Abstract
The association of the pap operon with the CS31A and F17 adhesins was studied with 255 Escherichia coli strains isolated from calves, lambs, or humans with diarrhea. The three classes of PapG adhesin with different receptor binding preferences were also screened. The pap operon was associated with 50 and 36% of human strains that produced CS31A and ovine strains that produced F17, respectively. Among the bovine isolates, the pap operon was detected in 61% of the CS31A-positive isolates and 72% of the strains that produce both CS31A and F17. The class II adhesin gene was present in bovine (20%) and ovine (71%) isolates. Both class II and III adhesins were genetically associated with 36% of the human strains. The highest prevalence of the pap operon was observed among E. coli strains that produce additional adhesins involved in the binding of bacteria to intestinal cells. Among the bovine isolates, the reference strain for CS31A and F17c was found to be positive for the pap operon. Phenotypic and genotypic characterizations were undertaken. Pap31A appeared as fine and flexible fimbriae surrounding the bacteria but did not mediate adhesion to calf intestinal villi. Pap31A production was optimal with bacteria cultured on minimal growth media and repressed by addition of exogenous leucine. The deduced amino acid sequence of the PapA31A structural subunit showed 57 to 97% identity with the different P-related structural subunits produced by E. coli strains isolated from pigs with septicemia or humans with urinary tract infections. None of the three papG allelic variants was detected, but a homologous papG gene was present in the chromosome of strain 31A.
The reference strain Escherichia coli 31A, isolated from the feces of a diarrheic calf, causes experimental septicemia in gnotobiotic calves and lambs (9). This strain produces the plasmid-encoded CS31A antigen, which is closely related to the K88 fimbriae produced by porcine enterotoxigenic E. coli (18, 35). More recently, a new CS31A-related adhesin was described in Klebsiella pneumoniae strains involved in nosocomial infections (12). CS31A binds to an N-acetylneuraminic acid-containing receptor present in human Int-407 cells (12). However, the CS31A antigen clearly differs from typical fimbriae and appears as a capsule-like material surrounding the bacteria (18). Strain 31A also produces the F17c fimbria (formerly called 20K), which is responsible for in vitro N-acetyl-d-glucosamine-dependent adhesion of bacteria to calf and lamb brush border villi (4, 10). The F17c fimbria belongs to the F17 family, which includes fimbriae expressed by bovine diarrheic and septicemic E. coli strains and by human uropathogenic E. coli strains (4, 5, 30, 37). Pathogenic F17-producing E. coli strains represent a significant part of the bacterial strains isolated from diarrheic calves in France and Belgium and from lambs with nephrosis in Scotland (4, 5).
P fimbriae are mannose-resistant hemagglutinins detected predominantly at the cell surface of E. coli strains associated with human urinary tract infection (UTI) (25). These fimbriae are closely associated with upper urinary tract colonization and pyelonephritis and bind to the kidney vascular endothelium (28). P-related fimbriae are also associated with UTIs in dogs and septicemia in pigs (16, 33). The adhesin subunit, termed PapG, is located at the tip of the P fimbriae and mediates the binding of the bacteria to α-d-galactosyl-(1-4)-β-galactopyranose (Gal-Gal)-containing receptors present in host tissues (31). Three allelic variants of the gene that encodes the P or Prs adhesin were described previously (39). All recognize glycolipids in the globoseries but differ in their binding to GbO3, GbO4, and GbO5 globosides (31, 39).
In the study described in this report, 255 pathogenic E. coli strains isolated from human, bovine, and ovine intestinal contents were screened for the pap operon that codes for the P-related fimbriae. The allelic variants that code for the three classes of P adhesins were also investigated. The association of the P-related fimbriae with CS31A antigen, F17c fimbriae, and other virulence factors was analyzed. In addition, we demonstrate that a 17.5-kDa protein produced by the 31A reference E. coli strain was a fimbrial structural subunit closely related to those of the Prs-like fimbria F1651 produced by E. coli strains isolated from piglets with septicemia and to P or Prs fimbriae produced by E. coli strains isolated from humans with UTIs.
MATERIALS AND METHODS
Bacterial strains.
E. coli 31A was isolated from the intestinal contents of a calf with diarrhea (9, 18). E. coli 31A/O6 is a plasmid-cured strain obtained from 31A (4, 18). Strain 31A/O6(20K−) is a spontaneous mutant defective in F17c production (4). Strain 5131 was isolated from the intestinal contents of a septicemic piglet and expresses F1651 fimbriae (21). Wild-type E. coli strain IA2 was isolated from a human with a UTI and produces P fimbriae (F11 serotype) (41). Strains 5131 and IA2 were kindly provided by J. Harel (Faculty of Veterinary Medicine, University of Montreal, Saint-Hyacinthe, Quebec, Canada) and M. Svensson (Department of Medical Microbiology, Lund, Sweden), respectively. E. coli HB101 strains that harbored recombinant plasmid pHRU845, pPILL110-35, or pJFK102, each of which contains the pap gene cluster that encodes the class I, class II, and class III adhesin, respectively, were used as positive controls. The recombinant strains were kindly provided by M. Svensson. The characteristics and origins of the E. coli strains are summarized in Table 1.
TABLE 1.
Characteristics and origins of the bacterial strains
| Strain | Characteristics | Phenotype | Reference(s) |
|---|---|---|---|
| 31A | E. coli strain from fecal flora of a diarrheic calf carrying plasmid p31A | CS31A, F17c, and Pap31A positive | 18 |
| 31A/O6 | p31A-cured variant of 31A | F17c and Pap31A positive | 4, 18 |
| 31A/O6(20K−) | F17c-negative mutant from 31A/O6 | Pap31A positive | 4 |
| 5131 | E. coli strain from a pig with septicemia | F1651 and F1652 positive | 21 |
| IA2 | E. coli strain from a human with a UTI | P (F11 serotype) positive | 41 |
| HB101(pHRU845) | Recombinant | Production of PapG class I adhesin | 26 |
| HB101(pPLILL110-35) | Recombinant | Production of PapG class II adhesin | 26 |
| HB101(pJFK102) | Recombinant | Production of PapG class III adhesin | 26 |
| HB101 | E. coli K-12 recipient strain |
Genotypic detection was performed with human, bovine, and ovine intestinal E. coli strains. A total of 118 E. coli strains (included in our bacterial collection) were isolated from calves with septicemia and/or diarrhea in France and Belgium. The 77 human strains were isolated from sporadic diarrheal stools of patients in the Centre Hospitalier Régional Universitaire of Clermont-Ferrand, France (23). The 60 ovine E. coli strains described elsewhere (4, 5) were isolated in Scotland from lambs with nephropathy (1).
Genotypic detection of the pap operon and the related adhesin genes.
The pap operon was detected by using a previously described PCR protocol (29). A 328-bp DNA fragment was amplified from the highly conserved papC gene. A multiplex-primer PCR assay was used to identify each of the three papG allelic variants that encode the Gal-Gal binding adhesin of P fimbriae (26). Use of the primer cocktail resulted in three DNA fragments of 461, 190, and 258 bp specific for the adhesin genes of classes I, II, and III, respectively. DNAs extracted from E. coli strains harboring recombinant plasmid pHRU845, pPILL110-35, or pJFK102 were used as positive controls. Amplification procedures were also performed to screen for the presence of the papI, papE, and papF genes located in different positions on the pap gene cluster (34, 43). A DNA extract from the IA2 reference strain was used as a positive control.
For all the amplification procedures, DNA extracted from the HB101 recipient strain was used as a negative control and was included in every PCR run. The PCR products obtained were electrophoresed on a 1.5% agarose gel and were visualized by staining with ethidium bromide.
Preparation of DNA probe and hybridization.
The bacterial strains were cultured overnight at 37°C on Luria-Bertani (LB) broth, and total chromosomal DNA was extracted and purified with the Easy-DNA kit (Invitrogen). The chromosomal DNAs were plotted on a Hybond N+ membrane (Amersham) and alkali fixed on the membrane according to the manufacturer's recommendations. A DNA probe was generated by PCR amplification as described previously (26). The amplified fragment was obtained from recombinant plasmid pHRU845 and was purified with the Wizard PCR Preps DNA purification system (Promega). The DNA probe was labeled with the Renaissance Random primer fluorescein kit under conditions recommended by the supplier (NEN Life Science Products) and then purified on a ProbeQuant G-50 microcolumn (Pharmacia). Membrane prehybridization, hybridization, and washes were performed in accordance with the manufacturer's recommendations (NEN). The enhanced chemiluminescence signal was detected on autoradiography film. Chromosomal DNAs extracted from recombinant strain HB101(pHRU845) and recipient strain HB101 were used as positive and negative controls, respectively.
Fimbrial purification and biochemical characterization.
After growth on Minca agar medium (19), the bacterial suspension was mechanically sheared (2 min in a Top mix blender at maximal speed) and centrifuged (20,000 × g for 10 min at 4°C). The resultant supernatant was precipitated with 20% ammonium sulfate, and the precipitate was collected by centrifugation (10,000 × g for 10 min at 4°C). Sodium dodecyl sulfate (SDS) was added to a final concentration of 2%, and the SDS-insoluble material was collected by ultracentrifugation (110,000 × g for 200 min at 20°C). After dissociation of the polymers (2 h of incubation at 37°C in 8.5 M guanidine hydrochloride), 5 mM Tris hydrochloride (pH 7.8) was added to obtain 6 M guanidine hydrochloride. The suspension was subjected to gel filtration chromatography on a Sephacryl S300 (Pharmacia) column, and the fractions containing the major peak were pooled, dialyzed at 4°C against ammonium acetate buffer, and then lyophilized. SDS-polyacrylamide gel electrophoresis (PAGE) analysis revealed two copurified polypeptide bands of 20 and 17.5 kDa.
The lyophilized material was dissolved in Laemmli sample buffer and was loaded on a preparative acrylamide gel. After electrophoresis, the band corresponding to the 17.5-kDa polypeptide was cut, and the protein was electroeluted, dialyzed, and lyophilized as described elsewhere (4). A 15-μg sample of the purified 17.5-kDa polypeptide was transferred electrophoretically to a polyvinylidene difluoride membrane (Immobilon-P; Millipore) as described previously (4), and the first NH2-terminal amino acid residues were determined by Edman degradation. The N-terminal amino acid sequence of the 17.5-kDa polypeptide was compared with the sequences of proteins listed in the National Biomedical Research Foundation (NBRF) protein sequence data bank by using the FASTA program.
Analysis of in vitro expression of the 17.5-kDa protein.
In vitro production of the 17.5-kDa protein was analyzed by culturing the E. coli strains on different growth media. In addition to a complex agar medium (LB), different synthetic agar media were used: Minca medium (19) supplemented with 0.1% glucose (MG-1), minimal Davis agar medium (Difco) supplemented with 0.1% Casamino Acids and 0.1% glucose (MD-1) (15), and minimal M9 agar medium supplemented with 0.2% glucose or 0.2% glycerol.
The putative repression by the leucine was investigated after growing the bacteria on the M9 agar medium with or without 1 mM leucine supplemented with valine and isoleucine (each at 50 μg ml−1). Overnight cultures were harvested in phosphate-buffered saline (pH 7.2) and adjusted to an optical density of 120 at 600 nm. The fimbrial suspensions were heated for 20 min at 60°C and centrifuged at 10,000 × g for 15 min. The resulting supernatants (2 μl) were plotted onto nitrocellulose membranes. Dot blotting assays were performed in triplicate with antibodies specific to the 17.5-kDa protein (dilution, 1:500). Images of the plots were obtained by using an Imager-Appligene, and density profiling was performed by using the Scion image-processing program.
Production of antisera.
Two antisera directed against denatured and native 17.5-kDa protein, respectively, were produced in mice as described previously (4). The 31A/O6(20K−) strain cultured on MD-1 medium was chosen to produce the native fimbriae. The antiserum raised against the native protein was repeatedly adsorbed with the 31A/O6(20K−) strain cultured at 18°C on MD-1. Antibodies directed against F1651 and P (F11 serotype) fimbriae were kindly provided by J. Harel and M. Svensson, respectively.
Electron microscopy.
Bacterial cells were placed on carbon-stabilized collodion-coated copper grids, negatively stained with 1% phosphotungstic (pH 6.8), and observed with a transmission electron microscope (HU 12A; Hitachi) operated at 75 kV. Gold immunolabeling was performed with specific antibodies directed against the native 17.5-kDa polypeptide (dilution, 1:15) and 10-nm colloidal goat anti-rabbit immunoglobulin G (Nordic) as described previously (4, 18).
Assay of in vitro adhesion on calf intestinal villi.
Adhesion to calf and lamb intestinal villi was tested as described previously (4, 17). Hemagglutination tests were performed in the presence or absence of 0.5% d-mannose. The villi were washed for 30 min in cold phosphate-buffered saline containing 1.5 mM 14-(2-aminoethyl)-benzensulfonyl-fluoride-hydrochloride to prevent proteolysis (4). Adhesion was scored by using a phase-contrast microscope at a magnification of ×1,000. A maximal attachment index was scored when 30 bacteria adhered to a 50-μm segment of a villus brush border.
Hemagglutination.
Hemagglutination tests were performed on glass slides at 4°C in the absence or presence of 0.5% d-mannose as described previously (4, 18). A bacterial suspension (50 μl, 109 cells ml−1) was mixed with an identical volume of a 3% (vol/vol) suspension of erythrocytes from group A negative human blood and from animals (calf, sheep, rabbit, goat, horse, and rat).
Nucleotide sequencing of the gene encoding the 17.5-kDa protein.
DNA to be amplified was released from whole organisms by boiling. Bacteria were harvested from 1 ml of an overnight LB broth culture, resuspended in 1 ml of sterile water, and incubated at 100°C for 10 min. To amplify the gene encoding the 17.5-kDa protein, primers were deduced from the published sequence of the f1651 operon (33). The sequences of the oligonucleotide primers located upstream and downstream of the f1651-A gene were 5′-CTGTCGATAAATAACCTGCCCTG-3′ and 5′-GTAATGTACTCCAGAAATACATCA-3′, respectively. The Expand High Fidelity PCR system containing thermostable Taq DNA and Pwo DNA polymerases was used to amplify the papA31A gene as recommended by the manufacturer (Boehringer Mannheim). The bacterial extracts were subjected to 25 cycles of amplification at an annealing temperature of 63°C (GenAmp 2400 thermal cycler; Perkin-Elmer). The DNA fragment obtained (760 bp) was purified by using the Prep-A-Gene DNA purification system (Bio-Rad) and was cloned into the Bluescript (KS+) vector (Stratagene Ltd.). The nucleotide sequence was determined with double-stranded DNA by the dideoxy chain termination method with a model 373A automatic DNA sequencer (Applied Biosystems Inc.).
Nucleotide sequence accession number.
The nucleotide sequence of papA31A has been deposited in GenBank under accession no. AF165981.
RESULTS
Genotypic detection of pap operon among bovine, ovine and human E. coli strains.
In order to investigate the putative association of P-related fimbriae with CS31A and/or F17-related adhesins, 255 E. coli strains isolated from the intestinal contents of calves, lambs, and hospitalized patients were screened for the papC gene (which is highly conserved in pap operons). The results are summarized in Table 2.
TABLE 2.
Detection of pap operon and three allelic variants of PapG adhesina
| Host | Phenotype
|
No. of strains | No. (%) of strains with pap operon | No. of strains with papG-related adhesin gene
|
|||
|---|---|---|---|---|---|---|---|
| CS31A antigen production | F17 fimbria production | Class I | Class II | Class III | |||
| Calf | + | + | 40 | 29 (72) | 0 | 10 | 0 |
| + | − | 21 | 8 (38) | 0 | 0 | 0 | |
| Subtotal | 61 | 37 (61) | |||||
| − | + | 32 | 7 (22) | 0 | 0 | 0 | |
| − | − | 25 | 5 (20) | 0 | 0 | 0 | |
| Total | 118 | 49 (42) | 10 (20)b | ||||
| Lamb | − | + | 31 | 11 (36) | 0 | 8 | 0 |
| − | − | 29 | 6 (21) | 0 | 4 | 1 | |
| Total | 60 | 17 (28) | 12 (71) | 1 (6) | |||
| Human | + | − | 26 | 13 (50) | 0 | 2 | 1 |
| − | − | 51 | 14 (27) | 0 | 3 | 4 | |
| Total | 77 | 27 (36) | 5 (18) | 5 (18) | |||
The genotypic detection of the pap operon and the three classes of PapG adhesin was performed by polymerase chain reaction as described previously (26, 29). The phenotypic detection of CS31A and F17 antigens was performed by Western blotting or dot blotting (4, 5, 18).
Values in parentheses are percentage of strains with the papG-related adhesin gene among the pap-positive isolates.
Among the CS31A-positive isolates, the pap operon was detected in 61% of E. coli strains isolated from the intestinal contents of calves with diarrhea and/or septicemia and 50% of strains isolated from human diarrheic stools specimens. This percentage increased to 72% among bovine strains that produced both the CS31A antigen and F17-related fimbriae. The prevalence of the pap operon was greatly reduced among bovine and human CS31A-negative strains (20 and 27%, respectively).
The association of the pap operon with F17-related fimbriae was also investigated among E. coli strains isolated from the intestinal contents of calves with diarrhea or lambs with nephropathy (E. coli strains that produce F17-related fimbriae are not isolated from human feces [4]). CS31A-negative strains that produced or that did not produce F17 isolated from diarrheic calves were weakly associated with the pap operon (22 and 20%, respectively). PCR analysis showed that 36% of F17-producing strains from the intestinal contents of lambs with nephropathy were positive for the pap operon. This percentage decreased to 21% among ovine isolates which did not express F17-related fimbriae.
In order to compare the detection of a pap operon and the production of fimbriae, phenotypic detection of P-related fimbriae was performed with pap-positive E. coli strains grown on Davis medium (MD-1). Analysis of Western blotting assays results revealed that 89% of the E. coli strains that harbored the pap operon produced P-related fimbriae under these growth conditions, suggesting the presence of a complete pap operon.
Detection of the pap-related adhesin gene among bovine, ovine, and human E. coli strains.
The PapG adhesin subunit occurs as three molecular variants (classes I to III) encoded by distinct allelic variants. Each class of the PapG adhesin exhibits subtly different receptor binding preferences (39). The 255 E. coli strains were investigated for the presence of the papG alleles by a multiplex PCR protocol. The results are summarized in Table 2.
The gene that encodes the class I adhesin was not detected among the human, ovine, or bovine strains tested, confirming that the class I adhesin is rare among pap-positive E. coli strains (24, 34).
The gene that encodes the class II adhesin gene was detected in 10 of the 49 (20%) strains isolated from calves that harbored the pap operon. Among the bovine E. coli strains, the class II allelic variant was exclusively associated with strains that produced both CS31A and F17 adhesins. The class III adhesin gene was not detected in DNAs extracted from the bovine isolates. Among the strains isolated from diarrheic stools of hospitalized patients, 10 of the 27 pap-positive E. coli strains (36%) exhibited a class II (18%) or a class III (18%) adhesin gene. Of the 17 pap-positive E. coli strains from lambs with nephropathy, 12 (71%) exhibited the class II adhesin gene (only 1 strain was found to have the class III adhesin gene).
Selection of bacterial strains.
The pap operon was found to be highly associated with the bovine E. coli strains of this study that produce CS31A adhesins and F17-related fimbriae. Both F17c and CS31A have been characterized from reference strain 31A isolated in our laboratory from a calf with diarrhea (4, 18). In this report, E. coli 31A was found to be positive for the pap operon. In order to determine whether strain 31A produced a new P-related fimbria, biochemical and immunological characterization of the fimbriae and genotypic characterization of the structural and adhesin subunits were undertaken.
Purification and characterization of Pap31A fimbriae.
A 17.5-kDa protein was first detected during the F17c purification procedure described elsewhere (4). The purification was performed from a crude fimbrial extract of plasmidless strain E. coli 31A/O6 (CS31A negative, F17c positive, pap positive) cultured on Minca medium (MG-1). The surface structures were harvested from whole cells by mechanical shearing, precipitated with ammonium sulfate, and solubilized with SDS. After gel filtration chromatography, SDS-PAGE analysis of the fractions eluted from the S300 column revealed two major polypeptides bands of 20 and 17.5 kDa. The 20-kDa polypeptide represents the F17c-A fimbrial major subunit described previously (4, 37). The 17.5-kDa polypeptide was further purified by preparative gel electrophoresis.
The first 15 N-terminal amino acid residues of the 17.5-kDa protein (APTTPQGQGRVTFNG) obtained by Edman degradation appeared to be identical to those of the major subunit of P-related fimbria F1651 produced by E. coli strains responsible for septicemia in piglets (33). A high degree of homology was also observed with the first amino acid residues of the PapAIA2 and PapAJ96 fimbrial subunits produced by the human uropathogenic E. coli strains IA2 (F11 serotype) and J96 (F13 serotype), respectively (3, 41). In view of these results, the 17.5-kDa polypeptide was termed PapA31A.
A crude fimbrial extract from strain 31A was subjected to Western blot assay. A single 17.5-kDa band reacted with specific antibodies raised against the purified PapA31A structural subunit (data not shown). In addition, a high degree of cross-reactivity was observed with fimbrial extracts obtained from E. coli 5131 and IA2, which produce the F1651 and the P (F11 serotype) fimbriae, respectively (data not shown).
In vitro expression of PapA31A structural subunit.
To determine whether the production of PapA31A depends on the culture medium, strain 31A was grown on different media (LB, MD-1, MG-1, and M9), and PapA31A production was quantified by a dot blot assay. The results are summarized in Fig. 1.
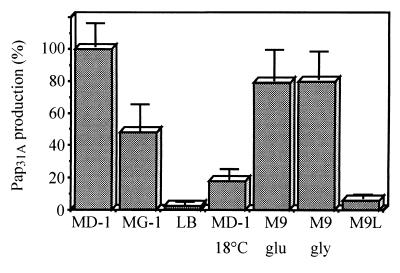
FIG. 1.
In vitro expression of PapA31A structural subunit. PapA31A production was analyzed by culturing E. coli 31A on different growth media at 37°C: minimal Davis medium supplemented with glucose and Casamino Acids (MD-1), semisynthetic Minca medium supplemented with glucose (MG-1), and the complex media LB and M9 supplemented with glucose (M9 glu) or glycerol (M9 gly). The repression by leucine was investigated after growing the bacteria on M9 medium supplemented with glucose and 1 mM leucine (M9L). Pap31A production was also determined on MD-1 at 18°C. Results were expressed as the percentages of PapA31A production related to maximal production (assigned as 100%). Fimbrial production was determined in triplicate by a quantitative dot blot assay.
The results were expressed as percentages of PapA31A production related to the maximal production (assigned value of 100%) when strain 31A was cultured on minimal Davis medium (MD-1) at 37°C. Fimbrial production decreased greatly when the bacteria were cultured on complex LB medium (2.5%) or on minimal MD-1 medium at 18°C (8%). Intermediate fimbrial production (48%) was obtained with bacteria cultured on semisynthetic Minca medium (MG-1) at 37°C.
A high level of production of PapA31A (79%) was observed when strain 31A was cultured on M9 medium supplemented with glycerol. A similar result was observed with bacteria grown on M9 medium with glucose as the carbon source, suggesting that PapA31A production was not subject to catabolic repression (Fig. 1).
Expression of some fimbriae (including F1651) is repressed by exogenous leucine in the growth medium, whereas expression of other fimbriae (including P fimbriae from strains from humans with UTIs) is not (22). PapA31A production decreased greatly (6%) when 1 mM leucine was added to M9 medium (Fig. 1), suggesting that the regulation of Pap31A clearly differs from that of P fimbriae produced by E. coli strains that cause UTIs in humans.
Morphological observations.
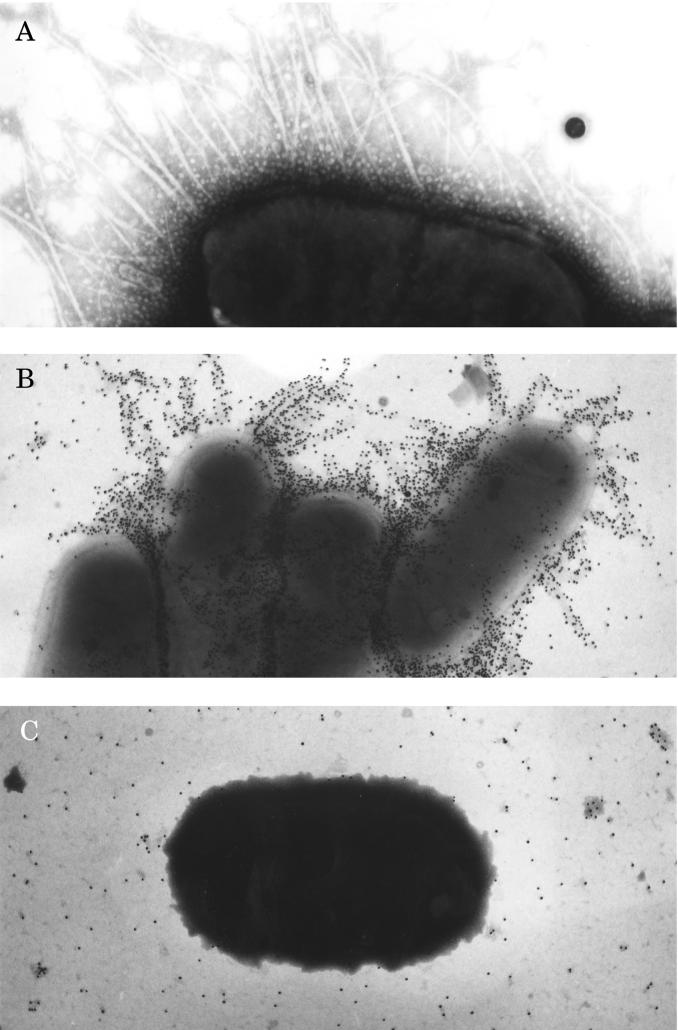
Negative-staining electron microscopy of whole cells of E. coli 31A/O6(20K−) cultured on MD-1 at 37°C revealed fine and flexible fimbria-like filaments (diameter, approximately 8 to 10 nm) (Fig. 2A). Gold immunolabeling assays showed that antibodies directed against the native PapA31A protein completely decorated the fimbriae on E. coli 31A/O6(20K−) grown on MD-1 (Fig. 2B), indicating that the purified PapA31A protein was the structural subunit of the fimbria-like filaments. These fimbriae were termed Pap31A.
FIG. 2.
Transmission electron micrographs of bacterial preparations. E. coli 31A/O6(20K−) cultured on minimal David medium (MD-1) at 37°C was negatively stained with 1% phosphotungstic acid (A) and labeled by the immunogold labeling technique with anti-PapA31A antibodies (B). (C) Strain 31A/O6(20K−) cultured on MD-1 at 18°C was labeled by immunogold labeling with anti-PapA31A antibodies. Magnifications: A, ×45,000; B, ×32,500; C, ×32,500.
The low level of production of the Pap31A fimbriae quantified as described above when the bacteria were cultured on MD-1 at 18°C (8% of the maximal production) was confirmed since the bacteria were not decorated (Fig. 2C). A similar result was obtained with the bacterial strain cultured on complex LB medium (data not shown).
In vitro adhesion on intestinal villi and hemagglutination.
In vitro adhesion to calf and lamb intestinal villi and agglutination of erythrocytes from different species were investigated in order to determine the putative adhesive ability of Pap31A fimbriae. E. coli 31A/O6(20K−) cultured on MD-1 medium at 37°C was unable to adhere in vitro to calf or lamb intestinal villi. This bacterial strain did not agglutinate with human blood (type A negative) or with erythrocytes from a calf, sheep, rabbit, horse, or rat. These results indicated that Pap31A fimbriae did not mediate hemagglutination or adhesion of the bacteria to calf or lamb intestinal villi.
Nucleotide sequence of gene encoding the structural subunit and amino acid comparison.
The gene that encodes the PapA31A structural subunit was amplified with a high-fidelity PCR system from E. coli 31A, and the papA31A nucleotide sequence was determined. Sequence analysis revealed that a polypeptide of 182 amino acid residues could be translated from the ATG codon at position 79 to the TAA codon at position 625. The signal peptidase cleavage site deduced from the NH2-terminal amino acid sequence of PapA31A was located between residues Ala-21 and Ala-22 (Fig. 3).
FIG. 3.
Comparison of the deduced amino acid sequence of the structural subunit PapA31A with those of F1651, PapIA2, and PapJ96 fimbriae. The first amino acid residue of the mature protein was numbered +1. Gaps (∗) were introduced to obtain a maximal alignment. Identical amino acid residues are indicated by hyphens. The nucleotide sequence of the gene encoding the PapA31A protein is available from the GenBank data library under accession no. AF165981.
Comparison of the deduced PapA31A amino acid sequence with those listed in the NBRF and SWISS-PROT data banks revealed significant homologies with different PapA-related fimbrial structural subunits. The highest degrees of homology were observed with the Prs-like F1651A (98%) and the PapAIA2 (F11 serotype) (96%) fimbrial structural subunits (Fig. 3). A high degree of homology (80%) was observed with the PapA fimbrial subunit produced by E. coli J96 (F13 serotype) from a human with a UTI (Fig. 3). A significant degree of identity was also observed with the sequence of the PapA subunit expressed by E. coli strains of serotypes F71 (61%), F72 (60%), and F9 (57%) from humans with UTIs (data not shown).
P-related adhesin gene of E. coli 31A.
E. coli 31A was negative by PCR for the three papG allelic variants. In order to determine whether strain 31A carries a papG gene on the chromosome, a nylon filter prepared with DNAs purified from strain 31A and the plasmidless 31A/O6 strain was hybridized with a labeled probe specific for the papGJ96 adhesin gene. The DNA probe hybridized with E. coli 31A and 31A/O6, suggesting that a sequence homologous to the papG sequence was indeed present on the chromosomes of the strains and that the sequence probably represents a new papG variant.
In addition, the presence of genes located in different positions on the pap gene cluster was investigated. The DNA extracted from E. coli 31A was found to be positive by amplification for sequences specific for the papI gene located at the 5′ end of the pap gene cluster and for the papE and papF genes located immediately upstream of papG at the 3′ end of the pap gene cluster. These results strongly suggested that a complete pap gene cluster seems to be present on the bacterial chromosome.
DISCUSSION
Many microorganisms have the genetic capacity to express different adhesins, providing access to multiple receptors and therefore increasing their pathogenicities. In this report, the pap operon that encodes the P fimbriae and the papG genes that code for the three classes of PapG adhesin were investigated among human, ovine, and bovine E. coli strains that produce CS31A and/or F17 adhesins.
It is well documented that pap-positive strains are present in the fecal flora of 10 to 20% of healthy adults and children (2, 8, 42). We observed a similar percentage of pap-positive strains among the CS31A-negative E. coli strains isolated from hospitalized patients with sporadic diarrhea. This percentage increased significantly among diarrheagenic CS31A-positive strains (50%). However, only 23% of the pap-positive E. coli strains that produced CS31A were genetically associated with a PapG adhesin subunit of class II or class III. These results are in agreement with those of a study of human pap-positive strains which showed that fecal isolates carry new variants or unexpressed papG genes to a greater extent than urinary tract isolates (14). The human CS31A-producing strains in this study did not belong to the enterotoxigenic E. coli, enteropathogenic E. coli, enteroinvasive E. coli, or enterohemorrhagic E. coli bacterial groups generally associated with human diarrhea and do not possess the adhesive factors commonly associated with these pathogenic bacterial groups (23). However, CS31A promotes adhesion of the bacteria to the Caco-2 and Int-407 cell lines (12), suggesting a role for CS31A in bacterial adhesion to the bacteria to the human intestinal tract. Furthermore, human CS31A-positive isolates produced a Dr-related adhesin that recognizes the decay-accelerating factor present in both brush border enterocytes and urinary tract cells (27). We speculate that the simultaneous presence of CS31A, P, and Dr adhesins in human E. coli strains increases the pathogenicity of the strains first by allowing the colonization of the intestinal tract and then by permitting the spread of the bacteria to extraintestinal sites.
The ovine isolates were obtained from the intestinal contents of lambs with diarrhea for which a fatal acute renal failure was diagnosed (1). In accordance with the E. coli strains from humans with UTIs (24) but in contrast to the human and bovine intestinal isolates in this study, most pap-positive ovine strains (76%) were associated with an allelic variant of the PapG adhesin. Except for one strain, all the pap-positive ovine strains were genetically associated with a class II adhesin. The adhesin of class II preferentially binds GbO4 globosides (the major isoreceptors on the human kidney) and is associated with acute pyelonephritis (24). It was of interest that the class II adhesin was associated with E. coli strains isolated both from lambs with severe renal tubular disease and from humans with acute pyelonephritis.
The highest prevalence of the pap operon (72%) was observed in bovine isolates that produced both CS31A and F17 adhesins. In contrast, 22 to 38% of the bovine E. coli strains that produced CS31A or F17 were associated with the pap operon. A high prevalence of P fimbriae has been described only among the strains that produced cytotoxic necrotizing factor type I (CNF1) and that were isolated from calves with septicemia or diarrhea (6, 32). The presence of a pathogenicity island (PAI) that included at least the cnf1 and pap operons explains the high prevalence of P fimbriae among bovine CNF1-producing strains (J. P. Nougareyde, F. Herault, E. Jacquemin, J. De Ryckes, J. Mainil, and E. Oswald, Abstr. 96th Gen. Meet. Am. Soc. Microbiol. 1996, abstr. B-77, p. 168, 1996). However, the presence of a similar PAI could not explain the close association between CS31A, F17, and a pap operon among the bovine strains included in the study described in this report. Indeed, the genetic information necessary for the synthesis of CS31A is located on a large conjugative plasmid (18, 35), and the bovine strains tested do not produce CNF1. While the papG genes are not detected among fecal isolates collected from healthy cows (34), the class II adhesin was found to be genetically associated with the bovine isolates studied. These results suggested a role of class II adhesin in the bacterial infectious process. In this report, we demonstrated the exclusive association of the class II adhesin gene with bovine E. coli strains that produce both CS31A and F17. In contrast, it has been demonstrated that the class III variant was found to be associated with CNF1-producing strains isolated from pigs and calves with septicemia or diarrhea (6, 13). The different PapG allelic variants seemed to be associated with particular virulence factors produced by pathogenic E. coli strains in domestic animals.
In view of these results, it seemed of interest to undertake additional experiments to study the organization of the pap operon and the biochemical properties of putative P-related fimbriae produced by diarrheagenic E. coli strains. The bovine reference strain E. coli 31A has been chosen for this study because (i) E. coli 31A isolated in our laboratory from a calf with diarrhea was the reference strain for both CS31A and F17c adhesins (4, 5, 18, 35, 37), (ii) the highest prevalence of the pap operon was observed among bovine isolates that produced both CS31A and F17, and (iii) strain 31A was found to be genetically associated with the pap operon.
The gene that encodes the P structural subunit was amplified from E. coli 31A, and the nucleotide sequence of the papA31A gene was determined. When the deduced amino acid sequences were compared, PapA31A showed the highest degree of homology with the structural subunit of F1651 and P (F11 serotype) fimbriae produced by E. coli strains from pigs with septicemia and humans with UTIs, respectively (33, 41). The presence of the papA, papE, papF, papI, and papG genes located in different positions in the gene cluster suggested the presence of a complete pap gene cluster on the 31A bacterial chromosome. However, the papG gene present on the chromosome of E. coli 31A was different from those of the three allelic variants described to date. This result strongly suggested the presence of additional papG variants different from those that have been described previously and that encode adhesin subunits able to recognize GbO3, GbO4, or GbO5 isoreceptors. Characterization of the binding properties of E. coli 31A and nucleotide sequence analysis of this new papG variant would be of great interest.
Despite their genetic relatedness, the optimal conditions for the expression of Pap31A were different from those for the expression of F1651 and P fimbriae. In contrast to Pap31A, the production of both P and F1651 is controlled by catabolic repression (22, 36). Furthermore, as described for F1651 (11) but not for P fimbriae of strains from humans with UTIs (7), Pap31A production is reduced by addition of exogenous leucine. These results indicated that the regulation of Pap31A production clearly differs from that of P-related fimbria production of strains from different hosts.
In addition to Pap31A, E. coli 31A produces CS31A and F17 adhesins. However, expression of the three surface structures was subject to differential regulation. Indeed, in contrast to F17c, expression of both CS31A (36) and Pap31A is regulated by addition of exogenous leucine and rich growth medium. Moreover, CS31A (but not Pap31A) production is subject to catabolic repression by glucose (36). These results suggested different roles for the three fimbriae in different locations in the host tissues or in different physiological states.
Reference strain 31A produced flexible Pap31A fimbria-like filaments. However, in vitro adhesion tests suggest the lack of functional receptors for P-related adhesins on bovine intestinal cells. Experimental oral infection of gnotobiotic calves with strain 31A causes septicemia with constant edema of the kidney and death of the animals in less than 48 h (9). These experiments demonstrate that strain 31A is able to persist in the bovine intestinal tract, colonize mucosal surfaces, and translocate to the mesenteric lymph nodes. It is well documented that F1651 fimbriae are required for the full pathogenicity of E. coli strains in gnotobiotic pigs, not for initial colonization of the intestinal mucosa but for systemic bacterial persistence and resistance to phagocytosis (38). Furthermore, among human uropathogenic E. coli strains, P fimbriae are not only responsible for binding to and colonization of the urinary mucosa by E. coli (20) but also protect E. coli strains from the bactericidal activity of human polymorphonuclear neutrophils (40). We speculate that Pap31A fimbriae could play a role in the resistance to phagocytosis or extraintestinal adherence of E. coli 31A.
In summary, the distribution of the pap operon among diarrheagenic E. coli strains that produce additional adhesive factors implicated in bacterial binding to intestinal cells suggested a role for P-related fimbriae in the spread of bacteria to extraintestinal sites. In addition, the pap operon was highly associated with bovine isolates that produce both CS31A and F17 adhesins. Therefore, a genotypic and phenotypic characterization of the P-related fimbriae produced by E. coli 31A assigned as the bovine reference strain for CS31A and F17c production was performed and demonstrated that (i) a complete pap gene cluster necessary for the synthesis of P-related fimbriae seems to be present in the bacterial chromosome, (ii) E. coli 31A contains a variant or a partial copy of the papG adhesin gene, and (iii) PapA31A is highly related to PapA and F1651-A subunits but the environmental conditions for optimal production are different.
ACKNOWLEDGMENTS
We thank M. Svensson for providing antibodies directed against P fimbriae, the wild-type E. coli IA2 strain, and the HB101 strains harboring recombinant plasmid pHRU845, pPILL110-35, or pJFK102. We thank Josée Harel for providing antibodies directed against F1651 fimbriae and E. coli 5131 from pigs with septicemia and Stéphanie Dutilloy for preparation of the graphics.
REFERENCES
- 1.Angus K W, Hodgson J C, Hosie B D, Low J C, Mitchell G B, Dyson D A, Holliman A. Acute nephropathy in young lambs. Vet Rec. 1989;124:9–14. doi: 10.1136/vr.124.1.9. [DOI] [PubMed] [Google Scholar]
- 2.Arthur M, Johnson C E, Rubin R H, Arbeit R D, Campanelli C, Kim C, Steinbach S, Agarwal M, Wilkinson R, Goldstein R. Molecular epidemiology of adhesin and hemolysin virulence factors among uropathogenic Escherichia coli. Infect Immun. 1989;57:303–313. doi: 10.1128/iai.57.2.303-313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bäga M, Normark S, Hardy J, O'Hanley P, Lark D, Olsson O, Schoolnick G, Falkow S. Nucleotide sequence of the papA gene encoding the Pap pilus subunit of human uropathogenic Escherichia coli. J Bacteriol. 1984;157:330–333. doi: 10.1128/jb.157.1.330-333.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertin Y, Girardeau J P, Darfeuille-Michaud A, Contrepois M. Characterization of 20K fimbria, a new adhesin of septicemic and diarrhea-associated Escherichia coli that belong to a family of adhesins with N-acetyl-d-glucosamine recognition. Infect Immun. 1996;64:332–342. doi: 10.1128/iai.64.1.332-342.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertin Y, Martin C, Oswald E, Girardeau J P. Rapid and specific detection of F17-related pilin and adhesin genes in diarrheic and septicemic Escherichia coli strains by multiplex polymerase chain reaction. J Clin Microbiol. 1996;34:2921–2928. doi: 10.1128/jcm.34.12.2921-2928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertin Y, Martin C, Girardeau J P, Pohl P, Contrepois M. Association of genes encoding P fimbriae, CS31A antigen and EAST 1 toxin among CNF1-producing Escherichia coli strains from cattle with septicemia and diarrhea. FEMS Microbiol Lett. 1998;162:235–239. doi: 10.1111/j.1574-6968.1998.tb13004.x. [DOI] [PubMed] [Google Scholar]
- 7.Braaten B A, Platko J V, van der Woude M W, Simons B H, de Graaf F K, Calvo J M, Low D A. Leucine-responsive regulatory protein controls the expression of both the pap and fan pili operons in Escherichia coli. Proc Natl Acad Sci USA. 1992;89:4250–4254. doi: 10.1073/pnas.89.10.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brauner A, Katouli M, Ostenson C G. P-fimbriation and haemolysin production are the most important virulence factors in diabetic patients with Escherichia coli bacteraemia: a multivariate statistical analysis of seven bacterial virulence factors. J Infect. 1995;31:27–31. doi: 10.1016/s0163-4453(95)91271-1. [DOI] [PubMed] [Google Scholar]
- 9.Contrepois M, Dubourguier H C, Parodi A L, Girardeau J P, Ollier J L. Septicaemic and experimental infection of calves. Vet Microbiol. 1986;12:109–118. doi: 10.1016/0378-1135(86)90073-8. [DOI] [PubMed] [Google Scholar]
- 10.Contrepois M, Bertin Y, Girardeau J P, Picard B, Goullet P. Clonal relationships among bovine pathogenic Escherichia coli producing surface antigen CS31A. FEMS Microbiol Lett. 1993;106:217–222. doi: 10.1111/j.1574-6968.1993.tb05962.x. [DOI] [PubMed] [Google Scholar]
- 11.Daigle F, Dozois C M, Jacques M, Harel J. Mutations in the f165(1)A and f165(1)E fimbrial genes and regulation of their expression in an Escherichia coli strain causing septicemia in pigs. Microb Pathog. 1997;22:247–252. doi: 10.1006/mpat.1996.0111. [DOI] [PubMed] [Google Scholar]
- 12.Di Martino P, Bertin Y, Girardeau J P, Livrelli V, Joly B, Darfeuille-Michaud A. Molecular characterization and adhesive properties of CF29K, an adhesin of Klebsiella pneumoniae strains involved in nosocomial infections. Infect Immun. 1995;63:4336–4344. doi: 10.1128/iai.63.11.4336-4344.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dozois C M, Clement S, Desautels C, Oswald E, Fairbrother J. Expression of P, S, and F1C adhesins by cytotoxic necrotizing factor 1-producing Escherichia coli from septicemic and diarrheic pigs. FEMS Microbiol Lett. 1997;152:307–312. doi: 10.1111/j.1574-6968.1997.tb10444.x. [DOI] [PubMed] [Google Scholar]
- 14.Ekbäck G, Mörner S, Lund B, Normark S. Correlation of genes in the pap gene cluster to expression of globoside-specific adhesin by uropathogenic Escherichia coli. FEMS Microbiol Lett. 1986;34:355–360. [Google Scholar]
- 15.Fairbrother J M, Lallier R, Leblans L, Jacques M, Lariviere S. Production and purification of Escherichia coli fimbrial antigen F165. FEMS Microbiol Lett. 1988;56:247–252. [Google Scholar]
- 16.Garcia E, Bergmans H E N, van den Bosch J F, Orskof I, van den Zeijst B A M, Gaastra W. Isolation and characterization of dog uropathogenic Escherichia coli strains and their fimbriae. Antonie Leeuwenhoek. 1988;54:149–151. doi: 10.1007/BF00419202. [DOI] [PubMed] [Google Scholar]
- 17.Girardeau J P. A new in vitro technique for attachment to intestinal villi using enteropathogenic Escherichia coli. Ann Inst Pasteur Microbiol. 1980;131:31–37. [PubMed] [Google Scholar]
- 18.Girardeau J P, Der Vartanian M, Ollier J L, Contrepois M. CS31A, a new K88-related fimbrial antigen on bovine enterotoxigenic and septicemic Escherichia coli strains. Infect Immun. 1988;56:2180–2188. doi: 10.1128/iai.56.8.2180-2188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guinee P A, Jansen W H, Agterberg C M. Detection of the K99 antigen by means of agglutination and immunoelectrophoresis in Escherichia coli isolates from calves and its correlation with enterotoxigenicity. Infect Immun. 1976;13:1369–1377. doi: 10.1128/iai.13.5.1369-1377.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagberg L, Jodal U, Korhonen T K, Lidin-Janson G, Lindberg U, Svanborg Eden C. Adhesion, hemagglutination, and virulence of Escherichia coli causing urinary tract infections. Infect Immun. 1981;31:564–570. doi: 10.1128/iai.31.2.564-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harel J, Forget M, Ngeleka M, Jacques M, Fairbrother J M. Isolation and characterization of adhesin-defective TnphoA mutants of septicemic porcine Escherichia coli of serotype O115:K−:F165.J. Gen Microbiol. 1992;138:2337–2345. doi: 10.1099/00221287-138-11-2337. [DOI] [PubMed] [Google Scholar]
- 22.Harel J, Martin C. Virulence gene regulation in pathogenic Escherichia coli. Vet Res. 1999;30:131–155. [PubMed] [Google Scholar]
- 23.Jallat C, Livrelli V, Darfeuille-Michaud A, Rich C, Joly B. Escherichia coli strains involved in diarrhea in France: high prevalence and heterogeneity of diffusely adhering strains. J Clin Microbiol. 1993;31:2031–2037. doi: 10.1128/jcm.31.8.2031-2037.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johanson M, Plos K, Marklund B I, Svanborg C. pap, papG and prsG DNA sequences in Escherichia coli from the fecal flora and the urinary tract. Microb Pathogen. 1993;15:121–129. doi: 10.1006/mpat.1993.1062. [DOI] [PubMed] [Google Scholar]
- 25.Johnson J R. Virulence factors in urinary tract infection. Clin Microbiol Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson J R, Brown J J. A novel multiply primed polymerase chain reaction assay for identification of variant papG genes encoding the Gal(alpha 1-4)Gal-binding PapG adhesins of Escherichia coli. J Infect Dis. 1996;173:920–926. doi: 10.1093/infdis/173.4.920. [DOI] [PubMed] [Google Scholar]
- 27.Kerneis S, Gabastou J M, Bernet-Camard M F, Coconnier M H, Nowicki B J, Servin A L. Human cultured intestinal cells express attachment sites for uropathogenic Escherichia coli bearing adhesins of the Dr adhesin family. FEMS Microbiol Lett. 1994;119:27–32. doi: 10.1111/j.1574-6968.1994.tb06862.x. [DOI] [PubMed] [Google Scholar]
- 28.Korhonen T K, Virkola R, Holthöfer H. Localization of binding sites for purified Escherichia coli P fimbriae in the human kidney. Infect Immun. 1986;54:328–332. doi: 10.1128/iai.54.2.328-332.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Bouguenec C, Archambaud M, Labigne A. Rapid and specific detection of the pap, afa, and sfa adhesin-encoding operons in uropathogenic Escherichia coli strains by polymerase chain reaction. J Clin Microbiol. 1992;30:1189–1193. doi: 10.1128/jcm.30.5.1189-1193.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Bouguenec C, Bertin Y. AFA and F17 adhesins produced by pathogenic Escherichia coli strains in domestic animals. Vet Res. 1999;30:317–342. [PubMed] [Google Scholar]
- 31.Lund B, Marklund B I, Strömberg N, Lindberg F, Karlsson K, Normark S. Uropathogenic Escherichia coli can express serologically identical pili of different receptor binding specificities. Mol Microbiol. 1988;2:255–263. doi: 10.1111/j.1365-2958.1988.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 32.Mainil J G, Jacquemin E, Herault F, Oswald E. Presence of pap-, sfa-, and afa-related sequences in necrotoxigenic Escherichia coli isolates from cattle: evidence for new variants of the AFA family. Can J Vet Res. 1997;61:193–199. [PMC free article] [PubMed] [Google Scholar]
- 33.Maiti S N, DesGroseillers L, Fairbrother J M, Harel J. Analysis of genes coding for the major and minor fimbrial subunits of the Prs-like fimbriae F1651 of porcine septicemic Escherichia coli strain 4787. Microb Pathog. 1994;16:15–25. doi: 10.1006/mpat.1994.1002. [DOI] [PubMed] [Google Scholar]
- 34.Marklund B I, Tennent J M, Garcia E, Hamers A, Baga M, Lindberg F, Gaastra W, Normark S. Horizontal gene transfer of the Escherichia coli pap and prs pili operons as a mechanism for the development of tissue-specific adhesive properties. Mol Microbiol. 1992;6:2225–2242. doi: 10.1111/j.1365-2958.1992.tb01399.x. [DOI] [PubMed] [Google Scholar]
- 35.Martin C, Bœuf C, Bousquet F. Escherichia coli CS31A fimbriae: molecular cloning, expression and homology with the K88 determinant. Microb Pathog. 1991;10:429–442. doi: 10.1016/0882-4010(91)90108-m. [DOI] [PubMed] [Google Scholar]
- 36.Martin C. The clp (CS31A) operon is negatively controlled by Lrp, ClpB, and l-alanine at the transcriptional level. Mol Microbiol. 1996;21:281–292. doi: 10.1046/j.1365-2958.1996.00651.x. [DOI] [PubMed] [Google Scholar]
- 37.Martin C, Rousset E, De Greve H. Human uropathogenic and bovine septicaemic Escherichia coli strains carry an identical F17-related adhesin. Res Microbiol. 1997;148:55–64. doi: 10.1016/S0923-2508(97)81900-6. [DOI] [PubMed] [Google Scholar]
- 38.Ngeleka M, Jacques M, Martineau-Doizé B, Daigle F, Harel J. Pathogenicity of an Escherichia coli O115:K“V165” mutant negative for F1651 fimbriae in septicemia of gnotobioic pigs. Infect Immun. 1993;61:836–843. doi: 10.1128/iai.61.3.836-843.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strongberg N, Nyholm P G, Pascher I, Normark S. Saccharide orientation at the cell surface affects glycolipid receptor function. Proc Natl Acad Sci USA. 1991;88:9340–9344. doi: 10.1073/pnas.88.20.9340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tewari R, Ikeda T, Malaviya R, Mac Gregor J I, Little J R, Hultgren S J, Abraham S N. The PapG tip adhesin of P fimbriae protects Escherichia coli from neutrophil bactericidal activity. Infect Immun. 1994;62:5296–5304. doi: 10.1128/iai.62.12.5296-5304.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Die I, Hoekstra W, Bergmans H. Analysis of the primary structure of P-fimbrillins of uropathogenic Escherichia coli. Microb Pathog. 1987;3:149–154. doi: 10.1016/0882-4010(87)90091-x. [DOI] [PubMed] [Google Scholar]
- 42.Wold A E, Caugant D A, Lidin-Janson G, de Man P, Svanborg C J. Resident colonic Escherichia coli strains frequently display uropathogenic characteristics. Infect Dis. 1992;165:46–52. doi: 10.1093/infdis/165.1.46. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto S, Terai A, Yuri K, Kurazono H, Takeda Y, Yoshida O. Detection of urovirulence factors in Escherichia coli by multiplex polymerase chain reaction. FEMS Immunol Med Microbiol. 1995;1:85–90. doi: 10.1111/j.1574-695X.1995.tb00179.x. [DOI] [PubMed] [Google Scholar]