Abstract
Despite numerous promising therapeutic targets, there are no proven medical treatments for calcific aortic stenosis (AS). Multiple stakeholders need to come together and several scientific, operational, and trial design challenges must be addressed in order to capitalize on the recent and emerging mechanistic insights into this prevalent heart valve disease. We briefly discuss the pathobiology and most promising pharmacological targets, screening, diagnosis and progression of AS, identification of sub-groups that should be targeted in clinical trials, and the need to elicit the patient voice earlier rather than later in clinical trial design and implementation. Potential trial endpoints and tools for assessment and approaches to implementation and design of clinical trials are reviewed. The efficiencies and advantages offered by a clinical trial network and platform trial approach are highlighted. The objective is to provide practical guidance that will facilitate a series of trials to identify effective medical therapies for AS resulting in expansion of therapeutic options to complement mechanical solutions for late-stage disease.
Condensed abstract
Progress in elucidating the pathobiology of aortic stenosis (AS) and identifying promising therapeutic targets has not yet translated into proven pharmacologic treatment for AS. This review offers practical guidance to address scientific, operational, and trial design challenges and proposes the development of a clinical trials research network to execute a platform trial as the optimal path to efficiently test multiple potential therapies to identify effective ones that can complement mechanical solutions for late-stage disease.
Introduction
Calcific aortic valve stenosis is widely prevalent among adults greater than 65 years of age; approximately 25% have aortic sclerosis, the pathological precursor to aortic stenosis (AS) which is itself observed in 5–10% (1–3). Given the strong relationship between aging and AS, it is anticipated that there will be a doubling in the prevalence of AS in the coming decades as life expectancy increases and the population ages (4). The only known effective treatment for AS is aortic valve replacement (AVR), which improves survival and quality of life for symptomatic patients with severe AS; approximately 150,000 surgical or transcatheter AVRs are performed annually in the United States (5,6). Worldwide, AS is responsible for ~125,000 deaths and ~350,000 AVRs with an associated loss of 1.8 million disability-adjusted life years per year (7). The high residual risk related to heart failure even after successful AVR in conjunction with low procedural complication rates and low morbidity from transcatheter AVR have motivated studies to test whether earlier AVR before symptom onset or with less severe stenosis might yield better outcomes (8–10). While these strategy trials may lead to important improvements in patient outcomes, they remain limited in their impact as they reinforce the long-standing paradigm that AS is simply a mechanical disease treated with a mechanical solution.
Ongoing research has led to significant progress in elucidating the pathobiology of AS and in identifying promising therapeutic targets. However, there are currently no recommended medical therapies and a dearth of actively enrolling clinical trials due to several barriers (11). Both medical therapies targeting the valve (to slow/halt progressive valve obstruction) and the ventricle (to prevent/reverse maladaptive cardiac remodeling and dysfunction) are needed to delay or avoid the development of heart failure and the need for AVR.
Given that valve obstruction is the primary stimulus for cardiac remodeling that occurs in response to pressure overload, this review focuses principally on medical therapies that target the pathobiology underlying progressive disease in the valve leaflets. Our over-arching objective is to provide a roadmap for efficiently testing promising medical therapies to slow AS progression. We hope that this practical guidance will facilitate a series of trials to identify effective medical therapies for treatment of AS resulting in expansion of therapeutic options to complement mechanical solutions for late-stage disease.
Pathobiology and Promising Therapeutic Targets for Aortic Stenosis
Pathobiology of AS
A detailed description of the pathobiology of AS and promising therapeutic targets is beyond the scope of this review but have been reviewed elsewhere (11–15). The pathophysiology of AS appears to have two phases. The earlier initiation phase demonstrates many similarities with atherosclerosis, with accumulation of apolipoprotein (apo) B-containing lipoproteins in the subendothelium, lipoprotein oxidation, activation of inflammatory pathways, production of angiotensin-converting enzyme (ACE), and upregulation of adhesion molecules and matrix metalloproteinases, and shear stress. Consequently, risk factors for the incidence of AS are also similar and include older age, dyslipidemia, high lipoprotein(a) (Lp[a]) levels, hypertension, smoking, obesity as well as bicuspid morphology.
In contrast, the later stages of AS result in progressive leaflet fibrosis and calcification and are dominated by alterations in complex, interrelated cellular and molecular pathways and perturbations in mineral metabolism. Together these drive increased leaflet thickening and valve stiffness that leads to progressive AS. Disease progression in this propagation phase is most closely linked to markers of valve calcification and valve stenosis severity (5,15,16). The molecular mechanisms involved in both the initiation and propagation phases of AS, as well as the gene candidates identified through genetic association studies, suggest potential therapeutic targets for AS prevention and treatment which may be phase-specific (Figure 1) (13,17–19).
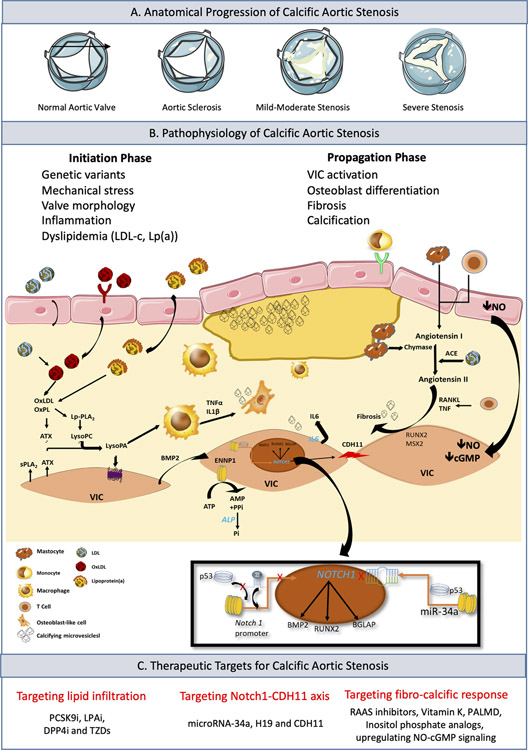
Figure 1. Disease Progression in Calcific Aortic Stenosis (CAS).
Panel A. Anatomical progression of CAS with valve anatomy viewed from the aortic side and open in systole. Panel B. Corresponding clinically relevant risk factors and histological development of CAS. Early lesion initiation by lipid infiltration into the subendothelium (LPA, FADS1/2). Infiltration initiation due to mechanical stress, dyslipidemia or abnormal valve morphology. Lp(a), LDL, OxLDL, and OxPLs enter into the subendothelial space triggering the recruitment, activation, and proliferation of monocytes and macrophages. At the molecular level, the calcification process driven by the oxidized phospholipid content of OxLDLs, leads to the enzymatic generation of LysoPA via ATX. In turn, LysoPA promotes osteogenic transition though the production of BMP2, which associates, with the expression of bone-related transcription factors like RUNX2 and MSX2. In addition, activation of macrophages in aortic valve by OxLDL promotes the production of TNFα and IL1β with pro-osteogenic properties. Chymase and ACE promote the production of angiotensin II, which increases the synthesis and secretion of collagen by VICs. This leads to an imbalance in metalloproteinase synthesis/inhibition pathway fibrous tissue begins to accumulate in the aortic valve. Early in the disease progression VICs and macrophages secret microvesicles initiating microcalcification. Overexpression of ENNP1 and ALP further increases osteogenic mineralization. Inset: Regulation of Notch1 transcription. H19 suppresses transcription of Notch1 by blocking the binding of p53 to the Notch1 promoter. The microRNA miR-34a is able to bind directly to the Notch1 transcript resulting in decreased translation of Notch1 mRNA. Both pathways of Notch1 suppression result in an increase CDH11 expression and calcification of the aortic valve. Panel C. Therapeutic modalities currently under investigation for CAS. ACE=angiotensin-converting enzyme; ALP=alkaline phosphatase; ATX=autotaxin; BMP2=bone morphogenetic protein 2; CAS=calcific aortic stenosis; CDH11=cadherin-11; ENNP1=ectonucleotidases; IL1β=Interleukin 1β; Lp(a)=lipoprotein(a); LDL=low-density lipoprotein; LysoPA=lysophosphatidic acid; MSX2=homeobox protein; NO-cGMP=nitric oxide cyclic guanosine monophosphate; OxLDL=oxidized LDL; OxPLs=oxidized phospholipids; RUNX2=runt-related transcription factor2; TNFα=tumor necrosis factor α; VIC=valve interstitial cell.
Although the focus of this manuscript is on valvular pathology, the functional consequences of AS on the myocardium are also important. Increased afterload due to valve obstruction induces a hypertrophic response in the left ventricle which restores wall stress and maintains cardiac performance for many years if not decades. Eventually this process decompensates, with progressive myocyte cell death and myocardial fibrosis driving the transition to heart failure, symptom development and adverse events.(20)
Promising Therapeutic Targets for Calcific Aortic Stenosis
Lipid-lowering therapy.
Despite the failure of statin therapy to influence AS progression (21,22), hypothesis-driven and hypothesis-free genetic association studies confirmed the role of apoB-containing atherogenic lipoprotein particles as being involved in the earliest steps of aortic valve micro and macro calcification (the initiation phase) (23–26). These include very-low-density, intermediate-density and low-density lipoprotein particles (VLDL, IDL and LDL) as well as Lp(a). Reduction of apoB-containing lipoprotein particles may exert beneficial effects in AS through the inhibition of leaflet mineralization, the inhibition of macrophage infiltration, the prevention of osteoblast-like phenotype transformation and the reduction of leaflet cholesterol accumulation. Importantly, patients with elevated Lp(a) levels also demonstrate faster disease progression suggesting an effect on the propagation as well as initiation phases of the disease (27). Proprotein convertase subtilisin/kexin type 9 (PCSK9) and Lp(a) inhibitors have been shown to provide unprecedented reductions in non-Lp(a) and Lp(a)-containing lipoprotein particles, respectively, and may be key in the prevention of AS if adequately tested in randomized controlled trials (RCT) of high-risk patients (28,29).
Renin-angiotensin-aldosterone system (RAAS).
The effects of the RAAS inhibitors in patients with AS on surrogate endpoints in small studies have been described elsewhere (12). Beyond their blood pressure effects, RAAS inhibition might reduce pro-fibrotic processes within the valve leaflets and myocardium in patients with AS, thereby potentially slowing valve narrowing and preventing/reversing maladaptive cardiac remodeling (12). The ARBAS RCT (NCT04913870) will investigate whether ARBs can slow disease progression in the valve and delay fibrosis accumulation in the myocardium.
Metabolic targets.
Drugs targeting glucose-insulin homeostasis such as dipeptidyl peptidase-4 (DPP-4) inhibitors and thiazolidinediones (TZDs) also represent potential therapies for AS. DPP-4 has many biological functions including a possible role in a wide range of heart diseases. Endothelial dysfunction in the aortic valve may lead to higher expression of DPP-4, which in turn, may promote osteogenic mechanisms in aortic valves (30). The beneficial impact of TZDs in the aortic valve may be due to the ability of these compounds to reduce the expression of the receptor for advanced glycation end products (RAGE), thereby exerting anti-inflammatory effects. However, the level of evidence for hypoglycemic drugs is currently only supported by retrospective and/or pre-clinical studies with RCTs awaited (31,32).
Nitric oxide cyclic guanosine monophosphate (NO-cGMP) signaling.
Preclinical studies have demonstrated the role of phosphodiesterase type 5 inhibition in preventing and reversing maladaptive cardiac remodeling and dysfunction in the setting of pressure overload (33,34). In a small clinical study, administration of sildenafil was associated with acute improvements in pulmonary and systemic hemodynamics and biventricular unloading in patients with severe AS, but longer term studies are needed to assess effects on cardiac remodeling (35). Oxidative stress, related in part to decreased expression and activity of antioxidant enzymes and uncoupling of nitric oxide synthase, has been linked to calcification in aortic valve leaflets (36). Among other sequelae, this converts soluble guanylate cyclase to its oxidized form, thereby reducing its activation, reducing cGMP production, and facilitating pro-calcific signaling. Ataciguat is an NO-independent soluble guanylate cyclase activator with particular affinity for the oxidized form of soluble guanylate cyclase (37). Preclinical studies demonstrate that ataciguat reduces aortic valve calcification and slows progression of valve dysfunction; early stage clinical studies (NCT02481258) also suggest that it may slow progression of aortic valve calcification (38).
NOTCH Pathway & non-coding RNAs.
Additional promising candidates, not related to lipid metabolism, focus upon the NOTCH1 pathway, which is a clear genetic driver of calcific aortic valve disease in humans (39). While NOTCH1 mutations are very rare, multiple modifiers of NOTCH1 protein expression have been identified that lead to hallmarks similar to genetic mutation. Specifically, the long non-coding RNA, H19, and microRNA-34a both suppress NOTCH1 synthesis at pre- and post-transcription stages, respectively (40–43). There are likely multiple other RNAs that alter NOTCH1 which is consequential because loss of NOTCH1 protein expression, via genetic mutation or transcription modification, leads to overexpression of cadherin-11 (CDH11) by aortic valve interstitial cells (40,42,44). CDH11 enrichment is found in human cases of AS (45), overexpression results in AS in mice (46), and genetic or pharmacological targeting of CDH11 prevents calcific aortic valve disease and AS in Notch1 mutant mice (47). These findings linking the NOTCH1-CDH11 axis provide hope for possibly identifying a unifying pathobiology of AS by examining modifiers of NOTCH1 expression and also a potential therapy. Unfortunately, further development of the monoclonal antibody to CDH11 (owned by Roche) has been halted after not meeting the primary outcome in a Phase IIb clinical trial for rheumatoid arthritis. Thus, new strategies for targeting the NOTCH1-CDH11 pathway, or the RNAs that modify it, are needed.
Mineral metabolism.
Calcification is the key process driving the propagation phase of the disease, with calcific deposits increasing mechanical stresses in the valve and leading to valve injury, inflammation and further calcification in a vicious cycle that increases valve stiffness and obstruction (48). Successful therapies will need to break this cycle if they are to prove effective. In addition, they will need to exert their anti-calcific effects on the valve while maintaining bone health in the elderly population of patients with AS. Given the link between osteoporosis and increased cardiovascular calcification there was hope that osteoporosis agents might slow AS progression. Indeed, bisphosphonates have anti-inflammatory and lipid-lowering properties. They also prevent myofibroblasts within the aortic valve from differentiating into an osteogenic phenotype, thereby preventing deposition of calcific material in the valve (48). However, the recent SALTIRE II RCT (NCT02132026) failed to demonstrate an effect of alendronate or denosumab on aortic valve calcification activity (18F-fluoride PET), progression of valve calcium burden (CT-AVC), nor hemodynamic assessments on echocardiography (49). Vitamin K2 supplementation to potentiate the anti-calcific effects of matrix-Gla protein is currently being investigated in the BASIK-2 trial, which is also using the same imaging endpoints (50). Other potential anti-calcific agents include inositol phosphate analogs which stabilize regions of microcalcification and inhibit vascular calcification in rodents and in vitro models (51).
Hypertension and heart rate.
Hypertension increases the diastolic transvalvular pressure gradient and mechanical stress as the valve closes, potentially leading to leaflet injury, inflammation and endothelial dysfunction. Accordingly, reducing hypertension might slow AS progression in addition to its other cardiovascular benefits (52,53). Related to this, higher resting heart rate may be associated with a faster progression of aortic stenosis, making it a potential therapeutic target (54).
Future research to identify additional targets and therapies.
Beyond use of established preclinical animal models, recent omics efforts that leverage human tissue and exquisite molecular phenotyping are particularly promising for identifying novel therapeutic targets (55). Additionally, meta-analyses or retrospective studies in large databases with reliable medication and serial quantitative echocardiographic data could provide hypothesis-generating insights regarding existing medical therapies associated with slower progression of AS to be tested in prospective RCTs.
Identification of Individuals with AS and Rate of Progression
An Unmet Need: Identification of Individuals with AS
An echocardiogram is indicated for diagnosis of AS, but only in individuals with signs or symptoms suggestive of AS (5), which usually results in diagnosis of AS at a late stage of disease. There is variability in whether cardiac auscultation is performed during a clinical encounter and it is neither sensitive nor specific for diagnosis of AS (56–60). Using auscultation, general practitioners had a sensitivity of 44% and specificity of 69% for detecting significant valvular heart disease; cardiologists were no better with a sensitivity of 31% and specificity of 81% (61). Among individuals ≥65 years of age without a prior diagnosis of valvular heart disease (OxValve cohort, n=2500), systematic echocardiography identified 51% with mild or more left-sided valvular heart disease or moderate or severe right-sided valvular heart disease, including 6.4% with significant (moderate or more) valvular heart disease. For AS specifically, based on the OxValve cohort and results from the Cardiovascular Health Study, AS is undetected in 1.3–2% of asymptomatic individuals >65 years of age without suspected valvular heart disease, which translates to approximately 1 million adults with undetected AS in the United States (4,62,63). Moreover, ascertainment of symptoms attributable to AS is not straightforward in older adults; early symptoms of heart failure (e.g. dyspnea on exertion) may be dismissed as deconditioning or age-related and only further evaluated when they are more overt and severe (e.g. orthopnea). The current approach to diagnosis of AS represents an important barrier to identification of patients with early stage AS who would be appropriate candidates for medical therapy trials.
Better tools to detect and diagnose early-stage AS are urgently needed. Although systematic screening with a complete echocardiogram in all individuals over 65 years of age, for example, would likely be cost prohibitive and strain capacity, point-of-care ultrasound (POCUS) could serve as a stethoscope-extender to provide a screening look to detect AS to be followed by a more detailed, complete examination in selected patients (64–67). Routine reporting of valve calcification observed on chest CT scans performed for other purposes may help identify some patients (68). Deep learning-based algorithms for detecting AS using an electrocardiogram show promise in preliminary studies (69). Investment in studies to identify and to validate reliable, scalable, and cost-effective tools to screen for and detect AS at an early stage of disease in diverse populations is critical to and would be synergistic with efforts to identify and test novel therapies and treatment strategies and eventually apply effective therapies to those who would benefit.
Rate of Progression of AS
Determining the sample size needed to ensure statistical power and defining the appropriate enrollment criteria for clinical trials testing medical therapy aimed at slowing disease progression depend on reliable data on average disease progression rates, variability in those estimates, and factors that affect the rate of disease progression in individual patients. Several prospectively and retrospectively conducted studies have informed current understanding of the rate of AS progression (22,70–94) (Table 1 and Supplementary Table 1). These studies typically have enrolled asymptomatic patients with mild to moderate AS and described the rates of progression of AS in all-comers, specific clinical subgroups, or in the setting of medical therapy interventions, such as cholesterol-lowering therapy with HMG-CoA reductase inhibitors (i.e. statins) or agents targeting vascular calcification (12,22,49,74,75). Severity and progression of AS has been defined by several key parameters, including aortic valve area (AVA), peak transvalvular velocity (Vpeak), and mean transvalvular pressure gradient by Doppler echocardiography. These studies show heterogeneous rates of AS progression and associated variance with an annualized increase in Vpeak ranging from 0.08 and 0.0.40 m/s/y and reduction in AVA between −0.03 and −0.16 cm2/y. Annualized rates of change in AVC score derived by CT also vary between patient populations (74,78–83,85,95).
Table 1.
Prospective Studies Evaluating Progression of Aortic Stenosis Severity over Time
| First Author | Year | N | Mean follow-up when available (years) | Baseline population entry criteria and study design | Modality to assess progression | Baseline aortic valve measurements (and % with mild, moderate, and severe when available) | Annualized progression rate of AS severity (AVA, Vpeak, ΔP, AVC score) |
|---|---|---|---|---|---|---|---|
| Otto et al. (70) | 1989 | 42 | 1.7 | Echocardiographic evidence of AS (including symptomatic and asymptomatic patients). Vpeak ≥2.6m/s | Echocardiography | Vpeak: 3.7 m/s ΔPmean: 35 mmHg |
AVA: −0.1 cm2/y Vpeak: 0.36 ± 0.31 m/s/y ΔPmean: 8 (−7–23) mmHg/y |
| Faggiano et al. (71) | 1992 | 45 | 1.5 | Physical exam and echocardiographic evidence of AS. Vpeak ≥2.5m/s | Echocardiography | AVA: 0.75 ± 0.3 cm2 Vpeak: 4.0 ± 0.7 m/s ΔPmax: 64 ± 30 mmHg Moderate (AVA): 16% Severe (AVA): 84% |
AVA: −0.1 ± 0.13 cm2/y Vpeak: 0.4 ± 0.3 m/s/y ΔPmax: 15 ± 10 mmHg/y |
| Peter et al. (72) | 1993 | 49 | 2.7 | Physical exam findings of AS and initial peak pressure gradient ≥16 mmHg on echocardiography | Echocardiography | ΔPmax: 38 ± 15 mmHg | ΔPmax: 11 ± 11 mmHg/y |
| Otto et al. (73) | 1997 | 123 | 2.5 | Asymptomatic, physical exam and echocardiographic evidence of AS. Vpeak ≥2.5 m/s | Echocardiography | AVA: 1.3 ± 0.5 cm2 Vpeak: 3.6 ± 0.6 m/s ΔPmean: 29 ± 11 mmHg |
AVA: −0.12 ± 0.19 cm2/y Vpeak: 0.32 ± 0.34 m/s/y ΔPmean: 7 ± 7 mmHg/y |
| Cowell et al. (74) | 2005 | 155 | 2.1* | Echocardiographic evidence of calcific AS (Vpeak ≥2.5 m/s). RCT (SALTIRE; Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression)) |
Echocardiography and CT | Placebo: AVA: 1.02 ± 0.41 cm2 Vpeak: 3.45 ± 0.67 m/s ΔPpeak: 49.5 ± 19.5 mmHg AVC: 6621 (3037–9575) AU Statin: AVA: 1.03 ± 0.4 cm2 Vpeak: 3.39 ± 0.62 m/s ΔPpeak: 47.8 ± 17.4 AVC: 5424 (2750–9689) AU |
All Patients : AVA: −0.081 ± 0.107 cm2/y Vpeak: 0.201 ± 0.208 m/s/y ΔPpeak: 6.52 ± 7.24 mmHg/y AVC: 1608 ± 1865 AU/y Placebo: AVA: −0.083 ± 0.107 cm2/y Vpeak: 0.203 ± 0.208 m/s/y ΔPpeak: 6.56 ± 7.10 mmHg/y AVC: 1648 ± 1790 AU/y Statin: AVA: −0.079 ± 0.107 cm2/y Vpeak: 0.199 ± 0.210 m/s/y ΔPpeak: 6.48 ± 7.43 mmHg/y AVC: 1564 ± 1956 AU/y |
| Rossebo et al. (22) | 2008 | 1873 | 4.4* | Echocardiographic evidence of mild to moderate AS (Vpeak 2.5–4 m/s) without symptoms RCT (SEAS; Simvastatin and Ezetimibe in Aortic Stenosis) |
Echocardiography | Placebo: AVA: 1.27 ± 0.46 cm2 Vpeak: 3.10 ± 0.54 m/s ΔPmean: 23.0 ± 8.7 mmHg Statin/ezetimibe: AVA: 1.29 ± 0.48 cm2 Vpeak: 3.09 ± 0.55 m/s ΔPmean: 22.7 ± 8.8 mmHg |
Placebo: AVA: −0.03 ± 0.1 cm2/y Vpeak: 0.16 ± 0.01 m/s/y ΔPmean: 2.8 ± 0.1 mmHg/y Statin/ezetimibe: AVA: −0.03 ± 0.1 cm2/y Vpeak : 0.15 ± 0.01 m/s/y ΔPmean: 2.7 ± 0.1 mmHg/y |
| Chan et al. (75) | 2010 | 269 | 3.5* | Echocardiographic evidence of mild to moderate AS (Vpeak 2.5–4.0m/s) without symptoms. RCT (ASTRONOMER; Aortic Stenosis Progression Observation: Measuring the Effects of Rosuvastatin) |
Echocardiography | Placebo: AVA: 1.56 ± 0.70 cm2 Vpeak: 3.19 ± 0.42 m/s ΔPmean: 23.1 ± 7.6 mmHg Statin: AVA: 1.49 ± 0.71 cm2 Vpeak: 3.16 ± 0.42 m/s ΔPmean: 22.5 ± 7.6 mmHg |
Placebo: AVA: −0.08 ± 0.21 cm2/y ΔPmean: 3.9 ± 4.9 mmHg/y Statin: AVA: −0.07 ± 0.15 cm2/y ΔPmean: 3.8 ± 4.4 mmHg/y |
| Hekimian et al. (76) | 2013 | 110 | 1 | Echocardiographic evidence of at least mild AS (ΔPmean ≥10 mmHg and aortic valve structural changes consistent with AS) without symptoms, and enrolled in the COFRASA study. Echocardiography follow-up completed at 1 year. | Echocardiography | Vpeak: 3.07 ± 0.82 m/s ΔPmean: 24 ± 16 mmHg |
Vpeak: 0.21 ± 0.30 m/s/y ΔPmean: 4 ± 6 mmHg/y |
| Kearney et al. | 2013 | 239 (147 included in progression analysis) | 6.5 | Patients treated at a single Australian tertiary university with baseline AS (ΔPmean >10 mmHg); patients with at least 2 echocardiograms >6 months apart were included in progression analysis | Echocardiography | Progression analysis cohort: ΔPmean: 21 ± 11mmHg Mild: 67% Moderate: 23% Severe: 10% |
Progression analysis cohort: AVA: −0.11 ± 0.20 cm2/y ΔPmean: 5.0 ± 5.2 mmHg/y Mild: AVA: −0.16 ± 0.20 cm2/y ΔPmean: 3.7 ± 4.0 mmHg/y Moderate: AVA: −0.11 ± 0.21 cm2/y ΔPmean: 6.4 ± 5.1 mm Hg/y Severe: AVA: −0.02 ± 0.12 cm2/y ΔPmean: 10.2 ± 7.7 mm Hg/y |
| Capoulade et al. (77) | 2015 | 183 | 2.5 | Echocardiographic evidence of at least mild AS (Vpeak >2 m/s), without symptoms, enrolled in the PROGRESSA study with 1-year follow-up and laboratory data (Lp-PLA2) available | Echocardiography | AVA: 1.26 ± 0.29 cm2 Vpeak: 2.80 ± 0.60 m/s ΔPmean: 19.0 ± 9.0 mmHg Mild (Vpeak): <3.0 m/s: 29% Moderate (Vpeak): 3.0 to 3.9 m/s: 29% Severe (Vpeak): ≥4.0 m/s: 4% |
All patients: Vpeak (LP-PLA2 <11.4): 0.12 ± 0.02 m/s/y Vpeak (LP-PLA2 >11.4): 0.17 ± 0.03 m/s/y Mild: Vpeak (LP-PLA2 <11.4): 0.09 ± 0.02 m/s/y Vpeak (LP-PLA2 >11.4): 0.16 ± 0.02 m/s/y Moderate-Severe: Vpeak (LP-PLA2 <11.4): 0.19 ± 0.04 m/s/y Vpeak (LP-PLA2 >11.4): 0.20 ± 0.06 m/s/y |
| Nguyen et al. (78) | 2016 | 203 | 3.2 | Echocardiographic evidence of at least mild AS without symptoms, enrolled in COFRASA/GENERAC, with echocardiography and CT at baseline (and yearly thereafter) and at least 2-year follow-up | Echocardiography and CT | AVA: 1.39 ± 0.37 cm2 Vpeak: 3.03 ± 0.65 m/s ΔPmean: 23 ± 11 mmHg AVC: 1168 ± 984 AU Mild: 47% Moderate: 44% Severe: 9% |
AVA: −0.08 ± 0.07 cm2y Vpeak: 0.17 ± 0.16 m/s/y ΔPmean: 3 ± 4 mmHg/y AVC: 218 ± 226 AU/y |
| Tastet et al. (95) | 2017 | 101 | 2 | Echocardiographic evidence of at least mild AS (Vpeak >2 m/s), without symptoms, enrolled in the PROGRESSA study, and with follow-up CT at 2 years | Echocardiography and CT | No systolic hypertension: Vpeak: 2.8 ± 0.5 m/s ΔPmean: 18 ± 7 mmHg AVC: 595 (339–1212) AU Systolic hypertension: Vpeak: 2.8 ± 0.4 m/s ΔPmean: 17 ± 7 mmHg AVC: 665 (406–1096) AU |
No systolic hypertension: AVC: 78.5 (29–151.5) AU/y Systolic hypertension: AVC: 185 (63–412) AU/y |
| Tastet et al. (79) | 2017 | 323 | 2.3 | Echocardiographic evidence of AS with echocardiography and MDCT within 3 months and second echocardiographic exam within ≥6 months | Echocardiography and CT | Women: ΔPmean: 24 ± 13 AVC: 531 (224–1098) Men: ΔPmean: 22 ± 10 AVC: 1019 (571–1921) |
Women with non-severe baseline AVC: ΔPmean: 2.8 (0.5–5.6) mmHg/y Women with severe baseline AVC: ΔPmean: 6.4 (3.4–16.3) mmHg/y Men with non-severe baseline AVC: ΔPmean: 2.0 (0.5–4.6) mmHg/y Men with severe baseline AVC: ΔPmean: 5.6 (2.4–8.8) mmHg/y |
| Tastet et al. (80) | 2018 | 159 | 2 | Echocardiographic evidence of at least mild AS (Vpeak >2 m/s), without symptoms, enrolled in the PROGRESSA study, and with laboratory data (including ApoB and ApoA-I levels) and 2-year follow-up data available | Echocardiography | All Patients: AVA: 1.25 ± 0.25 cm2 Vpeak: 2.7 ± 0.5 m/s ΔPmean: 18 ± 8 mmHg ApoB/ApoA-I <0.62: AVA: 1.23 ± 0.26 cm2 Vpeak: 2.7 ± 0.4 m/s ΔPmean: 17 ± 7 mmHg ApoB/ApoA-I ≥0.62: AVA: 1.28 ± 0.22 cm2 Vpeak: 2.8 ± 0.5 m/s ΔPmean: 18 ± 9 mmHg |
ApoB/ApoA-I <0.62: Vpeak: 0.08 (0.005–0.18) m/s ApoB/ApoA-I ≥0.62: Vpeak: 0.15 (0.0045–0.245) m/s |
| Kubota et al. (81) (81–85) | 2018 | 296 | 3 | Echocardiographic evidence of at least mild AS (ΔPmean ≥10 mmHg and aortic valve structural changes consistent with AS) without symptoms, enrolled in COFRASA/GENERAC, with echocardiography and CT at baseline (and yearly thereafter) and at least 1-year follow-up | Echocardiography and CT | AVA: 1.4 ± 0.4 cm2 Vpeak: 3.13 ± 0.76 m/s ΔPmean: 25 ± 16 mmHg AVC: 1339 ± 1217 AU Mild: 44% Moderate: 43% Severe: 13% |
AVA: −0.09 ± 0.09 cm2/y Vpeak: 0.18 ± 0.18 m/s/y ΔPmean: 4 ± 4 mmHg/y AVC: 223 ± 301 AU/y |
| Tastet et al. (82) | 2019 | 303 (CT N=220) | 2.6* (echocardiography) and 2* (CT) | Echocardiographic evidence of at least mild AS (Vpeak ≥2.0 m/s and/or AVA <2.0cm2) and with available repeat echocardiography or CT within >6 months. Prospective and retrospective data collection |
Echocardiography and CT | Warfarin: Vpeak: 2.5 ± 0.5 m/s AVC: 395 (63–991) AU DOAC: Vpeak: 2.3 ± 0.5 m/s AVC: 322 (64–820) AU No Anticoagulation: Vpeak: 2.7 ± 0.4 m/s AVC: 511 (254–1015) AU |
Warfarin: Vpeak: 0.14 (0.06–0.32) m/s/y AVC: 204 (48–317) AU/y DOAC: Vpeak: 0.10 (−0.01–0.20) m/s/y AVC: 66 (11–86) AU/y No Anticoagulation: Vpeak: 0.09 (0.04–0.19) m/s/y AVC: 94 (31–203) AU/y |
| Doris et al. (83) | 2020 | 114 (reproducibility cohort N=33, progression cohort N=81) | 2* | Evidence of aortic stenosis (peak velocity >2 m/s). Reproducibility cohort: repeat echocardiography or CT AVC scanning within 4 weeks. Disease progression cohort: repeat echocardiography, CT or both after at least 1 year. | Echocardiography and CT | Disease Progression Cohort: AVA: 1.1 (0.9–1.4) cm2 Vpeak: 3.4 (2.8–4.1) m/s ΔPmean: 25 (16–36) mmHg AVC: 1339 (553–2422) Mild: 31% Moderate: 41% Severe: 28% |
All Patients: AVA: −0.1 (−0.2–0.0) cm2/y Vpeak: 0.1 (0.0–0.3) m/s/y ΔPmean: 2 (0–4) mmHg/y AVC : 152 (65–375) AU/y Mild: AVA: −0.1 (−0.2–0.0) cm2/y Vpeak: 0.1 (0.0–0.2) m/s/y ΔPmean: 1 (0–2) mmHg/y AVC: 64 (48–134) AU/y Moderate: AVA: −0.1 (−0.1–0.0) cm2/y Vpeak: 0.2 (0.1–0.3) m/s/y ΔPmean: 3 (1–5) mmHg/y AVC: 289 (106–443) AU/y Severe: AVA: 0.0 (−0.1–0.0) cm2/y Vpeak: 0.1 (−0.1–0.2) m/s/y ΔPmean: 3 (0–5) mmHg/y AVC: 342 (163–583) AU/y |
| Tastet et al. (84) | 2020 | 162 | 3* | Echocardiographic evidence of at least mild AS (Vpeak >2 m/s), without symptoms, enrolled in the PROGRESSA study, and with dual energy x-ray absorptiometry (DEXA) available | Echocardiography | Bone mineral density (BMD) tertile 1: Vpeak: 2.76 ± 5.4 m/s BMD tertile 2: Vpeak: 2.73 ± 5.0 m/s BMD tertile 3: Vpeak: 2.77 ± 5.0 m/s |
Overall Vpeak: 0.11 ± 0.18 m/s/y |
| Shen et al. (85) | 2020 | 141 | 3.2 | Echocardiographic evidence of at least mild AS (Vpeak >2 m/s), without symptoms, and enrolled in the PROGRESSA study. | Echocardiography and CT | Tricuspid: AVA: 1.25 (1.10–1.43) cm2 Vpeak: 2.58 (2.35–2.86) m/s ΔPmean: 14.4 (12.5–18.0) mmHg AVC: 571 (320–938) AU Bicuspid: AVA: 1.23 (1.04–1.45) cm2 Vpeak: 2.68 (2.49–3.02) m/s ΔPmean: 17.0 (14.7–21.8) mmHg AVC: 411 (95–1280) AU |
Tricuspid: AVA: −0.05 (−0.095–0.0) cm2/y Vpeak: 0.085 (0.015–0.175) m/s/y ΔPmean: 1.3 (0.2–2.4) mmHg/y AVC: 93.5 (39.5–163) AU/y Bicuspid: AVA: −0.04 (−0.095–0.005) cm2/y Vpeak: 0.08 (0.00–0.20) m/s ΔPmean: 0.9 (−0.35–2.9) mmHg/y AVC: 64 (4.5–228.5) AU/y |
| Pawade (49) | 2021 | 150 | 2 | Echocardiographic evidence of AS (Vpeak >2.5 m/s and grade 2–4 AVC on echocardiography) Prospective, RCT (SALTIRE II; Study Investigating the Effect of Drugs Used to Treat Osteoprosis on the Progression of Calcific Aortic Stenosis) |
Echocardiography, CT, and 18F-NaF positron emission tomography | Vpeak: 3.36 (2.93–3.82) m/s ΔPmean: 23 (18–32) mmHg AVC: 1152 (655–2065) AU |
Placebo : AVA: −0.065 (−0.145– −0.02) cm cm2/y Vpeak: 0.165 (0.06–0.295) m/s/y ΔPmean: 2 (0.05–4.5) mmHg/y: AVC: 177 (38–337.5) AU/y Denosumab : AVA: −0.07 (−0.125– −0.04) cm cm2/y Vpeak: 0.245 (0.075–0.375) m/s/y ΔPmean: 3 (0.05–6) mmHg/y: AVC: 171.5 (99–402) Alendronic Acid : AVA: −0.045 (−0.125– −0.02) cm cm2/y Vpeak: 0.22 (0.055–0.315) m/s/y ΔPmean: 3 (0.05–4) mmHg/y: AVC: 163 (69–406.5) |
Modified from ACC/AHA Guideline for Management of Valvular Heart Disease by Otto et al (5).
Median follow-up.
Data are mean ± SD, median (IQR), or % (95% CI) as available. AS = aortic stenosis; AVA = aortic valve area; AVC = aortic valve calcification; CT = computed tomography; ΔP = transaortic pressure gradient; IQR = interquartile range; RCT = randomized clinical trial; SD = standard deviation; Vpeak = peak aortic valve velocity.
The available data suggest variability in terms of AS progression, with rates depending on severity at initial assessment and presence of other risk factors that contribute to rapid progression of disease. Limitations common to many prior studies include variable reporting of baseline and follow-up AS assessment by echocardiographic and CT measures, lack of standardized follow-up imaging and clinical protocols between studies, small sample sizes, and limited long-term follow-up duration extending beyond 5 years after baseline measurements. Consequently, robust estimates regarding average rates of progression (and associated variances of these rates) are lacking, and as such, power estimates for designing medical therapy trials with imaging endpoints are inherently limited. Future investigations will need to focus on standardizing eligibility criteria and imaging assessments (both echocardiography and CT) to allow for comparison of findings between studies to better inform estimates regarding progression of disease severity. Furthermore, larger studies and longer-term follow-up are required for annualized estimates of rates of progression of AS beyond the first few years of enrollment. Improvements in study design will help better define the natural history of AS and identify the patient populations at highest risk for rapid progression of disease.
AS Medical Therapy Trial Enrollment Population and Eliciting the Patient Voice
Trial Enrollment Population
The primary goal of medical therapy targeting the progression of valve obstruction is to delay or, ideally, obviate the need for AVR. However, despite completion of several RCTs evaluating the effects of medical therapy interventions for patients with AS (12,22,49,74,75), no effective therapies have been identified. In order to optimize the efficiency and increase the likelihood of successful matching of therapy with the appropriate target population, several eligibility criteria need to be considered and tailored to the specific intervention being evaluated, including severity of AS, age, sex, valve morphology, symptom status, comorbidities, and life expectancy (Table 2). Co-morbid conditions such as cardiomyopathy, other forms of valve disease, coronary artery disease, arrhythmias and other cardiovascular diseases may present challenges in assessing the efficacy of the medical therapies to treat AS because attributing study endpoints to AS versus comorbid conditions may not be possible.
Table 2.
Proposed Eligibility Criteria Considerations for Medical Therapy Trials Targeting the Valve in Individuals with AS.
| Eligibility Criteria | Considerations |
|---|---|
| Aortic valve-related | |
| Morphology (85,99) | • Progression rates and drug targets aimed at preventing progression of disease may differ between patients with a bicuspid or trileaflet aortic valve. |
| Severity of aortic stenosis (3,5,99,101) | • Mild to moderate AS is the most appropriate target for medical therapy trials. • Due to faster disease progression, it will be easier to detect a treatment effect in those with moderate AS, but mild AS may be more responsive to therapy to slow or halt disease progression. • Aortic valve sclerosis (pre-AS) is difficult to detect, progresses variably and often slowly, and difficult to measure reliably, making it costly and less efficient to study (at least initially). • Patients at later/severe stages of disease will be less likely to benefit from medical interventions to slow progressive valve obstruction. |
| Sex (96–98) | • Males exhibit more calcification in the leaflets, whereas females tend to have more fibrosis |
| Mixed aortic valve disease | • Significant concomitant aortic regurgitation may confound symptoms or mask potential benefits of AS therapies. |
| Severity of symptoms (5) | • Patients with severe symptoms are more likely to have hemodynamically significant valve obstruction and are less likely to benefit from medical therapy targeting valve obstruction. |
| Planned aortic valve intervention | • Patients with anticipated aortic valve replacement in the near future should generally be excluded from these trials. |
| Cardiomyopathy | • Temporal variability in myocardial function may confound measurement of the effect of therapy on hemodynamic progression (e.g. Vpeak may be influenced by variability in stroke volume rather than changes in valve obstruction). • Symptoms in patients with impaired myocardial function may be difficult to attribute to the valve versus underlying cardiomyopathy. |
| Concomitant cardiovascular comorbidities | |
| Other valvular heart disease (5) | • Assessing symptoms due to AS is challenging in the setting of other significant valvular lesions. • Patients may be less likely to benefit from medical therapy interventions for AS when other valve disease is present. |
| Coronary artery disease (5) | • Assessing symptoms due to AS may be challenging in the setting of significant coronary artery disease. • Because of overlap in the pathobiology of AS and atherosclerotic disease, patients with coronary artery disease may already be on medications affecting a pathway being targeted by an investigational therapy for AS. |
| Cerebrovascular disease | • Prior cerebrovascular disease may account for thrombotic complications in patients with AS. |
| Atrial arrhythmias (atrial fibrillation/atrial flutter) | • Thromboembolic events in patients with atrial arrhythmias might not be due to the study treatment. |
| Age (96) | • Depending on the therapeutic target, investigators may need to consider enrolling younger versus older patient populations. • Age cut-offs for studies should be determined based on mechanism of action and anticipated time period needed to observe benefit of the medical intervention for AS. |
| Frailty, limited life expectancy, or severe multimorbidity or end-stage organ dysfunction (5) | • Patients with significant frailty, limited life expectancy, or severe multimorbidity or end-stage organ dysfunction may be less likely to survive study duration or experience benefits attributed to medical therapies for AS. • Significant end-organ dysfunction (e.g. chronic kidney disease) may influence pathophysiology and the degree to which a prospective therapy may effectively slow AS progression. |
AVR is the standard of care for symptomatic patients with severe AS; thus, medical therapy trials will focus on patients who are not yet experiencing symptoms related to their AS. Until effective therapies are identified, enrolling individuals with aortic sclerosis is unlikely to provide useful results because progression from sclerosis to stenosis occurs in only a subset of patients over many years, thus requiring larger trial sample sizes and a longer study duration. On the opposite end of the spectrum, once AS has become more end-stage (severe or near severe), medical therapy to slow progression of valve obstruction is unlikely to be beneficial unless such a therapy is anticipated to rapidly reverse or halt calcification and hemodynamic progression.
Even within mild to moderate stages of AS, pathobiology is influenced by relative severity of AS, sex, age, and valve morphology, each of which may influence the likelihood of a given therapy slowing disease progression. Less severe AS is characterized more by inflammation whereas later stage disease is more fibro-calcific. Several studies have consistently demonstrated an association between sex and calcium burden in aortic valve leaflets. Females have lower amounts and density of calcification and more fibrosis in their leaflets than men for a given severity of AS (96–98). Older age, too, has been linked to greater leaflet calcification (96). Interestingly, the number of cardiac risk factors was associated with faster AS progression in patients with a bicuspid but not trileaflet valve (99). After adjustment for age and sex, a bicuspid valve may be associated with faster disease progression, but results are mixed (85,99).
Beyond accounting for the influence of the factors above on likelihood of responsiveness to a specific therapy, enriching study populations for patients at-risk for rapid progression of disease will improve efficiency and reduce the costs of these studies. The strongest predictors of disease progression are baseline assessments of valve calcification and hemodynamic severity (99–101). Patients with moderate AS progress faster than patients with milder disease, thereby making it easier to demonstrate a treatment effect in those with moderate disease. While historic examples of medical therapy trials have used echocardiographic or CT-based imaging findings to help select potential candidates for enrollment and follow changes in these parameters in response to study interventions, novel molecular imaging modalities may provide additive insights regarding aortic valve pathobiology and predisposition for faster progression, including information on processes that drive inflammation, remodeling, and calcification, all of which are critical components of AS (102). To date, 18F-sodium fluoride (18F-NaF) and 18F-fluorodeoxyglucose (18F-FDG) have been evaluated for their potential roles in evaluating calcification and inflammation in the setting of AS (103,104); 18F-NaF has demonstrated most potential to identify active disease and patients at risk for progression.
Other clinical factors and biomarkers associated with faster progression could serve as enrichment criteria for trials, including metabolic syndrome (105), diabetes mellitus (92), vitamin K antagonists (82), Lp-PLA2 levels (77), apoB/apoA-I ratio (80), Lp(a) (106), and oxidized phospholipids on apolipoprotein B-100 (106). A growing body of evidence suggests that a number of genetically-influenced factors may contribute to development and progression of AS (23,107,108). Lastly, machine learning algorithms have demonstrated great potential to use large-scale datasets to augment available risk-stratification and prediction models, as reported in a recent analysis which suggested the possibility for identifying distinct phenotypes of AS severity (109,110). Such strategies may allow for selection or enrichment of patients at highest risk for rapid disease progression.
Eliciting the Patient Voice
While shared decision making (SDM), including the process of identifying individual patient goals for therapy as defined by patients themselves, is recommended in current guidelines (5), this process is often quite perfunctory and is not widely implemented in a comprehensive manner with the use of specific skillsets and decision aids. Specifically, in patients with AS, little is known about which treatment goals are most important, how patients compare their options, and how their own preferences are integrated into decision making (111). It will be essential to include patients in the design of clinical trials of early medical interventions aimed to prevent progression of AS. Several patient advocacy organizations (e.g. Heart Valve Voice US, Mended Hearts) have developed programs to train patients with heart valve disease to participate in the design and conduct of research. Benefits of patient engagement include early understanding of which patient populations to target (i.e. stage of disease progression); patient views on medical interventions; clarity on potential implementation hurdles for trial enrollment; and impact on diverse populations. Furthermore, when designing clinical trials, patient input early in the process can assist in selecting trial outcomes that are meaningful to the patients for whom the intervention is intended; identifying trial operations acceptable to patients (i.e. number and type of study visits); and promoting recruitment and retention of diverse patients through patient-oriented materials (112,113). Interventions such as these are shown to improve patient engagement in trials,(113) improve adherence to study protocols, and are often cost-effective (112).
The Food and Drug Administration (FDA) has focused for several years on refining recommendations for the incorporation of the patient voice in both study design and drug and device approval. In 2016, the FDA published a Patient Preference Information (PPI) white paper (114), targeted at investigators and industry leading clinical trials. The FDA encourages submission of PPI and provides guidance on the conduct of PPI studies. PPI is most relevant in decisions that are “preference sensitive” as in the case of AS. Preference sensitive decisions are when: (1) there is more than one valid treatment option; (2) data supporting one option over another is limited; and (3) patient goals and preferences vary in how they weigh risks and benefits of the choices.
Specifically, PPI can assist investigators and other stakeholders in understanding how patients’ conceptualize the choice of a medical or device therapy; how they prioritize or weigh risks and benefits; and the extent to which these preferences may vary across diverse populations, particularly those traditionally under-represented in cardiovascular clinical trials (e.g., women, African American, Latinx populations, among others).
One of the central issues in which PPI may be informative when considering medical therapy is the willingness of the affected patient population to consider taking a medication for prevention of disease progression. Notably, barriers exist for well-established medications: 50% of patients are non-adherent to their medications in primary prevention for cardiovascular disease (115). In primary prevention for heart disease and stroke, researchers identified the burden of taking multiple medications and focused intervention studies on the role of polypill (116). Yet, when examining the effectiveness of such as intervention, researchers required 80% adherence prior to being randomized in the study; 1 in 4 patients initially screened did not go on to the randomized study.
Interventions that require patient action are inherently complex and related to patients’ unique context. Indeed, medication adherence trends are impacted by race and gender (117,118). In the area of stroke prevention in the setting of atrial fibrillation, disparities in adherence to medications are seen in some studies by gender, but not differing levels of health literacy (119). Poor adherence may be mitigated when the medication regimen is simplified, as in once-a-day dosing and no requirement for monitoring (120).
It is thus imperative to plan for PPI studies in trial design for studies testing therapies for AS, as patients will be weighing a trade-off of a) adhering to a medication for years to avoid (or delay the need for) an AVR versus b) avoiding an additional medication and face the decision of AVR as “bail out” therapy if their AS becomes severe. There remains uncertainty as to how many patients with mild AS will go on to need AVR with close follow-up (121) and how patients view this uncertainty will be important to understand. Several additional considerations to PPI studies in this context will need to be considered (Table 3).
Table 3.
Considerations for Patient Goals and Preferences for Medical Therapy Trials in AS
| Patient population |
| • Age • Comorbidities • Historically underrepresented patients receiving therapy or participating in trials (e.g. women, minorities, rural patients) • Type of valve disease (bicuspid vs. trileaflet aortic valve) • Stage of disease (sclerosis vs. mild vs. moderate AS) • Native valve disease only vs. post-AVR |
| Trade-offs |
| • Medication for an asymptomatic condition versus avoiding or delaying AVR • Varying types of side effects of medication: physical symptoms, inconvenience, economic • Uncertainty regarding whether AVR will eventually be indicated during remaining life years |
| Implementation barriers to trial enrollment and strategies to address |
| • Patient perception of randomization vs. observational study design • Reaching underserved populations • Trust in medical system • Education on disease process • Consistent methodology for measuring and incorporating patient preferences • Drug delivery (daily vs. multiple doses, oral vs. subcutaneous vs. intravenous) |
Abbreviations: AS, aortic stenosis; AVR, aortic valve replacement
Trial Endpoints and Assessment
Rationale for Imaging Endpoints Assessing Disease Progression
AS is a slowly developing condition, that is often monitored in the clinic for many years if not decades. Traditional clinical endpoints, like time to AVR or death, accrue over similarly long periods. RCTs based on clinical endpoints are therefore challenging, requiring very large patient populations and long durations of follow-up. This makes them expensive and unattractive to pharmaceutical companies and research funders. An alternative strategy is therefore required, at least initially.
Progressive valve narrowing is the hallmark of AS. Without it, patients do not develop left ventricular decompensation, symptoms or clinical events, nor are they considered for AVR. Given this tight linkage, any therapy that can effectively slow disease progression should also delay or obviate AS-related clinical events; in this sense, “disease progression” (i.e. progressive valve narrowing) is ostensibly a face-value surrogate for clinical events/outcomes. Accordingly, to the degree that an imaging modality can accurately measure disease progression, it seems warranted to perform RCTs testing medical therapy for AS with imaging endpoints evaluating progression of valve disease as the primary efficacy endpoint. Because imaging endpoints are measured on a continuous scale and can be assessed longitudinally (allowing for repeated measures analyses), statistical power for detecting an effect is substantially increased (with resulting decreases in sample size) compared with dichotomous endpoints (122). Accordingly, imaging endpoints would facilitate more rapid, efficient, and cost-effective identification of the most promising therapeutic candidates and discarding of the ineffective.
Potential Imaging Endpoints
Determining the optimal imaging endpoint to track disease progression and response to therapy depends on numerous factors including expense, precision, radiation exposure and availability. Importantly, when powering an RCT, two key imaging factors determine the required sample size and duration of follow-up: (1) the measurement error (noise) of the specific technique; and (2) its average progression over time. This leads to the concept of the progression-to-noise ratio (also known as Cohen’s statistic) (83).
International clinical guidelines recommend assessing AS severity with echocardiography as well as CT aortic valve calcium scoring (CT-AVC) when echo assessments are discordant (5,123). Currently these two imaging modalities are also the most attractive imaging endpoints for RCTs. In addition, novel assessments including contrast CT angiography (124) and molecular assessments of disease activity, such as 18F-NaF PET, (104) are promising, each with their own potential advantages and disadvantages (Table 4).
Table 4.
Imaging endpoints and tools for assessing aortic stenosis progression
| Imaging modality | Measure(s) of AS severity | Advantages | Limitations |
|---|---|---|---|
| VALVE | |||
| Echocardiography |
Hemodynamic assessment - Maximum velocity - Mean gradient - Aortic valve area |
- Most widely used in clinical practice - No radiation exposure - Relatively inexpensive - Main imaging method used to guide AVR |
- Poor progression to noise ratio - Relatively large patient sample sizes required to demonstrate a treatment effect - Most measures dependent upon LV flow (LV flow may change on follow-up) |
| CT aortic valve calcium scoring (CT-AVC) |
Structural assessment - Quantification of the aortic valve calcium burden |
- Favorable progression to noise ratio - Relatively small patient samples sizes to demonstrate an effect - Flow independent measurements - Widely available at most centers - Low per scan costs |
- Radiation exposure - Ignores the contribution of fibrosis to aortic stenosis severity and progression |
| Contrast CT angiography |
Structural assessment - Quantification of the aortic valve fibro-calcific burden |
- Equipment is widely available at most centers - Assesses the burden of both valve calcification and fibrosis - Flow independent measurement - Widely available at most centers |
- Novel technique that is currently not well validated. - Time consuming image analysis methodology - Progression to noise ratio not yet established - Requires contrast administration |
| PET/CT |
Disease activity assessment - 18F-NaF uptake as marker of calcification activity |
- Assessment of calcification activity – a pathophysiologic mechanism that could be targeted by therapeutics - Highly reproducible - As an assessment of disease activity may change more quickly than structural or hemodynamic assessments |
- Expensive - Radiation exposure - Only available at specialized centers - Ignores the contribution of fibrosis to aortic stenosis severity and progression |
| MYOCARDIUM | |||
| Echocardiography | - LV mass - Global longitudinal strain - Ejection fraction |
- Widely used in clinical practice - No radiation exposure - Low per scan costs - Main imaging method used to guide AVR |
- Poor precision in measurement - Progression to noise not known - Relatively high number of patients required to demonstrate a treatment effect |
| CMR | - LV Mass - Myocardial fibrosis - Ejection fraction |
- No radiation exposure - Improved precision in measurements compared to echocardiography - Relatively small number of patients required to demonstrate a treatment effect |
- Higher per scan costs than echo but lower sample sizes required - Requires contrast administration |
Echocardiography is the first line clinical imaging test for AS with decisions to proceed to AVR made on the basis of AVA and transvalvular gradients (5). For these reasons, echocardiography is a key endpoint in potential future trials. However, hemodynamic echocardiographic assessments are sensitive to small changes in flow status and misalignment of the Doppler beam with the aortic valve jet. Consequently, they are hampered by relatively poor measurement precision and progression-to-noise ratio (83).
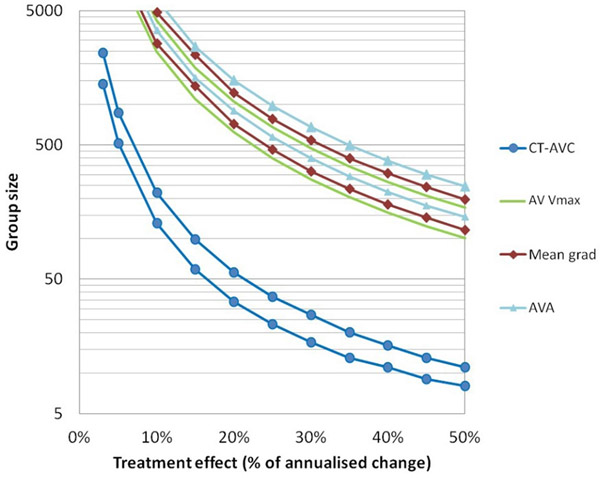
CT-AVC has emerged as an alternative assessment of AS severity, providing a structural, flow-independent, assessment of the calcium burden in the valve. Using sex-specific thresholds, CT-AVC demonstrates good accuracy versus echocardiography and provides powerful prognostic information (100,101,125). In the context of clinical trials, the progression-to-noise ratio is 4-fold better than for echocardiography, indicating a superior ability to detect changes in disease severity over time. Indeed, recent data suggest that >10-fold fewer patients would be required to detect 10%, 20% and 30% treatment effects using CT-AVC (165, 43 and 20 patients, respectively) compared with the best performing echocardiographic assessment (Vpeak: 3142, 787 and 351 patients, respectively) (Figure 2) (83). This makes a strong argument for using CT-AVC alongside echocardiography in phase 2 trials, although the proportion of non-interpretable CT scans (~10–15%) must also be considered in sample size calculations and it should be remembered that CT-AVC does not account for the effects of therapy on valve fibrosis.
Figure 2. Sample sizes for AS medical therapy trials by imaging modality.
Estimates are based upon the measurement error (noise) and the average progression observed for each imaging assessment (progression to noise or Cohens co-efficient). The number of participants required in a study to detect a given treatment effect size at different levels of power are plotted. For each modality an upper bound at 90% power and lower bound at 70% are plotted with α=0.05 for all. Nominal treatment effects up to 50% of the measured annualized progression for each modality are considered. Group size calculations should also consider the proportion of non- interpretable scans that may be encountered. AVA, aortic valve area; AV Vmax, maximum transvalvular velocity; CT-AVC, aortic valve calcification by computed tomography. (reproduced from Doris et al. Heart 2020 (83) Creative Commons Attribution 4.0 Unported (CC BY 4.0) license)
Clinical Event Endpoints for AS Trials
All clinical outcomes or events assessed should be patient-centered insofar as they are meaningful to patients—how they feel, function, or survive—either directly or indirectly. Several such outcomes could be considered for assessment of medical therapies targeting AS, each with potential advantages and disadvantages (Table 5). Beyond patient-centeredness, broadly there are two potential motivations for including a particular clinical feature in a medical therapy trial for AS: (1) the end point is thought to be affected by and related to progression of valve disease (e.g. hospitalization for heart failure, aortic valve intervention); or (2) it is a safety endpoint that is important to include even if not directly related to progressive valve obstruction (e.g. osteoporosis, type 1 myocardial infarction, stroke).
Table 5.
Potential clinical (event) endpoints, including advantages and limitations
| Clinical Endpoints | Details | Advantages | Limitations |
|---|---|---|---|
| AS-RELATED END POINTS | |||
| Aortic Valve Replacement | TAVI or SAVR with or without coronary bypass grafting / aortic root replacement | - Clinical endpoint attributable to the disease process being modified - Easy and accurate to capture – may be available from electronic patient records and data linkage - The major intervention that patients and physicians are keen to avoid |
- Requires large trials and/or prolonged follow-up (∼5–10 yrs) - Optimal timing of AVR not yet established (strategies may differ between institutions and likely to change within the follow up period of future trials) |
| Unplanned aortic stenosis related hospital admission | An unplanned hospital admission with syncope, heart failure, chest pain, or arrhythmia (ventricular arrhythmia or second- or third-degree heart block) attributed to AS.10 | - Clinical endpoint attributable to the disease process being modified | - Requires large trials and/or prolonged follow-up (∼5–10 yrs) - Patients with AS often have comorbid conditions that may account for the clinical events, rather than being due to AS. |
| AS-related death | Death related to aortic stenosis (sudden cardiac death, death due to heart failure, ventricular arrhythmia or AV conduction disease in a patient with severe aortic stenosis) | - Clinical endpoint attributable to the disease process being modified | - Requires large trials and/or prolonged follow-up (∼5–10 yrs) - Adjudication may be challenging in the context of co-morbidities such as coronary artery disease |
| SYMPTOMS, DISABILITY & QUALITY OF LIFE | |||
| Symptoms | Shortness of breath Chest pain Presyncope and syncope |
Important to patients | - Requires large trials and/or prolonged follow up (∼5–10 yrs) - May be attributable to co-morbidities rather than AS |
| Quality of Life Assessments | Kansas City Questionnaire 6-minute walk test |
Important to patients | - Requires large trials and/or prolonged follow-up (∼5–10 yrs) - Changes may be attributable to co-morbidities or frailty rather than AS |
| Disability Free Years | Number of years a patient lives free from disability | Important to patients | - Requires large trials and/or prolonged follow-up (∼5–10 yrs) - Changes may be attributable to co-morbidities or frailty rather than AS |
| SAFETY ENDPOINTS | |||
| Atherothrombotic events | Myocardial infarction Stroke |
Important safety data for drugs without a safety profile | - Requires large trials and/or prolonged follow-up (∼5–10 yrs) - Events are related to atherosclerosis not AS |
| Coronary Revascularization | Coronary artery bypass grafting Percutaneous coronary intervention |
- Important safety data for drugs without a safety profile - Decisions to proceed with revascularization may be triggered by progression in AS |
- Requires large trials and/or prolonged follow-up (∼5–10 yrs) - Events are related to atherosclerosis not AS |
| Death | All-cause mortality | - Important safety data for drugs without a safety profile - Potentially available from electronic patient records and data linkage - No need for adjudication |
- Requires large trials and/or prolonged follow-up (∼5–10 yrs) - Events may not be related to AS but rather co-morbidity |
The development of composite clinical endpoints that combine sequelae of AS is desirable. Greater power is achieved with more severity levels of the outcome assessment, more times at which it is assessed, and longer follow-up duration. These composite endpoints would include components such as AVR, AS-related hospital admission, and AS-related death. Definitions for the latter two are not well established but can be addressed (Table 5) and outlined in a future consensus document. Non-AS related clinical events (e.g. non-CV death, type 1 myocardial infarction, stroke) could be collected separately to assess the safety of any potential drug, particularly in those without an existing clinical and safety track record. Symptoms and quality of life assessments are important to patients, although symptoms will often reflect co-morbidities rather than AS. Selection of appropriate clinical endpoints will be influenced by the stage of AS enrolled in the trial. For example, if patients with aortic valve sclerosis and mild AS are enrolled quality of life related to valve function is unlikely to deteriorate and clinical endpoints such as death or AVR are unlikely to accrue at a meaningful rate over 2–3 years. In contrast, if patients with moderate AS are enrolled, then imaging endpoints along with patient-centered clinical outcomes should be included as the development of symptomatic severe AS will occur sooner.
Recommended Strategy
Ultimately imaging endpoints need to be agreed upon by multiple stakeholders including the FDA. Given the complementary strengths of echocardiography and CT-AVC, one potentially attractive strategy would be to measure disease progression with both CT-AVC and echocardiography. If an intervention demonstrates efficacy on CT calcium scoring, then further recruitment and follow up can ensue until there is sufficient power to detect an effect on echocardiographic endpoints. Addition of CT angiography to the CT protocol would be ideal to not only provide additional data on disease progression alongside other modalities but to also anticipate the possibility that future studies may demonstrate contrast CT to be the optimal way to track progression of valve pathology, given its ability to assess both calcific and fibrotic valve thickening in an integrated manner.
Given the current lack of any effective medical treatments for AS (large unmet need) and the mechanism by which AS causes clinical events—fundamentally and necessarily through progressive valve narrowing—we propose that a drug that slows AS progression based on imaging endpoints would warrant full FDA approval or at least accelerated approval (126). If full approval based on an imaging endpoint was acceptable, perhaps directional (but not statistically significant) benefits on clinical outcomes would be sufficient to reinforce the efficacy and benefit of the drug in the absence of any concerning safety data. Accelerated approval, however, is “subject to the requirement that the applicant study the drug further, to verify and describe its clinical benefit.” (126) Presumably this would entail demonstrating that the drug also reduces AS-related clinical events (e.g. death, valve intervention, AS-related hospitalization, quality of life). Because such evidence would need to come from a placebo-controlled randomized trial and due to the difficulty of enrolling in such a trial once accelerated approval is given, such a clinical outcome trial would likely need to be in the later stages of follow-up before accelerated approval was granted. Rather than running two separate and sequential trials, the first with an imaging endpoint and the second with a clinical endpoint, the most efficient way to meet the requirement to “verify and describe” the clinical benefit would be to run a single trial with one study population with an earlier (~1–2 year) imaging-based endpoint examining progression and a later endpoint examining clinical outcomes. If there was no beneficial effect of the drug on the imaging endpoint, the trial could be stopped; if the drug slowed AS progression by an imaging endpoint, then accelerated approval could be granted when the trial had reached adequate enrollment and follow-up to ensure that a determination on clinical benefit could be made. Such a trial would also enable investigators to test whether AS progression rate (assessed by imaging) is associated with subsequent clinical outcomes and test whether a drug’s reduction of AS progression rate mediates the benefit of the drug on clinical outcomes. This would reinforce the validity of AS progression as a clinically meaningful surrogate endpoint, thereby potentially allowing full approval (rather than accelerated approval) to be granted to drugs that demonstrate a beneficial effect on AS progression assessed by imaging in future trials. The safety data required by the FDA will likely depend on whether the drug is novel or has some prior track record.
Implementation and Design of Clinical Trials
There are many barriers to the development of an effective medical therapy for AS including a lack of funding partnerships, an international consortium of sites to conduct trials in this population efficiently, and interest from the pharmaceutical industry (11). Many of these challenges may be overcome by engaging key stakeholders to create a clinical trials network with the goal of efficiently designing and testing promising medical therapies to slow the progression of AS (Central Illustration). The power of clinical trial networks have been demonstrated in multiple domains including HF (127), acute lung injury (128), and cardiothoracic surgery (129) to name a few. For instance, since 2008 the NHLBI-funded Heart Failure Clinical Research Network (HFN) has completed 13 multicenter clinical trials which have rapidly informed contemporary HF management and provided strong evidence for the benefits of such clinical trial networks (127).
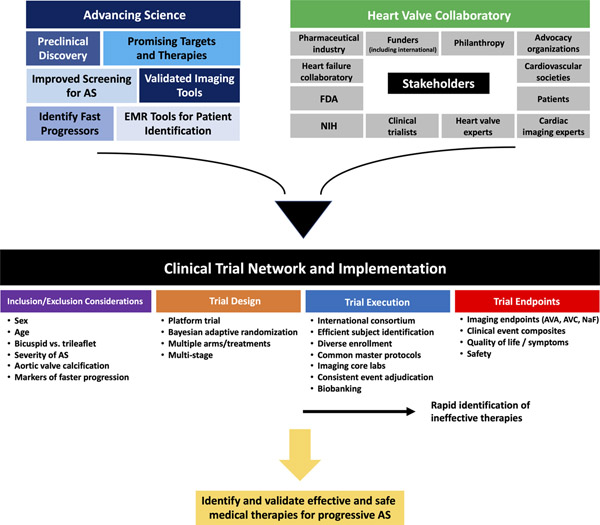
Central Illustration. Over-arching Approach to Evaluate Medical Therapy for Calcific Aortic Stenosis.
Scientific advances in key areas combined with collaboration across multiple stakeholders (facilitated by the Heart Valve Collaboratory) could establish a clinical trial network purpose-fit to implement a platform trial to identify and validate effective and safe medical therapies for progressive aortic stenosis. AS, aortic stenosis; AVA, aortic valve area; AVC, aortic valve calcification; EMR, electronic medical record; FDA, Food and Drug Administration; NaF, sodium fluoride; NIH, National Institutes of Health.
Given the prevalence of AS and the lack of proven medical therapies, there is enormous potential for the development of a clinical trial network in this domain. Such a network could reduce start-up delays and fatigue associated with launching clinical trials and would oversee the development and administration of common master protocols so that new candidate therapies in the pipeline may be tested in a rapid and efficient manner (Figure 3). Moreover, the clinical trial network could support and oversee central core labs for consistent and reliable evaluation of imaging endpoints (CT, echocardiography, etc.), clinical event adjudication, and biobanking. Finally, the selection of participating clinical sites would be dependent on several factors including the sites’ ability to identify and to recruit patients rapidly throughout the continuum of AS severity, the availability of multi-modality imaging expertise in AS including echocardiography, CT, and PET/CT, and the presence of diverse clinical champions to oversee the success of trials at each site. Sites would also be selected to help ensure enrollment of a diverse study population including patients traditionally under-represented in clinical trials such as women and minorities (130,131).
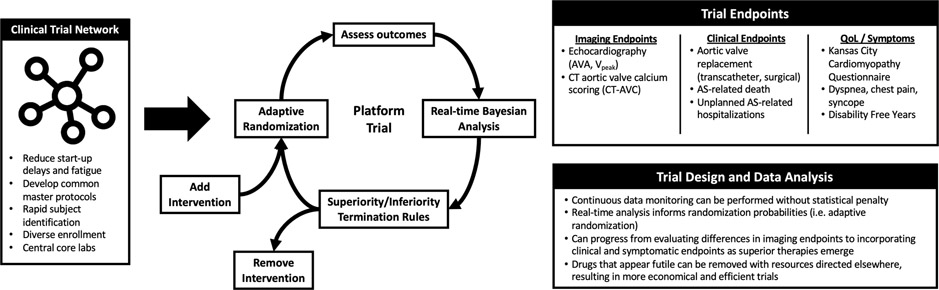
Figure 3. Proposed AS Medical Therapy Trial Endpoints and Design.
A clinical trial network could facilitate the execution of a platform trial with endpoints, design, and data analysis characteristics as shown. AS, aortic stenosis; AVA, aortic valve area; CT-AVC, aortic valve calcification on computed tomography; Vpeak, peak transvalvular velocity.
Concurrent with the identification of potential participating international clinical sites, engaging diverse funding agencies including international governmental agencies (i.e. National Institutes of Health [NIH], Centers for Medicare and Medicaid Services [CMS], Canadian Institutes of Health Research, etc.), cardiovascular societies, and philanthropic organizations will be critical to establishing a clinical trial network. Next, in collaboration with patient advocacy groups, pharmaceutical industry partners, and governmental regulatory agencies (i.e. FDA), clinical trials of promising medical therapies can be designed with the goal of efficiently identifying safe and effective therapies while providing sufficient evidence for their approval by international regulatory agencies.
Given the number of potential medical therapies for the treatment of AS (see Promising targets), a clinical trial network could consider novel trial designs such as adaptive multi-arm, multi-stage trial designs—platform trials—to efficiently allow for multiple medical therapies to be compared with a common control group as was demonstrated in the RECOVERY trial (NCT04381936) testing therapies for SARS-CoV-2 (132,133) (Figure 3). Recently, Pawade et. al. moved in this direction when they conducted a randomized controlled trial testing the efficacy of two individual drugs, denosumab and alendronic acid, to prevent the progression of AS by separately comparing these two agents against a common control group (49).
A platform trial could take advantage of biostatistical techniques such as Bayesian adaptive randomization where the probability of randomization may be altered based on continuous data monitoring to adjust the sample size needed to ensure futile studies end sooner and promising interventions reach a definitive conclusion (Figure 3) (134,135). As compared with frequentist trial designs, real-time continuous data monitoring can be performed without statistical penalty in Bayesian designs as frequent analysis of the data does not alter the method by which the probability of treatment effect is computed (122,136). Given that the vast majority of treatments that enter development are unsuccessful, adaptive platform trials represent a statistically efficient method of identifying successful therapies. If a drug appears to be futile, instead of continuing that arm of the trial, it can be removed and resources can be directed elsewhere. Indeed, the FDA has endorsed the design and implementation of adaptive clinical trials (137).
However, in contrast to some seminal platform trials such as I-SPY2 (138) and REMAP-CAP (139), where endpoints are assessed over the course of months, one of the challenges of adaptive trial designs for AS is that detectable progression of disease can be slow, often occurring over years. This long time-course may make it impractical to implement meaningful trial adaptations over short time periods. Nevertheless, depending on the chosen study population (those with early vs. late disease), primary endpoints selected, and the planned duration of the trial, a Bayesian adaptive clinical trial may be feasible and could result in a more efficient trial. Some of these challenges may be partially attenuated if the trial could be enriched with a population of patients that are most likely to progress quickly.
Building the infrastructure to operationalize a platform trial would require a commitment to and focus on the disease with the goal of finding effective treatment(s) as a higher priority than the evaluation of any particular experimental therapy (132). Coordination and financial support of multiple stakeholders would be essential. Given the societal costs of AS, there is strong rationale for government funding. Pharmaceutical companies may trade loss of control over the study design and execution for the substantially lower cost of testing the efficacy of their specific drug. The advantages of a platform trial would extend to patients who have access to a greater number of experimental therapies and benefit from response-adaptive randomization; and to investigators who can evaluate for potential synergy between treatments and leverage a larger and more heterogeneous study population to quantify differences in treatment effects in subgroups.
Conclusion
The aging of the population and the large and increasing number of individuals with AS combined with the current lack of any effective medical therapy to slow disease progression and the dearth of RCTs testing promising therapies provide a clarion call for action. A productive and effective path forward to test new drugs efficiently for AS will require alignment of multiple stakeholders, clarity and agreement on the relevant goalposts for approval, development of a clinical trial network with standardized imaging protocols and endpoint assessment, and the focus, resolve, and creativity to tackle and to solve the challenges that arise. Recent and ongoing advances in elucidating the pathobiology of AS have identified numerous promising targets and existing therapies to test. A fit-for-purpose infrastructure and over-arching plan now needs to be built and executed to evaluate these potential therapies for the betterment of the millions of individuals who now have or will develop AS (Central Illustration). To that end, we propose that a multistakeholder working group be convened including experts in valvular heart disease and representatives from National Institutes of Health, Centers for Medicare and Medicaid Services, FDA, industry, Heart Failure Collaboratory, patient advocacy groups, and others under the auspices of the Heart Valve Collaboratory to provide forward momentum for the ambitious goal of launching a clinical trials research network that could execute a platform trial as proposed to move the field from promising targets to proven therapies.
Supplementary Material
Highlights.
Despite numerous promising targets, no medical therapies for aortic stenosis have proven effective.
Better tools are needed to detect early-stage aortic stenosis and identify patients prone to rapid progression.
A clinical trial network and platform trial could accelerate discovery of improved methods of surveillance and effective therapies.
Sources of funding:
Dr. Madhavan has received support from an institutional grant by the National Institutes of Health/National Heart, Lung, and Blood Institute to Columbia University Irving Medical Center (T32 HL007854). Dr. Merryman is supported by the National Institutes of Health (R35-HL135790) and Fondation Leducq. Dr Harrell is supported by CTSA award No. UL1 TR002243 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Disclosures: Dr. Lindman has served on the scientific advisory board for Roche Diagnostics, has received research grants from Edwards Lifesciences and Roche Diagnostics. Dr. Arsenault has received investigator-initiated research contracts from Pfizer and Ionis pharmaceuticals and is a consultant for Novartis and Silence Therapeutics. Dr. Coylewright has research grants from Edwards LifeSciences and Boston Scientific and serves on consulting/advisory board for Abbott, Medtronic and Alleviant. Dr. Mack was co-primary investigator for the PARTNER Trial for Edwards Lifesciences and COAPT trial for Abbott and is study chair for the APOLLO trial for Medtronic. Dr. Leon reports institutional research support from Edwards Lifesciences, Medtronic, Boston Scientific, and Abbott, and consulting/advisory board participation for Medtronic, Boston Scientific, Gore, Meril Lifescience, and Abbott. Dr. Pibarot has research grants from Edwards Lifesciences and Medtronic for echo corelab analyses in transcatheter aortic valve replacement. The other authors have nothing to disclose.
Abbreviations:
- AS
aortic stenosis
- AVA
aortic valve area
- AVR
aortic valve replacement
- CT-AVC
computed tomography – aortic valve calcification
- FDA
Food and Drug Administration
- HVC
Heart Valve Collaboratory
- PET
positron emission tomography
- PPI
patient preference information
- RCT
randomized clinical trial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lindman BR, Clavel MA, Mathieu P et al. Calcific aortic stenosis. Nat Rev Dis Primers 2016;2:16006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osnabrugge RL, Mylotte D, Head SJ et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol 2013;62:1002–12. [DOI] [PubMed] [Google Scholar]
- 3.Coffey S, Cox B, Williams MJ. The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis. J Am Coll Cardiol 2014;63:2852–61. [DOI] [PubMed] [Google Scholar]
- 4.d'Arcy JL, Coffey S, Loudon MA et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J 2016;37:3515–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Writing Committee M, Otto CM, Nishimura RA et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2021;77:e25–e197. [DOI] [PubMed] [Google Scholar]
- 6.Baumgartner H, Falk V, Bax JJ et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 7.Roth GA, Mensah GA, Johnson CO et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J Am Coll Cardiol 2020;76:2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vemulapalli S, Carroll JD, Mack MJ et al. Procedural Volume and Outcomes for Transcatheter Aortic-Valve Replacement. N Engl J Med 2019;380:2541–2550. [DOI] [PubMed] [Google Scholar]
- 9.Lindman BR, Lindenfeld JA. Prevention and Mitigation of Heart Failure in the Treatment of Calcific Aortic Stenosis: A Unifying Therapeutic Principle. JAMA Cardiol 2021. [DOI] [PubMed] [Google Scholar]
- 10.Lindman BR, Dweck MR, Lancellotti P et al. Management of Asymptomatic Severe Aortic Stenosis: Evolving Concepts in Timing of Valve Replacement. JACC Cardiovasc Imaging 2020;13:481–493. [DOI] [PubMed] [Google Scholar]
- 11.Lindman BR, Merryman WD. Unloading the Stenotic Path to Identifying Medical Therapy for Calcific Aortic Valve Disease: Barriers and Opportunities. Circulation 2021;143:1455–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marquis-Gravel G, Redfors B, Leon MB, Genereux P. Medical Treatment of Aortic Stenosis. Circulation 2016;134:1766–1784. [DOI] [PubMed] [Google Scholar]
- 13.Zheng KH, Tzolos E, Dweck MR. Pathophysiology of Aortic Stenosis and Future Perspectives for Medical Therapy. Cardiol Clin 2020;38:1–12. [DOI] [PubMed] [Google Scholar]
- 14.Hutcheson JD, Aikawa E, Merryman WD. Potential drug targets for calcific aortic valve disease. Nat Rev Cardiol 2014;11:218–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 2005;111:3316–26. [DOI] [PubMed] [Google Scholar]
- 16.Otto CM, Prendergast B. Aortic-valve stenosis--from patients at risk to severe valve obstruction. N Engl J Med 2014;371:744–56. [DOI] [PubMed] [Google Scholar]
- 17.Theriault S, Dina C, Messika-Zeitoun D et al. Genetic Association Analyses Highlight IL6, ALPL, and NAV1 As 3 New Susceptibility Genes Underlying Calcific Aortic Valve Stenosis. Circ Genom Precis Med 2019;12:e002617. [DOI] [PubMed] [Google Scholar]
- 18.Theriault S, Gaudreault N, Lamontagne M et al. A transcriptome-wide association study identifies PALMD as a susceptibility gene for calcific aortic valve stenosis. Nature communications 2018;9:988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helgadottir A, Thorleifsson G, Gretarsdottir S et al. Genome-wide analysis yields new loci associating with aortic valve stenosis. Nature communications 2018;9:987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Treibel TA, Badiani S, Lloyd G, Moon JC. Multimodality Imaging Markers of Adverse Myocardial Remodeling in Aortic Stenosis. JACC Cardiovasc Imaging 2019;12:1532–1548. [DOI] [PubMed] [Google Scholar]
- 21.Chan KL, Teo K, Dumesnil JG, Ni A, Tam J. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 2010;121:306–14. [DOI] [PubMed] [Google Scholar]
- 22.Rossebo AB, Pedersen TR, Boman K et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008;359:1343–56. [DOI] [PubMed] [Google Scholar]
- 23.Thanassoulis G, Campbell CY, Owens DS et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med 2013;368:503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Despres AA, Perrot N, Poulin A et al. Lipoprotein(a), Oxidized Phospholipids, and Aortic Valve Microcalcification Assessed by 18F-Sodium Fluoride Positron Emission Tomography and Computed Tomography. CJC Open 2019;1:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith JG, Luk K, Schulz CA et al. Association of low-density lipoprotein cholesterol-related genetic variants with aortic valve calcium and incident aortic stenosis. JAMA 2014;312:1764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perrot N, Valerio V, Moschetta D et al. Genetic and In Vitro Inhibition of PCSK9 and Calcific Aortic Valve Stenosis. JACC Basic Transl Sci 2020;5:649–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng KH, Tsimikas S, Pawade T et al. Lipoprotein(a) and Oxidized Phospholipids Promote Valve Calcification in Patients With Aortic Stenosis. J Am Coll Cardiol 2019;73:2150–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I et al. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N Engl J Med 2020;382:244–255. [DOI] [PubMed] [Google Scholar]
- 29.Ray KK, Wright RS, Kallend D et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N Engl J Med 2020;382:1507–1519. [DOI] [PubMed] [Google Scholar]
- 30.Choi B, Lee S, Kim SM et al. Dipeptidyl Peptidase-4 Induces Aortic Valve Calcification by Inhibiting Insulin-Like Growth Factor-1 Signaling in Valvular Interstitial Cells. Circulation 2017;135:1935–1950. [DOI] [PubMed] [Google Scholar]
- 31.Lee S, Lee SA, Choi B et al. Dipeptidyl peptidase-4 inhibition to prevent progression of calcific aortic stenosis. Heart 2020;106:1824–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li F, Cai Z, Chen F et al. Pioglitazone attenuates progression of aortic valve calcification via down-regulating receptor for advanced glycation end products. Basic Res Cardiol 2012;107:306. [DOI] [PubMed] [Google Scholar]
- 33.Takimoto E, Champion HC, Li M et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med 2005;11:214–22. [DOI] [PubMed] [Google Scholar]
- 34.Nagayama T, Hsu S, Zhang M et al. Sildenafil stops progressive chamber, cellular, and molecular remodeling and improves calcium handling and function in hearts with pre-existing advanced hypertrophy caused by pressure overload. J Am Coll Cardiol 2009;53:207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindman BR, Zajarias A, Madrazo JA et al. Effects of phosphodiesterase type 5 inhibition on systemic and pulmonary hemodynamics and ventricular function in patients with severe symptomatic aortic stenosis. Circulation 2012;125:2353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol 2008;52:843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Z, Pyriochou A, Kotanidou A et al. Soluble guanylyl cyclase activation by HMR-1766 (ataciguat) in cells exposed to oxidative stress. Am J Physiol Heart Circ Physiol 2008;295:H1763–71. [DOI] [PubMed] [Google Scholar]
- 38.Zhang B, Roos C, Hagler M et al. Activation of Oxidized Soluble Guanylate Cyclase Slows Progression of Aortic Valve Calcification. Arteriosclerosis, Thrombosis, and Vascular Biology 2019;39:A123. [Google Scholar]
- 39.Garg V, Muth AN, Ransom JF et al. Mutations in NOTCH1 cause aortic valve disease. Nature 2005;437:270–4. [DOI] [PubMed] [Google Scholar]
- 40.Hadji F, Boulanger MC, Guay SP et al. Altered DNA Methylation of Long Noncoding RNA H19 in Calcific Aortic Valve Disease Promotes Mineralization by Silencing NOTCH1. Circulation 2016;134:1848–1862. [DOI] [PubMed] [Google Scholar]
- 41.Merryman WD, Clark CR. Lnc-ing NOTCH1 to Idiopathic Calcific Aortic Valve Disease. Circulation 2016;134:1863–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toshima T, Watanabe T, Narumi T et al. Therapeutic inhibition of microRNA-34a ameliorates aortic valve calcification via modulation of Notch1-Runx2 signalling. Cardiovasc Res 2020;116:983–994. [DOI] [PubMed] [Google Scholar]
- 43.Raddatz MA, Vander Roest MJ, Merryman WD. Notch1 suppression by microRNA-34a: a new mechanism of calcific aortic valve disease. Cardiovasc Res 2020;116:871–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen J, Ryzhova LM, Sewell-Loftin MK et al. Notch1 Mutation Leads to Valvular Calcification Through Enhanced Myofibroblast Mechanotransduction. Arterioscler Thromb Vasc Biol 2015;35:1597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hutcheson JD, Chen J, Sewell-Loftin MK et al. Cadherin-11 regulates cell-cell tension necessary for calcific nodule formation by valvular myofibroblasts. Arterioscler Thromb Vasc Biol 2013;33:114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sung DC, Bowen CJ, Vaidya KA et al. Cadherin-11 Overexpression Induces Extracellular Matrix Remodeling and Calcification in Mature Aortic Valves. Arterioscler Thromb Vasc Biol 2016;36:1627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clark CR, Bowler MA, Snider JC, Merryman WD. Targeting Cadherin-11 Prevents Notch1-Mediated Calcific Aortic Valve Disease. Circulation 2017;135:2448–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pawade TA, Newby DE, Dweck MR. Calcification in Aortic Stenosis: The Skeleton Key. J Am Coll Cardiol 2015;66:561–77. [DOI] [PubMed] [Google Scholar]
- 49.Pawade TA, Doris MK, Bing R et al. Effect of Denosumab or Alendronic Acid on the Progression of Aortic Stenosis: A Double-Blind Randomized Controlled Trial. Circulation 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peeters F, van Mourik MJW, Meex SJR et al. Bicuspid Aortic Valve Stenosis and the Effect of Vitamin K2 on Calcification Using (18)F-Sodium Fluoride Positron Emission Tomography/Magnetic Resonance: The BASIK2 Rationale and Trial Design. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schantl AE, Verhulst A, Neven E et al. Inhibition of vascular calcification by inositol phosphates derivatized with ethylene glycol oligomers. Nature communications 2020;11:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rieck AE, Cramariuc D, Boman K et al. Hypertension in aortic stenosis: implications for left ventricular structure and cardiovascular events. Hypertension 2012;60:90–7. [DOI] [PubMed] [Google Scholar]
- 53.Capoulade R, Clavel MA, Mathieu P et al. Impact of hypertension and renin-angiotensin system inhibitors in aortic stenosis. Eur J Clin Invest 2013;43:1262–72. [DOI] [PubMed] [Google Scholar]
- 54.de Oliveira Moraes AB, Stahli BE, Arsenault BJ et al. Resting heart rate as a predictor of aortic valve stenosis progression. Int J Cardiol 2016;204:149–51. [DOI] [PubMed] [Google Scholar]
- 55.Blaser MC, Kraler S, Luscher TF, Aikawa E. Multi-Omics Approaches to Define Calcific Aortic Valve Disease Pathogenesis. Circ Res 2021;128:1371–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Webb J, Thoenes M, Chambers JB. Identifying Heart Valve Disease in Primary Care: Differences Between Practice in Germany, France and the United Kingdom. European Journal of Cardiovascular Medicine 2014;3:388–392. [Google Scholar]
- 57.Thoenes M, Bramlage P, Zamorano P et al. Patient screening for early detection of aortic stenosis (AS)-review of current practice and future perspectives. J Thorac Dis 2018;10:5584–5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaede L, Di Bartolomeo R, van der Kley F, Elsasser A, Iung B, Mollmann H. Aortic valve stenosis: what do people know? A heart valve disease awareness survey of over 8,800 people aged 60 or over. EuroIntervention 2016;12:883–9. [DOI] [PubMed] [Google Scholar]
- 59.Lindman BR, Arnold SV, Bagur R et al. Priorities for Patient-Centered Research in Valvular Heart Disease: A Report From the National Heart, Lung, and Blood Institute Working Group. Journal of the American Heart Association 2020;9:e015975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McBrien ME, Heyburn G, Stevenson M et al. Previously undiagnosed aortic stenosis revealed by auscultation in the hip fracture population--echocardiographic findings, management and outcome. Anaesthesia 2009;64:863–70. [DOI] [PubMed] [Google Scholar]
- 61.Gardezi SKM, Myerson SG, Chambers J et al. Cardiac auscultation poorly predicts the presence of valvular heart disease in asymptomatic primary care patients. Heart 2018;104:1832–1835. [DOI] [PubMed] [Google Scholar]
- 62.Stewart BF, Siscovick D, Lind BK et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997;29:630–4. [DOI] [PubMed] [Google Scholar]
- 63.Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA. The Population 65 Years and Older in the United States: 2016. In: U.S. Department of Commerce EaSA, editor: U.S. Census Bureau, 2018. [Google Scholar]
- 64.Hammadah M, Ponce C, Sorajja P, Cavalcante JL, Garcia S, Gossl M. Point-of-care ultrasound: Closing guideline gaps in screening for valvular heart disease. Clin Cardiol 2020;43:1368–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kobal SL, Trento L, Baharami S et al. Comparison of effectiveness of hand-carried ultrasound to bedside cardiovascular physical examination. Am J Cardiol 2005;96:1002–6. [DOI] [PubMed] [Google Scholar]
- 66.Narula J, Chandrashekhar Y, Braunwald E. Time to Add a Fifth Pillar to Bedside Physical Examination: Inspection, Palpation, Percussion, Auscultation, and Insonation. JAMA Cardiol 2018;3:346–350. [DOI] [PubMed] [Google Scholar]
- 67.Nascimento BR, Beaton AZ, Nunes MCP et al. Integration of echocardiographic screening by non-physicians with remote reading in primary care. Heart 2019;105:283–290. [DOI] [PubMed] [Google Scholar]
- 68.Williams MC, Massera D, Moss AJ et al. Prevalence and clinical implications of valvular calcification on coronary computed tomography angiography. European heart journal cardiovascular Imaging 2021;22:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kwon JM, Lee SY, Jeon KH et al. Deep Learning-Based Algorithm for Detecting Aortic Stenosis Using Electrocardiography. Journal of the American Heart Association 2020;9:e014717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Otto CM, Pearlman AS, Gardner CL. Hemodynamic progression of aortic stenosis in adults assessed by Doppler echocardiography. J Am Coll Cardiol 1989;13:545–50. [DOI] [PubMed] [Google Scholar]
- 71.Faggiano P, Ghizzoni G, Sorgato A et al. Rate of progression of valvular aortic stenosis in adults. Am J Cardiol 1992;70:229–33. [DOI] [PubMed] [Google Scholar]
- 72.Peter M, Hoffmann A, Parker C, Luscher T, Burckhardt D. Progression of aortic stenosis. Role of age and concomitant coronary artery disease. Chest 1993;103:1715–9. [DOI] [PubMed] [Google Scholar]
- 73.Otto CM, Burwash IG, Legget ME et al. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997;95:2262–70. [DOI] [PubMed] [Google Scholar]
- 74.Cowell SJ, Newby DE, Prescott RJ et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 2005;352:2389–97. [DOI] [PubMed] [Google Scholar]
- 75.Chan KL, Teo K, Dumesnil JG, Ni A, Tam J, Investigators A. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 2010;121:306–14. [DOI] [PubMed] [Google Scholar]
- 76.Hekimian G, Boutten A, Flamant M et al. Progression of aortic valve stenosis is associated with bone remodelling and secondary hyperparathyroidism in elderly patients--the COFRASA study. Eur Heart J 2013;34:1915–22. [DOI] [PubMed] [Google Scholar]
- 77.Capoulade R, Mahmut A, Tastet L et al. Impact of plasma Lp-PLA2 activity on the progression of aortic stenosis: the PROGRESSA study. JACC Cardiovasc Imaging 2015;8:26–33. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen V, Mathieu T, Melissopoulou M et al. Sex Differences in the Progression of Aortic Stenosis and Prognostic Implication: The COFRASA-GENERAC Study. JACC Cardiovasc Imaging 2016;9:499–501. [DOI] [PubMed] [Google Scholar]
- 79.Tastet L, Enriquez-Sarano M, Capoulade R et al. Impact of Aortic Valve Calcification and Sex on Hemodynamic Progression and Clinical Outcomes in AS. J Am Coll Cardiol 2017;69:2096–2098. [DOI] [PubMed] [Google Scholar]
- 80.Tastet L, Capoulade R, Shen M et al. ApoB/ApoA-I Ratio is Associated With Faster Hemodynamic Progression of Aortic Stenosis: Results From the PROGRESSA (Metabolic Determinants of the Progression of Aortic Stenosis) Study. Journal of the American Heart Association 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kubota N, Testuz A, Boutten A et al. Impact of Fetuin-A on progression of calcific aortic valve stenosis - The COFRASA - GENERAC study. Int J Cardiol 2018;265:52–57. [DOI] [PubMed] [Google Scholar]
- 82.Tastet L, Pibarot P, Shen M et al. Oral Anticoagulation Therapy and Progression of Calcific Aortic Valve Stenosis. J Am Coll Cardiol 2019;73:1869–1871. [DOI] [PubMed] [Google Scholar]
- 83.Doris MK, Jenkins W, Robson P et al. Computed tomography aortic valve calcium scoring for the assessment of aortic stenosis progression. Heart 2020;106:1906–1913. https://creativecommons.org/licenses/by/4.0/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tastet L, Shen M, Capoulade R et al. Bone Mineral Density and Progression Rate of Calcific Aortic Valve Stenosis. J Am Coll Cardiol 2020;75:1725–1726. [DOI] [PubMed] [Google Scholar]
- 85.Shen M, Tastet L, Capoulade R et al. Effect of bicuspid aortic valve phenotype on progression of aortic stenosis. European heart journal cardiovascular Imaging 2020;21:727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roger VL, Tajik AJ, Bailey KR, Oh JK, Taylor CL, Seward JB. Progression of Aortic-Stenosis in Adults - New Appraisal Using Doppler Echocardiography. Am Heart J 1990;119:331–338. [DOI] [PubMed] [Google Scholar]
- 87.Brener SJ, Duffy CI, Thomas JD, Stewart WJ. Progression of aortic stenosis in 394 patients: relation to changes in myocardial and mitral valve dysfunction. J Am Coll Cardiol 1995;25:305–10. [DOI] [PubMed] [Google Scholar]
- 88.Bahler RC, Desser DR, Finkelhor RS, Brener SJ, Youssefi M. Factors leading to progression of valvular aortic stenosis. Am J Cardiol 1999;84:1044–8. [DOI] [PubMed] [Google Scholar]
- 89.Palta S, Pai AM, Gill KS, Pai RG. New insights into the progression of aortic stenosis: implications for secondary prevention. Circulation 2000;101:2497–502. [DOI] [PubMed] [Google Scholar]
- 90.Rosenhek R, Klaar U, Schemper M et al. Mild and moderate aortic stenosis. Natural history and risk stratification by echocardiography. Eur Heart J 2004;25:199–205. [DOI] [PubMed] [Google Scholar]
- 91.Ersboll M, Schulte PJ, Al Enezi F et al. Predictors and progression of aortic stenosis in patients with preserved left ventricular ejection fraction. Am J Cardiol 2015;115:86–92. [DOI] [PubMed] [Google Scholar]
- 92.Nishimura S, Izumi C, Nishiga M et al. Predictors of Rapid Progression and Clinical Outcome of Asymptomatic Severe Aortic Stenosis. Circ J 2016;80:1863–9. [DOI] [PubMed] [Google Scholar]
- 93.Kebed K, Sun D, Addetia K, Mor-Avi V, Markuzon N, Lang RM. Progression of aortic stenosis and echocardiographic criteria for its severity. European heart journal cardiovascular Imaging 2020;21:737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nayeri A, Xu M, Farber-Eger E et al. Initial changes in peak aortic jet velocity and mean gradient predict progression to severe aortic stenosis. Int J Cardiol Heart Vasc 2020;30:100592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tastet L, Capoulade R, Clavel MA et al. Systolic hypertension and progression of aortic valve calcification in patients with aortic stenosis: results from the PROGRESSA study. European heart journal cardiovascular Imaging 2017;18:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Voisine M, Hervault M, Shen M et al. Age, Sex, and Valve Phenotype Differences in Fibro-Calcific Remodeling of Calcified Aortic Valve. Journal of the American Heart Association 2020;9:e015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thaden JJ, Nkomo VT, Suri RM et al. Sex-related differences in calcific aortic stenosis: correlating clinical and echocardiographic characteristics and computed tomography aortic valve calcium score to excised aortic valve weight. Eur Heart J 2016;37:693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simard L, Cote N, Dagenais F et al. Sex-Related Discordance Between Aortic Valve Calcification and Hemodynamic Severity of Aortic Stenosis: Is Valvular Fibrosis the Explanation? Circ Res 2017;120:681–691. [DOI] [PubMed] [Google Scholar]
- 99.Yang LT, Boler A, Medina-Inojosa JR et al. Aortic Stenosis Progression, Cardiac Damage, and Survival: Comparison Between Bicuspid and Tricuspid Aortic Valves. JACC Cardiovasc Imaging 2021;14:1113–1126. [DOI] [PubMed] [Google Scholar]
- 100.Clavel MA, Pibarot P, Messika-Zeitoun D et al. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an international registry study. J Am Coll Cardiol 2014;64:1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pawade T, Clavel MA, Tribouilloy C et al. Computed Tomography Aortic Valve Calcium Scoring in Patients With Aortic Stenosis. Circ Cardiovasc Imaging 2018;11:e007146. [DOI] [PubMed] [Google Scholar]
- 102.Jung JJ, Jadbabaie F, Sadeghi MM. Molecular imaging of calcific aortic valve disease. J Nucl Cardiol 2018;25:1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dweck MR, Jenkins WS, Vesey AT et al. 18F-sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circ Cardiovasc Imaging 2014;7:371–8. [DOI] [PubMed] [Google Scholar]
- 104.Dweck MR, Jones C, Joshi NV et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation 2012;125:76–86. [DOI] [PubMed] [Google Scholar]
- 105.Capoulade R, Clavel MA, Dumesnil JG et al. Impact of metabolic syndrome on progression of aortic stenosis: influence of age and statin therapy. J Am Coll Cardiol 2012;60:216–23. [DOI] [PubMed] [Google Scholar]
- 106.Capoulade R, Chan KL, Yeang C et al. Oxidized Phospholipids, Lipoprotein(a), and Progression of Calcific Aortic Valve Stenosis. J Am Coll Cardiol 2015;66:1236–46. [DOI] [PubMed] [Google Scholar]
- 107.Nazarzadeh M, Pinho-Gomes AC, Smith Byrne K et al. Systolic Blood Pressure and Risk of Valvular Heart Disease: A Mendelian Randomization Study. JAMA Cardiol 2019;4:788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nazarzadeh M, Pinho-Gomes AC, Bidel Z et al. Plasma lipids and risk of aortic valve stenosis: a Mendelian randomization study. Eur Heart J 2020;41:3913–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sengupta PP, Shrestha S, Kagiyama N et al. A Machine-Learning Framework to Identify Distinct Phenotypes of Aortic Stenosis Severity. JACC Cardiovasc Imaging 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Quer G, Arnaout R, Henne M, Arnaout R. Machine Learning and the Future of Cardiovascular Care: JACC State-of-the-Art Review. J Am Coll Cardiol 2021;77:300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lytvyn L, Guyatt GH, Manja V et al. Patient values and preferences on transcatheter or surgical aortic valve replacement therapy for aortic stenosis: a systematic review. BMJ Open 2016;6:e014327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Levitan B, Getz K, Eisenstein EL et al. Assessing the Financial Value of Patient Engagement: A Quantitative Approach from CTTI's Patient Groups and Clinical Trials Project. Ther Innov Regul Sci 2018;52:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Poger JM, Mayer V, Duru OK et al. Network Engagement in Action: Stakeholder Engagement Activities to Enhance Patient-centeredness of Research. Medical care 2020;58 Suppl 6 Suppl 1:S66–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Patient Preference Information – Voluntary Submission, Review in Premarket Approval Applications, Humanitarian Device Exemption Applications, and De Novo Requests, and Inclusion in Decision Summaries and Device Labeling. U.S. Food and Drug Administration, 2016. [Google Scholar]
- 115.Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med 2012;125:882–7 e1. [DOI] [PubMed] [Google Scholar]
- 116.Yusuf S, Joseph P, Dans A et al. Polypill with or without Aspirin in Persons without Cardiovascular Disease. N Engl J Med 2021;384:216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bosworth HB, Dudley T, Olsen MK et al. Racial differences in blood pressure control: potential explanatory factors. Am J Med 2006;119:70 e9–15. [DOI] [PubMed] [Google Scholar]
- 118.Charles H, Good CB, Hanusa BH, Chang CC, Whittle J. Racial differences in adherence to cardiac medications. J Natl Med Assoc 2003;95:17–27. [PMC free article] [PubMed] [Google Scholar]
- 119.Bartolazzi F, Ribeiro ALP, de Sousa W, Vianna MS, da Silva JLP, Martins MAP. Relationship of health literacy and adherence to oral anticoagulation therapy in patients with atrial fibrillation: a cross-sectional study. Journal of thrombosis and thrombolysis 2021. [DOI] [PubMed] [Google Scholar]
- 120.Beyer-Westendorf J, Ehlken B, Evers T. Real-world persistence and adherence to oral anticoagulation for stroke risk reduction in patients with atrial fibrillation. Europace 2016;18:1150–7. [DOI] [PubMed] [Google Scholar]
- 121.Du Y, Gossl M, Garcia S et al. Natural history observations in moderate aortic stenosis. BMC Cardiovasc Disord 2021;21:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Harrell FE. General COVID-19 Therapeutics Trial Design. 2021. [Google Scholar]
- 123.Baumgartner H, Hung J, Bermejo J et al. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr 2017;30:372–392. [DOI] [PubMed] [Google Scholar]
- 124.Cartlidge TR, Bing R, Kwiecinski J et al. Contrast-enhanced computed tomography assessment of aortic stenosis. Heart 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pawade T, Sheth T, Guzzetti E, Dweck MR, Clavel MA. Why and How to Measure Aortic Valve Calcification in Patients With Aortic Stenosis. JACC Cardiovasc Imaging 2019;12:1835–1848. [DOI] [PubMed] [Google Scholar]
- 126.Administration USFaD. Drugs for Human Use Part 314 Applications for FDA Approval to Market a New Drug Subpart H Accelerated Approval of New Drugs for Serious or Life-Threatening Illnesses. 2020.
- 127.Heart Failure Network. https://hfnetwork.org/
- 128.PETAL Network. https://petalnet.org/
- 129.CTSN Current Trials. http://www.ctsurgerynet.org/currenttrials.html
- 130.Ortega RF, Yancy CW, Mehran R, Batchelor W. Overcoming Lack of Diversity in Cardiovascular Clinical Trials: A New Challenge and Strategies for Success. Circulation 2019;140:1690–1692. [DOI] [PubMed] [Google Scholar]
- 131.Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med 2002;162:1682–8. [DOI] [PubMed] [Google Scholar]
- 132.Berry SM, Connor JT, Lewis RJ. The platform trial: an efficient strategy for evaluating multiple treatments. JAMA 2015;313:1619–20. [DOI] [PubMed] [Google Scholar]
- 133.Group RC. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet 2021;397:2049–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Adaptive Platform Trials C Adaptive platform trials: definition, design, conduct and reporting considerations. Nat Rev Drug Discov 2019;18:797–807. [DOI] [PubMed] [Google Scholar]
- 135.Saville BR, Berry SM. Efficiencies of platform clinical trials: A vision of the future. Clin Trials 2016;13:358–66. [DOI] [PubMed] [Google Scholar]
- 136.Lee JJ, Chu CT. Bayesian clinical trials in action. Statistics in medicine 2012;31:2955–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Center for Drug Evaluation R. Adaptive Designs for Clinical Trials of Drugs and Biologics Guidance.
- 138.Barker AD, Sigman CC, Kelloff GJ, Hylton NM, Berry DA, Esserman LJ. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther 2009;86:97–100. [DOI] [PubMed] [Google Scholar]
- 139.Angus DC, Berry S, Lewis RJ et al. The REMAP-CAP (Randomized Embedded Multifactorial Adaptive Platform for Community-acquired Pneumonia) Study. Rationale and Design. Ann Am Thorac Soc 2020;17:879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.