Abstract
Background
Data in the literature about HSV reactivation in COVID-19 patients are scarce, and the association between HSV-1 reactivation and mortality remains to be determined. Our objectives were to evaluate the impact of Herpes simplex virus (HSV) reactivation in patients with severe SARS-CoV-2 infections primarily on mortality, and secondarily on hospital-acquired pneumonia/ventilator-associated pneumonia (HAP/VAP) and intensive care unit-bloodstream infection (ICU-BSI).
Methods
We conducted an observational study using prospectively collected data and HSV-1 blood and respiratory samples from all critically ill COVID-19 patients in a large reference center who underwent HSV tests. Using multivariable Cox and cause-specific (cs) models, we investigated the association between HSV reactivation and mortality or healthcare-associated infections.
Results
Of the 153 COVID-19 patients admitted for ≥ 48 h from Feb-2020 to Feb-2021, 40/153 (26.1%) patients had confirmed HSV-1 reactivation (19/61 (31.1%) with HSV-positive respiratory samples, and 36/146 (24.7%) with HSV-positive blood samples. Day-60 mortality was higher in patients with HSV-1 reactivation (57.5%) versus without (33.6%, p = 0.001). After adjustment for mortality risk factors, HSV-1 reactivation was associated with an increased mortality risk (hazard risk [HR] 2.05; 95% CI 1.16–3.62; p = 0.01). HAP/VAP occurred in 67/153 (43.8%) and ICU-BSI in 42/153 (27.5%) patients. In patients with HSV-1 reactivation, multivariable cause-specific models showed an increased risk of HAP/VAP (csHR 2.38, 95% CI 1.06–5.39, p = 0.037), but not of ICU-BSI.
Conclusions
HSV-1 reactivation in critically ill COVID-19 patients was associated with an increased risk of day-60 mortality and HAP/VAP.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-021-03843-8.
Keywords: HSV, Critically ill, Mortality, COVID-19, Pneumonia, Bacteremia
Background
Severe SARS-CoV-2 infection is a life-threatening disease associated with 10–50% mortality [1–8] responsible of respiratory failure and Acute Respiratory Distress Syndrome (ARDS), requiring oxygen support in intensive care unit (ICU). It is associated with immune alterations with profound lymphopenia and requires corticosteroid therapy [2, 9, 10] and immune modulators [11–15] which could affect the risk of superinfections [16]. Hospital-acquired pneumonia (HAP) and particularly ventilator-associated pneumonia (VAP) are frequent among COVID-19 patients, with rates ranging from 29 to 79% [6, 16–21].
Herpes simplex virus (HSV) reactivations have been already described and are common in critically ill patients with ARDS [22–24]. An oropharyngeal HSV reactivation was observed in 20 to 54% of critically ill patients [25–27], and HSV was detectable in lower respiratory tract of up to 64% of mechanically-ventilated patients [27, 28].
Several authors described an impact of HSV tracheobronchitis or pneumonia on mortality [29–31]. Moreover, HSV detection in lower respiratory tract and true HSV bronchopneumonitis were associated with poorer outcomes [27, 32]. Data on the association between HAP/VAP and HSV reactivation are scarce [27, 29]. In March 2020, during the first COVID-19 wave [8], we observed blood and respiratory samples positive for HSV in several patients. Therefore, we decided to systematically monitor on a weekly basis HSV reactivation in blood of critically ill COVID-19 patients admitted to our ICU. Furthermore, each on-demand respiratory samples was also processed for HSV detection.
Our primary objective was to describe patients with HSV reactivation and to evaluate its impact on mortality in critically ill COVID-19 patients. Our secondary objective was to investigate the impact of HSV reactivation on severe healthcare-associated ICU-infections (i.e., HAP/VAP and ICU-acquired bloodstream infections [ICU-BSI]) in COVID-19 patients.
Materials and methods
Study design and population
We conducted an observational study using prospectively collected data from patients hospitalized at the Bichat university hospital, a large reference center for COVID-19 patients in the Northern Paris Region.
We included all COVID-19 patients who underwent HSV tests in blood and lower respiratory tract (i.e., in mechanically-ventilated patients) samples on a regular basis.
Data collection
The study used data from the French prospectively collected multicenter OutcomeRea® database. In accordance with French law, the OutcomeRea® database was approved by the French Advisory Committee for Data Processing in Health Research and the French National Commission for Data Protection and Liberties (registration number 8999262). The database protocol was approved by the Institutional Review Board of the Clermont-Ferrand University Hospital, France. As the research objective of this study was not detailed in the original database protocol, this particular data analysis was then approved by the ethical committee of the Society of Resuscitation of French Language (SRLF), referenced as CE SRLF 21–83. Data storage and data analysis followed MR003 or MR004 methodology as appropriate and had been approved by French legal authorities. Informed consent was not required since the study did not modify patient management, and data were anonymously processed. Oral information was given to the patients or closest family about the possible use of data entered for research purposes, especially about determinants of prognostic of ICU patients.
Demographic, clinical and microbiological data on Polymerase Chain Reaction (PCR)-tested COVID-19 patients were prospectively collected at ICU admission and daily by clinicians.
The following variables at ICU admission were included: demographics, chronic disease/comorbidities, Sequential Organ Failure Assessment (SOFA) scores, body temperature, blood levels of C-Reactive Protein (CRP), D-Dimers, Lactate Dehydrogenase (LDH), and ferritin, ventilation status, and use of immune modulatory agents. Clinical and biological parameters were recorded daily. We also recorded daily data on antiviral agents (including acyclovir, ganciclovir, valganciclovir and valaciclovir) and immunomodulator (corticosteroids, Interleukin-6 receptor antagonists [IL6-ra] and interleukin-1 receptor antagonists [IL1-ra]) medications.
Patients were monitored daily during the ICU stay and followed after ICU discharge until day 60 after admission.
Study procedures
Blood HSV specimens were prospectively collected on ICU admission and weekly. All mechanically-ventilated COVID-19 patients underwent regularly lower respiratory tract samples for HSV test until removal of the endotracheal tube or death. Endotracheal aspirates, bronchoalveolar fluid, and samples through plugged telescoping catheter were all considered as lower respiratory tract samples. HSV-1 and HSV-2 were routinely screened. All specimens were sent to an accredited reference virology laboratory. Blood and lower respiratory HSV were detected by an automated real-time PCR technology “Simplexa® direct assay HSV1/HSV2 Diasorin®”. HSV-1 and HSV-2 DNA were detected by real-time PCR targeting the HSV polymerase genes. Results for HSV-1 and HSV-2 were provided as positive or negative with PCR cycle threshold (Ct) values.
Definitions and outcomes
HSV reactivation was defined as a HSV-positive PCR in blood or respiratory samples.
The primary outcome was the 60-day mortality. Secondary outcomes were HAP/VAP and ICU-BSI. HAP was defined as a pneumonia occurring in patients hospitalized for at least 48 h. VAP was defined as a pneumonia occurring in ICU patients mechanically ventilated for at least 48 h [33]. The following three criteria had to be fulfilled: (i) new or progressive and persistent infiltrates or consolidation or cavitation; (ii) pathological body temperature (< 36 °C or > 38 °C) or white blood cell count < 4 or > 12 × 103 cells/mm3; (iii) either the new onset/increase of purulent aspirates or worsening gas exchange [33–35]. Endotracheal aspirates, bronchoalveolar fluid, and plugged telescoping catheter samples with semi-quantitative cultures were assessed for microbiological diagnostic with positivity thresholds of 106, 104 and 103 cfu/mL, respectively.
ICU-BSI was defined as a bacteremic episode occurring > 48 h after ICU admission. Typical skin contaminants (i.e., coagulase-negative staphylococci [CoNS]) were included only if ≥ 2 blood cultures showed the same phenotype on separate samples drawn within a 48-h period, or ≥ 1 blood culture positive for clinical sepsis, without any other infectious process, and with an antibacterial agent just initiated by the attending physician [36].
Statistical analysis
Characteristics of patients were described as median (interquartile range [IQR]) or count (percent) for qualitative and quantitative variables, respectively. HSV positive and negative patients were compared with Chi-square, Fisher and Wilcoxon tests, as appropriate.
The association between HSV-1 reactivation and mortality was estimated using univariable and multivariable Cox models. The variable of interest (HSV-1 reactivation) was forced as a time-dependent variable in the multivariable model. The following covariates were included in the models: age, chronic disease, SOFA score, type of ventilation, use of corticosteroids (i.e., well-known mortality risk factors in critically ill COVID-19 patients). To avoid overfitting with mechanical ventilation variables, only extra-respiratory components of SOFA score were taken into account in the multivariate model. Then, a hazard ratio (HR) for HSV-1 reactivation was derived; a HR > 1 indicated an increased risk for HSV-1 reactivation. The follow-up lasted 60 days. We performed subgroup analyses for blood and respiratory HSV-1 reactivation, respectively. Moreover, we tested the impact of acyclovir therapy on day-60 mortality by sorting HSV-1 positive patients into two groups according to acyclovir treatment.
To evaluate the effect of HSV-1 reactivation on healthcare-associated infections (i.e., HAP/VAP and ICU-BSI), we used cause-specific hazards models. Time zero (T0) was the ICU admission. To determine the risk of healthcare-associated infections, the basis assumption was that censoring was not associated with an altered chance of the event occurring at any given moment. In the current analysis, ICU-mortality was considered as competing event with HAP/VAP or ICU-BSI. The risk of HSV-1 reactivation (versus non-HSV-1) as a time-dependent variable was then estimated using Cox cause-specific hazards models. Risk of HAP/VAP or ICU-BSI was expressed in cause-specific hazard ratios (csHR). We performed subgroup analysis for blood and respiratory HSV-1 reactivations, respectively. p-values < 0.05 were considered to be significant. Statistical analyses were performed using SAS 9.4 (Cary, North Carolina, USA).
Results
Study population
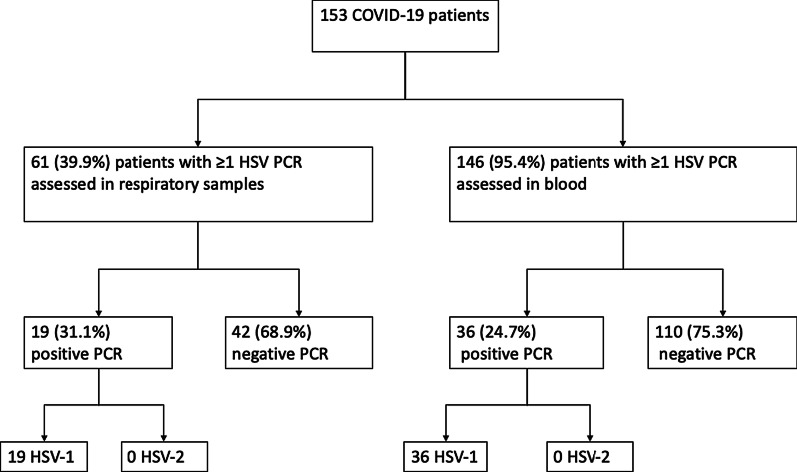
Of the 153 SARS-CoV2 patients admitted for more than 48 h from February 2020 to February 2021 and who underwent at least one HSV screening, respiratory and blood samples were tested from 61/153 (39.9%) and 146/153 (95.4%) patients, respectively (Fig. 1). HSV results were positive in respiratory samples for 19/61 (31.1%) patients and blood samples for 36/146 (24.7%) patients. Fifteen of 153 (9.8%) patients had a HSV-1 reactivation both in blood and respiratory tract: HSV-1 reactivation occurred first in blood for 7/15, in respiratory tract for 6/15 and simultaneously for 2/15 patients.
Fig. 1.
Flowchart. HSV Herpes simplex virus. PCR polymerase chain reaction
The median time from ICU admission to the first HSV-positive sample was 14 days (IQR 9.5–18). Importantly, HSV-1 was recovered from all HSV-positive specimens, no reactivation with HSV-2 was observed. Overall, 40/153 (26.1%) patients had at least one positive sample with HSV-1. In total, 481 samples were collected, including 120 respiratory samples: 91 (81.3%) from bronchoalveolar lavage, the other ones from upper tracheal secretions.
Baseline characteristics
The median age was 60.8 (IQR 50–70) years (Table 1). Patients were mostly men (n = 115, 75.2%) and seventy-three (47.7%) had at least one chronic disease; cardiovascular disease and obesity were the most frequently concomitant medical conditions, in 49 (32%) and 62 (40.5%) patients, respectively. The median SOFA score on admission was 5 (IQR 3–7). During the first two ICU days, 52 (33.4%) patients required mechanical ventilation, corticosteroids were administered to 108 (70.6%) patients and tocilizumab or anakinra to 31 (20.3%) patients.
Table 1.
Baseline characteristics of patients with and without HSV reactivations
| All patients (n = 153) | Without HSV reactivation (n = 113) | HSV reactivation (n = 40) | p-value | ||
|---|---|---|---|---|---|
| Female | Number (%) | 38 (24.8) | 28 (24.8) | 10 (25) | 1.000 |
| Age | Median (IQR) | 60.8 [50.2; 69.5] | 60.8 [50.1; 68.8] | 61.9 [50.9; 70.8] | 0.546 |
| BMI | Median (IQR) | 28.8 [25.3; 33] | 29 [25.4; 33] | 27.7 [24.4; 33.2] | 0.629 |
| Diabetes | Number (%) | 48 (31.4) | 40 (35.4) | 8 (20) | 0.078 |
| Other chronic diseases* | Number (%) | 73 (47.7) | 55 (48.7) | 18 (45) | 0.716 |
| SOFA score | Median (IQR) | 5 [3; 7] | 5 [3; 7] | 5 [4; 7] | 0.468 |
| Maximal body temperature (°C) | Median (IQR) | 38.2 [37.5; 39.1] | 38 [37.5; 39] | 38.9 [37.8; 39.3] | 0.029 |
| Mechanical ventilation | 0.009 | ||||
| None | Number (%) | 16 (10.5) | 14 (12.4) | 2 (5) | |
| CPAP/HFNO | Number (%) | 85 (55.6) | 69 (61.1) | 16 (40) | |
| IMV/PEEP < 10 mmHg | Number (%) | 0 | 0 | 0 | |
| IMV/PEEP > 10 mmHg | Number (%) | 40 (26.1) | 22 (19.5) | 18 (45) | |
| ECMO | Number (%) | 12 (7.8) | 8 (7.1) | 4 (10) | |
| Lymphocytes count (/mm3) | Median (IQR) | 885 [595; 1220] | 890 [610; 1230] | 880 [570; 1210] | 0.784 |
| Initial use of corticosteroid | Number (%) | 108 (70.6) | 86 (76.1) | 22 (55) | 0.016 |
| Tocilizumab/anakinra | Number (%) | 31 (20.3) | 25 (22.1) | 6 (15) | 0.492 |
| CRP (mg/L) | Median (IQR) | 138.5 [77.5; 204.5] | 125 [64.5; 183.5] | 180.5 [115.5; 252.5] | 0.001 |
| D-Dimers (mg/mL) | Median (IQR) | 962 [588; 2043] | 952 [564; 1768] | 1056.5 [682; 4376] | 0.195 |
| Ferritin (ng/mL) | Median (IQR) | 1159 [592; 1979] | 1060 [526; 1915] | 1348 [832; 2370] | 0.110 |
| LDH (UI/L) | Median (IQR) | 438.5 [337; 606.9] | 417 [331; 538] | 504.9 [388; 695] | 0.019 |
HSV-positive and -negative patients were compared with Chi-square, Fisher and Wilcoxon tests IQR Inter Quartile Range. These data were collected on ICU admission (day one or two)
HSV Herpes simplex virus; BMI Body Mass Index; SOFA Sequential Organ Failure Assessment; CPAP Continuous Positive Airway Pressure; HFNO High-Flow Nasal Oxygenotherapy; IMV Invasive Mechanical Ventilation; PEEP Positive End Expiratory Pressure; ECMO Extra-Corporeal Membrane Oxygenation; CRP C Reactive Protein; LDH Lactate Dehydrogenase
*Other chronic diseases were hepatic, heart, respiratory, renal or hematologic diseases, according to the Knaus definitions
HSV-positive and -negative patients were compared with Chi-square, Fisher and Wilcoxon tests IQR Inter Quartile Range. These data were collected on ICU admission (day one or two)
HSV Herpes simplex virus; BMI Body Mass Index; SOFA Sequential Organ Failure Assessment; CPAP Continuous Positive Airway Pressure; HFNO High-Flow Nasal Oxygenotherapy; IMV Invasive Mechanical Ventilation; PEEP Positive End Expiratory Pressure; ECMO Extra-Corporeal Membrane Oxygenation; CRP C Reactive Protein; LDH Lactate Dehydrogenase
*Other chronic diseases were hepatic, heart, respiratory, renal or hematologic diseases, according to the Knaus definitions
At admission, patients with HSV-1 reactivation during the hospitalization had higher body temperature (38.9 °C versus 38 °C; p = 0.001), higher CRP levels (180.5 mg/L versus 125 mg/L; p = 0.001) and higher LDH levels (504.9 UI/L versus 417 U/L, p = 0.02) than patients without HSV-1 reactivation. The median HSV Ct value of the first positive blood sample was 33.5 (IQR 31.2–35.6), whereas it was 28.2 (IQR 21.4; 33.4) for the first positive respiratory sample.
HSV-1 reactivation and mortality
Primary and secondary outcomes are described in Table 2. The median length of ICU stay was 23 (IQR 17–40) days and 9 (IQR 6–15) days for patients with and without HSV-1 reactivation (p = 0.001), respectively. Eighty-nine patients (58.2%) required invasive mechanical ventilation or Extra-Corporeal Membrane Oxygenation (ECMO). After ICU discharge, only one clinical HSV-1 infection (skin infection) was detected and treated with acyclovir.
Table 2.
Outcomes in HSV and non-HSV patients
| All patients (n = 153) | Without HSV reactivation (n = 113) | HSV reactivation (n = 40) | p-value | ||
|---|---|---|---|---|---|
| Length of stay ICU | Median (IQR) | 11.5 [7; 21.5] | 9 [6; 15] | 23 [17; 40] | < 0.001 |
| Death in ICU | Number (%) | 57 (37.3) | 36 (31.9) | 21 (52.5) | 0.024 |
| Death at day 60 | Number (%) | 61 (39.9) | 38 (33.6) | 23 (57.5) | 0.014 |
| HAP/VAP | Number (%) | 67 (43.8) | 34 (30.1) | 33 (82.5) | < 0.001 |
| ICU-BSI | Number (%) | 38 (24.8) | 20 (17.7) | 18 (45) | 0.001 |
| IMV/ECMO | Number (%) | 89 (58.2) | 54 (47.8) | 35 (87.5) | < 0.001 |
HSV-positive and -negative patients were compared with Chi-square, Fisher and Wilcoxon tests
HSV Herpes simplex virus; IQR Interquartile range; ICU Intensive Care Unit; HAP Hospital-acquired pneumonia; VAP Ventilator-associated pneumonia; ICU-BSI Intensive care unit bloodstream infection. IMV Invasive mechanical ventilation. ECMO Extra-Corporeal Membrane Oxygenation
Day-60 mortality in the whole cohort was 39.9%, higher in patients with HSV-1 reactivation (57.5% versus 33.6% in patients without HSV-1 reactivation, p = 0.001).
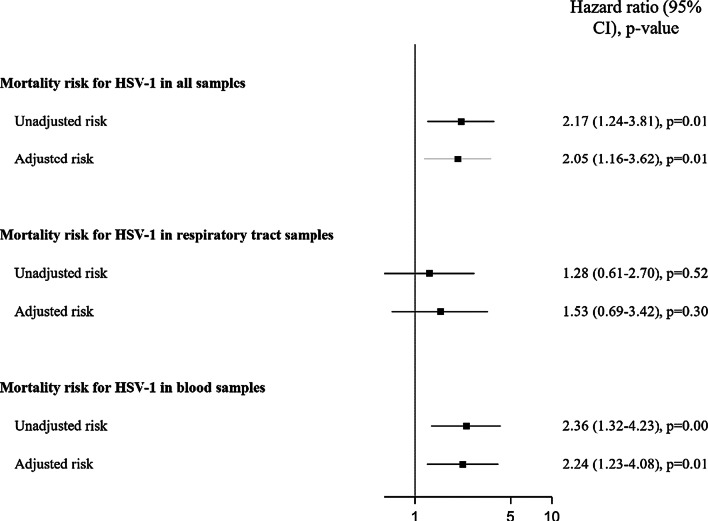
In univariable Cox models, HSV-1 reactivation was associated with an increased risk of mortality (HR 2.17, 95% CI 1.24–3.81; p = 0.007). After adjustment for mortality risk factors (age, oxygenation and ventilation characteristics, extra-respiratory components of SOFA score and corticosteroids therapy), multivariable Cox models showed an increased risk of mortality for HSV-1 reactivation (HR 2.05; 95% CI, 1.16–3.62; p = 0.01, Fig. 2, Additional file 1: Table E1).
Fig. 2.
Association between HSV-1 reactivation and mortality at day 60. HSV Herpes simplex virus; SOFA Sequential Organ Failure Assessment
Among respiratory samples, univariable and multivariable Cox models did not show any association between mortality and HSV-1 reactivation (Fig. 2 and Additional file 1: Table E2).
However, in blood samples, univariable (HR 2.36; 95% CI 1.32–4.23; p = 0.004) and multivariable (HR 2.24; 95% CI 1.23–4.08; p = 0.009, Fig. 2 and Additional file 1: Table E3) Cox models showed an association between HSV-1 reactivation and mortality.
Among patients with HSV-1 reactivation, acyclovir was administered to twenty-eight (70%) patients, 3 (IQR 2–5) days after positivity in median. After adjustment for mortality factors and using acyclovir as a time-dependent covariate, our Cox model showed an increased risk of mortality at day 60 for patients treated with acyclovir (HR 4.37, 95% CI 2.12–9.02, p < 0.001).
HSV-1 reactivation and healthcare-associated ICU infections
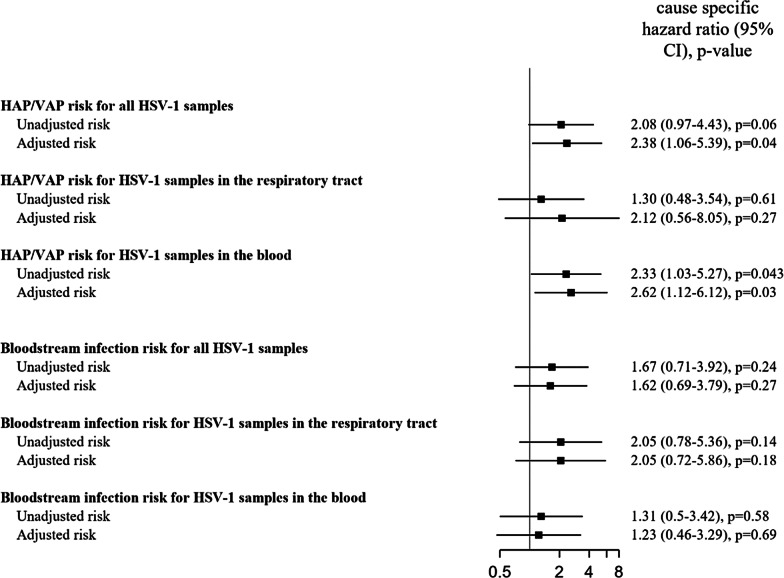
Sixty-seven (43.8%) patients developed a HAP/VAP. In univariable cause-specific models, HSV-1 reactivation was not significantly associated with an increased risk of HAP/VAP (csHR 2.08, 95% CI 0.97–4.43, p = 0.059). Multivariable specific cause models showed an increased risk of HAP/VAP (csHR 2.38, 95% CI 1.06–5.39, p = 0.037, Fig. 3 and Additional file 1: Table E4). Among respiratory samples, cause-specific models did not show any association between mortality and HSV-1 reactivation (data not shown). In blood samples, multivariable cause-specific models (HR 2.62; 95% CI 1.12–6.12; p = 0.027, Additional file 1: Table E5) showed an association between HSV-1 reactivation and HAP/VAP. We did not find a significant different distribution of pathogens identified in patients with or without HSV-1 reactivation in the respiratory tract (data not shown). We observed two HAPs after ICU discharge.
Fig. 3.
Association between HSV detection and nosocomial infections (hospital acquired pneumonia/ventilator associated pneumonia and bacteremia). NB: Adjustment factors were age, chronic disease, extra-respiratory SOFA score, type of ventilation, use of corticosteroids. To avoid overfitting with mechanical ventilation variables, only extra-respiratory components of SOFA score were taken into account. SOFA Sequential Organ Failure Assessment
ICU-BSI occurred in 38 (24.8%) patients, in 18 (45.0%) patients with HSV-1 reactivation versus 20 (17.7%) patients without, respectively. Univariable (csHR 1.67; 95% CI 0.71–3.92; p = 0.235) and multivariable (csHR 1.62; 95% CI 0.69–3.79; p = 0.269) cause-specific models did not show any increased risk for ICU-BSI (Fig. 3). Similar results were observed in the different subgroup analyses.
Discussion
Using high-quality prospectively collected data from all COVID-19 patients admitted to a large French COVID-19 reference center, we showed that mortality and HAP-VAP risks were increased in patients with HSV-1 reactivation. However, the risk for ICU-BSI was not increased in patients with HSV-1 reactivation. In our cohort none of the COVID-19 patients had HSV-2 reactivation.
Data on HSV reactivation in COVID-19 patients are scarce. Only one small study described HSV reactivation in lower respiratory tract of COVID-19 patients [37] without specifically investigating its impact on mortality or ICU-acquired infections. Furthermore, to our knowledge, no data on HSV detection in blood samples of COVID-19 patients were available. Interestingly, we observed higher rates of HSV positive PCR in the blood compared to previous studies including non-COVID-19 patients [38, 39]. This finding may be explained by immune alterations with profound leucopenia [2, 40] and a large use of immunomodulators in COVID-19 patients [11, 14].
We showed that HSV-1 reactivation in COVID-19 patients was associated with an increased mortality.
Impact of HSV reactivation on prognostic among critically ill non-COVID-19 patients is well known. Several authors showed an increased morbidity and mortality [25, 29, 30, 41]. However, data on HSV-1 reactivation among COVID-19 patients are scant [37]. Le Balch et al.showed that HSV-1 reactivation among severe COVID-19 patients was associated with a longer duration of invasive mechanical ventilation and a longer length of stay, but without any impact on mortality. However, due to the low number of included patients (n = 38), adjusting for confounding factors was impossible. To the best of our knowledge, we performed the first study that prospectively investigated HSV-1 reactivation in COVID-19 patients. The relevance of HSV-1 reactivation and its real impact on mortality remains controversial [42]. On the one hand, HSV could be a simple bystander and its presence not reflect a direct pathogenicity [43–45]. Due to the observational nature of our study, we could not determine causality between HSV and mortality since many confounders both related with HSV-1 reactivation and mortality might have been unmeasured. It may be especially the case for immune paralysis that might have induced both HSV-1 reactivation and increased the mortality risk [46–48]. In our study, most patients did not demonstrate reactivation until about two weeks. Patients who had HSV-1 reactivation had higher levels of inflammation at admission, suggesting a different immune response comparing to patients without HSV-1 reactivation. Recently, Seeßle et al. [49] showed an increase in activated CD8 cells followed by a drop in interferon stimulated gene expression occurring after HSV-1 reactivation, also occurring in a similar time frame to what was seen in our study. This suggests some form of immune failure leading to mortality with HSV-1 reactivation just being an epiphenomenon. However, since our analyses were adjusted for severity of critical illness, a direct pathogenic role of HSV may be hypothesized. A recent meta-analysis [50] showed a decreased mortality among HSV patients receiving acyclovir. In light of these considerations, it could be possible that HSV is a true pathogen inducing pulmonary parenchyma damages. However, the only randomized trial evaluating the use of acyclovir in HSV reactivation [28] showed a marginal and non-significant impact on mortality, thus weakening this hypothesis. Of note, our data showed that adequate early acyclovir therapy was not associated with a prognostic improvement of HSV-1 positive patients. Although our study was not designed to evaluate the impact of acyclovir and these data were issued from a complementary analysis, it suggested that HSV-1 reactivation was more a marker of poor immune status than a disease that would need specific therapy. Further studies are necessary to determine if early acyclovir therapy could improve outcomes.
We observed that HSV-1 reactivation was associated with an increased risk of HAP/VAP. Interestingly, only a few studies investigated the association between HSV reactivation and VAP in critically ill patients. Daubin et al. [51] performed a prospective observational study and determined the incidence of VAP among intubated patients in ICU. HSV-1 was recovered in only 31% of VAP patients. To date, the association between HAP/VAP and HSV has not been studied in COVID-19 patients.
We showed an association between HSV-1 reactivation and HAP/VAP which remained significant after adjustment on several factors. On the one hand, our findings suggested that HSV-1 reactivation could induce lung injuries which could promote bacterial superinfections. On the other hand, it is conceivable that lesions induced by superinfections may promote HSV reactivation in lung and/or blood. The causality relationship between HAP/VAP and HSV reactivation remains to be elucidated. Overall, COVID-19 exposes critically ill patients to an increased risk of superinfections and HSV reactivation by induced immunosuppression and severe lung damages. HSV-1 reactivation among COVID-19 patients could help clinicians to identify patients at risk of developing superinfections. Moreover, frequent microbiological samples may be collected in order to early identify HAP/VAP.
Our study has several limitations. First, we performed an observational study and residual confounding cannot be excluded. Therefore, associations between HSV-1 and mortality or HAP/VAP should be interpreted with caution. However, our multivariable models allowed us to adjust for several factors. It may be debatable to do not use IL1-ra or IL6-ra exposition as an adjustment covariate for multivariable Cox Models. Actually, Tocilizumab seems to improve outcomes in recent studies [13, 52]. Nevertheless, only 31 (20.3%) patients received Interleukin-receptor antagonists ([IL-ra] i.e., Anakinra or Tocilizumab) in the first two ICU days in our cohort. The association between IL-ra and mortality using univariables Cox models was not significant (HR 1.04, 95% CI 0.54–2.01, p = 0.90). Therefore, we did not include IL-ra as adjustment covariate. Second, HSV reactivation in respiratory tract was tested only in intubated patients, which may result in a selection of patients with poorer prognostic. Nevertheless, HSV reactivation in blood was also tested in non-intubated patients, thus mitigating this selection bias. Moreover, our subgroup analyses showed an increased risk of mortality and HAP/VAP for HSV-1 reactivation in blood, thus suggesting that blood HSV-1 reactivation played a predominant role (i.e., compared to respiratory HSV-1 reactivation). Third, in our main analysis, we pooled respiratory and blood samples, thus assuming that HSV positive blood PCR was mostly due to a pulmonary reactivation. Our analyses showed a predominant relevance of blood HSV reactivation. It may be debatable that a HSV reactivation in blood may be associated with a respiratory HSV reactivation. Fourth, median time to HSV-1 reactivation was shorter than median length of ICU stay for patients without HSV-1 reactivation (14 days versus 9 days, respectively). Some patients without HSV-1 reactivation leaved the ICU before HSV-1 reactivation could occur. HSV-1 was not routinely tested after ICU discharge so a selection bias may occur. Nevertheless, even if ICU HSV screening was not performed after ICU discharge, we reviewed all patient courses and we detected only one case with a HSV infection (HSV skin infection): we suppose that event did not substantially modify the results. Moreover, no clinical or biological signs of HSV-1 reactivation were described so we could reasonably assume that no patient had HSV-1 reactivation after ICU discharge. Fifth, our analysis suggested that blood sampling may be sufficient to monitor HSV-1 reactivation. Nevertheless, we have to nuance that notion. We had a potential selection bias caused by the lack of respiratory samples for non-intubated patients which may result in a selection of patients with poorer prognosis. Moreover, our subgroup analysis for HSV-1 reactivation in respiratory tract showed a non-significant trend for healthcare-associated ICU-infections with a HR > 1, particularly for bloodstream infection. Sixth, we performed a monocentric study, thus limiting the generalizability to other ICU settings. However, a large sample size with high quality data was collected. Finally, our findings should be limited to critically ill patients. The rate of respiratory HSV reactivation in non-critically ill patients remained unresolved and further studies are needed in this context.
Conclusion
Critically ill COVID-19 patients frequently reactivate HSV-1 but not HSV-2. HSV-1 reactivation in critically ill COVID-19 patients was associated with an increased risk of day-60 mortality and HAP/VAP occurrence. The association between HSV-1 reactivation and mortality may be useful for the clinician to identify patients with the worst prognosis. Further studies are necessary to determine the causality relationship.
Supplementary Information
Additional file 1. Table E1. Univariable and multivariable Cox models investigating the association between HSV-1 reactivation and mortality at day 60. Table E2. Univariable and multivariable Cox models investigating the association between HSV-1 reactivation and mortality at day 60 in respiratory samples. Table E3. Univariable and multivariable Cox models investigating the association between HSV-1 reactivation and mortality at day 60 in blood samples. Table E4. Univariable and multivariable cause specific models investigating the association between HSV-1 reactivation and HAP/VAP. Table E5. Univariable and multivariable cause specific models investigating the association between HSV-1 reactivation in blood and HAP/VAP.
Acknowledgements
The authors thank Céline Féger, M.D., (EMIBiotech) for her editorial support.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- CoNS
Coagulase-negative staphylococci
- CRP
C-reactive protein
- cs
Cause-specific
- Ct
Cycle threshold
- ECMO
Extra-corporeal membrane oxygenation
- HAP
Hospital-acquired pneumonia
- HR
Hazard risk
- HSV
Herpes simplex virus
- ICU
Intensive care unit
- ICU-BSI
Intensive care unit bloodstream infection
- IL-ra
Interleukin receptor antagonists
- IL1-ra
Interleukin-1 receptor antagonists
- IL6-ra
Interleukin-6 receptor antagonists
- IQR
Interquartile range
- LDH
Lactate dehydrogenase
- PCR
Polymerase chain reaction
- SOFA
Sequential organ failure assessment
- T0
Time 0
- VAP
Ventilator-associated pneumonia
Authors' contributions
JFT, SR, AM and NB designed the study. AM, NHF, JP, MAN, PJ, SP, TG, FS, PHW, EM, LB, RS, DD acquired the data. SR, NB and JFT did the statistical analysis. AM, NB and JFT analyzed and interpreted the data. AM, NB and JFT drafted the manuscript. All authors critically reviewed the manuscript and approved the final report.
Funding
This work was supported by the Swiss National Science Foundation. NB is currently receiving a Mobility Grant from the Swiss National Science Foundation (Grant Number: P4P4PM_194449).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the national French and regional ethics committees.
Consent for publication
Not applicable.
Competing interests
The authors have disclosed that they do not have conflict of interest. JFT received fees for lectures to 3M, MSD, Pfizer, and Biomerieux. JFT received research grants from Astellas, 3M, MSD, and Pfizer. JFT participated to advisory boards of 3M, MSD, Bayer Pharma, Nabriva, and Pfizer.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Investig. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;m1985. [DOI] [PMC free article] [PubMed]
- 6.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47(1):60‑73. [DOI] [PMC free article] [PubMed]
- 7.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouadma L, Lescure F-X, Lucet J-C, Yazdanpanah Y, Timsit J-F. Severe SARS-CoV-2 infections: practical considerations and management strategy for intensivists. Intensive Care Med. 2020;46(4):579–582. doi: 10.1007/s00134-020-05967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693‑704. [DOI] [PMC free article] [PubMed]
- 12.Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermine O, Mariette X, Tharaux P-L, Resche-Rigon M, Porcher R, Ravaud P, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honore PM, Barreto Gutierrez L, Kugener L, Redant S, Attou R, Gallerani A, et al. SARS-CoV-2 infection as a risk factor for herpesviridae reactivation: consider the potential influence of corticosteroid therapy. Crit Care. 2020;24(1):623. doi: 10.1186/s13054-020-03349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wicky P-H, Niedermann MS, Timsit J-F. Ventilator-associated pneumonia in the era of COVID-19 pandemic: How common and what is the impact? Crit Care. 2021;25(1):153. doi: 10.1186/s13054-021-03571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blonz G, Kouatchet A, Chudeau N, Pontis E, Lorber J, Lemeur A, et al. Epidemiology and microbiology of ventilator-associated pneumonia in COVID-19 patients: a multicenter retrospective study in 188 patients in an un-inundated French region. Crit Care. 2021;25(1):72. doi: 10.1186/s13054-021-03493-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27(1):83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Razazi K, Arrestier R, Haudebourg AF, Benelli B, Carteaux G, Decousser J-W, et al. Risks of ventilator-associated pneumonia and invasive pulmonary aspergillosis in patients with viral acute respiratory distress syndrome related or not to Coronavirus 19 disease. Crit Care. 2020;24(1):699. doi: 10.1186/s13054-020-03417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maes M, Higginson E, Pereira-Dias J, Curran MD, Parmar S, Khokhar F, et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit Care. 2021;25(1):25. doi: 10.1186/s13054-021-03460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rouzé A, Martin-Loeches I, Povoa P, Makris D, Artigas A, Bouchereau M, et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: a European multicenter cohort study. Intensive Care Med. 2021;47(2):188–198. doi: 10.1007/s00134-020-06323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonizzoli M, Arvia R, di Valvasone S, Liotta F, Zakrzewska K, Azzi A, et al. Human herpesviruses respiratory infections in patients with acute respiratory distress (ARDS) Med Microbiol Immunol. 2016;205(4):371–379. doi: 10.1007/s00430-016-0456-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hraiech S, Bonnardel E, Guervilly C, Fabre C, Loundou A, Forel J-M, et al. Herpes simplex virus and Cytomegalovirus reactivation among severe ARDS patients under veno-venous ECMO. Ann Intensive Care. 2019;9(1):142. doi: 10.1186/s13613-019-0616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luyt C-E, Bouadma L, Morris AC, Dhanani JA, Kollef M, Lipman J, et al. Pulmonary infections complicating ARDS. Intensive Care Med. 2020;46(12):2168–2183. doi: 10.1007/s00134-020-06292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuxen DV, Cade JF, McDonald MI, Buchanan MR, Clark RJ, Pain MC. Herpes simplex virus from the lower respiratory tract in adult respiratory distress syndrome. Am Rev Respir Dis. 1982;126(3):416–419. doi: 10.1164/arrd.1982.126.3.416. [DOI] [PubMed] [Google Scholar]
- 26.Bruynseels P, Jorens PG, Demey HE, Goossens H, Pattyn SR, Elseviers MM, et al. Herpes simplex virus in the respiratory tract of critical care patients: a prospective study. Lancet. 2003;362(9395):1536–1541. doi: 10.1016/S0140-6736(03)14740-X. [DOI] [PubMed] [Google Scholar]
- 27.Luyt C-E, Combes A, Deback C, Aubriot-Lorton M-H, Nieszkowska A, Trouillet J-L, et al. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am J Respir Crit Care Med. 2007;175(9):935–942. doi: 10.1164/rccm.200609-1322OC. [DOI] [PubMed] [Google Scholar]
- 28.Luyt C-E, Forel J-M, Hajage D, Jaber S, Cayot-Constantin S, Rimmelé T, et al. Acyclovir for mechanically ventilated patients with herpes simplex virus oropharyngeal reactivation: a randomized clinical trial. JAMA Intern Med. 2020;180(2):263–272. doi: 10.1001/jamainternmed.2019.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouza E, Giannella M, Torres MV, Catalán P, Sánchez-Carrillo C, Hernandez RI, et al. Herpes simplex virus: a marker of severity in bacterial ventilator-associated pneumonia. J Crit Care. 2011;26(4):432.e1–6. doi: 10.1016/j.jcrc.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Linssen CFM, Jacobs JA, Stelma FF, van Mook WNKA, Terporten P, Vink C, et al. Herpes simplex virus load in bronchoalveolar lavage fluid is related to poor outcome in critically ill patients. Intensive Care Med. 2008;34(12):2202–2209. doi: 10.1007/s00134-008-1231-4. [DOI] [PubMed] [Google Scholar]
- 31.Engelmann I, Gottlieb J, Meier A, Sohr D, Ruhparwar A, Henke-Gendo C, et al. Clinical relevance of and risk factors for HSV-related tracheobronchitis or pneumonia: results of an outbreak investigation. Crit Care. 2007;11(6):R119. doi: 10.1186/cc6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coisel Y, Bousbia S, Forel J-M, Hraiech S, Lascola B, Roch A, et al. Cytomegalovirus and herpes simplex virus effect on the prognosis of mechanically ventilated patients suspected to have ventilator-associated pneumonia. PLoS ONE. 2012;7(12):e51340. [DOI] [PMC free article] [PubMed] [Retracted]
- 33.Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) Eur Respir J. 2017;50(3):1700582. doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 34.Timsit J-F, Esaied W, Neuville M, Bouadma L, Mourvllier B. Update on ventilator-associated pneumonia. F1000Res. 2017;6:2061. [DOI] [PMC free article] [PubMed]
- 35.Chastre J, Fagon J-Y. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 36.Buetti N, Ruckly S, de Montmollin E, Reignier J, Terzi N, Cohen Y, et al. COVID-19 increased the risk of ICU-acquired bloodstream infections: a case-cohort study from the multicentric OUTCOMEREA network. Intensive Care Med. 2021;47(2):180–187. doi: 10.1007/s00134-021-06346-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Balc’h P, Pinceaux K, Pronier C, Seguin P, Tadié J-M, Reizine F. Herpes simplex virus and cytomegalovirus reactivations among severe COVID-19 patients. Crit Care. 28 2020;24(1):530. [DOI] [PMC free article] [PubMed]
- 38.Lepiller Q, Sueur C, Solis M, Barth H, Glady L, Lefebvre F, et al. Clinical relevance of herpes simplex virus viremia in Intensive Care Unit patients. J Infect. 2015;71(1):93–100. doi: 10.1016/j.jinf.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Ong DSY, Bonten MJM, Spitoni C, Verduyn Lunel FM, Frencken JF, Horn J, et al. Epidemiology of multiple herpes viremia in previously immunocompetent patients with septic shock. Clin Infect Dis. 01 2017;64(9):1204‑10. [DOI] [PubMed]
- 40.Bouadma L, Wiedemann A, Patrier J, Surénaud M, Wicky P-H, Foucat E, et al. Immune alterations in a patient with SARS-CoV-2-related acute respiratory distress syndrome. J Clin Immunol. 2020;40(8):1082–1092. doi: 10.1007/s10875-020-00839-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brenner T, Rosenhagen C, Hornig I, Schmidt K, Lichtenstern C, Mieth M, et al. Viral infections in septic shock (VISS-trial)-crosslinks between inflammation and immunosuppression. J Surg Res. 2012;176(2):571–582. doi: 10.1016/j.jss.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 42.Simoons-Smit AM, Kraan EM, Beishuizen A, Strack van Schijndel RJ, Vandenbroucke-Grauls CM. Herpes simplex virus type 1 and respiratory disease in critically-ill patients: Real pathogen or innocent bystander? Clin Microbiol Infect. 2006;12(11):1050‑9. [DOI] [PubMed]
- 43.Assink-de Jong E, Groeneveld ABJ, Pettersson AM, Koek A, Vandenbroucke-Grauls CMJE, Beishuizen A, et al. Clinical correlates of herpes simplex virus type 1 loads in the lower respiratory tract of critically ill patients. J Clin Virol. 2013;58(1):79–83. doi: 10.1016/j.jcv.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 44.van den Brink J-W, Simoons-Smit AM, Beishuizen A, Girbes ARJ, Strack van Schijndel RJM, Groeneveld ABJ. Respiratory herpes simplex virus type 1 infection/colonisation in the critically ill: marker or mediator? J Clin Virol. 2004;30(1):68‑72. [DOI] [PubMed]
- 45.Verheij J, Groeneveld ABJ, Beishuizen A, van Lingen A, Simoons-Smit AM, van Schijndel RJMS. Herpes simplex virus type 1 and normal protein permeability in the lungs of critically ill patients: a case for low pathogenicity? Crit Care. 2004;8(3):R139. doi: 10.1186/cc2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monneret G, Venet F. Sepsis-induced immune alterations monitoring by flow cytometry as a promising tool for individualized therapy. Cytom B Clin Cytom. 2016;90(4):376–386. doi: 10.1002/cyto.b.21270. [DOI] [PubMed] [Google Scholar]
- 47.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13(3):260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seeßle J, Hippchen T, Schnitzler P, Gsenger J, Giese T, Merle U. High rate of HSV-1 reactivation in invasively ventilated COVID-19 patients: Immunological findings. PLoS ONE. 2021;16(7):e0254129. [DOI] [PMC free article] [PubMed]
- 50.Hagel S, Scherag A, Schuierer L, Hoffmann R, Luyt C-E, Pletz MW, et al. Effect of antiviral therapy on the outcomes of mechanically ventilated patients with herpes simplex virus detected in the respiratory tract: a systematic review and meta-analysis. Crit Care. 29 2020;24(1):584. [DOI] [PMC free article] [PubMed]
- 51.Daubin C, Vincent S, Vabret A, du Cheyron D, Parienti J-J, Ramakers M, et al. Nosocomial viral ventilator-associated pneumonia in the intensive care unit: a prospective cohort study. Intensive Care Med. 2005;31(8):1116–1122. doi: 10.1007/s00134-005-2706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637‑45. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table E1. Univariable and multivariable Cox models investigating the association between HSV-1 reactivation and mortality at day 60. Table E2. Univariable and multivariable Cox models investigating the association between HSV-1 reactivation and mortality at day 60 in respiratory samples. Table E3. Univariable and multivariable Cox models investigating the association between HSV-1 reactivation and mortality at day 60 in blood samples. Table E4. Univariable and multivariable cause specific models investigating the association between HSV-1 reactivation and HAP/VAP. Table E5. Univariable and multivariable cause specific models investigating the association between HSV-1 reactivation in blood and HAP/VAP.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.