BACKGROUND:
The main symptoms of chemotherapy-induced peripheral neuropathy (CIPN) include pain and numbness. Neuronal G protein–coupled receptor kinase 2 (GRK2) plays an important role in various pain models. Cisplatin treatment can induce the activation of proinflammatory microglia in spinal cord. The purpose of this study was to investigate the role of spinal neuronal GRK2 in cisplatin-induced CIPN and in the prevention of CIPN by electroacupuncture (EA).
METHODS:
The pain and sensory deficit behaviors of mice were examined by von Frey test and adhesive removal test. The expression of neuronal GRK2 in the spinal cord is regulated by intraspinal injection of adeno-associated virus (AAV) containing neuron-specific promoters. The protein levels of GRK2, triggering receptor expressed on myeloid cells 2 (TREM2), and DNAX-activating protein of 12 kDa (DAP12) in spinal dorsal horn were detected by Western blot, the density of intraepidermal nerve fibers (IENFs) was detected by immunofluorescence, and microglia activation were evaluated by real-time polymerase chain reaction (PCR).
RESULTS:
In this study, cisplatin treatment led to the decrease of GRK2 expression in the dorsal horn of spinal cord. Overexpression of neuronal GRK2 in spinal cord by intraspinal injection of an AAV vector expressing GRK2 with human synapsin (hSyn) promotor significantly inhibited the loss of IENFs and alleviated the mechanical pain and sensory deficits induced by cisplatin. Real-time PCR analysis showed that the overexpression of neuronal GRK2 significantly inhibited the messenger RNA (mRNA) upregulation of proinflammatory cytokine interleukin (IL)-1β, IL-6, inducible nitric oxide synthase (iNOS), and M1 microglia marker cluster of differentiation (CD)16 induced by cisplatin. Furthermore, the TREM2 and DAP12, which has been demonstrated to play a role in microglia activation and in the development of CIPN, were also downregulated by overexpression of neuronal GRK2 in this study. Interestingly, preventive treatment with EA completely mimics the effect of overexpression of neuronal GRK2 in the spinal cord in this mouse model of cisplatin-induced CIPN. EA increased GRK2 level in spinal dorsal horn after cisplatin treatment. Intraspinal injection of AAV vector specifically downregulated neuronal GRK2, completely reversed the regulatory effect of EA on CIPN and microglia activation. All these indicated that the neuronal GRK2 mediated microglial activation contributed to the process of CIPN.
CONCLUSIONS:
Neuronal GRK2 in the spinal cord contributed to the preventive effect of EA on CIPN. The neuronal GRK2 may be a potential target for CIPN intervention.
KEY POINTS.
Question: What is the molecular mechanism of chemotherapy-induced peripheral neuropathy (CIPN)–induced pain and sensory deficit?
Findings: Overexpression of spinal neuronal G protein–coupled receptor kinase 2 (GRK2) can alleviate cisplatin-induced pain and sensory deficit, and specific downregulation of neuronal GRK2 can reverse the preventive effect of electroacupuncture (EA) on CIPN.
Meaning: Upregulation of spinal neuronal GRK2 can inhibit cisplatin-induced CIPN and inhibit the activation of spinal proinflammatory microglia, so GRK2 may be used as a molecular target for the prevention and treatment of CIPN.
Chemotherapy-induced peripheral neuropathy (CIPN) is one of the common dose-limiting adverse effect of chemotherapy.1 Symptoms of CIPN include numbness and hyperalgesia of the feet or hands in the distribution of stockings and gloves, accompanied with the loss of intraepidermal nerve fibers (IENFs). This seriously affects the quality of life of patients.2 Our previous results showed that microglia was activated in the spinal cord by cisplatin treatment.3 Activated microglia have 2 phenotypes, classic activation M1 and alternate activation M2 phenotype.4 M1 microglia secrete a variety of proinflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α.4,5 In contrast, M2 microglia produced anti-inflammatory cytokines such as IL-10, IL-4, and tumor growth factor (TGF)-β.4
G protein–coupled receptor kinase 2 (GRK2) is a serine/threonine kinase that inhibits signal transduction by promoting desensitization or internalization of G protein–coupled receptors (GPCRs) and/or by interacting with multiple components of intracellular signaling pathways.6 It has been documented that downregulation of GRK2 in spinal cord is involved in the regulation of pain signal transmission in different models.7–9 In a rat model of chronic constriction injury of sciatic nerve, neuronal GRK2 expression was decreased in spinal cord.7 In the inflammatory pain model of mice injected carrageenan,10 prostaglandin E2 (PGE2),8 IL-1β,11 or GPCR combined with C-C motif chemokine ligand 3 (CCL3),10 the pain duration of GRK2+/− transgenic mice was significantly longer than that of wild-type mice. Overexpression of neuronal GRK2 can inhibit the transition from acute inflammatory pain to chronic pain.6,12 However, there is no evidence that neuronal GRK2 plays a role in cisplatin-induced CIPN.
Electroacupuncture (EA) is a combination of acupuncture and electrical stimulation techniques.13 Clinical practice showed that acupuncture has significant efficacy and safety in the treatment of peripheral neuropathy caused by chemotherapy.14 Previous studies have shown that EA at “Zusanli” can alleviate the mechanical allodynia induced by paclitaxel and the cold hyperalgesia induced by oxaliplatin.15,16 Moreover, EA can inhibit the level of OX-42 (microglia marker) and the release of proinflammatory cytokines IL-1β, IL-6, and TNF-α in complete Freund’s adjuvant (CFA)-induced inflammatory pain of rats. It is suggested that EA may relieve pain by regulating M1 microglia.17 This study further explored the role of spinal neuronal GRK2 in EA effect in alleviating cisplatin-induced pain and sensory deficit.
METHODS
Animals
Animals were purchased from Shanghai SLAC Laboratory Animal Co, Ltd. Young adult male C57BL/6 mice (9–10 weeks, 22–25 g) were housed under a 12-h light/12-h dark cycle at a room temperature of 23 ± 0.5 °C with water and food available ad libitum. Mice were habituated at least 1 week before the experimental manipulations. All experiments were conducted in accordance with the National Institutes of Health guidelines and Use of Laboratory Animals and the Ethical Issues of the International Association for the Study of Pain. We obtained Animal Care and Use animal experimentation committee approval to perform this study.
Induction of CIPN Model in Mice
Cisplatin was obtained from Sigma-Aldrich. An accumulated dose of 23 mg/kg cisplatin was intraperitoneally (i.p.) injected by 2 rounds of single injection for 5 days delivery with 5 days break in between.3 The same volume of normal saline was injected in the control group.
Intraspinal Adeno-associated Virus Injections
The virus was injected into spinal dorsal horn 3 weeks before cisplatin injection (Supplemental Digital Content 1, Methods, Table 1, http://links.lww.com/AA/D706). The mice were deeply anesthetized and then placed in a mobile stereotactic frame, the spine was fixed with a pair of spinal adapters, and the surface of spinal cord between T1-L1 vertebrae was exposed. A microscopic needle (outer diameter of 1.14 mm, inner diameter of 0.53 mm) was inserted into the dorsal spinal cord at a depth of 200 to 300 μm. The rate of injection was controlled at 60 nL/min by using a motorized perfusion system (Nanoliter 2010, World Precision Instruments). The microscopic needle was left in place for 5 minutes after the injection. The wound was sutured and the animals were put on a heat mat until recovery from anesthesia. For overexpression of the neuronal GRK2 in spinal cord, mice were injected with a 2:1 volume mixture of an adeno-associated virus (AAV)2/8 carrying CMV-DIO-GRK2-2A-EGFP and an AAV2/8 carrying human synapsin-cyclization recombinase estrogen receptor tamoxifen 2 (hSyn-CreERT2) (OBiO Technology Co Ltd) by a CRE-dependent manner, and CRE activity was induced by single tamoxifen (1 mg in 100 μL, i.p.) injections for 5 days after 3 weeks; tamoxifen was first dissolved in 95% ethanol and then diluted to its final concentration in corn oil. For downregulation of the neuronal GRK2 expression in spinal cord, mice were injected with 600 nL of an AAV2/9 carrying GRK2 shRNA with a neuronal-specific promoter Syn (OBiO Technology Co Ltd).
Behavioral Test
Mechanical allodynia was measured by using a series of von Frey hairs (0.02, 0.04, 0.07, 0.16, 0.4, 0.6, 1.0, and 1.4 g; Stoelting), and adhesive removal test was performed to observe tactile sensitivity of mice as described previously.3,18
EA Treatment
As described previously,19 during the EA treatment, the mice were loosely immobilized by using a special designed holder, the head, and 4 limbs left free of movement. Two pairs of stainless steel needles (0.16 mm in diameter) were inserted into “Kunlun” (BL60, at the ankle joint level and between the tip of the external malleolus and tendo calcaneus) and “Zusanli” (ST36, 5 mm lateral to the anterior tubercle of tibia) at a depth of 2 and 3 mm, respectively. Alternating trains of dense-sparse frequencies (100 and 2 Hz each for 3 seconds alternately), 1 and 2 mA for 15 minutes, respectively, were delivered by Han’s Acupoint Nerve Stimulator (LH202, Huawei Co Ltd). The EA treatment was given once a day from the day before the injection of cisplatin. For sham EA treatment, the same manipulation was used, but there was no electrical stimulation or manual needle operation.
Western Blot
The spinal tissues were separated and then placed into cold lysis buffer and homogenized on ice using a tissue homogenizer. After homogenizing with the sonifier (Hielscher), the lysate was centrifuged at 12,000 rpm for 20 minutes at 4 °C and the total protein level was measured using the Pierce bicinchoninic acid (BCA) Protein Assay Kit (Thermo Scientific). Western blotting was performed according to a previously published method using primary antibodies (Supplemental Digital Content 1, Methods, Table 2, http://links.lww.com/AA/D706). After incubation with the corresponding secondary antibody (1:10,000, Proteintech), Western blot images were captured using an ImageQuant LAS4000 mini image analyzer (GE Healthcare) and analyzed using ImageJ software (version 1.47).
Immunofluorescence
IENFs Staining
IENFs staining was performed as described previously.20 Skin biopsies (3 × 3 mm2) from the central plantar surface of the hind paws were immediately placed in Zamboni’s fixative for 24 hours, and then transferred to 20% and 30% sucrose for at least 24 hours, respectively. The biopsies were sliced into 25-µm-thick sections, and the sections were incubated with rabbit anti-protein gene product (PGP) 9.5 and their corresponding fluorescent secondary antibodies (1:1000, Invitrogen). The sections were observed under laser confocal microscopy (FV10i, Olympus). Relative immunofluorescence density was evaluated by using ImageJ (version 1.47).
Spinal Cord Staining
The mice were anesthetized with 1% pentobarbital sodium and perfused with normal saline followed by 4% formaldehyde. The spinal cord of the L4-L6 segments were postfixed in 4% formaldehyde for 4 hours at 4 °C, then transferred in 20% and 30% sucrose in 0.1 M phosphate-buffered saline (PBS), respectively, at 4 °C overnight. Thirty-micrometer-thick spinal cord sections were prepared. The sections were blocked at room temperature for 2 hours in Superblock Buffer (Thermo). The sections were transferred from the blocking solution to the primary antibodies (Supplemental Digital Content 1, Methods, Table 2, http://links.lww.com/AA/D706) overnight at 4 °C in shaking incubator. Then it reacted with alexa-488 or 594-conjugated secondary antibodies (1:1000, Invitrogen) at room temperature for 2 hours after having been washed 2 times in phosphate-buffered saline Tween-20 (PBST). Then the sections were fixed onto the slides with sealing solution containing 4',6-diamidino-2-phenylindole (DAPI) (Southern Biotechnology, 0100-20). Images were taken with a confocal microscope (FV10i, Olympus).
Real-Time Polymerase Chain Reaction
Tissues of L4-6 spinal dorsal horn were homogenized using TRIzol reagent (Invitrogen LA) and total RNA was extracted. Reverse transcription was performed by using PrimeScript RT reagent Kit with gDNA Eraser (Takara) and SYBR Premix Ex Taq II (Takara) for real-time quantitative polymerase chain reaction (qPCR) analysis in the end (Supplemental Digital Content 1, Methods, Table 3, http://links.lww.com/AA/D706).
Statistical Analysis
All data are presented as mean ± standard error of the mean (SEM). The statistical significance of differences between groups was analyzed with Student t test, 1-way or 2-way analysis of variance (ANOVA) following Tukey post-test. A P value <.05 was considered to be statistically significant. The number of animals is determined based on the results of preliminary experiments. The dispersion of adhesive removal test behavior results is large, and it is difficult to count the difference when the number of animals is small, more animals may be needed for the result to be more reliable. To determine an appropriate sample size, we tested the number of animals used in our previous experiments (von Frey N = 5–12; adhesive removal test N = 6–14) and related literature (von Frey N = 6–16; adhesive removal test N = 7–16) using the G*power analysis program. We used G*power in our methods to calculate needed effect size (eg, per sample size calculations provided by G*power) and then cite G*power in our references using 1 of the 2 citations provided on this website: http://www.gpower.hhu.de/. Our analysis indicated a sample size of X was sufficient for 80% power and an α < .05 when examining.
RESULTS
Neuronal GRK2 Alleviated CIPN
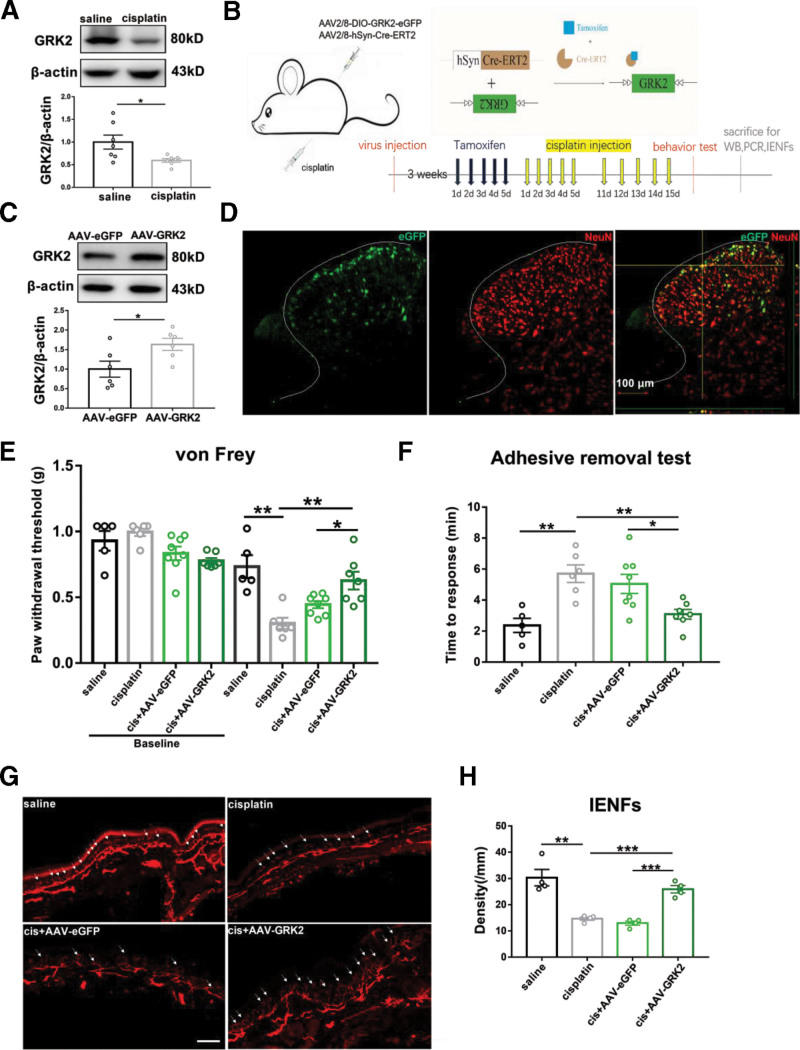
Previous studies have shown that overexpression of neuronal GRK2 significantly reduced hyperalgesia in a mouse model of inflammatory pain.8 In this study, the protein level of GRK2 in the spinal cord was declined after 5 injections of cisplatin (Figure 1A). Next, neuronal GRK2 expression was specifically upregulated by intraspinal virus injection and tamoxifen induction (Figure 1B). The animals are divided into 4 groups: namely saline, cisplatin, cisplatin + AAV–enhanced green fluorescent protein (eGFP), and cisplatin + AAV-GRK2. Intraspinal injection of AAV-GRK2 virus significantly increased the level of GRK2 in the spinal cord (Figure 1C), and AAV-GRK2 expressed eGFP (green) was colocated with neuronal nuclei (NeuN) labeled (red) neurons in the spinal cord (Figure 1D). At the third week after the first injection of cisplatin, overexpression of neuronal GRK2 significant attenuated cisplatin-induced mechanical allodynia and sensory deficits, and it also prevented the reduction in IENFs density (Figure 1E–H). The results suggested that the upregulation of neuronal GRK2 in spinal cord can prevent the neuropathy induced by cisplatin.
Figure 1.
Overexpression of neuronal GRK2 in spinal cord–alleviated cisplatin-induced peripheral neuropathy. A, Western blot analysis of GRK2 after 5 injections of cisplatin. Results are normalized to β-actin and shown as ratios to saline-treated mice. Values are mean ± SEM. *P < .05, P = .0259. B, Experimental strategy for neuronal GRK2 overexpression. Neuron-specific promoter hSyn targeted Cre-ERT2 recombination activated by tamoxifen. C, Tamoxifen-activated Cre-ERT2 upregulated the expression of GRK2 in the spinal cord. Results are normalized to β-actin. Values are mean ± SEM. *P < .05, P = .0334. D, Representative images show NeuN labeling (red) and eGFP-tagged (green) AAV-GRK2 in the spinal cord. Scale bar, 100 μm. E, Upregulation of neuronal GRK2 prevented cisplatin-induced reduction of PWT. Values are mean ± SEM. *P < .05, **P < .01. Saline versus cisplatin P = .0011; cis + AAV-eGFP versus cis + AAV-GRK2 P = .0198; cisplatin versus cis + AAV-GRK2 P = .0024. F, Upregulation of neuronal GRK2 shortened cisplatin-induced prolonged responsive time. Values are mean ± SEM. *P < .05, **P < .01. Saline versus cisplatin P = .0016; cis + AAV-eGFP versus cis + AAV-GRK2 P = .0178; cisplatin versus cis + AAV-GRK2 P = .0014. G, Immunofluorescence staining of PGP 9.5 positive nerve fibers (white arrow) in the hind paw (scale bar =100 mm). H, Upregulation of neuronal GRK2 inhibited cisplatin-induced IENFs loss. Values are mean ± SEM. **P < .01, ***P < .001. Saline versus cisplatin P = .0027; cis + AAV-eGFP versus cis + AAV-GRK2 P = .0002; cisplatin versus cis + AAV-GRK2 P = .0004. AAV indicates adeno-associated virus; Cre-ERT2, cyclization recombinase estrogen receptor tamoxifen 2; eGFP, enhanced green fluorescent protein; GRK2, G protein–coupled receptor kinase 2; hSyn, human synapsin; IENFs, intraepidermal nerve fibers; NeuN, neuronal nuclei; PCR, polymerase chain reaction; PGP, protein gene product; PWT, paw withdrawal threshold; SEM, standard error of the mean; WB, Western blot.
Neuronal GRK2 Inhibited Spinal Cord Inflammation and Microglia Activity
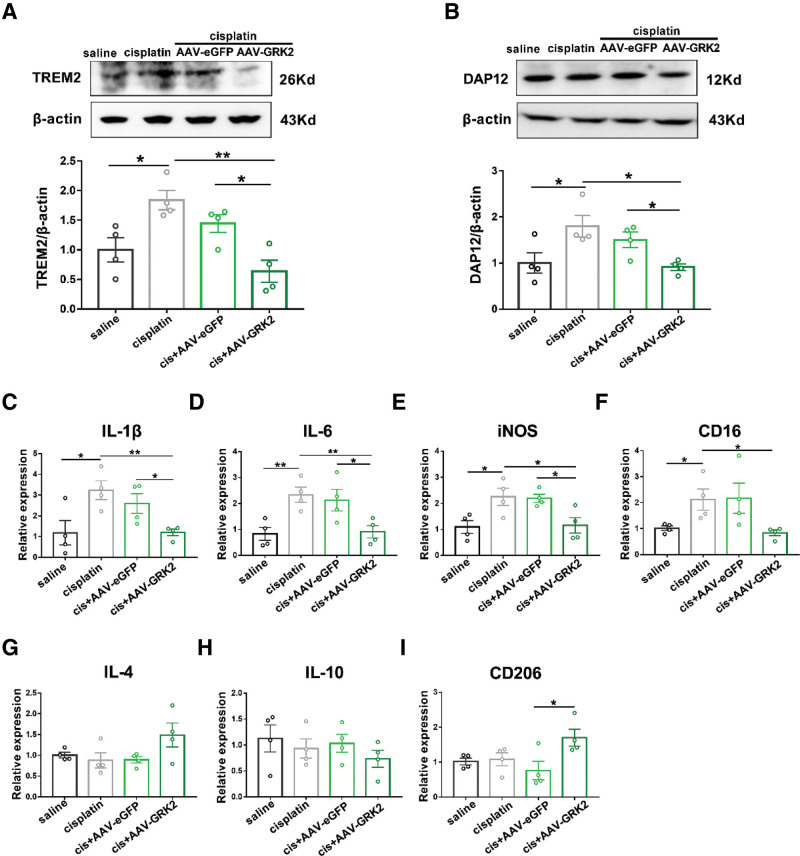
Study have shown that cisplatin treatment can induce spinal cord inflammation and microglia activation mediated by triggering receptor expressed on myeloid cells 2 (TREM2)/DNAX-activating protein of 12 kDa (DAP12) signaling pathway.3 Inhibition of TREM2 or DAP12 expression can reduce the inflammatory response of microglia and relieve pain.3,21 Consistent with previous studies,3 cisplatin increased the protein levels of TREM2 (Figure 2A) and DAP12 (Figure 2B) in the spinal cord. Overexpression of neuronal GRK2 significantly inhibited the upregulation of TREM2 (Figure 2A) and DAP12 (Figure 2B) protein induced by cisplatin in spinal dorsal horn. In this study, after cisplatin injection, the messenger RNA (mRNA) levels of proinflammatory cytokines IL-1β, IL-6, inducible nitric oxide synthase (iNOS), and proinflammatory microglial marker cluster of differentiation (CD)16 were significantly increased (Figure 2C–F). However, cisplatin did not change the mRNA levels of anti-inflammatory cytokines IL-4, IL-10, and anti-inflammatory microglia marker CD206 (Figure 2G–I). Upregulation of neuronal GRK2 significantly inhibited the increase of proinflammatory cytokines (IL-6, IL-1β, iNOS) and CD16 induced by cisplatin (Figure 2C–F), but it displayed no effect on anti-inflammatory cytokines (IL-4, IL-10) (Figure 2G, H). It should be noted that compared with AAV-eGFP mice, upregulation of neuronal GRK2 significantly increased the mRNA level of CD206 (Figure 2I). These results suggest that spinal neuronal GRK2 plays a role in the regulation of inflammation and microglial activity in cisplatin-induced CIPN.
Figure 2.
Upregulation of neuronal GRK2 altered the proinflammatory activity of spinal microglia and the expression of TREM2/DAP12. Western blot analysis of TREM2 (A) and DAP12 (B) on week 3 after the first cisplatin injection. Results are normalized to β-actin and shown as ratios to saline-treated mice. Values are mean ± SEM. *P < .05, **P < .01. A, Saline versus cisplatin P = .0149; cis + AAV-eGFP versus cis + AAV-GRK2 P = .0187; cisplatin versus cis + AAV-GRK2 P = .0012. B, saline versus cisplatin P = .0482; cis + AAV-eGFP versus cis + AAV-GRK2 P = .0168; cisplatin versus cis + AAV-GRK2 P = .0107. Real-time PCR analysis of pain-related molecules at week 3 after the first cisplatin injection. These molecules were included: IL-1β (C), IL-6 (D), iNOS (E), CD16 (F), IL-4 (G), IL-10 (H), and CD206 (I). Results are normalized to GAPDH. Values are mean ± SEM. *P < .05, **P < .01. C, Saline versus cisplatin P = .0321; cis + AAV-eGFP versus cis + AAV-GRK2 P = .0333; cisplatin versus cis + AAV-GRK2 P = .0054. D, Saline versus cisplatin P = .0078; cis + AAV-eGFP versus cis + AAV-GRK2 P = .0440; cisplatin versus cis + AAV-GRK2 P = .0092. E, Saline versus cisplatin P = .0293; cis + AAV-eGFP versus cis + AAV-GRK2 P = .0218; cisplatin versus cis + AAV-GRK2 P = .0485. F, Saline versus cisplatin P = .0395; cisplatin versus cis + AAV-GRK2 P = .0240. I, Cis + AAV-eGFP versus cis + AAV-GRK2 P = .0392. AAV indicates adeno-associated virus; CD, cluster of differentiation; DAP12, DNAX-activating protein of 12 kDa; eGFP, enhanced green fluorescent protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GRK2, G protein–coupled receptor kinase 2; IL, interleukin; iNOS, inducible nitric oxide synthase; PCR, polymerase chain reaction; SEM, standard error of the mean; TREM2, triggering receptor expressed on myeloid cells 2.
Repeated EA Alleviated Cisplatin-Induced Peripheral Neuropathy
Clinically, acupuncture can reduce the pain caused by CIPN and improve the quality of life.22 To observe the effect of EA on cisplatin-induced CIPN in mice, EA or sham EA treatment was given once a day from the day before cisplatin injection. Three weeks after the first cisplatin injection, the results showed that EA treatment significantly attenuated cisplatin-induced mechanical allodynia, sensory deficits and loss of IENFs, but sham EA treatment had no significant effect on it (Figure 3A–D). Moreover, the expression of GRK2 protein in spinal dorsal horn of mice treated with cisplatin was significantly increased after EA treatment (Figure 3E). Based on these, we speculated that EA treatment alleviated CIPN and prevented the reduction of IENFs by upregulating the expression of GRK2 protein in the spinal cord.
Figure 3.
Multiple EA treatment for prevention of cisplatin-induced peripheral neuropathy and regulation of GRK2 expression. A, EA prevented the decreased in paw withdrawal threshold induced by cisplatin in von Frey test. Values are mean ± SEM. *P < .05, **P < .01. Cis versus cis + EA P = .0021; cis + shEA versus cis + EA P = .0264. B, EA reduced the prolonged response time in adhesive removal test. Values are mean ± SEM. *P < .05, **P < .01. Cis versus cis + EA P = .0387; cis + shEA versus cis + EA P = .0040. C, Immunofluorescence staining of PGP 9.5 positive nerve fibers (white arrow) in the hind paw (scale bar = 100 μm). D, EA inhibited cisplatin-induced IENFs loss. Values are mean ± SEM. ***P < .001. Cis versus cis + EA P = .0002; cis + shEA versus cis + EA P = .0005. E, EA regulated the expression of GRK2 in spinal dorsal horn. Western blot analysis of GRK2 levels after EA. EA treatment reversed the decline of spinal GRK2 induced by cisplatin. Results are normalized to β-actin and shown as ratios to cisplatin-treated mice. Values are mean ± SEM. *P < .05. Cis versus cis + EA P = .0278. EA indicates electroacupuncture; GRK2, G protein–coupled receptor kinase 2; IENFs, intraepidermal nerve fibers; PGP, protein gene product; SEM, standard error of the mean; shEA, sham electroacupuncture.
EA Regulated Inflammatory Response and Microglia Activity in the Spinal Cord
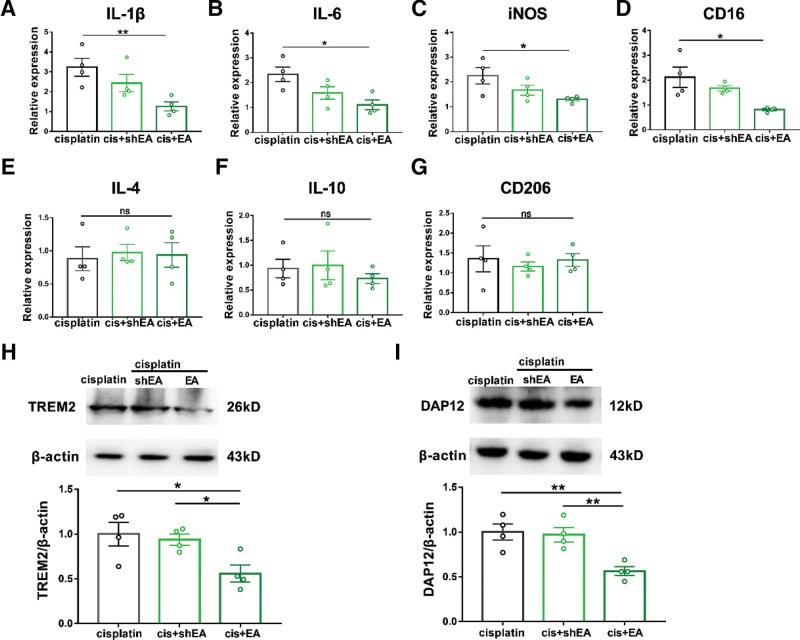
Studies have shown that acupuncture can regulate the secretion of proinflammatory cytokines (including IL-1β, IL-6, and TNF-α).23,24 To study the regulatory effect of EA on inflammatory response and microglia activity in cisplatin-treated mice, real-time polymerase chain reaction (PCR) was used to detect the expression of pain-related molecules in spinal dorsal horn after EA treatment. The results showed that EA significantly reduced the mRNA level of IL-1β, IL-6, and iNOS as well as CD16 in cisplatin-treated mice (Figure 4A–D). However, EA did not change the expression of IL-4, IL-10, and CD206 (Figure 4E–G). The Western blot analysis showed that the expression of TREM2 (Figure 4H) and DAP12 (Figure 4I) in spinal dorsal horn was significantly inhibited after EA treatment. Combined with the fact of preventive effect of minocycline on cisplatin-induced CIPN, our results suggested that the preventive effect of EA on cisplatin-induced CIPN may be related to its inhibitory effect on proinflammatory cytokines and M1 microglia.
Figure 4.
EA inhibited the production of proinflammatory cytokines in the spinal cord. Real-time PCR analysis of pain-related molecules: IL-1β (A), IL-6 (B), iNOS (C), CD16 (D), IL-4 (E), IL-10 (F), and CD206 (G). Results are normalized to GAPDH. Values are mean ± SEM. *P < .05, **P < .01. A, Cis versus cis + EA P = .0079. B, Cis versus cis + EA P = .0130. C, Cis versus cis + EA P = .0299. D, Cis versus cis + EA P = .0199. E, Cis versus cis + EA P = .8290. F, Cis versus cis + EA P = .3768. G, Cis versus cis + EA P = .9430. Western blot analysis of TREM2 (H) and DAP12 (I) on week 3 after the first cisplatin injection. Results are normalized to β-actin and shown as ratios to cisplatin-treated mice. Values are mean ± SEM. *P < .05, **P < .01. H, Cis versus cis + EA P = .0363; cis + shEA versus cis + EA P = .0165. I, Cis versus cis + EA P = .0056; cis + shEA versus cis + EA P = .0051. CD indicates cluster of differentiation; DAP12, DNAX-activating protein of 12 kDa; EA, electroacupuncture; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IL, interleukin; iNOS, inducible nitric oxide synthase; ns, nonsignificant; PCR, polymerase chain reaction; SEM, standard error of the mean; shEA, sham electroacupuncture; TREM2, triggering receptor expressed on myeloid cells 2.
Neuronal GRK2 Mediated EA Alleviating Cisplatin-Induced CIPN
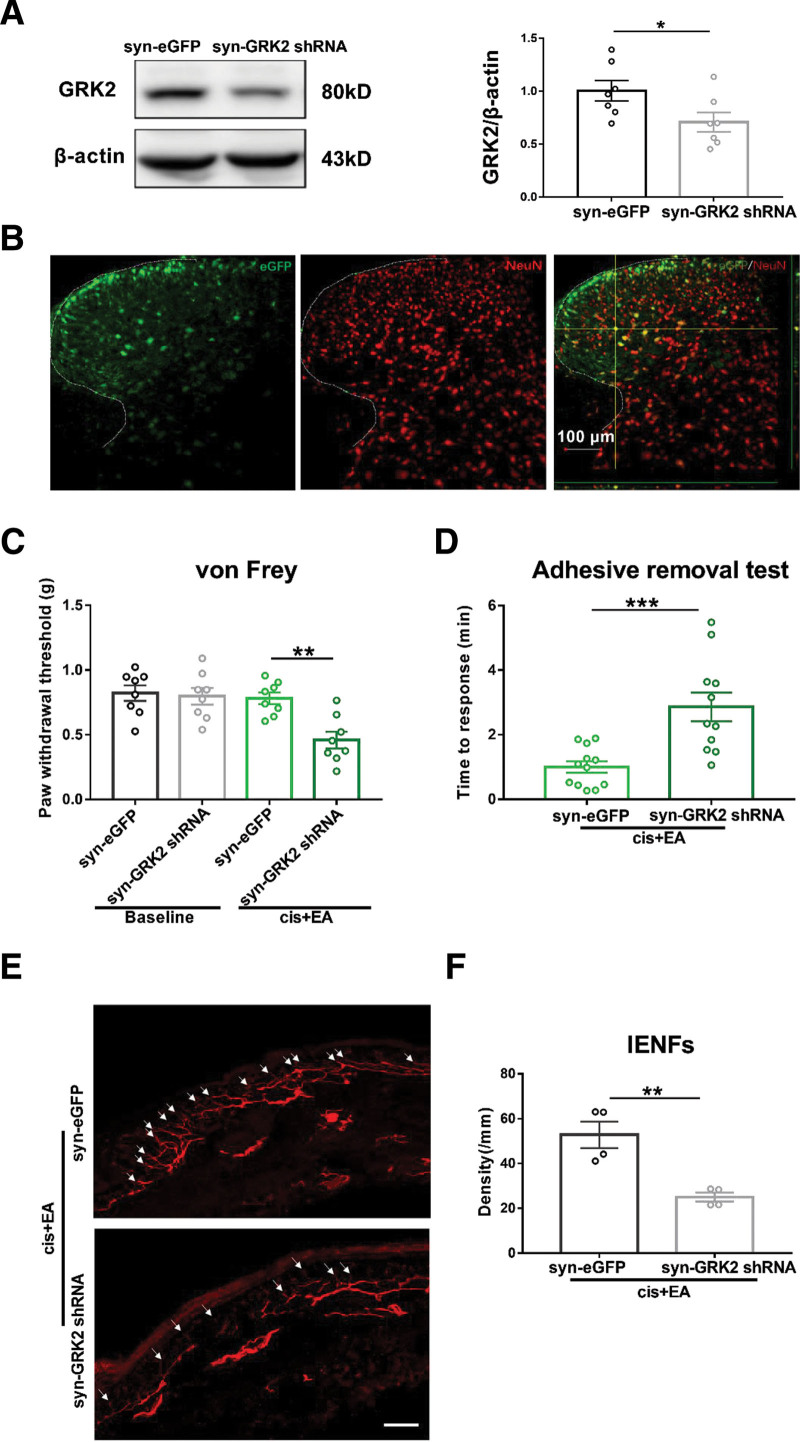
To further investigate whether neuronal GRK2 is involved in EA alleviating cisplatin-induced CIPN, downregulation of neuronal GRK2 in the spinal cord was generated by intraspinal injection with an AAV vector delivering GRK2 shRNA with a neuronal specific promoter synapsin. AAV virus injection reduced the level of GRK2 in the spinal cord (Figure 5A), and AAV expressed eGFP (green) was colocated with NeuN labeled (red) neurons in the spinal cord (Figure 5B). The behavioral tests showed that downregulation of neuronal GRK2 expression in the spinal cord significantly inhibited the effect of EA on mechanical allodynia, sensory deficit, and IENFs loss (Figure 5C–F). These results indicated that the downregulation of neuronal GRK2 significantly inhibited the alleviating effect of EA on cisplatin-induced CIPN.
Figure 5.
Neuronal GRK2 mediated EA alleviating cisplatin-induced CIPN. A, Western blot analysis of down regulated GRK2 protein expression in the spinal dorsal horn of mice 3 wk after the virus administration. Results are normalized to β-actin and shown as ratios to syn-eGFP-treated mice. Values are mean ± SEM. *P < .05. syn-eGFP versus syn-GRK2 shRNA P = .0441. B, Representative images showed NeuN labeling (red) and eGFP-tagged (green) AAV-GRK2 shRNA in the spinal cord. Scale bar = 100 μm. C, Downregulation of neuronal GRK2 in the spinal cord inhibited the analgesic effect of EA in cisplatin-induced mechanical allodynia. Values are mean ± SEM. **P < .01. Cis + EA + syn-eGFP versus cis + EA + syn-GRK2 shRNA P = .0010. D, Downregulation of neuronal GRK2 inhibited the alleviative effect of EA in cisplatin-induced sensory deficits. Values are mean ± SEM. ***P < .001. Cis + EA+ syn-eGFP versus cis + EA+ syn-GRK2 shRNA P = .0006. E, Immunofluorescence staining of PGP 9.5 positive nerve fibers (white arrow) in the hind paw (scale bar =100 mm). F, Downregulation of neuronal GRK2 inhibited the preventive effect of EA on cisplatin-induced IENFs loss. Values are mean ± SEM. **P < .01. Cis + EA+ syn-eGFP versus cis + EA + syn-GRK2 shRNA P = .0043. AAV indicates adeno-associated virus; CIPN, chemotherapy-induced peripheral neuropathy; EA, electroacupuncture; eGFP, enhanced green fluorescent protein; GRK2, G protein–coupled receptor kinase 2; IENFs, intraepidermal nerve fibers; NeuN, neuronal nuclei; PGP, protein gene product; SEM, standard error of the mean; shRNA, short hairpin RNA.
Neuronal GRK2 Mediated the Regulation of EA on Inflammatory Response and Microglia Activity
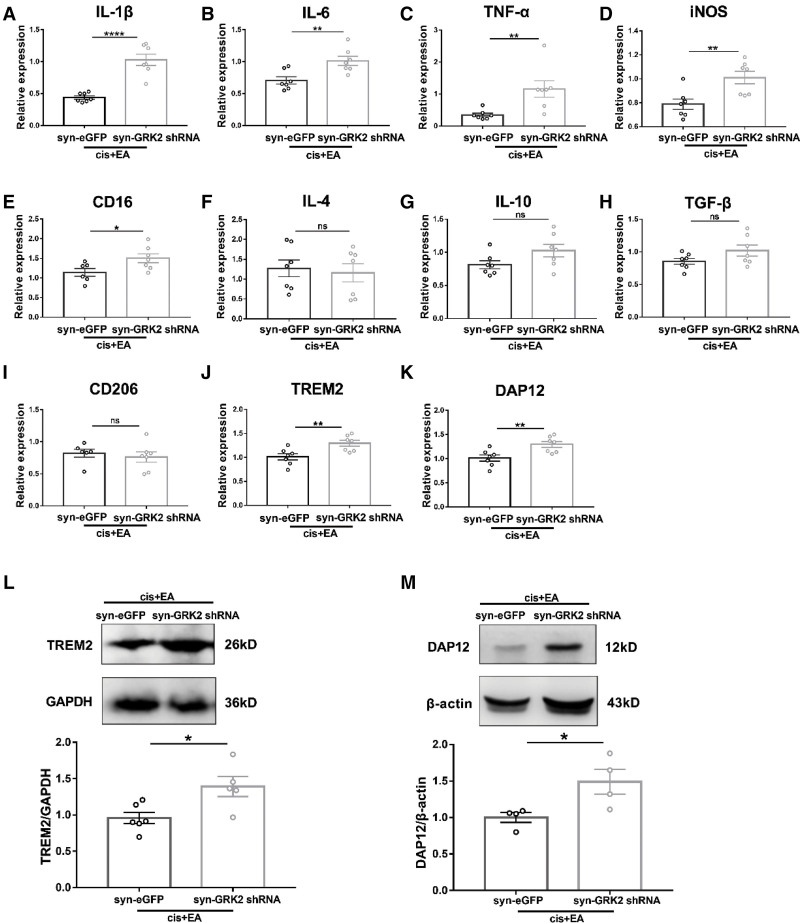
To further verify whether neuronal GRK2 mediates the regulation of EA on microglia inflammatory response, we detected the activation of microglia in spinal dorsal horn of mice treated with cisplatin by real-time PCR after downregulation of neuronal GRK2. The results indicated that downregulation of neuronal GRK2 significantly inhibited the down-regulatory effect of EA on IL-1β, IL-6, TNF-α, iNOS, and CD16 (Figure 6A–E), but it had no effect on IL-4, IL-10, TGF-β, and CD206 (Figure 6F–I). Consistent with this, downregulation of neuronal GRK2 also significantly inhibited the downregulation of mRNA and protein expression of TREM2 (Figure 6J, L) and DAP12 (Figure 6K, M) by EA treatment. These results suggested that downregulation of neuronal GRK2 in spinal cord significantly inhibited the regulation of EA on inflammatory response and microglia activity after cisplatin treatment.
Figure 6.
Neuronal GRK2 mediated the regulation of EA on microglia activation. Real-time PCR analysis of pain-related molecules at week 3 after the first cisplatin injection. These molecules were included IL-1β (A), IL-6 (B), TNF-α (C), iNOS (D), CD16 (E), IL-10 (F), IL-4 (G), and TGF-β (H). Results are normalized to GAPDH. Values are mean ± SEM. *P < .05, **P < .01, ****P < .0001. A, P < .0001. B, P = .0055. C, P = .0093. D, P = .0064. E, P = .0391. F, P = .7324. G, P = .0825. H, P = .1118. I, P = .5767. J, P = .0082. K, P = .0273. Downregulation of neuronal GRK2 inhibited the downregulation of EA on TREM2 (J) and DAP12 (K) mRNA. Downregulation of neuronal GRK2 inhibited the downregulation of EA on TREM2 (L) and DAP12 (M) protein expression. Results are normalized to GAPDH (TREM2) or β-actin (DAP12) and shown as ratios to syn-eGFP treated mice. Values are mean ± SEM. *P < .05. L, P = .0180. M, P = .0372. CD indicates cluster of differentiation; EA, electroacupuncture; eGFP,enhanced green fluorescent protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GRK2, G protein–coupled receptor kinase; IL, interleukin; iNOS, inducible nitric oxide synthase; ns, nonsignificant; PCR, polymerase chain reaction; SEM, standard error of the mean; shRNA, short hairpin RNA; TGF, tumor growth factor; TNF, tumor necrosis factor; TREM2, triggering receptor expressed on myeloid cells 2.
DISCUSSION
We demonstrated that the mechanical allodynia and sensory deficit induced by cisplatin were related to the decreased GRK2 expression in spinal dorsal horn. Overexpression of neuronal GRK2 or EA treatment alleviated cisplatin-induced CIPN, prevented the loss of IENFs, and regulated inflammatory response and microglia activation in spinal cord. Most importantly, EA increased the expression of GRK2 in spinal dorsal horn. Downregulation of neuronal GRK2 inhibited the regulatory effect of EA on CIPN and microglia activation. Therefore, the present study may provide evidence that modulation of the neuronal GRK2 pathway is a potential new and important therapeutic strategy for CIPN.
It has been reported that a link between mechanical allodynia and reduced neuronal GRK2 expression in spinal dorsal horn was observed in rats with a transection of the lumbar spinal nerve25 and in rats with a chronic constriction injury of the sciatic nerve.7 Our current findings show that a nerve injury induced by cisplatin produces significant mechanical allodynia and sensory deficits as well as reduces the density of IENFs. Cisplatin treatment also reduced the expression of GRK2 in spinal dorsal horn. Overexpression of neuronal GRK2 alleviated cisplatin-induced CIPN and prevented the loss of IENFs. The results suggested that the decrease of GRK2 was related to CIPN. Consistent with this, plantar injection of HSV-GRK2 to overexpress GRK2 in dorsal root ganglion (DRG) neurons can reduce inflammation pain.8 Studies have shown that the decrease of GRK2 expression makes cells sensitive to cisplatin-induced apoptosis, while the increase of GRK2 expression can reduce apoptosis.26 Therefore, we speculate that in the model of cisplatin-induced CIPN, the decrease of GRK2 in spinal cord may make neurons more sensitive to cisplatin stimulation, leading to neuronal injury and decrease of IENFs. Overexpression of neuronal GRK2 has protective effect on cisplatin-induced reduction of IENFs.
Acupuncture has been proved to enhance endogenous opiates, such as dynorphin, endorphin, and enkephalin, release corticosteroids, relieve pain, and promote healing process.27 In this study, EA alleviated cisplatin-induced mechanical allodynia and sensory deficit, as well as the decrease of the density of IENFs. Clinical studies have shown that EA can improve the pain, numbness, tingling, and nerve conduction rate of chemotherapy patients.22,28 Studies have shown that the decrease of spinal GRK2 completely reverses the therapeutic effect of EA on inflammatory pain.29 In this study, EA increased the expression of GRK2 protein in spinal dorsal horn after cisplatin treatment, and selective downregulation of neuronal GRK2 expression in spinal cord inhibits the regulatory effect of EA on CIPN.
Activation of glial cells is necessary for the induction of chronic pain.30 Nerve injury can induce the activation of microglia in spinal dorsal horn within a few hours.31 Activated microglia release TNF-α and IL-1β to induce CIPN in rats.32 Studies have shown that inflammation or neuronal injury can induce the decrease of neuronal GRK2 expression mediated by cytokines, thus promoting the increase of neuronal excitability, leading to mechanical allodynia.7 Our results showed that overexpression of neuronal GRK2 inhibited the increase of IL-1β, IL-6, iNOS, and CD16 mRNA levels induced by cisplatin, but did not change the mRNA expression of IL-4 and IL-10. It is worth noting that overexpression of neuronal GRK2 increases the mRNA expression of CD206. Studies have shown that GRK2 directly phosphorylates EPAC1, which can inhibit EPAC1-to-Rap1 signal transduction, thereby inhibiting persistent inflammatory pain.6 Low GRK2 in primary sensory neurons converts epinephrine (EPI)-induced signals from protein kinase A–dependent to protein kinase C ε (PKCε)-dependent pathways, prolonging EPI-induced hyperalgesia.12 In addition, GRK2 is an important regulator of immune cell response in the process of inflammation. The expression level of GRK2 in the brain also regulates the intensity of microglia-mediated inflammation, which may be determined by limiting the local release rate of inflammatory mediators.33 Low nociceptor GRK2 leads to prolonged inflammatory hyperalgesia via biased cyclic adenosine monophosphate (cAMP) signaling from protein kinase A (PKA) to Epac-Rap1, extracellular-regulated protein kinase (ERK)/PKCε pathways.34 The complex GRK2 “interaction group” has many signaling proteins involved in inflammation. The mechanism network of its activity and expression level indicates that GRK2 plays an important role in inflammation.
EA can reduce pain by regulating the activity of spinal microglia and inhibiting neuroinflammation. In collagen-induced arthritis of rats, EA reduced the release of TNF-α, and alleviated inflammation and hyperalgesia.35 EA has shown promise in inhibiting microglial proliferation coupled with improved functional recovery after spinal cord injury in rats.36 In this study, EA treatment also inhibited the increase of IL-1β, IL-6, iNOS, and CD16 mRNA levels after cisplatin treatment, but did not affect the expression of IL-4, IL-10, and CD206.
TREM2 mainly controls the function of 3 cell types, all derived from the myeloid lineage: microglia, osteoclasts, and immature dendritic cells (DCs).37 Microglia are the main cell type that produces TREM2 in the central nervous system (CNS).38 TREM2/DAP12 complex is associated with significant regulation of microglia activity in a variety of neurological diseases.39 TREM2-mediated signal transduction is an important inducer to activate microglia phenotype.40 Cisplatin treatment increased the expression of TREM2 and DAP12 in spinal cord; blocking the TREM2/DAP12 signaling inhibited the microglia activation and the development of CIPN.3 The present results showed that overexpression of neuronal GRK2 or EA treatment inhibited cisplatin-induced increase in TREM2 and DAP12 expression. Selective downregulation of neuronal GRK2 inhibited the regulatory effect of EA on cisplatin-induced inflammation and TREM2/DAP12 signaling pathway. The results indicate that the preventive effect of EA on CIPN may be mediated by spinal neuronal GRK2.
In summary, our study revealed that cisplatin treatment reduced the expression of GRK2 protein in the spinal cord. Upregulation of neuronal GRK2 in spinal cord or EA alleviated mechanical allodynia, sensory deficits, and loss of IENFs, and inhibited cisplatin-induced microglial activation. The specific downregulation of neuronal GRK2 completely reversed the regulatory effect of EA on CIPN and microglia activation. These results further support that neuronal GRK2 in the spinal cord may be an important molecular target for clinical CIPN intervention.
ACKNOWLEDGMENTS
We are grateful for the support from the Innovative Research Team of High-Level Local Universities in Shanghai, the Development Project of Shanghai Peak Disciplines-Integrated Chinese and Western Medicine, and the Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01) and ZJLab.
DISCLOSURES
Name: Xue Ma, BD.
Contribution: This author conceived the idea of experimental design, performed all the experiments, and significantly contributed to the research.
Name: Yu Chen, BD.
Contribution: This author helped with the technical guidance of the experiment and significantly contributed to the research.
Name: Xiao-Chen Li, BD.
Contribution: This author helped with animal experiments and significantly contributed to the research.
Name: Wen-Li Mi, PhD.
Contribution: This author provided some ideas, helped with manuscript revisions, and significantly contributed to the research.
Name: Yu-Xia Chu, PhD.
Contribution: This author provided some ideas, helped with manuscript revisions, and significantly contributed to the research.
Name: Yan-Qing Wang, PhD.
Contribution: This author provided some ideas, helped with manuscript revisions, and significantly contributed to the research.
Name: Qi-Liang Mao-Ying, PhD.
Contribution: This author developed the concept, supervised the project, conceived the experiments, revised the manuscript, and significantly contributed to the research.
This manuscript was handled by: Jianren Mao, MD, PhD.
Supplementary Material
GLOSSARY
- AAV
- adeno-associated virus
- ANOVA
- analysis of variance
- BCA
- bicinchoninic acid
- cAMP
- cyclic adenosine monophosphate
- CCL3
- C-C motif chemokine ligand 3
- CD
- cluster of differentiation
- CFA
- complete Freund’s adjuvant
- CIPN
- chemotherapy-induced peripheral neuropathy
- CNS
- central nervous system
- CRE
- cyclization recombinase
- Cre-ERT2
- CRE-estrogen receptor, tamoxifen 2
- DAP12
- DNAX-activating protein of 12 kDa
- DAPI
- 4',6-diamidino-2-phenylindole
- DCs
- dendritic cells
- DRG
- dorsal root ganglion
- EA
- electroacupuncture
- eGFP
- enhanced green fluorescent protein
- EPI
- epinephrine
- ERK
- extracellular regulated protein kinase
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GPCR
- G protein–coupled receptor
- GRK2
- G protein–coupled receptor kinase 2
- hSyn
- human synapsin
- i.p.
- intraperitoneally
- IENFs
- intraepidermal nerve fibers
- IL
- interleukin
- iNOS
- inducible nitric oxide synthase
- mRNA
- messenger RNA
- NeuN
- neuronal nuclei
- ns
- nonsignificant
- PBS
- phosphate-buffered saline
- PBST
- phosphate-buffered saline Tween-20
- PCR
- polymerase chain reaction
- PGE2
- prostaglandin E2
- PGP
- protein gene product
- PKA
- protein kinase A
- PKCε
- protein kinase C ε
- PWT
- paw withdrawal threshold
- qPCR
- quantitative polymerase chain reaction
- SEM
- standard error of the mean
- shEA
- sham electroacupuncture
- shRNA
- short hairpin RNA
- SYN
- synapsin
- TNF
- tumor necrosis factor
- TGF
- tumor growth factor
- TREM2
- triggering receptor expressed on myeloid cells 2
- WB
- Western blot
Published ahead of print October 15, 2021.
Funding: This study was supported by the National Natural Science Funds of China (81873101, 81473749, 81371247, 81771202, 81971056), the National Key R&D Program of China (2017YFB0403803).
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website.
Reprints will not be available from the authors.
REFERENCES
- 1.Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44:1507–1515. [DOI] [PubMed] [Google Scholar]
- 2.Flatters SJL, Dougherty PM, Colvin LA. Clinical and preclinical perspectives on chemotherapy-induced peripheral,neuropathy (CIPN): a narrative review. Br J Anaesth. 2017;119:737–749. [DOI] [PubMed] [Google Scholar]
- 3.Hu LY, Zhou Y, Cui WQ, et al. Triggering receptor expressed on myeloid cells 2 (TREM2) dependent microglial activation promotes cisplatin-induced peripheral neuropathy in mice. Brain Behav Immun. 2018;68:132–145. [DOI] [PubMed] [Google Scholar]
- 4.Hu X, Leak RK, Shi Y, et al. Microglial and macrophage polarization—new prospects for brain repair. Nat Rev Neurol. 2015;11:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol. 2016;142:23–44. [DOI] [PubMed] [Google Scholar]
- 6.Singhmar P, Huo X, Eijkelkamp N, et al. Critical role for Epac1 in inflammatory pain controlled by GRK2-mediated phosphorylation of Epac1. Proc Natl Acad Sci U S A. 2016;113:3036–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleibeuker W, Ledeboer A, Eijkelkamp N, et al. A role for G protein-coupled receptor kinase 2 in mechanical allodynia. Eur J Neurosci. 2007;25:1696–1704. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Heijnen CJ, van Velthoven CT, et al. Balancing GRK2 and EPAC1 levels prevents and relieves chronic pain. J Clin Invest. 2013;123:5023–5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Won KA, Kim MJ, Yang KY, et al. The glial-neuronal GRK2 pathway participates in the development of trigeminal neuropathic pain in rats. J Pain. 2014;15:250–261. [DOI] [PubMed] [Google Scholar]
- 10.Eijkelkamp N, Heijnen CJ, Willemen HL, et al. GRK2: a novel cell-specific regulator of severity and duration of inflammatory pain. J Neurosci. 2010;30:2138–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willemen HLDM, Eijkelkamp N, Wang H, et al. Microglial/macrophage GRK2 determines duration of peripheral IL-1beta-induced hyperalgesia: contribution of spinal cord CX3CR1, p38 and IL-1 signaling. Pain. 2010;150:550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Heijnen CJ, Eijkelkamp N, et al. GRK2 in sensory neurons regulates epinephrine-induced signalling and duration of mechanical hyperalgesia. Pain. 2011;152:1649–1658. [DOI] [PubMed] [Google Scholar]
- 13.Chai W, Tai Y, Shao X, et al. Electroacupuncture alleviates pain responses and inflammation in a rat model of acute gout arthritis. Evid Based Complement Alternat Med. 2018;2018:2598975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao T, Seidman AD, Piulson L, et al. A phase IIA trial of acupuncture to reduce chemotherapy-induced peripheral neuropathy severity during neoadjuvant or adjuvant weekly paclitaxel chemotherapy in breast cancer patients. Eur J Cancer. 2018;101:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng X, Zhang Y, Li A, et al. The effects of opioid receptor antagonists on electroacupuncture-produced anti-allodynia/hyperalgesia in rats with paclitaxel-evoked peripheral neuropathy. Brain Res. 2011;1414:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moon HJ, Lim BS, Lee DI, et al. Effects of electroacupuncture on oxaliplatin-induced neuropathic cold hypersensitivity in rats. J Physiol Sci. 2014;64:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao J, Wang L, Li Y. Electroacupuncture alleviates the inflammatory response via effects on M1 and M2 macrophages after spinal cord injury. Acupunct Med. 2017;35:224–230. [DOI] [PubMed] [Google Scholar]
- 18.Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav Immun. 2007;21:642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao-Ying QL, Cui KM, Liu Q, et al. Stage-dependent analgesia of electro-acupuncture in a mouse model of cutaneous cancer pain. Eur J Pain. 2006;10:689–694. [DOI] [PubMed] [Google Scholar]
- 20.Mao-Ying QL, Kavelaars A, Krukowski K, et al. The anti-diabetic drug metformin protects against chemotherapy-induced peripheral neuropathy in a mouse model. PLoS One. 2014;9:e100701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi M, Konishi H, Sayo A, Takai T, Kiyama H. TREM2/DAP12 signal elicits proinflammatory response in microglia and exacerbates neuropathic pain. J Neurosci. 2016;36:11138–11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong R, Sagar S. Acupuncture treatment for chemotherapy-induced peripheral neuropathy–a case series. Acupunct Med. 2006;24:87–91. [DOI] [PubMed] [Google Scholar]
- 23.Zijlstra FJ, van den Berg-de Lange I, Huygen FJ, Klein J. Anti-inflammatory actions of acupuncture. Mediators Inflamm. 2003;12:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arrieta Ó, Hernández-Pedro N, Fernández-González-Aragón MC, et al. Retinoic acid reduces chemotherapy-induced neuropathy in an animal model and patients with lung cancer. Neurology. 2011;77:987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleibeuker W, Gabay E, Kavelaars A, et al. IL-1 beta signaling is required for mechanical allodynia induced by nerve injury and for the ensuing reduction in spinal cord neuronal GRK2. Brain Behav Immun. 2008;22:200–208. [DOI] [PubMed] [Google Scholar]
- 26.Pathania AS, Ren X, Mahdi MY, Shackleford GM, Erdreich-Epstein A. GRK2 promotes growth of medulloblastoma cells and protects them from chemotherapy-induced apoptosis. Sci Rep. 2019;9:13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patil S, Sen S, Bral M, et al. The role of acupuncture in pain management. Curr Pain Headache Rep. 2016;20:22. [DOI] [PubMed] [Google Scholar]
- 28.Schroeder S, Meyer-Hamme G, Epplée S. Acupuncture for chemotherapy-induced peripheral neuropathy (CIPN): a pilot study using neurography. Acupunct Med. 2012;30:4–7. [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Liu SB, Li Q, Wang H, Wang YQ, Mao-Ying QL. Downregulation of spinal G protein-coupled kinase 2 abolished the antiallodynic effect of electroacupuncture. Evid Based Complement Alternat Med. 2015;2015:848603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milligan ED, Twining C, Chacur M, et al. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:40–52. [DOI] [PubMed] [Google Scholar]
- 32.Qin B, Li Y, Liu X, Gong D, Zheng W. Notch activation enhances microglial CX3CR1/P38 MAPK pathway in rats model of vincristine-induced peripheral neuropathy. Neurosci Lett. 2020;715:134624. [DOI] [PubMed] [Google Scholar]
- 33.Lombardi MS, van den Tweel E, Kavelaars A, Groenendaal F, van Bel F, Heijnen CJ. Hypoxia/ischemia modulates G protein-coupled receptor kinase 2 and beta-arrestin-1 levels in the neonatal rat brain. Stroke. 2004;35:981–986. [DOI] [PubMed] [Google Scholar]
- 34.Eijkelkamp N, Wang H, Garza-Carbajal A, et al. Low nociceptor GRK2 prolongs prostaglandin E2 hyperalgesia via biased cAMP signaling to Epac/Rap1, protein kinase Cepsilon, and MEK/ERK. J Neurosci. 2010;30:12806–12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye TS, Du ZH, Li ZH, et al. Repeated electroacupuncture persistently elevates adenosine and ameliorates collagen-induced arthritis in rats. Evid Based Complement Alternat Med. 2016;2016:3632168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang K, Wu H, Wang G, Li M, Zhang Z, Gu G. The effects of electroacupuncture on TH1/TH2 cytokine mRNA expression and mitogen-activated protein kinase signaling pathways in the splenic T cells of traumatized rats. Anesth Analg. 2009;109:1666–1673. [DOI] [PubMed] [Google Scholar]
- 37.Bouchon A, Hernández-Munain C, Cella M, Colonna M. A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J Exp Med. 2001;194:1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neumann H, Takahashi K. Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis. J Neuroimmunol. 2007;184:92–99. [DOI] [PubMed] [Google Scholar]
- 39.Konishi H, Kiyama H. Microglial TREM2/DAP12 signaling: a double-edged sword in neural diseases. Front Cell Neurosci. 2018;12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keren-Shaul H, Spinrad A, Weiner A, et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169:1276–1290. e17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.