Summary
Targeted muscle reinnervation (TMR) surgery has been shown to aid in prevention and treatment of neuropathic pain. Technical and anatomical descriptions of TMR surgery for upper extremity amputees (including transradial, transhumeral, and forequarter amputations) have been reported, yet such descriptions of TMR surgery for partial hand amputations are currently lacking. Herein we outline the technique of different types of partial hand amputation TMR surgeries to serve as a reference and guide. A retrospective review was performed by our multi-institutional team to identify clinical cases where partial hand TMR surgeries were performed. Patient demographics, characteristics, amputation subtype, nerve transfer, pain score, pain outcome, and functional outcome data were collected and analyzed. From January 2018 to September 2019, 13 patients underwent partial hand TMR procedures. Eight cases resulted from trauma, and 6 were secondary to oncologic procedures. The amputations consisted of 8 ray, 2 trans-metacarpal, 2 radial-sided hand, and 1 index finger amputation with recurrent painful neuromas. Twelve patients were weaned off narcotics completely and only 3 remained on a neuromodulator for ongoing pain control. Technical considerations for partial hand TMR surgery have been outlined, with early pilot data showing beneficial pain control outcomes.
INTRODUCTION
Targeted muscle reinnervation (TMR) is a nerve transfer technique where proximal stumps of the transected major peripheral nerves are transferred to recipient redundant motor nerves within adjacent muscles of the amputated limb.1–5 TMR purposefully redirects the major peripheral nerve into an intact nerve and accompanying neuromuscular junction that is acutely transected, thus providing a target for the regenerating major peripheral nerve to grow into rather than forming a painful neuroma.1–5
TMR provides additional benefits by increasing electromyography signal generators for advanced myoelectric prostheses.1,6,7 Following reinnervation, the targeted muscles then serve as biological amplifiers for motor nerve signals, providing increased electromyography signals and focused, intuitive control of prosthetic devices.1,6–12 TMR facilitates advanced prosthetic device use and desensitizes the amputation stump by decreasing symptomatic neuroma development. Subsequent studies demonstrated that TMR can also effectively reduce phantom limb pain (PLP) or phantom limb sensation.3–5,8
In this study, we describe the application of TMR to 4 different, but common types of partial hand amputations, and outline the technique for others to employ in their practices. This technique was applied to manage partial hand amputations in the clinical setting at multiple academic and community medical centers.
METHODS AND MATERIALS
Multiple partial hand amputations, including ray, radial- and ulnar-sided partial hand, and metacarpal/trans-metacarpal amputations, were performed. Coaptation of transected proximal major nerves from the amputation procedure was performed to end motor nerve targets in an end-to-end fashion following TMR principles.1,2
Clinical Correlate Cases
After institutional review board approval at our respective centers, the authors reviewed patients who had undergone partial hand amputations where TMR was performed. Thirteen cases were identified between January 2018 and September 2019. Six cases were completed for oncologic reasons, 6 cases were related to trauma, and 1 case was for recurrent neuropathic neuromas. Primary TMR was performed in 10 cases, and secondary TMR in 3 cases. Ten patients were adults and 3 were children, with an average age of 45 at the time of surgery. The distribution of cases consisted of 8 ray amputations, 2 trans-metacarpal level amputations, 2 radial sided partial hand amputations, and 1 index finger amputation with recurrent painful digital neuromas. Follow-up ranged from 5 to 35 months, with mean follow up of 19.8 months (See Table 1). Of these patients, 12/13 were able to be weaned off of narcotics and only 3/13 remained on a neuromodulator (eg, Gabapentin or Lyrica). No postoperative neuroma pain was reported in any of the cases. Postoperative PLP was reported as “none” in 8/13 cases and “mild” in 5/13 cases. A prosthetic device was not indicated in 8/13 cases, and tolerated in 4/4 cases where a prosthetic device was utilized. Although indicated, 1 patient declined the use of a prosthetic (Table 2). (See Video [online], which displays middle finger ray amputation.)
Table 1.
Patient Demographics and Characteristics
| No. Patients (%), N = 13 | |
|---|---|
| Demographics | |
| Men | 11 (85%) |
| Mean age, y (range) | 45.1 (18–72) |
| Mean follow-up, mo (range) | 19.8 (5–35) |
| Hand involved | |
| Right | 8 |
| Left | 5 |
| Partial hand amputation type | |
| Ray amputation | 8 |
| Thumb amputation | 1 |
| Radial hand | 2 |
| Trans-metacarpal | 2 |
| Type of TMR performed | |
| Primary TMR | 10 |
| Secondary TMR | 3 |
Table 2.
TMR Clinical Case Descriptions, with Nerve Transfer and Outcome Descriptions
| Category | Case | Amputation Sub-type | TMR Transfers Performed | Primary versus Secondary TMR | VAS Pain Scores | Pain Outcomes and Medication Usage | Functional Outcome |
|---|---|---|---|---|---|---|---|
| Oncologic | 1 | 3rd Ray amputation right hand | Proper digital-to-VIMMN x2 | Primary—neuroma prevention | 0/10 | Neuroma pain: none PLP: none Narcotics: none | Prosthetic not indicated |
| 2 | 3rd Ray amputation right hand | Proper digital-to-VIMMN x2 | Primary—neuroma prevention | 0/10 | Neuroma pain: none PLP: none Narcotics: none | Prosthetic not indicated | |
| 3 | 4th Ray amputation left hand | Proper digital-to-VIMMN x2 | Primary—neuroma prevention | 1/10 | Neuroma pain: none PLP: mild Narcotics: none | Prosthetic not indicated | |
| 4 | Thumb amputation/ radial partial left hand | (1) Thumb PDN-to-adductor pollicis MN; (2) Thumb PDN-to-FPB-MN (3) RSN to AIN MB | Primary—neuroma prevention | 0/10 | Neuroma pain: none PLP: none Narcotics: none | Tolerates prosthetic wear | |
| 5 | 3rd Ray amputation left hand | Proper digital-to-VIMMN x2 | Primary—neuroma prevention | 2/10 | Neuroma pain: none PLP: mild Narcotics: none +neuromodulator | Prosthetic not indicated | |
| 6 | 4th Ray amputation right hand | Proper digital-to-VIMMN x2 | Primary—neuroma prevention | 0/10 | Neuroma pain: none PLP: none Narcotics: none | Prosthetic not indicated | |
| Trauma | 7 | 3rd Ray amputation right hand | Proper digital-to-VIMMN x2 | Primary—neuroma prevention | 1/10 | Neuroma pain: none PLP: Mild Narcotics: none | Prosthetic not indicated |
| 8 | 3th Ray amputation left hand | Proper digital-to-VIMMN x2 | Primary—neuroma prevention | 1/10 | Neuroma pain: none PLP: mild Narcotics: none | Prosthetic not indicated | |
| 9 | Trans-metacarpal right hand | (1) RSN-to-AIN MB; (2) Proper digital MN-to-2nd & 3rd VIMMNs; (3) Proper digital Uln-to-4th VIMMN | Secondary—neuroma treatment | Pre-TMR: 6-7/10 Post-TMR: 2/10 | Neuroma pain: none PLP: mild Narcotics: +opioid +neuromodulator | Tolerates prosthetic | |
| 10 | Trans-metacarpal right hand | (1) RSN-to-MN-Thenar MB; (2) MN-to-2nd VIMMN; (3) Uln-to-3rd DIMMN | Primary—neuroma prevention | 0/10 | Neuroma pain: none PLP: None Narcotics: none | Declined prosthesis | |
| 11 | Radial-sided partial left hand | Thumb PDN-to-index LMMN | Secondary—neuroma treatment | Pre-TMR: 7-8/10 Post-TMR: 1/10 | Neuroma pain: none PLP: none Narcotics: +preoperative and none postoperative | Tolerates prosthetic | |
| 12 | Radial-sided partial right hand | (1) Thumb PDN-to-adductor pollicis MN; (2) Thumb PDN-to-FPB-MN | Primary—neuroma prevention | 0/10 | Neuroma pain: none PLP: none Narcotics: none | Tolerates prosthetic | |
| Recurrent neuroma | 13 | Index finger ray amputation of right hand | Proper digital-to-LMMN | Secondary —neuroma treatment | Pre-TMR: 7-8/10 Post-TMR: 2/10 | Neuroma pain: none PLP: none Narcotics: +preoperative and none postoperative neuromodulator: +preoperative and none postoperative | Prosthetic not indicated |
Video 1. Video 1 from ”Targeted Muscle Reinnervation in Partial Hand Amputations.”.
OPERATIVE TECHNIQUE
The first step is meticulous dissection and appropriate identification of the major peripheral nerves and the recipient target motor nerves. Nerve stimulators are useful in identifying target recipient motor nerve branches intraoperatively. Typically, we use an amperage of 0.5–2 mA and voltage of 50–100 and coordinate preoperatively with our Anesthesia colleagues to avoid long acting paralytic and muscle relaxant agents.
The nerve stumps must be resected sharply back to healthy fascicles. The nerve transfer coaptation site must be tension free; if this is not possible, nerve grafting or other coaptation aids may be indicated. The nerve transfers should be performed with the joints positioned under maximal stress to ensure the nerve coaptations are guaranteed tension-free in all positions. When nerve grafts are required, we prefer spare parts from the amputated extremity if available; otherwise, we consider processed nerve allograft or traditional autologous nerve.
For nerve preparations, we recommend transecting recipient target motor nerves proximally, and the transferred nerve stumps distally with neurorrhaphies performed using a hand-sewn interrupted epineurial technique using 8-0 nylon sutures. To limit regeneration times, shorten the distal target motor nerve stump as much as possible, while preserving a tension-free nerve coaptation. The neurorrhaphies are performed within 1–2 millimeters of where the recipient motor nerves enter the recipient muscles. Performing the coaptation close to the motor nerve entrance to the muscle belly permits the shortest possible reinnervation time and more rapid onset of reinnervation.
Nerve-nerve coaptation size mismatch frequently occurs when performing TMR in partial hand amputations, but does not seem to adversely affect the outcome.14 Following nerve transfer, the coaptation site is reinforced and wrapped using a cuff of local denervated muscle carefully raised from around the interface of the native target motor nerve and its corresponding muscle units.14 This denervated muscle cuff is gently wrapped around the nerve coaptation site and secured with interrupted 4-0 Vicryl suture.
Although tension-free TMR to a short recipient motor nerve with a muscle cuff is ideal, this is not always possible. If the motor branch to the target muscle is left long and the coaptation site too proximal for a muscle cuff to reach, nerve conduits, protectors or wraps can assist with coaptation size mismatches. Refer to Tables 3 and 4 for recommended nerve transfer options.
Table 3.
Nerve Transfers Available for Partial Hand Amputations
| Proximal Major Peripheral Nerve and Associated Distal Branches | Ulnar Nerve Branches | Median Nerve | Radial Sensory Nerve |
|---|---|---|---|
| Target motor nerves available for receipt of major peripheral nerve branch transfer | Volar/dorsal interossei | Volar/dorsal interossei | 1st dorsal interossei |
| Lumbricals 4/5 | Lumbricals 2/3 | Thenar motor branch | |
| AIN to PQ | AIN to PQ | AIN to PQ | |
| FCU | Thenar motor branch | Any other local motor branch | |
| Any other local motor branch | Any other local motor branch |
Table 4.
Specific Nerve Transfer Options for Each of the Various Partial Hand Amputation Subtypes
| Partial Hand Amputation Subtype | Nerve Transfer Options | ||
|---|---|---|---|
| Ulnar Nerve | Median Nerve | Radial Sensory Nerve | |
| Ray amputation | Utilize motor nerve target of residual muscles adjacent to resected ray: | ||
| • Volar interosseus muscle | |||
| • Dorsal interosseus muscle | |||
| • Lumbrical | |||
| Radial hand amputation: Transfer sensory nerve stumps to ulnar hand (away from prosthesis docking site) | N/A | Volar interossei 2/3 Dorsal interossei 3/4 Lumbricals 4/5 | Dorsal interossei 3/4 AIN to PQ |
| Ulnar hand amputation: Transfer sensory nerve stumps to radial hand (away from prosthesis docking site) | Volar interossei 1/2 Dorsal interossei 1/2 Lumbricals 2/3 | N/A | N/A |
| Trans-metacarpal ± thumb amputation | Volar interossei 2/3 Dorsal interossei 3/4 Lumbricals 4/5 | Volar interossei 1/2 Dorsal interossei 1/2 Lumbricals 2/3 | 1st dorsal interosseus thenar motor branch AIN to PQ |
| Trans-carpal amputation | Volar interossei 2/3 Dorsal interossei 3/4 Lumbricals 4/5 FCU AIN to PQ | Volar interossei 1/2 Dorsal interossei 1/2 Lumbricals 2/3 Thenar motor branch AIN to PQ | 1st dorsal interosseus thenar motor branch AIN to PQ |
| Starfish procedure** | Interossei dissected out, pedicled on the palmar neurovascular arch, and transposed to the dorsal hand | ||
**From Gaston et al.13
DISCUSSION
It is important that these procedures are performed without a paralytic or local nerve blockade. Adequate soft tissue coverage must be maintained over the sites of neurorrhaphy. Consideration should be given to utilization of spare parts for grafts. If a regional flap is to be performed, extended dissections into the recipient muscles targeted for reinnervation should be spared (ie, the TMR targets).
Postoperative therapy includes early mobilization of the remaining extremity, desensitization using neuromodulators such as gabapentin or pregabalin, mirror therapy, and exercises designed to trigger the nerve transfers. Patients must be preoperatively counseled on expectations and outcomes, including the importance of early therapy and neuromodulation. With well-performed TMR, minimal retraining is required for successful incorporation of a future myoelectric prosthesis.
CONCLUSIONS
We have described the specific steps and techniques necessary to perform TMR surgery for multiple partial hand amputation injuries. As reported in the literature, TMR yields improved pain control, decreased likelihood of PLP and phantom limb sensation, and a decrease in symptomatic neuroma formation. A decrease in symptomatic neuroma formation following TMR lends to greater ability to actively participate in aggressive postoperative therapy, improved tolerance of the prosthetic during rehabilitation and during the activities of daily living, and offers patients improved bioprosthetic function and potential.
Fig. 1.
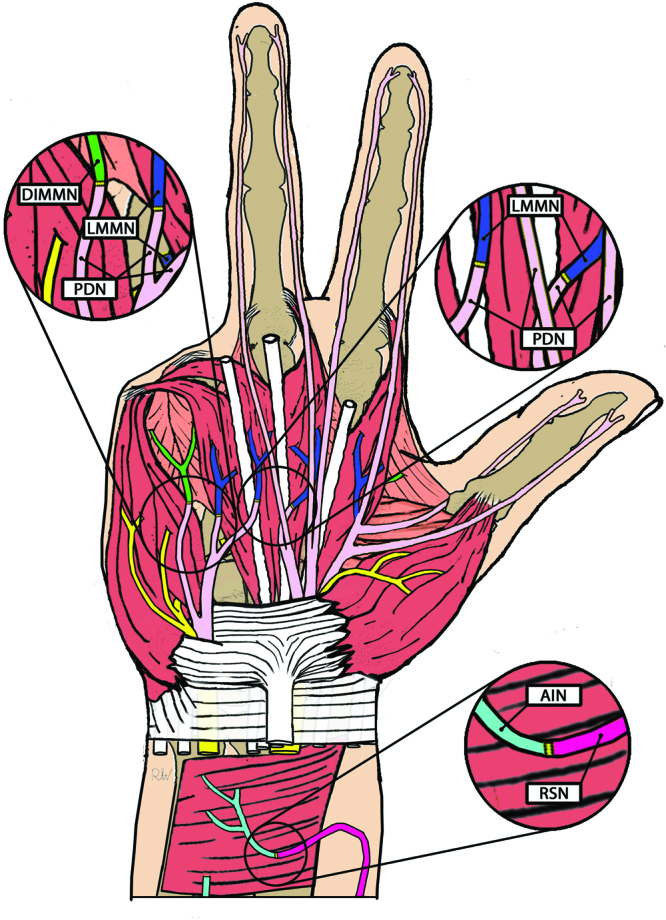
Partial hand amputation TMR schematic diagram illustrating various TMR donor/target combinations in the setting of partial hand amputation. LMMN, lumbrical muscle motor nerve; DIMMN, dorsal interosseus muscle motor nerve; PDN, proper digital nerve; AIN, anterior interosseus nerve; RSN, radial sensory nerve.
Fig. 2.
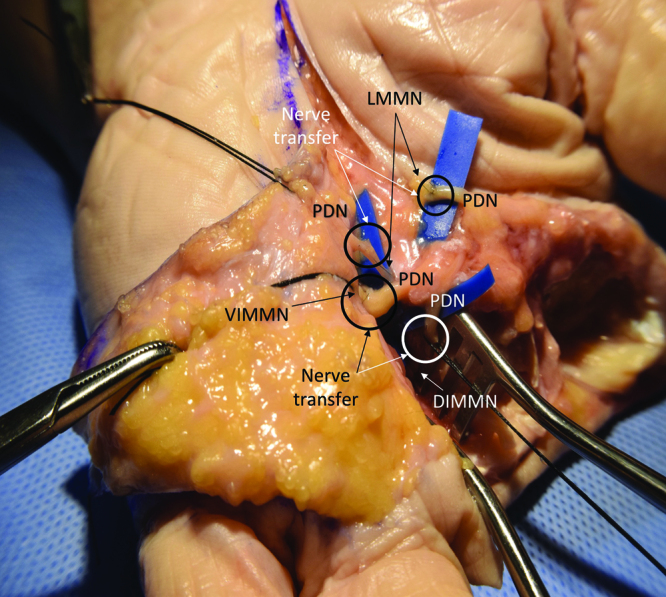
Radial hand amputation, TMR cadaver dissection, following completion of 4 TMR nerve transfers. LMMN, lumbrical muscle motor nerve; VIMMN, volar interosseus muscle motor nerve; PDN, proper digital nerve; DIMMN, dorsal interosseus muscle motor nerve.
Footnotes
Published online 28 May 2021.
Disclosure: Drs Eberlin and Valerio are Consultants for AxoGen Inc., Integra Lifesciences Inc., and Checkpoint Inc. All the other authors have nothing to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Cheesborough JE, Smith LH, Kuiken TA, et al. Targeted muscle reinnervation and advanced prosthetic arms. Semin Plast Surg. 2015; 29:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheesborough JE, Souza JM, Dumanian GA, et al. Targeted muscle reinnervation in the initial management of traumatic upper extremity amputation injury. Hand (N Y). 2014; 9:253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valerio IL, Dumanian GA, Jordan SW, et al. Preemptive treatment of phantom and residual limb pain with targeted muscle reinnervation at the time of major limb amputation. J Am Coll Surg. 2019; 228:217–226. [DOI] [PubMed] [Google Scholar]
- 4.Dumanian GA, Potter BK, Mioton LM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: A randomized clinical trial. Ann Surg. 2019; 270:238–246. [DOI] [PubMed] [Google Scholar]
- 5.Alexander JH, Jordan SW, West JM, et al. Targeted muscle reinnervation in oncologic amputees: Early experience of a novel institutional protocol. J Surg Oncol. 2019; 120:348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuiken TA, Barlow AK, Hargrove L, et al. Targeted muscle reinnervation for the upper and lower extremity. Tech Orthop. 2017; 32:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuiken TA, Li G, Lock BA, et al. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA. 2009; 301:619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowen JB, Wee CE, Kalik J, et al. Targeted muscle reinnervation to improve pain, prosthetic tolerance, and bioprosthetic outcomes in the amputee. Adv Wound Care (New Rochelle). 2017; 6:261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen A, Yao J, Kuiken T, et al. Cortical motor activity and reorganization following upper-limb amputation and subsequent targeted reinnervation. Neuroimage Clin. 2013; 3:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hargrove LJ, Miller LA, Turner K, et al. Myoelectric pattern recognition outperforms direct control for transhumeral amputees with targeted muscle reinnervation: A randomized clinical trial. Sci Rep. 2017; 7:13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapelner T, Jiang N, Holobar A, et al. Motor unit characteristics after targeted muscle reinnervation. PLoS One. 2016; 11:e0149772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y, Zhang D, Wang Y, et al. Two ways to improve myoelectric control for a transhumeral amputee after targeted muscle reinnervation: A case study. J NeuroEng Rehabil. 2018; 15: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaston RG, Bracey JW, Tait MA, et al. A novel muscle transfer for independent digital control of a myoelectric prosthesis: the starfish procedure. J Hand Surg Am.2019; 44:163.e1–163.e5.doi: 10.1016/j.jhsa.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Valerio IL, Schulz S, West JM, et al. Targeted muscle reinnervation combined with vascularized regenerative peripheral nerve interfaces. Plast Reconst Surg Glob Open. 2020; 8: e2689. [DOI] [PMC free article] [PubMed] [Google Scholar]


