Abstract
The aim of this study was to evaluate the effect of feed additives (pumpkin seed cake and cloves) on the egg excretion of gastrointestinal nematodes (GIN) in sheep. Thirty ewes naturally infected with GIN were randomly selected from a flock and assigned to the following groups of 10 animals each: clove group (received 1.8 g ground cloves/ewe/day, for 7 days), pumpkin seed cake group (200 g pumpkin seed cake/ewe/day, for 7 days) and control group. Before starting the study, on day 0, and 5 days after the 7-day supplementation, on day 12, the body condition and FAMACHA scores were assessed and individual faecal egg counts (FEC) were performed. The mean body condition and the FAMACHA scores did not change significantly between day 0 and 12 with the exception of a significantly deteriorated FAMACHA score in the clove group. The percentage reduction of FEC was 40.7% on day 12 in the clove group and 52.9% in the pumpkin seed cake group. In the control group, FEC increased by 8.7%. A coproculture of faecal samples from four of the most infected animals on day 0 revealed Trichostrongylus spp. larvae L3 in all four selected ewes, Ostertagia spp. and Cooperia spp. in three and Haemonchus contortus in one ewe. These results are promising and encourage further studies aimed to evaluate the possibility that these plant supplements could be a complementary method for parasite control, thus reducing the need for chemotherapy.
Keywords: Sheep, Pumpkin seed cake, Clove, Faecal egg count, Helminths, Feed supplement
Abstract
Le but de cette étude était d’évaluer l’effet des additifs alimentaires (tourteaux de graines de citrouille et clous de girofle) sur la production d’œufs par les nématodes gastro-intestinaux (NGI) chez le mouton. Trente brebis naturellement infectées par des NGI ont été sélectionnées au hasard dans un troupeau et réparties dans les groupes suivants de 10 animaux chacun : groupe clou de girofle (1,8 g de clous de girofle moulus / brebis / jour, pendant 7 jours), groupe tourteau de graines de citrouille (200 g de tourteau de graines de citrouille / brebis / jour, pendant 7 jours) et groupe témoin. Avant de commencer l’étude, au jour 0, et cinq jours après la supplémentation de 7 jours, au jour 12, l’état corporel et les scores FAMACHA ont été évalués et des dénombrements d’œufs fécaux (DOF) individuels ont été effectués. L’état corporel moyen et les scores FAMACHA n’ont pas changé de manière significative entre le jour 0 et le jour 12 à l’exception d’un score FAMACHA significativement détérioré dans le groupe clou de girofle. Le pourcentage de réduction du DOF était de 40,7 % au jour 12 dans le groupe clou de girofle et de 52,9 % dans le groupe tourteau de graines de citrouille. Dans le groupe témoin, le DOF a augmenté de 8,7 %. Une coproculture d’échantillons fécaux de quatre des animaux les plus infectés au jour 0 a révélé des larves L3 de Trichostrongylus spp. chez les quatre brebis sélectionnées, Ostertagia spp. et Cooperia spp. dans trois et Haemonchus contortus dans une. Ces résultats sont prometteurs et encouragent de nouvelles études visant à évaluer la possibilité que ces suppléments végétaux puissent être une méthode complémentaire de contrôle des parasites, réduisant ainsi le besoin en chimiothérapie.
Introduction
Grazing animals, particularly small ruminants, are frequently infected by gastrointestinal nematodes (GIN), resulting in reduced productivity and increased mortality [38]. For many years, parasitic diseases in ruminants have been controlled by the use of broad-spectrum and commonly available anthelmintics. However, the continuous and inappropriate use of anthelmintic drugs has led to a loss of effectiveness. Anthelmintic resistance has been reported in many European countries [16, 36] and worldwide [38].
The increase in resistance of GIN of small ruminants to conventional anthelmintic drugs implies the need for alternative strategies to reduce the parasite burden in the animals and the number of infective larvae in the pasture [18]. Alternative strategies are necessary not only because of resistance to anthelmintic drugs, but also due to consumer demand for reduced use of drugs in meat animals and for lower chemical residues in food and the environment [7]. One of the options could be to feed animals with plants that are not only a source of nutrients but also contain bioactive substances (tannins, etc.) with potential anthelmintic effects [8, 17–21, 29]. When discussing whether a drug or a medicinal plant extract is effective against parasites in vitro, it must be borne in mind that in order to be medically useful, such a substance must be bioavailable and should not intoxicate the patient [47].
Pumpkins belong to the Cucurbitaceae family, which comprises 130 genera and about 1000 species [9]. Among them are several medically important genera, one of which is Cucurbita [13]. Cucurbita pepo comprises three subspecies, C. pepo L. subsp. fraterna, C. pepo L. subsp. ovifera and C. pepo L. subsp. pepo [9, 34]. Cucurbita pepo L. subsp. pepo var. oleifera is an oil pumpkin with thin-coated seeds [43] and is cultivated for its seeds and seed oil. Pumpkin seed cake used in our study is a by-product in oil production. Cucurbit plant, which is native to the American continent, has been used in folk medicine worldwide for the treatment of gastrointestinal diseases and intestinal parasites, as well as other clinical conditions [37]. Pumpkin seeds (Cucurbita pepo L.) contain saturated and unsaturated fatty acids such as palmitic, palmitoleic, stearic, oleic, linoleic and α-linolenic acid and are rich sources of protein. They contain secondary metabolites such as terpenoids, quinones, saponins, steroids, phenols, tannins, alkaloids (berberine), cucurbitine, and palmatine [14, 17]. These secondary metabolites might have anthelmintic action [17]. Ethanol pumpkin seed extract, and hot and cold water pumpkin seed extracts significantly influenced the survival of L1 and L2 Heligmosomoides bakeri larvae compared to a negative control in vitro. An in vivo study showed that ethanol C. pepo seed extract administered to mice effectively reduced both the faecal egg count and the number of adult stages of H. bakeri [17]. Pumpkin seed treatment resulted in a significant reduction in the initial number of faecal worm eggs in sheep and showed a potential for parasite control [41].
The clove (Syzygium aromaticum L.), a member of the Myrtaceae family, is an aromatic tree native to the Moluccas and southern Philippines, but currently grown in many tropical areas including Africa, South America, Indonesia, Malaysia and Sri Lanka [40, 48]. It is an evergreen tree, 10–20 m tall, with spear-shaped leaves and cluster-like, yellowish flowers. Dried flower buds are commonly used in cooking, pharmacy, perfumery and cosmetics [40]. The main ingredient (up to 20%) is essential oil, characterised by the presence of eugenol, eugenol acetate and α- and β-caryophyllene [48]. Eugenol is the main bioactive compound [12]. From an aqueous acetone extract of dried flower buds of Syzygium aromaticum 18 hydrolysable tannins were isolated [4]. Anthelmintic effects of hydrolysable tannins have been evaluated in several studies [1, 11]. The clove is an important medicinal plant due to its broad spectrum of pharmacological effects (antioxidant, antimicrobial, antinociceptive, and antiviral), and has been used in traditional applications for centuries. Its medicinal uses are described in the literature [12]. Ethanol extract (0.5 mg/mL) of Syzygium aromaticum inhibited energy metabolism in the nematode H. contortus in vitro, resulting in reduced production of ATP, which leads to death of the parasite [29]. Clove extract showed in vitro dose- and time-dependent ovicidal and anthelmintic effects on H. contortus [8] and Fasciola gigantica [28].
The aim of the present study was to investigate the efficacy of diets with pumpkin seed cake and cloves on the egg excretion of GIN in sheep naturally infected with GIN under usual farming conditions.
Materials and methods
Ethics
The study was conducted at a sheep farm in central Slovenia between December 2019 and January 2020. All procedures complied with relevant Slovenian legislation (Animal Protection Act, Official Gazette of the Republic of Slovenia, No 38/2013).
Study animals
There were 120 sheep of the breed Jezersko-Solčava (autochthonous Slovenian breed) grazing on the farm from spring to autumn. This breed is annual poliestric and is used for meat production; the body weight of ewes is between 65 and 80 kg. After the grazing season, the animals were kept in a barn during the winter months. During the entire study, the animals were kept indoors. The winter ration consisted of hay (two thirds) and grass silage (one third), which was provided ad libitum. The sheep had unrestricted access to clean water.
The animals were not dewormed for 7 months prior to the study.
The study was performed on a total of 30 ewes aged between 1.5 and 13 years (median 3.8 years). Seven ewes lambed within 2 months before the beginning of the study, and others were pregnant. The ewes were randomly selected from the flock and randomly assigned to the following supplementation groups of 10 animals each: clove supplemented group, pumpkin seed cake supplemented group, and control group.
Groups supplemented with clove and pumpkin seed cake were kept in separate pens; the control group was kept with the flock.
Diet
The group supplemented with cloves received 1.8 g of freshly ground cloves/ewe/day for 7 days in addition to the usual ration. The cloves were bought in a local shop.
The group supplemented with pumpkin seed cake (Cucurbita pepo var. oleifera) received 200 g pumpkin seed cake/ewe/day divided into two meals (morning, evening) for 7 days with the usual ration. The composition of the pumpkin seed cake is shown in Table 1.
Table 1.
The composition of pumpkin seed cake.
| Component | Content (%) |
|---|---|
| Moisture | 6.04 |
| Raw protein | 57.0 |
| Raw oils and fats | 13.5 |
| Raw fibre | 3.50 |
| Raw ash | 9.43 |
Although the sheep were reluctant to eat cloves at the first day, they later got used to the taste and willingly ate the offered feed supplements.
The control group was fed with the usual ration. The owner received detailed instructions on how the animals in each group should be fed.
Measurements
At the beginning of the study, on day 0, and 5 days after 7-day supplementation on day 12, body condition score (5-point scale) [26] and colour of conjunctivas (FAMACHA score) [5, 24] were assessed in each ewe. Body condition score was assessed with palpation of the loin region (muscling, fat deposition on spinous and transverse processes): score one means emaciated and score five means obese.
Faecal egg counts were performed on each study animal on days 0 and 12. Individual faecal egg counts were determined by using McMaster technique [49].
A coprological culture of faecal samples from the four most infected animals was performed on day 0 to get information about helminth genera present in the flock. Sheep faecal samples collected in plastic bags were incubated for 12 days, at a temperature of 26–30° C. L3 larvae were obtained by baermannization and identified using identification keys [44].
Data analysis
Using the SPSS software package for Windows (version 22) [23], descriptive statistics for body condition score, FAMACHA score, and faecal egg count was calculated. To compare values of investigated variables between day 0 and day 12 for each group, a Wilcoxon signed-rank test for dependent samples was used. Faecal egg counts among the groups before and after supplementation were compared using an independent samples Kruskal-Wallis test and Dunett’s T3 post hoc test.
To determine the percentage of Strongylid eggs shedding reduction in samples after the supplementation, we calculated the mean number of eggs in the samples from each group before supplementation (T1) and after supplementation (T2), and used the equation [25, 27]:
The 95% confidence intervals were calculated with the eggCounts on-line analysis program [25, 46], using the Two samples paired model procedure (http://shiny.math.uzh.ch/user/furrer/shinyas/shiny-eggCounts/).
Eggs of other parasites and coccidian oocysts were found only in small numbers in some animals and were therefore not included in the statistical analysis.
Results
Body condition- and FAMACHA score
The results of the body condition assessments in clove-, pumpkin seed cake- and control group sheep are presented in Table 2. The mean body condition score values did not change significantly in any of the groups.
Table 2.
Body condition score before (day 0) and after supplementation (day 12).
| Group | Day 0 (mean) | Range | Day 12 (mean) | Range | p-value |
|---|---|---|---|---|---|
| Clove | 2.7 | 2.0–3.5 | 2.7 | 1.5–4.0 | 1.000 |
| Pumpkin seed cake | 3.0 | 2.0–4.5 | 2.9 | 1.5–4.0 | 0.317 |
| Control | 2.5 | 2.0–3.0 | 2.4 | 2.0–4.0 | 0.564 |
The results of conjunctiva colour score (FAMACHA) in the clove-, pumpkin seed cake- and control group sheep are presented in Table 3. The FAMACHA score values increased slightly on day 12 in all three groups, with a significant difference found only in the clove group.
Table 3.
FAMACHA score before (day 0) and after supplementation (day 12).
| Group | Day 0 (mean) | Range | Day 12 (mean) | Range | p-value |
|---|---|---|---|---|---|
| Clove | 2.3 | 1.0–4.0 | 2.8 | 2.0–4.0 | 0.025 |
| Pumpkin seed cake | 2.2 | 1.0–3.0 | 2.6 | 1.0–4.0 | 0.217 |
| Control | 2.5 | 1.0–4.0 | 2.8 | 2.0–4.0 | 0.366 |
Faecal egg counts
The results of faecal egg counts in the clove-, pumpkin seed cake- and control group sheep are presented in Table 4 and Figure 1. In the clove group, FEC decreased in eight ewes after the supplementation, while it remained unchanged in two ewes. The percentage decrease of FEC was 40.7% on day 12, with a 95% confidence interval of 0.32–0.47. In the pumpkin seed cake group, FEC decreased in eight ewes after the supplementation, increased in one, and remained unchanged in one ewe. The percentage reduction of FEC was 52.9%, with a 95% confidence interval of 0.45–0.60. In the control group, FEC decreased in six ewes after the supplementation, and increased in four ewes. The percentage increase of FEC was 8.7%.
Table 4.
Strongylid faecal egg counts in eggs per gram (EPG) and percentage reductions in faecal egg counts (FECR).
| Group | Day 0 – EPG (mean) | EPG (range) | Day 12 – EPG (mean) | EPG (range) | FECR (%) | p-value |
|---|---|---|---|---|---|---|
| Clove | 3270 | 50–9950 | 1940 | 50–7550 | 40.7 | 0.012 |
| Pumpkin seed cakes | 2365 | 50–7150 | 1115 | 0–2800 | 52.9 | 0.038 |
| Control | 3385 | 1500–7050 | 3680 | 1000–6900 | −8.7 | 0.959 |
Figure 1.
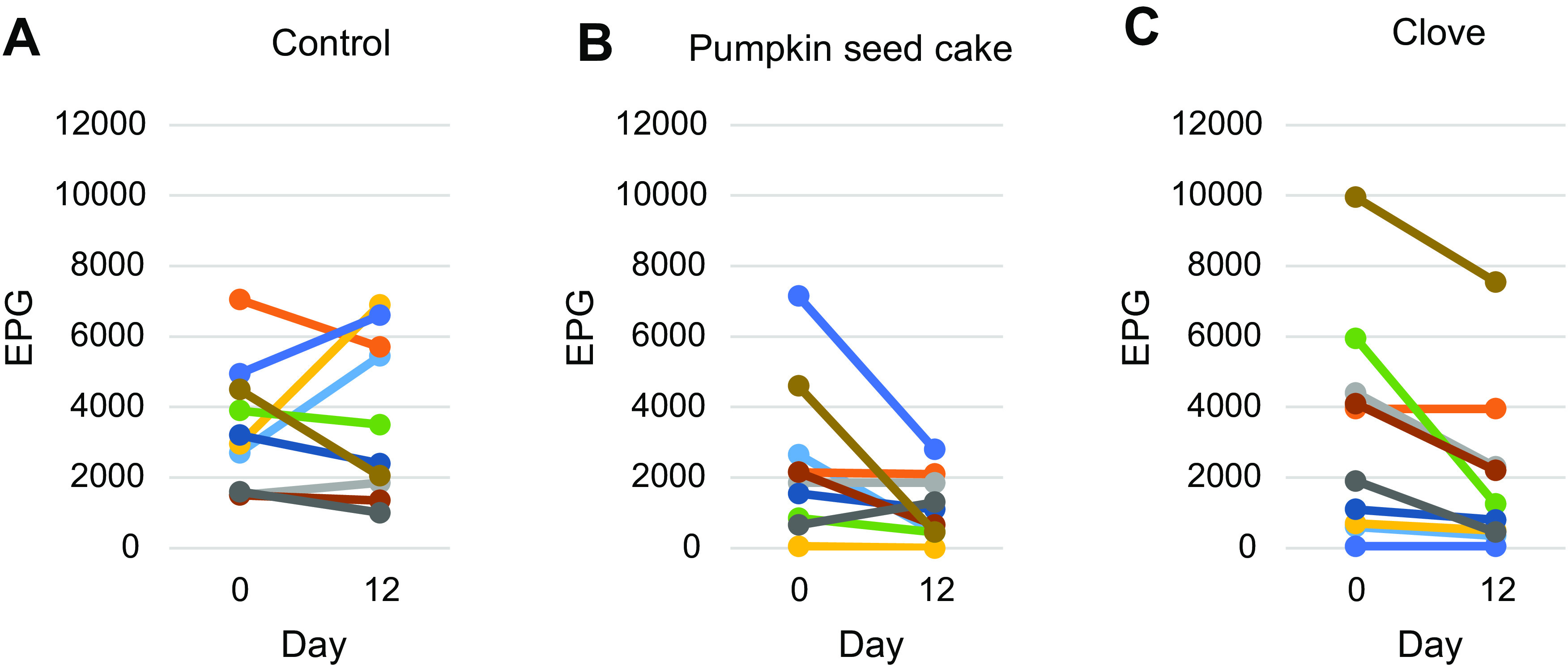
The results of Strongylid faecal egg counts in the control (A), pumpkin seed cake (B) and clove (C) group (the lines represent individual ewes).
The comparison of FEC between the groups before supplementation showed insignificant (p = 0.480) differences, and after supplementation, the differences between the groups were statistically significant (p = 0.020), the pumpkin seed cake group differed significantly from the control group (p = 0.018).
Coprological culture
Trichostrongylus spp. larvae L3 were found in all four selected ewes. L3 larvae of the genera Ostertagia spp. and Cooperia spp. were found in three ewes and only a few Haemonchus contortus larvae were found in one ewe.
Discussion
In this study, we explored the in vivo effect of pumpkin seed cake and clove supplements on nematode egg excretion in naturally infected ewes in farm conditions. In several published studies, there was evidence of anthelmintic properties of pumpkin seed and cloves in vitro and in vivo [8, 17, 28–31, 41]. Diet can influence the host’s ability to cope with the consequences of parasitism in different ways. It can increase the host’s ability to cope with the negative consequences of parasitism (resilience), it can improve host resistance, and it can directly affect the parasite population via ingestion of anthelmintic compounds [10].
To evaluate performance and detect clinical anaemia in the ewes with on-farm methods, we assessed body condition [26] and FAMACHA score [24]. The body condition of the ewes remained almost the same during supplementation in all groups studied. In most ewes, the body condition was within the recommended range of 2.0–3.5 [26]. Despite the high nutritional value of pumpkin seed cake (rich in protein), body condition score values did not improve in the group of ewes receiving this supplement. The ewes were fed with pumpkin seed cake for only seven days. The period between the two evaluations may have been too short for a noticeable improvement in body condition and to detect the differences in the mean body condition score between the groups. To change the body condition score in a sheep by one unit, the animal needs to gain 3.3–16.0 kg live weight depending on the breed and category of the animal (lamb after weaning, lactating ewe, dry ewe) [26]. Perhaps the weighing of the animals in our study could have led to different results, as we would have detected even small changes in live weight.
The mean conjunctiva score (FAMACHA) deteriorated slightly in all groups during the study. Although the results of the parasitological examination after supplementation were favourable in both supplemented groups, this may be partly due to the possible presence of hematophagous nematode species and the fact that the newly formed erythrocytes take some time to mature and become fully functional. Thus, they only appear in the bloodstream after 5 days [35]. Despite the high initial number of faecal nematode eggs in many ewes, the conjunctival score values in most ewes did not indicate serious anaemia; mean score values in all groups were between 2.2 and 2.5. Anaemia is considered at eye score of ≥3 [24]. This is consistent with the results of coproculture, where most of the larvae found were not from hematophagous nematode species.
The faecal egg counts changed differently between the groups after supplementation. In the control group, the average number of eggs increased (but the difference between day 0 and 12 was not statistically significant), which was to be expected since the animals did not receive any preparations or additives that would be effective against parasites. The juvenile forms of the parasites were able to mature and even started to produce eggs on their own. The number of eggs did not increase in some ewes, suggesting that some other factors (such as physiological status, etc.) might influence egg excretion by the parasites.
In the group of ewes that received a pumpkin seed cake supplement, the faecal egg count after supplementation decreased by an average of 52.9%. The decrease in the faecal egg count could be attributed to the active ingredients in pumpkin seed cake and partly to the high protein content (57% crude protein). It has been shown that adequate protein supplementation can help animals with pre-existing severe gastrointestinal worm infection by preventing or reducing clinical signs of H. contortus infection making pathological changes less pronounced [42]. A protein-rich diet in dairy goats resulted in a significantly lower faecal egg count, suggesting that resistance was enhanced by protein supplementation [15].
Anthelmintic properties in pumpkins are primarily attributed to the secondary metabolite cucurbitin in pumpkin seeds. This accelerates the excretion of the parasites from the host by weakening their ability to attach to the gastrointestinal wall [2], and also causes degenerative changes in the reproductive organs of the parasites [6]. Triterpenoid cucurbitacin also has anthelmintic activity [37]. Pumpkin seeds also contain tannins which have shown anthelmintic properties in several studies [19–21, 32, 33, 39] and could have contributed to decreased faecal egg counts. In another study, 4-month-old Merino lambs were infected with H. contortus larvae and treated with pumpkin seeds for two weeks. The treatment resulted in a 65.5% decrease in the initial faecal egg count, which, however, increased back to baseline as soon as the animals came off the treatment. The authors concluded that pumpkin seeds have a potential to control parasites by influencing parasite fertility [41]. In an experiment in which naturally infected goat kids were supplemented with ground pumpkin seeds (5 g/kg body weight), the researchers observed no effect on faecal egg count, but the treatment did affect the packed cell volume (p < 0.01), which was greater in the treated group than in the control group [31]. Although the faecal egg counts were similar among treatments, no animals treated with pumpkin showed clinical signs of infection (diarrhoea, bottle-jaw, anaemia) and were therefore not dewormed [31]. Pumpkin seeds have also shown anthelmintic effects in other animal species, e.g. in poultry [2] and in mice [3], where there was a reduction in the number of adult parasites and also in the number of excreted eggs. Bauri et al. [6] described the effect of pumpkin seed extract on H. contortus under in vitro conditions where the motility of adult parasites was reduced. Grzybek et al. [17] monitored blood and urine parameters as well as histopathology in pigs and rats during long-term feeding with pumpkin seed extract and found no abnormalities. Pumpkin seed cakes are a tasty feed for animals; they are easily accessible to farmers and can be used as high-protein concentrates in the diet. Not only are they a promising alternative to the use of antiparasitic drugs, but they can also be a source of protein. Results from Strickland et al. [41] also suggest that lambs can cope with high parasite loads in good nutrition and are still productive in the early stages of infection.
In the group of sheep supplemented with ground cloves, the number of strongylid eggs per gram of faeces decreased by 40.6% on average. The decrease in the number of excreted eggs can be attributed to bioactive substances in cloves (alkaloids, glycosides, essential oils, tannins, etc.), in particular eugenol [48], which is thought to act on the cuticle of the parasite, and tannins, which are known to have anthelmintic properties [19–21, 32, 33, 39]. Charitha et al. [8] investigated the in vitro effect of an acetone extract from cloves on egg hatching and on adult motility in H. contortus. The egg hatch test and adult motility assay revealed significant anthelmintic properties against H. contortus, namely that at a concentration of 10.0 mg/mL within 2 min after exposure, 100% mortality of the worms was achieved. The worm-killing effect of clove extracts could be attributed to their strong corrosive effect on the cuticle and tegument of helminths. In addition, clove extract inhibited the energy metabolism of H. contortus by inhibiting fumarate reductase and succinate dehydrogenase activity, which prevented the parasite from residing in the abomasum and being expelled by the host [29]. Manoj Dhanraj and Veerakumari [30] observed the effect of clove ethanol extract on acetylcholinesterase activity and motility in the paramphistome Cotylophoron cotylophorum. The results of these studies indicate a similar mode of action of the clove on helminths as with some anthelmintic drugs (energy metabolism, muscle paralysis).
Clove essential oil is generally recognised as a safe substance when consumed in quantities lower than 1500 mg/kg [12]. The administration of standardised polyphenolic extract of clove buds (Clovinol) to Wistar rats did not result in toxicologically significant changes in clinical observations or behaviour compared to the untreated control group of animals, ophthalmological examinations, body weight, organ weight, feed and water intake, urinalysis, haematological and clinical biochemical parameters, which all point to the no observed-adverse-effect level at the highest dose tested (1 g/kg body weight per day) [45].
No side effects were observed in our study. Cloves are easily accessible and easy to dose. The farmer told us that the sheep did not want to eat them on the first day, probably because of the specific taste and smell, but later they had no problems consuming cloves.
The results of this study suggest that pumpkin seed cake and cloves may have the potential to reduce faecal egg counts of GIN.
In vivo studies on the effect of pumpkin seeds and cloves on the excretion of gastrointestinal nematode eggs are sparse in small ruminants. To the best of our knowledge, no previous study has investigated the effect on gastrointestinal nematode eggs excretion of pumpkin seed cake or cloves added to the diet of naturally infected sheep. The results obtained are promising for the potential use of pumpkin seed cakes and cloves added to the diet of sheep for GIN control. Moreover, pumpkin seed cakes and cloves are available to farmers and are easy to use as a feed supplement and could therefore become a part of an integrated approach to parasite control, through which treatment with anthelmintic drugs could be reduced. Nonetheless, this study shows some weaknesses. Firstly, identification and description of the plant secondary metabolites were not performed, considering that their concentration may be highly variable [22, 37]. Additionally, the different diets were not analysed for macronutrients and were not balanced among groups, which could confound the real anthelmintic effects of the plant supplements. The feeding trial with pumpkin seed cake and cloves lasted for a very short period, while a longer period might have resulted in a greater anthelmintic effect. Therefore, further studies are needed to confirm the results obtained in this study and to evaluate the real possibility that these plant supplements could be used as a new method for the control of small ruminant GIN in the future. The exact mechanisms of their mode of action should be addressed in further research and the effect on different parasite species should be investigated as well. Further research would be needed on combined use of pumpkin seed cake and cloves to investigate their potential synergistic effects on GIN in small ruminants. The study should be repeated on different breeds of sheep in uniform groups, at different seasons and various basic diets (grazing, winter diet) in order to generalise the findings to any animal species.
Acknowledgments
The authors acknowledge the financial support from the Slovenian Research Agency (research core funding No. [P4-0092]). The authors would like to thank COST Action CA16230 COMBAR supported by COST (European Cooperation in Science and Technology, www.cost.eu) for inspiring this work. The authors have no conflicts of interest to declare.
Cite this article as: Ježek J, Mirtič K, Rešetič N, Hodnik JJ & Vergles Rataj A. 2021. The effect of pumpkin seed cake and ground cloves (Syzygium aromaticum) supplementation on gastrointestinal nematode egg shedding in sheep. Parasite 28, 78.
Footnotes

Special Issue – Combatting anthelmintic resistance in ruminants
Invited Editors: Johannes Charlier, Hervé Hoste, and Smaragda Sotiraki
References
- 1.Acevedo-Ramírez PMC, Hallal-Calleros C, Flores-Pérez I, Alba-Hurtado F, Mendoza-Garfías MB, Castro del Campo N, Barajas R. 2019. Anthelmintic effect and tissue alterations induced in vitro by hydrolysable tannins on the adult stage of the gastrointestinal nematode Haemonchus contortus. Veterinary Parasitology, 266, 1–6. [DOI] [PubMed] [Google Scholar]
- 2.Acorda JA, Mangubat IYE, Divina BP. 2019. Evaluation of the in vivo efficacy of pumpkin (Cucurbita pepo) seeds against gastrointestinal helminths of chickens. Turkish Journal of Veterinary and Animal Sciences, 43(2), 206–211. [Google Scholar]
- 3.Ayaz E, Gökbulut C, Coşkun H, Türker A, Özsoy S, Ceylan K. 2015. Evaluation of the anthelmintic activity of pumpkin seeds (Cucurbita maxima) in mice naturally infected with Aspiculuris tetraptera. Journal of Pharmacognosy and Phytotherapy, 7(9), 189–193. [Google Scholar]
- 4.Bao L, Eerdunbayaer Nozaki A, Takahashi E, Okamoto K, Ito H, Hatano T. 2012. Hydrolysable tannins isolated from Syzygium aromaticum: structure of a new C-glucosidic ellagitannin and spectral features of tannins with a tergalloyl group. Heterocycles, 85(2), 365–381. [Google Scholar]
- 5.Bath GF, Malan FS, van Wyk JA. 1996. The FAMACHA ovine anaemia guide to assist with the control of haemonchosis, in Proceedings of the 7th Annual Congress of the Livestock Health and Production Group of the South African Veterinary Association, Port Elizabeth. p. 5. [Google Scholar]
- 6.Bauri RK, Tigga MN, Kullu SS. 2015. A review on use of medicinal plants to control parasites. Indian Journal of Natural Products and Resources, 6(4), 268–277. [Google Scholar]
- 7.Burke JM, Miller JE. 2020. Sustainable approaches to parasite control in ruminant livestock. Veterinary Clinics Food Animal Practice, 36(1), 89–107. [DOI] [PubMed] [Google Scholar]
- 8.Charitha VG, Adeppa J, Malakondaiah P. 2017. In vitro anthelmintic activity of Syzigium aromaticum and Melia dubia against Haemonchus contortus of sheep. Indian Journal of Animal Sciences, 87(8), 968–970. [Google Scholar]
- 9.Chomicki G, Schaefer H, Renner SS. 2020. Origin and domestication of Cucurbitaceae crops: insights from phylogenies, genomics and archaeology. New Phytologist, 226, 1240–1256. [DOI] [PubMed] [Google Scholar]
- 10.Coop RL, Kyriazakis I. 2001. Influence of host nutrition on the development and consequences of nematode parasitism in ruminants. Trends in Parasitology, 17(7), 325–330. [DOI] [PubMed] [Google Scholar]
- 11.Corona-Palazuelos MB, Murillo-Ayala EX, Castro-del Campo N, Romo-Rubio JA, Cervantes-Pacheco BJ, Gaxiola-Camacho SM, Barajas-Cruz R. 2016. Influence of tannin extract addition on the amount of nematodes found in feedlot calves at the beginning of the fattening process. Agrociencia, 50, 1013–1025. [Google Scholar]
- 12.Cortés-Rojas DF, Fernandez de Souza CR, Oliveira WP. 2014. Clove (Syzygium aromaticum): a precious spice. Asian Pacific Journal of Tropical Biomedicine, 4(2), 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhiman K, Gupta A, Sharma DK, Gill NS, Goyal AA. 2012. A review on the medicinally important plants of the family Cucurbitaceae. Asian Journal of Clinical Nutrition, 4, 16–26. [Google Scholar]
- 14.Ethiraj S, Balasundaram J. 2016. Phytochemical and biological activity of Cucurbita seed extract. Journal of Advances in Biotechnology, 6(1), 813–821. [Google Scholar]
- 15.Etter E, Hoste H, Chartier C, Pors I, Koch C, Broqua C, Coutineau H. 2000. The effect of two levels of dietary protein on resistance and resilience of dairy goats experimentally infected with Trichostrongylus colubriformis: comparison between high and low producers. Veterinary Research, 31, 247–258. [DOI] [PubMed] [Google Scholar]
- 16.Geurden T, Hoste H, Jacquiet P, Traversa D, Sotiraki S, di Regalbono AF, Tzanidakis N, Kostopoulou D, Gaillac C, Privat S, Giangaspero A, Zanardello C, Noe L, Vanimisetti B, Bartram L. 2014. Anthelmintic resistance and multidrug resistance in sheep gastro-intestinal nematodes in France, Greece and Italy. Veterinary Parasitology, 201, 59–66. [DOI] [PubMed] [Google Scholar]
- 17.Grzybek M, Kukula-Koch W, Strachecka A, Jaworska A, Phiri AM, Paleolog J, Tomczuk K. 2016. Evaluation of anthelmintic activity and composition of pumpkin (Cucurbita pepo L.) seed extracts in vitro and in vivo studies. International Journal of Molecular Sciences, 17, 1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hördegen P, Hertzberg H, Heilmann J, Langhans W, Maurer V. 2003. The anthelmintic efficacy of five plant products against gastrointestinal trichostrongylids in artificially infected lambs. Veterinary Parasitology, 117, 51–60. [DOI] [PubMed] [Google Scholar]
- 19.Hoste H, Gaillard L, Le Frileux Y. 2005. Consequences of the regular distribution of sainfoin hay on gastrointestinal parasitism with nematodes and milk production in dairy goats. Small Ruminant Research, 59, 265–271. [Google Scholar]
- 20.Hoste H, Jackson F, Athanasiadou F, Thamsborg S, Hoskin SO. 2006. The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends in Parasitology, 32, 253–261. [DOI] [PubMed] [Google Scholar]
- 21.Hoste H, Martinez-Ortiz-De-Montellano C, Manolaraki F, Brunet S, Ojeda-Robertos N, Fourquaux I, Torres-Acosta JFJ, Sandoval-Castro CA. 2012. Direct and indirect effects of bioactive tannin-rich tropical and temperate legumes against nematode infections. Veterinary Parasitology, 186, 18–27. [DOI] [PubMed] [Google Scholar]
- 22.Hoste H, Torres-Acosta JFJ, Sandoval-Castro CA, Mueller-Harvey I, Sotiraki S, Louvandini H, Thamsborg SM, Terril TH. 2015. Tannin containing legumes as a model for nutraceuticals against digestive parasites in livestock. Veterinary Parasitology, 212, 5–17. [DOI] [PubMed] [Google Scholar]
- 23.IBM Corporation. 2013. IBM SPSS statistics 22 core system user’s guide. ftp://public.dhe.ibm.com/software/analytics/spss/documentation/statistics/22.0/en/client/Manuals/IBM_SPSS_Statistics_Core_System_User_Guide.pdf
- 24.Kaplan RM, Burke JM, Terrill TH, Miller JE, Getz WR, Mobini S, Valencia E, Williams MJ, Williamson LH, Larsen M, Vatta AF. 2004. Validation of the FAMACHA© eye color chart for detecting clinical anemia in sheep and goats on farms in the southern United States. Veterinary Parasitology, 123, 105–120. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan RM. 2020. Biology, epidemiology, diagnosis, and management of anthelmintic resistance in gastrointestinal nematodes of livestock. Veterinary Clinics Food Animal Practice, 36(1), 17–30. [DOI] [PubMed] [Google Scholar]
- 26.Kenyon PR, Maloney SK, Blache D. 2014. Review of sheep body condition score in relation to production characteristics. New Zealand Journal of Agricultural Research, 57(1), 38–64. [Google Scholar]
- 27.Kochapakdee S, Pandey V, Pralomkarn W, Choldumrongkul S, Ngampongsai W, Lewpethara A. 1995. Anthelmintic resistance in goats in southern Thailand. Veterinary Record, 137, 124–125. [DOI] [PubMed] [Google Scholar]
- 28.Kumar P, Singh DK. 2014. In vitro anthelmintic activity of Allium sativum, Ferula asafoetida, Syzygium aromaticum and their active components against Fasciola gigantica. Journal of Biology and Earth Sciences, 4(1), B57–B65. [Google Scholar]
- 29.Kumar SS, Veerakumari L. 2017. Effect of ethanol extracts of Syzigium aromaticum on fumarate reductase and succinate dehydrogenase of Haemonchus contortus. Journal of Global Trends in Pharmaceutical Scieneces, 8(4), 4648–4655. [Google Scholar]
- 30.Manoj Dhanraj K, Veerakumari L. 2014. In vitro effect of Syzygium aromaticum on the motility and acetylcholinesterase of Cotylophoron cotylophorum. Indian Journal of Veterinary and Animal Science Research, 43(3), 187–194. [Google Scholar]
- 31.Matthews KK, O’Brien DJ, Whitley NC, Burke JM, Miller JE. 2016. Investigation of possible pumpkin seeds and ginger effects on gastrointestinal nematode infection indicators in meat goat kids and lambs. Small Ruminant Research, 136, 1–6. [Google Scholar]
- 32.Paolini V, Bergeaud JP, Grisez C, Prevot F, Dorchies P, Hoste H. 2003. Effects of condensed tannins on goats experimentally infected with Haemonchus contortus. Veterinary Parasitology, 113, 253–261. [DOI] [PubMed] [Google Scholar]
- 33.Paolini V, Frayssines A, De La Farge F, Dorcheis P, Hoste H. 2003. Effects of condensed tannins on established populations and on incoming larvae of Trichostrongylus colubriformis and Teladorsagia circumcincta in goats. Veterinary Research, 34, 331–339. [DOI] [PubMed] [Google Scholar]
- 34.Paris HS, Nerson H. 2003. Seed dimensions in the subspecies and cultivar-groups of Cucurbita pepo. Genetic Resources and Crop Evolution, 50, 615–625. [Google Scholar]
- 35.Reece WO. 2015. Dukes’ physiology of domestic animals, 13th edn. Ames, Iowa: Blackwell Publishing. [Google Scholar]
- 36.Rose H, Rinaldi L, Bosco A, Mavrot F, de Waal T, Skuce P, Charlier J, Torgerson PR, Hertzberg H, Hendrickx G, Vercruysse J, Morgan ER. 2015. Widespread anthelmintic resistance in European farmed ruminants: a systematic review. Veterinary Record, 176(21), 546. [DOI] [PubMed] [Google Scholar]
- 37.Salehi B, Capanoglu E, Adrar N, Catalkaya G, Shaheen S, Jaffer M, Giri L, Suyal R, Jugran AK, Calina D, Docea AO, Kamiloglu S, Kregiel D, Antolak H, Pawlikowska E, Sen S, Acharya K, Selamoglu Z, Sharifi-Rad J, Martorell M, Rodrigues CF, Sharopov F, Martins N, Capasso R. 2019. Cucurbits plants: a key emphasis to its pharmacological potential. Molecules, 24(10), 1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salgado JA, Santos CP. 2016. Overview of anthelmintic resistance of gastrointestinal nematodes of small ruminants in Brazil. Brazilian Journal of Veterinary Parasitology, Jaboticabal, 25(1), 3–17. [DOI] [PubMed] [Google Scholar]
- 39.Saratsi K, Hoste H, Voutzourakis N, Tzanidakis N, Stefanakis A, Thamsborg SM, Mueller-Harvey I, Hadjigeorgiou I, Sotiraki S. 2020. Feeding of carob (Ceratonia siliqua) to sheep infected with gastrointestinal nematodes reduces faecal egg counts and worm fecundity. Veterinary Parasitology, 284(109200), 1–11. [DOI] [PubMed] [Google Scholar]
- 40.Singh J, Baghotia A, Goel SP. 2012. Eugenia caryophyllata Thunberg (Family Myrtaceae): A Review. International Journal of Research in Pharmaceutical and Biomedical Sciences, 3(4), 1469–1475. [Google Scholar]
- 41.Strickland VJ, Krebs GL, Potts W. 2009. Pumpkin kernel and garlic as alternative treatments for the control of Haemonchus contortus in sheep. Animal Production Science, 49, 139–144. [Google Scholar]
- 42.Sykes AR, Coop RL. 2001. Interactions between nutrition and gastrointestinal parasitism in sheep. New Zealand Veterinary Journal, 49(6), 222–226. [DOI] [PubMed] [Google Scholar]
- 43.Teppner H. 2000. Cucurbita pepo (Cucurbitaceae) – history, seed coat types, thin coated seeds and their genetics. Phyton, 40(1), 1–42. [Google Scholar]
- 44.Van Wyk JA, Mayhew E. 2013. Morphological identification of parasitic nematode infective larvae of small ruminants and cattle: a practical lab guide. Onderstepoort Journal of Veterinary Research, 80(1), 539. [DOI] [PubMed] [Google Scholar]
- 45.Vijayasteltar L, Nair GG, Maliakel B, Kuttan R, Krishnakumar IM. 2016. Safety assessment of a standardized polyphenolic extract of clovebuds: Subchronic toxicity and mutagenicity studies. Toxicology Reports, 3, 439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang C, Torgerson PR, Kaplan RM, George MM, Furrer R. 2018. Modelling anthelmintic resistance by extending eggCounts package to allow individual efficacy. International Journal for Parasitology: Drugs and Drug Resistance, 8, 386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wink M. 2012. Medicinal plants: a source of anti-parasitic secondary metabolites. Molecules, 17, 12771–12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organization. 2002. Flos Caryophylli, in WHO monographs on selected medicinal plants, Vol. 2. World Health Organization: Geneva. p. 45–54. [Google Scholar]
- 49.Zajac AM, Conboy GA. 2006. Veterinary Clinical Parasitology, 7th edn. Ames, Iowa: Blackwell Publishing. [Google Scholar]


