Abstract
We describe two individuals with progressive verbal difficulty who exhibited impairment of propositional language, with relatively well-preserved auditory comprehension, naming, and repetition—a profile that is consistent with dynamic aphasia. By providing a brief review of pertinent literature and the results from our neurologic, speech and language, neuropsychological, and neuroimaging testing, this report sheds light on the infrequently reported dynamic aphasia in the context of frontotemporal dementia. Our patients’ insights into their verbal difficulty tend to support the notion that dynamic aphasia results from interference at the stage where thoughts are converted into verbal messages—that is, the thought–verbal interface.
Keywords: primary progressive aphasia, dynamic aphasia, propositional language, left frontal lobe, frontotemporal dementia
Over the past 2 decades, there has been considerable advancement in the characterization of a group of neurodegenerative disorders that are referred to collectively as frontotemporal dementia (FTD) that, as the name implies, are predominantly associated with frontotemporal lobar degeneration. Subcategories of FTD include the behavioral variant of FTD (bvFTD), the semantic variant of primary progressive aphasia (PPA), the agrammatic variant of PPA (referred to as “nonfluent/agrammatic variant-nfvPPA” in Gorno-Tempini et al, 2011, p. 1009), corticobasal syndrome, progressive supranuclear palsy (PSP), and FTD associated with motor neuron disease (Finger, 2016).
The purpose of this report is to shed light on an underrecognized manifestation of FTD, known as dynamic aphasia (eg, Warren et al, 2003). Dynamic aphasia, which was initially reported in non-neurodegenerative cases (eg, stroke, tumor surgery) involving insults to the left frontal lobe, is characterized by primary difficulty with propositional language with well-preserved naming, repetition, and auditory comprehension (eg, Luria, 1970; Luria and Tsvetkova, 1968). In the context of FTD (although the term “FTD” was not used in their paper), Esmonde et al (1996) reported three cases of progressive dynamic aphasia. Conversational speech was diminished in the three individuals, and their propositional language (narrative/spontaneous verbal expression) and verbal fluency (letter and categorical fluency) were impaired, but their auditory comprehension of words and sentences, confrontation naming, repetition, and semantic memory were well preserved.
Single-case reports (Perez et al, 2013; Robinson, 2013; Robinson et al, 2005, 2006, 2015; Snowden et al, 1996; Warren et al, 2003) of progressive dynamic aphasia concur that the predominant difficulty is with propositional language and verbal fluency. The most common neuroimaging finding in these cases was left frontal lobe atrophy (Perez et al, 2013; Robinson, 2013; Snowden et al, 1996; Warren et al, 2003).
In the verbal domain, both isolated and co-occurring forms of dynamic aphasia have been reported: dynamic aphasia as the sole clinically relevant manifestation (Perez et al, 2013); as a co-occurring deficit with mild agrammatism and dysarthria (Robinson et al, 2015); as a co-occurring deficit with mild agrammatism and orofacial apraxia (Robinson, 2013); as a co-occurring deficit with agrammatism, dysarthria, and severe orofacial apraxia (probably representing apraxia of speech as opposed to nonverbal oral apraxia; Robinson et al, 2005); as a co-occurring deficit with mild dysarthria (eg, Esmonde et al, 1996); and as a co-occurring deficit with mild naming impairment (eg, Warren et al, 2003).
Outside the verbal domain, co-occurring personality/behavioral changes (eg, Esmonde et al, 1996) and deficits in intellectual, memory, and executive domains have been reported (eg, Robinson et al, 2006). Furthermore, Milano and Heilman (2015) reported a rarer manifestation of dynamic aphasia in two individuals with PPA who exhibited difficulty with propositional language but preserved auditory comprehension, naming, repetition, reading, and writing. Contrary to classic dynamic aphasia, however, these individuals also exhibited impaired automatic speech, which the authors attributed to a lack of initiation, which in turn was linked to the individuals’ bilateral medial frontal lobe atrophy. Recognizing “lack of will or initiative” as the primary problem, Milano and Heilman (2015, p. 744) preferred to use the term primary progressive speech abulia. Dynamic aphasia has also been reported as an initial/primary manifestation of PSP (presumed PSP diagnosis based on clinical symptoms: Esmonde et al, 1996; Robinson et al, 2006, 2015) (postmortem confirmed PSP diagnosis: Esmonde et al, 1996).
Given the heterogeneity in dynamic aphasia presentations and the underrecognition of it in the literature as a manifestation of FTD, additional case reports are needed to further our understanding of its clinical presentation. Accurate recognition/characterization of different neurodegenerative clinical syndromes provides opportunities to elucidate relevant cognitive/linguistic processes and their neuroanatomical/neurophysiological correlates. Accurate characterization also has important implications for appropriate patient counseling and management.
We report detailed neurologic, speech and language, neuropsychological, and neuroimaging (MRI, fluorodeoxyglucose [FDG]-PET, beta-amyloid-PET and tau-PET [in one]) characteristics of two individuals who presented with dynamic aphasia in the context of FTD. Even though propositional verbal difficulty has been recognized as the hallmark feature of dynamic aphasia, transcriptions of substantial samples of narrative verbal expression from individuals with dynamic aphasia are limited (Esmonde et al, 1996; Snowden et al, 1996). Here we include transcribed samples of propositional verbal and written expression. We also address the following questions.
In the context of FTD, where does a primary clinical presentation of dynamic aphasia fall under the current clinical classification systems? Dynamic aphasia is not currently considered a variant of FTD despite a few previously published relevant case reports. We evaluate the placement of our patients within FTD subclassification systems (ie, PPA, bvFTD, PSP) in order to expand the literature on the clinical presentation trend of dynamic aphasia.
Are written language (ie, reading or writing) impairments evident in individuals with dynamic aphasia? Although some studies (eg, Robinson et al, 2006, 2015; Snowden et al, 1996; Warren et al, 2003) have reported that oral reading is well preserved in individuals with aphasia, reading comprehension in individuals with dynamic aphasia has not been examined. Given that auditory verbal comprehension, auditory verbal repetition, word retrieval, and oral reading have been reported to be well preserved in individuals with dynamic aphasia, we hypothesized that reading comprehension would be well preserved as well. Written narrative/spontaneous expression in dynamic aphasia has been reported in only a couple of reports, and the findings have varied, ranging from preserved (eg, Perez et al, 2013) to severely impaired (eg, Warren et al, 2003).
Do motor speech disorders often co-occur in the progressive form of dynamic aphasia? Findings from previous case reports have varied from absence (eg, Perez et al, 2013) to presence of dysarthria (Esmonde et al, 1996; Robinson et al, 2005, 2006, 2015) and/or verbal apraxia (Robinson, 2013; Robinson et al, 2005).
Do individuals with the progressive form of dynamic aphasia demonstrate aberrant nonverbal interactive behaviors? Diminished nonverbal interactive behaviors in addition to diminished spontaneous speech could signal the presence of related disorders such as abulia, which is a domain-general disorder of diminished motivation (Marin and Wilkosz, 2005) rather than a specific verbal disorder such as dynamic aphasia. Nonverbal interactive behavior is an important area to explore given that Milano and Heilman (2015) reported two cases that appeared to have a clinical profile that straddled the boundary between abulia and dynamic aphasia. Based on our own clinical experience with individuals with disorders of diminished motivation (eg, apathy, abulia, and akinetic mutism) and a review of the relevant literature (eg, Mychack et al, 2001), we consider that impairment of nonverbal interactive behaviors results mainly from right frontal lobe dysfunction. Given that dynamic aphasia is associated with left frontal lobe dyfunction, we hypothesized that nonverbal interactive behaviors should be preserved in these individuals.
What is the underlying mechanism in dynamic aphasia? Investigating the underlying mechanism in dynamic aphasia in the context of neurodegenerative disease is challenging because other cognitive/linguistic domains in addition to propositional language usually become affected as the disease progresses. During the initial presentation, however, the individuals in our reports had primary difficulty in propositional language, but it was not severe enough to preclude them from providing some insights about the nature of their difficulty. Based on our interpretation of their insights, we add to the existing (but limited and inconclusive) literature on the underlying mechanisms of dynamic aphasia.
CASE REPORTS
Participants
Patient 1 was a 71-year-old, monolingual, right-handed Caucasian man with 20 years of education who had retired from his job as an attorney editor for a legal publishing company. He presented with an ~2½-year history of progressive verbal difficulty. His specific complaints included “failing to come up with the right words” and a lack of communication due to a preference for silence. He was unable to precisely express the nature of his verbal difficulty. He denied concerns with auditory comprehension and handwriting but thought he could be having issues with his short-term memory and with reading comprehension. He had no family history of neurodegenerative diseases. He completed one clinical visit during which he had interdisciplinary evaluation appointments across 2 days.
Patient 2 was a 60-year-old, right-handed Caucasian woman with 16 years of education who had retired from her job as a teacher. She presented with an ~2-year history of progressive verbal difficulty. She was bilingual (English–French) and reportedly had greater verbal difficulty with French than with general American English. Her only complaint was verbal difficulty, describing it as having trouble asking questions to others and answering others’ questions, which she admitted was a frustrating difficulty. She denied concerns with auditory comprehension, reading, and writing but admitted to having difficulty with thinking (ie, thinking related to answering others’ questions).
Per her sister and her husband, the amount of patient 2’s verbal output had declined. Her husband reported other symptoms such as anxiety/difficulty related to making decisions and some compulsion toward computer games. She had no family history of neurodegenerative diseases. She completed three clinical visits, the second ~3 years after the first, and the third ~3 years after that, when her cognition and communication were severely impaired, which severely limited her participation. Her first and second clinical visits each involved interdisciplinary evaluation appointments across 2 days.
ASSESSMENTS
Neurologic
A behavioral neurologist and movement disorders specialist (K.A.J.) performed pertinent examinations assessing global cognitive ability, behavioral and psychiatric features, executive control, limb praxis, and parkinsonism.
Speech, Language, and Nonverbal Communication
A speech-language pathologist (J.R.D) performed pertinent examinations assessing global language ability, auditory comprehension, naming, repetition, oral reading, reading comprehension, written output, surface versus deep alexia/agraphia, verbal fluency, receptive vocabulary, semantic association, comprehension of grammar/syntax, motor speech, and nonverbal oral praxis. Due to a change over time in the study protocol to better assess agrammatism and impairment of word knowledge, patient 2 received additional language measures during her second visit.
All of the speech and language evaluations were audio and video recorded for subsequent analysis. Conversational and narrative verbal samples were transcribed and analyzed. In addition to speech and language, nonverbal interactive behaviors such as eye contact, affect, social greetings, responsiveness to questions, and the presence of inappropriate behaviors were noted.
Neuropsychological
A neuropsychologist (M.M.M.) oversaw pertinent examinations assessing estimated premorbid intellectual function, verbal and visual memory, visuomotor processing speed, aspects of executive function, visuospatial reasoning, visuospatial perceptual processing, and visuospatial construction.
Neuroimaging
Both patients underwent a standardized MRI protocol at 3T that included a 3D MPRAGE (magnetization prepared rapid acquisition gradient echo) sequence (TR/TE/T1, 2300/3/900 msec; flip angle 8 degrees, 26-cm field of view; 256 × 256 in plane matrix with a phase field of view of 0.94, voxel sizes of 1 × 1 × 1.2 mm). All MPRAGE images were corrected for gradient nonlinearity (Jovicich et al, 2006) and intensity nonuniformity using a combination of N3 (Sled et al, 1998) and statistical parametric mapping (SPM12) normalization (www.fil.ion.ucl.ac.uk/spm). Regional gray matter volumes were calculated using the automated anatomical labeling atlas (Tzourio-Mazoyer et al, 2002). Using data from a control cohort (N = 120, 55–90 years old), age and total intracranial volume adjusted z scores were calculated for each brain region (for details, see Vemuri et al, 2011), and a brain region was considered atrophic if z ≥ 1 (eg, Utianski et al, 2019b).
FDG-PET scans at each visit were obtained using a PET/CT scanner (GE Healthcare) as detailed in a previous study (Josephs et al, 2014). Individual patterns of hypometabolism were assessed using the clinical tool of 3D stereotactic surface projections (Minoshima et al, 1995). Activity from the PET data was normalized to the pons and was compared with an age-matched normative database (N = 294, 31–89 years old), yielding a 3D stereotactic surface projections z-score image. CortexID (GE Healthcare) software packages were used for data analysis.
The patients also completed beta-amyloid PET imaging with Pittsburgh compound B (PiB). For the PiB PET, we defined a global PIB standard uptake value ratio (SUVR) as previously described (Jack et al, 2008). A global cut point of >1.48 (Jack et al, 2019) was used to define a patient as PiB positive.
Patient 2 also underwent tau PET scanning to assess for the presence of paired helical filament tau (Alzheimer tau) in the brain using the [18F]flortaucipir (previously referred to as [18F]AV-1451) ligand. For tau-PET, we calculated a temporal lobe meta-region of interest that has been shown to accurately capture the tau signal in individuals with Alzheimer disease (Jack et al, 2018). Uptake was calculated in the entorhinal cortex, fusiform, parahippocampal, and inferior temporal and middle temporal gyri, and values were divided by uptake in the cerebellar crus to calculate an SUVR. The pathologically validated cut point of 1.29 (Lowe et al, 2020) was used to determine whether a patient was flortaucipir positive.
RESULTS
Neurologic Assessment
The results of our patients’ neurologic assessments are summarized in Table 1.
TABLE 1.
Results of our Patients’ Neurologic Assessments Across Visits
| Domain Assessed | Demographic Data or Test Used | Patient 1 | Patient 2† | |
|---|---|---|---|---|
| Visit 1 | Visit 1 | Visit 2 | ||
| Age (years) | 71 | 62 | 65 | |
| Years since symptom onset | ~2½ | ~2 | ~5 | |
| Global Cognitive Ability | MMSE | 30/30 | 29/30 | DNT |
| MoCA | 24/30§ | 25/30‡ | 22/30§ | |
| Behavioral | FBI | 18/72§ | 19/72§ | DNT |
| CBI–R | DNT | DNT | 61/180‖ | |
| Executive Control | FAB | 15/18§ | 16/18 | 15/18§ |
| Psychiatric | NPI–Q | 4/36 | 4/36 | 14/36‖ |
| Praxis | WAB–R–Praxis | 59/60 | 57/60 | 50/60§ |
| Parkinsonism | MDS–UPDRS III Motor subsection | 8/132 | 4/132 | 2/132 |
| Calculation | Calculation subsection of MoCA | 3/3 | 3/3 | 3/3 |
| Prosopagnosia | Famous Faces Recognition | 10/10 | 10/10 | 10/10 |
At the third visit, patient 2 was not a candidate for assessment secondary to the severity of her deficits, but per her husband’s ratings, she was 52/180 on the CBI–R and 12/36 on the NPI–Q.
Minimal impairment.
Mild impairment.
Moderate impairment.
CBI–R = Cambridge Behavioral Inventory—Revised. DNT = did not test. FAB = Frontal Assessment Battery. FBI = Frontal Behavioral Inventory. MDS–UPDRS III = Movement Disorders Society-sponsored version of the Unified Parkinson's Disease Rating Scale. MMSE = Mini-Mental State Examination. MoCA = Montreal Cognitive Assessment. NPI–Q = Neuropsychiatric Inventory Questionnaire. WAB–R = Western Aphasia Battery—Revised.
Patient 1
Patient 1’s global cognitive ability was largely preserved (normal Mini-Mental State Examination [Folstein et al, 1975], mild impairment on the Montreal Cognitive Assessment [MoCA; Nasreddine et al, 2005]). On the MoCA, he lost 1 point for a visuospatial/executive item, 1 point for naming, 1 point for letter fluency, and 3 points for delayed recall. He showed mild frontal behavioral features (Frontal Behavioral Inventory [Kertesz et al, 1997], scoring 1 point for apathy and 2 points for logopenia) and mild executive dyscontrol (Frontal Assessment Battery [Dubois et al, 2000], losing 2 points for lexical fluency and 1 point for motor series programming). His ratings on the Neuropsychiatric Inventory Questionnaire (Kaufer et al, 2000) did not reveal substantial psychiatric features (he received 1 point each for apathy and anxiety, and 2 points for depression). No limb apraxia (Praxis subtest of the Western Aphasia Battery—Revised [WAB–R–Praxis; Kertesz and Raven, 2007]) or parkinsonism (Movement Disorders Society-sponsored version of the Unified Parkinson’s Disease Rating Scale [Goetz et al, 2008]) was appreciated. His calculation (MoCA) and face recognition (Famous Faces Recognition [Josephs et al, 2012] abilities were intact.
Patient 2
Patient 2’s global cognitive ability was intact during her first visit (normal Mini-Mental State Examination, minimal impairment on MoCA). On the MoCA, she lost 1 point for letter fluency, 1 point for abstraction, and 3 points for delayed recall. She showed mild frontal behavioral features (Frontal Behavioral Inventory, scoring 1 point each for apathy, spontaneity, indifference, disorganization, perseveration, irritability, poor judgment, restlessness, and utilization behavior; and 2 points each for inflexibility, inattention, logopenia, verbal apraxia, and hypersexuality). Apart from losing 2 points for lexical fluency, she performed well on executive control (Frontal Assessment Battery). Her ratings on the Neuropsychiatric Inventory Questionnaire did not reveal substantial psychiatric features (she received 1 point each for apathy, anxiety, eating disorder, and aberrant motor behavior). No limb apraxia (WAB–R–Praxis) or parkinsonism (Movement Disorders Society-sponsored version of the Unified Parkinson’s Disease Rating Scale) was appreciated. Her calculation (MoCA) and face recognition (Famous Faces Recognition) abilities were intact.
During patient 2’s second visit, she showed a mild impairment on the MoCA, moderate frontal behavioral features (Cambridge Behavioural Inventory—Revised [Wear et al, 2008]), mild executive dyscontrol (Frontal Assessment Battery), and moderate psychiatric features (Neuropsychiatric Inventory Questionnaire). She showed mild limb apraxia (WAB–R–Praxis) but no parkinsonism (Movement Disorders Society-sponsored version of the Unified Parkinson’s Disease Rating Scale). Her calculation (MoCA) and face recognition abilities were still intact.
Speech, Language, and Nonverbal Communication Assessment
The results of our patients’ speech and language assessments are summarized in Tables 2 and 3 and in Figure 1. Their nonverbal interactive behaviors are summarized in Table 4.
TABLE 2.
Results of our Patients’ Speech and Language Assessments Across Visits
| Domain/Area Assessed | Test/Task Used | Patient 1 | Patient 2† | |
|---|---|---|---|---|
| Visit 1 | Visit 1 | Visit 2 | ||
| Global Language Ability | WAB–R AQ | 94.4 | 97.2 | 64.9 |
| Information Content | WAB–R Information Content | 9/10 | 10/10 | 6/10 |
| Verbal Fluency | WAB–R Fluency | 9/10 | 9/10 | 4/10 |
| Spontaneous Speech | WAB–R Spontaneous Speech | 18/20 | 19/20 | 10/20 |
| Auditory Comprehension | WAB–R Auditory verbal comprehension | 10/10 | 10/10 | 8.65/10 |
| Verbal Repetition | WAB–R Repetition | 9.8/10 | 10/10 | 6.6/10 |
| Naming/Word Retrieval | WAB–R Naming | 9.4/10 | 9.6/10 | 7.2/10 |
| Oral Reading | WAB–R Reading Commands | 20/20 | 20/20 | 17/20 |
| Reading Comprehension | WAB–R Reading, Comprehension of Sentences | 40/40 | 40/40 | 32/40 |
| Surface versus Deep (phonological) Alexia Assessment | WAB–R Reading Irregular Words | 10/10 | 10/10 | 9/10 |
| WAB–R Reading Nonwords | 10/10 | 9/10 | 3/10 | |
| Writing Output | WAB–R Writing Output | 33.5/34 | 34/34 | 19/34 |
| Surface versus Deep (phonological) Agraphia Assessment | WAB–R Writing Irregular Words | 10/10 | 10/10 | 10/10 |
| WAB–R Writing Nonwords | 9/10 | 4/10 | 2/10 | |
| Verbal Fluency | WAB–R Animal Fluency (M = 18.2, SD = 4.2)‡ | 14 (<16th %tile)‡ | 15 (<23rd %tile)‡ | 7 (<1st %tile)‡ |
| Action Fluency | 5 (<2.5th %tile)§ | 9 (<2.5th %tile)§ | 2(<2.5th %tile)§ | |
| FAS Letter Fluency (M = 42, SD = 12.1)‡ | 10 (<1st %tile)‡ | 17 (<2nd %tile)‡ | DNT | |
| Single Word Receptive Vocabulary | PPVT | SS = 114 82nd %tile |
SS = 97 42nd %tile |
SS = 89 23rd %tile |
| Noun Confrontation Naming | BNT–SF | 15/15 | 13/15 | DNT |
| Associative Semantic knowledge | PPTT | 47/52 | 49/52 | 47/52 |
| Grammatic/Syntactic Comprehension | Token Test, Part V | 17/22 | 21/22 | DNT |
| Syntactic Expression | NAT | DNT | DNT | 2/10 |
| Comprehension of Syntax | BDAE Syntactic Processing | DNT | DNT | 9/12 |
| BDAE Reversible Possessives | DNT | DNT | 10/10 | |
| BDAE Embedded Sentences | DNT | DNT | 7/10 | |
| Sentence repetition | BDAE Repetition | DNT | DNT | 5/10 |
| Word Knowledge | SYDBAT Naming | DNT | DNT | 17/30 |
| SYDBAT Semantic Association | DNT | DNT | 28/30 | |
| Motor Speech‖ | Apraxia of Speech | None | None | None |
| Dysarthria | None | None | None | |
| Nonverbal Oral Praxis | NVOA | 32/32 | 26/32 | 8/32 |
Shading indicates abnormal or clinically impaired.
At the third visit, patient 2 was not a candidate for assessment secondary to the severity of her deficits (see Discussion).
Evaluation protocol described in Josephs et al (2013).
AQ = Aphasia Quotient. BDAE = Boston Diagnostic Aphasia Examination. BNT = Boston Naming Test, short form (Lansing et al, 1999). DNT = did not test. FAS = Letters “F”, “A” and “S”. NAT = Northwestern Anagram Test (Weintraub et al, 2009). NC = not completed. NVOA = nonverbal oral apraxia (Botha et al, 2014). PPTT = Pyramids and Palm Trees Test (Howard and Patterson, 1992). PPVT = Peabody Picture Vocabulary Test (Dunn and Dunn, 2006). SS = standard score (100 = average, 85 is −1 SD, 115 is +1 SD). SYDBAT = Sydney Language Battery. TT = Token Test. WAB–R = Western Aphasia Battery—Revised.
TABLE 3.
Patients’ Conversational Speech Sample and Narrative Formulation (Picture Description Task)
| Patient One | ||
| Discourse Context | Transcript | |
|
| ||
| Conversational language sample |
(Referring to his verbal difficulty) What was the first thing you noticed? (~4 second pause) uh (~2 second pause) lack of communication uh (~3 second) uh the first thing I noticed was uuh (~2 second pause) uuh (~1 second pause) just the lack of communication (nodding head) What do you mean by that? (~2 second pause) well (~3 second pause) I would prefer silence Okay, so you are talking less? I think so yeah am am I am I am positive of that (nodding head) And others have observed this as well? Yes (~1 second pause) and and they charged it off to the fact that I have hearing aids (pointing to his ear) uh when uh in fact uh I was communicating less (nodding his head) How long have you worn hearing aids? Since twenty o’ four |
|
| Description of the “Picnic” picture from the Western Aphasia Battery—Revised | Oh well um (~3 second pause) there is a picnic going on and the uff boy is flying a kite and uh (~1 second pause) uh uh a fisherman catching a fish and um (~5 second pause) a sculptor uh (~2 second pause) um fitting a being (or building) a sand sculpture and um (~1 second pause) hmm a car with a house (~1 second pause) and uh a boat. uh a boat um with that’s a (~1 second pause) um (~3 second pause) sailing along and um (~2 second pause) they have uh the the the picnic person has a picnic basket, and a radio and um (~1 second pause) some sandals otherwise there there there um (~1 second pause) she has shoes but um uh he is barefoot and um (~2 second pause) there is a dog following um (~2 second pause) the boy fuh flying the kite. *,a,b | |
|
| ||
| Patient Two | ||
| Discourse Context | Transcript Visit 1 | Transcript Visit 2 |
|
| ||
| Conversational language sample |
Tell me why you are here now. What kind of trouble have you been having? Um (~2 second pause) I I don’t get my words out, so How long has that been going on? Um, I think since like about 2012 Can you describe it for me a little bit more? Um (~5 second pause) well, people ask me questions and then I I I don’t I don’t know how to answer You have any trouble finding the words that you want to say? Um, no Are you having any trouble understanding what people say to you? Um I I don’t have any um (~3 second pause) I don’t have any misunderstandings Okay. Are you having any trouble understanding what you read? No. I don’t Do you read for pleasure? Yeah And tell me about writing, any difficulties with that? Um, no, no difficulties Okay. Are you having any problems other than not knowing how to answer things that people ask you about? Well (~3 second pause) um (~2 second pause) I wa I uh um um um before last year I I was trying to get people to talk to me, you know, if I would ask questions and then they’d answer me, so Okay. So you were able to do that before and it is harder to do it now? Yeah |
Why are you here today? What kind of trouble have you been having? (~4 second pause) I don’t know What’s been giving you difficulty? I don’t know (~5 second pause) I don’t know Are you having trouble with anything? Um hmmm What is that? No response in ~6 seconds |
| Description of the “Picnic” picture from the Western Aphasia Battery—Revised |
Um, they are at the beach and the boy is running with a kite. And there is a sailboat in the water. And (~3 second pause) the girl is um (~4 second pause) building a sandcastle and the dog is running behind the boy. And then the muh mom and dad are sitting on a blanket and um the dad is reading a book and the mom is pouring wine I think, a bottle of wine. (~2 second pause) And then there is a person on the dock who is fishing.*,a, b
(looked at the picture for ~3 seconds before looking at the examiner) Anything else? Um There is a flagpole (~2 second pause) um (~2 second pause) near the house, so, yeah (~4 second pause) And there is there is a radio next to the woman and sandals next to the man.^ (looked at the picture for ~10 seconds before turning the stimulus page) |
No verbal response to initial instruction. Examiner prompts (pointing to the picture) “what’s happening there?” (~3 second pause) (Pointing to the picture) the the this thing (looks at the examiner and then at his materials) What’s happening? Hmm (tries to turn the page in the stimulus booklet) Examiner stops her from turning the page saying “Stick with this… What’s happening there?” Not looking at the picture replies Yeah (~3 second pause) I don’t know Tell me, what’s he doing? He is doing (~14 second pause looking at the picture) bible Alright…And what is he doing? (~10 second pause looking at the picture) Kite (looks at the examiner for ~3 seconds) (looks at the picture for ~2 seconds, points to something in the picture) Yeah, what is happening there? Looking at the examiner says Um hm Tell me about that. (~6 seconds pause looking at the examiner) What’s going on there? Looking at the picture replies Yeah (no verbal response for ~10 seconds) I don’t know What is she doing? (~6 second pause looking at the picture) I don’t know Can you tell me what she is doing? Um Hmm (then looks at the examiner without verbal response for ~8 seconds) Alright |
The clinician’s questions and comments appear in bold, and the patients’ answers and comments appear in italics.
Time to generate description = 1 minute 23 seconds.
63 (abnormal) words per minute (normal M = 166, SD = 22; Nicholas and Brookshire, 1993).
Correct information units for 1 minute of this sample = 68.25% (normal range % CIU = 72–93; M = 86; SD = 6; Nicholas and Brookshire, 1993).
CIU = correct information unit.
The clinician’s questions and comments appear in bold, and the patients’ answers and comments appear in italics.
Time to generate description = *46 seconds and ^23 seconds.
88 (abnormal) words per minute (normal M = 166, SD = 22; Nicholas and Brookshire, 1993).
Correct information units for 1 minute of this sample = 80.68% (normal range % CIU = 72 – 93; M = 86; SD = 6; Nicholas and Brookshire, 1993).
CIU = correct information unit.
FIGURE 1.

Our patients’ written descriptions of the picnic scene from the Western Aphasia Battery—Revised. Allotted time for this task was 3 minutes. Both of the patients completed a verbal description of the picnic picture before a written description, except for patient 2 during visit 2. *Number of times writing was erased. +Used the entire 3 minutes but appeared to want to write further.
TABLE 4.
Our Patients’ Nonverbal Interactive Behaviors During the Speech and Language Assessment
| Behavior | Patient 1 | Patient 2 | ||
|---|---|---|---|---|
| Visit 1 | Visit 1 | Visit 2 | Visit 3† | |
| Eye contact | Appropriate | Appropriate | Inconsistent | Inconsistent |
| Nonverbal social greetings | Appropriate (smiled at the clinician during initial encounter) | Appropriate (smiled at the clinician during initial encounter) | NA | NA |
| Facial affect‡ | Equivocally flat or tendency toward flat affect§ | Equivocally flat or tendency toward flat affect§ | Frequent smiling | Smiling frequency and intensity reduced compared to previous visit |
| Responsiveness (or intent to respond) to questions/requests | Consistent | Consistent | Inconsistent due to inattention (often required reinstruction and prompts) | Profoundly impaired; rarely established joint attention with the clinician |
| Inappropriate behaviors | None | None | • Trying multiple times to look at what the examiner was writing • Inappropriate or unexplainable smiling/laughing • Turning the page of the stimulus book before responding to the task in hand |
|
| Other | Subtle intermittent rocking back and forth while sitting | Rocked torso sideways or back and forth frequently while sitting; intermittent hand stereotypy—rubbing hands | Rocked back and forth or sideways frequently while sitting; frequent hand stereotypy—rubbing hands | |
Due to a lack of substantial participation, this evaluation was based on observation of a limited video recording.
Rating scale used: minimal, mild, moderate, severe.
The tendency toward flat affect appeared like “lost in thought” facial expression.
NA = not assessed because the pertinent segment was not captured on video.
Patient 1
Patient 1 exhibited a lack of spontaneous verbal initiation (eg, asking questions, self-initiated comments) and impaired propositional verbal (conversational language and picture description) and written expression. He also struggled with word fluency measures. His performance in other areas of language, motor speech, and nonverbal interactive behaviors was well preserved. He had mild receptive (Token Test [De Renzi and Vignolo, 1962], Table 2) and expressive agrammatism (picture description task, Table 3). He did not have nonverbal oral apraxia. Our lab uses a 5-point scale to rate overall aphasia severity (0–4; 4 = severe; eg, Botha et al, 2015), which is based on well-known signs of aphasia such as agrammatism and word-finding difficulty. We rated patient 1’s severity as 0.5, which translates into minimal aphasia.
Patient 2
During her first visit, patient 2 exhibited a lack of spontaneous verbal initiation and impaired propositional verbal expression, written expression, word fluency, and ability to write nonwords. Her performance in other areas of language and her nonverbal interactive behaviors were well preserved. She exhibited subtle nonverbal oral apraxia. Overall, we rated patient 2 as not having classic aphasia characteristics due to a lack of explicit word retrieval difficulty or agrammatism. The percentage of correct information units (Table 3) in her picture description sample was comparable to that of individuals without brain damage. Interjections (nonword fillers) and pauses between words and utterances (Table 3) made her difficulty with propositional verbal expression evident.
During her second visit, patient 2 exhibited worse propositional verbal and written expression, word fluency, and ability to write nonwords. Deficits were evident in auditory comprehension, repetition, naming, receptive vocabulary, semantic association, reading comprehension, grammaticality, and reading nonwords. She exhibited marked nonverbal oral apraxia. Similar to her first visit, her ability to read and write irregular words was preserved. However, unlike in her first visit, she displayed clear deficits in nonverbal interactive behaviors, as indicated in Table 4. Because she was the only one who received additional measures (Boston Diagnostic Aphasia Examination—Third Edition [Goodglass et al, 2000] and Sydney Language battery [Savage et al, 2013]), and because these measures were administered to her during her second visit only, they will not be discussed here but are summarized in Table 2.
Neuropsychological Assessments
The results of our patients’ neuropsychological assessments are summarized in Table 5.
TABLE 5.
Results of our Patients’ Neuropsychological Assessments Across Visits
| Domain Assessed | Test Used | Patient 1 | Patient 2† | ||
|---|---|---|---|---|---|
| Visit 1 | Visit 1 | Visit 2 | |||
| Estimate of Premorbid Intellectual Function | WRAT–3 Reading‡ | 120 | 105 | DNT | |
| Memory | AVLT LOT§‖ | 10 | 7 | DNT | |
| AVLT Delayed Recall§‖ | 9 | 7 | DNT | ||
| WMS–III LM I§ | 3 | 11 | DNT | ||
| WMS–III LM II Recall§ | 6 | 12 | DNT | ||
| WMS–III VR I§ | 14 | 12 | DNT | ||
| WMS–III VR II§ | 10 | 9 | DNT | ||
| Visuomotor Processing Speed | TMT Part A§¶ | 7 | 11 | 6 | |
| Executive Function | Problem-Solving/Concept Formation | D-KEFS Sorting§ | 7 | 9 | DNT |
| Mental Set-Shifting | TMT Part B§¶ | 6 | 10 | 6 | |
| Visuospatial Reasoning | VOSP Cube Analysis# | 9 | 10 | 6 | |
| Visuospatial Perceptual Processing | VOSP Incomplete Letters# | 20 | 20 | 20 | |
| Visuospatial Construction | Rey-O§†† | 11 | 10 | 9 | |
At the third visit, patient 2 was not a candidate for assessment secondary to the severity of her deficits.
Standard score (M = 100, SD = 15).
Scaled score (M = 10, SD = 3).
Based on Ivnik et al, 1996; MOANS.
Based on Ivnik et al, 1992; MOANS.
Raw score Incomplete Letters out of 20/Cube Analysis out of 10.
Based on Machulda et al, 2007; MOANS.
AVLT = Auditory Verbal Learning Test (Rey, 1964). D-KEFS = Delis-Kaplan Executive Function System (Delis et al, 2001). DNT = did not test. LM = Logical Memory. LOT = Learning Over Trials. MOANS = Mayo’s Older Americans Normative Studies. Rey–O = Rey Osterrieth Complex Figure Drawing (Osterrieth, 1944). TMT = Trail Making Test (Spreen and Strauss, 1998). VOSP = Visual Object and Space Perception (Warrington and James, 1991). VR = Visual Reproduction. WMS = Wechsler Memory Scale (Wechsler, 1997). WRAT–3 = Wide Range Achievement Test—3rd rev (Wilkinson, 1993).
Patient 1
Patient 1’s word list encoding and recall ability were average, but his immediate and delayed recall of paragraphs were severely and mildly impaired, respectively. His immediate and delayed visual memory performances were superior and average, respectively. His visuomotor processing speed and problem-solving/concept formation were low average, which likely reflected a subtle decline from his estimated premorbid ability level (based on superior single-word reading). His mental set-shifting ability was mildly impaired, and his visuospatial reasoning, perception, and construction were intact.
Patient 2
On her first visit, patient 2’s single-word reading was average; her word list encoding and delayed recall abilities were low average; her immediate paragraph recall and delayed recall were average and high average, respectively; and her immediate and delayed visual memory performances were high average and average, respectively. Her visuomotor processing speed; problem-solving/concept formation; mental set-shifting; and visuospatial reasoning, perception, and construction were within normal limits. At her second visit, patient 2’s visuomotor processing speed, mental set-shifting, and visuospatial reasoning were severely impaired, and her visuospatial perception and construction were within normal limits.
Neuroimaging
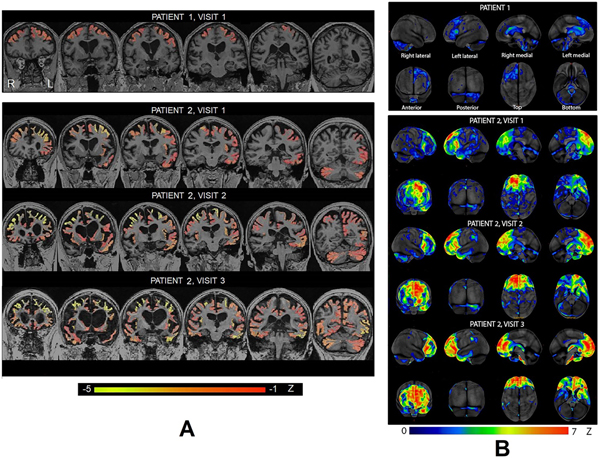
Our patients’ T1 brain MRI images are displayed in Figure 2A, and their FDG-PET scans are displayed in Figure 2B.
FIGURE 2.
Our patients’ A. T1 brain MRIs and B. FDG-PET scans. Cortex-ID z-score maps are displayed for each scan. L = left. R = right.
Patient 1
Patient 1’s MRI showed mild atrophy in the left inferior frontal gyrus and the bilateral middle and superior frontal gyri. His FDG-PET scan showed hypometabolism in the left lateral frontal lobe, particularly the inferior frontal gyrus, and the left anterior cingulate. His global PiB SUVR ratio was 1.41, indicating that he was beta-amyloid negative.
Patient 2
At her first visit, patient 2’s MRI showed moderate atrophy in the bilateral frontal lobes, particularly the left middle and superior frontal gyri, and mild atrophy in the left lateral temporal lobes, left insula, parietal–occipital lobes, and cerebellum. Striking hypometabolism was observed in the left medial and lateral prefrontal cortex and anterior cingulate, with additional involvement of the right prefrontal cortex, right anterior cingulate, left anterior lateral temporal lobe, and supramarginal gyrus. Her global PiB SUVR ratio was 1.24, indicating that she was beta-amyloid negative.
At her second visit, ~3 years later, patient 2’s MRI showed severe frontal atrophy, with worsening of atrophy across the regions that were involved on the first MRI and additional spread into the right insula, cingulate, precuneus, and parietal regions, as well as striking enlargement of the ventricles. Hypometabolism throughout the frontal lobe and anterior cingulate was also worse at the second visit. Her global PiB SUVR ratio was 1.19, indicating that she was beta-amyloid negative. Her flortaucipir meta-region of interest SUVR was 1.24, suggesting that she did not have Alzheimer pathology.
At her third visit, which occurred ~3 years after her second visit, patient 2’s MRI showed severe knife-edge atrophy of the frontal and anterior temporal lobes, with atrophy in the right temporal lobe. Hypometabolism also worsened from the second visit, particularly in the right medial and anterior cingulate cortices. Her global PiB SUVR ratio was 1.14, again indicating that she was beta-amyloid negative and showing no progression over time. Her flortaucipir meta-region of interest SUVR was 1.22.
DISCUSSION
In this report, we described two patients with progressive dynamic aphasia in the context of FTD. We will first discuss the findings of the interdisciplinary assessment and then address the specific questions raised in the Introduction.
Interdisciplinary Assessment
During their initial visits, both of the patients presented with diminished spontaneous verbal initiation and propositional language impairment, but their auditory comprehension, sentence repetition, and naming were relatively well preserved. This clinical pattern is consistent with what is known as dynamic aphasia (eg, Costello and Warrington, 1989; Esmonde et al, 1996; Luria, 1964, 1970, 1973; Luria and Tsvetkova, 1968; Perez et al, 2013; Robinson et al, 1998, 2006, 2015; Snowden et al, 1996; Warren et al, 2003).
The WAB–R measures of information content, fluency, and spontaneous speech underestimated our patients’ verbal propositional difficulty. Transcribing the patients’ verbal samples (conversational speech and picture description) and calculating the approximate duration of pauses between utterances (Table 3) provided a better estimate of the significant verbal propositional difficulty that the patients were experiencing. Their verbal propositional difficulty did not include explicit word retrieval difficulty (eg, paraphasic errors, circumlocutions), which supports relative preservation of lexical access. Also, agrammatism was not a notable feature of their verbal expression, although patient 1 did exhibit mild difficulty. Our patients presented without co-occurring perseverative verbal behavior (cf, Luria, 1970, “inertia,” p. 208). Both patients struggled with letter and categorical fluency, which has been reported in other cases of progressive (Esmonde et al, 1996; Perez et al, 2013; Robinson et al, 1998, 2006, 2015; Snowden et al, 1996; Warren et al, 2003) and nonprogressive forms (eg, Luria and Tsvetkova, 1968) of dynamic aphasia.
Nonlinguistic cognitive difficulties in individuals with progressive forms of dynamic aphasia are heterogeneous in the extent and type of neuropsychological abnormalities that have been reported in previous case studies (Esmonde et al, 1996; Perez et al, 2013; Robinson, 2013; Robinson et al, 2006, 2015; Snowden et al, 1996; Warren et al, 2003). In our patients, there were varying degrees of involvement on verbal memory tasks (word list or paragraph recall) at the first visit. Further research is required, however, to investigate whether the presence of dynamic aphasia could interfere with patients’ performance on verbal recall tasks.
Both of our patients had preserved delayed visual memory and visuospatial function. Patient 1 showed deficits in frontal executive functions outside the domain of verbal modality, as evidenced by mild impairment in mental set-shifting and problem-solving/concept formation. Furthermore, both patients showed mild frontal behaviors on the Frontal Behavioral Inventory during visit 1. Taken together, it is possible that although their dynamic aphasia was the primary problem/concern during the initial evaluation, a broader frontal lobe syndrome was emerging. This finding is not surprising given that it is uncommon to find exclusive impairment of one cognitive/linguistic domain (eg, propositional language only) in the context of progressive neurologic diseases.
Dynamic aphasia as the primary manifestation of atypical parkinsonism such as PSP has been reported in a few previous studies (Esmonde et al, 1996, Robinson et al, 2006, 2015). However, our patients did not show any signs of parkinsonism. Our patients’ global cognitive ability was relatively well preserved, and both were also beta amyloid negative (and tau negative in patient 2), which indicates that Alzheimer disease was not the underlying pathology.
Left greater than right frontal atrophy is a common finding in individuals with progressive dynamic aphasia (eg, Robinson, 2013; Snowden et al, 1996, Warren et al, 2003), and our patients showed left-sided frontal lobe atrophy, although patient 2 also exhibited atrophy in the temporal and parietal lobes. The only study to date that has included FDG-PET findings in progressive dynamic aphasia reported left anterior frontal hypometabolism in one patient (Perez et al, 2013). Our patients showed left-sided hypometabolism of the prefrontal cortex and anterior cingulate, with patient 2 exhibiting additional involvement of the temporal and parietal lobes. Our findings are consistent with the notion that the left frontal lobe plays a crucial role in narrative/propositional language abilities. For example, Alexander et al (1989) reviewed the pertinent clinical and experimental literature and concluded that “narrative capacity, abstract verbal skills,” and “paralinguistic functions” moderately lateralized to the left anterior frontal lobe (Table 2, p. 685).
Patient 1 was lost to follow-up. Evaluation of patient 2 during her second visit revealed that her clinical presentation had evolved to include significant frontal behavioral features (per husband’s report, she would ask permission to go to the bathroom, fail to complete everyday tasks, hug strangers, touch/pat people inappropriately, not show affection to family members including grandchildren, wear same clothing, etc.).
During patient 2’s third visit, her deficits were so severe that she could barely participate in the formal evaluations. At that time, she was nonverbal but was able to write her full name. Per her husband’s report, she had not spoken in 2 years, and she continued to be disinhibited and socially inappropriate. She had become completely dependent on him for all activities of her daily living. The term “bvFTD with mutism” appeared to best capture her clinical status. Her brain scan demonstrated widespread atrophy, with particularly severe knife-edge atrophy of the frontal and anterior temporal lobes. Based on these imaging findings, it was suspected that her underlying pathology could be Pick’s disease (Josephs, 2017).
Specific Questions
In the FTD context, where does a primary clinical presentation of dynamic aphasia fall under the current clinical classification systems?
Subcategories of FTD, namely, PPA, bvFTD, and PSP, are relevant to this discussion. Patient 1 in our study clearly met the core criteria (Gorno-Tempini et al, 2011) for a diagnosis of PPA because he presented with verbal difficulty as his primary complaint, and interdisciplinary assessments revealed that it was indeed his most prominent difficulty. His clinical profile, however, was not consistent with any of the three variants of PPA recognized by the international consensus (Gorno-Tempini et al, 2011). In fact, a substantial percentage of PPA cases cannot be classified by the international consensus criteria (Botha et al, 2015; Senaha et al, 2013; Utianski et al, 2019a; Wicklund et al, 2014). Thus, dynamic aphasia may represent one of the “unclassifiable” types. In addition to dynamic aphasia, patient 1 showed mild receptive and expressive agrammatism, a pattern that was also noted by Robinson et al (2005, 2013), possibly justifying a classification of mixed PPA (with dynamic aphasia and agrammatic features).
Assigning patient 2 to one of the existing clinical diagnostic subgroups of FTD is not as straightforward. Verbal difficulty was her only complaint, which was largely consistent with the results of the clinical assessment, and she accordingly met the core criteria for a PPA diagnosis. Her husband’s responses to the Frontal Behavioral Inventory and her neuroimaging findings (ie, bifrontal atrophy) provided cues to an evolving behavioral syndrome. Her behavioral concerns were prominent during her second visit, and her diagnosis was accordingly updated to bvFTD (Rascovsky et al, 2011). Although one could argue that her sparse verbal output and lack of affection represented generalized apathy, she also exhibited disinhibition and socially inappropriate behaviors. Overall, patient 2’s clinical story demonstrates that there are individuals whose clinical progression transitions from a PPA diagnosis to a bvFTD diagnosis.
Finally, dynamic aphasia can exist as an early/predominant manifestation of PSP (eg, Esmonde et al, 1996, Robinson et al, 2006, 2015). Although there were no other specific clinical signs to predict PSP pathology in our patients, there is a previous report of two autopsy-confirmed individuals with PSP who presented with the progressive form of dynamic aphasia (eg, Esmonde et al, 1996). Therefore, dynamic aphasia could be considered as another possible manifestation of the speech/language disorders variant of PSP (Höglinger et al, 2017). Taken together, dynamic aphasia can manifest either as PPA, as a prominent feature of evolving bvFTD, or as a prominent feature of PSP (Figure 3).
FIGURE 3.
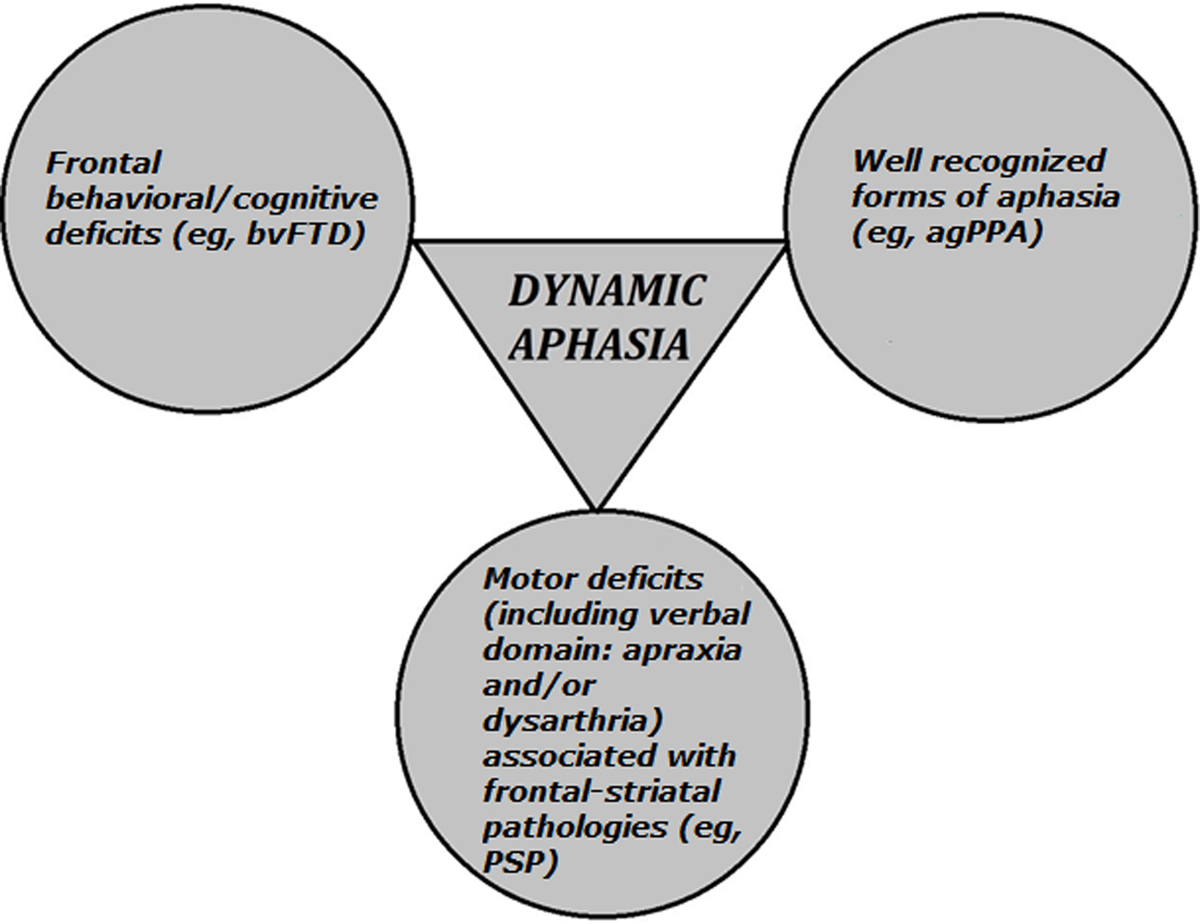
Dynamic aphasia in the context of frontotemporal dementia. The triangle indicates that dynamic aphasia can be a primary/predominant manifestation of frontotemporal dementia. Circles include disorders that can co-occur with, or eventually evolve from, dynamic aphasia. agPPA = agrammatic primary progressive aphasia. bvFTD = behavioral variant of frontotemporal dementia. PSP = progressive supranuclear palsy.
Are written language (ie, reading or writing) impairments evident in dynamic aphasia?
Although several studies have reported that oral reading is well preserved in individuals with progressive dynamic aphasia (eg, Robinson et al, 2006, 2015; Warren et al, 2003), reading comprehension ability has not been addressed. In this study, our patients’ performance in both oral reading and reading comprehension (WAB–R) was well preserved. Further research, however, is required to determine if reading (and auditory) comprehension suffers when tasks require higher order thought processing such as reasoning, inference, and deduction.
When it comes to writing disorders in PPA, surface dysgraphia, phonological and deep dysgraphia, graphemic buffer disorder, and disorders of handwriting have been documented (see Graham, 2014, for a review). However, literature on written propositional impairment is almost nonexistent. Our patients demonstrated written propositional impairment as evidenced by their hesitations (eg, erasing) and their struggle to complete the written narrative task within the time limit (3 minutes). In the case of their verbal samples, explicit verbal interjections (eg, nonword fillers) and pauses between words/utterances (Table 3) signaled underlying propositional language impairment in our patients. In the case of their written samples, however, the finished product appeared well framed, as our patients appeared to have taken time (accuracy over speed) to compensate for their propositional language impairment. This finding reveals that verbal self-monitoring may be successful during writing in individuals with dynamic aphasia.
We investigated written expression only in picture description; it is unknown how the patients would have performed in other structured tasks (eg, answering open-ended personal questions) or in more ecologically valid tasks (eg, composing an email or text message). Further study of spontaneous and narrative written formulation in individuals with dynamic aphasia is needed. Patient 2 also had difficulty writing nonwords, which may have been due to the demands of the novel, cognitively and attentionally heavy phonological task. In fact, difficulty with writing nonwords has been noted in other frontal lobe syndromes such as progressive agrammatic aphasia and progressive apraxia of speech (eg, Botha et al, 2015).
Do motor speech disorders co-occur in progressive dynamic aphasia?
Our patients did not exhibit dysarthria or apraxia of speech. Taken together with other case reports in the literature (Esmonde et al, 1996; Perez et al, 2013; Robinson, 2013; Robinson et al, 2005, 2006, 2015), motor speech disorders do not always co-occur with progressive dynamic aphasia.
Do patients with primary progressive dynamic aphasia demonstrate aberrant nonverbal interactive behaviors?
We raised this question to compare and contrast between dynamic aphasia and abulia. Abulia is a disorder of diminished motivation (Ghoshal et al, 2011; Marin and Wilkosz, 2005). Reduced spontaneous speech is a common feature of abulia and dynamic aphasia; but in the former, it presumably reflects diminished motivation, and in the latter, it reflects propositional language difficulty. Because subjectively judged eye contact, nonverbal social greetings, and responsiveness (intent to respond) to the examiner’s questions/tasks were well preserved in our patients during their first visit, we did not think that their reduced spontaneous speech was due to diminished internal motivation. Rather, reduced spontaneous speech in the context of propositional language difficulty appeared to best capture their clinical profile.
On a side note, characterizing facial affect, which is a component of our nonverbal interactive behavior assessment, was not straightforward in these patients. We have also encountered flat facial affect in individuals with disorders of diminished motivation (eg, apathy, abulia, akinetic mutism). Both of our patients showed a tendency toward flat affect, but it appeared more like a “lost in thought” expression than an emotionless facial expression. Further research is required to elucidate different possible types of facial affect disturbances (eg, motor deficit based, as in parkinsonism; internal motivation deficit based, as in abulia and akinetic mutism; emotional disturbance related, as in depression; and higher order cognitive/linguistic deficit based, such as in dynamic aphasia and autism).
What is the underlying mechanism in dynamic aphasia?
Dynamic aphasia is a condition that is “on the borderline between a speech disturbance and a disturbance of thought processes” (Luria, 1970, p. 208). Luria proposed that dynamic aphasia reflects a breakdown in inner verbalization, which he viewed as a stage that translates thought processes into a “linear scheme” of sentences (Luria, 1970; Luria and Tsvetkova, 1967, p. 302). Luria quoted complaints from his patients such as “emptiness in the head” to argue that these patients have difficulty with inner conceptualization of what they want to say.
Our patients’ complaints appear to support Luria’s hypothesis. Regarding verbal difficulty, patient 2 expressed “well, people ask me questions and then I I I don’t I don’t know how to answer” (Table 3). When patient 1 was asked what he meant by “lack of communication,” he replied “well…I would prefer silence” (Table 3). It is not clear whether he preferred silence due to the effort he had to put forth to overcome his propositional language difficulty, he was unable to precisely explain the nature of his difficulty, or he was having difficulty translating ideas into verbal messages. Warren et al (2003) also reported a case of dynamic aphasia in which they thought the breakdown was at the “pre-linguistic stage of sentence production” (p. 150). In Levelt’s (1999) blueprint of the speaker model, this prelinguistic stage corresponds to the conceptual preparation step, which is the first step in verbal expression. However, how this conceptual preparation stage is implemented is far from being understood (for a review, see Barker et al, 2020).
Several other theories regarding the underlying mechanism of dynamic aphasia have been postulated. These include selective impairment of verbal planning (Costello and Warrington, 1989), impaired temporal/sequential aspects of propositional language (Snowden et al, 1996), impaired semantic strategy formation (Gold et al, 1997), and impaired frontal attention processes required for language production (Alexander, 2006). Robinson and colleagues proposed two possible subtypes of dynamic aphasia—one where the underlying mechanism is impaired ability to choose between competing verbal responses (Robinson et al, 1998, 2005) and the other where the underlying mechanism is impaired ability to “generate a fluent sequence of novel thought”(Robinson et al, 2006, p. 1357).
Our study was retrospectively developed based on data from two patients; as such, we did not manipulate variables with the specific aim of discerning the underlying mechanisms of dynamic aphasia. Theoretical and clinical investigations focusing on narrative formulation or propositional language are scant in relation to representational (phonemes, semantics and lexicon) and rule-based (syntax/grammar) aspects of language. Because the concept of propositional language and associated variables is not well understood, it is quite challenging to come up with precise theoretical constructs that could explain the mechanisms underlying dynamic aphasia. We agree with Barker et al (2020) that “the investigation of idea formulation for spoken language at the sentence level (ie, propositional language) is broader than the stimulus–response mapping required for core language skills (eg, naming, reading), and therefore inherently more complex and ‘messy’, attracting fewer experimental studies” (p. 227). About dynamic aphasia, Luria (1970, p. 199) felt that “it is extremely difficult to explain this syndrome in neurological terms, for we are still far from understanding its mechanisms.”
In 2021, we are still far from understanding this intriguing syndrome. We hope that further investigations on progressive forms of dynamic aphasia, in addition to those resulting from acute/subacute causes (eg, stroke, brain tumor), might help elucidate the underlying mechanisms in this clinical manifestation.
CONCLUSION
Integrating findings of previous studies with that of our two cases suggest that dynamic aphasia is a distinct variant of PPA/FTD. The primary patient complaint is progressive verbal difficulty. Presenting clinical features include difficulty with verbal and written propositional expression and word fluency, with intact or well-preserved auditory comprehension, repetition, naming, oral reading, and reading comprehension. Nonverbal interactive behaviors and the intent/willingness to interact are well preserved.
If spontaneous verbal expression and conversational and narrative abilities are overlooked during clinical examination, the defining features of dynamic aphasia may be missed. Neuroimaging correlates include left (or left > right) frontal atrophy on MRI and/or left frontal hypometabolism on FDG-PET; additional involvement of temporal and parietal lobes could be present, especially as the disease progresses over time. The underlying mechanism in dynamic aphasia appears to be a breakdown in the thought–verbal interface.
ACKNOWLEDGMENTS
The authors thank the patients and their families for their time and dedication to research into primary progressive aphasia. We thank Rene L. Utianski, PhD, Mayo Clinic, for her assistance with data collection from patient 2 (third visit), and Sarah M. Boland, CCRP, Mayo Clinic, for her assistance as the study coordinator.
Supported in part by NIH grants (R01 DC010367 and R01 DC14942) to K.A.J. and (R01 DC12519) to J.L.W.
V.J.L consults for Bayer Schering Pharma, Piramal Life Sciences, Life Molecular Imaging, Eisai Inc., and Merck Research and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, and the National Institutes of Health. The remaining authors declare no conflicts of interest.
Glossary
- bv
behavioral variant
- FDG
fluorodeoxyglucose
- FTD
frontotemporal dementia
- MoCA
Montreal Cognitive Assessment
- PiB
Pittsburgh compound B
- PPA
primary progressive aphasia
- PSP
progressive supranuclear palsy
- SUVR
standard uptake value ratio
- WAB–R
Western Aphasia Battery—Revised
Footnotes
Portions of this work were presented at the 26th Annual Meeting of the Cognitive Neuroscience Society, March 2019, San Francisco, California.
REFERENCES
- Alexander MP. 2006. Impairments of procedures for implementing complex language are due to disruption of frontal attention processes. J Int Neuropsychol Soc. 12:236–247. doi: 10.1017/s1355617706060309 [DOI] [PubMed] [Google Scholar]
- Alexander MP, Benson DF, Stuss DT. 1989. Frontal lobes and language. Brain Lang. 37:656–691. doi: 10.1016/0093-934X(89)90118-1 [DOI] [PubMed] [Google Scholar]
- Barker MS, Nelson NL, Robinson GA. 2020. Idea formulation for spoken language production: the interface of cognition and language. J Int Neuropsychol Soc. 26:226–240. doi: 10.1017/S1355617719001097 [DOI] [PubMed] [Google Scholar]
- Botha H, Duffy JR, Strand EA, et al. 2014. Nonverbal oral apraxia in primary progressive aphasia and apraxia of speech. Neurology. 82:1729–1735. doi: 10.1212/WNL.0000000000000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha H, Duffy JR, Whitwell JL, et al. 2015. Classification and clinicoradiologic features of primary progressive aphasia (PPA) and apraxia of speech. Cortex. 69:220–236. doi: 10.1016/j.cortex.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello AL, Warrington EK. 1989. Dynamic aphasia: the selective impairment of verbal planning. Cortex. 25:103–114. doi: 10.1016/S0010-9452(89)80010-3 [DOI] [PubMed] [Google Scholar]
- De Renzi E, Vignolo LA. 1962. The Token Test: a sensitive test to detect receptive disturbances in aphasics. Brain. 85:665–678. doi: 10.1093/brain/85.4.665 [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. 2001. Delis-Kaplan Executive Function System (D-KEFS): Examiner’s Manual. San Antonio, Texas: Psychological. [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, et al. 2000. The FAB: a frontal assessment battery at bedside. Neurology. 55:1621–1626. doi: 10.1212/wnl.55.11.1621 [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM. 2007. Peabody Picture Vocabulary Test. 4th ed. Bloomington, Minnesota: NCS Pearson. [Google Scholar]
- Esmonde T, Giles E, Xuereb J, et al. 1996. Progressive supranuclear palsy presenting with dynamic aphasia. J Neurol Neurosurg Psychiatry. 60:403–410. doi: 10.1136/jnnp.60.4.403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC. 2016. Frontotemporal dementias. Continuum (Minneap Minn). 22(2 Dementia):464–489. doi: 10.1212/CON.0000000000000300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. 1975. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 12:189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Ghoshal S, Gokhale S, Rebovich G, et al. 2011. The neurology of decreased activity: abulia. Rev Neurol Dis. 8:e55–67. [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, et al. 2008. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 23:2129–2170. doi: 10.1002/mds.2234 [DOI] [PubMed] [Google Scholar]
- Gold M, Nadeau SE, Jacobs DH, et al. 1997. Adynamic aphasia: a transcortical motor aphasia with defective semantic strategy formation. Brain Lang. 57:374–393. doi: 10.1006/brln.1997.1750 [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, Barresi B. 2000. Boston Diagnostic Aphasia Examination—Third Edition. Short Form Record Booklet. Philadelphia, Pennsylvania: Lippincott Williams & Wilkins. [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, et al. 2011. Classification of primary progressive aphasia and its variants. Neurology. 76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham NL. 2014. Dysgraphia in primary progressive aphasia: characterisation of impairments and therapy options. Aphasiology. 28:1092–1111. doi: 10.1080/02687038.2013.869308 [DOI] [Google Scholar]
- Höglinger GU, Respondek G, Stamelou M, et al. 2017. Clinical diagnosis of progressive supranuclear palsy: the Movement Disorder Society criteria. Mov Disord. 32:853–864. doi: 10.1002/mds.26987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D, Patterson K. 1992. The Pyramids and Palm Trees Test: a Test of Semantic Access From Words and Pictures. Bury St. Edmunds, United Kingdom: Thames Valley Test. [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, et al. 1996. Neuropsychological tests’ norms above age 55: COWAT, BNT, MAE Token, WRAT–R Reading, AMNART, Stroop, TMT, and JLO. Clin Neuropsychol. 10:262–278. doi: 10.1080/13854049608406689 [DOI] [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, et al. 1992. Mayo’s older Americans normative studies: updated AVLT norms for ages 56 to 97. Clin Neuropsychol. 6:83–104. doi: 10.1080/13854049208401880 [DOI] [Google Scholar]
- Jack CR Jr, Lowe VJ, Senjem ML, et al. 2008. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 131:665–680. doi: 10.1093/brain/awm336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Botha H, et al. 2019. The bivariate distribution of amyloid-β and tau: relationship with established neurocognitive clinical syndromes. Brain. 142:3230–3242. doi: 10.1093/brain/awz268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Wiste HJ, Schwarz CG, et al. 2018. Longitudinal tau PET in ageing and Alzheimer’s disease. Brain. 141:1517–1528. doi: 10.1093/brain/awy059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA. 2017. Current understanding of neurodegenerative diseases associated with the protein tau. Mayo Clin Proc. 92:1291–1303. doi: 10.1016/j.mayocp.2017.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, et al. 2012. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 135:1522–1536. doi: 10.1093/brain/aws032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, et al. 2014. The evolution of primary progressive apraxia of speech. Brain. 137: 2783–2795. doi: 10.1093/brain/awu223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, et al. 2013. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology. 81:337–345. doi: 10.1212/WNL.0b013e31829c5ed5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, et al. 2006. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 30:436–443. doi: 10.1016/j.neuroimage.2005.09.046 [DOI] [PubMed] [Google Scholar]
- Kaufer DI, Cummings JL, Ketchel P, et al. 2000. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 12:233–239. doi: 10.1176/jnp.12.2.233 [DOI] [PubMed] [Google Scholar]
- Kertesz A, Davidson W, Fox H. 1997. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci. 24:29–36. doi: 10.1017/s0317167100021053 [DOI] [PubMed] [Google Scholar]
- Kertesz A, Raven JC. 2007. Western Aphasia Battery—Revised. San Antonio, Texas: Psychological. [Google Scholar]
- Lansing AE, Ivnik RJ, Cullum CM, et al. 1999. An empirically derived short form of the Boston Naming Test. Arch Clin Neuropsychol.14:481–487. [PubMed] [Google Scholar]
- Levelt WJM. 1999. Producing spoken language: a blueprint of the speaker. In: Brown CM, Hagoort P, eds. The Neurocognition of Language. Oxford, United Kingdom: Oxford University Press; 83–122. [Google Scholar]
- Lowe VJ, Lundt ES, Albertson SM, et al. 2020. Tau-positron emission tomography correlates with neuropathology findings. Alzheimers Dement. 16:561–571. doi: 10.1016/j.jalz.2019.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria AR. 1964. Factors and forms of aphasia. In: de Reuck AVS, O’Connor M, eds. Disorders of Language. London, Wisconsin: J. & A. Churchill; 143–161. [Google Scholar]
- Luria AR. 1970. Traumatic Aphasia: Its Syndromes, Psychology and Treatment. The Hague, Netherlands: Mouton. [Google Scholar]
- Luria AR. 1973. Chapter 12: Speech. In: The Working Brain: An Introduction to Neuropsychology. London, England: Penguin; 303–322. [Google Scholar]
- Luria AR, Tsvetkova LS. 1968. The mechanism of ‘dynamic aphasia.’ Found Lang. 4:296–307. [Google Scholar]
- Machulda MM, Ivnik RJ, Smith GE, et al. 2007. Mayo’s older Americans normative studies: visual form discrimination and copy trial of the Rey-Osterrieth Complex Figure. J Clin Exp Neuropsychol. 29:377–384. doi: 10.1080/13803390600726803 [DOI] [PubMed] [Google Scholar]
- Marin RS, Wilkosz PA. 2005. Disorders of diminished motivation. J Head Trauma Rehabil. 20:377–388. doi: 10.1097/00001199-200507000-00009 [DOI] [PubMed] [Google Scholar]
- Milano NJ, Heilman KM. 2015. Primary progressive speech abulia. J Alzheimers Dis. 46:737–745. doi: 10.3233/JAD-142112 [DOI] [PubMed] [Google Scholar]
- Minoshima S, Frey KA, Koeppe RA, et al. 1995. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med. 36:1238–1248. [PubMed] [Google Scholar]
- Mychack P, Kramer JH, Boone KB, et al. 2001. The influence of right frontotemporal dysfunction on social behavior in frontotemporal dementia. Neurology. 56:S11–S15. doi: 10.1212/wnl.56.suppl_4.s11 [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, et al. 2005. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Nicholas LE, Brookshire RH. 1993. A system for quantifying the informativeness and efficiency of the connected speech of adults with aphasia. J Speech Hear Res. 36:338–350. doi: 10.1044/jshr.3602.338 [DOI] [PubMed] [Google Scholar]
- Osterrieth P 1944. Test of copying a complex figure: contribution to the study of perception and memory. Arch Psychol (Geneve). 30:286–356. [Google Scholar]
- Perez DL, Dickerson BC, McGinnis SM, et al. 2013. You don’t say: dynamic aphasia, another variant of primary progressive aphasia? J Alzheimers Dis. 34:139–144. doi: 10.3233/JAD-121861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, et al. 2011. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 134:2456–2477. doi: 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A 1964. The Clinical Psychological Examination. Paris, France: Presses Universitaires de France. [Google Scholar]
- Robinson GA. 2013. Primary progressive dynamic aphasia and parkinsonism: generation, selection and sequencing deficits. Neuropsychologia. 51:2534–2547. doi: 10.1016/j.neuropsychologia.2013.09.038 [DOI] [PubMed] [Google Scholar]
- Robinson G, Blair J, Cipolotti L. 1998. Dynamic aphasia: an inability to select between competing verbal responses? Brain. 121:77–89. doi: 10.1093/brain/121.1.77 [DOI] [PubMed] [Google Scholar]
- Robinson G, Shallice T, Cipolotti L. 2006. Dynamic aphasia in progressive supranuclear palsy: a deficit in generating a fluent sequence of novel thought. Neuropsychologia. 44:1344–1360. doi: 10.1016/j.neuropsychologia.2006.01.002 [DOI] [PubMed] [Google Scholar]
- Robinson G, Shallice T, Cipolotti L. 2005. A failure of high level verbal response selection in progressive dynamic aphasia. Cogn Neuropsychol. 22: 661–694. doi: 10.1080/02643290442000239 [DOI] [PubMed] [Google Scholar]
- Robinson GA, Spooner D, Harrison WJ. 2015. Frontal dynamic aphasia in progressive supranuclear palsy: distinguishing between generation and fluent sequencing of novel thoughts. Neuropsychologia. 77:62–67. doi: 10.1016/j.neuropsychologia.2015.08.001 [DOI] [PubMed] [Google Scholar]
- Savage S, Hsieh S, Leslie F, et al. 2013. Distinguishing subtypes in primary progressive aphasia: application of the Sydney Language Battery. Dement Geriatr Cogn Disord. 35:208–218. doi: 10.1159/000346389 [DOI] [PubMed] [Google Scholar]
- Senaha MLH, Caramelli P, Brucki SMD, et al. 2013. Primary progressive aphasia: classification of variants in 100 consecutive Brazilian cases. Dement Neuropsychol. 7:110–121. doi: 10.1590/S1980-57642013DN70100017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. 1998. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 17:87–97. doi: 10.1109/42.668698 [DOI] [PubMed] [Google Scholar]
- Snowden JS, Griffiths HL, Neary D. 1996. Progressive language disorder associated with frontal lobe degeneration. Neurocase. 2:429–440. doi: 10.1080/13554799608402417 [DOI] [Google Scholar]
- Spreen O, Strauss E. 1998. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. 2nd ed. New York, New York: Oxford University Press. [Google Scholar]
- Strauss E, Sherman EMS, Spreen O. 2006. Chapter 8: Executive Functions. In: Strauss E, Sherman EMS, Spreen O, eds. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed. New York, New York: Oxford University Press; 401–545. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 15:273–289. doi: 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Utianski RL, Botha H, Martin PR, et al. 2019a. Clinical and neuroimaging characteristics of clinically unclassifiable primary progressive aphasia. Published online August 13. Brain Lang. 197:104676. doi: 10.1016/j.bandl.2019.104676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski R, Duffy JR, Clark H, et al. 2019b. Prominent auditory deficits in primary progressive aphasia: a case study. Cortex. 117:396–406. doi: 10.1016/j.cortex.2019.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Simon G, Kantarci K, et al. 2011. Antemortem differential diagnosis of dementia pathology using structural MRI: Differential-STAND. Neuroimage. 55:522–531. doi: 10.1016/j.neuroimage.2010.12.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JD, Warren JE, Fox NC, et al. 2003. Nothing to say, something to sing: primary progressive dynamic aphasia. Neurocase. 9:140–155. doi: 10.1076/neur.9.2.140.15068 [DOI] [PubMed] [Google Scholar]
- Warrington EK, James M. 1991. The Visual Object and Space Perception Battery. Bury St. Edmunds, United Kingdom: Thames Valley Test. [Google Scholar]
- Wear HJ, Wedderburn CJ, Mioshi E, et al. 2008. The Cambridge Behavioural Inventory Revised. Dement Neuropsychol. 2:102–107. doi: 10.1590/S1980-57642009DN20200005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D 1997. Wechsler Memory Scale—Third Edition Manual. San Antonio, Texas: Psychological. [Google Scholar]
- Weintraub S, Mesulam MM, Wieneke C, et al. 2009. The Northwestern Anagram Test: measuring sentence production in primary progressive aphasia. Am J Alzheimers Dis Other Demen. 24:408–416. doi: 10.1177/1533317509343104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicklund MR, Duffy JR, Strand EA, et al. 2014. Quantitative application of the primary progressive aphasia consensus criteria. Neurology. 82:1119–1126. doi: 10.1212/WNL.0000000000000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS. 1993. Wide Range Achievement Test. 3rd rev. Wilmington, Delaware: Jastak Associates. [Google Scholar]
- Woods SP, Scott JC, Sires DA, et al. 2005. Action (verb) fluency: test–retest reliability, normative standards, and construct validity. J Int Neuropsychol Soc. 11:408–415. [PubMed] [Google Scholar]