Abstract
Telocytes (TCs) are newly identified interstitial cells characterized by thin and long cytoplasmic processes, called telopodes, which exhibit a distinctive moniliform shape and, often, a sinuous trajectory. Telopodes typically organize in intricate networks within the stromal space of most organs, where they communicate with neighboring cells by means of specialized cell-to-cell junctions or shedding extracellular vesicles. Hence, TCs are generally regarded as supporting cells that help in the maintenance of local tissue homeostasis, with an ever-increasing number of studies trying to explore their functions both in physiological and pathological conditions. Notably, TCs appear to be part of stem cell (SC) niches in different organs, including the intestine, skeletal muscle, heart, lung, and skin. Indeed, growing evidence points toward a possible implication of TCs in the regulation of the activity of tissue-resident SCs and in shaping the SC niche microenvironment, thus contributing to tissue renewal and repair. Here, we review how the introduction of TCs into the scientific literature has deepened our knowledge of the stromal architecture focusing on the intestine and skeletal muscle, two organs in which the recently unveiled unique relationship between TCs and SCs is currently in the spotlight as potential target for tissue regenerative purposes.
Keywords: intestine, skeletal muscle, stem cell niches, stemness, stromal cells, telocytes, tissue microenvironment
Introduction
According to the current state of knowledge, telocytes (TCs) represent a novel type of interstitial cells recently identified in a variety of organs. 1 TCs are ultrastructurally identifiable as stromal cells with typical characteristics consisting in a small piriform-, spindle-, or triangular-shaped cell body giving rise to extremely slender cytoplasmic projections, named telopodes, reaching up to several hundred micrometers in length and often displaying a sinuous trajectory and a dichotomous branching pattern.1–4 Telopodes exhibit a distinctive moniliform shape, owing to the alternation of thin segments, referred to as podomers, and small dilations, termed podoms, accommodating different organelles as mitochondria, endoplasmic reticulum cisternae, and caveolae.1–4
Over the last decade, the above-mentioned ultrastructural criteria allowed to disclose the presence and distribution of TCs in the stromal compartment of an ever-growing number of organs by transmission electron microscopy (TEM).1,3–5 Moreover, both TEM and three-dimensional imaging, such as focused ion beam–scanning electron microscope (FIB-SEM) tomography, revealed that telopodes have an exceptional ability to build complex stromal networks by establishing multiple intercellular communications both between TCs, through homocellular junctions, and between TCs and other neighboring cell types, through heterocellular junctions.1,3–7 Hence, such intricate interstitial networks can serve to mediate cell-to-cell signaling and have been proposed to constitute a scaffold necessary to dictate the correct tissue organization during development and maintain tissue/organ structural integrity and function in postnatal life.1,4,8 In addition, TCs can release different types of extracellular vesicles which act as transporters involved in the intercellular exchange of a variety of molecular signals, including cytokines, growth factors, and mRNAs, as well as epigenetic regulators like miRNAs and other non-coding RNAs.1,4,9,10
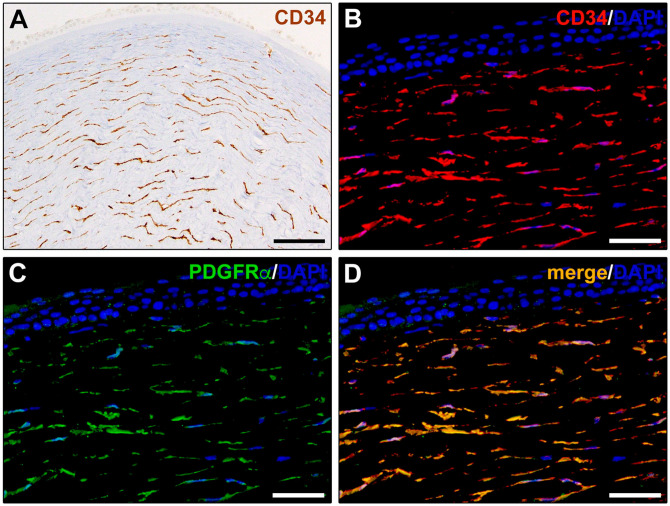
A growing number of studies have reported that immunohistochemical staining and light microscopy techniques are also suitable to identify TCs in the connective tissues of different organs.1,4,11–14 Currently, although TC-specific antigenic markers have yet to be determined, CD34 is widely accepted to immunolabel TCs that are, indeed, frequently referred to as TCs/CD34+ stromal cells (Figs. 1A and 2A).11,15,16 Platelet-derived growth factor receptor (PDGFR)α is also commonly employed as TC marker, and double immunolabeling for CD34 and PDGFRα can help in distinguishing TCs from other stromal cells in many organs (Fig. 1B–D).1,4,13,14 It has also been demonstrated that the immunophenotype of TCs may vary among organs and that TC subtypes having different immunohistochemical characteristics can often coexist in the stroma of the same organ. 14 For instance, TCs may be CD34+, PDGFRα+, or c-kit/CD117+ in the stromal compartment of some organs, such as the heart and the cornea, while they are CD34+ and PDGFRα+ but lack c-kit/CD117 expression in others, such as the gastrointestinal tract.1,4,14,17,18 Moreover, TCs surrounding the intestinal crypts have been shown to be characterized by the expression of the Foxl1 transcription factor. 19 In addition, when using CD34 as TC marker, it is important to consider that its expression is shared by TCs and endothelial cells of blood vessels in all vascularized connective tissues. Therefore, immunohistochemical detection of CD34 in combination with the endothelial cell-specific marker CD31, also known as platelet/endothelial cell adhesion molecule-1, has been largely applied to allow a clear discrimination of CD34+CD31− TCs from neighboring CD34+CD31+ vascular endothelial structures (Fig. 2B).17,20 Double immunostaining for CD34 and α-smooth muscle actin (αSMA) is also useful to distinguish CD34+αSMA− TCs from other often adjacent cell types that, depending on the kind of tissue, may be represented by myofibroblasts, myoid cells, periglandular myoepithelial cells, smooth muscle cells, and pericytes, all expressing αSMA but lacking CD34 (Fig. 2C).13,20 Furthermore, increasing evidence supports the notion that TCs display gene expression, proteomic profiles, and miRNA signatures rather different from those of “classical” fibroblasts and other tissue-resident stromal cells, such as mesenchymal stem cells (SCs).1,4,21–23 Table 1 presents a summary of the main immunophenotypic markers useful to identify TCs in different organs.
Figure 1.
Immunohistochemical localization of telocytes in human corneal stroma. (A) CD34 immunoperoxidase-based immunohistochemistry. Telocytes are identifiable as CD34+ stromal cells with a small cell body giving rise to long and thin moniliform cytoplasmic extensions (telopodes) characterized by the alternation of slender segments and small dilations along their length. (B–D) Double fluorescence immunohistochemistry for CD34 (red) and PDGFRα (green) with DAPI (blue) counterstain for nuclei. Telocytes are identifiable as CD34+PDGFRα+ stromal cells with characteristic telopodes. Scale bar: (A), 100 µm; (B–D), 50 µm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; PDGFRα, platelet-derived growth factor receptor α.
Figure 2.
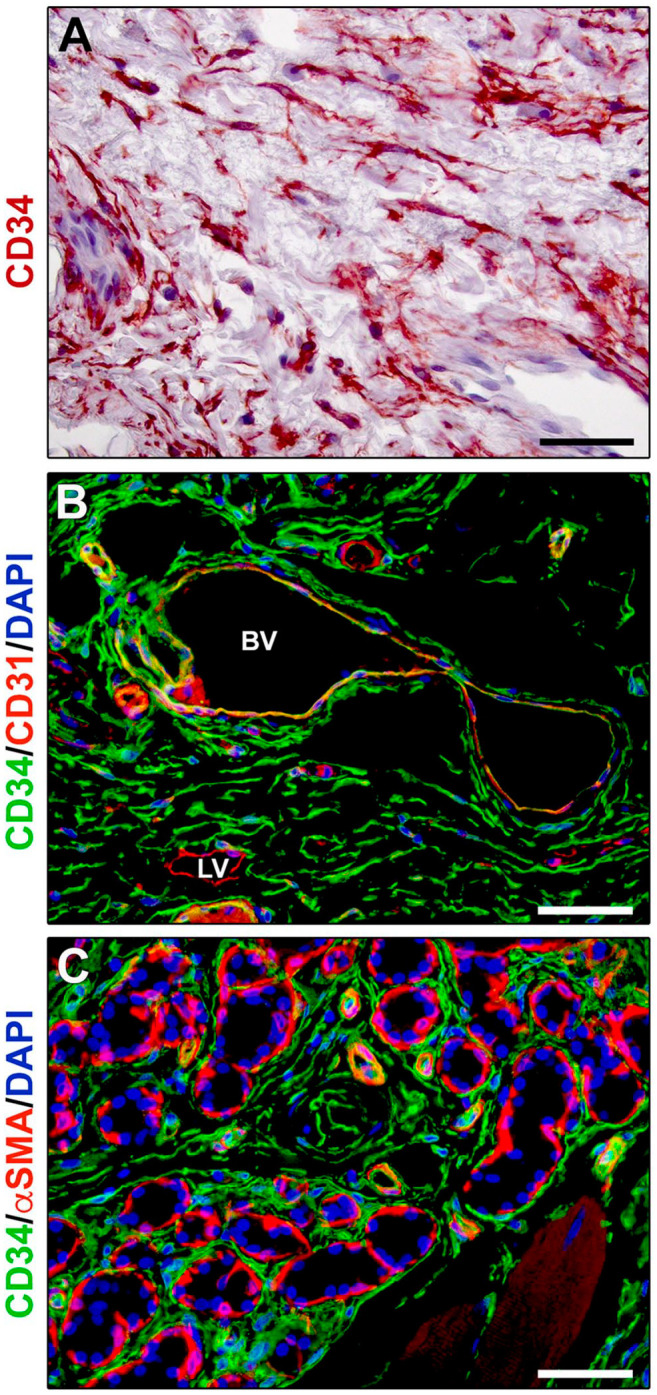
Immunohistochemical localization of telocytes in human tongue connective tissue. (A) CD34 immunoperoxidase-based immunohistochemistry with hematoxylin counterstain. By means of their long and moniliform prolongations (telopodes), CD34+ telocytes form an extensive stromal cell network throughout the tongue lamina propria. (B) Double fluorescence immunohistochemistry for CD34 (green) and CD31 (red) with DAPI (blue) counterstain for nuclei. Telocytes, identifiable as CD34+CD31− stromal cells, intimately surround blood vessels showing CD34+CD31+ endothelial cells, as well as the CD34−CD31+ endothelium of initial lymphatic vessels. (C) Double fluorescence immunohistochemistry for CD34 (green) and αSMA (red) with DAPI (blue) counterstain for nuclei. CD34+αSMA− telocytes intimately encircle secretory salivary gland units outside of αSMA+ myoepithelial cells, as well as the αSMA+ pericytes of capillary vessels and smooth muscle cell layer of arterioles. Scale bar: (A–C), 50 µm. Abbreviations: αSMA, α-smooth muscle actin; BV, blood vessel; DAPI, 4′,6-diamidino-2-phenylindole; LV, lymphatic vessel.
Table 1.
Summary of the Main Immunophenotypic Markers of TCs in Different Organs.
| Organ | Marker | References |
|---|---|---|
| Heart | CD34 c-kit/CD117 PDGFRα PDGFRβ |
Marini et al. 17 and Kondo and Kaestner 24 |
| Skeletal muscle | CD34 c-kit/CD117 PDGFRβ |
Marini et al. 17 |
| Synovium | CD34 PDGFRα |
Rosa et al. 12 |
| Airways and lung | CD34 c-kit/CD117 |
Marini et al. 17 and Kondo and Kaestner 24 |
| Gastrointestinal tract | CD34 PDGFRα Foxl1 (pericryptal) |
Vannucchi and Faussone-Pellegrini, 14 Marini et al., 17 Shoshkes-Carmel et al., 19 Cretoiu et al. 23 and Kondo and Kaestner 24 |
| Tongue | CD34 PDGFRα |
Rosa et al. 20 |
| Salivary glands | CD34 PDGFRα |
Vannucchi and Faussone-Pellegrini 14 and Rosa et al. 20 |
| Liver | CD34 PDGFRα |
Vannucchi and Faussone-Pellegrini 14 |
| Kidney | CD34 c-kit/CD117 |
Kondo and Kaestner 24 |
| Urinary bladder | PDGFRα (upper lamina propria) CD34 (lower lamina propria, detrusor) |
Vannucchi and Faussone-Pellegrini 14 and Marini et al. 17 |
| Uterus | CD34 c-kit/CD117 PDGFRα PDGFRβ |
Vannucchi and Faussone-Pellegrini 14 and Marini et al. 17 |
| Fallopian tube | CD34 c-kit/CD117 |
Marini et al. 17 |
| Testis | CD34 PDGFRα |
Marini et al. 13 |
| Cornea | CD34 PDGFRα c-kit/CD117 (some) |
Marini et al. 18 |
| Skin | CD34 | Romano et al. 16 and Kondo and Kaestner 24 |
| Mammary gland | CD34 | Kondo and Kaestner 24 |
Abbreviations: TC, telocyte; PDGFRα, platelet-derived growth factor receptor α; PDGFRβ, platelet-derived growth factor receptor β.
The TC roles are still not fully known, but numerous studies suggest that these peculiar stromal cells may have both general and location-specific heterogeneous functions.23,24 First, as already mentioned, the unique aptitude of telopodes to establish multiple connections with neighboring cells could provide structural support and cell-to-cell signaling cues necessary to coordinate tissue organization during morphogenesis and to preserve tissue homeostasis during postnatal life.8,23,24 Of note, the numerous abnormalities in the TC networks reported in a variety of diseased tissues further strengthen the idea that TCs may act locally as critical homeostatic regulators.8,11,25,26 Moreover, in organs that are subjected to constant physical stress, such as the gastrointestinal tract, urinary bladder, and skeletal muscle, it has been proposed that TCs may be involved in the modulation of mechanical sensing to favor the necessary tissue adaptation to the different physiological conditions.24,27 Besides, the findings that TCs express cytokines and form intercellular contacts with immune cells in different organs, including the heart, skin, and intestine, suggest that they may take part in the regulation of local immune responses. 24
In this context, growing evidence points toward a possible implication of TCs in tissue renewal and reparative processes through the regulation of survival, proliferation, differentiation, maturation, and guidance of SCs located in the niches of several organs.8,24 Indeed, a number of studies reported that TCs colocalize with tissue-resident SCs/progenitor cells and, therefore, are part of the SC niche microenvironment of different organs, such as the intestine, skeletal muscle, heart, lung, skin, and eye.8,24,28–33 SCs, as fundamental cells for tissue regeneration identified in most adult human organs, possess an intrinsic capacity for either long-term self-renewal or multi-lineage differentiation, but are strictly dependent on the microenvironment where they reside. 34 Hence, the term SC niche refers not only to SCs themselves and their progeny but also to the specific surrounding microenvironment consisting of multiple cell types (e.g., mesenchymal stromal cells, blood and lymphatic capillaries, nerve endings, adipocytes, and supporting interstitial cells), as well as a niche-specific extracellular matrix, rich in SC growth factors, chemokines, and other regulatory molecules, in which these cells are embedded. 34 Among the different cell types populating the niche, a variety of tissue-resident inflammatory/immune cells cooperating with SCs to protect tissue against damage and pathogens are also present. SC niches represent functional units for growth and regeneration in many tissues, where they hold a position of significant importance for preserving the proper tissue function. In particular, the rate and modality by which tissue-resident SCs maintain tissue local homeostasis and repair vary according to the tissue architecture and regenerative demands. 34 For instance, cellular replacements are constant in bone marrow, epidermis, and intestine; episodic in the hair follicle and mammary gland; while limited in brain and muscle tissue. Nevertheless, largely quiescent SCs can be activated when local tissue is injured. 34 Despite the aforementioned tissue differences, it appears that SCs often follow similar patterns in communicating with their niche microenvironment to transition between quiescent and regenerative states. 34 Within their niche, SCs are protected as much as possible from damage or loss by maintaining appropriate communications with their surroundings, which is also essential to ensure their proper responsiveness to physiological cues for cell replacement and repair.34,35 The primary function of a specialized niche microenvironment is to coordinate SC activities both temporally and spatially.34,35 In particular, cell–cell interactions within the niche provide structural support, regulate adhesive interactions, and produce soluble signals controlling SC function. Hence, the niche provides unique chemical, physical, mechanical, and topographical signals that are crucial in facilitating the renewal and regulating the fate of SCs.34,35 Although TCs have only recently entered this scenario, based on their peculiar features, they have increasingly attracted much attention as possible coordinators of intercellular signals within the SC niche microenvironment of different organs either through direct cell-to-cell junctions or through the release and transfer of extracellular vesicles, such as exosomes, ectosomes, and multivesicular cargos, to tissue-resident SCs.8–10,24,28,29,36,37 Interestingly, recent evidence further indicates that SCs are also capable of secreting small vesicles into the surrounding extracellular milieu, 38 thus promoting a close and steady crosstalk between TCs and SCs within the niche. In addition, as in some organs TCs have been reported to express SC markers such as c-kit/CD117, Sca-1, and Oct-4, some authors have suggested that TCs themselves could represent a subpopulation of mesenchymal SCs directly engaged on the frontline during tissue regenerative processes.8,29,31,39
In this review, we depict how the identification and characterization of TCs have contributed to deepen our knowledge of the stromal architecture focusing on the intestine and skeletal muscle, two organs in which the recently unveiled unique relationship between TCs and SCs is currently in the spotlight as potential target for tissue regenerative purposes. In particular, we provide an overview of the tissue-specific arrangement and complexity of SC niches and their reappraisal following the introduction of TCs as new components of the niche microenvironment, emphasizing the functional relevance of the TC-SC interplay and possible pathological implications.
TCs as New Components of Gastrointestinal SC Niche
The Gastrointestinal SC Niche Microenvironment Before the Identification of TCs
The gastrointestinal wall comprises four layers of specialized tissues. The innermost layer is the mucosa that surrounds the lumen of the tract and is made up of three sublayers, namely, the epithelium, the lamina propria consisting of loose connective tissue, and the muscularis mucosae (i.e., a thin layer of smooth muscle). The submucosa is located just beneath the mucosa and contains blood and lymphatic vessels, elastic fibers, and nerves including the submucous plexus, also called Meissner’s plexus. The underlying muscle coat surrounds the submucosa and comprises two sublayers of smooth muscle in circular and longitudinal orientation separated by the myenteric plexus, also called Auerbach’s plexus. The serosa/adventitia represents the outermost layer.
The single-layered columnar epithelium lining the lumen of both the small and large intestines performs nutrient digestion and absorption and acts as a primary defense against pathogenic agents and toxins. It presents the highest cellular turnover rate compared with any other tissue in the body, as it is continuously renewed within 3 to 5 days to maintain its morphofunctional integrity. 40 Such a rapid turnover depends on the constant replacement of epithelial cells by proliferating progenitors derived from multipotent intestinal SCs (ISCs) that are able to give rise to the various differentiated intestinal cell lineages on the basis of the specific gut region. In the small intestine, the epithelium is structured into repeating units consisting of two morphologically and functionally distinct compartments, namely, a finger-like villus projecting into the lumen and a pocket-like crypt (Lieberkuhn crypt) reaching the underlying stroma. Instead, the epithelium of the large intestine is characterized by deeper crypts and a flat luminal surface devoid of villi. The ISCs that are present in the small intestine are located at the bottom of the crypts, with each crypt containing from 12 to 16 ISCs. 41 Once left their staminal compartment, the ISCs quickly divide and give rise to highly proliferative progenitor cells in the so-called transit amplifying (TA) region located in the upper crypt. These TA cells migrate upward toward the villus, and while approaching the top of the crypt, they differentiate into distinct mature epithelial cell lineages, including absorptive enterocytes, mucus-secreting goblet cells, secretory Paneth cells, enteroendocrine cells, and tuft cells. Most of the differentiated cells continue to migrate upward to replace the shedding epithelial cells at the top of the villus, while Paneth cells come back to the staminal compartment, where they finally distribute interspersed with ISCs. 42
Since their first identification in 2007, two types of ISCs have been characterized. The first type is represented by the crypt base columnar (CBC) SCs, identified through genetic lineage tracing experiments and marked by the expression of leucine-rich repeat containing G protein-coupled receptor 5 (Lgr5), a receptor for R-spondin molecules, and Olfactomedin 4 gene that is a target of the Wnt/β-catenin pathway.43,44 CBC ISCs have been shown to be actively cycling and long-lived cells able to give rise to all the differentiated cell lineages of the intestinal epithelium and, thus, they are considered the active population of ISCs. 43 The second pool of ISCs instead comprises normally quiescent and more slowly cycling SCs residing above the crypt base around the so-called “+4” region, that is, four cells distal to the bottom of the crypt. These alternative ISCs express different cell markers and, even if they do not normally perform SC function, they appear to constitute a reserve staminal population that can be activated following stressing conditions.45–49 Indeed, it has been demonstrated that, despite the specific ablation of CBC ISCs in mice using a human diphtheria toxin receptor gene knocked into the Lgr5 locus, the epithelial homeostasis was not perturbed, as Bmi1-expressing SCs are able to compensate for the loss of CBC ISCs and repopulate the intestinal epithelium. 50 Interestingly, these quiescent ISCs have been proposed to be more likely the slow-cycling committed secretory progenitor cells located at around the +4 position, which would then be capable of dedifferentiating into CBC ISCs under certain conditions. 51 In support to this notion, Delta-like (Dll)1+ and Atoh1+ secretory progenitor cells have been shown to be able to convert into CBC ISCs following crypt damage through a dedifferentiation process accompanied by a dynamic chromatin reorganization.52–54 Besides slow-cycling secretory progenitor cells, highly proliferative and short-lived Alpi+ enterocyte progenitor cells, Lyz1+ Paneth cells, and differentiated enteroendocrine cells have also been found to have the potential of regaining stemness and reverting to CBC ISCs to regenerate the intestinal epithelium after ISC loss.55–59 Furthermore, recent single cell analyses of the ISC lineage demonstrated the existence of a damage-inducible population of multipotent slow-cycling and Clusterin+ ISCs referred to as revival SCs. 60 Although rare and with a very limited contribution to the maintaining of normal intestinal homeostasis, these cells are able to quickly expand and regenerate the intestinal epithelium after irradiation-induced epithelial injury. 60 Taken together, these observations indicate the high plasticity of numerous non-SCs of the ISC lineage. Of note, recent studies have additionally demonstrated that also villus epithelial cells, traditionally considered irreversibly committed to a functional cell state, actually can constantly change their functional state, expressing different genes on the basis of their position along the villus axis and, thus, exhibiting a great spatial heterogeneity.61–63
The self-renewal, long-term maintenance, and differentiation capabilities of ISCs rely on the intricate interaction among different intracellular pathways and extracellular signals provided by the neighboring epithelial and mesenchymal cells constituting the specialized niche microenvironment (i.e., the ISC niche).64,65 Consistent with this notion, in vitro expansion and growth of CBC ISCs into organoids containing proliferating and differentiated epithelial cells is, indeed, supported only if factors partly provided in vivo by the mesenchymal niche are administered.64,66
Among these factors, the canonical Wnt/R-spondin system represents one of the major mitogens. The Wnt/β-catenin pathway, which is activated by the binding of Wnt ligands to Frizzled receptors and LRP5/6 coreceptors, initiates a signaling cascade that finally leads to the inhibition of cytoplasmic β-catenin ubiquitination, with its consequent stabilization and translocation into the nucleus, where it interacts with the transcription factor T-cell factor (TCF) and other cofactors to activate the transcription of Wnt target genes. 67 R-spondins are the ligands for the orphan receptors Lgr4/5/6 and are considered Wnt agonists, as they can potentiate Wnt signaling by positively regulating the abundance of Wnt receptors on the cell surface. 68 Several experimental studies have demonstrated the pivotal role played by canonical Wnt signaling cascade in ISC function and crypt cell proliferation. Among these, the conditional knockout of the Wnt effectors TCF4 or β-catenin, as well as the transgenic overexpression of the Wnt inhibitor Dickkopf (Dkk)1 or the deletion of R-spondin receptors Lgr4/5 in the gut epithelium determined the disappearance of ISCs and a rapid loss of crypt proliferation.69–73 On the contrary, transgenic expression and injection of R-spondin1 led to hyperproliferation of the crypt cells. 74 It has also been recently demonstrated that the global blockade of Wnt production through the conditional ablation of Wntless, a transmembrane protein required for the secretion of mature Wnt proteins, causes a deep impairment in the homeostatic renewal of the intestinal epithelium. 75 Furthermore, Wnt signaling inhibition with the obligatory Wnt processing enzyme Porcupine (Porcn) inhibitor C59 determined a burst of mitogen-activated protein kinase (MAPK)-dependent proliferation of CBC ISCs that rapidly depleted the SC pool, as they continued to differentiate without replenishing the staminal compartment. 76 Another signaling pathway able to maintain ISC stemness is represented by Notch signaling, which starts with the binding of the ligands Jagged and Dll to the Notch receptor and leads to the proteolytic cleavage of the Notch intracellular domain that translocates into the nucleus and activates the transcription of target genes. In this context, the inhibition of Notch signaling caused ISC loss and secretory cell hyperplasia, whereas its overactivation led to intestinal progenitor cell expansion.77,78 ISC proliferation and self-renewal is additionally promoted by the epidermal growth factor receptor (EGFR) signal transduction cascade, as demonstrated by the fact that EGFR inhibition or the removal of epidermal growth factor (EGF) from the medium of the intestinal organoid culture system blocked the proliferation of ISCs and further induced them into quiescence.66,79 In contrast to the aforementioned signaling pathways, the bone morphogenetic protein (BMP) signaling cascade negatively regulates ISC stemness, as it inhibits their proliferation and promotes their differentiation through a Smad-mediated transcriptional repression of a large number of Wnt signature genes, including Lgr5.80–83 Indeed, the transgenic expression of the BMP antagonist Noggin in mice has been shown to cause ectopic crypt formation, 84 as well as the conditional inactivation of BMPR1A in mice led to intestinal polyposis. 81 Finally, two other pathways involved in the regulation of ISC function are represented by the Hippo and the Hedgehog (Hh) signaling pathways. 65 Collectively, the activity of all these signalings creates reverse gradients along the crypt–villus axis to orchestrate self-renewal and differentiation of ISCs.
Over the past decade, in the wake of the results obtained by Sato et al. 66 on the intestinal organoid culture system demonstrating that epithelial signaling production is entirely dispensable for ISC regulation, scientists have long debated about the identity of the intestinal niche cells.65,85 First, on the basis of their direct interaction with ISCs and their capability to secrete signaling molecules such as Wnt3 and EGF, postmitotic Paneth cells have been proposed as the ISC niche. 86 However, this hypothesis was soon questioned by the results of two independent studies showing no changes in ISC proliferation and maintenance upon ablation of Paneth cells.87,88 Moreover, Wnt3 deletion in Paneth cells showed no effect on crypt health, 89 as well as the elimination of Wnt production in the epithelial compartment by epithelium-specific gene knockout of Porcn had no evident effects on intestinal homeostasis.90,91 All these findings, together with the observation that the global blockade of Wnt secretion in both the epithelial and subepithelial mesenchymal intestinal compartment determined ISC loss, 75 led scientists to put forward stromal myofibroblasts, located in close proximity to ISCs, as an alternative source of mitogenic Wnt signals. This second hypothesis was investigated through the inhibition of Wnt signaling in myofibroblasts using myosin heavy chain 11 (Myh11)-CreER;PorcnloxP mice, but again no effects on intestinal morphogenesis and SC function were detected. 91
A Reappraisal of the Gastrointestinal SC Niche Microenvironment and Stromal Compartment After the Identification of TCs
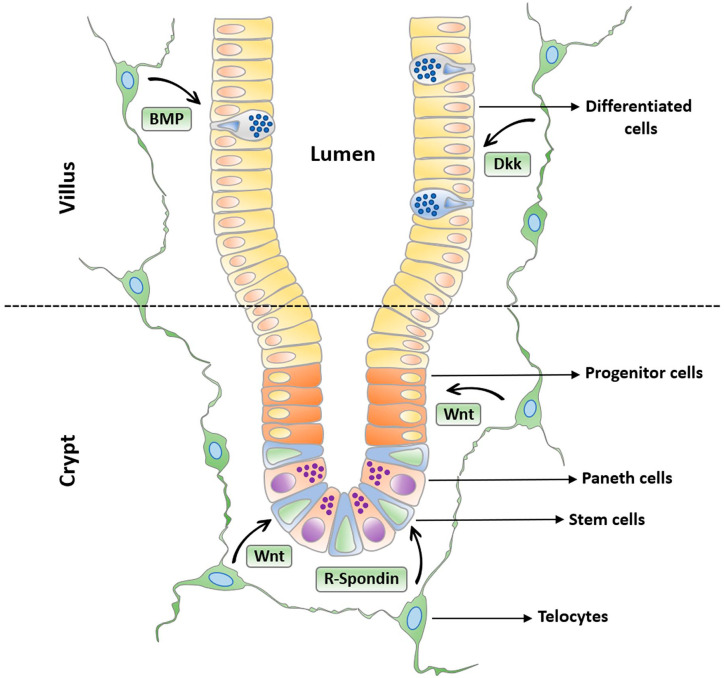
In recent years, several studies have identified TCs, characterized by the expression of the winged helix transcription factor Foxl1, the Hh signaling mediator Gli1, CD34, and PDGFRα, as the crucial source of mitogenic/morphogenic signals to the crypt staminal compartment.24,64 TCs are closely associated with the columnar epithelial cells and form a continuous plexus (pericryptal sheath) just underneath the epithelium via the protrusion of long and thin moniliform cytoplasmic extensions reaching several hundred micrometers in size (Fig. 3). In addition, TCs express multiple critical crypt signaling proteins such as the Wnt signaling molecules Wnt2B and Wnt5A, the coactivator R-spondin3, as well as the BMP antagonist gremlin 1 (Grem1). Of note, they are distinct from other mesenchymal cells such as myofibroblasts, as demonstrated by their immunonegativity for αSMA and Myh11.19,92 The first functional evidence that TCs constitute a critical component of the ISC niche, having a pivotal function in intestinal maintenance, came in 2016 from the experiments of Aoki and colleagues. 92 In this study, conditional diphtheria toxin-mediated ablation of Foxl1-expressing TCs in mice resulted in a rapid degeneration of the intestinal mucosal architecture, with the ceasing of epithelial proliferation within a few days after the administration of the toxin. 92 In particular, the length of both small and large bowels was shortened, villus length was halved, and the number of proliferating cells per crypt was reduced by more than 95%. Of note, even if the Wnt signaling to the epithelium was found to be critically reduced, the nature of the involved mitogenic pathway was not unequivocally demonstrated. 92 The question of whether Wnt signals secreted by TCs are effectively required for the crypt function was solved in a following experimental study in which Wnt production was specifically blocked in Foxl1+ TCs through the conditional gene ablation of Porcn, which is necessary for the functional maturation of all Wnt proteins. 19 Such an active Wnt signaling elimination led to the complete disappearance of the epithelial proliferation and renewal in both the small intestine and colon within 72 hr and to mice death in 3 to 4 days, providing clear evidence that the Wnt proteins produced by epithelial cells or other Foxl1– stromal cells are not able to compensate for the loss of Wnt signals from Foxl1+ TCs and definitely establishing Foxl1+ TCs as the critical source of Wnt signals. 19 In the same study, sorted Foxl1+ intestinal TCs were also used to perform a transcriptome profiling. Interestingly, this analysis not only demonstrated that these cells are characterized by an expression profile that is clearly different from Foxl1– mesenchymal or epithelial cells, but also that besides expressing Wnt signaling activators, including Wnt2b, Wnt5a, and R-spondin3, they also express Wnt signaling inhibitors such as Dkk2, Dkk3, and the decoy receptor sFRP1. Similarly, Foxl1+ TCs express BMP4, BMP5, BMP6, and BMP7, as well as the BMP inhibitors chordin-like1 and Grem1. 19 Such an apparently paradoxical observation led the authors to perform single molecule RNA-FISH (fluorescence in situ hybridization) experiments, through which they demonstrated that TCs are able to compartmentalize their transcripts depending on their position along the crypt–villus axis, releasing more Wnt activators at the bottom of the crypt, where CBC ISCs are situated, and producing more Wnt inhibitors near the crypt–villus junction and at the villus tip (Fig. 3). 19 By maintaining this different Wnt activators:inhibitors ratio along the crypt–villus axis, TCs may therefore contribute to the Wnt-BMP gradient formation that is known to be essential for ISC self-renewal and differentiation (Fig. 3). After being characterized in mice, Foxl1+ TCs have recently been described in the human colon as well by a single cell RNAseq analysis of the colonic stroma from patients with ulcerative colitis (UC) and healthy controls. 93 Interestingly, given the decrease in the proportion of these cells in patients with UC, it has been suggested that Foxl1+ TC impairment could play a role in the pathogenesis of inflammatory bowel diseases, probably by mediating the interactions of luminal antigens with the immune system after failure of the intestinal barrier. 93 Finally, it has been recently reported that Foxl1+ TCs of the intestinal niche are evolutionarily conserved among terrestrial vertebrates and can be induced by thyroid hormone through sonic Hh signaling during amphibian metamorphosis for SC development. 94
Figure 3.
Schematic representation of the continuous plexus formed by telocytes with their long and thin moniliform extensions (telopodes) appositioned just underneath the intestinal epithelium. The telocyte plexus compartmentalizes the production of crucial signaling molecules contributing to the formation of the Wnt-BMP gradient essential for intestinal stem cell self-renewal and differentiation along the crypt–villus axis. Telocytes surrounding the bottom of the crypt release more Wnt activators such as canonical Wnt ligands and R-Spondin, which promote stem cell proliferation, while those bordering the epithelium at the crypt–villus junction and at the villus tip release more Wnt inhibitors, such as BMP and Dkk family members, thus orchestrating progressive cell differentiation along the crypt–villus axis. Abbreviations: BMP, bone morphogenetic protein; Dkk, Dickkopf.
Although not specifically referred to as TCs but presumably identifiable as so, populations of pericryptal stromal cells expressing different markers such as CD34, Gp38 (podoplanin), PDGFRα, and Gli1 have been described by different groups as major components of the ISC niche.95–97 In 2017, bulk RNA-sequencing analysis revealed that CD34+ and Gp38+ cells express Wnt2b in normal intestine and present a dramatic upregulation of R-spondin1 and Grem1 after dextran sodium sulfate–induced intestinal inflammation, thus playing a crucial role in regulating ISC proliferation during both physiological homeostasis and intestinal repair. 95 In addition, these cells have been shown to support ISC proliferation in the organoid culture system, as their addition to the culture caused the transformation of organoids with budding crypts to spherically shaped organoids, a phenotype due to increased ISC proliferation and impaired epithelial differentiation. 95 Subsequently, Greicius et al. 96 reported that conditional knockout of Porcn using a PDGFRα-Cre line in the small intestine impaired crypt formation, revealing that the PDGFRα+ mesenchymal cells provide Wnt signals necessary for ISC maintenance. Furthermore, PDGFRα+ mesenchymal cells were also found to express R-spondin3 and to support the growth of organoids from Wnt-deficient intestines. 96 Similarly, another group reported the crucial role of cells expressing the Hh pathway transcription factor Gli1 in the regulation of ISC niche signals. 97 In this study, conditional ablation of Wntless, a protein that like Porcn is required for the production of all Wnt proteins, through an inducible Gli1-CreER driver caused ISC loss and consequent crypt collapse only in the colon and not in the small intestine, unless such an ablation was combined with a Villin-CreER driver, which thus removed any Wnt proteins emanating from the intestinal epithelium. 97 On the basis of these results, the authors concluded that Gli1+ stromal cells are essential Wnt producing cells for ISC maintenance only in the colon, while in the small intestine, they provide redundant Wnt signals together with epithelial cells. 97 Because (1) Foxl1+ TCs represent the subepithelial subset of PDGFRα+ gastrointestinal stromal cells, (2) Gli1+ cells include both Foxl1+ TCs and CD34+ stromal cells, and (3) gastrointestinal TCs have been identified as CD34+PDGFRα+ stromal cells,19,64,98 it is tempting to speculate that there may be a substantial overlap among all these different cell populations and that each of these studies identified indeed the same stromal cell population, namely, the TCs. As previously mentioned, TCs reside broadly beneath the entire intestinal epithelium. Interestingly, by combining laser capture microdissection and single cell RNA-sequencing, a subpopulation of TCs localized at the villus tip and marked by the epithelial staminal marker Lgr5 has been recently characterized. 99
In contrast to ISC niches, stomach SC niches have been so far poorly investigated. In this regard, by using single cell transcriptome and mouse genetic analyses, a very recent study has identified conserved stromal cell populations that are Hh responsive and express Wnt ligands and TC markers. 100 Such stromal cell populations have been shown to play a critical role in gastrointestinal regeneration, while during development and adult homeostasis, their action was found to be redundant with other stromal cell populations. 100 Taken together, these results demonstrated for the first time that gastric stromal cells constitute a critical Wnt SC niche and that Hh- and Gli2-mediated transcriptional activation of SC niche signals is conserved in both the stomach and the intestine. 100
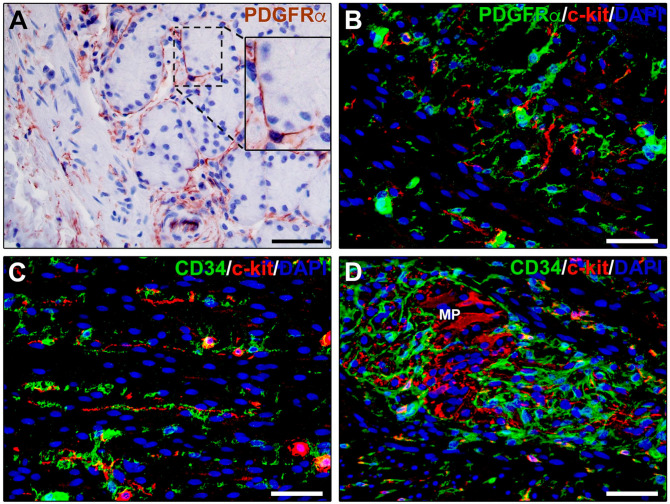
Besides being located in the lamina propria of the mucosa, where they form nets bordering the funds of gastric glands, the intestinal crypts, and the entire abluminal side of the epithelium, TCs are widely distributed throughout all the other layers of the gastrointestinal wall (Fig. 4A–D).98,101 In the submucosa, CD34+PDGFRα+ TCs have been shown to be organized in a dense network that surrounds large vessels, envelops local ganglia, forms tight meshes in the loose connective tissue, and constitutes an almost continuous monolayer embracing the muscularis mucosae. 98 Such a spatial organization likely guarantees mechanical support to the connective components during peristalsis and helps in maintaining the integrity of the ganglia during bowel movements. Moreover, the TC scaffold might be implicated in the regional distribution of other stromal cells and play a significant role in regulating the arrangement and maintenance of the extracellular matrix. In the muscularis propria, similar complex networks of CD34+PDGFRα+ TCs have been described at the submucosal border of the circular muscle layer, in the myenteric plexus region where they surround ganglia and the enteric nerve strands, and among the smooth muscle bundles and cells of both the circular and longitudinal muscle layers. 98
Figure 4.
Immunohistochemical localization of telocytes in human gastrointestinal tract. (A) PDGFRα immunoperoxidase-based immunohistochemistry with hematoxylin counterstain. PDGFRα+ telocytes form a plexus closely surrounding gastric glands. Inset: higher magnification of the boxed area showing a telocyte with its moniliform prolongations (telopodes) juxtaposed underneath gastric gland cells. (B–D) Double fluorescence immunohistochemistry for PDGFRα (B, green) or CD34 (C and D, green) and c-kit (red) with DAPI (blue) counterstain for nuclei. In the muscularis propria, PDGFRα+ and CD34+ telocytes and c-kit+ interstitial cells of Cajal form interconnected networks in muscle layers and at the myenteric plexus. Scale bar: (A–D), 50 µm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; MP, myenteric plexus; PDGFRα, platelet-derived growth factor receptor α.
In the different layers of the gastrointestinal wall, TCs interact with a large variety of neighboring cells by establishing functional communications through both vesicles/exosomes and cell-to-cell contacts. 7 In this context, in the gut muscle coat, it is important to take into account the spatial and cellular interaction of TCs with the specialized gut pacemaker interstitial cells of Cajal (ICCs), with which they are supposed to regulate gastrointestinal motility. The networks of TCs and ICCs have been described to run parallel and sometimes intermingled, forming cell-to-cell contacts with each other, within both the circular and longitudinal muscle layers, as well as at the myenteric plexus region (Fig. 4B–D).98,102,103 By contrast, TCs but no ICCs have been found within and around interganglionic nerve fascicles, submucosal nerves, and mesenteric nerves. 103 ICCs are constantly innervated and participate in the control of peristalsis, ensuring coordinated patterns of smooth muscle cell activity by mediating neurotransmission.102,104–106 Conversely, although under light microscopy nerve fibers are usually seen in the proximity of TCs,7,107,108 the observation of these stromal cells by using TEM has unequivocally demonstrated that TCs never establish cell-to-cell contacts with nerve endings. 7 Consequently, it has been proposed that through their contacts with both ICCs and smooth muscle cells, TCs might help to coordinate smooth muscle cell activity by spreading the slow waves generated by the ICCs or amplifying the electrical signal generated in the ICCs.101,107,108 Such an integrated circuit comprising smooth muscle cells, ICCs, and PDGFRα-expressing TCs is commonly referred to as “SIP syncytium.”109,110 On the basis that PDGFR signaling plays pivotal roles in both organogenesis and morphogenesis,111,112 another possible explanation of the close relationship between TCs and ICCs is that, in postnatal life, CD34+PDGFRα+ TCs might represent mesenchymal progenitor cells able to differentiate into ICCs. 113 Of note, even if initially considered the same cell type, ICCs and TCs can be easily distinguished within the gut muscle coat according to their different immunophenotypes, as ICCs are c-kit+CD34−PDGFRα−, while TCs are c-kit−CD34+PDGFRα+ (Fig. 4B–D).1,98,114 The possibility that TCs might represent local progenitors of ICCs is also supported by the evidence that, after ICC apoptosis, mitoses are frequently detected in TCs but not in ICCs, as well as the downregulation or even loss of CD34 expression and the acquisition of the ICC marker c-kit by TCs in culture. 8 Besides ICCs and smooth muscle cells, TCs are also often closely apposed to immune cells such as plasma cells, lymphocytes, mast cells, eosinophils, and basophils. In this regard, it has been assumed that TCs might have a role in both immune-regulation and immune-surveillance. In particular, it has been proposed that TCs could secrete soluble chemoattractant molecules along their telopodes, acting as guides for the immune cells, as well as behaving as dendritic-like cells by presenting tissue-derived antigens to immune cells either by intercellular contacts or the release of exosomes.1,4,7,14 Among immune cells, gastrointestinal macrophages regulated by enteric neurons and with an anti-inflammatory phenotype seem to play a significant role in the muscularis propria of the enteric wall, being directly involved in intestinal homeostasis and functionality.115–117 Through immunohistochemical and TEM analyses, TCs and macrophages have been reported to establish extended cell-to-cell contacts in both human and mouse gut, with TCs almost encircling macrophages by their long and thin processes. Such a close and extended spatial relationship might guarantee an adequate distribution of macrophages in the different layers of the gut wall and could represent a support for these cells when the meshes are stretched during the contractile activity. Of note, it has also recently been demonstrated that TCs are frequently intercalated between macrophages and smooth muscle cells, making contacts with both cell types. 118 This finding led to propose TCs as mediators of the macrophage actions on smooth muscle cells. 118 Finally, as it has been reported that the TCs located in the tunica muscularis of the gut express both purine receptors P2Y1 and apamin-sensitive SK3 channels and respond to agonists and antagonists to these receptors,107,119–122 an additional role raised for gastrointestinal TCs is that of representing targets of neural signals. Interestingly, changes in the functionality of such receptors have been associated with different gastrointestinal diseases.123–125 Of note, as enteric TCs also express soluble guanylyl cyclase, it has been suggested that they may even act as neural transducers responding to ATP and nitric oxide.107,110
Implications of TCs in Gastrointestinal Diseases
On the basis of the aforementioned intriguing roles proposed for TCs in the digestive tract, a growing number of studies have investigated their possible involvement in different gastrointestinal pathological processes.25,26,126–130 Changes in the distribution of TCs have been first reported in surgical specimens obtained from patients affected by Crohn’s disease (CD) and UC, two inflammatory bowel diseases characterized by chronic inflammation, extensive tissue fibrosis, and gastrointestinal dysmotility.126,127 In the terminal ileum of patients suffering from CD, in which fibrosis commonly involves the entire bowel wall and the most peculiar histopathological feature is represented by discontinuous signs of inflammation and fibrosis referred to as “skip lesions,” TCs displayed a similar distribution to normal control specimens in disease-unaffected segments, whereas they were significantly reduced in disease-affected portions, particularly in the areas displaying the most severe fibrotic changes and derangement of the intestinal wall architecture. 126 Interestingly, in the muscularis propria of CD lesions, the TC network was discontinuous or even completely absent both among smooth muscle bundles and around myenteric plexus ganglia. 126 A similar reduction in TCs strictly related to the gastrointestinal tissue fibrotic changes has also been reported in UC. 127 Indeed, a significant reduction in TCs was detected in the affected muscularis mucosae and submucosa of both early and advanced fibrotic UC specimens. 127 Furthermore, the TC network was preserved in the spared muscularis propria of early fibrotic UC cases, while being greatly compromised in fibrotic areas of muscle layers and around myenteric ganglia in advanced fibrotic UC cases. 127 Of note, CD34/c-kit double immunostaining clearly revealed that in the muscularis propria of both CD and UC, the impairment in the TC network was paralleled by the loss of the ICC network, suggesting that the concomitant reduction in both interstitial cell types may significantly contribute to gastrointestinal dysmotility in these pathological conditions.126,127 Abnormalities in TC networks accompanied by a significant reduction of intermingling ICCs might play a pivotal role also in gastroparesis, a pathological condition characterized by delayed or absent emptying of the stomach in the absence of mechanical obstruction. 130 Indeed, TCs and ICCs may have a pivotal role in the coordination of antral contractions that are required to fragment and liquefy the food before emptying into the duodenum, by modulating excitatory and inhibitory impulses and allowing gastric smooth muscle cells to act as a syncytium. 131 As already mentioned, it also appears that in inflammatory bowel diseases, the decrease in the pericryptal Foxl1+ TC network may lead to ISC niche impairment and consequent failure of the intestinal barrier. 93 As TCs are believed to be primarily committed to the maintenance of local tissue homeostasis through intercellular signaling, it has also been suggested that the disruption of the TC network might favor an uncontrolled activation of neighboring fibroblasts with their transition to profibrotic myofibroblasts, as supported by the concomitance of the increase in the number of αSMA+ myofibroblasts and disappearance of CD34+ TCs in UC tissue specimens. 127 Nevertheless, it seems that TCs might even act as myofibroblast precursors by changing their immunophenotype, namely, by losing CD34 and gaining αSMA expression, in certain conditions such as during tissue repair and formation of granulation tissue or in the tumor-associated stroma by differentiating into cancer-associated fibroblasts.8,11,132 As far as cancer is concerned, it has been recently shown that loss of PTEN signaling in Foxl1+ TCs is sufficient to initiate spontaneous colonic neoplasia in mice. 129 Finally, although most authors support the notion that gastrointestinal stromal tumors (GISTs) derive exclusively from or differentiate toward the ICC lineage, a hypothesis corroborated by the fact that they frequently express the c-kit/CD117 antigen, the existence of PDGFRα-mutant GISTs or familial PDGFRα-mutation syndromes has been recently demonstrated, suggesting that PDGFRα+ TCs also may be related to this kind of pathological conditions.26,128
TCs as New Components of Skeletal Muscle SC Niche
The Skeletal Muscle SC Niche Microenvironment Before the Identification of TCs
The skeletal muscle tissue responsible for all voluntary movements is made of long tube-like multinucleated cells, called skeletal myofibers, which are formed through the fusion of hundreds of mononucleated precursors during development. 133 Each myofiber is structured with finely organized bundles of actin and myosin filaments contributing to the muscle contractile function and, thus, to generate the force needed for locomotion or postural control. 133 In fact, age-related loss of skeletal muscle mass, strength, and function, termed sarcopenia, contributes to decreased independence and autonomy heavily compromising the quality of life of older people. 34 Besides contractile myofibers, muscle tissue is also constituted by perimysial and endomysial connective tissue, vasculature, and the innervating motor and sensory nerves. 133
Skeletal muscle is a high plasticity tissue which enables the adaptation to physiological demands such as postnatal growth or training. 134 Indeed, it contains muscle SCs allowing regenerative processes throughout most of adult life, especially after injury or excessive physical training as they ultimately provide myogenic precursors that can rebuild damaged muscle tissue.135,136 Muscle SCs, which spend most of their lifetime in a dormant state, reside as individual cells in circumscribed niches scattered throughout the tissue and, because of their location just outside the plasma membrane of the myofibers, are commonly referred to as satellite cells.34,135,137 Hence, during adulthood, under physiological conditions, satellite cells are retained in a quiescent state, but upon stimulation (e.g., physical exercise or muscle damage), they activate and re-enter the cell cycle undergoing proliferation and differentiation, a series of processes that mainly recapitulates the steps of embryonic and fetal myogenesis.138,139 Satellite cells sustain skeletal muscle growth and repair through two mechanisms, either by increasing the size of a preexisting myofiber (hypertrophy) or by new myofiber formation (hyperplasia).135,136,138
Despite different origin, as limb and trunk muscle satellite cells have somitic origin while those of head muscles derive from cranial mesoderm,140,141 all muscle resident satellite cells appear to own a universal potential to perform myogenic function. Moreover, these cells continue to exhibit heterogeneity in their gene expression signature during postnatal growth, but such differences are subsequently lost when they assume the role of adult SCs. 133 Even if many markers can be used to identify satellite cells, the paired box transcription factor 7 (Pax7) is considered the main specific marker necessary for their survival and function. 142 It is generally expressed during quiescence, activation, and proliferation phases, excepting myoblasts. Other markers include the myogenic regulatory factors, such as Myf5, MyoD, and MRF4.143–146 In particular, Pax7 and Myf5 are coexpressed in the majority of these cells in the quiescent state, as well as during their activation and proliferation phases. 143 The transcription factor MyoD is another important marker that is highly expressed during early activation of satellite cells and is subsequently maintained in differentiating myonuclei, whereas MRF4 expression is augmented mainly during cell differentiation stage. 147 Once activated, satellite cells undergo asymmetric division following this scheme: a daughter cell is committed to myogenesis, while the other one reverts back to quiescence to maintain a constant SC pool. 148 In such a process, the transcription factors MyoD and myogenin exhibit asymmetric expression within daughter cells. In fact, the committed ones upregulate MyoD and myogenin, while MyoD− and myogenin− cells remain as reservoir.144,148 Taken together, in the adult skeletal muscle, quiescent satellite cells are characterized by the expression of Pax7 and Myf5, but not MyoD or myogenin. After muscle tissue injury, activated satellite cells undergo mitotic division to generate a progeny of proliferating adult myoblasts coexpressing Pax7, Myf5, and MyoD. Afterward, adult myoblasts downregulate Pax7, acquire myogenin and MRF4 expression, and progress toward differentiation into skeletal myocytes that fuse and form multinucleated myofibers.143,149 Besides the aforementioned markers, satellite cells also express Pax3 that seems to play an important role in the initial stages of myogenesis, but its expression is downregulated once the cell is committed to myodifferentiation. Other described markers, which however are not exclusive to satellite cells, include Barx2 transcription factor that is coexpressed with Pax7 and regulates muscle growth and maintenance, CD34 selectively expressed in quiescent cells, transmembrane heparan sulfate proteoglycans syndecan-3 and syndecan-4, chemokine receptor CXCR4, and neural cell adhesion molecule-1.143,148,150–153 Moreover, although satellite cells have been classically considered a homogeneous group of SCs accumulated by the expression of Pax7, subsequent studies suggested that the pool of satellite cells might be composed of two different subpopulations. One larger subpopulation would consist of SCs with a higher myogenic differentiation potential that readily undergo activation and proliferation after injury, while the other one would be mainly responsible for SC pool maintenance to ensure tissue regeneration in case of subsequent injuries.154,155
In their quiescent state, satellite cells are specifically located between the basal lamina, which protects them from the surrounding milieu, and the sarcolemma of skeletal myofibers. 136 Muscle repair/regeneration upon damage is a tightly orchestrated process that begins with an inflammatory response followed by tissue necrosis, and then satellite cell activation, expansion, migration, differentiation, and fusion to restore injured myofibers or generate new myofibers.34,148 First, the activation of satellite cells implies that a profound transition takes place into their nucleus from a low/absent to a high transcriptional activity. 156 Increasing evidence indicates that such an activation of satellite cells and their subsequent commitment are regulated through a cascade of complex signaling pathways that are strictly influenced by multiple extrinsic factors within the surrounding microenvironment. 157 Indeed, this complex niche microenvironment may control all aspects of satellite cell biology, as their quiescence, activation, proliferation, differentiation, or renewal and return to quiescence. In particular, satellite cell functionality and fate direction appear to be tightly regulated by a microenvironment composed of a wide variety of factors, such as numerous secreted molecules and different cell types, including capillary endothelial cells, pericytes, oxygen levels, hormones, motor neurons, immune cells, cytokines, fibroblasts, growth factors, myofibers, myofiber metabolism, and the extracellular matrix.139,157
A Reappraisal of the Skeletal Muscle SC Niche Microenvironment and Stromal Compartment After the Identification of TCs
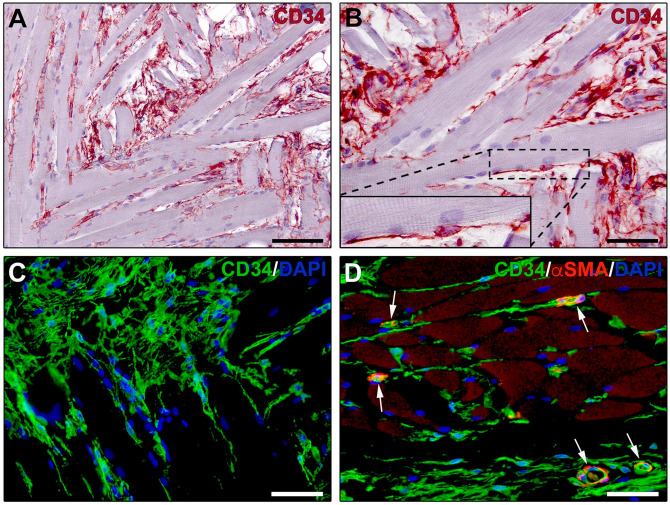
In this context, TCs have been identified as a peculiar type of interstitial cells widely distributed in the perimysial and endomysial compartments of adult skeletal muscle tissue only in recent years (Fig. 5A–D).17,31,139,158,159 Hence, although the exact role of muscle TCs is still under investigation, growing evidence suggests that these stromal cells represent previously neglected components of the skeletal muscle SC niche that may participate in repair/regenerative processes. In early studies, several hypotheses on the putative functions exerted by muscle TCs have been mainly assumed on the basis of their morphological assessment by TEM and light microscopy.158–160 Indeed, it has been described that with their characteristic telopodes, TCs constitute an extensive and complex interstitial network located remarkably close to myofibers (Fig. 5B), microvessels (Fig. 5D), nerve fibers, satellite cells, or other putative progenitor cells. 158 As they communicate with their long telopodes with multiple cell components of the SC niche, it has been proposed that such long-distance intercellular connections might help in integrating different signals within the niche to regulate skeletal muscle homeostasis, remodeling, and regeneration.31,160 In particular, it is believed that the network of telopodes might constitute not only an essential physical scaffold to guide myogenic progenitor cells during their migration and differentiation after activation, but also an important source of regulatory paracrine signals within the SC niche.31,160 For instance, skeletal muscle TCs have been shown to highly express vascular endothelial growth factor (VEGF), a trophic factor with known regulatory effects on small vessels and satellite cells.139,158,161 These early assumptions have recently been strengthened by two experimental studies that investigated changes in tissue distribution of TCs in a mouse model of skeletal muscle damage due to forced eccentric contraction, highlighting important morphofunctional interactions between TCs and satellite cells.139,162 Compared with uninjured control skeletal muscles, damaged muscles exhibited an extended network of CD34+CD31− TCs throughout the endomysium surrounding myofibers.139,162 Notably, myofiber injury and related redistribution and extension of the TC network were significantly attenuated when forced eccentric contraction was applied to the muscles in the presence of bone marrow–mesenchymal stromal cell secretome. 162 Moreover, in the injured muscles, the network of telopodes was preferentially arranged around activated satellite cells displaying MyoD nuclear positivity. 139 Besides light microscopy, TEM further revealed the very close intercellular communication between TCs and satellite cells upon injury-induced activation, as demonstrated by the presence of telopodes crossing the broken myofiber basal lamina and contacting the underlying activated satellite cells incompletely covered by the basal lamina. 139 Of note, the breakdown of the myofiber basal lamina is essential to allow activated satellite cell migration toward the injury site,163–165 and activated satellite cells may contribute themselves to such a process. In fact, satellite cells are capable of secreting functional proteolytic enzymes selectively digesting individual components of the extracellular matrix, such as matrix metalloproteinase-2 and metalloproteinase-9, as well as their specific tissue inhibitors.165,166 Interestingly, as TCs also express matrix metalloproteinases, 22 it has been suggested that they might cooperate with satellite cells in the digestion of the basal lamina or even mediate the release of promyogenic factors sequestered in the extracellular matrix, thus actively contributing to satellite cell activation. 139 The same study also analyzed the reciprocal behavior of TCs and satellite cells isolated from single living endomysial sheath-covered myofibers, which confirmed the preferential interaction of TCs and satellite cells and further revealed an increased expression of VEGF in TCs from damaged muscles. 139 Collectively, these experimental findings strengthen the notion that TCs may be novel key components of the skeletal muscle SC niche endowed with specific “nursing cell” roles in the induction of satellite cell activation and promotion of myogenic differentiation through both intercellular contacts and paracrine mechanisms. 139
Figure 5.
Immunohistochemical localization of telocytes in skeletal muscle interstitium. (A, B) CD34 immunoperoxidase-based immunohistochemistry with hematoxylin counterstain. (C) Fluorescence immunohistochemistry for CD34 (green) with DAPI (blue) counterstain for nuclei. CD34+ telocytes form a complex reticular network in the perimysium and endomysium. Inset in (B): higher magnification of the boxed area showing a CD34+ telocyte projecting two long and thin moniliform processes (telopodes) in close relationship with a skeletal muscle fiber. (D) Double fluorescence immunohistochemistry for CD34 (green) and αSMA (red) with DAPI (blue) counterstain. CD34+αSMA− telocytes with their telopodes surround skeletal muscle fibers and microvessels showing αSMA+ pericytes/smooth muscle cells (arrows). Scale bar: (A), 100 µm; (B–D), 50 µm. Abbreviations: αSMA, α-smooth muscle actin; DAPI, 4′,6-diamidino-2-phenylindole.
Another recent study, which specifically investigated the presence of TCs in human fetal skeletal muscle tissue from lower limbs, found that these stromal cells may be mainly involved in the early myogenic period, possibly guiding muscle tissue organization and compartmentalization, as well as angiogenesis and myotube maturation. 167 Indeed, relevant differences in the distribution of TCs within the fetal skeletal muscle tissue have been detected when comparing different gestational ages. In particular, TCs seem to increase in number in muscles from 9 to 11.5 weeks of gestation, where their telopodes form an extensive reticular network in close relationship with primary and secondary myotubes undergoing maturation. 167 Instead, at 12 weeks of gestation, when mature myotubes become evident, the number of skeletal muscle TCs appears to be strongly reduced. 167 Taken together, it seems therefore that this peculiar stromal cell population residing in the skeletal muscle tissue SC niche might exert promyogenic functions during both morphogenesis and development and in postnatal life.
In addition to the satellite cell lineage, the skeletal muscle SC niche appears to harbor other non-satellite progenitor cells such as bone marrow–derived mesenchymal SCs, pericytes, vessel-associated progenitors of mesodermal origin called mesoangioblasts, and CD133+ cells, but their contribution to the myoblast pool appears to be minor.34,133,168–173 In particular, pericytes, which are supposed to give rise to mesenchymal SCs, are able to support several aspects of tissue regeneration. 174 Another population of interstitial cells long known to be equipped with muscle regeneration capability is represented by the PDGFRα+ stromal cells, which appear to include mesenchymal progenitor cells and fibro/adipogenic progenitor cells that have the potential to develop into fibroblasts and adipogenic cells.175,176 As PDGFRα has been only recently recognized as one of the marker of TCs in a variety of tissues, there is the possibility that such PDGFRα+ progenitors may be, indeed, TCs.4,8,17 Of note, even the landmark study by Bojin et al., 31 first describing TCs in the human skeletal muscle SC niche, suggested that these cells might also function as progenitor cells during muscle tissue repair/regeneration, an observation mainly based on the expression of the proliferative marker Ki67 and the SC marker Oct-4 by these TCs. Besides, it appears that TCs might widely behave as progenitor cells in a number of tissue reparative and pathological processes.8,11,132 Finally, a very recent immunohistochemical study employing different progenitor cell markers identified an interstitial cell population coexpressing CD10, CD34, CD271, and PDGFRα, which seems to correspond to the TCs, in the endomysium of the human skeletal muscle. 177 Indeed, TCs are well known to be identifiable as CD34+PDGFRα+ stromal cells, and immunoelectron microscopy clearly showed that the CD10+ interstitial cells possess the distinctive ultrastructural features of TCs, namely, very long and thin moniliform telopodes formed by the alternation of podomers and podoms.1,4,17,177 Interestingly, the expression of CD10 is evocative of an intrinsic capacity of these cells to take part in myogenesis and muscle tissue regeneration. In muscle lesions, endomysial CD10+ interstitial cells were found to be increased in number. 177 Moreover, some CD10+ cells expressed the proliferation marker Ki67, while coexpression of CD10 and Ki67 was not found in healthy muscle tissue. 177 The marker profile of these cells also seems to change dynamically in response to muscle damage and atrophy. 177 In particular, in skeletal muscle affected by focal single myofiber damage, CD10 was upregulated as well as CD34 and CD271, but PDGFRα was less expressed. Instead, in severe and widespread muscle lesions, the endomysial CD10+ interstitial cells seem to lose CD34 expression and to increase PDGFRα positivity. 177 Furthermore, it was noted that CD10 is downregulated in the endomysium of atrophic myofibers, while being abundantly expressed around the neighboring hypertrophic myofibers, which further suggests that these CD10+ interstitial cells/TCs are endowed with great plasticity and may be implicated in skeletal muscle tissue repair/regeneration processes. 177
Future Directions
To conclude, here we have mainly summarized the emerging evidence for TCs as novel components of the SC niches in the gastrointestinal wall and skeletal muscle, thus specifically focusing on two tissues with high regenerative capacity and for which a considerable amount of morphofunctional findings has been collected over the last few years. Nevertheless, as already mentioned, to date, the presence of TCs has been described in several SC niches of different tissues/organs, including the epicardium, heart valves, skin, respiratory tree, urinary system, limbus, uvea, meninges, and choroid plexus. Regardless of the different anatomic locations, TCs are currently in the spotlight because they are thought to contribute to both tissue morphogenesis during development and tissue renewal/repair in postnatal life at least in two ways, namely, acting as SC modulators supporting proliferation and maturation of neighboring SCs, or having themselves mesenchymal progenitor properties. Hence, it is being increasingly reported that abnormalities in TCs may impair tissue homeostasis and regenerative abilities in different conditions, suggesting that in future, they may provide a cell source for regenerative medicine. No less, it is believed that TCs may represent one of the main precursors of tumor-associated fibroblasts in different type of cancers. For these reasons, an in-depth understanding of the molecular mechanisms underlying the TC-SC crosstalk in SC niches of multiple tissues/organs, as well as of the presumed aptitude of TCs to function as mesenchymal progenitors, is a pressing research need in the regenerative medicine arena.
Footnotes
Author Contributions: MManetti planned the review structure. IR and MMarini drafted the manuscript. MMarini and MManetti finalized the manuscript. IR and MManetti prepared the figures. All authors have read and approved the final manuscript.
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by MIUR (Ministry of Education, University and Research, Italy)-University of Florence research funds granted to M.Manetti.
ORCID iD: Mirko Manetti  https://orcid.org/0000-0003-3956-8480
https://orcid.org/0000-0003-3956-8480
Contributor Information
Irene Rosa, Section of Anatomy and Histology, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy.
Mirca Marini, Section of Anatomy and Histology, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy.
Mirko Manetti, Section of Anatomy and Histology, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy.
Literature Cited
- 1. Cretoiu D, Vannucchi MG, Bei Y, Manetti M, Faussone-Pellegrini MS, Ibba-Manneschi L, Xiao J, Cretoiu SM. Telocytes: new connecting devices in the stromal space of organs. In: Loewy Z, editor. Innovations in cell research and therapy. London: IntechOpen; 2020. p. 69–94. doi: 10.5772/intechopen.89383. [DOI] [Google Scholar]
- 2. Popescu LM, Faussone-Pellegrini MS. TELOCYTES: a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-like Cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010;14(4):729–40. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Faussone Pellegrini MS, Popescu LM. Telocytes. Biomol Concepts. 2011;2(6):481–9. doi: 10.1515/BMC.2011.039. [DOI] [PubMed] [Google Scholar]
- 4. Cretoiu SM, Popescu LM. Telocytes revisited. Biomol Concepts. 2014;5(5):353–69. doi: 10.1515/bmc-2014-0029. [DOI] [PubMed] [Google Scholar]
- 5. Cantarero I, Luesma MJ, Alvarez-Dotu JM, Muñoz E, Junquera C. Transmission electron microscopy as key technique for the characterization of telocytes. Curr Stem Cell Res Ther. 2016;11(5):410–4. doi: 10.2174/1574888x10666150306155435. [DOI] [PubMed] [Google Scholar]
- 6. Cretoiu D. The third dimension of telocytes revealed by FIB-SEM tomography. Adv Exp Med Biol. 2016;913:325–34. doi: 10.1007/978-981-10-1061-3_21. [DOI] [PubMed] [Google Scholar]
- 7. Faussone-Pellegrini MS, Gherghiceanu M. Telocyte’s contacts. Semin Cell Dev Biol. 2016;55:3–8. doi: 10.1016/j.semcdb.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 8. Díaz-Flores L, Gutiérrez R, Díaz-Flores L, Jr, Goméz MG, Sáez FJ, Madrid JF. Behaviour of telocytes during physiopathological activation. Semin Cell Dev Biol. 2016;55:50–61. doi: 10.1016/j.semcdb.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 9. Cretoiu D, Xu J, Xiao J, Cretoiu SM. Telocytes and their extracellular vesicles: evidence and hypotheses. Int J Mol Sci. 2016;17(8):1322. doi: 10.3390/ijms17081322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marini M, Ibba-Manneschi L, Manetti M. Cardiac telocyte-derived exosomes and their possible implications in cardiovascular pathophysiology. Adv Exp Med Biol. 2017;998:237–54. doi: 10.1007/978-981-10-4397-0_16. [DOI] [PubMed] [Google Scholar]
- 11. Díaz-Flores L, Gutiérrez R, García MP, Sáez FJ, Díaz-Flores L, Jr, Valladares F, Madrid JF. CD34+ stromal cells/fibroblasts/fibrocytes/telocytes as a tissue reserve and a principal source of mesenchymal cells. Location, morphology, function and role in pathology. Histol Histopathol. 2014;29(7):831–70. doi: 10.14670/HH-29.831. [DOI] [PubMed] [Google Scholar]
- 12. Rosa I, Marini M, Guasti D, Ibba-Manneschi L, Manetti M. Morphological evidence of telocytes in human synovium. Sci Rep. 2018;8(1):3581. doi: 10.1038/s41598-018-22067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marini M, Rosa I, Guasti D, Gacci M, Sgambati E, Ibba-Manneschi L, Manetti M. Reappraising the microscopic anatomy of human testis: identification of telocyte networks in the peritubular and intertubular stromal space. Sci Rep. 2018;8(1):14780. doi: 10.1038/s41598-018-33126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vannucchi MG, Faussone-Pellegrini MS. The telocyte subtypes. Adv Exp Med Biol. 2016;913:115–26. doi: 10.1007/978-981-10-1061-3_7. [DOI] [PubMed] [Google Scholar]
- 15. Díaz-Flores L, Gutiérrez R, García MP, González-Gómez M, Carrasco JL, Alvarez-Argüelles H, Díaz-Flores L., Jr. Telocytes/CD34+ stromal cells in pathologically affected white adipose tissue. Int J Mol Sci. 2020;21(24):9694. doi: 10.3390/ijms21249694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Romano E, Rosa I, Fioretto BS, Lucattelli E, Innocenti M, Ibba-Manneschi L, Matucci-Cerinic M, Manetti M. A two-step immunomagnetic microbead-based method for the isolation of human primary skin telocytes/CD34+ stromal cells. Int J Mol Sci. 2020;21(16):5877. doi: 10.3390/ijms21165877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marini M, Rosa I, Ibba-Manneschi L, Manetti M. Telocytes in skeletal, cardiac and smooth muscle interstitium: morphological and functional aspects. Histol Histopathol. 2018;33(11):1151–65. doi: 10.14670/HH-11-994. [DOI] [PubMed] [Google Scholar]
- 18. Marini M, Mencucci R, Rosa I, Favuzza E, Guasti D, Ibba-Manneschi L, Manetti M. Telocytes in normal and keratoconic human cornea: an immunohistochemical and transmission electron microscopy study. J Cell Mol Med. 2017;21(12):3602–11. doi: 10.1111/jcmm.13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shoshkes-Carmel M, Wang YJ, Wangensteen KJ, Tóth B, Kondo A, Massasa EE, Itzkovitz S, Kaestner KH. Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature. 2018;557(7704):242–6. doi: 10.1038/s41586-018-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosa I, Taverna C, Novelli L, Marini M, Ibba-Manneschi L, Manetti M. Telocytes constitute a widespread interstitial meshwork in the lamina propria and underlying striated muscle of human tongue. Sci Rep. 2019;9(1):5858. doi: 10.1038/s41598-019-42415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Song D, Cretoiu D, Zheng M, Qian M, Zhang M, Cretoiu SM, Chen L, Fang H, Popescu LM, Wang X. Comparison of Chromosome 4 gene expression profile between lung telocytes and other local cell types. J Cell Mol Med. 2016;20(1):71–80. doi: 10.1111/jcmm.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zheng Y, Zhang M, Qian M, Wang L, Cismasiu VB, Bai C, Popescu LM, Wang X. Genetic comparison of mouse lung telocytes with mesenchymal stem cells and fibroblasts. J Cell Mol Med. 2013;17(4):567–77. doi: 10.1111/jcmm.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cretoiu D, Radu BM, Banciu A, Banciu DD, Cretoiu SM. Telocytes heterogeneity: from cellular morphology to functional evidence. Semin Cell Dev Biol. 2017;64:26–39. doi: 10.1016/j.semcdb.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 24. Kondo A, Kaestner KH. Emerging diverse roles of telocytes. Development. 2019;146(14):dev175018. doi: 10.1242/dev.175018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ibba-Manneschi L, Rosa I, Manetti M. Telocyte implications in human pathology: an overview. Semin Cell Dev Biol. 2016;55:62–9. doi: 10.1016/j.semcdb.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 26. Díaz-Flores L, Gutiérrez R, García MP, Gayoso S, Gutiérrez E, Díaz-Flores L, Jr, Carrasco JL. Telocytes in the normal and pathological peripheral nervous system. Int J Mol Sci. 2020;21(12):4320. doi: 10.3390/ijms21124320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vannucchi MG, Traini C. The telocytes/myofibroblasts 3-D network forms a stretch receptor in the human bladder mucosa. Is this structure involved in the detrusor overactive diseases? Ann Anat. 2018;218:118–23. doi: 10.1016/j.aanat.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 28. El Maadawi ZM. A tale of two cells: telocyte and stem cell unique relationship. Adv Exp Med Biol. 2016;913:359–76. doi: 10.1007/978-981-10-1061-3_23. [DOI] [PubMed] [Google Scholar]
- 29. Bei Y, Wang F, Yang C, Xiao J. Telocytes in regenerative medicine. J Cell Mol Med. 2015;19(7):1441–54. doi: 10.1111/jcmm.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gherghiceanu M, Popescu LM. Cardiomyocyte precursors and telocytes in epicardial stem cell niche: electron microscope images. J Cell Mol Med. 2010;14(4):871–7. doi: 10.1111/j.1582-4934.2010.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bojin FM, Gavriliuc OI, Cristea MI, Tanasie G, Tatu CS, Panaitescu C, Paunescu V. Telocytes within human skeletal muscle stem cell niche. J Cell Mol Med. 2011;15(10):2269–72. doi: 10.1111/j.1582-4934.2011.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Popescu LM, Gherghiceanu M, Suciu LC, Manole CG, Hinescu ME. Telocytes and putative stem cells in the lungs: electron microscopy, electron tomography and laser scanning microscopy. Cell Tissue Res. 2011;345(3):391–403. doi: 10.1007/s00441-011-1229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luesma MJ, Gherghiceanu M, Popescu LM. Telocytes and stem cells in limbus and uvea of mouse eye. J Cell Mol Med. 2013;17(8):1016–24. doi: 10.1111/jcmm.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fuchs E, Blau HM. Tissue stem cells: architects of their niches. Cell Stem Cell. 2020;27(4):532–56. doi: 10.1016/j.stem.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wagers AJ. The stem cell niche in regenerative medicine. Cell Stem Cell. 2012;10(4):362–9. doi: 10.1016/j.stem.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 36. Cismaşiu VB, Popescu LM. Telocytes transfer extracellular vesicles loaded with microRNAs to stem cells. J Cell Mol Med. 2015;19(2):351–8. doi: 10.1111/jcmm.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Albulescu R, Tanase C, Codrici E, Popescu DI, Cretoiu SM, Popescu LM. The secretome of myocardial telocytes modulates the activity of cardiac stem cells. J Cell Mol Med. 2015;19(8):1783–94. doi: 10.1111/jcmm.12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nawaz M, Fatima F, Vallabhaneni KC, Penfornis P, Valadi H, Ekström K, Kholia S, Whitt JD, Fernandes JD, Pochampally R, Squire JA, Camussi G. Extracellular vesicles: evolving factors in stem cell biology. Stem Cells Int. 2016;2016:1073140. doi: 10.1155/2016/1073140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Galiger C, Kostin S, Golec A, Ahlbrecht K, Becker S, Gherghiceanu M, Popescu LM, Morty RE, Seeger W, Voswinckel R. Phenotypical and ultrastructural features of Oct4-positive cells in the adult mouse lung. J Cell Mol Med. 2014;18(7):1321–33. doi: 10.1111/jcmm.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simons BD, Clevers H. Stem cell self-renewal in intestinal crypt. Exp Cell Res. 2011;317(19):2719–24. doi: 10.1016/j.yexcr.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 41. Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143(1):134–44. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 42. van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–60. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 43. Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 44. van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137(1):15–7. doi: 10.1053/j.gastro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 45. Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, Breault DT. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci USA. 2011;108(1):179–84. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40(7):915–20. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334(6061):1420–4. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR, Kuo CJ. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci USA. 2012;109(2):466–71. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yousefi M, Li L, Lengner CJ. Hierarchy and plasticity in the intestinal stem cell compartment. Trends Cell Biol. 2017;27(10):753–64. doi: 10.1016/j.tcb.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478(7368):255–9. doi: 10.1038/nature1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495(7439):65–9. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- 52. van Es JH, Sato T, van de Wetering M, Lyubimova A, Yee Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, Martens ACM, Barker N, van Oudenaarden A, Clevers H. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol. 2012;14(10):1099–104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tomic G, Morrissey E, Kozar S, Ben-Moshe S, Hoyle A, Azzarelli R, Kemp R, Chilamakuri CSR, Itzkovitz S, Philpott A, Winton DJ. Phospho-regulation of ATOH1 is required for plasticity of secretory progenitors and tissue regeneration. Cell Stem Cell. 2018;23(3):436–43.e7. doi: 10.1016/j.stem.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jadhav U, Saxena M, O’Neill NK, Saadatpour A, Yuan GC, Herbert Z, Murata K, Shivdasani RA. Dynamic reorganization of chromatin accessibility signatures during dedifferentiation of secretory precursors into Lgr5+ intestinal stem cells. Cell Stem Cell. 2017;21(1):65–77.e5. doi: 10.1016/j.stem.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jones JC, Dempsey PJ. Enterocyte progenitors can dedifferentiate to replace lost Lgr5+ intestinal stem cells revealing that many different progenitor populations can regain stemness. Stem Cell Investig. 2016;3:61. doi: 10.21037/sci.2016.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tetteh PW, Basak O, Farin HF, Wiebrands K, Kretzschmar K, Begthel H, van den Born M, Korving J, de Sauvage F, van Es JH, van Oudenaarden A, Clevers H. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell. 2016;18(2):203–13. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 57. Schmitt M, Schewe M, Sacchetti A, Feijtel D, van de Geer WS, Teeuwssen M, Sleddens HF, Joosten R, van Royen ME, van de Werken HJG, van Es J, Clevers H, Fodde R. Paneth cells respond to inflammation and contribute to tissue regeneration by acquiring stem-like features through SCF/c-Kit signaling. Cell Rep. 2018;24(9):2312–28.e7. doi: 10.1016/j.celrep.2018.07.085. [DOI] [PubMed] [Google Scholar]
- 58. Yu S, Tong K, Zhao Y, Balasubramanian I, Yap GS, Ferraris RP, Bonder EM, Verzi MP, Gao N. Paneth cell multipotency induced by Notch activation following injury. Cell Stem Cell. 2018;23(1):46–59.e5. doi: 10.1016/j.stem.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yan KS, Gevaert O, Zheng GXY, Anchang B, Probert CS, Larkin KA, Davies PS, Cheng ZF, Kaddis JS, Han A, Roelf K, Calderon RI, Cynn E, Hu X, Mandleywala K, Wilhelmy J, Grimes SM, Corney DC, Boutet SC, Terry JM, Belgrader P, Ziraldo SB, Mikkelsen TS, Wang F, von Furstenberg RJ, Smith NR, Chandrakesan P, May R, Chrissy MAS, Jain R, Cartwright CA, Niland JC, Hong YK, Carrington J, Breault DT, Epstein J, Houchen CW, Lynch JP, Martin MG, Plevritis SK, Curtis C, Ji HP, Li L, Henning SJ, Wong MH, Kuo CJ. Intestinal enteroendocrine lineage cells possess homeostatic and injury-inducible stem cell activity. Cell Stem Cell. 2017;21(1):78–90.e6. doi: 10.1016/j.stem.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ayyaz A, Kumar S, Sangiorgi B, Ghoshal B, Gosio J, Ouladan S, Fink M, Barutcu S, Trcka D, Shen J, Chan K, Wrana JL, Gregorieff A. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature. 2019;569(7754):121–5. doi: 10.1038/s41586-019-1154-y. [DOI] [PubMed] [Google Scholar]
- 61. Moor AE, Harnik Y, Ben-Moshe S, Massasa EE, Rozenberg M, Eilam R, Bahar Halpern K, Itzkovitz S. Spatial reconstruction of single enterocytes uncovers broad zonation along the intestinal villus axis. Cell. 2018;175(4):1156–67.e15. doi: 10.1016/j.cell.2018.08.063. [DOI] [PubMed] [Google Scholar]
- 62. Beumer J, Artegiani B, Post Y, Reimann F, Gribble F, Nguyen TN, Zeng H, Van den Born M, Van Es JH, Clevers H. Enteroendocrine cells switch hormone expression along the crypt-to-villus BMP signalling gradient. Nat Cell Biol. 2018;20(8):909–16. doi: 10.1038/s41556-018-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gehart H, van Es JH, Hamer K, Beumer J, Kretzschmar K, Dekkers JF, Rios A, Clevers H. Identification of enteroendocrine regulators by real-time single-cell differentiation mapping. Cell. 2019;176(5):1158–73.e16. doi: 10.1016/j.cell.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 64. Kaestner KH. The intestinal stem cell niche: a central role for Foxl1-expressing subepithelial telocytes. Cell Mol Gastroenterol Hepatol. 2019;8(1):111–7. doi: 10.1016/j.jcmgh.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhu G, Hu J, Xi R. The cellular niche for intestinal stem cells: a team effort. Cell Regen. 2021;10(1):1. doi: 10.1186/s13619-020-00061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–5. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 67. Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 68. de Lau W, Peng WC, Gros P, Clevers H. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev. 2014;28(4):305–16. doi: 10.1101/gad.235473.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol. 2007;27(21):7551–9. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. van Es JH, Haegebarth A, Kujala P, Itzkovitz S, Koo BK, Boj SF, Korving J, van den Born M, van Oudenaarden A, Robine S, Clevers H. A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Mol Cell Biol. 2012;32(10):1918–27. doi: 10.1128/MCB.06288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ireland H, Kemp R, Houghton C, Howard L, Clarke AR, Sansom OJ, Winton DJ. Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: effect of loss of beta-catenin. Gastroenterology. 2004;126(5):1236–46. doi: 10.1053/j.gastro.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 72. Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA. 2004;101(1):266–71. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17(14):1709–13. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, Funk WD, Tomizuka K. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309(5738):1256–9. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- 75. Valenta T, Degirmenci B, Moor AE, Herr P, Zimmerli D, Moor MB, Hausmann G, Cantù C, Aguet M, Basler K. Wnt ligands secreted by subepithelial mesenchymal cells are essential for the survival of intestinal stem cells and gut homeostasis. Cell Rep. 2016;15(5):911–8. doi: 10.1016/j.celrep.2016.03.088. [DOI] [PubMed] [Google Scholar]
- 76. Kabiri Z, Greicius G, Zaribafzadeh H, Hemmerich A, Counter CM, Virshup DM. Wnt signaling suppresses MAPK-driven proliferation of intestinal stem cells. J Clin Invest. 2018;128(9):3806–12. doi: 10.1172/JCI99325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Carulli AJ, Keeley TM, Demitrack ES, Chung J, Maillard I, Samuelson LC. Notch receptor regulation of intestinal stem cell homeostasis and crypt regeneration. Dev Biol. 2015;402(1):98–108. doi: 10.1016/j.ydbio.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435(7044):964–8. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 79. Basak O, Beumer J, Wiebrands K, Seno H, van Oudenaarden A, Clevers H. Induced quiescence of Lgr5+ stem cells in intestinal organoids enables differentiation of hormone-producing enteroendocrine cells. Cell Stem Cell. 2017;20(2):177–90.e4. doi: 10.1016/j.stem.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 80. Chen L, Toke NH, Luo S, Vasoya RP, Fullem RL, Parthasarathy A, Perekatt AO, Verzi MP. A reinforcing HNF4-SMAD4 feed-forward module stabilizes enterocyte identity. Nat Genet. 2019;51(5):777–85. doi: 10.1038/s41588-019-0384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36(10):1117–21. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 82. Kosinski C, Li VS, Chan AS, Zhang J, Ho C, Tsui WY, Chan TL, Mifflin RC, Powell DW, Yuen ST, Leung SY, Chen X. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci USA. 2007;104(39):15418–23. doi: 10.1073/pnas.0707210104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Qi Z, Li Y, Zhao B, Xu C, Liu Y, Li H, Zhang B, Wang X, Yang X, Xie W, Li B, Han JDJ, Chen YG. BMP restricts stemness of intestinal Lgr5(+) stem cells by directly suppressing their signature genes. Nat Commun. 2017;8:13824. doi: 10.1038/ncomms13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Haramis AP, Begthel H, van den Born M, van Es J, Jonkheer S, Offerhaus GJ, Clevers H. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303(5664):1684–6. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 85. Samuelson LC. Debate over the identity of an intestinal niche-cell population settled. Nature. 2018;558(7710):380–1. doi: 10.1038/d41586-018-05281-z. [DOI] [PubMed] [Google Scholar]
- 86. Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469(7330):415–8. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Durand A, Donahue B, Peignon G, Letourneur F, Cagnard N, Slomianny C, Perret C, Shroyer NF, Romagnolo B. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1). Proc Natl Acad Sci USA. 2012;109(23):8965–70. doi: 10.1073/pnas.1201652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kim TH, Escudero S, Shivdasani RA. Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc Natl Acad Sci USA. 2012;109(10):3932–7. doi: 10.1073/pnas.1113890109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Farin HF, Van Es JH, Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143(6):1518–29.e7. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 90. Kabiri Z, Greicius G, Madan B, Biechele S, Zhong Z, Zaribafzadeh H, Edison Aliyev J, Wu Y, Bunte R, Williams BO, Rossant J, Virshup DM. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development. 2014;141(11):2206–15. doi: 10.1242/dev.104976. [DOI] [PubMed] [Google Scholar]
- 91. San Roman AK, Jayewickreme CD, Murtaugh LC, Shivdasani RA. Wnt secretion from epithelial cells and subepithelial myofibroblasts is not required in the mouse intestinal stem cell niche in vivo. Stem Cell Rep. 2014;2(2):127–34. doi: 10.1016/j.stemcr.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Aoki R, Shoshkes-Carmel M, Gao N, Shin S, May CL, Golson ML, Zahm AM, Ray M, Wiser CL, Wright CV, Kaestner KH. Foxl1-expressing mesenchymal cells constitute the intestinal stem cell niche. Cell Mol Gastroenterol Hepatol. 2016;2(2):175–88. doi: 10.1016/j.jcmgh.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kinchen J, Chen HH, Parikh K, Antanaviciute A, Jagielowicz M, Fawkner-Corbett D, Ashley N, Cubitt L, Mellado-Gomez E, Attar M, Sharma E, Wills Q, Bowden R, Richter FC, Ahern D, Puri KD, Henault J, Gervais F, Koohy H, Simmons A. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell. 2018;175(2):372–86.e17. doi: 10.1016/j.cell.2018.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hasebe T, Fujimoto K, Ishizuya-Oka A. Thyroid hormone-induced expression of Foxl1 in subepithelial fibroblasts correlates with adult stem cell development during Xenopus intestinal remodeling. Sci Rep. 2020;10(1):20715. doi: 10.1038/s41598-020-77817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Stzepourginski I, Nigro G, Jacob JM, Dulauroy S, Sansonetti PJ, Eberl G, Peduto L. CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc Natl Acad Sci USA. 2017;114(4):E506–13. doi: 10.1073/pnas.1620059114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Greicius G, Kabiri Z, Sigmundsson K, Liang C, Bunte R, Singh MK, Virshup DM. PDGFRα+ pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo. Proc Natl Acad Sci USA. 2018;115(14):E3173–81. doi: 10.1073/pnas.1713510115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Degirmenci B, Valenta T, Dimitrieva S, Hausmann G, Basler K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature. 2018;558(7710):449–53. doi: 10.1038/s41586-018-0190-3. [DOI] [PubMed] [Google Scholar]
- 98. Vannucchi MG, Traini C, Manetti M, Ibba-Manneschi L, Faussone-Pellegrini MS. Telocytes express PDGFRalpha in the human gastrointestinal tract. J Cell Mol Med. 2013;17(9):1099–108. doi: 10.1111/jcmm.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bahar Halpern K, Massalha H, Zwick RK, Moor AE, Castillo-Azofeifa D, Rozenberg M, Farack L, Egozi A, Miller DR, Averbukh I, Harnik Y, Weinberg-Corem N, de Sauvage FJ, Amit I, Klein OD, Shoshkes-Carmel M, Itzkovitz S. Lgr5+ telocytes are a signaling source at the intestinal villus tip. Nat Commun. 2020;11(1):1936. doi: 10.1038/s41467-020-15714-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kim JE, Fei L, Yin WC, Coquenlorge S, Rao-Bhatia A, Zhang X, Shi SSW, Lee JH, Hahn NA, Rizvi W, Kim KH, Sung HK, Hui CC, Guo G, Kim TH. Single cell and genetic analyses reveal conserved populations and signaling mechanisms of gastrointestinal stromal niches. Nat Commun. 2020;11(1):334. doi: 10.1038/s41467-019-14058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Vannucchi MG. The telocytes: ten years after their introduction in the scientific literature. An update on their morphology, distribution, and potential roles in the gut. Int J Mol Sci. 2020;21(12):4478. doi: 10.3390/ijms21124478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Vannucchi MG, Traini C. Interstitial cells of Cajal and telocytes in the gut: twins, related or simply neighbor cells? Biomol Concepts. 2016;7(2):93–102. doi: 10.1515/bmc-2015-0034. [DOI] [PubMed] [Google Scholar]
- 103. Veress B, Ohlsson B. Spatial relationship between telocytes, interstitial cells of Cajal and the enteric nervous system in the human ileum and colon. J Cell Mol Med. 2020;24(6):3399–406. doi: 10.1111/jcmm.15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ward SM, Sanders KM, Hirst GD. Role of interstitial cells of Cajal in neural control of gastrointestinal smooth muscles. Neurogastroenterol Motil. 2004;16(Suppl 1):112–7. doi: 10.1111/j.1743-3150.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- 105. Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–43. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- 106. Chaudhury A. Furthering the debate on the role of interstitial cells of Cajal in enteric inhibitory neuromuscular neurotransmission. Am J Physiol Cell Physiol. 2016;311(3):C479–81. doi: 10.1152/ajpcell.00067.2016. [DOI] [PubMed] [Google Scholar]
- 107. Kurahashi M, Nakano Y, Hennig GW, Ward SM, Sanders KM. Platelet-derived growth factor receptor alpha-positive cells in the tunica muscularis of human colon. J Cell Mol Med. 2012;16(7):1397–404. doi: 10.1111/j.1582-4934.2011.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kurahashi M, Nakano Y, Peri LE, Townsend JB, Ward SM, Sanders KM. A novel population of subepithelial platelet-derived growth factor receptor α-positive cells in the mouse and human colon. Am J Physiol Gastrointest Liver Physiol. 2013;304(9):G823–34. doi: 10.1152/ajpgi.00001.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev. 2014;94(3):859–907. doi: 10.1152/physrev.00037.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Blair PJ, Rhee PL, Sanders KM, Ward SM. The significance of interstitial cells in neurogastroenterology. J Neurogastroenterol Motil. 2014;20(3):294–317. doi: 10.5056/jnm14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hoch RV, Soriano P. Roles of PDGF in animal development. Development. 2003;130(20):4769–84. doi: 10.1242/dev.00721. [DOI] [PubMed] [Google Scholar]
- 112. Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22(10):1276–312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Vannucchi MG, Bani D, Faussone-Pellegrini MS. Telocytes contribute as cell progenitors and differentiation inductors in tissue regeneration. Curr Stem Cell Res Ther. 2016;11(5):383–9. doi: 10.2174/1574888x10666150528142741. [DOI] [PubMed] [Google Scholar]
- 114. Pieri L, Vannucchi MG, Faussone-Pellegrini MS. Histochemical and ultrastructural characteristics of an interstitial cell type different from ICC and resident in the muscle coat of human gut. J Cell Mol Med. 2008;12(5B):1944–55. doi: 10.1111/j.1582-4934.2008.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. De Schepper S, Verheijden S, Aguilera-Lizarraga J, Viola MF, Boesmans W, Stakenborg N, Voytyuk I, Schmidt I, Boeckx B, Dierckx de Casterlé I, Baekelandt V, Gonzalez Dominguez E, Mack M, Depoortere I, De Strooper B, Sprangers B, Himmelreich U, Soenen S, Guilliams M, Vanden Berghe P, Jones E, Lambrechts D, Boeckxstaens G. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell. 2018;175(2):400–15.e13. doi: 10.1016/j.cell.2018.07.048. [DOI] [PubMed] [Google Scholar]
- 116. Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell. 2016;164(3):378–91. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Muller PA, Koscsó B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, Stanley ER, Dahan S, Margolis KG, Gershon MD, Merad M, Bogunovic M. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158(2):300–13. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ji S, Traini C, Mischopoulou M, Gibbons SJ, Ligresti G, Faussone-Pellegrini MS, Sha L, Farrugia G, Vannucchi MG, Cipriani G. Muscularis macrophages establish cell-to-cell contacts with telocytes/PDGFRα-positive cells and smooth muscle cells in the human and mouse gastrointestinal tract. Neurogastroenterol Motil. 2021;33(3):e13993. doi: 10.1111/nmo.13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Traserra S, Villarte S, Traini C, Palacin S, Vergara P, Vannucchi MG, Jimenez M. The asymmetric innervation of the circular and longitudinal muscle of the mouse colon differently modulates myogenic slow phasic contractions. Neurogastroenterol Motil. 2020;32(4):e13778. doi: 10.1111/nmo.13778. [DOI] [PubMed] [Google Scholar]
- 120. Sanders KM, Kito Y, Hwang SJ, Ward SM. Regulation of gastrointestinal smooth muscle function by interstitial cells. Physiology. 2016;31(5):316–26. doi: 10.1152/physiol.00006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yeoh JW, Corrias A, Buist ML. A mechanistic model of a PDGFRα(+) cell. J Theor Biol. 2016;408:127–36. doi: 10.1016/j.jtbi.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 122. Lu C, Huang X, Lu HL, Liu SH, Zang JY, Li YJ, Chen J, Xu WX. Different distributions of interstitial cells of Cajal and platelet-derived growth factor receptor-α positive cells in colonic smooth muscle cell/interstitial cell of Cajal/platelet-derived growth factor receptor-α positive cell syncytium in mice. World J Gastroenterol. 2018;24(44):4989–5004. doi: 10.3748/wjg.v24.i44.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Grover M, Bernard CE, Pasricha PJ, Parkman HP, Abell TL, Nguyen LA, Snape W, Shen KR, Sarr M, Swain J, Kendrick M, Gibbons S, Ordog T, Farrugia G. Platelet-derived growth factor receptor α (PDGFRα)-expressing “fibroblast-like cells” in diabetic and idiopathic gastroparesis of humans. Neurogastroenterol Motil. 2012;24(9):844–52. doi: 10.1111/j.1365-2982.2012.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Coyle D, O’Donnell AM, Puri P. Altered distribution of small-conductance calcium-activated potassium channel SK3 in Hirschsprung’s disease. J Pediatr Surg. 2015;50(10):1659–64. doi: 10.1016/j.jpedsurg.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 125. Song NN, Lu HL, Lu C, Tong L, Huang SQ, Huang X, Chen J, Kim YC, Xu WX. Diabetes-induced colonic slow transit mediated by the up-regulation of PDGFRα+ cells/SK3 in streptozotocin-induced diabetic mice. Neurogastroenterol Motil. 2018;30:e13326. doi: 10.1111/nmo.13326. [DOI] [PubMed] [Google Scholar]
- 126. Milia AF, Ruffo M, Manetti M, Rosa I, Conte D, Fazi M, Messerini L, Ibba-Manneschi L. Telocytes in Crohn’s disease. J Cell Mol Med. 2013;17(12):1525–36. doi: 10.1111/jcmm.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Manetti M, Rosa I, Messerini L, Ibba-Manneschi L. Telocytes are reduced during fibrotic remodelling of the colonic wall in ulcerative colitis. J Cell Mol Med. 2015;19(1):62–73. doi: 10.1111/jcmm.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ricci R, Giustiniani MC, Gessi M, Lanza P, Castri F, Biondi A, Persiani R, Vecchio FM, Risio M. Telocytes are the physiological counterpart of inflammatory fibroid polyps and PDGFRA-mutant GISTs. J Cell Mol Med. 2018;22(10):4856–62. doi: 10.1111/jcmm.13748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Langlois MJ, Servant R, Reyes Nicolás V, Jones C, Roy SAB, Paquet M, Carrier JC, Rivard N, Boudreau F, Perreault N. Loss of PTEN signaling in Foxl1+ mesenchymal telocytes initiates spontaneous colonic neoplasia in mice. Cell Mol Gastroenterol Hepatol. 2019;8(3):530–3.e5. doi: 10.1016/j.jcmgh.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Usai-Satta P, Bellini M, Morelli O, Geri F, Lai M, Bassotti G. Gastroparesis: new insights into an old disease. World J Gastroenterol. 2020;26(19):2333–48. doi: 10.3748/wjg.v26.i19.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Camilleri M, Chedid V, Ford AC, Haruma K, Horowitz M, Jones KL, Low PA, Park SY, Parkman HP, Stanghellini V. Gastroparesis. Nat Rev Dis Primers. 2018;4(1):41. doi: 10.1038/s41572-018-0038-z. [DOI] [PubMed] [Google Scholar]
- 132. Díaz-Flores L, Gutiérrez R, García MP, González M, Sáez FJ, Aparicio F, Díaz-Flores L, Jr, Madrid JF. Human resident CD34+ stromal cells/telocytes have progenitor capacity and are a source of αSMA+ cells during repair. Histol Histopathol. 2015;30(5):615–27. doi: 10.14670/HH-30.615. [DOI] [PubMed] [Google Scholar]
- 133. Sambasivan R, Tajbakhsh S. Adult skeletal muscle stem cells. Results Probl Cell Differ. 2015;56:191–213. doi: 10.1007/978-3-662-44608-9_9. [DOI] [PubMed] [Google Scholar]
- 134. Matsakas A, Patel K. Skeletal muscle fibre plasticity in response to selected environmental and physiological stimuli. Histol Histopathol. 2009;24(5):611–29. doi: 10.14670/HH-24.611. [DOI] [PubMed] [Google Scholar]
- 135. Chang NC, Rudnicki MA. Satellite cells: the architects of skeletal muscle. Curr Top Dev Biol. 2014;107:161–81. doi: 10.1016/B978-0-12-416022-4.00006-8. [DOI] [PubMed] [Google Scholar]
- 136. Shi X, Garry DJ. Muscle stem cells in development, regeneration, and disease. Genes Dev. 2006;20(13):1692–708. doi: 10.1101/gad.1419406. [DOI] [PubMed] [Google Scholar]
- 137. Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132(4):598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Almeida CF, Fernandes SA, Ribeiro Junior AF, Keith Okamoto O, Vainzof M. Muscle satellite cells: exploring the basic biology to rule them. Stem Cells Int. 2016;2016:1078686. doi: 10.1155/2016/1078686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Manetti M, Tani A, Rosa I, Chellini F, Squecco R, Idrizaj E, Zecchi-Orlandini S, Ibba-Manneschi L, Sassoli C. Morphological evidence for telocytes as stromal cells supporting satellite cell activation in eccentric contraction-induced skeletal muscle injury. Sci Rep. 2019;9(1):14515. doi: 10.1038/s41598-019-51078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Murphy M, Kardon G. Origin of vertebrate limb muscle: the role of progenitor and myoblast populations. Curr Top Dev Biol. 2011;96:1–32. doi: 10.1016/B978-0-12-385940-2.00001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Harel I, Nathan E, Tirosh-Finkel L, Zigdon H, Guimarães-Camboa N, Evans SM, Tzahor E. Distinct origins and genetic programs of head muscle satellite cells. Dev Cell. 2009;16(6):822–32. doi: 10.1016/j.devcel.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. von Maltzahn J, Jones AE, Parks RJ, Rudnicki MA. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc Natl Acad Sci USA. 2013;110(41):16474–9. doi: 10.1073/pnas.1307680110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Dumont NA, Wang YX, Rudnicki MA. Intrinsic and extrinsic mechanisms regulating satellite cell function. Development. 2015;142(9):1572–81. doi: 10.1242/dev.114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Relaix F, Bencze M, Borok MJ, Der Vartanian A, Gattazzo F, Mademtzoglou D, Perez-Diaz S, Prola A, Reyes-Fernandez PC, Rotini A, Taglietti. Perspectives on skeletal muscle stem cells. Nat Commun. 2021;12(1):692. doi: 10.1038/s41467-020-20760-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Kang JS, Krauss RS. Muscle stem cells in developmental and regenerative myogenesis. Curr Opin Clin Nutr Metab Care. 2010;13(3):243–8. doi: 10.1097/MCO.0b013e328336ea98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2(1):22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 147. Günther S, Kim J, Kostin S, Lepper C, Fan CM, Braun T. Myf5-positive satellite cells contribute to Pax7-dependent long-term maintenance of adult muscle stem cells. Cell Stem Cell. 2013;13(5):590–601. doi: 10.1016/j.stem.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93(1):23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Le Grand F, Rudnicki MA. Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol. 2007;19(6):628–33. doi: 10.1016/j.ceb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Said RS, Mustafa AG, Asfour HA, Shaqoura EI. Myogenic satellite cells: biological milieu and possible clinical applications. Pak J Biol Sci. 2017;20(1):1–11. doi: 10.3923/pjbs.2017.1.11. [DOI] [PubMed] [Google Scholar]
- 151. Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151(6):1221–34. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Cornelison DD, Filla MS, Stanley HM, Rapraeger AC, Olwin BB. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol. 2001;239(1):79–94. doi: 10.1006/dbio.2001.0416. [DOI] [PubMed] [Google Scholar]
- 153. Ratajczak MZ, Majka M, Kucia M, Drukala J, Pietrzkowski Z, Peiper S, Janowska-Wieczorek A. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells. 2003;21(3):363–71. doi: 10.1634/stemcells.21-3-363. [DOI] [PubMed] [Google Scholar]
- 154. Tierney MT, Sacco A. Satellite cell heterogeneity in skeletal muscle homeostasis. Trends Cell Biol. 2016;26(6):434–44. doi: 10.1016/j.tcb.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Nguyen JH, Chung JD, Lynch GS, Ryall JG. The microenvironment is a critical regulator of muscle stem cell activation and proliferation. Front Cell Dev Biol. 2019;7:254. doi: 10.3389/fcell.2019.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Malatesta M, Costanzo M, Cisterna B, Zancanaro C. Satellite cells in skeletal muscle of the hibernating dormouse, a natural model of quiescence and re-activation: focus on the cell nucleus. Cells. 2020;9(4):1050. doi: 10.3390/cells9041050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Dinulovic I, Furrer R, Handschin C. Plasticity of the muscle stem cell microenvironment. Adv Exp Med Biol. 2017;1041:141–69. doi: 10.1007/978-3-319-69194-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Popescu LM, Manole E, Serboiu CS, Manole CG, Suciu LC, Gherghiceanu M, Popescu BO. Identification of telocytes in skeletal muscle interstitium: implication for muscle regeneration. J Cell Mol Med. 2011;15(6):1379–92. doi: 10.1111/j.1582-4934.2011.01330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Suciu LC, Popescu BO, Kostin S, Popescu LM. Platelet-derived growth factor receptor-β-positive telocytes in skeletal muscle interstitium. J Cell Mol Med. 2012;16(4):701–7. doi: 10.1111/j.1582-4934.2011.01505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ceafalan LC, Popescu BO, Hinescu ME. Cellular players in skeletal muscle regeneration. Biomed Res Int. 2014;2014:957014. doi: 10.1155/2014/957014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Sassoli C, Pini A, Chellini F, Mazzanti B, Nistri S, Nosi D, Saccardi R, Quercioli F, Zecchi-Orlandini S, Formigli L. Bone marrow mesenchymal stromal cells stimulate skeletal myoblast proliferation through the paracrine release of VEGF. PLoS ONE. 2012;7(7):e37512. doi: 10.1371/journal.pone.0037512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Squecco R, Tani A, Chellini F, Garella R, Idrizaj E, Rosa I, Zecchi-Orlandini S, Manetti M, Sassoli C. Bone marrow-mesenchymal stromal cell secretome as conditioned medium relieves experimental skeletal muscle damage induced by ex vivo eccentric contraction. Int J Mol Sci. 2021;22(7):3645. doi: 10.3390/ijms22073645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Montarras D, L’honoré A, Buckingham M. Lying low but ready for action: the quiescent muscle satellite cell. FEBS J. 2013;280(17):4036–50. doi: 10.1111/febs.12372. [DOI] [PubMed] [Google Scholar]
- 164. Ceafalan LC, Fertig TE, Popescu AC, Popescu BO, Hinescu ME, Gherghiceanu M. Skeletal muscle regeneration involves macrophage-myoblast bonding. Cell Adh Migr. 2018;12(3):228–35. doi: 10.1080/19336918.2017.1346774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Thomas K, Engler AJ, Meyer GA. Extracellular matrix regulation in the muscle satellite cell niche. Connect Tissue Res. 2015;56(1):1–8. doi: 10.3109/03008207.2014.947369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Sassoli C, Formigli L, Bini F, Tani A, Squecco R, Battistini C, Zecchi-Orlandini S, Francini F, Meacci E. Effects of S1P on skeletal muscle repair/regeneration during eccentric contraction. J Cell Mol Med. 2011;15(11):2498–511. doi: 10.1111/j.1582-4934.2010.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Marini M, Manetti M, Rosa I, Ibba-Manneschi L, Sgambati E. Telocytes in human fetal skeletal muscle interstitium during early myogenesis. Acta Histochem. 2018;120(5):397–404. doi: 10.1016/j.acthis.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 168. Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9(3):255–67. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 169. Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, Cossu G. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- 170. Sancricca C, Mirabella M, Gliubizzi C, Broccolini A, Gidaro T, Morosetti R. Vessel-associated stem cells from skeletal muscle: from biology to future uses in cell therapy. World J Stem Cells. 2010;2(3):39–49. doi: 10.4252/wjsc.v2.i3.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111(4):589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 172. Mitchell KJ, Pannérec A, Cadot B, Parlakian A, Besson V, Gomes ER, Marazzi G, Sassoon DA. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol. 2010;12(3):257–66. doi: 10.1038/ncb2025. [DOI] [PubMed] [Google Scholar]
- 173. Ambrosi TH, Longaker MT, Chan CKF. A revised perspective of skeletal stem cell biology. Front Cell Dev Biol. 2019;7:189. doi: 10.3389/fcell.2019.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Crisan M, Corselli M, Chen WC, Péault B. Perivascular cells for regenerative medicine. J Cell Mol Med. 2012;16(12):2851–60. doi: 10.1111/j.1582-4934.2012.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Uezumi A, Fukada S, Yamamoto N, Ikemoto-Uezumi M, Nakatani M, Morita M, Yamaguchi A, Yamada H, Nishino I, Hamada Y, Tsuchida K. Identification and characterization of PDGFRalpha+ mesenchymal progenitors in human skeletal muscle. Cell Death Dis. 2014;5(4):e1186. doi: 10.1038/cddis.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12(2):153–63. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Hejbøl EK, Hajjaj MA, Nielsen O, Schrøder HD. Marker expression of interstitial cells in human skeletal muscle: an immunohistochemical study. J Histochem Cytochem. 2019;67(11):825–44. doi: 10.1369/0022155419871033. [DOI] [PMC free article] [PubMed] [Google Scholar]