Abstract
Xanthones from the tropical fruit mangosteen (Garcinia mangostana) display anti-inflammatory and anti-oxidative activities. Here, we isolate and identify potential inducers of the aryl hydrocarbon receptor (AhR) and nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathways from mangosteen using a bioassay-guided strategy. Mangosteen fruit pericarp extracts were subjected to sequential solvent extractions, followed by chromatography coupled with NMR spectroscopy and mass spectrometric analyses for identification and isolation of pure compounds. Isolation of active fractions led to seven prenylated xanthones that were identified and subsequently evaluated for bioactivity. In vitro luciferase reporter cellular assays using H1L6.1c3 (AhR induction) and HepG2-ARE (Nrf2 induction) were used to identify AhR and Nrf2 activators. All seven prenylated xanthones displayed AhR inducing activity, whereas only five xanthones activated Nrf2. Garcinone D (GarD) significantly upregulated AhR/Cyp1a1 and Nrf2/HO-1 protein expression and enhanced zonula occludens-1 and occludin protein levels in HT-29 cells. In addition, GarD inhibited oxidative stress-induced intestinal epithelial barrier dysfunction by enhancing tight junction (TJ) proteins and inhibition of reactive oxygen species production. Inhibition of AhR by pretreating cells with an AhR antagonist revealed that the AhR pathway is required for the improved epithelial barrier functions of GarD. These results highlight a dual mechanism by GarD that confers protection against intestinal epithelial barrier dysfunction.
Keywords: mangosteen, xanthones, garcinone D, AhR receptor, Nrf2, tight junction
1. Introduction
The tropical fruit mangosteen (Garcinia mangostana) is reported to exert biological effects related to risk reduction of various diseases, including cancer, diabetes, neurological disorders, and oxidative/inflammation-associated pathology [1–3]. These biological effects are primarily associated with a class of polyphenols, known as xanthones, that are widely distributed throughout the whole mangosteen plant. At least 70 xanthones have been identified in the mangosteen plant, 50 of which were isolated from the pericarp of the fruit in significantly higher concentrations than in the pulp [4, 5]. Xanthones are a structurally unique class of polyphenols composed of a tricyclic scaffold (C6-C3-C6) that is modified by the addition of isoprene, methoxyl and hydroxyl groups in the A and B rings [2]. This tricyclic planar scaffold is associated with their biological properties, although variations in bioactivities are also dependent on the nature or position of the different functional groups [6–8].
In vitro studies have revealed that mangosteen xanthones are bioaccessible and are metabolized into phase II conjugates [9, 10]. In human intestinal cells lines (i.e., Caco-2 and HT-29 cells), α-mangostin, the most abundant xanthone in mangosteen, is transported across the apical membrane and converted into phase II metabolites suggesting that xanthones are absorbed through the intestinal mucosa. Bioavailability of xanthones has also been reported in various in vivo studies in mice and humans showing unconjugated and conjugated (glucuronated/sulfated) derivative in plasma, serum and liver [11–15]. Since xanthones are absorbed across intestinal epithelial cells (IECs) in the intestinal mucosa, it is possible that these compounds interact with signaling pathways in IECs. Two signaling pathways associated with cellular metabolism in the gastrointestinal tract including the nuclear factor erythroid 2-related factor 2 (Nrf2) and aryl hydrocarbon receptor (AhR) transcriptional pathways have recently attracted considerable attention due to their modulatory roles in gut inflammatory and oxidative stress-related responses [16–19]. Several studies have demonstrated that natural products [20–22] can modulate the AhR and Nrf2 pathways resulting in enhanced intestinal barrier function and inhibition of gut inflammation. Therefore, we hypothesized that, when absorbed into the cells/tissues of the epithelium, xanthones could activate the AhR and Nrf2 pathways and play a role in resisting oxidative stress- and inflammation-induced intestinal epithelial barrier dysfunction.
Prenylated xanthones from mangosteen have been shown to exhibit modulatory effects on Nrf2 signaling in in vitro [23, 24] and in vivo studies [24, 25], albeit none of these studies focused on the possible interaction between xanthones and the Nrf2 pathway in IECs. On the other hand, there is virtually no report on mangosteen-derived or other dietary xanthones functioning as AhR activators. Studies indicate that AhR activation may be a potential approach to restoring tight junction (TJ) barrier function and that AhR activating agents may have potential as therapeutic agents against intestinal pathologies including inflammatory bowel disease (IBD) [19, 26, 27]. Therefore, we tested the hypothesis that mangosteen xanthones could interact with both the Nrf2 and AhR pathways and confer protective effect for intestinal barrier integrity. This study aimed to provide in vitro mechanistic evidence on the ability of xanthones to modulate the Nrf2 and AhR pathways in HT-29 cells, a cell line with intestinal epithelial morphology [28]. We employed a bioassay-guided isolation of mangosteen components to isolate candidates with potential to activate the AhR and Nrf2 pathways. We report, for the first time, that mangosteen xanthones may act as bifunctional activators of the AhR and Nrf2 pathways and enhance intestinal barrier function.
2. Materials and methods
2.1. Chemicals
Freeze-dried powder from mangosteen rind (pericarp) originally produced in China was purchased from a local commercial source. Mangosteen powder was stored in sealed bags at −20°C in darkness until use. Dulbecco’s Minimum Essential medium (DMEM; with L-glutamine), trypsin–ethylenediaminetetraacetic acid (EDTA) and penicillin-streptomycin solutions were purchased from Life Technologies Co. (Grand Island, NY, USA). RIPA (10x) lysis buffer were purchased from Cell Signaling Technology (CST, Danvers, MA, USA). 2’,7’-Dichlorofluorescin diacetate (Cas. 4091-99-0) was purchased from Sigma-Aldrich (St. Louis. MO, USA). Fetal bovine serum (FBS) was obtained from Atlanta Biologicals Inc (Lawrenceville, GA, USA). Prolong Gold Antifade with DAPI (Cat # 8961S) was purchased from CST. Silica gel (6 nm, 70–230 mesh and 230–400 mesh) was obtained from Fisher Scientific (Fair Lawn, NJ, USA). Silica gel 60 F254 pre-coated thin layer chromatography (TLC) plates were obtained from EMD Chemicals Inc. (Gibbstown, NJ, USA). All other chemicals and solvents were of analytical grade and were purchased either from Sigma-Aldrich or Fisher Scientific.
2.2. Cell culture and MTT assay
HT-29 cells (ATCC® HTB-38™) were obtained from ATCC (Manassas, VA). Recombinant mouse hepatoma cells (H1L6.1c3) was a gift from Gregory
Kennedy (University of Alabama at Birmingham) to Jan-Peter van Pijkeren. The antioxidant response element reporter-HepG2 cell line (HepG2-ARE) designed to monitor the Nrf2 antioxidant response pathway activation was purchased from BPS Bioscience, Inc. (San Diego, USA). Cell cultures were maintained in 75-cm2 tissue culture flasks in DMEM supplemented with 10 % v/v FBS, 1% v/v penicillin-streptomycin (10,000 U/ml) and incubated at 37°C with 5% CO2 humidified atmosphere. Cells (80–90% confluence) were subcultured every 3 days, passaging at a 1:5 split ratio. Cell viability was measured using a standard MTT assay [29].
2.3. In vitro AhR and Nrf2 activation assays
AhR activation was assayed using H1L6.1c3 cells following a procedure previously described [30]. Expression of the luciferase gene in H1L6.1c3 cells is driven by dioxin responsive element (DRE) in an AhR-dependent manner [31, 32]. Nrf2 antioxidant response was measured using a recombinant HepG2-ARE with stably integrated luciferase firefly gene under the control of ARE promoters. Briefly, cells (2 × 104 cells/well) were seeded into two 96-well plates (flat clear bottom white polystyrene, Corning) for 24 h (80% confluence); one plate was used to determine AhR/ARE activation and the other to measure cell viability following a standard MTT assay. Cells were then exposed to titrated or fixed concentrations of xanthones (0–25 μM) or mangosteen extracts (0–50 μg/mL) dissolved in DMSO (0.1% final concentration) for 24 h. Controls were DMEM with 0.1% DMSO, the AhR ligand 6-formylindolo[3,2-b] carbazole (FICZ, 500 nM) and the Nrf2 activator tert-butyl hydroquinone (tBHQ, 25 μM). For assessment of AhR and Nrf2 activation, luciferase assay reagent (Bright-Glo Luciferase Assay, Promega) was directly added to the well containing treatment medium at 1:1 ratio (100 μL per well containing 100 μL treatment media). The Bright-Glo reagent causes cell lysis and generates a luminescent signal, which is proportional to AhR or ARE activation. Total luminescence was measured after 2 min using a luminometer (BD moonlight 3010, BD Biosciences). AhR and Nrf2 activation (expressed in relative luminescence units, RLU) were calculated as the fold increase in luminescence relative to the DMSO control after having normalized to cell viability.
2.4. Extraction and solvent partitioning
The steps carried out to extract and direct a semipreparative scale isolation of AhR and ARE-inducing components from mangosteen are summarized in Fig. S1. Mangosteen powder (100 g) was repeatedly (at least 5x) extracted with methylene chloride:methanol (CH:ME, 1:1 v/v). Solvents were removed by rotary evaporation and the resulting residue (45.2 g) was resuspended in water (HPLC grade). This crude water extract was then subjected to sequential liquid partitioning using analytical grade n-hexane (1:1 v/v), ethyl acetate (1:1 v/v), and butanol (1:1 v/v) five times each to afford a hexane-soluble fraction (CHME:HEX), an ethyl acetate-soluble fraction (CHME-EA), a butanol-soluble fraction (CHME-BU) and a residual water-soluble fraction (CHME-WA). Solvents were removed by rotary evaporation at 35°C under vacuum or freeze-drying.
2.5. Bioassay-guided semi-preparative isolation of XRE-inducing xanthones
The AhR-luciferase bioassay (section 2.3) was used to determine fractions that activate AhR. Results indicated that CHME-HEX, CHME:EA, and CHME:BU were sufficiently active with concentration to quadruple (CQ) AhR of 0.43–0.98 μg ml−1 (Table S1) to warrant further fractionation. All three active crude extracts were dissolved again in methanol and subjected to silica column chromatography. Based on TLC separations (samples eluted in 40–60% ethyl acetate in hexane), CHME-HEX (8.1 g) and CHME-EA (30 g) had qualitatively similar TLC patterns and were subsequently pooled (HEX-EA) and concentrated on a rotary evaporator. The dried HEX-EA and CHME-BU residues were then loaded onto a silica gel (70–230 mesh) column (5.5 × 40 cm) and eluted stepwise with 40%, 50%, 60% and 70% MeOH in hexane. Eluates were collected based on the color of eluting portions. Eluates showing qualitatively similar TLC patterns were pooled and concentrated on the rotary evaporator under vacuum at 40°C, yielding five fractions (HEX-EAs F1-F3, BU F1, and BU F2). Further qualitative determinations revealed that TLC patterns of HEX-EA F3 were similar to those of BUs F2 and F2 so that these fractions were combined (HEX-EA-BU). Solvents were removed by rotary evaporation and dry matter from each isolate was tested for AhR induction. All fractions sufficiently activated AhR (Table S1) and were, therefore, subjected to further purifications.
Dried extracts were dissolved again in MeOH and subjected to preparative HPLC separations performed on a Reveleris® Prep purification system (Grace Co., Columbia, MD). Separation was conducted on a 25 cm × 21.2 mm, 5 μm Discovery® C18 column (Supelco, Bellefonte, PA). The mobile phases consisted of A: 0.1% formic acid in water and B: methanol and optimal separations were achieved using a gradient of 65 to 90% B over 0–30 min at a flow rate of 1.0 mL/min and injection volume of 1.5 mL. Peak resolution was monitored by UV (254 and 210 nm) and evaporative light-scattering detectors (ELSD). Separations of HEX-EAs F1 and F2 afforded six major fractions: FI-FVI (50–200 mg) whereas three fractions, each comprising one major peak, were collected from HEX-EA-BU: FVII-FIX (20–60 mg). These semi-pure fractions were dried in a rotary evaporator and residues were subjected once more to preparative HPLC isolation until substantial amounts of highly pure compounds were collected. Solvents were removed by rotary evaporation or lyophilization and stored at −20°C until further use.
2.6. Structure analysis and compound identification
1H and 13C nuclear magnetic resonance (NMR) spectroscopic analysis of the isolated compounds were recorded on a Bruker Avance III 600 spectrometer (operating at 600.01 MHz for 1H) equipped with a 5 mm cryogenic probe (National Magnetic Resonance Facility at Madison, University of Wisconsin-Madison, WI). Electrospray ionization-mass spectrometry (ESI-MS/MS) via direct infusion were performed on an Agilent (Palo Alto, CA) LC-MSD TOF mass spectrometer (Biotechnology Center, UW-Madison, WI) in positive ion mode. Spectroscopic data (Fig. S2) obtained were analyzed and/or compared to previous reports [33–38] to confirm the structures and identities of compounds in Table 1.
Table 1.
Observed masses of mangosteen xanthones obtained by ESI-MS (cationic mode).
| Cpd no. | Compound ID | m/z [M+H]+ | MS2 fragmentationions | Identification method* |
|---|---|---|---|---|
| 1 | 8-desoxygartanin | 381 | 353 | [32, 33] |
| 2 | gartanin | 397 | 381, 353, 376 | [33, 34] |
| 3 | α-mangostin | 411 | 381, 353, 385, 348 | [33, 35] |
| 4 | 9-hydroxycalabaxanthone | 409 | 381, 353 | [33] |
| 5 | β-mangostin | 425 | 381, 353 | [33, 36] |
| 6 | garcinone D | 429 | 411, 353 | [37] |
| 7 | γ-mangostin | 397 | 381, 353 | [36] |
Methods of identification: MS = interpretation of mass spectra; 1H, 13C NMR = interpretation of NMR data; Literature comparison with mass spectra and NMR data: [31]-Nguyen et al (2003), [32]-Han et al (2009), [33]-Bennet et al (1990), [34]-Guo et al (2016), [35]-Aisha et al (2012), [36]-Xu et al (2014).
2.7. Extraction of cytosolic and nuclear proteins
HT-29 cell cytoplasmic and nuclear protein extracts were obtained using the Thermo Scientific NE-PER nuclear and cytoplasmic extraction kit (Cat. 78833, Thermo Fisher Scientific, IL, USA), following the manufacturer’s protocol, before being used for immunoblotting.
2.8. Western blot analysis
Protein concentration was measured with a Pierce™ BCA protein assay (Thermo Fisher Scientific, IL, USA) according to the manufacturer’s instructions. Proteins in cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described previously [39]. After transfer, membranes were blocked by 5% non-fat milk (Cat. M7409, Sigma Aldrich) for 1 h at 20–21°C. Blocked membranes were then incubated overnight at 4°C with primary antibodies from ProteinTech (Rosemont, IL, USA): zonula occludens-1 (ZO-1, 20742–1-AP), ZO-2 (18900–1-AP), ZO-3 (22116–1-AP), occludin (13409–1-AP), claudin-1 (13050–1-AP), claudin-4 (16195–1-AP), Cyp1a1 (13241–1-AP), heme oxygenase-1 (HO-1, 10701–1-AP), Nrf2 (16396–1-AP), beta-actin (20536–1-AP) and lamin A/C (10298–1-AP) or from Santa Cruz Biotechnology (Dallas, TX, USA): AhR (sc-133088). After a 1-h incubation with anti-rabbit IgG, horseradish peroxidase (HRP)-conjugated (#7074, CST) or anti-mouse IgG, HRP-conjugated (7076, CST) secondary antibodies, blot images were captured using a FluorChem E imager (ProteinSimple, San Jose, CA). The intensities were quantified by densitometric analysis using AlphaView software for FluorChem™ systems.
2.9. In vitro permeability and ROS assays
HT-29 cells were seeded (1.5 × 105 cells/cm2) into 12-well transwell inserts (ThinCerts™, 0.4 um pore size, Greiner Bio-one) for differentiation following a method previously described [40]. Only monolayers with transepithelial electrical resistance (TEER) values above 200 Ω cm−2 [28] measured using an epithelial volt-ohm meter Millicell ERS-2 (Millipore Sigma, Burlington, MA) were used. Flux of fluorescein isothiocyanate-dextran (FITC-dextran, 4 kDa, Sigma-Aldrich) through HT-29 monolayers was measured as previously described [27], with some modifications. After treatments, FITC-dextran (0.2 ml, 0.5 mg mL−1 in HBSS/HEPES) and HBSS (1.0 ml) were added to the apical and basolateral compartments, respectively. Aliquots (150 μL) were taken from the basolateral chamber at 0.5, 1, 2, 3, and 4 h and placed into a black, clear bottom 96-well plate. The fluorescence intensity of the samples was measured immediately at excitation/emission wavelengths of 485/530 nm using a BioTek™ Synergy™ (BioTek, VT, USA) microplate reader. Data were normalized using the control values and results were expressed as % of control.
Total ROS was measured using 2’,7’-dichlorofluorescin diacetate (DCFH-DA), a cell-permeable fluorogenic probe that oxidizes to fluorescent dichlorofluorescein (DCF) in the presence of ROS, as described previously [29]. Briefly, cells pre-treated with GarD (0–25 μM) for 6 h were washed with PBS followed by a 40-min incubation with 15 μM DCFH-DA in serum- and phenol red free DMEM. Cells were then washed twice with PBS and treated with 2.5 mM tert-butyl hydroperoxide (tBHP) for 4 h. Endpoint fluorescence was measured using a BioTek™ Synergy™ (BioTek, VT, USA) microplate reader with excitation and emission settings of 485 nm and 535 nm, respectively. Data were normalized using the control values and results were expressed as % of control.
2.11. Immunofluorescence
HT-29 cells were seeded in a Milli EZ 4-well chamber slides (Cat. PEZGS0416, Millipore Sigma) at a density of 1.4 × 104/cm2 and allowed to differentiate for 10 days [40]. After treatments, cells were incubated in freshly prepared 4% paraformaldehyde solution (in PBS) at room temperature for 20 min, permeabilized with 0.2% TritonX-100 (in PBS) for 15 min and blocked with 5% BSA (in PBS) for 1 h. This was followed by primary antibody incubation (rabbit anti-ZO-1, 1:200) for 1.5 h at room temperature and by a 2-h incubation with Alexa Fluor 488-conjugated goat anti-rabbit IgG (H+L) secondary antibody (Cat. 111–545-144, Jackson ImmunoResearch, PA, USA) at room temperature and protected from light. Slides were then washed with PBS and mounted with Prolong Gold Antifade with DAPI (Cat. 8961S, CST) and allowed to dry at 4°C for 24 h in complete darkness. Microscopy of the mounted slides was performed on a Zeiss LSM 710 Meta Confocal Laser Scanning Microscope (Carl Zeiss AG, Oberkochen, Germany). Scan areas, at least four fields of view from each treatment group, were chosen based on intact cell structures and even staining. Instrument parameters and settings are further described in Table S2. Image processing and intensity quantification were carried out using ZEN 2011 (blue edition) software (Carl Zeiss AG, Oberkochen, Germany). Because the amount of TJ in the paracellular space is highly dependent on the number of cells [41], the fluorescence intensity was divided by the number of cells shown in the image area analyzed. Data was expressed as fluorescence intensity per cell relative to the control.
2.12. Statistical analysis
Statistical analyses were performed using SigmaPlot 13 software (Systat Software, Inc., San Jose, CA). Data from at least three independent experiments were subjected to one-way ANOVA followed by a post hoc analysis with the Tukey’s test to determine significant differences among treatments. Differences at P ≤ 0.05 were considered statistically significant.
3. Results
3.1. Isolation and identification of AhR inducers from mangosteen
Final purification procedures utilized semi-preparative HPLC to isolate compounds 1–7 (Table 1) from FI-FIX (as described in the Materials and Methods). Fractions III, VI, and IX were identified as the same compound. Structure and identity were confirmed by LC-MS/MS and NMR (1H and 13C NMR) analyses by interpretation of spectroscopic data and by comparing data from previous reports [33–38]. All compounds that exhibited potent AhR-inducing activity were identified as prenylated xanthones whose chemical structures, identities and bioactivities have previously been reported [33–38, 42, 43]. These compounds include 8-desoxygartanin (1), gartanin (2), α-mangostin (3), 9-hydroxycalabaxanthone (9-OHCal, 4), β-mangostin (5), GarD (6), and γ-mangostin (7) (Fig. 1).
Figure 1.
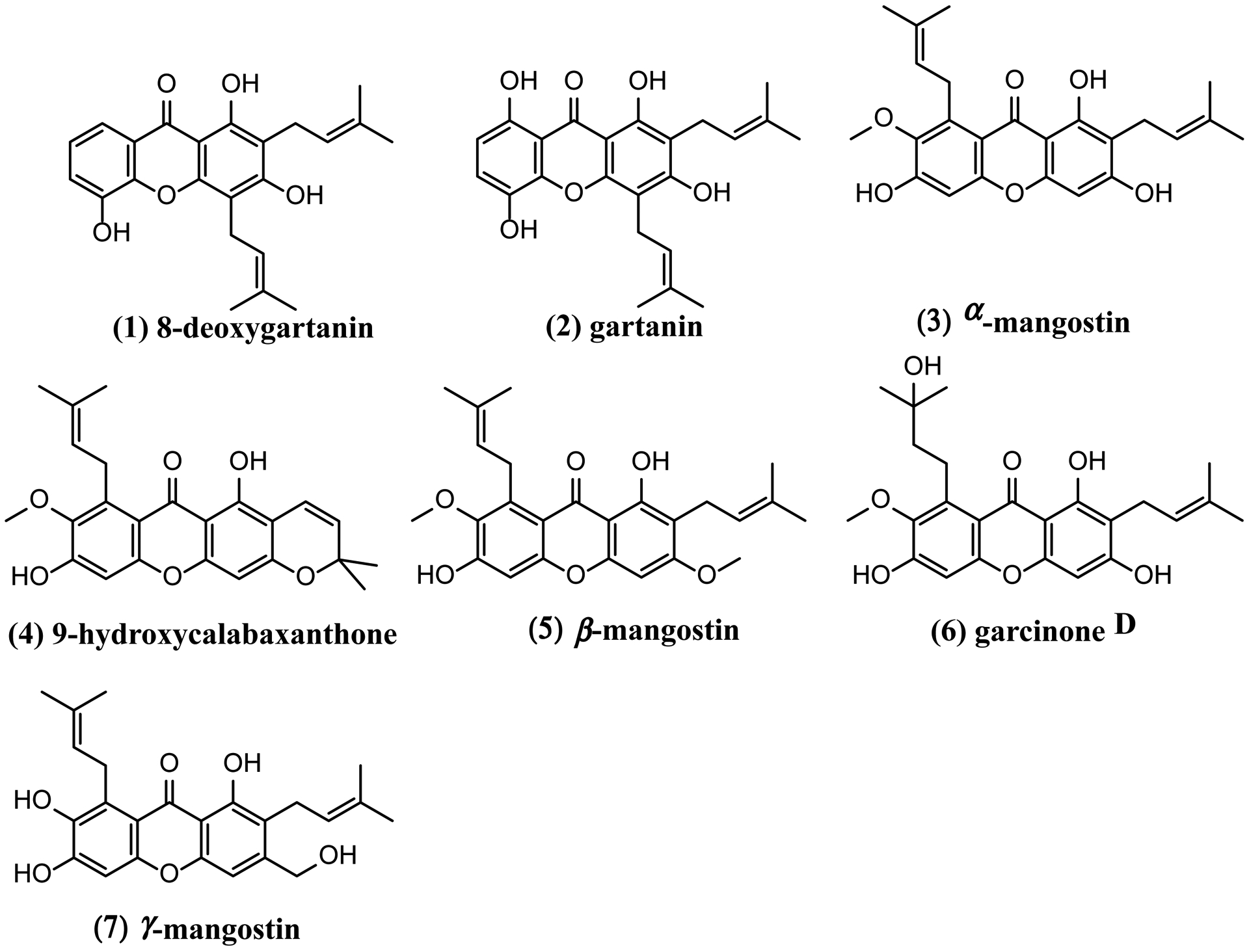
Chemical structures of prenylated xanthones isolated from Garcinia mangostana.
3.2. Mangosteen xanthones dose-dependently activate the dioxin response element
To validate the AhR-luciferase reporter assay, FICZ, a prototypical AhR activator was tested in H1L6 cells. Twenty-four hours after treatment, FICZ (500 nM) significantly increased the luminescence signal (43-fold of control) with a CQ of 0.042 μM (Table 2). All compounds (1–7) were tested for their toxicity and ability to activate the AhR pathway at 0.0–100 μM. Cytotoxicity of individual compounds were compared using calculated IC50 values (Table 2). Compounds 2 and 4–7 did not exhibit significant toxicity (% cell viability > 90%) against H1L6 at 0 to 12.5 μM. At concentrations greater than 25 μM, all compounds exhibited significant toxicity on H1L6 cells with compound 3 being the most toxic (IC50 = 26.3 μM) and compound 7 (IC50 = 65 μM) the least. Based on the cytotoxicity profile and preliminary results of the AhR luciferase assay, 12.5 μM was selected as the maximum concentration for definitive testing of AhR activation. All xanthones significantly activated the AhR pathway at low doses (CQ range = 0.14–5.1 μM) in a concentration-dependent manner (Fig. 2). With a CQ value of 0.142 μM, compound 6 showed the highest potency as an AhR activator without exhibiting >50% H1L6 cell toxicity at the concentration range tested (Fig. 2). Compounds 1, 2, 3, 4 and 7 also exhibited potent AhR-inducing effect (CQ = 0.16–0.51 μM), but their toxicity (IC50 = 26.3–65 μM) against H1L6 cells were higher than that of compound 6. The CQ value of compound 5 was 10–36 times higher than the rest of the compounds suggesting that compound 5 may be a relatively weaker AhR activator. The AhR-driven luciferase signals in cells treated with compounds 1,2,3,4,6, and 7 started to decrease after reaching their peaking values (Fig. 2). Overall, these results suggest that six out of the seven xanthones isolated are potent AhR activators at low micromolar concentration range, with compound 6 as the most potent activator.
Table 2.
AhR and Nrf2 activation characteristics of pure xanthones isolated from mangosteen.
| AhR activation | Nrf2 activation | ||||
|---|---|---|---|---|---|
| Cpd No. | Identity | CQ (μM) | IC50 (μM) | CQ (μM) | IC50 (μM) |
| + control | FICZ | 0.006 ± 0.000 | - | - | - |
| 1 | 8-desoxygartanin | 0.162 ± 0.001 | 36.00 ± 1.70 | 22.19 ± 1.24 | 49.91 ± 3.81 |
| 2 | gartanin | 0.412 ± 0.005 | 36.50 ± 0.71 | 9.43 ± 1.90 | 63.55 ± 1.94 |
| 3 | α-mangostin | 0.510 ± 0.007 | 26.30 ± 0.08 | 16.25 ± 0.46 | 24.11 ± 0.28 |
| 4 | 9-OHCal | 0.380 ± 0.004 | 47.60 ± 0.56 | - | 52.25 ± 3.96 |
| 5 | β-mangostin | 5.125 ± 0.006 | - | - | - |
| 6 | Garcinone D | 0.142 ± 0.008 | - | 8.08 ± 0.07 | 38.50 ± 7.27 |
| 7 | γ-mangostin | 0.825 ± 0.008 | 65.00 ± 3.50 | 14.93 ± 0.10 | 32.59 ± 3.96 |
CQ and IC50 are mean values ± SD of at least 2 separate experiments, each performed in triplicate. Maximum test concentrations of samples were 100 μM and 25 μM for MTT and AhR assays of pure compounds, respectively.
CQ-concentration of quadrupling; IC50- inhibitory concentration of pure compounds where the cell viability is reduced by half. Compounds with no IC50 values (−) means the compound did not exhibit >50% H1L6/HepG2-ARE cell toxicity at the concentration range tested. Compounds with no CQ values (−) for Nrf2 activation means the concentration range tested did not quadruple Nrf2 activation in HepG2-ARE cells.
Figure 2.
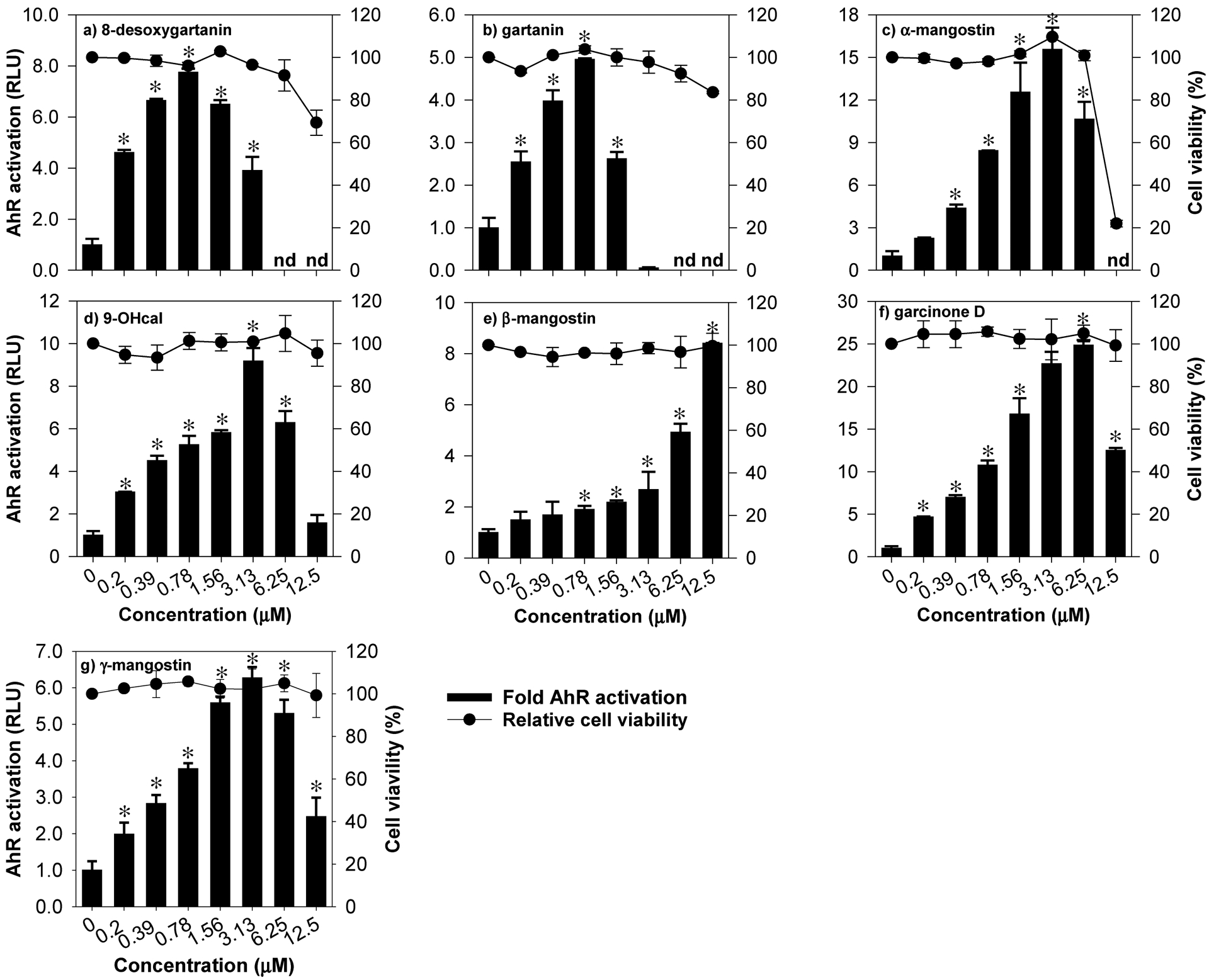
Activation of AhR by mangosteen-derived prenylated xanthones in H1L6.1c3 cells with stably integrated luciferase firefly gene driven by dioxin responsive element (DRE). Data are expressed as mean values ± SD from triplicate experiments. Bars bearing * were significantly different by one-way ANOVA followed by Tukey-HSD test if P ≤ 0.01 vs control. n.d. = not detected.
3.3. Mangosteen xanthones differentially activate the antioxidant response element
Using the ARE-luciferase reporter cell line HepG2-ARE, the ability of mangosteen xanthones to activate the Nrf2 pathway was evaluated. A non-cytotoxic concentration range was determined by exposing cells to 0–100 μM of test compounds followed by a standard MTT test. The IC50 values ranged from 24–52 μM (Table 2), suggesting that xanthones are only moderately cytotoxic in HepG2-ARE cells. Since the relative viability of cells was not significantly affected by 0–25 μM treatments (Fig. 3), this concentration range was used to quantify the extent of Nrf2 activation. The CQ the induction of luciferase gene ranged from 8 to 22 μM (Table 2). Compounds 2 (9.4 μM) and 6 (8.0 μM) exhibited relatively more potent Nrf2 induction ability compared to the rest of the compounds tested. The CQ values of compounds 1, 3 and 7 ranged from 15 to 22 μM. CQ values cannot be calculated for compounds 4 and 5 at the concentration range tested, suggesting that these compounds may be weak Nrf2 inducers.
Figure 3.
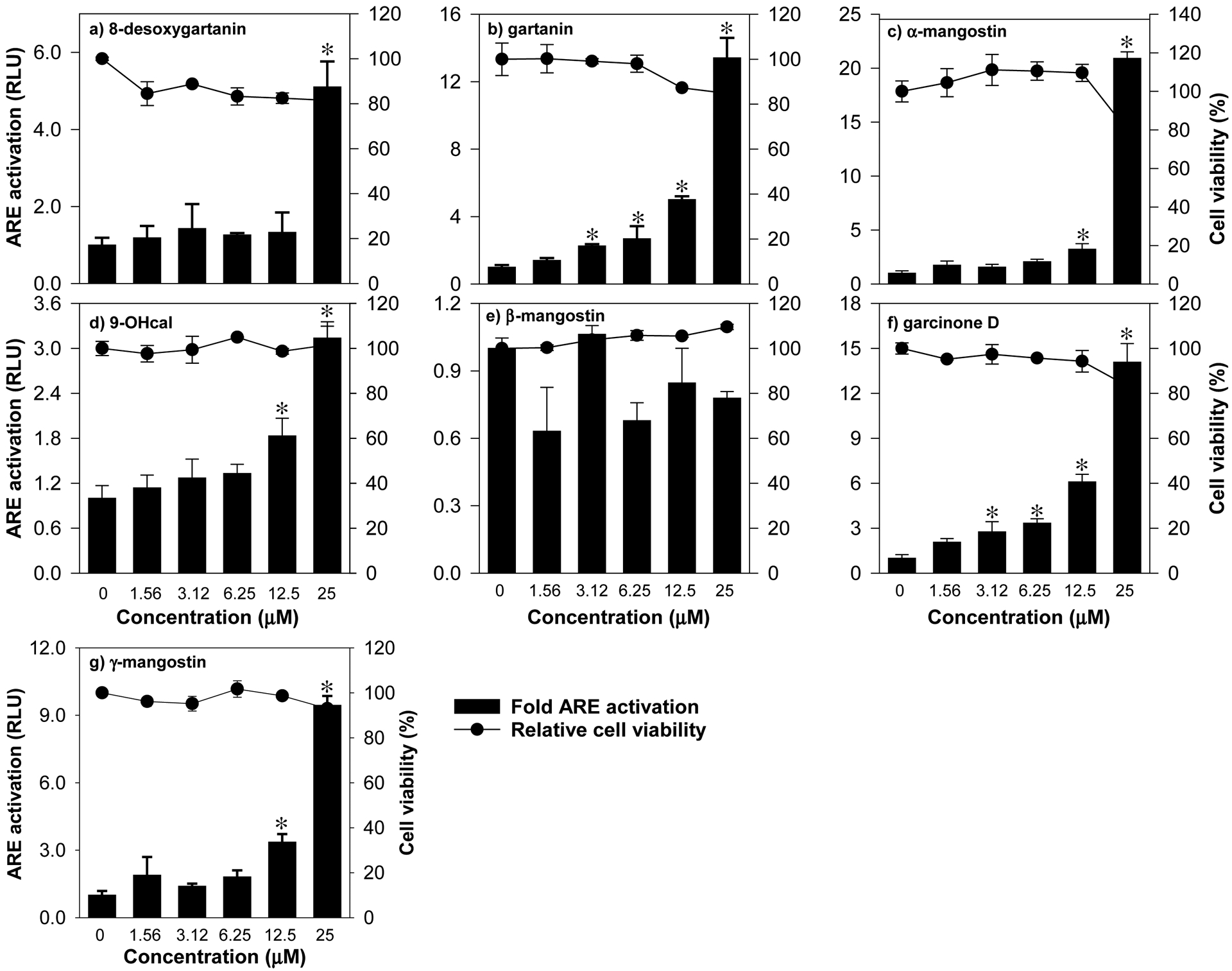
Activation of ARE by mangosteen-derived prenylated xanthones in HepG2-ARE cells with stably integrated luciferase firefly gene under the control of ARE promoters. Data are expressed as mean values ± SD from triplicate experiments. Bars bearing * were significantly different by one-way ANOVA followed by Tukey-HSD test if P ≤ 0.01 vs control.
3.4. Garcinone D is a bifunctional activator
The AhR- and ARE-luciferase reporter assays revealed that mangosteen xanthones, including α-mangostin, 9-OHCal, gartanin, and GarD are inducers of the AhR and Nrf2 pathways. Overall, GarD exhibited the greatest potency of induction for both Nrf2 and AhR pathways compared to the rest of the compounds tested. Based on this result, GarD was selected to further evaluate bifunctional activation of phase 1 and phase 2 responses in HT-29 cells. Immunoblotting of nuclear proteins revealed that exposure of HT-29 cells to 12.5 μM GarD resulted in nuclear translocation of AhR that peaked (3.8-fold increase vs control) as early as 1 h and gradually returned to baseline levels (similar to controls) at 3 to 6 h (Fig. 4a). Exposure of cells to FICZ (positive control), a potent tryptophan-derived endogenous AhR ligand [44] also demonstrated nuclear translocation of AhR reaching 4.6-fold of AhR accumulation in the nucleus as early as 1 h relative to the control. This nuclear accumulation of AhR was accompanied by a significant decrease in cytosolic levels of AhR showing the same trend for both GarD and FICZ. AhR induction was further demonstrated by the observed dose-dependent increase in AhR (up to 4.3-fold) and CyP1a1 (up to 3.2-fold), an AhR-mediated enzyme, in HT-29 cells exposed 12.5 μM GarD (Fig.5a,b). FICZ (500 nM) induced 2.8- and 3.5-fold increase in AhR and Cyp1a1, respectively. These results demonstrate that GarD may induce AhR and AhR-mediated enzymes in epithelial cells.
Figure 4.
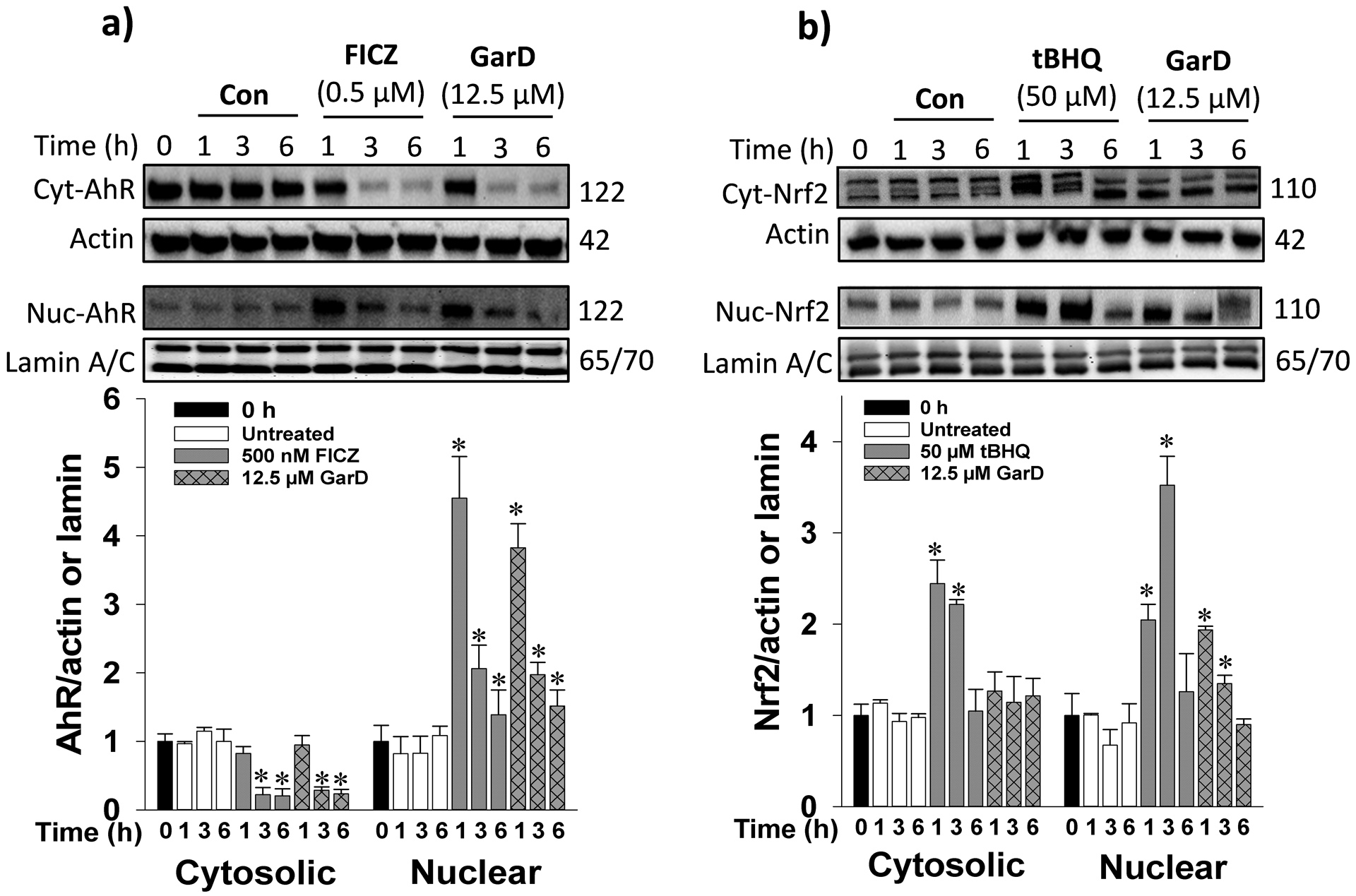
Garcinone D induces AhR and Nrf2 nuclear translocation. Cells were lysed and immunoblotted with anti-AhR (a) or anti-Nrf2 (b) antibodies. Cells were treated with 12.5 μM GarD, 50 μM tert-butyl hydroquinone (tBHQ, known Nrf2 activator) or 0.5 μM FICZ (known AhR activator) over a 6-h period of. Cytosolic and nuclear proteins were collected at the selected time points and immunoblotted with the antibodies. Relative protein densities were normalized to β-actin or lamin A/C. The blots shown are representatives of at least three independent experiments. Data are expressed as mean values ± SD from triplicate experiments. Bars bearing * were significantly different by one-way ANOVA followed by Tukey-HSD test if P ≤ 0.01 vs control.
Figure 5.
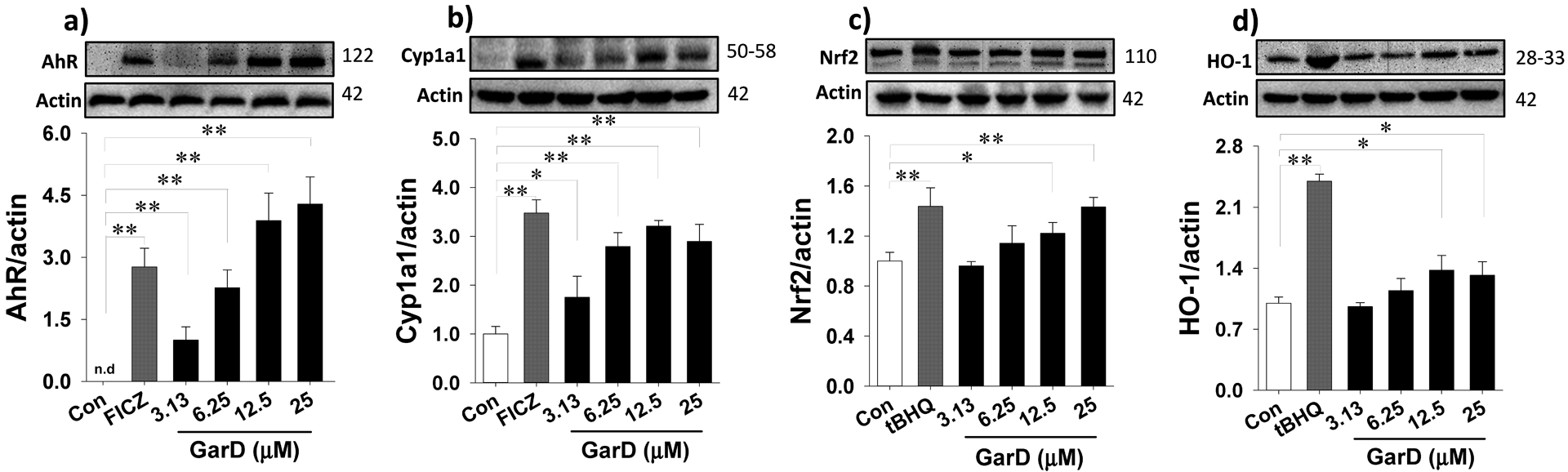
Garcinone D upregulates AhR (a), Cyp1a1 (b), Nrf2 (c), and HO-1 (d) in HT-29 cells. Cells were treated with 12.5 μM GarD, with 50 μM tBHQ (Nrf2 activator), or with 0.5 μM FICZ (AhR activator) over a 24-h period. Cell lysates were collected and immunoblotted with anti-AhR, anti-Nrf2, anti-HO-1, anti-Cyp1a1, and anti-Actin antibodies; relative protein densities normalized to β-actin. Data are expressed as mean values ± SD from triplicate experiments. Bars bearing * (P ≤ 0.05) and ** (P ≤ 0.01) were significantly different by one-way ANOVA followed by Tukey-HSD test vs control.
To determine whether the observed GarD-induced ARE-luciferase gene activation is a result of Nrf2 stabilization and cyto-nuclear translocation, nuclear protein extracts were probed for Nrf2 protein. Time-course experiments using 12.5 μM GarD revealed significant increase in nuclear Nrf2 levels as early as 1 h, reaching 1.9-fold greater than the control and gradually returning to baseline levels at 3 and 6 h (Fig.4b). Nuclear translocation of Nrf2 was also demonstrated in cells treated with 50 μM tBHQ (positive control), a known Nrf2 inducer [45]. Furthermore, GarD (0–25 μM) treatment dose-dependently increased total Nrf2 (by 40% vs control) and heme oxygenase-1 (HO-1, by 37% vs control) protein levels (in whole cell lysates, Fig. 5c,d).
To investigate whether GarD induces ROS production in HT-29 cells, total intracellular ROS was quantified using the oxidation-activated fluorescent dye DCF-DA. Exposure of HT-29 cells to GarD (0–25 μM) for 12 h caused a dose-dependent moderate increase in DCF fluorescence that reached 2-fold of the basal level (untreated control) at 25 μM, indicating increased generation of ROS (Fig. 6a). Exposure to 25 μM tBHP, a known oxidative stress inducer (positive control), caused a 4-fold increase in ROS levels relative to the control. A 24-h time course experiment using 25 μM GarD revealed that ROS in negative controls (cells in DMEM without the test compound) increased in a time-dependent manner. At 12h and 24h of incubation, ROS levels in cells treated with GarD were significantly higher compared to those of the controls, suggesting that GarD induces ROS production in HT-29 cells.
Figure 6.
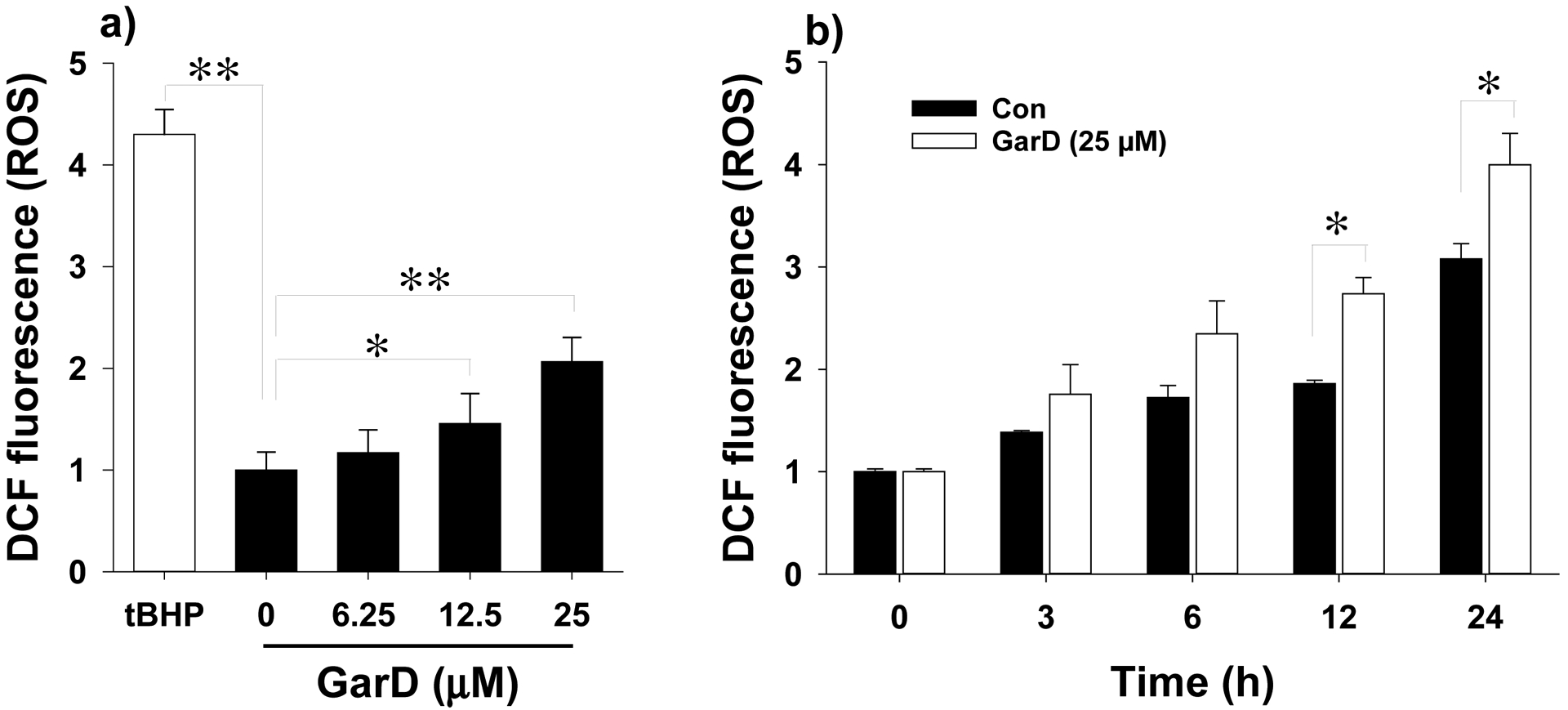
Effects of Garcinone D on ROS formation in HT-29 cells. For the DCF-DA assay, cells were treated with 0–25 μM GarD for 12 h in the presence of 15 μM DCF-DA. Fluorescence was measured at excitation and emission wavelengths of 485 and 535 nm, respectively. Data are expressed as mean values ± SD from triplicate experiments. Bars bearing * (P ≤ 0.05) and ** (P ≤ 0.01) were significantly different by one-way ANOVA followed by Tukey-HSD test vs control.
3.5. GarD induces TJ proteins
TJ proteins equip intestinal epithelial cells with the ability to maintain barrier integrity [46]. To test the hypothesis that GarD induces expression of TJ proteins, HT-29 cells were exposed to the same concentrations of GarD (0–25 μM) that promoted AhR and Nrf2 activation (Fig. 4,5). GarD dose-dependently increased protein levels of ZO-1 and occludin (Fig. 7) after a 24-h exposure. At the concentration range tested, GarD increased ZO-1 by 34–83% and occludin by 22–91% relative to untreated controls (Fig. 7a,b). GarD had no inducing effect on other TJ proteins, including ZO-2, ZO-3, claudin-1 and claudin-4 (data not shown).
Figure 7.
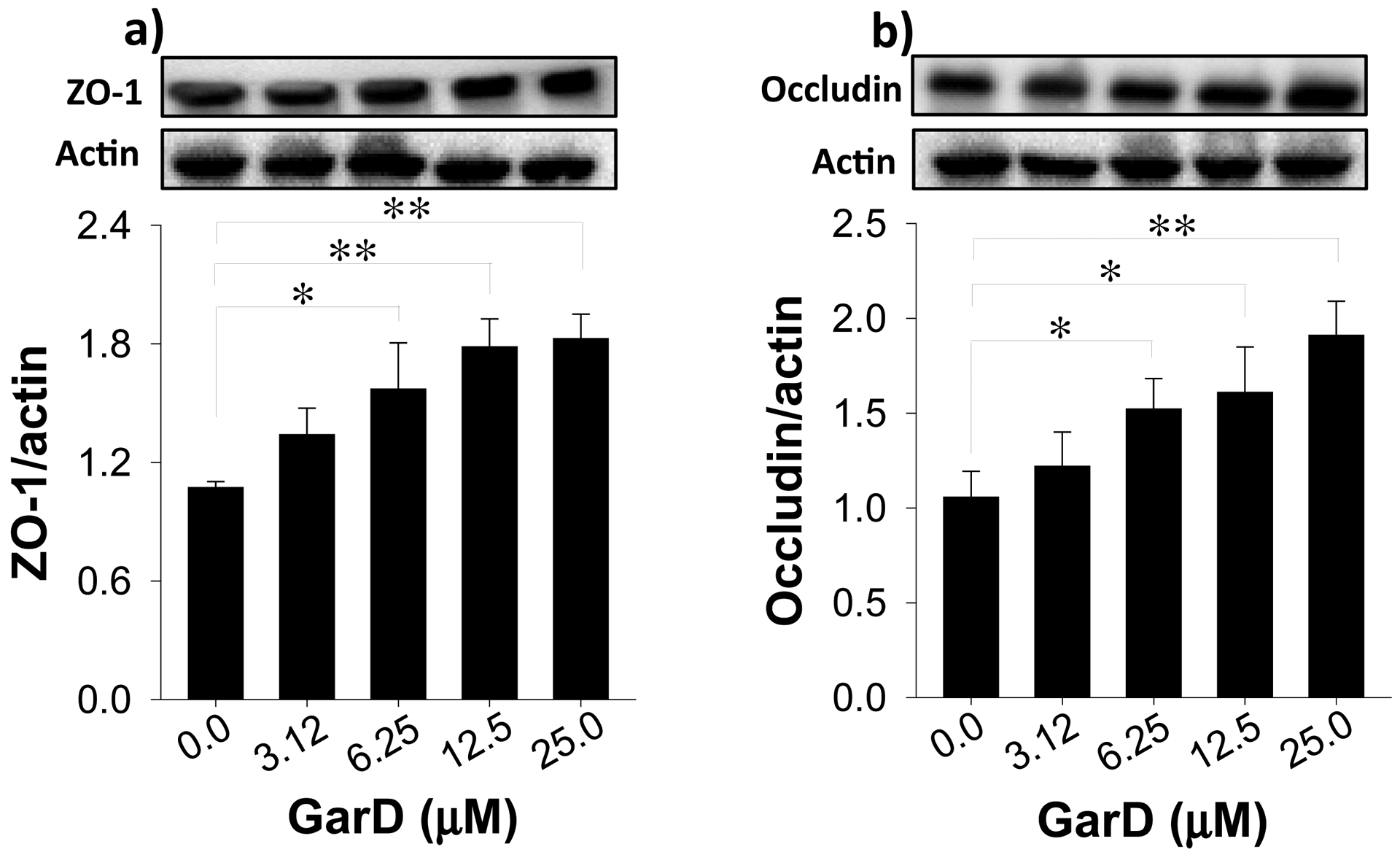
Garcinone D upregulates TJ proteins in HT-29 cells. Cells were treated with 12.5 μM GarD over a 24-h period. Cell lysates were collected and immunoblotted with anti-ZO-1 (a) and anti-occludin (b); relative protein densities normalized to β-actin. Data are expressed as mean values ± SD from triplicate experiments. Bars bearing * (P ≤ 0.05) and ** (P ≤ 0.01) were significantly different by one-way ANOVA followed by Tukey-HSD test vs control.
3.6. Garcinone D protects against oxidative stress-induced TJ disruption
Oxidative stress induced by excessive ROS (i.e., hydrogen peroxide, peroxynitrite, etc.) production disrupts the epithelial and endothelial barrier function by destabilizing TJ proteins [47, 48]. To test the hypothesis that GarD inhibits oxidative stress-induced TJ disruption, TJ protein expression in GarD pre-treated HT-29 cells exposed to non-toxic levels of tBHP was analyzed by immunoblotting. tBHP (50 μM, 16 h) exposure caused 68, 28, and 48% reduction in ZO-1, occludin, and claudin-1, respectively compared to untreated controls (Fig. 8a,b,c). GarD (12.5 μM) rescued about 28, 60, and 85% of ZO-1, occludin, and claudin-1 protein lost due tBHP exposure. Although GarD exposure did not increase claudin-1 expression (data not shown), it conferred HT-29 cells protection against tBHP exposure.
Figure 8.
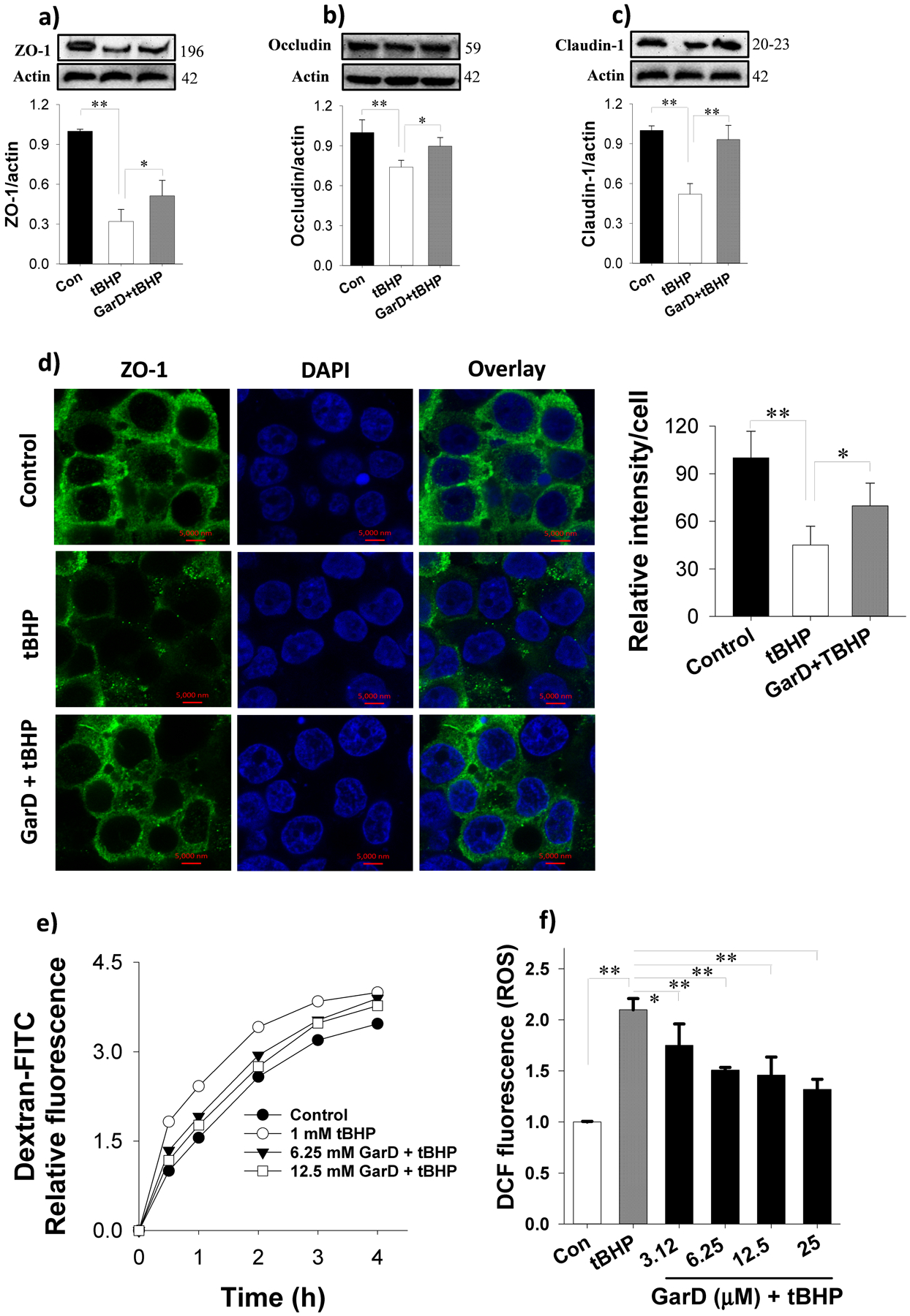
Garcinone D rescues TJ proteins from oxidative stress-induced downregulation in HT-29 cells. Garcinone D inhibited tBHP-induced ROS production in HT-29 cells. Pre-treatment with 12.5 μM Garcinone D followed by a 16-h co-incubation with 50 μM tBHP (oxidative stress inducer) increased ZO-1 (a), occludin (b) and claudin-1 (c) compared to tBHP alone. (d) Immunofluorescence images showing the protective effect of Garcinone D against tBHP-induced ZO-1 protein losses. Cells were pretreated without or with 12.5 μM Garcinone D for 2 h, followed by exposure to 50 μM tBHP for 16 h. Immunofluorescence protocol (described in section 2.11) was followed to probe for ZO-1 proteins using a confocal microscope. (e) Leakage of FITC-dextran into the bottom chamber expressed in relative fluorescence intensity. Cells were pre-treated with or without Garcinone D (added to the apical compartment) for 6 h followed by a 16-h incubation with 100 μM tBHP to establish oxidative stress and disrupt expression of TJ. (f) Garcinone D inhibited tBHP-induced ROS production. Bars bearing * (P ≤ 0.05) and ** (P ≤ 0.01) were significantly different by one-way ANOVA followed by Tukey-HSD test vs control.
Immunofluorescence microscopy of ZO-1 protein further demonstrated the protective effect of GarD against tBHP-induced TJ protein disruption (Fig. 8d). Intact nuclei and a mesh-like pattern surrounding adjacent cells indicated membrane localization of ZO-1 between cells occurred. tBHP (50 μM, 16 h) reduced ZO-1 fluorescence intensity to 45% of the control, but 2-h pre-treatment with GarD (12.5 μM) and subsequent co-incubation with tBHP for 16 h rescued 45% of ZO-1 intensity lost to tBHP treatment. The functional consequence of GarD mediated upregulation of TJ proteins was examined using in vitro permeability assay of FITC-dextran in transwell plates. Pretreatment of HT-29 cells with 6.25 or 12.5 μM GarD dose-dependently inhibited tBHP induced leakage of FITC-dextran into the bottom of chambers (Fig. 8e).
Since GarD caused a moderate upregulation of Nrf2 and the cytoprotective HO-1 enzyme (Fig. 5c,d), GarD was tested for inhibition of ROS production in HT-29 cells. Exposure of cells to 2 mM tBHP for 4 h induced a 2-fold increase in total ROS levels relative to controls (Fig. 8f). Pretreatment of cells with GarD dose-dependently suppressed (by 25 to 68% vs tBHP) ROS production induced by tBHP.
3.7. GarD-induced TJ protection against oxidative stress requires AhR
The AhR pathway has been shown to protect against IBD by maintaining TJ barrier integrity [19]. To determine whether the protection conferred by GarD against tBHP-induced TJ disruption is associated with the AhR pathway, HT-29 cells were treated with a non-toxic concentration (MTT data not shown) of CH-223191 (CAS # 301326–22-7), a known selective and potent AhR antagonist [49]. Cells exposed to 50 μM tBHP caused significant reductions in ZO-1 and occludin protein levels (Fig. 9). Pretreatment with 12.5 μM GarD (GarD + tBHP) significantly abolished the effect of tBHP, rescuing 65% and 72% of ZO-1 and occludin lost to tBHP exposure. This protective effect of GarD against tBHP was attenuated in cells exposed to the AhR inhibitor prior to GarD treatment (CH-223191 + GarD + tBHP), suggesting that TJ upregulation by GarD may require the AhR pathway.
Figure 9.
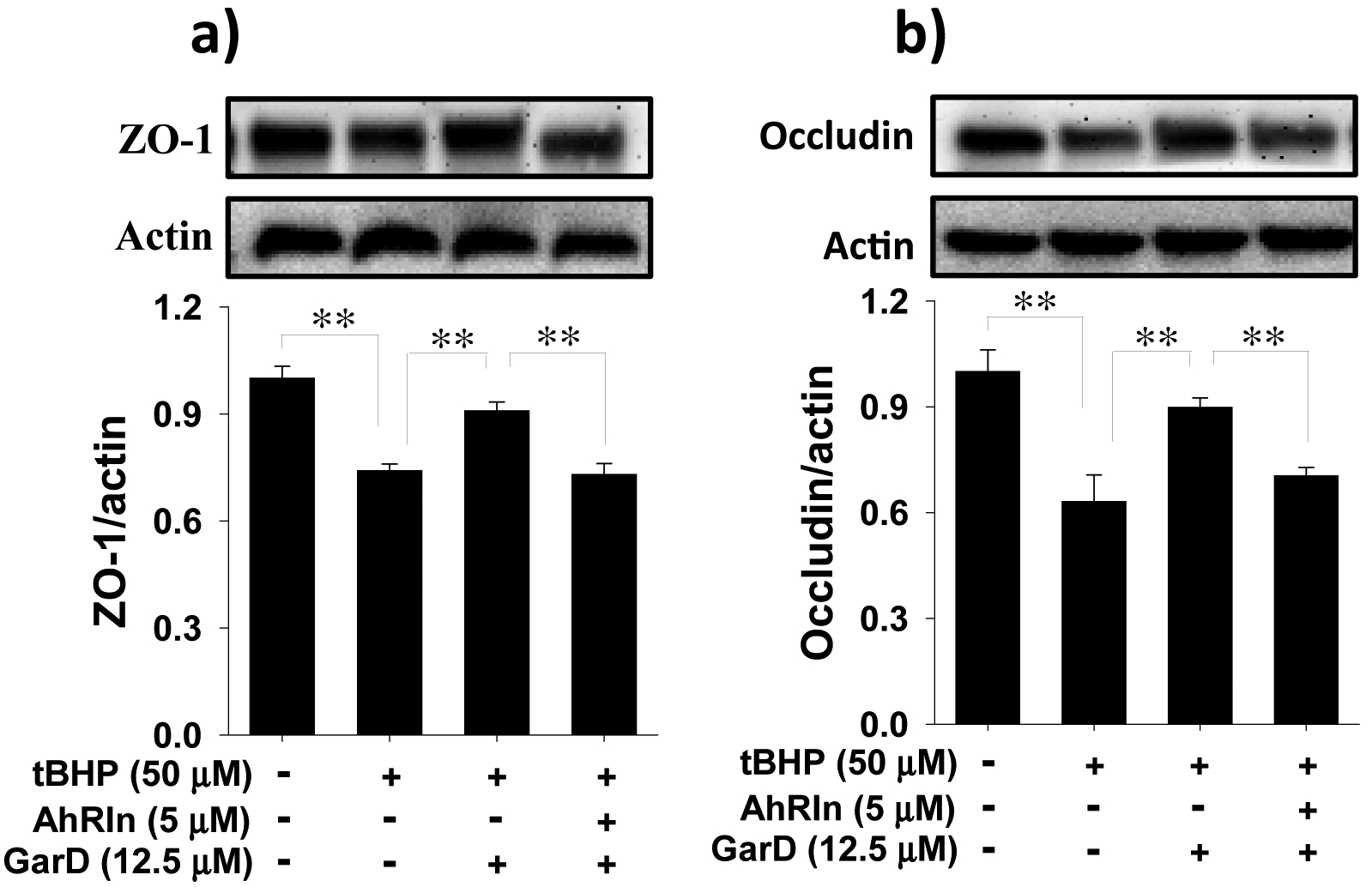
Protection against oxidative stress induced TJ disruption in HT-29 cells by Garcinone D requires AhR. (a,b) Representative immunoblots for ZO-1 and occludin. Cells were either untreated or treated with 5 μM CH-223191 (a potent and selective AhR antagonist) and subsequently co-incubated with 12.5 μM Garcinone D and 50 μM tBHP. Cell lysates were collected and immunoblotted with ZO-1 and anti-occludin antibodies; relative protein densities normalized to β-actin. Data are expressed as mean values ± SD from triplicate experiments. Bars bearing * (P ≤ 0.05) and ** (P ≤ 0.01) were significantly different by one-way ANOVA followed by Tukey-HSD test vs control.
4. Discussion
In the present study, the potential effect of mangosteen-derived xanthones in modulating intestinal barrier function was investigated. Results revealed that six of seven prenylated xanthones (except 4) from mangosteen pericarp are potential bifunctional inducers of AhR and Nrf2 and may offer protective and therapeutic effects through activation of the AhR and Nrf2 pathways in intestinal epithelial cells. Bifunctional inducers are compounds that can activate phase I and the phase II metabolic pathways, particularly the AhR/xenobiotic response element (XRE) and Nrf2/antioxidant response element (ARE) pathways [50, 51]. GarD, one of the xanthones isolated enhances expression of TJ proteins and confers barrier function protective effects that appear to involve activation of both phase I (AhR) and phase II (Nrf2) pathways.
Despite several reports showing absorption and retro-transport of xanthones across the intestinal lining [9–12], the direct signaling effects and molecular targets of many of these compounds in IECs are largely unknown. It has been reported that α-mangostin, a major xanthone in mangosteen, exacerbates dextran sulfate sodium (DSS)-induced ulcerative colitis in mice by promoting intestinal dysbiosis [52]. Contrastingly, mangosteen extract was found to alleviate colonic pathology in mice with DSS-induced colitis via inhibition of the NF-ĸB pathway [53], suggesting that components in the extract possibly interact with the NF-ĸB pathway or other signaling pathways to inhibit colitis. This is consistent with the results from our initial screening that showed potent AhR activation by mangosteen extracts (Table S1). Our in vitro studies revealed that xanthones are optimally effective AhR activators at low micromolar concentrations and this effect diminished at higher doses. Therefore, further in vivo studies evaluating dose-dependent effects may clarify discrepancies in previous findings. Clearly, limited and contrasting findings on the effects of mangosteen on colitis necessitate further research on the action mechanism(s) of xanthones relevant to chronic intestinal disorders. The premise of the current study is that xanthones from the mangosteen fruit may modulate signaling pathways in intestinal epithelium and confer gut health related benefits.
Experiments using H1L6.1c3 cells enabled comprehensive screening of crude fractions in solvents of varying polarities that sufficiently activated the AhR pathway (Table S1) via a luciferase reporter assay. Semi-preparative fractionations coupled with NMR spectroscopy and mass spectrometry enabled identification and isolation of highly pure (>90%) AhR inducing prenylated xanthones (Fig. 2, Table 1, Fig. S2). Phytochemicals considered to be hormetic compounds demonstrate nonmonotonic concentration-response, also called biphasic dose-response. Examples of such compounds are resveratrol [54], curcumin [55], and sulforaphane [56]. The observed AhR activation profile of the xanthones tested, except for compound 5 (β-mangostin, Fig. 2), suggest that these compounds could activate AhR in a biphasic manner. Compounds 1, 2, 3, 4, 6, and 7 can induce biologically opposite effects at different doses: stimulatory effect at low doses and an inhibitory effect at high doses. To our knowledge, such trend in AhR activation has not been reported previously for xanthones or for any dietary phytochemicals. We currently do not have experimental data to explain this trend; however, a possible AhR antagonistic effect of xanthones at high concentrations can be explored in future experiments to explain the biphasic dose response observed in the present study.
The xanthones isolated from the mangosteen are characterized by a tricyclic polycyclic aromatic hydrocarbon core with varying number of functional groups such as prenyl (-C6H5), hydroxyl (-OH), and methoxyl (-OCH3) groups. The predominantly planar tricyclic core appears to be the main structural characteristic that allow xanthones to bind to AhR. This is consistent with the fact that potent AhR activators such as dioxins and polyaromatic hydrocarbons (PAHs) are planar compounds, although structure-activity relationship (SAR) analysis of polychlorinated biphenyls suggested that absolute planarity is not a requirement for receptor binding [57, 58]. The presence of −C6H5 group may also contribute to the affinity of xanthones for AhR binding. Prenylation of biomolecules generally increases hydrophobicity [59]. Planarity and hydrophobicity are main chemical characteristics of classical AhR activators such as dioxins and β-naphthoflavones [60]. Six of the xanthones isolated (except 9-OHCal) have two −C6H5 groups. Studies that applied regression analyses to similar sets of test compounds identified electronegativity, hydrophobicity, and hydrogen-bonding as properties that could also contribute to AhR interaction [61, 62]. Therefore, presence of −OH groups, which can facilitate intermolecular hydrogen bonding, may also contribute to the ability of xanthones to bind to AhR. In addition, −OCH3 group may increase electronegativity and possibly contributes to AhR binding of xanthones. All xanthones identified contain two to four OH groups, whereas only three contain −OCH3 groups. Overall, there are no clear patterns that correlate functional groups and potency of AhR activation by xanthones, although it appears that −OH and −C6H5 groups are the main functional groups that differentiate the xanthones in terms of their potency to activate AhR. To our knowledge, this is the first evidence of AhR activation by mangosteen-derived xanthones. Further SAR studies may clarify how structural variations affect binding of xanthones to AhR.
Mangosteen xanthones have previously been shown to induce quinone reductase (QR) [63], whose induction is dependent on Nrf2 activation. We tested activation of Nrf2 using stably transfected HepG2 cells and found that five of the seven xanthones are moderate Nrf2 activators (Fig. 3). Almost all currently known small-molecule activators of Nrf2 are considered indirect activators [64], which form covalent adducts (i.e., Michael acceptors, disulfides) with the cysteine sulfhydryl groups of Keap1 protein, a negative repressor of Nrf2 [65]. In the present study, Nrf2 activation by xanthones can be partially explained by their ability to induce basal ROS, as shown in the case of GarD (Fig. 6a,b). Elevation of ROS levels in HT-29 cells treated with GarD was likely an effect of the catalytic activity of cytochromes P450 enzymes, including Cyp1A1 [66–68], releasing highly reactive quinones and ROS (i.e., H2O2) in the process. It can be noted that Cyp1a1 protein was significantly induced by GarD treatment supporting a Cyp1a1-mediated ROS production (Fig. 5b). Additional studies are required to provide further evidence on this possibility and to define precise role of AhR-Cyp1A1 pathway activation in GarD-mediated Nrf2 induction.
The gastrointestinal tract is the first barrier against oxidative agents in the colonic luminal environment and is continually exposed to recurrent oxidative stress. Perturbed redox homeostasis is a common feature of IBD characterized mainly by uncontrolled ROS levels and impaired antioxidant defenses [69]. To maintain low oxidative state, IECs coordinately activate a redox-sensitive signaling pathway mainly controlled by Nrf2, a member of the cap’n’collar subfamily of transcription factors that regulate an evolutionarily conserved transcriptional activation of detoxification responses [70]. GarD induced Nrf2 protein expression, as well as its target gene HO-1 (Fig. 5c,d) in HT-29. At the concentration tested (0 to 25 μM), Nrf2 response was dose-dependent while HO-1 showed an indication of an ensuing plateauing of response. A possible explanation is that even after 24 h, GarD continually activates Nrf2, resulting in Nrf2 stabilization and, therefore, increasing protein level. However, HO-1 response may no longer be dose-dependent beyond 12.5 μM. Overall, induction of Nrf2 and HO-1 by GarD can be considered as moderate to weak relative to well-known Nrf2 inducers such as sulforaphane which, at a concentration a low as 10 μM, can induce Nrf2 in HT-29 cells by 200% relative to the control [71]. Additionally, GarD exhibited inhibitory effect against tBHP-induced ROS generation (Fig. 8f), which is consistent with its ability to induce HO-1, an oxidant-inducible stress protein with pleiotropic antioxidant and anti-inflammatory effects [72]. These results are in agreement with those of previous studies that demonstrated activation of Nrf2 pathway by mangosteen-derived xanthones including γ-mangostin, α-mangostin, tovophyllin A, and GarD [23–25, 73].
Our approach of searching for an epithelial function for xanthones by bioassay-guided isolations and immunoblotting revealed additional clues for potential AhR-mediated mechanisms. AhR is recognized historically for its role in carcinogenesis [74]; however, recent evidence demonstrates that activation of AhR protects against IBD [75, 76]. AhR−/− mice develop severe colitis compared to wild type mice [77]. Moreover, dietary compounds present in cruciferous vegetables [78] and endogenous AhR activators derived from indole or tryptophan metabolism [79] have been shown to promote AhR-mediated intestinal immune function. Our studies revealed that GarD (0–25 μM) induces the nuclear translocation and expression of AhR (Fig. 4,5a), and upregulates Cyp1A1 expression (Fig. 5b) without exhibiting toxicity. Furthermore, GarD mediated upregulation of TJ proteins, including ZO-1 and occludin (Fig. 7) suggesting that GarD exhibit modulatory effects on TJ proteins in intestinal epithelial cells. TJs composed of transmembrane and peripheral proteins (e.g., ZO-1, claudin, occludin and adhesion molecules) connect adjacent epithelial cells, regulate paracellular permeability, and maintain barrier integrity [80]. Current understanding of the exact molecular mechanism involved in the modulatory effect of AhR and TJ upregulation is limited. A possible role of the AhR-Notch-1 signaling axis in the regulation of intestinal barrier function has been suggested. A recent study demonstrated that increase in TJ expression in FICZ-treated Caco-2 cells was dependent on the upregulation epithelial Notch-1 signaling as inhibition of Notch-1 abrogated the effect of FICZ [26]. In another study, loss of Notch-1 expression in Caco-2 cells correlated with decreased trans-epithelial resistance and dysregulated localization and expression of TJ proteins [81].
To test the hypothesis that GarD protects epithelial barrier function by enhancing TJ protein expression, HT-29 cells were exposed to an inflammatory cocktail [41] composed of IL-1β, TNF-α, IFN-γ and lipopolysaccharide to disrupt TJ proteins. Contrary to results from previous reports [41, 82], our experiments using the inflammatory cocktail did not cause downregulation of TJ proteins (data not shown) in HT-29 cells. Consequently, an alternative way of disrupting TJ protein expression by induction of oxidative stress was explored. Extensive amount of evidence implicates oxidative stress caused by uncontrolled levels of cellular ROS [47] in the disruption of TJ that leads to barrier dysfunction, a characteristic feature of IBD [83]. We demonstrated this oxidative stress related effect on barrier function by treating HT-29 cells with tBHP, a widely used toxic agent to study cellular response to oxidative stress [45]. tBHP disrupted the expression of TJ proteins (e.g., ZO-1, occludin, and claudin-1, Fig. 8a–d) and increased permeability of FITC-dextran through HT-29 monolayers (Fig. 8e), but GarD attenuated these effects by upregulating TJ protein expression that resulted in reduced passage of FITC-dextran through the monolayer. Mechanistically, blocking AhR by CH-223191, a known AhR antagonist [84], abolished the protective effect of GarD on ZO-1 and claudin-1 against tBHP (Fig. 9) suggesting that AhR is required in the upregulation of TJ proteins by GarD. Furthermore, GarD inhibited tBHP induced ROS production (Fig. 8f), which is consistent with the expected functional effect of Nrf2 and HO-1 upregulation (Fig. 4,5). These results suggest a two-pronged mechanism of action by GarD that involves activation of the AhR and Nrf2 pathways and ultimately leads to protection of intestinal barrier function.
AhR plays an important role in the maintenance of intestinal mucosal homeostasis [85, 86]. This has been demonstrated by diet-derived ligands that are linked to AhR activation. For example, activation of AhR by FICZ, a tryptophan metabolite suppressed DSS-induced disruption of intestinal epithelial barrier function in mice and attenuated reduction in transepithelial resistance caused by TNF-α/IFN-γ in Caco-2 cells [19]. This barrier integrity protective effect of FICZ via AhR activation can be explained, in part, through upregulation of TJ protein expression and inhibition of disrupted TJ localization as demonstrated in Caco-2 cells and mice [19, 87]. Our results that revealed upregulation of TJ proteins (Fig. 7) and protection of barrier integrity (Fig. 8) in HT-29 cells by GarD are consistent with these observations. Recently, a paper by Singh et al [27] convincingly demonstrated a two-pronged mechanism of epithelial barrier function enhancement via the Nrf2 and AhR pathways. Urolithin A, a major microbial metabolite derived from polyphenols of berries and pomegranate was shown to enhance TJ expression, protect intestinal epithelial barrier function and reduce inflammation in mice with DSS and 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis and in cell cultures (Caco-2 and HT-29 cells).
In conclusion, this study highlights a potential mechanism that underlie the barrier function-associated beneficial effect of mangosteen-derived xanthones. Results of this study indicates that GarD exerts intestinal epithelial barrier protective activities through a two-pronged mechanism involving the AhR and Nrf2 pathways. The NF-ĸB inhibitory effect of mangosteen extract demonstrated by Chae et al [53], could be a direct effect of the activation of Nrf2 by xanthones, which could inhibit the NF-ĸB pathway via induction of HO-1 [73, 88] and other cytoprotective proteins. Results of the current study did not find α-mangostin as a potent inducer of either AhR or Nrf2 pathways. Aside from promoting dysbiosis, the exacerbation of DSS-induced colitis in mice administered with a-mangostin reported by Gutierrez-Orozco et al [52] may, in part, be due to the lack of modulatory activity of α-mangostin on signaling pathways such as AhR and Nrf2 whose activation may confer protection against colitis. We currently do not have data to show whether mangosteen-derived xanthones activate AhR in a ligand-like manner (similar to the prototypical AhR ligand FICZ) or via a non-ligand/indirect mechanism. Additional studies are required to provide further evidence and to define the precise role of Nrf2 pathway in GarD induced enhancement of epithelial barrier function. Clarifying both mechanisms requires further in-depth studies using AhR and Nrf2 knockout mice to determine in vivo translatability of our findings. Overall, results of the current study revealed important clues on barrier function protective effects of mangosteen consumption.
Supplementary Material
Acknowledgement
Jeremy Johnson is supported by an NIH MERIT award (R37CA227101) and United States Department of Agriculture/National Institute of Food and Agriculture award # 2017-67017-26364. Kirk Parkin and Jan-Peter van Pijkeren are supported provided by the University of Wisconsin Vilas Trust Funds, the College of Agricultural and Life Sciences, in part through a Hatch Grant (WIS01792), and the United States Department of Agriculture/National Institute of Food and Agriculture award # 2018-67017-27523.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- [1].Ashton MM, Dean OM, Walker AJ, Bortolasci CC, Ng CH, Hopwood M, Harvey BH, Möller M, McGrath JJ, Marx W, Turner A, Dodd S, Scott JG, Khoo J-P, Walder K, Sarris J, Berk M, The therapeutic potential of mangosteen pericarp as an adjunctive therapy for bipolar disorder and schizophrenia, Front. Psychiatry 10 (2019) 115–115. 10.3389/fpsyt.2019.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gutierrez-Orozco F, Failla LM, Biological activities and bioavailability of mangosteen xanthones: A critical review of current evidence, Nutrients 5(8) (2013) 3163–3183. 10.3390/nu5083163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Watanabe M, Gangitano E, Francomano D, Addessi E, Toscano R, Costantini D, Tuccinardi D, Mariani S, Basciani S, Spera G, Gnessi L, Lubrano C, Mangosteen extract shows a potent insulin sensitizing effect in obese female patients: A prospective randomized controlled pilot study, Nutrients 10(5) (2018) 586. 10.3390/nu10050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Obolskiy D, Pischel I, Siriwatanametanon N, Heinrich M, Garcinia mangostana L. : a phytochemical and pharmacological review, Phytother. Res 23(8) (2009) 1047–1065. 10.1002/ptr.2730. [DOI] [PubMed] [Google Scholar]
- [5].Pedraza-Chaverri J, Cárdenas-Rodríguez N, Orozco-Ibarra M, Pérez-Rojas JM, Medicinal properties of mangosteen (Garcinia mangostana), Food Chem. Toxicol 46(10) (2008) 3227–3239. 10.1016/j.fct.2008.07.024. [DOI] [PubMed] [Google Scholar]
- [6].Jiang D-J, Dai Z, Li Y-J, Pharmacological effects of xanthones as cardiovascular protective agents, Cardiovasc. Drug Rev 22(2) (2004) 91–102. 10.1111/j.1527-3466.2004.tb00133.x. [DOI] [PubMed] [Google Scholar]
- [7].Peres V, Nagem TJ, de Oliveira FF, Tetraoxygenated naturally occurring xanthones, Phytochemistry 55(7) (2000) 683–710. 10.1016/S0031-9422(00)00303-4. [DOI] [PubMed] [Google Scholar]
- [8].Vemu B, Nauman MC, Veenstra JP, Johnson JJ, Structure activity relationship of xanthones for inhibition of Cyclin Dependent Kinase 4 from mangosteen (Garcinia mangostana L.), Int. J. Nutr 4(2) (2019) 38–45. [PMC free article] [PubMed] [Google Scholar]
- [9].Bumrungpert A, Kalpravidh RW, Suksamrarn S, Chaivisuthangkura A, Chitchumroonchokchai C, Failla ML, Bioaccessibility, biotransformation, and transport of α-mangostin from Garcinia mangostana (Mangosteen) using simulated digestion and Caco-2 human intestinal cells, Mol. Nutr. Food Res 53(S1) (2009) S54–S61. 10.1002/mnfr.200800260. [DOI] [PubMed] [Google Scholar]
- [10].Gutierrez-Orozco F, Chitchumroonchokchai C, Lesinski GB, Suksamrarn S, Failla ML, α-Mangostin: anti-inflammatory activity and metabolism by human cells, J. Agric. Food Chem 61(16) (2013) 3891–3900. 10.1021/jf4004434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chitchumroonchokchai C, Riedl KM, Suksumrarn S, Clinton SK, Kinghorn AD, Failla ML, Xanthones in mangosteen juice are absorbed and partially conjugated by healthy adults, J. Nutr 142(4) (2012) 675–680. 10.3945/jn.111.156992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chitchumroonchokchai C, Thomas-Ahner JM, Li J, Riedl KM, Nontakham J, Suksumrarn S, Clinton SK, Kinghorn AD, Failla ML, Anti-tumorigenicity of dietary α-mangostin in an HT-29 colon cell xenograft model and the tissue distribution of xanthones and their phase II metabolites, Mol. Nutr. Food Res 57(2) (2013) 203–211. 10.1002/mnfr.201200539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kondo M, Zhang L, Ji H, Kou Y, Ou B, Bioavailability and antioxidant effects of a xanthone-rich mangosteen (Garcinia mangostana) product in humans, J. Agric. Food Chem 57(19) (2009) 8788–8792. 10.1021/jf901012f. [DOI] [PubMed] [Google Scholar]
- [14].Ramaiya A, Li G, Petiwala S, Johnson J, Single dose oral pharmacokinetic profile of α-mangostin in mice, Curr. Drug Targets 13(14) (2012) 1698–1704. 10.2174/138945012804545524. [DOI] [PubMed] [Google Scholar]
- [15].Petiwala SM, Li G, Ramaiya A, Kumar A, Gill RK, Saksena S, Johnson JJ, Pharmacokinetic characterization of mangosteen (Garcinia mangostana) fruit extract standardized to α-mangostin in C57BL/6 mice, Nutr. Res 34(4) (2014) 336–345. 10.1016/j.nutres.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cuadrado A, Rojo AI, Wells G, Hayes JD, Cousin SP, Rumsey WL, Attucks OC, Franklin S, Levonen A-L, Kensler TW, Dinkova-Kostova AT, Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases, Nat. Rev. Drug Discov 18(4) (2019) 295–317. 10.1038/s41573-018-0008-x. [DOI] [PubMed] [Google Scholar]
- [17].Muku GE, Murray IA, Perdew GH, Activation of the Ah receptor modulates gastrointestinal homeostasis and the intestinal microbiome, Curr. Pharmacol. Rep 5(5) (2019) 319–331. 10.1007/s40495-019-00197-2. [DOI] [Google Scholar]
- [18].Khor TO, Huang M-T, Kwon KH, Chan JY, Reddy BS, Kong A-N, Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium–induced colitis, Cancer Res 66(24) (2006) 11580–11584. 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- [19].Yu M, Wang Q, Ma Y, Li L, Yu K, Zhang Z, Chen G, Li X, Xiao W, Xu P, Yang H, Aryl hydrocarbon receptor activation modulates intestinal epithelial barrier function by maintaining tight junction integrity, Int. J. Biol. Sci 14(1) (2018) 69–77. 10.7150/ijbs.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yang L, Shen L, Li Y, Li Y, Yu S, Wang S, Hyperoside attenuates dextran sulfate sodium-induced colitis in mice possibly via activation of the Nrf2 signalling pathway, J. Inflamm. (Lond), 14 (2017) 25. 10.1186/s12950-017-0172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bai X, Gou X, Cai P, Xu C, Cao L, Zhao Z, Huang M, Jin J, Sesamin enhances Nrf2-mediated protective defense against oxidative stress and inflammation in colitis via AKT and ERK activation, Oxid. Med. Cell. Longev (2019). 10.1155/2019/2432416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Murray IA, Perdew GH, Ligand activation of the Ah receptor contributes to gastrointestinal homeostasis, Curr. Opin. Toxicol 2 (2017) 15–23. 10.1016/j.cotox.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fang Y, Su T, Qiu X, Mao P, Xu Y, Hu Z, Zhang Y, Zheng X, Xie P, Liu Q, Protective effect of alpha-mangostin against oxidative stress induced-retinal cell death, Sci. Rep 6(1) (2016) 21018. 10.1038/srep21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang A, Li D, Wang S, Zhou F, Li P, Wang Y, Lin L, γ-Mangostin, a xanthone from mangosteen, attenuates oxidative injury in liver via NRF2 and SIRT1 induction, J. Funct. Foods 40 (2018) 544–553. 10.1016/j.jff.2017.11.047. [DOI] [Google Scholar]
- [25].Ibrahim SRM, El-Agamy DS, Abdallah HM, Ahmed N, Elkablawy MA, Mohamed GA, Protective activity of tovophyllin A, a xanthone isolated from Garcinia mangostana pericarps, against acetaminophen-induced liver damage: role of Nrf2 activation, Food Funct 9(6) (2018) 3291–3300. 10.1039/C8FO00378E. [DOI] [PubMed] [Google Scholar]
- [26].Liu Z, Li L, Chen W, Wang Q, Xiao W, Ma Y, Sheng B, Li X, Sun L, Yu M, Yang H, Aryl hydrocarbon receptor activation maintained the intestinal epithelial barrier function through Notch1 dependent signaling pathway, Int. J. Mol. Med 41(3) (2018) 1560–1572. 10.3892/ijmm.2017.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Singh R, Chandrashekharappa S, Bodduluri SR, Baby BV, Hegde B, Kotla NG, Hiwale AA, Saiyed T, Patel P, Vijay-Kumar M, Langille MGI, Douglas GM, Cheng X, Rouchka EC, Waigel SJ, Dryden GW, Alatassi H, Zhang H-G, Haribabu B, Vemula PK, Jala VR, Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway, Nat. Commun 10(1) (2019) 89. 10.1038/s41467-018-07859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Martínez-Maqueda D, Miralles B, Recio I, HT29 cell line, in: Verhoeckx K, Cotter P, López-Expósito I, Kleiveland C, Lea T, Mackie A, Requena T, Swiatecka D, Wichers H (Eds.), The impact of food bioactives on health: in vitro and ex vivo models, Springer International Publishing, Cham, 2015, pp. 113–124. 10.1007/978-3-319-16104-4. [DOI] [PubMed] [Google Scholar]
- [29].Tocmo R, Parkin K, S-1-propenylmercaptocysteine protects murine hepatocytes against oxidative stress via persulfidation of Keap1 and activation of Nrf2, Free Radic. Biol. Med 143 (2019) 164–175. 10.1016/j.freeradbiomed.2019.07.022. [DOI] [PubMed] [Google Scholar]
- [30].Özçam M, Tocmo R, Oh J-H, Afrazi A, Mezrich JD, Roos S, Claesen J, van Pijkeren J-P, Gut symbionts Lactobacillus reuteri R2lc and 2010 encode a polyketide synthase cluster that activates the mammalian aryl hydrocarbon receptor, Appl. Environ. Microbiol 85(10) (2019) e01661–18. 10.1128/AEM.01661-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Seidel SD, Winters GM, Rogers WJ, Ziccardi MH, Li V, Keser B, Denison MS, Activation of the Ah receptor signaling pathway by prostaglandins, J. Biochem. Mol. Toxicol 15(4) (2001) 187–196. 10.1002/jbt.16 [DOI] [PubMed] [Google Scholar]
- [32].Han D, Nagy SR, Denison MS, Comparison of recombinant cell bioassays for the detection of Ah receptor agonists, BioFactors 20(1) (2004) 11–22. 10.1002/biof.5520200102. [DOI] [PubMed] [Google Scholar]
- [33].Nguyen L-HD, Vo HT, Pham HD, Connolly JD, Harrison LJ, Xanthones from the bark of Garcinia merguensis, Phytochemistry 63(4) (2003) 467–470. 10.1016/S0031-9422(02)00433-8. [DOI] [PubMed] [Google Scholar]
- [34].Han A-R, Kim J-A, Lantvit DD, Kardono LBS, Riswan S, Chai H, Carcache de Blanco EJ, Farnsworth NR, Swanson SM, Kinghorn AD, Cytotoxic xanthone constituents of the stem bark of Garcinia mangostana (mangosteen), J. Nat. Prod 72(11) (2009) 2028–2031. 10.1021/np900517h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bennett GJ, Lee H-H, Lee L-P, Synthesis of minor xanthones from Garcinia mangostana, J. Nat. Prod 53(6) (1990) 1463–1470. 10.1021/np50072a010. [DOI] [Google Scholar]
- [36].Guo M, Wang X, Lu X, Wang H, Brodelius PE, α-Mangostin extraction from the native mangosteen (Garcinia mangostana L.) and the binding mechanisms of α-mangostin to HSA or TRF, PLOS ONE 11(9) (2016) e0161566. 10.1371/journal.pone.0161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Aisha AFA, Abu-Salah KM, Ismail Z, Majid AMSA, In vitro and in vivo anti-colon cancer effects of Garcinia mangostana xanthones extract, BMC Complement. Altern. Med 12(1) (2012) 104. 10.1186/1472-6882-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [38].Xu Z, Huang L, Chen X-H, Zhu X-F, Qian X-J, Feng G-K, Lan W-J, Li H-J, Cytotoxic prenylated xanthones from the pericarps of Garcinia mangostana, Molecules 19(2) (2014). 1820–1827. 10.3390/molecules19021820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yan M, Vemu B, Veenstra J, Petiwala SM, Johnson JJ, Carnosol, a dietary diterpene from rosemary (Rosmarinus officinalis) activates Nrf2 leading to sestrin 2 induction in colon cells, Integr. Mol. Med 5(4) (2018). 10.15761/IMM.1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gout S, Marie C, Lainé M, Tavernier G, Block MR, Jacquier-Sarlin M, Early enterocytic differentiation of HT-29 cells: biochemical changes and strength increases of adherens junctions, Exp. Cell Res 299(2) (2004) 498–510. 10.1016/j.yexcr.2004.06.008. [DOI] [PubMed] [Google Scholar]
- [41].Putt KK, Pei R, White HM, Bolling BW, Yogurt inhibits intestinal barrier dysfunction in Caco-2 cells by increasing tight junctions, Food Funct 8(1) (2017) 406–414. 10.1039/C6FO01592A. [DOI] [PubMed] [Google Scholar]
- [42].Li P, Yang Z, Tang B, Zhang Q, Chen Z, Zhang J, Wei J, Sun L, Yan J, Identification of xanthones from the mangosteen pericarp that inhibit the growth of Ralstonia solanacearum, ACS Omega 5(1) (2019) 334–343. 10.1021/acsomega.9b02746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wittenauer J, Falk S, Schweiggert-Weisz U, Carle R, Characterisation and quantification of xanthones from the aril and pericarp of mangosteens (Garcinia mangostana L.) and a mangosteen containing functional beverage by HPLC–DAD–MSn, Food Chem 134(1) (2012) 445–452. 10.1016/j.foodchem.2012.02.094. [DOI] [Google Scholar]
- [44].Wincent E, Amini N, Luecke S, Glatt H, Bergman J, Crescenzi C, Rannug A, Rannug U, The suggested physiologic Aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans, J. Biol. Chem 284(5) (2009) 2690–2696. 10.1074/jbc.M808321200. [DOI] [PubMed] [Google Scholar]
- [45].Rush GF, Gorski JR, Ripple MG, Sowinski J, Bugelski P, Hewitt WR, Organic hydroperoxide-induced lipid peroxidation and cell death in isolated hepatocytes, Toxicol. Appl. Pharmacol 78(3) (1985) 473–483. 10.1016/0041-008X(85)90255-8. [DOI] [PubMed] [Google Scholar]
- [46].Chelakkot C, Ghim J, Ryu SH, Mechanisms regulating intestinal barrier integrity and its pathological implications, Exp. Mol. Med 50(8) (2018) 103. 10.1038/s12276-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rao R, Oxidative stress-induced disruption of epithelial and endothelial tight junctions, Front. Biosci, 13 (2008) 7210–7226. 10.2741/3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Samak G, Gangwar R, Meena AS, Rao RG, Shukla PK, Manda B, Narayanan D, Jaggar JH, Rao R, Calcium channels and oxidative stress mediate a synergistic disruption of tight junctions by ethanol and acetaldehyde in Caco-2 cell monolayers, Sci. Rep 6(1) (2016) 38899. 10.1038/srep38899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Attignon EA, Distel E, Le-Grand B, Leblanc AF, Barouki R, de Oliveira E, Aggerbeck M, Blanc EB, Down-regulation of the expression of alcohol dehydrogenase 4 and CYP2E1 by the combination of α-endosulfan and dioxin in HepaRG human cells, Toxicol. In Vitro 45 (2017) 309–317. 10.1016/j.tiv.2017.06.029. [DOI] [PubMed] [Google Scholar]
- [50].Prochaska HJ, Talalay P, Regulatory mechanisms of monofunctional and bifunctional anticarcinogenic enzyme inducers in murine liver, Cancer Res 48(17) (1988) 4776–4782. [PubMed] [Google Scholar]
- [51].Hayes JD, Dinkova-Kostova AT, McMahon M, Cross-talk between Transcription Factors AhR and Nrf2: Lessons for cancer chemoprevention from dioxin, Toxicol. Sci 111(2) (2009) 199–201. 10.1093/toxsci/kfp168. [DOI] [PubMed] [Google Scholar]
- [52].Gutierrez-Orozco F, Thomas-Ahner JM, Berman-Booty LD, Galley JD, Chitchumroonchokchai C, Mace T, Suksamrarn S, Bailey MT, Clinton SK, Lesinski GB, Failla ML, Dietary α-mangostin, a xanthone from mangosteen fruit, exacerbates experimental colitis and promotes dysbiosis in mice, Mol. Nutr. Food Res 58(6) (2014) 1226–1238. 10.1002/mnfr.201300771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chae H-S, You BH, Song J, Ko HW, Choi YH, Chin Y-W, Mangosteen extract prevents dextran sulfate sodium-induced colitis in mice by suppressing NF-κB activation and inflammation, J. Med. Food 20(8) (2017) 727–733. 10.1089/jmf.2017.3944. [DOI] [PubMed] [Google Scholar]
- [54].Calabrese EJ, Mattson MP, Calabrese V, Resveratrol commonly displays hormesis: Occurrence and biomedical significance, Hum. Exp. Toxicol 29(12) (2010) 980–1015. 10.1177/0960327110383625. [DOI] [PubMed] [Google Scholar]
- [55].Moghaddam NSA, Oskouie MN, Butler AE, Petit PX, Barreto GE, Sahebkar A, Hormetic effects of curcumin: What is the evidence?, J. Cell. Physiol 234(7) (2019) 10060–10071. 10.1002/jcp.27880. [DOI] [PubMed] [Google Scholar]
- [56].Pal S, Konkimalla VB, Hormetic potential of sulforaphane (SFN) in switching cells’ fate towards survival or death, Min Rev. Med. Chem 16(12) (2016) 980–995. 10.2174/1389557516666151120115027. [DOI] [PubMed] [Google Scholar]
- [57].Andrysík Z, Vondráček J, Marvanová S, Ciganek M, Neča J, Pěnčíková K, Mahadevan B, Topinka J, Baird WM, Kozubík A, Machala M, Activation of the aryl hydrocarbon receptor is the major toxic mode of action of an organic extract of a reference urban dust particulate matter mixture: The role of polycyclic aromatic hydrocarbons, Mutat. Res 714(1) (2011) 53–62. 10.1016/j.mrfmmm.2011.06.011. [DOI] [PubMed] [Google Scholar]
- [58].McKinney JD, Singh P, Structure-activity relationships in halogenated biphenyls: Unifying hypothesis for structural specificity, Chem. Biol. Interact 33(2) (1981) 271–283. 10.1016/0009-2797(81)90046-6. [DOI] [PubMed] [Google Scholar]
- [59].Hodges-Loaiza HB, Parker LE, Cox AD, Chapter 3 - Prenylation and phosphorylation of Ras superfamily small GTPases, in: Hrycyna CA, Bergo MO, Tamanoi F (Eds.), The Enzymes, Academic Press; 2011, pp. 43–69. 10.1016/B978-0-12-415922-8.00003-3. [DOI] [Google Scholar]
- [60].Nguyen LP, Bradfield CA, The search for endogenous activators of the aryl hydrocarbon receptor, Chem. Res. Toxicol 21(1) (2008) 102–116. 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Arulmozhiraja S, Morita M, Structure–activity relationships for the toxicity of polychlorinated dibenzofurans: approach through density functional theory-based descriptors, Chem. Res. Toxicol 17(3) (2004) 348–356. 10.1021/tx0300380. [DOI] [PubMed] [Google Scholar]
- [62].Ashek A, Lee C, Park H, Cho SJ, 3D QSAR studies of dioxins and dioxin-like compounds using CoMFA and CoMSIA, Chemosphere 65(3) (2006) 521–529. 10.1016/j.chemosphere.2006.01.010. [DOI] [PubMed] [Google Scholar]
- [63].Chin Y-W, Jung H-A, Chai H, Keller WJ, Kinghorn AD, Xanthones with quinone reductase-inducing activity from the fruits of Garcinia mangostana (Mangosteen), Phytochemistry 69(3) (2008) 754–758. 10.1016/j.phytochem.2007.09.023. [DOI] [PubMed] [Google Scholar]
- [64].Magesh S, Chen Y, Hu L, Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents, Med. Res. Rev 32(4) (2012) 687–726. 10.1002/med.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].McMahon M, Itoh K, Yamamoto M, Hayes JD, Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression, J. Biol. Chem 278(24) (2003) 21592–21600. 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- [66].Bondy SC, Naderi S, Contribution of hepatic cytochrome P450 systems to the generation of reactive oxygen species, Biochem. Pharmacol 48(1) (1994) 155–159. 10.1016/0006-2952(94)90235-6. [DOI] [PubMed] [Google Scholar]
- [67].Morel Y, Mermod N, Barouki R, An autoregulatory loop controlling CYP1A1 gene expression: role of H(2)O(2) and NFI, Mol. Cell Biol 19(10) (1999) 6825–6832. 10.1128/mcb.19.10.6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Foti RS, Pearson JT, Rock DA, Wahlstrom JL, Wienkers LC, In vitro inhibition of multiple cytochrome P450 isoforms by xanthone derivatives from mangosteen extract, Drug Metab. Dispos 37(9) (2009) 1848–1855. 10.1124/dmd.109.028043. [DOI] [PubMed] [Google Scholar]
- [69].Piechota-Polanczyk A, Fichna J, Review article: the role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases, Naunyn Schmiedebergs Arch. Pharmacol 387(7) (2014) 605–620. 10.1007/s00210-014-0985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Nguyen T, Nioi P, Pickett CB, The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress, J. Biol. Chem 284(20) (2009) 13291–13295. 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hao Q, Wang M, Sun NX, Zhu C, Lin YM, Li C, Liu F, Zhu WW, Sulforaphane suppresses carcinogenesis of colorectal cancer through the ERK/Nrf2-UDP glucuronosyltransferase 1A metabolic axis activation, Oncol. Rep 43(4) (2020) 1067–1080. 10.3892/or.2020.7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ryter SW, Choi AMK, Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation, Transl. Res 167(1) (2016) 7–34. 10.1016/j.trsl.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Yang X, Wang S, Ouyang Y, Tu Y, Liu A, Tian Y, He M, Pi R, Garcinone D, a natural xanthone promotes C17.2 neural stem cell proliferation: Possible involvement of STAT3/Cyclin D1 pathway and Nrf2/HO-1 pathway, Neurosci. Lett 626 (2016) 6–12. 10.1016/j.neulet.2016.05.012. [DOI] [PubMed] [Google Scholar]
- [74].Dietrich C, Kaina B, The aryl hydrocarbon receptor (AhR) in the regulation of cell–cell contact and tumor growth, Carcinogenesis 31(8) (2010) 1319–1328. 10.1093/carcin/bgq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Goettel JA, Gandhi R, Kenison JE, Yeste A, Murugaiyan G, Sambanthamoorthy S, Griffith AE, Patel B, Shouval DS, Weiner HL, Snapper SB, Quintana FJ, AHR activation is protective against colitis driven by T cells in humanized mice, Cell Rep 17(5) (2016) 1318–1329. 10.1016/j.celrep.2016.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Benson JM, Shepherd DM, Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn’s disease, Toxicol. Sci 120(1) (2011) 68–78. 10.1093/toxsci/kfq360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Fukumoto S, Toshimitsu T, Matsuoka S, Maruyama A, Oh-oka K, Takamura T, Nakamura Y, Ishimaru K, Fujii-Kuriyama Y, Ikegami S, Itou H, Nakao A, Identification of a probiotic bacteria-derived activator of the aryl hydrocarbon receptor that inhibits colitis, Immunol. Cell Biol 92(5) (2014) 460–465. 10.1038/icb.2014.2 [DOI] [PubMed] [Google Scholar]
- [78].Hooper LV, You AhR what you eat: Linking diet and immunity, Cell 147(3) (2011) 489–491. 10.1016/j.cell.2011.10.004. [DOI] [PubMed] [Google Scholar]
- [79].Hubbard TD, Murray IA, Perdew GH, Indole and tryptophan metabolism: Endogenous and dietary routes to Ah receptor activation, Drug Metab. Dispos 43(10) (2015) 1522–1535. 10.1124/dmd.115.064246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Schulzke JD, Ploeger S, Amasheh M, Fromm A, Zeissig S, Troeger H, Richter J, Bojarski C, Schumann M, Fromm M, Epithelial tight junctions in intestinal inflammation, Ann. N Y Acad. Sci 1165(1) (2009) 294–300. 10.1111/j.1749-6632.2009.04062.x. [DOI] [PubMed] [Google Scholar]
- [81].Dahan S, Rabinowitz KM, Martin AP, Berin MC, Unkeless JC, Mayer L, Notch-1 signaling regulates intestinal epithelial barrier function, through interaction with CD4+ T cells, in mice and humans, Gastroenterology 140(2) (2011) 550–559. 10.1053/j.gastro.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wang J, Ghosh SS, Ghosh S, Curcumin improves intestinal barrier function: modulation of intracellular signaling, and organization of tight junctions, Am. J. Physiol. Cell Physiol 312(4) (2017) C438–C445. 10.1152/ajpcell.00235.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Förster C, Tight junctions and the modulation of barrier function in disease, Histochem. Cell Biol 130(1) (2008) 55–70. 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhao B, Degroot DE, Hayashi A, He G, Denison MS, CH223191 is a ligand-selective antagonist of the Ah (Dioxin) receptor, Toxicol Sci 117(2) (2010) 393–403. 10.1093/toxsci/kfq217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lamas B, Natividad JM, Sokol H, Aryl hydrocarbon receptor and intestinal immunity, Mucosal Immunol 11(4) (2018) 1024–1038. 10.1038/s41385-018-0019-2. [DOI] [PubMed] [Google Scholar]
- [86].Leavy O, The ‘AHR diet’ for mucosal homeostasis, Nat. Rev. Immunol 11(12) (2011) 806–806. 10.1038/nri3115. [DOI] [PubMed] [Google Scholar]
- [87].Yu K, Ma Y, Zhang Z, Fan X, Li T, Li L, Xiao W, Cai Y, Sun L, Xu P, Yu M, Yang H, AhR activation protects intestinal epithelial barrier function through regulation of Par-6, J. Mol. Histol 49(5) (2018) 449–458. 10.1007/s10735-018-9784-1. [DOI] [PubMed] [Google Scholar]
- [88].Ahmed SMU, Luo L, Namani A, Wang XJ, Tang X, Nrf2 signaling pathway: Pivotal roles in inflammation, Biochim. Biophys. Acta Mol. Basis Dis 1863(2) (2017) 585–597. 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


