Abstract
Objective
COVID-19 is a rapidly spreading disease and many people have been infected in a short time. Favipiravir is under investigation for the treatment of COVID-19 and given to patients in many countries following emergency use approval. Based on data from animal studies, favipiravir use is contraindicated during pregnancy. Currently, there is no human data except for a single case report on use of favipiravir in pregnancy.
Study design
This article includes the outcomes of 29 pregnancies reported to the Clinical Pharmacology and Toxicology Unit regarding favipiravir use in pregnancy. For drug risk assessment, maternal characteristics were obtained at first contact. After the expected day of delivery, follow-up is conducted by phone call and all relevant data regarding pregnancy and newborn outcome were documented.
Results
Of the 29 pregnancies exposed to favipiravir, 5 were electively terminated and 24 resulted in live birth. There were no miscarriages or no stillbirths. There were 25 live births including one pair of twins. Three children were born premature, and one infant had patent foramen ovale. Birth weights, lengths and head circumferences of all infants were within normal range.
Conclusion
The results of the study indicate that favipiravir is unlikely to be a major human teratogen, but experience is still limited for a well-grounded risk assessment. Although these findings may be useful for the physicians and patients, larger studies are needed due to small number of cases.
Keywords: Pregnancy outcome, Favipiravir, Coronavirus, Newborn, Teratogen
Introduction
Millions of people have died of COVID-19 since the pandemic began. During the past two years, many drug and vaccine studies have been conducted against the novel type of coronavirus. However, at the time of this writing, there are still serious questions about the prophylaxis and pharmacotherapy options, and unfortunately the deaths continue to climb.
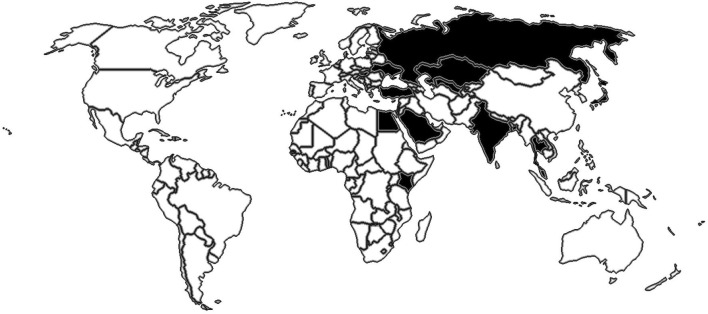
Favipiravir is the antiviral drug of choice for the treatment of COVID-19 in some countries (Fig. 1 ), including Russia, India, Japan, Saudi Arabia, and Turkey [1]. Treatment protocol varies from country to country. Based on data from animal reproduction studies, use is contraindicated in pregnancy [2]. Favipiravir increases malformations and decreases fetal viability in experimental animals. During and after the treatment period, the most effective method of contraception should be used by both man and woman during and after the treatment period, because favipiravir can also disperse into the sperm [3]. Favipiravir should not be used in women who are pregnant or suspected of becoming pregnant, and pregnancy test should be done before the drug is given [4].
Fig. 1.
Geographical distribution of the use of favipiravir.
Currently, there is no human data except for a single case report on the effect of favipiravir in pregnancy [5]. A pregnant woman who was given favipiravir due to Ebola infection in the third trimester of pregnancy gave birth to a live baby, Ebola infection was detected in the baby without congenital anomalies, but the baby recovered. The pregnant woman who gave birth died due to postpartum hemorrhage [6].
To date, there are no prospective data on favipiravir use in pregnancy and counseling of favipiravir is difficult. Given that unplanned pregnancies as well as the contraindications of favipiravir in pregnancy, there is a need to evaluate the effects of favipiravir on pregnancy outcome. Therefore, this study aims to investigate the maternal and neonatal characteristics of favipiravir exposed pregnancies referred to our hospital for clinical pharmacology consultation.
Methods
The study enrolled women who contacted the Clinical Pharmacology and Toxicology Unit (CPTU) in a tertiary Turkish hospital, the Izmir Ataturk Research Hospital, regarding favipiravir exposure in pregnancy. These were accidental exposures and women discontinued favipiravir use after discovering the pregnancy. Following clinical practice guidelines for COVID-19 proposed by the Turkish Ministry of Health [7], favipiravir was given to patients by the filiation teams. Consisting of three members (physician, health professional, and assistant personnel), the filiation team is responsible for following up each case and visiting households to take history and initiate favipiravir treatment in Turkey [8].
CPTU offers drug risk assessment to pregnant women and health care providers. For drug risk assessment, the following information is routinely obtained at the first contact: obstetric and medical history, consanguineous marriage, details of drug exposures (dose, duration, timing in pregnancy, concomitant medication), alcohol or illegal drugs and smoking. Following the face-to-face risk counseling, the pregnant woman receives a written report. After the expected day of delivery, follow-up is usually conducted by phone call with the woman and all relevant data regarding pregnancy and newborn outcome are documented.
In this study, maternal data and the outcomes of pregnant women contacted CPTU regarding favipiravir exposure between 1 September 2020 and 31 December 2020 were evaluated retrospectively. Pregnancy complications (such as preeclampsia, oligohydramnios, gestational diabetes, etc.), type of delivery (normal or cesarean section), whether a congenital defect was observed, postpartum neonatal diseases and/or need for intensive care, gestational week, gender, birth weight, birth length and head circumference of the newborn were questioned. Pregnancies resulting in premature birth, miscarriage and elective termination were also recorded. The newborn checkup findings of the family physician or infant’s pediatrician were examined in detail.
Each case was evaluated individually in terms of pregnancy outcomes (healthy birth, miscarriage, premature birth, and elective termination), and it was mainly focused on whether there was a structural defect in the babies. To assess teratogenicity and well-being of infants, clinical examinations including blood test, pulse oximetry, hearing screen and ultrasounds were done by pediatricians at hospital within 72 h of birth. Neonatal congenital anomalies were registered according to the International Classification of Diseases (ICD-10, Q00-Q99) [9].
Secondary outcomes were data on birth weight, length, and head circumference of preterm (<37 weeks) and term babies. Follow-up controls were monthly performed by the family physicians (two times in first month). Weight, supine length, and head circumference measurements were recorded at the periodic visits, plotted, and analyzed on the growth chart. Additionally, cognitive, fine- gross motor, social- emotional and language development were routinely assessed at each visit. The newborn percentile values were assessed in accordance with gender and gestational week using Fenton’s (preterm) and Neyzi’s (term) infant growth charts [10], [11]. In case of multiple pregnancies, each newborn was considered as one case.
Categorical data were presented as number and percentages. Numerical data were described using median and range (minimum–maximum). Because of the limited number of cases, a control group was not used, and detailed calculations of significances were not performed.
The study was approved by the university ethics committee (#20–088, November 2020). All patients were informed that their medical data will be stored and used for scientific research.
Results
From 1 September 2020 until 31 December 2020 a total of 30 pregnancies with favipiravir exposure were identified. After excluding one case with favipiravir exposure four months before pregnancy, 29 exposures occurred during the contraindicated period (i.e., from 7 days before conception and/or during pregnancy) were included in this study. In 2 of these 29 cases favipiravir was taken until 2nd and 3rd day before pregnancy (case 16 and case 24). There was no case lost to follow-up. Pregnant women received favipiravir treatment for COVID-19 and they were not given a pregnancy test before treatment. During face-to-face risk counselling, it was observed that the pregnant women exposed to favipiravir had fears about the drug’s risk.
Maternal characteristics are presented in Table 1 . Most of the pregnant women were young (≤35 years) and did not smoke or use alcohol. One in four women was nulliparous and most women have never had a miscarriage before. There was only one pregnant woman who reported consanguineous marriage and birth defect (musculoskeletal anomaly and Down syndrome) in her previous child (case 20). This woman gave birth to a healthy baby girl (3390 g, 50 cm) at week 39 by cesarean section.
Table 1.
Maternal characteristics and obstetrical history of favipiravir exposed women (n = 29).
| Number (%)or Median (min/max) | |
|---|---|
| Age | 29 (20–39) |
| ≤35 years | 24 (82.7%) |
| >35 years | 5 (6.8%) |
| Smoking | |
| No | 22 (75.9%) |
| ≤ 5 cig/day | 4 (13.8%) |
| > 5 cig/day | 3 (10.3%) |
| Alcohol | |
| No | 27 (93.1%) |
| ≤1 drink/day | 2 (6.9%) |
| > 1 drink/day | 0 (0.0%) |
| Chronic disease | |
| No | 22 (75.9%) |
| Yes | 7 (24.1%) |
| Epilepsy | 2 (28.5%) |
| Hypothyroidism | 2 (28.5%) |
| Hyperthyroidism | 1 (14.3%) |
| Allergic rhinitis | 1(14.3%) |
| Sjögren syndrome | 1(14.3%) |
| Asthma | 1 (14.3%) |
| Consanguineous marriage | |
| No | 28 (96.6%) |
| Yes | 1 (3.4%) |
| Previous pregnancies | |
| 0 | 7 (24.1%) |
| 1 | 11 (37.9%) |
| 2 | 8 (27.6%) |
| 3 | 3 (10.4%) |
| Previous parities | |
| 0 | 10 (34.5%) |
| 1 | 10 (34.5%) |
| 2 | 8 (27.5%) |
| 3 | 1 (3.5%) |
| Previous miscarriages | |
| 0 | 23 (79.3%) |
| 1 | 5 (17.3%) |
| 2 | 1 (3.4%) |
| Previous children with birth defect | |
| 0 | 28 (96.6%) |
| 1 | 1 (3.4%) |
| Gestational week at first contact CPTU | 8 (4–16) |
Approximately one out of every four women had a history of chronic disease. The most common chronic diseases in pregnant women were thyroid diseases (3/29), epilepsy (2/29) and allergic diseases (2/29). These pregnant women also used drugs for their chronic diseases along with favipiravir. Non-steroidal anti-inflammatory drugs (NSAID), hydroxychloroquine and paracetamol were mostly taken as additional drugs. These are the drugs chosen to support the treatment of COVID-19. In addition, it was determined that about one in three pregnant women used natural products such as thyme, mint lemon, cinnamon, ginger, cherry stalk, corn silk, propolis. Three of the pregnant women reported taking various antidepressant and anxiolytic medications without a prescription after being diagnosed with COVID-19.
Data on favipiravir exposures during pregnancy are given in Table 2 . All pregnancies were unplanned and the first CPTU contact of almost all pregnant women was immediately after realizing that they were pregnant (between weeks 4–16). In pregnant women, the dose of favipiravir and the duration of treatment were as described in national treatment protocol (3200 mg followed by 1200 mg/day for 5 days) [7]. However, three pregnant women reported that they could not continue the medication for 5 days.
Table 2.
Patterns of favipiravir exposure (n = 29).
| Number (%) orMedian (min/max) | |
|---|---|
| Time of exposure | |
| Preconception * | 2 (6.9%) |
| 1st trimester | 25 (86.2%) |
| 0- 3rd week | 3 (12.0%) |
| 3rd- 4th week | 7 (28.0%) |
| 4th- 5thweek | 12 (48.0%) |
| 5th- 6th week | 3 (12.0%) |
| 2nd trimester | 2 (6.9%) |
| Dose of favipiravir | |
| 3200 mg followed by 1200 mg/day | 26 (89.6%) |
| 1200 mg/day | 3 (10.3%) |
| 400 mg/day | 1 (3.4%) |
| Duration of exposure (day) | 5 (5–14) |
| Comedication | |
| No | 9 (31.0%) |
| Yes | 20 (69.0%) |
| NSAID | 8 (40.0%) |
| Hydroxychloroquine | 7 (35.0%) |
| Natural products | 6 (30.0%) |
| Paracetamol | 5 (25.0%) |
* Favipiravir was taken until 2nd and 3rd day before conception.
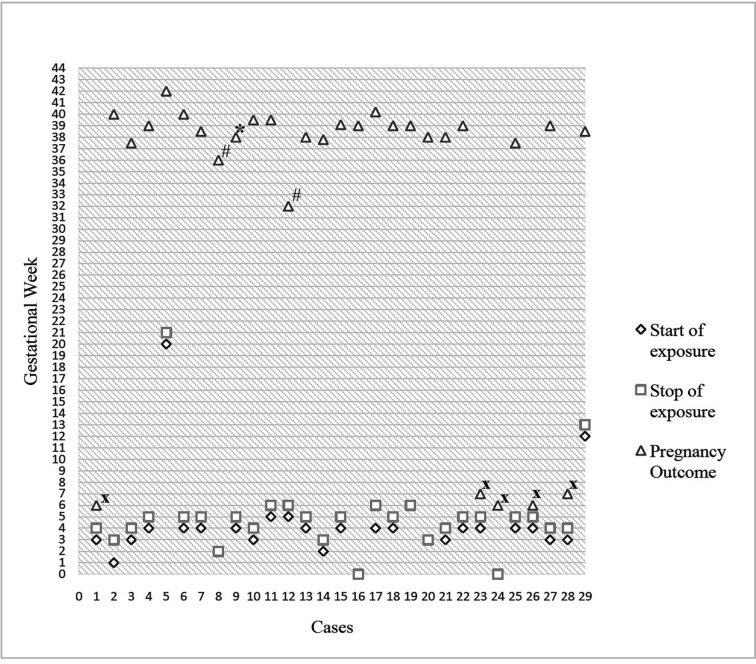
Of the 29 pregnancies exposed to favipiravir, 5 were electively terminated and 24 resulted in live birth. There were no miscarriages or no stillbirths. Fig. 2 presents data on favipiravir exposure and pregnancy outcome. When the cases were evaluated in a chronological order, it was remarkable that the consultation dates of the pregnancies resulting in elective termination were on the first and last days of the study. The absence of similar pregnancies with favipiravir exposure was the cause of the elective terminations in first cases. In last cases, it was caused by the lack of sufficient data on the drug. Three out of 5 pregnant women who decided to terminate their pregnancies were nulliparous. Approximately half of the pregnant women who decided to continue their pregnancies (11/24) reported that their doctors had also recommended elective terminations to them.
Fig. 2.
Favipiravir exposure and pregnancy outcome * Birth defect, # preterm birth, x elective termination.
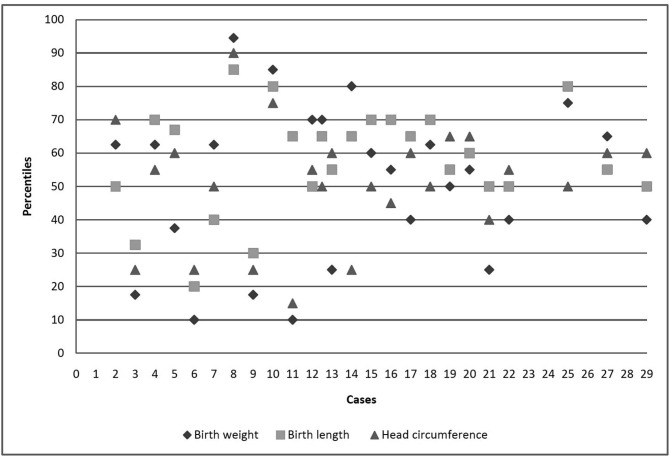
There were 25 live births (12 males, 13 females) including one pair of twins. Caesarean section was performed in more than half of the live births (13/24). Two children were born premature (gestational age at birth < 37 weeks). One of the 25 live born infants showed minor congenital anomaly (case 9, patent foramen ovale). The mother had oligohydramnios at week 35 and gave birth at term. Birth weights, lengths and head circumferences of all infants including one with birth defect and two preterm babies were within normal range after correction for sex and gestational age. The physical growth and neurological development were uneventful for the infants at 4 months of age. Further details are given in Fig. 3 .
Fig. 3.
Child characteristics.
Discussion
In this study, 29 pregnancies with favipiravir exposure were evaluated. No spontaneous abortions and no miscarriages were observed. While the rates of birth defects and preterm births were not higher when compared with the general population [12], [13], there was a considerably higher rate of elective termination [14]. Most pregnant women were young (24/29), had no chronic disease (22/29) and did not use alcohol (27/29) or tobacco (22/29). Except for one pregnant woman with consanguineous marriage, there was no history of childbearing with birth defects. They took no additional teratogenic drugs. So, the study group was well suited to observe the effects of the drug in pregnancy. On the other hand, the small number of cases and the inability to compare the data of the study group with a control group are limitations of the study. However, while preparing this article, it was aimed to present the first data on favipiravir exposure as soon as possible, because COVID-19 and new drugs used in its treatment are issues that need urgent evaluation.
There is currently no specific medication for COVID-19. Only an antiviral drug called remdesivir has been approved by the United States Food and Drug Administration (FDA), but remdesivir is unavailable in many other countries of the world [15]. Drugs used in the treatment vary from country to country and over time. In this study, pregnant women took favipiravir with/without hydroxychloroquine when referred to CPTU for drug risk assessment. However, hydroxychloroquine has no place in the treatment of COVID-19 today [7], [15]. Other drugs most taken with favipiravir were NSAID and paracetamol. This is not surprising because COVID-19 often causes fever and pain. Nearly one in four pregnant women reported that they used herbal or other natural products additionally. Three of the pregnant women took various psychotropic drugs after being diagnosed with COVID-19. Pathogenic and therapeutic uncertainties cause patients to feel depressed and to seek alternative treatments.
All pregnancies were unplanned and the first CPTU contact was immediately after discovering the pregnancy. While most of the exposures occurred within the first weeks of pregnancy, fetuses may be not yet at great risk of birth defects. Elimination half-life of favipiravir is shorter (2.5–5 h) when compared with major teratogens such as thalidomide or isotretinoin [2]. However, when evaluating drug exposure, it should be considered that the critical period of organogenesis extends from about 20–70 days after the first day of last menstrual period, or from 1 week before the missed menstruation until the 44th day [16].
The most striking findings of the study were neonatal outcomes of women exposed to favipiravir. There were neither stillbirths nor spontaneous abortions, and all ongoing pregnancies resulted in live births. No major congenital malformation was observed, but one child had patent foramen ovale (PFO). PFO is a minor congenital malformation that affects about one in four newborns [17]. Most patients with a PFO do not have any symptoms. PFO usually closes 6 months to a year after the baby's birth [18].
Two pregnancies resulted in preterm delivery, and these were planned cesarean sections. One of them was due to twin pregnancy at week 32 (case 12) and the other cesarean section was due to abnormal fetal presentation at week 36 (case 8). The possibility of premature birth in twin pregnancies is higher than in singleton pregnancies [19]. Several publications have shown that cesarean delivery can reduce mortality and morbidity for preterm twins [20], [21]. Preterm birth is also associated with fetal malpresentation like breech presentation and most breech fetuses are born by planned cesarean delivery [22].
The rate of elective terminations (5/29, 17.2%) was much more than reported in the country (approximately 3%) where the study was conducted [23]. There was no termination for embryonal pathology. Pregnant women decided to terminate their pregnancies at the earliest stage because of fear of the drug’s risk. Interestingly, nearly half of the women (11/24) who wished to continue pregnancy reported that their gynecologists or consultant physicians had told they could “not” have a healthy baby and they “should” terminate the pregnancy. If drug data is not enough, it should be explained that drug effects on human pregnancy cannot be evaluated absolutely based on experimental animal studies. As well as about the risk of teratogenicity of the drug, physicians should inform pregnant women about the possibility of giving birth to healthy children (chance versus risk).
Loss to follow-up is very important in determining a study’s validity [24]. There was no loss to follow-up in the study, because the pregnant women were referred for risk counseling only 11–14 months before the date of this writing. This demonstrates the importance of early first contact for follow-up in studies. It is also important to routinely follow-up in units where drug counseling is provided during pregnancy. Abnormal pregnancy results that can be detected in routine follow-up and provide information about the effects of the drug in pregnancy.
COVID-19 is a rapidly spreading disease and many people have been infected in a short time. Adverse symptoms of disease and side effects of drugs used in the treatment should be followed closely. The focus should be on drug effects and particular attention should be paid to special patient groups such as pregnant women, the elderly, and children in the use of newly tested drugs. Since these groups, especially pregnant women, are excluded from clinical trials for drug efficacy, there is no way to evaluate accidental exposures other than patient follow-up.
In addition, the findings obtained by the follow-up of the patients should be shared rapidly, because the terms such as “uncertainty”, “no single case” and “rare medical conditions” can cause fear and elective pregnancy terminations [25]. Drugs identified as “X” or “contraindicated” based on experimental studies or insufficient human data are of concern to both physician and pregnant woman. High risk perceptions of teratogenic drug effects lead to mismanagement of pregnancy [26]. Medication counseling in pregnancy is not just a literature review and its presentation to the patient. It is necessary to communicate in a way that the pregnant woman can understand and to pay attention to her fears and thoughts about pregnancy. The decision to terminate the pregnancy should be left to pregnant woman and her family.
This study has some limitations. Favipiravir is not approved in most countries all around the world but only in a few such as Turkey. While there is an urgency for a pilot study about the relationship between favipiravir and pregnancy, to counsel patients in the countries where this drug is allowed, the small number of observations does not allow to draw firm conclusion to be adopted in other countries.
Conclusion
The results of the study indicate that favipiravir is unlikely to be a major human teratogen, but experience is still limited for a well-grounded risk assessment. Each case with inadvertent favipiravir exposure in pregnancy should be followed up and reported. Although these findings are useful for the physicians and patients, the number of observations is too small to draw firm conclusion. Nevertheless, it is important that an individual risk assessment and face-to-face counseling should be done to avoid high risk perceptions of teratogenic effect and mismanagement of pregnancy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The author thanks all contributing patients, their physicians, and families as well as all the colleagues in the CPTU.
References
- 1.Sood S., Bhatia G.K., Seth P., Kumar P., Kaur J., Gupta V., et al. Efficacy and safety of new and emerging drugs for COVID-19: Favipiravir and dexamethasone. Curr Pharmacol Rep. 2021;7(2):49–54. doi: 10.1007/s40495-021-00253-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joshi S., Parkar J., Ansari A., Vora A., Talwar D., Tiwaskar M., et al. Role of favipiravir in the treatment of COVID-19. Int J Infect Dis. 2021;102:501–508. doi: 10.1016/j.ijid.2020.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagata T., Lefor A.K., Hasegawa M., Ishii M. Favipiravir: a new medication for the Ebola virus disease pandemic. Disaster Med Public Health Prep. 2015;9(1):79–81. doi: 10.1017/dmp.2014.151. [DOI] [PubMed] [Google Scholar]
- 4.Delang L., Abdelnabi R., Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral Res. 2018;153:85–94. doi: 10.1016/j.antiviral.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Louchet M., Sibiude J., Peytavin G., Picone O., Tréluyer J.-M., Mandelbrot L. Placental transfer and safety in pregnancy of medications under investigation to treat coronavirus disease 2019. Am J Obstet Gynecol. 2020;2(3):100159. doi: 10.1016/j.ajogmf.2020.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caluwaerts S. Nubia’s mother: Being pregnant in the time of experimental vaccines and therapeutics for Ebola. Reprod Health. 2017;14(s3):157. doi: 10.1186/s12978-017-0429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Republic of Turkey Ministry of Health, COVID-19 adult patient treatment [updated 2021 May 7] [cited 2021 August 10] Available from https://covid19.saglik.gov.tr/TR-66926/eriskin-hasta-tedavisi.html
- 8.Demirtaş T., Tekiner H. Filiation: A historical term the COVID-19 outbreak recalled in Turkey. Erciyes Med J. 2020;42(3):354–358. [Google Scholar]
- 9.ICD-10 Version:2019 [cited 2021 August 10] Available from https://icd.who.int/browse10/2019/en#/Q90-Q99
- 10.Fenton T.R., Kim J.H. A systematic review and metaanalysis to revise the Fenton growth chart for premature infants. BMC Pediatr. 2013;13:59. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neyzi O., Günöz H., Furman A., et al. Reference values for body weight, height, head circumference and body mass index in Turkish children. Cocuk Sagligi ve Hastaliklari Derg. 2008;51(1–14):Turkish. [Google Scholar]
- 12.Schaefer C., Peters P., Miller R.K. Academic Press; London: 2015. Drugs During Pregnancy and Lactation: Treatment Options and Risk Assessment; p. 159. [Google Scholar]
- 13.Blencowe H., Cousens S., Chou D., Oestergaard M., Say L., Moller A.-B., et al. Born too soon preterm birth action group. Born too soon: theglobal epidemiology of 15 million preterm births. Reprod Health. 2013;10(S1) doi: 10.1186/1742-4755-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sedgh G., Singh S., Hussain R. Intended and unintended pregnancies worldwide in 2012 and recent trends. Stud Fa. Plann. 2014;45(3):301–314. doi: 10.1111/j.1728-4465.2014.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines [cited 2021 August 10] Available from https://covid19treatmentguidelines.nih.gov/ [PubMed]
- 16.Schaefer C., Peters P., Miller R.K. Academic Press; London: 2015. Drugs During Pregnancy and Lactation: Treatment Options and Risk Assessment; p. 10. [Google Scholar]
- 17.Asrress K.N., Marciniak M., Marciniak A., Rajani R., Clapp B. Patent foramen ovale: the current state of play. Heart. 2015;101(23):1916–1925. doi: 10.1136/heartjnl-2015-307639. [DOI] [PubMed] [Google Scholar]
- 18.Yuan Z., Zhang L.-Z., Li B., Chung H.-T., Jiang J.-X., Chiang J.Y., et al. Investigation of echocardiographic characteristics and predictors for persistent defects of patent foramen ovale or patent ductus arteriosus in Chinese newborns. Biomed J. 2021;44(2):209–216. doi: 10.1016/j.bj.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizrahi M., Furman B., Shoham-Vardi I., Vardi H., Maymon E., Mazor M. Perinatal outcome and peripartum complications in preterm singleton and twins’ deliveries: a comparative study. Eur J Obstet Gynecol Reprod Biol. 1999;87(1):55–61. doi: 10.1016/s0301-2115(99)00075-5. [DOI] [PubMed] [Google Scholar]
- 20.Sentilhes L., Oppenheimer A., Bouhours A.-C., Normand E., Haddad B., Descamps P., et al. Neonatal outcome of very preterm twins: policy of planned vaginal or cesarean delivery. Am J Obstet Gynecol. 2015;213(1):73.e1–73.e7. doi: 10.1016/j.ajog.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Smith G.C.S., Shah I., White I.R., Pell J.P., Dobbie R. Mode of delivery and the risk of delivery-related perinatal death among twins at term: a retrospective cohort study of 8073 births. BJOG. 2005;112(8):1139–1144. doi: 10.1111/j.1471-0528.2005.00631.x. [DOI] [PubMed] [Google Scholar]
- 22.Herbst A., Thorngren-Jerneck K. Mode of delivery in breech presentation at term: increased neonatal morbidity with vaginal delivery. Acta Obstet Gynecol Scand. 2001;80:731–737. doi: 10.1034/j.1600-0412.2001.080008731.x. [DOI] [PubMed] [Google Scholar]
- 23.Huber-Krum S, Karadon D, Kurutas S, et al. Estimating abortion prevalence and understanding perspectives of community leaders and providers: Results from a mixed-method study in Istanbul, Turkey. Women's Health 2020;16: 1745506520953353. [DOI] [PMC free article] [PubMed]
- 24.Dettori J. Loss to follow-up. Evid Based Spine Care J. 2011;2(01):7–10. doi: 10.1055/s-0030-1267080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozturk Z., Olmez E., Gurpınar T., Vural K. Birth outcomes after inadvertent use of category X drugs contraindicated in pregnancy: Where is the real risk? Turk J Pediatr. 2018;60(3):298–305. doi: 10.24953/turkjped.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Damase-Michel C., Pichereau J., Pathak A., Lacroix I., Montastruc J.L. Perception of teratogenic and foetotoxic risk by health professionals: a survey in Midi-Pyrenees area. Pharm Pract. 2008;6(1) doi: 10.4321/S1886-36552008000100003. [DOI] [PMC free article] [PubMed] [Google Scholar]