Abstract
The Electrophysiology Professional Interest Area (EPIA) and Global Brain Consortium endorsed recommendations on candidate EEG measures for Alzheimer’s disease (AD) clinical trials. The Panel reviewed the field literature. As most consistent findings, AD patients with mild cognitive impairment and dementia showed abnormalities in peak frequency, power, and “interrelatedness” at posterior alpha (8-12 Hz) and widespread delta (<4 Hz) and theta (4-8 Hz) rhythms in relation to disease progression and interventions. The following consensus statements were subscribed: (i) Standardization of instructions to patients, rsEEG recording methods, and selection of artifact-free rsEEG periods are needed; (ii) Power density and “interrelatedness” rsEEG measures (e.g., directed transfer function, phase lag index, linear lagged connectivity, etc.) at delta, theta, and alpha frequency bands may be use for stratification of AD patients and monitoring of disease progression and intervention; and (iii) International multisectoral initiatives are mandatory for regulatory purposes.
Keywords: The Alzheimer’s Association International Society to Advance Alzheimer’s Research and Treatment (ISTAART), Alzheimer’s disease (AD), Dementia, Electroencephalography (EEG), Eyes-closed Resting State Condition, Clinical Trials, Biomarkers
1. BACKGROUND
1.1. Qualified biomarkers for the diagnosis and monitoring of Alzheimer’s disease (AD) patients in clinical trials
The International Working Group (IWG) and the US National Institute on Aging–Alzheimer’s Association Research Framework (NIA-AA Research Framework) have recently proposed and refined recommendations and research criteria for the diagnosis and monitoring of Alzheimer’s disease (AD) at the preclinical, prodromal (with objective mild cognitive impairment, ADMCI), and overt dementia (ADD) stages for clinical trials, based on in-vivo fluid and neuroimaging biomarkers [1; 2; 3; 4]. According to these diagnostic criteria, AD status is associated with (i) a reduction of cerebrospinal (CSF) Aβ42 and an increase in amyloid at the brain level as revealed by amyloid positron emission tomography (PET) mapping, and (ii) an increase of phospho-tau in CSF and of tau PET tracer retention. Neurodegeneration over the course of disease progression may be revealed by 18Fluorodeoxyglucose PET (FDG-PET), total tau in CSF, and magnetic resonance imaging (MRI) of brain atrophy in temporoparietal cortex and the medial temporal lobes (including the hippocampi).
1.2. Measures of eyes-closed resting state electroencephalographic (rsEEG) rhythms
The use of the above neuroimaging techniques for serial recordings in longitudinal AD clinical trials may be limited due to their high costs and invasiveness, especially in lower- and middle-income countries. Apart from the indirect role played by FDG-PET as a marker of synaptic integrity, none of these biomarkers reflects effects of AD neuropathology on neurophysiology of brain neural signal transmission underpinning cognitive processes. It is the “gap” of physiology in the amyloidosis, tauopathy, and neurodegeneration framework of biomarkers in AD research [4].
To fill that gap, measures of eyes-closed resting state electroencephalographic (rsEEG) rhythms are quite promising as they are non-invasive, reproducible (without learning effects) until severe dementia, cost-effective, and based on recording techniques largely available worldwide including lower- and middle-income countries [5, 6;7]. These measures may probe effects of AD on ascending activating systems and reciprocal thalamocortical circuits in which oscillatory (de)synchronizing signals dynamically underpin cortical arousal in the regulation of quiet vigilance [5, 6; 8;7]. This phase synchronization/desynchronization of cortical neural activity may occur in an interrelated way in multiple cortical areas, gating transmissions and communications of action potentials within both local and long-range neural networks [9; 10; 11; 12]. Animal studies elucidated the cellular and molecular basis of on-going EEG activity at cortical and subcortical level [13; 14; 15]. Furthermore, they demonstrated that AD neuropathology may cause disconnection among neural cells, damage to cortico-cortical and cortico-subcortical pathways, and loss of myelinated axons possibly associated with cortical neural hyperexcitability and hypersynchronization as well as reduced neurotransmission, neural signaling and synaptic activity [16; 17]
In clinical contexts, rsEEG activity is typically recorded from 19-25 to > 80 scalp electrodes placed according to 10-10 montage system [18; Figure 1] while patients are in quiet wakefulness with their eyes closed. They are instructed to stay psycho-physiologically relaxed with no cognitive demand and to allow their mind to wander freely (Panel 1).
Figure.1:
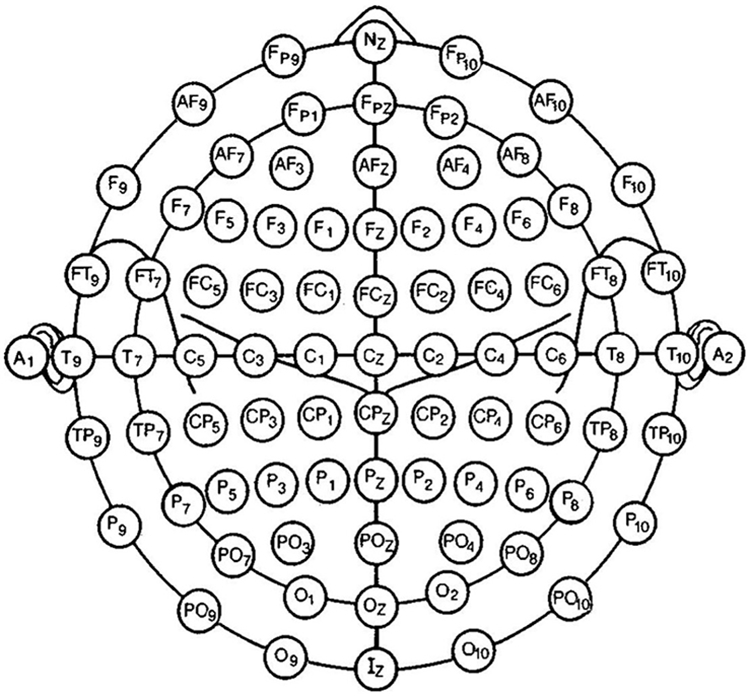
Modified combinatorial nomenclature of the 10–10-system of scalp electrode montage for clinical electroencephalographic (EEG) recordings, extended with anterior and posterior electrodes in the inferior chain. Adapted from [18], (courtesy from the Publisher).
Panel 1: Experimental conditions of recordings of electroencephalographic (EEG) activity in quiet vigilance (resting state) in humans.
Neurophysiological mechanisms, duration, and instructions
Neurophysiological mechanisms keeping the state of low vigilance with eyes-closed for several minutes (i.e., 5-15 minutes or more). It also probes the transition to drowsiness and sleep, hence the experimenter (or trained technologist) should not alert the subject in case of sleep. The instructions invite the subject to sit quietly, stay relaxed in a state of mind wandering (i.e., no goal-oriented mental activity), and keep the eyes closed.
Neurophysiological mechanisms regulating the increase and decrease in the vigilance level while opening and closing the eyes sequentially (i.e., 5-10 minutes). The periods of eyes-open and -closed in response to the experimenter’s cue are short (i.e., 1 min), and the sequence of eyes-open and -closed is repeated (i.e., 2-4 times). The instructions to the subject are like those of the first condition. The experimenter will have to alert the subject in case of sleep to have enough EEG data related to the proper mental state.
The third condition tests the neurophysiological mechanisms underlying the steady maintenance of low vigilance at eyes-closed (i.e., 3-5 min) and moderate vigilance at eyes-open (i.e., 3-5 min). The instructions to the subject are like those of the second condition.
Due to the resistive features of skull and scalp, rsEEG rhythms recorded at the scalp level is mainly represented by frequencies spanning about from 1 to 100 Hz, detectable by the high temporal resolution (< 1 ms) of EEG recording systems [19; 20, 21].
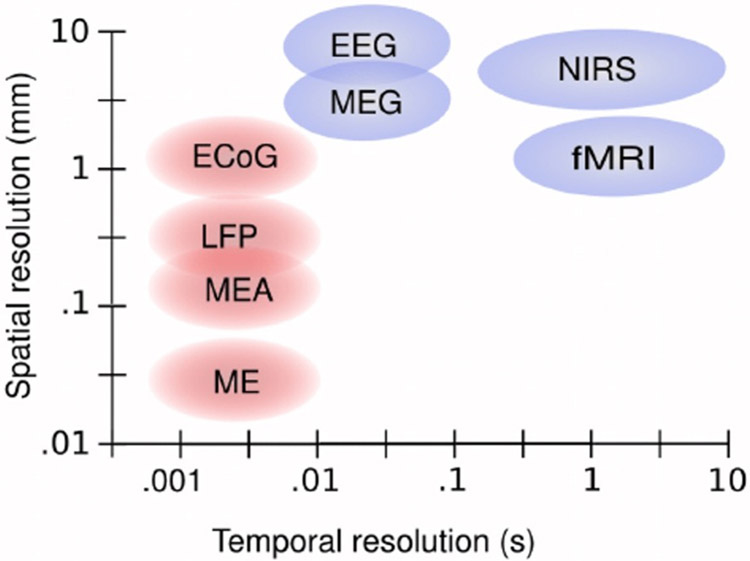
Spatial resolution of EEG techniques is low to moderate (i.e., few centimeters) as compared to the neuroimaging techniques mentioned above. Indeed, rsEEG activity measured at a scalp electrode may results from cortical sources located several centimeters apart or distributed into large regions due to head volume conduction effects [> 10 cm2; 20]. This resolution depends on the number of scalp EEG electrodes and mathematical techniques for EEG source estimation (Figure 2).
Figure 2:
Schematic overview of the scale of spatial and temporal resolution of measurement methods used for electrophysiology and functional neuroimaging. Measurement methods are EEG, magnetoencephalography (MEG), near-infrared spectroscopy (NIRS), functional magnetic resonance imaging (fMRI), electrocorticography (ECOG), local field potential (LFP) recordings, micro-electrode array (MEA) recordings, and microelectrode (ME) recordings. Non-invasive methods are shown in blue and invasive methods are shown in red. Adapted from [192] (courtesy from the Publisher).
In artifact-free rsEEG activity, posterior (“dominant”) alpha rhythms (about 8-12 Hz) are the dominant oscillations and reduce in amplitude in the transition from eyes closed to open condition, due to activation of visual-spatial cortical systems [5, 6; 22]. In quiet vigilance (no task demands), high amplitude of low-frequency alpha rhythms (about 8-10 Hz) may reflect low levels of general brain arousal, attention, and readiness [23; 24], while high amplitude of high-frequency alpha (about 10-13 Hz) and low-frequency beta (about 12-20 Hz) rhythms may reflect low levels of perceptual, somatomotor, and memory processes [23; 25].
During sensorimotor and cognitive events, alpha rhythms are replaced by faster cortical rsEEG oscillations, namely high-frequency beta (20-30 Hz) and gamma (30-70 Hz) rhythms, mainly prompted by (i) forebrain cholinergic direct inputs to hippocampus and cerebral cortex and (ii) thalamocortical projections [15; 20].
In healthy adults, rsEEG activity at delta (1-4 Hz) and theta (4-7 Hz) rhythms may typically show small amplitude and exhibit complex patterns of changes during sensorimotor and cognitive events [26; 20]. Therefore, abnormally prominent theta or delta rhythms in the resting state condition are considered as signs of brain dysfunctions [5, 6].
Several methods can probe information embedded in artifact-free scalp rsEEG waveforms (see Supplementary materials: Main features of resting state EEG measures used in studies on Alzheimer’s disease). Fast Fourier Transform (FFT) assumes a linearity of rsEEG signals and returns “local “power density of rsEEG voltage time series at each scalp electrode, frequency-bin-by-frequency bin (Figure 3). Other methods estimate “interrelatedness of rsEEG activity at scalp electrode pairs or EEG source connectivity,” based on an assumption of linearity or nonlinearity of rsEEG signals [5; 27, 28, 29] and graph theoretical indices represent general topological features of those estimates as network nodes connected (or not) by edges [30; 31; 32; 33; Panel 2].
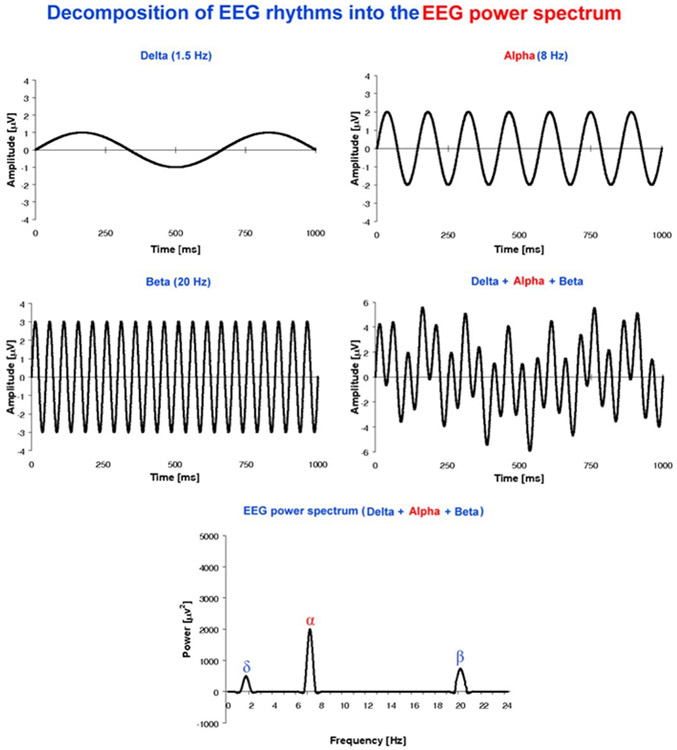
Figure 3:
Decomposition of EEG rhythms into an EEG power spectrum. A sketch illustrates example of sinusoidal EEG waveforms at frequency binds od delta, alpha and beta rhythms and how they can be represented (i) when summed each other at given phases or (ii) used as an input for the calculation of an EEG power density spectrum. Modified [193].
The inclusion of rsEEG measures in AD clinical trials (e.g., patients’ stratification, monitoring disease progression, efficacy of therapeutics, etc.) as enriching neurophysiological biomarkers has to steam on a preliminary demonstration that those measures are reliable, consistent, and sensitive (e.g., test-retest reliability, effect and sample sizes, etc.). For example, the statistical power and the variability of effect-sample sizes of a given rsEEG measure are relevant parameters to evaluate if that candidate biomarker can be included in clinical trial protocols of phases > 1 designed for AD patients, namely protocols testing the neurobiological efficacy and therapeutic effects of new drugs against AD. It should be considered that clinical trials of phases 2 and 3 typically involve 100-300 and 300-3,000 patients for each patients’ group, respectively [34]. Therefore, a suitable rsEEG biomarker is expected to be reliable, consistent, and have a sample size substantially lower than n=300 patients for group.
Concerning the issue of test-retest reliability, previous studies reported that the power density of artifact-free rsEEG rhythms is stable at 12-40 months retest performed in healthy adults [35; 36]. Namely, those studies showed high test-retest correlations (r = 0.92 at 5 min, 0.84 at 12–14 weeks, [37]; intra-class correlation coefficients = 0.8-0.9 at 4 weeks, [36]. Furthermore, the absolute and relative power densities of rsEEG activity, including dominant posterior alpha rhythms during the eyes-closed condition, were quite consistent when computed from artifact-free rsEEG data lasting 20 s, 40 s, 60 s to 4 minutes [36; 37; 38] which are the typical durations of rsEEG datasets used for the quantitative analysis performed in Clinical Neurophysiology [5].
In general, results of the previous test-retest studies showed that the rsEEG relative power density was slightly more repeatable than the absolute power density, probably for the general property of normalization procedures to reduce measure variabilities [e.g., 36]. Furthermore, the reproducibility of spectral rsEEG measures over sessions was higher in the eyes-closed than -open condition, possibly due to the effects of residual blinking and saccades in the data used for rsEEG spectral analyses [39; 36]. Moreover, the reproducibility of rsEEG measures was higher in rsEEG power density than interrelatedness (connectivity) estimates at both sensor and source levels [36]. Finally, such a reproducibility was slightly higher at sensor than source levels, but the findings encouraged the use of both levels of analysis for clinical applications [36; 40].
Keeping in mind the above data and considerations, the reliability and consistency of typical rsEEG measures derived from healthy adults seem to be generally suitable for applications in Clinical Neurophysiology [5]. However, those statistical features should be carefully taken into account to interpret the statistical effects reported in the studies using rsEEG measures to compare AD and control individuals and to investigate AD progression and therapy responses.
Panel 2: Graph theory modeling the topology of measures of rsEEG interrelatedness Activity.
Gore graph indexes in rsEEG research
“Clustering coefficient” as the degree to which nodes in a graph tend to cluster together.
“Path length” as the mean number of edges any node needs to reach the others of the graph.
“Betweenness centrality” as number of edges one node needs to reach all the other nodes of the graph (the lower the number, the higher the centrality).
“Clustering coefficient” as the degree to which nodes in a graph tend to cluster together.
“Local efficiency” is computed on node neighborhoods and is related to the clustering coefficient (“global efficiency”) as a measure of how efficiently it exchanges information among nodes of the network.
“Modularity” as the degree to which the network may be subdivided into delineated groups.
“Small-worldness” is defined as a good balance between an intense local connectivity and effective “hubs” connecting far nodes.
“Robustness and adaptation” are the ability of a network to maintain its connectivity when a fraction of nodes (links) is damaged.
1.3. Aims and methodology
Although international panels of experts have emphasized the merits of rsEEG measures in AD research [6;7], there are still uncertainties about the specific biomarker value (e.g., disease status, progression, etc.) of different rsEEG measures for their use in AD clinical trials, possibly due to the heterogeneity of methodological approaches that are available. To clarify these uncertainties, the Electrophysiology Professional Interest Area (EPIA) of Alzheimer’s Association and Global Brain Consortium endorsed this article written by a multidisciplinary Expert Panel to provide recommendations about candidate rsEEG measures for AD clinical trials. The core question was “Are there rsEEG measures that reveal consistent effects across previous studies carried out in AD patients for use in future clinical trials for patients’ stratification, monitoring of disease progression, and study of the efficacy of interventions?” Expertise in the Panel covers several relevant disciplines (i.e., Neurology, Psychiatry, Neuroimaging of Dementias; Clinical Neurophysiology; Quantitative Analysis of rsEEG rhythms in Dementias; Cognitive Neurosciences; Public Health) strictly related to the core question.
The Panel formulated the recommendations based on a comprehensive review of the field literature performed through Web of Science, Scopus, and MEDLINE, using several appropriate combinations of the following key words.
For the “populations of interest,” the following key words were used: “Subjective memory Complaint” (SMC), “Subjective Cognitive Complaint” (SCC), “Mild Cognitive Impairment” (MCI), “Mild Neurocognitive Disorder”, “Alzheimer’s neuropathology”, “Alzheimer’s Disease” (AD), “Dementia of Alzheimer’s Type” (DAT), “Major Cognitive Impairment”, “Major Neurocognitive Disorder”, and “Dementia.”
For the “EEG techniques of interest,” the following key words were used: “Electroencephalography (EEG)”, “Resting State EEG”, “Ongoing EEG”, “Background EEG”, “Quantitative EEG (qEEG)”, “Brain rhythms”, and “Brain Oscillations.”
For the “EEG measures of interest,” we used the following key words: “Waveforms (i.e., visual analysis and description of well-known graphoelements observed on ongoing rsEEG traces), “EEG Power Density,” “Relative Power Density,” “Alpha peak frequency,” “Complexity and Irregularity (referring to the dynamics of temporal patterns at one scalp electrode or spatiotemporal patterns in many scalp electrodes) [40],” “Interrelatedness of EEG activity or EEG source connectivity,” “Spectral Coherence, ” “Partial Coherence, ” “Entropy, ” “Chaos, ” “Information Theory, ” “Granger Causality,” “Directed Transfer Function,” “Phase-Amplitude Coupling,” “Synchronization likelihood (SL),” “Phase Lag Index (PLI),” “Phase Locking Value (PLV),” “Amplitude Envelope Correlation (AEC),” “Graph Theory,” “Clustering coefficient,” “Characteristic path length,” “Modularity index,” “Linear Source Estimation,” “Nonlinear Source Estimation,” “Functional Connectivity,” and “Inverse problem.”
For the “Experimental design of interest,” the following key words were used: “Longitudinal studies” (cohorts of AD patients followed over time), “Cross-sectional studies” (samples of AD patients with different severity and disease duration), “Classification studies” (accuracy of EEG measures in the discrimination between controls, patients with AD and/or other types of cognitive impairment at individual level).
Only papers published in international journals with peer-review and impact factor were selected (−2020). Some papers could not be downloaded from available Internet resources and were not considered. All selected abstracts/papers were critically reviewed by selected members of the Expert Panel (i.e., C.B., L.B., C.D.P, B.G., R.L., F.T., S. L. G.N., and G.Y.) taking into account the criteria reported in Jelic and Kowalski [42] namely original article published in English with 10 or more persons per diagnostic group, diagnosed according to the established consensus clinical diagnostic criteria used as a "gold standard." The validity of the rsEEG measures was mainly based on the guidelines for rsEEG recordings and data analysis in Clinical Neurophysiology [5]. The selected rsEEG papers were distinguished in three arbitrary classes with increasing weight in the formulation of the recommendations. They were as follows:
1). Class A.
ADD or ADMCI patients mostly diagnosed using qualified CSF or PET diagnostic biomarkers of AD [1; 2; 3; 4, 43] and > 10 participants for each group. When the data were available in the Class-A paper, we reported the effect and sample sizes of the main rsEEG measures characterizing AD patients over controls (see Tables 1, 2, and 3).
Table 1.
Measures of eyes-closed resting state electroencephalographic (rsEEG) rhythms for patients’ stratification in Alzheimer’s disease (AD) clinical trials. These biomarkers refer to cross-sectional and longitudinal rsEEG studies of the so-called “Class A” in which AD patients with dementia (ADD), amnesic mild cognitive impairment (ADMCI) or subjective memory complaint (SMC) / subject cognitive impairment (SCI) were compared with old cognitively unimpaired (CU) persons with intact cognition (CU persons) resting in quiet wakefulness for a few minutes. In “Class A” studies, AD patients received a diagnosis based on qualified biomarkers of AD derived from cerebrospinal fluid (CSF) or amyloid positron emission tomography (PET) in line with recent international guidelines [1; 2; 3; 4]. Patients’ stratification may be based on rsEEG measures showing either differences between AD patients and CU control persons at the group or individual level (i.e., classification studies including those calculating discriminant accuracy by area under the receiving operating characteristic -AUROC- curve) or significant accuracy in the statistical prediction of their cognitive decline at a follow-up of 12 months or later. Noteworthy, to test the generalizability of the findings of the articles with the symbol “*” [129, 147], the reported classification accuracy should be cross-validated using the same (trained) classifiers and rsEEG measures in fully independent individual rsEEG datasets obtained from clinical recording units not involved in the original experiments. Frequency bands of rsEEG rhythms were standard, namely delta, theta, alpha, beta, and gamma. More literature evidence and the meaning of rsEEG measures in the table (i.e., rsEEG power density, “spectral coherence”, estimates of cortical source activity generating scalp rsEEG rhythms, etc.) are reported in the main text. When the data were available in the “Class-A” paper, the effect and sample sizes of the main rsEEG measures characterizing AD patients over controls are reported. Other explanations and considerations are reported in the “Supplementary materials”.
| Purpose | Design | Patient samples | EEG biomarkers | Main findings | Selected References (Class A) |
|---|---|---|---|---|---|
| Disease Status | Cross-Sectional | SMC seniors (N= 318, age= 76) | Delta, theta, alpha and beta power density and sources | The most prominent effects of neurodegeneration on EEG metrics were localized in frontocentral regions with an increase in high frequency oscillations (higher beta and gamma power) and a decrease in low frequency oscillations (lower delta power) | Gaubert et al., 2019 |
| Disease Status | Cross-sectional | ADD (N= 117, age= 70), ADMCI (N= 138, age= 75), CU persons (N= 138, age= 66) |
Delta, theta, alpha and beta power density and sources | Findings suggest that the increase in relative theta power may be the first change in patients with dementia due to AD. | Musaeus et al., 2018 |
| Disease Status | Cross-sectional | HC (N= 17, age= 76), AD (N= 26, age= 76), |
Alpha and theta power density | Compared to CU persons, the patients’ groups showed lower alpha “phase lag index” and lower dominant frequency (that showing maximum rsEEG power density in alpha range). The effect size was 1.23 calculated by Cohen’s method (Cohen’s d) and the sample size per group was 10. | Peraza et al., 2018 |
| Disease Status | Cross-sectional | ADD (N= 197, age= 67), MCI (N= 230, age= 65), Significant cognitive deficit (SCD) (N= 210, age= 60) |
Delta, theta, alpha and beta global field power (GFP) and global field synchronization (GFS) | Decreased CSF amyloid b 42 significantly correlated with increased theta and delta GFP, whereas increased p- and t-tau with decreased alpha and beta GFP. | Smailovic et al., 2018 |
| Disease Status | Cross-Sectional | SMC seniors (N= 318, age=76) | Delta, theta, alpha and beta power density and sources | Statistical trend in the relationship between amyloid deposition and posterior alpha power density. | Teipel et al., 2018 |
| Disease status | Cross-sectional | CU persons (N= 66, age= 70), ADD (N= 66, age= 70) |
Beta power and global rsEEG power density | rsEEG variables showed that the trained random forest learning machine reached 62% of classification accuracy in the discrimination between the CU persons and ADD individuals | Dauwan et al., 2016 |
| Disease status | Cross-sectional | CU persons (N= 60, age= 69) ADMCI (N= 70, age= 69) |
Alpha power density | As compared to ADMCI group, the CU group showed greater alpha sources activations. The ADMCI subgroup with high education attainment showed a lower alpha source activation topographically widespread as compared to the ADMCI subgroup with low education. The effect sizes were 0.49 for the global alpha 2 source activity and 0.65 for the global alpha 3 source activity. The sample sizes were 53 ADMCI patients (for each subgroup) for the global alpha 2 source activity and 30 ADMCI patients for the global alpha 3 source activity. | Babiloni et al., 2020c |
| Disease status | Cross-sectional | ADD (N= 372, age= 71), CU persons (N= 146, age= 66) |
Delta, theta, alpha and beta power density and sources | In receiver operating characteristic curve analyses obtained using statistical pattern recognition (SPR) method, the EEGs separated AD patients from healthy elderly individuals with an area under the curve (AUC) of 0.90, representing a sensitivity of 84% and a specificity of 81%. | Engedal et al., 2015 |
| Disease status | Cross-sectional | ADD (N= 31, age= 60), CU persons (N= 17, age= 60) |
Alpha “spectral coherence” and global theta power density | In AD patients, global theta power was increased, left temporal alpha “spectral coherence” and interhemispheric theta coherence were decreased. The effect size (Cohen’s d) was 0.88 for the theta power and the sample size was 22 for each group. | Adler et al., 2003 |
Table 2.
Biomarkers of eyes-closed rsEEG rhythms for patients’ stratification in AD clinical trials. These biomarkers refer to rsEEG measures showing significant accuracy in the statistical prediction of their cognitive decline at a follow-up of 12 months or later. Noteworthy, to test the generalizability of the findings of the articles with the symbol “*” [106], the reported classification accuracy should be cross-validated using the same (trained) classifiers and rsEEG measures in fully independent individual rsEEG datasets obtained from clinical recording units not involved in the original experiments. Frequency bands of rsEEG rhythms were standard, namely delta, theta, alpha, beta, and gamma. More literature evidence and the meaning of rsEEG measures in the table (i.e., rsEEG power density, “spectral coherence”, estimates of cortical source activity generating scalp rsEEG rhythms, etc.) are reported in the main text. When the data were available in the “Class-A” paper, the effect and sample sizes of the main rsEEG measures characterizing AD patients over controls are reported. Other explanations and considerations are reported in the “Supplementary materials”.
| Purpose | Design | Patient samples | EEG biomarkers | Main findings | Selected References (Class A) |
|---|---|---|---|---|---|
| Prediction | Longitudinal | ADMCI (N= 72, age= 69), noADMCI (N= 54, age=68) |
Delta, theta, and alpha source activities | Compared to the noADMCI patients, the ADMCI patients were characterized by greater global delta and theta source activity and lower ratio between posterior delta-theta and alpha source activity. The output of the Linear Mixed Models was presented in terms of standardized coefficient, corresponding p-value and, for the interaction factor only, effect size (pseudo h2) calculated as the ratio of explained variability of interaction effect on the total variability of each model. | Jovicich et al., 2019 |
| Prediction | Longitudinal | SMC seniors (N= 318, age= 76) | Alpha and Delta power density and sources | Brain β-amyloidosis had not affected behavior and cognition when measured at 30 months. β-amyloidosis alone is insufficient to identify patients at high risk of rapid progression to prodromal Alzheimer's disease. It was calculated the sample size for achieving sufficient confidence around a positive and a negative likelihood ratio. These likelihood ratios incorporated the sensitivity and specificity of the predictive model, providing a direct estimate of how much the combination of predictors would change the odds of a progression to prodromal Alzheimer’s disease. Based on Rowe and colleagues reported variable progression of 14% over 3 years, and the use of 95% CIs, sample size was 82. | Dubois et al., 2018 |
| Prediction | Longitudinal | ADD (N= 32, age= 69), MCI (N= 56, age= 68), CU persons (N= 41, age= 65) |
Delta, theta, alpha and beta power density and spectral coherence | AUROC showed a low discriminative ability of SPR method: 60% for ADD vs. MCI, 66% for ADD vs. CU persons, and 56% for MCI vs. CU persons. | Nielsen et al., 2018 |
| Prediction | Longitudinal | SCD amypos (N= 25, age= 67) SCD amyneg (N= 38, age 67) MCI (N= 142, age 68) |
Delta and theta power density and sources | High delta-theta and lower alpha power density predicted clinical worsening over time in SCD seniors with amyloid deposition in the brain. The effect size (Cohen’s d) was 0.78 for the theta power and the sample size was 27 for each group. | Gouw et al., 2017 |
| Prediction | Longitudinal | ADMCI (N= 27, age= 58) | Alpha and theta power density and sources | Alpha and theta relative power density combined with mean frequency from left temporo-occipital derivation (T5-O1) correctly classified at 85% ADMCI using logistic regression model applied on baseline EEG variables by means of chunk test | Jelic et al., 2000 |
| Prediction | Longitudinal | AD (N= 38, age= 62), MCI (N= 31, age= 63), CU persons (N= 24, age= 61) |
Delta, theta, alpha, EEG mean frequency, gamma coherence, network topology |
Predictors of conversion from MCI to AD:
The effect size (Cohen’s d) was 0.64 for the alpha power and the sample size was 40 for each group. |
Huang et al.,2000 |
Table 3.
Measures of rsEEG rhythms for monitoring AD progression and response to interventions in AD clinical trials. These biomarkers refer to longitudinal rsEEG studies of the so-called “Class A” (see Table 1 for definitions). The qualification of those measures was based on studies showing differences in rsEEG measures at baseline vs. follow-up recordings or before vs. after a pharmacological intervention in relation to placebo. For example, in the PQ912 study, ADMCI and ADD patients formed an experimental group (n=60) receiving an inhibitor of the glutaminyl cyclase enzyme (PQ912) that plays a central role in the formation of synaptotoxic pyroglutamate-A-beta oligomers for 12 weeks, while a placebo group (n=60) received hypocaloric beverage for the same period [181]. In other studies, ADD and ADMCI patients received Acetylcholinesterase inhibitors (AChEIs) for weeks/months. Frequency bands of rsEEG rhythms were standard, namely delta, theta, alpha, beta, and gamma. When the data were available in the “Class-A” paper, the effect and sample sizes of the main rsEEG measures characterizing AD patients over controls are reported. More literature evidence and the meaning of rsEEG measures in the table are reported in the main text.
| Purpose | Design | Patient samples | EEG biomarkers | Main findings | Selected References (Class A) |
|---|---|---|---|---|---|
| Disease progression and monitoring | Longitudinal | ADMCI (N= 72, age= 69), noADMCI (N= 54, age= 68) |
Delta, theta, and alpha source activities | ADMCI patients showed increased limbic theta source activity and greater cognitive decline at a 24-month follow-up in relation to reduced functional connectivity. The output of the Linear Mixed Models was presented in terms of standardized coefficient, corresponding p-value and, for the interaction factor only, effect size (pseudo h2) calculated as ratio of explained variability of interaction effect on total variability of each model. | Jovicich et al., 2019 |
| Disease progression and monitoring | Longitudinal | ADMCI (N= 27, age= 58) | Alpha and theta power density and sources | ADMCI showed increased temporal and occipital theta-delta power density and decreased beta power density at an averaged follow-up of 21 months | Jelic et al., 2000 |
| Assessment of treatment effects | Longitudinal | ADMCI (N= 47, age=70), ADD (N= 56, age= 72) |
Delta, theta, alpha, dominant frequency, functional connectivity | ADMCI receiving a glutaminyl cyclase inhibitor involved in the amyloid production (PQ912) showed enhanced interrelatedness of alpha rhythms as revealed by an improved phase lag index. | Briels et al., 2020 |
| Assessment of treatment effects | Longitudinal | ADMCI (N= 60, age= 72), ADD (N= 60, age= 71) |
Delta, theta, and alpha source activities |
Results showed an improvement of memory and a reduction of global theta power density across all electrodes in the Souvenaid PQ912 as compared with the placebo group. The effect size was 0.37 for theta power, while the sample size was 116 for the protocol group (PP), 0.29 and 188 for the intent to treat (ITT) group, and 0.32 and 155 for the modified ITT group.
PQ912 intervention did not affect the “phase lag index” as measure of rsEEG “interrelatedness” |
Scheltens et al., 2018 |
| Assessment of treatment effects | Longitudinal | ADD (N= 15, age= 65), ADD placebo (N= 10, age= 61) |
Delta and theta GFP | AChEIs (tacrine) reduced theta GFP after 3 and 12 months, while both delta and theta GFP reduced after 6 months | Jelic et al., 1998 |
2). Class B.
ADD or ADMCI patients enrolled using traditional criteria for the clinical diagnosis of AD as inclusion criteria [e.g., 44] and large populations (> 100 participants) of AD and control CU persons.
3). Class C.
As Class B but with smaller populations (< 100 participants). In the article, Class C papers were described without reporting the “Class C” label and without the specification of the number of persons for the sake of brevity.
The selected members of the Expert Panel (i.e., C.B., L.B., C.D.P, B.G., R.L., F.T., S. L. G.N., and G.Y.) produced a first draft of the article. This draft has been sent to the other co-Authors (January 6th, 2020) for further discussions looking towards a unanimous consensus about the recommendations to be released. It was finalized in March 2020.
Significant caveats and intrinsic limitations of this article include (i) the potentially restrictive criteria used for the literature revision and classification; (ii) the inclusion of studies that have applied the heterogeneous diagnostic criteria for AD used for decades, which may not exclude the presence of moderate cerebrovascular, non-AD hippocampal impairment (TDP-43), and Lewy body co-pathology, especially in older ages; (iii) the blurring effects of head as a volume conductor spreading scalp rsEEG activity (Figure 4), and (iv) heterogeneous procedures for the detection of artifacts in preliminary rsEEG data analysis. See more details in the Supplementary materials: Caveats and intrinsic limitations.
Figure 4.
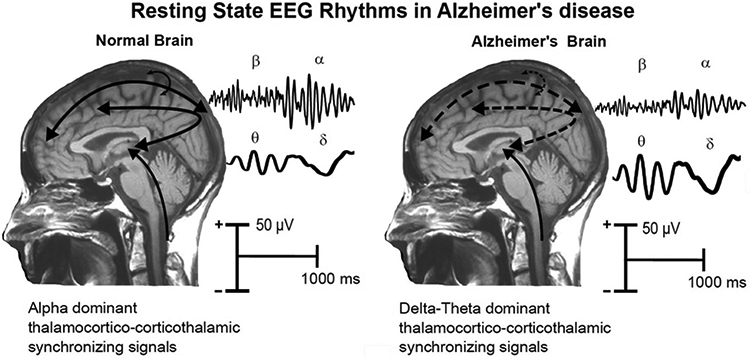
Tentative physiological model of the generation of resting state eyes-closed EEG (rsEEG) rhythms in the brain of age-matched old cognitively unimpaired (CU) persons and Alzheimer’s disease (AD) patients. In the normal brain, dominant EEG rhythms are observed at alpha frequencies (8-12 Hz), which would denote the background, spontaneous synchronization around 10 Hz of neural networks regulating the fluctuation of the subject’s global arousal and consciousness states. These networks would span neural populations of cerebral cortex, thalamus, basal forebrain and brainstem, including glutamatergic, cholinergic, dopaminergic and serotoninergic parts of the reticular ascending systems. In the brain of AD patients, the amplitude of these rhythms is reduced (i.e., tonic background desynchronization) together with an amplitude increase of the pathological rsEEG slow frequencies spanning delta (< 4 Hz) and theta (4-7 Hz) rhythms. This “slowing” of rsEEG rhythms would mainly reflect a sort of thalamocortical “disconnection mode”.
2. RsEEG MEASURES FOR AD PATIENTS’ STRATIFICATION
2.1. “Local” measures of rsEEG rhythms in AD patients
ADD and ADMCI patients repeatedly showed marked changes in linear measures of rsEEG power density at delta, theta, and alpha bands in relation to CSF, neuroimaging, and clinical markers. The main results are reported below.
In two experiments of the international multicentric North Baltic (NORDEEG) study (Class A), as compared to age-matched old cognitively unimpaired (CU) persons (n=138), ADD (n=117) and ADMCI (n=138) patients showed increased global theta power density and lower cognitive performance as well as a positive relationship between posterior alpha-beta power density and cognitive performance [45]. Furthermore, decreased CSF amyloid β42 significantly correlated with increased theta and delta power density averaged across all electrodes (global field power, GFP) in patients diagnosed with subjective cognitive decline (SCD, n = 210), MCI (n=230), and ADD (n=197). In contrast, increased p- and t-tau correlated with decreased alpha and beta GFP [46]. Moreover, decreased CSF amyloid β42 and increased p- and t-tau were significantly associated with decreased alpha and beta global field synchronization (GFS) at zero voltage across all alectrodes as a function of cognitive status [46].
European multicentric PHARMACOG study (Class A) compared rsEEG measures in amnesic MCI seniors with diagnostic CSF markers incompatible (noADMCI, n = 54) and compatible (ADMCI, n = 72) with AD diagnosis [47]. A statistical model incorporated rsEEG source estimates from recordings performed every 6 months for 2 years. Results displayed that as compared to noADMCI patients, ADMCI patients were characterized by greater global delta and theta source activity and greater ratio between posterior delta-theta and alpha source activity [47]. Furthermore, ADMCI patients who could be evaluated until the last follow-up (n=63) exhibited an association between occipital theta/alpha source activity and global cognitive status, as measured by ADAS-cog13 score, and reduced connectivity within the default mode network in resting state functional MRI markers [47].
In an ITALIAN-TURKEY rsEEG study (Class A), as compared to CU persons (n=60), ADMCI patients with high (n=35) and low (n=35) education attainments presented greater alpha source activations topographically widespread [48]. On the contrary, in relation to the ADMCI subgroup with the low education attainment, the ADMCI subgroup with the high education attainment displayed lower alpha source activations topographically widespread (notably, the two ADMCI subgroups had matched cerebrospinal AD diagnostic biomarkers, APOEε4 genotyping, brain gray-white matter measures, and neuropsychological scores).
Another study [41; Class A] examined the association between CSF biomarkers and EEG parameters in AD patients (n=14). Those patients showed significant negative correlation between CSF beta-amyloid (Aβ)-42 concentration and the logarithms of CSD over the right temporal area in the theta band. Total tau concentration was negatively correlated with the lagged phase synchronization (LPS) between the left frontal eye field and the right auditory area in the alpha-2 band in patients with AD.
The effects of AD neuropathology on the amplitude of delta, theta, and alpha rhythms are corroborated by the following studies of independent worldwide consortia (Class B).
In a study based on the NEWYORK (n=264) and the STOCKHOLM (n=155) rsEEG databases, ADD and ADMCI patients were characterized by decreased alpha, beta, and gamma Global Field Synchronization (GFS), and increased delta GFS as compared to CU individuals with intact cognition [49]. In another STOCKHOLM rsEEG study, ADD (n=38) patients showed decreased alpha and beta GFP, and anteriorization of dipole source location fitting scalp alpha and beta rhythms as compared to ADMCI (n=31) and age-matched CU persons (n=24) [50].
In some experiments of the ITALIAN rsEEG study, as compared to CU persons (n=126), ADD (n=193) and ADMCI (n=155) patients presented a widespread increase of delta source activity and a decrease of posterior alpha source activity in relation to cognitive performance, as revealed by mini mental state evaluation (MMSE) score and other neuropsychological tests [51]. Furthermore, those abnormalities in rsEEG source activities were related to structural (e.g., hippocampal and cortical gray matter atrophy) [52, 53, 54] and functional (e.g., poor FDG-PET metabolism in posterior cortical regions) [55] neuroimaging markers. Findings at source level were cross validated by GFP markers at the scalp level. In fact, ADMCI patients (n=85) showed that the ratio between theta and gamma power density and the ratio between high- and low-frequency alpha power density were related to gray matter atrophy in hippocampus, thalamus, and basal ganglia in relation to cognitive status [56, 57, 58, 59, 60].
In the European DESCRIPA rsEEG study, when compared to non-amnesic MCI (n=51), SMC (n=53) not tested for amyloid deposition in the brain, and age-matched CU (n=79) persons, ADMCI patients (n=92) exhibited increased occipital-frontal theta source activity and lower posterior alpha source activity [61].
In the study by Roh and colleagues, as compared to CU persons (n=39), ADD (n=41) and ADMCI (n=38) patients presented (1) higher temporal and parieto-occipital theta power density and a decrease of posterior alpha source activity and (2) lower parieto-occipital alpha and frontal and temporal beta 2 power density in relation with cognitive deficits [62].
Other rsEEG studies of Class C showed similar results about delta, theta, and alpha power density in relation to cognitive functions [56, 59; 63].
Furthermore, beta power density (13-25 Hz) was positively correlated with good cognitive functions in ADD patients [64].
Other studies of Class B and C explored the relationship between rsEEG power density and neuroimaging measures in ADD patients. As compared to age-matched CU persons, ADD patients showed abnormal resting state functional MRI connectivity and decreased regional cortical blood perfusion and or metabolism by 99mTc-HMPAO SPECT or FDG-PET and scans, respectively, in relation to spatially widespread delta power density or cortical source activity [65 Class C; 66 Class B; 67 Class B; 68 Class C; 69 Class C; 70 Class C].
Age and genetic risk factors
The above effects were modulated by age and genetic risk factors, as shown by the following rsEEG studies (Class B), while gender factor was matched or used as a covariate. In the rsEEG study of VU Amsterdam University Medical Center (AMSTERDAM study), abnormalities in delta and alpha power density were more pronounced in young (≤ 65 years) than old (> 65 years) seniors with ADD (n=320) and intact cognition (n=246) [71].
In the ITALIAN rsEEG study, posterior alpha source activity was lower in ADMCI patients (n=84) than in age-matched CU persons (n=89), especially in those patients carrying the haplotype B of cystatin C genotyping [72]. A similar effect on posterior alpha source activity was also observed in ADMCI (n=89) and ADD patients (n=103) with ApoEϵ4 genotyping over non-carriers [73]. This effect was replicated in ADD (n=125) and CU persons (n=60) in an independent study using a similar methodology [74]. However, it was not replicated in the AMSTERDAM study carried out with ADD (n=320) and CU persons (n=246) using a methodological approach based on scalp rsEEG power density measures [75].
The above rsEEG measures (e.g., increased delta-theta power density and decreased posterior alpha power density) differed in ADD and ADMCI patients in other studies, as reported by the following studies (Class B). As compared to age-matched CU persons (n=35), ADD patients (n=61) were characterized by abnormalities in widespread scalp delta power density [76, 77].
A rsEEG study by the European Consortium of Lewy Body Dementia (E-DLB study) was performed in CU (n=42) and ADD (n=42) patients, Results displayed greater abnormalities in posterior alpha source activity and fewer abnormalities in delta source activity estimated in AD patients over controls [78].
In the ITALIAN study, there was greater delta and theta GFP and lower alpha GFP in ADD (n=60) than age-matched CU persons (n=30) [79], in line with previous relevant evidence [80; 81]. Furthermore, as compared to age-matched CU persons (n=38), ADD patients (n=48) showed reduction in posterior alpha source activities and increase in spatially widespread delta source activities [82].
In another rsEEG study [83], the ratio between alpha and delta+theta power density, and the global rsEEG mean frequency, were lower in ADD patients (n=62) than CU persons (n=14).
Preclinical AD
Previous rsEEG studies showed mixed results in SMC seniors with significant amyloid brain deposition (preclinical Alzheimer’s neuropathology).
In the French INSIGHT-preAD study (Class A), SMC seniors (n=318) 70-85 years old were tested by18F-florbetapir PET SUVR as marker of amyloid deposition in the brain and received an extensive data collection including rsEEG recordings [84]. Three articles reported the rsEEG findings. In the first study [85], there was only a negative trend in the relationship between amyloid deposition and posterior alpha power density (no effect in beta power density). In the second study [86], results unveiled a nonlinear U-shaped relationship between Alzheimer’s neuropathology and delta, beta, and gamma power density, while the relationship with alpha rhythms remained unclear.
In the third study [87], high values of Alzheimer neuropathology and education attainment were related to posterior alpha power density (no effect in the other standard frequency bands). Taken together, those findings suggest that the preclinical Alzheimer’s neuropathology may affect rsEEG rhythms in SMC seniors but with variable consequences on posterior alpha rhythms or other rsEEG measures.
Nonlinear rsEEG measures
Nonlinear rsEEG measures demonstrated an overall loss of EEG complexity in ADD patients [88;89; 90; 91; 41; 92, 93; 27; 94; see 95 for a review], especially at alpha rhythms [96] and even in patients with autopsy-confirmed neuropathology [97]. Furthermore, ADD and VaD patients showed different EEG complexity values [98], with the general limitation of a relatively low number of AD patients and the lack of control on the severity of cognitive dysfunctions in the patients’ groups. Overall, the following considerations motivate further validation studies before the use of nonlinear complexity rsEEG measures in regular AD clinical trials: (i) the heterogeneity of the above reference theories and methodological procedures (i.e., information theory, entropy, chaos, etc.); (ii) experimental databases with relatively small groups of AD patients; (iii) variable findings at rsEEG frequency bands [99]; (iv) sensitivity of those measures to instrumental and biological noise; and (v) the lack of validations with neuroimaging and diagnostic biomarkers of AD.
2.2. RsEEG measures of “interrelatedness/source connectivity”
In the INSIGHT-PreAD project (Class A), as compared to SMC seniors (n=175) without significant amyloid accumulation in the brain, those with preclinical Alzheimer’s neuropathology (n=25) showed that neurodegeneration, as revealed by FDG-PET hypometabolism, was associated with increased frontocentral beta and gamma power density, decreased delta power density, higher spectral entropy, higher complexity, and increased functional connectivity measured by weighted symbolic mutual information in theta rhythms Interestingly, if persons with neurodegeneration exceeded a certain threshold of amyloid load, the whole trend of rsEEG metrics reversed with increased delta power and the decrement of the following variables: beta and gamma power, median spectral frequency, spectral entropy, complexity and others typically characterizing ADMCI and ADD [86].
A bulk of previous studies have shown convergent evidence of differences between CU persons and ADD patients in measures of “interrelatedness” of rsEEG rhythms at electrode pairs or estimates of cortical source connectivity. Many of them were performed using “spectral coherence” at electrode pairs, a very popular linear rsEEG technique. As compared with age-matched CU persons, ADD patients pointed to lower “spectral coherence” between electrode pairs at alpha (8-12 Hz) and beta (13-20 Hz) rhythms [41; 100; 101; 102; 103; 104; 105; 106,107; 108; 109, 110; 111; 112; 113]. However, these effects were topographically variable being observed in temporo-parieto-occipital electrode pairs in some investigations [100; 106; 111] and frontocentral [104] and fronto-parietooccipital electrode pairs in others [41, 109; 103].
In the AMSTERDAM study (Class B), global alpha and beta “synchronization likelihood” across all combinations of electrode pairs (sensitive to both linear and nonlinear “interrelatedness”) was lower in ADD (n=109) and ADMCI (n=88) patients as compared to age-matched CU persons [29]. Furthermore, the ADD patients exhibited decreased global alpha “phase lag index” in relation to disease severity [114].
In the same line, the ITALIAN multicentric rsEEG study (Class B) reported a reduction of the global alpha “spectral coherence” in ADMCI patients (n=57) as a function of the cerebrovascular impairment in cholinergic tracts from basal forebrain to cerebral cortex as revealed by MRIs [115]. These effects were spatially specified in ADD (n=109), VaD (n=25), and ADMCI (n=88) patients as compared to CU (n=69) [116, 117]. In relation to the CU persons, the ADD and ADMCI patients showed a reduction in fronto-parietal alpha “synchronization likelihood” [116, 117]. Furthermore, “directed transfer function” (DTF; a multivariate measure of directional rsEEG “interrelatedness” derived from Granger causality) was lower from parietal to frontal electrodes in ADD and ADMCI patients as compared to age-matched CU persons [118, 119; 120]. This effect was confirmed in independent investigations [121, 122].
In a monocentric rsEEG study (Class B), another measure related to “spectral coherence” (namely, “phase synchronization”) showed decreased alpha “interrelatedness” between temporal and parietal electrodes in ADD (n=125) patients compared with age-matched CU persons (n=60) [123].
In the European E-DLB study (Class B), another rsEEG measure called “linear lagged connectivity” (removing the zero-lag “interrelatedness” more sensitive to head volume conduction effects) exhibited widespread lowering of alpha inter-hemispherical alpha source connectivity in ADD (n=120) and ADMCI patients (n=70) as compared to age-matched CU persons (n=100) [124, 125, 126].
The effect of AD on the above rsEEG “interrelatedness” was less clear at delta (< 4 Hz) and theta (4-7 Hz) bands. Compared with age-matched CU persons, ADD patients showed decreased “spectral coherence” at low frequencies, especially at central theta rhythms [100; 108]. In contrast, other rsEEG studies reported widespread increases of “spectral coherence” at delta rhythms [115] or quite complex topographical patterns showing increases and decreases of “spectral coherence” values at different electrode pairs [127].
Specifically, Sankari et al. [127] observed (i) an increase in left intrahemispheric frontal coherence in alpha, theta, and delta rhythms; (ii) an increase in left intrahemispheric temporo-parietal coherence in all bands; and (iii) a decrease in right intrahemispheric temporal-parietal-central coherence in all bands [127].
Other measures of rsEEG “interrelatedness” showed the following results.
In a large monocentric rsEEG study (Class B), global theta “phase synchronization” was higher in ADD patients (n=125) over CU (n=60) [74].
In the ITALIAN rsEEG study (Class B), “linear lagged connectivity” exhibited higher delta inter-hemispherical connectivity at occipital sources and higher theta intra-hemispherical connectivity at occipital-temporal sources in ADD patients (n=120) compared with age-matched CU persons (n=100) [124, 118], whereas “synchronization likelihood” showed widespread increased interrelatedness at delta rhythms in ADD (n=82) and ADMCI patients (n=88) over CU persons (n=69) [116, 117].
In contrast, a national monocentric rsEEG study displayed decreased “phase lag index” (removing the influence of zero-lag coherence) at the delta and theta rhythms within the frontal and between the frontal and temporal/parietal electrodes in ADMCI patients (n=9) as compared to age-matched CU persons (n=14) [128].
Some divergent results in the above studies may be due to different applied rsEEG measures of “interrelatedness” (“spectral coherence, synchronization likelihood, phase lag index, lagged connectivity, DTF”) and their different sensitivity to head volume conduction and common drive effects (see Supplementary materials: Main features of rsEEG measures used in AD studies for more discussion).
2.3. “Local and interrelatedness” measures of rsEEG rhythms in the classification of AD individuals
RsEEG measures may be used to quantify brain neurophysiological dysfunctions in ADD and ADMCI individuals for their sub-group stratification in observational and intervention clinical trials. This use may imply a classification rate > 80% in the discrimination between AD and control individuals based on rsEEG measures.
In the NORDEEG study (Class A), temporal theta power density showed a classification rate of 73% in the discrimination between ADD (n=117) and CU persons (n=138) [45]. In the same study, combined alpha and theta GFP reached 84% in the discrimination between ADD (n=38) and CU (n=24) persons as well 78% between ADD and ADMCI patients [50]. Furthermore, alpha and theta relative power density combined with mean frequency from left temporo-occipital electrodes (T5-O1) correctly predicted ADMCI (n=27) patients progressing to dementia with an accuracy of 85% [106].
In the AMSTERDAM study (Class A), global rsEEG power density, pair-wise “interrelatedness” (“phase transfer entropy”), and minimum spanning tree graph indexes at several frequency bands were used to train random forest learning machines (i.e., highest degree, leaf number, and tree hierarchy) for classifications between ADD (n=66) and age-matched CU persons (n=66) individuals [129]. This approach reached 62% of classification accuracy in the discrimination between the CU persons and ADD individuals.
In the ITALIAN rsEEG study (Class B), the ratio between parieto-occipital delta and alpha cortical source activities reached discriminated ADD (n=127) and age-matched CU persons (n=121) individuals with 75% of area under the receiver operating characteristic (ROC) curve [130]. The clinical significance of those results was also tested by a correlation analysis between the activity of parieto-occipital cortical delta or alpha 1 sources and the MMSE score in all Nold and AD subjects as a whole group [130]. Results showed that the higher the activity of parieto-occipital delta sources, the lower the MMSE scores (i.e., global cognitive status). Furthermore, the higher the activity of dominant parieto-occipital alpha 1 sources, the higher the MMSE scores.
Furthermore, a first-order polynomial regression of graph small-worldness index reached an area under the ROC curve of 61% in discriminating between stable (n=74) and progressing (n=71) ADMCI patients, while considering ApoEϵ4 allele it reached 97% accuracy [131].
In the E-DLB rsEEG study (Class B), delta and alpha cortical source estimates reached 85-90% of the area under the ROC curve of in the discrimination between age-matched CU persons vs. ADD patients (n=42 each group) [78]. Such accuracy dropped under 80% in the classification of individuals with prodromal (i.e., MCI) stages of AD (n=75) [132].
These findings extended those of other smaller national rsEEG studies. Global delta and alpha “spectral coherence” between electrode pairs successfully classified ADD (n=64) compared with CU people (n=54) with a classification accuracy > 80% [133]. Furthermore, a stepwise logistic regression analyses reached a classification accuracy of 82% between ADD (n=31) and CU persons (n=17) using left temporal alpha “spectral coherence” and global theta power density [100].
A seminal national study (Class B) was very important from a methodological point of view. Two rsEEG databases were used, one formed by ADMCI (n=25) and CU (n=56) persons [122] while the other was based on ADD (n=17) and CU (n=17) persons [134]. In the first experiment [122], mean-square and phase coherence, Granger causality principle (e.g., partial coherence, DTF, direct DTF, full-frequency DTF), phase synchrony indices, information-theoretic divergence, state space based indices, and stochastic event synchrony (SES)1 were comparatively used to discriminate ADMCI and CU persons. Most synchrony measures indicated decreased EEG synchrony in MCI patients. However, this effect was statistically significant for only two measures (ffDTF and ρ of SES method). The SES reached 68% and 75% of classification accuracy as measured by linear and quadratic discriminant analyses, respectively. Instead, the ff-DTF reached 70% by both linear and quadratic discriminant analyses. Combined measures reached 83% of classification accuracy.
In the second experiment [134], ff-DTF and SES reached classification rates of 83% and 98% using ADMCI and ADD patients, respectively. These results were replicated by other national monocentric studies showing > 90% of accuracy in the discrimination between AD and control CU persons with a combination of those procedures on theta and alpha rhythms [135; 136].
In European DECIDE rsEEG study (Class B), non-normalized DTF and spectral coherences of rsEEG activity mainly combining delta, theta, and alpha rhythms reached an area under ROC curve of 86%-88% in the discrimination between ADD and control CU persons [120].
Bennys and colleagues [137] reported that the ratio between parieto-temporo occipital theta and alpha + beta calculated from absolute power EEG bands reached an area under the ROC curve of 86 % in the discrimination between ADD (n=35) and age-matched CU (n=35) persons with a significant decrease in fast activities in mildly impaired patients.
The above results were corroborated using more advanced mathematical classifiers based on learning classifiers (see caveats in the Supplementary materials: The risk of “inflated” discrimination accuracy in classification studies) in the following large national monocentric rsEEG studies (Class B).
A classification rate of 90% was obtained through an artificial neural network as a classifier in the discrimination between patients with dementia (n=111) and age-matched CU persons (n=56) using the topographic distributions of absolute delta and theta power density as input rsEEG measures [138]. Furthermore, a similar discrimination accuracy between ADD patients and CU persons was reached giving rsEEG power density and measures of “interrelatedness” as inputs to several mathematical classifiers including principal component linear discriminant analysis, partial least squares linear discriminant analysis, principal component logistic regression, partial least squares logistic regression, bagging, random forest, support vector machines and feed-forward neural network (10-fold cross-validation runs) [139]. As best results, random forests reached 81.5% classification accuracy in the discrimination between mild ADD patients (n=116) and age-matched CU persons (n=45), while neural networks reached 88.5% classification accuracy between moderate ADD (n=81) and CU persons [139].
The ITALIAN rsEEG study (Class B) tested the discrimination among ADD (n=180), ADMCI (n=115), and CU individuals (n=171) using multichannel rsEEG voltage time series as inputs to advanced “IFAST” artificial neural networks [140,142;141]. This approach showed classification accuracies > 90% between ADD and CU persons as well as between ADD and ADMCI patients [140; 141]. Based on rsEEG rhythms (0-12 Hz) recorded at baseline and 1-year clinical follow up, the ADMCI patients were retrospectively classified with 86% accuracy in the discrimination between those progressing to ADD and those with a stable ADMCI condition [142]. Notably, the classification accuracy did not improve using the most discriminant cortical source activity such as posterior alpha in relation to delta/theta sources as an input to backpropagation artificial neural networks [77% accuracy; 143].
In other studies, high classification accuracies of 94-98% were obtained with novel rsEEG "interrelatedness" measures based on Sugihara causality analysis in CU (n=15), MCI (n=16), and ADD (n=17) persons [144; Class C] and based on inter-regional transfer entropy analysis in CU (n=15), MCI (n=16), and ADD (n=17) persons [145; Class C].
In the NORDEEG study (Class A), a stepwise classification procedure using support vector machines as a statistical pattern recognition (SPR) produced 5 values from 0 to 1 for each person, based on the analysis of 20 selected rsEEG measures (i.e., power density and “spectral coherence”) extracted from the original EEG recording. These 5 values referred to the following diagnostic labels: “NRM” (CU persons index), “sMCI” (MCI index), “AD” (AD index), “ADms” (AD, moderate/severe index), and “LP” (Lewy body/Parkinson’s disease index). A graph represented these values within their confidence intervals to support the diagnosis. A seminal experiment tested the diagnostic accuracy in the evaluation of clinicians based only on that EEG-based graph, using as a gold standard clinical diagnosis obtained by an experienced multidisciplinary team with the agreement of at least 2 experienced physicians (specialist level in dementia). The team gave the clinical diagnosis using all available examination results (e.g., clinical, neuropsychological, fluid biomarkers, neuroimaging markers), but independently (“blind”) of the EEG results [146]. Five clinical units using harmonized EEG procedures were involved. This procedure was followed in the diagnosis of ADD (n=32), MCI (n=56, 65% of them having CSF diagnostic biomarker values compatible with AD), and CU individuals (n=41) who had received the evaluation of CSF diagnostic biomarkers [146]. The diagnostic accuracy of the EEG-based graph was expressed as a percentage of correct diagnosis, using the mentioned clinical diagnosis as a gold standard. Results exhibited the following relatively low-moderate diagnostic accuracy based on the EEG-based graph: 60% for ADD vs. MCI, 66% for ADD vs. CU, and 56% for MCI vs. CU persons [146].
In precedence, the same Consortium had developed other experiments (Class B) in larger populations in which not all persons had received CSF diagnostic biomarker analysis. In an interesting experiment, accuracy in the correct classification of individuals between two groups, based on the mentioned SPR index from 0 to 1, was tested in CU individuals (n=146), ADD (n=135), and LP (n=15) seniors [147]. The SPR index was used as an input for the ROC curve analysis. Results displayed the following correct binary classifications: 90% for ADD (n=135) vs. CU (n=146) persons [147]. These findings outperformed the same classification exercise based on a standard visual assessment of neuroimaging [148] and agreed with previous evidence obtained by the same classification procedure applied in ADD (n=226), ADMCI (n=41), and CU persons (n=226) [149] from the same NORDEEG cohort. Similar results were obtained using an independent SPR procedure based on support vector machines carried out in small populations of CU individuals and patients with dementia [150].
Keeping in mind the above rsEEG results at the individual level, combined “synchronization” and “interrelatedness” measures across rsEEG frequency bands could repeatedly produce binary classifications of ADD and ADMCI over control CU persons with an accuracy ranging from 90% to 70%, thus potentially being useful for patient stratification purposes in AD clinical trials. Notably, the use of these binary classifications for mono-modal diagnostic purposes provided modest accuracy around 60%.
2.4. Topology of the “interrelatedness/functional connectivity” measures of rsEEG rhythms in ADD and ADMCI patients: the new wave of the graph theory indices
The popular Graph theory probed the topology of rsEEG “interrelatedness” at electrode or source pairs in the comparison between groups of ADD/ADMCI patients and CU individuals [9; 151; 32; 31; see also 152 for a review]. As compared to age-matched CU persons, ADD and ADMCI patients were characterized by a more random topology of rsEEG “interrelatedness” at electrode or source pairs, possibly due a reduction in “small worldness” properties of the underlying cortical neural networks [9; 10; 153; 154; 155, 156; 157]. However, it should be remarked that the rsEEG studies lending support to such an interpretation exhibited inconsistent findings about the single graph indices forming the “small world” construct (i.e., “clustering coefficient” and “path length”) and unclear effects on delta, theta, alpha, beta, and gamma bands [32; 9; 154; 155,156]. This inconsistency can be explained at least in part by (i) different rsEEG measures of “interrelatedness” used in those studies; (ii) the application of bivariate measures of such “interrelatedness,” which are more sensitive to volume conduction and common drive effects than multivariate measures are (see Supplement 2.2); (iii) the use of sensor vs. source level; and (iv) diverse statistical thresholds used 5).
Beyond small worldness, further graph measures were tested (see Panel 2 for the definitions). In the NORTH-EAST ENGLAND study (Class A), minimum spanning trees (MSTs) of “phase lag index” of rsEEG rhythms at electrode pairs were used to model the hierarchical clustering organization of cortical networks as topology of rsEEG “interrelatedness” between CU individuals (n=17) and ADD (n=26) [158]. Compared to age-matched CU persons, the ADD patients showed lower alpha “phase lag index” and lower dominant frequency (maximum rsEEG power density at alpha range) [158].
In the AMSTERDAM study (Class B), MSTs of “phase lag index” of rsEEG rhythms at electrode pairs were also used to model the topology of rsEEG “interrelatedness” between ADD (n=133) and CU individuals (n=115) [114]. Compared to age-matched CU persons, ADD patients presented decreases of alpha “phase lag index” with increasing disease severity and a shift of the “betweenness centrality” center of mass from posterior to more anterior scalp regions (“nodes”) with increasing disease severity [114]. Concerning the specificity of those results, frontal delta “phase lag index” was selectively affected in bvFTD patients (n=48) against the background of preserved “global efficiency”, whereas parietal and occipital loss of network organization and “global efficiency” was observed in ADD patients (n=69) in relation to decreased alpha “phase lag index” [159].
Keeping in mind the above results, the graph topology of the “interrelatedness” of rsEEG rhythms may enrich our understanding of AD as a “disconnection” syndrome [6; 160; 11; 12]. However, the most consistent topographic patterns and rsEEG measures to model this abnormality for systematic applications in AD clinical trials has not yet been determined. More international research is needed to improve the promising techniques computing the “interrelatedness” of rsEEG activity based on multivariate models and source estimations [5].
2.5. Predictive value of baseline rsEEG measures in CU persons and AD seniors
Previous longitudinal studies in CU persons and AD patients tested the value of rsEEG measures derived from baseline datasets to predict their cognitive status at follow-ups.
In the AMSTERDAM rsEEG study (Class A), high delta-theta and lower alpha power density predicted clinical worsening over time in SCD (n=63) and MCI patients (n=142) with amyloid deposition in the brain [161].
In the STOCKHOLM rsEEG study (Class A), combined alpha and theta power density and mean frequency from left temporal-occipital regions predicted cognitive decline in ADMCI patients (n=27) at 1-year follow-up [106]. In a parallel rsEEG study, anterior localization of alpha sources predicted the cognitive decline in ADMCI patients (n=31) at about 2-year follow-up [50].
In another national rsEEG study (Class B), low posterior alpha power density predicted the cognitive decline in ADMCI (n=88) and ADD (n=42) patients at 1-year follow-up [162].
In the ITALIAN rsEEG study (Class B), ADMCI patients (n=74) with high alpha3/alpha2 frequency power density ratio at scalp electrodes presented greater cortical atrophy and lower perfusion rate in the temporo-parietal cortex as revealed by neuroimaging markers and conversion to ADD status at 3-year follow up [163]. Furthermore, high temporal delta source activity predicted marked cognitive decline in ADMCI patients (n=69) at an average of 14-month follow-up [164].
In the NEWYORK rsEEG study, high temporoparietal theta power density and slowing of mean rsEEG frequency predicted cognitive decline from SMC (n=44) to significant cognitive deficits at 7-9-year follow-up with an overall predictive accuracy of 90%, thus extending previous evidence of the same Workgroup in CU persons, SMC, ADMCI, and ADD individuals [165,166,167].
In another national rsEEG study, baseline high theta power density and cognitive performance predicted cognitive decline at an average of 20-month follow-up with an overall predictive accuracy of 93% in CU persons ranging from intact cognition (n=24) to ADMCI (n=20) and ADD (n=14) status [63;168].
Tables 1 and 2 report the most consistent findings of the rsEEG studies of Class A reviewed in this chapter. They lead support to the value of rsEEG measures of “cortical neural synchronization” and “interrelatedness/connectivity” in the characterization of AD status and prediction of cognitive decline for patients’ stratification purposes in clinical trials.
3. MEASURES OF rsEEG RHYTHMS REFLECTING AD PROGRESSION AND EFFECTS OF INTERVENTION
3.1. Value of rsEEG measures of disease progression in ADMCI and ADD patients
Several studies on AD and CU persons tested the value of rsEEG measures to monitor progression of brain dysfunctions comparing those measures derived from baseline and follow-up recordings. Core results are reported in the following.
In the STOCKHOLM rsEEG study (Class A), ADMCI (n=27) seniors showed increased temporal and occipital theta-delta power density and decreased beta power density at an averaged follow-up of 21 months [106].
In the international multicentric PHARMACOG rsEEG study (Class A), as compared to noADMCI patients (n=54), ADMCI patients (n=72) presented increased limbic theta source activity and greater cognitive decline at 24-month follow-up in relation to reduced functional connectivity within cortical default mode network as revealed by resting state fMRI [47].
In the ITALIAN rsEEG study (Class B), in relation to age-matched CU persons (n=35), ADD patients (n=88) showed increased delta and reduced parieto-occipital alpha and beta source activities at an averaged follow-up of 13 months [24]. In the same study, similar effects were observed at the prodromal stage of ADMCI [169]. Specifically, the ADMCI patients (n=55) displayed reduced alpha source activities in posterior regions at an averaged follow-up of 13 months.
Other national monocentric studies on AD and control CU persons tested the value of rsEEG measures to monitor progressive brain dysfunctions. ADD patients (n=40) presented increased parietal and occipital theta-delta power density and reduced alpha-beta power density at an averaged follow-up of 30 months [170]. In another study, half of ADD patients (n=40) were characterized by increased temporal and occipital delta-theta power density at an averaged follow-up of 12 months [171]. In another study, ADD patients showed increased delta-theta power density and decreased alpha power density at an averaged follow-up of 2 years while no changes were observed in VaD and functional psychiatric patients [80]. Finally, measures of “interrelatedness” of rsEEG rhythms revealed progressive brain dysfunctions in AD patients. Compared with age-matched CU persons (n=14), ADMCI patients (n=9) exhibited decreased delta and theta “phase lag index” within frontal and between frontal and temporal/parietal areas at 1-year follow-up [128].
3.2. Value of rsEEG measures in monitoring disease progression and intervention in ADMCI and ADD patients
Several national studies presented effects of Acetylcholinesterase inhibitors (AChEIs), enhancing the cholinergic tone, on rsEEG measures obtained in ADD patients.
In the STOCKHOLM rsEEG study (Class A), as compared to untreated ADD patients, AD patients (n=15) receiving AChEIs (tacrine) had a reduction of theta GFP after 3 and 12 months of therapy, while both delta and theta GFP reduced after 6 months (n=10) [107]. These results extended previous evidence [172;173].
In the GERMAN study (Class B), rsEEG measures were sensitive to AChEIs in ADD patients. In a group of AD patients (n=15), alpha-theta power density ratio responded to a single dose of AChEIs (tetrahydroaminoacridine) predicting clinical effects of a chronic treatment of 7 weeks [174]. In other ADD patients (n=15), a significant reduction in spatially widespread delta and theta power density was observed after AChEI treatment (rivastigmine) of 5 days [175], while after treatment of 1-2 weeks (rivastigmine) only decreased theta power density (n=35) was observed [176]. In another group of ADD patients (n=20), decreased theta power density after 1 week and short-term memory performance did predict treatment response (rivastigmine) at 6-month follow-up [177].
In the ITALIAN rsEEG study (Class B), in relation to CU individuals (n=65), ADD patients (n=58) presented a reduction in posterior alpha source activity at 1-year follow-up, which was less marked in those patients (n=28) clinically responding to concomitant treatment with AChEIs (donepezil), as revealed by global cognitive status (i.e., MMSE score), when compared to those who did not respond (n=30) [178].
Other smaller national rsEEG studies reported similar effects in ADD patients. Specifically, AD patients (n=18) showed a significant reduction in temporal delta power density and an increase in power density at other frequency ranges including temporal and centroparietal theta after AChEIs (donepezil) administered for 6 months [179]. This effect was in line with other evidence [180,181]. Furthermore, another group of ADD patients (n=16) displayed a significant reduction in widespread delta and theta power density after AChEIs (rivastigmine) given for 3 months [182]. Cortical source estimation of those data pointed to significant effects in a network that included left fronto-parietal regions, posterior cingulate cortex, bilateral parahippocampal regions, and the hippocampus [182].
Other pharmacological interventions presented significant effects on rsEEG measures in ADMCI and ADD patients. Core results are reported in the following.
In the PQ912 study (Class A), ADMCI and ADD patients formed an experimental group (n=60) receiving a 12-week treatment with an inhibitor of the glutaminyl cyclase enzyme (PQ912) that plays a central role in the formation of synaptotoxic pyroglutamate-A-beta oligomers while a placebo group (n=60) received a hypocaloric beverage for the same period. Results showed an improvement of memory and a reduction of theta GFP in the experimental group as compared with the increase of that rsEEG measure in the placebo group [183]. Of note, the PQ912 intervention did not affect the “phase lag index” as measure of rsEEG “interrelatedness” [183]. In a re-analysis of those rsEEG data, a new measure of rsEEG “interrelatedness” at electrode pairs comprising the amplitude envelope correlation with leakage correction (AEC-c) increased more in the alpha frequency band of the experimental group (n=47) compared to the placebo group (n=56) [184].
In the international SOUVENAID study II (Class B), ADD patients formed an experimental group receiving Souvenaid functional food for 24 weeks (n=86) while a placebo group received a hypocaloric beverage for the same period (n=93). Results displayed an improvement of memory and an increase in global delta “phase lag index” across all electrode pairs in the Souvenaid group as compared to the placebo group [185]. A re-analysis of “phase lag index” data presented a stable graph index at 24-month follow-up of local brain network connectivity at beta rhythms in a subsample of the experimental group (n=70-66) as compared to the placebo group (n=77-75) [186].
Table 3 reports the most consistent findings of the rsEEG studies of Class A reviewed in this chapter. They lend support to the value of rsEEG measures in the characterization of AD progression and pharmacological intervention in clinical trials.
4. NEUROPATHOPHYSIOLOGICAL BASIS OF EEG MEASURES IN AD PATIENTS
In the review of the literature, AD patients showed the most consistent abnormalities in (eyes-closed) rsEEG rhythms featured as changes in delta-theta and alpha power density at scalp electrodes or estimates of cortical source activity. It can be speculated that these abnormalities may reflect, directly or indirectly, the effect of AD neuropathology on distributed brain neural networks involved in the generation of cortical rsEEG rhythms and the regulation of general brain arousal, balance of cortical inhibition/excitation, and vigilance [6; 11;7]. Those networks might be formed by subcortical and cortical neural circuits [187; 14; 188], with a special role of thalamocortical functional connectivity during active event-related information processing [25].
However, more literature relating AD neuropathology to beta and gamma rhythms is emerging. It was recently shown that once the amyloid load exceeds a certain level, the power spectrum level of beta and gamma decreased markedly [86]. Other bodies of evidence have demonstrated that gamma power as well as gamma coherence is markedly affected in human patients with AD [49; 29; 189].
More discussion about the neurophysiological underpinnings of the rsEEG findings reviewed in this article can be found in the Supplementary materials (Physiological basis of abnormal rsEEG measures in AD patients).
5. RECOMMENDATIONS
5.1. Diagnostic specificity of stand-alone rsEEG measures
Despite historical efforts at evidence-based reviews [6,7], the diagnostic usefulness of rsEEG measures is still controversial. Using an evidence-based technique, Jelic and Kowalski [42] performed a systematic review of the literature regarding the diagnostic accuracy of rsEEG in dementing disorders published from 1980 until 2009. They concluded that although the classification accuracy values were in general high (> 80%), the evidence for the diagnostic utility of rsEEG markers in AD was insufficient to suggest its use alone in memory clinics routines, also considering that they do not provide a direct measure of the underlying AD neuropathology. Along the same line, international guidelines on AD diagnosis did not recommend the use of rsEEG biomarkers for diagnostic purposes; rather, they target molecular and structural measures of AD neuropathology [2; 3; 4]. Notably, the present review did not find consistent novel findings to change this position to date. However, novel techniques including rsEEG measures based on local, “interrelatedness/connectivity”, and improved/standardized graph theory markers as inputs to machine learning algorithms may hold the promise in increasing the diagnostic utility of quantitative EEG in future, especially in lower- and middle-income countries [7].
5.2. Stratification of AD patients in clinical trials based on rsEEG measures
The present article reports that ADD and ADMCI patients may be characterized by consistent changes in rsEEG measures.
The most consistent findings were obtained using linear rsEEG measures pointing to reduced relative power density at scalp electrodes or estimates of source activity as well as reduced “interrelatedness” (e.g., “spectral coherence” and “phase lag index” at electrode pairs or linear lagged source connectivity) at dominant alpha rhythms in posterior regions in ADD and ADMCI patients as compared to age-matched CU persons and patients with matched cognitive deficits due to other neurodegenerative and cerebrovascular causes (Table 1). Furthermore, the same linear rsEEG measures of amplitude and “interrelatedness” of posterior theta rhythms were repeatedly associated with higher probability to develop a significant cognitive decline in CU persons and AD patients (Table 2). Also, rsEEG measures at delta frequencies were often reported as significant markers (Tables 1 and 2).
Those linear rsEEG measures are recommended for the stratification of ADD and ADMCI patients in a stepwise triage procedure for the enrollment of patients in clinical trials. In the first step, probable ADD and ADMCI patients may be selected based on the traditional NINCDS-ADRDA criteria for the clinical diagnosis of AD [44; 2]. In the second step, these patients may receive standard non-invasive and cost-effective rsEEG recordings and be sub-grouped in those with lowest vs. highest abnormalities in rsEEG measures (i.e., threshold based on median values). In the third step, the ADD and ADMCI patients with the highest abnormalities of rsEEG measures may receive invasive and relatively expensive procedures to extract CSF and neuroimaging diagnostic biomarkers of AD [3; 4]. Only the ADD and ADMCI patients who are positive for those diagnostic biomarkers would then be used in the observational or intervention clinical trials. This procedure would confer some benefit to financial resources, reducing the number of patients “negative” to the CSF and neuroimaging diagnostic biomarkers of AD in the enrollment phase. Furthermore, the enrolled patients having highest abnormalities of rsEEG measures may be ideal to test new diagnostic procedures and therapeutic interventions in ADD and ADMCI patients.
Alternatively, should the above clinical trials aim to recruit prodromal AD people in the very early stages of disease, rsEEG recordings may be helpful to those with normal or minimally abnormal local or “interrelatedness” rsEEG measures.
Discussion about costs of rsEEG measures in AD clinical trials can be found in Supplementary materials (Physiological basis of abnormal rsEEG measures in AD patients).
5.3. RsEEG measures of AD progression and efficacy of interventions
Disease progression biomarkers are clearly important in both observational and intervention AD clinical trials. In the monitoring of AD progression and response to therapies, the most consistent findings were obtained using linear rsEEG measures pointing to reduced power density at scalp electrodes or estimates of source activity as well as reduced “interrelatedness” (e.g., “spectral coherence”, DTF, and “phase lag index” at electrode pairs or linear lagged source connectivity) at delta, theta, alpha and gamma rhythms (Table 3).
In this respect, those rsEEG measures may be used as secondary endpoints of interventions and have a complementary value compared with neuroimaging biomarkers providing more direct measurements of progressive AD neuropathology and neurodegeneration [2; 3; 4].
5.4. International initiatives for research on rsEEG measures in AD
In general, the findings reviewed in the present article raise the need for international consensus initiatives to develop or refine a multi-center standardization of instructions to patients, rsEEG recordings, and selection of artifact-free rsEEG periods in line with the standards of clinical trials in AD. First attempts can be found in initiatives of International Federation of Clinical Neurophysiology [5;7]. Future double-blind, prospective, multicenter clinical trials may also carry out comparisons of different linear and non-linear rsEEG measures (e.g., “local”, “interrelatedness/connectivity” and Graph theory) of disease monitoring, progression, and intervention to reach consensus about optimal standard operating computational procedures and their validity and reliability. Ideally, these procedures will be based on well-established open-access Internet-based software platforms to ensure future replicability [see two interesting examples in 190; 191].
We also recommend that all efforts should be made to make neurophysiological datasets (with accompanying anonymized data) open access. This open science approach would also ensure replicability of reported results, in addition to enabling datasets to be pulled (allowing big data analytics), and algorithms/classification models to be developed/tested against one another, accelerating the data science in this field. For this purpose, repositories could be used such as OpenNEURO (https://openneuro.org/).
In those initiatives, an important role should be played by regulatory authorities (e.g. Food and Drug Administration, European Medicine Agency, etc.), international scientific Societies of Clinical Neurophysiology and Neurology, and Alzheimer’s Association as validated rsEEG measures are expected to be used as biomarkers in clinical trials relevant for drug regulation and licensing in the future.
A summary of the recommendations of the present expert panel is reported in Panel 3.
Panel 3: Recommendations
Summary
“Data acquisition” after careful multi-center standardization and harmonization of instructions to patients and rsEEG recordings.
“Data analysis” based on standardized selection of artifact-free rsEEG periods and extraction of linear “local” (e.g., rsEEG power spectral density) and “interrelatedness/connectivity” rsEEG measures by open-access Internet-based software platforms for replicability.
“Use” for stratification of AD patients and monitoring of disease progression and intervention.
“Endorsement” for multi-sectoral international initiatives for further validation of rsEEG measures in AD
6. CONCLUSIONS
What can be the added value of rsEEG measures for the instrumental assessment and monitoring of disease status and progression in ADD and ADMCI patients?
In precedence, IWG-2 suggested two classes of biomarkers for the assessment of ADD, namely the “diagnostic” biomarkers (i.e., those measuring the pathophysiological hallmarks of the disease such as the cerebral amyloidosis and the amount of total or phospho-tau in the cerebrospinal fluid or directly within the brain by PET), and the “progression or topographical” biomarkers (i.e., those measuring progression of region-specific neurodegeneration with characteristic “AD-signatures”, such as FDG-PET and structural MRI). More recently, NIA-AA Research Framework [4] suggested three classes of biomarkers for the assessment of AD. They named “diagnostic disease” biomarkers as those measuring cerebral amyloidosis (i.e., “A” biomarkers) and phospho-tau (i.e., “T” biomarkers) by CSF or PET techniques. They also named “neurodegenerative/progression” biomarkers (i.e., “N” biomarkers) those measuring total tau by CSF sampling, FDG-PET hypometabolism, and structural MRI markers of brain atrophy.
Keeping in mind the “physiologic” meaning of rsEEG measures reviewed in the present article, we posit that the term “physiologic (P) biomarker”. In this line, the AD biomarker framework [4] may become “A”, “T”, “P”, and “N.” This “P” biomarker (e.g., + or -) may possibly reflect vulnerability or resilience of subcortical and thalamocortical loops and the ascending activating systems as additional and supplementary relevant information concerning the AD status and progression.
Supplementary Material
HIGHLIGHTS.
Resting-state electroencephalographic (rsEEG) measures may reflect synchronization of cortical neurons in humans.
Patients with Alzheimer’s disease (AD) show consistent abnormalities in rsEEG measures at both the group and individual level.
Linear EEG measures may be used as neurophysiological markers in AD clinical trials.
Acknowledgements
The present paper was facilitated by the Alzheimer’s Association International Society to Advance Alzheimer's Research and Treatment (ISTAART), through the Electrophysiology professional interest area (PIA). EPIA is committed to (1) exploit EEG biomarkers for improving the understanding of neurophysiological mechanisms underlying Alzheimer’s disease and age-related dementing disorders at micro, meso, and macro spatial scale and (2) promoting clinical applications. Of note, the views and opinions expressed by authors in this publication represent those of the authors and do not necessarily reflect those of the PIA membership, ISTAART or the Alzheimer's Association.
Furthermore, this manuscript was facilitated by the Global Brain Consortium (https://globalbrainconsortium.org). The Global Brain Consortium is committed to achieving the vision of improved and more equitable health outcomes worldwide by strengthening linkages between neuroscientists across borders and disciplines. Quantitative EEG techniques and biomarkers are considered as an important resource for brain research and clinical applications in neurologic and psychiatric diseases, especially in lower- and middle-income countries.
We thank Prof. Philip Scheltens, Dr. Cornelius Stam, Dr. Wilhelm de Haan, and Dr. Alida Gouw of Center at the VU University Medical Center in Amsterdam for the constructive and helpful revision of an original version of the manuscript.
We also thank Dr. Claire Sexton of ISTAART for the same reasons.
Conflict of interests
Dr. Claudio Babiloni is supported by European Committee (H2020-EU.1.3.1.H2020-MSCA-ITN-ETN-2016 project with short title “BBDiag”).
Dr. Flavio Nobili: This work was developed within the framework of the DINOGMI Department of Excellence of MIUR 2018-2022 (legge 232 del 2016).
Dr. Petra Ritter is supported by H2020 Research and Innovation Action grants VirtualBrainCloud 826421, Human Brain Project 785907, 945539 and ERC 683049; German Research Foundation CRC 1315, CRC 936 and RI 2073/6-1; Berlin Institute of Health & Foundation Charité, Johanna Quandt Excellence Initiative.
Dr. Andrea Vergallo is an employee of Eisai Inc.
Dr. Harald Hampel is an employee of Eisai Inc., serves as Senior Associate Editor for the Journal Alzheimer’s & Dementia. Before May 2019, he had received lecture fees, travel funding, and research grants from several Pharmacological Companies.
Dr. Maria C. Carrillo, Dr. Rebecca M. Edelmayer, and Dr. Keith N. Fargo are full-time employees of the Alzheimer's Association.
Dr. Brendan Lucey is supported by the National Institute on Aging (K76 AG054863).
Lists of abbreviations
- AA
Alzheimer’s Association
- AChEIs
Acetylcholinesterase inhibitors
- AD
Alzheimer’s Disease
- ADD
AD dementia
- ADMCI
Alzheimer’s disease mild cognitive impairment
- ADms
AD, moderate/severe index
- AEC
Amplitude Envelope Correlation
- ANNs
Artificial neural networks
- ATN
Amyloid, tau and neurodegeneration
- bvFTD
Behavioral variant Frontotemporal dementia
- CSF
Cerebrospinal
- CU
Cognitively unimpaired
- D2
Global correlation dimension
- DAT
Dementia of Alzheimer’s Type
- DLB
Lewy Body
- DSM-IV and DSM-V
Diagnostic and Statistical Manual of Mental Disorders
- DTF
Directed transfer function
- ECOG
Electrocorticography
- EEG
Electroencephalography
- EMG
Electromyographic
- EPIA
Electrophysiology Professional Interest Area
- EOG
Electrooculographic
- FDG-PET
Fluorodeoxyglucose PET
- ff-DTF
Full frequency DTF
- FFT
Fast Fourier Transform
- fMRI
Functional magnetic resonance imaging
- GDC
Global Dimensional Complexity
- GFP
Global field power
- GFS
Global field synchronization
- ICD-10
10th revision of the International Classification of Diseases
- IFAST
Implicit Function as Squashing Time (as an artificial neural network)
- IFCN
International Federation of Clinical Neurophysiology
- ISTAART
Alzheimer’s Association International Society to Advance Alzheimer’s Research and Treatment
- IWG
The International Working Group
- K2
Kolmogorov entropy
- LFP
Local field potential
- LP
DLB/PDD index
- LPS
Lagged phase synchronization
- MCI
Mild Cognitive Impairment
- MEA
Micro-electrode array MEG-Magnetoencephalography
- MMSE
Mini mental state evaluation
- MRI
Magnetic resonance imaging
- NIA-AA Research Framework
US National Institute on Aging–Alzheimer’s Association Research Framework
- NINCDS-ADRDA
National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association
- NIRS
Near-infrared spectroscopy
- NRM
CU persons’ index
- P
physiologic
- PET
Positron emission tomography
- PET SUVR
positron emission tomography standardized uptake value ratio
- PIA
Electrophysiology professional interest area
- PLI
Phase Lag Index
- RFMs
Random forest learning machine classifiers
- ROC
Receiver operating characteristic
- rsEEG
Resting-state electroencephalographic
- SCC
Subjective Cognitive Complaint
- SCD
Subjective cognitive decline
- SES
Stochastic event synchrony
- SL
Synchronization likelihood
- SMC
Subjective memory Complaint
- sMCI
Stable MCI
- SVD
Subcortical vascular dementia
- SVMs
Support vector machines
- qEEG
Quantitative EEG
- TVB
The Virtual Brain
- VaD
Vascular dementia
- WMH
White matter hyperintensities
Footnotes
SES method relies on i) identification of so-called “bumps” in the time–frequency space and ii) aligning the bumps. The discrimination is based on parameters: ρ: fraction of non-coincident bumps, δt and δf : average time and frequency offsets respectively between coincident bumps. However the problem of aligning is ambiguous and depends on arbitrary choices.
Electrophysiology Professional Interest Area (EPIA) https://action.alz.org/personifyebusiness/Membership/ISTAART/PIA/Electrophysiology.aspx
The Alzheimer's Association International Society to Advance Alzheimer's Research and Treatment (ISTAART) https://action.alz.org/personifyebusiness/default.aspx?tabid=1516
Alzheimer’s Association https://www.alz.org/
Global Brain Consortium (GBC) https://globalbrainconsortium.org/
REFERENCES
- 1.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011. May; 7(3):270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011; 7(3):263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, Cappa S, Crutch S, Engelborghs S, Frisoni GB, Fox NC, Galasko D, Habert MO, Jicha GA, Nordberg A, Pasquier F, Rabinovici G, Robert P, Rowe C, Salloway S, Sarazin M, Epelbaum S, de Souza LC, Vellas B, Visser PJ, Schneider L, Stern Y, Scheltens P, Cummings JL. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014; 13, 614–29. [DOI] [PubMed] [Google Scholar]
- 4.Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14,535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babiloni C, Barry RJ, Başar E, Blinowska KJ, Cichocki A, Drinkenburg WHIM, Klimesch W, Knight RT, Lopes da Silva F, Nunez P, Oostenveld R, Jeong J, Pascual-Marqui R, Valdes-Sosa P, Hallett M. International Federation of Clinical Neurophysiology (IFCN) - EEG research workgroup: Recommendations on frequency and topographic analysis of resting state EEG rhythms. Part 1: Applications in clinical research studies. Clin Neurophysiol. 2020a. January;131(1):285–307. [DOI] [PubMed] [Google Scholar]
- 6.Babiloni C, Blinowska K, Bonanni L, Cichocki A, De Haan W, Del Percio C, Dubois B, Escudero J, Fernández A, Frisoni G, Guntekin B, Hajos M, Hampel H, Ifeachor E, Kilborn K, Kumar S, Johnsen K, Johannsson M, Jeong J, LeBeau F, Lizio R, Lopes da Silva F, Maestú F, McGeown WJ, McKeith I, Moretti DV, Nobili F, Olichney J, Onofrj M, Palop JJ, Rowan M, Stocchi F, Struzik ZM, Tanila H, Teipel S, Taylor JP, Weiergräber M, Yener G, Young-Pearse T, Drinkenburg WH, Randall F. What electrophysiology tells us about Alzheimer's disease: a window into the synchronization and connectivity of brain neurons. Neurobiol Aging. 2020b; 85:58–73. [DOI] [PubMed] [Google Scholar]
- 7.Rossini PM, Di Iorio R, Vecchio F, Anfossi M, Babiloni C, Bozzali M, Bruni AC, Cappa SF, Escudero J, Fraga FJ, Giannakopoulos P, Guntekin B, Logroscino G, Marra C, Miraglia F, Panza F, Tecchio F, Pascual-Leone A, Dubois B. Early diagnosis of Alzheimer's disease: the role of biomarkers including advanced EEG signal analysis. Report from the IFCN-sponsored panel of experts. Clin Neurophysiol. 2020; 131(6):1287–1310. [DOI] [PubMed] [Google Scholar]
- 8.Pfurtscheller G and Lopes da Silva. Spatiotemporal analysis of alpha frequency components with the ERD technique. Brain Topography. 1989; 2(1-2):3–8. [DOI] [PubMed] [Google Scholar]
- 9.de Haan W, Pijnenburg YA, Strijers RL, van der Made Y, van der Flier WM, Scheltens P, Stam CJ. Functional neural network analysis in frontotemporal dementia and Alzheimer's disease using EEG and graph theory. BMC Neurosci. 2009; 21; 10:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stam CJ, Jones BF, Nolte G, Breakspear M, Scheltens P. Small-world networks and functional connectivity in Alzheimer's disease. Cereb Cortex. 2007a;17(1):92–9. [DOI] [PubMed] [Google Scholar]
- 11.Pievani M, de Haan W, Wu T, Seeley WW, Frisoni GB. Functional network disruption in the degenerative dementias. Lancet Neurol. 2011. September;10(9):829–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teipel S, Grothe MJ, Zhou J, Sepulcre J, Dyrba M, Sorg C, Babiloni C. Measuring Cortical Connectivity in Alzheimer's Disease as a Brain Neural Network Pathology: Toward Clinical Applications. J Int Neuropsychol Soc. 2016. ;22(2):138–63. [DOI] [PubMed] [Google Scholar]
- 13.Buzsáki G, Logothetis N, Singer W. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron. 2013;80(3):751–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crunelli V, David F, Lőrincz ML, Hughes SW. The thalamocortical network as a single slow wave-generating unit. Curr Opin Neurobiol. 2015; 31:72–80. [DOI] [PubMed] [Google Scholar]
- 15.Steriade M, Timofeev I. Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron. 2003;37(4):563–76. [DOI] [PubMed] [Google Scholar]
- 16.Ahnaou A, Walsh C, Manyakov NV, Youssef SA, Drinkenburg WH. Early Electrophysiological Disintegration of Hippocampal Neural Networks in a Novel Locus Coeruleus Tau-Seeding Mouse Model of Alzheimer's Disease.Neural Plast. 2019. ;2019:6981268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah D, Praet J, Latif Hernandez A, Höfling C, Anckaerts C, Bard F, Morawski M, Detrez JR, Prinsen E, Villa A, De Vos WH, Maggi A, D'Hooge R, Balschun D, Rossner S, Verhoye M, Van der Linden A. Early pathologic amyloid induces hypersynchrony of BOLD resting-state networks in transgenic mice and provides an early therapeutic window before amyloid plaque deposition. Alzheimers Dement. 2016;12(9):964–976. [DOI] [PubMed] [Google Scholar]
- 18.Seeck M, Koessler L, Bast T, Leijten F, Michel C, Baumgartner C, Beniczky S. The standardized EEG electrode array of the IFCN. Clinical Neurophysiology. 2017;128(10), 2070–2077. [DOI] [PubMed] [Google Scholar]
- 19.Nunez PL. Toward a quantitative description of large-scale neocortical dynamic function and EEG. Behav Brain Sci. 2000; 23, 371–398. [DOI] [PubMed] [Google Scholar]
- 20.Nunez PL, Srinivasan R Electric Fields of the Brain: The Neurophysics of EEG, 2nd edition, Oxford University Press. 2006. [Google Scholar]
- 21.Nunez PL, Srinivasan R, Fields RD. EEG functional connectivity, axon delays and white matter disease, Clinical Neurophysiology. 2015; 126, 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan L, Huang H, Schwab N, Tanner J, Rajan A, Lam NB, Zaborszky L, Li CR, Price CC, Ding M. From eyes-closed to eyes-open: Role of cholinergic projections in EC-to-EO alpha reactivity revealed by combining EEG and MRI. Human Brain Mapping. 2019; 40(2), 566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klimesch W, Doppelmayr M, Hanslmayr S. Upper alpha ERD and absolute power: Their meaning for memory performance. Progress in Brain Research. 2006; 159, 151–165. [DOI] [PubMed] [Google Scholar]
- 24.Babiloni C, Lizio R, Del Percio C, Marzano N, Soricelli A, Salvatore E, Ferri R, Cosentino FI, Tedeschi G, Montella P, Marino S, De Salvo S, Rodriguez G, Nobili F, Vernieri F, Ursini F, Mundi C, Richardson JC, Frisoni GB, Rossini PM. Cortical sources of resting state EEG rhythms are sensitive to the progression of early stage Alzheimer's disease. J Alzheimers Dis. 2013b;34(4):1015–35. [DOI] [PubMed] [Google Scholar]
- 25.Pfurtscheller G, Da Silva FL. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clinical Neurophysiology. 1999; 110(11),1842–1857. [DOI] [PubMed] [Google Scholar]
- 26.Başar E, Başar-Eroglu C, Karakaş S, Schürmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. International Journal of Psychophysiology. 2001; 39(2), 241–248. [DOI] [PubMed] [Google Scholar]
- 27.Stam C, Jelles B, Achtereekte H, Rombouts S, Slaets J, Keunen R. Investigation of EEG non-linearity in dementia and Parkinson's disease. Electroencephalogr Clin Neurophysiol. 1995;95(5):309–317. [DOI] [PubMed] [Google Scholar]
- 28.Stam CJ, Jelles B, Achtereekte HA, van Birgelen JH, Slaets JP. Diagnostic usefulness of linear and nonlinear quantitative EEG analysis in Alzheimer's disease. Clin Electroencephalogr. 1996;27(2):69–77. [DOI] [PubMed] [Google Scholar]
- 29.Stam CJ, van der Made Y, Pijnenburg YA, Scheltens P. EEG synchronization in mild cognitive impairment and Alzheimer's disease. Acta Neurol Scand. 2003;108(2):90–6. [DOI] [PubMed] [Google Scholar]
- 30.Bullmore E, Sporns O Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009; 10, 186–98. [DOI] [PubMed] [Google Scholar]
- 31.Reijneveld JC, Ponten SC, Berendse HW, Stam CJ. The application of graph theoretical analysis to complex networks in the brain. Clin Neurophysiol. 2007;118(11):2317–31. [DOI] [PubMed] [Google Scholar]
- 32.Stam CJ, Nolte G, Daffertshofer A. Phase lag index: assessment of functional connectivity from multichannel EEG and MEG with diminished bias from common sources. Hum Brain Mapp. 2007b; 28, 1178–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Diessen E, Numan T, van Dellen E, van der Kooi AW, Boersma M, Hofman D, van Lutterveld R, van Dijk BW, van Straaten EC, Hillebrand A, Stam CJ. Opportunities and methodological challenges in EEG and MEG resting state functional brain network research. Clin Neurophysiol. 2015; 126, 1468–81. [DOI] [PubMed] [Google Scholar]
- 34.DeMets D, Friedman L, Furberg C (2010). Fundamentals of Clinical Trials (4th ed.). Springer. ISBN 978-1-4419-1585-6. [Google Scholar]
- 35.Näpflin M, Wildi M, Sarnthein J. Test-retest reliability of resting EEG spectra validates a statistical signature of persons. Clin Neurophysiol. 2007. November;118(11):2519–24. [DOI] [PubMed] [Google Scholar]
- 36.Duan W, Chen X, Wang YJ, Zhao W, Yuan H, Lei X. Reproducibility of power spectrum, functional connectivity and network construction in resting-state EEG. J Neurosci Methods. 2020. October 24:108985. [DOI] [PubMed] [Google Scholar]
- 37.Salinsky MC, Oken BS, Morehead L. Test-retest reliability in EEG frequency analysis. Electroencephalogr Clin Neurophysiol. 1991. November;79(5):382–92. [DOI] [PubMed] [Google Scholar]
- 38.Smit DJ, Posthuma D, Boomsma DI, Geus EJ. Heritability of background EEG across the power spectrum. Psychophysiology. 2005. November;42(6):691–7. [DOI] [PubMed] [Google Scholar]
- 39.Corsi-Cabrera M, Galindo-Vilchis L, del-Río-Portilla Y, Arce C, Ramos-Loyo J. Within-subject reliability and inter-session stability of EEG power and coherent activity in women evaluated monthly over nine months. Clin Neurophysiol. 2007. January;118(1):9–21. [DOI] [PubMed] [Google Scholar]
- 40.Moezzi B, Hordacre B, Berryman C, Ridding MC, Goldsworthy MR. Test-retest Reliability of Functional Brain Network Characteristics Using Resting-state EEG and Graph Theory. BioRxiv (2018), p. 385302. Cold Spring Harbor Laboratory. [Google Scholar]
- 41.Jeong J EEG dynamics in patients with Alzheimer's disease. Clin Neurophysiol. 2004;115(7):1490–505. [DOI] [PubMed] [Google Scholar]
- 42.Jelic V, Kowalski J. "Evidence-based evaluation of diagnostic accuracy of resting EEG in dementia and mild cognitive impairment." Clinical EEG and Neuroscience. 2009; 40.2: 129–142. [DOI] [PubMed] [Google Scholar]
- 43.Hata M, Tanaka T, Kazui H, Ishii R, Canuet L, Pascual-Marqui RD, Aoki Y, Ikeda S, Sato S, Suzuki Y, Kanemoto H, Yoshiyama K, Iwase M. Cerebrospinal Fluid Biomarkers of Alzheimer's Disease Correlate With Electroencephalography Parameters Assessed by Exact Low-Resolution Electromagnetic Tomography (eLORETA). Clin EEG Neurosci. 2017;48(5):338–347. [DOI] [PubMed] [Google Scholar]
- 44.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–44. [DOI] [PubMed] [Google Scholar]
- 45.Musaeus CS, Engedal K, Høgh P, Jelic V, Mørup M, Naik M, Oeksengaard AR, Snaedal J, Wahlund LO, Waldemar G, Andersen BB. EEG Theta Power is an Early Marker of Cognitive Decline in Dementia due to Alzheimer's Disease. J Alzheimers Dis. 2018;64(4):1359–1371. [DOI] [PubMed] [Google Scholar]
- 46.Smailovic U, Koenig T, Kåreholt I, Andersson T, Kramberger MG, Winblad B, Jelic V. Quantitative EEG power and synchronization correlate with Alzheimer's disease CSF biomarkers. Neurobiol Aging. 2018; 63:88–95. [DOI] [PubMed] [Google Scholar]
- 47.Jovicich J, Babiloni C, Ferrari C, Marizzoni M, Moretti DV, Del Percio C, Lizio R, Lopez S, Galluzzi S, Albani D, Cavaliere L, Minati L, Didic M, Fiedler U, Forloni G, Hensch T, Molinuevo JL, Bartrés Faz D, Nobili F, Orlandi D, Parnetti L, Farotti L, Costa C, Payoux P, Rossini PM, Marra C, Schönknecht P, Soricelli A, Noce G, Salvatore M, Tsolaki M, Visser PJ, Richardson JC, Wiltfang J, Bordet R, Blin O, Frisoniand GB; and the PharmaCog Consortium. Two-Year Longitudinal Monitoring of Amnestic Mild Cognitive Impairment Patients with Prodromal Alzheimer's Disease Using Topographical Biomarkers Derived from Functional Magnetic Resonance Imaging and Electroencephalographic Activity. J AlzheimersDis.2019;69(1):15–35. [DOI] [PubMed] [Google Scholar]
- 48.Babiloni C, Ferri R, Noce G, Lizio R, Lopez S, Lorenzo I, Panzavolta A, Soricelli A, Nobili F, Arnaldi D, Famà F, Orzi F, Buttinelli C, Giubilei F, Cipollini V, Marizzoni M, Güntekin B, Aktürk T, Hanoğlu L, Yener G, Özbek Y, Stocchi F, Vacca L, Frisoni GB, Del Percio C. Abnormalities of Cortical Sources of Resting State Alpha Electroencephalographic Rhythms are Related to Education Attainment in Cognitively Unimpaired Seniors and Patients with Alzheimer's Disease and Amnesic Mild Cognitive Impairment.CerebCortex.2020c.doi: 10.1093/cercor/bhaa356. [DOI] [PubMed] [Google Scholar]
- 49.Koenig LB1, McGue M, Krueger RF, Bouchard TJ Jr. Genetic and environmental influences on religiousness: findings for retrospective and current religiousness ratings. J Pers. 2005; 73(2):471–88. [DOI] [PubMed] [Google Scholar]
- 50.Huang C, Wahlund L, Dierks T, Julin P, Winblad B, Jelic V. Discrimination of Alzheimer's disease and mild cognitive impairment by equivalent EEG sources: a cross-sectional and longitudinal study. Clin Neurophysiol. 2000;111(11):1961–7. [DOI] [PubMed] [Google Scholar]
- 51.Babiloni C, Binetti G, Cassetta E, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Frisoni G, Hirata K, Lanuzza B, Miniussi C, Moretti DV, Nobili F, Rodriguez G, Romani GL, Salinari S, Rossini PM. Sources of cortical rhythms change as a function of cognitive impairment in pathological aging: a multicenter study. Clin Neurophysiol. 2006b;117(2):252–68. [DOI] [PubMed] [Google Scholar]
- 52.Babiloni C, Frisoni GB, Pievani M, Vecchio F, Lizio R, Buttiglione M, Geroldi C, Fracassi C, Eusebi F, Ferri R, Rossini PM. Hippocampal volume and cortical sources of EEG alpha rhythms in mild cognitive impairment and Alzheimer disease. Neuroimage. 2009a;44(1):123–35. [DOI] [PubMed] [Google Scholar]
- 53.Babiloni C, Carducci F, Lizio R, Vecchio F, Baglieri A, Bernardini S, Cavedo E, Bozzao A, Buttinelli C, Esposito F, Giubilei F, Guizzaro A, Marino S, Montella P, Quattrocchi CC, Redolfi A, Soricelli A, Tedeschi G, Ferri R, Rossi-Fedele G, Ursini F, Scrascia F, Vernieri F, Pedersen TJ, Hardemark HG, Rossini PM, Frisoni GB. Resting state cortical electroencephalographic rhythms are related to gray matter volume in subjects with mild cognitive impairment and Alzheimer's disease. Hum Brain Mapp. 2013a; 34, 1427–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Babiloni C, Del Percio C, Boccardi M, Lizio R, Lopez S, Carducci F, Marzano N, Soricelli A, Ferri R, Triggiani AI, Prestia A, Salinari S, Rasser PE, Basar E, Famà F, Nobili F, Yener G, Emek-Savaş DD, Gesualdo L, Mundi C, Thompson PM, Rossini PM, Frisoni GB. Occipital sources of resting-state alpha rhythms are related to local gray matter density in subjects with amnesic mild cognitive impairment and Alzheimer's disease. NeurobiolAging. 2015;36(2), 556–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Babiloni C, Del Percio C, Caroli A, Salvatore E, Nicolai E, Marzano N, Lizio R, Cavedo E, Landau S, Chen K, Jagust W, Reiman E, Tedeschi G, Montella P, De Stefano M, Gesualdo L, Frisoni GB, Soricelli A. Cortical sources of resting state EEG rhythms are related to brain hypometabolism in subjects with Alzheimer's disease: an EEG-PET study. Neurobiol Aging. 2016a; 48:122–134. [DOI] [PubMed] [Google Scholar]
- 56.Moretti DV, Miniussi C, Frisoni GB, Geroldi C, Zanetti O, Binetti G & Rossini PM. Hippocampal atrophy and EEG markers in subjects with mild cognitive impairment. Clinical neurophysiology. 2007; 118(12), 2716–2729. [DOI] [PubMed] [Google Scholar]
- 57.Moretti DV, Pievani M, Fracassi C, Binetti G, Rosini S, Geroldi C & Frisoni GB. Increase of theta/gamma and alpha3/alpha2 ratio is associated with amygdalo-hippocampal complex atrophy. Journal of Alzheimer's Disease. 2009; 17(2), 349–357. [DOI] [PubMed] [Google Scholar]
- 58.Moretti DV, Frisoni GB, Binetti G & Zanetti O. Anatomical substrate and scalp EEG markers are correlated in subjects with cognitive impairment and Alzheimer's disease. Frontiers in psychiatry. 2011; 1, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moretti DV, Prestia A, Fracassi C, Binetti G, Zanetti O & Frisoni GB. Specific EEG changes associated with atrophy of hippocampus in subjects with mild cognitive impairment and Alzheimer's disease. International Journal of Alzheimer's disease, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moretti DV. Increase of EEG Alpha3/Alpha2 Power Ratio Detects Inferior Parietal Lobule Atrophy in Mild Cognitive Impairment. Curr Alzheimer Res. 2018; 15(5):443–451. [DOI] [PubMed] [Google Scholar]
- 61.Babiloni C, Visser PJ, Frisoni G, De Deyn PP, Bresciani L, Jelic V, Nagels G, Rodriguez G, Rossini PM, Vecchio F, Colombo D, Verhey F, Wahlund LO, Nobili F. Cortical sources of resting EEG rhythms in mild cognitive impairment and subjective memory complaint. Neurobiol Aging. 2010a; 31(10):1787–98. [DOI] [PubMed] [Google Scholar]
- 62.Roh JH, Park MH, Ko D, Park KW, Lee DH, Han C, Jo SA, Yang KS, Jung KY. Region and frequency specific changes of spectral power in Alzheimer's disease and mild cognitive impairment. Clin Neurophysiol. 2011;122(11):2169–76. [DOI] [PubMed] [Google Scholar]
- 63.Van der Hiele K, Vein AA, Reijntjes RHAM, Westendorp RGJ, Bollen ELEM, Van Buchem MA & Middelkoop HAM. EEG correlates in the spectrum of cognitive decline. Clinical neurophysiology. 2007; 118(9),1931–1939. [DOI] [PubMed] [Google Scholar]
- 64.Kim JS, Lee SH, Park G, Kim S, Bae SM, Kim DW, Im CH. Clinical implications of quantitative electroencephalography and current source density in patients with Alzheimer’s disease. Brain Topography. 2012; 25(4):461–474. [DOI] [PubMed] [Google Scholar]
- 65.Stigsby B, Jóhannesson G, Ingvar DH. Regional EEG analysis and regional cerebral blood flow in Alzheimer's and Pick's diseases. Electroencephalogr Clin Neurophysiol. 1981; 51(5):537–47. [DOI] [PubMed] [Google Scholar]
- 66.Brenner RP, Ulrich RF, Spiker DG, Sclabassi RJ, Reynolds CF 3rd, Marin RS, Boller F. Computerized EEG spectral analysis in elderly normal, demented and depressed subjects. Electroencephalogr Clin Neurophysiol. 1986; 64(6):483–92. [DOI] [PubMed] [Google Scholar]
- 67.Rae-Grant A, Blume W, Lau C, Hachinski VC, Fisman M, Merskey H. The electroencephalogram in Alzheimer-type dementia. A sequential study correlating the electroencephalogram with psychometric and quantitative pathologic data. Arch Neurol. 1987; 44(1):50–4. [DOI] [PubMed] [Google Scholar]
- 68.Kwa V, Weinstein HC, Posthumus Meyjes EF, van Royen EA, Bour LJ, Verhoeff PN, Ongerboer de Visser BW. Spectral analysis of the EEG and 99m-Tc-HMPAO SPECT-scan in Alzheimer's disease. Biol Psychiatry. 1993; 33(2):100–7. [DOI] [PubMed] [Google Scholar]
- 69.Rodriguez G, Nobili F, Copello F, Vitali P, Gianelli MV, Taddei G, Catsafados E, Mariani G. 99mTc-HMPAO regional cerebral blood flow and quantitative electroencephalography in Alzheimer's disease: a correlative study. J Nucl Med. 1999; 40(4):522–9. [PubMed] [Google Scholar]
- 70.Peraza LR, Taylor JP & Kaiser M. Divergent brain functional network alterations in dementia with Lewy bodies and Alzheimer's disease. Neurobiology of aging. 2015; 36(9), 2458–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Waal H, Stam CJ, de Haan W, van Straaten EC, Scheltens P, van der Flier WM. Young Alzheimer patients show distinct regional changes of oscillatory brain dynamics. Neurobiol Aging. 2012; 33(5):1008. [DOI] [PubMed] [Google Scholar]
- 72.Babiloni C, Benussi L, Binetti G, Bosco P, Busonero G, Cesaretti S, Dal Forno G, Del Percio C, Ferri R, Frisoni G, Ghidoni R, Rodriguez G, Squitti R, Rossini PM. Genotype (cystatin C) and EEG phenotype in Alzheimer disease and mild cognitive impairment: a multicentric study. Neuroimage. 2006c; 29, 948–64. [DOI] [PubMed] [Google Scholar]
- 73.Babiloni C, Ferri R, Binetti G, Cassarino A, Dal Forno G, Ercolani M, Ferreri F, Frisoni GB, Lanuzza B, Miniussi C, Nobili F, Rodriguez G, Rundo F, Stam CJ, Musha T, Vecchio F, Rossini PM. Fronto-parietal coupling of brain rhythms in mild cognitive impairment: A multicentric EEG study. Brain Res Bull. 2006d; 69, 63–73. [DOI] [PubMed] [Google Scholar]
- 74.Canuet L, Tellado I, Couceiro V, Fraile C, Fernandez-Novoa L, Ishii R & Cacabelos R. Resting-state network disruption and APOE genotype in Alzheimer's disease: a lagged functional connectivity study. PloS one. 2012; 7(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Waal H, Stam CJ, de Haan W, van Straaten EC, Blankenstein MA, Scheltens P, van der Flier WM. Alzheimer's disease patients not carrying the apolipoprotein E ε4 allele show more severe slowing of oscillatory brain activity. Neurobiol Aging. 2013; 34(9):2158–63. [DOI] [PubMed] [Google Scholar]
- 76.Walker MP, Ayre GA, Cummings JL, Wesnes K, McKeith IG, O'Brien JT, Ballard CG. The Clinician Assessment of Fluctuation and the One Day Fluctuation Assessment Scale. Two methods to assess fluctuating confusion in dementia. Br J Psychiatry. 2000a; 177:252–6. [DOI] [PubMed] [Google Scholar]
- 77.Walker MP, Ayre GA, Cummings JL, Wesnes K, McKeith IG, O’brien JT, Ballard CG. Quantifying fluctuation in dementia with Lewy bodies, Alzheimer’s disease, and vascular dementia. Neurology. 2000b; 54(8),1616–1625. [DOI] [PubMed] [Google Scholar]
- 78.Babiloni C, Del Percio C, Lizio R, Noce G, Cordone S, Lopez S, Soricelli A, Ferri R, Pascarelli MT, Nobili F, Arnaldi D, Aarsland D, Orzi F, Buttinelli C, Giubilei F, Onofrj M, Stocchi F, Stirpe P, Fuhr P, Gschwandtner U, Ransmayr G, Caravias G, Garn H, Sorpresi F, Pievani M, Frisoni GB, D'Antonio F, De Lena C, Güntekin B, Hanoğlu L, Başar E, Yener G, Emek-Savaş DD, Triggiani AI, Franciotti R, De Pandis MF, Bonanni L. Abnormalities of cortical neural synchronization mechanisms in patients with dementia due to Alzheimer's and Lewy body diseases: an EEG study. Neurobiol Aging. 2017a; 55, 143–158. [DOI] [PubMed] [Google Scholar]
- 79.Moretti DV, Babiloni C, Binetti G, Cassetta E, Dal Forno G, Ferreric F & Rodriguez G. Individual analysis of EEG frequency and band power in mild Alzheimer's disease. Clinical Neurophysiology. 2004; 115(2), 299–308. [DOI] [PubMed] [Google Scholar]
- 80.Sloan EP, Fenton GW. EEG power spectra and cognitive change in geriatric psychiatry: a longitudinal study. Electroencephalography and clinical neurophysiology. 1993; 86(6), 361–367. [DOI] [PubMed] [Google Scholar]
- 81.Szelies B, Mielke R, Herholz K & Heiss WD. Quantitative topographical EEG compared to FDG PET for classification of vascular and degenerative dementia. Electroencephalography and clinical neurophysiology. 1994; 91(2), 131–139. [DOI] [PubMed] [Google Scholar]
- 82.Babiloni C, Ferri R, Moretti DV, Strambi A, Binetti G, Dal Forno G, Ferreri F, Lanuzza B, Bonato C, Nobili F, Rodriguez G, Salinari S, Passero S, Rocchi R, Stam CJ, Rossini PM. Abnormal fronto-parietal coupling of brain rhythms in mild Alzheimer's disease: a multicentric EEG study. Eur J Neurosci. 2004a; 19(9):2583–90. [DOI] [PubMed] [Google Scholar]
- 83.Gawel M, Zalewska E, Szmidt-Sałkowska E, Kowalski J. The value of quantitative EEG in differential diagnosis of Alzheimer's disease and subcortical vascular dementia. J Neurol Sci. 2009; 283(1-2):127–33. [DOI] [PubMed] [Google Scholar]
- 84.Dubois B, Epelbaum S, Nyasse F, Bakardjian H, Gagliardi G, Uspenskaya O & Bertrand A. Cognitive and neuroimaging features and brain β-amyloidosis in individuals at risk of Alzheimer's disease (INSIGHT-preAD): a longitudinal observational study. The Lancet Neurology. 2018; 17(4), 335–346. [DOI] [PubMed] [Google Scholar]
- 85.Teipel S, Bakardjian H, Gonzalez-Escamilla G, Cavedo E, Weschke S, Dyrba M, Grothe MJ, Potier MC, Habert MO, Dubois B, Hampel H. INSIGHT-preAD study group. No association of cortical amyloid load and EEG connectivity in older people with subjective memory complaints. Neuroimage Clin. 2018; 17: 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gaubert S, Raimondo F, Houot M, Corsi MC, Naccache L, Diego Sitt J, Hermann B, Oudiette D, Gagliardi G, Habert MO, Dubois B, De Vico Fallani F, Bakardjian H, Epelbaum S. Alzheimer’s Disease Neuroimaging Initiative. EEG evidence of compensatory mechanisms in preclinical Alzheimer's disease. Brain. 2019. July 1;142(7):2096–2112. [DOI] [PubMed] [Google Scholar]
- 87.Babiloni C, Susanna Lopez, Claudio Del Percio, Giuseppe Noce, Maria Teresa Pascarelli, Roberta Lizio, Teipel Stefan J, Gabriel González-Escamilla, Hovagim Bakardjian, Nathalie George, Enrica Cavedo, Simone Lista, Chiesa Patrizia A, Andrea Vergallo, Pablo Lemercier, Giuseppe Spinelli, Grothe Michel J, Marie-Claude Potier, Fabrizio Stocchi, Raffaele Ferri, Marie-Odile Habert, Fraga Francisco J, Bruno Dubois, Hampel H. Resting-state posterior alpha rhythms are abnormal in subjective memory complaint seniors with preclinical Alzheimer’s neuropathology and high education level: the INSIGHT-PreAD study. Neurobiol Aging. 2020d. (in press). [DOI] [PubMed] [Google Scholar]
- 88.Besthorn C, Sattel H, Geiger-Kabisch C, Zerfass R, Förstl H. Parameters of EEG dimensional complexity in Alzheimer's disease. Electroencephalogr Clin Neurophysiol. 1995; 95(2):84–9. [DOI] [PubMed] [Google Scholar]
- 89.Hornero R, Abásolo D, Escudero J, Gómez C. Nonlinear analysis of electroencephalogram and magnetoencephalogram recordings in patients with Alzheimer's disease. Philos Trans A Math Phys Eng Sci. 2009; 367(1887):317–36. [DOI] [PubMed] [Google Scholar]
- 90.Jelles B, van Birgelen JH, Slaets JP, Hekster RE, Jonkman EJ, Stam CJ. Decrease of non-linear structure in the EEG of Alzheimer patients compared to healthy controls. Clin Neurophysiol. 1999; 110(7):1159–67. [DOI] [PubMed] [Google Scholar]
- 91.Jeong J, Kim SY, Han SH. Non-linear Dynamical Analysis of the EEG in Alzheimer's Disease With Optimal Embedding Dimension. Electroencephalogr Clin Neurophysiol. 1998; 106(3):220–8. [DOI] [PubMed] [Google Scholar]
- 92.Pritchard WS. The Brain in Fractal Time: 1/f-like Power Spectrum Scaling of the Human Electroencephalogram. Int J Neurosci. 1992; 66(1-2):119–29 [DOI] [PubMed] [Google Scholar]
- 93.Pritchard WS, Duke DW, Coburn KL, Moore NC, Tucker KA, Jann MW, Hostetler RM. EEG-based, Neural-Net Predictive Classification of Alzheimer's Disease Versus Control Subjects Is Augmented by Non-Linear EEG Measures. Electroencephalogr Clin Neurophysiol. 1994; 91(2):118–30. [DOI] [PubMed] [Google Scholar]
- 94.Yagyu T, Wackermann J, Shigeta M, Jelic V, Kinoshita T, Kochi K, et al. Global dimensional complexity of multichannel EEG in mild Alzheimer's disease and age-matched cohorts. Dement Geriatr Cogn Disord. 1997; 8(6):343–347. [DOI] [PubMed] [Google Scholar]
- 95.Dauwels J, Vialatte F & Cichocki A. Diagnosis of Alzheimer's disease from EEG signals: where are we standing? Current Alzheimer Research. 2010a; 7(6), 487–505. [DOI] [PubMed] [Google Scholar]
- 96.Jelles B, Scheltens P, van der Flier WM, Jonkman EJ, da Silva FH, Stam CJ. Global dynamical analysis of the EEG in Alzheimer's disease: frequency-specific changes of functional interactions. Clin Neurophysiol. 2008; 119(4):837–41. [DOI] [PubMed] [Google Scholar]
- 97.Woyshville MJ, Calabrese JR. Quantification of occipital EEG changes in Alzheimer's disease utilizing a new metric: the fractal dimension. Biol Psychiatry. 1994; 35(6):381–387. [DOI] [PubMed] [Google Scholar]
- 98.Jeong J, Chae JH, Kim SY, Han SH. Nonlinear dynamic analysis of the EEG in patients with Alzheimer's disease and vascular dementia. J Clin Neurophysiol. 2001; 18(1):58–6;7. [DOI] [PubMed] [Google Scholar]
- 99.Sun J, Wang B, Niu Y, Tan Y, Fan C, Zhang N, Xiang J Complexity Analysis of EEG, MEG, and fMRI in Mild Cognitive Impairment and Alzheimer's Disease: A Review. Entropy. 2020; 22(2), 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adler G, Brassen S, Jajcevic A. EEG coherence in Alzheimer's dementia. J Neural Transm (Vienna). 2003; 110(9):1051–8. [DOI] [PubMed] [Google Scholar]
- 101.Anghinah R, Kanda PA, Jorge MS, Lima EE, Pascuzzi L, Melo AC. Alpha band coherence analysis of EEG in healthy adult's and Alzheimer's type dementia patients. Arq Neuropsiquiatr. 2000; 58(2A):272–5. [DOI] [PubMed] [Google Scholar]
- 102.Besthorn C, Förstl H, Geiger-Kabisch C, Sattel H, Gasser T, Schreiter-Gasser U. EEG coherence in Alzheimer disease. Electroencephalogr Clin Neurophysiol. 1994; 90(3):242–5. [DOI] [PubMed] [Google Scholar]
- 103.Dunkin JJ, Leuchter AF, Newton TF, Cook IA. Reduced EEG coherence in dementia: state or trait marker? Biol Psychiatry. 1994; 35(11):870–9. [DOI] [PubMed] [Google Scholar]
- 104.Fonseca LC, Tedrus GM, Fondello MA, Reis IN, Fontoura DS. EEG theta and alpha reactivity on opening the eyes in the diagnosis of Alzheimer's disease. Clin EEG Neurosci. 2011; 42(3):185–9. [DOI] [PubMed] [Google Scholar]
- 105.Fonseca LC, Tedrus GM, Carvas PN, Machado EC. Comparison of quantitative EEG between patients with Alzheimer's disease and those with Parkinson's disease dementia. Clin Neurophysiol. 2013; 124(10):1970–4. [DOI] [PubMed] [Google Scholar]
- 106.Jelic V, Johansson SE, Almkvist O, Shigeta M, Julin P, Nordberg A, Winblad B, Wahlund LO. Quantitative electroencephalography in mild cognitive impairment: longitudinal changes and possible prediction of Alzheimer's disease. Neurobiol Aging. 2000; 21(4):533–40. [DOI] [PubMed] [Google Scholar]
- 107.Jelic V, Dierks T, Amberla K, Almkvist O, Winblad B, Nordberg A. Longitudinal changes in quantitative EEG during long-term tacrine treatment of patients with Alzheimer’s disease. Neurosci Lett. 1998; 254:85–88. 28. [DOI] [PubMed] [Google Scholar]
- 108.Knott V, Engeland C, Mohr E, Mahoney C, Ilivitsky V. Acute nicotine administration in Alzheimer's disease: an exploratory EEG study. Neuropsychobiology. 2000; 41(4):210–20. [DOI] [PubMed] [Google Scholar]
- 109.Leuchter AF, Newton TF, Cook IA, Walter DO, Rosenberg-Thompson S & Lachenbruch PA. Changes in brain functional connectivity in alzheimer-type and multi-infarct dementia. Brain: A Journal of Neurology. 1992; 115 (Pt 5), 1543–1561. [DOI] [PubMed] [Google Scholar]
- 110.Leuchter AF, Dunkin JJ, Lufkin RB, Anzai Y, Cook IA & Newton TF. Effect of white matter disease on functional connections in the aging brain. Journal of Neurology, Neurosurgery, and Psychiatry. 1994; 57(11), 1347–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Locatelli T, Cursi M, Liberati D, Franceschi M, Comi G. EEG coherence in Alzheimer's disease. Electroencephalogr Clin Neurophysiol. 1998; 106(3):229–37. [DOI] [PubMed] [Google Scholar]
- 112.Pogarell O, Teipel SJ, Juckel G, Gootjes L, Möller T, Bürger K & Hampel H. EEG coherence reflects regional corpus callosum area in Alzheimer’s disease. Journal of Neurology. Neurosurgery & Psychiatry 2005; 76(1), 109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sloan EP, Fenton GW, Kennedy NS, MacLennan JM. Neurophysiology and SPECT cerebral blood flow patterns in dementia. Electroencephalography and Clinical Neurophysiology. 1994; 91(3), 163–170. [DOI] [PubMed] [Google Scholar]
- 114.Engels MM, Stam CJ, van der Flier WM, Scheltens P, de Waal H, van Straaten EC. Declining functional connectivity and changing hub locations in Alzheimer's disease: an EEG study. BMC Neurol. 2015; 15:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Babiloni C, Frisoni GB, Vecchio F, Pievani M, Geroldi C, De Carli C, Ferri R, Vernieri F, Lizio R, Rossini PM. Global functional coupling of resting EEG rhythms is related to white-matter lesions along the cholinergic tracts in subjects with amnesic mild cognitive impairment. J Alzheimers Dis. 2010b; 19(3):859–71. [DOI] [PubMed] [Google Scholar]
- 116.Babiloni C, Miniussi C, Moretti DV, Vecchio F, Salinari S, Frisoni G, Rossini PM. Cortical networks generating movement-related EEG rhythms in Alzheimer's disease: an EEG coherence study. Behav Neurosci. 2004b;118(4):698–706. [DOI] [PubMed] [Google Scholar]
- 117.Babiloni C, Cassetta E, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Lanuzza B, Miniussi C, Moretti DV, Nobili F, Pascual-Marqui RD, Rodriguez G, Romani GL, Salinari S, Zanetti O, Rossini PM. Donepezil effects on sources of cortical rhythms in mild Alzheimer's disease: Responders vs. Non-Responders. Neuroimage. 2006e; 31,1650–65. [DOI] [PubMed] [Google Scholar]
- 118.Babiloni C, Frisoni GB, Pievani M, Vecchio F, Infarinato F, Geroldi C, Salinari S, Ferri R, Fracassi C, Eusebi F, Rossini PM. White matter vascular lesions are related to parietal-to-frontal coupling of EEG rhythms in mild cognitive impairment. Hum Brain Mapp. 2008a; 29(12):1355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Babiloni C, Ferri R, Binetti G, Vecchio F, Frisoni GB, Lanuzza B, Miniussi C, Nobili F, Rodriguez G, Rundo F, Cassarino A, Infarinato F, Cassetta E, Salinari S, Eusebi F, Rossini PM. Directionality of EEG synchronization in Alzheimer's disease subjects. Neurobiol Aging. 2009b; 30(1):93–102. [DOI] [PubMed] [Google Scholar]
- 120.Blinowska KJ, Rakowski F, Kaminski M, De Vico Fallani F, Del Percio C, Lizio R, Babiloni C. Functional and effective brain connectivity for discrimination between Alzheimer's patients and healthy individuals: A study on resting state EEG rhythms. Clin Neurophysiol. 2017; 128(4):667–680. [DOI] [PubMed] [Google Scholar]
- 121.Dauwels J, Vialatte F, Latchoumane C, Jeong J & Cichocki A. EEG synchrony analysis for early diagnosis of Alzheimer's disease: a study with several synchrony measures and EEG data sets. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2009; pp. 2224–2227. [DOI] [PubMed] [Google Scholar]
- 122.Dauwels J, Vialatte F, Musha T, Cichocki A. A comparative study of synchrony measures for the early diagnosis of Alzheimer's disease based on EEG. Neuroimage. 2010b; 49(1):668–93. [DOI] [PubMed] [Google Scholar]
- 123.Canuet L, Pusil S, López ME, Bajo R, Pineda-Pardo J, Cuesta P, Galvez G, Gaztelu JM, Lourido D, García-Ribas G, Maestú F. Network Disruption and Cerebrospinal Fluid Amyloid-Beta and Phospho-Tau Levels in Mild Cognitive Impairment. J Neurosci. 2015; 35, 10325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Babiloni C, Triggiani AI, Lizio R, Cordone S, Tattoli G, Bevilacqua V, Soricelli A, Ferri R, Nobili F, Gesualdo L, Millán-Calenti JC, Buján A, Tortelli R, Cardinali V, Barulli MR, Giannini A, Spagnolo P, Armenise S, Buenza G, Scianatico G, Logroscino G, Frisoni GB, Del Percio C. Classification of Single Normal and Alzheimer's Disease Individuals from Cortical Sources of Resting State EEG Rhythms. Front Neurosci. 2016b; 23;10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Babiloni C, Del Percio C, Lizio R, Noce G, Lopez S, Soricelli A, Ferri R, Nobili F, Arnaldi D, Famà F, Aarsland D, Orzi F, Buttinelli C, Giubilei F, Onofrj M, Stocchi F, Stirpe P, Fuhr P, Gschwandtner U, Ransmayr G, Garn H, Fraioli L, Pievani M, Frisoni GB, D’Antonio F, De Lena C, Güntekin B, Hanoğlu L, Başar E, Yener G, Emek-Savaş DD, Triggiani AI, Franciotti R, Taylor JP, Vacca L, De Pandis MF, Bonanni L. Abnormalities of resting-state functional cortical connectivity in patients with dementia due to Alzheimer’s and Lewy body diseases: an EEG study. Neurobiol Aging. 2018a; 65, 18–40. [DOI] [PubMed] [Google Scholar]
- 126.Babiloni C, Del Percio C, Pascarelli MT, Lizio R, Noce G, Lopez S, Rizzo M, Ferri R, Soricelli A, Nobili F, Arnaldi D, Famà F, Orzi F, Buttinelli C, Giubilei F, Salvetti M, Cipollini V, Franciotti R, Onofrj M, Stirpe P, Fuhr P, Gschwandtner U, Ransmayr G, Aarsland D, Parnetti L, Farotti L, Marizzoni M, D'Antonio F, De Lena C, Güntekin B, Hanoğlu L, Yener G, Emek-Savaş DD, Triggiani AI, Taylor JP, McKeith I, Stocchi F, Vacca L, Hampel H, Frisoni GB, De Pandis MF, Bonanni L. Abnormalities of functional cortical source connectivity of resting-state electroencephalographic alpha rhythms are similar in patients with mild cognitive impairment due to Alzheimer's and Lewy body diseases. Neurobiol Aging. 2019a; 77:112–127. [DOI] [PubMed] [Google Scholar]
- 127.Sankari Z, Adeli H, Adeli A. Intrahemispheric, interhemispheric, and distal EEG coherence in Alzheimer’s disease. Clinical Neurophysiology, 2011; 122.5: 897–906. [DOI] [PubMed] [Google Scholar]
- 128.Tóth B, File B, Boha R, Kardos Z, Hidasi Z, Gaál ZA, Csibri E, Salacz P, Stam CJ, Molnár M. EEG network connectivity changes in mild cognitive impairment - Preliminary results. Int J Psychophysiol. 2014; 92(1):1–7. [DOI] [PubMed] [Google Scholar]
- 129.Dauwan M, van der Zande JJ, van Dellen E, Sommer IE, Scheltens P, Lemstra AW, Stam CJ Random forest to differentiate dementia with Lewy bodies from Alzheimer's disease. Alzheimers Dement (Amst). 2016; 19, 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lizio R, Del Percio C, Marzano N, Soricelli A, Yener GG, Başar E, Mundi C, De Rosa S, Triggiani AI, Ferri R, Arnaldi D, Nobili FM, Cordone S, Lopez S, Carducci F, Santi G, Gesualdo L, Rossini PM, Cavedo E, Mauri M, Frisoni GB, Babiloni C. Neurophysiological assessment of Alzheimer's disease individuals by a single electroencephalographic marker. J Alzheimers Dis. 2016; 49(1):159–77. [DOI] [PubMed] [Google Scholar]
- 131.Vecchio F, Di Iorio Riccardo, Miraglia F, Granata G, Romanello R, Bramanti P, Rossini PM. Transcranial Direct Current Stimulation Generates a Transient Increase of Small-World in Brain Connectivity: An EEG Graph Theoretical Analysis. Exp Brain Res. 2018; 236(4):1117–1127. [DOI] [PubMed] [Google Scholar]
- 132.Babiloni C, Del Percio C, Lizio R, Noce G, Cordone S, Lopez S, Soricelli A, Ferri R, Pascarelli MT, Nobili F, Arnaldi D, Famà F, Aarsland D, Orzi F, Buttinelli C, Giubilei F, Onofrj M, Stocchi F, Stirpe P, Fuhr P, Gschwandtner U, Ransmayr G, Caravias G, Garn H, Sorpresi F, Pievani M, D'Antonio F, De Lena C, Güntekin B, Hanoğlu L, Başar E, Yener G, Emek-Savaş DD, Triggiani AI, Franciotti R, Frisoni GB, Bonanni L, De Pandis MF. Abnormalities of Cortical Neural Synchronization Mechanisms in Subjects with Mild Cognitive Impairment due to Alzheimer's and Parkinson's Diseases: An EEG Study. J Alzheimers Dis. 2017b; 59(1):339–358. [DOI] [PubMed] [Google Scholar]
- 133.Andersson M, Hansson O, Minthon L, Rosén I, Londos E. Electroencephalogram variabiliy in dementia with lewy bodies, Alzheimer’s disease and controls. Dement Geriatr Cogn Disord. 2008; 26(3):284–90. [DOI] [PubMed] [Google Scholar]
- 134.Dauwels J, Srinivasan K, Ramasubba RM, Musha T, Vialatte FB, Latchoumane C, Jeong J, Cichocki A. Slowing and Loss of Complexity in Alzheimer's EEG: Two Sides of the Same Coin? Int J Alzheimers Dis. 2011; 539621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gallego-Jutglà E, Solé-Casals J, Vialatte FB, Dauwels J, Cichocki A. A theta-band EEG based index for early diagnosis of Alzheimer's disease. J Alzheimer’s Dis. 2015; 43(4):1175–84. [DOI] [PubMed] [Google Scholar]
- 136.Knyazeva MG, Jalili M, Brioschi A, Bourquin I, Fornari E, Hasler M, Meuli R, Maeder P, Ghika J. Topography of EEG multivariate phase synchronization in early Alzheimer's disease. Neurobiol Aging. 2010; 31(7):1132–44. [DOI] [PubMed] [Google Scholar]
- 137.Bennys K, Rondouin G, Vergnes C, Touchon J. Diagnostic value of quantitative EEG in Alzheimer's disease. Neurophysiol Clin. 2001;31(3):153–60. [DOI] [PubMed] [Google Scholar]
- 138.Anderer P, Saletu B, Klöppel B, Semlitsch H, Werner H. Discrimination between demented patients and normals based on topographic EEG slow wave activity: Comparison between z statistics, discriminant analysis and artificial neural network classifiers. Electroencephalography and Clinical Neurophysiolog. 1994; 91(2), 108–117. [DOI] [PubMed] [Google Scholar]
- 139.Lehmann C, Koenig T, Jelic V, Prichep L, John RE, Wahlund LO, Dodge Y, Dierks T. Application and comparison of classification algorithms for recognition of Alzheimer’s disease in electrical brain activity (EEG). J Neurosci Methods. 2007; 161, 342–350. [DOI] [PubMed] [Google Scholar]
- 140.Buscema M, Rossini P, Babiloni C, Grossi E. The IFAST model, a novel parallel nonlinear EEG analysis technique, distinguishes mild cognitive impairment and Alzheimer's disease patients with high degree of accuracy. Artif Intell Med. 2007; 40(2):127–41. [DOI] [PubMed] [Google Scholar]
- 141.Rossini PM, Buscema M, Capriotti M, Grossi E, Rodriguez G, Del Percio C, Babiloni C. Is it possible to automatically distinguish resting EEG data of normal elderly vs. mild cognitive impairment subjects with high degree of accuracy? Clin Neurophysiol. 2008; 119(7):1534–45. [DOI] [PubMed] [Google Scholar]
- 142.Buscema M, Grossi E, Capriotti M, Babiloni C, Rossini P. The I.F.A.S.T. model allows the prediction of conversion to Alzheimer disease in patients with mild cognitive impairment with high degree of accuracy. Curr Alzheimer Res. 2010; 7(2):173–87. [DOI] [PubMed] [Google Scholar]
- 143.Triggiani AI, Bevilacqua V, Brunetti A, Lizio R, Tattoli G, Cassano F, Soricelli A, Ferri R, Nobili F, Gesualdo L, Barulli MR, Tortelli R, Cardinali V, Giannini A, Spagnolo P, Armenise S, Stocchi F, Buenza G, Scianatico G, Logroscino G, Lacidogna G, Orzi F, Buttinelli C, Giubilei F, Del Percio C, Frisoni GB, Babiloni C. Classification of Healthy Subjects and Alzheimer's Disease Patients with Dementia from Cortical Sources of Resting State EEG Rhythms: A Study Using Artificial Neural Networks. Front Neurosci. 2017; 10:604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McBride J, Zhao X, Munro N, Jicha G, Schmitt F, Kryscio R, Smith C, Jiang Y. Sugihara causality analysis of scalp EEG for detection of early Alzheimer’s disease. submitted. Neuroimage Clin. 2015; 7: 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McBride J, Zhao X, Munro N, Jicha G, Smith C MD, Jiang Y. Discrimination of Mild Cognitive Impairment and Alzheimer’s Disease Using Transfer Entropy Measures of Scalp EEG. J Healthc Eng. 2015; 6(1): 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schjønning Nielsen M, Simonsen AH, Siersma V, Engedal K, Jelic V, Andersen BB, Naik M, Hasselbalch SG, Høgh P. Quantitative Electroencephalography Analyzed by Statistical Pattern Recognition as a Diagnostic and Prognostic Tool in Mild Cognitive Impairment: Results from a Nordic Multicenter Cohort Study. Dement Geriatr Cogn Dis Extra. 2018; 8(3):426–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Engedal K, Snaedal J, Hoegh P, Jelic V, Bo Anderson B, Naik M, Wahlund LO, Oeksendgaard AR. Quantitative EEG applying the statistical recognition pattern method: A useful tool in dementia diagnostic workup. Dement Geriatr Cogn Disord. 2015; 40, 1–12. [DOI] [PubMed] [Google Scholar]
- 148.Ferreira D, Jelic V, Cavallin L, Oeksengaard AR, Snaedal J, Høgh P, Andersen BB, Naik M, Engedal K, Westman E, Wahlund LO. Electroencephalography Is a Good Complement to Currently Established Dementia Biomarkers. Dement Geriatr Cogn Disord. 2016; 42(1-2):80–92. [DOI] [PubMed] [Google Scholar]
- 149.Snaedal J, Johannesson GH, Gudmundsson TE, Blin NP, Emilsdottir AL, Einarsson B, et al. Diagnostic accuracy of statistical pattern recognition of electroencephalogram registration in evaluation of cognitive impairment and dementia. Dement Geriatr Cogn Disord. 2012; 34(1):51–60. [DOI] [PubMed] [Google Scholar]
- 150.Garn H, Coronel C, Waser M, Caravias G, Ransmayr G. Differential diagnosis between patients with probable Alzheimer's disease, Parkinson's disease dementia, or dementia with Lewy bodies and frontotemporal dementia, behavioral variant, using quantitative electroencephalographic features. J Neural Transm (Vienna). 2017; 124(5):569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Miraglia F, Vecchio F, Bramanti P, Rossini PM. EEG characteristics in “eyes-open” versus “eyes-closed” conditions: small-world network architecture in healthy aging and age-related brain degeneration. Clinical Neurophysiology. 2016; 127(2), 1261–1268. [DOI] [PubMed] [Google Scholar]
- 152.Tijms BM, Wink AM, de Haan W, van der Flier WM, Stam CJ, Scheltens P, Barkhof F. Alzheimer's disease: connecting findings from graph theoretical studies of brain networks. Neurobiol Aging. 2013; 34(8):2023–36. [DOI] [PubMed] [Google Scholar]
- 153.Supekar K, Menon V, Rubin D, Musen M, Greicius MD. Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Comput. Biol 2008; 4 p. e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Frantzidis CA, Vivas AB, Tsolaki A, Klados MA, Tsolaki M, Bamidis PD. Functional disorganization of small-world brain networks in mild Alzheimer's Disease and amnestic Mild Cognitive Impairment: an EEG study using Relative Wavelet Entropy (RWE). Front Aging Neurosci. 2014; 6:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vecchio F, Miraglia F, Bramanti P, Rossini PM. Human brain networks in physiological aging: a graph theoretical analysis of cortical connectivity from EEG data. J Alzheimer’s Dis. 2014; 41(4):1239–49. [DOI] [PubMed] [Google Scholar]
- 156.Vecchio F, Miraglia F, Quaranta D, Granata G, Romanello R, Marra C, Bramanti P, Rossini PM. Cortical connectivity and memory performance in cognitive decline: A study via graph theory from EEG data. Neuroscience. 2016; 316:143–50. [DOI] [PubMed] [Google Scholar]
- 157.Franciotti R, Falasca NW, Arnaldi D, Famà F, Babiloni C, Onofrj M, Nobili FM, Bonanni L. Cortical Network Topology in Prodromal and Mild Dementia Due to Alzheimer's Disease: Graph Theory Applied to Resting State EEG. Brain Topogr. 2019; 32(1):127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Peraza LR, Cromarty R, Kobeleva X, Firbank MJ, Killen A, Graziadio S, Thomas AJ, O'Brien JT, Taylor JP. Electroencephalographic derived network differences in Lewy body dementia compared to Alzheimer's disease patients. Sci Rep. 2018; 8(1):4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yu M, Gouw AA, Hillebrand A, Tijms BM, Stam CJ, van Straaten EC, Pijnenburg YA. Different functional connectivity and network topology in behavioral variant of frontotemporal dementia and Alzheimer's disease: an EEG study. Neurobiol Aging. 2016; 42:150–62. [DOI] [PubMed] [Google Scholar]
- 160.D'Amelio M, Rossini PM. Brain excitability and connectivity of neuronal assemblies in Alzheimer's disease: from animal models to human findings. Prog Neurobiol. 2012; 99(1):42–60. [DOI] [PubMed] [Google Scholar]
- 161.Gouw AA, Alsema AM, Tijms BM, Borta A, Scheltens P, Stam CJ, van der Flier WM. EEG spectral analysis as a putative early prognostic biomarker in nondemented, amyloid positive subjects. Neurobiol Aging. 2017; 57:133–142. [DOI] [PubMed] [Google Scholar]
- 162.Luckhaus C, Grass-Kapanke B, Blaeser I, Ihl R, Supprian T, Winterer G, Zielasek J, Brinkmeyer J. Quantitative EEG in progressing vs stable mild cognitive impairment (MCI): results of a 1-year follow-up study. Int J Geriatr Psychiatry. 2008; 23,1148–55. [DOI] [PubMed] [Google Scholar]
- 163.Moretti DV. Conversion of mild cognitive impairment patients in Alzheimer's disease: prognostic value of Alpha3/Alpha2 electroencephalographic rhythms power ratio. Alzheimers Res Ther. 2015; 7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rossini PM, Del Percio C, Pasqualetti P, Cassetta E, Binetti G, Dal Forno G, Ferreri F, Frisoni G, Chiovenda P, Miniussi C, Parisi L, Tombini M, Vecchio F, Babiloni C. Conversion from mild cognitive impairment to Alzheimer's disease is predicted by sources and coherence of brain electroencephalography rhythms. Neuroscience. 2006; 143,793–803. [DOI] [PubMed] [Google Scholar]
- 165.Prichep LS, John ER, Ferris SH, Reisberg B, Almas M, Alper K, Cancro R. Quantitative EEG correlates of cognitive deterioration in the elderly. Neurobiol Aging. 1994. January-Feb,15(1):85–90. [DOI] [PubMed] [Google Scholar]
- 166.Prichep LS, John ER, Ferris SH, Rausch L, Fang Z, Cancro R, Torossian C, Reisberg B. Prediction of longitudinal cognitive decline in normal elderly with subjective complaints using electrophysiological imaging. Neurobiol Aging. 2006; 27(3):471–81. [DOI] [PubMed] [Google Scholar]
- 167.Prichep LS, Ghosh Dastidar S, Jacquin A, Koppes W, Miller J, Radman T, O'Neil B, Naunheim R, Huff JS. Classification algorithms for the identification of structural injury in TBI using brain electrical activity. Comput Biol Med. 2014; 53:125–33. [DOI] [PubMed] [Google Scholar]
- 168.Nobili F, Copello F, Vitali P, Prastaro T, Carozzo S, Perego G, Rodriguez G. Timing of disease progression by quantitative EEG in Alzheimer' s patients. J Clin Neurophysiol. 1999; 16(6):566–73. [DOI] [PubMed] [Google Scholar]
- 169.Babiloni C, Del Percio C, Lizio R, Marzano N, Infarinato F, Soricelli A, Salvatore E, Ferri R, Bonforte C, Tedeschi G, Montella P, Baglieri A, Rodriguez G, Famà F, Nobili F, Vernieri F, Ursini F, Mundi C, Frisoni GB, Rossini PM. Cortical sources of resting state electroencephalographic alpha rhythms deteriorate across time in subjects with amnesic mild cognitive impairment. Neurobiol Aging. 2014; 35(1):130–42. [DOI] [PubMed] [Google Scholar]
- 170.Coben LA, Danziger W, Storandt M. A Longitudinal EEG Study of Mild Senile Dementia of Alzheimer Type: Changes at 1 Year and at 2.5 Year. Electroencephalogr Clin Neurophysiol. 1985;61(2):101–12. [DOI] [PubMed] [Google Scholar]
- 171.Soininen H, Partanen J, Laulumaa V, Helkala EL, Laakso M, Riekkinen PJ. Longitudinal EEG spectral analysis in early stage of Alzheimer's disease. Electroencephalogr Clin Neurophysiol. 1989; 72(4):290–7. [DOI] [PubMed] [Google Scholar]
- 172.Alhainen K, Riekkinen PJ. Discrimination of Alzheimer’s patients responding to cholinesterase inhibitor therapy. Acta Neurol Scand. 1993; 149:16–21. [DOI] [PubMed] [Google Scholar]
- 173.Shigeta M, Persson A, Vitanen M, Winblad B, Nordberg A. EEG regional changes during long-term treatment with tetrahydroaminoacridine (THA) in Alzheimer’s disease. Acta Neurol Scand. 1993; 149:58–61. [DOI] [PubMed] [Google Scholar]
- 174.Alhainen K, Partanen J, Reinikainen K, Laulumaa V, Soininen H, Airaksinen M, Riekkinen P. Discrimination of tetrahydroaminoacridine responders by a single dose pharmaco-EEG in patients with Alzheimer's disease. Neurosci Lett. 1991; 127(1):113–6. [DOI] [PubMed] [Google Scholar]
- 175.Adler G, Brassen S. Short-term rivastigmine treatment reduces EEG slow-wave power in Alzheimer patients. Neuropsychobiology. 2001; 43(4), 273–276. [DOI] [PubMed] [Google Scholar]
- 176.Brassen S, Adler G. Short-term effects of acetylcholinesterase inhibitor treatment on EEG and memory performance in Alzheimer patients: an open, controlled trial. Pharmacopsychiatry. 2003; 36(6):304–8. [DOI] [PubMed] [Google Scholar]
- 177.Adler G, Brassen S, Chwalek K, Dieter B, Teufel M. Prediction of treatment response to rivastigmine in Alzheimer's dementia. J Neurol Neurosurg Psychiatry. 2004; 75,292–4. [PMC free article] [PubMed] [Google Scholar]
- 178.Babiloni C, Cassetta E, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Lanuzza B, Miniussi C, Moretti DV, Nobili F, Pascual-Marqui R, Rodriguez G, Romani G, Salinari S, Zanetti O, Rossini PM. Donepezil effects on sources of cortical rhythms in mild Alzheimer's disease: Responders vs. Non-Responders. Neuroimage. 2006;31(4):1650–65. [DOI] [PubMed] [Google Scholar]
- 179.Balkan S, Yaras N, Mihci E, Dora B, Agar A, Yargicoglu P. Effect of donepezil on EEG spectral analysis in Alzheimer's disease. Acta Neurol Belg. 2003; 103,164–169. [PubMed] [Google Scholar]
- 180.Kogan EA, Korczyn AD, Virchovsky RG, Klimovizky SS, Treves TA,Neufeld MY. EEG changes during long-term treatment with donepezil in Alzheimer's disease patients. Journal of neural transmission. 2001; 108(10),1167–1173. [DOI] [PubMed] [Google Scholar]
- 181.Kogan EA, Verchovsky RG, Neufeld MY, Klimovitsky SSh, Treves TA, Korczyn AD. Long-term tetrahydroaminoacridine treatment and quantitative EEG in Alzheimer's disease. J Neural Transm Suppl. 2007; (72):203–6. [DOI] [PubMed] [Google Scholar]
- 182.Gianotti LR, Künig G, Faber PL, Lehmann D, Pascual-Marqui RD, Kochi K, Schreiter-Gasser U. Rivastigmine effects on EEG spectra and three-dimensional LORETA functional imaging in Alzheimer's disease. Psychopharmacology (Berl). 2008; 198,323–32. [DOI] [PubMed] [Google Scholar]
- 183.Scheltens P, Hallikainen M, Grimmer T, Duning T, Gouw AA, Teunissen CE, Wink AM, Maruff P, Harrison J, van Baal CM, Bruins S, Lues I, Prins ND. Safety, tolerability and efficacy of the glutaminyl cyclase inhibitor PQ912 in Alzheimer's disease: results of a randomized, double-blind, placebo-controlled phase 2a study. Alzheimer’s Res Ther. 2018; 10(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Briels CT, Stam CJ, Scheltens P, Bruins S, Lues I, Gouw AA. In pursuit of a sensitive EEG functional connectivity outcome measure for clinical trials in Alzheimer's disease. Clin Neurophysiol. 2020; 131(1):88–95. [DOI] [PubMed] [Google Scholar]
- 185.Scheltens P, Twisk JW, Blesa R, Scarpini E, von Arnim CA, Bongers A, Harrison J, Swinkels SH, Stam CJ, de Waal H, Wurtman RJ, Wieggers RL, Vellas B, Kamphuis PJ. Efficacy of Souvenaid in mild Alzheimer's disease: results from a randomized, controlled trial. J Alzheimer’s Dis. 2012; 31(1):225–36. [DOI] [PubMed] [Google Scholar]
- 186.de Waal H, Stam CJ, Lansbergen MM, Wieggers RL, Kamphuis PJ, Scheltens P, Maestú F, van Straaten EC. The effect of souvenaid on functional brain network organisation in patients with mild Alzheimer's disease: a randomised controlled study. PLoS One. 2014; 9(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hughes SW, Crunelli V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. The Neuroscientist. 2005; 11(4),357–372. [DOI] [PubMed] [Google Scholar]
- 188.Dey AK, Stamenova V, Turner G, Black SE, Levine B. Pathoconnectomics of cognitive impairment in small vessel disease: A systematic review. Alzheimer’s & Dement. 2016; 12(7):831–45. [DOI] [PubMed] [Google Scholar]
- 189.Guillon J, Attal Y,Colliot O, La Corte V, Dubois B,Schwartz D,Chavez M, De Vico F. Loss of brain inter-frequency hubs in Alzheimer's disease. Sci Rep. 2017; 7:10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Colclough GL, Woolrich MW, Tewarie PK, Brookes MJ, Quinn AJ,Smith SM. How reliable are MEG resting-state connectivity metrics?. Neuroimage.2016; 138,284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mahjoory K, Nikulin VV, Botrel L, Linkenkaer-Hansen K, Fato MM, Haufe S. Consistency of EEG source localization and connectivity estimates.Neuroimage. 2017; 152:590–601. [DOI] [PubMed] [Google Scholar]
- 192.van Gerven M, Bahramisharif A, Heskes T, Jensen O. Selecting features for BCI control based on a covert spatial attention paradigm. Neural Netw. 2009; 22(9):1271–7. [DOI] [PubMed] [Google Scholar]
- 193.Pizzagalli D. Electroencephalography and High-Density Electrophysiological Source Localization. Physics. 2007; Corpus ID: 5813608 [Google Scholar]
- 194.Ahnaou A, Raeymaekers L, Biermans R, Moechars D, Peeraer E, Manyakov N, … Drinkenburg WH. Dynamic alterations of brain network oscillations in a Tau seeding mouse model of Alzheimer’s disease. Clinical Neurophysiology. 2016; 127(3),e74. [Google Scholar]
- 195.Theiler S, Eubank S, Longtin A, Galdrikian B, Farmer D. Testing for nonlinearity in time series: the method of surrogate data. Physica D: Nonlinear Phenomena. 1992; Volume 58, Issues 1–4, 15 [Google Scholar]
- 196.Toledo JB, Cairns NJ, Da X, Chen K, Carter D, Fleisher A, Householder E, Ayutyanont N, Roontiva A, Bauer RJ, Eisen P, Shaw LM, Davatzikos C, Weiner MW, Reiman EM, Morris JC, Trojanowski JQ; Alzheimer’s Disease Neuroimaging Initiative (ADNI). Clinical and multimodal biomarker correlates of ADNI neuropathological findings. Acta Neuropathol Commun. 2013; 1:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Srinivasan R, Winter WR, Nunez PL. Source analysis of EEG oscillations using high-resolution EEG and MEG. Progress in Brain Research. 2006; 159,29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Blinowska KJ. Review of the methods of determination of directed connectivity from multichannel data. Medical & biological engineering & computing. 2011; 49(5),521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Blinowska KJ, Kaminski M. Functional brain networks: random, “small world” or deterministic? PloS one. 2013; 8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Brunner C,Billinger M,Seeber M,Mullen TR,Makeig S. Volume Conduction Influences Scalp-Based Connectivity Estimates. Front Comput Neurosci. 2016; 10:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Van de Steen F, Faes L, Karahan E, Songsiri J, Valdes-Sosa PA, Marinazzo D. Critical comments on EEG sensor space dynamical connectivity analysis. Brain Topogr. 2016; 1–12. [DOI] [PubMed] [Google Scholar]
- 202.Babiloni C, Binetti G, Cassarino A, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Frisoni G, Galderisi S, Hirata K, Lanuzza B, Miniussi C, Mucci A, Nobili F, Rodriguez G, Luca Romani G, Rossini PM. Sources of cortical rhythms in adults during physiological aging: a multicentric EEG study. Hum Brain Mapp. 2006a; 27(2):162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gaál ZA, Boha R, Stam CJ, Molnár M. Age-dependent features of EEG-reactivity--spectral, complexity, and network characteristics. Neurosci Lett. 2010;479(1):79–84. [DOI] [PubMed] [Google Scholar]
- 204.Caplan JB, Bottomley M, Kang P, Dixon RA. Distinguishing rhythmic from non-rhythmic brain activity during rest in healthy neurocognitive aging. NeuroImage. 2015; 112:341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Klass DW, Brenner RP. Electroencephalography of the elderly. J Clin Neurophysiol. 1995; 12(2):116–31. [DOI] [PubMed] [Google Scholar]
- 206.Hartikainen P, Soininen H, Partanen J, Helkala EL, Riekkinen P. Aging and spectral analysis of EEG in normal subjects: a link to memory and CSF AChE. Acta Neurol Scand. 1992; 86(2):148–55. [DOI] [PubMed] [Google Scholar]
- 207.Finnigan S, Robertson IH. Resting EEG theta power correlates with cognitive performance in healthy older adults. Psychophysiology. 2011; 48(8),1083–1087. [DOI] [PubMed] [Google Scholar]
- 208.Roca-Stappung M, Fernández T, Becerra J, Mendoza-Montoya O, Espino M,Harmony T. Healthy aging: relationship between quantitative electroencephalogram and cognition. Neuroscience letters. 2012; 510(2),115–120. [DOI] [PubMed] [Google Scholar]
- 209.Cook IA, Leuchter AF, Morgan M, Witte E, Stubbeman WF, Abrams M, Rosenberg S, Uijtdehaage SH. Early Changes in Prefrontal Activity Characterize Clinical Responders to Antidepressants. Neuropsychopharmacology. 2002; 27:120–13. [DOI] [PubMed] [Google Scholar]
- 210.Giaquinto S, Nolfe G, Vitali S. EEG changes induced by oxiracetam on diazepam-medicated volunteers. Clin Neuropharmacol. 1986; 9 Suppl 3:S79–84. [PubMed] [Google Scholar]
- 211.Rossini PM, Rossi S, Babiloni C, Polich J. Clinical neurophysiology of aging brain: from normal aging to neurodegeneration. Prog Neurobiol. 2007; 83(6):375–400. [DOI] [PubMed] [Google Scholar]
- 212.Klimesch W EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999; 29(2-3):169–95. [DOI] [PubMed] [Google Scholar]
- 213.Dustman RE, LaMarche JA, Cohn NB, Shearer DE, Talone JM. Power spectral analysis and cortical coupling of EEG for young and old normal adults. Neurobiology of aging. 1985; 6.3:193–198. [DOI] [PubMed] [Google Scholar]
- 214.Vysata O, Kukal J, Prochazka A, Pazdera L, Simko J, Valis M. Age-related changes in EEG coherence. Neurol Neurochir Pol. 2014; 48(1):35–8. [DOI] [PubMed] [Google Scholar]
- 215.Smit DJ, Boomsma DI, Schnack HG, Hulshoff Pol HE, de Geus EJ. Individual differences in EEG spectral power reflect genetic variance in gray and white matter volumes. Twin Res Hum Genet. 2012; 15(3):384–92. [DOI] [PubMed] [Google Scholar]
- 216.Zhu H, Sun Y, Zeng J, Sun H. Mirror neural training induced by virtual reality in brain-computer interfaces may provide a promising approach for the autism therapy. Med Hypotheses. 2011; 76(5):646–7. [DOI] [PubMed] [Google Scholar]
- 217.Li S, Franken P, Vassalli A. Bidirectional and context-dependent changes in theta and gamma oscillatory brain activity in noradrenergic cell-specific Hypocretin/Orexin receptor 1-KO mice. Sci Rep. 2018; 8(1):15474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Vorobyov V, Bakharev B, Medvinskaya N, Nesterova I, Samokhin A, Deev A, Tatarnikova O, Ustyugov AA, Sengpiel F, Bobkova N. Loss of Midbrain Dopamine Neurons and Altered Apomorphine EEG Effects in the 5xFAD Mouse Model of Alzheimer's Disease. J Alzheimer’s Dis. 2019; 70(1):241–256. [DOI] [PubMed] [Google Scholar]
- 219.Schirner M, McIntosh AR, Jirsa V, Deco G, Ritter P. Inferring multi-scale neural mechanisms with brain network modelling. Elife. 2018; 7: e28927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ritter P, Schirner M, McIntosh AR, Jirsa VK. The virtual brain integrates computational modeling and multimodal neuroimaging. Brain Connect. 2013; 3(2):121–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Stefanovski L, Triebkorn P, Spiegler A, Diaz-Cortes MA, Solodkin A, Jirsa V, McIntosh AR, Ritter P, Alzheimer's Disease Neuroimaging Initiative. Linking Molecular Pathways and Large-Scale Computational Modeling to Assess Candidate Disease Mechanisms and Pharmacodynamics in Alzheimer's Disease. Front Comput Neurosci. 2019; 13:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Babiloni C, Del Percio C, Bordet R, Bourriez JL, Bentivoglio M, Payoux P, Derambure P, Dix S, Infarinato F, Lizio R, Triggiani AI, Richardson JC, Rossini PM. Effects of acetylcholinesterase inhibitors and memantine on resting-state electroencephalographic rhythms in Alzheimer's disease patients. Clin Neurophysiol. 2013c; 124(5):837–50. [DOI] [PubMed] [Google Scholar]
- 223.Zimmermann J, Perry A, Breakspear M, Schirner M, Sachdev P, Wen W, Kochan NA, Mapstone M, Ritter P, McIntosh AR, Solodkin A. Differentiation of Alzheimer's disease based on local and global parameters in personalized Virtual Brain models. Neuroimage Clin. 2018; 19:240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.