Abstract
Background:
Preeclampsia is associated with increased risk of future heart failure (HF), but the relationship between preeclampsia and HF subtypes are not well-established.
Objectives:
The objective of this analysis was to identify the risk of HF with preserved ejection fraction (HFpEF) following a delivery complicated by preeclampsia/eclampsia.
Methods:
A retrospective cohort study using the New York and Florida state Healthcare Cost and Utilization Project State Inpatient Databases identified delivery hospitalizations between 2006 – 2014 for women with and without preeclampsia/eclampsia. We identified women admitted for HF after discharge from index delivery hospitalization until 9/30/2015 using ICD-9-CM diagnosis codes. Patients were followed from discharge to the first instance of primary outcome (HFpEF hospitalization), death, or end of study period. Secondary outcomes included hospitalization for any HF and HFrEF, separately. The association between preeclampsia/eclampsia and HFpEF was analyzed using Cox proportional hazards models.
Results:
There were 2,532,515 women included in the study: 2,404,486 without and 128,029 with preeclampsia/eclampsia. HFpEF hospitalization was significantly more likely among women with preeclampsia/eclampsia, after adjusting for baseline hypertension and other covariates (aHR 2.09 [1.80 – 2.44]). Median time to onset of HFpEF was 32.2 months (IQR 0.3 – 65.0 months), and median age at HFpEF onset was 34.0 years (IQR 29.0 – 39.0 years). Both traditional (hypertension, diabetes mellitus) and sociodemographic (Black race, rurality, low income) risk factors were also associated with HFpEF and secondary outcomes.
Conclusions:
Preeclampsia/eclampsia is an independent risk factor for future hospitalizations for heart failure with preserved ejection fraction.
Keywords: Preeclampsia, HFpEF, Pregnancy, Women, Heart Failure
CONDENSED ABSTRACT:
This retrospective cohort study using the New York and Florida state Healthcare Cost and Utilization Project State Inpatient Databases identified delivery hospitalizations between 2006 – 2014 for women with and without preeclampsia/eclampsia using ICD-9-CM diagnosis codes. Patients were followed from discharge to the first instance of primary outcome (HFpEF hospitalization), death, or end of study period. HFpEF hospitalization was significantly more likely among women with preeclampsia/eclampsia, after adjusting for baseline hypertension and other covariates (aHR 2.09 [1.80 – 2.44]). Both traditional (hypertension, diabetes mellitus) and sociodemographic (Black race, rurality, low income) risk factors were also associated with HFpEF and secondary outcomes.
Introduction
Hypertensive disorders of pregnancy affect 5–10% of pregnancies and are a leading cause of maternal morbidity and mortality.(1) Preeclampsia, defined as new onset hypertension associated with end organ damage after the 20th week of gestation, is a risk factor for future cardiovascular disease, and is associated with a 3–4-fold risk of developing heart failure (HF).(2–5) However, the reason for the increased risk of HF among women with a history of preeclampsia is not known, and prior studies that have examined the long-term risk for HF in women with a history of preeclampsia do not distinguish between HF with reduced ejection fraction (HFrEF) or HF with preserved ejection fraction (HFpEF).(2,3,5)
HFpEF, characterized by a left ventricular ejection > 50%, is a disease that predominantly affects middle-aged and older women and is associated with chronic inflammation, traditional risk factors such as hypertension, diabetes mellitus and obesity, and nontraditional risk factors such as autoimmune disease.(6,7) The prevalence of HFpEF is increasing, now accounting for half of heart failure hospital admissions, and carries a high five-year mortality.(8) Diastolic dysfunction and asymptomatic left ventricular concentric remodeling are increased in preeclamptic women years after pregnancy.(9) Patients with abnormal cardiac remodeling, particularly concentric hypertrophy with diastolic dysfunction, are at increased risk for adverse cardiovascular events including the development of clinical heart failure;(10,11) this raises the possibility that preeclampsia could be associated with HFpEF through these pathways.
The goal of this retrospective cohort study of state inpatient billing databases from the Healthcare Cost and Utilization Project (HCUP) was to characterize the relationship between preeclampsia and long-term risk for HFpEF hospital admission. We hypothesized that preeclampsia is associatd with a high incidence of HFpEF hospitalizations.
Methods
Data and Patient Sample
Through the Center for Administrative Data Research at Washington University School of Medicine in St. Louis, we obtained access to the Statewide Inpatient Databases (SID) for New York and Florida between 2005 and 2015 from the Heathcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality. The SID include approximately 97% of all acute care community hospital discharges (including teaching and nonteaching hospitals). Data elements available in the SID include ICD-9-CM diagnosis and procedure codes, demographics, length of stay, and payment source, among others.
Women aged 16–50 years discharged from a delivery hospitalization between 1/2006 and 9/2014 were identified based on evidence for a vaginal delivery or cesarean delivery using the algorithm of Kuklina et al based on ICD-9-CM diagnosis and procedure codes.(12) Hospitalizations were excluded from the analysis if the admission was coded only for an antepartum or postpartum condition but no delivery was identified. The first eligible delivery during the study period for each woman was considered the index hospitalization. Women with preeclampsia, preeclampsia with severe features and eclampsia were identified by ICD-9-CM codes (Appendix) and compared to women who delivered without preeclampsia, preeclampsia with severe features or eclampsia during the index hospitalization. Women with gestational hypertension were included in the control group unless they also carried a diagnosis of preeclampsia or eclampsia, as the association with subsequent HF was not expected to be as strong as in the preeclampsia/eclampsia group.(13–15)
Among women who had an index delivery hospitalization, we identified the first subsequent event of the following: the first hospitalization coded for HF, death, subsequent delivery hospitalization coded for preeclampsia or eclampsia, or the end of the data (9/30/2015). The primary outcome, maternal hospitalization for HFpEF after the index delivery, was identified based on ICD-9-CM diagnosis codes for HFpEF (Supplemental Table 1). Subsequent hospitalizations for women after delivery were tracked through 9/30/2015 using the encrypted identifier in the SID.
In order to reduce the risk of confounding due to patients who may be at increased risk of developing HF unrelated to their preeclampsia diagnosis, patients were excluded if they had any of the following diagnoses in the 6 months prior to or during the index delivery hospitalization: chronic kidney disease, systemic lupus erythematous, ischemic heart disease, hypertensive heart disease, pulmonary embolism, myocardial infarction, and heart failure, including cardiomyopathies and peripartum cardiomyopathy (Supplemental Appendix). Patients who died during the index delivery hospitalization were also excluded.
Clinical and sociodemographic variables included in our analysis included age (<35, 35–39 and ≥40), race (White, Black or other), tobacco use, diabetes mellitus, gestational diabetes, chronic hypertension, gestational hypertension, preeclampsia (mild, superimposed on chronic hypertension, severe, eclampsia), preterm delivery, cesarean delivery, median household income quartile based on zip code, Medicaid insurance, urban/rural locality (large metropolitan, small metropolitan, micropolitan, and non-metropolitan/non-micropolitan) and selected Elixhauser comorbidities.(16,17) Elixhauser comorbidities are a well-established method for identifying common medical conditions using claims data,(16) and have been used in many prior studies in this and other datasets.
Outcomes
The primary outcome was hospitalization for HFpEF after index delivery hospitalization for women with vs. without preeclampsia/eclampsia. Prespecified secondary analyses were performed for time tohospitalization for any HF (HFpEF, HFrEF or unspecified) and HFrEF for women with vs. without preeclampsia/eclampsia.
Analyses
The effect of preeclampsia/eclampsia on the primary outcome of HFpEF hospitalization was evaluated in time to event analysis. Analysis was performed for HFpEF admissions and for the secondary outcomes of any HF and HFrEF hospitalizations. Observations were censored at the earliest of death, end of follow-up, or preeclampsia/eclampsia in a subsequent pregnancy. In addition, for HFpEF, observations were censored at the time of HFrEF. For HFrEF, observations were censored at the time of HFpEF. The outcome was first compared between subjects with and without preeclampsia using the log-rank test with Kaplan-Meier curves. Proportionality assumptions were tested by Schoenfeld residuals for both individual covariates and the global Schoenfeld test. This revealed time-dependency in the association of preeclampsia/eclampsia with overall HF and HFrEF outcomes. Therefore, two separate Cox proportional hazards models were fit for these outcomes, one for the early (0–90 days post index delivery) and one for the late (91 or more days post index delivery). The 90 day time point was chosen due to a natural break identified on visual examination of the curves, and as this was a clinically logical timepoint, given that 97% of peripartum cardiomyopathy (HFrEF) hospitalizations occur within 3 months of delivery.(18) No violation of proportionality assumption was found for HFpEF. The models were adjusted for relevant demographic and clinical comorbidities. Observations with missing covariate information were excluded from the models. To account for within-state correlation, observations were clustered by state in the models. As a sensitivity analysis we evaluated the primary outcome after excluding control subjects with gestational hypertension. Sensitivity analyses were also performed for the primary outcome of HFpEF considering both potential extreme scenarios for unspecified HF admissions. One sensitivity analysis was performed censoring each unspecified HF event (ie assuming all were non-events) and one analysis was performed considering each unspecified HF event as an event (ie assuming all were HFpEF events).
This study was considered exempt by the Washington University Human Research Protection Office and the requirement for informed consent was waived due to the de-identified nature of the data (IRB #201810063). Statistical analyses was done with SAS v9.4 and R version 4.0.
Results
Patient Population
Of the 2,532,515 women with a delivery hospitalization between 2006 and 2014 who met the inclusion criteria (Figure 1), 128,029 (5.1%) had preeclampsia/eclampsia during the index delivery hospitalization. Median follow up time was 72 months (range 0–120, IQR 45–99 months). A total of 2,320 (0.1%) records did not have information on HF type. Of the remaining records, 180,086 (7.1%) were missing information on race or income and were excluded from the multivariate models. There were 569 women with hospitalizations for HFpEF and 985 women with hospitalizations for HFrEF. The baseline characteristics of the cohort are summarized in Table 1 and Supplemental Table 2. Women with preeclampsia/eclampsia during the index delivery hospitalization were more likely to have underlying chronic hypertension (13.9% vs 2.1%), diabetes mellitus (3.4% vs 0.8%) and gestational diabetes (10.3% vs 5.8%). Women with preeclampsia/eclampsia were more likely to be ≥ 40 years old (5.6% vs 3.7%) and Black (27.9% vs 18.1%) compared to those without preeclampsia/eclampsia. Women with preeclampsia/eclampsia during the index delivery hospitalization were also more likely to have Medicaid insurance and to live in a zip code with median household income less than the 25th percentile compared to women without preeclampsia/eclampsia.
Figure 1. Attrition Diagram.
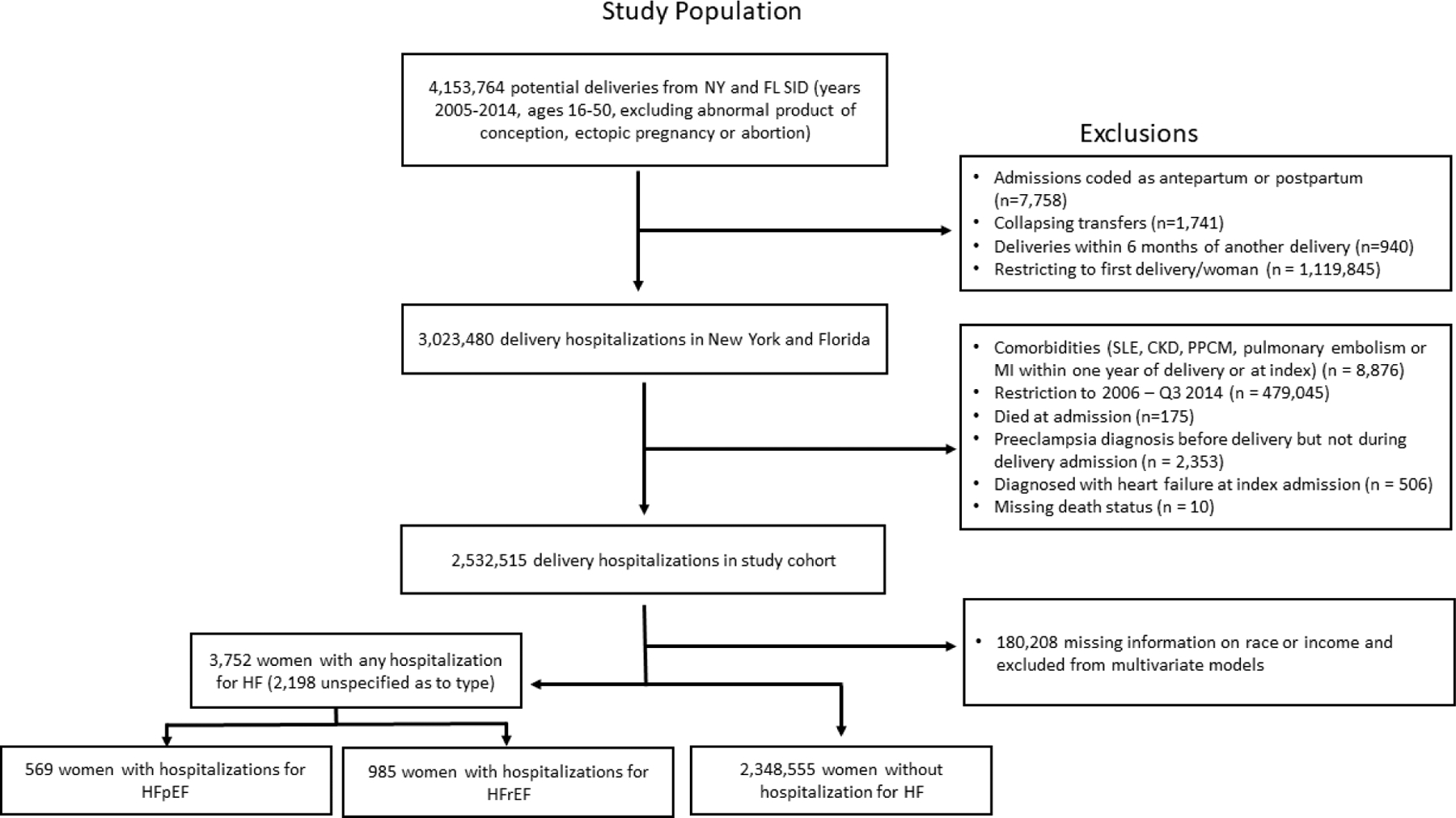
The above algorithm was used to arrive at the final population included in this analysis. NY – New York, FL – Florida, SID – State Inpatient Database, SLE - Systemic Lupus Erythematosus, CKD – Chronic Kidney Disease, PPCM – peripartum cardiomyopathy, Q3 – third quarter
Table 1.
Baseline Demographics
| No Preeclampsia | Preeclampsia or Eclampsia | |
|---|---|---|
| n | 2,404,486 | 128,029 |
| Number from State FL/NY (%) | 1,069,512/1,334,974 (44.5/55.5) | 59,471/68,558 (46.5/53.5) |
| Age (%) | ||
| Under 35 | 1,992,541 (82.9) | 102,364 (80.0) |
| 35–39 | 322,078 (13.4) | 18,448 (14.4) |
| 40 + | 89,867 (3.7) | 7,217 (5.6) |
| Race (%) | ||
| White | 1,139,319 (47.4) | 52,342 (40.9) |
| Black | 435,219 (18.41 | 35,698 (27.9) |
| Other | 791,896 (32.9) | 38,298 (29.9) |
| Missing | 38,052 (1.6) | 1,691 (1.3) |
| Elixhauser Score (%) | ||
| 0 | 2,060,473 (85.7) | 85, 480 (66.8) |
| 1 | 292,110 (12.1) | 30, 964 (24.2) |
| ≥2 | 51,903 (2.2) | 11, 585 (9.0) |
| Diabetes Mellitus (%) | 19,657 (0.8) | 4, 386 (3.4) |
| Gestational Diabetes (%) | 139,988 (5.8) | 13, 170 (10.3) |
| Chronic Hypertension (%) | 50,658 (2.1) | 17, 842 (13.9) |
| Gestational Hypertension (%) | 85,362 (3.6) | 3,066 (2.4) |
| Tobacco Use (%) | 98,896 (4.1) | 4,787 (3.7) |
| Income Quartile (%) | ||
| Lowest income quartile (1) | 647,372 (26.9) | 39,022 (30.5) |
| Quartile 2 | 560,180 (23.3) | 28,342 (22.1) |
| Quartile 3 | 567,633 (23.6) | 28,589 (22.3) |
| Highest income quartile (4) | 497,816 (20.7) | 22,271 (17.4) |
| Missing | 131,485 (5.5 | 9,805 (7.7) |
| Medicaid Insurance (%) | 1,089,637 (45.3) | 61,262 (47.9) |
| Urban/Rural (%) | ||
| Large Metro | 1,776,519 (73.9) | 96,864 (75.7) |
| Small Metro | 483,034 (20.1) | 24,269 (19.0) |
| Micropolitan | 102,852 (4.3) | 4,790 (3.7) |
| Non metro or micro | 41,407 (1.7) | 2,070 (1.6) |
| Missing | 674 (0.0) | 36 (0.0) |
| Post-index Mortality (%) | 0.06 | 0.15 |
FL – Florida, NY – New York
Preeclampsia/Eclampsia and Heart Failure with Preserved Ejection Fraction
There were 603 women with subsequent hospitalization(s) for HFpEF (Central Illustration). Preeclampsia/eclampsia during the index hospitalization was independently associated with increased risk of HFpEF compared to women without preeclampsia/eclampsia, with an aHR of 2.09 (95% CI 1.80 – 2.44), median time to onset of 32.2 months (IQR 0.3 – 65.0 months), and median age at diagnosis of HFpEF of 34 years (IQR 29 – 39). Chronic hypertension, diabetes mellitus and Black race were the most significant additional risk factors for hospitalization for HFpEF (aHR 4.36 (3.18–5.98), 5.35 (5.08–5.63), and 2.89 (2.51–3.32), respectively, Table 2). Rural locality (aHR 1.59 (1.38–1.84)) and markers of poverty, including the lowest income quartile (aHR 1.22 (1.07–1.38) and Medicaid insurance (aHR 1.41 (1.31–1.51)) were also associated with increased risk of HFpEF.
Central Illustration. Heart Failure Hospitalizations Following Delivery Complicated by Preeclampsia/Eclampsia.
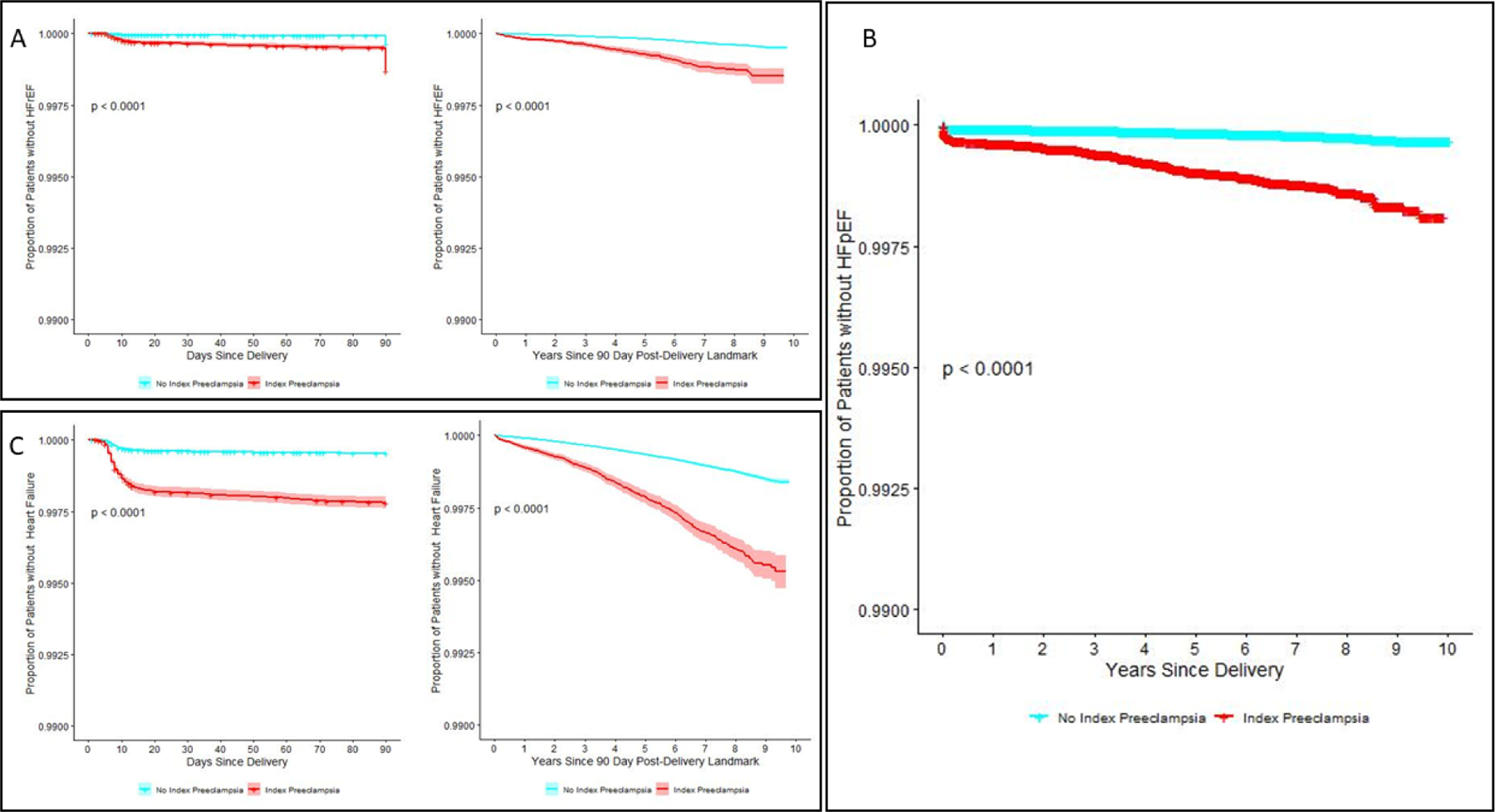
Kaplan-Meier curves for rehospitalizations for heart failure with reduced ejection fraction (A) following delivery within the first 90 days and between 90 days and 10 years postpartum. Kaplan-Meier curve for rehospitalizations for heart failure with preserved ejection fraction following delivery (B). Kaplan-Meier curves for any heart failure rehospitalization (C) within the first 90 days and between 90 days and 10 years postpartum.
Table 2.
Risk of Subsequent Hospitalization for Heart Failure with Preserved Ejection Fraction Following Delivery
| Hazard Ratio | Confidence Interval | |
|---|---|---|
| Preeclampsia or Eclampsia | 2.09 | 1.80–2.44 |
| Age 35–39 vs Under 35 | 1.78 | 1.55–2.06 |
| Age ≥40 vs Under 35 | 1.59 | 1.51–1.67 |
| Black Race | 2.89 | 2.51–3.32 |
| Diabetes Mellitus | 5.35 | 5.08–5.64 |
| Gestational Diabetes | 1.67 | 1.61–1.74 |
| Cesarean Delivery | 1.79 | 1.69–1.89 |
| Chronic Hypertension | 4.36 | 3.18–5.98 |
| Preterm Delivery | 1.51 | 1.48–1.53 |
| Medicaid Insurance | 1.41 | 1.31–1.51 |
| Income Q1 vs Q4 (highest) | 1.22 | 1.07–1.38 |
| Income Q2 vs Q4 (highest) | 0.93 | 0.72–1.19 |
| Income Q3 vs Q4 (highest) | 0.97 | 0.90–1.05 |
| Small Metro vs Large Metro | 1.30 | 1.07–1.58 |
| Micropolitan vs Large Metro | 1.42 | 1.27–1.59 |
| Non metro or micropolitan vs Large Metro | 1.59 | 1.38–1.84 |
Q4 - Highest income quartile
Q1 - Lowest income quartile
Model includes all variables listed in table.
A sensitivity analysis was performed excluding control subjects with gestational hypertension. In this subsample of 2,269,814 women, preeclampsia/eclampsia during the index hospitalization was independently associated with increased risk of HFpEF compared to women without preeclampsia/eclampsia, with an aHR of 2.20 (1.97 – 2.46, P<0.001) (Supplemental Table 3) Sensitivity analyses were performed to account for the unspecified HF hospitalizations, and did not change the results in a clinically meaningful way (Supplemental Table 4). Preeclampsia/eclampsia during the index hospitalization remained independently associated with increased risk of HFpEF compared to women without preeclampsia/eclampsia, both considering unspecified HF hospitalizations as HFpEF events (aHR 1.91 [1.89–1.93]) and considering unspecified HF hospitalizations as HFrEF events (aHR 2.09 [1.79–2.44]).
Preeclampsia/Eclampsia and Heart Failure with Reduced Ejection Fraction
There were 1072 women with subsequent hospitalization(s) due to HFrEF, with median time to onset of 31.5 months (Central Illustration and Supplemental Table 5) and median age at diagnosis of 33.4 years. Preeclampsia/eclampsia during the index hospitalization was independently associated with increased risk of 90-day HFrEF, with an aHR of 2.13 (95% CI 2.08 – 2.18) and of late HFrEF, with an aHR of 1.92 (95% CI 1.76 – 2.10) compared to women without preeclampsia/eclampsia. Other major risk factors for 90-day HFrEF included older age, Black race, chronic hypertension, diabetes, and cesarean delivery. Other major risk factors for late HFrEF included Black race, diabetes mellitus and chronic hypertension. Markers of poverty including Medicaid insurance and median income in the lowest three quartiles were also associated with increased risk of late HFrEF.
Relationship between Hospitalization for Preeclampsia/Eclampsia and Any Heart Failure
There were 3,995 women who developed HF of any type. Women with a history of preeclampsia/eclampsia were at increased risk of developing both 90-day (short-term) and late HF, with median time to onset of HF hospitalization of 20.8 months and median age at diagnosis of 33.0 years. In the first 90 days post-index delivery, the risk of HF was more than two-fold higher for women with eclampsia/preeclampsia (aHR 2.65, 95% CI 2.61–2.70, Central Illustration and Supplemental Table 4) compared to women without preeclampsia/eclampsia. Age, Black race, chronic hypertension, diabetes mellitus, cesarean delivery, Medicaid enrollment and rural locality were also associated with increased risk of short-term HF. Preeclampsia/eclampsia, age ≥40 and Black race were the strongest predictors of 90-day HF. Preeclampsia/eclampsia remained a significant predictor for late HF after adjusting for other clinical and demographic factors (aHR 1.66, 95% CI 1.65–1.66). The strongest predictors of late HF were diabetes mellitus, chronic hypertension and Black race.
Comparison between HFpEF and HFrEF
There were a total of 1072 reported HFrEF hospitalizations and 603 HFpEF hospitalizations. There were no significant differences in age, race, insurance type, or urbanicity/rurality between the HFpEF and HFrEF groups. Importantly, women diagnosed with HFpEF were more likely than those with HFrEF to carry a diagnosis of chronic hypertension (24.9% vs 20.0%, p=0.02) or diabetes mellitus (14.4% vs 8.5%, p <0.001%).
Discussion
This large retrospective study identified a strong, independent association of preeclampsia/eclampsia with HFpEF in the decade following pregnancy after adjusting for other major HF risk factors including diabetes mellitus and chronic hypertension. These findings support current literature linking hypertensive disorders of pregnancy with future cardiovascular disease, and specifically HF.(2) Importantly, preeclampsia/eclampsia was independently associated with both HFrEF and HFpEF, an important distinction given the female sex-predominance of HFpEF. The reasons for the sex-disparities in HFpEF remain elusive, though risk factors including obesity, hypertension, and diabetes mellitus, which are more common in women than in men and are also risk factors for preeclampsia/eclampsia, have been proposed to contribute to the development of HFpEF.(19) Thus this sex-specific risk factor may in part explain the predominance of HFpEF in women.
While prior studies investigating the relationship between preeclampsia/eclampsia and future HF have not specified HF type, based on the current study it can be speculated that at least one third of the reported cases are due to HFpEF (Figure 1). Many hospitalizations for HF in this study did not specify HF type, thus all HF hospitalizations were also analyzed together as “any heart failure”. Preeclampsia/eclampsia remained a significant independent predictor of any HF in this analysis, consistent with prior studies investigating hypertensive disorders of pregnancy (HDP) and future HF risk.(2,3,5) Based upon the breakdown of HFpEF and HFrEF in the specified HF analyses and the number of unspecified HF hospitalizations, it is likely that the incidence of HFpEF is under-reported in this study.
HDP are an emerging, sex-specific risk factor for future cardiovascular disease in women. HDP cover a broad range of hypertensive disorders including gestational hypertension, chronic hypertension, chronic hypertension with superimposed preeclampsia, preeclampsia (with or without severe features), eclampsia and the HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome.(20,21) Though it has previously remained unclear whether HDP were a marker or contributor to future cardiovascular risk, an increasing number of studies suggest an independent association of HDP to future cardiovascular events.(14,15,22–24)
The presentation of HFpEF in this study occurred with a median time to event of 32.2 months and median age of 34.0 years, consistent with onset of HF during the reproductive years for the majority of the patients included in the study. Preeclampsia/eclampsia have previously been associated with increased risk of short-term HF in the form of peripartum cardiomyopathy, a distinct subtype of HFrEF which occurs within the first five months after delivery.(25,26) More recently, several studies have also identified HDP as a risk factor for acute peripartum HFpEF, suggesting that peripartum HF may present along the spectrum of ejection fractions.(27,28) While Briller et al. recently identified that preeclampsia/eclampsia is associated with increased risk of HFpEF during pregnancy and the early postpartum period, the present study importantly indicates that women with preeclampsia/eclampsia also remain at risk for longer-term HFpEF even after the physiologic changes of pregnancy have resolved. When compared to age and sex matched controls, patients < 55 years of age with HFpEF have a 3-fold increase in death at one year compared to controls.(29) Thus our study likely identifies a particularly high-risk cohort of women with HF who could potentially benefit from early identification and risk factor modification.
Chronic hypertension and diabetes mellitus were also identified as independent risk factors for HFpEF hospitalization in this study, and were found to be more common among women developing HFpEF than HFrEF. These traditional cardiovascular risk factors are known risk factors for the development of HFpEF.(19) Women with preexisting hypertension or diabetes mellitus are at increased risk of developing preeclampsia/eclampsia, and conversely women with preeclampsia/eclampsia are also at four-fold risk of developing future hypertension and two-fold risk of developing future diabetes mellitus.(4,21,30,31) Thus the findings that both traditional and non-traditional cardiovascular risk factors were independently associated with the development of early HFpEF in this cohort is consistent with what is already known about both HFpEF and preeclampsia, and supports a multi-risk model of phenotype development.
In the present study, hospital admissions for HFpEF after a diagnosis of preeclampsia/eclampsia disproportionately affected Black women, women with low income, women living in rural locations and women receiving Medicaid insurance. It is also known that Black patients have a 50% higher incidence of HF than white patients, and it occurs at an earlier age.(32) These findings highlight previously recognized sociodemographic disparities in maternal morbidity and mortality.(33–37) Potential contributors to these disparities include lack of postpartum access to healthcare services, structural racism, and increased burden of underlying cardiovascular risk factors. These findings, in light of the knowledge that cardiovascular disease is the leading cause of maternal mortality as well as the leading cause of overall mortality in women in the United States, support the need for post-partum cardiovascular risk stratification and counseling, bias reduction efforts, and continued access to healthcare for women with adverse pregnancy outcomes.
There are limitations to this study. We used ICD-9-CM diagnosis codes to identify hospitalizations for heart failure which are subject to coding error. Importantly, “HFpEF” was added as a new definition to the ACCF/AHA Heart Failure Guidelines in October 2013.(6) It is therefore likely that many women with a diagnosis of HFpEF were not coded as such, consistent with the large number of “unspecified” HF admissions. However, our results remained robust after multiple sensitivity analyses. We did not have access to outpatient data and thus were not able to identify those who developed HF in the outpatient setting. Given these two factors, it is likely that the present study underestimates the true risk of HFpEF in women following a pregnancy complicated by preeclampsia/eclampsia. While our follow-up was long compared to many prior studies, longer-term follow-up (i.e., 30 – 50 years) is needed to develop a complete picture of the relationship between preeclampsia and women’s lifetime cardiovascular risk for HFpEF. Despite these limitations, our study provides an important contribution to the literature through further delineation of HF phenotypes, suggesting development of both HFrEF and HFpEF are increased in women with a history of preeclampsia.
Conclusions
Preeclampsia/eclampsia is an independent risk factor for future heart failure hospitalizations for heart failure with preserved ejection fraction in the decade following a pregnancy complicated by preeclampsia/eclampsia. Additional risk factors include traditional cardiovascular risk factors such as hypertension and diabetes mellitus, as well as sociodemographic risk factors including Black race, markers of poverty, and living in a rural setting. These findings suggest that preeclampsia/eclampsia may share common pathophysiologic mechanisms in women with HFpEF and HFrEF.
Supplementary Material
Clinical Perspectives:
Competency in Patient Care and Procedural Skills:
Hypertensive disorders, including pre-eclampsia and eclampsia, occur in up to 10% of all pregnancies and are associated with adverse pregnancy outcomes and long-term cardiovascular effects, including hypertension, dyslipidemia, insulin resistance and heart failure with preserved ejection fraction (HfpEF), beginning within the first few years after delivery.
Translational Outlook:
Future research should address the mechanisms, prevention, and treatment of pre-eclampsia to reduce the subsequent development of cardiovascular complications, including HFpEF.
Acknowledgements:
The writing group would like to thank Joanna Buss for data programming.
Sources of Funding
This study was supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) and Grant Number R24 HS19455 through the Agency for Healthcare Research and Quality (AHRQ) and The Longer Life Foundation Grant # 2020-005.
ABBREVIATIONS:
- CKD
Chronic Kidney Disease
- FL
Florida
- HCUP
Healthcare Cost and Utilization Project
- HF
Heart failure
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- NY
New York
- PPCM
Peripartum cardiomyopathy
- SID
Statewide Inpatient Databases
- SLE
Systemic Lupus Erythematosus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no financial disclosures to report.
References
- 1.Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. European journal of obstetrics, gynecology, and reproductive biology 2013;170:1–7. [DOI] [PubMed] [Google Scholar]
- 2.Wu P, Haththotuwa R, Kwok CS et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circulation Cardiovascular quality and outcomes 2017;10. [DOI] [PubMed] [Google Scholar]
- 3.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. American heart journal 2008;156:918–30. [DOI] [PubMed] [Google Scholar]
- 4.Stuart JJ, Tanz LJ, Missmer SA et al. Hypertensive Disorders of Pregnancy and Maternal Cardiovascular Disease Risk Factor Development: An Observational Cohort Study. Ann Intern Med 2018;169:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brouwers L, van der Meiden-van Roest AJ, Savelkoul C et al. Recurrence of pre-eclampsia and the risk of future hypertension and cardiovascular disease: a systematic review and meta-analysis. BJOG : an international journal of obstetrics and gynaecology 2018;125:1642–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 7.Nieminen MS, Harjola VP, Hochadel M et al. Gender related differences in patients presenting with acute heart failure. Results from EuroHeart Failure Survey II. European journal of heart failure 2008;10:140–8. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin EJ, Virani SS, Callaway CW et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 9.Ghossein-Doha C, van Neer J, Wissink B et al. Pre-eclampsia: an important risk factor for asymptomatic heart failure. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2017;49:143–149. [DOI] [PubMed] [Google Scholar]
- 10.Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. Journal of the American College of Cardiology 1995;25:879–84. [DOI] [PubMed] [Google Scholar]
- 11.Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovascular imaging 2011;4:98–108. [DOI] [PubMed] [Google Scholar]
- 12.www.hcup-us.ahrq.gov/sidoverview.jsp. HCUP State Inpatient Databases (SID) Healthcare Cost and Utilization Project (HCUP) 2005–2015 Agency for Healthcare Research and Quality, Rockville, MD. [PubMed] [Google Scholar]
- 13.Ackerman CM, Platner MH, Spatz ES et al. Severe cardiovascular morbidity in women with hypertensive diseases during delivery hospitalization. American journal of obstetrics and gynecology 2019;220:582.e1–582.e11. [DOI] [PubMed] [Google Scholar]
- 14.Honigberg MC, Riise HKR, Daltveit AK et al. Heart Failure in Women With Hypertensive Disorders of Pregnancy: Insights From the Cardiovascular Disease in Norway Project. Hypertension 2020;76:1506–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen SN, Cheng CC, Tsui KH et al. Hypertensive disorders of pregnancy and future heart failure risk: A nationwide population-based retrospective cohort study. Pregnancy hypertension 2018;13:110–115. [DOI] [PubMed] [Google Scholar]
- 16.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 17.Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying Increased Risk of Readmission and In-hospital Mortality Using Hospital Administrative Data: The AHRQ Elixhauser Comorbidity Index. Med Care 2017;55:698–705. [DOI] [PubMed] [Google Scholar]
- 18.Elkayam U, Akhter MW, Singh H et al. Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation 2005;111:2050–5. [DOI] [PubMed] [Google Scholar]
- 19.Pepine CJ, Merz CNB, El Hajj S et al. Heart failure with preserved ejection fraction: Similarities and differences between women and men. International journal of cardiology 2020;304:101–108. [DOI] [PubMed] [Google Scholar]
- 20.ACOG Practice Bulletin No. 203: Chronic Hypertension in Pregnancy. Obstetrics and gynecology 2019;133:e26–e50. [DOI] [PubMed] [Google Scholar]
- 21.Hypertension Gestational and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstetrics and gynecology 2020;135:e237–e260. [DOI] [PubMed] [Google Scholar]
- 22.Leon LJ, McCarthy FP, Direk K et al. Preeclampsia and Cardiovascular Disease in a Large UK Pregnancy Cohort of Linked Electronic Health Records: A CALIBER Study. Circulation 2019;140:1050–1060. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbloom JI, Lewkowitz AK, Lindley KJ et al. Expectant Management of Hypertensive Disorders of Pregnancy and Future Cardiovascular Morbidity. Obstetrics and gynecology 2020;135:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenbloom JI, Stwalley D, Lindley KJ, Michael Nelson D, Olsen MA, Stout MJ. Latency of preterm hypertensive disorders of pregnancy and subsequent cardiovascular complications. Pregnancy hypertension 2020;21:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bello N, Rendon IS, Arany Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: a systematic review and meta-analysis. Journal of the American College of Cardiology 2013;62:1715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindley KJ, Conner SN, Cahill AG, Novak E, Mann DL. Impact of Preeclampsia on Clinical and Functional Outcomes in Women With Peripartum Cardiomyopathy. Circulation Heart failure 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindley KJ, Williams D, Conner SN, Verma A, Cahill AG, Davila-Roman VG. The Spectrum of Pregnancy-Associated Heart Failure Phenotypes: An Echocardiographic Study. The international journal of cardiovascular imaging 2020;36:1637–1645. [DOI] [PubMed] [Google Scholar]
- 28.Briller JE, Mogos MF, Muchira JM, Piano MR. Pregnancy Associated Heart Failure With Preserved Ejection Fraction: Risk Factors and Maternal Morbidity. Journal of cardiac failure 2021;27:143–152. [DOI] [PubMed] [Google Scholar]
- 29.Tromp J, MacDonald MR, Tay WT et al. Heart Failure With Preserved Ejection Fraction in the Young. Circulation 2018;138:2763–2773. [DOI] [PubMed] [Google Scholar]
- 30.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. Bmj 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Timpka S, Markovitz A, Schyman T et al. Midlife development of type 2 diabetes and hypertension in women by history of hypertensive disorders of pregnancy. Cardiovasc Diabetol 2018;17:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis EF, Claggett B, Shah AM et al. Racial Differences in Characteristics and Outcomes of Patients With Heart Failure and Preserved Ejection Fraction in the Treatment of Preserved Cardiac Function Heart Failure Trial. Circulation Heart failure 2018;11:e004457. [DOI] [PubMed] [Google Scholar]
- 33.Luke AA, Huang K, Lindley KJ, Carter EB, Joynt Maddox KE. Severe Maternal Morbidity, Race, and Rurality: Trends Using the National Inpatient Sample, 2012–2017. J Womens Health (Larchmt) 2020. [DOI] [PubMed] [Google Scholar]
- 34.Admon LK, Winkelman TNA, Zivin K, Terplan M, Mhyre JM, Dalton VK. Racial and Ethnic Disparities in the Incidence of Severe Maternal Morbidity in the United States, 2012–2015. Obstetrics and gynecology 2018;132:1158–1166. [DOI] [PubMed] [Google Scholar]
- 35.Prevention” CfDCa. Trends in pregnancy-related mortality in the United States: 1987–2016. https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm#trends: “Centers for Disease Control and Prevention”, Year of publication: 2019. [Google Scholar]
- 36.Leonard SA, Main EK, Scott KA, Profit J, Carmichael SL. Racial and ethnic disparities in severe maternal morbidity prevalence and trends. Ann Epidemiol 2019;33:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen EE, Davis NL, Goodman D et al. Vital Signs: Pregnancy-Related Deaths, United States, 2011–2015, and Strategies for Prevention, 13 States, 2013–2017. MMWR Morb Mortal Wkly Rep 2019;68:423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


