Summary
Objective:
To examine the efficacy of a family-tailored education and problem-solving behavioral intervention, Supporting Treatment Adherence Regimens (STAR), in young children (2–12 years old) with new-onset epilepsy compared to an attention control (i.e., education only; EO) intervention. Participants randomized to the STAR intervention were hypothesized to demonstrate significantly improved adherence at post-intervention, 3-month, 6-month, and 12-month follow-up visits compared to the EO intervention. Seizure and health-related quality of life (HRQOL) outcomes were also examined.
Methods:
Two hundred children with new-onset epilepsy and their caregivers were recruited during routine epilepsy clinic visits. Baseline questionnaires were completed, and electronic adherence monitors were provided. Participants with adherence < 95% during the run-in period were randomized to either STAR or EO intervention. Active intervention was provided to both groups for four months. Questionnaires were completed at conclusion of the active intervention phase and three follow-up time points (3, 6, and 12-months). Group differences in adherence, seizure outcomes and HRQOL were examined using regression-based ANCOVAs and longitudinal mixed effect linear or logistical models.
Results:
Adherence at 12-month follow-up was significantly different between the STAR (M=82.34, SD=21.29) and EO intervention groups (M=61.77, SD=28.29), with the STAR group demonstrating 20.6% greater adherence (b=19.11, p=0.04, 95% CI=1.00, 37.22, d=0.83). No significant differences were found between groups on seizure and HRQOL outcomes.
Significance:
A family-based behavioral adherence intervention demonstrated sustained adherence improvements one year following epilepsy diagnosis compared to an epilepsy-specific education intervention. STAR is an efficacious adherence intervention that can be easily implemented into routine epilepsy care.
Keywords: antiepileptic drugs, compliance, youth, behavioral intervention
Introduction
Epilepsy is a common childhood condition affecting approximately 470,000 youth in the United States.1 Initial treatment for epilepsy includes anti-seizure medications (ASMs). However, the efficacy of ASMs is compromised if children do not take their ASM as prescribed, known as non-adherence. Approximately 60% of youth with epilepsy are non-adherent to ASMs, with devastating consequences, including a 3-fold increased risk of seizures,2, 3 suboptimal health-related quality of life (HRQOL),4 inaccurate clinical decision-making,5 and higher health care utilization6, 7 and costs.7 Thus, improving ASM adherence is of critical importance to optimize health and psychosocial outcomes in youth with epilepsy.
A recent Cochrane review indicated a lack of well-designed randomized controlled clinical trials (RCT) to improve adherence in epilepsy, especially in young children.8 To address this gap, we developed an education and problem-solving intervention for young children with epilepsy and their families, known as Supporting Treatment Adherence Regimens (STAR). STAR was initially tested in a series of pilot trials, which indicated high feasibility, acceptability, and family satisfaction.9, 10 Preliminary efficacy data also indicated that compared to treatment as usual, participants who received STAR had improved adherence over time.9 Capitalizing on these findings, the next logical step is to test the efficacy of the STAR intervention to improve ASM adherence in children with epilepsy via a RCT.
The primary aim was to examine the efficacy of a family-tailored adherence intervention (STAR) on electronically monitored ASM adherence in children with new-onset epilepsy compared to an education only (EO) intervention. Participants in the STAR intervention were hypothesized to demonstrate a statistically significant increase in ASM adherence at post-intervention, 3-month, 6-month, and 12-month follow-up visits compared to participants receiving EO. An exploratory and related aim examined intervention group differences in longitudinal adherence trajectories. A secondary aim examined the efficacy of the STAR intervention on seizure freedom and HRQOL in children with epilepsy compared to the EO intervention. Participants in the STAR intervention were hypothesized to demonstrate statistically significant improvements in seizure control and HRQOL at the 6- and 12-month follow-up compared to the EO intervention. Six and 12-month outcomes were selected because three months was originally considered insufficient to detect changes in more distal outcomes of seizure freedom and HRQOL. Similar to the primary outcome, an exploratory and related aim examined intervention group differences in longitudinal HRQOL and seizure trajectories.
Methods
Participants
Participants included children with epilepsy, 2–12 years old, and their caregivers recruited from the Comprehensive Epilepsy Center at Cincinnati Children’s Hospital Medical Center from April 2013 to December 2018. To be study eligible, the child would be: 1) between two and 12 years of age, 2) diagnosed with epilepsy in the past seven months, and 3) prescribed only one ASM. Children and caregivers were also required to read and speak English. The seven-month new diagnosis time frame was chosen because studies have documented that suboptimal adherence patterns are established within 6–7 months of diagnosis3, 11 and those patterns are associated with long-term seizure outcomes.2 Exclusion criteria included non-epilepsy medical disorders requiring daily medications for the child, with the exception of allergies and asthma, significant child developmental delay (i.e., autism), and the family living further than 75 miles of the Center.
Eligible participants were approached during routine epilepsy clinic visits by trained research coordinators. Once families were explained study procedures and had their questions answered, consent and assent (11 years and older) forms were reviewed and then signed.
Standard protocol, approvals, registrations, and patient consents
This study was approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board and the trial was registered in clinicaltrials.gov (NCT01851057). Anonymized data will be shared by request from any qualified investigator.
Procedures
Overall Study Design
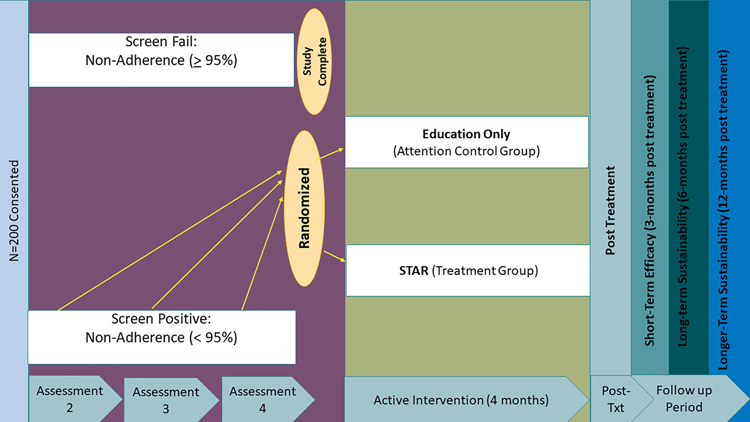
The overall study is an enrichment RCT comparing family-tailored education and problem-solving adherence intervention (STAR) to an attention control intervention (Education Only). An enrichment design ensures that only individuals who demonstrate non-adherence receive an intervention. Thus, participants were enrolled and initially screened for non-adherence. Participants were monitored for non-adherence (defined as <95%) for seven months and had the opportunity to be randomized at three different time points (Assessment 2, 3, and 4). Assessment 2 was one-month following the baseline visit and Assessment 3 and 4 were 4 and 7 months following baseline, respectively. If they screen failed at all three assessment points, trial participation ended due to near-perfect adherence (≥ 95%). Participants who were randomized received eight intervention sessions over a 4-month period and completed three follow-up visits (3, 6, and 12 months following intervention; See Figure 1 for study protocol). Detailed trial procedures have been previously published.12
Figure 1.
STAR Trial Study Design
Assessments
Families completed baseline paper-pencil questionnaires and were provided adherence electronic monitors: Medication Event Monitoring System (MEMS®) TrackCap or Vaica SimpleMed+ pillboxes. Families were asked to place their ASM in the electronic monitor and research staff called within 3 days of their enrollment to ensure use of the electronic monitor. Some participants were on liquid formulations and thus families were asked to place droppers or dispensing cups in the MEMS TrackCap as a proxy for monitoring adherence.
At subsequent assessment points (A2, A3, A4; See Figure 1), adherence data were downloaded, caregivers completed questionnaires, and medical chart reviews were conducted. If participant adherence was <95% at any of these assessment points, they were randomized to one of two intervention arms: STAR or EO. Participants were compensated for their time and effort using an incentivized schedule, ranging from $10-$50. Families could earn up to $405 if all study procedures were completed.
Randomization Method:
Stratified block randomization with six strata based on two stratification variables was used: baseline adherence (i.e., two levels; adherence for 1-month > 80% or < 80%) and phase of randomization (i.e., three levels; A2, A3, A4). Blocks of two or four were chosen randomly within each stratum. A pseudorandom number generator was used to generate the blocks and random group assignments within each strata via the statistical software, R (©The R Foundation) by the biostatistician. The randomization list was held by a research coordinator independent of the study to reduce any potential biases. Randomized participants were notified of their randomization status within 48 hours and were scheduled for their first intervention session (STAR or EO) within one week.
Intervention
Both STAR and EO intervention groups received eight total sessions, six face-to-face and two via phone. Face-to-face sessions were approximately ~45 minutes and phone calls were ~15 minutes for both groups. Sessions occurred biweekly for the first eight weeks and then two booster sessions occurred at Week 12 and Week 16. Content of both intervention arms are briefly reviewed below. Interventionists for both arms were trained by a licensed psychologist (SG) who provided monthly supervision to interventionists, which included review of audio sessions and feedback to interventionists. Training for both arms included review of treatment manuals, live role plays for clinical content in sessions, and shadowing experiences with more experienced interventionists. For both intervention arms of the trial, the first session focused on addressing epilepsy knowledge deficits and providing feedback on the child’s adherence patterns. Use of questionnaire data allowed interventionists to provide key information to families based on their epilepsy knowledge gaps. For the STAR intervention group, adherence education was provided. Adherence feedback from the electronic monitors was provided to both groups via paper and indicated missed and taken doses in the past month. Of note, the STAR interventionists reviewed the paper adherence feedback report and helped families identify adherence patterns. Notably, the PI and statistician were blinded to treatment allocation throughout the study. In addition, the healthcare team was not actively told which group participants were randomized to; however, it is possible that participants shared this information during routine clinical care.
STAR Intervention Sessions.
Interventionists for STAR sessions included masters and doctoral level psychologists/trainees. Six STAR interventionists were trained and only one interventionist was assigned to each family. Following the first session, subsequent sessions used a problem-solving approach to address the family’s individualized adherence barriers. This problem solving approach included 1) Identification of the adherence barrier experienced by the family, 2) Generation of 8–10 creative solutions by all family members involved in the session, 3) Evaluation of the solutions by family members, with each member rating the solution as + or −, 4) Choice of one or two combined solutions to implement, and 5) Provision of detailed information on how the solution will be implemented (who, what, when, where, and how). Sessions 2, 4, and 6 focused on problem-solving around adherence barriers. Sessions 3 and 5 involved brief telephone contacts to follow-up on how the solution was being implemented and tweaking solutions if needed between face-to-face sessions. Sessions 7 and 8 were booster sessions to review information, problem-solve any new identified barriers, and plan for the future.
EO Intervention Sessions.
EO interventionists included nurse practitioners, medical fellows, and master’s level health-related professionals (e.g., health services field) who were independent from the STAR interventionists. Six EO interventionists were trained and only one interventionist was assigned to each family. Following the first session, face to face sessions covered the following topics: seizure safety, sleep hygiene, communication and psychosocial comorbidities, and school-based issues. Telephone calls reviewed completion of a seizure safety checklist at home or implementation of sleep routines at home. Session 9 summarized all of the information from prior sessions and allowed families to ask any final questions.
Post-intervention and follow up study visits
All participants completed questionnaires and had adherence electronic monitors downloaded at mid-intervention, post-intervention, 3-month, 6-month, and 12-month follow-up. Medical chart reviews were also completed at each of these visits.
Measures
Background Information Form.
Parents completed a background and demographics form, which elicited information regarding the child’s age, sex, race/ethnicity, and caregiver occupation. Caregiver occupation was used to calculate revised Duncan scores,13 a proxy measure of socioeconomic status, with higher scores representing higher socioeconomic status (range 15–97). For households with two caregivers, the higher Duncan score was used in analyses.
Medical Chart Review.
A medical chart review was conducted by clinical research coordinators using the electronic health record. Information regarding seizure type/etiology, treatment, seizure history was extracted at each assessment point.
Seizure History Form.
Caregivers completed a form regarding the frequency and types of seizures experienced by the child and any medication changes between study visits. Data from the seizure history form and medical chart review were used to assess the presence or absence of seizures in the past three months. Given the heterogeneity of seizures (e.g. absence, generalized tonic-clonic, myoclonic), seizures were coded as absent (0) or present (1) in the past three months and this variable was used for analyses.
Adherence Electronic Monitors.
Two types of adherence electronic monitors were used in the study: MEMS®) TrackCap or Vaica SimpleMed+ pillboxes. The MEMS® 6 TrackCap is an electronic pill cap and bottle and the SimpleMed+ pillbox is an electronic pillbox. Both devices allow for the measurement of daily ASM adherence. MEMS® TrackCap data were downloaded at each clinic visit while SimpleMed pillboxes have real-time data collection, which were extracted routinely.
Daily data from electronic monitors was used to calculate adherence, our primary outcome. Adherence rates were calculated on a monthly basis (e.g., 30 days) by dividing the number of doses taken by the number of doses prescribed and multiplying by 100% (e.g., 40 doses taken/60 doses prescribed * 100%=66.7%). Depending on when patients were randomized, baseline adherence was the immediate 30 days preceding randomization, which could have occurred at A2, A3, or A4. Following randomization, monthly adherence rates were calculated for the entire 16-month study, with months 1–4 representing active intervention, month 5 representing post-intervention, month 7 representing 3-month follow-up, month 10 representing 6-month follow-up and month 16 representing 12-month follow-up.
HRQOL measures.
Two different HRQOL measures were used in the study, a generic (i.e., PedsQL™ 14) and epilepsy-specific (i.e., Quality of Life in Childhood Epilepsy Questionnaire; QOLCE) measure. The PedsQL™ is a 23-item generic measure of HRQOL that assesses the following domains of functioning: Physical, Emotional, Social, and School. Both parent-proxy and child-report forms were used. Children ages eight years and older completed the measure in the current study. A total score can also be calculated with scores ranging from 0–100 (higher scores represent better HRQOL). The PedsQL™ has excellent reliability and validity.14 The QOLCE is a 79-item parent-proxy measure of epilepsy-specific HRQOL for children 4–18 years.15 The measure assesses 15 domains of functioning, with higher scores representing better HRQOL (range 0–100). Internal consistency and validity are strong for this measure.15 For both the PedsQL and QOLCE, only total scores were used for analyses.
Treatment fidelity.
A treatment fidelity checklist was created for all STAR and EO sessions. Approximately 37% of the face-to-face sessions were reviewed for treatment fidelity (n=62 EO sessions and n=61 STAR) by two clinical psychologists who were not the study PI. Treatment fidelity for STAR sessions was 98% and for EO sessions was 97%.
Statistical Analyses
To verify that our intervention arms were equal on demographic and clinical characteristics, we conducted χ2 tests of independence for the categorical variables and independent samples t-tests for the continuous variables. We also assessed whether missingness on adherence at any timepoint varied by group or other demographic variables to test whether the missing-at-random assumption could be met for data missing on our primary outcome. We examined the data for outliers or influential data points using Cook’s d. If any influential observations were identified, sensitivity analyses were conducted to assess the impact of these observations on parameter estimates. A robust variance estimator was used for all analyses to account for minor deviations from normality in the residuals in our models.
All analyses assumed intent-to-treat and retained all participants with available data within their randomized intervention arm. Analyses were performed in Stata version 16 using maximum likelihood estimation. For our primary aim, we conducted four regression-based ANCOVA models to assess whether there were intervention group differences in adherence at our study endpoints: post-intervention, 3-month, 6-month, and 12-month follow-ups. These models covaried for baseline adherence levels and wave, plus any covariates that are associated with the missing data were included to ensure valid inferences under the missing at random assumption. We also examined whether the overall adherence trajectories across all 12 months of data varied by intervention group (again controlling for wave and any covariates associated with missingness) using longitudinal mixed effect models.
For secondary outcomes (the presence of seizures and HRQOL), the analyses were the same as those used for our primary outcomes: regression-based ANCOVAs with maximum likelihood estimation controlling for baseline outcome levels and wave. As hypothesized, we only looked at the 6 and 12-month follow-up periods for these outcomes. In post-hoc analyses, we examined post-treatment and 3-month follow-up seizure outcomes. For the HRQOL models, we assumed a continuous outcome using regression models. For the presence of seizures (0=no; 1=yes), our models assumed a binary outcome using logistic regression models. We also examined whether the overall HRQOL and seizure trajectories across all time points of data varied by intervention group, controlling for wave, using longitudinal mixed effect linear models for HRQOL and longitudinal mixed effect logistic models for seizures. HRQOL and seizure outcomes were assessed at baseline, mid-intervention, post-intervention, and at the 3-month, 6-month, and 12-month follow-ups.
A power analysis was conducted, a priori, for our primary hypothesis that participants in the STAR intervention will exhibit a significantly greater increase in adherence at post-intervention compared to participants in the EO intervention. We assumed a significance level of 0.05, a regression-based analysis of covariance model that included all available data based on the intent-to-treat principle, a minimum effect size of d=0.57, and equal sample size across groups. We estimated that we would need to randomize n=132 participants, with n=48 per group after adjusting for attrition to achieve at least 80% power to detect a significant treatment group difference at post-treatment.
Results
Participants
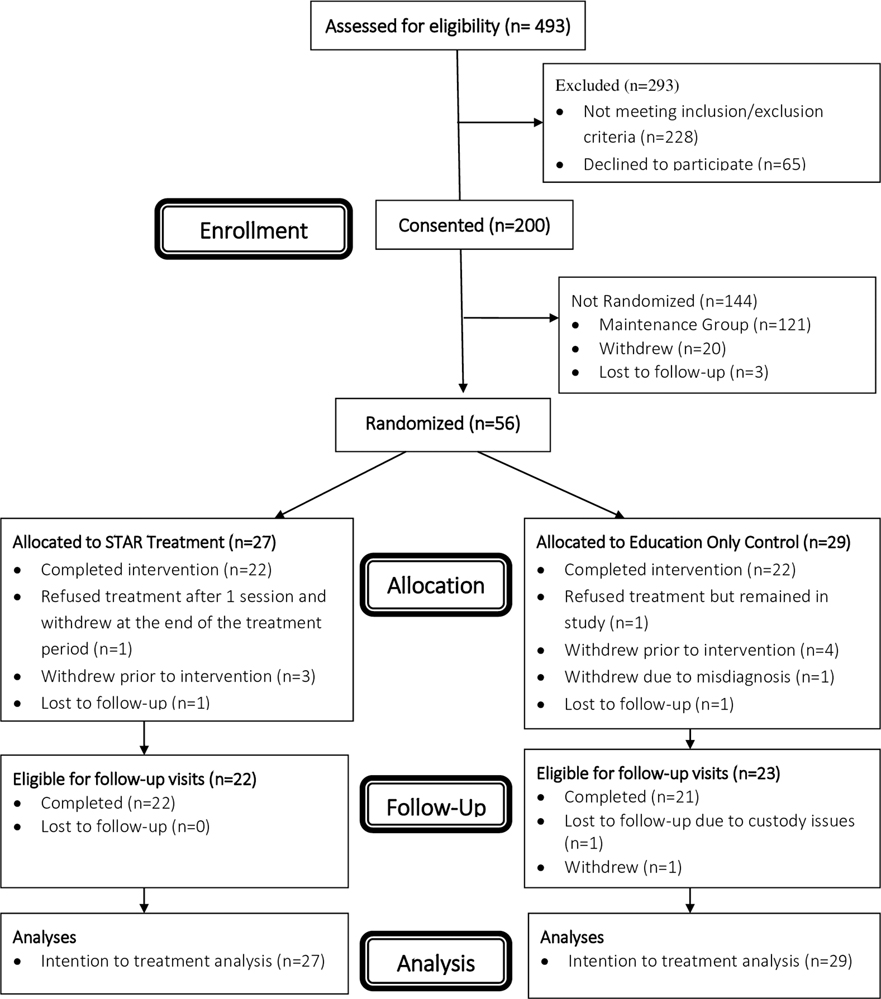
Participant demographic and medical characteristics are contained in Table 1. CONSORT reporting guidelines were followed including a CONSORT diagram (See Figure 2). Examination of outliers indicated the presence of four possible influential data points. However, sensitivity analyses revealed that the parameter estimates and statistical significance were not impacted by the inclusion or exclusion of these data points, so no data was excluded from the final analyses. Missing data on our primary outcome was associated with some of our baseline covariates. However, sensitivity analyses again revealed that inclusion or exclusion of these covariates did not impact any of the parameter estimates or statistical significance, so no additional covariates (aside from baseline outcome levels and wave) were included in the final analyses. The intervention groups did not differ significantly on any baseline variables or missing data at any time point on our primary outcome with the exception of epilepsy type (X2 =12.5, p=0.03). A total of n=56 participants (n=27 in STAR and n=29 in EO) were included in the analyses. Notably, no significant differences were noted in demographic variables between those who withdrew and those randomized. Descriptive data regarding trial engagement (e.g., sessions completed, time to first session) and primary and secondary outcome measures are presented in Table 2.
Table 1.
Demographic data on children with epilepsy and their caregivers (n = 200)
| Education Only Group (n=29) | STAR Intervention Group (n=27) | Withdraw Group (n=23) | Non-Randomized Group (High Adherence; n=121) | |
|---|---|---|---|---|
|
| ||||
| M±SD | M±SD | M±SD | M±SD | |
|
| ||||
| Child Age (years) | 8.15±3.30 | 7.13±2.80 | 7.58±2.89 | 7.05±2.98 |
| Months since epilepsy diagnosis | 2.63±2.64 | 2.49±2.04 | 2.58±1.95 | 2.76±2.32 |
| Duncan score | 48.99±22.5 | 49.94±19.64 | 65.41±19.00 | 52.27±20.82 |
| N (%) | N (%) | N (%) | N (%) | |
| Child Sex | ||||
| • Girls | 14 (48%) | 15 (56%) | 65 (54%) | 11 (48%) |
| • Boys | 15 (52%) | 12 (44%) | 56 (46%) | 12 (52%) |
| Child Race | ||||
| • White: Non-Hispanic | 21 (72%) | 18 (67%) | 102 (84%) | 18 (78%) |
| • White: Hispanic | 0 (0%) | 0 (0%) | 3 (2.5%) | 0 (0%) |
| • Black: Non-Hispanic | 5 (17%) | 5 (18%) | 5 (4%) | 4 (17%) |
| • Black: Hispanic | 1 (3.4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| • More than one race: Non-Hispanic | 1 (3.4%) | 4 (15%) | 7 (6%) | 1 (4%) |
| • More than one race: Hispanic | 1 (3.4%) | 0 (0%) | 1 (1%) | 0 (0%) |
| • Asian | 0 (0%) | 0 (0%) | 3 (2.5%) | 0 (0%) |
| Seizure Type | ||||
| • Focal Onset | 8 (27.5%) | 3 (11%) | 45 (37%) | 4 (17%) |
| • Generalized Onset | 11 (38%) | 20 (74%) | 47 (39%) | 9 (39%) |
| • Unknown Onset (e.g., unclassified) | 10 (34.5%) | 4 (15%) | 29 (24%) | 10 (44%) |
| Initial Prescribed Anti-epileptic Drug | ||||
| • Carbamazapine | 6 (21%) | 0 (0%) | 18 (15%) | 3 (13%) |
| • Ethosuximide | 6 (21%) | 12 (44%) | 32 (26%) | 4 (17%) |
| • Levetiracetam | 7 (24%) | 5 (18.5%) | 39 (32%) | 9 (39%) |
| • Oxcarbazepine | 2 (7%) | 1 (3.7%) | 11 (9%) | 1 (4%) |
| • Lamotrigine | 0 (0%) | 0 (0%) | 3 (3%) | 0 (0%) |
| • Topiramate | 0 (0%) | 1 (3.7%) | 1 (1%) | 0 (0%) |
| • Valproic Acid | 8 (27%) | 8 (30%) | 17 (14%) | 6 (26%) |
| Primary Caregiver | ||||
| • Mother/Stepmother | 27 (93%) | 26 (96%) | 113 (93%) | 18 (78%) |
| • Father/Stepfather | 0 (0%) | 0 (0%) | 7 (6%) | 3 (13%) |
| • Other (e.g., grandmother, uncle/aunt) | 2 (7%) | 1 (4%) | 1 (1%) | 2 (9%) |
| Caregiver Marital Status | ||||
| • Single | 6 (21%) | 11 (40.7%) | 13 (11%) | 5 (21.7%) |
| • Married | 18 (62%) | 11 (40.7%) | 101 (83%) | 13 (56.5%) |
| • Divorced/Separated | 5 (17%) | 4 (15%) | 5 (4%) | 5 (21.7%) |
| • Widowed | 0 (0%) | 1 (3.7%) | 2 (2%) | 0 (0%) |
Note: No significant differences were found between those who withdrew and those randomized on child age, time since diagnosis, family Duncan scores, race/ethnicity, or sex.
Figure 2.
STAR Trial Consort Diagram
Table 2.
STAR versus EO Treatment Descriptives
| Education Only Group (n=29) | STAR Intervention Group (n=27) | |
|---|---|---|
| M±SD or n (%) | M±SD or n (%) | |
| Number who initiated treatment | 22 (76%) | 23 (85%) |
| Time from consent to Treatment Session 1 | 147.4 ± 119 days | 125.1 ± 82 days |
| # of face-to-face sessions completed * | 6.0 + 0 | 5.8 ± 1.0 |
| # of telephone sessions completed | 1.9 ± 0.35 | 1.7 ± 0.62 |
| Monthly Adherence Percentage | ||
| • Baseline | 72.95 + 20.1 | 76.25 + 19.3 |
| • Month 1 | 78.63 + 24.6 | 78.78 + 20.8 |
| • Month 2 | 77.33 + 23.5 | 81.19 + 21.3 |
| • Month 3 | 78.48 + 18.2 | 83.84 + 24.3 |
| • Month 4 | 77.73 + 22.1 | 80.36 + 26.9 |
| • Month 5 | 78.25 + 23.3 | 74.24 + 31.5 |
| • Month 6 | 74.77 + 26.0 | 71.47 + 32.0 |
| • Month 7 | 65.00 + 28.7 | 73.08 + 30.9 |
| • Month 8 | 68.06 + 37.2 | 73.69 + 27.2 |
| • Month 9 | 68.74 + 34.0 | 72.94 + 30.5 |
| • Month 10 | 67.57 + 31.2 | 80.0 + 21.5 |
| • Month 11 | 63.51 + 32.2 | 79.73 + 20.8 |
| • Month 12 | 61.81 + 33.3 | 81.13 + 14.6 |
| • Month 13 | 62.43 + 33.8 | 79.68 + 17.8 |
| • Month 14 | 67.19 + 31.8 | 75.92 + 27.1 |
| • Month 15 | 61.67 + 34.2 | 71.71 + 31.6 |
| • Month 16 | 61.77 + 28.3 | 82.34 + 21.3 |
| Presence of Seizures in the Past Three Months (% Yes) | ||
| • Baseline | 16 (55%) | 14 (52%) |
| • Post-treatment | 5 (24%) | 1 (4%) |
| • 3-month follow-up | 9 (41%) | 6 (26%) |
| • 6-month follow-up | 6 (30%) | 6 (27%) |
| • 12-month follow-up | 7 (35%) | 8 (38%) |
| PedsQL Total Score: Caregiver Report | ||
| • Baseline | 81.15 ± 14.6 | 84.23 ± 10.8 |
| • Post-treatment | 79.84 ± 15.2 | 84.08 ± 12.5 |
| • 3-month follow-up | 85.37 ± 11.5 | 79.10 ± 15.3 |
| • 6-month follow-up | 81.03 ± 16.4 | 85.00 ± 9.8 |
| • 12-month follow-up | 81.01 ± 12.8 | 82.93 ± 10.8 |
| PedsQL Total Score: Child Report | ||
| • Baseline | 77.13 ± 13.9 | 73.00 ± 12.8 |
| • Post-treatment | 75.96 ± 12.9 | 73.15 ± 18.9 |
| • 3-month follow-up | 78.23 ± 15.7 | 73.00 ± 19.8 |
| • 6-month follow-up | 80.40 ± 12.5 | 78.06 ± 16.3 |
| • 12-month follow-up | 82.04 ± 13.2 | 80.32 ± 11.1 |
| Quality of Life in Childhood Epilepsy Total Score (Caregiver Report) | ||
| • Baseline | 74.08 ± 12.2 | 72.31 ± 12.0 |
| • Post-treatment | 75.41 ± 12.2 | 75.28 ± 11.6 |
| • 3-month follow-up | 76.86 ± 13.3 | 73.51 ± 14.6 |
| • 6-month follow-up | 75.42 ± 14.0 | 76.68 ± 11.3 |
| • 12-month follow-up | 77.86 ± 13.0 | 77.81 ± 11.4 |
Note:
One STAR participant initiated Session 1 but did not complete additional sessions due to multiple family stressors.
Primary Aim Analysis
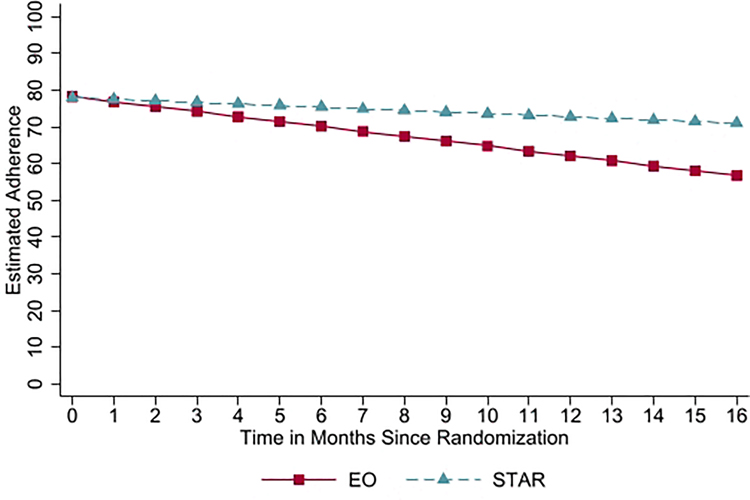
Regression-based ANCOVA models revealed that the intervention groups were not significantly different on adherence at post-intervention (b=−4.97, p=0.51, 95% CI = −19.70, 9.76, d=0.14), 3-month follow-up (b=7.09, p=0.41, 95% CI = −9.94, 24.12, d=0.27), or 6-month follow-up (b=10.87, p=0.22, 95% CI=−6.63, 28.36, d=0.46). However, the groups were significantly different in their adherence rates at the 12-month follow-up, b=19.11, p=0.04, 95% CI=1.00, 37.22, d=0.83, such that those in the STAR intervention arm (mean adherence at 12 month follow-up=82.34, sd=21.29) had, on average, 20.6% higher adherence at this time point than those in the EO intervention arm (mean adherence at 12 month follow-up=61.77, sd=28.29; see Figure 3).
Figure 3.
Group Differences on Adherence
Overall longitudinal trajectories, including all months of adherence (17 time points), indicated that the average intervention group adherence trajectories were not significantly different, b=0.91, p=0.20 (See Table 2 for means).
Secondary Aim Analysis
Intervention groups on caregiver reported HRQOL did not significantly differ at the 6-month follow up, as measured by QOLCE total scores (b=0.80, p=0.77, 95% CI=−4.61, 6.22, d=0.10) or PedsQL total scores (b=2.27, p=0.48, 95% CI=−4.07, 8.62, d=0.30). Similarly, there were no significant intervention group differences on the caregiver-reported HRQOL measures at the 12-month follow-up (QOLCE: b = 0.98, p=0.71, 95% CI=−4.18, 6.13, d=0.004; PedsQL: b=1.22, p=0.71, 95% CI= −5.27, 7.71, d=0.16). There were no significant differences between intervention groups on child-reported PedsQL total scores at the 6-month follow-up (b=−2.38, p=0.61, 95% CI=−11.57, 6.80, d=0.16), or the 12-month follow-up (b=−1.33, p=0.66, 95% CI=−7.31, 4.64, d=0.14) (See Table 2).
For the seizure outcome, we observed no significant differences between the intervention groups in the odds of seizures at the 6-month follow-up (OR=0.97, p=0.97, 95% CI=0.21, 4.48) or the 12-month follow-up (OR=1.23, p=0.80, 95% CI=0.25, 6.11). Post-hoc analyses revealed that there were also no statistically significant differences between groups at the post-treatment (OR=0.14, p=0.10, 95% CI = 0.01, 1.45) or 3-month follow-up (OR=0.47, p=0.26, 95% CI = 0.12, 1.76) (See Table 2).
Overall longitudinal trajectories, including baseline, mid-intervention, post-intervention and all three follow-ups, indicated that the average intervention group trajectories were not significantly different for HRQOL (PedsQL caregiver total: b=−0.23, p=0.70; PedsQL child total: b=−0.52, p=0.54; QOLCIE total: b=0.30, p=0.55) or seizure outcomes (OR=1.03, p=0.89).
Discussion
STAR is an RCT of a family-based behavioral intervention to improve adherence over 12 months in young children with newly-diagnosed epilepsy and their caregivers compared to a robust attention control group. Although the trial failed to reach group differences on the primary endpoint of electronically-monitored adherence at post-treatment, adherence improvements were noted over time, with moderate effects (d=0.46) at the 6-month follow-up and large statistically significant effects (d=0.83) at 12-months post-treatment. Families who received STAR demonstrated sustained adherence compared to a progressive adherence decline for EO. It is notable that in both treatment arms, participants who initiated treatment completed all face-to-face sessions (with the exception of one STAR participant) and most telephone sessions highlighting high treatment engagement. Effectively, these results partially support our pilot and feasibility trials.
Regarding our adherence outcomes, lack of significant group differences on adherence at post-intervention and short-term follow-up is surprising. One potential reason is the role of attention in improving adherence for both groups in the short-term; however, such attention effects are not long-lasting compared to the execution of problem-solving strategies (e.g., STAR intervention) that may consolidate over time with practice. Alternatively, adherence variability became more heterogeneous and wider over time for the attention control EO intervention arm and more homogenous for the STAR intervention group. This level of variability may have contributed to the non-significant findings early on but does suggest less variability in adherence and more consistent individual adherence patterns for STAR over time. Further, while adherence science has not established a benchmark for clinically meaningful change, prior research demonstrates the important contribution of both biology and behavior (i.e., adherence) in seizure outcomes, with less adherence variability contributing to better seizure outcomes.3 Overall, these data highlight the importance of having attention-control (i.e., EO) groups in behavioral trials.
We predicted significant group differences on two secondary outcomes (i.e., seizures and HRQOL) at 6- and 12-months following intervention. Surprisingly, significant differences were not noted in the presence or absence of seizures between groups at these timepoints. This may be due to the lack of a linear relationship between adherence and seizures in epilepsy,3 where both biology and behavior play key roles in seizure outcomes. In fact, animal models have demonstrated that 0% and 50% adherence have similarly worse seizure severity and frequency in rats compared to a 100% adherence group.16 Originally, we chose these later time points (i.e., 6- and 12-months post intervention) to provide ample time to observe the impact of STAR on seizures. However, our post-hoc analyses for the seizure outcome revealed that the post-treatment time point may be more optimal for future trials, as evidenced by the larger odds ratio at this time point, indicating that the STAR group had 86.1% lower chance of seizures than EO at post-treatment.
No significant group differences were found for HRQOL at 6 and 12-month follow-up time points or HRQOL trajectories over time. One main contributing factor to the lack of HRQOL group differences is likely that the EO attention control group received a comprehensive series of sessions focused on salient epilepsy-related topics associated with HRQOL. For example, topics focused on improving sleep, seizure safety, school and learning, and psychosocial comorbidities are known to influence HRQOL.17–19
One interesting finding from this study is that fewer participants were randomized to intervention than anticipated. Based on published adherence data, we anticipated that approximately 60% of participants would be randomized to one of our two intervention groups. However, only 28% were randomized due to 61% demonstrating high adherence through the course of the entire 7-month baseline period. This finding is likely secondary to a notable feature of our Comprehensive Epilepsy Center, which includes an interdisciplinary psychosocial service embedded in routine epilepsy care. This psychosocial service was developed in 2011 and uses a standardized approach to screen all patients with epilepsy and provide proactive psychosocial intervention, targeting adjustment to illness, psychological comorbidities (e.g., anxiety, depression, behavior, learning, development), and management of epilepsy, including treatment adherence.20, 21 Participants in this trial would have been receiving psychosocial care during trial recruitment; thus, fewer participants demonstrated adherence rates below 95%, which was required to be randomized. While the psychosocial service may have contributed to a lower number of participants being randomized, these data highlight the clinical utility of adherence assessment and intervention into routine interdisciplinary clinical care.
Study limitations and directions for future research should also be noted. First, participants were recruited from a single pediatric epilepsy center and thus results may not be generalizable to patients from other pediatric centers, where recruitment of diverse participants is more accessible. Second, STAR was designed for children with newly diagnosed epilepsy, but should also be tested in children with treatment-resistant epilepsy, for whom adherence may be more complicated due to multiple medications and changing doses. Third, our study focused on a broad developmental period from toddlerhood to school-aged children, which may have resulted in variable child involvement during sessions. While the unique barriers of children are different based on development, the problem-solving strategies that were taught and implemented were tailored to the unique needs of each family. We promoted the child’s involvement in sessions regardless of age to facilitate family communication and engagement by all family members, which is known to facilitate better intervention outcomes. Fourth, this RCT required families to attend sessions at the hospital with the interventionist. Given the catchment area for our Center, inclusion criteria required residency within 75 miles of our hospital, which prevented approximately 100 families from study participation.12 Further, STAR required this travel every other week over a 4-month period, which may have been quite difficult for those who most needed the intervention. Face-to-face behavioral adherence interventions may be less desired and feasible, especially in the context of convenience and recent COVID-19 pandemic restrictions.
Finally, while STAR treatment was tailored to address family-specific individual adherence barriers, all STAR treatment group families were provided with education, individualized adherence feedback reports, and problem-solving training. Some families may have benefited from even one individual component of STAR treatment. For example, for families demonstrating more minor adherence difficulties, individualized adherence feedback reports may have been sufficient. To address some of these limitations, we have created a mobile Health (mHealth) adherence intervention (i.e., epilepsy Adherence in Children and Technology; eACT study), derived from STAR, in which we use an adaptive design. Specifically, for this active clinical trial across four pediatric epilepsy centers, we provide stepped up care based on how families respond to intervention components, with all families in the intervention group receiving micro-learning education sessions and individualized adherence feedback reports first. Only those families who continue to demonstrate non-adherence following implementation of these strategies will have the opportunity to be re-randomized to receive problem-solving sessions with an interventionist. Because eACT is a mHealth adherence intervention, it allows for broader inclusion/exclusion criteria with a focus on recruiting families who live in rural and medically underserved areas, which will increase generalizability of findings.
STAR, a family-based behavioral intervention targeting problem-solving, may optimize adherence across the first year of epilepsy treatment for children with new-onset epilepsy and be integrated into routine epilepsy care. Specifically, identifying adherence barriers, which can change over time, and teaching families problem-solving strategies may allow for a more proactive approach to addressing adherence in the clinic setting. Future consideration of whether adherence interventions vary depending on where they are during the course of their epilepsy diagnosis is warranted. Specifically, adherence interventions for families experiencing initial adjustment difficulties may look different compared to those families experiencing greater psychosocial adherence barriers later in the disease course. In effect, greater tailoring of adherence interventions warrants more attention and is currently being tested in our eACT trial.
Key Points.
An adherence problem-solving intervention improved adherence at 12-month follow up for young children with epilepsy and their families.
No quality of life or seizure differences were noted for those randomized to treatment versus attention control (i.e., education).
Increasing access to adherence interventions and examining which adherence strategies most yield adherence changes is a key next step.
Acknowledgements
We thank the families and children who participated in this study, as well as all the providers who care for these children. We would also like to thank all the research assistants, predoctoral interns, and postdoctoral fellows who helped to facilitate this study.
Study funding: This research was funded by a grant from the National Institutes of Health-Eunice Kennedy Shriver National Institute of Child Health and Human Development awarded to the first author (R01HD073115).
Footnotes
Disclosures
Drs. Modi, Mara, and Guilfoyle have no conflicts of interest to disclose. Dr. Glauser has received support from, and/or has served as a paid consultant for Eisai, SK Life Sciences, Supernus, Neurelis. He has intellectual property and stock options for Clarigent Health.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines
Clinical Trials Number: NCT01851057
References
- 1.Zack MM, Kobau R. National and State Estimates of the Numbers of Adults and Children with Active Epilepsy—United States, 2015. Morbidity and Mortality Weekly Report 2017;66:821–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Modi AC, Rausch JR, Glauser TA. Early pediatric antiepileptic drug nonadherence is related to lower long-term seizure freedom. Neurology 2014;82:671–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modi AC, Wu YP, Rausch JR, Peugh JL, Glauser TA. Antiepileptic drug nonadherence predicts pediatric epilepsy seizure outcomes. Neurology 2014;83:2085–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu YP, Follansbee-Junger K, Rausch J, Modi A. Parent and family stress factors predict health-related quality in pediatric patients with new-onset epilepsy. Epilepsia 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Modi AC, Wu YP, Guilfoyle SM, Glauser TA. Uninformed Clinical Decisions Resulting From Lack of Adherence Assessment in Children with New Onset Epilepsy. Epilepsy and Behavior 2012;25:481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samsonsen C, Reimers A, Brathen G, Helde G, Brodtkorb E. Nonadherence to treatment causing acute hospitalizations in people with epilepsy: an observational, prospective study. Epilepsia 2014;55:e125–128. [DOI] [PubMed] [Google Scholar]
- 7.Faught RE, Weiner JR, Guerin A, Cunnington MC, Duh MS. Impact of nonadherence to antiepileptic drugs on health care utilization and costs: findings from the RANSOM study. Epilepsia 2009;50:501–509. [DOI] [PubMed] [Google Scholar]
- 8.Al-Aqeel S, Gershuni O, Al-Sabhan J, Hiligsmann M. Strategies for improving adherence to antiepileptic drug treatment in people with epilepsy. Cochrane Database Syst Rev 2017;2:CD008312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Modi AC, Guilfoyle SM, Mann KA, Rausch JR. A pilot randomized controlled clinical trial to improve antiepileptic drug adherence in young children with epilepsy. Epilepsia 2016;57:e69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Modi AC, Guilfoyle SM, Rausch J. Preliminary Feasibility, Acceptability, and Efficacy of an Innovative Adherence Intervention for Children With Newly Diagnosed Epilepsy. J Pediatr Psychol 2013;38:605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Modi AC, Rausch JR, Glauser TA. Patterns of non-adherence to antiepileptic drug therapy in children with newly diagnosed epilepsy. JAMA 2011;305:1669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modi AC, Glauser TA, Guilfoyle SM. Supporting Treatment Adherence Regimens in young children with epilepsy and their families: Trial design and baseline characteristics. Contemp Clin Trials 2020;90:105959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens G, Featherman DL. A revised socioeconomic index of occupational status. Soc Sci Res 1981;10:364–395. [Google Scholar]
- 14.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 Generic Core Scales in healthy and patient populations. Med Care 2001;39:800–812. [DOI] [PubMed] [Google Scholar]
- 15.Sabaz M, Lawson JA, Cairns DR, et al. Validation of the quality of life in childhood epilepsy questionnaire in American epilepsy patients. Epilepsy Behav 2003;4:680–691. [DOI] [PubMed] [Google Scholar]
- 16.Thomson KE, Modi AC, Glauser TA, Rausch JR, Steve White H. The impact of nonadherence to antiseizure drugs on seizure outcomes in an animal model of epilepsy. Epilepsia 2017;58:1054–1062. [DOI] [PubMed] [Google Scholar]
- 17.Loiselle KA, Ramsey RR, Rausch JR, Modi AC. Trajectories of Health-Related Quality of Life Among Children With Newly Diagnosed Epilepsy. J Pediatr Psychol 2016;41:1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferro MA. Risk factors for health-related quality of life in children with epilepsy: a meta-analysis. Epilepsia 2014;55:1722–1731. [DOI] [PubMed] [Google Scholar]
- 19.Loiselle KA, Rausch JR, Modi AC. Behavioral predictors of medication adherence trajectories among youth with newly diagnosed epilepsy. Epilepsy & Behavior 2015;50:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guilfoyle SM, Follansbee-Junger K, Modi AC. Development and preliminary implementation of a psychosocial service into standard medical care for pediatric epilepsy. Clin Pract Pediatr Psychol 2013;1:276–288. [Google Scholar]
- 21.Guilfoyle SM, Monahan S, Wesolowski C, Modi AC. Depression screening in pediatric epilepsy: Evidence for the benefit of a behavioral medicine service in early detection. Epilepsy Behav 2015;44C:5–10. [DOI] [PubMed] [Google Scholar]