 has created a major health crisis across the globe. Invasion of SARS-CoV-2 into the lungs causes acute respiratory distress syndrome (ARDS) that result in the damage of lung alveolar epithelial cells. Currently, there is no standard treatment available to treat the disease and the resultant lung scarring is irreversible even after recovery. This has prompted researchers across the globe to focus on developing new therapeutics and vaccines for the treatment and prevention of COVID-19. Mesenchymal stem cells (MSCs) have emerged as an efficient drug screening platform and MSC-derived organoids has found applications in disease modeling and drug discovery. Perinatal tissue derived MSC based cell therapies have been explored in the treatment of various disease conditions including ARDS because of their enhanced regenerative and immunomodulatory properties. The multi-utility properties of MSCs have been described in this review wherein we discuss the potential use of MSC-derived lung organoids in screening of novel therapeutic compounds for COVID-19 and also in disease modeling to better understand the pathogenesis of the disease. This article also summarizes the rationale behind the development of MSC-based cell- and cell-free therapies and vaccines for COVID-19 with a focus on the current progress in this area. With the pandemic raging, an important necessity is to develop novel treatment strategies which will not only alleviate the disease symptoms but also avoid any off-target effects which could further increase post infection sequelae. Naturally occurring mesenchymal stem cells could be the magic bullet which fulfil these criteria.
Keywords: SARS-CoV2, Mesenchymal stem cells, COVID-19, Cell therapy, Exosomes
1. Introduction
The severe respiratory consequences of the coronavirus disease 2019 (COVID-19) pandemic are a major cause of morbidity and mortality among patients. Extreme manifestation of respiratory failure and long lasting lung damage in COVID-19 infection results in acute respiratory distress syndrome (ARDS) which entails invasive therapies and respiratory support. This has prompted urgent and critical need for novel therapies to avoid, mitigate or recover from ARDS. At present, the treatment is mostly symptomatic and supportive, mainly by anti-inflammatory and antiviral strategies [1]. Several efforts are underway to make vaccines for this deadly disease and in recent months many vaccines are approved across the globe but it will take longer to vaccinate people around the world due to limitation in production capacity of vaccines. New mutant strains are emerging from countries such as India, UK, Brazil and Africa and the efficacy of these vaccines against the mutant strains needs to be completely evaluated [2]. Presently, there are no definitive treatments available around the world which provides recovery from ARDS. It is evident that there is a time-bound urgency to screen, develop and deploy novel vaccines and drugs to bring the COVID-19 pandemic under control. In this context cell-based approaches, primarily using mesenchymal stem (stromal) cells (MSCs) are emerging as the potential screening tool for drug discovery process. Furthermore, MSCs can be a therapeutic approach option to treat ARDS and other inflammatory responses caused by COVID-19 infection.
Mesenchymal stem cells are multipotent stem cells that possess high proliferation and self-renewal properties. Although initially MSCs were only obtained from bone marrow, they are now isolated and culture-expanded from a variety of tissues sources such as dental pulp, adipose tissue, placenta, amniotic membrane, Wharton jelly, umbilical cord blood and menstrual blood [3,4]. These stem cells have the ability to differentiate into multiple tissue types including cartilage, bone, muscle, cardiomyocyte, keratocytes, hepatocytes, renal tubular cells, neural cells, retina and others [[5], [6], [7], [8], [9], [10]]. MSCs isolated from most tissues commonly express CD73, CD90, and CD105 and but lack expression of CD45, CD34, CD14 or CD11b, CD79a or CD19, and HLA-DR [[11], [12], [13], [14], [15]]. Recent preclinical and clinical models of transplanted MSCs have strongly endorsed the potential role of MSCs on tissue regeneration and homeostasis [16,17]. During the past decade, MSCs have been used in several clinical trials to treat diseases including musculoskeletal, cardiovascular, neurodegenerative and metabolic diseases [[18], [19], [20], [21]].
Pharmaceutical industry primarily employs tumor cells or immortalized cells to screen drug candidates in vitro. These cell lines have many advantages because of high scalability, however immortalized cell based models are not ideal for pharmacology and toxicity studies. The major disadvantage is that they do not represent a differentiated cell type present in the human tissue and therefore result in high rate attrition rate in late stage drug development after being tested originally in tumor cell lines. Immortalized cell lines are generally used in toxicity testing of drug candidates by growing in reduced oxygen atmosphere because their metabolism is often anaerobic. The anaerobic metabolism of transformed cell lines makes them less sensitive to mitochondrial toxicants and thus mitochondrial toxicity is systematically underreported in drug toxicity studies [[22], [23], [24]]. Hence, there is a pertinent need to develop human cell model based on normal cells; the ideal candidate for this are mesenchymal stem cells as they are natural, non-genetically manipulated cell types obtained from various human sources and are multipotent and therefore make an excellent cell types for modeling disease for drug discovery [25]. Furthermore, MSCs isolated from perinatal tissues such as placenta, amnion, umbilical cord and Wharton jelly are especially attractive as they are naïve and not subject to imprints from infection in the course of life. Monolayer cultures and organoids from MSCs are a great tool to study host-virus interactions and model respiratory infections, such as influenza and enteric infections. These organoids are also used to investigate pathogenesis of HIV and dengue virus [26]. Researchers are focusing on generating MSCs from induced pluripotent stem cells (iPSCs) which will help in the production of disease-specific MSCs that will go a long way in understanding the pathogenesis of the disease and its phenotype. This will also facilitate drug development by evaluating efficacy across multiple genetic backgrounds [27,28]. However, the potential of MSCs in developing infectious disease models and in drug screening have been underutilized so far.
2. COVID-19 and challenges
COVID-19 is caused by the inhalation of SARS-CoV-2 via the nasal tract where the virus attaches to the epithelial cells present in the nasal cavity by the interaction with viral spike protein and starts replicating. The virus enters the upper respiratory tract along the conducting airways and the main receptor for this virus is angiotensin-converting enzyme 2 (ACE2) which is abundantly expressed in both the nasal epithelial cells and lung alveolar epithelial cells [29,30]. At this point there is an increased expression of interferons such as CXCL10. In majority of the cases the virus only affects the upper respiratory tract but in few cases the virus moves to the gas exchange units of the lung where it infects alveolar type II cells. The virus replicates in these cells resulting in apoptosis with the release of huge number of viral particles causing severe scarring and fibrosis eventually leading to ARDS, a condition in which most of the patients suffering need mechanical ventilation support [[31], [32], [33]]. In ARDS, there is an inflammation of lungs by hypoxia wherein lungs will also lose its elasticity because of increased pulmonary vascular permeability. The condition induces pro-inflammatory cytokines such as tumor necrosis factor and interleukins that will lead to the damage the alveolar epithelium and capillary endothelium resulting in alveolar edema. Most of the patients suffering from severe ARDS need mechanical ventilation support [34].
The lack of in vitro platforms is one of the challenges in the development of new drugs for COVID-19 treatment despite the fact there are large libraries of anti-viral compounds which needs to be screened for that anti- SARS-CoV2 activity. To circumvent this problem, MSCs could be an ideal solution by virtue of their ability to form lung organoids which could be an efficient in vitro screening system for novel drugs against COVID-19 [35].
Another major factor hindering the development of anti-COVID vaccine is the limited availability of suitable cell lines which can express the target viral antigen effectively. Currently many vaccines are approved across the globe and several more are in clinical development. Rapid emergence of new viral strains would require the quick development of effective vaccines against new strains as the currently available vaccines would have limited efficacy against emerging mutant strains. The potential of MSCs as novel vaccine platform have been explored in the prevention and inhibition of cancer [36] but very few studies have explored the potential of MSC as a novel platform to develop prophylactic vaccine for infectious disease. In an HIV study, Tomchuck et al., [37] conducted a proof of concept study where MSC was modified to express a foreign antigen using a plasmid encoding gp120 which was able to induce antibody-mediated immune response in C57Bl/6 mice without the need for additional adjuvants or boosting. Although an initial report have attempted to develop MSC based vaccine platform for COVID-19 [38], it is necessary to utilize the untapped potential of MSC based platforms in COVID-19 vaccine development, which would provide an effective alternative to the existing cell line based platforms.
Currently there is no standard treatment available for COVID-19 which is the main reason for increasing mortality associated with the disease compared to seasonal flu. Few antiviral medications like remdesivir is being used in the treatment regimen of patients but the efficacy and safety of these drugs in patients is still uncertain [39]. COVID-19 affects the lungs by destroying its fundamental framework and the damage to the lung tissue due to fibrosis is irreversible. Currently there are no therapies that can reverse the damage of lungs caused by COVID-19 [40]. Cytokine storm triggered during the pathological course of the condition leads to lung fibrosis and ARDS that eventually results in multi-organ failure [41,42]. In the context of lung injury Kavianpour et al., and Yen et al., [43,44] outlined two most promising features of MSCs which makes it unique - first their immunomodulatory property caused by downregulating pro-inflammatory mediators and second, their ability to differentiate into alveolar cells. It warrants studying the therapeutic potential of MSCs in SARS-CoV2 infection because the disease manifestation is typically characterized by the release of pro-inflammatory cytokines leading to lung damage. Especially, MSCs from perinatal tissues are naïve, have a high yield and have an enhanced ability to rapidly differentiate in multiple cell types, when compared to their adult counterpart.
The properties of MSCs can be used in a three pronged strategy for COVID-19 that includes the prevention (vaccine development), drug development (by creating MSC based in vitro models for drug screening) and in treatment of disease (cell therapy and cell free therapy).
3. Mesenchymal stem cells in screening of SARS-CoV2 DRUGS
Although researchers are exploring the therapeutic potential of MSCs in treating lung injury and ARDS but there are not many studies exploring their role in disease modeling and drug screening with respect to COVID-19. Previously, in the case of Zika virus (ZIKV), a virus that mainly targets developing foetus and causes microcephaly, brain organoids have been developed to study its pathogenesis. As stem cell-derived brain organoids are similar to the foetal brain, they have been exploited to study ZIKV infection. It was identified that early-stage organoids are particularly susceptible to destruction by the ZIKV, leading to thinner neuronal layer, a hallmark of ZIKV infection [[45], [46], [47]], making these stem cell derived model a perfect platform for studying the pathogenesis of ZIKV in vitro.
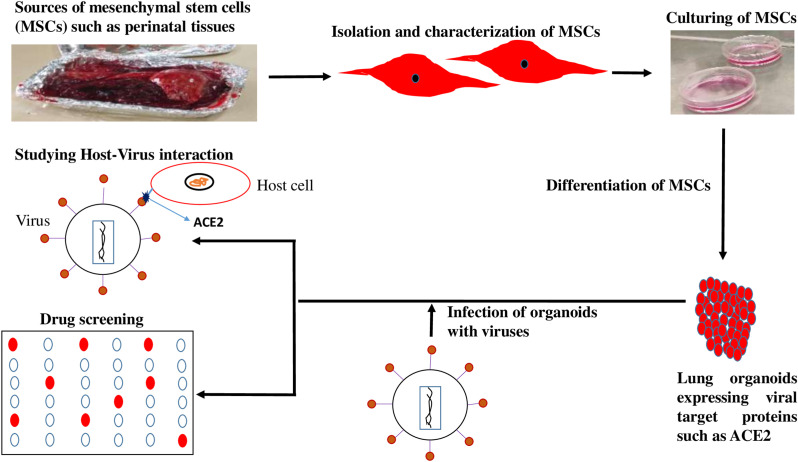
It has been established that MSCs can differentiate into type II alveolar epithelial cells in vitro [48] which makes it ideal to develop MSC derived COVID-19 lung organoid model to analyse disease pathogenesis and for drug screening. Some initial studies have shown that lung organoids, particularly aveolar type II cells that express ACE2 are permissive to SARS-CoV-2 infection. SARS-CoV-2 infection into lung organoids indicated a robust induction of chemokines and cytokines similar to that observed amongst human COVID-19 pulmonary infections. Screening of stem cell-derived lung organoids with FDA-approved drug candidates such as imatinib and mycophenolic acid showed that there was a physiologically relevant decrease in COVID-19 infection in the stem cell-derived lung organoids [49]. Using mini lungs and mini colons derived from stem cells to test around 1000 drugs, researchers have identified seven promising candidates including the antiviral drug remdesivir [50]. Furthermore, when lung organoids expressing ACE2 derived from stem cells was used to study the efficacy of anti-androgenic drugs against SARS-CoV-2 infected organoids, it was seen these drugs reduced of the number of infected cells along with the decrease in ACE2 levels [51]. In another study, when lung stem cell derived alveolospheres expressing ACE2 was infected with virus, the infected alveolospheres showed similar features of COVID-19 including interferon (IFN)-mediated inflammatory responses, loss of surfactant proteins, and apoptosis [52]. Lung organoids have also been developed from adult stem cells isolated from human lungs surgically removed due to lung cancer. These organoids had cells that make up both the upper and lower airways of human lungs, including specialized alveolar cells known as AT2 using a special cocktail of growth factors. When these organoids were infected with SARS-CoV-2, it was seen that the upper airway cells are critical for the virus to establish infection, whereas the lower airway cells are important for the immune response. Both cell types were observed to contribute to cytokine storm seen in severe cases of COVID-19 [53]. It has been shown that lung bud tip organoids can be developed from human foetal lung epithelial stem cells which displayed alveolar differentiation potential in 3D. This was used to establish air–liquid interface cultures in combination with foetal fibroblasts to differentiate into bronchioalveolar-like cultures. This mix of cell types allows to study the interactions of SARS-CoV-2 in multiple areas in the airways that would help in the detailed understanding of the disease [54,55]. Other than respiratory symptoms, 50% of patients show gastrointestinal symptoms like diarrhea or nausea associated with disease severity. Stem cell derived intestinal organoids have been used to study the pathogenesis and to screen drugs to mitigate the gastrointestinal symptoms associated with COVID-19. One such study has shown viral infected intestinal organoids, when treated with remdesivir inhibited viral replication [56]. Further studies will help to develop this agent as a potential drug to treat COVID-19 induced gastrointestinal damage and soothe gastrointestinal symptoms. These studies provide sufficient confidence that MSCs-derived models could provide a good system to study disease pathogenesis and develop novel drugs against SARS-CoV2. A schematic representation, indicating the role of MSC in drug screening and disease modeling is summarized in Fig. 1 .
Fig. 1.
Role of mesenchymal stem cells (MSCs) in drug screening. Lung organoids expressing viral target proteins such as ACE2 generated by the differentiation of MSCs isolated from perinatal tissues are excellent model systems in novel drug screening and to study host-virus interaction in the pathogenesis of Covid-19.
4. SARS-CoV2 vaccine development using mesenchymal stem cells
Vaccines are important for the prevention of COVID-19 and countries across the globe are investing heavily to develop effective vaccines. Currently many vaccines are approved and several more are in advanced stages of clinical trials. The failure of some of the vaccines under development is due to low tolerability, efficiency, immune adaptability and safety which opens a dire need for novel vaccine testing platforms. MSC based vaccine platforms have been explored in cancer and in infectious diseases such as HIV [37]. Many studies have shown that genetically engineered MSCs expressing viral proteins/antigens were able to induce viral antigen specific antibody response from the host species. When MSC expressing HPV proteins E6/E7 in combination with modified E7 fusion protein vaccine was administered into mice, there was an E7-specific antibody response from the mice against the MSC expressing HPV proteins along with substantial reduction in the growth of the tumor [36,57]. Recently, a group in China used a novel approach for the development of COVID-19 vaccine based on genetically modified human MSCs [38]. They transfected plasmid expressing SARS-CoV2 nucleocapsid (N) protein in MSCs isolated from umbilical cord. The engineered MSC cells when injected into the mice showed a rapid and effective antibody response against the MSC expressing viral antigen. The injected MSCs were also degraded by immune system after 20 days of administration. These studies are a forerunner for the use of MSCs for the development of vaccines for COVID-19.
5. Use of mesenchymal stems cells in SARS-CoV2 therapy
5.1. Cell therapy
Infectious respiratory diseases caused by different viruses can cause respiratory symptoms that range from common cold to severe acute respiratory syndrome [58]. Although, antibacterial and antiviral drug treatments used currently can successfully eliminate the virus, full recovery from viral induced pneumonia depends on the resistance of the patient. In recent years stem cell-based therapy has become a potential approved system for the treatment of virus-induced lung injury [59], and MSCs is considered to be one of the major therapeutic cellular tools because of their non-immunogenic, non-manipulated properties.
Influenza A viral (IAV) infection, the most common cause of viral pneumonia and antiviral drugs are used as the primary treatment strategy for influenza-induced pneumonia. There are no antiviral drugs that are approved in the market can repair damaged lung cells. Studies on IAV-infected animal models have shown the beneficial effects of the administration of tissue-derived MSCs [60]. Bone marrow derived MSCs when cocultured with H5N1 infected human alveolar epithelial cells has shown to restore alveolar fluid clearance and alveolar protein permeability which are significantly altered during viral infection [61]. Similar effects were also demonstrated using umbilical cord blood-derived MSC (UC-MSC) [62]. In a COPD mouse model, human Wharton's jelly-derived MSCs (WJ-MSCs) showed pulmonary regenerative effects. The COPD-induced mice had increased emphysema volume which was significantly decreased in the WJ-MSC treated group [63]. Another clinical study analyzed the effect of UC-MSC in bronchopulmonary dysplasia (BPD), a chronic lung disease of preterm infants that compromises the pulmonary function. This study showed that transplantation of UC-MSC decreased BPD severity along with the reduction in the levels of interleukin-6, interleukin-8, tumor necrosis factor α, matrix metalloproteinase-9, and transforming growth factor β1 in tracheal aspirates [64]. In several ARDS models, various tissue derived MSCs such as UC-MSCs and BM-MSCs were shown to improve lung function by promoting differentiation of pulmonary endothelial cells and alveolar epithelial cells, and also increased the secretion of alveolar surfactant [[65], [66], [67], [68]]. These studies indicating the advantages of perinatal derived MSCs to improve pulmonary function in various lung related disorders, suggest that they could be ideal therapeutic candidates for treating COVID-19 induced lung damage.
Several clinical trials have been registered using stem cells for COVID-19 treatment that aim to use different cell sources, dosage and importantly diverse targeted patient group. A majority of registered stem cell clinical trials to treat COVID-19 have proposed the use of MSCs as a treatment modality. MSCs are a well characterized type of adult stem cells with ideal proliferative, differentiation, immunomodulatory and non-tumorigenic properties. In addition, MSCs are also without ethical issues and are available for allogenic or autologous use [[69], [70], [71], [72]]. Recently, a small group of critically ill COVID-19 patients in China were given MSCs and subsequently FDA approved Emergency Use Authorization of stem cells to Athersys and GIOSTAR (Global Institute of Stem Cell Therapy and Research) that has created excitement among medical community. Administration of UC-MSCs has shown to improve whole lung lesion volume in COVID-19 patients in a clinical trial [73]. In another pilot trial, four rounds of UC-MSCs were given to 16 severely ill COVID-19 patients wherein there was significant improvement in oxygenation index with the mortality rate of just 6.25% compared to standard mortality rate of 45.4% in severely ill patients [74]. Another clinical study on 31 severe or critical COVID-19 pneumonia patients using UC-MSC therapy showed that there was a reduction in cytokine storm along with oxygen restoration. Regulation of inflammatory response and promotion of tissue repair by the transplanted UC-MSCs are the possible mode of action for improved lung microenvironment, function and pulmonary fibrosis in these studies [75,76]. As these trials were conducted in smaller patient population to study feasibility and safety of UC-MSCs in treating COVID-19, it is further necessary to conduct larger trials to ascertain and study the detailed therapeutic effects of UC-MSCs.
Other than tissue regeneration, MSCs are known to play a role in immunomodulation through the interaction of immune cells in both innate and adaptive immune systems, leading to immunosuppression of many effector activities. MSCs can reduce the cytokine storm produced during many viral infections including coronavirus [43]. As described by Bailey et al., and Roche et al. [77,78], MSCs are also known for its resistance to viral infections when compared to more differentiated cells. This ability is conferred by the presence of IFN-stimulated genes (ISG) that can target at many stages during viral cycle, thereby avoiding viruses to overpass cell membrane, nuclear import of mRNAs, genome integration/amplification, blocking endocytic route, mRNA transcription, protein translation, viral assembly and release. Avian influenza virus (AIV) causes disease among birds species, including chickens, ducks, and turkeys. There are several AIV subtypes such as H5N1, H7N9, and H9N2 that can cross species barriers and become infectious to mammals [79,80]. Although antiviral drugs can reduce the severity and duration of symptoms, it does not eliminate flu symptoms or repair the tissue injury caused by virus associated inflammation. It has been shown that anti-inflammatory therapies may attenuate viral-induced lung injury in mice [81]. Given that MSCs possess immunomodulatory and regenerative properties and ability to secrete endothelial and epithelial growth factors, Li et al. demonstrated that treatment with MSCs greatly improved acute lung injury induced by the H9N2 virus in mice [82]. These properties of MSCs in treating viral induced diseases suggest a wide scope to develop these cells as a potential therapeutic agent in the treatment of COVID-19.
5.2. Cell free therapy
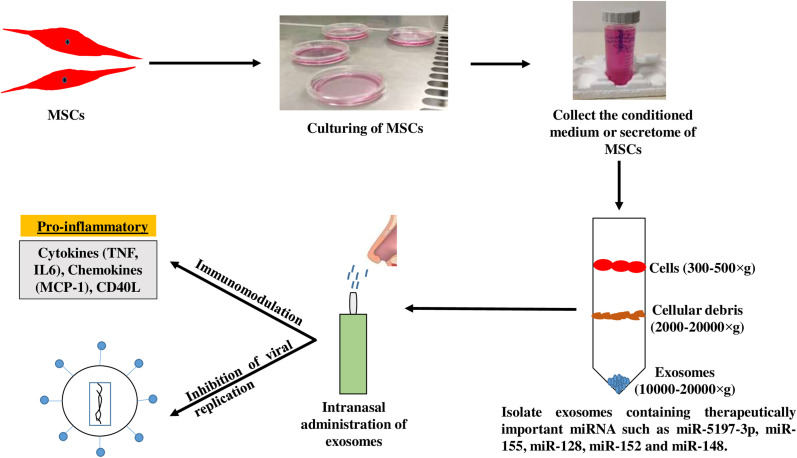
Mesenchymal stem cells not only have efficient differentiation potency but also secrete a wide range of paracrine factors and extracellular vesicles that play a key role in tissue regeneration and repair. We have reported previously that the secretions of MSCs encapsulated in sodium alginate may be promising anti-cancer agents [83]. In recent years, exosomes have emerged as a promising cell-free strategy in the treatment of various disease conditions that includes inflammatory diseases, cancer and myocardial infarction [84,85]. Biancone et al., and others [[86], [87], [88]] have explained that the functional mechanism of MSCs mainly rely on paracrine signaling, and exosomes are likely to be the key constituents of this process. Bobrie et al., and O'Loughlin et al., [89,90] define exosomes as membrane vesicles of 50–100 nm in size, which can be released from all kinds of cells. They deliver specific functional RNAs (miRNA and siRNA), proteins, and lipids from donor to recipient cells by direct fusion or active uptake [91,92]. Because of this shuttling property, exosomes take part in many physiological or pathological processes, including intracellular communication, modulation of immune responses, and tumorigenesis [[93], [94], [95]]. It has been shown that exosomes could shuttle both infectious cargo and protective host molecules between cells [[96], [97], [98], [99]], but their roles in pathogen infection is still in an early stage. MSCs can produce high amount of exosomes (MSC-Exo) compared with other cell types [100], and MSC-Exo exert an influence on various diseases similar to that of MSCs. It was reported that the main form of functional RNA in exosomes is miRNA [101,102]. The expression profile of miRNA has a signature of the origin parental cells as determined by RNA sequencing. Increasing evidence suggests that exosome-packaged miRNAs are maintained in stable condition and play important regulatory roles in recipient cells [103]. As reviewed by Yin et al. [104], the use of MSC-derived exosomes as cell-free therapeutics offers several advantages compared to their cellular counterparts such as high stability, low immunogenicity, easy storage, and ability to cross the blood-brain barrier. Sarvar et al., [105] described that these exosomes have bilipid membrane composition, convenient off the shelf availability, and biocompatibility that make them ideal as drug delivery vehicle. It has been shown that exosomes secreted from UC-MSCs inhibited HCV infection in vitro, especially viral replication, with low cell toxicity [106]. It was shown that miRNAs from UC-MSC-derived exosomes (UC-MSC-Exo) had unique expression profiles, mainly represented by let-7f, miR-145, miR-199a, and miR-221 which largely contributed to the suppression of HCV RNA replication. In addition, UC-MSC-Exo therapy showed synergistic effect when combined with FDA approved drugs such as interferon-α or telaprevir, enhancing their anti-HCV ability and thus improving their clinical significance for future application as optimal adjuvants of anti-HCV therapy [106]. Chu and colleagues used UC-MSC-Exo in the treatment of mild COVID-19 pneumonia. The exosomes were in the nebulized form and did not trigger any allergic reactions. These exosomes were able to promote the absorption of pulmonary lesions and reduced the hospitalization time for mild cases of SARS-CoV2 induced pneumonia [107]. These features of exosomes have gained sufficient interest in evaluating their potential role as a therapeutic and pharmacological intervention in addressing COVID-19 pandemic. Exosome therapy could prevent the cytokine storm elicited by the immune system as shown in recent studies indicating a promotion of endogenous repair by exosomes [108]. Emerging evidence clearly support the possibility of using MSC-Exo as a new form of therapy for treating various viral diseases including COVID-19. The process of developing MSC based cell free therapy for COVID-19 is represented in Fig. 2 .
Fig. 2.
Diagrammatic representation of cell free therapy. Exosomes isolated from mesenchymal stem cells (MSCs) can be delivered via intranasal route that will act against SARS-CoV-2 through immunomodulation and inhibiting viral replication.
Drugs have shown limited efficacy in the management of COVID-19 and is predominantly supportive in nature. In this context there is a dire emergency to fast-track development of vaccines and therapeutic agents. MSC based organoid models fit the requirement both as a reliable screening tool and also as therapeutic agents. These organoids which overexpress viral target proteins may emerge as a promising tool in screening an array of novel drug candidates against COVID-19. Furthermore, using perinatal tissue MSCs have fewer ethical implications when compared to animal models and embryonic stem cells. Conventional in vitro culture systems and animal models have been useful in studying the pathogenesis of viral infections and to develop vaccines but models that can accurately recapitulate human responses to infection are still lacking. Organ-on-a-chip is an emerging technology that can effectively mimic in vivo conditions. They recapitulate tissue–tissue interfaces, fluid flows, mechanical cues, and organ-level physiology that accurately mimic human pathophysiology. Using MSC derived lung-on-a-chip system, the pathogenesis of coronavirus can be studied and drug candidates screened. Furthermore, MSCs have also the potential to be developed into novel vaccine platform where they can be manipulated to overexpress immunogenic COVID-19 components and be used to induce efficient antigen specific immune responses.
6. Conclusion
Special features of MSCs to differentiate into various cell types including alveolar lung epithelial cells without a risk of tumorigenesis, make it attractive therapeutic agents to reverse COVID-19 induced lung damage. Furthermore, in a cell-free format, the exosomes of MSCs have significant therapeutic value as they are also known to play a key role in subduing the cytokine storm in SARS-CoV2 infection. The emergence of mutant strains is posing a significant challenge for healthcare across the globe. There are presently four reported variants of concern (VOCs) -Alpha(B.1.1.7); Beta(B.1.351); and Gamma (P.1) and Delta(B.1.617.2) of SARS-CoV2 to which currently available vaccines and drugs have varying efficacy. MSC-derived organoids present a good model to study pathogenesis and to understand the molecular mechanisms of these new mutant strains. Importantly, naïve stem cells derived from placenta and other perinatal tissues would definitely have an added advantage over adult stem cells by virtue of their high yield and easy flexibility to differentiate. These stem cells would have an efficient therapeutic effect across multiple variants as their mode of action is by regeneration of damaged tissue and viral clearance irrespective of the mutant forms. A fast acting therapy which is also capable of reverting the havoc caused by the deadly SARS-CoV2 virus is the pressing need of the hour. Mesenchymal stem cell based approaches will hopefully be able to fit the bill as the perfect antidote to the present and the continuously evolving forms of SARS-CoV2.
Declaration of competing interest
Authors declare no conflict of interest for the article titled- ‘Stalling SARS-CoV2 infection with stem cells: can regenerating perinatal tissue mesenchymal stem cells offer a multi-tiered therapeutic approach to COVID-19?’.
Acknowledgements
MS is thankful for the TMA Pai scholarship and fellowship from the UGC, Govt of India. Conflicts of Interest: The authors declare no conflict of interest.
References
- 1.Wu Y.-C., Chen C.-S., Chan Y.-J. The outbreak of COVID-19: an overview. J. Chin. Med. Assoc. 2020;83(3):217. doi: 10.1097/JCMA.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirby T. New variant of SARS-CoV-2 in UK causes surge of COVID-19. Lancet Respir. Med. 2021;9(2):e20–e21. doi: 10.1016/S2213-2600(21)00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seifrtova M., Havelek R., Ćmielová J., Jiroutova A., Soukup T., Brůčková L., Mokrý J., English D., Řezáčová M. The response of human ectomesenchymal dental pulp stem cells to cisplatin treatment. Int. Endod. J. 2012;45(5):401–412. doi: 10.1111/j.1365-2591.2011.01990.x. [DOI] [PubMed] [Google Scholar]
- 4.Ullah I., Subbarao R.B., Rho G.J. Human mesenchymal stem cells-current trends and future prospective. Biosci. Rep. 2015;35(2) doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park S.H., Kim K.W., Chun Y.S., Kim J.C. Human mesenchymal stem cells differentiate into keratocyte-like cells in keratocyte-conditioned medium. Exp. Eye Res. 2012;101:16–26. doi: 10.1016/j.exer.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Huo D.-M., Dong F.-T., Yu W.-H., Gao F. Differentiation of mesenchymal stem cell in the microenvironment of retinitis pigmentosa. Int. J. Ophthalmol. 2010;3(3):216. doi: 10.3980/j.issn.2222-3959.2010.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aurich H., Sgodda M., Kaltwaßer P., Vetter M., Weise A., Liehr T., Brulport M., Hengstler J.G., Dollinger M.M., Fleig W.E. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut. 2009;58(4):570–581. doi: 10.1136/gut.2008.154880. [DOI] [PubMed] [Google Scholar]
- 8.Golpanian S., Wolf A., Hatzistergos K.E., Hare J.M. Rebuilding the damaged heart: mesenchymal stem cells, cell-based therapy, and engineered heart tissue. Physiol. Rev. 2016;96(3):1127–1168. doi: 10.1152/physrev.00019.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kangari P., Talaei-Khozani T., Razeghian-Jahromi I., Razmkhah M. Mesenchymal stem cells: amazing remedies for bone and cartilage defects. Stem Cell Res. Ther. 2020;11(1):1–21. doi: 10.1186/s13287-020-02001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu W., Hong X., Le Bras A., Nowak W.N., Bhaloo S.I., Deng J., Xie Y., Hu Y., Ruan X.Z., Xu Q. Smooth muscle cells differentiated from mesenchymal stem cells are regulated by microRNAs and suitable for vascular tissue grafts. J. Biol. Chem. 2018;293(21):8089–8102. doi: 10.1074/jbc.RA118.001739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Augello A., Kurth T.B., De Bari C. Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur. Cell. Mater. 2010;20(121):e33. doi: 10.22203/ecm.v020a11. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain G., Fox J., Ashton B., Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem cells. 2007;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 13.Crisan M., Yap S., Casteilla L., Chen C.-W., Corselli M., Park T.S., Andriolo G., Sun B., Zheng B., Zhang L. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell stem cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. Int. Soc. Cell. Ther. Position Stat. Cytother. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 15.Uccelli A., Moretta L., Pistoia V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008;8(9):726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 16.Lv F.-J., Tuan R.S., Cheung K.M., Leung V.Y. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem cells. 2014;32(6):1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 17.Le Blanc K. Mesenchymal stromal cells: tissue repair and immune modulation. Cytotherapy. 2006;8(6):559–561. doi: 10.1080/14653240601045399. [DOI] [PubMed] [Google Scholar]
- 18.Sid-Otmane C., Perrault L.P., Ly H.Q. Mesenchymal stem cell mediates cardiac repair through autocrine, paracrine and endocrine axes. J. Transl. Med. 2020;18(1):1–9. doi: 10.1186/s12967-020-02504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zumwalt M., Reddy A.P. Stem cells for treatment of musculoskeletal conditions-orthopaedic/sports medicine applications. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2020;1866(4):165624. doi: 10.1016/j.bbadis.2019.165624. [DOI] [PubMed] [Google Scholar]
- 20.Hernández R., Jiménez-Luna C., Perales-Adán J., Perazzoli G., Melguizo C., Prados J. Differentiation of human mesenchymal stem cells towards neuronal lineage: clinical trials in nervous system disorders. Biomol. Therapeut. 2020;28(1):34. doi: 10.4062/biomolther.2019.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dave S., Vanikar A., Trivedi H., Thakkar U., Gopal S., Chandra T. Novel therapy for insulin-dependent diabetes mellitus: infusion of in vitro-generated insulin-secreting cells. Clin. Exp. Med. 2015;15(1):41–45. doi: 10.1007/s10238-013-0266-1. [DOI] [PubMed] [Google Scholar]
- 22.Fröhlich E. Comparison of conventional and advanced in vitro models in the toxicity testing of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018;46(sup2):1091–1107. doi: 10.1080/21691401.2018.1479709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez‐Enríquez S., Juárez O., Rodríguez‐Zavala J.S., Moreno‐Sánchez R. Multisite control of the Crabtree effect in ascites hepatoma cells. Eur. J. Biochem. 2001;268(8):2512–2519. doi: 10.1046/j.1432-1327.2001.02140.x. [DOI] [PubMed] [Google Scholar]
- 24.Marroquin L.D., Hynes J., Dykens J.A., Jamieson J.D., Will Y. Circumventing the Crabtree effect: replacing media glucose with galactose increases susceptibility of HepG2 cells to mitochondrial toxicants. Toxicol. Sci. 2007;97(2):539–547. doi: 10.1093/toxsci/kfm052. [DOI] [PubMed] [Google Scholar]
- 25.Zuba-Surma E.K., Wojakowski W., Madeja Z., Ratajczak M.Z. Stem cells as a novel tool for drug screening and treatment of degenerative diseases. Curr. Pharmaceut. Des. 2012;18(18):2644–2656. doi: 10.2174/138161212800492859. [DOI] [PubMed] [Google Scholar]
- 26.Giani A.M., Chen S. Human pluripotent stem cell-based organoids and cell platforms for modelling SARS-CoV-2 infection and drug discovery. Stem Cell Res. 2021;53:102207. doi: 10.1016/j.scr.2021.102207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan M.A., Alanazi F., Ahmed H.A., Shamma T., Kelly K., Hammad M.A., Alawad A.O., Assiri A.M., Broering D.C. iPSC-derived MSC therapy induces immune tolerance and supports long-term graft survival in mouse orthotopic tracheal transplants. Stem Cell Res. Ther. 2019;10(1):1–15. doi: 10.1186/s13287-019-1397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao C., Ikeya M. Generation and applications of induced pluripotent stem cell-derived mesenchymal stem cells. Stem Cell. Int. 2018:2018. doi: 10.1155/2018/9601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. e00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mason R.J. Eur Respiratory Soc; 2020. Pathogenesis of COVID-19 from a Cell Biology Perspective. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mossel E.C., Wang J., Jeffers S., Edeen K.E., Wang S., Cosgrove G.P., Funk C.J., Manzer R., Miura T.A., Pearson L.D., Holmes K.V., Mason R.J. SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells. Virology. 2008;372(1):127–135. doi: 10.1016/j.virol.2007.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian Z., Travanty E.A., Oko L., Edeen K., Berglund A., Wang J., Ito Y., Holmes K.V., Mason R.J. Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am. J. Respir. Cell Mol. Biol. 2013;48(6):742–748. doi: 10.1165/rcmb.2012-0339OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rawal G., Yadav S., Kumar R. Acute respiratory distress syndrome: an update and review. J. Transl. Int. Med. 2018;6(2):74–77. doi: 10.1515/jtim-2016-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss D.J. Cell-based therapy for chronic obstructive pulmonary disease. Rebuilding the lung. Ann. Am. Thorac. Soc. 2018;15(Supplement 4):S253–S259. doi: 10.1513/AnnalsATS.201808-534MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei H.-J., Wu A.T., Hsu C.-H., Lin Y.-P., Cheng W.-F., Su C.-H., Chiu W.-T., Whang-Peng J., Douglas F.L., Deng W.-P. The development of a novel cancer immunotherapeutic platform using tumor-targeting mesenchymal stem cells and a protein vaccine. Mol. Ther. 2011;19(12):2249–2257. doi: 10.1038/mt.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomchuck S.L., Norton E.B., Garry R.F., Bunnell B.A., Morris C.A., Freytag L.C., Clements J.D. Mesenchymal stem cells as a novel vaccine platform. Front. Cell. Infect. Microbiol. 2012;2:140. doi: 10.3389/fcimb.2012.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J., Jiao H., Yin X. bioRxiv; 2020. Engineered Human Mesenchymal Stem Cells as New Vaccine Platform for COVID-19. [Google Scholar]
- 39.Piscoya A., Ng-Sueng L.F., Parra del Riego A., Cerna-Viacava R., Pasupuleti V., Roman Y.M., Thota P., White C.M., Hernandez A.V. Efficacy and harms of remdesivir for the treatment of COVID-19: a systematic review and meta-analysis. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0243705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ojo A.S., Balogun S.A., Williams O.T., Ojo O.S. Pulmonary fibrosis in COVID-19 survivors: predictive factors and risk reduction strategies. Pulmonary Med. 2020;2020 doi: 10.1155/2020/6175964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mustafa M.I., Abdelmoneim A.H., Mahmoud E.M., Makhawi A.M. Cytokine storm in COVID-19 patients, its impact on organs and potential treatment by QTY code-designed detergent-free chemokine receptors. Mediat. Inflamm. 2020:2020. doi: 10.1155/2020/8198963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kavianpour M., Saleh M., Verdi J. The role of mesenchymal stromal cells in immune modulation of COVID-19: focus on cytokine storm. Stem Cell Res. Ther. 2020;11(1):1–19. doi: 10.1186/s13287-020-01849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yen B.L., Yen M.L., Wang L.T., Liu K.J., Sytwu H.K. Current status of mesenchymal stem cell therapy for immune/inflammatory lung disorders: gleaning insights for possible use in COVID‐19. Stem Cells Transl. Med. 2020;9(10):1163–1173. doi: 10.1002/sctm.20-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcez P.P., Loiola E.C., Madeiro da Costa R., Higa L.M., Trindade P., Delvecchio R., Nascimento J.M., Brindeiro R., Tanuri A., Rehen S.K. Zika virus impairs growth in human neurospheres and brain organoids. Science (New York, N.Y.) 2016;352(6287):816–818. doi: 10.1126/science.aaf6116. [DOI] [PubMed] [Google Scholar]
- 46.Dang J., Tiwari S.K., Lichinchi G., Qin Y., Patil V.S., Eroshkin A.M., Rana T.M. Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell. 2016;19(2):258–265. doi: 10.1016/j.stem.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qian X., Nguyen H.N., Song M.M., Hadiono C., Ogden S.C., Hammack C., Yao B., Hamersky G.R., Jacob F., Zhong C., Yoon K.J., Jeang W., Lin L., Li Y., Thakor J., Berg D.A., Zhang C., Kang E., Chickering M., Nauen D., Ho C.Y., Wen Z., Christian K.M., Shi P.Y., Maher B.J., Wu H., Jin P., Tang H., Song H., Ming G.L. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165(5):1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma N., Gai H., Mei J., Ding F.B., Bao C.R., Nguyen D.M., Zhong H. Bone marrow mesenchymal stem cells can differentiate into type II alveolar epithelial cells in vitro. Cell Biol. Int. 2011;35(12):1261–1266. doi: 10.1042/CBI20110026. [DOI] [PubMed] [Google Scholar]
- 49.Han Y., Yang L., Duan X., Duan F., Nilsson-Payant B.E., Yaron T.M., Wang P., Tang X., Zhang T., Zhao Z. Identification of candidate COVID-19 therapeutics using hPSC-derived lung organoids. BioRxiv. 2020;(05.05.079095) doi: 10.1101/2020.05.05.079095. [DOI] [Google Scholar]
- 50.Han Y., Duan X., Yang L., Nilsson-Payant B.E., Wang P., Duan F., Tang X., Yaron T.M., Zhang T., Uhl S., Bram Y., Richardson C., Zhu J., Zhao Z., Redmond D., Houghton S., Nguyen D.T., Xu D., Wang X., Jessurun J., Borczuk A., Huang Y., Johnson J.L., Liu Y., Xiang J., Wang H., Cantley L.C., tenOever B.R., Ho D.D., Pan F.C., Evans T., Chen H.J., Schwartz R.E., Chen S. Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature. 2021;589(7841):270–275. doi: 10.1038/s41586-020-2901-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samuel R.M., Majd H., Richter M.N., Ghazizadeh Z., Zekavat S.M., Navickas A., Ramirez J.T., Asgharian H., Simoneau C.R., Bonser L.R., Koh K.D., Garcia-Knight M., Tassetto M., Sunshine S., Farahvashi S., Kalantari A., Liu W., Andino R., Zhao H., Natarajan P., Erle D.J., Ott M., Goodarzi H., Fattahi F. Androgen signaling regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men. Cell Stem Cell. 2020;27(6):876–889. doi: 10.1016/j.stem.2020.11.009. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katsura H., Sontake V., Tata A., Kobayashi Y., Edwards C.E., Heaton B.E., Konkimalla A., Asakura T., Mikami Y., Fritch E.J. Human lung stem cell-based alveolospheres provide insights into SARS-CoV-2-mediated interferon responses and pneumocyte dysfunction. Cell stem cell. 2020;27(6):890–904. doi: 10.1016/j.stem.2020.10.005. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tindle C., Fuller M., Fonseca A., Taheri S., Ibeawuchi S.R., Beutler N., Katkar G., Claire A., Castillo V., Hernandez M., Russo H., Duran J., Crotty Alexander L.E., Tipps A., Lin G., Thistlethwaite P.A., Chattopadhyay R., Rogers T.F., Sahoo D., Ghosh P., Das S. 2021. Adult Stem Cell-Derived Complete Lung Organoid Models Emulate Lung Disease in COVID-19, bioRxiv : the Preprint Server for Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamers M.M., van der Vaart J., Knoops K., Riesebosch S., Breugem T.I., Mykytyn A.Z., Beumer J., Schipper D., Bezstarosti K., Koopman C.D., Groen N., Ravelli R.B.G., Duimel H.Q., Demmers J.A.A., Verjans G., Koopmans M.P.G., Muraro M.J., Peters P.J., Clevers H., Haagmans B.L. An organoid-derived bronchioalveolar model for SARS-CoV-2 infection of human alveolar type II-like cells. EMBO J. 2021;40(5) doi: 10.15252/embj.2020105912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nikolić M.Z., Caritg O., Jeng Q., Johnson J.A., Sun D., Howell K.J., Brady J.L., Laresgoiti U., Allen G., Butler R., Zilbauer M., Giangreco A., Rawlins E.L. Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids. eLife. 2017;6 doi: 10.7554/eLife.26575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krüger J., Groß R., Conzelmann C., Müller J.A., Koepke L., Sparrer K.M., Weil T., Schütz D., Seufferlein T., Barth T.F. Drug inhibition of SARS-CoV-2 replication in human pluripotent stem cell–derived intestinal organoids. Cell. Mol. Gastroenterol. Hepatol. 2021;11(4):935–948. doi: 10.1016/j.jcmgh.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomchuck S.L., Zwezdaryk K.J., Coffelt S.B., Waterman R.S., Danka E.S., Scandurro A.B. Toll‐like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem cells. 2008;26(1):99–107. doi: 10.1634/stemcells.2007-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baker S.C. Coronaviruses: from common colds to severe acute respiratory syndrome. Pediatr. Infect. Dis. J. 2004;23(11):1049–1050. doi: 10.1097/01.inf.0000145815.70485.f7. [DOI] [PubMed] [Google Scholar]
- 59.Han J., Li Y., Li Y. Strategies to enhance mesenchymal stem cell-based therapies for acute respiratory distress syndrome. Stem Cell. Int. 2019;2019 doi: 10.1155/2019/5432134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yudhawati R., Amin M., Rantam F.A., Prasetya R.R., Dewantari J.R., Nastri A.M., Poetranto E.D., Wulandari L., Lusida M.I., Koesnowidagdo S., Soegiarto G., Shimizu Y.K., Mori Y., Shimizu K. Bone marrow-derived mesenchymal stem cells attenuate pulmonary inflammation and lung damage caused by highly pathogenic avian influenza A/H5N1 virus in BALB/c mice. BMC Infect. Dis. 2020;20(1):823. doi: 10.1186/s12879-020-05525-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chan M.C., Kuok D.I., Leung C.Y., Hui K.P., Valkenburg S.A., Lau E.H., Nicholls J.M., Fang X., Guan Y., Lee J.W., Chan R.W., Webster R.G., Matthay M.A., Peiris J.S. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 2016;113(13):3621–3626. doi: 10.1073/pnas.1601911113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Loy H., Kuok D.I., Hui K.P., Choi M.H., Yuen W., Nicholls J.M., Peiris J.M., Chan M.C. Therapeutic implications of human umbilical cord mesenchymal stromal cells in attenuating influenza A (H5N1) virus–associated acute lung injury. J. Infect. Dis. 2019;219(2):186–196. doi: 10.1093/infdis/jiy478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cho J.W., Park K.S., Bae J.Y. Effects of Wharton's jelly-derived mesenchymal stem cells on chronic obstructive pulmonary disease. Regenrat. Ther. 2019;11:207–211. doi: 10.1016/j.reth.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang Y.S., Ahn S.Y., Yoo H.S., Sung S.I., Choi S.J., Oh W.I., Park W.S. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J. Pediatr. 2014;164(5):966–972. doi: 10.1016/j.jpeds.2013.12.011. e6. [DOI] [PubMed] [Google Scholar]
- 65.Walter J., Ware L.B., Matthay M.A. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir. Med. 2014;2(12):1016–1026. doi: 10.1016/S2213-2600(14)70217-6. [DOI] [PubMed] [Google Scholar]
- 66.Zhang X., Chen J., Xue M., Tang Y., Xu J., Liu L., Huang Y., Yang Y., Qiu H., Guo F. Overexpressing p130/E2F4 in mesenchymal stem cells facilitates the repair of injured alveolar epithelial cells in LPS-induced ARDS mice. Stem Cell Res. Ther. 2019;10(1):74. doi: 10.1186/s13287-019-1169-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Yang Y., Chen Q.H., Liu A.R., Xu X.P., Han J.B., Qiu H.B. Synergism of MSC-secreted HGF and VEGF in stabilising endothelial barrier function upon lipopolysaccharide stimulation via the Rac1 pathway. Stem Cell Res. Ther. 2015;6:250. doi: 10.1186/s13287-015-0257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ren H., Zhang Q., Wang J., Pan R. Comparative effects of umbilical cord- and menstrual blood-derived MSCs in repairing acute lung injury. Stem Cell. Int. 2018;2018:7873625. doi: 10.1155/2018/7873625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khoury M., Cuenca J., Cruz F.F., Figueroa F.E., Rocco P.R., Weiss D.J. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur. Respir. J. 2020;55(6) doi: 10.1183/13993003.00858-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Musiał-Wysocka A., Kot M., Majka M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant. 2019;28(7):801–812. doi: 10.1177/0963689719837897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choudhery M.S., Harris D.T. Stem cell therapy for COVID‐19: possibilities and challenges. Cell Biol. Int. 2020;44(11):2182–2191. doi: 10.1002/cbin.11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fričová D., Korchak J.A., Zubair A.C. Challenges and translational considerations of mesenchymal stem/stromal cell therapy for Parkinson's disease. NPJ Regenerat. Med. 2020;5(1):1–10. doi: 10.1038/s41536-020-00106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shi L., Huang H., Lu X., Yan X., Jiang X., Xu R., Wang S., Zhang C., Yuan X., Xu Z. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Trans. Target. Ther. 2021;6(1):1–9. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feng Y., Huang J., Wu J., Xu Y., Chen B., Jiang L., Xiang H., Peng Z., Wang X. Safety and feasibility of umbilical cord mesenchymal stem cells in patients with COVID-19 pneumonia: a pilot study. Cell Prolif. 2020;53(12) doi: 10.1111/cpr.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo Z., Chen Y., Luo X., He X., Zhang Y., Wang J. Administration of umbilical cord mesenchymal stem cells in patients with severe COVID-19 pneumonia. Crit. Care. 2020;24(1):420. doi: 10.1186/s13054-020-03142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S., Fan J., Wang W., Deng L., Shi H., Li H., Hu Z., Zhang F., Gao J., Liu H., Li X., Zhao Y., Yin K., He X., Gao Z., Wang Y., Yang B., Jin R., Stambler I., Lim L.W., Su H., Moskalev A., Cano A., Chakrabarti S., Min K.J., Ellison-Hughes G., Caruso C., Jin K., Zhao R.C. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bailey C.C., Zhong G., Huang I.-C., Farzan M. IFITM-family proteins: the cell's first line of antiviral defense. Annu. Rev. Virol. 2014;1:261–283. doi: 10.1146/annurev-virology-031413-085537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rocha J.L.M., de Oliveira W.C.F., Noronha N.C., Dos Santos N.C.D., Covas D.T., Picanço-Castro V., Swiech K., Malmegrim K.C.R. Mesenchymal stromal cells in viral infections: implications for COVID-19. Stem Cell Rev. Rep. 2021;17(1):71–93. doi: 10.1007/s12015-020-10032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin Y., Shaw M., Gregory V., Cameron K., Lim W., Klimov A., Subbarao K., Guan Y., Krauss S., Shortridge K. Avian-to-human transmission of H9N2 subtype influenza A viruses: relationship between H9N2 and H5N1 human isolates. Proc. Natl. Acad. Sci. Unit. States Am. 2000;97(17):9654–9658. doi: 10.1073/pnas.160270697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu Q., Liu D.-y., Yang Z.-q. Characteristics of human infection with avian influenza viruses and development of new antiviral agents. Acta Pharmacol. Sin. 2013;34(10):1257–1269. doi: 10.1038/aps.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharma G., Champalal Sharma D., Hwei Fen L., Pathak M., Bethur N., Pendharkar V., Peiris M., Altmeyer R. Reduction of influenza virus-induced lung inflammation and mortality in animals treated with a phosophodisestrase-4 inhibitor and a selective serotonin reuptake inhibitor. Emerg. Microb. Infect. 2013;2(1):1–9. doi: 10.1038/emi.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Y., Xu J., Shi W., Chen C., Shao Y., Zhu L., Lu W., Han X. Mesenchymal stromal cell treatment prevents H9N2 avian influenza virus-induced acute lung injury in mice. Stem Cell Res. Ther. 2016;7(1):1–11. doi: 10.1186/s13287-016-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mandal S., Arfuso F., Sethi G., Dharmarajan A., Warrier S. Encapsulated human mesenchymal stem cells (eMSCs) as a novel anti-cancer agent targeting breast cancer stem cells: development of 3D primed therapeutic MSCs. Int. J. Biochem. Cell Biol. 2019;110:59–69. doi: 10.1016/j.biocel.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 84.Katakowski M., Buller B., Zheng X., Lu Y., Rogers T., Osobamiro O., Shu W., Jiang F., Chopp M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335(1):201–204. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu J., Jiang M., Deng S., Lu J., Huang H., Zhang Y., Gong P., Shen X., Ruan H., Jin M., Wang H. miR-93-5p-Containing exosomes treatment attenuates acute myocardial infarction-induced myocardial damage, molecular therapy. Nucl. Acids. 2018;11:103–115. doi: 10.1016/j.omtn.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Biancone L., Bruno S., Deregibus M.C., Tetta C., Camussi G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol. Dial. Transplant. 2012;27(8):3037–3042. doi: 10.1093/ndt/gfs168. [DOI] [PubMed] [Google Scholar]
- 87.Yu B., Zhang X., Li X. Exosomes derived from mesenchymal stem cells. Int. J. Mol. Sci. 2014;15(3):4142–4157. doi: 10.3390/ijms15034142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Armstrong J.P., Holme M.N., Stevens M.M. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano. 2017;11(1):69–83. doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bobrie A., Colombo M., Raposo G., Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12(12):1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 90.O'Loughlin A.J., Woffindale C.A., JA Wood M. Exosomes and the emerging field of exosome-based gene therapy. Curr. Gene Ther. 2012;12(4):262–274. doi: 10.2174/156652312802083594. [DOI] [PubMed] [Google Scholar]
- 91.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 92.Mathivanan S., Fahner C.J., Reid G.E., Simpson R.J. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40(D1):D1241–D1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang B., Yin Y., Lai R.C., Tan S.S., Choo A.B.H., Lim S.K. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cell. Dev. 2014;23(11):1233–1244. doi: 10.1089/scd.2013.0479. [DOI] [PubMed] [Google Scholar]
- 94.Lai F.W., Lichty B.D., Bowdish D.M. Microvesicles: ubiquitous contributors to infection and immunity. J. Leukoc. Biol. 2015;97(2):237–245. doi: 10.1189/jlb.3RU0513-292RR. [DOI] [PubMed] [Google Scholar]
- 95.Pant S., Hilton H., Burczynski M.E. The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem. Pharmacol. 2012;83(11):1484–1494. doi: 10.1016/j.bcp.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cosset F.-L., Dreux M. HCV transmission by hepatic exosomes establishes a productive infection. J. Hepatol. 2014;60(3):674–675. doi: 10.1016/j.jhep.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 97.Kalamvoki M., Du T., Roizman B. Cells infected with herpes simplex virus 1 export to uninfected cells exosomes containing STING, viral mRNAs, and microRNAs. Proc. Natl. Acad. Sci. Unit. States Am. 2014;111(46):E4991–E4996. doi: 10.1073/pnas.1419338111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liang G., Liu G., Kitamura K., Wang Z., Chowdhury S., Monjurul A.M., Wakae K., Koura M., Shimadu M., Kinoshita K. TGF-β suppression of HBV RNA through AID-dependent recruitment of an RNA exosome complex. PLoS Pathog. 2015;11(4) doi: 10.1371/journal.ppat.1004780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Madison M.N., Jones P.H., Okeoma C.M. Exosomes in human semen restrict HIV-1 transmission by vaginal cells and block intravaginal replication of LP-BM5 murine AIDS virus complex. Virology. 2015;482:189–201. doi: 10.1016/j.virol.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yeo R.W.Y., Lai R.C., Zhang B., Tan S.S., Yin Y., Teh B.J., Lim S.K. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 2013;65(3):336–341. doi: 10.1016/j.addr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 101.Zhang J., Li S., Li L., Li M., Guo C., Yao J., Mi S. Exosome and exosomal microRNA: trafficking, sorting, and function. Dev. Reprod. Biol. 2015;13(1):17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alexander M., Hu R., Runtsch M.C., Kagele D.A., Mosbruger T.L., Tolmachova T., Seabra M.C., Round J.L., Ward D.M., O'Connell R.M. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat. Commun. 2015;6(1):1–16. doi: 10.1038/ncomms8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cui Y., Luan J., Li H., Zhou X., Han J. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett. 2016;590(1):185–192. doi: 10.1002/1873-3468.12024. [DOI] [PubMed] [Google Scholar]
- 104.Yin K., Wang S., Zhao R.C. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark. Res. 2019;7(1):8. doi: 10.1186/s40364-019-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sarvar D.P., Shamsasenjan K., Akbarzadehlaleh P. Mesenchymal stem cell-derived exosomes: new opportunity in cell-free therapy. Adv. Pharmaceut. Bull. 2016;6(3):293. doi: 10.15171/apb.2016.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qian X., Xu C., Fang S., Zhao P., Wang Y., Liu H., Yuan W., Qi Z. Exosomal microRNAs derived from umbilical mesenchymal stem cells inhibit hepatitis C virus infection. Stem Cells Transl. Med. 2016;5(9):1190–1203. doi: 10.5966/sctm.2015-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chu M., Wang H., Bian L., Huang J., Wu D., Fei F., Zhang R., Chen Y., Xia J. 2021. Nebulization Therapy with Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes for COVID-19 Pneumonia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cell. Dev. 2020;29(12):747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]