Abstract
Light guiding and manipulation in photonics have become ubiquitous in events ranging from everyday communications to complex robotics and nanomedicine. The speed and sensitivity of light-matter interactions offer unprecedented advantages in biomedical optics, data transmission, photomedicine, and detection of multi-scale phenomena. Recently, hydrogels have emerged as a promising candidate for interfacing photonics and bioengineering by combining their light-guiding properties with live tissue compatibility in optical, chemical, physiological, and mechanical dimensions. Herein, we review the latest progress over hydrogel photonics and its applications in guiding and manipulation of light. We discuss physics of guiding light through hydrogels and living tissues and existing technical challenges in translating these tools into biomedical settings. We provide a comprehensive and thorough overview of the materials, fabrication protocols, and design architectures used in hydrogel photonics. Finally, we describe recent examples of applying structures such as hydrogel optical fibers, living photonic constructs and their use as light-driven hydrogel robots, photomedicine tools, and organ-on-a-chip models. By providing a critical and selective evaluation of the field’s status, we set a foundation for the next generation of hydrogel photonics research.
Keywords: Hydrogel Photonics, Optical Sensing, Multi-Responsive Hydrogels, 3D Printing, Light-Driven Robots, Organ-on-chip, Photomedicine
1. Introduction
Looking into their past and current applications, hydrogel materials are proven to be versatile tools in biomedicine [1–4]. Since the earliest reports on hydrogel fabrication and their initial applications, their use and applications have been growing considerably and continues to do so. They are opening new avenues of research and development in fields such as tissue engineering and regenerative medicine, not only as biomaterials [5–8] but also creating more advanced and recent applications such as hydrogel machines [9] and 3D organ-on-a-chip technologies [10,11]. Among the different fields where hydrogels have been used, one can find a more recent niche of applications in guiding and manipulating light. From the time Leonardo da Vinci first schematized the most primitive contact lenses in the 1500s, it would take until the 1960s for their soft versions to be fabricated using synthetic hydrogels, such as poly(2- hydroxyethylmethacrylate) (pHEMA). In fact, this was likely the first true biomedical use of hydrogels as we know them today [12]. From that point onwards, hydrogel research gained gradually increasing momentum and the complexity of these materials grew accordingly. The first studied hydrogels (1960s) were mainly employed as cross-linked meshes with interesting swelling and mechanical properties, which then gave birth to responsive hydrogels (1970s), whose swelling behavior can change as a response to environmental conditions, such as pH [13]. It did not take long for scientists to combine these highly hydrated meshes with sound nutrient diffusion as supportive environments for cells. The 1980s were particularly transformative in that regard, as Langer and Vacanti established the concept of tissue engineering [14] and Lim and Sun reported seminal work on cell encapsulation within hydrogel structures [15]. In the tissue engineering field, hydrogels were increasingly employed, wet, dried, or cryo-modified, to fabricate the most distinct structures: ranging from nanoscale [16,17] to macroscale dimensions [18,19], with increasing complexity, stimuli responsiveness, swelling, and degradation properties. With time, these types of hydrogels were translated both commercially and clinically, finding many applications as reviewed recently elsewhere [20,21]. Consequentially, the hydrogel market has also grown considerably and is predicted to reach a value of over 15 Billion USD by 2022 [21].
Overall, what makes hydrogels so unique is the fact that these are highly hydrophilic, viscoelastic self-supporting meshes, composed mostly of water like so many biological tissues [22] and also have mechanical properties in the relevant ranges for living tissues [23]. These properties make hydrogels some of the most suitable candidates for supporting, transporting, and releasing cells and other biological entities such as growth factors and proteins [24–27].
Further, in parallel to hydrogels’ direct biological uses, these materials revealed themselves as essential tools in other fields [28–30]. Of note, it did not take long for these highly hydrated, responsive meshes - with excellent transparency – to populate the optical field with exciting applications. After the advent of laser light and the simultaneous emergence of the first hydrogel soft lenses (1960), the first 3D holograms were obtained using Gelatin [31,32]. This very early advance likely represents the first optical application of a natural hydrogel. Despite this report of natural hydrogel holograms dating 60 years ago, the combination of optics and hydrogels directly applied to the biomedical field is a recent development. Henceforth, this topic constitutes the scope of this review.
Initially, we review hydrogels’ properties in biomedical photonics, specifically focusing on the behavior of light within biological interfaces and the requirements for hydrogels to serve as photonic entities, i.e., to guide and manipulate light. Within that context, we establish a comparison between hydrogels and classic solid-state optical materials, focusing on integrating such structures within biological environments, evaluating their compatibility with living entities such as cells and tissues. From there, we review what techniques have been employed in the past to fabricate optically relevant hydrogel structures. From earlier film assembly techniques to more recent 3D fabrication approaches, we also comment on how advances in different fields such as tissue engineering and bioprinting can be translated or combined with optical hydrogel architectures. Then, we cover the up-to-date hydrogel optical structures and their respective biomedical applications. Starting with the earliest thin hydrogel-film-photonics, we gradually move to more advanced systems such as optical hydrogel fibers and bioinspired structural hydrogels. These different arrangements and designs have been explored for different purposes within the biomedical field. They have proven to be powerful tools in optical sensing, light guiding, and other optical applications, as reviewed in section 4.
Previous reviews have summarized advances in the biomedical applications of hydrogels from various perspectives [33–36], some of which have recently evaluated previous applications of hydrogel photonic constructs [37]. In this review, we cover some of these topics, provide an update on recent advances, and mostly focus this knowledge on how such photonic systems might evolve in the coming years and integrate distinct fields in a cross disciplinary manner with parallel advances, informed by current trends. In that sense, we discuss possible avenues to increase such systems’ functionality through multi-responsive constructs, where optical properties can be combined with electrical or magnetic responsiveness for increasing sensing capabilities and/or manipulation and structuring of hydrogel constructs. Additionally, we delve into the optical interface between engineered hydrogels and live cells, where photonics might be combined with both entities to create next-generation in vitro platforms. In these systems, we discuss how tissue emulation or disease reconstruction might be combined with real time, label free sensing or light actuation, namely, for inducing motion in hydrogels – the basal requirement for creating and controlling movement in micro-sized architectures (microrobots).
Additionally, it is crucial to consider that light might not be simply of interest for stimulating and sensing different phenomena but rather be delivered to specific locations within the body for therapeutic purposes. With that in mind, we look at the current landscape of hydrogel-based photomedicine, where exciting new progress on light-based multifactorial tumor therapies and tissue regeneration have come to light in the past few years. On a smaller scale, one might not only consider the full living organism as an essential target but also state of the art in recreation and micro-modeling of biological tissues and organs. Through that perspective, we explore the role of hydrogels and their interactions with light using organ-on-a-chip models, where optical signals might be employed towards the fabrication of complex 3D architectures on-chip. Simultaneously, we discuss the possibility of integrating photonic hydrogel sensing components within those micro-sized biological environments, where small and fast optical readouts might lead to advances with transformative potential.
Bringing together all these distinct topics, we outline the current state of the art for hydrogels in biomedical photonics critically and selectively. We discuss the current caveats of these novel applications of hydrogel structures and outline which new research avenues might arise soon, considering the ongoing technological advances. By connecting seemingly distinct fields through an optics-focused perspective, we set a roadmap for integrating advanced light-hydrogel materials, constructs, and designs in a broad array of novel biomedical platforms and applications.
2. Hydrogel-based photonics
Photonics can be briefly described as the guidance and manipulation of light, regardless of its varying characteristics, mostly through specific structures that interact with light [38–40]. Naturally, light guidance cannot be achieved without particular settings, e.g., transparency, as opaque materials will not allow photons to move through but rather block them. Therefore, several photonics applications use surfaces with distinct topographies, in which air is the medium of light-wave propagation. In most cases, light waves’ interaction with surface structures such as edges leads to diffraction or plasmonic resonances and consequent changes in properties such as wavelength (colors in the visible range). This process is the basis for several surface-based nanophotonic systems, which have been reported in the past decade for biomedical applications such as sensing and diagnostics [41,42]. In that context, a specific readout marks the presence of a particular analyte of interest. As such, there is a need to guide light and ensure that an analyte’s presence will lead to detectable changes in the incident light properties.
On the other hand, to simply guide light, there doesn’t need to be a specific change in light properties but rather a way to contain and direct it across space with, e.g., optical fibers. Therefore, the type of structure and its interactions with light will depend on the intended application. Their different properties have led to their use for various purposes in hydrogels, from structural photonics to more general fiber optic light-guiding. In the following sections, we discuss the behavior of light in the hydrogel and living tissue environments. We describe which parameters should be taken into account for balancing different optical events, such as transmission, loss, or scattering, to design hydrogels as complementary alternatives to the classical materials in the biomedical optics field.
2.1. Optical coupling losses at the material/tissue interface
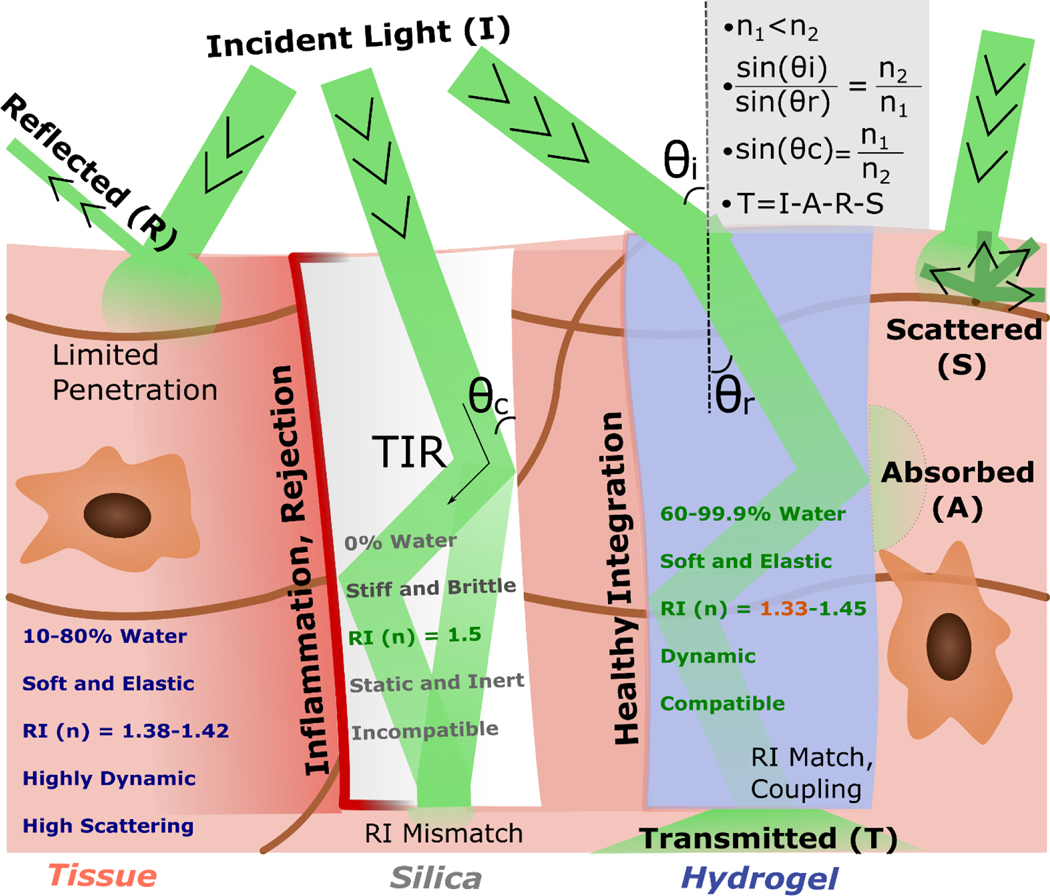
Hydrogels, just as any other material, interact with light leading to multiple fates upon such contact. The light might either be transmitted or reflected, scatter after colliding with specific structures that will change its direction of propagation, leave the medium, or be contained within via the phenomenon of total internal reflection (TIR) (Figure 1). Even though hydrogels and biological tissues share similar water-based environments, biological tissues’ complexity goes far beyond simple polymeric meshes. As a very complex and multi-organized structure, biological tissues are lossy for light propagation: as waves interact with tissues, they suffer from absorption and multiple scattering [43], which significantly reduce light intensity over traveled distance. It takes less than 1 mm of penetration in soft tissues for light intensity to reduce to 37% (1/e) [44]. Hence, the power required for light to reach as deep as human subcutaneous tissue might already have to be above the safety thresholds (4 W cm−2), naturally depending also on wavelength and exposure [44]. For a millimeter of penetration depth, soft biological tissues have losses in the range of tens if not hundreds of dB cm−1. For comparison, solid-state materials such as silica used in optical fibers can have optical losses as low as 0.2 dB Km−1 [45]. However, despite their favorable light-guiding properties, solid-state materials have a critical biomedical limitation, i.e., they are far from compatible when interacting with biological tissues [46]. This challenge has led the scientific community to look for alternatives that could bring together biological compatibility with proper light guidance in biological tissues.
Figure 1: Overview of Hydrogel Optics:
Schematic of the main events behind light going through biological tissues, solid-state silica, and hydrogels. The denser media have inherently higher refractive indexes (n2), leading to a slowing down of light going through and an angle of refraction (ɵr) inferior to that of incidence (ɵi). The refractive index difference allows for total internal reflection (TIR) when the incident angle is equal or below the critical angle (ɵc), containing light within the denser material. This process leads to coupling losses when there is a high refractive index difference (mismatch), as with silica versus living tissues. The light which is transmitted (T) results from the Incident light (I) minus the light that is reflected (R) and/or scattered (S). The general mathematical equations that connect these different concepts, namely Snell’s Law, are represented on the right panel. An overview of the main properties that make hydrogels more like biological tissues than classical solid-state alternatives is also outlined.
Other than light to travel through materials, one other relevant optical parameter is the refractive index, i.e., how light slows down in a particular medium compared to vacuum [47]. As a baseline reference, the refractive index for water (n) is 1.33. While classical solid-state materials have a considerably higher refractive index (up to 1.55 for silica oxide-based materials), biological tissues, most of which have high portions of water [22], have n ranging from 1.38 to 1.42 [48]. This significant mismatch also makes classic solid-state materials problematic in an optical context: high refractive index differences lead to inherent coupling losses as light tends to reflect to its origin, as observed in optical fiber-air interfaces. When combined, both the low biological compatibility and refractive index mismatch make solid-state materials sub-optimal for integration with biological tissues. Even though solid-state optical materials can be found in the biomedical field in the form of optical fibers such as in endoscopes, they are mainly employed to look at inner cavities and surfaces [49,50]. So far, these materials have not been directly integrated into biological tissues for potential long-term, real-time monitoring applications. The search for a material that would combine optical guiding capabilities with biological compatibility (both without toxicity and optical mismatch) points to hydrogels as a potential candidate (Figure 1).
2.2. Hydrogel requirements as photonic entities
Naturally, for hydrogels to perform well in optical settings, the requirement that these materials need to fulfill are different from those commonly looked after for applications in tissue engineering and regenerative medicine [4,51]. On the one hand, it is essential to maintain these materials’ cytocompatibility to the greatest extent possible to introduce them in biological environments. However, beyond biological compatibility, other characteristics might be needed depending on whether the goal is to guide light or rather to engineer a way to manipulate changes in the incident light. These changes are relevant when considering the need to obtain a responsive system (e.g., sensing and diagnostics).
When pursuing sensing applications, a shift in a detectable response marks the target entity’s presence to be detected. In optical terms, a change in light properties happens as a response to the target molecule/structure’s presence to be detected. Throughout the literature, different approaches for engineering such responsive systems, several of which are connected with structural coloration, are described ahead. As previously referred to, light propagates as waves that interact with specific structures (e.g., edges or slits), leading to diffraction (bending), refraction (changing direction), or plasmonics (resonance). Typically, edges, slits, and other geometric structures are not commonly present in hydrogels. In fact, hydrogels are naturally isotropic structures with no specific shape or orientation unless engineered to obtain anisotropic features such as mesh alignment or space-varying porosity [52,53]. As such, one of the main challenges for hydrogel photonics is engineering structures into the hydrogels, either in bulk or on the surface. Using distinct patterning and structuring techniques, hydrogels can have optical interactions that alter when the hydrogel contacts the target analyte. Frequently, hydrogels and analytes’ interactions lead to a swelling/deswelling behavior that will change (expand/contract) the patterned structure, leading to a shift in the characteristics of incident light and therefore creating a readout signal [54,55].
In a distinct context, one’s goal might be to guide light and not necessarily have it change properties. In this case, the main challenge then becomes the length at which light guiding is effectively done with minimal loss. Earlier [44], simple rectangular hydrogel slabs were used to encapsulate cells and transmit light. Taking advantage of the material’s transparency and the 1.35 refractive index of PEG 10wt% being higher than that of air (n close to 1), light traveled throughout the gel in a zig-zag fashion. This phenomenon, which we discussed in section 2, is due to total internal reflection (TIR) and is also present in standard optical fibers that are coated with a material (cladding) with a lower refractive index than that of the silica core [56]. However, biological tissues have a refractive index closer to those of hydrogels (Figure 1), making light-guiding within tissues and across more extensive lengths a considerable challenge, which led to the development of hydrogel optical fibers - explored later in this review.
Overall, one can bring hydrogels’ requirements for light guiding and manipulation down to two essential parameters. The first is transparency, which ensures that light can move through the material. The second is structure, which provides that either light can be guided throughout the material over significant lengths (e.g., through layers with different refractive indices) or interact with light, giving rise to measurable changes as a response to the presence of target analytes. As such, when devising any optical hydrogel architecture, there are two essential points to reflect upon. (i) The choice of polymer, with all its accompanying characteristics in terms of transparency and refractive index, and (ii) the fabrication technique, which will be responsible for giving the material a specific structure. Both points are reviewed in the following sections.
2.3. Common polymers in optical hydrogels
The choice of polymers to fabricate hydrogel structures in the optical context needs to be informed by the hydrogel’s distinct characteristics and requirements for specific applications, which can vary greatly (Table 1).
Table 1:
Commonly Used Polymers in Hydrogel-based Photonics
| Material | Source | Type of Structure | Exploited Property | Application | Refs. |
|---|---|---|---|---|---|
| Alginate | Natural (Seaweed) | Optical Fiber Shell (Cladding) | Low Refractive Index | Optical Fiber Sensing (Metals, Strain, Glucose) | [59,64–66] |
| Alginate | Natural (Seaweed) | Optical Bioinks | Adequate Printing Rheology, Particle Encapsulation | O2 Imaging | [80] |
| Agarose | Natural (Algae) | Thin Films | Electrostatic Interaction with Carbon dots | Heavy-Metal Sensing | [81] |
| Agarose | Natural (Algae) | Linear Fibers, Cladding layers | Cytocompatibility | Waveguide | [70,74] |
| Acrylamide | Synthetic | No-Core Fiber | Analyte-induced Refractive index changes | Ph Sensing, Glucose sensing | [61,62] |
| Acrylamide | Synthetic | Optical Fiber Core | Mechanical Stretchability | Strain Sensing | [59] |
| Poly-Acrylamide | Synthetic | Thin Films | Easy Functionalization | Glucose Sensing | [60] |
| Acrylamide/Methacrylamide | Synthetic | Classic Hydrogel | High Refractive index, high water content | Ophthalmological | [63] |
| Polymethacrylic Acid (PMAA) | Synthetic | Thin Films | Ph Sensitivity | Ph Sensing | [82] |
| Poly(ethylene glycol) Diacrylate (PEGDA) | Synthetic | Optical Fiber Core | High refractive index | Glucose Sensing, Metal Sensing | [62,64–66] |
| Poly(2-vinyl pyridine) P2VP | Synthetic | Thin Films | pH Responsiveness, dynamic swelling | pH Sensing, molecule-induced holographic changes | [83,84] |
| Poly(hydroxy-ethyl methacrylate) (PHEMA) | Synthetic | Thin Films | UV Crosslinking, Ion perfusion | Holographic pH Sensing | [85] |
| Chitosan | Natural (Chitin) | Hydrogel Photonic Crystals | No particular property | pH Sensing | [86] |
| Chitosan | Natural (Chitin) | Lbl films with Dextran | Charge Interactions with Dextran | Glucose Sensing | [77] |
| Gelatin | Natural (Collagen) | Uniform Layer Core | Thermal Crosslinking, Transparency | Waveguide | [70] |
| Gelatin | Natural (Collagen) | Thin Films | Thermal Crosslinking | Holographic Retroreflection | [71] |
| Gelatin/Alginate | Natural | Thin Films | Tunable pH Swelling | pH Sensing | [55] |
| Gelatin Methacryloyl (GelMA) | Natural Modified (Collagen) | Inverse Opal Scaffold | UV crosslinking on templated structures | Structural Colored Hydrogels | [69,87] |
| Cellulose | Natural (Plants) | Multi-core Fibers | Degradability | Waveguide | [75] |
| Silk Fibroin | Natural (Silk) | Core (film) – Clad (hydrogel) structures | Biological Compatibility, Transparency | Waveguide | [76,88] |
Abbreviations: Lbl – Layer-by-layer
When using hydrogels for optical applications, the material’s interaction with light is likely the most critical parameter to consider, together with properties like transparency or particular refractive indices, as previously discussed. Nevertheless, the complexity of optical hydrogel systems in the biomedical field might require different characteristics, such as biological compatibility, which also need to be considered when deciding the material to use.
Overall, both natural and synthetic polymers have been used for different optical applications (Table 1). Synthetic polymers are frequently used to fabricate hydrogels due to the ease of their modification and the ability to obtain tunable responses with a higher degree of reproducibility in terms of material consistency, mechanical properties, degradation rates, and optical transparency [57,58]. Acrylamide-based hydrogels are among the most commonly used for optical applications, being employed in the core of hydrogel optical fibers and thin hydrogel films that are discussed later in detail in Sections 3 and 4 [59,60]. These acrylamide-based structures can be functionalized with chemical moieties, e.g., phenylboronic acid towards glucose sensing, or simply take advantage of swelling/de-swelling events for detecting changes in pH and other target analytes [61,62]. Interestingly, acrylamide/methacrylamide blends were proposed to overcome one significant limitation of hydrogels, i.e., their high water content and consequent low refractive indices (close to water). Reducing hydrogels’ water content leads to an increase in the refractive index but brings significant cytocompatibility challenges. By manipulating the ratio of both acrylamide variants, researchers tuned these hydrogels to achieve a high, silica-like refractive index (n=1.53) in a structure still containing major water content (66%), remaining transparent and compatible with skin cells [63]. Overall, acrylamide has been reported as a versatile platform for optical hydrogel development, further capable of enduring considerable mechanical stretching, which can be employed for strain sensing [59]. Poly(ethylene glycol) (PEG) has been used as a versatile hydrogel in similar settings, namely within optical fiber cores [62,64–66]. PEG is modified for sensing applications such as glucose and metal detection, more frequently in its PEGDA (PEG diacrylate) form, which allows for UV crosslinking within molds [65]. In fact, crosslinking is also an important parameter, which depending on the experimental setting, might weigh heavily on the polymer choice [67,68]. While UV crosslinking is useful for obtaining fiber-like PEGDA structures [65] or complex structural colored gelatin methacryloyl (GelMA) hydrogels [69], thermal crosslinking might be easier to employ for fabricating gelatin layers and films [70,71]. Ionic crosslinking might be a quick and straightforward way to create alginate cladding layers in optical fibers, bringing the advantage of its high water content and low refractive index [59,64–66]. Alginate and gelatin are two examples of natural polymers used extensively in the optical context, starting with the early hydrogel optical experiments with gelatin [31,32]. Nevertheless, other natural polymers were shown to perform just as well in optical terms, combining transparency and light guiding capabilities with similar natural degradability and even biological compatibility [72,73]. Examples of such polymers are agarose [70,74], cellulose [75], silk fibroin [76] and chitosan [77]. All of these polymers have been classically used for tissue engineering and regenerative medicine applications [78,79]. The fact that they have also been validated within the optical context suggests that future innovation might bring together 3D cellular engineered structures with light guiding and manipulation, with interesting possibilities for in vitro tissue/disease modelling. Simultaneously, it can allow for the integration of real-time sensing and detection of relevant events such as pharmacological responses and overall drug testing, as discussed in the organ-on-chip section 9.
Globally, the choice of polymer for biomedical hydrogel photonics needs to integrate distinct properties depending on how closely the construct is designed to integrate into biological environments. For biomedical platforms used to screen analytes at the bench and not integrate into tissues, the choice might be of synthetic polymers that can be widely modified without concerns for biological compatibility. In order to integrate optical constructs into biological tissues, other properties such as biocompatibility need to be considered. In this context, naturally derived polymers can potentially receive increased interest for applications where intricate cell-material interactions might be wanted.
Similarly, some naturally derived polymers can easily be degraded in the tissues, which is relevant for designing implantable constructs that degrade with time, avoiding removal surgery if no longer needed. Naturally, degradability is a very elaborate process involving changes in structure, mechanics, and optical properties. While it is not the scope of this review to delve into the complex changes of biodegradability in biomaterials, it is essential to highlight that the process of degradability is most likely to comprise optical performance of the biomedical constructs. As such, engineering degradable photonic devices depends on the stability required for the material to perform as desired, delaying any considerable degradation to the point where the construct is no longer needed, and removal surgery can therefore be avoided.
Nevertheless, the material alone is not always sufficient to create complex optical/biological architectures, fabrication steps are also essential to give shape and structure to the final construct, as discussed in the fabrication techniques section 3.
3. Techniques for Fabricating Photonic Hydrogel Constructs
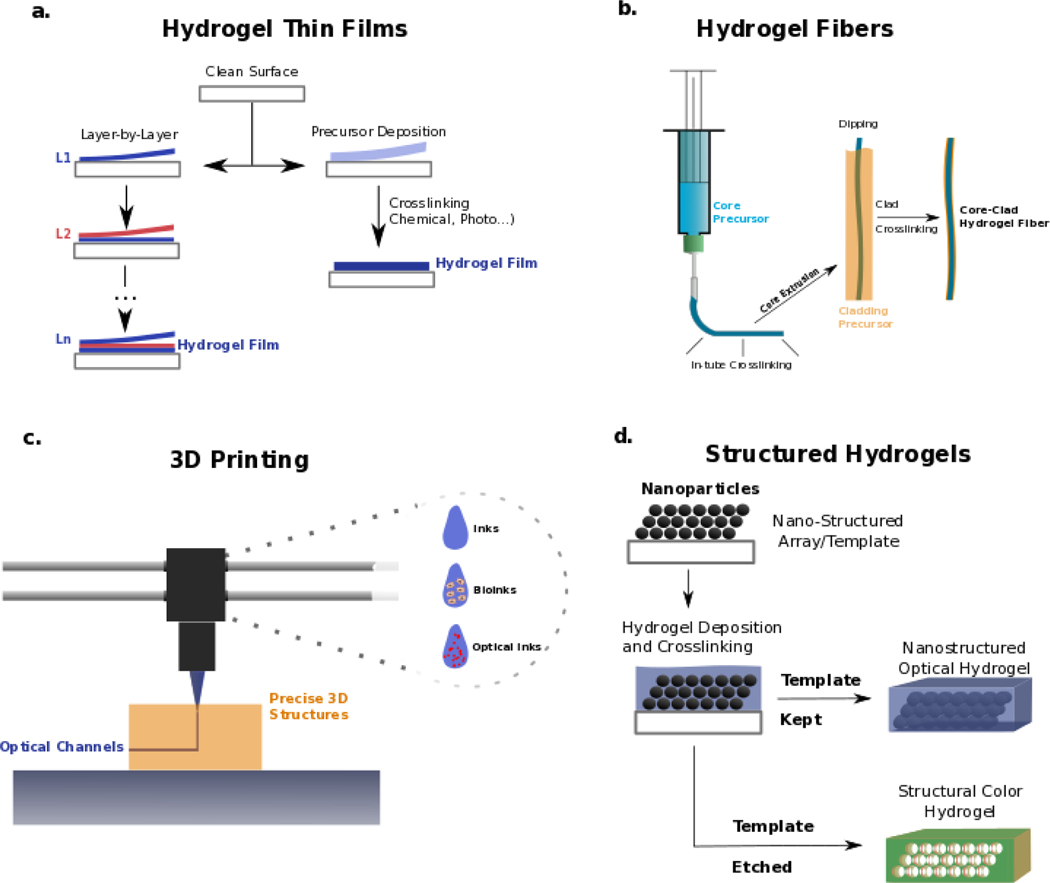
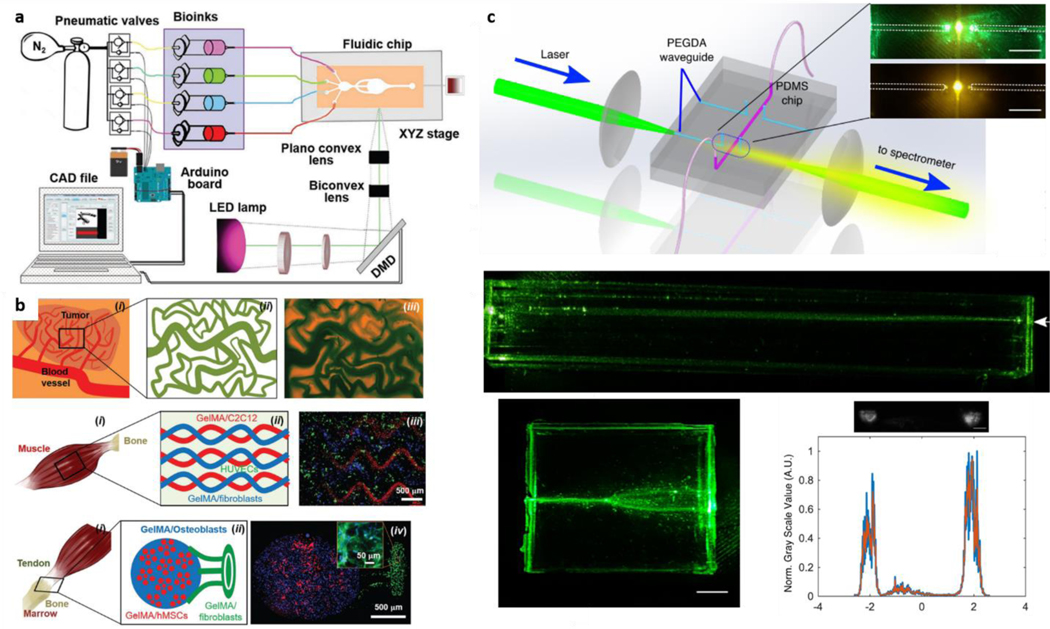
Different fabrication strategies have been adopted to create photonic hydrogel structures (Figure 2), with the earliest ones focusing on creating thin hydrogel films by deposition onto clean surfaces. Even though there might be slight variations in the specific protocols to develop these films depending on the processing requirements of the materials (e.g. temperature, type of crosslinking), two main fabrication routes are used to create hydrogel thin films (Figure 2a). The first and most straightforward one consists of depositing the hydrogel precursor onto a clean surface and then crosslink the precursor through various means, including chemical, thermal, or light-based (UV) stimuli. This type of approach has been used for fabricating synthetic films such as acrylamide ones [60] or natural gelatin-alginate films [55]. In this technique, there are essentially two critical steps for obtaining the thin film configuration. The first is the attachment of the hydrogel to the working surface [89] with the deposition of a thin layer, which is frequently done through spin coating techniques that use centrifugal forces to uniformly deposit materials on a surface, leading to hydrogel films ranging from tens to hundreds of micrometers in thickness [89]. The second widely used approach to fabricate hydrogel thin films is based on layer-by-layer assembly. This method uses sequential and alternated deposition of differently charged (positive versus negative) polymer solutions, which will gradually assemble on top of each other, growing towards a thin film [90]. The number of deposited bilayers will then dictate the thickness of this film. Each molecular bilayer is naturally very thin (nanometer-sized). As such, hundreds of bilayers are needed to reach thicknesses of hundreds of micrometers [91] as those obtained by spin-coating deposition. In both cases (single-step or layer-by-layer), hydrogel films can swell/de-swell depending on distinct stimuli. Swelling is frequently the main change leading to optical shifts, often exploited for different biomedical applications, as discussed in the thin film section. Further details on hydrogel thin film fabrication specifics, types of crosslinking, and surface manipulation have been reviewed elsewhere [92,93].
Figure 2: Fabrication techniques for obtaining hydrogel structures with relevant photonic properties.
a | Hydrogel thin films can be produced by deposition on a surface of either layer-by-layer molecular structures or single-step precursor deposition and crosslinking. b | Hydrogel fibers for optical applications are usually fabricated through UV crosslinking materials, structured in fiber-like shapes inside a tube, and then pushed out. These fibers can then be coated with a different hydrogel by, e.g., ionic crosslinking, as frequently pursued core-clad architectures. c | 3D printing techniques allow precise deposition of other materials and be further explored in the optical context. These techniques facilitate the fabrication of complex 3D structures where inks and bioinks are combined with novel types of optical inks that can interact with light differently in, e.g., optical channels within constructs. d | Structured hydrogels are frequently fabricated by depositing these materials within a nano-structured template, which can then be kept inside the hydrogel (e.g., spaced particles) to add further optical interactions (e.g., plasmonic resonance). Alternatively, these nanostructures can be etched out (by, e.g., thermal or chemical processes), leaving only their shape in the hydrogel, as used to obtain hydrogels with structural colors (i.e., the color given by the interaction of light with nanostructures and not by the presence of dyes)
Hydrogel films have been demonstrated as powerful devices for photonics in the biomedical field in detection and sensing applications, and are usually employed at laboratory benchtop experiments and not directly integrated into biological tissues. In these films, physiologically relevant molecules and fluids can be tested for analyte detection [77] as within other types of surface-based assays such as lateral flow chips [94,95], which are not required to integrate into biological tissues. For that purpose, different kinds of structures have been fabricated, where 3D complexity adds to the optical capabilities and biological compatibility of constructs. One such type of construct is hydrogel fibers (Figure 2b). Hydrogel fibers and microfibers are truly versatile structures in the fields of biofabrication and tissue engineering, being highly suitable to recapitulate biological-like tissue structures with meter-long lengths [96,97]. They have seen gradually increasing shape and architectural complexity [98–100]. In the optical context, fiber-like designs are also motivated by the paradigm-shifting invention of optical fibers, representing the fastest and more efficient solution for transporting information [45]. As previously discussed, optical fibers are built with non-biocompatible materials, namely solid-state silica. As such, hydrogels have been increasingly studied as candidates for fabricating biologically relevant optical fibers. The fabrication and complexity of these hydrogel architectures in the photonic field are still limited using relatively simple techniques. Mostly, hydrogel fibers for optical applications have been fabricated by injecting precursors into a transparent tube that facilitates light-based crosslinking of the material which is then extruded towards a fiber-shaped gel, frequently made of PEGDA [65,66] or Acrylamide [62]. This single-material fiber represents a core structure, which can then be coated by another material of low refractive index as a cladding structure for approaching Total Internal Reflection (TIR) and improved light propagation. Recently, alginate has been used for cladding optical fiber cores, mainly due to the ease of dipping the core material into the alginate solution and then crosslinking the alginate shell by dipping it into a divalent cationic solution such as CaCl2+ [64,66]. This simple strategy appears to dominate recent advances in optical hydrogel fiber fabrication strategies and could still evolve by taking advantage of the progress happening in hydrogel fiber biofabrication [101–103]. The translation of these technologies, which allow multi-material and multi-compartment hydrogel fiber biofabrication towards the field of optical fibers, can lead to the creation of faster single-step methods for fabricating optical hydrogel fibers. Such methods bring not only higher fabrication efficiency. Further, they can also facilitate the encapsulation of micro and nanostructures in different compartments for, e.g., multi-responsive characteristics or inclusion of living cells. Nevertheless, the simple core-clad optical hydrogel fibers so far fabricated are interesting complementary tools to solid state fibers, performing well in the biomedical field as we discuss in the optical hydrogel fiber section 4.
One other fabrication technique that has truly grown in biofabrication is 3D printing that enables integrating complex shapes, precise deposition of distinct materials and cells. Due to this precise control on cell/material deposition, this technique facilitates the recapitulation of biological tissue complexity, topics discussed in other reviews [104,105]. However, while 3D bioprinting has already reached outer space [106], bridging those advances to the photonics field is still an emerging area of study. Nevertheless, the capabilities of 3D printing allow envisioning interesting applications for matching complex 3D shapes and tissue-like architectures with optically functional structures. 3D bioprinting employs different biomaterials as inks, which in the biomedical field are frequently hydrogel-based bioinks, named so due to their ability to support cells and maintain their viability while having favorable rheological properties. [107,108]. Even though the combination of hydrogel photonics and 3D printing has not yet been extensively explored, optical inks can also emerge soon, where hydrogels are structured or combined with optically-relevant entities such as particles for plasmonic resonance. 3D printing can then be employed to introduce these shapes and materials within complex environments. Printing optical inks could enable biofabrication of optical channels within a tissue-like construct that can guide light and report on different relevant events such as cellular proliferation and ECM remodeling (Figure 2c). Researchers recently created bioinks of alginate and cells, containing optical sensor particles which responded to oxygen levels by imageable fluorescent changes [80]. This work can be seen as one of the first examples of an optical bioink. Similar approaches can be further tuned to not only derive fluorescent signals but guide and manipulate light within 3D printed channels and shapes.
Additionally, hydrogels for optical applications can depend on the introduction of 3D structuring, which allows for more complex light interactions such as structural colors. These interactions can be used for either sensing, holographic imaging, or biomechanical-optical transduction, as discussed in the structured hydrogel section 4. Once again, the distinct techniques employed to fabricate structural hydrogels revolve around simple concepts that can be brought down to one essential step. Usually, structuring starts by depositing hydrogel precursors on a previously structured array, frequently with shapes on the nanometer size (Figure 2d). Once the hydrogel is crosslinked around this template, two distinct paths can be followed to fabricate different structures.
On one hand, this template might be kept within the hydrogel, creating an array within the 3D water mesh that can interact with light and even be arranged optically [109]. This type of structure is highly relevant for holographic-related applications. Even if not directly employed in biological environments, it could still be used for biomedical-data encoding and sensing applications [109,110]. On the other hand, once the hydrogel is crosslinked around the nanostructured template, the latter can be etched out, leaving only the hydrogel containing the negative shape of the structure that was once within. These structures, frequently named inverse opal scaffolds, obtain structural colors, in which the interaction of physical patterns with light will give rise to varying shades [69,87]. Changes in these colors can be employed for highly relevant biomedical sensing through real-time optical transduction of biological events, as discussed in the structured hydrogel applications section 4.
As we have outlined in section 3, different techniques with allow fabricating hydrogel-based structures with interesting optical properties. Some of these approaches still have room for development, such as 3D printing and more complex optical hydrogel fibers. The combination of distinct fabrication techniques such as 3D printing and optical fiber integration within constructs is not impossible. This approach might be explored to combine advanced in vitro tissue emulation with real-time sensing and reporting of relevant responses through light-based readouts. Overall, the fabrication method depends on the optical structure required by the target application, and such examples are discussed in the next section.
4. Photonic applications of hydrogel structures
Applying hydrogels as optical and photonic entities in the biomedical field is related to the type, architecture and materials of the construct that is fabricated, as previously discussed. Next, we review the recent applications of hydrogels by focusing on the main types of optical constructs with validated applications: Hydrogel Thin Films, Optical Fibers and Waveguides, and Bioinspired Nanostructured Hydrogels.
4.1. Thin hydrogel films
Thin hydrogel films are one of the earliest types of photonic constructs used in biomedical sensing applications—some of the first examples of such platforms date back to the early 2000s [111,112]. Hydrogel thin films rely mostly on polymer mesh swelling/deswelling changes, causing the film thickness/height to shift leading to changes in its interaction with light [54]. Functionalized hydrogels can have selective molecule binding and drive a shift in water content where they can be employed as a sensing platform. Most optical thin films have also been assembled with other structures, e.g., nanoparticles, to integrate hydrogel film properties with plasmonic-like sensing or even holographic arrangements [71,85].
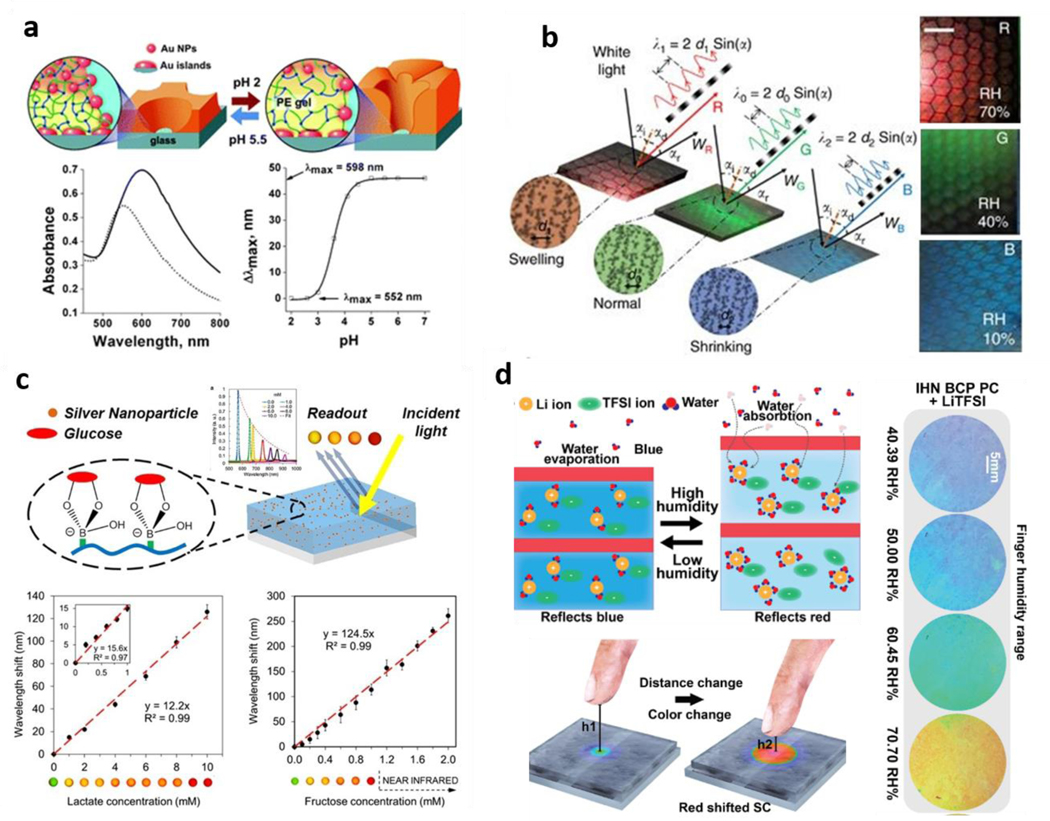
Some interesting approaches to engineer hydrogels where swelling alone can be used for thin film optical sensing showed that the combination with nanostructures might not be necessary to achieve detectable shifts in light behavior. Researchers used a layer-by-layer approach to obtain phenylboronic-acid-functionalized, acrylamide-based hydrogel films with sub-micrometer thickness, where the direct binding of glucose molecules could lead to hydrogel swelling. This swelling caused a shift in incident light wavelength and allowed analyte detection without further structuring [54]. One other study reported the engineering of block-copolymer lamellar hydrogel films, which could bind to distinct counterions and lead to varying degrees of hydration, changing the wavelength of interacting light and hydrogel film color accordingly [84]. Even though these films were not directly used for biomedical applications, they represent an exciting advance on pure swelling-based optical thin film applications. A more recent example is that where layer-by-layer assembly was used to form hydrogel films of dextran, chitosan, and glucose oxidase, creating a multi-step change in film characteristics resulting in the shift of light properties. Glucose oxidase converts glucose to gluconic acid, lowering the film’s local pH, which, being pH-sensitive, swells, leading to a shift in optical properties that was be used for detection [77]. pH sensing has also been approached with hydrogels containing nanostructures, such as the inclusion of nanoparticles on a pH-sensitive P2VP hydrogel, in which pH-induced changes in swelling altered the distance between particles leading to a pH-dependent shift in wavelength [83] (Figure 3a). pHEMA hydrogels were also used for structured pH sensing, where researchers introduced nanoparticles in the hydrogel through diffusing silver ions and then reducing these ions to nanoparticles. By then slightly tilting the film and backing it with a mirror, the authors obtained a holographic system where pH-induced changes in swelling led to changes in the wavelength of light resulting in distinct colors across a broad range of pH [85]. The strategy of taking advantage of holographic changes was also more recently bridged to natural, biocompatible gelatin films. Silver nanostructures could also be embedded in the films and lead to swelling-induced changes in detectable wavelength [71] (Figure 3b). The inclusion of nanostructures was combined with phenylboronic functionalization to obtain glucose-induced swelling in the hydrogel and subsequent optical shifts based on analyte concentration [60]. This responsiveness was further expanded to sense not only glucose but other sugars such as lactate and fructose, representing a versatile, adaptable system [60] (Figure 3c).
Figure 3: Thin Hydrogel Films –
a| Engineered hydrogel films incorporating silver particles for pH sensing. Adapted with permission [116]. Copyright 2008, Wiley. b| Gelatin-based holographic films with swelling-shrinking shifts in wavelength and color. Adapted under the terms of CC BY License [71]. Copyright 2016, the authors. c| Thin-Film holographic sensors with phenylboronic modifications for glucose and another analyte sensing. Reproduced with permission from reference [60]. Copyright 2014, American Chemical Society. d| Block co-polymer hydrogels with varying hydration levels respond to proximity to the human finger as touchless response platforms. Adapted under the terms of CC-BY-NC license [115]. Copyright 2020, the authors.
These hydrogel film platforms have also been engineered to sense other molecules such as alcohols and heavy metals [113,114]. Even though thin-film research has been around for some time, it is still possible to see significant advances in their use towards increasingly complex applications such as smart displays. Very recently, researchers showed how PEGDA/P2VP hydrogel films could be combined with hygroscopic ionic liquids to obtain variations in structural colors on a surface depending on the relative humidity [115]. This principle was then used to obtain a touchless display where a human finger could trigger a response from a distance [115] (Figure 3d).
Overall, thin films have seen significant evolution in the past decade and are undoubtedly powerful platforms for sensing distinct analytes based on label-free, fast optical shifts. Nevertheless, despite some advances in including biologically compatible, natural, and degradable materials in these structures, their use in close contact with biological systems is still limited. This limitation is either due to toxic components or simply because these films are engineered as surfaces to be used in an air/film interface for sensing. The thin film sensors are not integrated into biological tissues. These sensors have potential to be integrated at the skin-air interface [117,118]. In addition to the thin films, other hydrogel architectures and constructs (e.g., hydrogel fibers) have been used to take optical hydrogel engineering closer to biological environments, i.e., from cell-material constructs to living tissues. Such structures are discussed in the next section.
4.2. Optical hydrogel fibers and waveguides
It is essential to start this discussion by differentiating optical fibers from waveguides. Generally, optical waveguides include a broad range of structures capable of guiding light across space. Optical fibers can be included in that definition as a type of waveguide [119]. However, optical fibers represent a more sophisticated structure that improves light-guiding, such as fiber-like geometry when compared to the optical waveguides. Therefore, optical fibers typically perform better than more rudimentary waveguide structures. In the text, we will refer to optical fibers and waveguides independently, where we see waveguides as more general structures with an asymmetrical shape that can guide light but do not present a fiber-like shape.
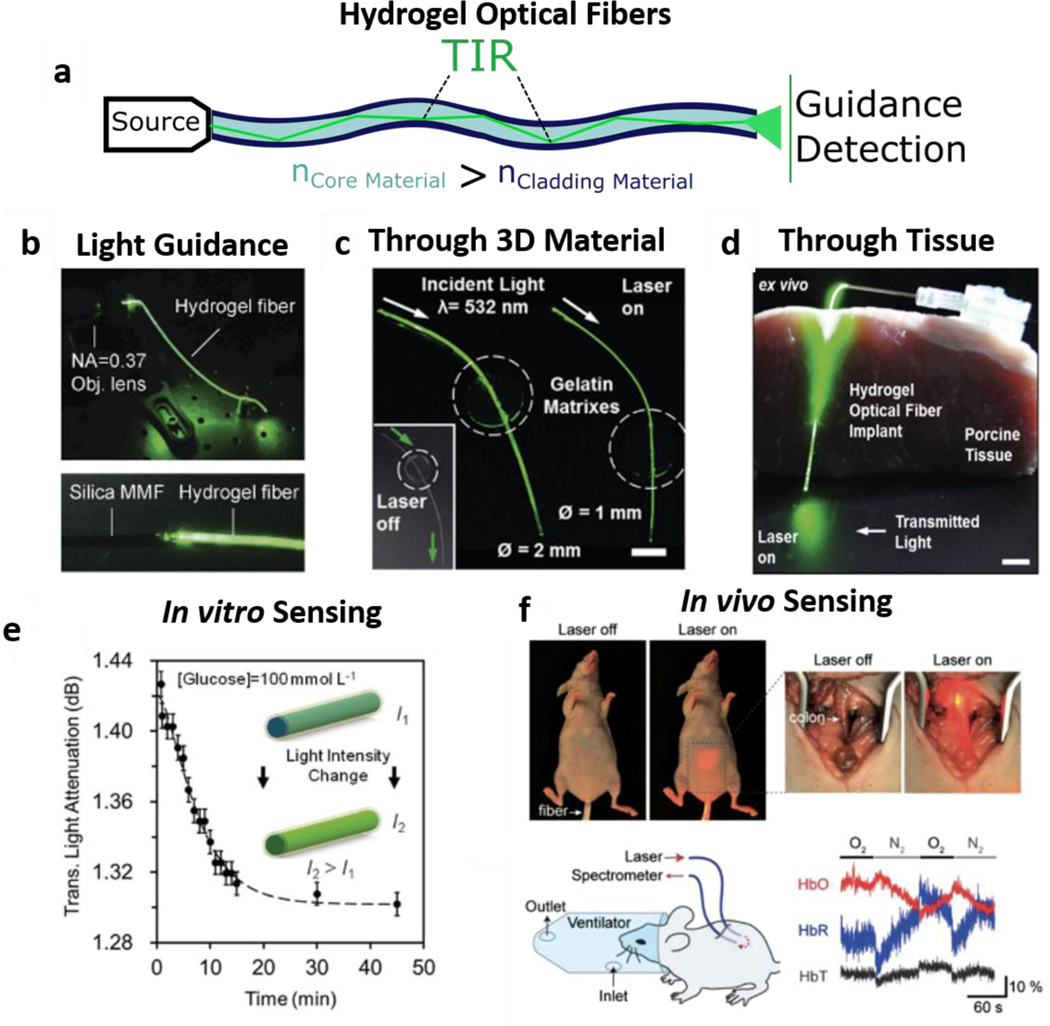
Focusing on waveguide structures, a strategy was reported to obtain a core of gelatin within two agarose layers (cladding) to guide light through gelatin, as one of the earliest degradable waveguides reported [70]. This study presented a simple planar structure, i.e., a core with a lower refractive index than the cladding leading to light being conducted across the space inside the core via total internal reflection (TIR). Others also resorted to agarose to create optical waveguide structures combined with live-cell encapsulation [74]. Further, synthetic PEG hydrogels were used to create planar hydrogel waveguides and encapsulated cells to screen cytotoxicity in vitro and in vivo [44]. Similar PEGDA waveguides were also used more recently to detect heavy metal ions [120]. Other natural materials have also been used to fabricate waveguides, such as silk fibroin, which not only brings exciting compatibility and cell interaction properties but can also be genetically engineered for optimized light-guiding [88]. Although all these waveguide studies represent significant advances in light guiding with demonstrated applicability to sense target analytes and screen cytotoxicity, these structures of planar or tubular shape have limited efficiency in guidance for light to travel. This limitation is due to increased loss via asymmetrical cladding as light moves through the planar architectures, making it such that these structures frequently are designed to be no more than 4 cm in length [44,120]. As a more efficient alternative, optical fibers have been fabricated to overcome this limitation, granting much more efficient light-guiding due to the fiber-like geometry where the tubular organization of different material layers ensures that the core is fully covered with cladding material. Therein, a beam of light can be conducted in a confined space due to TIR, leading to low losses and amplifying the possibility of transporting light across more considerable lengths (Figure 4a).
Figure 4 -. Optical Hydrogel Fibers:-.
a | Step-Index Optical Hydrogel Fiber Light Guiding due to refractive index differences b |Light Coupling and Guidance through an engineered hydrogel optical fiber. Adapted with permission from ref. [59]. Copyright 2016, Wiley c| Core-Clad light-guiding across 3D Gelatin matrixes and d| Porcine Tissue. Adapted under the terms of CC-BY license from ref. [66] Copyright 2017, the authors. e| In Vitro Hydrogel Optical Fiber for Glucose Sensing. Adapted under the terms of CC-BY license from ref.[66] Copyright 2017, the authors. f| In Vivo hydrogel optical fiber implementation and Oxygen Level Real-Time Detection. Reproduced with permission from ref. [65] Copyright 2015, Wiley.
Solid-state silica optical fibers have been around for a long time, but complex core-clad hydrogel optical fibers are more recent. In that work, researchers used two hydrogels to obtain a step-index optical fiber with a PEG core and an alginate shell, to guide light due to refractive index differences (around 1.45 for PEG and 1.33 for alginate) [65]. Here, authors showed that while core-only fibers enabled only tissue penetration depths of around 5 cm, the core-shell optical fiber provides a penetration depth of up to 15 cm depending on the wavelength. Shortly after, by replacing the PEG core with acrylamide, researchers obtained mechanically resilient optical fibers (Figure 4b), which were able to report on applied strain levels upon tensile deformation [59]. Both technologies were later combined when a PEGDA-Acrylamide core that were shelled with alginate and combined with phenylboronic acid functionalization for glucose sensing [66]. These fibers were shown to guide light through 3D hydrogel environments (Figure 4c) as well as in biological tissues (Figure 4d). The change in swelling due to glucose-phenylboronic acid interactions was then used to sense glucose levels in vitro (Figure 4e). Previously, researchers evaluated oxygen levels in vivo showing how these fibers could be directly implanted into biological tissues (Figure 4f) [65].
Other labs have also focused on similar strategies, such as the use of quantum-dot-doped PEGDA core-alginate Shell fibers for metal sensing [121]. Researchers also created a smartphone-based approach to detect glucose levels, again using PEGDA fibers [62]. Acrylamide/PEGDA core, alginate clad fibers were also adapted for laser photomedicine purposes. [122]. Recently, scientists combined similar PEG-based optical fiber structures with 3D printing to guide light into tissues, demonstrating its capability to trigger the exposure of RGD (arg-gly-asp) amino acidic motifs and control cellular adhesion [123]. Recently, agarose was used to create optical fibers in a slightly different manner in fabrication. By pouring agarose in a mold with rods, authors fabricated cylindrical fibers with a core surrounded by air-filled holes. The core was able to guide light and generate speckle fields due to being surrounded by these holes. Solutions could be perfused into these holes and lead to light behavior changes for potential sensing applications [124].
Overall, hydrogel optical fibers have demonstrated exemplary performance in guiding light in terms of distance and coupling with living tissues. This property adds to their advantages over solid-state fibers in terms of improved biological compatibility. Nevertheless, the materials explored so far for these structures and their fabrication methods are still limited. One can expect that future studies on this topic, will take advantage of different materials and crosslinking methods. These methods can lead to faster throughput in fiber fabrication, possibly taking advantage of current developments in core-shell microfluidics and bioprinting [98,100,125]. Adapting this knowledge to the optical context will potentiate core-cladding fabrication and light guiding properties with unprecedented efficiency while maintaining or further improving biological performance and compatibility using polymers capable of improved cell-material interactions.
4.3. Bioinspired structured hydrogels
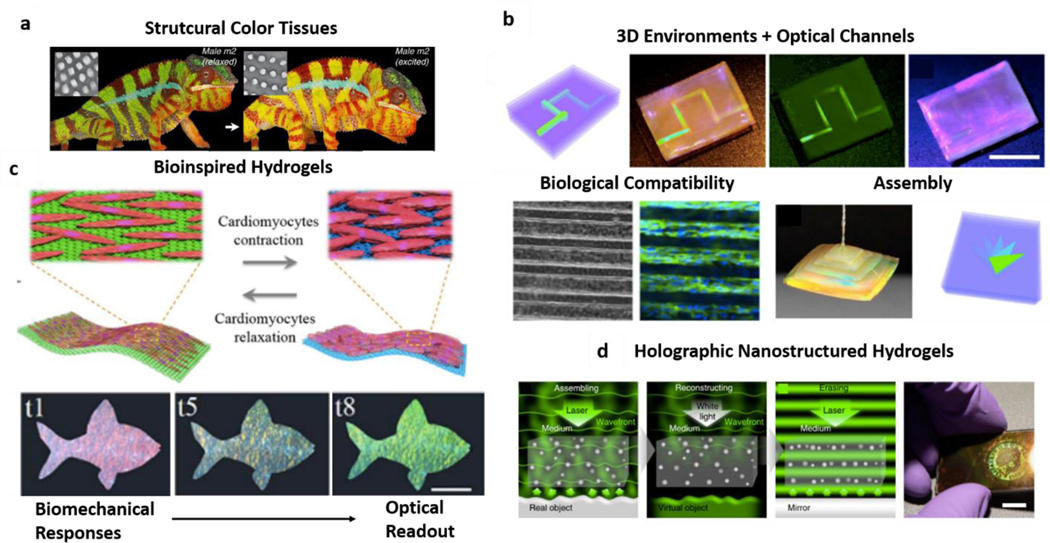
Among the various pigments and colors found in nature, structural colors are amongst the most fascinating. Unlike dies or pigments, these are observable colors due to light interaction with sub-micron shapes present in structures as those found in plants, flowers, bird feathers, or insect shells [126–128]. Structural colors are the basis of chameleon’s changing colors due to alterations in organized 3D lattices present on the skin (Figure 5a) [129]. In an approach to mimic nature, scientists have used similar strategies to generate and engineer structurally colored materials [130]. Also, in hydrogels, fabrication of reverse opal scaffolds - as discussed in the fabrication section (section 3) - has been pursued to obtain intricate shapes leading to structural coloration. In the earliest examples, the materials used lacked biological compatibility, hence limiting their biomedical applications. An excellent example of this concept is the vast body of literature focusing on the use of poly-dodecyl glyceryl itaconate/polyacrylamide networks (PDGI/PAAM ) [131–133], which have been successfully engineered for various color properties combined with their mechanical responsiveness. Even if these materials have potential use in devices for sensing and diagnostics outside of living tissues, they are not particularly compatible with biological entities. On the other hand, inverse opal scaffolds have been engineered with materials widely known to be cyto-compatible and were further employed as direct scaffolds for tissue engineering due to their intricate shapes that provide cues for cell adhesion and orientation [134]. These studies, however, neglect the photonic properties of the opal hydrogels.
Figure 5 -. Structured Hydrogels:
a| Naturally inspired biomimetic structural tissues. Adapted under the terms of CC-BY license. [129] Copyright 2015, the authors. b| Structural color hydrogels as channels and assembled structures with cyto-compatible properties. Adapted, with permission, from references [69,87]. Copyright 2017, National Academy of Sciences and Copyright 2018, AAAS. c| Structural hydrogels use for biomechanical-optical transduction through cardiomyocyte-induced hydrogel contraction and color change. Adapted with permission from reference [87]. Copyright 2018, AAAS. d| Hydrogels with nanostructures as assembled by light and holographic image generation for data encoding. Adapted under the terms of CC-BY license from reference [109]. Copyright 2016, the authors.
Most of the studies on structured hydrogels where biological compatibility and optical properties are reported in the recent years. In this period, researchers used a nanostructured template to introduce colors in gelatin methacryloyl (GelMA) hydrogels, combined with enzymatic glutaraldehyde crosslinking to give them self-healing properties [69]. Here, authors demonstrated that these hydrogels could exhibit exuberant structural colors, be arranged in complex 3D architectures, and had no negative impact on cell viability (Figure 5b). Almost at the same time, it was reported that silk hydrogels could also be templated similarly, and could have remarkable light interactions, and be suitable for smart contact lenses that could potentially grant night vision [135]. Even though the authors did not evaluate biological compatibility of the constructs, silk fibroin is long known to be biocompatible and is widely used as a biomaterial for various applications [136]. Both studies are examples of the combination of biologically compatible materials and structural coloration. Later, a group of researchers took the nanostructured GelMA inverse opal scaffolds one step forward by engineering living structural color hydrogels [87]. By combining the structural-colored scaffolds with cardiomyocytes, authors obtained a construct where the mechanical forces induced by cardiac cell contraction could lead to changes in the hydrogel lattice (Figure 5c). Then, just like the color changes in chameleons’ skin, this shape change led to color shifting in the engineered hydrogel - direct transduction of physiological response (cardiomyocyte contraction) to an optical signal (change in structural color) [87]. This work represents hydrogel photonics for fast, label-free detection of cellular events by a simple analysis of the material’s interaction with light and subsequent changes in color wavelength. Exciting future studies might outline the potential of these rapidly detectable changes to elucidate the mechanics of single cells and multi-cellular structures, much of which yet remains to be investigated fully [23,137].
Further, organized nanostructures not only serve as a template for structurally colored architectures but can also remain within hydrogel meshes and interact with light in such a way that their organization forms a holographic representation of a particular image (Figure 5d) [109]. These structures are sometimes also combined with hydrogel film technology and are discussed in section 4.1. The holographic hydrogels can potentially be used for coding relevant information and data can be used in the biomedical data context, ever-growing in complexity [138].
5. Active optics at the cell/hydrogel interface
As previously discussed, the various ways to engineer hydrogels and obtain optically relevant architectures for guiding and manipulating light in close contact with biological tissues have developed significantly over the past years. Nevertheless, the direct combination of hydrogels, cellular structures, and light-guidance is still under development and can further lead to new exciting applications. Initial approaches from different labs on light-guiding hydrogels have shown that light guiding can be simultaneously associated with cell encapsulation, such as in agarose waveguides with encapsulated cancer cells [74] or light-guiding PEG hydrogels with HeLa cells [44]. These reports represent two of the earliest studies where there was a direct combination between light-guiding hydrogels and cellular components. Simultaneously, on the other side of the spectrum, researchers have assembled E. Coli to obtain cell-only waveguides [139] (Figure 6a). More recently, it was demonstrated that cells in suspension could also guide light by obtaining cyanobacteria waveguides (Figure 6b). The suspended cells were shown to trap light beams, guiding light with reduced diffraction [140]. These experiments demonstrate that, unlike studies where cells had no influence on the construct’s light-guiding properties, they can take an active role by trapping and directing light.
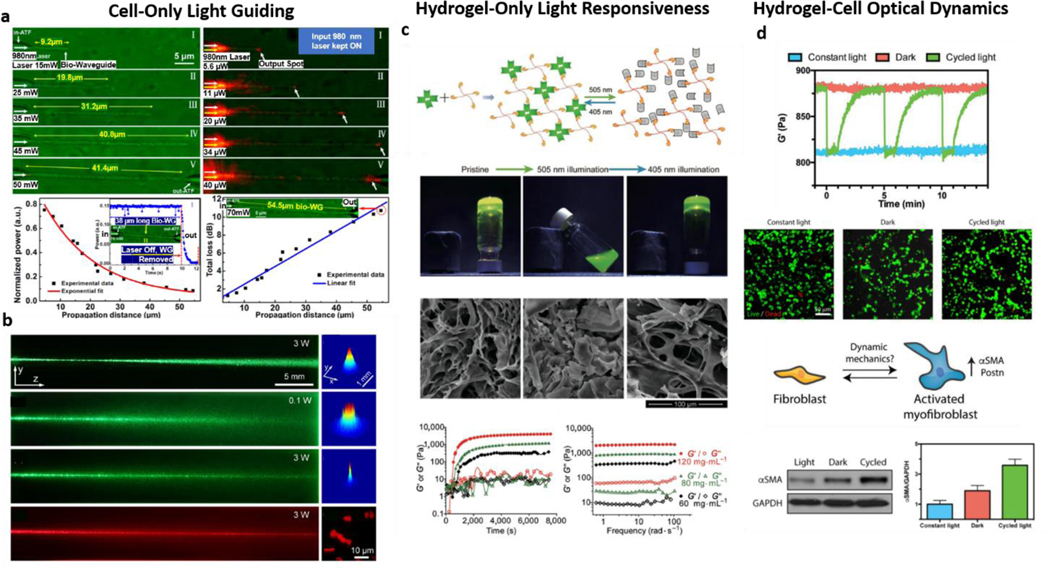
Figure 6: Active Cell/Hydrogel interfaces:
a| Cell-only optical waveguide structures made from E. Coli. Adapted with permission from ref. [139]. Copyright 2013, American Chemical Society. b| Cell-only waveguide of cyanobacteria in suspension. Adapted with permission from ref. [140]. Copyright 2017, American Physical Society. c| Hydrogel-only light-responsiveness can be engineered as demonstrated for polymeric gels, with light-induced gelling and mechanical switching. Adapted with permission from ref. [141]. Copyright 2018, Springer. d| Light effects in hydrogel and cells can be combined to translate optical signals into hydrogel mechanical dynamics and tune the phenotype of fibroblasts as an example of a living, light-responsive construct. Adapted with permission from ref [142]. Copyright 2018, Wiley.
Moreover, similar to hydrogels being highly hydrated meshes, one can envision that cells can be used to guide light in hydrogels, as demonstrated in water and glycerol suspensions [140]. Additionally, these studies resorted mostly to the use of bacteria. It was also recently shown that eukaryotic cells and their intracellular constituents also have very particular interactions with light [143]. Light-cell interaction becomes even more interesting as we consider that cells are highly active structures. As such, they might not only be able to manipulate light passively but also simultaneously respond to it, as observed by the capability of polarized light to affect cellular cytoskeleton organization [144]. Very recent evidence further hints at the possibility of genetically manipulating the optical properties (refractive index) of living cells by taking advantage of reflectins (proteins), which are natively responsible for the structural coloration of cephalopods [145]. These results suggest that exciting discoveries and applications of cell-light interactions will likely become a reality in the near future.
Further, hydrogels themselves can also be engineered to respond to light actively, leading to changes in crosslinking and influencing encapsulated cells. An excellent example of this process happens with hydrogels based on the photoswitchable protein dronpa. Light-based crosslinking changes can dynamically control the hydrogel formation and mechanical properties (Figure 6c), influencing the migration of cells [141,146]. Another recent example of protein-based, light-responsive environments was reported, where light-induced hydrogel mechanical changes could function as a cyclic mechanical loading platform [142]. Therein, this mechanical responsiveness to light induced myofibroblast activation (Figure 6d) [142]. Overall, there are very distinct ways to approach the interface between light guidance, cells, and hydrogels, and engineering active responses from both entities to light. The transition from passive light manipulation to the dynamic interaction between light, cells, and materials can lead to more exciting developments such as live hydrogel photonic structures. These constructs might inform on changes within the biological environment, simultaneously responding to it from a mechanical point of view and subsequent cell mechanotransduction.
Moreover, engineered hydrogel systems can present additional degrees of complexity and functionality, especially if they are also engineered to respond to other types of stimuli that can be combined with light interactions, as explored next.
6. Multi-responsive hydrogels in light-based applications
Hydrogels have been engineered to respond to a wide variety of stimuli, a topic which has been broadly discussed in the literature by others [147–150]. Here, we focus on the combination of hydrogel responsiveness with photonic applications. We previously discussed some responsive hydrogels and their swelling behavior in response to changes in, e.g., pH or upon the presence of analytes such as glucose. However, there are other stimuli that hydrogels have been engineered to respond to such as electrical, thermal, and magnetic, which can be combined with their optical characteristics. These stimuli can be interesting complementary approaches for multi-responsive detection and also for the fabrication and assembly of optically-relevant hydrogel constructs. [151]
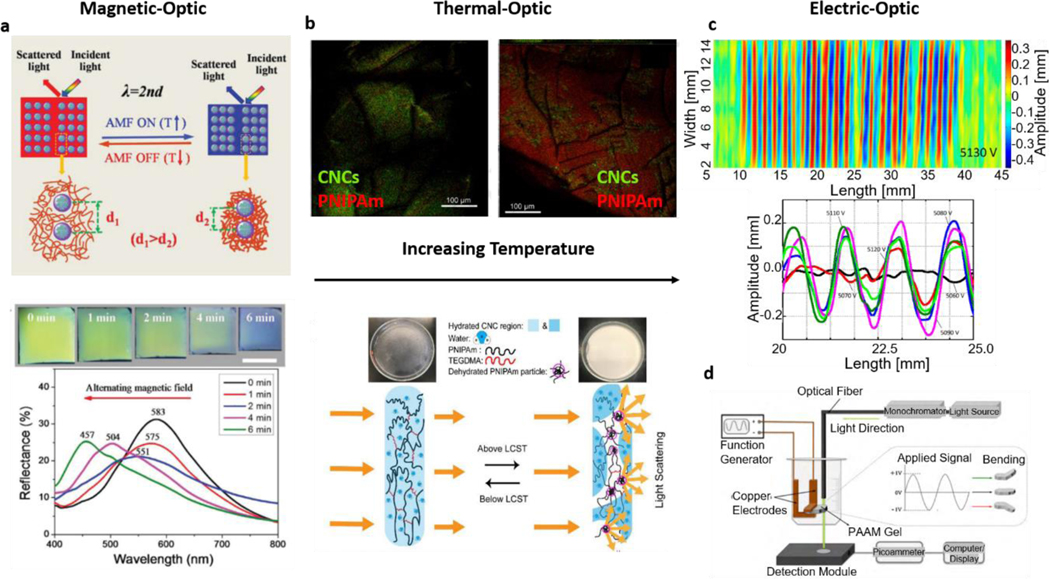
Regarding the combination of hydrogel photonics and magnetic responsiveness, there have been recent advances where magnetic-based manipulation of hydrogel structures was proven to be relevant for tuning optical properties. It was shown that by nanostructuring the hydrogels with magnetic particles, it was possible to arrange their orientation and lead to distinct shifts in the wavelength of the incident light, obtaining a photonic hydrogel structure which responded to alternating magnetic fields (Figure 7a) [152]. In this study, the previously discussed photonic hydrogel structures were combined with multi-responsiveness strategies. Similarly, it was shown that hydrogels can be patterned with magnetic regions, becoming inherently responsive to magnetic fields and simultaneously capable of changing the polarization plane of incident light [153]. In another study on polarization, coupled gelatin hydrogels with magnetically responsive bicelles were manipulated to polarize light via temperature changes. This work represents an engaging multi-responsive platform integrating distinct stimuli (magnetic, thermal, and optical) into a single construct [154]. Further, a multi-responsive platform that combined thermo-responsive hydrogels with gold nanorods and magnetic silica particles was reported to employ magnetic stimuli to control swelling/de-swelling states causing a detectable shift in the plasmonic light resonance [155]. Indeed, these studies present optically engineered hydrogels that might benefit from additional responsiveness, where distinct stimuli can then be recognized and translated into an optical change. Additionally, magnetic manipulation of hydrogels has been used for assembling complex structures in the biofabrication field [156,157], which can also be applied for hydrogel optical structuring. This magnetic manipulation can potentially be used to assemble different parts with heterogenous properties, leading to platforms with multi-stimuli responsiveness actuated by optical, magnetic, thermal, or chemical stimuli.
Figure 7: Multi-Responsive Hydrogel Photonics:
a| The conjugation of hydrogel magnetic responsiveness and optical structuring allows to modulate incident light wavelength by changing the structure via the changing magnetic field. Adapted with permission from ref. [152]. Copyright 2017, Wiley. b| Thermo-responsive PNIPAAm (Poly(N-isopropyl acrylamide) hydrogel films with cellulose nanocrystals can change their structure and shift their interaction with the light on temperatures above or below the critical value. Adapted with permission from ref. [158]. Copyright 2019, Elsevier. c| Electro-responsive hydrogels can change their shape and light interactions with varying voltage. Adapted under the terms of CC-BY license from ref. [159]. Copyright 2018, the authors. d| Electro-responsive hydrogels in devices capable of optical sensing electric field variations such as those in cardiac muscle contraction. Adapted with permission from ref. [160]. Copyright 2010, Elsevier.
Similarly, thermoresponsive hydrogels have been mostly used for temperature-induced gelation changes and consequent applications for drug and cell delivery [161,162]. However, temperature as a response-triggering stimulus has also been combined with photonic hydrogel engineering. For example, thin-film structures described in this review can be combined with thermoresponsive hydrogels leading to films where temperature can also affect the photonic properties and lead to changes in interacting light characteristics [163,164]. This property might be further applied to distinct hydrogel structures, namely particle-based systems [165] and films with transparency/opacity transitions above or below certain temperature levels (Figure 7b). Further, the fact that temperature can also play a role in the performance of optical-fiber structures suggests it can also be explored for additional functionality, e.g., in the proximity of tissues at body temperature levels [166].
Electrically responsive hydrogels have also gathered significant attention, namely as muscle tissue engineering platforms where electrical stimulation and conductivity are important [167,168]. Nevertheless, electro-responsive hydrogels brings another dimension to the range of stimuli that photonic hydrogel structures can respond to. Even though not widely explored yet, some interesting examples of this combination, such as temperature-responsive polymers with conductive particles, allowed researchers to obtain conducting hydrogel thin films [169]. Further, scientists combined hydrogel photonics and electric responsiveness towards applications in direct biomedical sensing [160]. This work reported electroactive polyacrylamide hydrogels that could respond to an electric field leading to a change in the light passing through the construct and facilitating the transduction of electrical signals for wireless optical detection (Figure 7d). More recently, researchers have also shown that hydrogel surface topography [159] and swelling states [170] could also lead to electrically-induced changes in the interaction with incident light (Figure 7c). This kind of electrical-induced changes can be coupled with well-established systems where surface topography is employed for highly sensitive and specific plasmonic sensing [171–173]. All these results show that the combination of electrically responsive hydrogels and photonics is relevant for approaches to analyze electrical changes such as those happening in muscle tissues. Alternatively, it can allow using electrical fields to manipulate light-hydrogel interactions within sensing devices and platforms. Simultaneously, progress is being made on incorporating graphene - known for extraordinary electrical and optical performance [174,175] – within hydrogels [176]. Exciting advances in electro-optical platforms will likely derive new application through these advances in the future.
7. Light-driven hydrogel robots
Engineering hydrogels can translate optical signals not only into bulk mechanics or structural changes but also into motion, i.e., hydrogel robots - particularly microrobots. Inducing controlled movement in small structures is very interesting for biomedical applications due to the possibility of moving and transporting biologically-relevant cargo to locations that are typically hard to reach within an organism [177]. Additionally, integration of cells with hydrogels and their manipulation towards motion can further lead to living robots [178,179]. Overall, these are very lively fields of research, and the topic of soft microrobots has been extensively reviewed throughout recent years [180–183]. As such, here, we focus our discussion on the recent advances in light-driven hydrogel robots.
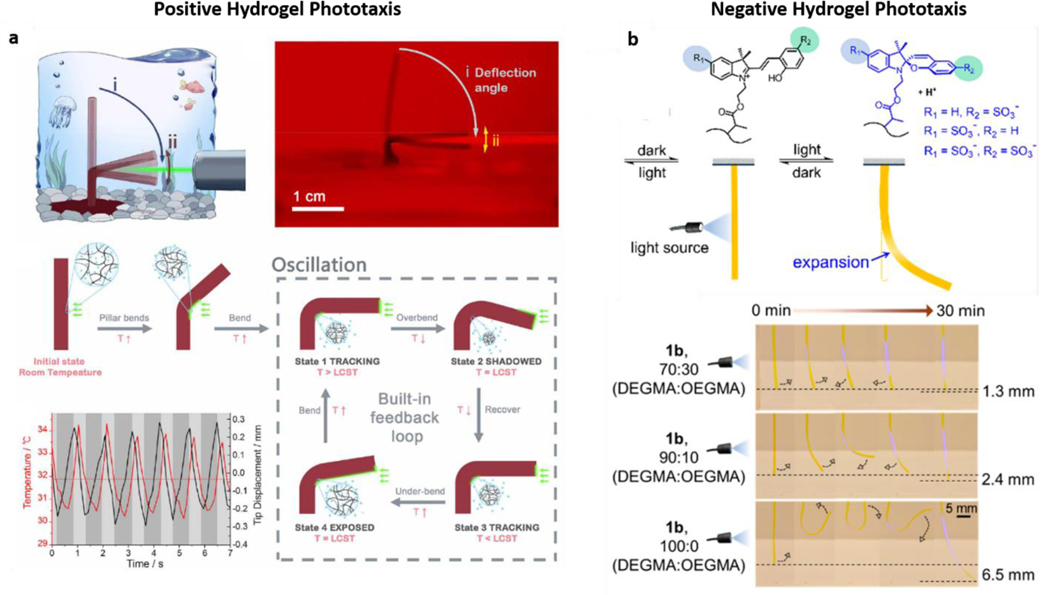
As previously described, hydrogels can respond to multiple stimuli, sometimes even in a single structure. Naturally, these distinct responses have been exploited to create motion in hydrogel structures via, e.g., pH responsiveness and magnetic stimuli [184,185]. However, it is possible to observe cases where light and hydrogel interaction is the main driving force for hydrogel motion and robotic function. Moreover, motion can be obtained with light alone, coupled with thermal responsiveness and light-triggered plasmonic heating. Indeed, a large volume of recent research has focused on combining thermo-responsive hydrogels with optically active structures, such as gold nanoparticles, for inducing light-triggered changes in the local hydrogel environment. The most common example of such systems employs PNIPAAm (Poly(N-isopropyl acrylamide) hydrogels, which encapsulate gold particles that lead to an increase in temperature upon exposure to visible light. This temperature increase causes the hydrogel to contract towards the light (positive phototaxis) (Figure 8a) [186]. Different variations of this strategy have been reported in the past year, such as gold nanopatterned-disks that wrinkle at the air-water interface when exposed to light [187], spiral-shaped structures which move through light-controlled spinning [188], and photo-responsive microcrawlers motioned by light-modulated friction [189]. Researchers have recently combined these advances with 3D printing technology to print PNIPAAm/Gold nanorod actuators with designer shape and improved resolution [190]. Similar hydrogels have also been previously integrated within microfluidic devices [191,192].
Figure 8: Light-Induced Hydrogel Motion:
a| Thermo-responsive PNIPAAm hydrogels containing plasmonic gold structures can respond to light by the local increase in temperature through gold-photon interactions, contracting the gel and leading to positive (towards light) phototaxis. Reproduced with permission from ref. [186]. Copyright 2019, AAAS. b| Distinctly, specific molecular switches can be integrated within hydrogels, directly responding to light and causing a local expansion of the hydrogel mesh, leading to motion against the light source (negative phototaxis). The integration of both behaviors can lead to 180o motion ranges capable of moving both towards and away from light. Adapted with permission from ref. [193]. Copyright 2020, American Chemical Society.
Overall, combination of PNIPAAm hydrogels and heat-generating gold plasmonic structures dominates the current landscape of light-driven hydrogel robots. However, there are also exciting advances in developing gels that can directly respond to light without plasmonic-based photothermal changes. This possibility was explored by using molecular switches that, unlike the previously discussed examples, can trigger a volumetric expansion in the hydrogels (instead of contraction) [193]. This difference in behavior leads to a relatively novel type of negative phototaxis, where motion happens away from and not towards light [193] (Figure 8b). On another exciting approach, scientists took inspiration from aquatic invertebrates to create liquid crystal gels (LCGs), which can molecularly respond to light and bend through photothermal changes that can be translated into crawling, walking, jumping, and swimming [194]. Even though these structures differ significantly from classical hydrogels, it is interesting to see how the combination of specific molecular changes and their incorporation into hydrogel meshes can be employed. These approaches can soon replace the need to incorporate plasmonic structures or even bypass the need for thermal changes and obtain purely light-responsive hydrogel robots. Finally, it is also relevant to mention that light-responsive hydrogels might not necessarily be used to fabricate microrobots but rather serve as the 3D environments for motion. This phenomenon was demonstrated with light-controlled local hydrogel melting and consequent induction of traveling-wave motions in elastomer robots [195].
8. Hydrogels in photomedicine
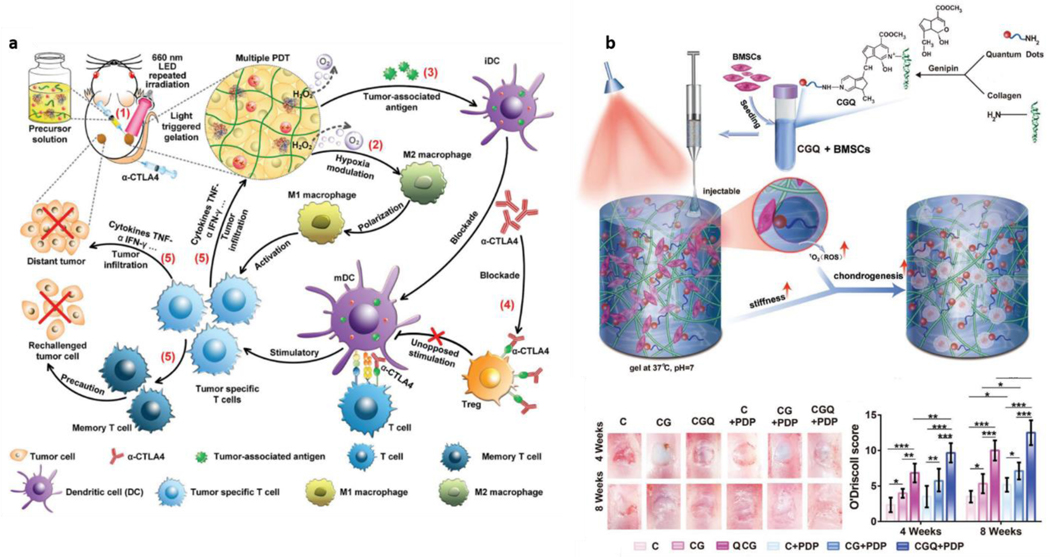
Focusing on the biomedical use of hydrogels as light-interacting entities, we also discuss the field of photomedicine, where hydrogels are used as entities that can aid or potentiate light-driven therapeutics. These therapeutic strategies usually gravitate around photodynamic events, i.e., events that can be modulated by light, such as Photodynamic Therapy (PDT), already established at the clinical level for cancer treatment [196,197]. Photodynamic strategies integrate a broad set of approaches that aim to destroy unwanted biological entities, such as bacteria or tumor cells. Recent advances in this field have taken advantage of hydrogels as structures that can carry other photo-responsive and photo-active structures, respond themselves to light, or combine both. The topic of photodynamic therapies was broadly reviewed elsewhere [198–201]. Here, we will explore how hydrogels have been engineered as a tool for optical modulation of therapeutically relevant strategies.
In the case of antibacterial- and antimicrobial-focused approaches, hydrogels are frequently used as a passive carrier for the transport and release of photosensitizers - molecules that can increase the sensitivity of specific bacterial strains to light (e.g., methylene blue) [202–204]. These approaches can take advantage of hydrogels such as chitosan, with natural anti-bacterial properties [205]. However, more interesting for this review’s scope are strategies wherein hydrogel interactions with light can be weaponized to kill unwanted microbes and promote healthy tissue healing. An excellent example of this duality is a study that combined thermoresponsive hydrogels with copper/silica nanoparticles. Upon interaction with near-infrared (NIR) light, these constructs experienced an increase in temperature (photothermal) combined with a release of radical oxygen species (ROS) (photodynamic) and of copper ions, which directly stimulated cell proliferation and angiogenesis (pro-regenerative) [206]. This work is an example of a complete synergy between killing bacteria and promoting healthy cell responses. This process was controlled by NIR light and consequent volumetric changes on the hydrogel composites [206]. Photodynamic anti-bacterial therapies have also been approached by combining peptide self-assembly with photosensitizer molecules. In such strategies, the hydrogel structure itself is reinforced by the photosensitizer’s presence, allowing the latter to be locally delivered for improving responses, e.g., wound-healing outcomes [205]. As reviewed elsewhere, self-assembling biomolecular hydrogels have gained significant traction for phototherapy in recent years [207].
In anti-tumor strategies, a vast body of literature focuses on using various hydrogels to enhance light-driven therapeutics. Similar to anti-bacterial approaches, some of these strategies share standard features and employ hydrogels to efficiently deliver photosensitizers for improving light-induced killing of cancer cells [208–213]. Further, another recent avenue of studies focuses on killing cancer cells and stimulating anti-tumor immunogenic responses. In these approaches, hydrogels were used to transport structures of interest, such as drug-carrying nanosheets [214], or taking advantage of oxygen-generating events to revert intratumoral hypoxia [215]. Light-driven changes in gelation have also been recently studied to enhance therapeutic approaches. With that in mind, researchers engineered PEGDA-based solutions, which could be injected in the vicinity of solid tumors, and posteriorly form a gel upon light actuation. The authors were then able to fixate the whole structure in the desired region to deliver toxic radical oxygen species (ROS) and pro-immunogenic nanoparticles simultaneously [216], resulting in a complex network of tumor-suppressive events (Figure 9a). On another approach, researchers created a method where the light was delivered to the tumor site by integrating luminescent materials within calcium alginate hydrogels [217]. These hydrogels fixated in the desired region, persistently releasing light and leading to continuous ROS production and significant tumor size decreases [217].
Figure 9: New Paradigms in Hydrogel Photomedicine:
a | Engineered PEGDA precursor solutions with pro-immunogenic entities can be locally immobilized through light-induced hydrogel crosslinking to modulate a wide variety of tumor-suppressing events. Reproduced with permission from ref. [216]. Copyright 2019, Wiley. b | Similar light-hydrogel interactions can be employed on collagen-based hydrogels to direct mechanical stiffening and ROS production towards cell proliferation, chondrogenic differentiation, and improve cartilage healing. Adapted under the terms of CC-BY license from ref. [218]. Copyright 2019, the authors.
Even though most of the discussed studies use hydrogel-light interactions to kill certain biological entities, there are other recent approaches where the goal is quite the opposite: to potentiate cell proliferation and differentiation towards achieving benign phenotypic conversion for tissue engineering and regeneration purposes. In two distinct studies, collagen hydrogels were engineered to change in mechanical properties upon a light trigger, causing an increase in hydrogel stiffness combined with moderate ROS release. ROS were shown not to be cytotoxic at that level but instead favored the chondrogenic commitment of mesenchymal stem cells [218,219], significantly improving cartilage healing (Figure 9b). These two recent studies demonstrate that light-responsive structures can synergize with the hydrogels for complex chemical changes, mechanics, and release of molecules. As shown by these advances, this process can have applications that go far beyond killing unwanted cells and instead be tuned so that cytotoxic effects turn into cyto-compatible and bioactive events. Future studies exploring these concepts will likely yield exciting phototherapy solutions where light stimuli and hydrogels can heal damaged tissues. This process might not only happen by removing unwanted biological players but also by actively promoting phenotypic reversion or regeneration through cell proliferation and guided stem cell differentiation.
9. Hydrogel-light interactions for organ-on-chip applications
Recent advances in hydrogels and microfluidics enable recapitulating critical physiological events in miniaturized platforms. These platforms frequently referred to as organ-on-chip or even body-on-chip, are fascinating alternatives to simpler models such as 2D static cultures that lack important architectural and biological tissue dynamics. Organ-on-chip models present potential advantages over more complex animal models, as animals have fundamentally different physiological characteristics compared to human organs and tissues.
In this field, hydrogels have been used as important components to miniaturize cell culture compartments with 3-dimensional arrangements as complex and vascularized structures or bone marrow niches [220–222]. Within this field, hydrogel light interactions can be employed for two essential processes: The first is the fabrication of such intricate architectures and biologically relevant 3D niches. The second focuses on integrating the fast, label-free optical on-chip sensing to detect and track critical biological events.
Fabrication-wise, the most exciting advances in light-based hydrogel uses hydrogel precursors within microfluidic chips and takes advantage of light-based crosslinking to generate 3D structures with controlled shape and architecture. Using this approach, PEGDA and GelMA hydrogels were arranged through flow alone and led to intricate shapes without needing light-covering masks [223]. By shining light to crosslink hydrogel precursors in specific stages of flow, researchers were able to mimic 3D arrangements of distinct tissues such as tumor, muscle, and tendon-bone interfaces (Figure 10a). Indeed, the potential for light-based laser-writing might have further applications in the organ-on-chip context. Recently, optical manipulation of drug-containing particles combined with light-based hydrogel writing was employed to engineer tunable drug cocktails [224]. This strategy can be utilized to obtain on-chip drug depots with varying characteristics allowing the combination of 3D environment, cell culture, and drug testing, all with controllable shape and positioning. Further, light-based hydrogel crosslinking and manipulation have proven to be valuable tools for fabricating 3D gradients that combine ranges of chemical and mechanical properties for faster (high-throughput) screening of cellular responses to the surrounding environment [225,226]. Such approaches to modulate the 3D microenvironment of cells can be further explored to recreate naturally space-varying niches such as hematopoietic stem cells within microfluidic devices [227]. Additionally, hydrogels might be manipulated by light to recreate environments within which cells can be integrated and serve as translucent 3D platforms wherein cells can be exposed to light to promote differentiation towards specific phenotypes. This idea was earlier utilized to obtain neural-muscular motor units on-a-chip [228].
Figure 10: Light-hydrogel interactions for on-chip applications:
a| Manipulation of hydrogel precursors within microfluidic devices combined with light-based crosslinking to fabricate on-chip biologically relevant architectures such as b| Tumor, muscle, and tendon-bone interfaces. Adapted with permission from ref. [223]. Copyright 2018, Wiley. c| Integration of hydrogel waveguides within PDMS chips for light guiding and multi-output detection. Adapted under the terms of CC-BY license from ref. [229]. Copyright 2019, the authors.
Even though light-hydrogel interactions have led to exciting advances in microfabricating 3D environments for chip-based cell culture, hydrogel photonics might have a more significant role to play. Indeed, it can facilitate integration of fast, label-free optical sensing and detection within microfluidic chips of higher design complexity. At present, microfluidics and organ-on-chip techniques rely mostly on imaging, image processing, and analysis to derive quantitative data from biological assays [230,231]. Nevertheless, this process is likely to change once hydrogel photonic structures come into play to obtain real-time readouts without needing for fixation, staining, or other sample-destructive procedures. Even if this possibility is still explored little, recent studies demonstrate its feasibility. Some of these studies are close to the field of optofluidics, where micro-optical events are combined with microfluidic structures. On such an approach, researchers introduced PEGDA microlenses on microfluidic chips, showing that these hydrogel structures could react to presence of salts, changing their shape and leading to a shifting of focal planes, which could be used to evaluate the concentrations of analytes of interest [232]. It was also demonstrated that PEGDA waveguides could be integrated into PDMS structures [229]. Therein, the authors showed that PEGDA waveguides were capable of guiding light through the 3D PDMS structures; function as beam splitters with multiple outputs, and possibly facilitate the measurement of relevant on-chip events (Figure 10b).
Overall, even if some of these recent approaches for responsive hydrogel structures are integrated within microfluidic chips and have been effectively used to obtain fast optical readouts, they are not yet integrated within organ-on-chip systems. Together, these earlier microfluidic studies pave the way for combining photonic structures with miniaturized 3D biological architectures. The possibility of having real-time sensing through optical hydrogel components in organ-on-chip applications is likely to emerge in the near future.
10. Conclusions and outlook
Over the past decade, we have observed considerable advances in engineering of hydrogels towards photonic biomedical applications. These hydrogels emerge as complementary materials within the biomedical scope to the solid-state materials that are classically used for light guiding and manipulation applications. Hydrogels represent a safer, biocompatible, and adaptable platform technology for efficient optical interactions and contact with cells, tissues, and organs.
Overall, a wide range of polymers has been utilized for this purpose, both from synthetic and natural origins (Table 1). On the one hand, synthetic polymers have a higher degree of purity and capability to be modified and tuned to enhance optical or biological performance. On the other hand, natural polymers are more variable and harder to modify, but were also presented as good candidates in photonic structures due to potential advantages related to degradability and biological compatibility. Nevertheless, both branches of polymers have been shown to have equally important roles in hydrogel photonics. As we keep moving forward, we will potentially see some of these polymers being replaced by smarter engineered alternatives that can further push the optical performance of photonic systems, such as guiding characteristics. This advance can occur by modifying refractive indexes by decreasing water content to lower levels while maintaining cytocompatibility. Also, in a hydrogel-fabrication landscape that is still dominated by UV-based crosslinking, future studies will likely focus on distinct crosslinking methods. For example, visible-light crosslinking can lead to similar structures like those reported with UV but with higher biological safety, e.g., for encapsulating cells within constructs [233,234]. Other types of crosslinking, such as ionic and supramolecular assembly, represent more recent alternatives that can become substantial players in hydrogel photonics.
In addition to the choice of material, the construct architecture dictates the capabilities of these materials to interact with light for guiding and manipulating its properties towards sensing. Here, we identified four main types of optical hydrogel structures and respective fabrication techniques (Figure 2). Thin films are the earliest used constructs for hydrogel photonics, and these structures can be obtained mainly through either layer-by-layer or single-step deposition techniques. Thin hydrogel films have been engineered and adapted to various scenarios but commonly represent platforms used on surfaces interfacing air outside of biological environments. However, thin films are still extremely efficient in detecting biologically relevant targets such as glucose, changes in pH, or heavy metals. Despite being a part of the hydrogel photonic landscape for a long time, there are recent creative ways to use thin films and to combine distinct responsiveness for fabricating, e.g., touchless screens. These recent breakthroughs will potentially have broad implications for the future of electronic devices and interactive surfaces and their multitude of applications.
Hydrogel optical fibers pose more complex 3D structures than these films and they can transport light across considerable lengths. They have been engineered to respond and shift light properties from their interaction with several analytes of interest. Interestingly, most hydrogel optical fibers have focused on similar materials and relatively simple fabrication approaches. On that note, we expect significant advances in hydrogel fiber-optics as complex hydrogel fiber biofabrication methods merge towards the optical field. This translation will likely improve optical fiber performance and increase complexity in both shape and stimuli responsiveness. Similarly, 3D printing of hydrogels has presented remarkable developments in tissue engineering and regenerative medicine in recent years. Few studies have explored the possibility of combining the precise material deposition and organization of 3D printing technologies with optically relevant shapes, where novel types of optical inks can introduce light-guiding channels and conduits within complex 3D printed constructs [69,80].
As an alternative at smaller dimensions, nanostructured hydrogels have been shown to recapitulate the structural coloring found in nature. Similarly, by engineering hydrogels, scientists created structurally colored arrangements that can be assembled into complex optical structures that can actively respond to deformation. These constructs have been explored for tracking forces such as those made by contractile cells in novel real-time mechano-optical transduction platforms and living robots. Further, structuring of hydrogels and their active optical arrangement have been applied for holographic imaging and recapitulating shapes and information, which can be useful for bio medically relevant data storage and encryption.
One other important point we outlined is the need for hydrogel photonics to be combined with living entities such as encapsulated cells. Even though cells are considered optically inert in multiple studies, there have been others where cells are considered as an integral part of the active optical entity, e.g., biological waveguides. Simultaneously, hydrogels might not only passively guide light but also respond to it, as in reversible cross-linking events. Combined, the possibility of having light not only guided but also actively shaping the hydrogel/cell interface will likely lead to exciting advances in hydrogel optics. When these optics are fully integrated with live constructs, light can direct materials and cells towards distinct mechanical states and cellular phenotypes. At the same time, optical readouts might facilitate real-time screening. These readouts can inform on complex cellular events such as proliferation, differentiation, and matrix deposition, or even assess drug efficacy in 3D engineered tissues.
Further, we explored the possibility of combining hydrogel photonics with multi-responsive hydrogel systems, taking advantage of the years of scientific research in the topic. Therein, we presented examples of hydrogel photonic responsiveness combined with magnetic, thermal, and electric stimuli. The combination of these distinct responses in a single structure is shown to be possible and will likely allow systems to respond to and inform on a plethora of stimuli. These can be further leveraged towards assembling and structuring of complex hydrogel constructs as sensors.
Moreover, combination of multiple responses in hydrogel structures has also enabled impressive light-driven locomotion capabilities. Hydrogel robots have emerged as capable tools with motion omnidirectionality and exciting possibilities in micro-robotics which might reach clinical applications soon. Overcoming the challenge of combining both negative and positive hydrogel phototaxis within the same structure is likely the next step to obtain more comprehensive motion ranges of these soft, biocompatible structures.
We also discussed how the combination and engineering of multi-responsive hydrogel systems led to recent advances in photomedicine. In this scope, light actuation as a therapeutic trigger has been shown to modulate complex tumor microenvironmental events. Further, light-based therapies can go far beyond simple cancer cell death. These complex photosystems with aggressive anti-cancer effects might be tuned down and converted into healthy stem-cell friendly tools. Also, the possibility of actively promoting tissue regeneration with these systems is opening up an entirely new avenue of investigations.
As a final topic, we explored the field of organ-on-a-chip technologies, which have yielded complex biological models in recent years, with proven applications in drug testing and optimization. Hydrogels, commonly used as 3D environments within these systems, can be leveraged for designing intricate shapes and architectures on-chip via hydrogel precursor flow and controlled light-based crosslinking. Moreover, the integration of photonic hydrogel structures within on-chip models can potentially reveal a game-changing addition to the field by enabling rapid, label-free detection of on-chip events in real-time. This advance is likely to add entirely a new time-dependent dimension to the collected information, bringing the science ever closer to modelling the actual physiological microenvironment as a complementary alternative to animal models.
Globally, under the light of all recent advances and the broadening scope of emerging ideas and applications, the field of hydrogel photonics has a promising long path forward as an emerging field with broad applications that span from biosensing to tissue models. Additionally, the close relationship between the materials and the fabrication techniques, accelerated by advances in these fields across interdisciplinary areas, will likely bring along exciting new engineered photonic structures and innovative applications. Optical platforms are likely to remain - as light itself - the fastest and most efficient solutions for biomedical detection, sensing, and screening. Transposing these platforms towards living environments is becoming a reality through engineering hydrogels as photonic materials cutting across multiple disciplines. As we observe the advances in this promising field currently, hydrogels are continuing to emerge as biologically compatible alternatives or complementary tools for the creation of next generation of living tissue photonics, spanning broad applications from scientific experiments on the benchtop to serving the needs of patients at the clinic or at the point of care in the future.
Acknowledgments
The authors acknowledge support from the Stanford Clinical and Translational Science Award (CTSA) to Spectrum. The CTSA program is led by the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH). The authors would further acknowledge NIH grants number U01FD005978, R01 EB029805, R01 DE024971, R01 AI120683. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors acknowledge further support from Stanford RISE COVID-19 Crisis Response Faculty Seed Grant Program.
CFG acknowledges support from Fundação para a Ciência e Tecnologia (Grant no. PD/BD/135253/2017) as well as Fundação Luso-Americana para o Desenvolvimento (FLAD).
Prof. Utkan Demirci (UD) is a founder of and has an equity interest in: (i) DxNow Inc., a company that is developing microfluidic IVF tools and imaging technologies, (ii) Koek Biotech, a company that is developing microfluidic technologies for clinical solutions, (iii) Levitas Inc., a company focusing on developing microfluidic sorters using magnetic levitation, and (iv) Hillel Inc., a company bringing microfluidic cell phone tools to home settings.
Biography

Carlos Guimarães is a Bioengineer (M.Eng.) pursuing his PhD at 3B’s Research Group, I3Bs – Research Institute on Biomaterials, Biodegradables and Biomimetics, University of Minho (Portugal), in collaboration with Canary Center at Stanford for Cancer Early Detection, Stanford University School of Medicine (USA). He obtained his Master’s degree in Biotechnology from the University of Porto in 2017. His current research focuses on the microfluidic manipulation of hydrogels as a versatile fabrication tool for micro tissue-engineering, high-throughput biomaterial discovery, and biomedical detection applications.

Rajib Ahmed is currently working as a postdoctoral fellow at Stanford University School of Medicine, Canary Center at Stanford for Cancer Early Detection. He received his B.Sc. and M.Sc. degrees from the university of Dhaka in 2010 and 2012, and also studied a double degree M.Sc. in Aston University and Technische Universitat Berlin 2013-2014. He received his Ph.D. degree from the University of Birmingham in 2018. His current Research Focuses on micro- and nanotechnologies-based biomedical optical devices for virus and cancer detection.

Rui L. Reis is a Full Professor of Tissue Engineering, Regenerative Medicine, Biomaterials and Stem Cells at University of Minho (UMinho). He is also the Director of the 3B’s Research Group of I3Bs – Research Institute on Biomaterials, Biodegradables and Biomimetics, Portugal. His group has been working on the development of biomaterials from natural polymers for engineering the regeneration of several tissues, as well as biofabrication and tissue engineering approaches for complex 3D models of health and disease.

Utkan Demirci is a Professor with tenure at Stanford University School of Medicine and serves as the co-director at the Canary Center for Cancer Early Detection of the Department of Radiology. His group focuses on developing innovative microfluidic biomedical platforms with broad applications to multiple diseases. Some of his inventions have already been translated into FDA approved products serving patients. He has mentored and trained many successful scientists, entrepreneurs, and academicians.
Footnotes
Conflicts of Interest
UD’s interests were viewed and managed in accordance with the conflict of interest policies.
References
- [1].Zhang YS, Khademhosseini A, Science (80-. ). 2017, DOI 10.1126/science.aaf3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Peppas NA, Hilt JZ, Khademhosseini A, Langer R, Adv. Mater 2006, DOI 10.1002/adma.200501612. [DOI] [Google Scholar]
- [3].Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A, Biomaterials 2015, DOI 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA, Adv. Mater 2009, 21, 3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang Y, Biomaterials 2018, 178, 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kamata H, Li X, Il Chung U, Sakai T, Adv. Healthc. Mater 2015, 4, 2360. [DOI] [PubMed] [Google Scholar]
- [7].Seliktar D, Science (80-. ). 2012, 336, 1124. [DOI] [PubMed] [Google Scholar]
- [8].Geckil H, Xu F, Zhang X, Moon S, Demirci U, Nanomedicine 2010, 5, 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu X, Liu J, Lin S, Zhao X, Mater. Today 2020, 36, 102. [Google Scholar]
- [10].Liu H, Wang Y, Cui K, Guo Y, Zhang X, Qin J, Adv. Mater 2019, 31, 1. [DOI] [PubMed] [Google Scholar]
- [11].Verhulsel M, Vignes M, Descroix S, Malaquin L, Vignjevic DM, Viovy JL, Biomaterials 2014, 35, 1816. [DOI] [PubMed] [Google Scholar]
- [12].Kirschner CM, Anseth KS, Acta Mater. 2013, 61, 931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rizwan M, Yahya R, Hassan A, Yar M, Azzahari AD, Selvanathan V, Sonsudin F, Abouloula CN, Polymers (Basel). 2017, 9, DOI 10.3390/polym9040137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Langer R, Vacanti JP, Science (80-. ). 1993, 260, 920. [DOI] [PubMed] [Google Scholar]
- [15].Lim F, Sun AM, Science (80-. ). 1980, DOI 10.1126/science.6776628. [DOI] [Google Scholar]
- [16].Raemdonck K, Demeester J, De Smedt S, Soft Matter 2009, 5, 707. [Google Scholar]
- [17].Nochi T, Yuki Y, Takahashi H, Sawada SI, Mejima M, Kohda T, Harada N, Kong IG, Sato A, Kataoka N, Tokuhara D, Kurokawa S, Takahashi Y, Tsukada H, Kozaki S, Akiyoshi K, Kiyono H, Nat. Mater 2010, 9, 572. [DOI] [PubMed] [Google Scholar]
- [18].Zhao C, Yuan G, Jia D, Han CC, Soft Matter 2012, 8, 7036. [Google Scholar]
- [19].Lyon LA, Meng Z, Singh N, Sorrell CD, John AS, Chem. Soc. Rev 2009, 38, 865. [DOI] [PubMed] [Google Scholar]
- [20].Eggermont LJ, Rogers ZJ, Colombani T, Memic A, Bencherif SA, Trends Biotechnol. 2020, 38, 418. [DOI] [PubMed] [Google Scholar]
- [21].Cascone S, Lamberti G, Int. J. Pharm 2020, 573, 118803. [DOI] [PubMed] [Google Scholar]
- [22].Naghii MR, Nutr. Health 2000, 14, 127. [DOI] [PubMed] [Google Scholar]
- [23].Guimarães CF, Gasperini L, Marques AP, Reis RL, Nat. Rev. Mater 2020, 5, 351. [Google Scholar]
- [24].Hoffman AS, Adv. Drug Deliv. Rev 2012, 64, 18. [Google Scholar]
- [25].Nicodemus GD, Bryant SJ, Tissue Eng. - Part B Rev 2008, 14, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tan WH, Takeuchi S, Adv. Mater 2007, 19, 2696. [Google Scholar]
- [27].Vermonden T, Censi R, Hennink WE, Chem. Rev 2012, 112, 2853. [DOI] [PubMed] [Google Scholar]
- [28].Eddington DT, Beebe DJ, Adv. Drug Deliv. Rev 2004, 56, 199. [DOI] [PubMed] [Google Scholar]
- [29].Karadaǧ E, Saraydin D, Çaldiran Y, Güven O, Polym. Adv. Technol 2000, 11, 59. [Google Scholar]
- [30].Hoffman AS, in Am. Chem. Soc. Polym. Prepr. Div. Polym. Chem, 1990. [Google Scholar]
- [31].Leith EN, Upatnieks J, J. Opt. Soc. Am 1962, DOI 10.1364/josa.52.001123. [DOI] [Google Scholar]
- [32].Denisyuk YN, Dokl. Akad. Nauk SSSR 1962, 144, 1275. [Google Scholar]
- [33].Caló E, Khutoryanskiy VV, Eur. Polym. J 2015, 65, 252. [Google Scholar]
- [34].Tavakoli J, Tang Y, Polymers (Basel). 2017, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Singh SR, Biomed. J. Sci. Tech. Res 2018, 5, 4302. [Google Scholar]
- [36].Choi JR, Yong KW, Choi JY, Cowie AC, Biotechniques 2019, 66, 40. [DOI] [PubMed] [Google Scholar]
- [37].Umar M, Min K, Kim S, APL Photonics 2019, 4, DOI 10.1063/1.5122780. [DOI] [Google Scholar]
- [38].Yan R, Gargas D, Yang P, Nat. Photonics 2009, 3, 569. [Google Scholar]
- [39].Lu L, Joannopoulos JD, Soljačić M, Nat. Photonics 2014, 8, 821. [Google Scholar]
- [40].Yao J, J. Light. Technol 2009, 27, 314. [Google Scholar]
- [41].Li Z, Leustean L, Inci F, Zheng M, Demirci U, Wang S, Biotechnol. Adv 2019, 37, 107440. [DOI] [PubMed] [Google Scholar]
- [42].Mejía-Salazar JR, Oliveira ON, Chem. Rev 2018, 118, 10617. [DOI] [PubMed] [Google Scholar]
- [43].Kim M, Choi Y, Yoon C, Choi W, Kim J, Park QH, Choi W, Nat. Photonics 2012, 6, 581. [Google Scholar]
- [44].Choi M, Choi JW, Kim S, Nizamoglu S, Hahn SK, Yun SH, Nat. Photonics 2013, 7, 987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gambling WA, IEEE J. Sel. Top. Quantum Electron 2000, 6, 1084. [Google Scholar]
- [46].Nizamoglu S, Gather MC, Yun SH, Adv. Mater 2013, 25, 5943. [DOI] [PubMed] [Google Scholar]
- [47].Born M, Wolf E, Hecht E, Phys. Today 2000, DOI 10.1063/1.1325200. [DOI] [Google Scholar]
- [48].Bolin FP, Preuss LE, Taylor RC, Ference RJ, Appl. Opt 1989, DOI 10.1364/ao.28.002297. [DOI] [PubMed] [Google Scholar]
- [49].Taffoni F, Formica D, Saccomandi P, Di Pino G, Schena E, Sensors (Switzerland) 2013, 13, 14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cibula E, Donlagic D, Stropnik C, Proc. IEEE Sensors 2002, 1, 711. [Google Scholar]
- [51].Khademhosseini A, Langer R, Biomaterials 2007, 28, 5087. [DOI] [PubMed] [Google Scholar]
- [52].Canadas RF, Ren T, Tocchio A, Marques AP, Oliveira JM, Reis RL, Demirci U, Biomaterials 2018, 181, 402. [DOI] [PubMed] [Google Scholar]
- [53].Canadas RF, Ren T, Marques AP, Oliveira JM, Reis RL, Demirci U, Adv. Funct. Mater 2018, 28, DOI 10.1002/adfm.201804148. [DOI] [Google Scholar]
- [54].Zhang X, Guan Y, Zhang Y, Biomacromolecules 2012, 13, 92. [DOI] [PubMed] [Google Scholar]
- [55].Tokarev I, Tokareva I, Gopishetty V, Katz E, Minko S, Adv. Mater 2010, 22, 1412. [DOI] [PubMed] [Google Scholar]
- [56].Knight JC, Birks TA, Russell PSJ, Atkin DM, Opt. Lett 1997, 22, 484. [DOI] [PubMed] [Google Scholar]
- [57].Cruz-Acuña R, Quirós M, Farkas AE, Dedhia PH, Huang S, Siuda D, García-Hernández V, Miller AJ, Spence JR, Nusrat A, García AJ, Nat. Cell Biol 2017, 19, 1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cruz-Acuña R, García AJ, Matrix Biol. 2017, 57–58, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Guo J, Liu X, Jiang N, Yetisen AK, Yuk H, Yang C, Khademhosseini A, Zhao X, Yun SH, Adv. Mater 2016, 28, 10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yetisen AK, Montelongo Y, Da Cruz Vasconcellos F, Martinez-Hurtado JL, Neupane S, Butt H, Qasim MM, Blyth J, Burling K, Carmody JB, Evans M, Wilkinson TD, Kubota LT, Monteiro MJ, Lowe CR, Nano Lett. 2014, 14, 3587. [DOI] [PubMed] [Google Scholar]
- [61].Pathak AK, Singh VK, Opt. Fiber Technol 2017, 39, 43. [Google Scholar]
- [62].Elsherif M, Hassan MU, Yetisen AK, Butt H, Biosens. Bioelectron 2019, 137, 25. [DOI] [PubMed] [Google Scholar]
- [63].Zhou C, Heath DE, Sharif ARM, Rayatpisheh S, Oh BHL, Rong X, Beuerman R, Chan-Park MB, Macromol. Biosci 2013, 13, 1485. [DOI] [PubMed] [Google Scholar]
- [64].Zhou M, Guo J, Yang C, Sensors Actuators, B Chem. 2018, 264, 52. [Google Scholar]
- [65].Choi M, Humar M, Kim S, Yun SH, Adv. Mater 2015, 27, 4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yetisen AK, Jiang N, Fallahi A, Montelongo Y, Ruiz-Esparza GU, Tamayol A, Zhang YS, Mahmood I, Yang SA, Kim KS, Butt H, Khademhosseini A, Yun SH, Adv. Mater 2017, 29, DOI 10.1002/adma.201606380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hu W, Wang Z, Xiao Y, Zhang S, Wang J, Biomater. Sci 2019, 7, 843. [DOI] [PubMed] [Google Scholar]
- [68].Lu L, Yuan S, Wang J*, Shen Y, Deng S, X. L. and Yang Q, Curr. Stem Cell Res. Ther 2018, 13, 490. [DOI] [PubMed] [Google Scholar]
- [69].Fu F, Chen Z, Zhao Z, Wang H, Shang L, Gu Z, Zhao Y, Proc. Natl. Acad. Sci. U. S. A 2017, 114, 5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Manocchi AK, Domachuk P, Omenetto FG, Yi H, Biotechnol. Bioeng 2009, 103, 725. [DOI] [PubMed] [Google Scholar]
- [71].Ahmed R, Yetisen AK, Yun SH, Butt H, Light Sci. Appl 2017, 6, e16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Jeon O, Bouhadir KH, Mansour JM, Alsberg E, Biomaterials 2009, 30, 2724. [DOI] [PubMed] [Google Scholar]
- [73].Bouhadir KH, Lee KY, Alsberg E, Damm KL, Anderson KW, Mooney DJ, Biotechnol. Prog 2001, 17, 945. [DOI] [PubMed] [Google Scholar]
- [74].Jain A, Yang AHJ, Erickson D, Opt. Lett 2012, 37, 1472. [DOI] [PubMed] [Google Scholar]
- [75].Dupuis A, Guo N, Gao Y, Godbout N, Lacroix S, Dubois C, Skorobogatiy M, Opt. Lett 2007, 32, 109. [DOI] [PubMed] [Google Scholar]
- [76].Applegate MB, Perotto G, Kaplan DL, Omenetto FG, Biomed. Opt. Express 2015, 6, 4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Wang X, Li Q, Guan Y, Zhang Y, Mater. Today Chem 2016, 1–2, 7. [Google Scholar]
- [78].Mano JF, Silva GA, Azevedo HS, Malafaya PB, Sousa RA, Silva SS, Boesel LF, Oliveira JM, Santos TC, Marques AP, Neves NM, Reis RL, J. R. Soc. Interface 2007, 4, 999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gasperini L, Mano JF, Reis RL, Soc JR Interface 2014, 11, 20140817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Trampe E, Koren K, Akkineni AR, Senwitz C, Krujatz F, Lode A, Gelinsky M, Kühl M, Adv. Funct. Mater 2018, 28, 1. [Google Scholar]
- [81].Gogoi N, Barooah M, Majumdar G, Chowdhury D, ACS Appl. Mater. Interfaces 2015, 7, 3058. [DOI] [PubMed] [Google Scholar]
- [82].Kozlovskaya V, Kharlampieva E, Khanal BP, Manna P, Zubarev ER, Tsukruk VV, Chem. Mater 2008, 20, 7474. [Google Scholar]
- [83].Tokarev I, Tokareva I, Minko S, Adv. Mater 2008, 20, 2730. [DOI] [PubMed] [Google Scholar]
- [84].Lim HS, Lee JH, Walish JJ, Thomas EL, ACS Nano 2012, 6, 8933. [DOI] [PubMed] [Google Scholar]
- [85].Yetisen AK, Butt H, da Cruz Vasconcellos F, Montelongo Y, Davidson CAB, Blyth J, Chan L, Carmody JB, Vignolini S, Steiner U, Baumberg JJ, Wilkinson TD, Lowe CR, Adv. Opt. Mater 2014, 2, 250. [Google Scholar]
- [86].Men D, Zhang H, Hang L, Liu D, Li X, Cai W, Xiong Q, Li Y, J. Mater. Chem. C 2015, 3, 3659. [Google Scholar]
- [87].Fu F, Shang L, Chen Z, Yu Y, Zhao Y, Sci. Robot 2018, 3, eaar8580. [DOI] [PubMed] [Google Scholar]
- [88].Qiao X, Qian Z, Li J, Sun H, Han Y, Xia X, Zhou J, Wang C, Wang Y, Wang C, ACS Appl. Mater. Interfaces 2017, 9, 14665. [DOI] [PubMed] [Google Scholar]
- [89].Prucker O, Brandstetter T, Rühe J, Biointerphases 2018, 13, 010801. [DOI] [PubMed] [Google Scholar]
- [90].Kozlovskaya V, Kharlampieva E, Erel I, Sukhishvili SA, Soft Matter 2009, 5, 4077. [Google Scholar]
- [91].Wang R, Zhang M, Guan Y, Chen M, Zhang Y, Soft Matter 2019, 15, 6107. [DOI] [PubMed] [Google Scholar]
- [92].Mateescu A, Wang Y, Dostalek J, Jonas U, Membranes (Basel). 2012, 2, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Yetisen AK, Butt H, Volpatti LR, Pavlichenko I, Humar M, Kwok SJJ, Koo H, Kim KS, Naydenova I, Khademhosseini A, Hahn SK, Yun SH, Biotechnol. Adv 2016, 34, 250. [DOI] [PubMed] [Google Scholar]
- [94].Jiang N, Ahmed R, Damayantharan M, Ünal B, Butt H, Yetisen AK, Adv. Healthc. Mater 2019, 8, 1. [DOI] [PubMed] [Google Scholar]
- [95].Li J, Macdonald J, Biosens. Bioelectron 2016, 83, 177. [DOI] [PubMed] [Google Scholar]
- [96].Onoe H, Okitsu T, Itou A, Kato-Negishi M, Gojo R, Kiriya D, Sato K, Miura S, Iwanaga S, Kuribayashi-Shigetomi K, Matsunaga YT, Shimoyama Y, Takeuchi S, Nat. Mater 2013, 12, 584. [DOI] [PubMed] [Google Scholar]
- [97].Mirani B, Pagan E, Shojaei S, Dabiri SMH, Savoji H, Mehrali M, Sam M, Alsaif J, Bhiladvala RB, Dolatshahi-Pirouz A, Radisic M, Akbari M, ACS Appl. Mater. Interfaces 2020, 12, 9080. [DOI] [PubMed] [Google Scholar]
- [98].Pi Q, Maharjan S, Yan X, Liu X, Singh B, van Genderen AM, Robledo-Padilla F, Parra-Saldivar R, Hu N, Jia W, Xu C, Kang J, Hassan S, Cheng H, Hou X, Khademhosseini A, Zhang YS, Adv. Mater 2018, 1706913, 1706913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Cheng Y, Zheng F, Lu J, Shang L, Xie Z, Zhao Y, Chen Y, Gu Z, Adv. Mater 2014, 26, 5184. [DOI] [PubMed] [Google Scholar]
- [100].Shao L, Gao Q, Zhao H, Xie C, Fu J, Liu Z, Xiang M, He Y, Small 2018, 14, 1. [DOI] [PubMed] [Google Scholar]
- [101].Davoodi E, Sarikhani E, Montazerian H, Ahadian S, Costantini M, Swieszkowski W, Willerth SM, Walus K, Mofidfar M, Toyserkani E, Khademhosseini A, Ashammakhi N, Adv. Mater. Technol 2020, DOI 10.1002/admt.201901044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Dai X, Liu L, Ouyang J, Li X, Zhang X, Lan Q, Xu T, Sci. Rep 2017, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Attalla R, Puersten E, Jain N, Selvaganapathy PR, Biofabrication 2018, 11, 15012. [DOI] [PubMed] [Google Scholar]
- [104].Arslan-Yildiz A, El Assal R, Chen P, Guven S, Inci F, Demirci U, Biofabrication 2016, 8, 0. [DOI] [PubMed] [Google Scholar]
- [105].Mota C, Camarero-Espinosa S, Baker MB, Wieringa P, Moroni L, Chem. Rev 2020, DOI 10.1021/acs.chemrev.9b00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Parfenov VA, Khesuani YD, Petrov SV, Karalkin PA, Koudan EV, Nezhurina EK, DAS Pereira F, Krokhmal AA, Gryadunova AA, Bulanova EA, Vakhrushev IV, Babichenko II, Kasyanov V, Petrov OF, Vasiliev MM, Brakke K, Belousov SI, Grigoriev TE, Osidak EO, Rossiyskaya EI, Buravkova LB, Kononenko OD, Demirci U, Mironov VA, Sci. Adv 2020, 6, eaba4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Skardal A, Devarasetty M, Kang HW, Mead I, Bishop C, Shupe T, Lee SJ, Jackson J, Yoo J, Soker S, Atala A, Acta Biomater. 2015, 25, 24. [DOI] [PubMed] [Google Scholar]
- [108].Chimene D, Kaunas R, Gaharwar AK, Adv. Mater 2020, 32, 1. [DOI] [PubMed] [Google Scholar]
- [109].Montelongo Y, Yetisen AK, Butt H, Yun SH, Nat. Commun 2016, 7, DOI 10.1038/ncomms12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Ahmed R, Yetisen AK, Yun SH, Butt H, Light Sci. Appl 2017, 6, e16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Lee Y-J, Braun PV, Adv. Mater 2003, 15, 563. [Google Scholar]
- [112].Suri JT, Cordes DB, Cappuccio FE, Wessling RA, Singaram B, Angew. Chemie - Int. Ed 2003, 42, 5857. [DOI] [PubMed] [Google Scholar]
- [113].Yetisen AK, in Hologr. Sensors, Springer, 2015. [Google Scholar]
- [114].Yetisen AK, in Hologr. Sensors, Springer, 2015. [Google Scholar]
- [115].Kang HS, Han SW, Park C, Lee SW, Eoh H, Baek J, Shin D, Park TH, Huh J, Lee H, Kim D, Ryu DY, Thomas EL, Koh W, Park C, Sci. Adv 2020, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Tokarev I, Minko S, Soft Matter 2009, 5, 511. [Google Scholar]
- [117].Lei Z, Wang Q, Wu P, Mater. Horizons 2017, 4, 694. [Google Scholar]
- [118].Lei Z, Wang Q, Sun S, Zhu W, Wu P, Adv. Mater 2017, 29, 1. [DOI] [PubMed] [Google Scholar]
- [119].Maurer RD, Appl. Phys. Lett 1975, 27, 220. [Google Scholar]
- [120].Guo J, Zhou M, Yang C, Sci. Rep 2017, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Zhou M, Guo J, Yang C, Sensors Actuators B Chem. 2018, 264, 52. [Google Scholar]
- [122].Jiang N, Ahmed R, Rifat AA, Guo J, Yin Y, Montelongo Y, Butt H, Yetisen AK, Adv. Opt. Mater 2018, 6, 1. [Google Scholar]
- [123].Feng J, Zheng Y, Bhusari S, Villiou M, Pearson S, Campo A, Adv. Funct. Mater 2020, 2004327, 1. [Google Scholar]
- [124].Fujiwara E, Cabral TD, Sato M, Oku H, Cordeiro CMB, Sci. Rep 2020, 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Xu P, Xie R, Liu Y, Luo G, Ding M, Liang Q, Adv. Mater 2017, 29, 1. [DOI] [PubMed] [Google Scholar]
- [126].Parker AR, Opt J. A Pure Appl. Opt 2000, 2, DOI 10.1088/1464-4258/2/6/201. [DOI] [Google Scholar]
- [127].Burg SL, Parnell AJ, J. Phys. Condens. Matter 2018, 30, DOI 10.1088/1361-648X/aadc95. [DOI] [PubMed] [Google Scholar]
- [128].Guidetti G, Sun H, Marelli B, Omenetto FG, Sci. Adv 2020, 6, DOI 10.1126/sciadv.aba8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Teyssier J, Saenko SV, Van Der Marel D, Milinkovitch MC, Nat. Commun 2015, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Kim H, Ge J, Kim J, Choi SE, Lee H, Lee H, Park W, Yin Y, Kwon S, Nat. Photonics 2009, 3, 534. [Google Scholar]
- [131].Haque MA, Kurokawa T, Kamita G, Yue Y, Gong JP, Chem. Mater 2011, 23, 5200. [Google Scholar]
- [132].Haque MA, Kamita G, Kurokawa T, Tsujii K, Gong JP, Adv. Mater 2010, 22, 5110. [DOI] [PubMed] [Google Scholar]
- [133].Yue Y, Kurokawa T, Haque MA, Nakajima T, Nonoyama T, Li X, Kajiwara I, Gong JP, Nat. Commun 2014, 5, DOI 10.1038/ncomms5659. [DOI] [PubMed] [Google Scholar]
- [134].Zhang YS, Zhu C, Xia Y, Adv. Mater 2017, 29, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Min K, Kim S, Kim S, Proc. Natl. Acad. Sci. U. S. A 2017, 114, 6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Zhang W, Chen L, Chen J, Wang L, Gui X, Ran J, Xu G, Zhao H, Zeng M, Ji J, Qian L, Zhou J, Ouyang H, Zou X, Adv. Healthc. Mater 2017, 6, 1. [DOI] [PubMed] [Google Scholar]
- [137].Latorre E, Kale S, Casares L, Gómez-González M, Uroz M, Valon L, Nair RV, Garreta E, Montserrat N, del Campo A, Ladoux B, Arroyo M, Trepat X, Nature 2018, 563, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Vivian J, Rao AA, Nothaft FA, Ketchum C, Armstrong J, Novak A, Pfeil J, Narkizian J, Deran AD, Musselman-Brown A, Schmidt H, Amstutz P, Craft B, Goldman M, Rosenbloom K, Cline M, O’Connor B, Hanna M, Birger C, Kent WJ, Patterson DA, Joseph AD, Zhu J, Zaranek S, Getz G, Haussler D, Paten B, Nat. Biotechnol 2017, 35, 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Xin H, Li Y, Liu X, Li B, Nano Lett. 2013, 13, 3408. [DOI] [PubMed] [Google Scholar]
- [140].Bezryadina A, Hansson T, Gautam R, Wetzel B, Siggins G, Kalmbach A, Lamstein J, Gallardo D, Carpenter EJ, Ichimura A, Morandotti R, Chen Z, Phys. Rev. Lett 2017, 119, 1. [DOI] [PubMed] [Google Scholar]
- [141].Wu X, Huang W, Wu WH, Xue B, Xiang D, Li Y, Qin M, Sun F, Wang W, Bin Zhang W, Cao Y, Nano Res. 2018, 11, 5556. [Google Scholar]
- [142].Liu L, Shadish JA, Arakawa CK, Shi K, Davis J, DeForest CA, Adv. Biosyst 2018, 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Humar M, Yun SH, Optica 2017, 4, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Constant C, Bergano A, Sugaya K, Dogariu A, J. Biophotonics 2018, 11, 1. [DOI] [PubMed] [Google Scholar]
- [145].Ogawa J, Iwata Y, Tonnu NU, Gopinath C, Huang L, Itoh S, Ando R, Miyawaki A, Verma IM, Pao GM, bioRxiv 2020, 2020.07.09.196436. [Google Scholar]
- [146].Lyu S, Fang J, Duan T, Fu L, Liu J, Li H, Chem. Commun 2017, 53, 13375. [DOI] [PubMed] [Google Scholar]
- [147].Koetting MC, Peters JT, Steichen SD, Peppas NA, Mater. Sci. Eng. R Reports 2015, 93, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Sharifzadeh G, Hosseinkhani H, Adv. Healthc. Mater 2017, 6, 1. [DOI] [PubMed] [Google Scholar]
- [149].Hoque J, Sangaj N, Varghese S, Macromol. Biosci 2019, 19, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Zhao X, Kim J, Cezar CA, Huebsch N, Lee K, Bouhadir K, Mooney DJ, Proc. Natl. Acad. Sci. U. S. A 2011, 108, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Soto F, Wang J, Deshmukh S, Demirci U, Adv. Intell. Syst 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Wang W, Fan X, Li F, Qiu J, Umair MM, Ren W, Ju B, Zhang S, Tang B, Adv. Opt. Mater 2018, 6, 1. [Google Scholar]
- [153].Gui Q, Zhou Y, Liao S, He Y, Tang Y, Wang Y, Soft Matter 2019, 15, 393. [DOI] [PubMed] [Google Scholar]
- [154].Isabettini S, Stucki S, Massabni S, Baumgartner ME, Reckey PQ, Kohlbrecher J, Ishikawa T, Windhab EJ, Fischer P, Kuster S, ACS Appl. Mater. Interfaces 2018, 10, 8926. [DOI] [PubMed] [Google Scholar]
- [155].Rittikulsittichai S, Kolhatkar AG, Sarangi S, Vorontsova MA, Vekilov PG, Brazdeikis A, Randall Lee T, Nanoscale 2016, 8, 11851. [DOI] [PubMed] [Google Scholar]
- [156].Zhao LB, Pan L, Zhang K, Guo SS, Liu W, Wang Y, Chen Y, Zhao XZ, Chan HLW, Lab Chip 2009, 9, 2981. [DOI] [PubMed] [Google Scholar]
- [157].Tasoglu S, Yu CH, Gungordu HI, Guven S, Vural T, Demirci U, Nat. Commun 2014, 5, DOI 10.1038/ncomms5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Sun X, Tyagi P, Agate S, Lucia L, McCord M, Pal L, Carbohydr. Polym 2019, 208, 495. [DOI] [PubMed] [Google Scholar]
- [159].Tang C, Li B, Zou C, Liu L, Chen H, Polymers (Basel). 2018, 10, DOI 10.3390/polym10070697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Fernandes MS, Dias NS, Silva AF, Nunes JS, Lanceros-Méndez S, Correia JH, Mendes PM, Biosens. Bioelectron 2010, 26, 80. [DOI] [PubMed] [Google Scholar]
- [161].Klouda L, Mikos AG, Eur. J. Pharm. Biopharm 2008, 68, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Taylor M, Tomlins P, Sahota T, Gels 2017, 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Seo S-W, Enemuo AN, Azmand HR, IEEE Sensors Lett. 2018, DOI 10.1109/lsens.2018.2832006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Junk MJN, Ilke A, Menges B, Jonas U, Langmuir 2010, 26, 12253. [DOI] [PubMed] [Google Scholar]
- [165].Zadražil A, Štěpánek F, Colloids Surfaces A Physicochem. Eng. Asp 2010, 372, 115. [Google Scholar]
- [166].Zhao Y, Lei M, Liu SX, Zhao Q, Sensors Actuators B Chem. 2018, 261, 226. [Google Scholar]
- [167].Ramón-Azcón J, Ahadian S, Estili M, Liang X, Ostrovidov S, Kaji H, Shiku H, Ramalingam M, Nakajima K, Sakka Y, Khademhosseini A, Matsue T, Adv. Mater 2013, 25, 4028. [DOI] [PubMed] [Google Scholar]
- [168].Ahadian S, Ramón-Azcón J, Estili M, Liang X, Ostrovidov S, Shiku H, Ramalingam M, Nakajima K, Sakka Y, Bae H, Matsue T, Khademhosseini A, Sci. Rep 2014, 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Janovák L, Dékány I, Appl. Surf. Sci 2010, 256, 2809. [Google Scholar]
- [170].Lee SN, Bin Baek Y, Shin DM, J. Nanosci. Nanotechnol 2014, 14, 6053. [DOI] [PubMed] [Google Scholar]
- [171].Ahmed R, Ozen MO, Karaaslan MG, Prator CA, Thanh C, Kumar S, Torres L, Iyer N, Munter S, Southern S, Henrich TJ, Inci F, Demirci U, Adv. Mater 2020, 1907160, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Hamon C, Novikov S, Scarabelli L, Basabe-Desmonts L, Liz-Marzán LM, ACS Nano 2014, 8, 10694. [DOI] [PubMed] [Google Scholar]
- [173].Guay JM, Calà Lesina A, Côté G, Charron M, Poitras D, Ramunno L, Berini P, Weck A, Nat. Commun 2017, 8, DOI 10.1038/ncomms16095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Kim J, Son H, Cho DJ, Geng B, Regan W, Shi S, Kim K, Zettl A, Shen YR, Wang F, Nano Lett. 2012, 12, 5598. [DOI] [PubMed] [Google Scholar]
- [175].Jo G, Choe M, Lee S, Park W, Kahng YH, Lee T, Nanotechnology 2012, 23, DOI 10.1088/0957-4484/23/11/112001. [DOI] [PubMed] [Google Scholar]
- [176].Song HS, Kwon OS, Kim JH, Conde J, Artzi N, Biosens. Bioelectron 2017, 89, 187. [DOI] [PubMed] [Google Scholar]
- [177].Sitti M, Ceylan H, Hu W, Giltinan J, Turan M, Yim S, Diller E, Proc. IEEE. Inst. Electr. Electron. Eng 2015, 103, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Ren T, Chen P, Gu L, Ogut MG, Demirci U, Adv. Mater 2020, 32, 1. [DOI] [PubMed] [Google Scholar]
- [179].Wang J, Ahmed R, Zeng Y, Fu K, Soto F, Sinclair B, Soh HT, Demirci U, Small 2020, 20055185. [DOI] [PubMed] [Google Scholar]
- [180].Palagi S, Singh DP, Fischer P, Adv. Opt. Mater 2019, 7, 1. [Google Scholar]
- [181].Zeng H, Wasylczyk P, Wiersma DS, Priimagi A, Adv. Mater 2018, 30, 1. [DOI] [PubMed] [Google Scholar]
- [182].Nocentini S, Parmeggiani C, Martella D, Wiersma DS, Adv. Opt. Mater 2018, 6, 1. [Google Scholar]
- [183].Soto F, Wang J, Ahmed R, Demirci U, Adv. Sci. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Li J, Li X, Luo T, Wang R, Liu C, Chen S, Li D, Yue J, Cheng SH, Sun D, Sci. Robot 2018, 3, 1. [DOI] [PubMed] [Google Scholar]
- [185].Li H, Go G, Ko SY, Park J-O, Park S, Smart Mater. Struct 2016, 25, 27001. [Google Scholar]
- [186].Zhao Y, Xuan C, Qian X, Alsaid Y, Hua M, Jin L, He X, Sci. Robot 2019, 4, 1. [DOI] [PubMed] [Google Scholar]
- [187].Kim H, Kang JH, Zhou Y, Kuenstler AS, Kim Y, Chen C, Emrick T, Hayward RC, Adv. Mater 2019, 31, DOI 10.1002/adma.201900932. [DOI] [PubMed] [Google Scholar]
- [188].Zhang H, Koens L, Lauga E, Mourran A, Möller M, Small 2019, 15, DOI 10.1002/smll.201903379. [DOI] [PubMed] [Google Scholar]
- [189].Rehor I, Maslen C, Moerman PG, van Ravensteijn BGP, van Alst R, Groenewold J, Eral HB, Kegel WK, Soft Robot. 2020, 00, 1. [DOI] [PubMed] [Google Scholar]
- [190].Nishiguchi A, Zhang H, Schweizerhof S, Schulte MF, Mourran A, Möller M, ACS Appl. Mater. Interfaces 2020, 12, 12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Gurkan UA, Tasoglu S, Akkaynak D, Avci O, Unluisler S, Canikyan S, MacCallum N, Demirci U, Adv. Healthc. Mater 2012, 1, 661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Gurkan UA, Anand T, Tas H, Elkan D, Akay A, Keles HO, Demirci U, Lab Chip 2011, 11, 3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Li C, Iscen A, Palmer LC, Schatz GC, Stupp SI, J. Am. Chem. Soc 2020, 142, 8447. [DOI] [PubMed] [Google Scholar]
- [194].Shahsavan H, Aghakhani A, Zeng H, Guo Y, Davidson ZS, Priimagi A, Sitti M, Proc. Natl. Acad. Sci. U. S. A 2020, 117, 5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Palagi S, Mark AG, Melde K, Qiu T, Zeng H, Parmeggiani C, Martella D, Wiersma DS, Fischer P, Int. Conf. Manip. Autom. Robot. Small Scales, MARSS 2017 - Proc. 2017, 1. [Google Scholar]
- [196].Dolmans D, Fukumura D, Jain R, Nat. Rev. Cancer 2003, 3, 375. [DOI] [PubMed] [Google Scholar]
- [197].Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J, CA. Cancer J. Clin 2011, 61, 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Solban N, Rizvi I, Hasan T, Lasers Surg. Med 2006, 38, 522. [DOI] [PubMed] [Google Scholar]
- [199].Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, Pogue BW, Hasan T, Chem. Rev 2010, 110, 2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Mallidi S, Anbil S, Bulin AL, Obaid G, Ichikawa M, Hasan T, Theranostics 2016, 6, 2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Spring BQ, Rizvi I, Xu N, Hasan T, Photochem. Photobiol. Sci 2015, 14, 1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Leung B, Dharmaratne P, Yan W, Chan BCL, Lau CBS, Fung KP, Ip M, Leung SSY, J. Photochem. Photobiol. B Biol 2020, 203, 111776. [DOI] [PubMed] [Google Scholar]
- [203].González-Delgado JA, Castro PM, Machado A, Araújo F, Rodrigues F, Korsak B, Ferreira M, Tomé JPC, Sarmento B, Int. J. Pharm 2016, 510, 221. [DOI] [PubMed] [Google Scholar]
- [204].Mao C, Xiang Y, Liu X, Cui Z, Yang X, Yeung KWK, Pan H, Wang X, Chu PK, Wu S, ACS Nano 2017, 11, 9010. [DOI] [PubMed] [Google Scholar]
- [205].Zhang Y, Zhang H, Zou Q, Xing R, Jiao T, Yan X, J. Mater. Chem. B 2018, 6, 7335. [DOI] [PubMed] [Google Scholar]
- [206].Li M, Liu X, Tan L, Cui Z, Yang X, Li Z, Zheng Y, Yeung KWK, Chu PK, Wu S, Biomater. Sci 2018, 6, 2110. [DOI] [PubMed] [Google Scholar]
- [207].Xing R, Liu Y, Zou Q, Yan X, Nanoscale 2019, 11, 22182. [DOI] [PubMed] [Google Scholar]
- [208].Liu C, Ruan C, Shi R, Jiang BP, Ji S, Shen XC, Biomater. Sci 2019, 7, 1705. [DOI] [PubMed] [Google Scholar]
- [209].Xu X, Zeng Z, Huang Z, Sun Y, Huang Y, Chen J, Ye J, Yang H, Yang C, Zhao C, Carbohydr. Polym 2020, 229, 115394. [DOI] [PubMed] [Google Scholar]
- [210].Luo L, Zhang Q, Luo Y, He Z, Tian X, Battaglia G, J. Control. Release 2019, 298, 99. [DOI] [PubMed] [Google Scholar]
- [211].Xing R, Liu K, Jiao T, Zhang N, Ma K, Zhang R, Zou Q, Ma G, Yan X, Adv. Mater 2016, 28, 3669. [DOI] [PubMed] [Google Scholar]
- [212].Meesaragandla B, Sarkar D, Mahalingam V, ACS Omega 2019, 4, 3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Chang G, Zhang H, Li S, Huang F, Shen Y, Xie A, Mater. Sci. Eng. C 2019, 99, 1392. [DOI] [PubMed] [Google Scholar]
- [214].Jin R, Yang J, Ding P, Li C, Zhang B, Chen W, Di Zhao Y, Cao Y, Liu B, Nanotechnology 2020, 31, DOI 10.1088/1361-6528/ab72b9. [DOI] [PubMed] [Google Scholar]
- [215].Zhou TJ, Xing L, Fan YT, Cui PF, Jiang HL, J. Control. Release 2019, 309, 82. [DOI] [PubMed] [Google Scholar]
- [216].Meng Z, Zhou X, Xu J, Han X, Dong Z, Wang H, Zhang Y, She J, Xu L, Wang C, Liu Z, Adv. Mater 2019, 31, 1. [DOI] [PubMed] [Google Scholar]
- [217].Sun SK, Wu JC, Wang H, Zhou L, Zhang C, Cheng R, Kan D, Zhang X, Yu C, Biomaterials 2019, 218, 119328. [DOI] [PubMed] [Google Scholar]
- [218].Zheng L, Liu S, Cheng X, Qin Z, Lu Z, Zhang K, Zhao J, Adv. Sci 2019, 6, DOI 10.1002/advs.201900099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Lu Z, Liu S, Le Y, Qin Z, He M, Xu F, Zhu Y, Zhao J, Mao C, Zheng L, Biomaterials 2019, 218, 119190. [DOI] [PubMed] [Google Scholar]
- [220].Chou DB, Frismantas V, Milton Y, David R, Pop-Damkov P, Ferguson D, MacDonald A, Vargel Bölükbaşı Ö, Joyce CE, Moreira Teixeira LS, Rech A, Jiang A, Calamari E, Jalili-Firoozinezhad S, Furlong BA, O’Sullivan LR, Ng CF, Choe Y, Marquez S, Myers KC, Weinberg OK, Hasserjian RP, Novak R, Levy O, Prantil-Baun R, Novina CD, Shimamura A, Ewart L, Ingber DE, Nat. Biomed. Eng 2020, 4, 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Herland A, Maoz BM, Das D, Somayaji MR, Prantil-Baun R, Novak R, Cronce M, Huffstater T, Jeanty SSF, Ingram M, Chalkiadaki A, Benson Chou D, Marquez S, Delahanty A, Jalili-Firoozinezhad S, Milton Y, Sontheimer-Phelps A, Swenor B, Levy O, Parker KK, Przekwas A, Ingber DE, Nat. Biomed. Eng 2020, 4, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Novak R, Ingram M, Marquez S, Das D, Delahanty A, Herland A, Maoz BM, Jeanty SSF, Somayaji MR, Burt M, Calamari E, Chalkiadaki A, Cho A, Choe Y, Chou DB, Cronce M, Dauth S, Divic T, Fernandez-Alcon J, Ferrante T, Ferrier J, FitzGerald EA, Fleming R, Jalili-Firoozinezhad S, Grevesse T, Goss JA, Hamkins-Indik T, Henry O, Hinojosa C, Huffstater T, Jang KJ, Kujala V, Leng L, Mannix R, Milton Y, Nawroth J, Nestor BA, Ng CF, O’Connor B, Park TE, Sanchez H, Sliz J, Sontheimer-Phelps A, Swenor B, Thompson G, Touloumes GJ, Tranchemontagne Z, Wen N, Yadid M, Bahinski A, Hamilton GA, Levner D, Levy O, Przekwas A, Prantil-Baun R, Parker KK, Ingber DE, Nat. Biomed. Eng 2020, 4, 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Miri AK, Nieto D, Iglesias L, Goodarzi Hosseinabadi H, Maharjan S, Ruiz-Esparza GU, Khoshakhlagh P, Manbachi A, Dokmeci MR, Chen S, Shin SR, Zhang YS, Khademhosseini A, Adv. Mater 2018, 30, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Chen YS, Chung KC, Huang WY, Bin Lee W, Fu CY, Wang CH, Bin Lee G, Lab Chip 2019, 19, 1764. [DOI] [PubMed] [Google Scholar]
- [225].Guimarães CF, Gasperini L, Ribeiro RS, Carvalho AF, Marques AP, Reis RL, Mater. Horizons 2020, DOI 10.1039/C7MH00043J. [DOI] [Google Scholar]
- [226].Vega SL, Kwon MY, Song KH, Wang C, Mauck RL, Han L, Burdick JA, Nat. Commun 2018, 9, 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Mahadik BP, Wheeler TD, Skertich LJ, Kenis PJA, Harley BAC, Adv. Healthc. Mater 2014, 3, 449. [DOI] [PubMed] [Google Scholar]
- [228].Uzel SGM, Platt RJ, Subramanian V, Pearl TM, Rowlands CJ, Chan V, Boyer LA, So PTC, Kamm RD, Sci. Adv 2016, 2, DOI 10.1126/sciadv.1501429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Torres-Mapa ML, Singh M, Simon O, Mapa JL, Machida M, Günther A, Roth B, Heinemann D, Terakawa M, Heisterkamp A, Sensors (Switzerland) 2019, 19, DOI 10.3390/s19194333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Wu J, Zheng G, Lee LM, Lab Chip 2012, 12, 3566. [DOI] [PubMed] [Google Scholar]
- [231].Mencattini A, Mattei F, Schiavoni G, Gerardino A, Businaro L, Di Natale C, Martinelli E, Front. Pharmacol 2019, 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Lv C, Xia H, Guan W, Sun YL, Tian ZN, Jiang T, Wang YS, Zhang YL, Chen QD, Ariga K, De Yu Y, Sun HB, Sci. Rep 2016, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Wang Z, Abdulla R, Parker B, Samanipour R, Ghosh S, Kim K, Biofabrication 2015, 7, DOI 10.1088/1758-5090/7/4/045009. [DOI] [PubMed] [Google Scholar]
- [234].Nele V, Wojciechowski JP, Armstrong JPK, Stevens MM, Adv. Funct. Mater 2020, 2002759, 1. [Google Scholar]