Abstract
Objectives
To estimate the seroprevalence of SARS-CoV-2 infection in patients with rheumatic diseases and to specify the proportion of asymptomatic and symptomatic forms of COVID-19.
Methods
We screened for SARS-CoV-2 infection among spondyloarthritis (SpA, n = 143) or rheumatoid arthritis (RA, n = 140) patients in our outpatient clinic at Cochin Hospital in Paris between June and August 2020. We performed a qualitative SARS-CoV-2 serological test which detects IgG directed against the N nucleocapsid protein (anti-N) and, for some patients, against the Spike protein (anti-S). Descriptive analyses were managed.
Results
During June–August 2020, the SARS-CoV-2 seroprevalence rate in our population was 2.83% (8/283 patients) without significant difference between RA and SpA patients (2.14% and 3.5%, respectively). We report 11 out of 283 patients (3.8%) with a diagnosis of SARS-CoV-2 infection. Among these 11 patients, 1 patient was asymptomatic (9%) with a confirmed diagnosis of COVID-19 by anti-S serology. Of the 283 patients, 85% were under bDMARDs, mainly on rituximab (RTX) (n = 44) and infliximab (IFX) (n = 136).
Conclusions
The seroprevalence of SARS-CoV-2 in patients with rheumatic diseases, mainly under bDMARDs treatments, was 2.83%. Among infected patients, 9% were asymptomatic. Detecting SARS-CoV-2 infections could be based on the strategy using patients’ interview and anti-N serology.
Keywords: COVID-19, Serology, Rheumatoid arthritis, Spondyloarthritis, DMARDs
1. Introduction
Since early 2020, the SARS-CoV-2 disease rapidly spread around the world leading to a global pandemic. According to data collected by the COVID-19 Global Rheumatology Alliance on 600 patients with rheumatic diseases worldwide infected by SARS-CoV-2, 46% of patients were hospitalized and 9% died from complications of SARS-CoV-2 [1]. Because of this case-reported approach, asymptomatic patients were not captured in this study. Two studies in France and in Italy noticed a low incidence of SARS-CoV-2 disease during the first wave in patient with rheumatic disease without evaluating the seroprevalence [2], [3]. Two other studies in Germany and in Northern Italy reported a low seroprevalence of SARS-CoV-2 among patients treated with bDMARDs or tsDMARDs (2.27%, and 2%, respectively) during the first wave, including symptomatic and asymptomatic patients [4], [5]. Such data are still lacking in France.
During the pandemic, patients with rheumatic diseases and under immunosuppressive (IS) drugs were considered to be at higher risk of severe infection by SARS-CoV-2 given the increased risk of infection generally observed under these treatments [6], [7], [8]. Patients were aware of this increased risk and have been reported to be almost twice as likely to adhere to strict isolation measures compared with healthy controls [9]. On the other hand, the impact of IS treatments on the humoral response against SARS-CoV-2 has been questioned and only few reported cases were published to date, mostly for patients treated with ocrelizumab in a context of multiple sclerosis [10], [11]. Those reports suggest a potential decreased immunogenicity of SARS-CoV-2 in patients treated with B-cell depleting treatments, but more conflicting results emerge from the rheumatologic literature and rituximab (RTX) [12], [13], [14]. More information on IS treatments and SARS-CoV-2 antibody response are necessary to drive the management of the vaccination policy in this specific population.
In this context, the objective of the present work was to assess the seroprevalence of SARS-CoV-2 infection in patients with rheumatic disease and specifying the proportion of asymptomatic and symptomatic forms of COVID-19.
2. Methods
2.1. Patients
We performed a systematic screening for COVID-19 infection among SpA or RA patients in our outpatient clinic at Cochin Hospital in Paris between June 1st and August 31st, 2020. Patients were systematically questioned about the occurrence of symptoms suggestive of COVID-19 since January 2020. Our study was performed before vaccination availability so the anti-SARS-CoV-2 antibodies results were unbiased. Patients were informed of the test and oral non-opposition was collected.
Diagnosis of SARS-CoV-2 infection was based on the presence of typical symptoms of SARS-CoV-2 infection or a positive PCR or evocative chest CT-scan. COVID-19 infection was suspected if patients developed non-specific signs of upper airway respiratory infection during the first wave period. Criteria for clinical severity of confirmed COVID-19 infection were applied [15].
2.2. Serological test
The serological tests were performed routinely on fresh, unfrozen samples. Consecutive assessments were performed to avoid selection bias. The serological test used was the qualitative SARS-CoV-2 serological test (microparticulate chemiluminescent immunoassay, Abbott, USA) which detects IgG directed against the N nucleocapsid protein (anti-N). IgM detection was not performed. The result was considered positive if the antibody level was greater than 1.4 and undetermined if it was between 0.49 and 1.4 as recommended by the company. A complementary serological test, detecting IgG directed against the SARS-CoV-2 Spike protein (anti-S), was performed if the anti-N serology was positive, or undetermined or inconclusive (with an index between 0.1 and 0.49) and/or if the patient had suggestive symptoms of COVID-19 disease. All these serological tests were previously validated by the COVID-19 Task Force at Pasteur Institute (Paris) and by the French Society of Virology.
2.3. Statistical analysis
Only descriptive analyses were managed. Binary values were expressed as number and percentage, continuous values as mean and standard deviation.
3. Results
3.1. Seroprevalence anti-N against SARS-Cov-2 and COVID-19 disease
In total, 283 patients were included in the study among which 140 RA and 143 SpA. The characteristics of the patients are described in Table 1 . The global SARS-CoV-2 seroprevalence rate was 2.83% (8/283 patients) without significant difference between RA and SpA patients (2.14% and 3.5%, respectively). Three patients had overt SARS-CoV-2 infection according to typical symptoms and/or positive PCR testing and/or positive CT-scan but no detectable seroconversion by using anti-N serology (Table 1). Taken together, we report 11 (RA n = 6, SpA n = 5) out of 283 patients (3.8%) with a diagnosis of SARS-CoV-2 infection whose characteristics are described in Table 2 . Among these 11 patients, 1 patient was asymptomatic corresponding to 9% of RA or SpA patients with SARS-Cov-2 infection who would not have been diagnosed by a patient-reported exclusive screening strategy and a non-systematic serology assessment. Nevertheless, relative to the whole screened population, the asymptomatic form of COVID-19 remained negligible (0.36%), even among patients widely treated with bDMARDs.
Table 1.
Patients’ characteristics.
| All patients (n = 283) |
Patients with RA (n = 140) |
Patients with SpA (n = 143) |
||||
|---|---|---|---|---|---|---|
| Seropositive |
Seronegative |
Seropositive |
Seronegative |
Seropositive |
Seronegative |
|
| n = 8 | n = 275 | n = 3 | n = 137 | n = 5 | n = 138 | |
| Age, y ± SD | 51 ± 9 | 55 ± 14.9 | 54 ± 1 | 60 ± 14,2 | 49 ± 12 | 50 ± 14.2 |
| Male sex, n (%) | 4 (50) | 101 (37) | 0 (0) | 22 (16) | 4 (80) | 79 (57) |
| Comorbidities | ||||||
| BMI kg/m2, ±SD | 28.4 ± 3.3 | 26.2 ± 9.5 | 27.8 ± 4.6 | 25.7 ± 6.1 | 28.8 ± 2.9 | 26.8 ± 5.8 |
| Hypertension, n (%) | 1 (12.5) | 80 (29) | 0 (0) | 45 (33) | 1 (20) | 35 (25) |
| Diabetes, n (%) | 1 (12.5) | 26 (9) | 0 (0) | 14 (10) | 1 (20) | 12 (9) |
| Chronic lung disease, n (%) | 1 (12.5) | 58 (21) | 0 (0) | 37 (27) | 1 (20) | 21 (15) |
| Symptoms of COVID | 7 (87.5) | 34 (12) | 2 (67) | 12 (9) | 5 (100) | 22 (15) |
| RF positive | 2 (67) | 118 (86) | ||||
| ACPA positive | 3 (100) | 118 (86) | ||||
| Erosive or destructive | 2 (67) | 103 (75) | ||||
| DAS 28, mean ± SD | 1.7 ± 0.5 | 2.7 ± 1.1 | ||||
| △ DAS 28a, mean ± SD | −0.3 ± 0.1 | −0.01 ± 0.6 | ||||
| Axial | 5 (100) | 120 (87) | ||||
| Peripheral | 4 (80) | 101 (73) | ||||
| X-ray sacroilitis | 4 (80) | 76 (55) | ||||
| HLA B27 | 3 (60) | 79 (57) | ||||
| BASDAI, mean ± SD | 25.9 ± 22.3 | 31.2 ± 19.5 | ||||
| △ BASDAIb, mean ± SD | −0.9 ± 0.2 | −0.3 ± 0.7 | ||||
| Glucocorticoids, n (%) | 1 (12.5) | 84 (31) | 1 (33) | 74 (54) | 0 (0) | 10 (7) |
| Dose < 10 mg per day, n (%) | 1 (100) | 75 (89) | 1 (100) | 67 (91) | 0 (0) | 8 (80) |
| Dose ≧ 10 mg per day, n (%) | 0 (0) | 9 (11) | 0 (0) | 7 (9) | 0 (0) | 2 (20) |
| csDMARDs, n (%) | 7 (87.5) | 176 (64) | 4 (100) | 108 (79) | 3 (60) | 68 (49) |
| Methotrexate | 5 (62.5) | 150 (55) | 3 (100) | 87 (63) | 2 (40) | 63 (46) |
| Leflunomide | 0 (0) | 12 (4) | 0 (0) | 11 (8) | 0 (0) | 1 (1) |
| Azathioprine | 0 (0) | 4 (1) | 0 (0) | 4 (3) | 0 (0) | 0 (0) |
| Sulfazalazine | 1 (12.5) | 4 (1) | 0 (0) | 0 (0) | 1 (20) | 4 (3) |
| Hydroxychloroquine | 1 (12.5) | 6 (2) | 1 (33) | 6 (4) | 0 (0) | 0 (0) |
| bDMARDs, n (%) | 7 (87.5) | 235 (85) | 3 (100) | 110 (80) | 4 (80) | 125 (91) |
| Anti-TNF | ||||||
| Etanercept alone | 0 (0) | 1 (0.5) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| With MTX | 0 (0) | 4 (2) | 0 (0) | 2 (2) | 0 (0) | 2 (2) |
| With other csDMARD | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Infliximab alone | 1 (12.5) | 55 (20) | 0 (0) | 0 (0) | 1 (20) | 55 (40) |
| With MTX | 3 (37.5) | 69 (25) | 1 (33) | 13 (9.5) | 2 (40) | 56 (41) |
| With other csDMARD | 1 (12.5) | 7 (3) | 0 (0) | 4 (3) | 1 (20) | 3 (2) |
| Adalimumab alone | 0 (0) | 1 (0.5) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| With MTX | 0 (0) | 3 (1) | 0 (0) | 1 (1) | 0 (0) | 2 (1.5) |
| With other csDMARD | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Golimumab alone | 0 (0) | 2 (1) | 0 (0) | 0 (0) | 0 (0) | 2 (1.5) |
| With MTX | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| With other csDMARD | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Others | 0 (0) | 9 (3) | 0 (0) | 8 (6) | 0 (0) | 1 (1) |
| Abatacept alone | ||||||
| With MTX | 0 (0) | 12 (4) | 0 (0) | 12 (9) | 0 (0) | 0 (0) |
| With other csDMARD | 0 (0) | 2 (1) | 0 (0) | 2 (1.5) | 0 (0) | 0 (0) |
| Rituximab alone | 0 (0) | 10 (4) | 0 (0) | 10 (7) | ||
| With MTX | 2 (25) | 25 (9) | 2 (67) | 26 (19) | ||
| With other csDMARD | 0 (0) | 6 (2) | 0 (0) | 6 (4.5) | ||
| With other bDMARD | 0 (0) | 1 (0.5) | 0 (0) | 1 (1) | ||
| ocilizumab alone | 0 (0) | 9 (3) | 0 (0) | 9 (7) | ||
| With MTX | 0 (0) | 9 (3) | 0 (0) | 9 (7) | ||
| With other csDMARD | 0 (0) | 4 (1.5) | 0 (0) | 4 (3) | ||
| With other bDMARD | 0 (0) | 1 (0.5) | 0 (0) | 1 (1) | ||
| Secukinumab alone | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| With MTX | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| With other csDMARD | 0 (0) | 1 (0.5) | 0 (0) | 1 (1) | ||
| JAK inhibitor | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Tofacitinib alone | ||||||
| With MTX | 0 (0) | 3 (1) | 0 (0) | 3 (2) | 0 (0) | 0 (0) |
| Baricitinib alone | 0 (0) | 1 (0.5) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| With MTX | 0 (0) | 1 (0.5) | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
RA: rheumatoid arthritis; SpA : Spondyloarthritis; BMI: Body mass index; RF: rheumatoid factor; ACPA: Anti Citrullinated Peptides Antibodies; DAS: Disease Activity Score; SD standard deviation; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; csDMARDs: Conventional synthetic disease-modifying antirheumatic drugs; bDMARDs: biologic DMARDs; MTX: methotrexate; TOCI: tocilizumab.
△ DAS 28 = {(DAS 28 in July) − (DAS 28 in February)}/(DAS 28 in February).
△ BASDAI = {(BASDAI in July) − (BASDAI in February)}/(BASDAI in February).
Table 2.
Characteristics of the 11 patients with a confirmed diagnosis of SARS-CoV-2 infection.
| Patient | COVID confirmed | IgG antinucleocapside | Anti-N index | IgG anti-Spike | Disease | Treatment | COVID-19 infection reported by interview | PCR | CT | Care support | Severity | Need of oxygen |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1a | Serology + symptoms + PCR + CT | Positive | 1.77 | Positive | RA | MTX + HCQ + RTX | Headache, myalgia, diarrhea, arthralgia, dry cough, rhinorrhea, anosmia, agueusia | Positive | Typical | Ambulatory | Moderate | No |
| Patient 2a | Serology + symptoms + PCR + CT | Positive | 2.78 | Positive | RA | MTX + RTX | Fever, headache, odynophagia, cough, expectoration, dyspnea, chest pain, myalgia, diarrhea, anosmia, agueusia | Positive | Typical | Medicine department | Severe | Yes |
| Patient 3 | Serology + symptoms | Positive | 1.48 | Positive | SpA | MTX + IFX | Cough, fever, myalgia | Suspicious | NA | Ambulatory | Low | No |
| Patient 4 | Serology + symptoms | Positive | 2.26 | Positive | SpA | SSZ + IFX | Fever, cough, headache, anosmia, agueusia | NA | NA | Ambulatory | Low | No |
| Patient 5 | Serology + symptoms | Positive | 2.02 | Positive | SpA | NSAID | Anosmia, agueusia, headache | NA | NA | Ambulatory | Low | No |
| Patient 6 | Serology + symptoms | Positive | 6.41 | Positive | SpA | MTX + IFX | Odynophagia, cough, expectoration, rhinorrhea, arthralgia, myalgia | Negative | NA | Ambulatory | Low | No |
| Patient 7 | Serology + symptoms | Positive | 3.28 | Positive | SpA | IFX | Anosmia, agueusia, chills | NA | NA | Ambulatory | Low | No |
| Patient 8 | Serology + no symptoms | Positive | 1.44 | Positive | RA | MTX + IFX | Asymptomatic | NA | NA | Ambulatory | Low | No |
| Patient 9 | Serology and anti S positive | Undetermined | 0.58 | Positive | RA | Corticosteroids | Asymptomatic | NA | NA | Ambulatory | Low | No |
| Patient 10 | Symptoms + PCR + CT | Inconclusive | 0.21 | Negative | RA | ABA | Fever, asthenia, agueusia | Positive | Typical | Medecine department | Moderate | No |
| Patient 11 | Symptoms + PCR + CT | Negative | 0.01 | Negative | RA | MTX + RTX | Diarrhea, cough, dyspnea | Positive | Typical | Medecine department | Moderate | Yes |
NSAID: non-steroidal anti-inflammatory drugs; MTX: methotrexate; HCQ: hydroxychloroquin; RTX: rituximab; SLZ: sulfasalazine; IFX: infliximab.
Patients treated with Rituximab.
3.2. Comparison between anti-N and anti-S serology
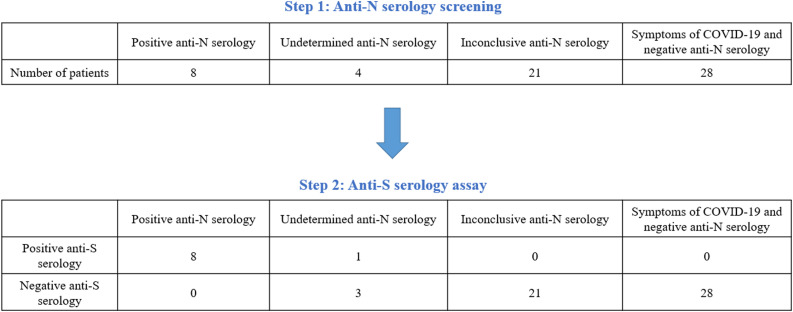
In total, 61 patients underwent additional anti-S antibody test among whom 8 were anti-N positive, 4 anti-N undetermined, 21 anti-N inconclusive, 28 had symptoms of COVID-19 disease but were anti-N negative. All patients with positive anti-N serology had a positive anti-S serology. Of the 28 patients with symptoms of SARS-CoV-2 infection and anti-N negative test, none had a positive anti-S test. Anti-S serology only allowed confirmation of the diagnosis for 1 patient with undetermined anti-N serology (Table 1 and Fig. 1 ).
Fig. 1.
Anti-S serology among the 61 patients with anti-N positive or undetermined or inconclusive serology or symptoms of COVID-19 with negative anti-N serology. Anti-N serology refers to IgG directed against the N nucleocapsid protein of the SARS-CoV-2. Threshold for positive anti-N test: > 1.4. Range for undetermined anti-N test: 0.49 to 1.4. Range for inconclusive test: 0.1 to 0.49. Threshold for negative anti-N test: < 0.1. Anti-S serology refers to IgG directed against the SARS-CoV-2 Spike protein.
3.3. Effects of biological DMARDs on the humoral response
Of the 283 patients followed in our outpatient clinic, 85% were under bDMARDs with a large proportion of patients on RTX (n = 44) and Infliximab (IFX) (n = 136) (Table 1). Among the 11 patients with SARS-CoV-2 infection, 6 were on methotrexate (MTX) combination therapy (54%), 5 on IFX (45%), 1 on Abatacept (ABA) (9%), 3 on RTX (27%), 1 on corticosteroid therapy alone (9%), and 1 on NSAIDs alone (9%). Three patients were hospitalized in a department of medicine for their SARS-CoV-2 infection. Of note, 2 of them were treated with RTX, required oxygen therapy, one with a severe disease (Fig. 1).
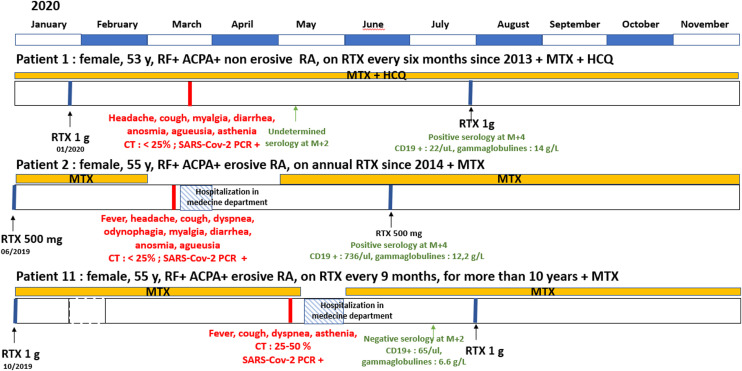
Two patients did not develop a humoral response (anti-N negative or inconclusive, anti-S negative), one was treated by RTX in combination with MTX, and one was treated with ABA monotherapy. One patient with a long-standing corticotherapy had an undetermined anti-N serology but a positive anti-S serology. Because RTX has been reported to potentially alter the humoral response, we focused our descriptive analysis on the 3 patients with SARS-CoV-2 infection and treated with RTX (Fig. 2 ). Patient #1, treated with RTX since 2013 at a dose of 1 g/6 months, got infected 2 months after her last perfusion and had detectable anti-N antibody quantified 4 months after the infection. Patient #2 treated since 2014 at a dosage of 500 mg/year, got infected 9 months after her last perfusion and had detectable anti-N antibody quantified 3 months after the infection. Patient #11, treated for more than 10 years at a dosage of 1 g/9 months, got infected 7 months after her last perfusion but anti-N antibodies were not detectable 2 months after the infection. Each patient had a measurable CD19 level at the time of the serology.
Fig. 2.
Descriptive analysis of the three patients treated with Rituximab (RTX).
4. Discussion
The anti-N seroprevalence of SARS-CoV-2 infection in patients with rheumatic disease during the first wave was relatively low (2.83%) in our population compared with healthy controls assessed between January and May 2020 in the Île de France region according to the EPICOV study (5.7% using anti-N serology) [16]. Nevertheless, such seroprevalence rate is consistent with those obtained in other department of rheumatology in Germany (2.27%) and in Italy (2.03%) [4], [5]. This relatively low seroprevalence in the population of patients with rheumatic diseases mostly treated with bDMARDs compared to the general population may be explained by the great respect for lock-down and protection measures as previously reported [9] or by the use of bDMARD which could reduce the humoral response against the SARS-CoV-2 [10], [11], [12]. By this systematic approach, we were able to identify 1 asymptomatic patient (9% of the infected patients) meaning that the rate of infection with COVID-19 may be underestimated in this specific population of RA and SpA patients mostly treated with bDMARDs. At the same exposure period, only 3.7% of the IDF healthy population had an anti-S positive serology and were asymptomatic [16]. This observation suggests that a systematic serologic screening could be important among patients treated with DMARDs, if we consider that vaccination policy and number of boots depends on the COVID-19 infection status. However, this low seroconversion rate supports the importance of vaccination in this largely unprotected population.
In this study, we showed that the use of a serological test detecting IgG antibodies against the nucleocapsid of the SARS-CoV-2 is an effective strategy for the diagnosis of COVID-19 disease given that anti-N antibodies, unlike anti-S antibodies, are not increased by currently available vaccines. Additional screening by an anti-S serology did not seem to improve the accuracy of the diagnosis for most patients and was conclusive only for one patient with undetermined anti-N serology. Nevertheless, because we did not systematically use anti-S antibody, we assumed that some asymptomatic patients may have been undiagnosed due to a positive test for anti-S but negative for anti-N as previously shown [16]. In this hypothesis, the percentage of asymptomatic patients would be even higher and different from what is observed among healthy controls. Asymptomatic patients might be those with a weaker immune response [17]. To what extend the immunomodulatory drugs can reduce the humoral response is of particular interest and has been recently assessed [12], [13], [14]. In our study, negative, undetermined or inconclusive anti-N serology was found for one patient on RTX (33%), one patient on ABA (33%) and one on glucocorticoids (33%). ABA, and RTX have been shown to reduce immune response after vaccination [18]. Recently, it has been found that patients treated by RTX were more prone to develop severe disease [19], [20] and may be at risk of reinfection [21]. An altered immune response due to RTX may be responsible for such poor outcomes. D'Silva et al. suggest that duration of RTX treatment, dose, and time between the last dose of RTX and SARS-CoV-2 infection may have an impact on the SARS-CoV-2 immune response and severity [12], [14]. In another study including 5 patients treated with RTX, all but one had developed a humoral response despite undetectable circulating CD19+ B-cells for two of them [13]. In our study, the 2 out of 3 patients with long-standing treatment with RTX developed a humoral response whatever the dose, the rhythm and the duration between last infusion and onset of SARS-CoV-2 infection, and despite profound B-cell depletion for one patient (Fig. 2). The third patient under RTX and without seroconversion had both B-cell depletion and hypogammaglobulinemia, two biological parameters that might be of prime importance to determine seroconversion, but not necessarily the severity of the infection. All patients treated with RTX had lung involvement but only patient 2 had a severe disease with mild lung involvement (25–50%) despite seroconversion, no hypogammaglobulinemia and no B-cell depletion quantified 3 months after SARS-CoV-2 infection. Further studies on a larger sample of patients are needed to look into detail the immune response against SARS-CoV-2 in patients on IS drugs. Importantly, the blood humoral response discussed above does not summarize the entire immune response, the T-cell response and the nasopharyngeal humoral response, mainly driven by IgA secretion, should also be of main and complementary importance.
In conclusion, the seroprevalence of SARS-CoV-2 in patients with rheumatic disease, mainly under bDMARDs treatments, was 2.83%. Among infected patients 9% were asymptomatic. The diagnostic strategy combining patients’ interview and anti-N serology seems to be effective in detecting SARS-CoV-2 infections. Seroconversion under RTX seems to depend on both B-cell depletion and hypogammaglobulinemia but studies on larger number of patients are needed. Regarding the severity of SarS-Cov-2 infection, special care should be taken in patients treated with RTX, regardless of seroconversion, hypogammaglobulinemia and B-cell depletion.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Gianfrancesco M., Hyrich K.L., Al-Adely S., et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costantino F., Bahier L., Tarancón L.C., et al. COVID-19 in French patients with chronic inflammatory rheumatic diseases: clinical features, risk factors and treatment adherence. Joint Bone Spine. 2021;88:105095. doi: 10.1016/j.jbspin.2020.105095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quartuccio L., Valent F., Pasut E., et al. Prevalence of COVID-19 among patients with chronic inflammatory rheumatic diseases treated with biologic agents or small molecules: a population-based study in the first two months of COVID-19 outbreak in Italy. Joint Bone Spine. 2020;87:439–443. doi: 10.1016/j.jbspin.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon D., Tascilar K., Krönke G., et al. Patients with immune-mediated inflammatory diseases receiving cytokine inhibitors have low prevalence of SARS-CoV-2 seroconversion. Nat Commun. 2020;11:3774. doi: 10.1038/s41467-020-17703-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benucci M., Damiani A., Giannasi G., et al. Serological tests confirm the low incidence of COVID-19 in chronic rheumatic inflammatory diseases treated with biological DMARD. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218214. [annrheumdis-2020-218214. Epub ahead of print. PMID: 32632035] [DOI] [PubMed] [Google Scholar]
- 6.Toussirot É., Pertuiset É., Sordet C., et al. Safety of rituximab in rheumatoid arthritis patients with a history of severe or recurrent bacterial infection: observational study of 30 cases in everyday practice. Joint Bone Spine. 2010;77:142–145. doi: 10.1016/j.jbspin.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Isvy A., Meunier M., Gobeaux-Chenevier C., et al. Safety of rituximab in rheumatoid arthritis: a long-term prospective single-center study of gammaglobulin concentrations and infections. Joint Bone Spine. 2012;79:365–369. doi: 10.1016/j.jbspin.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Singh J.A., Cameron C., Noorbaloochi S., et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet. 2015;386:258–265. doi: 10.1016/S0140-6736(14)61704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooijberg F., Boekel L., Vogelzang E.H., et al. Patients with rheumatic diseases adhere to COVID-19 isolation measures more strictly than the general population. Lancet Rheumatol. 2020;2:e583–e585. doi: 10.1016/S2665-9913(20)30286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucchini M., Bianco A., Del Giacomo P., et al. Is serological response to SARS-CoV-2 preserved in MS patients on ocrelizumab treatment? A case report. Mult Scler Relat Disord. 2020;44:102323. doi: 10.1016/j.msard.2020.102323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thornton J.R., Harel A. Negative SARS-CoV-2 antibody testing following COVID-19 infection in Two MS patients treated with ocrelizumab. Mult Scler Relat Disord. 2020;44:102341. doi: 10.1016/j.msard.2020.102341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Silva K.M., Serling-Boyd N., Hsu T.Y.-T., et al. SARS-CoV-2 antibody response after COVID-19 in patients with rheumatic disease. Ann Rheum Dis. 2021;80:817–819. doi: 10.1136/annrheumdis-2020-219808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.C S.K., Ahmed S., Shenoy V., et al. Correspondence on SARS-CoV-2 antibody response after COVID-19 in patients with rheumatic disease. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220148. [annrheumdis-2021-220148. Epub ahead of print. PMID: 33692021] [DOI] [PubMed] [Google Scholar]
- 14.D’Silva K.M., Serling-Boyd N., Hsu T.Y.-T., et al. Response to: Correspondence on SARS-CoV-2 antibody response after COVID-19 in patients with rheumatic disease by Shanoj et al. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220166. [annrheumdis-2021-220166. Epub ahead of print. PMID: 33692020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zu Z.Y., Jiang M.D., Xu P.P., et al. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology. 2020;296:E15–E25. doi: 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrat F, de Lamballerie X, Rahib D, et al. Seroprevalence of SARS-CoV-2 among adults in three regions of France following the lockdown and associated risk factors: a multicohort study. Preprint, Infectious Diseases (except HIV/AIDS). Epub ahead of print 18 September 2020. DOI: 10.1101/2020.09.16.20195693.
- 17.Long Q.-X., Tang X.-J., Shi Q.-L., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 18.Rondaan C., Furer V., Heijstek M.W., et al. Efficacy, immunogenicity and safety of vaccination in adult patients with autoimmune inflammatory rheumatic diseases: a systematic literature review for the 2019 update of EULAR recommendations. RMD Open. 2019;5:e001035. doi: 10.1136/rmdopen-2019-001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avouac J., Drumez E., Hachulla E., et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol. 2021;3:e419–e426. doi: 10.1016/S2665-9913(21)00059-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loarce-Martos J., García-Fernández A., López-Gutiérrez F., et al. High rates of severe disease and death due to SARS-CoV-2 infection in rheumatic disease patients treated with rituximab: a descriptive study. Rheumatol Int. 2020;40:2015–2021. doi: 10.1007/s00296-020-04699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pascale Daniel, Marc Raad, Rami Waked, et al. COVID-19 in a Patient Treated for Granulomatosis with Polyangiitis: Persistent Viral Shedding with No Cytokine Storm. Eur J Case Rep Intern Med. Epub ahead of print 24 September 2020. DOI: 10.12890/2020_001922. [DOI] [PMC free article] [PubMed]