Supplemental Digital Content is Available in the Text.
Key Words: HIV pre-exposure prophylaxis/prevention, intravaginal ring, tenofovir, emtricitabine
Objective:
To describe and compare systemic and local pharmacokinetics (PK) and cervicovaginal (CV) pharmacodynamics (PD) of oral tenofovir disoproxil fumarate (TDF) in combination with emtricitabine (FTC) with tenofovir (TFV) intravaginal ring (IVR).
Design:
Phase I, randomized, parallel-group study. Women (n = 22) used TDF/FTC oral tablets daily or TFV IVR continuously and were assessed at baseline and 14 days.
Methods:
TFV and FTC concentrations were measured in plasma, CV fluid (CVF), and CV tissue. TFV-diphosphate and FTC-triphosphate were assessed in CV tissue. In vitro PD antiviral activities of TFV and FTC (using in vivo concentration ranges) were modeled in the CVF and by infecting CV tissue explants ex vivo with HIV-1BaL.
Results:
Adverse events (AEs) were more common with oral TDF/FTC use (P < 0.01). The median CVF TFV concentrations were 106 ng/mL after use of TFV IVR vs. 102 ng/mL for TDF/FTC. The median TFV and TFV-diphosphate concentrations in CV tissue were >100-fold higher among IVR users. The median CVF FTC concentrations were 103 ng/mL. FTC and FTC-triphosphate were detected in all CV tissues from TDF/FTC users. HIV inhibitory activity of CVF increased significantly with treatment in both cohorts (P < 0.01) but was higher in TFV IVR users (P < 0.01). In vitro inhibition of tissue infection with ex vivo administration of TFV and FTC was dose dependent, with maximal efficacy achieved with 10 µg/mL TFV, 1 µg/mL FTC, and 0.1 µg/mL of TFV and FTC combined.
Conclusions:
Both products were safe and increased mucosal HIV inhibitory activity. In addition to systemic protection, oral TDF/FTC displays a PK/PD profile compatible with CV mucosal antiviral activity. TFV IVR resulted in fewer AEs, lower TFV plasma concentrations, higher CVF and tissue TFV and TFV-DP concentrations, and greater anti-HIV activity in CVF.
INTRODUCTION
More than 37 million people worldwide are infected with HIV type 1 (HIV-1).1 Women, particularly adolescent girls and young women (AGYW), bear the burden of the HIV-1 pandemic, with more than 59% of new infections occurring in women in sub-Saharan Africa.1 Currently, 300 mg of tenofovir disoproxil fumarate (TDF) in combination with 200 mg of emtricitabine (FTC) is approved for the prevention of HIV-1 acquisition in men and women in the United States and several other countries worldwide2–4 (reviewed in Ref. 5). However, the daily dosing requirement and systemic side effects, primarily gastrointestinal (GI), have made it difficult, particularly for AGYWs, to adhere to daily TDF/FTC for HIV-1 prevention.6,7
Topical tenofovir (TFV), as a 1% vaginal gel, was effective in reducing the incidence of HIV-18 and herpes simplex virus type 2 (HSV-2)9 in the CAPRISA 004 prevention trial. However, adherence to both daily6 and pericoital dosing regimens10 of TFV 1% gel was poor, particularly among AGYW in subsequent phase IIb/III trials. Topical HIV-1 prevention may be significantly improved by sustained delivery of antiretroviral drugs through vaginal rings. Vaginal administration of pharmaceuticals and pre-exposure prophylaxis (PrEP) products through an IVR provides local absorption of drug, resulting in enhanced bioavailability with high mucosal drug concentrations, reduced dosing frequency, and decreased systemic side effects, which likely increases adherence.11–15 The 30-day dapivirine IVR was shown to reduce the acquisition of HIV-1 among women enrolled in 2 large, placebo-controlled trials.14,16 The TFV and TFV/levonorgestrel (LNG) IVRs were developed as multipurpose prevention technologies to be used for up to 90 days to prevent HIV-1, HSV-2, and or unintended pregnancies.17 Unlike dapivirine, TFV is converted into its active metabolite, TFV-diphosphate (TFV-DP), intracellularly, remaining in the tissue at protective concentrations for several days,18 making the TFV IVR more forgiving of removals and inconsistent use, potentially decreasing adherence problems. This is important given that data support that many women may want to remove an IVR intermittently.14,16,19–21
Oral tablets containing TDF and FTC have proven to be very effective in preventing HIV infection in men and women, after rectal and vaginal exposure.2–4 As opposed to IVRs and other topical prevention products, plasma and peripheral blood mononuclear cell (PBMC) concentrations of parent molecules and active metabolites are high, exceeding estimated systemic prevention benchmarks.22–25
The main objective of this study was to compare the multicompartmental pharmacokinetics (PK), pharmacodynamics (PD), and safety of the oral TDF/FTC vs. TFV IVR after 14 days of use. It is unclear how much of the observed in vivo protection against HIV cervicovaginal (CV) mucosal infection for TDF/FTC users comes from active concentrations of these molecules at the CV mucosal level. This study characterized and correlated TDF/FTC mucosal concentrations and antiviral activity and compared them with those observed during topical delivery of TFV by the ring. Data on the relative mucosal and systemic contributions of TFV, TDF, and FTC and their active metabolites TFV-diphosphate (TFV-DP) and FTC-triphosphate (FTC-TP) to HIV protection are missing. This detailed multicompartmental comparison of PK and modeled PD of topical vs. systemic delivery of TFV-based products in healthy women provides this information, which has not been previously reported.
We used oral TDF/FTC tablets as the systemic administration comparator because this combination product, as opposed to TDF alone, is approved for HIV-1 prevention in women.2 PK was evaluated in plasma, cervicovaginal fluids (CVFs), ectocervical and vaginal tissues for TFV and FTC, and ectocervical and vaginal tissues for the active metabolites (TFV-DP and FTC-TP) and their competing nucleotides (deoxyadenosine triphosphate (dATP) and deoxycytidine triphosphate (dCTP)).
METHODS
Clinical Study
CONRAD 140 is an open-label, randomized, parallel-group study conducted at the CONRAD Intramural Clinical Research Center at Eastern Virginia Medical School (EVMS) (Norfolk, VA). The study was approved by the Chesapeake Institutional Review Board (IRB) (Pro00016706) and registered with ClinicalTrials.gov (#NCT02722343). All participants signed written informed consent before the performance of any study procedures. We screened healthy women aged 18–50 years, who were protected from pregnancy during the study, by heterosexual abstinence, nonhormonal contraceptives, or combined oral contraceptive pills (OCPs), and reported a history of regular menstrual cycles. We excluded women who self-reported using depot medroxyprogesterone acetate (DMPA) within the last 10 months or current use of the levonorgestrel (LNG) intrauterine system (IUS), had treatment for sexually transmitted infections in the past 3 months, or who tested positive for Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis, HIV-1, or active hepatitis B at screening. Safety laboratories were drawn at screening to assure normal creatinine clearance (>60 mL/min) and exclude anemia. Study visits are summarized in Table 1, Supplemental Digital Content, http://links.lww.com/QAI/B738, but, briefly, participants underwent 4 visits. Once eligibility was confirmed, we used a vaginal speculum to collect CVF. One ectocervical biopsy and 1 vaginal biopsy were obtained using a 3 × 5-mm Tischler biopsy forceps in the luteal phase of the menstrual cycle at visit 2 (V2). At visit 3 (V3), in the follicular phase of the menstrual cycle, we randomized participants to TDF/FTC vs. TFV IVR. Participants either took the first TDF/FTC pill in the clinic, under direct observation, or underwent insertion of the TFV IVR in the clinic. Visit 4 (V4) occurred in the luteal phase of the menstrual cycle on the 14th day of product use. At this final visit, we collected blood for PK analyses and used a vaginal speculum to collect CV fluid, 2 ectocervical biopsies, and 2 vaginal biopsies. Based on internal quality control data from several previous CONRAD studies, ectocervical and vaginal biopsies weigh, on average, approximately 25 and 35 mg, respectively.
Adherence
Participants in both groups were sent text message (SMS) adherence reminders. Those randomized to TDF/FTC received a daily reminder to take their pill at the same time each day. Participants randomized to the TFV IVR got a daily reminder to not remove the IVR and to notify the clinic if they were no longer using the IVR or if they were having any issues with the IVR, such as expulsion.
Treatment Assignment
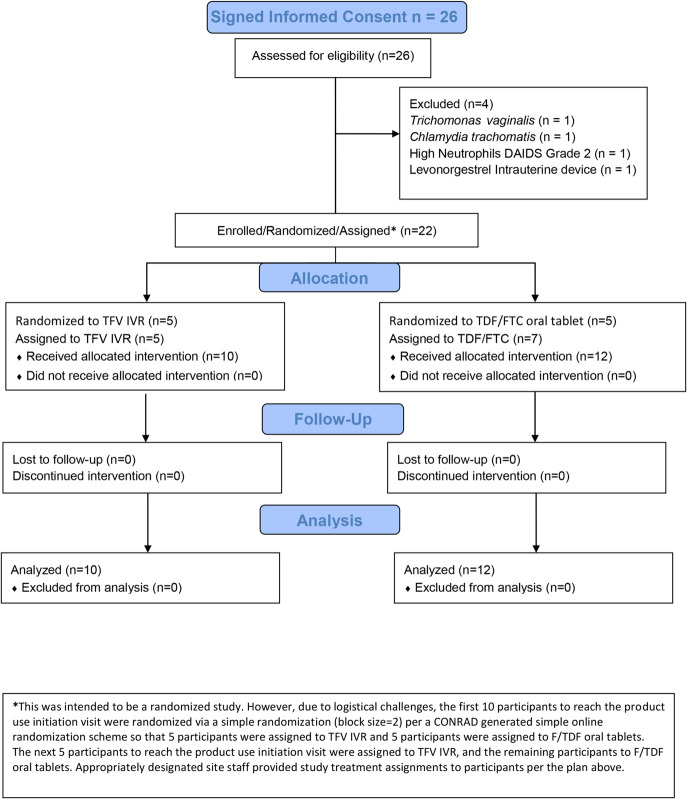
This was intended to be a randomized study. However, because of logistical challenges (TFV IVR product expiration dates), the first 10 participants to reach the product use initiation visit were randomized through a simple randomization (block size = 2) per a CONRAD-generated online randomization scheme so that 5 participants were assigned to TFV IVR and 5 participants were assigned to TDF/FTC. The next 5 participants to V3 were assigned to TFV IVR and the remaining participants to TDF/FTC. Appropriately designated site staff provided study treatment assignments to participants per the plan mentioned earlier. There were no allocation errors. The data manager and collaborating laboratories were blinded to treatment assignment.
Study Products
TFV IVRs were manufactured under good manufacturing practices at DPT Laboratories (San Antonio, TX) as previously described17,26 with the active pharmaceutical ingredient supplied by Gilead Sciences (Foster City, CA). The unit TFV dose for the IVRs was designed to be approximately 8–10 mg/d of TFV for 90 days of release, and we previously reported the estimated in vivo release over approximately 15 days as 11.32 ± 8.88 mg/d.18 The TDF/FTC 300/200 mg tablet is an FDA-approved product manufactured by Gilead Sciences and marketed as Truvada. The tablets were purchased from a local pharmacy (Hague Pharmacy, Norfolk, VA).
Safety Assessments
Safety was assessed by treatment-emergent adverse events (TEAEs). TEAEs were recorded by each participant in a diary and collected by the research coordinator at each study visit after genital sampling at baseline (visit 2) and graded for severity and relationship to study product or study procedures by the study investigators based on the NIH/NIAID Division of AIDS (DAIDS) severity scale.
Assessment of Plasma, Ectocervical and Vaginal Fluids, and Tissue PK Parameters and Endogenous Nucleotides
Ectocervical and vaginal tissue biopsies were collected in sterile cryovials and immediately flash frozen in liquid nitrogen. Whole blood was collected in a 3-mL K-EDTA vacutainer and centrifuged at 4°C for plasma. Ectocervical and vaginal fluids were collected with Dacron swabs. Tissues, CVF, and plasma were stored at −80°C and then shipped as 1 batch under frozen conditions to the University of North Carolina Center for AIDS Research, Clinical Pharmacology and Analytical Chemistry Core for analysis. The detailed PK analyses are included in the Methods section, Supplemental Digital Content, http://links.lww.com/QAI/B738. Tissue biopsies with below the level of quantification (BLQ) concentrations were expressed as ½ the lower limit of quantification (LLOQ) for analyses.
PD in vitro Modeling of Inhibition of HIV-1 Infection in Cells by Ectocervical and Vaginal Fluids
TZM-bl cells27 were plated and CVF (1:3 final dilution), with or without HIV-1BaL, was applied to the appropriate wells. For toxicity testing, 100 µL of medium, with or without nonoxynol-9 (N9) or CVF, was added to each well for 48 hours. The media were removed and replaced with 20 µL of CellTiter 96 Aqueous 1 Solution Cell Proliferation Assay (Promega, Madison, WI) and 100 µL of cDMEM media for 3–4 hours. Absorbance was read at 490 nm. For efficacy (inhibition) testing, we used the Bright-Glo Luciferase Assay System (Promega) following the manufacturer's instructions. In brief, 100 µL of medium ± CVF containing HIV-1BaL (5 × 103 TCID50) was added to each well. After 48 hours, the cells were lysed with 100 μL of Glo Lysis buffer. Lysate (50 µL) was transferred into a 96-well black microtiter plate, and 50 μL of Bright-Glo assay reagent was added and the luminescence was measured. The average percentage inhibition of HIV-1BaL growth in 3 wells with application of ectocervical or vaginal fluid, compared with control application of growth medium, was reported for each participant.
Explant Tissues Obtained for P24 Antigen Production Subset Study
To determine minimal inhibitory concentrations for TFV/TFV-DP and FTC/FTC-TP, we modeled mucosal HIV infection in ectocervical and vaginal tissues obtained from healthy, HIV-1 uninfected women, undergoing routine elective surgeries, for benign indications, under an EVMS IRB–approved protocol (09-09-FB-0175). All women donating their genital tissue specimens signed an informed consent form. Explants were obtained fresh from the operating room and taken immediately to our laboratory in chilled RPMI (cRPMI) 1640 media (Life Technologies, Carlsbad, CA) containing 10% fetal bovine serum (FBS) (ATCC, Manassas, VA) and 100 U/mL penicillin and 100ug/mL streptomycin (Thermo Fisher Scientific, Waltham, MA) (cRPMI). We treated the tissues with concentrations of TFV and FTC, in the range observed in tissues in vivo, and infected them ex vivo with HIV-1BaL. as previously described by our group28 and others.29,30 Aliquots of tissue culture media were obtained on days 4, 7, 11, 14, 18, and 21, and culture dishes were replenished with equal volumes of fresh media. We also used ectocervical explants obtained from National Development and Research Institutes, which were shipped overnight to our laboratory on ice.
Ex vivo Antiviral Activity Assessment of TFV and FTC Concentrations Detected in Plasma and Tissues From TFV IVR and TDF/FTC Cohorts
Cervical explants were exposed overnight to HIV-1BaL (104 TCID50 per explant) in the presence of different concentrations of TFV, FTC, and a combination of both, corresponding to median tissue concentrations of TFV and FTC detected after 14 days of either TDF/FTC or TFV IVR use in this study. Unpublished data from our laboratory indicate that in the unpolarized tissue culture system, media ARV concentrations tend to equilibrate overnight and are similar to tissue concentrations. The following day, explants were washed to remove virus and then cultured in cRPMI media. We collected half of the tissue culture supernatant every 3–4 days with replenishment of media at each collection for 21 days. HIV-1 P24 antigen expression in the supernatant was evaluated by ELISA (PerkinElmer, Waltham, MA) in pg/mL. Area under the curve (AUC) and cumulative (CUM) P24 antigen production were calculated from 4 to 21 days of culture as reported.30
Objective Biomarkers of IVR Adherence
Procedures for processing the IVRs and measuring glycerin concentrations remaining in the IVR after in vivo use and penetration of biologic analytes into the IVR from vaginal fluids have been previously described.31 Glycerin was measured in water extracts of the inner core of the IVRs using an enzymatic, colorimetric assay according to manufacturer's instructions (Sigma-Aldrich, St. Louis, MO). Bioanalytes that could penetrate the IVRs were quantitated using the 3-(4carboxybenzoyl) quinoline-2-carboxaldehyde) (CBQCA) assay (Thermo Fisher Scientific). RIPA (1% Igepal-630, 0.5% Deoxycholate, 0.1% SDS in PBS) extracts of IVRs were diluted 1:100 before addition of the fluorescent CBQCA reagent according to the manufacturer's instructions. The reagent reacted with any biological material containing free amine groups to generate a fluorescent signal. The standard curve was generated using bovine serum albumin (BSA) as instructed.
Sample Size and Statistical Methods
For this phase I study, sample size was based on similarly sized studies. We expected the PK profiles of the topical and oral regimens to be sufficiently different that statistical comparisons could be made. There was no imputation of missing data. Independent TEAEs were summarized by treatment group and compared by the Fisher exact test. Normality of continuous data were tested using the PROC univariate command in SAS software, version 9.4 (Carey, NC) and then the normal quantile plot distribution and the Shapiro–Wilk test statistic were examined. We compared continuous end points from the TDF/FTC vs. TFV IVR cohorts using an independent sample t test for normally distributed data (eg, age, creatinine clearance) or Wilcoxon–Mann–Whitney test for nonnormally distributed data (PK concentrations). For categorical variables from independent samples (eg, race, ethnicity, and contraceptive use) we used the χ2 statistic or Fisher exact tests as indicated by expected cell size. The mean values from the 2 cohorts were compared using an analysis of covariance model with the PD end point as the dependent variable, treatment group (consisting of 2 levels: TDF/FTC and TFV IVR) as a factor, and baseline value of the PD end point as a covariate. All tests were 2-sided and performed to the 0.05 alpha level of significance.
Comparisons of end points from the same cohort in ectocervical vs. vaginal samples were performed using a paired t test or Wilcoxon signed rank sum test on difference variables (eg, changes in HIV-1BaL inhibition between baseline and posttreatment). For the linear correlations between PD and PK data, a Spearman correlation coefficient was calculated for nonnormally distributed data. Additional statistical modeling of PD and PK relationships was performed with R Project software (University of Auckland), using the 4-parameter log-logistic regression32,33 to fit log-transformed PD end point (HIV inhibition in vitro) vs. TFV and FTC in CVF and TFV-DP and FTC-TP in tissue. In the fitted dose response curve, the concentration of TFV, FTC, TFV-DP, and FTC-TP required to reach 50% inhibition was estimated by extrapolation from the curve. Statistical significance was determined at the level of alpha = 0.05.
RESULTS
Patient Disposition
The first patient was screened for the study in May 2016, and the last patient visit was in July 2016. Twenty-six women provided informed consent and were screened, and 22 women completed all study visits (Fig. 1). Table 1 demonstrates that there were no significant differences between the 2 treatment groups. Each participant was exposed to treatment for 14 days overall, and all participants self-reported 100% compliance with the assigned treatment and activity restrictions.
FIGURE 1.
CONRAD A15-140 flow diagram.
TABLE 1.
Demographics of the 2 Drug Treatment Groups
| Variable | TFV IVR Cohort (n = 10) | TDF/FTC Cohort (N = 12) | P | ||||
| Mean | SD | Median | Mean | SD | Median | ||
| Age, yrs | 39.8 | 6.39 | 39.0 | 39.4 | 5.57 | 40.5 | 0.93 |
| BMI (kg/m2) | 31.46 | 6.82 | 30.12 | 30.55 | 7.62 | 29.39 | 0.77 |
| Creatinine clearance (mL/min) | 144.07 | 59.24 | 157.85 | 144.26 | 36.46 | 130.18 | 0.99 |
| Hemoglobin (g/dL) | 12.96 | 0.79 | 13.25 | 12.77 | 1.12 | 12.85 | 0.65 |
| Ethnicity | N | % | N | % | 1.00 | ||
| Hispanic | 0 | 0 | 2 | 17 | |||
| Non-Hispanic | 10 | 100 | 10 | 83 | |||
| Race | N | % | N | % | 1.00 | ||
| Black or African American | 4 | 40 | 4 | 33 | |||
| White | 6 | 60 | 8 | 67 | |||
| Contraception | 0.86 | ||||||
| Abstinence | 1 | 10 | 1 | 8 | |||
| Tubal ligation or tubal occlusion | 8 | 80 | 8 | 67 | |||
| Condoms | 0 | 0 | 2 | 17 | |||
| Oral contraceptive pills | 1 | 10 | 1 | 8 | |||
Safety
Overall, 9 (75%) participants in the TDF/FTC group reported 14 TEAEs vs. 2 (20%) participants in the TFV IVR group (P < 0.01). Six of the 12 (50%) women in the TDF/FTC group vs. no women in the TFV IVR group reported GI TEAEs (P < 0.01). All GI TEAEs (nausea, diarrhea, and vomiting) were assessed as related to TDF/FTC use. One (10%) participant in the TFV IVR cohort reported bladder discomfort, which was assessed as related to IVR use, and 1 (10%) TFV IVR user experienced mild vaginal pain after 1 genital biopsy procedure. All TEAEs were assessed as mild or moderate. There were no TEAEs that led to participant withdrawal or modification of study treatment. The TEAEs assessed as moderate included nausea reported by 2 (17%) participants and diarrhea reported by 1 (8%) participant in the TDF/FTC group. All TEAEs in the TFV IVR group were assessed as mild.
PK of Topically Delivered Antiretrovirals vs. Orally Delivered Antiretrovirals (ARVs)
Table 2 demonstrates that oral TDF/FTC users had significantly higher plasma concentrations of TFV, whereas TFV IVR users had significantly higher local ectocervical and vaginal tissues and CVF concentrations of TFV. In the TFV IVR treatment group, median TFV concentrations in ectocervical and vaginal tissues were >104 ng/g and median TFV ectocervical and vaginal fluid concentrations were >106 ng/mL. There was a nonsignificant trend (P = 0.08) toward higher concentrations of TFV in vaginal vs. ectocervical fluids (Table 2). The median TFV-DP concentrations in cervical and vaginal tissues were >106 fmol/g with IVR use, with significantly higher concentrations of TFV and TFV-DP in vaginal vs. ectocervical tissues (P values < 0.01) (Table 2).
TABLE 2.
Systemic and Local Pharmacokinetics (PK) of Tenofovir and Emtricitabine, Active Metabolites, and Competing Nucleotides After 14 days of TDF/FTC or TFV IVR Use
| Matrix | Oral TDF/Emtricitabine Cohort (n = 12) | Tenofovir Intravaginal Ring Cohort (n = 10) | P | ||||||
| N | Mean | CoVa (%) | Median | N | Mean | CoVa (%) | Median | ||
| Plasma (ng/mL) | |||||||||
| TFV | 12 | 166.70 | 44.4 | 168.50 | 10 | 1.68 | 93.9 | 1.78 | <0.01 |
| FTC | 12 | 909.69 | 84.9 | 1140 | NA | NA | NA | NA | NA |
| Ectocervical fluid (ng/mL) | |||||||||
| TFV | 12 | 506.76 | 995.25 | 72.85 | 10 | 809,389 | 555,626 | 710,508 | <0.01 |
| FTC | 12 | 2468 | 2792 | 1523 | NA | NA | NA | NA | NA |
| Vaginal fluid (ng/mL) | |||||||||
| TFV | 12 | 1136 | 1867 | 435.05 | 10 | 1,401,801 | 788,231 | 1,299,804 | <0.01 |
| FTC | 12 | 5758 | 8294 | 3199 | NA | NA | NA | NA | NA |
| Ectocervical tissue (ng/g) | |||||||||
| TFV | 12 | 102.76 | 38.1 | 97.80 | 10 | 13,609 | 138.2 | 11,809 | <0.01 |
| FTC | 12 | 965.69 | 39.70 | 962.36 | NA | NA | NA | NA | NA |
| Vaginal tissue (ng/g) | |||||||||
| TFV | 12 | 87.37 | 50.4 | 82.59 | 10 | 35,718 | 119.6 | 25,988 | <0.01 |
| FTC | 12 | 903.79 | 51.9 | 1037.40 | NA | NA | NA | NA | NA |
| Active metabolite in ectocervical tissue (fmol/g) | |||||||||
| TFV-DP | 12 | 19,743 | 106.6 | 14,695 | 10 | 1,960,243 | 154 | 1,846,671 | <0.01 |
| FTC-TP | 12 | 281,518 | 79.3 | 343,256 | NA | NA | NA | NA | NA |
| Active metabolite in vaginal tissue (fmol/g) | |||||||||
| TFV-DP | 12 | 17,085 | 57.8 | 15,044 | 10 | 12,348,111 | 105.1 | 11,044,730 | <0.01 |
| FTC-TP | 12 | 310,363 | 81.3 | 300,092 | NA | NA | NA | NA | NA |
| Competing nucleotides in ectocervical tissue (fmol/g) | |||||||||
| dATP | 12 | 126,182 | 51.7 | 131,825 | 10 | 131,205 | 65.2 | 134,950 | 0.71 |
| dCTP | 12 | 92,656 | 52.6 | 97,769 | 10 | 103,812 | 55.2 | 99,354 | 0.61 |
In the oral dosing cohort, TFV was quantifiable in all plasma and most (9/12 75%) CV fluid samples (LLOQ = 0.3 ng/mL). FTC was quantifiable in all plasma and all genital tissues and fluids (Table 2). The median TFV and FTC concentrations in CV tissues were around 90 and 1000 ng/g, respectively. TFV, FTC, and FTC-TP were quantifiable in all ectocervical and vaginal tissues after 14 days of oral dosing (Table 2). TFV-DP tissue concentrations were below the limits of quantification (BLQ) in 18 of 24 (75%) cervical and vaginal samples (sample-specific LLOQ range 16,839–87,813 fmol/g). TDF/FTC users had significantly higher concentrations of TFV and FTC in vaginal vs. ectocervical fluids (P values 0.04 and 0.01, respectively; data displayed in Table 2). There were no significant differences in TFV-DP or FTC-TP concentrations between ectocervical and vaginal tissues, however (P values 0.98 and 0.37, respectively; data displayed in Table 2). Regarding competing endogenous nucleotides, dATP and dCTP, there were no significant differences between the 2 dosing cohorts (Table 2, and see Fig. 1, Supplemental Digital Content, http://links.lww.com/QAI/B738).
Inhibition of HIV-1 in vitro by Ectocervical and Vaginal Fluids
There were no statistically significant differences between the inhibitory activity of vaginal vs. ectocervical fluids against HIV-1BaL at baseline or after treatment for participants taking TDF/FTC (all P values > 0.33) or using the TFV IVR (all P values > 0.29) (data displayed in Table 2, Supplemental Digital Content, http://links.lww.com/QAI/B738), and so inhibition data for both vaginal and ectocervical fluids are combined in Figure 2A, B. Both TDF/FTC and TFV IVR cohorts experienced significant increases in the inhibitory capacity of ectocervical or vaginal fluids against HIV-1BaL in vitro with treatment compared with that at baseline (all P values < 0.01) (Fig. 2A). The posttreatment inhibitory capacity of CVF was significantly higher with TFV IVR use compared with TDF/FTC use (95.3 vs. 49.8% inhibition; P < 0.01) (Fig. 2B). One participant assigned to TDF/FTC and 2 TFV IVR users had contamination of the in vitro cultures with yeast or bacteria and were excluded, so the analysis was conducted for 11 of the 12 participants in the TDF/FTC group and for 8 of the 10 participants in the TFV IVR group.
FIGURE 2.
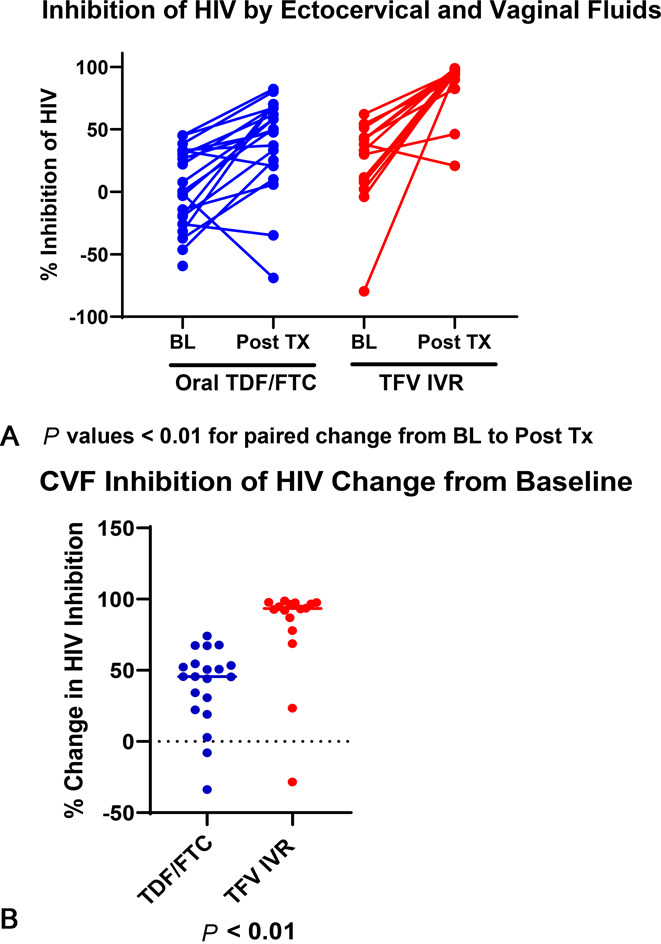
A, Inhibition of HIV by ectocervical and vaginal fluids. B, CVF inhibition of HIV change from baseline.
Correlation Between Drug Concentrations (PK) and in vitro HIV Inhibition (PD)
In the TDF/FTC cohort, when ectocervical and vaginal fluid concentrations were combined, FTC concentrations in CV fluid were significantly correlated with HIV-1BaL inhibition by CVFs (Spearman R = 0.49, P = 0.04) (see Fig. 2a, Supplemental Digital Content, http://links.lww.com/QAI/B738). TFV concentrations in CVF, however, were not correlated with HIV-1 inhibition in vitro (R = 0.26, P = 0.29) in oral pill users (see Fig. 2b, Supplemental Digital Content, http://links.lww.com/QAI/B738). Using a log-logistic regression curve, the concentrations of TFV and FTC required to reach 50% HIV inhibition in CVF in vitro were similar to those found in vaginal fluids of oral TDF/FTC users (Table 2) and in agreement with the observed magnitude of HIV inhibition in their CVFs (see Table 2, Digital Content, http://links.lww.com/QAI/B738).
Both ectocervical and vaginal fluid concentrations of TFV (R = 0.65, P < 0.001) (see Fig. 2c, Supplemental Digital Content, http://links.lww.com/QAI/B738) and ectocervical and vaginal tissue concentrations of TFV (R = 0.49, P = 0.05) and TFV-DP (R = 0.59, P = 0.02) had significant linear correlations with HIV inhibition in CVF in TFV IVR users.
Correlation Between Drug Concentrations and Ex Vivo HIV Inhibition in Ectocervical Explants
We used tissue explants from 4 donors from our local explant protocol and 5 from NDRI. The mean (SD) age of the 9 donors was 45.3 (±6.7) years (range 37–56 years). Tissues cultured for 21 days showed a dose response for antiviral activity (Fig. 3A, B) as measured by cumulative P24 production in culture. Compared with explants exposed to HIV-1BaL only, there was a significant decrease in P24 antigen production (P < 0.05) when explants were infected in the presence of 10 µg/mL of TFV alone. This concentration, 10,000 ng/g, is comparable with the TFV tissue concentrations observed in TFV IVR arm of the clinical study (Table 2). Ex vivo treatment of explants with FTC 0.1 µg/mL and 1 µg/mL partially and completely, respectively, inhibited, HIV tissue infection measured by P24 antigen production when compared with HIV only control (Fig. 3A, B). These concentrations, 100 and 1000 ng/g, are comparable with those observed in tissues obtained from women in the daily oral TDF/FTC arm of the clinical study (Table 2). A cooperative inhibitory effect on P24 antigen production was seen when explants were treated with both TFV and FTC at the lowest dose of 0.1 µg/mL (100 ng/g TFV and 100 ng/g FTC; Fig. 3A, B). No protection was found for explants exposed to 0.1 µg/mL of TFV alone. This TFV concentration, 100 ng/g, was comparable with that observed in tissues of women in the TDF/FTC cohort (Table 2).
FIGURE 3.
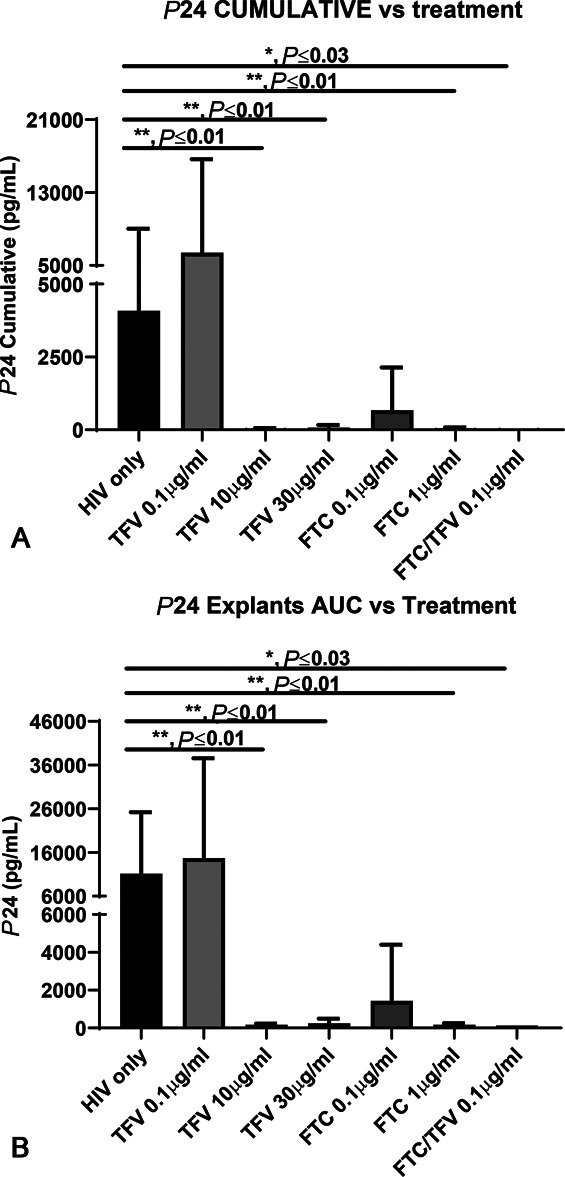
A, p24 CUMULATIVE vs. treatment. B, p24 explants AUC vs. treatment.
Objective Biomarkers of IVR Adherence
All 10 (100%) returned TFV IVRs were able to be processed. The mean residual glycerin concentration in the IVR core was 0.51 mM (±0.45 mM) with a median concentration of 0.31 mM (range 0–1.28 mM). The mean penetrated CBQCA concentration in the IVR core was 5536 µg/mL (±6521 µg/mL) with a median concentration of 3545 µg/mL (range 1920–23,590 µg/mL). Based on previous validation data,31 these values are consistent with proper, per protocol, use of the IVR.
DISCUSSION
It is well recognized that adherence to oral PrEP, particularly among AGYW, remains key to preventing vaginal acquisition of HIV-1. Although randomized controlled trials have demonstrated that taking 4 doses per week of oral TDF/FTC is strongly protective for men who have sex with men, multiple PK studies demonstrate that concentrations of active metabolite, TFV-DP, are about 100 times higher in rectal tissues compared with vaginal tissues.24,34,35 Thus, more robust adherence to oral PrEP is probably needed to protect women from vaginal acquisition of HIV-1.34,36 Indeed, PK/PD modeling using a PD target derived from an in vitro infection model suggests that 6–7 doses of TDF/FTC per week are needed to protect 100% of a simulated population from vaginal acquisition of HIV-1.36
Both HIV-1 PrEP products used in this clinical study, TDF/FTC oral tablets and TFV IVR, were safe during 14 consecutive days of use. Although no treatment had to be discontinued because of TEAEs, there were more GI TEAEs and product-related and moderate TEAEs with systemically delivered oral TDF/FTC compared with topically applied TFV. In previous studies demonstrating efficacy of TDF/FTC in preventing HIV-1 infection,2–4 participants receiving active study drug (TDF/FTC2–4 and TDF2) reported GI TEAEs more frequently compared with placebo users, particularly in the first 4 weeks of use.3
This study's data are consistent with previous data by our group and others on the PK of topically applied TFV (TFV and TFV/LNG IVRs18 and TFV gel37–41). As expected, topically applied TFV resulted in lower TFV plasma concentrations, significantly below therapeutic and prophylactic levels,22–24 and concomitantly higher CV tissue TFV-DP concentrations compared with those measured with TDF/FTC39,41 use. CV tissue concentrations of TFV-DP and fluid concentrations of TFV in IVR users exceeded those associated with protection in both nonhuman primate42,43 (TFV-DP tissue concentrations > 1000 fmol/mg) and clinical studies34,44 (TFV CVF concentrations > 1000 ng/mL). Of importance, concentrations associated with protection remain high for several days after IVR removal.18,45
Among oral PrEP users, our PK data are consistent with previous studies of TDF/FTC given to women as a single dose24,35 or daily for 14 days28 or 5–6 weeks.23,39 Oral intake of TDF/FTC resulted in high levels of plasma analytes, which have been associated with therapeutic and prophylactic activity.22,25,36 In CV tissues, however, TFV and TFV-DP were below prophylactic concentrations, whereas FTC concentrations were compatible with HIV inhibition.36 In a previous study of 14 days of TDF/FTC dosing (CONRAD 137 study, ClinicalTrials.gov NCT02904369), we found CVF and tissue concentrations of TFV, FTC, TFV-DP, and FTC-TP similar to those measured in this study.28 Of note, in the MTN 001 study, a cross-over, multicompartmental PK study, using 6 weeks of daily TDF/FTC and/or TFV vaginal gel, 81% of vaginal tissue samples had TFV-DP concentrations below the LLOQ39 after 6 weeks of TDF/FTC use in 144 US and African women. This rate of below the LLOQ samples is similar to what we found in this study (75%) and in our previous PK study (63%). Similarly, in the daily dosing arm of HPTN 066, TFV-DP concentrations in vaginal tissue homogenate were mostly below LLOQ with a maximum of 41 fmol/mg after 5 weeks of daily, directly observed, dosing.23 FTC-TP was quantifiable in all tissues in this study. FTC-TP was not measured in the MTN 001 study.39 We report higher concentrations of FTC in vaginal tissue compared with the HPTN 066 study, but similar FTC CVF concentrations.23
There is debate regarding whether systemically delivered HIV PrEP products act locally in lower genital tract tissues or systemically in the blood compartment (ie, PBMCs) and at more distant lymphoid tissues. Although our study provides modeled PD data to support local mucosal activity of both TDF/FTC and TFV IVR, the direct comparison of PK profiles suggests a larger role for FTC at the CV mucosal level in TDF/FTC users. TDF may play a more important role in systemic and rectal protection. For the systemic compartment, TFV benchmarks were established from the Partners PrEP study, which demonstrated a 75% overall efficacy of TDF/FTC and TDF in reducing HIV-1 acquisition compared with placebo.25 In a post hoc analysis of seroconverters (n = 29) vs. matched controls (n = 196), 70% of uninfected individuals had plasma TFV concentrations of > 40 ng/mL, consistent with steady state dosing vs. 17% of seroconverters.25 The estimated protective effect of PrEP against HIV, based on concentrations >40 ng/mL, was 88% for individuals receiving TDF and 91% for participants receiving TDF/FTC.25 As expected, we found plasma TFV concentrations exceeding this plasma benchmark among oral TDF/FTC users but not TFV IVR users.
CVF concentrations of TFV and FTC delivered orally through TDF/FTC tablets were correlated with in vitro functional modeling of HIV-1 inhibition in CVF. Although early HIV-1 infection of the lower female genital tract occurs at the CV tissue level and not in CV luminal fluid,46 these data serve as a surrogate of antiviral activity in the CV compartment. Because it is not feasible to obtain tissue biopsies from seroconverters in large phase III studies of HIV-1 PrEP products, CVF concentrations of TFV34,44 have been used as benchmarks for HIV-1 prevention.
In addition, the range of median concentrations of FTC found in CVF (1.5–3.2 ug/mL) is consistent with other groups' demonstrations of partial HIV inhibitory activity in vitro.24,36 High CVF concentrations of FTC with corresponding anti-HIV activity are consistent with detailed PK studies in women demonstrating high penetration of FTC into CV tissue.24 On the contrary, among TDF/FTC users, we found that CVF concentrations of TFV were not correlated with local antiviral activity. The median concentrations of TFV found in CVF (0.07–0.4 ug/mL) in this study are not sufficient, alone, to display complete HIV inhibitory activity in vitro,36 as shown by our PK/PD correlations and confirmed by our collaborators in vitro modeling system.36 The situation is different in the rectal compartment, where, as demonstrated in a previous study, unlike FTC, TFV-DP concentrations after 1 dose of TDF/FTC are 100 times higher in rectal than in CV tissue.24
We also modeled local antiviral activity in tissue by treating ectocervical explants with TFV and FTC at concentrations seen after in vivo TDF/FTC or TFV IVR use (Table 2) and confirmed that FTC seems to be key to the local action of oral TDF/FTC. TFV alone at 0.1 µg/mL did not inhibit P24 antigen production after ex vivo HIV exposure (Fig. 3). Conversely, FTC at 1 ug/mL, a concentration found in CV tissues and fluids after oral administration of TDF/FTC (Table 2), showed complete protection against HIV tissue infection ex vivo (Fig. 3). TFV and FTC showed cooperative effect, apparently better than additive, at 0.1 ug/mL in the ex vivo tissue explant system, together being able to completely inhibit HIV infection.
The synergistic activity of these drugs is consistent with other reports describing that the metabolism of TFV to TFV-DP and FTC to FTC-TP, in human T leukemic cell line and human PBMCs, was more efficient, resulting in higher antiviral inhibition, when cells were treated in vitro with the combination of TFV and FTC compared with either drug alone.47 When human ectocervical explants were exposed ex vivo to median TFV concentrations detected in tissues from study participants in both cohorts, we observed that 10,000 ng/g of TFV, a concentration seen in tissues of TFV IVR users, was able to block HIV-1 infection. These data suggest that 14 days of TFV IVR use is enough to protect CV tissues against HIV-1 infection. HIV-1 infection was almost completely inhibited in explants treated with 1000 ng/mL of FTC or the combination of 100 ng/mL of FTC and TFV, suggesting that adherent TDF/FTC users would be protected from HIV-1 infection at the CV tissue level, in addition to any protection derived from high plasma concentrations.
A limitation of the explant ex vivo treatment and infection assay is that we were not able to confirm the actual concentrations of TFV, FTC, TFV-DP, and FTC-TP in the treated explants. However, we have internal unpublished and reported data that support a good correlation between culture medium and tissue concentrations of parent and active metabolites after ex vivo treatment of ectocervical and vaginal tissues with TFV and FTC.48 In addition, explants are obtained from consenting women who are undergoing indicated hysterectomies. Although explants are an excellent source of lower genital tract tissue, there are no exclusion criteria based on concomitant medications and coexisting medical conditions at the time of surgery, so in general, explants come from women who are generally less healthy and older than the participant population enrolled in this study.
Our PK/PD modeling data support that daily oral TDF/FTC exerts antiviral activity at the CV mucosal level. The mucosal activity of TDF/FTC is likely mainly due to the FTC component, with a possible additive effect by TFV. This is consistent with previous single dose and steady state PK studies in women showing high genital tract concentrations of FTC, compared with plasma concentrations49 or rectal concentrations.24 In this study, FTC had higher concentrations in genital tract tissues after oral dosing, compared with TFV.49 However, the Partner's PrEP study supported a 71% efficacy of TDF alone compared with placebo among women,2 which may suggest an additional systemic component in the protection mediated by TDF. Lower concentrations of TFV in the CV compartment, however, do not rule out a partially inhibitory or synergistic antiviral effect at the mucosal level.
TFV concentrations in regional draining lymph nodes with TFV IVR use in humans is unknown but have been detected in rabbits50 and might offer a second line of protection for topical TFV delivery. Our data also support that the TFV IVR, but not TDF/FTC use, achieved ectocervical and vaginal fluid concentrations (0.7–1.3 mg/mL), which are expected to prevent HSV-2 acquisition (>10,000–200,000 ng/mL).51,52 These high levels of mucosal TFV lead to persistently high levels of TFV-DP,34,39,44 which confer protection for several days endowing the TFV IVR with the forgiveness that other prevention methods, including oral TDF/FTC, cannot afford.
As stated earlier, one of the limitations of this study is the lack of direct PD data using CV biopsies from trial participants and the inability to verify the concentrations of active metabolites, TFV-DP and FTC-TP, in the ex vivo treated surgical explants before exposure to HIV-1BaL. Other limitations include having to change the randomization scheme because of expiration dates of the TFV IVR batch. In spite of this change, the 2 cohorts did not differ regarding demographics. This was an open-label study, but the collaborating laboratories did not know the participant's product assignment, and therefore, this randomization issue likely did not affect study end point evaluations. We included combined oral contraceptive pill (OCP) users but excluded women using DMPA because previous data from our group supported that DMPA, but not OCP use, affects TFV mucosal PK after TFV gel use, increasing the concentration of TFV-DP in vaginal tissues.53 We also excluded levonorgestrel intrauterine system users because we did not have data on the impact of this contraceptive on mucosal TFV PK.
The strengths of this study include longitudinal follow-up with systemic and local PK characterization and PD modeling of both topical and oral TFV among a cohort of well-screened healthy women. In addition to measuring adherence by self-report, which may be subject to social desirability bias,54,55 our group developed objective biomarkers of IVR use,31 which demonstrated good IVR adherence in this study. Plasma PK values for TDF/FTC users suggested high adherence as well.
These data demonstrate that both oral and topical PrEP showed in vitro efficacy against HIV-1BaL and resulted in measurable concentrations of parent compounds (TFV, FTC) and active metabolites (TFV-DP and FTC-TP) in the genital tract. The PK/modeled PD correlations point to FTC as the most active component, locally at the CV mucosal level, in oral TDF/FTC users, whereas TFV may add protection systemically and rectally and is the only active component locally in TFV IVR users. Although PK/modeled PD correlations and inferences have limitations, discussed earlier, our data are consistent with other group's suggestions of the mucosal contributions of TFV-DP and FTC-TP in the lower female genital tract.24,36,49
In conclusion, based on the described PK/PD profiles of TFV IVR and TDF/FTC oral tablets in women, both approaches would show antiviral activity at the CV mucosal level, in addition to any possible systemic protection conferred by high ARV levels in blood after oral TDF/FTC. TFV levels in the CV compartment were much higher than those of TDF/FTC. Although both PrEP approaches have pros and cons, they provide users with choice, a factor of paramount importance in driving uptake of and adherence to HIV prevention methods.
Supplementary Material
ACKNOWLEDGMENTS
This study was made possible by the generous support of the American people through the United States Agency for International Development (USAID) and the US President's Emergency Plan for AIDS Relief (PEPFAR) under Cooperative Agreement AID-OAA-A-14-00011. The contents of this article are the sole responsibility of CONRAD and do not necessarily reflect the views of USAID, PEPFAR, or the US Government. Tenofovir active pharmaceutical ingredient for manufacture of the intravaginal ring was provided by Gilead Sciences (Foster City, CA).
Footnotes
Supported by CONRAD/EVMS with funds from the United States Agency for International Development (USAID) and the US President's Emergency Plan for AIDS Relief (PEPFAR) under a Cooperative Agreement (AID-OAA-A-14-00011) with EVMS. The contents of this article do not necessarily reflect the views of USAID, PEPFAR, or the US Government.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
Contributor Information
Louise A. Ouattara, Email: YaoAL@evms.edu.
Terry A. Jacot, Email: jacotta@evms.edu.
Mackenzie Cottrell, Email: mlcottre@email.unc.edu.
Craig Sykes, Email: craig_sykes@unc.edu.
Kimberly Blake, Email: khblake1024@gmail.com.
Xi Fang, Email: xifang@uwm.edu.
Susan Ju, Email: shjuxx@gmail.com.
Nikolas C. Vann, Email: vannnc@evms.edu.
Gustavo F. Doncel, Email: doncelgf@evms.edu.
REFERENCES
- 1.United Nations. Global AIDS Update 2018Miles to Go: Closing Gaps, Breaking Barriers, Righting Injustices. Available at: http://wwwunaidsorg/sites/default/files/media_asset/miles-to-go_enpdf. 2018. Accessed January 10, 2021. [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. [DOI] [PubMed] [Google Scholar]
- 5.Riddell Jt, Amico KR, Mayer KH. HIV preexposure prophylaxis: a Review. JAMA. 2018;319:1261–1268. [DOI] [PubMed] [Google Scholar]
- 6.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrazzo J, Rabe L, Kelly C, et al. Association of tenofovir (TFV) detection with reduced risk of herpes simplex virus type-2 (HSV-2) acquisition in the VOICE (MTN 003) study. AIDS Res Hum Retroviruses. 2014;30(suppl 1):A31. [Google Scholar]
- 10.Delany-Moretlwe S, Lombard C, Baron D, et al. Tenofovir 1% vaginal gel for prevention of HIV-1 infection in women in South Africa (FACTS-001): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2018;18:1241–1250. [DOI] [PubMed] [Google Scholar]
- 11.Hussain A, Ahsan F. The vagina as a route for systemic drug delivery. J Control Release. 2005;103:301–313. [DOI] [PubMed] [Google Scholar]
- 12.Alexander NJ, Baker E, Kaptein M, et al. Why consider vaginal drug administration? Fertil Steril. 2004;82:1–12. [DOI] [PubMed] [Google Scholar]
- 13.Nel A, Kapiga S, Bekker LG, et al. Safety and Efficacy of Dapivirine Vaginal Ring for HIV-1 Prevention in AFrican Women. Vol. 2016. Boston, MA: CROI; 2016. [Google Scholar]
- 14.Baeten JM, Palanee-Phillips T, Brown ER, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016;375:2121–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thurman AR, Clark MR, Hurlburt JA, et al. Intravaginal rings as delivery systems for microbicides and multipurpose prevention technologies. Int J Womens Health. 2013;5:695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nel A, van Niekerk N, Kapiga S, et al. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med. 2016;375:2133–2143. [DOI] [PubMed] [Google Scholar]
- 17.Clark JT, Clark MR, Shelke NB, et al. Engineering a segmented dual-reservoir polyurethane intravaginal ring for simultaneous prevention of HIV transmission and unwanted pregnancy. PLoS One. 2014;9:e88509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thurman AR, Schwartz JL, Brache V, et al. Randomized, placebo controlled phase I trial of safety, pharmacokinetics, pharmacodynamics and acceptability of tenofovir and tenofovir plus levonorgestrel vaginal rings in women. PloS One. 2018;13:e0199778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koetsawang S, Ji G, Krishna U, et al. Microdose intravaginal levonorgestrel contraception: a multicentre clinical trial. II. Expulsions and removals. World health organization. Task force on long-acting systemic agents for fertility regulation. Contraception. 1990;41:125–141. [DOI] [PubMed] [Google Scholar]
- 20.Montgomery ET, van der Straten A, Cheng H, et al. Vaginal ring adherence in sub-Saharan Africa: expulsion, removal, and perfect use. AIDS Behav. 2012;16:1787–1798. [DOI] [PubMed] [Google Scholar]
- 21.Smith DJ, Wakasiaka S, Hoang TD, et al. An evaluation of intravaginal rings as a potential HIV prevention device in urban Kenya: behaviors and attitudes that might influence uptake within a high-risk population. J Womens Health (Larchmt). 2008;17:1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendrix CW, Andrade A, Bumpus NN, et al. Dose frequency ranging pharmacokinetic study of tenofovir-emtricitabine after directly observed dosing in healthy volunteers to establish adherence benchmarks (HPTN 066). AIDS Res Hum Retroviruses 2016;32:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patterson KB, Prince HA, Kraft E, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3:112re114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donnell D, Baeten JM, Bumpus NN, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr. 2014;66:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson TJ, Clark MR, Albright TH, et al. A 90-day tenofovir reservoir intravaginal ring for mucosal HIV prophylaxis. Antimicrob Agents Chemother. 2012;56:6272–6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei X, Decker JM, Liu H, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46:1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thurman AR, Schwartz JL, Cottrell ML, et al. Safety and pharmacokinetics of a tenofovir alafenamide fumarate-emtricitabine based oral antiretroviral regimen for prevention of HIV acquisition in women: a randomized controlled trial. EClinicalMedicine. 2021;36:100893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson-Harman N, Hendrix CW, Bumpus NN, et al. Correlation between compartmental tenofovir concentrations and an ex vivo rectal biopsy model of tissue infectibility in the RMP-02/MTN-006 phase 1 study. PloS One. 2014;9:e111507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richardson-Harman N, Lackman-Smith C, Fletcher PS, et al. Multisite comparison of anti-human immunodeficiency virus microbicide activity in explant assays using a novel endpoint analysis. J Clin Microbiol. 2009;47:3530–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacot TA, Clark MR, Adedipe OE, et al. Development and clinical assessment of new objective adherence markers for four microbicide delivery systems used in HIV prevention studies. Clin Transl Med. 2018;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VØlund A. Application of the four-parameter logistic model to bioassay: comparison with slope ratio and parallel line models. Biometrics. 1978:357–365. [PubMed] [Google Scholar]
- 33.Prinz H. Hill coefficients, dose–response curves and allosteric mechanisms. J Chem Biol. 2010;3:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karim SS, Kashuba AD, Werner L, et al. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet. 2011;378:279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louissaint NA, Cao YJ, Skipper PL, et al. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses. 2013;29:1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cottrell ML, Yang KH, Prince HM, et al. A translational Pharmacology approach to predicting outcomes of preexposure prophylaxis Against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis.. 2016;214:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz JL, Rountree RW, Kashuba ADM, et al. A multi-compartment, single and multiple dose pharmacokinetic study of the vaginal candidate microbicide 1% tenofovir gel. PLoS One. 2011;6:e25974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thurman AR, Chandra N, Yousefieh N, et al. Differences in local and systemic TFV PK among premenopausal versus postmenopausal women exposed to TFV 1% vaginal gel. J Acquir Immune Defic Syndr. 2018;78:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hendrix CW, Chen BA, Guddera V, et al. MTN-001: randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PloS one. 2013;8:e55013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herold BC, Chen BA, Salata RA, et al. Impact of sex on the pharmacokinetics and pharmacodynamics of 1% tenofovir gel. Clin Infect Dis. 2016;62:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thurman AR, Schwartz JL, Brache V, et al. Effect of hormonal contraception on pharmacokinetics of vaginal tenofovir in healthy women: increased tenofovir diphosphate in injectable depot medroxyprogesterone acetate users. J Acquir Immune Defic Syndr. 2019;80:79–88. [DOI] [PubMed] [Google Scholar]
- 42.Dobard C, Sharma S, Martin A, et al. Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue. J Virol. 2011;86:718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parikh UM, Dobard C, Sharma S, et al. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J Virol. 2009;83:10358–10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kashuba AD, Gengiah TN, Werner L, et al. Genital tenofovir concentrations correlate with protection against HIV infection in the CAPRISA 004 trial: importance of adherence for microbicide effectiveness. J Acquir Immune Defic Syndr. 2015;69:264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thurman AR, Schwartz JL, Ravel J, et al. Vaginal microbiota and mucosal pharmacokinetics of tenofovir in healthy women using tenofovir and tenofovir/levonorgestrel vaginal rings. PLoS One. 2019;14:e0217229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–139. [DOI] [PubMed] [Google Scholar]
- 47.Borroto-Esoda K, Vela JE, Myrick F, et al. In vitro evaluation of the anti-HIV activity and metabolic interactions of tenofovir and emtricitabine. Antivir Ther. 2006;11:377–384. [PubMed] [Google Scholar]
- 48.Ouattara A, Clark M, Doncel G, et al. HIVR4P 2016 Conference. In: Time of Addition Studies of Elvitegravir/TFV Prodrug combinations Demonstrate Extended Window of prophylactic Activity in Cell and tissue Models. Vol. P08.01. Chicago, IL; 2016. [Google Scholar]
- 49.Dumond JB, Yeh RF, Patterson KB, et al. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS. 2007;21:1899–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clark MR, Friend DR. Pharmacokinetics and topical vaginal effects of two tenofovir gels in rabbits. AIDS Res Hum Retroviruses. 2012;28:1458–1466. [DOI] [PubMed] [Google Scholar]
- 51.Abdool Karim SS, Abdool Karim Q, Kharsany AB, et al. Tenofovir gel for the prevention of herpes simplex virus type 2 infection. N Engl J Med. 2015;373:530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andrei G, Lisco A, Vanpouille C, et al. Topical tenofovir, a microbicide effective against HIV, inhibits herpes simplex virus-2 replication. Cell Host Microbe. 2011;10:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thurman A, Schwartz JL, Brache V, et al. The effect of hormonal contraception on pharmacokinetics of vaginal tenofovir in healthy women: increased tenofovir diphosphate in injectable depot medroxyprogesterone acetate users. J Acquir Immune Defic Syndr. 2018;80:79–88. [DOI] [PubMed] [Google Scholar]
- 54.Mensch BS, Brown ER, Liu K, et al. Reporting of adherence in the VOICE trial: did disclosure of product nonuse increase at the termination visit? AIDS Behav. 2016;20:2654–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mngadi KT, Maarschalk S, Grobler AC, et al. Disclosure of microbicide gel use to sexual partners: influence on adherence in the CAPRISA 004 trial. AIDS Behav. 2014;18:849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.