Abstract
Working memory acts as a key bridge between perception, long-term memory, and action. The brain regions, connections, and neurotransmitters that underlie working memory undergo dramatic plastic changes during the life span, and in response to injury. Early life reliance on deep gray matter structures fades during adolescence as increasing reliance on prefrontal and parietal cortex accompanies the development of executive aspects of working memory. The rise and fall of working memory capacity and executive functions parallels the development and loss of neurotransmitter function in frontal cortical areas. Of the affected neurotransmitters, dopamine and acetylcholine modulate excitatory-inhibitory circuits that underlie working memory, are important for plasticity in the system, and are affected following preterm birth and adult brain injury. Pharmacological interventions to promote recovery of working memory abilities have had limited success, but hold promise if used in combination with behavioral training and brain stimulation. The intense study of working memory in a range of species, ages and following injuries has led to better understanding of the intrinsic plasticity mechanisms in the working memory system. The challenge now is to guide these mechanisms to better improve or restore working memory function.
Keywords: working memory, plasticity, neurodevelopment, aging, brain injury, neurotransmitters, dopamine, acetylcholine, MRI, preterm birth
Introduction
One of the most important neuroscience discoveries of the past century is that the brain’s structure is not fixed following development, but is constantly altering its connections. The changeable nature of brain systems that underlie cognitive functions affects how these abilities develop and are maintained throughout the life span. Working memory is a system by which information can be temporarily stored “online” in the brain, so that it is available for further cognitive processing, and as such acts as a bridge between perception, long-term memory, and action (Baddeley 2000). Arguably no higher cognitive function has been more intensely investigated across species than working memory, which has allowed for insight into how a variety of neurobiological factors on different scales (from neurotransmitters and dendritic spines to cognitive networks) interact to influence cognitive function. Here we examine how working memory abilities and the brain networks that underlie them plastically adapt throughout the lifetime and in response to brain injury, and how we may promote adaptive plastic changes in the working memory system.
Working Memory throughout the Life Span
Life Span Changes to Working Memory Abilities
Short-term memory is at the core of working memory, and is the first aspect to develop. At 7½ months of age, infants can maintain the memory for the location of a toy hidden in one of two wells for about 2 seconds. This capacity increases to 10 seconds by 12 months of age (Diamond 1985). The basic modular structure of working memory (see Box 1) is present by 4 years of age, and improves in capacity from childhood through to adolescence (Alloway and others 2006).
Box 1. Psychological Theories of Working Memory.
Working memory is an essential cognitive function that allows us to link perception with action. Working memory can be divided into the temporary maintenance of information and its “online” manipulation. Short-term memory is a function closely related to working memory and represents the maintenance aspect of working memory. Generally, animal studies of working memory focus on short-term memory, but occasionally assess other aspects of the system, including distractor-resistance, particularly in non-human primates.
In 1974, Baddeley and Hitch formulated a multicomponent model of working memory (Baddeley and Hitch 1974). They proposed that working memory be composed of three distinct subsystems: two slave systems, one specialized for language (phonological loop) and the other responsible for processing visuospatial information (visuospatial sketchpad), which function under the supervision of a central executive control system. Briefly, the phonological loop comprises (1) a storage system that allows verbal information to be held in memory for a short period of time, and (2) an active element, the articulatory rehearsal, that refreshes memory to slow memory decay. The visuospatial sketchpad represents temporary storage for visual information that persists for longer than equivalent auditory stimuli in the phonological loop.
The central executive is the least well-defined aspect of Baddeley’s model, and is responsible for managing and regulating maintained information. This comprises several attentional and executive processes that are often considered distinct executive functions in their own right. The executive processes that may act during maintenance including distractor resistance, resistance to intrusion from irrelevant memories, shifting attention within working memory and updating the contents of working memory, among others. Baddeley (2000) further introduced a new component of working memory, the episodic buffer, a temporary, limited capacity multimodal store that links information from various sources to create integrated episodes. It is controlled by the central executive and assimilates information from the subsidiary systems and from long-term memory (Baddeley 2000).
An alternative model is the “embedded processes model” proposed by Cowan (1999). In this model, the focus is placed on attentional control, and it is posited that working memory is subserved by a single store that is equated to long-term memory. Information becomes accessible by two mechanisms: (1) a reactivation process that acts as a rehearsal system and (2) an attentional process that puts information in a temporarily accessible state and provides a basis for ongoing cognitive processing. Similarly to Baddeley’s multicomponent model, Cowan proposes a central executive for voluntary executive processes in combination with an involuntary attentional orienting system (Cowan 1999). In this review, we do not subscribe to a particular model of working memory, but will state explicitly when we are referring to specific executive functions that are thought to contribute to the working memory system.
Improvements to executive aspects of working memory occur later in development. Developmental changes appear to be more dramatic when analyzing the ability to manipulate (rather than simply maintain) items in working memory (Bunge and Wright 2007). The use of changing strategies (such as increased rehearsal) contributes to the increase in performance in working memory in early childhood, but linear increases in performance from about 6 years of age indicate quantitative changes in capacity rather than further changes in strategy (Gathercole and Baddeley 2014). Increases in performance continue during adolescence (Brockmole and Logie 2013).
Working memory ability peaks around early adulthood and declines with age, with different components decaying at different rates (Figure 1). Visual working memory decays faster than verbal working memory, and by age 55 adults display poorer visual working memory capacity than 8-year-olds (Brockmole and Logie 2013). In contrast, verbal working memory remains robust and 70-year-old adults’ performance is comparable to that of 20-year-olds’ (Alloway and Alloway 2013). Age-related decline in working memory maintenance is less severe than in tasks that require manipulation of the remembered information (Reuter-Lorenz and Sylvester 2005).
Figure 1.
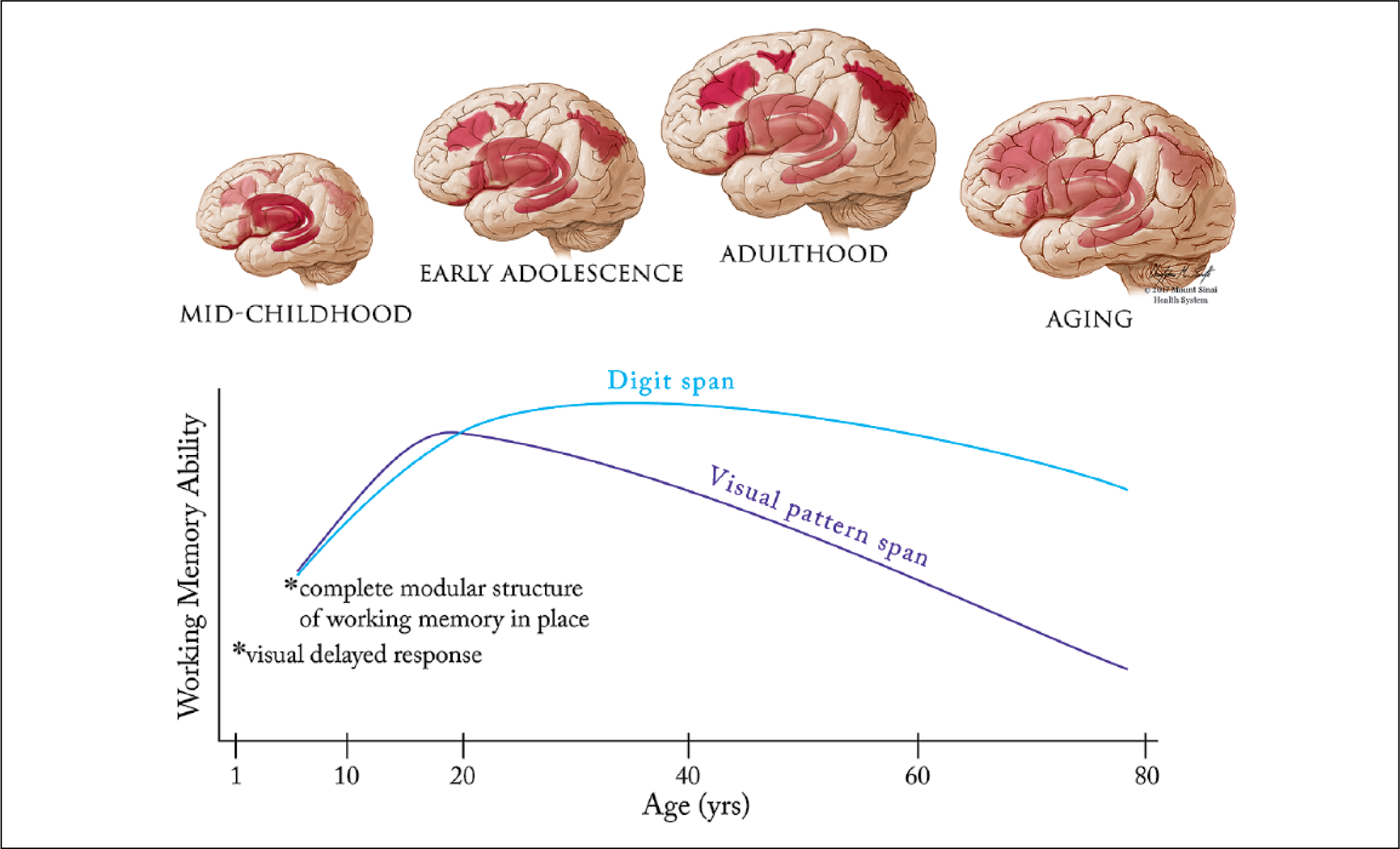
Lifespan development of the working memory system. Top: Early in development working memory depends on subcortical and ventral cortical structures such as the striatum, thalamus, hippocampus and insula. After a transition phase in late childhood/early adolescence, working memory gradually begins to settle upon a network of frontoparietal cortical regions (dorsolateral and ventrolateral prefrontal cortex, frontal eye fields and posterior parietal cortex), centered on the dorsolateral prefrontal cortex. Finally, associations between cortical structures (in particular the parietal cortex) and working memory ability decline during aging. The reliance of working memory on specific structures at different stages in development is represented on an opacity scale (more opaque = greater involvement of the area in working memory). Bottom: Not all aspects of working memory show the same trajectory during development and aging. By one year of age, infants can perform basic visual delayed response tasks, while the complete modular structure (from Baddeley’s model – see Box 1) is in place by at least age 4. Adults at age 55 perform visual pattern span tasks worse than 8 year olds, but can perform digit span tasks on a par with young adults (Brockmole and Logie, 2013).
Importantly, the rise and fall in working memory abilities during development and aging is not symmetrical, but is analogous to the changes in height that take place during over the same period. The remarkable increase in working memory ability observed between 5 and 19 years of age is about 23 times greater than the decline during aging (Alloway and Alloway 2013).
Plasticity in the Working Memory System due to Development and Aging
Developmental Plasticity in the Working Memory System.
The degree to which different brain areas contribute to working memory changes dramatically throughout the life span. One crucial difference between the mature and immature working memory systems is emphasized by an early study of Goldman. Ablation of dorsolateral prefrontal cortex (dlPFC) in adolescent monkeys causes severe working memory deficits, but the same lesion in infant monkeys causes only minor impairments (Goldman 1971). This suggests a fundamental difference between the anatomy underlying the developing and mature working memory systems.
The Striatum, Thalamus, Hippocampus, and Insula Are Critical to the Childhood Working Memory System.
Subcortical and ventral cortical structures and their connections to developing dorsal frontal and parietal cortical regions have a greater role in the developing working memory system and may undertake some of the functions that frontal and parietal cortex will later assume (Goldman and Rosvold 1972), and also drive the specialization of those cortical areas for working memory (Johnson and De Haan 2015). Lesions to monkey caudate (Goldman and Rosvold 1972), mediodorsal thalamus (Goldman 1974), and orbitofrontal cortex (Miller and others 1973) during infancy affect working memory performance to a greater extent than dlPFC lesions. In humans, the development of early functional connections between the thalamus and the salience network (anterior cingulate, insula and caudate nucleus) is correlated with greater spatial working memory ability at 2 years old (Alcauter and others 2014). Electroencephalography (EEG) and functional near-infrared spectroscopy (fNIRS) studies of very young infants suggest the developing frontoparietal network may also contribute, albeit to a lesser degree, to early working memory function (Fitch and others 2016), indicating that development of the working memory system may entail quantitative, rather than qualitative changes to the regions involved in working memory.
Children generally show less activation during working memory tasks in the frontoparietal network compared with adolescents and adults, with more reliance on the caudate nucleus, thalamus, and anterior insula (Scherf and others 2006). By early adolescence, spatial working memory ability begins to depend on the dlPFC (Alexander and Goldman 1978), and the contribution of subcortical regions and the salience network begins to fade (Scherf and others 2006; Simmonds and others 2017). During adolescence, future working memory capacity is best predicted by subcortical activation in the striatum and thalamus, which supports the hypothesis that basal ganglia-cortical loops might promote the slower Hebbian learning of the frontal cortex (Darki and Klingberg, 2015, Ullman and others 2014). In contrast, current working memory capacity is more strongly associated with frontal and parietal cortex gray matter volume and blood oxygen level–dependent (BOLD) activation (Darki and Klingberg 2015; Ullman and others 2014). Increases in dlPFC activation during adolescence are not noted in working memory tasks that do not tax the executive aspects of working memory (Simmonds and others 2017).
White matter connectivity in the working memory network continues to develop well into adolescence, when structural measures of corticostriatal and frontoparietal connections are correlated with both future and current working memory capacity (Darki and Klingberg 2015). Increases in fractional anisotropy (FA) and decreases in radial diffusivity (sensitive, but nonspecific markers of white matter microstructure) in frontal and parietal cortices and the superior longitudinal fasciculus (which connects them) are related to increases in working memory performance during adolescence (Figure 2), independently from cortical changes (Østby and others 2011).
Figure 2.
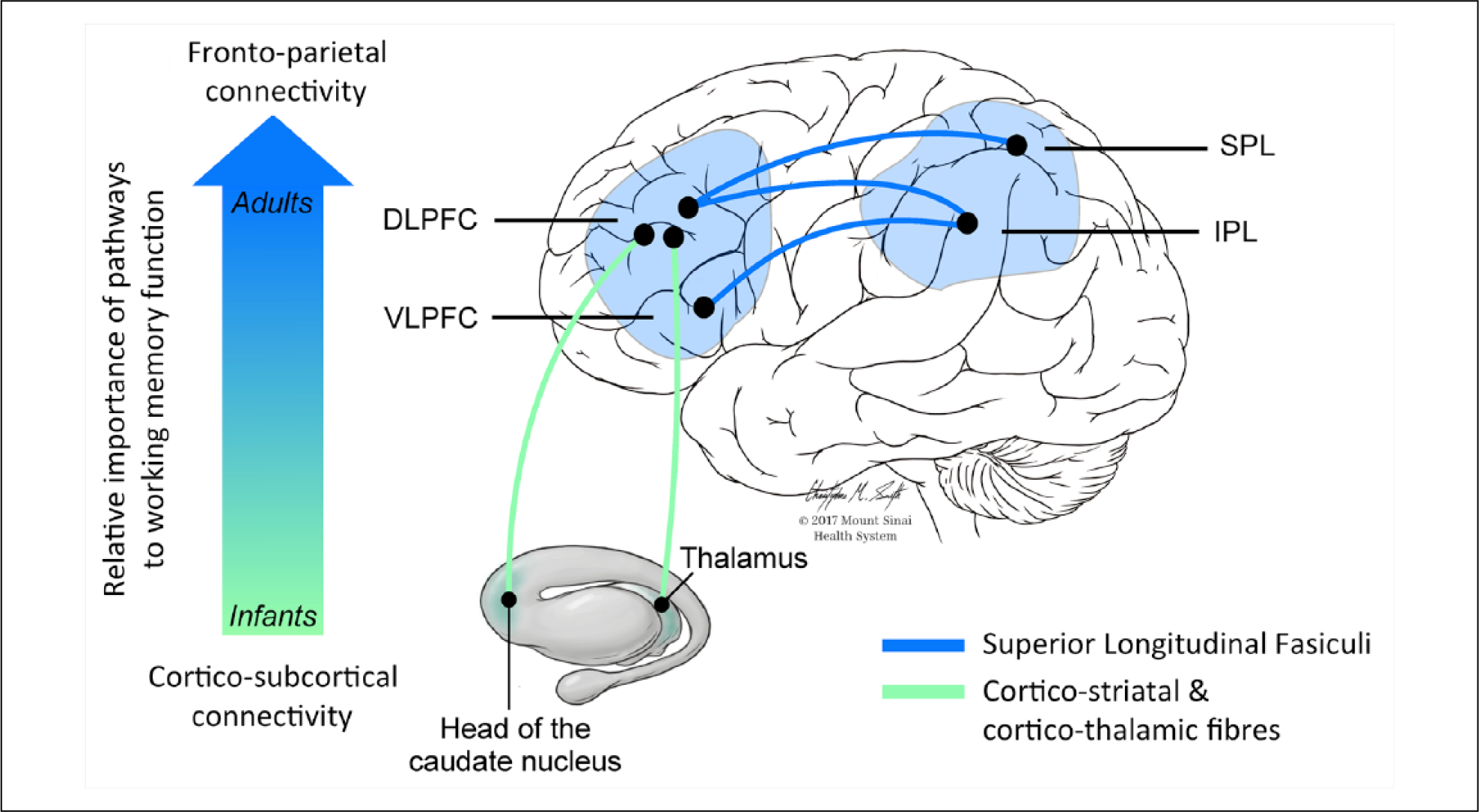
Schematic illustration of the white matter tracts mainly implicated in working memory processes at different points in the lifespan. The vertical arrow indicates how infants’ working memory functions rely initially on subcortical connections due to immature frontoparietal connections, while later in life there is a progressively greater involvement of the dorsolateral and ventrolateral prefrontal cortices connected to the parietal lobe through the three branches of the superior longitudinal fasciculus (SLF).
The importance of the hippocampus in working memory appears to diminish during adolescence, except during particularly demanding or temporally complex tasks. In rodents and non-human primates neonatal excitotoxic lesions of the hippocampus impair adult performance on working memory tasks that are not affected by adult hippocampal lesions, highlighting the effect that disruption of the early working memory system can have on development of the mature system (Heuer and Bachevalier 2011; Lipska and others 2002). In humans, correlation between hippocampal activation and working memory performance appears early, but fades during adolescence, as a correlation between prefrontal cortex (PFC) activation and working memory performance increases (Finn and others 2010). Hippocampal and PFC activity are highly correlated early on in adolescence, but only co-activate on particularly demanding working memory loads later in adolescence (Finn and others 2010). Across the extended adolescent period (8–27 years), age is negatively correlated with posterior hippocampal activity, and positively correlated with posterior parietal activity during visual short-term memory (von Allmen and others 2014).
Adolescent Changes to Prefrontal Neurotransmitter Function May Facilitate Development of Executive Functions.
The neurotransmitter milieu in the dlPFC undergoes profound changes during adolescence. Adult PFC relies on an excitatory-inhibitory circuit, which signals through pyramidal neurons acting on N-methyl-d-aspartate (NMDA) receptors, and parvalbumin-expressing GABA-ergic interneurons, and is modulated by cholinergic projections signaling through nicotinic α−7 receptors and dopaminergic projections signaling principally through D1 receptors (Box 2, Figure 3). Transmission via the NMDA GluN2B-type receptor, which is crucial for non-human primate working memory function (Wang and others 2013), emerges in the PFC in adolescence (Flores-Barrera and others 2014). Parvalbumin-expressing GABA-ergic interneurons, which are hypothesized to be a critical inhibitory part of the circuit, upregulate their expression during adolescence (Caballero and others 2014). Nicotinic acetylcholine α−7 receptors, in addition to the modulatory role in adult working memory (Yang and others 2013) are also required for the normal development of glutamatergic neurons, NMDA receptors and GABA-ergic parvalbumin-expressing (PV+) interneurons in the cortex (Lin and others 2014). Dopaminergic innervation of the PFC peaks around adolescence, at least in layer III neurons (Rosenbergand Lewis 1994), and its action on the excitatory-inhibitory circuit changes. D1 receptor stimulation can cause persistent NMDA-dependent depolarizations in the PFC, but only from adolescence in the rat (Tseng and O’Donnell 2005). In the juvenile rat, activation of D1 receptors but not D2 receptors increases the excitability of fast-spiking interneurons, but by adolescence D2 action on PFC GABA-ergic activity emerges (Tseng and O’Donnell 2007). It is unclear how readily this can be related to human development, where the concentration of D2 receptors in cortex is small. The increased dopaminergic facilitation of NMDA and GABA-ergic transmission in the PFC that occurs during adolescence likely makes PFC recurrent activity more robust and resistant to distractors (Brunel and Wang 2001).
Box 2. The Role of Neurotransmitters and Their Interaction in the Healthy, Mature Working Memory System.
Experimental and computational investigations of the working memory system have identified a critical role for a range of neurotransmitters in working memory function. By analogy with the visual system, Goldman-Rakic proposed that local networks of interacting excitatory and inhibitory neurons may account for the maintenance of specific stimuli (and inhibition of others) across a delay (Goldman-Rakic 1995). Computational models extended this view, showing that networks of interacting excitatory and inhibitory neurons are more stable if there is a predominance of NMDA (over AMPA) receptors (Wang 2001) and a dominance of inhibition over excitation (Compte and others 2000). This widespread inhibition is likely undertaken by the fast-spiking parvalbumin-expressing GABA-ergic interneurons (Wang and others 2004), which are highly sensitive to NMDA receptor antagonism (Behrens and others 2007) and underlie the gamma oscillations that are associated with maintaining a memory trace in the PFC (Bartos and others 2007). The importance of NMDA receptors has since been verified experimentally (Wang and others 2013). This circuit appears to be highly sensitive to modification via a number of factors affecting neurotransmission, such as the relative prevalence of NMDA or AMPA receptors, and modulation of neurotransmitter release via dopamine or acetylcholine function (Brunel and Wang 2001).
The relationship between neuromodulator function and working memory performance follows an inverted-U pattern, with deficits associated with both too little, and too much dopamine and acetylcholine transmission (Cools and D’Esposito 2011; Yang and others 2013). Originating from brainstem nuclei and the basal forebrain these neurotransmitters ascend through well-defined pathways to innervate different sectors of the cerebral cortex and subcortical nuclei (Figure 3). Primate PFC has particularly strong dopaminergic inputs, with dopamine terminals modulating glutamatergic transmission (Goldman-Rakic and others 1989) and preferentially contacting parvalbumin-expressing GABA-ergic cells (Sesack and others 1998). Prefrontal acetylcholine modulates the working memory circuit via the action of α7 nicotinic acetylcholine receptors in facilitating transmission via NMDA receptors (Yang and others 2013). Regional depletion of dopamine or acetylcholine in the primate PFC causes severe working memory impairments (Brozoski and others 1979; Croxson and others 2011).
There is a correspondence between the regional distribution of neurotransmitter receptors and the architectonics of cortical areas important for working memory, and single brain regions are innervated by several neuromodulators. By virtue of being anatomically and functionally interrelated, the cholinergic and dopaminergic systems influence each other. For example, in the striatum DA modulates the activity of cholinergic interneurons, which in turn trigger DA release. Furthermore, dopaminergic and cholinergic neurons commonly co-release more than one type of neurotransmitter. Neuromodulators can also alter the circuits that underlie working memory by modulating plasticity. Dopamine affects axonal and dendritic outgrowth, dendritic spine formation and synapse creation (Money and Stanwood 2013). Acetylcholine can promote long-term potentiation through its effects on facilitating NMDA transmission onto both glutamatergic and dopaminergic cells, and the interaction of dopamine, acetylcholine, and nitric oxide controls plasticity in key corticostriatal synapses (Centonze and others 2003). Brain damage, whether circumscribed or diffuse, affects the activity of several neurotransmitters. Altering the availability of neurotransmitters at synapses is a useful strategy to boost cognition in healthy subjects and brain-damaged patients (Berthier and Pulvermüller 2011). Drugs used to modulate the activity of a single neurotransmitter to enhance a cognitive function such as working memory, probably influence the function of several neurotransmitters. Therefore, attributing behavioral deficits to abnormal regulation of a single transmitter system may be misleading. Altogether, these arguments suggest that future pharmacological studies aimed to enhance working memory capacity in health or disease might examine if benefits already obtained by modulating a single transmitter system can be boosted further by combining agents acting on more than one neurotransmitter system.
Figure 3.
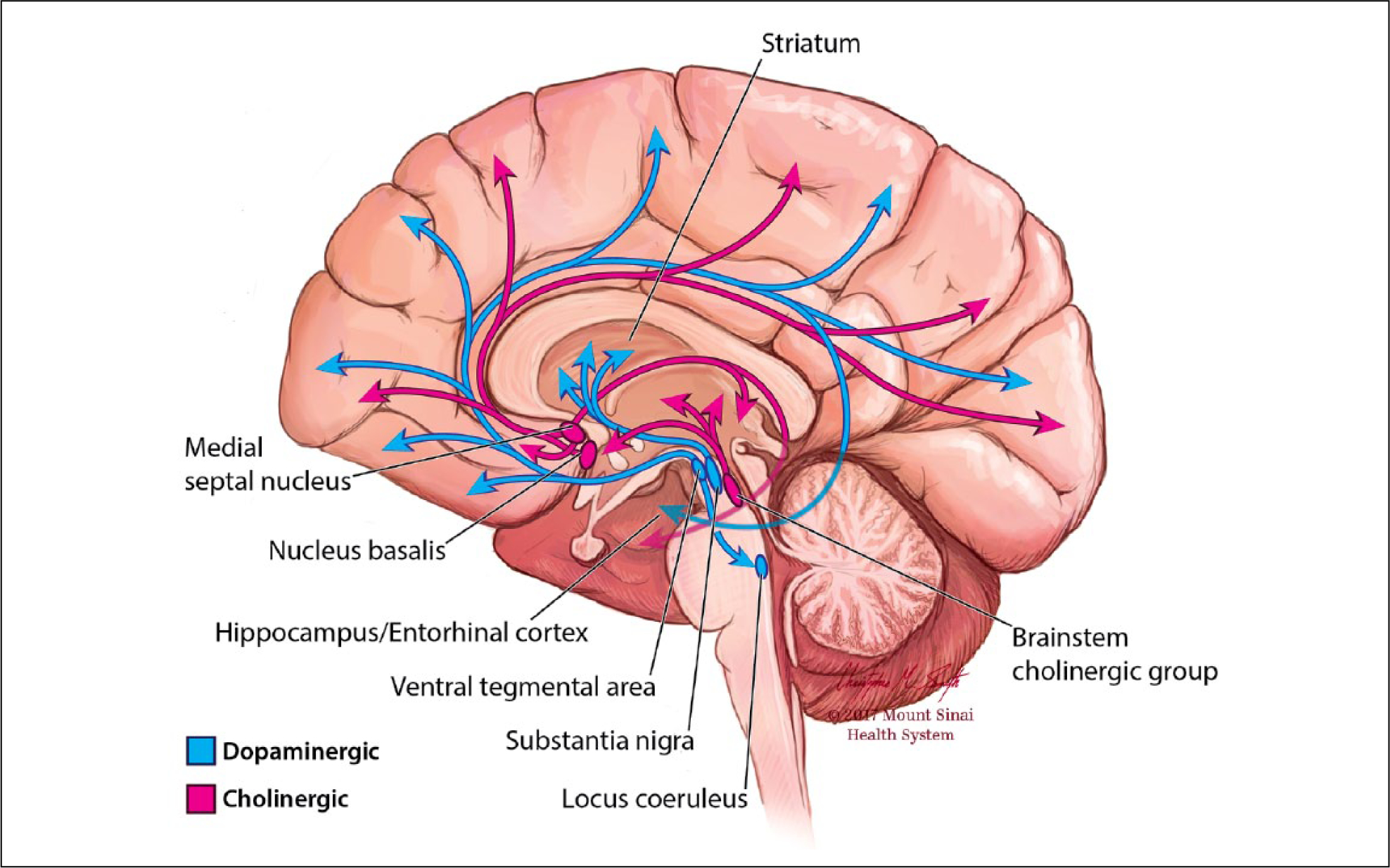
Dopamine and acetylcholine. Ascending projections of the dopaminergic and cholinergic systems from midbrain and basal forebrain innervate diverse cortical and subcortical targets.
Thus, as the executive components contributing to working memory become more elaborate, the prefrontal and parietal cortices become increasingly influential. Computational and experimental work has suggested that working memory that is robust in the face of distractors depends on a particular combination of neurotransmitters and cell types in the PFC, which undergo dramatic changes around the time working memory becomes more dependent on this region. This suggests that naturally occurring neurodevelopmental plasticity affecting neurotransmitter function may drive the adolescent shift of the working memory system toward the PFC, or alternatively that the requirement for more complex working memory processes may itself drive these changes.
Working Memory Relies on Communication between Cognitive Networks during Adulthood.
The adult working memory system relies on lateral PFC cortex, frontal eye fields, and posterior parietal cortex as well as subcortical regions, including the caudate nucleus, parts of the thalamus, the white matter tracts connecting these regions, and a range of neurotransmitters (Box 2, Figures 1, 2 and 3) (see Owen and others 2005 for a review). Other regions and pathways may be recruited under when working memory capacity is exceeded or under strain (Jeneson and others 2012; Lopez-Barroso and others 2011). The frontoparietal cortical regions in this network are also known as the executive control network. Successful working memory performance also induces suppression of the default mode network (posterior cingulate gyrus, inferior parietal lobes, and ventromedial prefrontal cortex) and involvement of the salience network (described above), possibly acting as a switch between the frontoparietal executive control network and the default mode network (Sridharan and others 2008).
In Aging, Plasticity in Frontal and Parietal Cortex Affects Working Memory Function.
Successful maintenance of working memory abilities during aging may either rely on compensatory changes for the inevitable decline in brain structure, or on maintaining youthful activation patterns (Samu and others 2017). Connectivity generally decreases within cognitive networks, yet older adults show more symmetrical prefrontal activation and a posterior to anterior shift in activation during working memory tasks (Figure 4) (Ansado and others 2012; Cabeza 2002). Reduced connectivity within the default mode and salience networks, and increased connectivity in the executive control network is associated with worse working memory performance during aging (Charroud and others 2016). This suggests that the salience network, which is important during the early development, either still contributes to working memory during healthy aging, or is temporarily able to compensate for the loss of function of frontoparietal cortex before this ability also deteriorates.
Figure 4.
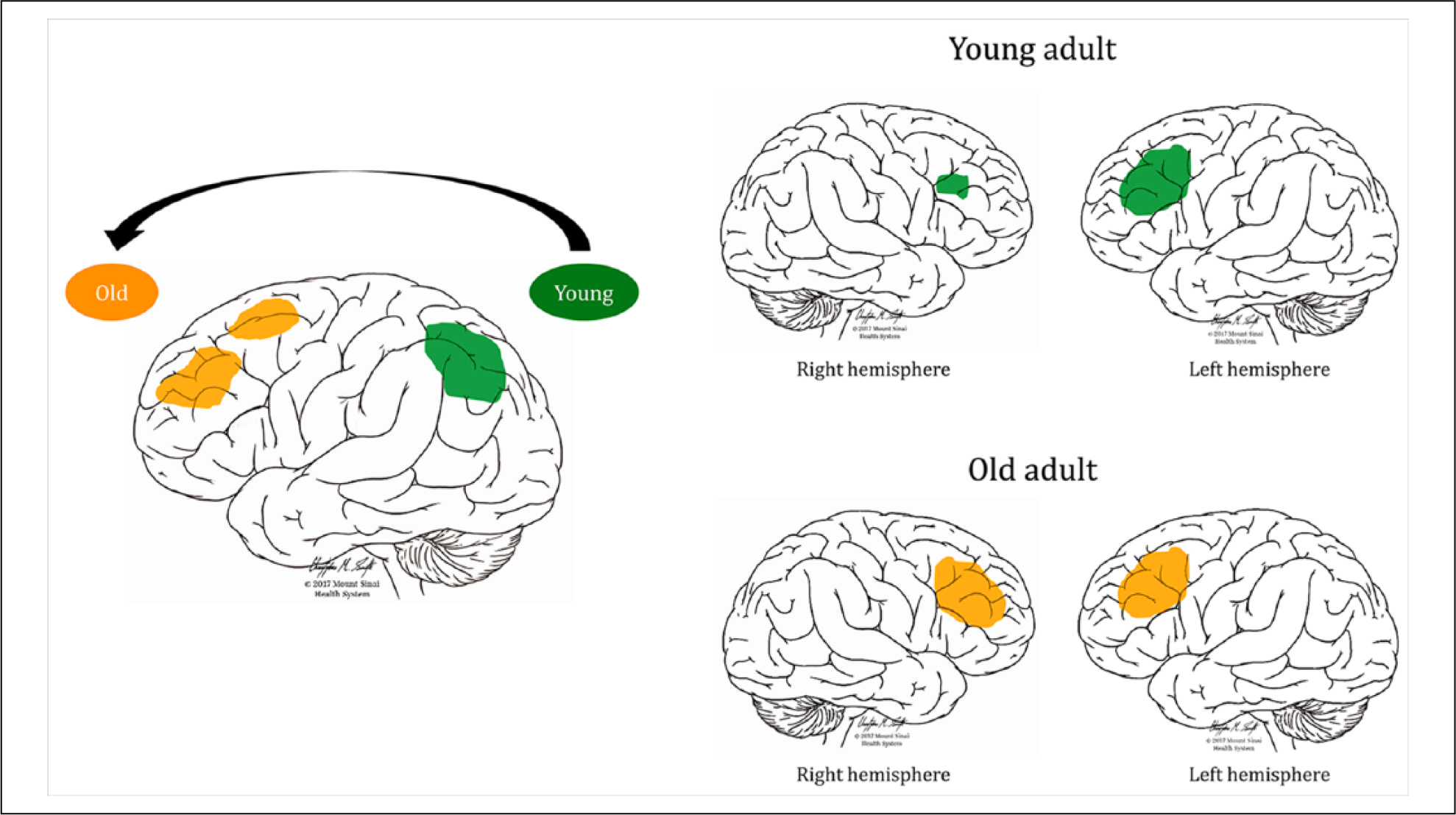
Examples of working memory adaptations during healthy aging. The left image shows a posterior-anterior shift in activation during a complex visual selective attention task (Ansado and others 2012). Younger subjects show greater activation in the posterior parietal cortex bilaterally, whereas older subjects shift their activation to more frontal regions (Davis and others 2008; Ansado and others 2012). The right image shows the usual left-dominant asymmetric activation in young adults during a verbal working memory task (top) and more symmetrical bilateral activation of prefrontal brain regions in older adults during the same task to compensate for age-related neural deficits (bottom, Cabeza, 2002).
These changes in activation may represent adaptive changes in response to structural decline, or an increasing reliance on “neural reserve.” Some researchers point to a reduction of inhibitory processes eventually causing decrements in working memory control, whereas others propose that changes in activation patterns are to enhance cognitive control. It has been suggested that changes in the pattern of activation in frontoparietal and frontostriatal networks during working memory tasks may reflect aging-related biological alterations, including neuronal degeneration, loss of dendrites and synapses, decreased length of myelinated axons, and vascular changes as well as dysregulation of dopaminergic and cholinergic systems that innervate these pathways (Störmer and others 2012; Toepper and others 2014).
Loss of Prefrontal Dendritic Spines, and Neurotransmitter Function May Limit Adaptive Plasticity in the Aging Working Memory System.
Non-human primate studies have shown that aging-related structural degradation is highly dependent on the brain region in question. Neuronal loss has been observed in the frontal eye fields (Smith and others 2004) but not the dlPFC (Peters and others 1994). PFC connectivity is affected through reduced dendritic branching, demyelination, and a loss of thin dendritic spines (Cupp and Uemura 1980; Dumitriu and others 2010). Thinner spines express less AMPA, and likely a greater proportion of NMDA receptors than thicker, mushroom spines (Kasai and others 2003) and are hypothesized to underlie the rapid changes in stored content that occur in new learning and working memory (Arnsten and others 2010; Morrison and Baxter 2012). These structural changes may lead to the observed loss of persistent delay-period firing (a potential functional marker of distractor-resistant working memory), but not cue-related firing in the dlPFC during working memory maintenance in aging (Wang and others 2011).
NMDA receptors are particularly vulnerable to the effects of aging (compared with cholinergic and GABA-ergic changes) in rats and monkeys, with much of the loss occurring in PFC in monkeys (Wenk and others 1991). Reductions in dopamine concentrations are also most drastic in PFC, whereas cortical norepinephrine and serotonin levels remain essentially unchanged (Goldman-Rakic and Brown 1981). Although cholinergic projections to the dlPFC (area 46) may be relatively preserved in normal aging, projections to the neighboring frontal eye fields are not (Smith and others 2004), potentially influencing the vulnerability to cell death in that area.
Thus, during aging, the prefrontal cortex loses dendritic spines and neuromodulatory input, that affects not only the local and long-range connectivity required for robust working memory but also the ability of the brain to plastically react to structural changes associated with aging (Calabresi and others 2007; Croxson and others, 2012). This likely constrains the compensatory response to only be effective until a certain degree of degradation has occurred.
Plasticity of the Working Memory System in Response to Injury in Humans
Preterm Birth as a Human Model of Developmental Disruption to the Working Memory System
Although it is difficult to study developmental lesions with great anatomical specificity in humans, some clinical groups, such as those born very preterm, frequently suffer early developmental injuries to structures in the developing working memory system. Working memory is often greatly affected during childhood following preterm birth, and there has been mixed evidence as to whether working memory deficits persist into adulthood (Nosarti and Froudist-Walsh 2016), with visual working memory perhaps more affected than other modalities (Menegaux and others 2017) (as is the case with normal aging). This provides an opportunity to study plasticity following early disruption to the working memory system.
Early Disruption of Deep Gray Matter Structures and Their Connections Affects Childhood Working Memory and Later Neurodevelopment.
Deep gray matter and white matter alterations are apparent following very preterm birth and associated with working memory deficiencies early in life, while working memory deficits associated with fronto-parietal alterations emerge later (Nosarti and Froudist-Walsh 2016). Hippocampal volume, but not PFC or parietal cortex volume assessed at the date of expected birth in very preterm infants is associated with working memory performance at 2 years of age (Beauchamp and others 2008). Adult hippocampal volumes, and microstructural alterations in the fornix (its major subcortical white matter connection) in those born very preterm are also correlated with working memory ability (Aanes and others 2015; Caldinelli and others 2017) supporting the idea that early damage to the hippocampus can alter later cortical development, even in humans.
Neonatal injury to the thalamus and striatum is predictive of working memory performance at 7 years of age in children born very preterm (Omizzolo and others 2014). Thalamocortical and corticostriatal connectivity is affected neonatally (Ball and others 2013), in childhood (Fischi-Gómez and others 2015) and in adulthood (Karolis and others 2016). This damage leads to prioritized development of connections between hub areas, possibly at the expense of minor connections (Karolis and others 2016). In adulthood, the connectivity between the executive control network, default mode network and salience network is altered, with connectivity between the striatum and the default mode network particularly affected (White and others 2014).
Increased Insula Activity Can Compensate for Disrupted Development of the Frontoparietal Network.
Disrupted connectivity due to white matter damage is associated both with reductions in short-term memory performance and compensatory plasticity in the working memory network. Adults born very preterm have both reduced FA in part of the splenium of the corpus callosum, and exhibit a positive correlation between splenium FA and visual short-term memory that is not seen the control group (Menegaux and others 2017). In other words, the more similar the splenium is to controls’, the smaller the memory impairments. This linear association may only be detectable in the preterm group as they sample the lower end of an inverted-U shaped splenium FA–working memory distribution (similar to the neuromodulatory system—Box 2). Those born very preterm who have macroscopic perinatal brain injury (as diagnosed by neonatal ultrasound scans) have volumetric reductions in the cingulum bundle in adulthood, and show reduced activation of the typical frontoparietal network during a challenging working memory task (3-back). However, they show significantly greater activation of the insula and surrounding perisylvian regions than controls on harder levels of the task. Greater activation of the insula was associated both with greater damage to the cingulum bundle, and positively with task performance. That is, the more preterm-born adults with perinatal brain injury differed from controls in their brain activation patterns, the better they performed the working memory task (Froudist-Walsh and others 2015). These results provide substantial evidence of successful compensatory plasticity (Cabeza and Dennis 2012). This compensatory plasticity was not found in very preterm adults (mostly) without brain injury, on an easier variant of the n-back task (Daamen and others 2015), suggesting that this pattern of functional compensation may be either specific to those with macroscopic perinatal brain injury, or only detectable on very challenging working memory tasks (Cabeza and Dennis 2012).
Disruption to GABA, Acetylcholine, and Dopamine Systems after Preterm Birth Suggest Possible Plasticity-Promoting Working Memory Interventions.
The function of several neurotransmitters that are critical to working memory function may be affected following very preterm birth, including GABA, acetylcholine, and dopamine. Preclinical studies in rodents suggest that hippocampal and cortical parvalbumin-expressing GABA-ergic interneuron development is particularly affected (Salmaso and others 2014), which may affect the stability of working memory representations (Box 2). A recent structural MRI study in adults born preterm focused on the basal forebrain, an area rich in cortically projecting cholinergic neurons. Basal forebrain volume was decreased in the preterm group, was related to the degree of neonatal complications, and mediated the association between neonatal complications and cognitive function (Grothe and others 2016). Apart from the direct effect that reduced cholinergic transmission has on working memory function in the adult brain (Croxson and others 2011), altered cholinergic transmission can also affect the degree to which the injured brain can plastically recover (Croxson and others 2012), and could have implications for successful brain aging. Indeed, premature brain aging may occur in adults born preterm (Karolis and others 2017).
The only direct in vivo evidence of neurotransmitter dysfunction following preterm birth in humans comes from the dopamine system. Reduced dopamine synthesis in the striatum was found in individuals born preterm with perinatal brain injury, and was correlated with the volume of the hippocampus (Froudist-Walsh and others 2017). This provides a crucial link between animal models of neonatal hippocampal lesions, which result in dopamine dysfunction, altered prefrontal cortex development and working memory impairments (Tseng and others 2009), and human studies showing an inverted-U shaped relationship between striatal dopamine synthesis and working memory performance (Cools and D’Esposito 2011). As individuals with low striatal dopamine synthesis capacity respond better to pro-dopaminergic drugs as cognitive enhancers (Cools and others 2009), it raises the possibility of a pharmacological intervention in those born very preterm with working memory impairments.
Intrinsic and Induced Plasticity of the Working Memory System in Brain-Injured Adults
Acquired brain injury (ABI) refers to damage to the brain after birth that is not related to congenital disorders, developmental disabilities, or progressive degeneration. The majority of ABI cases result from either traumatic brain injury (TBI) or hemorrhagic or ischemic stroke. Despite treatment focusing on motor and language recovery in stroke, a study focused on “hidden” cognitive symptoms found that almost 90% of subacute stroke patients suffered from working memory deficits (Jaillard and others 2009). Traumatic brain injury survivors also have deficits on a variety of working memory measures (Dunning and others 2016).
Spontaneous Plasticity after Adult Brain Injury.
Recovery of working memory in neurological patients with ABI depends on the amount of tissue spared by the lesion in areas contributing to working memory function, plus the functional status of areas beyond structurally damaged cerebral tissue. The reemergence of functional activity in areas deprived from neural input and/or deranged neurovascular coupling, whether close or remote to the focal brain injury, is crucial for recovery of cognitive function (Voytek and others 2010) (Box 3). Spontaneous plastic changes follow brain damage (Figure 5), which can restore working memory activity without any specific intervention (Cramer 2008). Patients with TBI often show changes in functional activation during working memory tasks that mimic those seen during aging, such as increased right dlPFC activation during verbal working memory (Turner and others 2011). Alterations to the salience and default mode networks (Sharp and others 2014) are also likely to affect working memory. While some studies have reported hub regions to be particularly affected by TBI (Sharp and others 2014), other have reported increased connectivity between these regions (Hillary and others 2014), similar to what is found following very preterm birth.
Box 3. Types of Neuroplasticity after Focal Brain Injury.
Loss of cognitive function after brain injury is caused by cell death in the lesioned area, cell malfunction in the perilesional area, and loss of input in remote areas from the same network. Recovery of function can occur spontaneously from weeks to months after injury (Cramer 2008). It depends not only on the restoration of the lesioned and perilesional areas but also on compensatory brain responses involving changes in the properties of neural pathways. Beyond spontaneous recovery, other forms of neuroplasticity that can be modulated with behavioral training, pharmacological interventions or brain stimulation play an important role in functional recovery.
At least four forms of neuroplasticity after focal brain injury have been described (Grafman 2000): (1) Homologous area adaptation refers to the shifting of a particular function from the damaged brain area to other brain areas, usually the homologous region of the contralateral hemisphere. This adaptation is more prevalent during childhood and increases the likelihood of dual-task interference between the original cognitive operation of the homologous area and the “reassigned” operation. (2) Cross-modal reassignment occurs when an area that has been deprived from its main inputs, receives input from new areas. This type of plasticity has limitations related to the type of computations the area can perform on the new input. (3) Map expansion denotes the enlargement of the size of cortical maps dedicated to a particular process due to training or repetitive exposure, which can be interpreted as the recruitment of new neurons into the network. (4) Compensatory masquerade is the adoption of a new strategy or processes in order to perform a task that previously depended on an impaired cognitive process.
Figure 5.
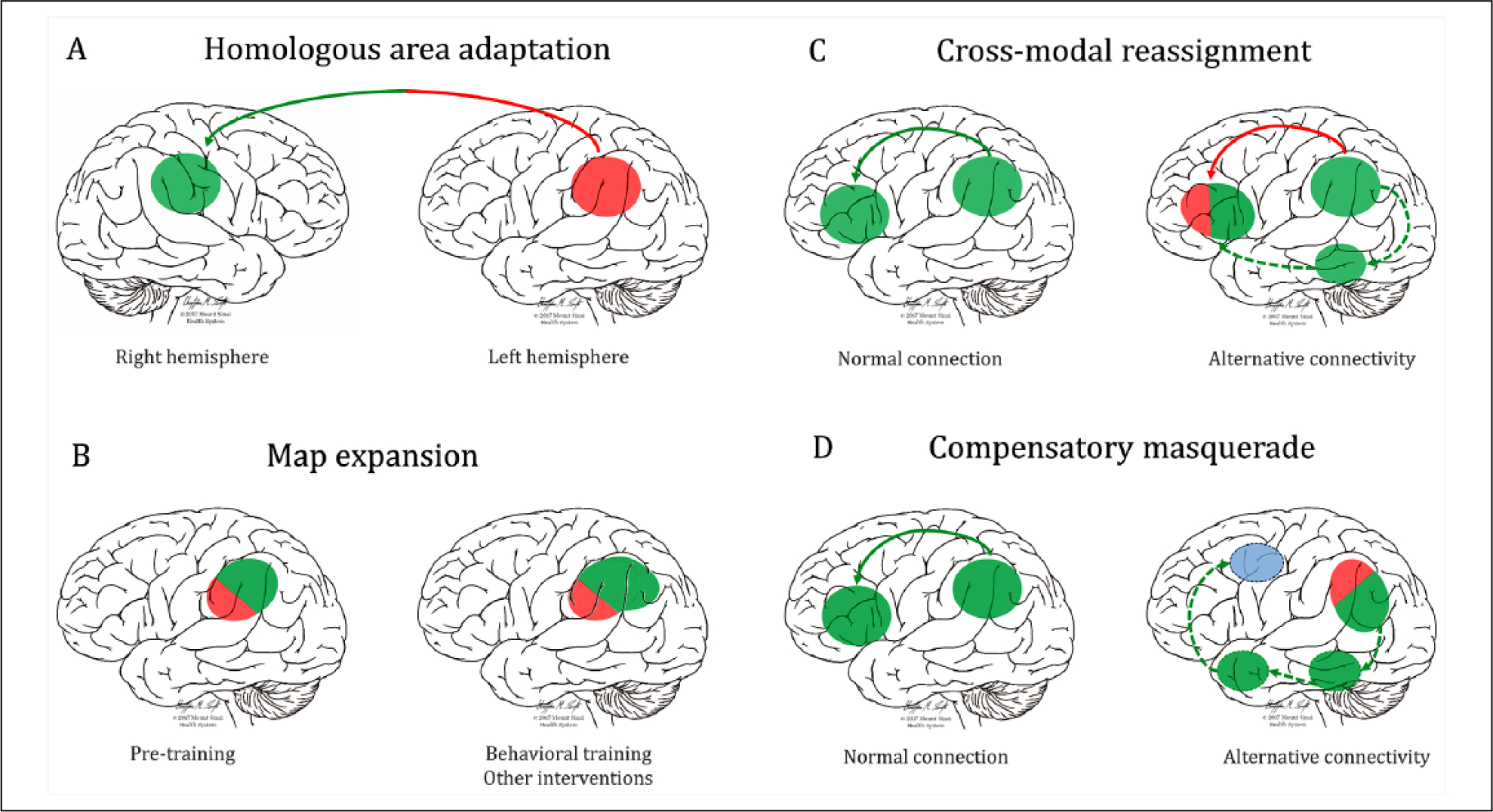
Different types of brain plasticity following lesions. Lesions or dysfunctions of a brain area alter the interaction between structurally normal regions and between normal and dysfunctional regions. (A) Interhemispheric transfer of a particular function from one lesioned area to its homotopic counterpart. (B) A normally functioning area can enlarge its volume in response to repetitive training or other treatments (drugs, brain stimulation). Dysfunctional unlesioned areas (as can occur during aging) can also show greater than normal activity during demanding tasks to in order maintain normal performance. (C) When an area is deprived from its main inputs (e.g. due to hearing loss, blindness, tract hypoplasia) patients can maintain normal task performance by receiving input from new areas. (D) New strategies using alternative spared regions and connections can be employed to undertake the affected task. Note that more than one type of plasticity (i.e., homologous area adaptation and ipsilateral map extension) can co-occur in a single subject. Green circles represent preserved brain areas, red circles fully lesioned/dysfunctional areas, and mixed red/green circles partially lesioned-dysfunctional areas. The blue circle represents vicarious compensation by an area not previously implicated in the function. Red lines represent interrupted connections, green lines normal connections, and dotted green lines new, alternative connections. See further information in Box 3 and text.
Plasticity-Promoting Interventions for Recovery of Working Memory Function.
Intrinsic changes occur mainly during the first 6 months after the lesion and tend to wane afterward, reaching a plateau 1 year after onset (Kleim and Jones 2008). At this point, behavioral training and restorative therapies emerge as good candidates to potentiate further reorganization in brain structure, function and connectivity beyond spontaneous recovery. Working memory training can induce changes in the neurotransmitters systems and cognitive networks underlying normal working memory performance. Plasticity in the density of D1 receptors in prefrontal cortex and increased dopaminergic release in the striatum occur after training (Bäckman and others 2011; McNab and others 2009), while improvements are more likely to transfer to other tasks if both tasks engage the same cognitive networks (Dahlin and others 2008). As a wide range of tasks recruit frontoparietal regions, plasticity associated with working memory training could have wide-reaching implications.
Although working memory training in subjects with brain injury has had some success (Weicker and others 2016), the benefits of behavioral training alone are being increasingly questioned, and gains are not always transferred to other cognitive domains (Melby-Lervåg and Hulme 2013). Drugs targeting the neuromodulatory systems are becoming popular to modulate working memory capacity in combination with behavioral training.
Pharmacological Treatments Have Had Limited Success in Promoting Working Memory Recovery.
Dopamine stimulation with the D2 receptor agonist bromocriptine improves working memory performance and increases frontostriatal connectivity in healthy subjects with low baseline working capacity, but has the opposite effect on those with high baseline capacity (Kimberg and others 1997; Wallace and others 2011). An early bromocriptine study in TBI patients found an increase in digit span in all nine patients assessed (Powell and others 1996). Later trials in patients with traumatic brain injury of mixed severity had no beneficial effects on working memory evaluated with reading span and spatial delayed-response tasks (McDowell and others 1998) or with a visually presented verbal n-back task (McAllister and others 2011). In one such study, only healthy subjects improved (McAllister and others 2011). fMRI revealed increased activation of working memory networks in healthy controls, but the lack of response to bromocriptine in traumatic patients was associated with increased activation in brain regions not related to working memory. Although in healthy subjects D1, but not D2, dopamine receptor agonists facilitate spatial working memory (Müller and others 1998), treatment with rotigotine (a high affinity D1 receptor agonist) failed to improve spatial working memory in patients with stroke-related hemispatial neglect following right hemisphere involvement (Gorgoraptis and others 2012). Similarly, methylphenidate, which blocks dopamine and noradrenaline transporters and thus increases dopamine and noradrenaline transmission, has had limited success in improving working memory function in TBI patients (Kim and others 2006; Kim and others 2012; Willmott and Ponsford 2009). This suggests that strategies that focus pharmacological treatment to act primarily on areas critical to working memory function may be needed.
Brain Stimulation for Promoting Working Memory Plasticity.
In addition to pharmacological approaches and behavioral training, non-invasive brain stimulation techniques, including transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS), have emerged as promising tools to enhance working memory capacity in healthy and patient populations. Brain stimulation modulates cortical excitability, affecting the release of neurotransmitters in both the directly stimulated and connected areas. For instance, high-frequency repetitive TMS over the dlPFC, induces incremental changes in endogenous dopamine in ipsilateral striatum (Strafella and others 2001), anterior cingulate, and medial orbitofrontal cortex (Cho and Strafella 2009). Both (excitatory) repetitive TMS and tDCS to left dlPFC have had initial success in improving working memory performance in healthy participants and those with brain injury (Brunoni and Vanderhasselt 2014; Jo and others 2009).
The individual actions of pharmacological treatment, brain stimulation, and behavioral treatment on the working memory network may be augmented if used in combination. The plasticity-promoting effects of pharmacological agents may effectively enhance experience-dependent plasticity if combined with behavioral training (Berthier and others 2014; Forsyth and others 2015), or if guided to the desired neural circuits using brain stimulation (Faingold and Blumenfeld 2015). The negative results of pharmacological treatment for brain-damaged patients may be due to low, and often single doses, as well as a lack of concurrent behavioral therapy. Furthermore, different pharmacotherapy strategies may be more effective at different stages post-injury due to the evolving nature of the injury and associated plasticity (Hoskison and others 2009).
Conclusions
Convergent evidence from animal lesion studies and neuroimaging studies in normally developing children suggest that the juvenile working memory system is dramatically different from the adult, with less reliance on dlPFC, and greater involvement of subcortical and ventral cortical areas including the hippocampus, striatum, thalamus, and insula. It is important to emphasize that, although the dlPFC may be less central to the working memory system at this point, it is not idly waiting to be brought online, and in fact shows adult-like function on other prefrontal dependent tasks at a younger age (Bunge and Wright 2007). A transition to a more frontoparietal dependent network occurs in late childhood and early adolescence, with further refinements in the frontoparietal network associated with improvements in working memory capacity and the executive aspects of working memory. The relatively late reliance of the working memory system on the dlPFC may be related to the protracted development of neurotransmitter function in this area of cortex, the optimal balance of which is crucial for the increasingly complex executive functions associated with working memory during adolescence. Changes to the action of neuromodulators on excitatory and inhibitory neurotransmission, and in receptor expression enhance the ability of the PFC to express persistent firing, which likely facilitates maintenance of memories across a delay in the face of distraction. Although some aspects of working memory are relatively robust to aging (such as verbal short-term memory), the visuospatial and executive components greatly decline. This decline of the executive components is accompanied by declining levels of neurotransmitters in frontal regions during aging. The dopaminergic projections to the PFC, cholinergic projections to the frontal eye fields, and prefrontal NMDA receptor expression are particularly vulnerable to aging and are accompanied by more symmetrical frontal and reduced parietal activation, and disconnectivity of cognitive networks. To some extent the aging process, as far as working memory is concerned, mirrors the developmental process, albeit with much less dramatic changes. This may be the result of distinct processes having opposing effects on working memory abilities at different points in the life span.
Following brain injury, plasticity can be yet more dramatic, and there are several routes to adaptive, or maladaptive plasticity (Figure 5). In this review, we highlight perinatal brain injury resulting from very preterm birth as providing a unique insight into how brain injury-induced plasticity interacts with natural developmental plasticity. Early life injury to deep grey matter structures leads to impaired working memory function during childhood, and altered development of the frontoparietal cortical structures and neurotransmitter systems required for the mature working memory system. In some cases, cortical structures that normally become less central to working memory function during development, such as the insula can compensate for these early injuries. This highlights a potential need for future studies explicitly comparing plasticity in working memory systems between the developing and adult brain, in order to establish whether subcortical and cortical damage leads to the same effects across the life span and how to differently promote plasticity at different stages of life. Because the juvenile and adult human brain tend to experience different injuries, animal studies investigating plasticity patterns following damage to the same structures at different ages may play an important role here.
An interesting possibility that is yet to be investigated is that the degree to which compensatory plasticity after injury may occur depends on the degree of spared dopaminergic and cholinergic projections. Behavioral and pharmacological interventions have been attempted in order to boost adaptive plasticity following adult brain injury, but have so far achieved mixed success. The possibility of combinations of behavioral, pharamacological, and brain stimulation interventions holds promise as a way to enhance the naturally occurring experience-dependent plasticity. Nonetheless, much work needs to be done in order to identify the optimal drug type, time of administration following injury, dosage, and combination in order to boost working memory abilities following different types of brain injury. We are beginning to understand why different areas become important to working memory at different stages of development and aging. The challenge going forward is to hone in on the optimal ways to commandeer the brain’s intrinsic plasticity mechanisms, in order to improve or restore working memory function.
Acknowledgments
The authors would like to thank Christopher Smith for figure preparation and Mark Baxter for helpful comments on the manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: M.J.T.-P. has been funded by a PhD scholarship from the Spanish Ministry of Education, Culture and Sport under the FPU program (FPU14/04021). D.L.B. has been supported by the “Juan de la Cierva” program of the Spanish Ministry of Economy and Competitiveness (FJCI-2014-22953). P.L.C. is supported by NINDS grant 1R21NS096936-01A1, NARSAD Young Investigator Grant 25102 and the Friedman Brain Institute.
Footnotes
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.L.B. has received honoraria from Pfizer, Eisai, Janssen-España, Novartis, Lundbeck, and Nutricia and consultancy for fees from Merz, Eli Lilly, and GlaxoSmithKline. He has received speaking fees from Pfizer, Eisai, Janssen-España, Novartis, Lundbeck, and Nutricia. All other authors report no conflicts of interest.
References
- Aanes S, Bjuland KJ, Skranes J, Løhaugen GCC. 2015. Memory function and hippocampal volumes in preterm born very-low-birth-weight (VLBW) young adults. Neuroimage 105:76–83. [DOI] [PubMed] [Google Scholar]
- Alcauter S, Lin W, Smith JK, Short SJ, Goldman BD, Reznick JS, and others. 2014. Development of thalamocortical connectivity during infancy and its cognitive correlations. J Neurosci 34:9067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Goldman PS. 1978. Functional development of the dorsolateral prefrontal cortex: an analysis utilizing reversible cryogenic depression. Brain Res 143:233–49. [DOI] [PubMed] [Google Scholar]
- Alloway TP, Alloway RG. 2013. Working memory across the lifespan: a cross-sectional approach. J Cogn Psychol 25:84–93. [Google Scholar]
- Alloway TP, Gathercole SE, Pickering SJ. 2006. Verbal and visuospatial short-term and working memory in children: are they separable? Child Dev 77:1698–716. [DOI] [PubMed] [Google Scholar]
- Ansado J, Monchi O, Ennabil N, Faure S, Joanette Y. 2012. Load-dependent posterior–anterior shift in aging in complex visual selective attention situations. Brain Res 1454:14–22. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Paspalas CD, Gamo NJ, Yang Y, Wang M. 2010. Dynamic network connectivity: a new form of neuroplasticity. Trends Cogn Sci 14:365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Soveri A, Johansson J, Andersson M, Dahlin E, and others. 2011. Effects of working-memory training on striatal dopamine release. Science 333:718. [DOI] [PubMed] [Google Scholar]
- Baddeley A 2000. The episodic buffer: a new component of working memory? Trends Cogn Sci 4:417–23. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch G. 1974. Working memory. Psychol Learn Motiv 8:47–89. [Google Scholar]
- Ball G, Boardman JP, Aljabar P, Pandit A, Arichi T, Merchant N, and others. 2013. The influence of preterm birth on the developing thalamocortical connectome. Cortex 49:1711–21. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. 2007. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8:45–56. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Thompson DK, Howard K, Doyle LW, Egan GF, Inder TE, and others. 2008. Preterm infant hippocampal volumes correlate with later working memory deficits. Brain 131:2986–94. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, and others. 2007. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science 318:1645–7. [DOI] [PubMed] [Google Scholar]
- Berthier ML, Dávila G, Green-Heredia C, Torres IM, Juárez y Ruiz de Mier R, De-Torres I, and others. 2014. Massed sentence repetition training can augment and speed up recovery of speech production deficits in patients with chronic conduction aphasia receiving donepezil treatment. Aphasiology 28:188–218. [Google Scholar]
- Berthier ML, Pulvermüller F. 2011. Neuroscience insights improve neurorehabilitation of poststroke aphasia. Nat Rev Neurol 7:86–97. [DOI] [PubMed] [Google Scholar]
- Brockmole JR, Logie RH. 2013. Age-related change in visual working memory: a study of 55,753 participants aged 8–75. Front Psychol 4:12. 10.3389/fpsyg.2013.00012/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. 1979. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science 205:929–32. [DOI] [PubMed] [Google Scholar]
- Brunel N, Wang X-J. 2001. Effects of neuromodulation in a cortical network model of object working memory dominated by recurrent inhibition. J Comput Neurosci 11:63–85. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Vanderhasselt M-A. 2014. Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: a systematic review and meta-analysis. Brain Cogn 86:1–9. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wright SB. 2007. Neurodevelopmental changes in working memory and cognitive control. Curr Opin Neurobiol 17:243–50. [DOI] [PubMed] [Google Scholar]
- Caballero A, Thomases DR, Flores-Barrera E, Cass DK, Tseng KY. 2014. Emergence of GABAergic-dependent regulation of input-specific plasticity in the adult rat prefrontal cortex during adolescence. Psychopharmacology (Berl) 231: 1789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R 2002. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging 17:85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dennis NA. 2012. Frontal lobes and aging: deterioration and compensation. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. 2nd ed. New York, NY: Oxford University Press. p. 628–52. [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. 2007. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci 30:211–9. [DOI] [PubMed] [Google Scholar]
- Caldinelli C, Froudist-Walsh S, Karolis V, Tseng C-E, Allin MP, Walshe M, and others. 2017. White matter alterations to cingulum and fornix following very preterm birth and their relationship with cognitive functions. Neuroimage 150:373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Gubellini P, Pisani A, Bernardi G, Calabresi P. 2003. Dopamine, acetylcholine and nitric oxide systems interact to induce corticostriatal synaptic plasticity. Rev Neurosci 14:207–16. [DOI] [PubMed] [Google Scholar]
- Charroud C, Le Bars E, Deverdun J, Steffener J, Molino F, Abdennour M, and others. 2016. Working memory performance is related to intrinsic resting state functional connectivity changes in community-dwelling elderly cohort. Neurobiol Learn Mem 132:57–66. [DOI] [PubMed] [Google Scholar]
- Cho SS, Strafella AP. 2009. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One 4:e6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compte A, Brunel N, Goldman-Rakic PS, Wang X-J. 2000. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex 10:910–23. [DOI] [PubMed] [Google Scholar]
- Cools R, D’Esposito M. 2011. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 69:e113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M. 2009. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci 29:1538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N 1999. An embedded-processes model of working memory. In: Miyake A, Shah P, editors. Models of working memory: mechanisms of active maintenance and executive control. Cambridge, England: Cambridge University Press. p. 62–101. [Google Scholar]
- Cramer SC. 2008. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol 63:272–87. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Browning PGF, Gaffan D, Baxter MG. 2012. Acetylcholine facilitates recovery of episodic memory after brain damage. J Neurosci 32:13787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxson PL, Kyriazis DA, Baxter MG. 2011. Cholinergic modulation of a specific memory function of prefrontal cortex. Nat Neurosci 14:1510–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupp CJ, Uemura E. 1980. Age-related changes in prefrontal cortex of Macaca mulatta: quantitative analysis of dendritic branching patterns. Exp Neurol 69:143–63. [DOI] [PubMed] [Google Scholar]
- Daamen M, Bäuml JG, Scheef L, Sorg C, Busch B, Baumann N, and others. 2015. Working memory in preterm-born adults: load-dependent compensatory activity of the posterior default mode network. Hum Brain Mapp 36:1121–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin E, Neely AS, Larsson A, Bäckman L, Nyberg L. 2008. Transfer of learning after updating training mediated by the striatum. Science 320:1510–2. [DOI] [PubMed] [Google Scholar]
- Darki F, Klingberg T. 2015. The role of fronto-parietal and fronto-striatal networks in the development of working memory: a longitudinal study. Cereb Cortex 25:1587–95. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. 2008. Qué PASA? The posterior–anterior shift in aging. Cereb Cortex 18:1201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A 1985. Development of the ability to use recall to guide action, as indicated by infants’ performance on AB. Child Dev 56:868–83. [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WGM, Lou W, and others. 2010. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci 30:7507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning DL, Westgate B, Adlam A-LR. 2016. A meta-analysis of working memory impairments in survivors of moderate-to-severe traumatic brain injury. Neuropsychology 30:811–9. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Blumenfeld H. 2015. Targeting neuronal networks with combined drug and stimulation paradigms guided by neuroimaging to treat brain disorders. Neuroscientist 21:460–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn AS, Sheridan MA, Kam CLH, Hinshaw S, D’Esposito M. 2010. Longitudinal evidence for functional specialization of the neural circuit supporting working memory in the human brain. J Neurosci 30:11062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischi-Gómez E, Vasung L, Meskaldji D-E, Lazeyras F, Borradori-Tolsa C, Hagmann P, and others. 2015. Structural brain connectivity in school-age preterm infants provides evidence for impaired networks relevant for higher order cognitive skills and social cognition. Cereb Cortex 25:2793–805. [DOI] [PubMed] [Google Scholar]
- Fitch A, Smith H, Guillory SB, Kaldy Z. 2016. Off to a good start: the early development of the neural substrates underlying visual working memory. Front Syst Neurosci 10:68. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4989029/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Barrera E, Thomases DR, Heng L-J, Cass DK, Caballero A, Tseng KY. 2014. Late adolescent expression of GluN2B transmission in the prefrontal cortex is input-specific and requires postsynaptic protein kinase A and D1 dopamine receptor signaling. Biol Psychiatry 75:508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth JK, Bachman P, Mathalon DH, Roach BJ, Asarnow RF. 2015. Augmenting NMDA receptor signaling boosts experience-dependent neuroplasticity in the adult human brain. Proc Natl Acad Sci U S A 112:15331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froudist-Walsh S, Bloomfield MA, Veronese M, Kroll J, Karolis V, Jauhar S, and others. 2017. The effect of perinatal brain injury on dopaminergic function and hippocampal volume in adult life. bioRxiv 128710. doi: 10.1101/128710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froudist-Walsh S, Karolis V, Caldinelli C, Brittain PJ, Kroll J, Rodríguez-Toscano E, and others. 2015. Very early brain damage leads to remodeling of the working memory system in adulthood: a combined fMRI/tractography study. J Neurosci 35:15787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole SE, Baddeley AD. 2014. Working memory and language. New York, NY: Psychology Press. [Google Scholar]
- Goldman PS. 1971. Functional development of the prefrontal cortex in early life and the problem of neuronal plasticity. Exp Neurol 32:366–87. [DOI] [PubMed] [Google Scholar]
- Goldman PS. 1974. An alternative to developmental plasticity: heterology of CNS structures in infants and adults. In: Stein DG, Rosen JJ, Butters N, editors. Plasticity and recovery of function in the central nervous system. New York, NY: Academic Press. p. 149–74. [Google Scholar]
- Goldman PS, Rosvold HE. 1972. The effects of selective caudate lesions in infant and juvenile rhesus monkeys. Brain Res 43:53–66. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. 1995. Cellular basis of working memory. Neuron 14:477–85. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Brown RM. 1981. Regional changes of monoamines in cerebral cortex and subcortical structures of aging rhesus monkeys. Neuroscience 6:177–87. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M. 1989. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proc Natl Acad Sci U S A 86:9015–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoraptis N, Mah Y-H, Machner B, Singh-Curry V, Malhotra P, Hadji-Michael M, and others. 2012. The effects of the dopamine agonist rotigotine on hemispatial neglect following stroke. Brain 135:2478–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafman J 2000. Conceptualizing functional neuroplasticity. J Commun Disord 33:345–56. [DOI] [PubMed] [Google Scholar]
- Grothe MJ, Scheef L, Bäuml J, Meng C, Daamen M, Baumann N, and others. 2016. Reduced cholinergic basal forebrain integrity links neonatal complications and adult cognitive deficits after premature birth. Biol Psychiatry Epub Dec 15. doi: 10.1016/j.biopsych.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Heuer E, Bachevalier J. 2011. Neonatal hippocampal lesions in rhesus macaques alter the monitoring, but not maintenance, of information in working memory. Behav Neurosci 125:859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillary FG, Rajtmajer SM, Roman CA, Medaglia JD, Slocomb-Dluzen JE, Calhoun VD, and others. 2014. The rich get richer: brain injury elicits hyperconnectivity in core subnetworks. PLoS One 9:e104021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskison MM, Moore AN, Hu B, Orsi S, Kobori N, Dash PK. 2009. Persistent working memory dysfunction following traumatic brain injury: evidence for a time-dependent mechanism. Neuroscience 159:483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillard A, Naegele B, Trabucco-Miguel S, LeBas JF, Hommel M. 2009. Hidden dysfunctioning in subacute stroke. Stroke 40:2473–9. [DOI] [PubMed] [Google Scholar]
- Jeneson A, Wixted JT, Hopkins RO, Squire LR. 2012. Visual working memory capacity and the medial temporal lobe. J Neurosci 32:3584–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo JM, Kim Y-H, Ko M-H, Ohn SH, Joen B, Lee KH. 2009. Enhancing the working memory of stroke patients using tDCS. Am J Phys Med Rehabil 88:404–9. [DOI] [PubMed] [Google Scholar]
- Johnson MH, De Haan M. 2015. Developmental cognitive neuroscience: an introduction. Hoboken, NJ: Wiley [Google Scholar]
- Karolis VR, Froudist-Walsh S, Brittain PJ, Kroll J, Ball G, Edwards AD, and others. 2016. Reinforcement of the brain’s rich-club architecture following early neurodevelopmental disruption caused by very preterm birth. Cereb Cortex 26:1322–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolis VR, Froudist-Walsh S, Kroll J, Brittain PJ, Tseng C-EJ, Nam K-W, and others. 2017. Volumetric grey matter alterations in adolescents and adults born very preterm suggest accelerated brain maturation. bioRxiv 127365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. 2003. Structure–stability–function relationships of dendritic spines. Trends Neurosci 26:360–8. [DOI] [PubMed] [Google Scholar]
- Kim J, Whyte J, Patel S, Europa E, Wang J, Coslett HB, and others. 2012. Methylphenidate modulates sustained attention and cortical activation in survivors of traumatic brain injury: a perfusion fMRI study. Psychopharmacology (Berl) 222:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-H, Ko M-H, Na S-Y, Park S-H, Kim K-W. 2006. Effects of single-dose methylphenidate on cognitive performance in patients with traumatic brain injury: a double-blind placebo-controlled study. Clin Rehabil 20:24–30. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, D’esposito M, Farah MJ. 1997. Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport 8:3581–5. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Jones TA. 2008. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res 51:S225–39. [DOI] [PubMed] [Google Scholar]
- Lin H, Hsu F-C, Baumann BH, Coulter DA, Anderson SA, Lynch DR. 2014. Cortical parvalbumin GABAergic deficits with α7 nicotinic acetylcholine receptor deletion: implications for schizophrenia. Mol Cell Neurosci 61:163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska BK, Aultman JM, Verma A, Weinberger DR, Moghaddam B. 2002. Neonatal damage of the ventral hippocampus impairs working memory in the rat. Neuropsychopharmacology 27:47–54. [DOI] [PubMed] [Google Scholar]
- Lopez-Barroso D, de Diego-Balaguer R, Cunillera T, Camara E, Münte TF, Rodriguez-Fornells A. 2011. Language learning under working memory constraints correlates with microstructural differences in the ventral language pathway. Cereb Cortex 21:2742–50. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Flashman LA, McDonald BC, Ferrell RB, Tosteson TD, Yanofsky NN, and others. 2011. Dopaminergic challenge with bromocriptine one month after mild traumatic brain injury: altered working memory and BOLD response. J Neuropsychiatry Clin Neurosci 23:277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell S, Whyte J, D’Esposito M. 1998. Differential effect of a dopaminergic agonist on prefrontal function in traumatic brain injury patients. Brain 121:1155–64. [DOI] [PubMed] [Google Scholar]
- McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, and others. 2009. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science 323:800–2. [DOI] [PubMed] [Google Scholar]
- Melby-Lervåg M, Hulme C. 2013. Is working memory training effective? A meta-analytic review. Dev Psychol 49:270. [DOI] [PubMed] [Google Scholar]
- Menegaux A, Meng C, Neitzel J, Bäuml JG, Müller HJ, Bartmann P, and others. 2017. Impaired visual short-term memory capacity is distinctively associated with structural connectivity of the posterior thalamic radiation and the splenium of the corpus callosum in preterm-born adults. Neuroimage 150:68–76. [DOI] [PubMed] [Google Scholar]
- Miller EA, Goldman PS, Rosvold HE. 1973. Delayed recovery of function following orbital prefrontal lesions in infant monkeys. Science 182:304–6. [DOI] [PubMed] [Google Scholar]
- Money KM, Stanwood GD. 2013. Developmental origins of brain disorders: roles for dopamine. Front Cell Neurosci 7:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG. 2012. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci 13:240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U, von Cramon DY, Pollmann S. 1998. D1- versus D2-receptor modulation of visuospatial working memory in humans. J Neurosci 18:2720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C, Froudist-Walsh S. 2016. Alterations in development of hippocampal and cortical memory mechanisms following very preterm birth. Dev Med Child Neurol 58:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omizzolo C, Scratch SE, Stargatt R, Kidokoro H, Thompson DK, Lee KJ, and others. 2014. Neonatal brain abnormalities and memory and learning outcomes at 7 years in children born very preterm. Memory 22:605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østby Y, Tamnes CK, Fjell AM, Walhovd KB. 2011. Morphometry and connectivity of the fronto-parietal verbal working memory network in development. Neuropsychologia 49:3854–62. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. 2005. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp 25:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Leahu D, Moss MB, McNally KJ. 1994. The effects of aging on area 46 of the frontal cortex of the rhesus monkey. Cereb Cortex 4:621–35. [DOI] [PubMed] [Google Scholar]
- Powell JH, al-Adawi S, Morgan J, Greenwood RJ. 1996. Motivational deficits after brain injury: effects of bromocriptine in 11 patients. J Neurol Neurosurg Psychiatry 60:416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Sylvester C-YC. 2005. The cognitive neuroscience of working memory and aging. In: Cabeza R, Nyberg L, Park D, editors. Cognitive neuroscience of aging. Oxford, England: Oxford University Press. p. 186–217. [Google Scholar]
- Rosenberg DR, Lewis DA. 1994. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: a tyrosine hydroxylase immunohistochemical study. Biol Psychiatry 36:272–7. [DOI] [PubMed] [Google Scholar]
- Salmaso N, Jablonska B, Scafidi J, Vaccarino FM, Gallo V. 2014. Neurobiology of premature brain injury. Nat Neurosci 17:341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samu D, Campbell KL, Tsvetanov KA, Shafto MA, Tyler LK, others. 2017. Preserved cognitive functions with age are determined by domain-dependent shifts in network responsivity. Nat Commun 8:14743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf KS, Sweeney JA, Luna B. 2006. Brain basis of developmental change in visuospatial working memory. J Cogn Neurosci 18:1045–58. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Melchitzky DS, Lewis DA. 1998. Dopamine innervation of a subclass of local circuit neurons in monkey prefrontal cortex: ultrastructural analysis of tyrosine hydroxylase and parvalbumin immunoreactive structures. Cereb Cortex 8:614–22. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Scott G, Leech R. 2014. Network dysfunction after traumatic brain injury. Nat Rev Neurol 10:156–66. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Hallquist MN, Luna B. 2017. Protracted development of executive and mnemonic brain systems underlying working memory in adolescence: a longitudinal fMRI study. Neuroimage. Epub Apr 26. doi: 10.1016/j.neuroimage.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Rapp PR, McKay HM, Roberts JA, Tuszynski MH. 2004. Memory impairment in aged primates is associated with focal death of cortical neurons and atrophy of subcortical neurons. J Neurosci 24:4373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. 2008. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A 105:12569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Störmer VS, Passow S, Biesenack J, Li S-C. 2012. Dopaminergic and cholinergic modulations of visual-spatial attention and working memory: insights from molecular genetic research and implications for adult cognitive development. Dev Psychol 48:875. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A. 2001. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci 21:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toepper M, Gebhardt H, Bauer E, Haberkamp A, Beblo T, Gallhofer B, and others. 2014. The impact of age on load-related dorsolateral prefrontal cortex activation. Front Aging Neurosci 6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. 2009. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res 204:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. 2005. Post-pubertal emergence of prefrontal cortical up states induced by D1–NMDA co-activation. Cereb Cortex 15:49–57. [DOI] [PubMed] [Google Scholar]
- Tseng K-Y, O’Donnell P. 2007. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex 17:1235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner GR, McIntosh AR, Levine B. 2011. Prefrontal compensatory engagement in TBI is due to altered functional engagement of existing networks and not functional reorganization. Front Syst Neurosci 5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman H, Almeida R, Klingberg T. 2014. Structural maturation and brain activity predict future working memory capacity during childhood development. J Neurosci 34:1592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Allmen DY, Wurmitzer K, Klaver P. 2014. Hippocampal and posterior parietal contributions to developmental increases in visual short-term memory capacity. Cortex 59:95–102. [DOI] [PubMed] [Google Scholar]
- Voytek B, Davis M, Yago E, Barceló F, Vogel EK, Knight RT. 2010. Dynamic neuroplasticity after human prefrontal cortex damage. Neuron 68:401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DL, Vytlacil JJ, Nomura EM, Gibbs SE, D’Esposito M. 2011. The dopamine agonist bromocriptine differentially affects fronto-striatal functional connectivity during working memory. Front Hum Neurosci 5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang X-J, Laubach M, and others. 2011. Neuronal basis of age-related working memory decline. Nature 476:210–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yang Y, Wang C-J, Gamo NJ, Jin LE, Mazer JA, and others. 2013. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron 77:736–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-J. 2001. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci 24:455–63. [DOI] [PubMed] [Google Scholar]
- Wang X-J, Tegnér J, Constantinidis C, Goldman-Rakic PS. 2004. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci U S A 101:1368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weicker J, Villringer A, Thöne-Otto A. 2016. Can impaired working memory functioning be improved by training? A meta-analysis with a special focus on brain injured patients. Neuropsychology 30:190–212. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Walker LC, Price DL, Cork LC. 1991. Loss of NMDA, but not GABA-A, binding in the brains of aged rats and monkeys. Neurobiol Aging 12:93–8. [DOI] [PubMed] [Google Scholar]
- White TP, Symington I, Castellanos NP, Brittain PJ, Froudist Walsh S, Nam K-W, and others. 2014. Dysconnectivity of neurocognitive networks at rest in very-preterm born adults. Neuroimage Clin 4:352–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmott C, Ponsford J. 2009. Efficacy of methylphenidate in the rehabilitation of attention following traumatic brain injury: a randomised, crossover, double blind, placebo controlled inpatient trial. J Neurol Neurosurg Psychiatry 80:552–7. [DOI] [PubMed] [Google Scholar]
- Yang Y, Paspalas CD, Jin LE, Picciotto MR, Arnsten AFT, Wang M. 2013. Nicotinic α7 receptors enhance NMDA cognitive circuits in dorsolateral prefrontal cortex. Proc Natl Acad Sci U S A 110:12078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


